Introduction
Cardiovascular disease is a significant threat to
human health and is the second leading cause of mortality following
malignant tumors (1). Acute
myocardial infarction is one of the major causes of morbidity and
mortality in coronary artery cardiovascular disease, which may lead
to myocardial cell damage and heart dysfunction. Timely restoration
of coronary artery blood perfusion by antiplatelet and
anticoagulant therapy, percutaneous coronary intervention, and
angioplasty treatment technology are the most effective treatments
for myocardial ischemia at present (2-4).
However, the accompanying ischemia/reperfusion (I/R) injury, which
is associated with poor prognosis, may cause cell death and
aggravate myocardial injury. Therefore, methods for effectively
preventing and mitigating I/R injury have been a major topic of
discussion in the field of myocardial protection studies.
Fibroblast growth factor (FGF-21) is a member of the
FGF family (5). It regulates the
metabolism of glucose and lipids and serves important roles in
obesity, diabetes mellitus, metabolic syndrome and nonalcoholic
fatty liver disease (6,7). It may also antagonize the
development of a number of cardiovascular diseases, including
atherosclerosis, coronary heart disease, myocardial ischemia,
myocardial hypertrophy, diabetes, cardiomyopathy and hypertension
(8-11). A previous study indicated that
FGF-21 may protect the heart from ischemia (12). Other studies have suggested that
FGF21 protects the heart from myocardial ischemia and I/R injury by
activating the protein kinase B (AKT) and 5-adenosine
monophosphate-activated protein kinase (AMPK) signaling pathway
through its binding with the FGF receptor 1, and that the
underlying mechanisms are associated with anti-apoptotic and
anti-inflammatory effects, antioxidant stress, and myocardial cell
energy metabolism and mitochondrial function regulation, among
others (13). However, the
mechanisms underlying the protective effect of FGF-21 are not
completely explained by these aforementioned interactions.
Autophagy is a process that involves the degradation
and digestion of mature proteins and cell structures through
lysosomal degradation pathways, to maintain cellular homeostasis
and organelle recycling (13,14). In selective autophagy, targets are
recognized by autophagy receptors, including Sequestosome 1 (p62),
that initiate membrane recruitment through interaction with
microtubule-associated proteins 1A/1B light chain 3A (LC3) and
other autophagy-associated proteins. Zhao et al (15) demonstrated that acetylcholine may
increase the tolerance of the myocardium to hypoxia/reoxygenation
(H/R) injury by activating autophagy though the AMPK-mechanistic
target of rapamycin (mTOR) pathway. Previous studies have indicated
that, by inducing autophagy, FGF-21 may ameliorate nonalcoholic
fatty liver disease and alleviate inflammation and fibrosis in type
1 diabetic mouse heart (7,16).
Rühlmann et al (17)
suggested that FGF-21 exerts neuroprotective effects on
apolipoprotein E-knockout (ApoE-KO) mice with long-term restricted
caloric intake by prolonged activation of the AMPK-mTOR signaling
pathway. However, the role of FGF-21 in autophagy during H9c2
cardiomyocyte H/R remains unclear. The present study aimed to
investigate the effect of FGF-21 on H9c2 cardiomyocyte injury
induced by H/R and the mechanism associated with changes in
autophagy. Cultured H9c2 cardiomyocytes subjected to hypoxia were
treated with a vehicle or FGF-21 during reoxygenation. The
viability of H9c2 rat cardiomyocytes was measured using Cell
Counting Kit-8 (CCK-8) and trypan blue exclusion assays. The
contents of creatine kinase (CK) and CK isoenzymes (CK-MB), cardiac
troponin I (cTnT), cardiac troponin T (cTnI) and lactate
dehydrogenase (LDH) in culture medium were detected with a CK,
CK-MB, cTnT, cTnI and LDH assay kits. The protein levels were
examined by western blot analysis. Autophagic flux was detected by
Ad-mCherry-GFP-LC3B autophagy fluorescent adenovirus reagent.
Materials and methods
Cell culture
H9c2 rat cardiomyocyte cells were purchased from the
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China). They were cultured in Dulbecco's
modified Eagle's medium (DMEM; HyClone, Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified incubator supplied with 5% CO2. When the
cells grew to 70-80% confluence, they were treated with serum-free
DMEM for 12 h for synchronization, following which the experiments
were conducted.
H/R cell model and drug
administration
Serum medium was removed from the synchronized
cardiomyocytes, they were rinsed three times with PBS, deoxygenated
Hanks' balanced salt solution was added (69 mM NaCl, 5 mM KCl, 0.3
mM KH2PO4, 4 mM NaHCO3, 0.3 mM
Na2HPO4, 0.5 mM MgCl2, 0.4 mM
MgSO4 and 1.3 mM CaCl2) and the culture flask
or plate (6-, 24- or 96-well plate) was maintained for 120 min at
37°C in an oxygen-deficient container (95% N2 and 5%
CO2, O2 concentration ≤1%). Then, the
solution was replaced with DMEM supplemented with 10% FBS in a
suitable environment (5% CO2, 37°C) supplemented with 0,
12.5, 25, 50 or 100 ng/ml FGF-21 (ProteinTech Group, Inc., Chicago,
IL USA) with or without 5 mmol/l 3-methyladenine (3-MA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and incubated at
37°C for 60 min for completion of the H/R model.
Viability assays
The viability of H9c2 rat cardiomyocytes was
measured using CCK-8 (Vazyme Biotech Co., Ltd., Nanjing, China) and
trypan blue exclusion assays (Beyotime Institute of Biotechnology,
Haimen, China). A total of 2×103 cells were seeded into
each well of a 96-well plate following H/R treatment. In accordance
with the protocol of the manufacturer, 10 µl CCK-8 solution
was added and the cells were incubated at 37°C for 4 h, following
which the optical density (OD) was measured using a microplate
reader (λ=450 nm). The average OD of 5 wells was recorded,
and cell viability was calculated. The procedure was repeated at
least three times, and cells in the control group were considered
to be 100% viable. The trypan blue exclusion assay was performed as
follows: 1×106 cells were cultured in each well of a
6-well plate. Following the H/R experiment, the culture medium was
discarded and cells were washed three times with PBS, followed by
dilution with trypan blue stock in PBS (4% final concentration).
Then, 0.04% diluted trypan blue solution was added to the 6-well
plate (500 µl/well). After 3 min, the stained cells were
observed under a light microscope at ×10 magnification, the dead
cells were dyed blue and the live cells were colorless and
transparent.
Measurement of LDH, CK and CK-MB
Following the H/R experiment, the cell culture
medium was collected at the end of the reoxygenation stage, and
levels of LDH, CK and CK-MB in the cell culture were measured by
LDH, CK and CK-MB assay kits (all from Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), in accordance with the
manufacturer's protocol.
Measurement of cTnI and cTnT
Following the H/R experiment, the cell culture
medium was collected at the end of the reoxygenation stage, and
levels of cTnT and cTnI were measured with a cTnT assay kit (Roche
Diagnostics GmbH, Mannheim, Germany) and a cTnI assay kit (Roche
Diagnostics GmbH) with Roche Elecsys Analyser.
Ad-mCherry-green fluorescent protein
(GFP)-microtubule- associated proteins 1A/1B light chain 3β (LC3B)
autophagy fluorescence double labeling adenovirus autophagy
assay
Ad-mCherry-GFP-LC3B autophagy fluorescent adenovirus
reagent (Beyotime Institute of Biotechnology) at a multiplicity of
infection of 80 was added to H9c2 cardiomyocytes that were cultured
in 24-well plates (50 µl/well). After 12 h transfection of
8×106 pfu (Plaque forming units) adenovirus, H/R was
performed. Following establishment of the H/R model, the expression
of mCherry and GFP was visualized with confocal fluorescence
microscopy at ×100 magnification. Autophagy flux was evaluated by
calculating the number of yellow and red puncta (18) with Image J (version 1.8.0;
National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Cells were rinsed with cold PBS twice and lysed in
radioimmunoprecipitation buffer containing phenylmethylsulfonyl
fluoride (all from Beyotime Institute of Biotechnology). Cell
protein concentrations were measured using the BCA method (Beyotime
Institute of Biotechnology). A total of 20 µg of protein was
loaded on 10% SDS-PAGE gels and then transferred to polyvinylidene
fluoride membranes (Roche Diagnostics Co., Ltd., Shanghai, China).
Following blocking in 5% non-fat milk for 1 h at room temperature,
the membranes were probed with primary antibodies (all dilutions
were 1:1,000) at 4°C overnight; the following primary antibodies
were used: Anti-Beclin-1 (11306-1-AP; ProteinTech Group, Inc.),
anti-LC3 (14600-1-AP; ProteinTech Group, Inc.), anti-P62 (ab5641;
Epitomics; Abcam, Cambridge, UK), anti-phosphatidylinositol
3-kinase (PI3K) catalytic subunit type 3 (Vps34; 20584-1-AP;
ProteinTech Group, Inc.), Phospho-mTOR (Ser2448) antibody (2971;
Cell Signaling Technology, Danvers, MA, USA), mTOR antibody (2972;
Cell Signaling Technology) and anti-GAPDH (13937-1-AP; ProteinTech
Group, Inc.). The membranes were then incubated for 2 h with
secondary antibodies at 37°C (HRP-conjugated Affinipure goat
anti-rabbit IgG; 1:7,000; BA1056; Wuhan Boster Biological
Technology Ltd., Wuhan, China). Bands were visualized using an ECL
chemiluminescent agent (CW0048; CWBio, Beijing, China). An
automatic chemical luminous imaging analysis system was used for
capturing images. The image analysis software ImageJ (version
1.8.0; National Institutes of Health) was used for analyzing the
results, and expression of the protein was determined relative to
that of GAPDH.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
All experimental data are expressed as mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference, as determined using one-way analysis of variance with
Tukey's range test.
Results
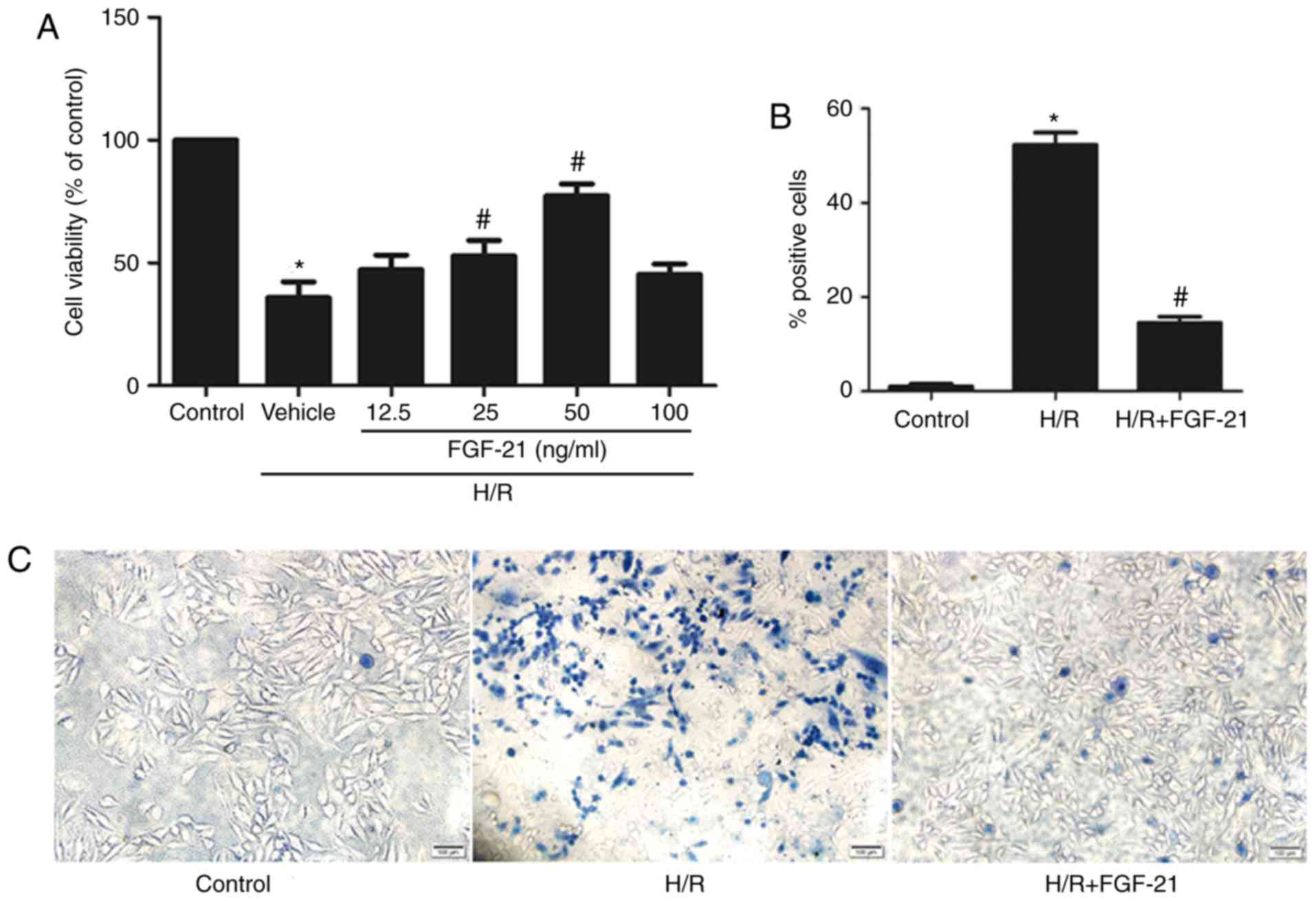
Cytoprotective effects of FGF-21 in cells
during H/R injury
After 2 h of hypoxia treatment, H9c2 cells were
incubated in 0, 12.5, 25, 50, and 100 ng/ml FGF-21 for 1 h at the
time of reoxygenation, and the viability of the cells was measured
using the CCK-8 assay. The results demonstrated that, compared with
the control group, cell viability was significantly decreased in
the H/R group; treatment with 25 or 50 ng/ml FGF-21 increased the
viability of cells with H/R [H/R vehicle group = 35.77±6.514%; H/R
+ FGF-21 (25 ng/ml) group = 52.77±6.394%; and H/R + FGF-21 (50
ng/ml) group = 77.30±4.899%; n=3; P<0.05; Fig. 1A]. These data suggest that an
appropriate concentration of FGF-21 may improve cell survival.
Subsequent experiments were conducted with 50 ng/ml FGF-21.
The trypan blue exclusion assay indicated that,
compared with the control group, the rate of damaged cells was
significantly increased in the H/R group; however, FGF-21 (50
ng/ml) significantly decreased the number of dead cells following
exposure to H/R (Fig. 1B and
C).
H/R increased levels of LDH, CK, CK-MB, cTnT and
cTnI in the cell culture medium at the end of reoxygenation, which
was rescued by FGF-21 (Fig. 2).
These results suggest that FGF-21 significantly alleviates
H/R-induced H9c2 myocardial cell injury.
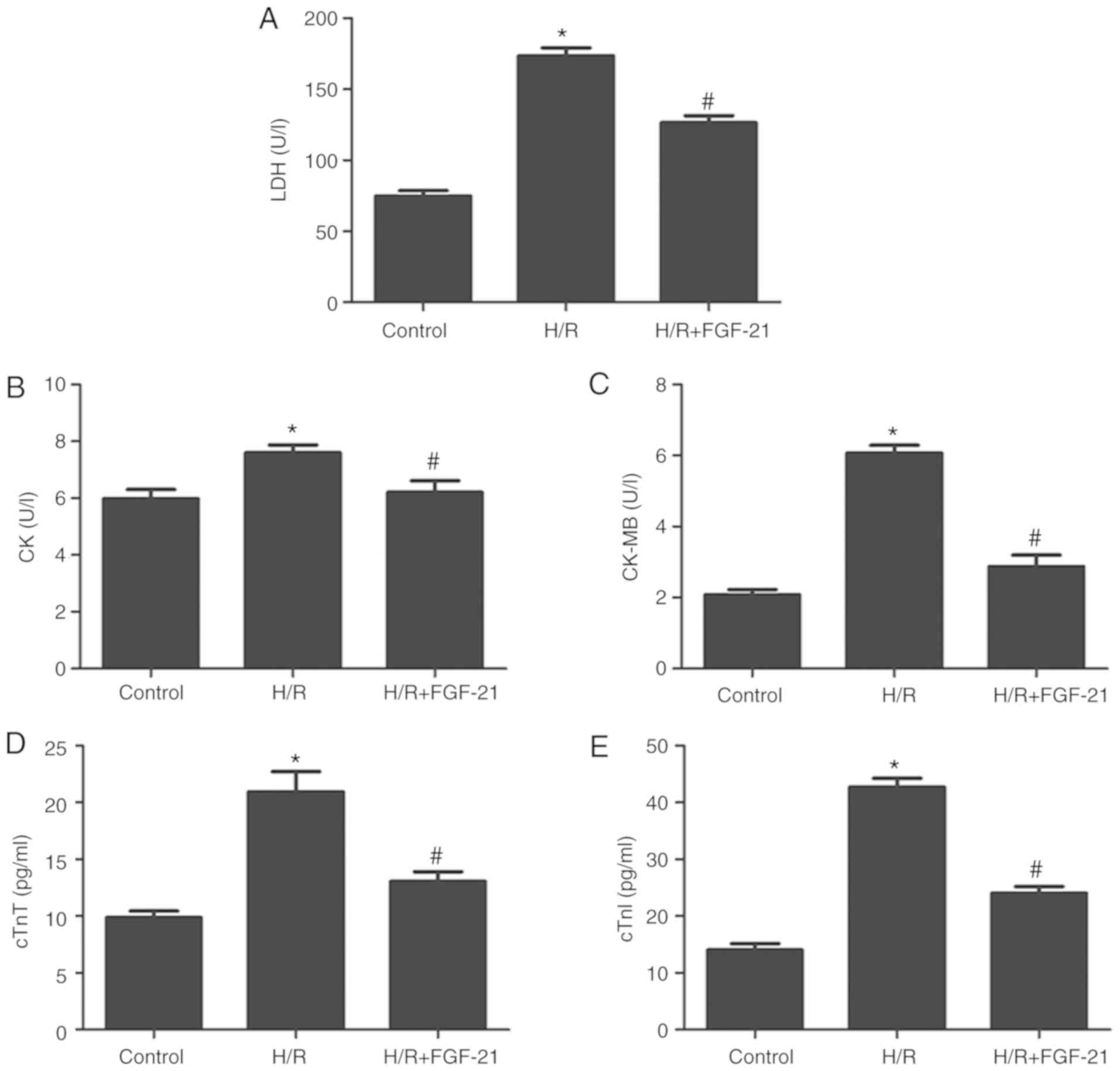 | Figure 2Effects of FGF-21 on signatures of
cell injury. Cell injury was measured by (A) LDH, (B) CK, (C)
CK-MB, (D) cTnT and (E) cTnI release. *P<0.05 vs.
control group and #P<0.05 vs. H/R group. Values are
expressed as mean ± standard deviation. Experiments were repeated
in triplicate. FGF-21, fibroblast growth factor-21; H/R,
hypoxia/reoxygenation; LDH, lactate dehydrogenase; CK, creatine
kinase; CK-MB, creatine kinase isoenzymes; cTnT, cardiac troponin
T; cTnI, cardiac troponin I. |
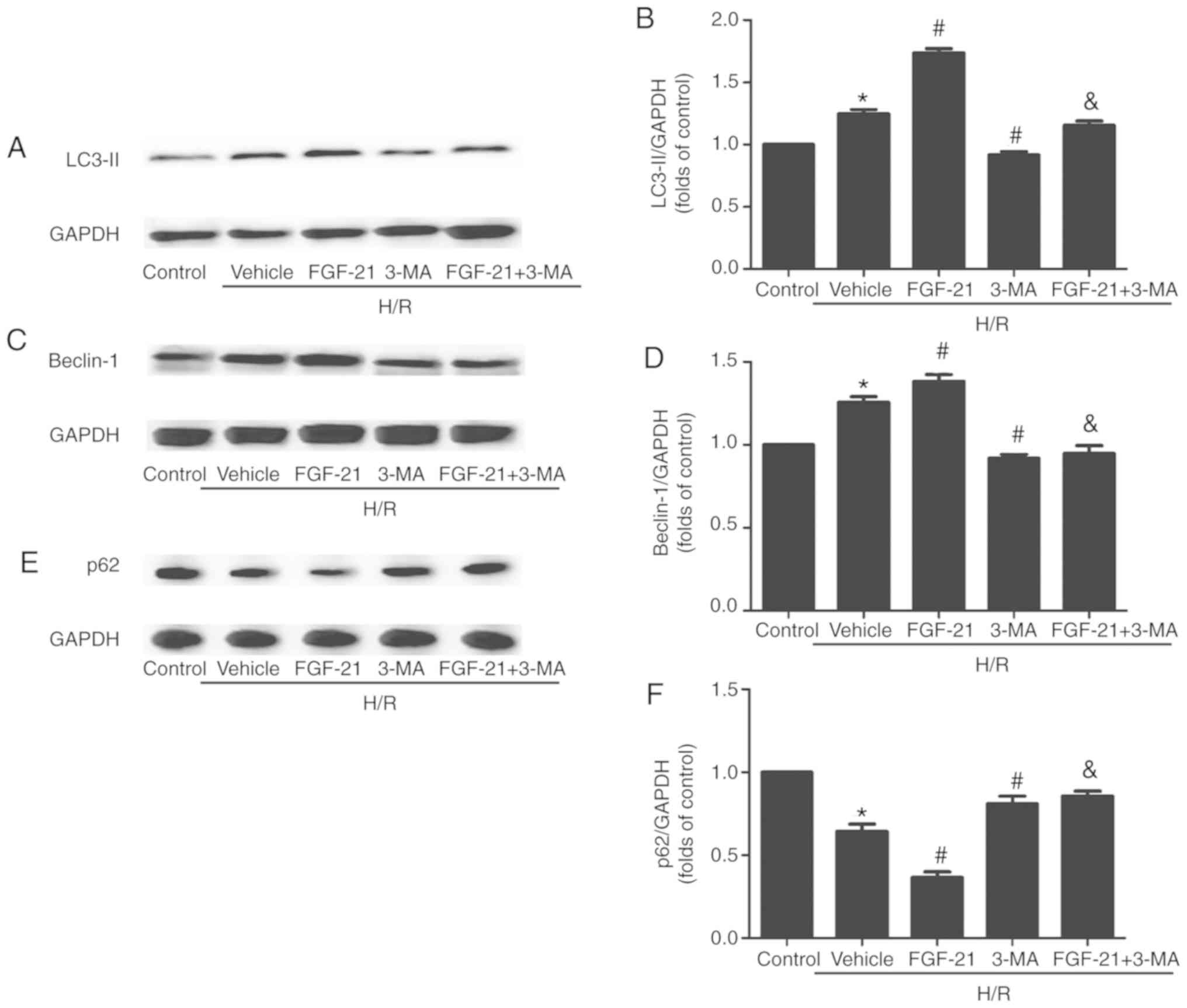
FGF-21 enhances autophagic flux of
cardiomyocytes during H/R
The western blot analysis results demonstrated that,
compared with the control group, the formation of lipid modified
microtubule-associated proteins 1A/1B light chain (LC3-II) and
expression levels of the Beclin-1 protein in cardiomyocytes were
significantly increased in the H/R group. The expression of p62 was
significantly decreased, compared with the control group. p62 is a
receptor that facilitates selective autophagy by interacting
simultaneously with cargoes and the LC3 protein on the
autophagosome to maintain cellular homeostasis. The formation of
LC3-II and expression levels of Beclin-1 were additionally
increased and the expression of p62 was also additionally decreased
in the FGF21-treated group compared with those in the H/R group.
However, this effect was partly abolished by 3-MA. Following
addition of the autophagic inhibitor 3-MA, the formation of LC3-II
and expression levels of Beclin-1 were downregulated and the
expression of p62 was upregulated compared with those in the
FGF-21-treated group (Fig.
3).
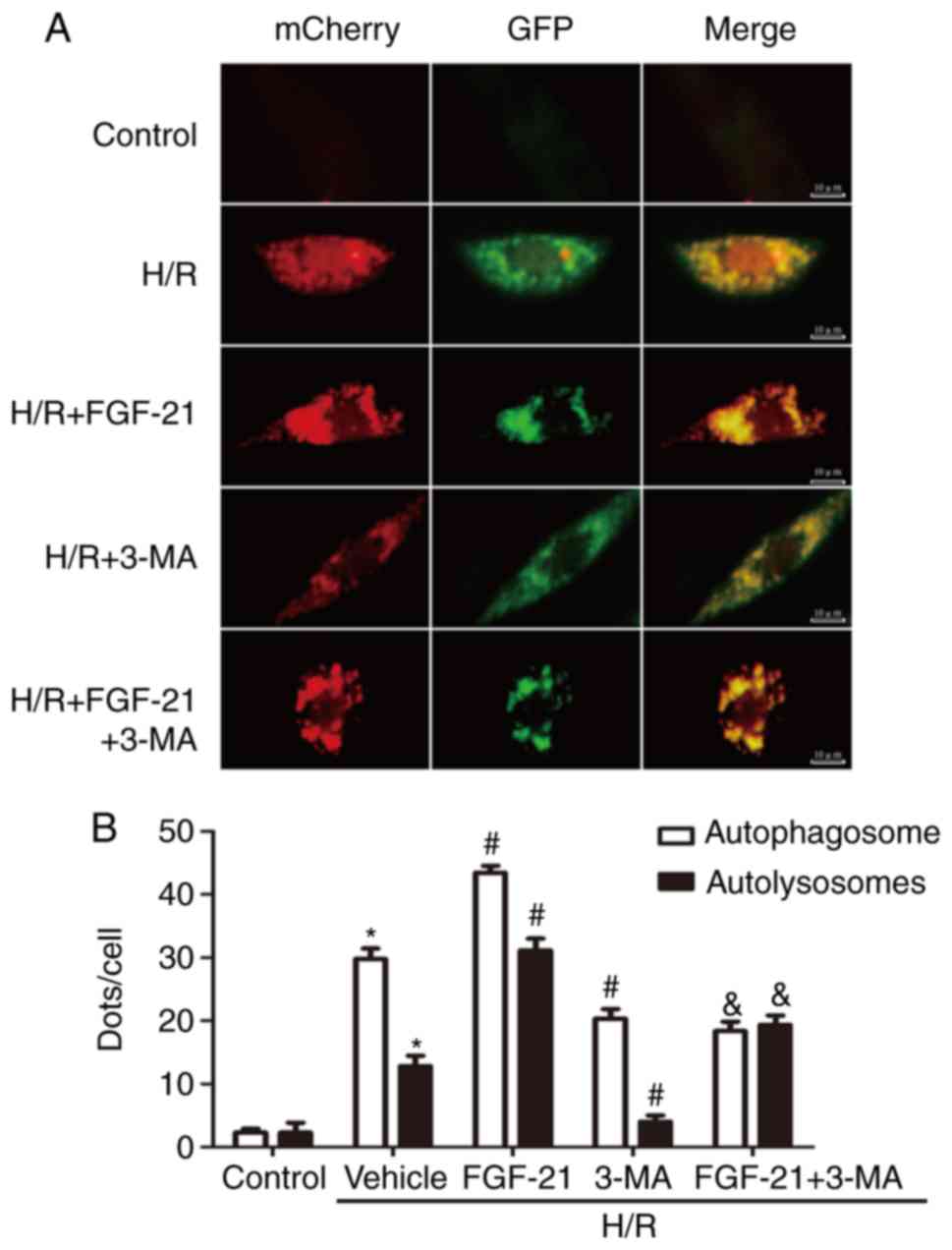
To monitor autophagic flux, tandem fluorescent
mCherry-GFP-LC3B was performed on H9c2 cardiomyocytes
(Ad-LC3-H9c2). Normal Ad-LC3-H9c2 cells exhibited basal autophagy
with few autophagosomes and autolysosomes. Ad-LC3-H9c2 cells
subjected to H/R exhibited increased autophagosomes and few
autolysosomes, suggesting that autophagic flux was increased during
myocardial H/R. In the FGF-21-treated group, Ad-LC3-H9c2 cells
subjected to H/R possessed more autophagosomes and autolysosomes
compared with those in the untreated group, indicating that FGF-21
treatment additionally enhanced autophagic flux. However,
co-treatment with FGF-21 and the autophagic inhibitor 3-MA
decreased autophagosomes and autolysosomes compared with those in
the FGF-21-treated group. In addition, in the 3-MA-treated group,
Ad-LC3-H9c2 cells subjected to H/R exhibited fewer autophagosomes
and autolysosomes compared with that in the untreated group,
indicating that FGF-21 treatment attenuated autophagic flux
(Fig. 4). These data suggest that
FGF-21 induced upregulation of autophagic flux during H/R
injury.
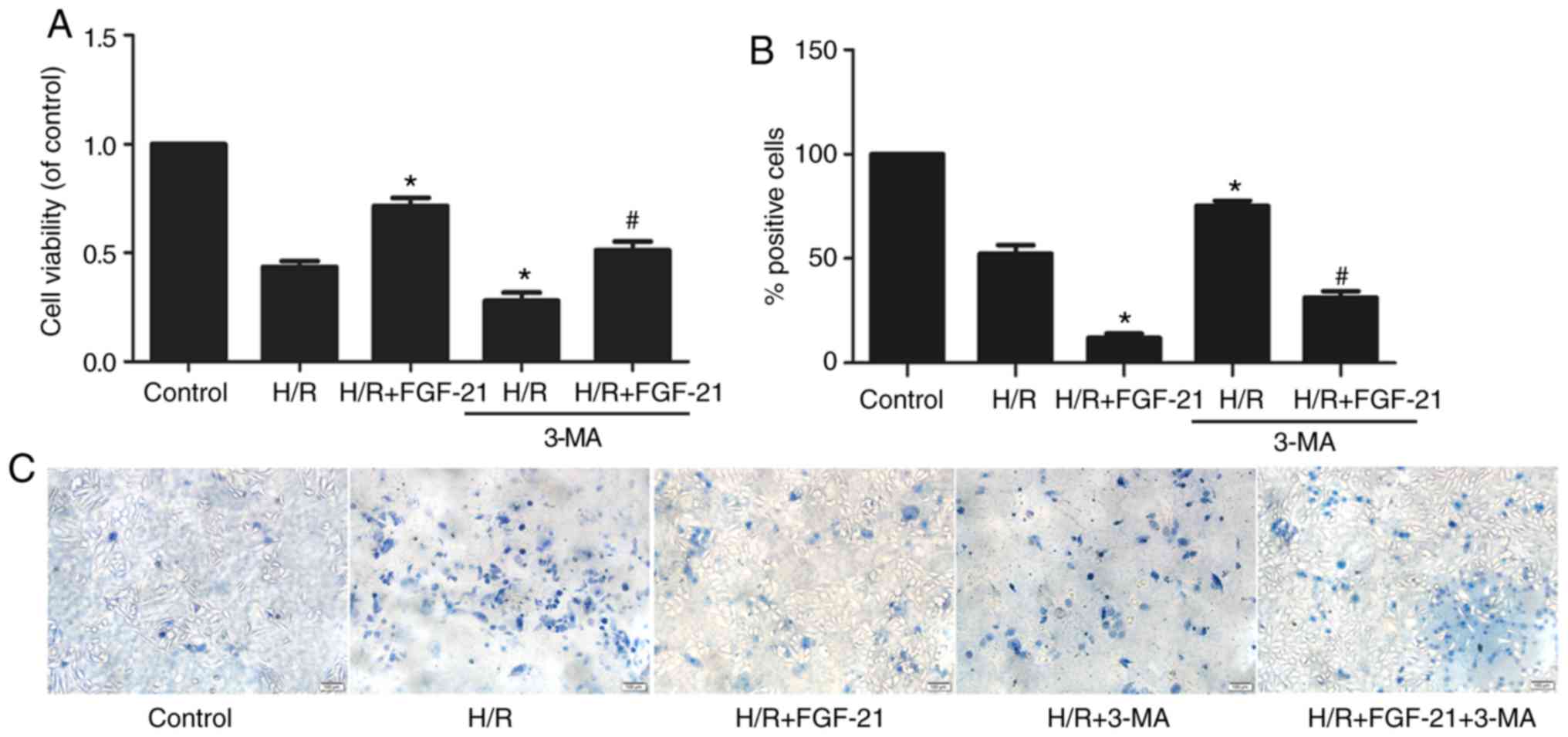
FGF-21-mediated autophagy enhances cell
survival
CCK-8 and trypan blue exclusion assays revealed that
3-MA partly abolished the effect of FGF-21 on the myocardial
viability rate in the H/R + FGF-21 group (Fig. 5). These results suggest that 3-MA
may reverse the protective effect of FGF-21 against H9c2
cardiomyocyte survival during H/R injury.
Compared with the H/R + FGF-21 group, levels of LDH,
CK, CK-MB, cTnT and cTnI in cell culture medium at the end of
reoxygenation in the H/R + FGF-21+3-MA group were significantly
increased (Fig. 6), which
additionally supports the hypothesis that 3-MA may reverse the
protective effect of FGF-21 against H9c2 cardiomyocyte H/R
injury.
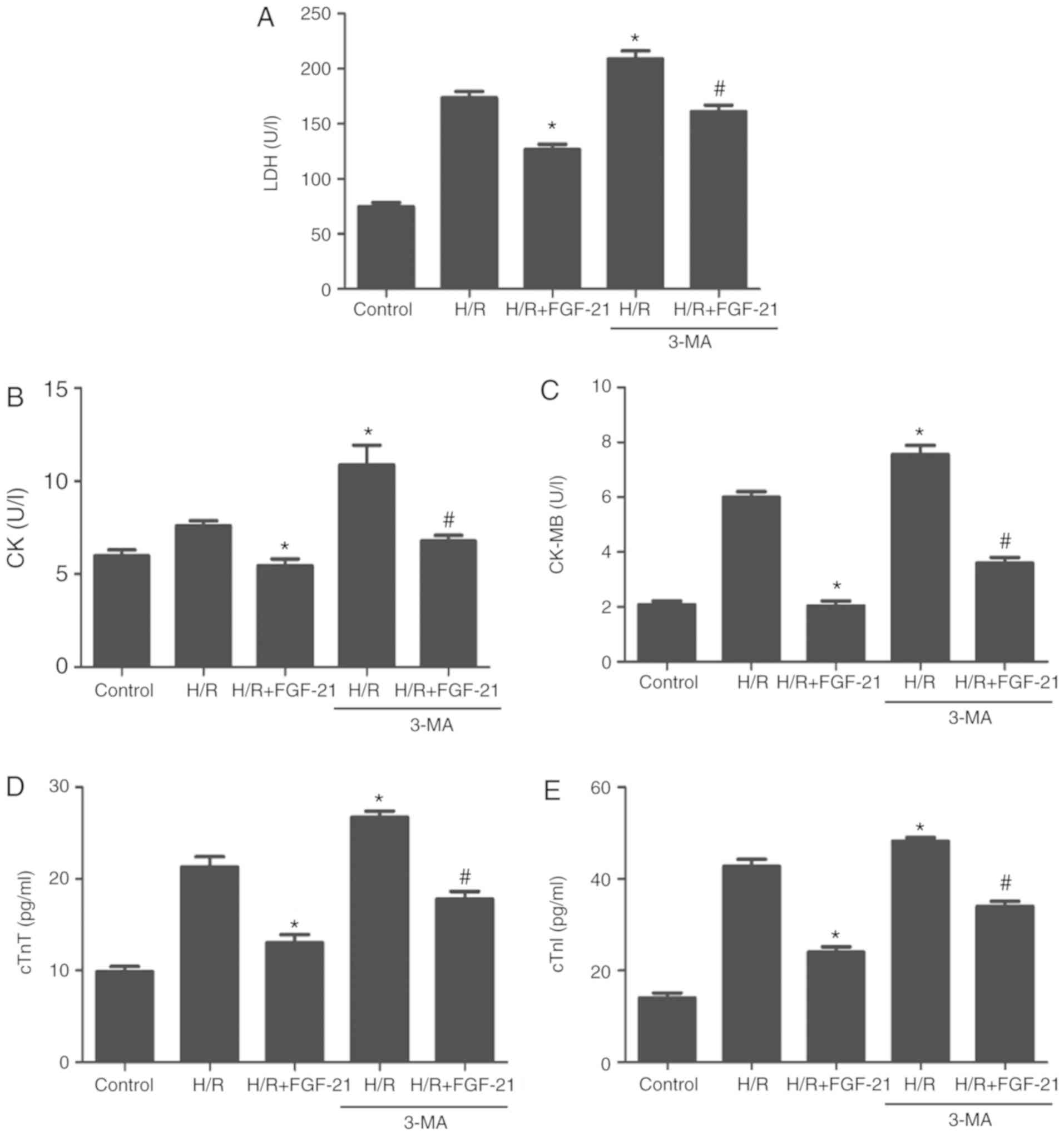 | Figure 6Effects of autophagy on
FGF-21-induced decrease of signatures of cell injury. Cell injury
was measured by (A) LDH, (B) CK, (C) CK-MB, (D) cTnT and (E) cTnI
release. *P<0.05 vs. H/R group and
#P<0.05 vs. H/R + FGF-21 group. Values are expressed
as mean ± standard deviation. Experiments were repeated in
triplicate. FGF-21, fibroblast growth factor-21; H/R,
hypoxia/reoxygenation; 3-MA, 3-methyladenine; LDH, lactate
dehydrogenase; CK, creatine kinase; CK-MB, creatine kinase
isoenzymes; cTnT, cardiac troponin T; cTnI, cardiac troponin I. |
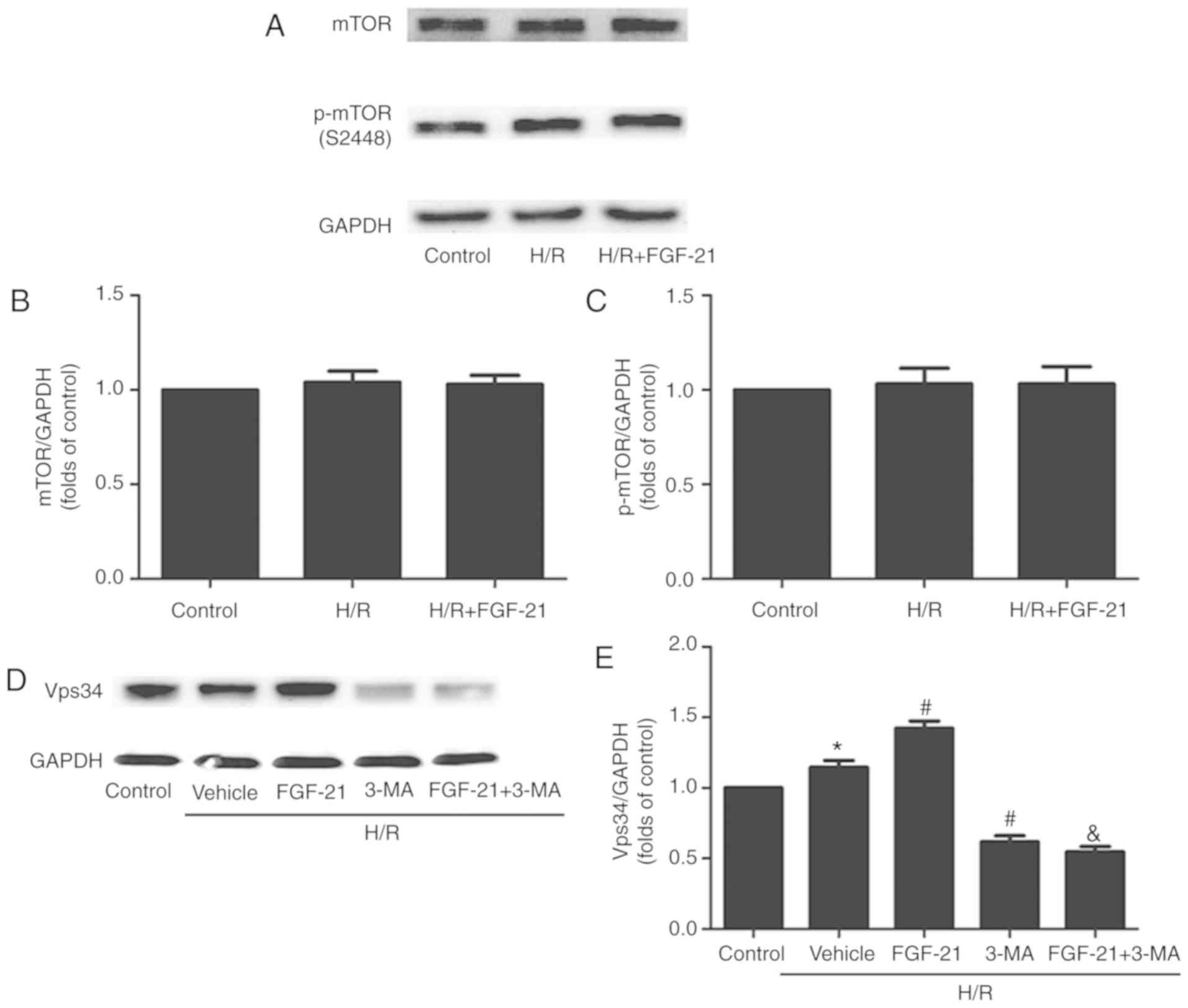
Effect of FGF-21 on H/R-induced changes
in mTOR and Vps34 signaling
Acetylcholine may increase the tolerance of the
myocardium to H/R injury by activating autophagy through the
AMPK-mTOR pathway (15). A
previous study demonstrated that FGF21 exerted neuroprotective
effects on ApoE-KO mice with long-term restricted caloric intake by
prolonging activation of the AMPK-mTOR signaling pathway (17). The present study examined the
expression of the phosphorylated (p)-mTOR protein and total mTOR
protein in each group of cells: No significant differences between
the control, H/R and H/R + FGF-21 groups were observed (Fig. 7A-C), indicating that
FGF-21-induced autophagy in H/R cardiomyocytes may not occur
through the mTOR signaling pathway.
The Beclin-1/Vps34 complex formed by Vps34 and
Beclin-1 may modulate the formation of the autophagic membrane. The
present study suggested that FGF-21 increased the expression level
of the Beclin-1 protein in H/R cardio-myocytes (Fig. 3). The autophagy inhibitor 3-MA
used in the present study is an inhibitor of Vps34; therefore,
expression levels of the Vps34 protein were examined in each group.
The results demonstrated that, compared with the control group, the
expression levels of the Vsp34 protein were significantly increased
in the H/R group, and it was additionally increased in the H/R +
FGF-21 group. However, co-treatment with FGF-21 and the autophagic
inhibitor 3-MA decreased the expression level of the Vsp34 protein
compared with that in the H/R + FGF-21 group. In addition, compared
with the H/R group, the expression level of the Vsp34 protein was
significantly attenuated in the H/R + 3-MA group (Fig. 7D and E). These results suggest
that the Vps34 protein serves a role in FGF-21-enhanced autophagy
in H/R cardiomyocytes.
Discussion
Cardiomyocyte H/R models are designed to mimic in
vivo myocardial I/R. At present, a number of previous studies
have used this cell model for studying the mechanism of H/R injury
(19-21). In the present study, compared with
the control group, the survival rate of cardiomyocytes in the H/R
group was significantly decreased and levels of CK, CK-MB, cTnT,
cTnI and LDH were significantly increased in the culture medium,
indicating that the H/R model was successfully established.
Numerous studies have demonstrated that levels of
autophagy increase in the heart during I/R in animal models and in
isolated cardiomyocytes during H/R (22,23). Whether autophagy has a protective
or deleterious role in the I/R injury process in the heart is
unclear at present. Low levels of autophagy triggered by
mild-to-moderate hypoxia or ischemia are protective and appear to
prevent activation of apoptosis by degrading and removing damaged
mitochondria (24,25). It has also been suggested that
autophagy enhances and exacerbates myocardial injury during
reperfusion, indicating that excessive autophagy is detrimental to
the heart (14). Ling et
al (26) identified that H/R
blocked autophagic flux in cardiomyocytes, causing accumulation of
autophagosomes. However, the experiments in the present study
indicated that autophagic flux was improved in cardiomyocytes
during H/R, which may be associated with the duration of the H/R
stages. In previous studies, the time intervals selected for
establishing the H/R models were varied (23,27,28); for example, each stage of the
hypoxia and reoxygenation model described by Ling et al
(26) was 3 h. In our preliminary
experiment, the highest survival rate was observed in the H9c2
cardiomyocytes treated with hypoxia for 2 h and reoxygenation for 1
h; therefore, the 2/1 h model was selected. The results from this
model indicated that prolonged H/R may inhibit cardiomyocyte
autophagic flux. Besides, in the model in the present study, the
application of 3-MA during reoxygenation inhibited autophagic flux,
aggravated cell damage and increased cell death, indicating that
reoxygenation-induced autophagic flux in cardiomyocytes may occur
as a compensatory response to H/R injury and serve a protective
role in cells.
Previous studies have suggested that several FGFs
may protect the myocardium from ischemic and/or I/R injury: Palmen
et al (29) identified
that FGF-1 may enhance ischemic tolerance of the myocardium,
promote recovery of cardiac function following I/R injury and
decrease the level of myocardial cell death; in addition, earlier
studies by our group have detected that FGF-2 may antagonize
myocardial apoptosis, decrease the area of infarction, ameliorate
impaired heart function, improve arrhythmia and protect
cardiomyocytes against myocardial ischemia and I/R injury (30-32). Singla et al (33) revealed that FGF-9 may protect mice
with diabetes from myocardial infarction. The cardioprotective
effects of FGF-21 have recently attracted considerable attention: A
previous study has identified that the myocardial infarct size is
significantly increased in FGF-21-KO mice compared with that in
wild-type mice (34). In
addition, injection of the FGF-21 adenovirus expression vector
(Ad-FGF-21) into wild-type mice skeletal muscle with myocardial
infarction increased the density of capillaries in the infarct
zone, decreased myocardial apoptosis and improved the ventricular
systolic and diastolic function (35). Upon injection of FGF-21 small
interfering RNA, the study identified that the aforementioned
myocardial protective effect of FGF-21 was reversed (12). In addition, intravenous injection
of FGF-21 in FGF-21-KO mice antagonized myocardial apoptosis
induced by I/R, decreased the infarct size and improved cardiac
function (34). The effect of
exogenous FGF-21 on infarct size and cardiac function was also
demonstrated in an isolated heart perfusion model (36). Cell experiments have also
suggested that exogenous FGF-21 may alleviate morphological changes
in H9c2 cardiomyocytes, depress nuclear fragmentation and decrease
the rate of apoptosis (37).
Underlying mechanisms include anti-apoptosis activities, regulation
of energy metabolism, antioxidant stress and regulation of
mitochondrial function (34-39). Consistent with the results of a
previous study (11), the present
study identified that FGF-21 attenuated cardiomyocyte H/R injury
and that co-treatment with FGF-21 and 3-MA during reoxygenation
significantly attenuated autophagic flux and the protective effect
on cardiomyocytes, indicating that the protective effect of FGF-21
may be associated with enhanced autophagy.
Autophagy may be regulated by the Beclin-1/Vps34
complex, AMPK/mTOR, and PI3K/AKT/mTOR signaling pathways. Rühlmann
et al (17) demonstrated
that FGF-21 may serve a neuroprotective role by activating the
AMPK/mTOR signaling pathway in ApoE-KO mice with long-term
restricted caloric intake. In addition, Zhao et al (15) identified that acetylcholine
increased the tolerance of the myocardium to H/R injury, which was
activated through autophagy by the AMPK-mTOR pathway. However, in
the experimental results of the present study, no significant
change occurred in p-mTOR and mTOR proteins following the
application of FGF-21 during reoxygenation, suggesting that
FGF-21-enhanced autophagy and protection of the myocardium from H/R
injury does not occur through the mTOR pathway.
Beclin-1 was the earliest mammalian autophagy gene
identified, which is localized on human chromosome 17q21 (40). Beclin-1 is expressed in the Golgi
apparatus and primarily regulates autophagy-associated proteins
through the formation of the Vps34 complex, which localizes these
autophagy-associated proteins into the structure of precursor
precursors, thereby regulating autophagy (41). Ling et al (26) identified that the application of
3-MA or short hairpin-Beclin-1 decreased the expression of the
autophagy-associated gene Beclin-1, partly abolishing the
protective effect of polydatin against cardiomyocyte death during
H/R, and myocardial infarct size during I/R. Similar results were
also observed in the present study using 3-MA to inhibit the
expression of the autophagy-associated genes Beclin-1 and Vps34.
The results revealed that H9c2 cardiomyocyte injury was
significantly increased in the H/R + FGF-21 + 3-MA group compared
with that in the H/R + FGF-21 treatment group, suggesting that
FGF-21-associated cardioprotection occurs through the
Beclin-1/Vps34 complex pathway to enhance autophagy. However,
mechanisms of cross-talk between autophagy and Vps34 remain unclear
and should be explored in future studies.
Initiation of autophagy causes the conversion of
LC3-I to LC3-II. An increase in LC3-II band intensity and a
decrease in LC3-I expression are considered to be hallmarks of
autophagy. In the present study, the LC3-I levels were not
examined, which was a limitation. 3-MA is an autophagy inhibitor
and used widely, but Bafilomycin A may also stop autophagosomes
degradation; this reagent will be included in future studies. The
PI3K-AKT pathway has been indicated to serve a critical role in
autophagy. Previous studies have suggested that FGFs activates AKT
and increases the level of p-AKT (31,42). As AKT is a regulator of
autophagy, how FGF-21 affects p-AKT will be observed in future
studies. The aim of the present study was to investigate the
protective effect of exogenous FGF21 on the myocardium; as no
specific agonists or inhibitors of FGF21 were identified, the
protein and mRNA expression levels of FGF21 in H/R-treated H9c2
cardiomyocytes were not examined. The present study also lacks
in vivo experiments to improve understanding of the role of
FGF21 in heart disease; this will be explored in future
studies.
Funding
The present study was supported by the National
Natural Scientific Foundation of China (81670429 and 81470435), the
Educational Department of Hunan Province Foundation (13C795), the
Innovation team of Basic Medicine of University of South China and
Aid Program for Science and Technology Innovative Research Team in
Higher Educational Institutions of Human Province (2008-244).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZSJ, ZR, WX, ZHT and GHL conceived and designed the
study. WX, ZR, YZ, MHL, ZR, HQY and YMH performed the experiments.
WX, YZ and ZR wrote the paper. SLQ and ZSJ reviewed and edited the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
World Health Organization (WHO): World
health statistics 2007. WHO; Geneva: 2007
|
|
2
|
Przyklenk K, Dong Y, Undyala VV and
Whittaker P: Autophagy as a therapeutic target for
ischaemia/reperfusion injury? Concepts, controversies, and
challenges. Cardiovasc Res. 94:197–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jennings RB: Historical perspective on the
pathology of myocardial ischemia/reperfusion injury. Circ Res.
113:428–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y and Ren J: Targeting autophagy for
the therapeutic application of histone deacetylase inhibitors in
ischemia/reper-fusion heart injury. Circulation. 129:1088–1091.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Planavila A, Redondo-Angulo I and
Villarroya F: FGF21 and cardiac physiopathology. Front Endocrinol
(Lausanne). 6:1332015. View Article : Google Scholar
|
|
6
|
Cheung BM and Deng HB: Fibroblast growth
factor 21: A promising therapeutic target in obesity-related
diseases. Expert Rev Cardiovasc Ther. 12:659–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu S, Wu Y, Ye X, Ma L, Qi J, Yu D, Wei
Y, Lin G, Ren G and Li D: FGF21 ameliorates nonalcoholic fatty
liver disease by inducing autophagy. Mol Cell Biochem. 420:107–119.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanajak P, Sa-Nguanmoo P, Wang X, Liang G,
Li X, Jiang C, Chattipakorn SC and Chattipakorn N: Fibroblast
growth factor 21 (FGF21) therapy attenuates left ventricular
dysfunction and metabolic disturbance by improving FGF21
sensitivity, cardiac mitochondrial redox homoeostasis and
structural changes in pre-diabetic rats. Acta Physiol (Oxf).
217:287–299. 2016. View Article : Google Scholar
|
|
9
|
Luo F, Guo Y, Ruan G and Li X: Metformin
promotes cholesterol efflux in macrophages by up-regulating FGF21
expression: A novel anti-atherosclerotic mechanism. Lipids Health
Dis. 15:1092016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng P, Zhang F, Yu L, Lin X, He L, Li X,
Lu X, Yan X, Tan Y and Zhang C: Physiological and pharmacological
roles of FGF21 in cardiovascular diseases. J Diabetes Res.
2016:15402672016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Planavila A, Redondo-Angulo I, Ribas F,
Garrabou G, Casademont J, Giralt M and Villarroya F: Fibroblast
growth factor 21 protects the heart from oxidative stress.
Cardiovasc Res. 106:19–31. 2015. View Article : Google Scholar
|
|
12
|
Liu SQ, Tefft BJ, Roberts DT, Zhang LQ,
Ren Y, Li YC, Huang Y, Zhang D, Phillips HR and Wu YH:
Cardioprotective proteins upregulated in the liver in response to
experimental myocardial ischemia. Am J Physiol Heart Circ Physiol.
303:H1446–H1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lavandero S, Troncoso R, Rothermel BA,
Martinet W, Sadoshima J and Hill JA: Cardiovascular autophagy:
Concepts, controversies, and perspectives. Autophagy. 9:1455–1466.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao M, Sun L, Yu XJ, Miao Y, Liu JJ, Wang
H, Ren J and Zang WJ: Acetylcholine mediates AMPK-dependent
autophagic cytoprotection in H9c2 cells during
hypoxia/reoxygenation injury. Cell Physiol Biochem. 32:601–613.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Cheng Y, Gu J, Wang S, Zhou S,
Wang Y, Tan Y, Feng W, Fu Y, Mellen N, et al: Fenofibrate increases
cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and
inflammation in the hearts of Type 1 diabetic mice. Clin Sci
(Lond). 130:625–641. 2016. View Article : Google Scholar
|
|
17
|
Rühlmann C, Wölk T, Blümel T, Stahn L,
Vollmar B and Kuhla A: Long-term caloric restriction in
ApoE-deficient mice results in neuroprotection via Fgf21-induced
AMPK/mTOR pathway. Aging. 8:2777–2789. 2016. View Article : Google Scholar :
|
|
18
|
Yu P, Zhang C, Gao CY, Ma T, Zhang H, Zhou
MM, Yang YW, Yang L and Kong LY: Anti-proliferation of
triple-negative breast cancer cells with physagulide P: ROS/JNK
signaling pathway induces apoptosis and autophagic cell death.
Oncotarget. 8:64032–64049. 2017.PubMed/NCBI
|
|
19
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar :
|
|
20
|
Lin CH, Wu WS, Lin MT, Liu WP, Hsu RB and
Chang CP: Attenuating ischemia-induced H9c2 myoblasts apoptosis by
therapeutic hypothermia. Am J Med Sci. 339:258–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Au KW, Kou CY, Woo AY, Chim SS, Fung KP,
Cheng CH, Waye MM and Tsui SK: Calcyclin binding protein promotes
DNA synthesis and differentiation in rat neonatal cardiomyocytes. J
Cell Biochem. 98:555–566. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: Enhancing macroautophagy protects against ischemia/reperfusion
injury in cardiac myocytes. J Biol Chem. 281:29776–29787. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Decker RS and Wildenthal K: Lysosomal
alterations in hypoxic and reoxygenated hearts. I. Ultrastructural
and cytochemical changes. Am J Pathol. 98:425–444. 1980.PubMed/NCBI
|
|
25
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: The interplay between pro-death and pro-survival signaling
pathways in myocardial ischemia/reperfusion injury: Apoptosis meets
autophagy. Cardiovasc Drugs Ther. 20:445–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ling Y, Chen G, Deng Y, Tang H, Ling L,
Zhou X, Song X, Yang P, Liu Y, Li Z, et al: Polydatin
post-treatment alleviates myocardial ischaemia/reperfusion injury
by promoting autophagic flux. Clin Sci (Lond). 130:1641–1653. 2016.
View Article : Google Scholar
|
|
27
|
Sagrillo-Fagundes L, Assunção Salustiano
EM, Ruano R, Markus RP and Vaillancourt C: Melatonin modulates
autophagy and inflammation protecting human placental trophoblast
from hypoxia/reoxygenation. J Pineal Res. 4:e125202018. View Article : Google Scholar
|
|
28
|
Liu F, Zhang J, Qian J, Wu G and Ma Z:
Baicalin attenuates liverhypoxia/reoxygenation injury by inducing
autophagy. Exp Ther Med. 2:657–664. 2018.
|
|
29
|
Palmen M, Daemen MJ, De Windt LJ, Willems
J, Dassen WR, Heeneman S, Zimmermann R, Van Bilsen M and Doevendans
PA: Fibroblast growth factor-1 improves cardiac functional recovery
and enhances cell survival after ischemia and reperfusion: A
fibroblast growth factor receptor, protein kinase C, and tyrosine
kinase-dependent mechanism. J Am Coll Cardiol. 44:1113–1123. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang ZS, Padua RR, Ju H, Doble BW, Jin Y,
Hao J, Cattini PA, Dixon IM and Kardami E: Acute protection of
ischemic heart by FGF-2: Involvement of FGF-2 receptors and protein
kinase C. Am J Physiol Heart Circ Physiol. 282:H1071–H1080. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang ZS, Srisakuldee W, Soulet F, Bouche
G and Kardami E: Non-angiogenic FGF-2 protects the ischemic heart
from injury, in the presence or absence of reperfusion. Cardiovasc
Res. 62:154–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu MH, Li GH, Peng LJ, Qu SL, Zhang Y,
Peng J, Luo XY, Hu HJ, Ren Z, Liu Y, et al: PI3K/Akt/FoxO3a
signaling mediates cardioprotection of FGF-2 against hydrogen
peroxide-induced apoptosis in H9c2 cells. Mol Cell Biochem.
414:57–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singla DK, Singla RD, Abdelli LS and Glass
C: Fibroblast growth factor-9 enhances M2 macrophage
differentiation and attenuates adverse cardiac remodeling in the
infarcted diabetic heart. PLoS One. 10:e01207392015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu SQ, Roberts D, Kharitonenkov A, Zhang
B, Hanson SM, Li YC, Zhang LQ and Wu YH: Endocrine protection of
ischemic myocardium by FGF21 from the liver and adipose tissue. Sci
Rep. 3:27672013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joki Y, Ohashi K, Yuasa D, Shibata R, Ito
M, Matsuo K, Kambara T, Uemura Y, Hayakawa S, Hiramatsu-Ito M, et
al: FGF21 attenuates pathological myocardial remodeling following
myocardial infarction through the adiponectin-dependent mechanism.
Biochem Biophys Res Commun. 459:124–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patel V, Adya R, Chen J, Ramanjaneya M,
Bari MF, Bhudia SK, Hillhouse EW, Tan BK and Randeva HS: Novel
insights into the cardio-protective effects of FGF21 in lean and
obese rat hearts. PLoS One. 9:e871022014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chanoit G, Lee S, Xi J, Zhu M, McIntosh
RA, Mueller RA, Norfleet EA and Xu Z: Exogenous zinc protects
cardiac cells from reperfusion injury by targeting mitochondrial
permeability transition pore through inactivation of glycogen
synthase kinase-3beta. Am J Physiol Heart Circ Physiol.
295:H1227–H1233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cong WT, Ling J, Tian HS, Ling R, Wang Y,
Huang BB, Zhao T, Duan YM, Jin LT and Li XK: Proteomic study on the
protective mechanism of fibroblast growth factor 21 to
ischemia-reperfusion injury. Can J Physiol Pharmacol. 91:973–984.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han MM, Wang WF, Liu MY, Li DS, Zhou B, Yu
YH and Ren GP: FGF-21 protects H9c2 cardiomyoblasts against
hydrogen peroxide-induced oxidative stress injury. Yao Xue Xue Bao.
49:470–475. 2014.In Chinese. PubMed/NCBI
|
|
40
|
Aita VM, Liang XH, Murty VV, Pincus DL, Yu
W, Cayanis E, Kalachikov S, Gilliam TC and Levine B: Cloning and
genomic organization of beclin 1, a candidate tumor suppressor gene
on chromosome 17q21. Genomics. 59:59–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang S, Guo Y, Zhang W, Zhang J, Zhang Y
and Xu P: Effect of FGF-21 on implant bone defects through
hepatocyte growth factor (HGF)-mediated PI3K/AKT signaling pathway.
Biomed Pharmacother. 109:1259–1267. 2019. View Article : Google Scholar
|