Introduction
Epithelial ovarian cancer (EOC) has the highest
mortality rate and the poorest prognosis among female malignant
tumors (1); however, a clear and
reliable biological marker for early treatment is lacking (2). Understanding the mechanism
underlying tumor metastasis and invasion may provide novel
strategies and therapeutic targets for the early testing and
diagnosis of EOC. There is a close correlation between
glycoconjugates of the cell membrane and biological
characteristics, including cell canceration, invasion and
metastasis (3). Glycoconjugates
are involved in several important biological processes, including
adhesion, recognition of cells and signal transduction (4). The Lewis y antigen is an
oligosaccharide containing two fucoses that belongs to the blood
group A, B and H antigen family. In ~75% of EOC cases, Lewis y
antigen is overexpressed to varying degrees, and its expression is
associated with prognosis (5). In
our previous studies, the genetic transfection technique was used
to transfect the α1,2-fucosyltransferase (α1,2-FT, FUT1) gene into
the RMG-I ovarian cancer cell line to establish a cell line
exhibiting high expression of Lewis y antigen (RMG-I-hFUT); this
cell line was used to show that Lewis y can promote malignant cell
behavior by increasing proliferation, adhesion, invasion,
metastasis, drug resistance and in vitro tumor rate
(6-8). In addition, it was shown that the
Lewis y antigen serves an important role in the occurrence,
development, invasion and metastasis of EOC.
The invasion and metastasis of tumor cells involves
cell adhesion molecules and protease-mediated degradation of the
extracellular matrix. The extracellular matrix metalloproteinase
inducer EMMPRIN or CD147 can alter the microenvironment of
carcinoma cells by inducing matrix metalloproteinases (MMPs),
angiogenic factors of carcinoma and substratum cells. It can also
modulate the anchor-independent growth of carcinoma cells. Previous
studies have shown that CD147 is involved in several processes,
including promoting the metastasis of carcinoma cells, drug
resistance, invasion and other aspects of malignancy (9-11).
CD147 has been identified as an important marker of an unfavorable
prognosis in ovarian carcinoma. Its expression is significantly
correlated with cell signaling molecules, including Akt and
extracellular signal-regulated kinase (ERK). CD147 promotes the
development of ovarian carcinoma by inducing the production of MMPs
and modulating tumor growth, angiogenesis, signal transduction and
drug-resistance (12-14).
The molecular weight of CD147 varies between 31 and
65 kDa depending on the degree of glycosylation and the level of
Lewis x antigen (15,16). CD147 glycosylation is required for
inducing the expression of MMP (15,17,18). However, the mechanism underlying
the effect of glycosylation on regulating CD147 function remains to
be fully elucidated. The present study examined the expression and
correlation between the Lewis y antigen and CD147 in EOC using
immunohistochemical staining of tissue specimens, and examined the
function and mechanism of Lewis y in CD147-mediated cell adhesion.
The RMG-I-hFUT cell line stably overexpressing Lewis y was used to
investigate the molecular basis of the pathogenesis, progression
and biological treatment of ovarian cancer.
Materials and methods
Ethics statement
Samples were fully encoded to protect patient
confidentially. The present study was approved by the Ethical
Committee of Shengjing Hospital of China Medical University
(Shenyang, China; approval no. 2013PS66K). The Ethics Committee
waived the requirement for patient consent, as the patient
information was withheld.
Patients and tissue samples
A total of 140 paraffin-embedded ovarian tissue
samples were obtained from surgical procedures performed between
2000 and 2012 in the Department of Obstetrics and Gynecology of
China Medical University Shengjing Hospital. All tissue sections
were diagnosed by two specialists independently. There were 60
cases of primary EOC, including 30 serous, 22 mucinous, three
endometrioid and five clear-cell carcinoma; in addition to 30
ovarian borderline tumors, 30 ovarian benign tumors and 20 normal
ovarian tissues (from normal ovarian specimens resected following
cervical carcinoma surgery). The average age of the patients was
46.97 (16-81) years. The average age of the malignant group was
50.62 (16-73) years with a median age of 53 years. The average age
of the borderline group was 39.41 (22-77) years with a median age
of 36 years. The average age of the benign group was 46.00 (22-81)
years with a median age of 44 years. The average age of the normal
group was 48.71 (37-59) years with a median age of 50 years. There
were no statistically significant differences between the groups
(Table I; P>0.05). According
to the pathological grading, there were 21 well-differentiated, 21
moderately differentiated and 18 poorly differentiated cases. The
group included 39 patients with stage I-II disease and 21 with
stage III-IV disease, according to the International Federation of
Gynecology and Obstetrics staging system for ovarian cancer
(19); 12 patients had lymph node
metastases. All cases were primary tumors with complete clinical
pathological data and without chemotherapy prior to surgery.
 | Table IOvarian tissue patient features. |
Table I
Ovarian tissue patient features.
| Feature | Overall | Malignant | Borderline | Benign | Normal |
|---|
| Cases (n) | 140 | 60 | 30 | 30 | 20 |
| Age, years (mean ±
SD)a | 46.97±10.2 | 50.62±13.7 | 39.41±8.6 | 46.00±11.3 | 48.71±12.2 |
| Age, years [median
(range)] | 51 (16-81) | 53 (16-73) | 36 (22-77) | 44 (22-81) | 50 (37-59) |
Immunohistochemical staining and
quantification
All ovarian tissue samples were obtained as
successive 5-µm-thick sections. The expression of Lewis y
and CD147 in ovarian carcinoma tissues was analyzed by
immunohistochemical streptavidin-biotin-peroxidase (SP) staining.
Positive and negative immunohistochemistry controls were routinely
used. Primary antibodies against Lewis y and CD147 (both from
Abcam, Cambridge, UK; cat. no. F3, ab3359; cat. no. ab666) were
used at a dilution of 1:100. Staining was performed according to
the instructions of the SP kit (Boshide Biotech Co., Ltd. Wuhan,
China). The samples were considered positive if there were buffy
granules in the cell membrane and cytoplasm. Immunohistochemical
signals were calculated by quantifying positively stained cells
under a light microscope (Olympus Corporation, Tokyo, Japan).
According to the chromatosis intensity, no pigmentation, light
yellow, buffy and brown were scored as 0, 1, 2 and 3, respectively.
The number of cells with chromatosis was scored in five high-power
fields from each section as follows: <5% = 0, 5-25% = 1, 26-50%
= 2, 51-75% = 3, and >75% = 4. The number of cells with
chromatosis was multiplied by the intensity to yield the following
scores: 0-2 = (−), 3-4 = (+), 5-8 = (++) and 9-12 = (+++). Two
observers examined the sections independently.
Cell line and cell culture
The RMG-I-hFUT cell line, which is characterized by
high expression of the FUT1 gene and Lewis y antigen, was
established by transfecting the pcDNA3.1(−)-HFUT-H expression
vector (containing the FUT1 gene) into RMG-I cells (a human ovarian
clear cell carcinoma cell line, donated by Professor Iwamori Masao
of Tokyo University (Tokyo, Japan) (7). The RMG-I and RMG-I-hFUT cells were
cultured in DMEM (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) containing 10% FBS (HyClone; GE Healthcare Life Sciences) at
37°C in 5% CO2 and saturated humidity.
Cells in the exponential growth phase were used in
the subsequent experiments. A total of 1×105 cells in 1
ml were inoculated into a 6-well plate in serum-free medium. For
the inhibition assay, the final concentration of Lewis y antibody
was 20 µg/ml, the duration of treatment was 1 h at 37°C in
5% CO2. In the case of CD147 antibody treatment, the
CD147 antibody was added to the culture medium at 10 µg/ml
and the cells were incubated for 1 h at 37°C in 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RMG-I and RMG-I-hFUT cells at an exponential phase
of growth were treated with TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA; 1 ml per 1×107
cells) to extract total RNA. Complementary DNA (cDNA) was
synthesized according to the manufacturer's protocol of the RNA
reverse transcription kit (Invitrogen; Thermo Fisher Scientific,
Inc.). The reaction conditions were as follows: 37°C for 15 min,
85°C for 5 sec, 4°C for 5 min. The primers used were as follows:
CD147, forward 5'-GACTGGGTACAAGATCAC-3' and reverse 5'-GCC TCC ATG
TTC AGG TTC TCA A-3'; FUT1, forward 5'-AGG TCA TCC CTG AGC TGA AAC
GG-3' and reverse 5'-CGC CTG CTT CAC CAC CTT CTT G-3'. The
real-time PCR reaction conditions were as follows: Denature at 95°C
for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for 30 sec in a
20-µl reaction mixture containing 10 µl
SYBR® Premix Ex Taq™ (2X), 0.4 µl PCR forward
primer (10 µmol/l), 0.4 µl PCR reverse primer (10
µmol/l), 2 µl cDNA and 7.2 µl dH2O.
The Light Cycler PCR system (Roche Diagnostics, Mannheim, Germany)
was used for real-time PCR amplification and Cq value detection.
The melting curves were analyzed following amplification. All PCR
was performed in triplicate. The data were analyzed using the Cq
method (20). The results were
considered significant when at least a 2-fold difference in
expression levels was detected.
Western blot analysis
The RMG-I-hFUT and RMG-I cells were washed twice
with cold PBS, treated with cell lysis buffer [50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 0.5% NP40, 100 mM NaF, 200 µM
Na3VO4 and 10 µg/ml each aprotinin,
leupeptin, phenylmethanesulfonyl fluoride and pepstatin] and
centrifuged at 14,000 × g for 15 min at 4°C. The protein
concentration in the supernatant was detected using the Coomassie
brilliant blue method. The supernatant was treated with 1X SDS-PAGE
loading buffer at 100°C for 5 min for protein denaturation.
Subsequently, 50 µg of each sample was separated by 10%
SDS-PAGE, transferred onto a polyvinylidene difluoride membrane,
blocked with 5% fat-free milk powder at room temperature for 2 h,
and incubated with primary antibody in TBST/1% non-fat milk at 4°C
overnight, followed by incubation with the appropriate secondary
HRP-labeled IgG at room temperature for 2 h and visualization using
an ECL reagent. The experiment was repeated three times. The
protein bands were visualized using the Molecular Imager system
GDS8000b (UVP, Inc., Upland, CA, USA). Total protein levels were
normalized to the expression of GAPDH on the same membrane, and the
bands were quantified using ImageJ software v1.8.0 (National
Institutes of Health, Bethesda, MD, USA).
The primary antibodies were as follows: Mouse
anti-human CD147 monoclonal antibody (cat. no. ab666, 1:1,000) and
rabbit anti-human MMP-2 monoclonal antibody (cat. no. ab92536,
1:1,000) from Abcam. Mouse anti-human GAPDH monoclonal antibody
(cat. no. sc-47724, 1:2,000) from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The secondary HRP-labeled antibodies (goat
anti-mouse IgG-HRP, cat. no. sc-2005, 1:2,000; goat anti-rabbit
IgG-HRP, cat. no. sc-2004, 1:2,000) were from Santa Cruz
Biotechnology, Inc.
Co-immunoprecipitation assay
The protein was extracted from the cells prior to
and following transfection. Following protein quantification, 6,000
µg of each lysate was added to 1 µg of CD147
monoclonal antibody and agitated at 4°C overnight, followed by the
addition of 40 µl Protein A+G-agarose and agitation at 4°C
for 2 h. The samples were then centrifuged at 2,500 g for 5 min at
4°C and washed three times with lysis buffer as described above to
collect the precipitate. The precipitated protein was mixed with 60
µl of 2X SDS-PAGE loading buffer at 100°C for 5 min for
denaturation. The supernatant (20 µl) was then subjected to
SDS-PAGE. The Lewis y (cat. no. F3, ab3359, 1:500; Abcam)/Lewis x
(cat. no. ab20137, 1:500; Abcam)/sLewis x (cat. no. sc-32243,
1:500; Santa Cruz Biotechnology, Inc.) antibodies were used to
detect the antigens. The remaining steps were the same as described
for the western blot analysis above. The protein for cellular
location was extracted from the cells prior to and following
transfection according to the Membrane Protein Extraction kit's
instructions. The other steps were the same as described above.
Mouse anti-human CD147 monoclonal antibody (cat. no. ab666, 1:200;
Abcam) was used to detect the antigen. The densitometry of the
protein bands was performed using ImageJ software v1.8.0 (National
Institutes of Health).
Confocal laser scanning microscopy
In brief, mouse anti-human Lewis y antibody (cat.
no. F3, ab3359; Abcam) and rabbit anti-human CD147 antibody (cat.
no. ab188190; Abcam) were diluted to 1:100 as primary antibody
solutions; goat anti-rabbit tetramethylrhodamine red
fluorescence-labeled secondary antibody (cat. no. sc-2492; Santa
Cruz Biotechnology, Inc.) and goat anti-mouse fluorescein
isothiocyanate green fluorescence-labeled secondary antibody (cat.
no. sc-2859; Santa Cruz Biotechnology, Inc.) were diluted to 1:200.
The cells were blocked using normal goat serum for 30 min, treated
with primary antibody solutions at 37°C for 1 h, and cultured at
room temperature overnight. Following washing with PBS, the cells
were incubated with secondary antibody solution at 37°C for 1 h,
stained with 4,6-diamidino-2-phenylindole (DAPI) for 5 min, and
then observed under a confocal laser scanning microscope (C1-SI;
Nikon Corporation, Tokyo, Japan). The data were collected by a
computer for digital imaging. For the negative controls, PBS
replaced the primary antibodies.
Cell adhesion assay
The 96-well plates were coated with 60 µg/ml
collagen IV or 12 µg/ml laminin (50 µl/well). The
plates coated with 3 mg/ml polylysine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 1% BSA (Sigma-Aldrich; Merck KGaA) were
used as maximal and minimal adhesion controls, respectively.
Following incubation for 2 h at 37°C, the plates were washed twice
with PBS, and blocked again with 1% BSA for 2 h. The cells were
digested with 0.25% trypsin, centrifuged at 1,000 × g for 5 min at
room temperature, and mixed with serum-free DMEM culture medium to
prepare single-cell suspensions. The cells were diluted to
5×104/ml, added to coated plates (100 µl/well)
and cultured at 37°C in 5% CO2 for 2 h. Following
washing to remove non-adherent cells, the plates were fixed with 4%
paraformaldehyde for 30 min, stained with 0.5% crystal violet (100
µl/well) for 2 h, and then washed twice with cold PBS. The
absorbance at 597 nm (A597 absorbance represents the
adhesive cells) was detected using a microplate reader. The
experiment was repeated three times.
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Quantitative data are
presented as the mean ± standard deviation, and qPCR data are
expressed as the mean ± standard error of the mean. Positive ratios
were evaluated using the χ2 test. Student's t-test was
used for comparisons between two groups and one-way analysis of
variance with the LSD or Bonferroni post hoc test was used for
comparisons between more than two groups. The correlation between
Lewis y antigen and CD147 in ovarian cancer was examined using a
χ2 test. Survival was analyzed using Kaplan-Meier
curves, and significant differences among clinicopathological
variants and immunomarkers were tested using the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of Lewis y antigen and CD147
in the groups of ovarian tissues
Lewis y antigen was upregulated in the 60 EOC
samples analyzed, the expression of which was high in the membrane
and occasional in the cytoplasm. The positive expression rate was
88.33%, which was higher than that of the borderline group (60.00%;
P<0.05) and the benign group (33.33%; P<0.01);
the expression rate of Lewis y in the borderline group was higher
than that of the benign group, however, the difference did not
reach statistical significance (P>0.05). The expression of Lewis
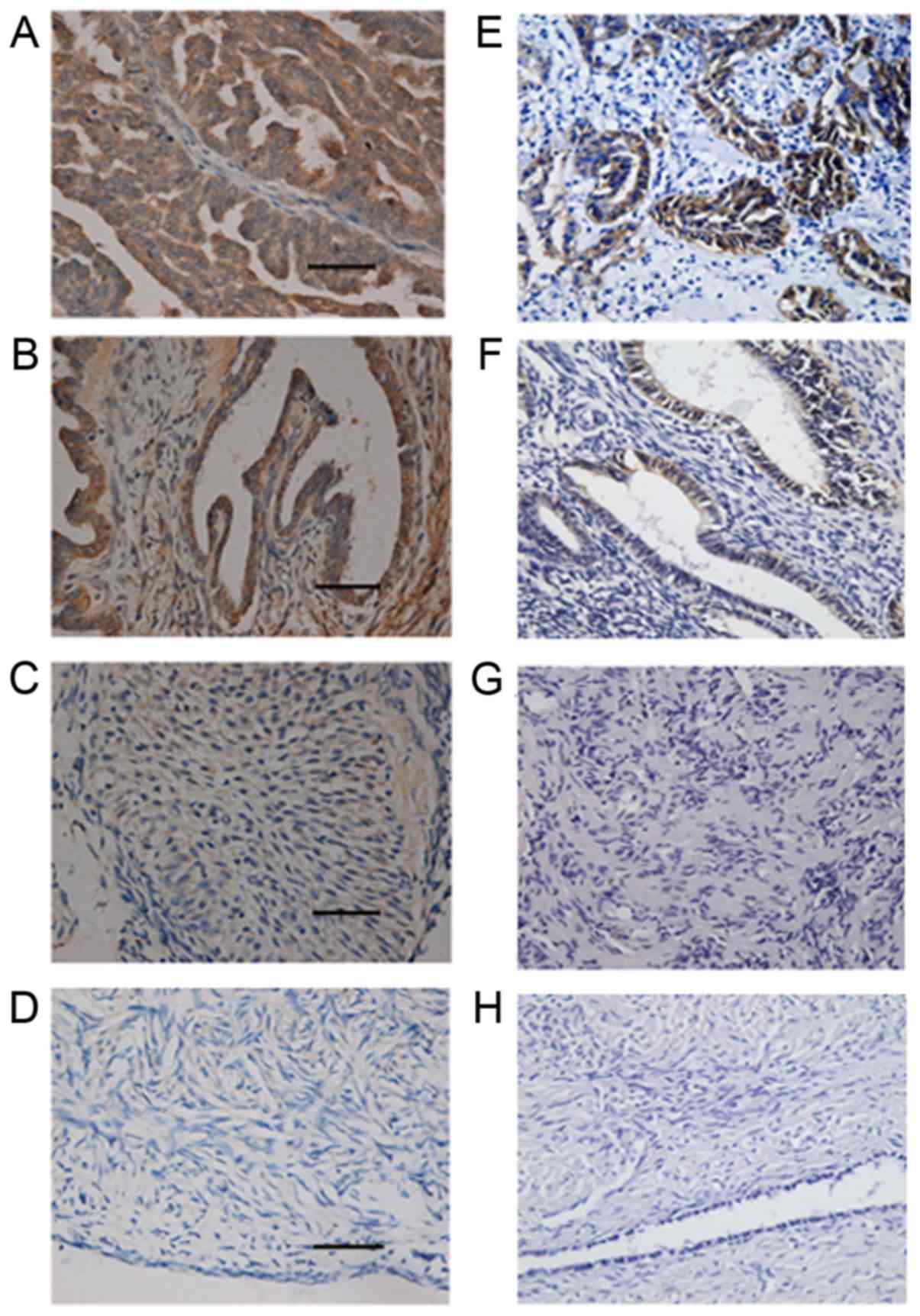
y antigen was negative in the 20 normal ovarian tissues (Fig. 1A-D; Table II).
 | Table IIExpression of Lewis y and CD147 in
different ovarian tissues. |
Table II
Expression of Lewis y and CD147 in
different ovarian tissues.
| Group | Cases (n) | Lewis y antigen
| CD147
|
|---|
| − | + | ++ | +++ | Positive (%) | − | + | ++ | +++ | Positive (%) |
|---|
| Malignant | 60 | 7 | 15 | 20 | 18 | 53 (88.33)a | 12 | 15 | 17 | 16 | 48 (80.00)a |
| Borderline | 30 | 12 | 6 | 11 | 1 | 18 (60.00)b | 15 | 6 | 7 | 2 | 15 (50.00)b |
| Benign | 30 | 20 | 6 | 4 | 0 | 10 (33.33) | 23 | 4 | 3 | 0 | 7 (23.33) |
| Normal | 20 | 20 | 0 | 0 | 0 | 0 (0) | 19 | 1 | 0 | 0 | 1 (5.00) |
The expression pattern of CD147 was similar to that
of Lewis y antigen, with high expression in the cell membrane and
occasional expression in the cytoplasm. The positive expression
rates were 80.00, 50.00, 23.30 and 5.00% in the malignant,
borderline, benign and normal groups, respectively. The highest
positive rate was that of the malignant group, which was higher
than the rates of the borderline, benign and normal ovarian groups
(P<0.05); the positive expression rate of the borderline group
was higher than that of the benign and normal groups (P<0.05);
there was no significant difference in the positive expression rate
between the benign and normal groups (P>0.05) (Fig. 1E-H; Table II).
Correlation between the expression of
Lewis y antigen and CD147 and the clinicopathological parameters of
ovarian cancer
The positive expression rate of the Lewis y antigen
was 90.00% in ovarian serous cystadenocarcinoma and 81.82% in
ovarian mucinous cystadenocarcinoma, with no significant difference
between the two (P>0.05). In endometrioid and clear cell
carcinomas, high expression rates of Lewis y were observed.
Positive expression of Lewis y was present in 95.24% of patients
with stages III-IV ovarian cancer, and was higher than that of
patients with stage I-II ovarian cancer (84.62%), however, this
difference was statistically significant (P>0.05). The positive
expression rates of Lewis y antigen in the well-, moderate, and
poorly differentiated groups were 80.95, 85.71 and 100%,
respectively. The degree of differentiation was inversely
correlated with the positive expression rate, although the
differences between the groups were not statistically significant
(P>0.05). The positive rate of Lewis y in the lymphatic node
metastasis group (100.0%) was higher than that in the
non-metastasis group (85.43%), although the difference was not
significant (P>0.05) (Table
III).
 | Table IIIAssociation between Lewis y and CD147
expression and pathological features. |
Table III
Association between Lewis y and CD147
expression and pathological features.
| Feature | Cases (n) | Lewis y antigen
| CD147
|
|---|
| Positive cases
(n) | Rate (%) | P-value | Positive cases
(n) | Rate (%) | P-value |
|---|
| Pathological
type | | | | | | | |
| Serous | 30 | 27 | 90.00 | >0.05 | 26 | 86.67 | >0.05 |
| Mucous | 22 | 18 | 81.82 | | 14 | 63.64 | |
| Endometrioid | 3 | 3 | 100.00 | | 3 | 100.00 | |
| Clear cell | 5 | 5 | 100.00 | | 5 | 100.00 | |
| FIGO stage | | | | | | | |
| I-II | 39 | 33 | 84.62 | >0.05 | 28 | 71.79 | <0.05a |
| III-Ⅳ | 21 | 20 | 95.24 | | 20 | 95.24 | |
| Differentiation
level | | | | | | | |
| Well | 21 | 17 | 80.95 | >0.05 | 15 | 71.43 | >0.05 |
| Moderate | 21 | 18 | 85.71 | | 16 | 76.19 | |
| Poor | 18 | 18 | 100.00 | | 17 | 94.44 | |
| Lymphatic
metastasis | | | | | | | |
| No | 48 | 41 | 85.42 | >0.05 | 36 | 75.00 | <0.05a |
| Yes | 12 | 12 | 100.00 | | 12 | 100.00 | |
The positive expression rates of CD147 in ovarian
serous cystadenocarcinoma and ovarian mucinous cystadenocarcinoma
were 86.67 and 73.33%, respectively, which were not significantly
different (P>0.05). CD147 was detected in 20 cases of stage
III-IV EOC (95.24%), and its expression was significantly higher
than that of stage I-II EOC (71.79%) (P<0.05). The positive
expression rates of CD147 in the well-, moderate, and poorly
differentiated groups were 71.43, 76.19 and 94.24, respectively.
The degree of differentiation was inversely correlated with the
positive expression rate of CD147, although the difference was not
statistically significant (P>0.05). The positive rate of CD147
in the lymphatic node metastasis group (100.0%) was higher than
that in the non-metastasis group (75.00%) and this difference was
statistically significant (P<0.05) (Table III).
Relevance of the expression of Lewis y
and CD147 in ovarian cancer
Of the 60 ovarian cancer tissues samples, 46 were
positive for the expression of both Lewis y and CD147 and five were
negative for both. A positive, significant correlation between
Lewis y and CD147 was observed in ovarian cancer
(χ2=9.71, P<0.01; Table IV).
 | Table IVRelevance of the expression of Lewis
y and CD147 in ovarian cancer. |
Table IV
Relevance of the expression of Lewis
y and CD147 in ovarian cancer.
| Lewis y | CD 147
| Total (n) |
|---|
| Positive (n) | Negative (n) |
|---|
| Positive | 46 | 7 | 53 |
| Negative | 2 | 5 | 7 |
| Total | 48 | 12 | 60 |
Survival analysis
In the 60 patients with EOC, four were lost to
follow-up, and the remaining 56 patients were regularly followed up
to April 2017, with a follow-up time of 12-86 months, and 27 cases
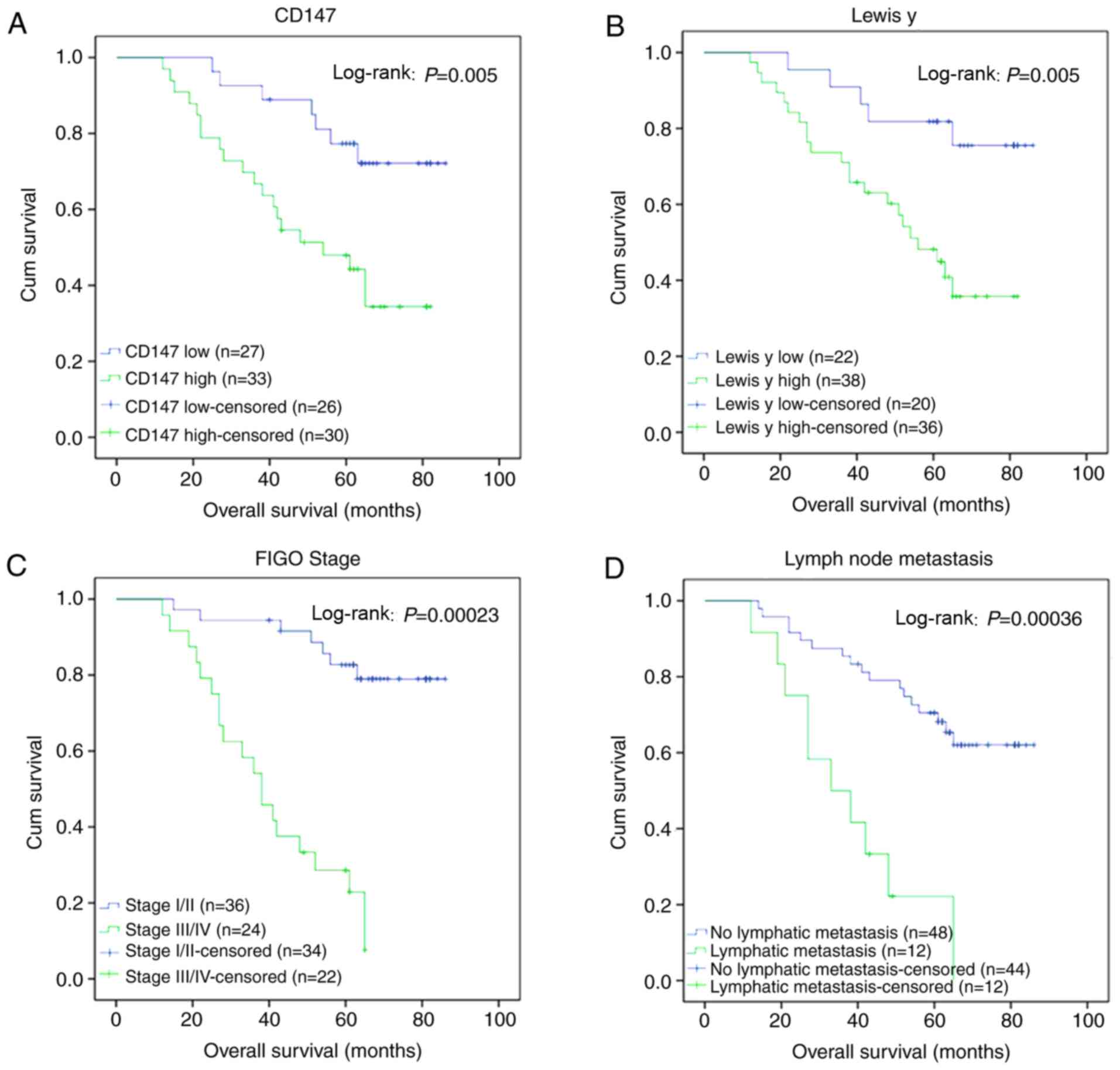
of mortality. Kaplan-Meier analysis of the patient survival rates
showed that the survival rate of patients with a high expression of
CD147 was lower than that of patients with a low expression of
CD147 (log-rank P=0.005, Fig.
2A). Similarly, the survival rate of patients with a higher
expression of Lewis y antigen was lower than of patients with lower
expression (log-rank P=0.005, Fig.
2B). The mortality rate of patients with pathological stages
III-IV (Fig. 2C) and lymph node
metastasis (Fig. 2D) was
significantly higher than that of patients with pathological stages
I-II and without lymph node metastasis (P=0.00023 and 0.00036,
respectively).
Expression of Lewis y antigen and CD147
in ovarian cancer cells
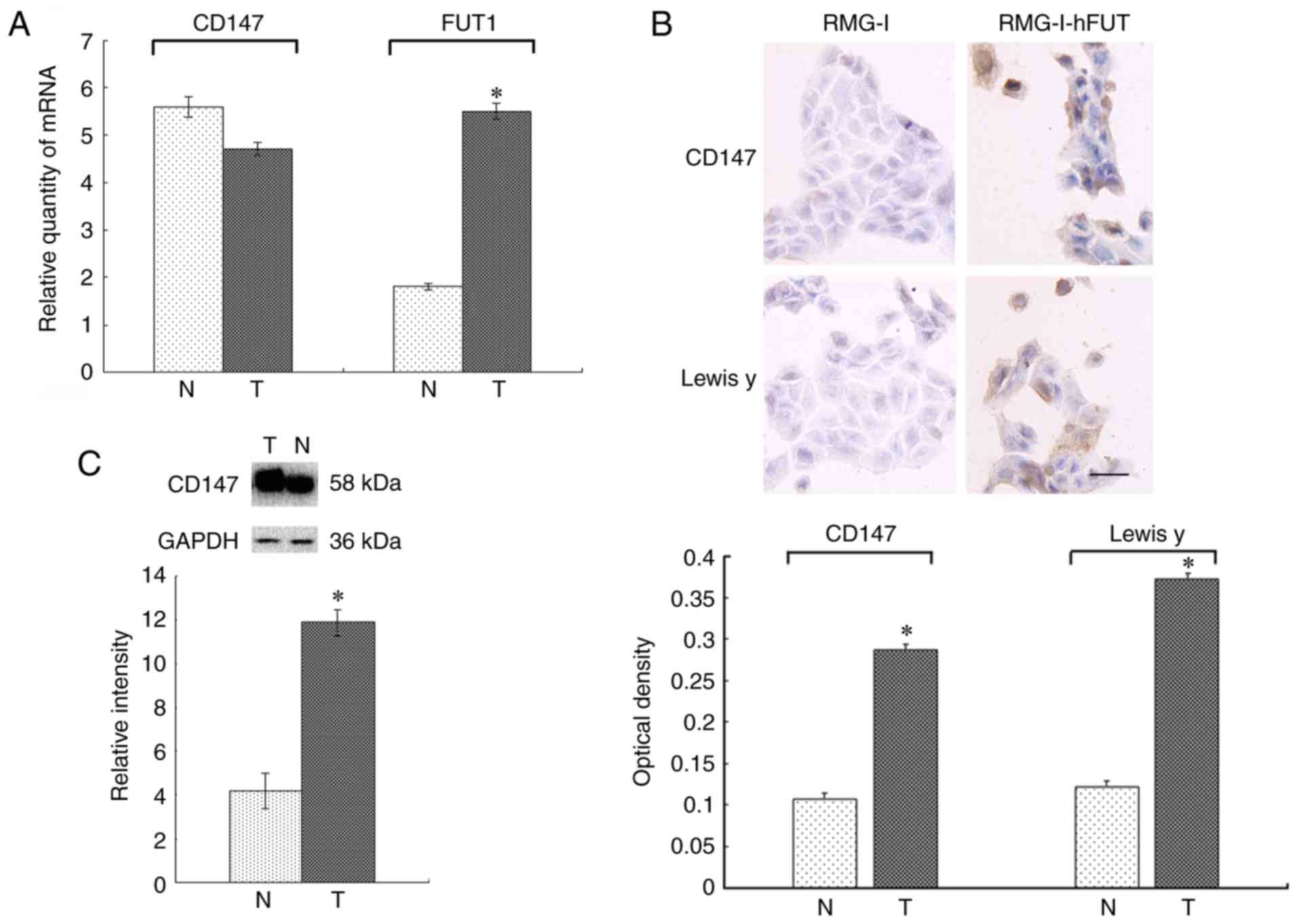
The RT-qPCR results are shown in Fig. 3A. The mRNA expression of CD147 was
lower in the RMG-I-hFUT cells than in the RMG-I cells, although the
difference was not significant (P>0.05). The mRNA expression of
FUT1 was 3.07-fold higher in the RMG-I-hFUT cells than in the RMG-I
cells (P<0.05).
The immunocytochemical staining revealed that
positive CD147 staining was predominantly located in the cell
membrane of the RMG-I cells, where it was detected as light-yellow
granules. The average optical density was 0.107±0.001. Positive
CD147 staining in the RMG-I-hFUT cells was widely located in the
membrane and cytoplasm, and was detected as brown granules. The
average optical density was 0.287±0.002, which was significantly
higher than that of the RMG-I cells (P<0.05; Fig. 3B). The expression pattern was
comparable between Lewis y and CD147, with expression mainly in the
cell membrane and occasionally in the cytoplasm. The expression of
Lewis y antigen was significantly higher in the RMG-I-hFUT cells
than in the RMG-I cells (P<0.05; Fig. 3B).
The expression of CD147 determined by western
blotting was similar to that detected by immunocytochemical
staining. The expression of CD147 was 2.43-fold higher in the
RMG-I-hFUT cell line than in the RMG-I cells (P<0.05; Fig. 3C).
Co-expression of Lewis y antigen and
CD147 in ovarian cancer cells
Co-expression of Lewis y antigen and CD147 in the
RMG-I cell line was detected using immunoprecipitation. The Lewis y
antigen was predominantly expressed in the highly glycosylated form
of CD147 (40-60 kDa, Fig. 4A). An
unidentified 26-kDa form of CD147 was also found containing the
Lewis y antigen structure (Fig.
4A). No expression of CD147 or Lewis y antigen was present in
the negative control (Fig.
4A).
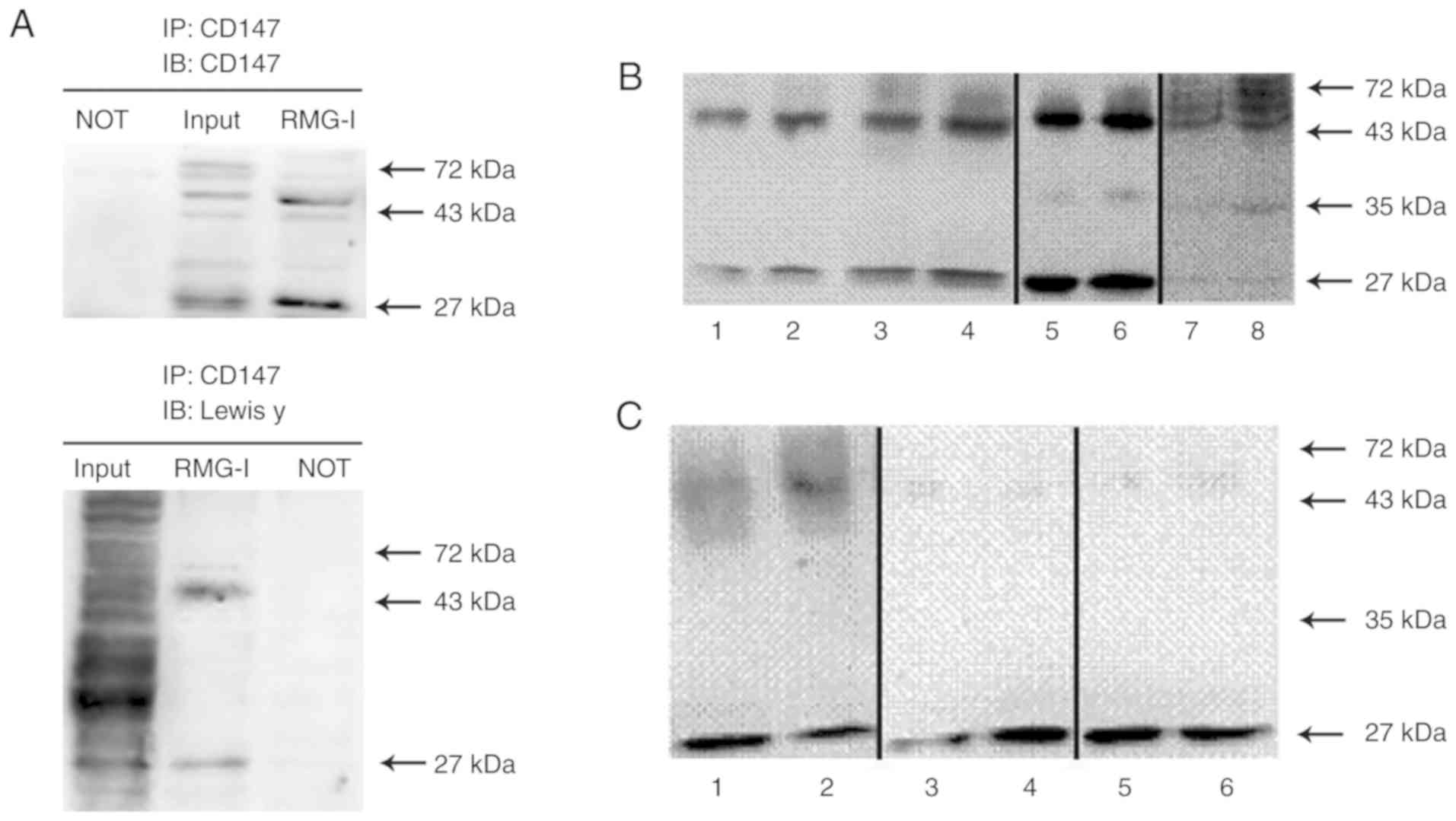 | Figure 4Co-expression of Lewis y antigen and
CD147 in ovarian cancer cells. Bands 1, 3, 5 and 7, RMG-I cells; 2,
4, 6, and 8, RMG-I-hFUT cells. (A) Cell lysates from RMG-I cells
were immunoprecipitated with anti-CD147 antibody, then
immunoblotted with anti-CD147 and anti-Lewis y antibodies. (B)
Bands 1-6, CD147 levels in precipitation samples following the
addition of 1 µg anti-CD147 antibody to 1,000 µg of
protein. Bands 1 and 2, CD147 levels in equal cytoplasmic
precipitation samples; bands 3 and 4, CD147 levels in equal
membrane precipitation samples; bands 5 and 6, CD147 levels in
total protein precipitation samples; bands 7 and 8, CD147 levels in
150 µg total protein precipitation samples. The samples of
bands 1-4, 5/6, and 7/8 were fresh samples collected at different
times, and the experiment was conducted immediately following
sample collection. (C) Bands 1-6, levels of glycosylated CD147
following the addition of 1 µg CD147 antibody to 6,000
µg of protein. The samples of bands 1/2, 3/4, and 5/6 were
transferred onto polyvinylidene difluoride membranes and the
membranes were incubated with different primary antibodies. Bands 1
and 2, Lewis y antigen level; bands 3 and 4, Lewis × antigen level;
bands 5 and 6, sLewis × antigen level. NOT, anti-IgG antibody
(negative control). |
The expression level of CD147 was higher following
transfection than prior to transfection. The unidentified 26-kDa
band detected in all samples showed changes in expression in
accordance with the highly glycosylated form of CD147 (40-60 kDa;
P<0.05, Fig. 4B). The lower
glycosylated form of CD147 (36 kDa) was weakly expressed in the
total protein lysates and in the CD147 immunoprecipitation samples
(Fig. 4B, lanes 5-8), whereas it
was undetected in the protein membrane and cytoplasmic
immunoprecipitation samples and in the Lewis y antigen
immunoprecipitation samples (Fig.
4B, lanes 1-4). Cellular colocalization experiments showed that
the highly glycosylated form and the 26-kDa form of CD147 were
expressed in the cell membrane and cytoplasm in the RMG-I and
RMG-I-hFUT cell lines. In addition, the expression of CD147 was
higher in the membrane and cytoplasm of the RMG-I-hFUT cells than
in the RMG-I cells (P<0.05; Fig.
4B, lanes 1-4).
The expression levels of the glycosylated antigen
CD147 in cells prior to and following transfection were examined by
immunoprecipitation. The results showed that Lewis y antigen was
predominantly expressed in the highly glycosylated form of CD147
and its expression was higher following cell transfection than
prior to transfection (P<0.05; Fig. 4C, lanes 1 and 2). Under the same
conditions, Lewis x and sialyl Lewis x showed weak expression in
the highly glycosylated form of CD147 (Fig. 4C, lanes 3-6). The Lewis y, Lewis
x, and sialyl Lewis x antigens were expressed at high levels in the
26 kDa form of CD147. The expression of Lewis y antigen in the 26
kDa CD147 was lower in the RMG-I-hFUT cells than in the RMG-I cells
(P<0.05, Fig. 4C, lanes 1 and
2). Both sialyl Lewis x and Lewis y showed higher expression levels
in the 26 kDa form of CD147 in cells prior to transfection, whereas
Lewis x showed higher expression levels in cells following
transfection (Fig. 4C, lanes
3-6).
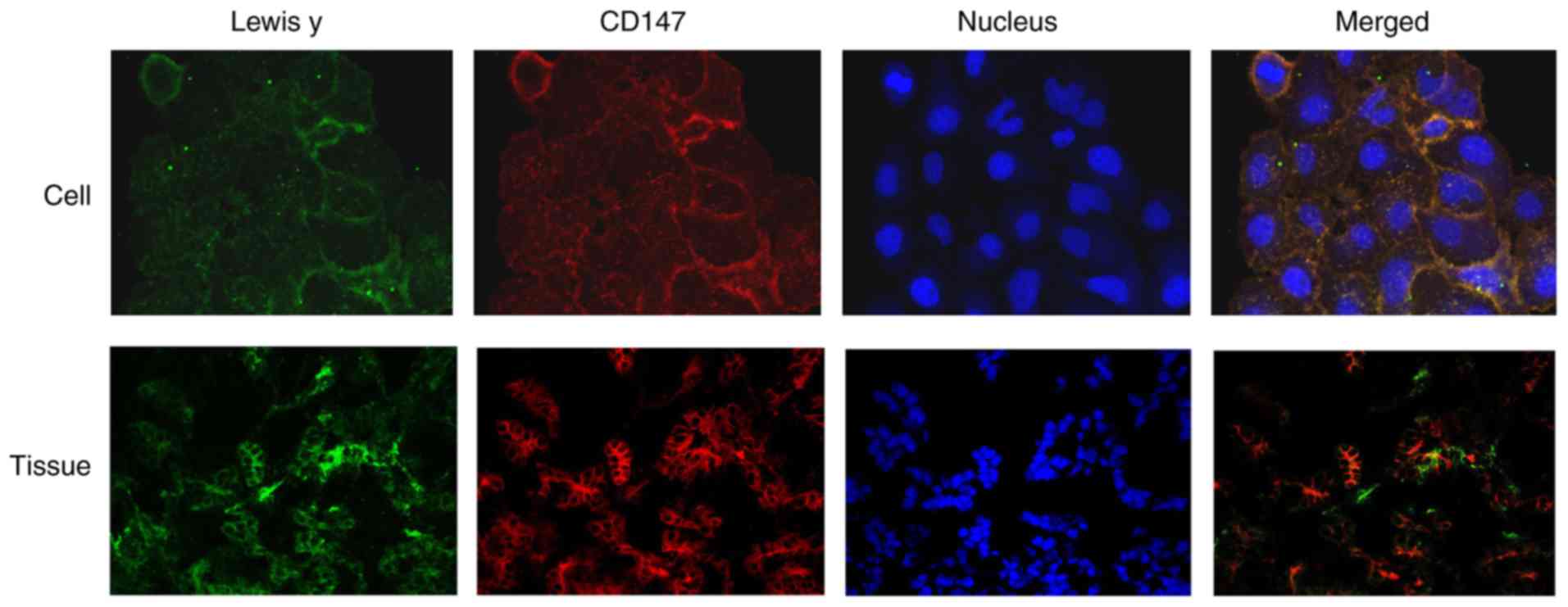
In the double fluorescence confocal experiment,
Lewis y (green) and CD147 (red) were predominantly located in the
cell membrane and partly in the cytoplasm; the green and red
fluorescent signals were higher at the edge of the cells. As shown
in Fig. 5, most of the green and
red fluorescence overlapped, as shown by the yellow fluorescence,
indicating the colocalization of CD147 and Lewis y.
Immunofluorescence double labeling showed the CD147
antigen as red fluorescence in EOC tissues, and the fluorescence
was primarily detected in the cell membrane. The green fluorescence
corresponded to Lewis y, which was also primarily detected in the
cell membrane, with occasional signal in the cytoplasm. The blue
fluorescence indicated the nuclei stained by DAPI. Image analysis
software was used to analyze the three fluorescence signals, and
yellow fluorescence appeared in the position of the red and green
signals, indicating the colocalization of Lewis y antigen and CD147
(Fig. 5).
Determination of the adhesive ability of
the RMG-I and RMG-I-hFUT cell lines on collagen IV and laminin
To examine the adhesive ability of the RMG-I and
RMG-I-hFUT cells, 96-well plates were coated with 60 µg/ml
of collagen IV or 12 µg/ml of laminin. The adhesive values
of the RMG-I-hFUT cell line on collagen IV and on laminin were
2.191±0.042 and 2.403±0.047, respectively. These values were
significantly higher than those of the RMG-I cell line, which were
1.198±0.090 and 1.582±0.142, respectively (P<0.05; Table V). However, treatment with the
anti-Lewis y monoclonal antibody significantly decreased the
adhesive abilities of the RMG-I and RMG-I-hFUT cells to 46.0 and
27.2%, respectively, on collagen IV (P<0.05; Table V), and to 36.2 and 24.7%,
respectively, on laminin (P<0.05; Table V). Treatment with anti-CD147
monoclonal antibody yielded similar results. The adhesive abilities
of the RMG-I and RMG-I-hFUT cells on collagen IV decreased to 55.7
and 31.4%, respectively (P<0.05, Table V), and on laminin to 55.4 and
38.5%, respectively (P<0.05, Table
V). Compared with the corresponding controls, there was no
significant difference in the cell adhesive abilities prior to or
following treatment (P>0.05, Table
V).
 | Table VDetermination of the adhesive
abilities, represented by the absorbance at 597 nm, of the RMG-I
and RMG-I-hFUT cell lines on collagen IV and laminin. |
Table V
Determination of the adhesive
abilities, represented by the absorbance at 597 nm, of the RMG-I
and RMG-I-hFUT cell lines on collagen IV and laminin.
| Group | Adhesive ability
(collagen IV)
| Adhesive ability
(laminin)
|
|---|
| RMG-I | RMG-I-hFUT | RMG-I | RMG-I-hFUT |
|---|
| Negative
control | 1.198±0.090 | 2.191±0.042a | 1.582±0.142 | 2.403±0.047a |
| Lewis y
antibody | 0.550±0.011b | 0.595±0.023b | 0.573±0.009b | 0.594±0.036b |
| CD147 antibody | 0.667±0.050b | 0.689±0.040b | 0.877±0.026b | 0.926±0.034b |
| IgM control | 1.549±0.113 | 2.068±0.076a | 1.416±0.082 | 2.259±0.151a |
Expression of MMP-2 in ovarian cancer
cells
The extracellular matrix (ECM) is a major barrier to
tumor metastasis. MMPs are important enzymes that degrade the ECM.
MMP-2, which can hydrolyze the main component type IV collagen,
serves an important role in the invasion and metastasis of
malignant tumors. As shown in Fig. 6A
and B, the expression of MMP-2 was upregulated by 3.64-fold
over the untransfected value in the RMG-I-hFUT cells (P<0.01).
To determine whether the upregulation of MMP-2 was associated with
increased expression of the Lewis y antigen on the cell surfaces of
CD147 and CD147, the cells were treated with anti-Lewis y antibody
and anti-CD147 antibody, respectively. As shown in Fig. 6A and B, in the presence of the
anti-Lewis y antibody and CD147 antibody, the expression of MMP-2
and the differences in expression intensities between the two cell
lines were significantly decreased.
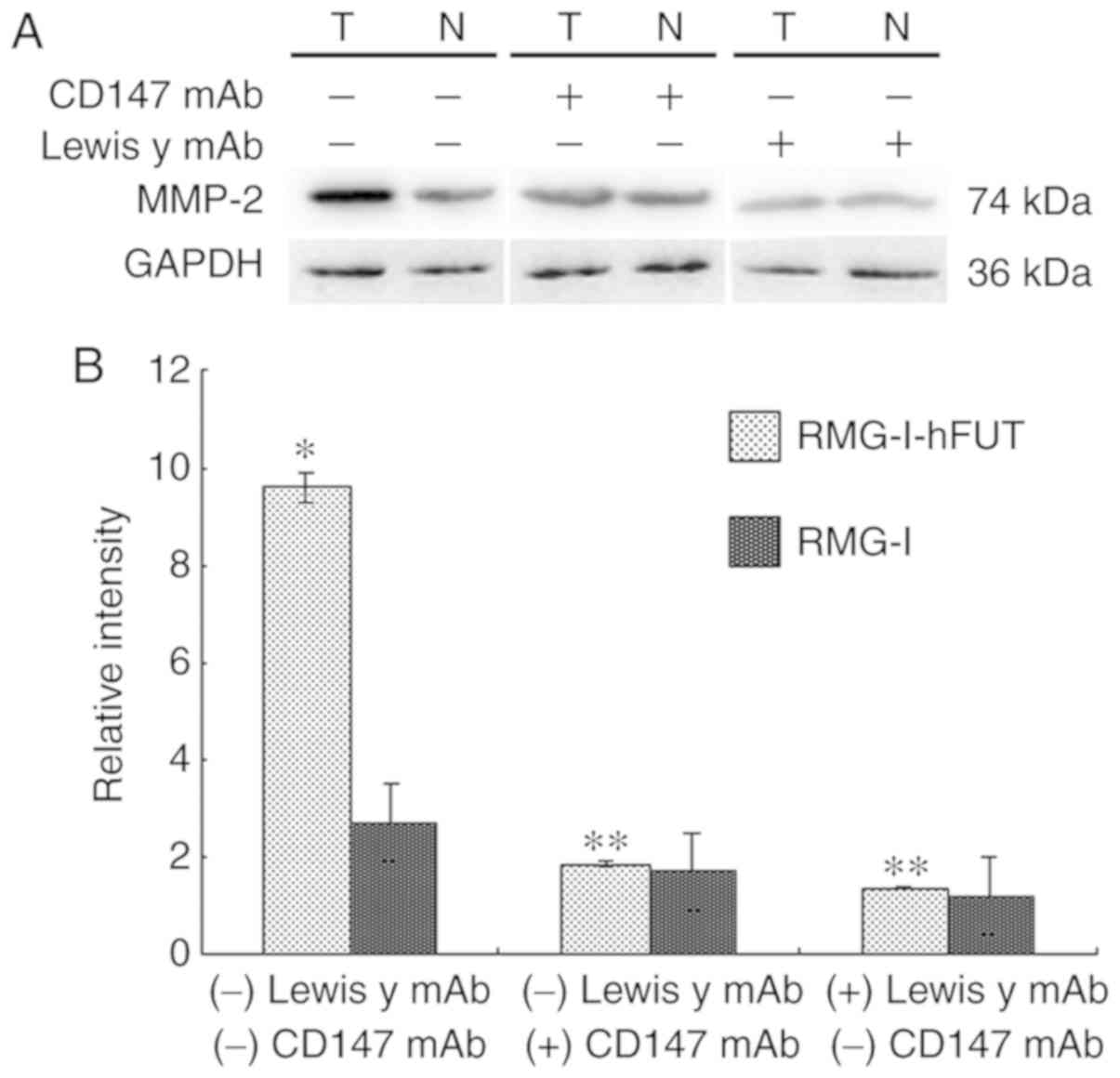 | Figure 6Effect of FUT1 transfection on the
expression of MMP-2, and the effect of anti-Lewis y antibody and
anti-CD147 antibody on the expression of MMP-2. Western blot
detection of the expression of MMP-2 in RMG-I and RMG-I-hFUT cells,
and in the absence and presence of anti-Lewis y antibody and
anti-CD147 antibody, respectively. (A) Representative western blots
of MMP-2 in the cell lines. (B) Densitometric quantification of the
protein expression (n=3). For the inhibition assay, the final
concentration of anti-Lewis y antibody was 20 µg/ml and the
final concentration of anti-CD147 antibody was 10 µg/ml. The
duration of treatment was 1 h. *P<0.05, vs. RMG-I;
**P<0.05, vs. RMG-I or RMG-I-hFUT cells without
anti-Lewis y antibody or anti-CD147 antibody treatment. FUT1,
α1,2-fucosyltransferase; MMP-2, matrix metalloproteinase; mAb,
monoclonal antibody; N, RMG-I cells; T, RMG-I-hFUT cells. |
Discussion
The main metastatic pathway of ovarian cancer is
intraperitoneal dissemination and adhesion. Invasion and metastasis
of malignant carcinoma are complex processes with multiple elements
and steps, including infiltration of the primary neoplasm,
degradation of the basement membrane, invasion into the blood
vessels of tumor cells and the invasion of tumor cells into target
tissues (21). CD147 is not only
a cell surface adhesion molecule that mediates cell adhesion, it is
also an inducer or extracellular MMPs, which are important in tumor
invasion and metastasis. Zhang et al (22) showed that CD147 can stimulate the
expression of MMP in hepatocellular carcinoma cells, modulate the
secretion of MMPs from surrounding fibroblasts, and promote the
infiltration and metastasis of tumor cells. CD147 is upregulated in
several types of tumor, including endometrial carcinoma, bone giant
cell tumor and urinary tumors. The expression of CD147 in certain
tumors increases in correlation with the malignancy of tumors, and
CD147 is correlated with the infiltration and metastasis of tumors
(23). Jin et al (24) reported that CD147 is upregulated
in malignant ovarian carcinoma and is closely associated with stage
and differentiation of serous cystadenocarcinoma. Sillanpää et
al (25) showed that, in
contrast to other types of ovarian carcinoma, serous
cystadenocarcinoma exhibits a low expression of CD147 that is
associated with tumor stage. In the present study, the expression
of CD147 was positively correlated with the malignancy of tumors,
and the positive expression rate of CD147 increased with clinical
stage, although it was not associated with the histological type or
degree of differentiation.
Carbohydrate chains in the cell membrane are an
important medium of communication among cells and between cells and
the external environment, and they are involved in cell signal
transduction pathways. Fucose is the final form in the synthesis of
carbohydrates, and following the glycosylation of fucose,
carbohydrate chains usually stop synthesis. The Lewis y antigen is
a difucosylated oligosaccharide and an important marker for in
determining the diagnosis and prognosis of several types of cancers
(26). Our previous and present
studies have been committed to examination of the role of Lewis y
antigen in the development of ovarian cancer and its mechanism of
action. Our previous studies showed that Lewis y antigen, a
tumor-associated antigen, promotes the proliferation, adhesion,
invasion, metastasis and drug resistance in ovarian cancer cell
lines (6-8). In the present study, the analysis of
tissue specimens showed that Lewis y antigen was overexpressed in
ovarian carcinoma, with a positive expression rate of 88.33%, which
was higher than that in the borderline and benign groups (60.00 and
33.33%; P<0.05 and P<0.01, respectively). In addition, the
expression of Lewis y was positively correlated with the grade of
malignancy (P<0.05) and disease stage. The results were not only
consistent with our previous results (27), but also further verified the
original results on the original basis. In addition to the
recollection of samples, the follow-up time of the patients was
extended. The present study focused on the association between the
levels and structures of CD147 and Lewis y in ovarian tissues,
determining whether CD147 has Lewis y glycosylation modification,
and examining the role of co-expressed CD147 and Lewis y in the
development of ovarian cancer.
CD147, which was originally cloned as a carrier of
the Lewis x antigen (28), is
involved in a series of biological processes as a main substrate of
N-acetylglucosamine glycosyltransferase V (9). The present results showed that the
expression of CD147 increased significantly in correlation with the
upregulation of Lewis y antigen following transfection with FUT1
(P<0.05). The results of the immunofluorescence and
immunoprecipitation assays demonstrated that Lewis y was one of the
components of CD147. In ovarian cancer cell lines, Lewis y was
predominantly detected in the highly glycosylated 26 kDa form of
CD147. In addition, compared with the parental cell lines, Lewis y
was significantly upregulated in the highly glycosylated form of
CD147 in the RMG-I-hFUT transfected cell line, whereas it was
significantly downregulated in the 26 kDa form (P<0.05). Under
the same conditions, Lewis x and sLewis x antigens were expressed
at low levels in the highly glycosylated form of CD147. This
indicates that, in the highly glycosylated form of CD147, most of
the Lewis x antigen was changed into Lewis y antigen under the
catalytic action of FUT1. Compared with sialytransferase, FUT1
exhibited a higher catalytic ability toward the substrate Lewis x,
resulting in the formation of the main product Lewis y and low
levels of sLewis x. This suggested that FUT1 had modification
priority towards the highly glycosylated form of CD147 but not the
26 kDa form, due to the enrichment of the lactosaminoglycan
constituent of the highly glycosylated form (29).
An unidentified 26 kDa protein band was detected in
the total protein lysate of the RMG-I and RMG-I-hFUT cell lines.
This band was observed in the cell membrane and the cytoplasm. As
the molecular weight of the CD147 core protein is 27 kDa, this
unknown 26 kDa protein band may be a form of membrane shedding
(30) or the subtype basigin-3
(31). Loss of solubility is an
important process in the functional regulation of several membrane
proteins. A 22 kDa form of CD147 detected in the HT1080 and A431
human cancer cell lines was suggested to be a form of membrane
shedding. Highly glycosylated CD147, which has a molecular weight
of 22 kDa (the molecular weight is ~10 kDa post-deglycosylation),
can still induce the production of MMPs (30). Belton et al (31) suggested that the 25 kDa form of
basigin-3 is a critical subtype of basigin. In human tumor cells,
the expression level of basigin-3 is <3% of that of basigin-2, a
main subtype of basigin. In immunoprecipitation samples, basigin-3
is mainly expressed in the cytoplasm. Following treatment with
recombinant human basigin, basigin-3 is expressed in the cell
membrane and cytoplasm (31). The
results of the present study showed that, although the 26 kDa form
of CD147 was expressed in the cell membrane and cytoplasm, it was
weakly expressed in the total protein samples. The
immunoprecipitation assays showed that this protein was highly
glycosylated and expressed the Lewis y, Lewis x and sLewis x
antigens. As previously reported, CD147 is differentially expressed
in different tissues and cell types (15). The membrane shedding form may be a
soluble form of basigin-3, which requires further
investigation.
The present cellular colocalization assay showed
that both the highly glycosylated form and the 26 kDa form of CD147
were expressed in the cell membrane and in the cytoplasm. In
addition, the two forms of CD147 were expressed at higher levels in
the RMG-I-hFUT cells than in the RMG-I cells (P<0.05). Taylor
et al (32) demonstrated
that the release of a small proportion of activated CD147 from the
cell surface of breast cancer into the culture medium was not
associated with proteinase shearing action. As with cellular CD147,
soluble CD147 maintained the original C- and N- termini.
A previous study suggested that soluble CD147 is
released as a mechanism of cystic shedding (33). The tumor promoter phorbol
12-myristate 13-acetate, which activates the protein kinase
C/Ca2+ and ERK1/2 signaling pathways, can significantly
induce the expression of soluble CD147, suggesting that the cystic
release of CD147 is controlled by cellular signal transduction
(33). Our previous study also
demonstrated that the Lewis y antigen can induce the
phosphorylation of ERK, resulting in the malignant progression of
ovarian cancer (34). In tumor
cells, the production of CD147 is a positive feedback cascade
response. Soluble CD147 may have other biological functions, as it
is important in the production of distant fibroblasts and
endothelial cells (35).
The results of the present study showed that the
mRNA expression of CD147 was marginally decreased (P>0.05) in
the transfected cells, whereas the protein expression of CD147 was
significantly upregulated (P<0.05). This suggests that the
changes in the expression levels of CD147 may be due to protein
N-glycosylation rather than regulation at the transcriptional
level. This may be associated with glycosylation-mediated changes
in the function of relevant transport proteins (36) and the ubiquitin proteasome-induced
inhibition of protein degradation (37).
The role of FUT1 glycosylation in the sugar chain of
CD147 remains to be elucidated. Lewis y antigen was expressed at
high levels in the highly glycosylated form of CD147, which can
induce the expression of MMP (15). This suggests that Lewis y
regulates the expression of CD147. The present study demonstrated
that the expression of MMP-2 was significantly higher in the
RMG-I-hFUT cell line than in the RMG-I cells, whereas its
expression decreased significantly following treatment with Lewis y
antibodies and CD147 antibodies.
Previous studies have shown that CD147 promotes
tumor invasion by inducing tumor cell adhesion and spreading in
integrin-dependent or anchorage-independent growth (38,39). In our previous study, it was
demonstrated that Lewis y, as an important component of integrin
α5β1 and CD44, is involved in the process of cell spreading and
promotes the adhesion and spread of transfected cells on
fibronectin and hyaluronic acid (8,40).
It has been suggested that the CD147 molecular chaperone MCT4 and
integrin β1 can interact with each other and contribute to tumor
metastasis. In addition, CD147, MCT4 and integrin β1 can regulate
cell adhesion and migration by forming a supramolecular complex
(41). The basement membrane
serves important roles in tumor progression. Its main components,
collagen IV and laminin, have been used as substrates in cell
adhesion experiments. The adhesive ability of FUT1-transfected
cells on collagen IV or laminin improved significantly (P<0.05)
and was markedly inhibited by anti-Lewis y and CD147 antibodies
(P<0.05). In addition, the suppressive effect of anti-Lewis y
was more marked than that of anti-CD147. This suggests that the
effect of Lewis y antigen on upregulating the expression of CD147
is accompanied by the upregulation of relevant adhesive molecules,
including integrins, which are involved in the regulation of cell
adhesion.
In conclusion, the Lewis y antigen and CD147 were
significantly upregulated in ovarian tumors, suggesting that they
promote the development of each other. Lewis y antigen is an
important component of the highly glycosylated CD147 molecule and
can therefore induce the expression of CD147 and CD147-mediated
MMP-2 in the RMG-I ovarian cancer cell line, resulting in increased
tumor adhesion and metastasis. The overexpression of Lewis y
antigen on the surface of ovarian cancer cells is a potential
therapeutic target for the treatment of ovarian tumors.
Funding
This study was supported by grants from The National
Natural Science Foundation of China (grant nos. 81172491, 81101527,
81472437 and 81672590) and the Outstanding Scientific Fund of
Shengjing Hospital (grant no. 201303).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and BL contributed to conception and design of
the study. JL, QL and YW contributed to the acquisition, analysis
and interpretation of the data, and were major contributors in
writing the manuscript. QL, ML and YQ contributed to the
acquisition of the data. QL and JG collected the clinical
specimens. JL, YW and BL contributed to the revision of the
manuscript. QL, ML, YQ and JG contributed to analysis and
interpretation of the data and to revision of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Samples were fully encoded to protect patient
confidentially. The study and its protocols were approved by the
Research Ethics Committees of Shengjing Hospital Affiliated with
China Medical University (no. 2013PS66K). The Ethics Committee
waived the requirement for patient consent, as the patient
information was withheld.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
McKenzie AJ, Campbell SL and Howe AK:
Protein kinase A activity and anchoring are required for ovarian
cancer cell migration and invasion. PLoS One. 6:e265522011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lorkova L, Pospisilova J, Lacheta J,
Leahomschi S, Zivny J, Cibula D, Zivny J and Petrak J: Decreased
concentrations of retinol-binding protein 4 in sera of epithelial
ovarian cancer patients: A potential biomarker identified by
proteomics. Oncol Rep. 27:318–324. 2012.
|
|
3
|
Phillips ML, Nudelman E, Gaeta FC, Perez
M, Singhal AK, Hakomori S and Paulson JC: ELAM-1 mediates cell
adhesion by recognition of a carbohydrate ligand, sialyl-Lex.
Science. 250:1130–1132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crucho CI, Correia-da-Silva P, Petrova KT
and Barros MT: Recent progress in the field of glycoconjugates.
Carbohydr Res. 402:124–132. 2015. View Article : Google Scholar
|
|
5
|
Wang ST, Liu JJ, Wang CZ, Lin B, Hao YY,
Wang YF, Gao S, Qi Y, Zhang SL and Iwamori M: Expression and
correlation of Lewisy antigen and TGF-β1 in ovarian epithelial
carcinoma. Oncol Rep. 27:1065–1071. 2012. View Article : Google Scholar
|
|
6
|
Liu J, Lin B, Hao Y, Qi Y, Zhu L, Li F,
Liu D, Cong J, Zhang S and Iwamori M: Lewisy antigen promotes the
proliferation of ovarian carcinoma-derived RMG-I cells through the
PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 28:1542009.
View Article : Google Scholar
|
|
7
|
Iwamori M, Tanaka K, Kubushiro K, Lin B,
Kiguchi K, Ishiwata I, Tsukazaki K and Nozawa S: Alterations in the
glycolipid composition and cellular properties of ovarian
carcinoma-derived RMG-I cells on transfection of the alpha
1,2-fucosyltransferase gene. Cancer Sci. 96:26–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan LM, Lin B, Zhu LC, Hao YY, Qi Y, Wang
CZ, Gao S, Liu SC, Zhang SL and Iwamori M: Enhancement of the
adhesive and spreading potentials of ovarian carcinoma RMG-1 cells
due to increased expression of integrin alpha5beta1 with the Lewis
Y-structure on transfection of the alpha1,2-fucosyltransferase
gene. Biochimie. 92:852–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanekura T and Chen X: CD147/basigin
promotes progression of malignant melanoma and other cancers. J
Dermatol Sci. 57:149–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nabeshima K, Iwasaki H, Koga K, Hojo H,
Suzumiya J and Kikuchi M: Emmprin (basigin/CD147): Matrix
metalloproteinase modulator and multifunctional cell recognition
molecule that plays a critical role in cancer progression. Pathol
Int. 56:359–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gabison EE, Hoang-Xuan T, Mauviel A and
Menashi S: EMMPRIN/CD147, an MMP modulator in cancer, development
and tissue repair. Biochimie. 87:361–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Wang L, Beretov J, Hao J, Xiao W
and Li Y: Co-expression of CD147/EMMPRIN with monocarboxylate
transporters and multiple drug resistance proteins is associated
with epithelial ovarian cancer progression. Clin Exp Metastasis.
27:557–569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou W, Yang H, Hou X, Zhang W, Chen B and
Xin X: Inhibition of CD147 gene expression via RNA interference
reduces tumor cell invasion, tumorigenicity and increases
chemosensitivity to paclitaxel in HO-8910 pm cells. Cancer Lett.
248:211–218. 2007. View Article : Google Scholar
|
|
14
|
Davidson B, Goldberg I, Berner A,
Kristensen GB and Reich R: EMMPRIN (extracellular matrix
metalloproteinase inducer) is a novel marker of poor outcome in
serous ovarian carcinoma. Clin Exp Metastasis. 20:161–169. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hakuma N, Betsuyaku T, Kinoshita I, Itoh
T, Kaga K, Kondo S, Nishimura M and Dosaka-Akita H: High incidence
of extracellular matrix metalloproteinase inducer expression in
non-small cell lung cancers. Association with clinicopathological
parameters. Oncology. 72:197–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
18
|
Jia L, Zhou H, Wang S, Cao J, Wei W and
Zhang J: Deglycosylation of CD147 down-regulates Matrix
Metalloproteinase-11 expression and the adhesive capability of
murine hepatocarcinoma cell HcaF in vitro. IUBMB Life. 58:209–216.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prat J; FIGO Committee on Gynecologic
Oncology: Staging classification for cancer of the ovary, fallopian
tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5. 2014.
View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: An imbalance of positive and negative
regulation. Cancer Res. 51:s5054–s5059. 1991.
|
|
22
|
Zhang Q, Zhou J, Ku XM, Chen XG, Zhang L,
Xu J, Chen GS, Li Q, Qian F, Tian R, et al: Expression of CD147 as
a significantly unfavorable prognostic factor in hepatocellular
carcinoma. Eur J Cancer Prev. 16:196–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weidle UH, Scheuer W, Eggle D, Klostermann
S and Stockinger H: Cancer-related issues of CD147. Cancer Genomics
Proteomics. 7:157–169. 2010.PubMed/NCBI
|
|
24
|
Jin JS, Yao CW, Loh SH, Cheng MF, Hsieh DS
and Bai CY: Increasing expression of extracellular matrix
metalloprotease inducer In ovary tumors: Tissue microarray analysis
of immunostaining score with clinicopathological parameters. Int J
Gynecol Pathol. 25:140–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sillanpää S, Anttila M, Suhonen K,
Hämäläinen K, Turpeenniemi-Hujanen T, Puistola U, Tammi M, Sironen
R, Saarikoski S and Kosma VM: Prognostic significance of
extracellular matrix metalloproteinase inducer and matrix
metalloproteinase 2 in epithelial ovarian cancer. Tumour Biol.
28:280–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mandal PK and Turnbull WB: Studies on the
synthesis of Lewis-y oligosaccharides. Carbohydr Res.
346:2113–2120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu L, Feng H, Jin S, Tan M, Gao S, Zhuang
H, Hu Z, Wang H, Song Z and Lin B: High expressions of BCL6 and
Lewisy antigen are correlated with high tumor burden and poor
prognosis in epithelial ovarian cancer. Tumour Biol.
39:10104283177116552017. View Article : Google Scholar
|
|
28
|
Kato N, Yuzawa Y, Kosugi T, Hobo A, Sato
W, Miwa Y, Sakamoto K, Matsuo S and Kadomatsu K: The E-selectin
ligand basigin/CD147 is responsible for neutrophil recruitment in
renal ischemia/reperfusion. J Am Soc Nephrol. 20:1565–1576. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prieto PA, Larsen RD, Cho M, Rivera HN,
Shilatifard A, Lowe JB, Cummings RD and Smith DF: Expression of
human H-type alpha1,2-fucosyltransferase encoding for blood group
H(O) antigen in Chinese hamster ovary cells. Evidence for
preferential fucosylation and truncation of polylactosamine
sequences. J Biol Chem. 272:2089–2097. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Egawa N, Koshikawa N, Tomari T, Nabeshima
K, Isobe T and Seiki M: Membrane type 1 matrix metalloproteinase
(MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix
metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J
Biol Chem. 281:37576–37585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Belton RJ Jr, Chen L, Mesquita FS and
Nowak RA: Basigin-2 is a cell surface receptor for soluble basigin
ligand. J Biol Chem. 283:17805–17814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taylor PM, Woodfield RJ, Hodgkin MN,
Pettitt TR, Martin A, Kerr DJ and Wakelam MJ: Breast cancer
cell-derived EMMPRIN stimulates fibroblast MMP2 release through a
phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene.
21:5765–5672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sidhu SS, Mengistab AT, Tauscher AN,
LaVail J and Basbaum C: The microvesicle as a vehicle for EMMPRIN
in tumor-stromal interactions. Oncogene. 23:956–963. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li FF, Liu JJ, Liu DW, Lin B, Hao YY, Cong
JP, Zhu LC, Gao S, Zhang SL and Iwamori M: Lewis Y regulates
signaling molecules of the transforming growth factor β pathway in
ovarian carcinoma derived RMG-I cells. Int J Oncol. 40:1196–1202.
2012. View Article : Google Scholar
|
|
35
|
Tang Y, Kesavan P, Nakada MT and Yan L:
Tumor-stroma interaction: Positive feedback regulation of
extracellular matrix metalloproteinase inducer (EMMPRIN) expression
and matrix metalloproteinase-dependent generation of soluble
EMMPRIN. Mol Cancer Res. 2:73–80. 2004.PubMed/NCBI
|
|
36
|
Benting JH, Rietveld AG and Simons K:
N-Glycans mediate the apical sorting of a GPI-anchored,
raft-associated protein in Madin-Darby canine kidney cells. J Cell
Biol. 146:313–320. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang WJ, Li QQ, Xu JD, Cao XX, Li HX, Tang
F, Chen Q, Yang JM, Xu ZD and Liu XP: Interaction between CD147 and
P-glycoprotein and their regulation by ubiquitination in breast
cancer cells. Chemotherapy. 54:291–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Curtin KD, Meinertzhagen IA and Wyman RJ:
Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular
architecture. J Cell Sci. 118:2649–2660. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marieb EA, Zoltan-Jones A, Li R, Misra S,
Ghatak S, Cao J, Zucker S and Toole BP: Emmprin promotes
anchorage-independent growth in human mammary carcinoma cells by
stimulating hyaluronan production. Cancer Res. 64:1229–1232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao L, Yan L, Lin B, Gao J, Liang X, Wang
Y, Liu J, Zhang S and Iwamori M: Enhancive effects of Lewis y
antigen on CD44-mediated adhesion and spreading of human ovarian
cancer cell line RMG-I. J Exp Clin Cancer Res. 30:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gallagher SM, Castorino JJ and Philp NJ:
Interaction of mono-carboxylate transporter 4 with beta1-integrin
and its role in cell migration. Am J Physiol Cell Physiol.
296:C414–C421. 2009. View Article : Google Scholar
|