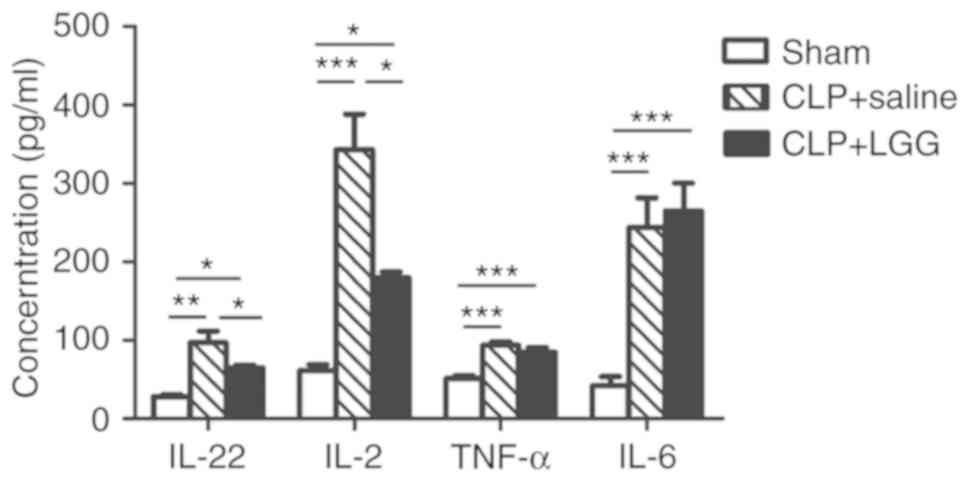
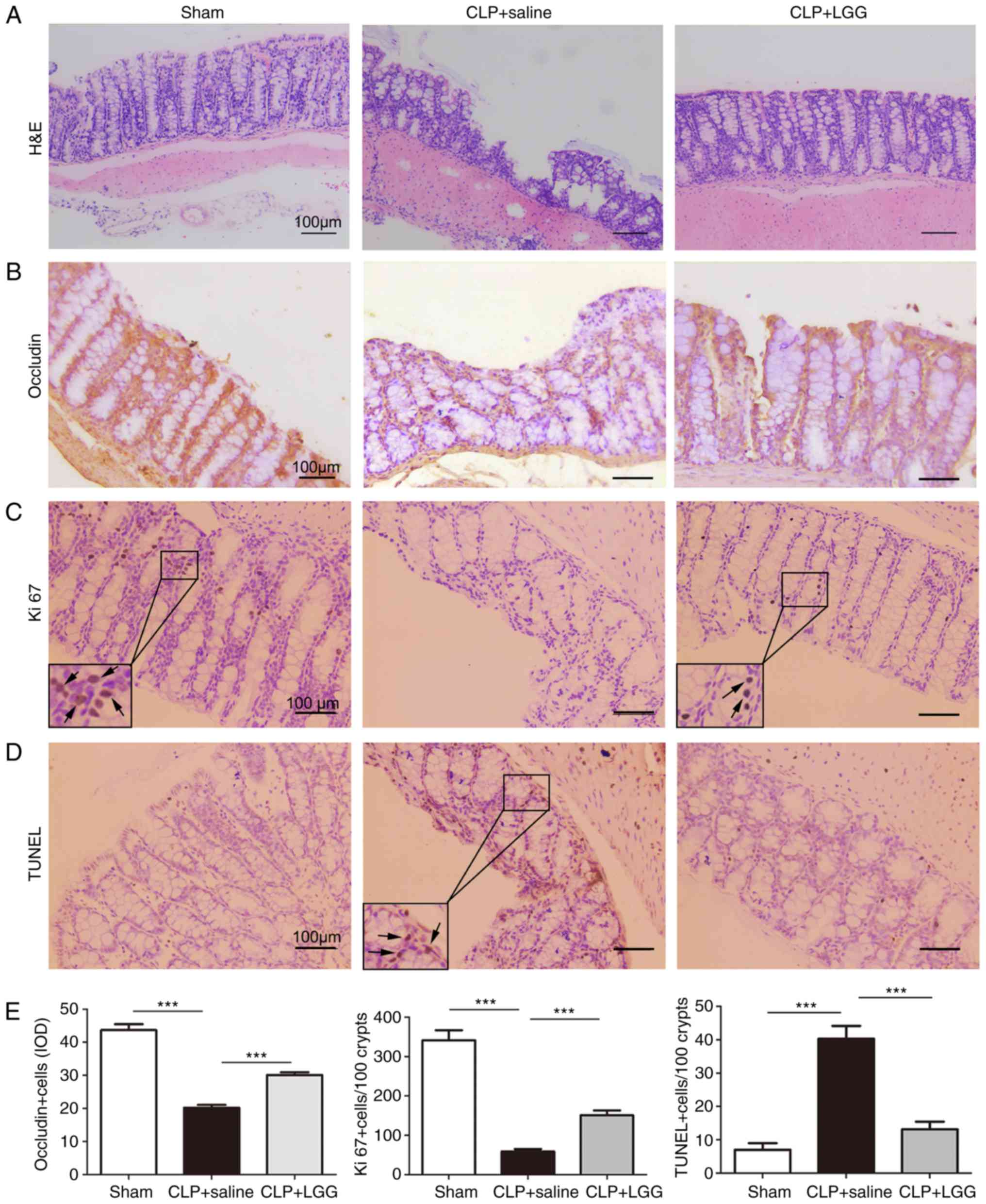
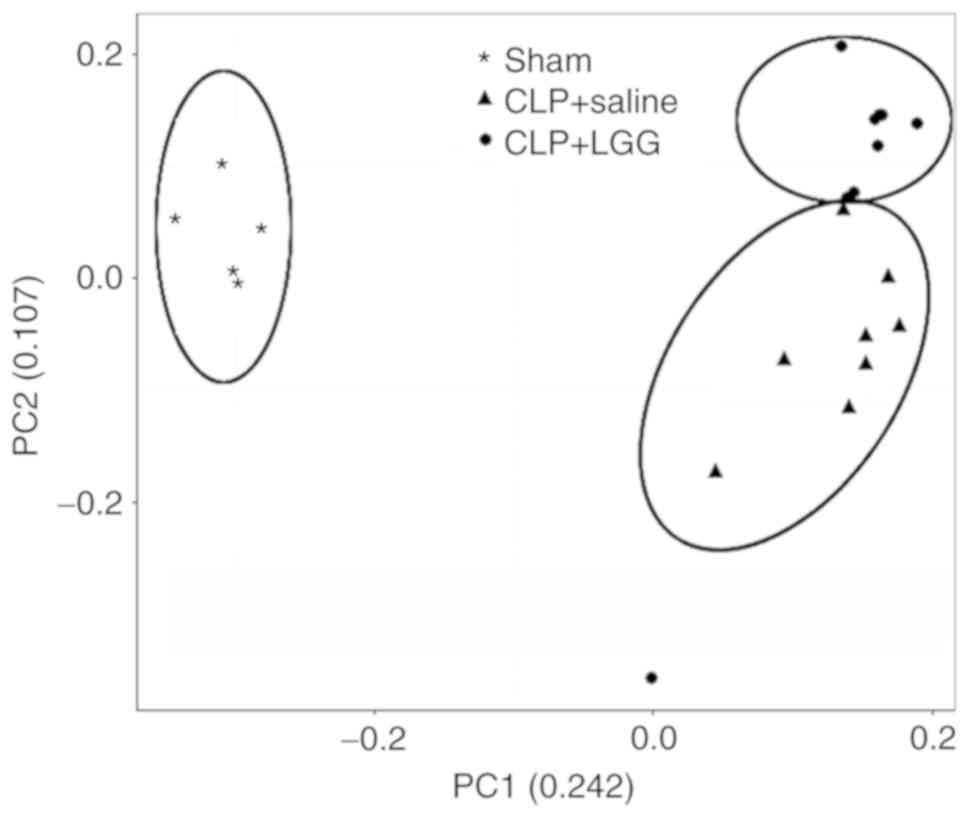
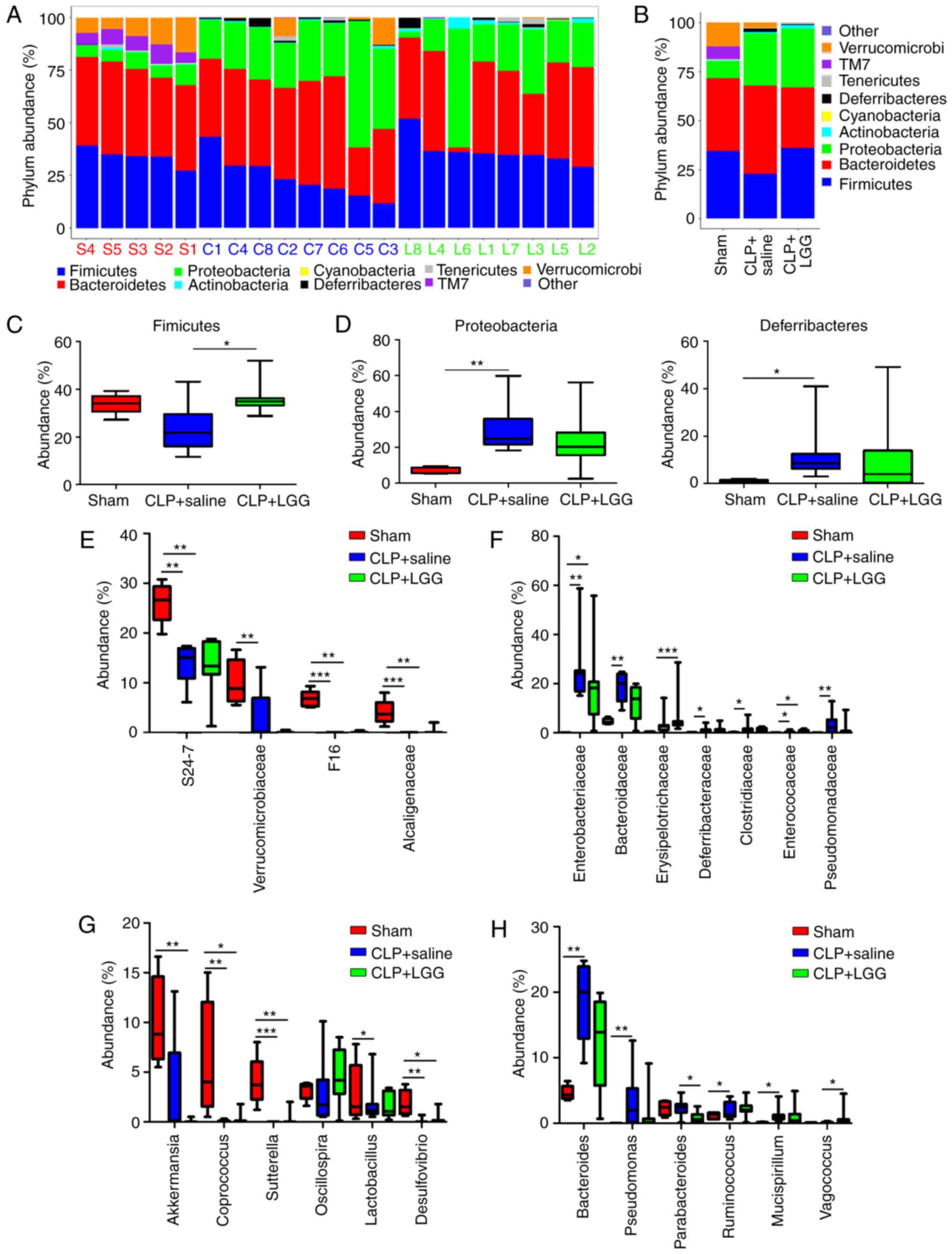
|
1
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dickson RP: The microbiome and critical
illness. Lancet Respir Med. 4:59–72. 2016. View Article : Google Scholar :
|
|
3
|
Klingensmith NJ and Coopersmith CM: The
gut as the motor of multiple organ dysfunction in critical illness.
Crit Care Clin. 32:203–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sánchez B, Delgado S, Blanco-Míguez A,
Lourenço A, Gueimonde M and Margolles A: Probiotics, gut
microbiota, and their influence on host health and disease. Mol
Nutr Food Res. 61:2017. View Article : Google Scholar
|
|
5
|
Krumbeck JA, Maldonado-Gomez MX,
Ramer-Tait AE and Hutkins RW: Prebiotics and synbiotics: Dietary
strategies for improving gut health. Curr Opin Gastroenterol.
32:110–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arboleya S, Watkins C, Stanton C and Ross
RP: Gut bifidobacteria populations in human health and aging. Front
Microbiol. 7:12042016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakai F, Hosoya T, Ono-Ohmachi A, Ukibe K,
Ogawa A, Moriya T, Kadooka Y, Shiozaki T, Nakagawa H, Nakayama Y
and Miyazaki T: Lactobacillus gasseri SBT2055 induces TGF-β
expression in dendritic cells and activates TLR2 signal to produce
IgA in the small intestine. PLoS One. 9:e1053702014. View Article : Google Scholar
|
|
8
|
Jo SG, Noh EJ, Lee JY, Kim G, Choi JH, Lee
ME, Song JH, Chang JY and Park JH: Lactobacillus curvatus WiKim38
isolated from kimchi induces IL-10 production in dendritic cells
and alleviates DSS-induced colitis in mice. J Microbiol.
54:503–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Besselink MG, van Santvoort HC, Buskens E,
Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen
TL, van Ramshorst B, Witteman BJ, et al: Probiotic prophylaxis in
predicted severe acute pancreatitis: A randomised, double-blind,
placebo-controlled trial. Lancet. 371:651–659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Xu K, Gui Q, Chen Y, Chen D and
Yang Y: Probiotic pre-administration reduces mortality in a mouse
model of cecal ligation and puncture-induced sepsis. Exp Ther Med.
12:1836–1842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
von Martels JZ, Sadaghian Sadabad M,
Bourgonje AR, Blokzijl T, Dijkstra G, Faber KN and Harmsen HJM: The
role of gut microbiota in health and disease: In vitro modeling of
host-microbe interactions at the aerobe-anaerobe interphase of the
human gut. Anaerobe. 44:3–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das S, Dash HR, Mangwani N, Chakraborty J
and Kumari S: Understanding molecular identification and polyphasic
taxonomic approaches for genetic relatedness and phylogenetic
relationships of microorganisms. J Microbiol Methods. 103:80–100.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caporaso JG, Kuczynski J, Stombaugh J,
Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich
JK, Gordon JI, et al: QIIME allows analysis of high-throughput
community sequencing data. Nat Methods. 7:335–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glöckner FO, Yilmaz P, Quast C, Gerken J,
Beccati A, Ciuprina A, Bruns G, Yarza P, Peplies J, Westram R and
Ludwig W: 25 years of serving the community with ribosomal RNA gene
reference databases and tools. J Biotechnol. 261:169–176. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Albenberg L, Esipova TV, Judge CP,
Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis
JD, Li H, et al: Correlation between intraluminal oxygen gradient
and radial partitioning of intestinal microbiota. Gastroenterology.
147:1055–1063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winter SE, Winter MG, Xavier MN,
Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J,
Lawhon SD, et al: Host-derived nitrate boosts growth of E. coli in
the inflamed gut. Science. 339:708–711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lupp C, Robertson ML, Wickham ME, Sekirov
I, Champion OL, Gaynor EC and Finlay BB: Host-mediated inflammation
disrupts the intestinal microbiota and promotes the overgrowth of
Enterobacteriaceae. Cell Host Microbe. 2:119–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honda K and Littman DR: The microbiome in
infectious disease and inflammation. Annu Rev Immunol. 30:759–795.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grootjans J, Lenaerts K, Derikx JP,
Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH
and Buurman WA: Human intestinal ischemia-reperfusion-induced
inflammation characterized: Experiences from a new translational
model. Am J Pathol. 176:2283–2291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alverdy JC, Laughlin RS and Wu L:
Influence of the critically ill state on host-pathogen interactions
within the intestine: Gut-derived sepsis redefined. Crit Care Med.
31:598–607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mittal R and Coopersmith CM: Redefining
the gut as the motor of critical illness. Trends Mol Med.
20:214–223. 2014. View Article : Google Scholar :
|
|
23
|
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini
V, Mardis ER and Gordon JI: An obesity-associated gut microbiome
with increased capacity for energy harvest. Nature. 444:1027–1031.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vollaard EJ and Clasener HA: Colonization
resistance. Antimicrob Agents Chemother. 38:409–414. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu K, Ogura H, Goto M, Asahara T,
Nomoto K, Morotomi M, Yoshiya K, Matsushima A, Sumi Y, Kuwagata Y,
et al: Altered gut flora and environment in patients with severe
SIRS. J Trauma. 60:126–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Boyle CJ, MacFie J, Mitchell CJ,
Johnstone D, Sagar PM and Sedman PC: Microbiology of bacterial
translocation in humans. Gut. 42:29–35. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lynch SV and Pedersen O: The human
intestinal microbiome in health and disease. N Engl J Med.
375:2369–2379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shreiner AB, Kao JY and Young VB: The gut
microbiome in health and in disease. Curr Opin Gastroenterol.
31:69–75. 2015. View Article : Google Scholar :
|
|
29
|
Donohoe DR, Garge N, Zhang X, Sun W,
O'Connell TM, Bunger MK and Bultman SJ: The microbiome and butyrate
regulate energy metabolism and autophagy in the mammalian colon.
Cell Metab. 13:517–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Furusawa Y, Obata Y, Fukuda S, Endo TA,
Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et
al: Commensal microbe-derived butyrate induces the differentiation
of colonic regulatory T cells. Nature. 504:446–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Everard A, Belzer C, Geurts L, Ouwerkerk
JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne
NM, et al: Cross-talk between Akkermansia muciniphila and
intestinal epithelium controls diet-induced obesity. Proc Natl Acad
Sci USA. 110:9066–9071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie G, Wang X, Liu P, Wei R, Chen W,
Rajani C, Hernandez BY, Alegado R, Dong B, Li D and Jia W:
Distinctly altered gutmicrobiota in the progression of liver
disease. Oncotarget. 7:19355–19366. 2016.PubMed/NCBI
|