Introduction
The thoracic ossification of the posterior
longitudinal ligament (T-OPLL) can cause thoracic spinal stenosis,
which results in intractable myelopathy and radiculopathy. T-OPLL
is a rare disease of the spine; however, it has a high disability
rate and is progressively aggravating. The disability rate of
patients with T-OPLL is much higher than that of patients with
cervical spinal cord disease caused by the cervical ossification of
the posterior longitudinal ligament (C-OPLL). The prevalence of
T-OPLL in individuals of Japanese ethnicity has been shown to be
1.6-1.9%. Among patients with T-OPLL, the most frequently
encountered type is the liner type, the most common level is T3-T4,
and the mean age of onset is >40 years (1,2).
Surgery is the only effective treatment for T-OPLL;
however, surgery is complex and the risk level is high. Although a
variety of surgical treatment methods have been reported, there is
currently no standard method available for the effective treatment
of T-OPLL. Furthermore, the post-operative complication rate is
9.6-40.8% (3–5), and the cause of neurological
deterioration in the early post-operative period is unclear.
Therefore, numerous studies have focused on elucidating the
pathogenesis of T-OPLL. Although the pathogenesis of T-OPLL remains
unclear, studies on C-OPLL have suggested that genetic factors play
an important role in the pathogenesis of OPLL (6,7).
Previous studies have reported >10 susceptibility genes/loci
that are linked to OPLL susceptibility, which include R-spondin 2
(8,9), bone morphogenetic protein (BMP)2
(10,11), BMP4 (12), BMP9 (13), runt-related transcription factor 2
(14,15), collagen (COL) type VI α 1 chain
(16–18), COL type XVII α 1 chain (19), transforming growth factor β1
(TGF-β1) (20), Toll-like
receptor 5 (21), nucleotide
pyrophosphatase/phosphodiesterase 1 (22) and interleukin (IL)15 receptor α
(23).
Due to the limited movement of the thoracic spine,
the stability of the thoracic vertebrae is higher than that of the
cervical vertebrae. Furthermore, the local mechanical stress that
affects degenerative disease of the spine is small. Therefore, we
hypothesized that genetic factors may play a greater role in the
pathogenesis of T-OPLL than environmental factors. Our recent
whole-genome sequencing study identified that rs199772854 in the
IL17 receptor C (IL17RC) gene is a potentially pathogenic
loci for T-OPLL (24). In
addition, our case-controlled association study confirmed that this
mutation was potentially associated with susceptibility to T-OPLL
in individuals of Chinese Han ethnicity (25).
The IL17RC gene is primarily responsible for
encoding type I transmembrane proteins (26). The majority of studies on the
pathogenic role of the IL17RC gene have demonstrated that it
mainly functions through the IL-17 signaling axis. The dysfunction
of the IL-17 signaling axis has been implicated in numerous human
diseases (27,28). The IL-17 signaling axis plays a
central role in the regulation of inflammation (29). Recent studies have demonstrated
that the IL-17 signaling axis also affects bone formation and
remodeling, and can protect bone mass in the case of bone loss due
to infection or hormone imbalance. IL17RC serves an
indispensable role in the development of osteoblasts, which
accelerates the differentiation of osteoblasts (30,31). OPLL results in increased bone
formation in the ligament tissues, and there is also an association
between OPLL disease and increased bone mineral density throughout
the body (7). These studies
indicate the potential role of IL17RC in the pathogenesis
and progression of osteogenic diseases.
In the present study, mouse embryonic osteoblast
cells were induced to differentiate into osteoblasts by
transfection of the IL17RC gene carrying the rs199772854A
site lentivirus, rs199772854C site lentivirus and empty lentivirus
into 3T3-E1 mouse embryonic osteoblasts. Furthermore, whether the
mutation loci causes the abnormal expression of the IL17RC
gene was analyzed and differences in the ability to induce
osteogenesis were detected in order to provide an experimental
basis for the potential role of the IL17RC gene in the
pathogenesis of T-OPLL.
Materials and methods
Cell lines and cell culture
The 3T3-E1 mouse embryonic osteoblast cell line
(American Type Culture Collection, Manassas, VA, USA) was cultured
in minimum Essential medium (MEM)-α with ribonucleosides,
deoxyribonucleosides, 2 mM L-glutamine and 1 mM sodium pyruvate,
and without ascorbic acid (cat no. A1049001; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cells were incubated at 37°C
with 5% CO2 in a 95% humidified atmosphere, and the
culture medium was replaced every 2 days. 293 cells (American Type
Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's
modified Eagle medium (DMEM; cat. no. 10564-037; Life Technologies;
Thermo Fisher Scientific, Inc.). The complete growth medium was
prepared by the addition of fetal bovine serum (FBS) to the base
medium to reach a final concentration of 10% under 37°C and a
humidified atmosphere containing 5% CO2, and the culture
medium was replaced every 2 days.
Construction of the IL17RC gene
vector
The primers were designed and synthesized according
to the human IL17RC sequence information. The upstream
HpaI restriction site and the downstream EcoRI
restriction site were added, and the primer sequences are presented
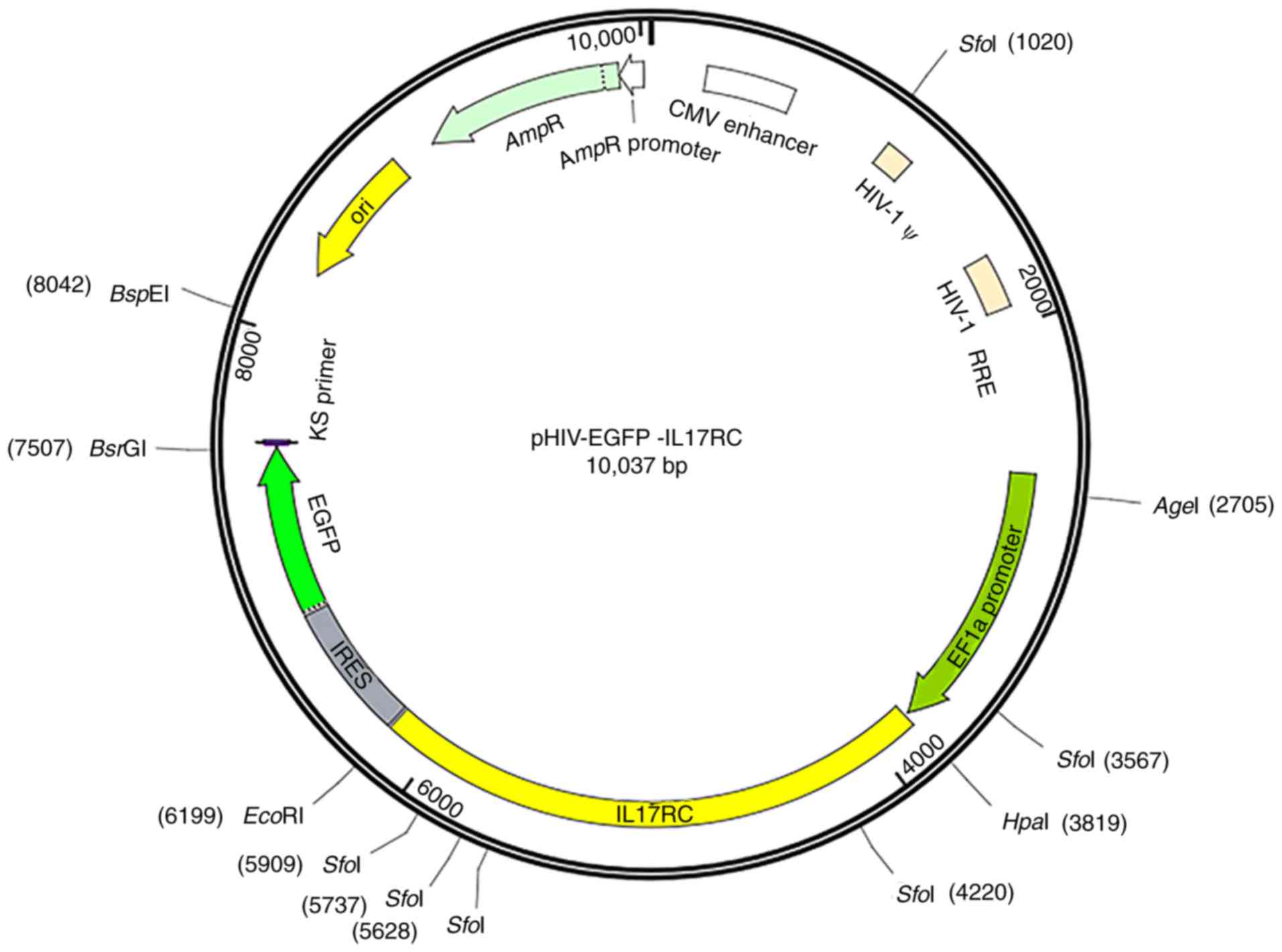
in Table I. The pHIV-EGFP vector
contained the EF1a-MCS-IRES-EGFP component sequence (Addgene,
Watertown, MA, USA), HpaI and EcoRI enzyme cleavage
sites and the following control insert: TAT TCT AGG GAT CCA ACC CTC
GAG TGA CCC GTC TAG AGG GCT AG. The vector map is presented in
Fig. 1. GFP is located downstream
of the target gene separated by the IRES sequence. The positive
bacterial Sanger sequencing primer sequences are shown in Table II. Mutant primers were designed
based on the human IL17RC gene sequence information, and
wild-type plasmids were used as templates to design mutant primers
according to the primer design principles of the Site-Directed
Mutagenesis kits (cat. no. 210514; Agilent Technologies, Inc.,
Santa Clara, CA, USA). The mutant and wild-type IL17RC gene
primer sequences are presented in Table III. The IL17RC gene
wild-type and mutant plasmids were subjected to endotoxin plasmid
extraction and extracted using Plasmid DNA Purification kits (cat.
no. K210006; Thermo Fisher Scientific, Inc.).
 | Table IIL17RC gene primer sequences. |
Table I
IL17RC gene primer sequences.
| Gene | Primer
sequence | Length (bp) |
|---|
| IL17RC-F |
5′-ATCGGTTAACATGAGGGCGGCCCGTGCTCTGCT-3′ | 2,376 |
| IL17RC-R |
5′-CGGAATTCTTAGCCCAGCGCCACCTTCCTGGA-3′ | |
 | Table IISanger sequencing primer
sequences. |
Table II
Sanger sequencing primer
sequences.
| Gene | Primer
sequence | Length (bp) |
|---|
| IL17RC-F |
5′-AAGCCTCAGACAGTGGTTCAAAG-3′ | 24 |
| IL17RC-R |
5′-CAAGCGGCTTCGGCCAGTAACGT-3′ | 25 |
 | Table IIIMutant and wild-type gene primer
sequences. |
Table III
Mutant and wild-type gene primer
sequences.
| Gene | Primer
sequence | Length (bp) |
|---|
|
IL17RC-rs199772854 C-F |
5′-gtgtcccgggcccttcagccagccctggat-3′ | 2,376 |
|
IL17RC-rs199772854 C-R |
5′-ggctggctgaagggcccgggacacttgctc-3′ | |
|
IL17RC-rs199772854 A-F |
5′-gtgtcccgggccattcagccagccctggat-3′ | 2,376 |
|
IL17RC-rs199772854 A-R |
5′-ggctggctgaatggcccgggacacttgctc-3′ | |
Transfection of the
pHIV-IL17RC-rs199772854A, pHIV-IL17RC-rs199772854C and pHIV-GFP
expression vectors into the 3T3-E1 cells
A total of 5 ml poly-D-lysine (PDL; cat. no. P7280;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was pre-added to a
10-cm dish, which was incubated at 37°C for 30 min. The PDL was
aspirated, and the dish was washed twice with phosphate-buffered
saline (cat. no. AM9625; Thermo Fisher Scientific, Inc.) and stored
in a 37°C incubator for 7 days. 293 cells within 20 passages were
adjusted to the logarithmic growth phase, and the cells were
divided into the 10-cm dishes to a density of ~70% following
trypsin digestion. The cells were cultured in DMEM at 37°C
overnight, and then the medium was discarded and a small amount of
Opti-MEM medium (cat. no. 51985034; Thermo Fisher Scientific, Inc.)
was added to rinse the dish. Subsequently, 6 ml Opti-MEM medium
were added to the dish and it was placed in an incubator for use.
The helper plasmid pMD2G, pSPAX2 and the wild-type or mutant
expression plasmid pHIV-EGFP-IL17RC (Addgene) were added to 500
μl Opti-MEM medium at a ratio of 3:6:9 μg, and 15
μl PLUS reagent (cat. no. 11514015; Thermo Fisher
Scientific, Inc.) was added to the mixture. In another tube
containing 500 μl Opti-MEM medium, 30 μl Lipofectin
Transfection Reagent (cat. no. 18292037; Thermo Fisher Scientific,
Inc.) was added and mixed well. After being allowed to stand for 5
min at room temperature, the two tubes were mixed in a volume of 1
ml. The liposomes were mixed with the plasmid and then coated at
room temperature for 30 min. The liposome and plasmid mixture was
added to the 3T3-E1 cells that had been added to the medium
containing Opti-MEM, and DMEM complete medium containing 10% FBS
was replaced after 6-8 h. The cells were cultured for 48 h at 37°C
in a 5% CO2 incubator. The virus solution was
concentrated and purified, and the cells were infected with the
high quality virus solution of pHIV-IL17RC-rs199772854C (titer:
6.4×108 TU/ml) and pHIV-IL17RC-rs199772854A (titer:
7.0×108 TU/ml) for 24 h. The viral supernatant was
aspirated and fresh DMEM was added. The 3T3-E1 cells were plated in
6-well plates at a density of ~30% and after 12 h, the concentrated
virus solution was added to infect the cells, at an MOI of 50 for
each group infection. GFP and target gene expression were monitored
at nearly the same time using a fluorescence microscope 48 h after
exposure. The cells with GFP expression were sorted using a
CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA) 72 h after
exposure. The GFP-positive cells sorted by FACS and part of the
sorted cells were examine by western blot analysis and cultured for
further analysis. Once the cells had grown to a confluence of
80-90%, they were subcultured.
Differentiation of 3T3-E1 cells into
osteoblasts
pHIV-I L17RC-rs199772854C (wild-type group),
pHIV-IL17RC-rs199772854A (mutation group), pHIV-GFP infected 3T3-E1
cells (empty lentivirus control group) and the 3T3-E1 cells without
adding any vector (mock group). 3T3-E1 cells were cultured to the
logarithmic growth phase and then divided into 6-well plates at a
density of ~70%. The plates were incubated at 37°C for 16-18 h.
When the cells were close to fusion, osteogenic induction medium
(low-glucose DMEM (cat. no. 11885-084), 10% FBS (cat no. 10099141;
both Gibco; Thermo Fisher Scientific, Inc.), 10 mmol/l
β-glycerophosphate (cat. no. G9422), 100 nmol/l dexamethasone (cat.
no. D4902), 50 μmol/l ascorbyl phosphate (cat. no. A4544;
all Sigma-Aldrich, Inc.), 100 U/ml penicillin and 0.1 mg/ml
streptomycin (cat no. 15140-122; Gibco; Thermo Fisher Scientific,
Inc.) was added. The cells were harvested at 21 days following
osteogenic induction.
Alkaline phosphatase (ALP) activity assay
and Alizarin red staining
After the cells were seeded in 24-well plates at a
density of 1×105 cells/well and cultured in osteogenic
medium for 21 days, osteogenic identification was performed using
an ALP activity staining kit (cat. no. GMS80033.1; Genmed
Scientific, Shanghai, China) as follows: i) The culture solution
was carefully removed from the 24-well plates; ii) this was
followed by the addition of 400 μl cleaning solution
(Reagent A) to clean the surface of the cells, and this was then
removed; iii) 400 μl fixative (Reagent B) was then added
followed by incubation for 2 min at room temperature, and removal;
iv) 400 μl cleaning solution (Reagent A) was then added for
washing and this was then removed; this process was repeated 2
times to wash the fixative residue; v) 400 μl staining
working solution was then added followed by incubation for 5-15 min
in the dark at room temperature, and the staining solution was then
removed; vi) 400 μl cleaning solution (Reagent A) was then
added followed by incubation for 5 min at room temperature for
washing which was then removed; this was repeated 2 times to wash
the stain residue; vii) 200 μl cleaning solution (Reagent A)
or PBS were then added to cover the cell surface, and observe the
cell staining by the naked eye.
In addition, Mineralization was assessed using an
Alizarin Red S kit (cat. no. GMS80046.3; Genmed Scientific) as
follows: i) The culture solution was carefully removed the from the
24-well plates; ii) 400 μl cleaning solution (Reagent A)
were then added to clean the surface of the cells, and this was
then removed; iii) 400 μl fixative (Reagent B) was then
added followed by incubation for 10 min at room temperature, and
this was then removed; iv) 400 μl cleaning solution (Reagent
A) were added for washing and this was then removed; this process
was repeated 2 times to wash the fixative residue; v) 400 μl
staining solution (Reagent C) were then added followed by
incubation at room temperature until orange-red color became
visible, drain, and drying at room temperature; vi) 400 μl
cleaning solution (Reagent A) were added for washing and this was
then removed; this was repeated 2 times to wash the stain residue;
vii) 200 μl cleaning solution (Reagent A) or PBS were then
added to cover the cell surface, and observe the cell staining by
the naked eye.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.), and reverse transcription
was performed using a M-MLV Reverse Transcriptase kit (Promega
Corp., Madison, WI, USA) according to the manufacturer's
instructions. qPCR was applied to quantify the mRNA levels of
IL17RC, ALP and GAPDH using SYBR-Green Real-Time PCR Master mix on
the LightCycler 480 Real-Time System (Roche Diagnostics, Basel,
Switzerland) under 2-Step Cycling (95°C for 10 min hold, 40 cycles
of 95°C for 15 sec and 60°C for 60 sec). All experiments were
performed in triplicate and normalized to GAPDH, and the relative
gene expression was calculated based on 2-ΔΔCq method
(29). The primers are listed in
Table IV.
 | Table IVPrimer sequences for reverse
transcription-quantitative PCR. |
Table IV
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence | Length (bp) |
|---|
| IL17RC-RT-F |
5′-CTGCCCTTGTGCAGTTTGG-3′ | 85 |
| IL17RC-RT-R |
5′-CAGATTCGTACCTCACTCCCTA-3′ | |
| ALP-RT-F |
5′-GTGAACCGCAACTGGTACTC-3′ | 81 |
| ALP-RT-R |
5′-GAGCTGCGTAGCGATGTCC-3′ | |
| GAPDH-RT-F |
5′-TGGGTGTGAACCATGAGAAGT-3′ | 126 |
| GAPDH-RT-R |
5′-TGAGTCCTTCCACGATACCAA-3′ | |
Western blot analysis
Cell lysates were obtained using ice-cold RIPA lysis
buffer (Beyotime Institute of Biotechnology, Shanghai, China)
containing 10 mM PMSF as a protease inhibitor. The total
concentration of the extracted proteins was determined using an
Enhanced BCA Protein Assay kit (Beyotime Institute of
Biotechnology). A total of 30 μg protein was subjected to
12% SDS-PAGE and transferred onto a nitrocellulose membrane. The
membrane was incubated in 1% bovine serum albumin containing
primary rabbit anti-human polyclonal antibodies at 4°C overnight.
Subsequently, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit antibody at room temperature
for 1 h and the protein was detected using
electro-chemiluminescence (Merck Millipore, Darmstadt, Germany).
The following corresponding primary and secondary antibodies were
used: Anti-IL17RC (1:1,000; cat. no. ab69673; Abcam, Cambridge, MA,
USA); anti-tumor necrosis factor receptor (TNFR)-associated factor
6 (TRAF6; 1:2,000; cat. no. ab33915; Abcam); anti-nuclear factor
(NF)-κB p65 (1:1,000; cat. no. 8242; Cell Signaling Technology,
Inc., Danvers, MA, USA); anti-IKKe (1:2,500; cat. no. ab210927);
anti-GFP (1:10,000; cat. no. ab183734); and anti-GAPDH (1:2,500;
cat. no. ab9485; all Abcam) antibodies; and goat anti-mouse
antibody (1:2,500; cat. no. CW0102M; Kangwei Biotech Co. Ltd.,
Beijing, China); and goat anti-rabbit antibody (1:2,500; cat. no.
CW0103M; Kangwei Biotech Co. Ltd.). The blots were detected using a
Kodak film developer (Fujifilm, Tokyo, Japan). The protein levels
were quantified by densitometric analysis using Image-Pro Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
GAPDH was used as the endogenous control.
Statistical analysis
All statistical analyses were performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Descriptive
data for continuous variables are presented as the means ± standard
deviation. The Student's t-test was used to compare the means
between 2 groups. For the comparisons with the control group,
statistical analyses were performed using one-way analysis of
variance (ANOVA) with the post hoc Dunnett's test. A value of
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed 3 times.
Results
Wild-type and mutant IL17RC gene bacteria
Sanger sequencing
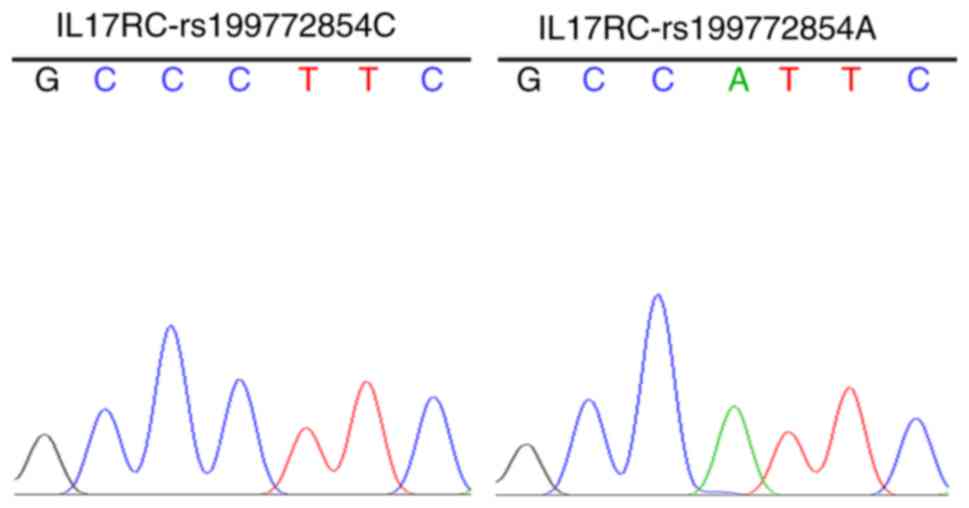
The strains that were identified as positive using
the electrophoresis of the PCR products were sequenced to obtain
the wild-type and mutant IL17RC gene clones (Fig. 2), indicating that the wild-type
and mutant IL17RC gene lentiviral packaging vectors were
successfully constructed.
Lentivirus infection of the 3T3-E1
cells
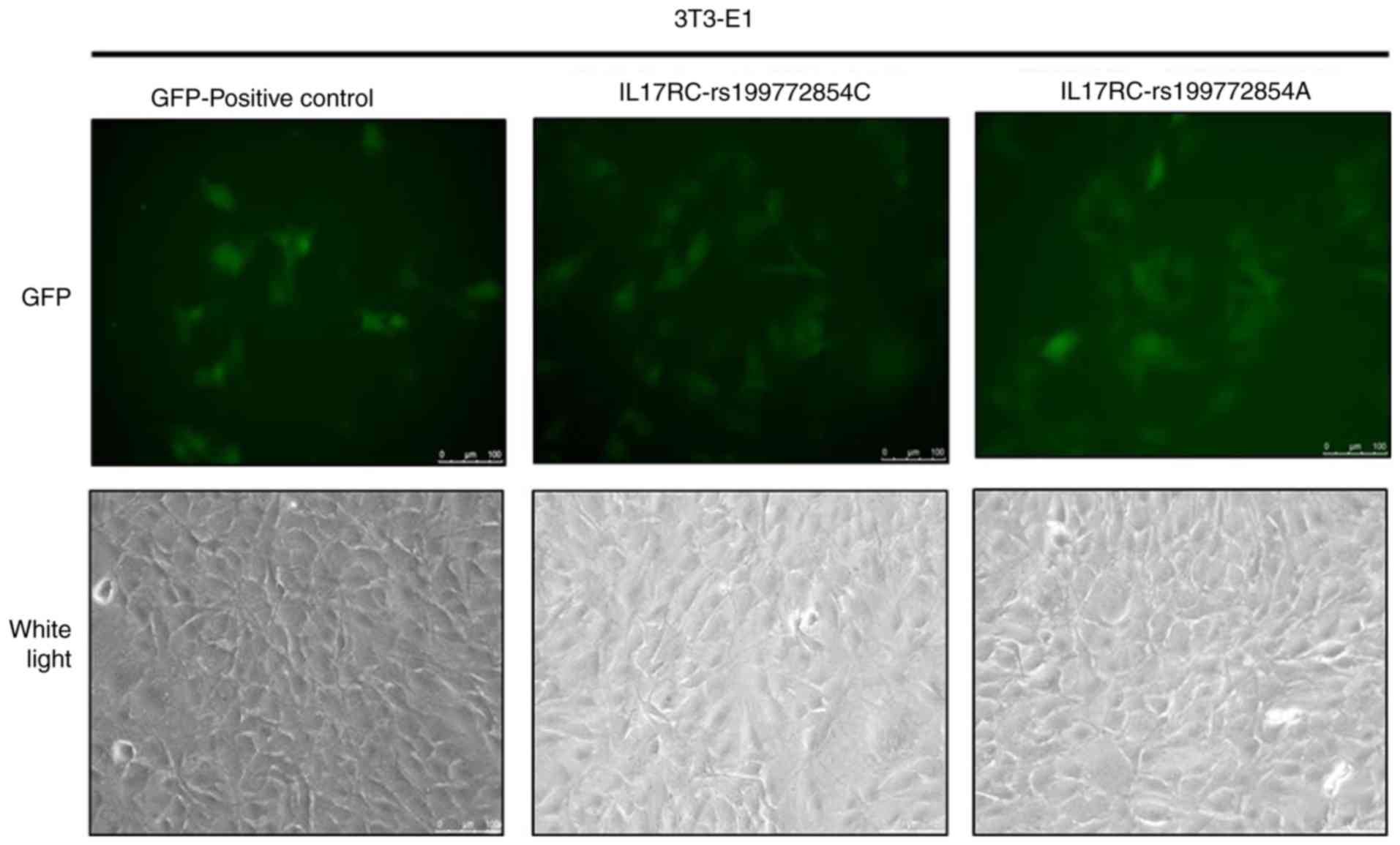
Significant levels of GFP expression were observed
using fluorescence microscopy at 48 h after cell exposure (Fig. 3), which indicated that the 3T3-E1
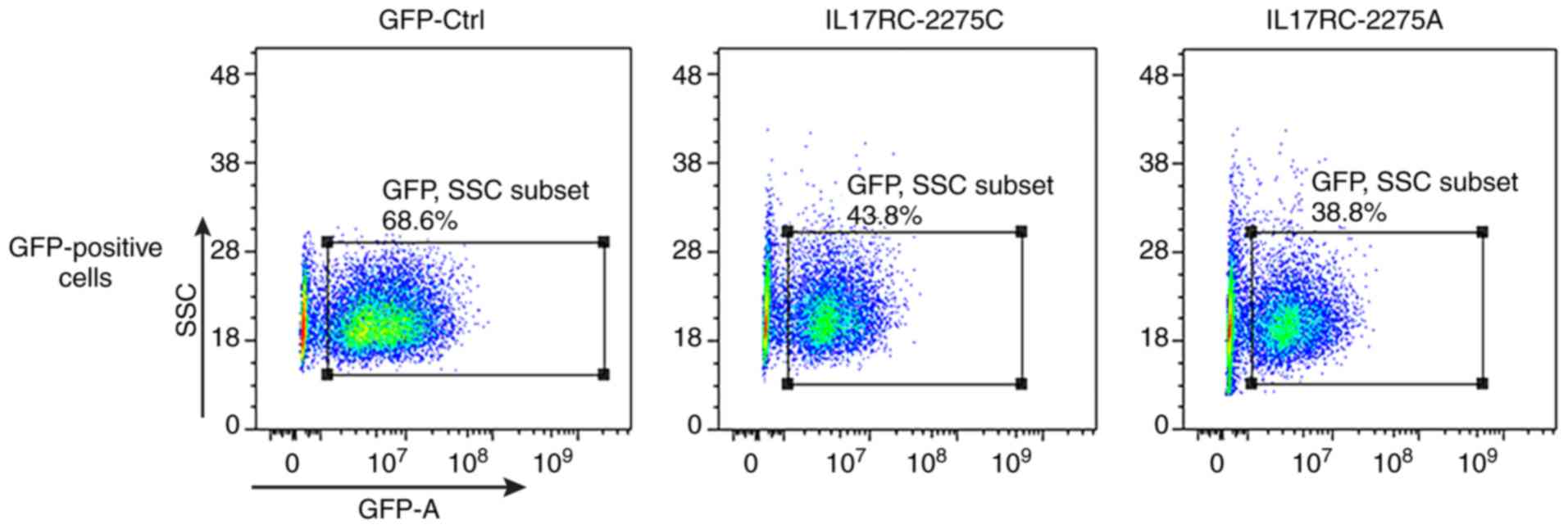
cells were successfully infected. The 3T3-E1 cells were trypsinized
at 72 h after they were infected, and the GFP-positive cells were
sorted by flow cytometry, as shown in Fig. 4. The positive cells were plated
and cultured, and then subjected to the subsequent osteogenesis
induction experiments. Cell lines expressing the wild-type and
mutant IL17RC gene were therefore obtained.
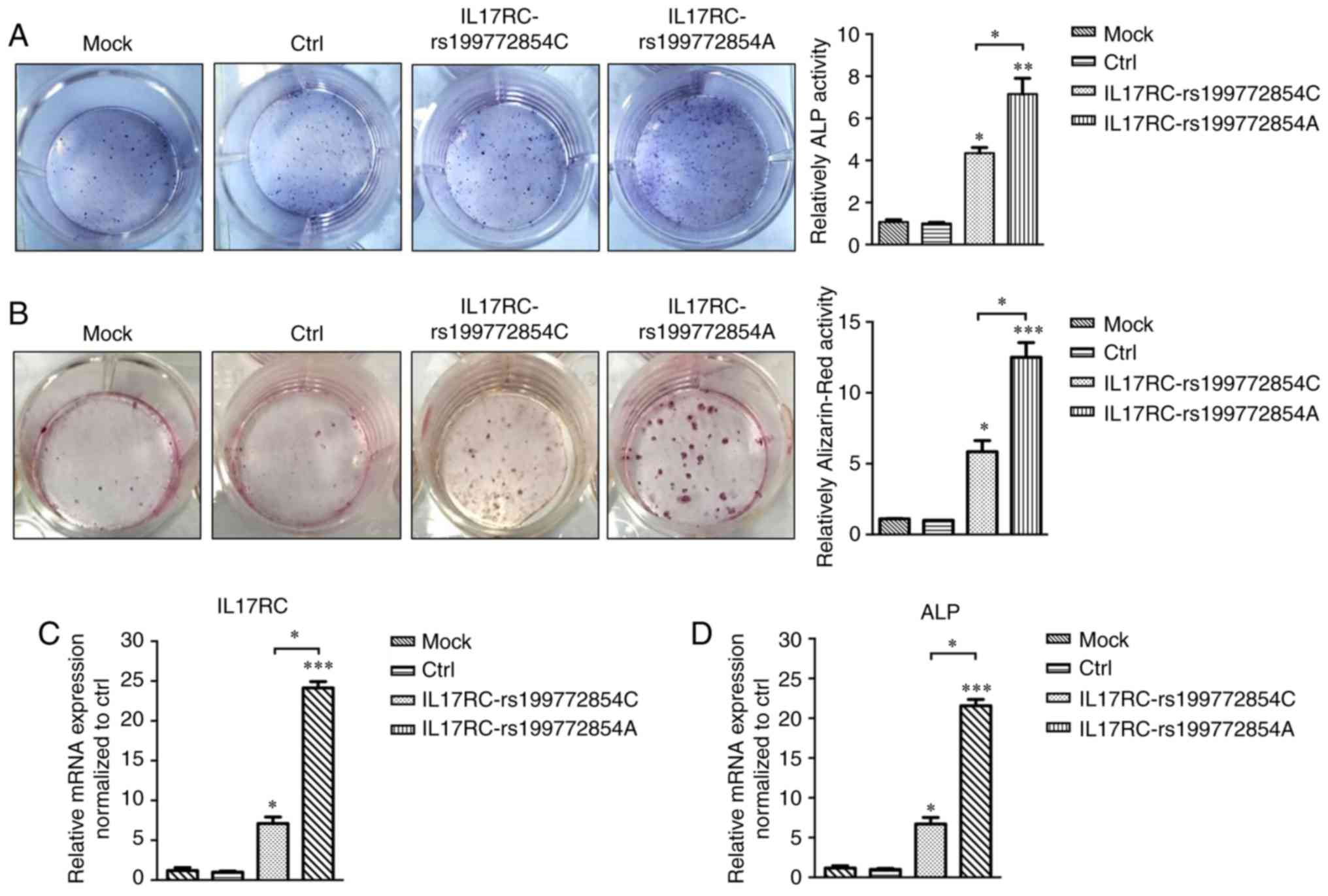
IL17RC gene rs199772854A site mutation
induces the osteogenic differentiation of 3T3-E1 cells
Following 21 days of osteogenic induction, the ALP
content of the IL17RC gene mutation group or the wild-type
group was significantly higher than that of the mock group and the
empty lentivirus control group. The ALP content of the
IL17RC gene mutation group was significantly higher than
that of the wild-type group (Fig.
5A). The Alizarin red content in the IL17RC gene
mutation group or the wild-type group was significantly higher than
that of the mock group and the empty lentivirus control group. The
Alizarin red content of the IL17RC gene mutation group was
significantly higher than that of the wild-type group (Fig. 5B). The results of RT-qPCR revealed
that the mRNA expression of IL17RC in the rs199772854A
transgenic IL17RC gene 3T3-E1 cell group was 18-, 24.1- and
3.4-fold higher than that of the mock group and the empty
lentivirus control group along with wild-type group, respectively.
The expression in the wild-type group was 6- and 7.1-fold higher
than that in the mock group and the empty lentivirus control group,
respectively (Fig. 5C). The mRNA
expression of ALP in the rs199772854A transgenic IL17RC gene
3T3-E1 cell group was 20-, 21.6- and 3.2-fold higher than that of
the mock group and the empty lentivirus control group along with
wild-type group, respectively. The expression in the wild-type
group was 5.6- and 7.1-fold higher than that in the mock group and
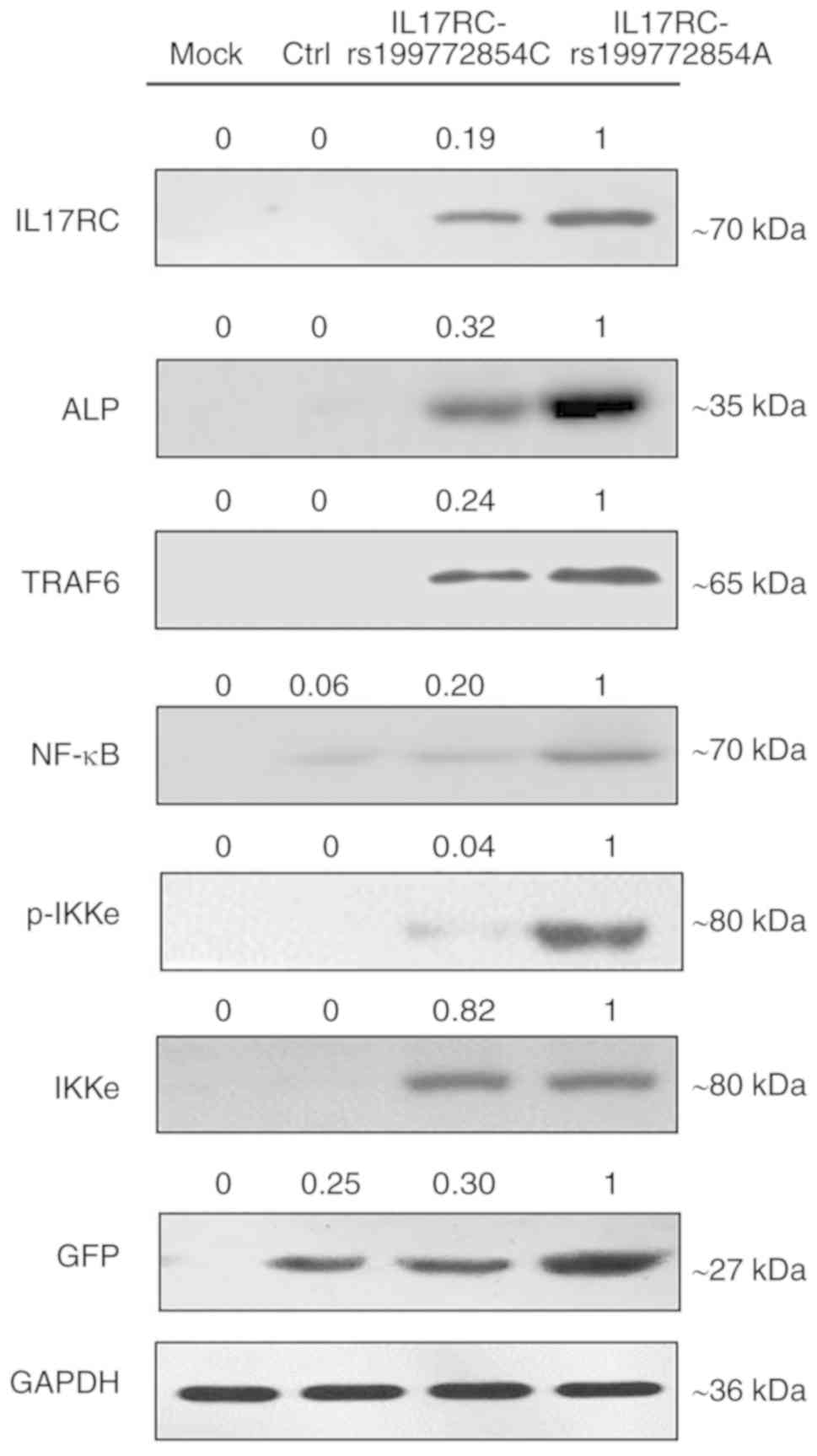
the empty lentivirus control group, respectively (Fig. 5D). At the protein level, the
expression of exogenous IL17RC in the cells was confirmed by
western blot analysis of GFP tags. The expression levels of
IL17RC, ALP, TRAF6, NF-κB and p-IKKe in the rs199772854A
transgenic IL17RC gene 3T3-E1 cell group were higher than
those in the mock group and the empty lentivirus control group
along with rs199772854C transgenic IL17RC gene 3T3-E1 cell
group, respectively. The protein expression of IKKe in the
rs199772854A transgenic IL17RC gene 3T3-E1 cell group was
higher than that in the mock group and control group, although no
significance was observed between the mutation group and the
wild-type group (Fig. 6). The
expression of IKKe was enhanced in the mutation group and the
wild-type group, whereas the expression of p-IKKe was further
enhanced in the mutant group. As the lentivirus (MOI) was added to
the cells in the different groups and the lentivirus-infected cells
used in our study for ALP, mRNA and protein detection were
collected after FACS sorting, the expression levels of ALP, mRNA
and protein were not affected by MOI. These results suggest that
the IL17RC gene mutation promotes the osteogenic
differentiation of 3T3-E1 cells and may increase IL17RC gene
expression by regulating the IL17 family signaling pathway.
Discussion
The pathological process of T-OPLL involves the
differentiation of posterior longitudinal ligament fibroblasts into
osteoblasts. To verify the pathogenic potential of a mutation site
that was identified in our previous study (24), IL17RC-induced mouse embryonic
osteoblasts (3T3-E1) cells were established in the present study.
In the present study, in the osteogenic differentiation model, the
IL17RC gene carrying the rs199772854A and rs199772854C sites
was transfected into mouse embryonic osteoblast (3T3-E1) cells, and
osteogenic identification, western blot analysis and RT-qPCR were
conducted at 21 days following osteogenic induction.
The rs199772854 locus of the IL17RC gene is
located in the promoter region. SNPs of the promoter region can
aggravate or attenuate transcriptional activity by affecting the
binding efficiency of transcription factors and various action
elements, consequently interfering with gene expression and
potentially causing disease. The sequence of IL17RC mRNA exhibits
no difference between the wild-type and mutant. Thus, RT-qPCR and
the western blot analysis cannot distinguish between the wild-type
and mutant IL17RC. The mutation in the promoter region just
enhances the expression level of the IL17RC gene mRNA which
further translates more IL17RC protein.
The results of the present study demonstrated that
the IL17RC gene rs199772854A mutation significantly
increased the expression of its own gene. Therefore, these results
suggest that this mutation site exerts its biological functions
through the overexpression of its own genes and the different
influences of downstream signaling were enhanced by the mutation of
IL17RC.
Previous studies have suggested that IL17RC
plays a major role in disease pathogenesis through its function in
the IL-17 signaling axis (32,33). IL17RC plays an
indispensable role in the development of osteoblasts and
accelerates the differentiation of osteoblasts (34). OPLL results in increased bone
formation in the ligament tissue, and there is also an association
between OPLL and increased bone mineral density in the body. Thus,
IL17RC plays a potential role in the pathogenesis and
progression of osteogenic diseases. IL17RC may act directly
in the disease pathology or may have an indirect effect due to its
function as a receptor for IL-17F and as a subunit of the IL-17R
complex. Therefore, IL17RC is a potential therapeutic target
in IL-17-dependent diseases (35).
TRAF6 is involved in the regulation of multiple
signaling pathways as a key upstream regulatory molecule, including
the IL-17 family signaling pathway (36). The overexpression of IL17RC
can affect TRAF6 and increase the expression of NF-κB (37,38). The transcription factor NF-κB is a
key factor in the expression of various genes that are regulated by
the immune system and inflammatory response, proliferation,
tumorigenesis and survival (39,40). The current study demonstrated that
the ratio of phosphorylated IKKe versus total protein in the
IL17RC gene mutation group was 18.5-fold higher than that of
the wild-type group. IKKe is required for the activation of NF-κB
through the phosphorylation and further degradation of IKKe. The
inadequate regulation of NF-κB is associated with cancer,
inflammation and autoimmune diseases, septic shock, viral infection
and immune dysfunction. Therefore, NF-κB is a key signaling
molecule between bone and the immune response. It has previously
been demonstrated that environmental factors can cause PDGA-BB and
TGF-β1 in ligament cells to stimulate NF-κB, which then influences
the differentiation of undifferentiated mesenchymal cells into
osteoblasts (41).
In the present study, mouse embryonic osteoblast
(3T3-E1) cells were induced to differentiate into osteoblasts by
transfecting the IL17RC gene carrying the rs199772854A site
mutation into these cells. The potential mechanism of the
IL17RC gene in T-OPLL was further investigated to confirm
our hypothesis that the IL17RC gene regulates the
development of T-OPLL through the IL17 family signaling pathway.
Western blot analysis and RT-qPCR were used to analyze the in
vitro model of osteogenesis. The results demonstrated that the
expression levels of TRAF6 and NF-κB in the mouse embryonic
osteoblast (3T3-E1) cells that were carrying the IL17RC gene
rs199772854A site mutation were higher than those in the unmutated
group.
There were some limitations to the present study.
Due to the fact that the surgical removal of the posterior
longitudinal ligament of the thoracic spine is complex and the risk
level is high, the resected non-ossified posterior longitudinal
ligament is too small. Hence, we were not able to carry out the
primary cell culture of thoracic posterior longitudinal ligament
during the current period. Although the classic cellular line
utilized in osteogenic differentiation was the 3T3-E1 by its great
osteogenic differentiation potential (42–44), using mouse cell lines may have
some differences in the activation of the signaling pathway.
Therefore, we will try to use posterior longitudinal ligament cell
as target cell studying T-OPLL in the future. For the lentivirus
system utilized in the establishment of wild-type and mutant
cellular lines, the random insertion of the target is also a
limitation of this study. In further studies, other systems will be
considered for use in our research, such as AAV system.
Furthermore, to the best of our knowledge, there are currently no
reports available on the IL17RC gene related to C-OPLL. To ac
complish this issue, we are currently collecting data from patients
with C-OPLL and aim to verify whether the IL17RC gene is
associated with C-OPLL in the future.
In conclusion, the present study suggests that the
IL17RC gene rs199772854A site mutation causes significantly
higher levels of IL17RC gene expression and TRAF6 and NF-κB
protein expression compared to the rs199772854C site, indicating
that the IL17RC gene rs199772854A loci mutation induces mouse
embryonic osteoblasts towards osteogenic differentiation and may
play a role in the pathogenesis of T-OPLL, and the IL17 signal axis
may be involved in the regulation of T-OPLL disease. Therefore, the
present study provides a theoretical basis for the early detection
and diagnosis of T-OPLL diseases and the investigation of
treatments other than surgery.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81672201).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW, SL and XiaogL conceived the study and designed
the experiments. PW, YM, CK, and CL performed the experiments. ZT,
LY, XiaoL and GH analyzed the data. PW wrote the manuscript. All
authors reviewed the manuscript and all authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Fujimori T, Watabe T, Iwamoto Y, Hamada S,
Iwasaki M and Oda T: Prevalence, concomitance, and distribution of
ossification of the spinal ligaments: Results of whole spine CT
scans in 1500 Japanese patients. Spine (Phila Pa 1976).
41:1668–1676. 2016. View Article : Google Scholar
|
|
2
|
Mori K, Imai S, Kasahara T, Nishizawa K,
Mimura T and Matsusue Y: Prevalence, distribution, and morphology
of thoracic ossification of the posterior longitudinal ligament in
Japanese: Results of CT-based cross-sectional study. Spine (Phila
Pa 1976). 39:394–399. 2014. View Article : Google Scholar
|
|
3
|
Yu LJ, Li WJ, Guo SG and Zhao Y:
Transforaminal thoracic interbody fusion: Treatment of thoracic
myelopathy caused by anterior compression. Orthopade. 47:985–991.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Imagama S, Ando K, Takeuchi K, Kato S,
Murakami H, Aizawa T, Ozawa H, Hasegawa T, Matsuyama Y, Koda M, et
al: Perioperative complications after surgery for thoracic
ossification of posterior longitudinal ligament: Nationwide
multicenter prospective study. Spine (Phila Pa 1976).
43:E1389–E1397. 2018.
|
|
5
|
Hu P, Yu M, Liu X, Liu Z and Jiang L: A
circumferential decompression- based surgical strategy for
multilevel ossification of thoracic posterior longitudinal
ligament. Spine J. 15:2484–2492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikegawa S: Genetics of ossification of the
posterior longitudinal ligament of the spine: A mini review. J Bone
Meta. 21:127–132. 2014. View Article : Google Scholar
|
|
7
|
Ikegawa S: Genomic study of ossification
of the posterior longitudinal ligament of the spine. Proc Jpn Acad
Ser B Phys Biol Sci. 90:405–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakajima M, Kou I, Ohashi H; Genetic Study
Group of the Investigation Committee on the Ossification of Spinal
Ligaments; Ikegawa S: Identification and functional
characterization of RSPO2 as a susceptibility gene for ossification
of the posterior longitudinal ligament of the spine. Am J Hum
Genet. 99:202–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakajima M, Takahashi A, Tsuji T, Karasugi
T, Baba H, Uchida K, Kawabata S, Okawa A, Shindo S, Takeuchi K, et
al: A genome- wide association study identifies susceptibility loci
for ossification of the posterior longitudinal ligament of the
spine. Nat Genet. 46:1012–1016. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Guo J, Cai T, Zhang F, Pan S,
Zhang L, Wang S, Zhou F, Diao Y, Zhao Y, et al: Targeted next-
generation sequencing reveals multiple deleterious variants in
OPLL- associated genes. Sci Rep. 6:269622016. View Article : Google Scholar
|
|
11
|
Wang H, Liu D, Yang Z, Tian B, Li J, Meng
X, Wang Z, Yang H and Lin X: Association of bone morphogenetic
protein- 2 gene polymorphisms with susceptibility to ossification
of the posterior longitudinal ligament of the spine and its
severity in Chinese patients. Eur Spine J. 17:956–964. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren Y, Feng J, Liu Z, Wan H, Li J and Lin
X: A new haplotype in BMP4 implicated in ossification of the
posterior longitudinal ligament (OPLL) in a Chinese population. J
Orhop Res. 30:748–756. 2012. View Article : Google Scholar
|
|
13
|
Ren Y, Liu ZZ, Feng J, Wan H, Li JH, Wang
H and Lin X: Association of a BMP9 haplotype with ossification of
the posterior longitudinal ligament (OPLL) in a Chinese population.
PLoS One. 7:e405872012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Shi L, Shi G, Guo Y, Chen D, Chen
D and Shi J: Connexin 43 affects osteogenic differentiation of the
posterior longitudinal ligament cells via regulation of ERK
activity by stabilizing Runx2 in ossification. Cell Physiol
Biochem. 38:237–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Zhao Y, Chen Y, Shi G and Yuan W:
RUNX2 polymorphisms associated with OPLL and OLF in the Han
population. Clin Orthop Relat Res. 468:3333–3341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu C, Chen Y, Zhang H, Chen Y, Shen X, Shi
C, Liu Y and Yuan W: Integrated microRNA- mRNA analyses reveal OPLL
specific microRNA regulatory network using high- throughput
sequencing. Sci Rep. 6:215802016. View Article : Google Scholar
|
|
17
|
Kong Q, Ma X, Li F, Guo Z, Qi Q, Li W,
Yuan H, Wang Z and Chen Z: COL6A1 polymorphisms associated with
ossification of the ligamentum flavum and ossification of the
posterior longitudinal ligament. Spine (Phila Pa 1976).
32:2834–2838. 2007. View Article : Google Scholar
|
|
18
|
Tanaka T, Ikari K, Furushima K, Okada A,
Tanaka H, Furukawa K, Yoshida K, Ikeda T, Ikegawa S, Hunt SC, et
al: Genomewide linkage and linkage disequilibrium analyses identify
COL6A1, on chromosome 21, as the locus for ossification of the
posterior longitudinal ligament of the spine. Am J Hum Genet.
73:812–822. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei W, He HL, Chen CY, Zhao Y, Jiang HL,
Liu WT, Du ZF, Chen XL, Shi SY and Zhang XN: Whole exome sequencing
implicates PTCH1 and COL17A1 genes in ossification of the posterior
longitudinal ligament of the cervical spine in Chinese patients.
Genet Mol Res. 13:1794–1804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamiya M, Harada A, Mizuno M, Iwata H and
Yamada Y: Association between a polymorphism of the transforming
growth factor-beta1 gene and genetic susceptibility to ossification
of the posterior longitudinal ligament in Japanese patients. Spine
(Phila Pa 1976). 26:1266–1267. 2001. View Article : Google Scholar
|
|
21
|
Chung WS, Nam DH, Jo DJ and Lee JH:
Association of toll- like receptor 5 gene polymorphism with
susceptibility to ossification of the posterior longitudinal
ligament of the spine in Korean population. J Korean Neurosurg Soc.
49:8–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Z, Zhu H, Ding L, Xiao H, Chen D and
Xue F: Association of NPP1 polymorphism with postoperative
progression of ossification of the posterior longitudinal ligament
in Chinese patients. Genet Mol Res. 12:4648–4655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo Q, Lv SZ, Wu SW, Tian X and Li ZY:
Association between single nucleotide polymorphism of IL15RA gene
with susceptibility to ossification of the posterior longitudinal
ligament of the spine. J Orthop Surg Res. 9:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang P and Liu X, Zhu B, Ma Y, Yong L,
Teng Z, Wang Y, Liang C, He G and Liu X: Identification of
susceptibility loci for thoracic ossification of the posterior
longitudinal ligament by whole- genome sequencing. Mol Med Rep.
17:2557–2564. 2018.
|
|
25
|
Wang P and Liu X, Zhu B, Ma Y, Yong L,
Teng Z, Liang C, He G and Liu X: Association of IL17RC and COL6A1
genetic polymorphisms with susceptibility to ossification of the
thoracic posterior longitudinal ligament in Chinese patients. J
Orthop Surg Res. 13:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ho AW and Gaffen SL: IL- 17RC: A partner
in IL- 17 signaling and beyond. Semin Immunopathol. 32:33–42. 2010.
View Article : Google Scholar
|
|
27
|
Dhaouadi T, Chahbi M, Haouami Y, Sfar I,
Abdelmoula L, Ben Abdallah T and Gorgi Y: IL- 17A, IL- 17RC
polymorphisms and IL17 plasma levels in Tunisian patients with
rheumatoid arthritis. PLoS One. 13:e01948832018. View Article : Google Scholar
|
|
28
|
Roos AB, Mori M, Gura HK, Lorentz A,
Bjermer L, Hoffmann HJ, Erjefält JS and Stampfli MR: Increased IL-
17RA and IL- 17RC in end- stage COPD and the contribution to mast
cell secretion of FGF- 2 and VEGF. Respir Res. 18:482017.
View Article : Google Scholar
|
|
29
|
Righetti RF, Dos Santos TM, Camargo LDN,
Aristóteles LRCRB, Fukuzaki S, de Souza FCR, Santana FPR, de Agrela
MVR, Cruz MM, Alonso-Vale MIC, et al: Protective effects of anti-
IL17 on acute lung injury induced by LPS in mice. Front Pharmacol.
9:10212018. View Article : Google Scholar
|
|
30
|
Sato K, Suematsu A, Okamoto K, Yamaguchi
A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y,
et al: Th17 functions as an osteoclastogenic helper T cell subset
that links T cell activation and bone destruction. J Exp Med.
203:2673–2682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kotake S, Udagawa N, Takahashi N,
Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N,
Gillespie MT, et al: IL- 17 in synovial fluids from patients with
rheumatoid arthritis is a potent stimulator of osteoclastogenesis.
J Clin Invest. 103:1345–1352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Campfield BT, Eddens T, Henkel M, Majewski
M, Horne W, Chaly Y, Gaffen SL, Hirsch R and Kolls JK: Follistatin-
like protein 1 modulates IL- 17 signaling via IL- 17RC regulation
in stromal cells. Immunol Cell Biol. 95:656–665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Luca A, Pariano M, Cellini B,
Costantini C, Villella VR, Jose SS, Palmieri M, Borghi M, Galosi C,
Paolicelli G, et al: The IL- 17F/IL- 17RC axis promotes respiratory
allergy in the proximal airways. Cell Rep. 20:1667–1680. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou S, Qiu XS, Zhu ZZ, Wu WF, Liu Z and
Qiu Y: A single- nucleotide polymorphism rs708567 in the IL- 17RC
gene is associated with a susceptibility to and the curve severity
of adolescent idiopathic scoliosis in a Chinese Han population: A
case- control study. BMC Musculoskelet Disord. 13:1812012.
View Article : Google Scholar
|
|
35
|
Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang
SJ, Kim HM, Lee Y and Kim HH: IL- 17 stimulates the proliferation
and differentiation of human mesenchymal stem cells: Implications
for bone remodeling. Cell Death Differ. 16:1332–1343. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin Y, Li F, Shi J, Li S, Cai J and Jiang
Y: MiR- 146a regulates inflammatory infiltration by macrophages in
polymyositis/dermatomyositis by targeting TRAF6 and affecting IL-
17/ICAM-1 pathway. Cell Physiol Biochem. 40:486–498. 2016.
View Article : Google Scholar
|
|
37
|
Qu F, Gao H, Zhu S, Shi P, Zhang Y, Liu Y,
Jallal B, Yao Y, Shi Y and Qian Y: TRAF6- dependent Act1
phosphorylation by the IκB kinase- related kinases suppresses
interleukin- 17- induced NF-κB activation. Mol Cell Biol.
32:3925–3937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang SH and Dong C: Signaling of
interleukin- 17 family cytokines in immunity and inflammation. Cell
Signal. 23:1069–1075. 2011. View Article : Google Scholar
|
|
39
|
Wang C, Petriello MC, Zhu B and Hennig B:
PCB 126 induces monocyte/macrophage polarization and inflammation
through AhR and NF-κB pathways. Toxicol Appl Pharmacol. 367:71–81.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng Y, Bi R, Guo H, Yang J, Du Y, Wang C
and Wei W: Andrographolide enhances TRAIL- induced apoptosis via
p53- mediated death receptors up- regulation and suppression of the
NF-κB pathway in bladder cancer cells. Int J Biol Sci. 15:688–700.
2019. View Article : Google Scholar :
|
|
41
|
Kosaka T, Imakiire A, Mizuno F and
Yamamoto K: Activation of nuclear factor kappaB at the onset of
ossification of the spinal ligaments. J Orthop Sci. 5:572–578.
2000. View Article : Google Scholar
|
|
42
|
Damsongsang P, Chaikiawkeaw D,
Phoolcharoen W, Rattanapisit K, Kaewpungsup P, Pavasant P and Hoven
VP: Surface- immobilized plant- derived osteopontin as an effective
platform to promote osteoblast adhesion and differentiation.
Colloids Surf B Biointerfaces. 173:816–824. 2019. View Article : Google Scholar
|
|
43
|
Ye M and Shi B: Zirconia nanoparticles-
induced toxic effects in osteoblast- like 3T3- E1 cells. Nanoscale
Res Lett. 13:3532018. View Article : Google Scholar
|
|
44
|
Lee YH, Bhattarai G, Park IS, Kim GR, Kim
GE, Lee MH and Yi HK: Bone regeneration around N- acetyl cysteine-
loaded nano-tube titanium dental implant in rat mandible.
Biomaterials. 34:10199–10208. 2013. View Article : Google Scholar : PubMed/NCBI
|