Introduction
Postoperative cognitive dysfunction (POCD) is a
postoperative complication in the central nervous system,
characterized by disorders including anxiety, personality changes
and impaired memory (1,2). For patients who undergo heart
surgery, the occurrence of POCD was 59 and 21% at 1 and 3 months
subsequent to undergoing surgery with anesthesia, respectively
(3,4). The incidence and course of POCD is
different depending on the age of the patient. Symptoms of patients
over 15 years old last for weeks to months, and the incidence of
POCD is higher compared with those younger than 15 (5). POCD delays recovery time and
prolongs hospitalization, and patients may even develop permanent
cognitive impairment and decreased quality of life following
anesthesia and surgery (6).
Currently, the pathological mechanism of POCD
remains unclear. A number of studies have reported that the central
inflammatory response, apoptosis-associated initiation factors,
neuronal apoptosis and a decrease in the total number of neurons
serve an important role in the occurrence and development of POCD
(7,8). Previously, one study demonstrated
that the ubiquitin proteasome system (UPS), a class of specific
proteins and protein degradation pathways in cells, serves a
notable function in memory (9).
In addition, UPS inhibitors may increase the number of
acetylcholine receptors (neurotransmitters that exist widely in the
central nervous system) in the neuronal cell membrane and affect
cognitive function (10). The
mechanisms of POCD have been indicated to be involved in excessive
neuroapoptosis. Therefore, a hypoxia/reoxygeneration (H/R) model
was established in the present study to evaluate the effect of ring
finger protein 41 (Nrdp1) on hippocampus neuron apoptosis.
Nrdp1, a newly discovered ubiquitin ligase, has a
ubiquitination function for a variety of substrates including
erb-b2 receptor tyrosine kinase (ErbB)3 and is involved in numerous
physiological and pathological processes that regulate cell
proliferation, inflammation and apoptosis (11,12). ErbB2, ErbB3 and ErbB4 mRNA
transcripts were identified throughout the cortical, hippocampal
and subcortical areas of the primate brain (13). It was reported that the
Nrdp1/ErbB3 signaling pathway regulated cognitive function and the
suicidal tendency of depression (14,15). However, the function and mechanism
of Nrdp1 in cardiopulmonary bypass operation (CPB)-induced
cognitive dysfunction remains unclear.
Therefore, in the present study, whether Nrdp1 was
involved in CPB-induced cognitive dysfunction in vivo and
in vitro was investigated. In addition, the present study
investigated the pathological mechanisms of Nrdp1 in this process.
This may identify a novel target for the prevention and treatment
of POCD.
Materials and methods
Animals
Sprague-Dawley (SD) male rats (n=30), 18 months old,
weighing 700-800 g, were purchased from the Chongqing Medical
University (Chongqing, China) and randomly divided into the control
group, sham group and model group (n=10 for each group). Rats were
kept in rooms maintained at 22±1°C and 55% humidity in a 12 h
light/dark cycle with access to food and water ad libitum.
Male newborn SD rats (n=20; age, <24 h; weighing 5-10 g) were
purchased from Chongqing Medical University and kept in the same
environment as described above. All experiments involving animals
were approved by the Animal Care Committee of Chongqing Medical
University and were performed according to the guidelines of the
National Institutes of Health on Animal Care (16). The protocol was ethically approved
by the Ethics Committee of Chongqing Medical University. All aged
rats were assessed for baseline measurements (using a Morris water
maze test) prior to the experiment, and the Morris water maze test
was measured following the experiment to evaluate the rat
behavior.
CPB model
A total of 60 rats were randomly divided into 6
groups including control, sham, model, model+normal control (NC),
model+Nrdp1 overexpression and CPB+Nrdp1 knockdown groups. Rats in
the model, model+NC, model+Nrdp1 overexpression and CPB+Nrdp1
knockdown groups were subjected to the CPB operation. Briefly, rats
were anesthetized using a intraperitoneal injection with 50 mg/kg
pentobarbital. The abdominal wall medial anterior artery, caudal
artery and right jugular vein were isolated. The abdominal wall
medial anterior artery was used to monitor arterial blood pressure,
while the caudal artery and right jugular vein were used to
establish CPB. The CPB mainly consisted of a blood reservoir, roll
pump and experimental animal membrane lung and vein. The pre-charge
liquid was 4 ml, composed of ringer lactate solution: 6%
hydroxyethyl starch: Mannitol: Sodium bicarbonate in a 11:7:1:1
ratio. When the activated clotting time (ACT) time was >480 sec,
CPB began. The starting perfusion flow was 20-40 ml/kg/min and
gradually increased to 160-180 ml/kg/min. The mean flow remained at
100-120 ml/kg/min, and the mean arterial pressure was maintained at
60-100 mmHg. Following 90 min of flow, the CPB was stopped, and the
residual machine pre-congestion or colloidal fluid was returned.
Then, 1 mg protamine was injected, the catheter was removed and the
blood vessel and incision were sutured. Subsequent to the
operation, a heating pad and a temperature control lamp were used
to maintain the body temperature of the rat at ~37°C (17). The rats from the sham-operation
group were anesthetized using pentobarbital, and then the abdominal
wall medial anterior artery, caudal artery and right jugular vein
were isolated, but not subjected to CPB operation. Control rats
received no treatment.
For lentivirus treatment, the rats received an
intracerebral ventricular injection of 5 µl 1×109
TU/ml lentivirus in each side of the hippocampus. Len-Nrdp1 was
administrated to the H/R+Nrdp1 group, len-control (ctrl) RNA was
administered to the H/R+NC group and len-shNrdp1 to the H/R+shNrdp1
group, which were all produced as described below (Tianjin Bioco
Biotechnology Co., Ltd., Tianjin, China). After 10 days, the model
was established.
Morris water-maze test
A Morris water-maze test was performed. The
stainless steel pool was divided into 4 quadrants, and the movable
platform was located in the first quadrant. Each rat was trained 4
times a day for 5 days to record the mean daily escape latency. On
the first day, the rats were placed on the platform for 30 sec. All
rats (8 in each group) were allowed to adapt to the environment
subsequent to the completion of the formal experiment. If the rat
did not reach the platform in 120 sec, they would be guided onto
the platform and remained on the platform for 10 sec. The training
record of the rats was 120 sec. Following training, the platform
was removed. Rats were placed in the opposite quadrant from the
location of the original platform as the point of entry, and the
swimming speed, platform quadrant latency distance and time
percentage were recorded in a 120 sec time frame. ANY-maze system
5.26 (Stoelting Co., Wood Dale, IL, USA) was used to analyze the
behavioral indicators running on a computer that automatically
recorded the time and movement route data in real time.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) staining
The aged rats in each group were anesthetized using
an intraperitoneal injection of 1% phenobarbital sodium (40 mg/kg)
and sacrificed by decapitation. The brain tissue was then isolated
and fixed in 4% paraformaldehyde for 24 h at room temperature,
soaked with 100% ethanol and xylene at room temperature for 24 h
and embedded in paraffin at 65°C for 2 h. Brain paraffin sections
were washed and rehydrated, washed with phosphate-buffered saline
(PBS) and incubated with 50 µl TUNEL reaction mixture for 1
h at 37°C in the dark. Sections were also incubated with 50
µl converter-peroxidase at 37°C for 30 min. Finally, the
sections were rinsed with PBS and stained with DAB substrate at
room temperature for 10 min. The In situ Cell Death
Detection kit (Roche Diagnostics GmbH, Mannheim, Germany) was used
for TUNEL staining, according to the manufacturer’s protocol. A
light microscope and LEICA QWin Plus software version 2.0 (Leica
Microsystems GmbH, Wetzlar, Germany) were used to analyze TUNEL
staining.
Primary hippocampus neuron cells
cultures
A total of 20 male newborn SD rats (age <24 h
old, weighing 5-10 g) were purchased from Chongqing Medical
University. Primary hippocampus neuron cells were separated from
the hippocampus of the newborn SD rats, and the cells from 20 rats
were selected. In brief, the newborn SD rats were decapitated, and
subsequently the skull was removed carefully and the brain was
extracted. The entire hippocampus was isolated and sliced into 1
mm3 thick sections. These sections were placed in a 10
cm dish and dissociated using 0.25% trypsin solution at 37°C for 10
min. Then, 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., MA, USA) was used to culture the cells. Subsequent to
centrifugation (1,000 × g for 5 min at 37°C), hippocampus neuron
cells were resuspended and plated in 6-well plates with cell
culture medium, containing poly-D-lysine (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), neurobasal media (Gibco; Thermo Fisher
Scientific, Inc.), 500 µM glutamine and 2% B27 supplement
(Gibco; Thermo Fisher Scientific, Inc.) in 5% CO2 at
37°C. Following culturing for 15 days, the hippocampus neuron cells
were used for the following experiments. The cell culture medium
was replaced on days 3 and 5 in vitro.
H/R model establishment
In brief, neuron cells were cultured in hypoxic
conditions for 2 h in a hypoxic chamber (1% O2,
5%CO2 and 94%N2; Biospherix, Ltd., Parish,
NY, USA) and then reoxygenated by incubation in a standard 5%
CO2 incubator at 37°C for 4 h. The cells in the control
group were incubated in a standard 5% CO2 incubator for
4 h as previously described (18).
Overexpression or knockdown of Nrdp1
expression in primary hippocampus neuron cells
The Nrdp1 coding fragment was cloned using a reverse
transcription-polymerase chain reaction with EcoRI and
BamHI sites. RNA was extracted using TRIzol®
reagent and reverse transcribed with a PrimeScript RT kit (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer’s instructions. The primers used in gene amplification
were as follows: Nrdp1 sense, 5′-CGG AAT TCA TGG GGT ATG ATG TAA
CCC GGT-3′ and antisense, 5′-CGG GAT CCA ATC TCC TCC ACA CCA TGT
GCA-3′. Nrdp1 was inserted into the lentivirus expression vector,
pLVX-IRES-Puro (Invitrogen; Thermo Fisher Scientific, Inc.),
forming a recombinant plasmid named pLVX-nrdp1-puro, which was
verified by sequencing. Overexpression of Nrdp1 in primary neuron
cells was achieved through lentivirus infection. 293 cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco’s modified Eagle’s medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) with 5%
CO2 at 37°C. Cells (2×105) were resuspended
and plated in 6-well plates with cell culture medium. The
lentiviral plasmids pLVX-nrdp1-Puro or pLVX-IRES-Puro, pLP/VSVG,
and pCMV-dR8.2 dvpr (Hanbio Technology Co., Ltd., Shanghai, China)
were co-transfected for 48 h at 37°C into 293 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Lentivirus supernatant was collected at 48 and
72 h after transfection. Primary hippocampus neurons were infected
via lentivirus supernatant for 48 h.
To obtain Nrdp1-deficient primary hippocampus neuron
cells, two Nrdp1-specific shRNAs as follows: 5′-GGT ATG ATG TAA CCC
GGT TCC-3′ and 5′-GGA TCA TGC GGA ACA TGT TGT-3′, were designed to
block Nrdp1 mRNA, and a scrambled shRNA as follows: 5′-GCG ATG GGC
GAA CTG ACA CG-3′, was used as a negative control. The shRNA and
scrambled shRNA were cloned into plasmid pLKO.1, forming
pLKO.1-shRNA or pLKO.1-shNC. Neurons (5×105) were seeded
in 6-well plates. Knockdown of Nrdp1 expression in neuron cells was
induced through transfection using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37°C.
MTT assay
The viability of the neuronal cells was determined
using an MTT assay. Hippocampus neurons were seeded into 96-well
plates (1×104 cells/well). Then, 0.2 mg/ml MTT salt
(Sigma-Aldrich; Merck KGaA) was added into each well, and the cells
were incubated in 5% CO2 for 4 h at 37°C. Then, dimethyl
sulfoxide was used to dissolve the formazan crystals for 20 min.
Finally, the Safire 2 microplate reader (Tecan Group, Ltd.,
Mannedorf, Switzerland) was used to measure the number of viable
hippocampus neuron cells by the absorbance at 490 nm. Each
experiment was repeated 6 times.
Flow cytometry
The fluorescein isothiocyanate (FITC)-Annexin V and
propidium iodide (PI) double staining were used to examine the
apoptosis of the neuron cells. A FITC-Annexin V/PI apoptosis
detection kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used
according to the manufacturer’s protocol. In brief, neuron cells
were cultured in 6-well plates (1×106 cells/well) and
treated with the above mentioned detection kit. The neuronal cells
were collected, and flow cytometry was conducted to assess the
apoptosis level of the cells. Each experiment was repeated 6
times.
Western blot analysis
The hippocampal tissues and neurons were collected
and proteins were extracted with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). Protein concentration was determined with a bicinchoninic
acid protein assay. Subsequently, 40 µg proteins were
separated by 10% SDS-PAGE. The PageRuler™ Plus Prestained Protein
Ladder (Thermo Fisher Scientific, Inc.) was used for loading as a
protein marker and to estimate the molecular weight of the samples.
Protein was transferred to a polyvinylidene fluoride (PVDF)
membrane. Following blocking with 5% non-fat milk at room
temperature for 2 h, the PVDF membranes were incubated with the
following primary antibodies: Cytochrome c (cyto c; 1:500;
cat. no. ab110325; Abcam, Cambridge, MA, USA), BCL2-associated X,
apoptosis regulator (Bax; 1:500; cat. no. ab77566; Abcam), cleaved
caspase-3 (1:500; cat. no. ab2302; Abcam), BCL2, apoptosis
regulator (Bcl-2; 1:500; cat. no. ab194583; Abcam), Nrdp1 (1:300;
cat. no. ab235336; Abcam), ErbB3 (1:500; cat. no. ab5470; Abcam),
protein kinase B (Akt; 1:500; cat. no. ab18785; Abcam),
phosphorylated (p)-Akt (1:400; cat. no. ab183556; Abcam), β-actin
(1:800; cat. no. ab227387; Abcam) at 4°C overnight. The membranes
were then incubated with appropriate horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibodies (1:1,000;
cat. no. ab205718; Abcam) at room temperature for 1 h. Finally, the
bands were visualized using chemiluminescence. Each experiment was
repeated 6 times.
Statistical analysis
The experimental data were assessed using SPSS 19
statistical software (SPSS, Inc., Chicago, IL, USA). The data were
expressed as the mean ± standard deviation. Multiple samples data
were compared using analysis of variance, comparisons among groups
were made using least significant difference post hoc tests, and
data between groups were compared using a χ2 test
including platform quadrant latency distance and time percentage.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cognitive function in aged rats prior to
and following CPB
Prior to the establishment of CPB, a Morris
water-maze test was performed. The results indicated that the
escape latency for 5 continuous days, swimming speed, platform
quadrant latency distance and quadrant latency time percentage were
not significantly different among the groups as presented in
Fig. 1A–D. However, following the
CPB, the escape latency was significantly increased (P<0.05),
while the platform quadrant latency distance and time percentage
were significantly decreased (P<0.05) in the model group
compared with the control and sham-operation as presented in
Fig. 1E–H.
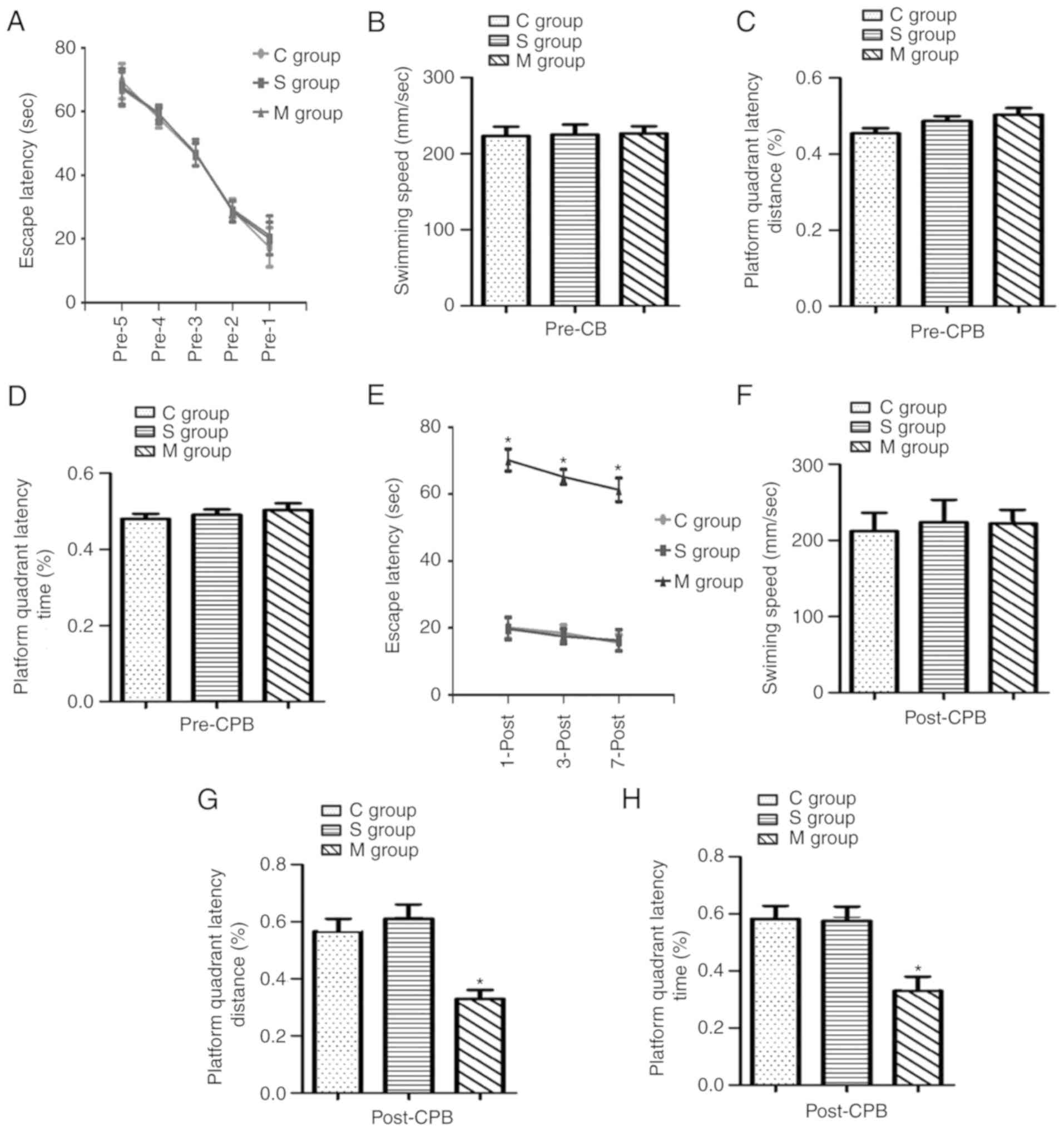 | Figure 1Cognitive function in aged rats prior
to and following a CPB experiment. A Morris water-maze test was
used to analyze the behavioral indicators. Cognitive function was
analyzed 5 continual days prior to the CPB experiment, measuring
(A) escape latency, (B) swimming speed, (C) platform quadrant
latency distance and (D) platform quadrant latency time. Cognitive
function was analyzed 3 continual days following the CPB
experiment, measuring (E) escape latency, (F) swimming speed, (G)
platform quadrant latency distance and (H) platform quadrant
latency time. C group, control group; S group, sham-operation
group; M group, model group; CPB, cardiopulmonary bypass operation.
*P<0.05 vs. sham group. |
Apoptosis in the hippocampus of aged rats
following CPB
Following CPB, TUNEL staining was used to detect the
apoptosis level in the hippocampus. The results revealed that
apoptosis in the hippocampus was significantly increased
(P<0.05) in the model group compared with the control and
sham-operation groups as presented in Fig. 2A and B. Furthermore, apoptosis
relative protein levels were investigated by western blot analysis.
The expression of cyto c, Bax and c-caspase-3 were notably
increased in the model group, while the expression of Bcl-2 was
decreased in the model group compared with the control group
(Fig. 2C).
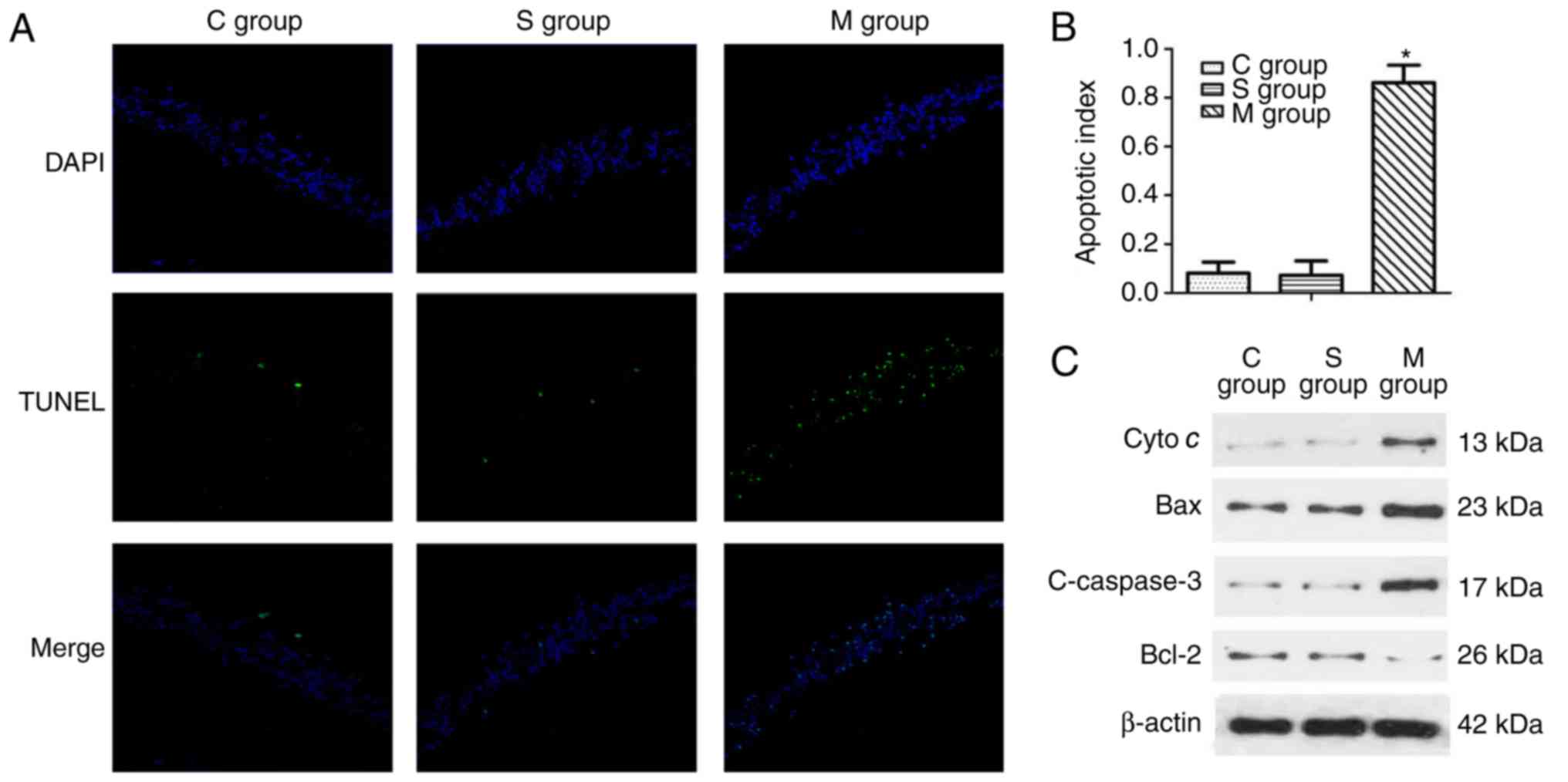 | Figure 2Apoptosis in hippocampus of aged rats
following a cardiopulmonary bypass operation. (A) A TUNEL assay was
used to detect the apoptosis in the hippocampus (the green dots
indicate the positive apoptotic cell) and (B) the results were
quantified. (C) Western blot analysis was used to evaluate the
protein expression level including cyto c, Bax, Bcl-2 and
c-caspase-3. C group, control group; S group, sham-operation group;
M group, model group; cyto c, cytochrome c; Bax,
BCL2-associated X, apoptosis regulator; Bcl-2, BCL2, apoptosis
regulator; c-, cleaved. *P<0.05 vs. sham group. |
Expression of Nrdp1, ErbB3 and p-AKT in
hippocampus of aged rats following CPB
Western blot analyses were used to detect the
expression of Nrdp1 and ErbB3 in the hippocampus of aged rats
subsequent to CPB (Fig. 3A). No
significant difference was identified between the control and the
sham group. The protein expression levels of Nrdp1were
significantly increased in the hippocampus following CPB compared
with that in the control and sham-operation group (P<0.05;
Fig. 3B). However, the expression
level of ErbB3 and the phosphorylation of AKT was significantly
decreased in the model group following CPB compared with the
control and sham group (P<0.05; Fig. 3C-E).
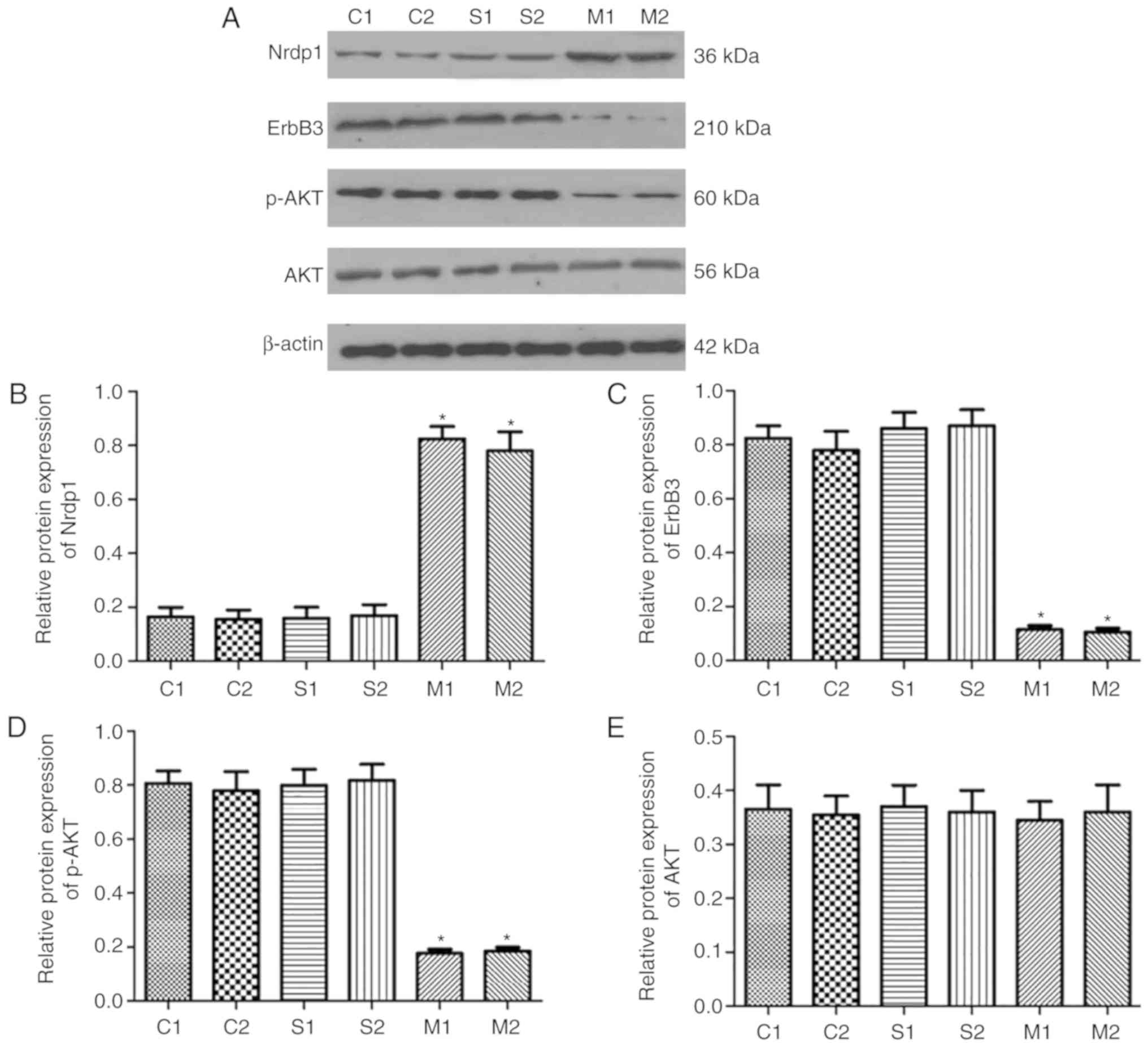 | Figure 3Protein expression of Nrdp1 and ErbB3
in the hippocampus of aged rats. (A) Protein expression of Nrdp1,
ErbB3, AKT and p-AKT in the hippo-campus of aged rats as assessed
using western blot analysis in different groups. Quantified protein
levels of (B) Nrdp1, (C) ErbB3, (D) p-AKT and (E) AKT. C group,
control group; S group, sham-operation group; M group, model group;
Nrdp1, ring finger protein 41; ErbB3, erb-b2 receptor tyrosine
kinase 3; p-, phosphorylated; AKT, protein kinase B.
*P<0.05 vs. sham group. |
Nrdp1 regulated the viability and
apoptosis of primary hippocampus neuron cells under H/R
To investigate the effect of Nrdp1 in the
hippocampus neuron cells, loss and gain of function studies were
performed and a H/R model was established. Primary hippocampus
neuronal cells were infected with overexpressing and knockdown
Nrdp1 lentivirus, and MTT and flow cytometry were used to detect
the cell viability and cell apoptosis. As shown in Fig. 4A and B, H/R treatment
significantly induced cell apoptosis, compared with the control
group (P<0.05). Nrdp1 overexpression significantly increased
cell apoptosis, while Nrdp1 knockdown significantly reduced
apoptosis, compared with the H/R group (P<0.05). H/R treatment
significantly inhibited cell viability compared with the control
group (P<0.05). Nrdp1 overexpression significantly reduced the
cell viability (P<0.05) while Nrdp1 knockdown significantly
increased the viability (P<0.05) compared with that in the H/R
group (Fig. 4C). Furthermore, H/R
treatment significantly increased the expression levels of
apoptotic proteins including cyto c, Bax and c-caspase-3
compared with the control group (P<0.05), and simultaneously
significantly decreased the expression levels of Bcl-2 compared
with the control group (P<0.05). Nrdp1 overexpression
significantly increased the expression levels of cyto c,
Bax, c-caspase-3 and significantly decreased Bcl-2 expression
levels compared with that in the H/R group (P<0.05). Nrdp1
knockdown significantly decreased the level of cyto c, Bax,
c-caspase-3 and significantly increased Bcl-2 level compared with
that in the H/R group (P<0.05; Fig. 4D and E).
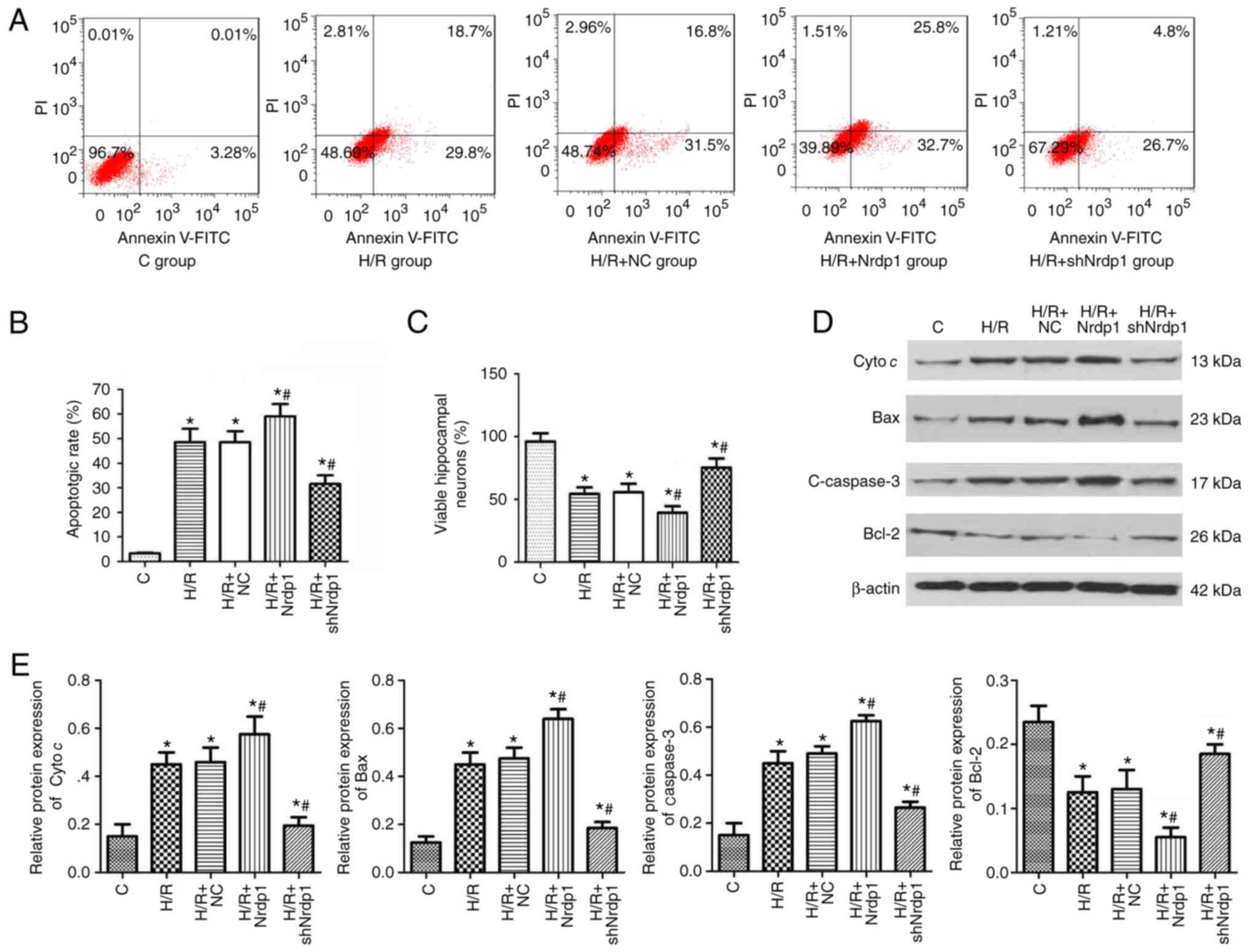 | Figure 4Nrdp1 regulated the viability and
apoptosis of primary hippocampus neuron cells. (A) Apoptosis of the
hippocampus neuron cells was detected using Annexin V-FITC/PI
staining and flow cytometry, and (B) quantified. (C) Subsequent to
treatment with H/R along with shNrdp1 or Nrdp1 overexpression
lentivirus infection, the hippocampus neuron cell viability was
detected using an MTT assay. (D) Western blot analysis was used to
evaluate the protein expression levels of cyto c, Bax, Bcl-2 and
c-caspase3, and (E) quantified. *P<0.05 vs. control
group, #P<0.05 vs. H/R+NC group. FITC, fluorescein
isothiocyanate; PI, propidium iodide; sh-, short hairpin RNA;
Nrdp1, ring finger protein 41; H/R, hypoxia/reoxygeneration; NC,
negative control; C, control group; cyto c, cytochrome c; Bax,
BCL2-associated X, apoptosis regulator; Bcl-2, BCL2, apoptosis
regulator; c-, cleaved. |
Nrdp1 regulated the protein expression in
hippocampus neuron cells subjected to H/R
Initially the present study evaluated the effect of
lentivirus infection. The western blot analysis results indicated
that Nrdp1 overexpression significantly elevated the expression
levels of Nrdp1 and shNrdp1 significantly reduced the levels of
Nrdp1 compared with the control (P<0.05; Fig. 5A and B). H/R treatment
significantly promoted the expression levels of Nrdp1 and also
significantly inhibited the levels of ErbB3 and the phosphorylation
of AKT compared with the control group (P<0.05). Nrdp1
overexpression significantly inhibited the expression levels of
ErbB3 and the phosphorylation of AKT, meanwhile promoting the
expression levels of Nrdp1compared with the control group
(P<0.05). Furthermore, knockdown of Nrdp1 significantly promoted
the levels of ErbB3 and the phosphorylation of AKT, meanwhile
significantly inhibiting the expression of Nrdp1 (P<0.05;
Fig. 5C-G).
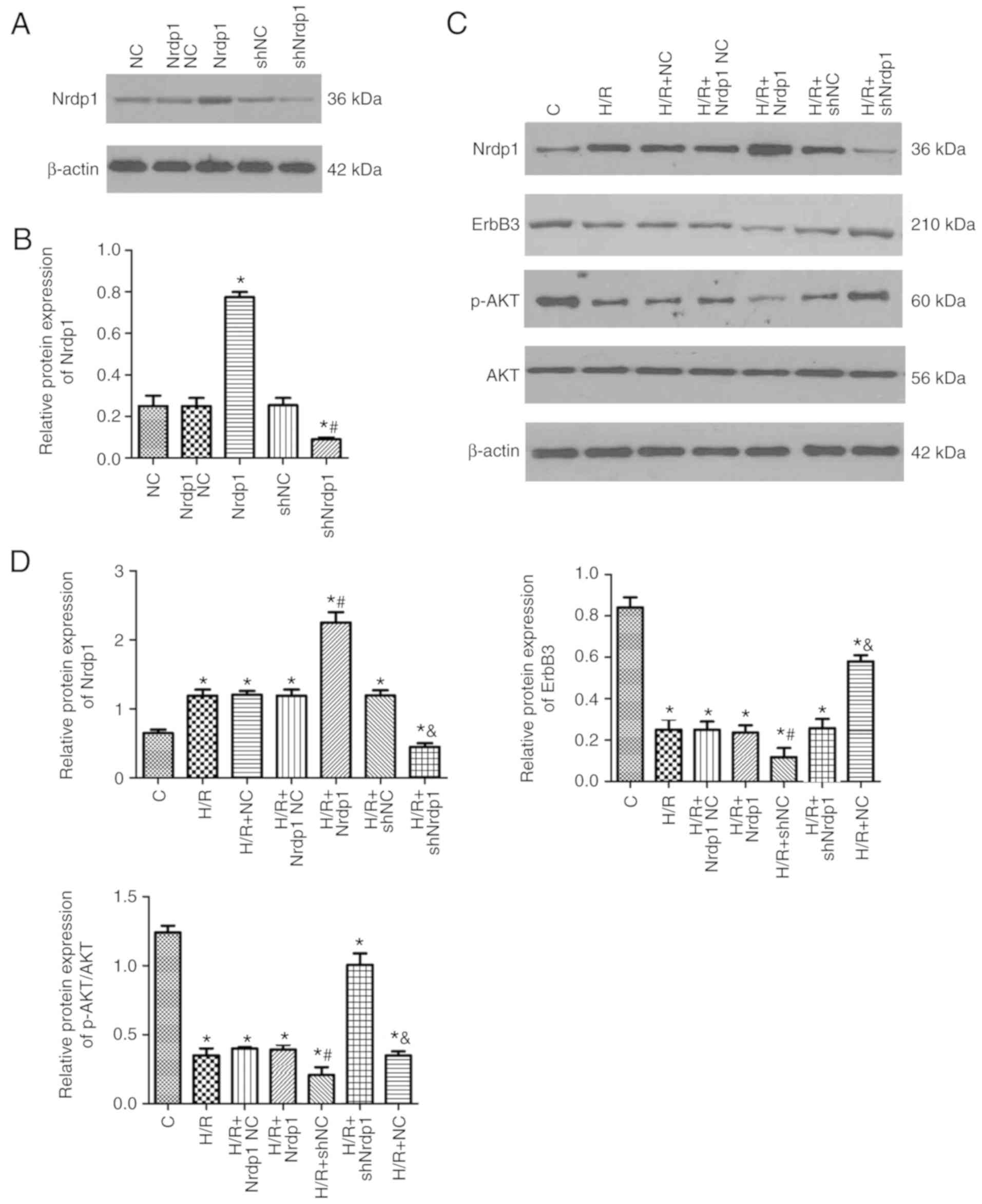 | Figure 5Protein expression of Nrdp1 and ErbB3
in the hippocampus of primary hippocampus neuron cells. (A and B)
Subsequent to treatment with H/R along with shNrdp1 or Nrdp1
overexpression lentivirus in addition to the control lentivirus
infection, the protein expression of (A) Nrdp1 was assessed and (B)
quantified. (C and D) Western blot analysis was further used to
determine the protein expression levels of (C) ErbB3 and p-AKT in
the hippocampus neuron cell of primary hippocampus neuron cells and
(D) quantified. *P<0.05 vs. control group,
#P<0.05 vs. H/R+NC group, &P<0.05
vs. H/R+shNC group. Nrdp1, ring finger protein 41; ErbB3, erb-b2
receptor tyrosine kinase 3; p-, phosphorylated; AKT, protein kinase
B; H/R, hypoxia/reoxygeneration; sh-, short hairpin RNA; C,
control; NC, negative control. |
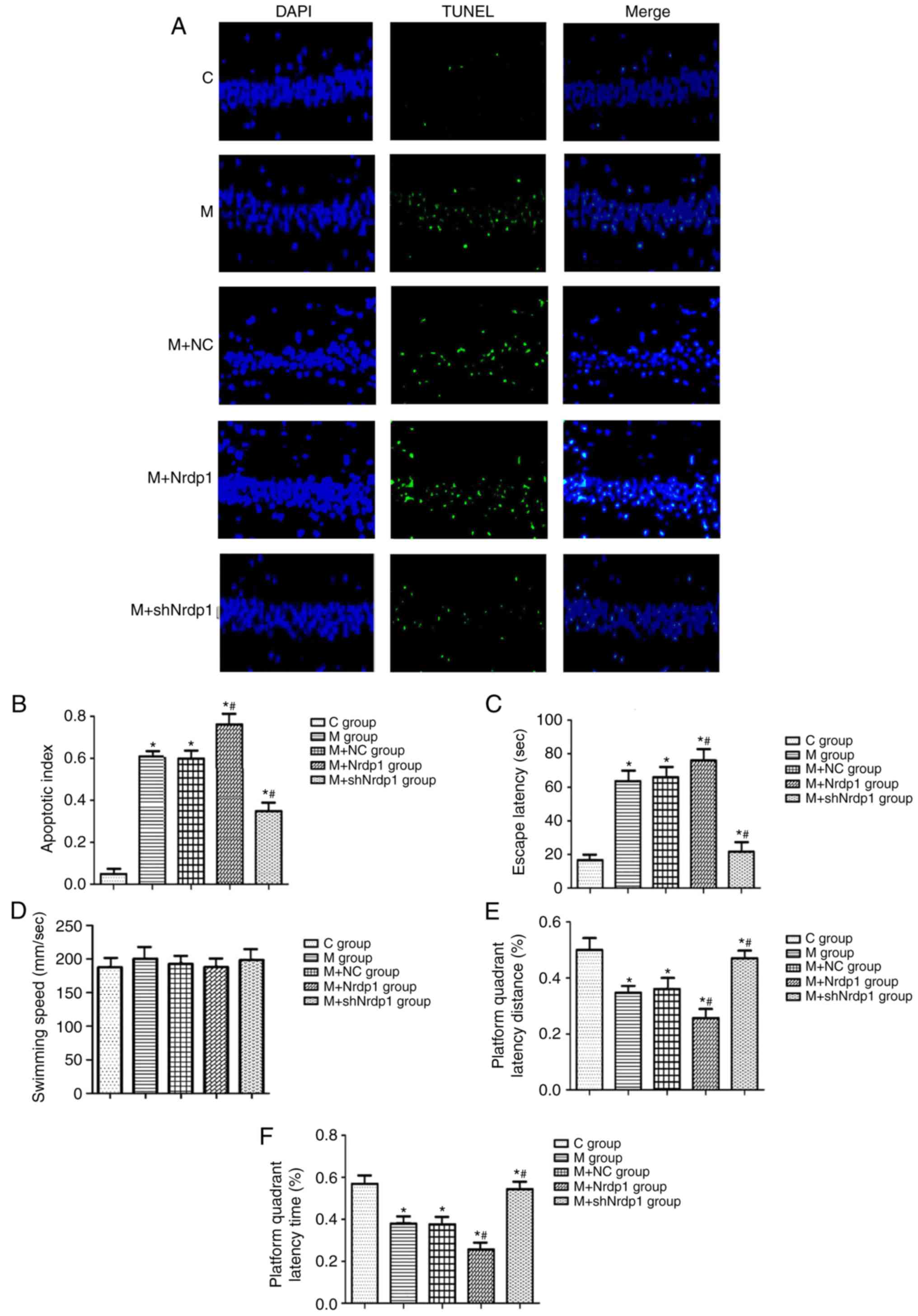
Nrdp1 regulated the cognitive function
and hippocampus neuron cell apoptosis
In order to investigate whether Nrdp1 expression
change results in cognitive function and cell apoptosis alteration,
lentivirus infection (lenti-Nrdp1 and lenti-shNrdp1) was used in a
Morris water-maze test. The results indicated that the
overexpression of Nrdp1 significantly increased the hippocampus
neuron cell apoptosis induced by CPB treatment compared with that
in the model groups (P<0.05). Additionally, the knockdown of
Nrdp1 significantly decreased the hippo-campus neuron cell
apoptosis compared with that in the model groups (P<0.05;
Fig. 6A and B). Overexpression of
Nrdp1 significantly increased the escape latency and decreased the
platform quadrant latency distance and time percentage in the
lenti-Nrdp1 treatment group compared with that in the model groups
(P<0.05). Correspondingly, the knockdown of Nrdp1 significantly
decreased the escape latency and increased the platform quadrant
latency distance and time percentage in the lenti-shNrdp1 treatment
group (P<0.05; Fig. 6C-F).
Nrdp1 regulated protein expression in the
hippocampus following CPB
To further investigate the mechanism, the present
study assessed the expression levels of apoptosis-associated
proteins. ACPB experiment significantly promoted the expression
levels of Nrdp1 and apoptotic proteins including cyto c, Bax
and c-caspase-3 and significantly inhibited the levels of ErbB3,
Bcl-2 and the phosphorylation of AKT compared with the control
group (P<0.05). Nrdp1 overexpression significantly inhibited the
expression of ErbB3, Bcl-2 and the phosphorylation of AKT, and
significantly promoted the expression of cyto c, Bax and
c-caspase-3 compared with that in the model group (P<0.05).
Knockdown of Nrdp1 significantly increased the levels of ErbB3,
Bcl-2 and the phosphorylation of AKT, and significantly decreased
the expression levels of cyto c, Bax and c-caspase-3
compared with that in the model group (P<0.05; Fig. 7A and B).
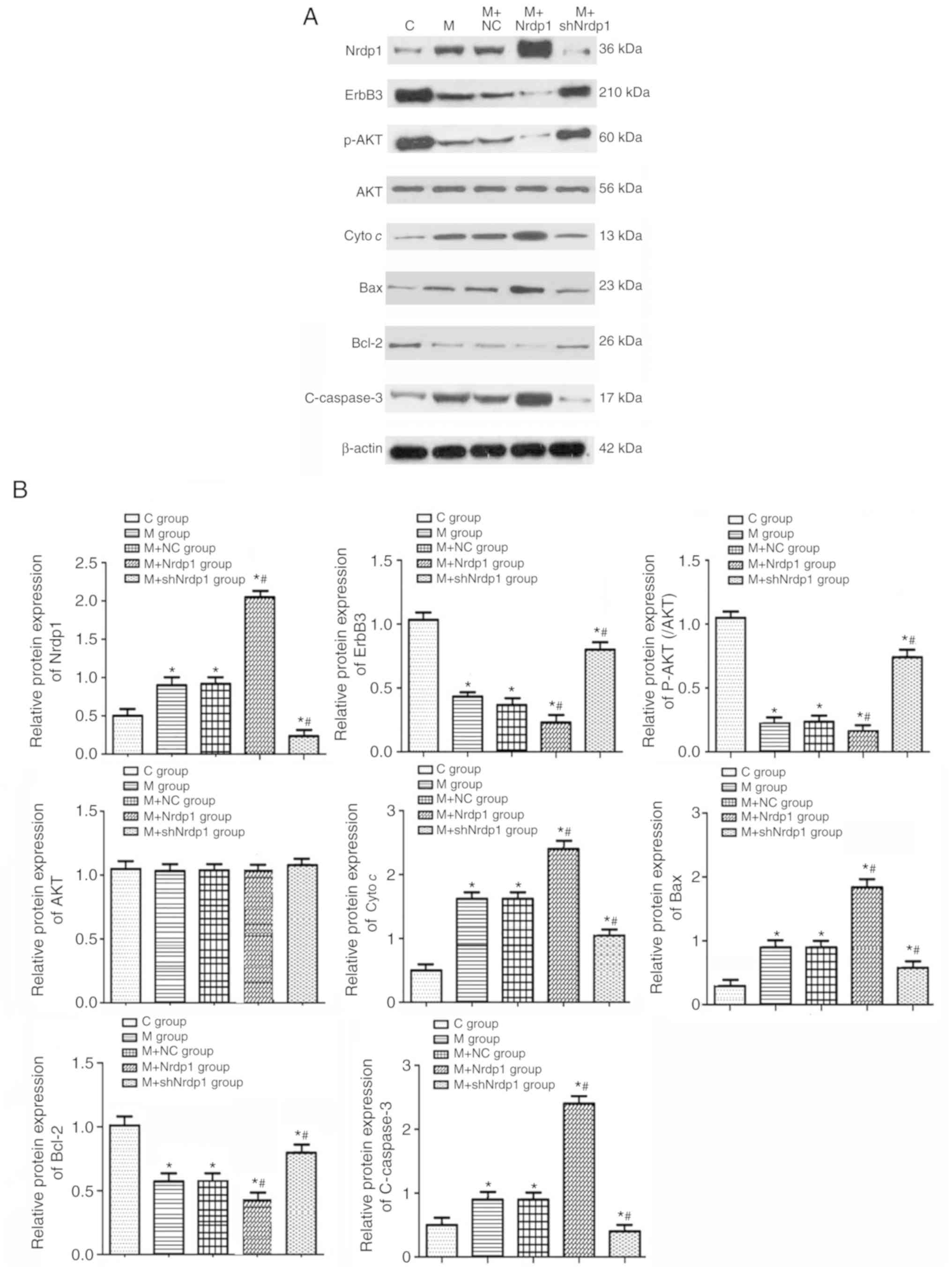 | Figure 7Protein expression of Nrdp1 and ErbB3
in the hippocampus of aged rats. (A) Subsequent to treatment with a
cardiopulmonary bypass operation along with shNrdp1 or Nrdp1
overexpression lentivirus infection, the protein expression of
Nrdp1, ErbB3, p-AKT, cyto c, Bax, Bcl-2 and c-caspase3 in the
hippo-campus neuron cells of aged rats was assessed using western
blot analysis and (B) quantified. *P<0.05 vs. control
group, #P<0.05 vs. M+NC group. C group, control
group; M group, model group; NC, negative control; Nrdp1, ring
finger protein 41; sh-, short hairpin RNA; ErbB3, erb-b2 receptor
tyrosine kinase 3; p-, phosphorylated; AKT, protein kinase B; cyto
c, cytochrome c; Bax, BCL2-associated X, apoptosis regulator;
Bcl-2, BCL2, apoptosis regulator; c-, cleaved. |
Discussion
A number of studies have reported that when POCD
occurs, apoptosis is observed in the hippocampus; this serves an
important role in occurrence and development of POCD (19,20). In the present study, the results
demonstrated that a CPB operation altered the cognitive function in
aged rats and resulted in apoptosis in the hippocampus. Certain
studies have demonstrated that the information from vertebral cells
in the hippocampus region may be projected into the cerebral cortex
through the cerebral foot and fimbria of the fornix, forming
connections among the neurons and a closed loop for learning and
memory (21,22). In addition, cell proliferation,
migration and differentiation in hippocampus were revealed to be
closely associated with spatial and associative learning and memory
(23). Apoptosis is a complex
intracellular cascade, with multiple signals and pathways involved
in the initiation of cellular apoptosis. However, the upstream
mechanism of neuronal apoptosis remains unclear.
Emerging evidence suggests that neuronal cell
apoptosis is a predominant pathological issue in numerous
neurological disorders, including Alzheimer’s disease and
Parkinson’s disease (24-26). Under various stimuli, mature
neurons are involved in neurodegeneration through the apoptosis
pathway (27). Certain studies
have indicated that neuronal death and decreased hippocampus size
result in cognitive dysfunction, and corresponding drug therapy may
improve cognitive function by reducing the levels of hippocampus
apoptosis (28,29). For example, Lycium barbarum
polysaccharides may improve cognitive function following traumatic
stress by regulating the regeneration and apoptosis balance of
neurons in the hippocampus (30),
and dexmedetomidine may improve the cognitive function in aged rats
by inhibiting the excessive excitability of neurons and decreasing
the apoptosis of hippocampus neurons (31). Therefore, apoptosis in the
hippocampus serves a notable function in the development and
progression of cognitive dysfunction. Hypoxia/reoxygenation serve a
crucial function in physiological and psychological disorders
including dizziness, insomnia, nausea and retrograde cognitive
function deficits. In the present study, a hippocampus neuron cell
H/R model was established and used to simulate the condition of the
neuron cells in the POCD brain.
In addition, Nrdp1 is involved in numerous
physiological and pathological processes and regulates cell
proliferation, inflammation and apoptosis (32). At present, a number of studies
have confirmed that in tumor cells and myocardial
ischemia-reperfusion animal models, Nrdp1 promotes the
ubiquitination of the substrate protein ErbB3, reduces the
expression of ErbB3, inhibits downstream signaling pathways
including those of signal transducer and activator of transcription
3, mitogen-activated protein kinases and AKT, and promotes the
occurrence of apoptosis (33-35). Additionally, in an animal model of
inflammation induced by lipopolysaccharides, Nrdp1 was revealed to
be associated with the apoptosis of cortical neurons (36). When the expression of Nrdp1 was
decreased using small interfering RNA, neuronal apoptosis in the
cortical areas was decreased (37).
In the present study, it was revealed that in the
hippocampus neuron cells of aged rats following CPB, the apoptosis
and the expression of Nrdp1 were increased. Additionally, the
expression of ErbB3 protein was decreased. In vitro and
in vivo studies indicated that Nrdp1 was involved in
regulating the cell viability and apoptosis of hippocampus neuron
cells. Furthermore, alterations in the cognitive function of aged
rats following CPB were observed. Mechanism studies demonstrated
that Nrdp1 decreased the expression of ErbB3 and p-AKT while
increasing the expression of c-caspase-3. Therefore, Nrdp1was
determined to be involved in hippocampus apoptosis in CPB-induced
cognitive dysfunction by regulating the ErbB3 protein level. The
results of the present study may provide a novel target for the
prevention and treatment of POCD.
The results of the present study demonstrated that a
cardiopulmonary CPB may induce apoptosis in the hippocampus by
causing POCD, and Nrdp1 served an important function in this
process by regulating the ErbB3 protein level.
Funding
The present study was supported by the Joint
Research Project of Southwest Medical University (grant no.
2016LZXNYD-J15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JZ performed the western blotting and flow cytometry
experiments, and wrote the manuscript. SM conceived and designed
the research. BH and QL performed the in vivo experiments.
YW performed the TUNEL assays.
Ethics approval and consent to
participate
All experiments involving animals were approved by
the Animal Care Committee of Chongqing Medical University and were
performed according to the guidelines of the National Institutes of
Health on Animal Care.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Benson RA, Ozdemir BA, Matthews D and
Loftus IM: A systematic review of postoperative cognitive decline
following open and endovascular aortic aneurysm surgery. Ann R Coll
Surg Engl. 99:97–100. 2017. View Article : Google Scholar :
|
|
2
|
Bilotta F, Qeva E and Matot I: Anesthesia
and cognitive disorders: A systematic review of the clinical
evidence. Expert Rev Neurother. 16:1311–1320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Funder KS, Steinmetz J and Rasmussen LS:
Cognitive dysfunction after cardiovascular surgery. Minerva
Anestesiol. 75:329–332. 2009.PubMed/NCBI
|
|
4
|
Liu C and Han JG: Advances in the
mechanisms and early warning indicators of the postoperative
cognitive dysfunction after the extracorporeal circulation.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 37:101–107. 2015.PubMed/NCBI
|
|
5
|
Newman MF, Kirchner JL, Phillips-Bute B,
Gaver V, Grocott H, Jones RH, Mark DB, Reves JG and Blumenthal JA;
Neurological Outcome Research Group and the Cardiothoracic
Anesthesiology Research Endeavors Investigators: Longitudinal
assessment of neurocognitive function after coronary-artery bypass
surgery. N Engl J Med. 344:395–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS; ISPOCD Group: Long-term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang
JM, Han L, Peng YM, Jiang JH and Wang QD: Dexmedetomidine improves
early postoperative cognitive dysfunction in aged mice. Eur J
Pharmacol. 746:206–212. 2015. View Article : Google Scholar
|
|
8
|
Zhang X, Dong H, Li N, Zhang S, Sun J,
Zhang S and Qian Y: Activated brain mast cells contribute to
postoperative cognitive dysfunction by evoking microglia activation
and neuronal apoptosis. J Neuroinflammation. 13:1272016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong B, Radulovic M, Figueiredo-Pereira ME
and Cardozo C: The ubiquitin-proteasome system: Potential
therapeutic targets for Alzheimer’s disease and spinal cord injury.
Front Mol Neurosci. 9:42016. View Article : Google Scholar
|
|
10
|
Lip PZ, Demasi M and Bonatto D: The role
of the ubiquitin proteasome system in the memory process. Neurochem
Int. 102:57–65. 2017. View Article : Google Scholar
|
|
11
|
Diamonti AJ, Guy PM, Ivanof C, Wong K,
Sweeney C and Carraway KL III: An RBCC protein implicated in
maintenance of steady-state neuregulin receptor levels. Proc Natl
Acad Sci USA. 99:2866–2871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Chen T, Zhang J, Yang M, Li N, Xu
X and Cao X: The E3 ubiquitin ligase Nrdp1 ‘preferentially’
promotes TLR-mediated production of type I interferon. Nat Immunol.
10:744–752. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahar I, MacIsaac A, Kim JJ, Qiang C,
Davoli MA, Turecki G and Mechawar N: Effects of neuregulin-1
administration on neurogenesis in the adult mouse hippocampus, and
characterization of immature neurons along the septotemporal axis.
Sci Rep. 6:304672016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahar I, Labonte B, Yogendran S, Isingrini
E, Perret L, Davoli MA, Rachalski A, Giros B, Turecki G and
Mechawar N: Disrupted hippocampal neuregulin-1/ErbB3 signaling and
dentate gyrus granule cell alterations in suicide. Transl
Psychiatry. 7:e11612017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson M, Lauderdale S, Webster MJ,
Chong VZ, McClintock B, Saunders R and Weickert CS: Widespread
expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain.
Brain Res. 1139:95–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen Y, Qin H, Chen J, Mou L, He Y, Yan Y,
Zhou H, Lv Y, Chen Z, Wang J, et al: Postnatal activation of TLR4
in astrocytes promotes excitatory synaptogenesis in hippocampal
neurons. J Cell Biol. 215:719–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee GM, Welsby IJ, Phillips-Bute B, Ortel
TL and Arepally GM: High incidence of antibodies to protamine and
protamine/heparin complexes in patients undergoing cardiopulmonary
bypass. Blood. 121:2828–2835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian Wu QF, Zhao C, Dong N, Li Q, Wang J,
Chen BB, Yu L, Han L, Du YM B, et al: Activation of transient
receptor potential vanilloid 4 involves in hypoxia/reoxygenation
injury in cardio-myocytes. Cell Death Dis. 8:e28282017. View Article : Google Scholar
|
|
19
|
Qi Z, Tianbao Y, Yanan L, Xi X, Jinhua H
and Qiujun W: Pre-treatment with nimodipine and 7.5% hypertonic
saline protects aged rats against postoperative cognitive
dysfunction via inhibiting hippocampal neuronal apoptosis. Behav
Brain Res. 321:1–7. 2017. View Article : Google Scholar
|
|
20
|
Tian A, Ma H, Zhang R, Cui Y and Wan C:
Edaravone improves spatial memory and modulates endoplasmic
reticulum stress-mediated apoptosis after abdominal surgery in
mice. Exp Ther Med. 14:355–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho N, Sommers MS and Lucki I: Effects of
diabetes on hippocampal neurogenesis: Links to cognition and
depression. Neurosci Biobehav Rev. 37:1346–1362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Machado VM, Lourenco AS, Florindo C,
Fernandes R, Carvalho CM and Araujo IM: Calpastatin overexpression
preserves cognitive function following seizures, while maintaining
post-injury neurogenesis. Front Mol Neurosci. 10:602017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong RF, Zhang B, Tai LW, Liu HM, Shi FK
and Liu NN: The neuroprotective role of miR-124-3p in a
6-hydroxy-dopamine-induced cell model of parkinson’s disease via
the regulation of ANAX5. J Cell Biochem. 119:269–277. 2018.
View Article : Google Scholar
|
|
25
|
Malik B, Currais A and Soriano S: Cell
cycle-driven neuronal apoptosis specifically linked to amyloid
peptide Aβ1-42 exposure is not exacerbated in a mouse model of
presenilin-1 familial Alzheimer’s disease. J Neurochem.
106:912–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xing HY, Li B, Peng D, Wang CY, Wang GY,
Li P, Le YY, Wang JM, Ye G and Chen JH: A novel monoclonal antibody
against the N-terminus of Aβ1–42 reduces plaques and improves
cognition in a mouse model of Alzheimer’s disease. PLoS One.
12:e1800762017. View Article : Google Scholar
|
|
27
|
Cordeiro MF, Normando EM, Cardoso MJ,
Miodragovic S, Jeylani S, Davis BM, Guo L, Ourselin S, A’Hern R and
Bloom PA: Real-time imaging of single neuronal cell apoptosis in
patients with glaucoma. Brain. 140:1757–1767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Saucedo-Cuevas L, Shresta S and
Gleeson JG: The neurobiology of zika virus. Neuron. 92:949–958.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schallner N, Pandit R, LeBlanc R III,
Thomas AJ, Ogilvy CS, Zuckerbraun BS, Gallo D, Otterbein LE and
Hanafy KA: Microglia regulate blood clearance in subarachnoid
hemorrhage by heme oxygenase-1. J Clin Invest. 125:2609–2625. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao J, Chen C, Liu Y, Li Y, Long Z, Wang
H, Zhang Y, Sui J, Wu Y, Liu L, et al: Lycium barbarum
polysaccharide improves traumatic cognition via reversing imbalance
of apoptosis/regeneration in hippocampal neurons after stress. Life
Sci. 121:124–134. 2015. View Article : Google Scholar
|
|
31
|
Xiong B, Shi Q and Fang H: Dexmedetomidine
alleviates postoperative cognitive dysfunction by inhibiting neuron
excitation in aged rats. Am J Transl Res. 8:70–80. 2016.PubMed/NCBI
|
|
32
|
Hamburger AW: The role of ErbB3 and its
binding partners in breast cancer progression and resistance to
hormone and tyrosine kinase directed therapies. J Mammary Gland
Biol Neoplasia. 13:225–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi H, Gong H, Cao K, Zou S, Zhu B, Bao H,
Wu Y, Gao Y, Tang Y and Yu R: Nrdp1-mediated ErbB3 degradation
inhibits glioma cell migration and invasion by reducing cytoplasmic
localization of p27Kip1. J Neurooncol. 124:357–364.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y, Wang L, Bao H, Zou S, Fu C, Gong H,
Gao Y, Tang Y, Yu R and Shi H: Nrdp1S, short variant of Nrdp1,
inhibits human glioma progression by increasing Nrdp1-mediated
ErbB3 ubiquitination and degradation. J Cell Mol Med. 20:422–429.
2016. View Article : Google Scholar
|
|
35
|
Zhang Y, Zeng Y, Wang M, Tian C, Ma X,
Chen H, Fang Q, Jia L, Du J and Li H: Cardiac-specific
overexpression of E3 ligase Nrdp1 increases ischemia and
reperfusion-induced cardiac injury. Basic Res Cardiol. 106:371–383.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Yang K, Wang T, Li W, Jin X and
Liu W: Nrdp1 increases ischemia induced primary rat cerebral
cortical neurons and pheochromocytoma cells apoptosis via
downregulation of HIF-1α protein. Front Cell Neurosci. 11:2932017.
View Article : Google Scholar
|
|
37
|
Shen J, Song Y, Lin Y, Wu X, Yan Y, Niu M,
Zhou L, Huang Y, Gao Y and Liu Y: Nrdp1 is associated with neuronal
apoptosis in lipopolysaccharide-induced neuroinflammation.
Neurochem Res. 40:971–979. 2015. View Article : Google Scholar : PubMed/NCBI
|