Introduction
Hypocretin/orexin is a hypothalamic neuropeptide,
which is involved in various physiological processes, including
emotion, energy homeostasis, feeding behavior, reward, and
sleep/vigilance maintenance and regulation (1). Hypocretin expression is almost
exclusively localized in the lateral hypothalamus (2,3).
Furthermore, two conserved promoter regions, orexin regulatory
element (OE)1 and OE2, have been identified within a 3.2-kb
fragment located upstream of the prepro-hypocretin gene. These
regions have been reported to target specific expression within the
lateral hypothalamus (3).
To detect and characterize the proliferation of
hypocretin neurons during embryonic development,
5-bromo-2-deoxyuri-dine was administered into the developing
ventral diencephalon of embryos of pregnant rats; this region is
where the embryonic hypothalamus is located (4). Hypocretin neuron proliferation
peaked sharply on day 12 of embryonic development. In mice, our
previous study detected prepro-hypocretin transcription from
embryonic day (E)10, using reverse transcription-polymerase chain
reaction (PCR) (5). A recent
study using mice expressing enhanced green fluorescent protein
(EGFP) under the control of the prepro-hypocretin promoter
demonstrated that EGFP and the hypocretin peptide are detectable
from E12 and E14, respectively (6). These findings suggest that
translational repression persists until the hypocretin neurons
migrate to a predetermined position, similar to other neurons
(7). In utero
electroporation of an EGFP expression vector into the third
ventricle at E12 revealed that some hypocretin neurons co-localize
with EGFP in the adult hypothalamus (8). These signals suggest that some
hypocretin neurons originate from the progenitor cells of the third
ventricular zone, at least at E10-E12. Following migration to the
designated area, these cells probably differentiate into
hypocretin-producing cells with the help of various factors,
including the hedgehog protein and its downstream transcription
factors, which are essential for anterior hypothalamic patterning
(9).
The co-expressed transcription factors in hypocretin
neurons regulate hypocretin transcriptional activities under
several conditions. Forkhead box A2 (FOXA2) regulates
prepro-hypocretin expression in the lateral hypothalamus during
fasting (10). Furthermore,
co-expression with LIM homeobox 9 (LHX9) has been observed in a
subset of developmental hypocretin-positive cells in the
hypothalamus (9) and adult murine
hypocretin neurons (11). LHX9 is
important for the normal development of a subset of hypocretin
neurons and canonical sleep behavior; however, to the best of our
knowledge, no direct effect on adult hypocretin expression has been
found (11). In our previous
study, several transcription factors were identified that are
downregulated in the hypothalamus of hypocretin neuron-ablated
transgenic mice (12);
specifically, early B-cell factor 2 (Ebf2); fifth ewing sarcoma
variant (Fev); insulin-like growth factor-binding protein 3
(Igfbp3); pleomorphic adenoma gene-like 1 (Plagl1); POU domain
class 2, transcription factor 1 (Pou2f1); paired-related homeobox
gene 1 (Prrx1); nuclear receptor subfamily 6 group A member 1
(Nr6a1); and visual system homeobox gene 2 (Vsx2). Ebf2-null mice
exhibit a significant reduction in hypocretin neurons in the
lateral hypothalamus (13), and
EBF2 modulates hypocretin promoter activities via the olf-1 motif
(14). Igfbp3, Plagl1 and Pou2f1
have also been identified as co-localized genes using comprehensive
translational profiling of all ribosome-bound transcripts (11). Our previous study revealed that
IGFBP3 reduces promoter activity of prepro-hypocretin (12), and NR6A1 upregulates hypocretin
transcription in embryos (8).
The present study aimed to examine the involvement
of these downregulated transcription factors (12) in hypo-cretin transcription. This
study further identified PLAGL1 as a co-expressed transcription
factor in hypothalamic hypocretin neurons. PLAGL1 has seven zinc
fingers of the C2H2 class (15),
recognizes DNA and RNA, and possesses transcriptional activity
(16). In humans, PLAGL1
undergoes parental genomic imprinting and is paternally expressed
(17). Plagl1-null mice exhibit
intrauterine growth restriction, altered bone formation and
neonatal lethality (18);
therefore, null mouse lines are difficult to obtain. In the present
study, the functional relevance of PLAGL1 in hypocretin
transcription was investigated and the results revealed that PLAGL1
may be associated with the canonical development and modulation of
various physiological processes in hypocretin neurons via newly
identified PLAGL1 binding sites
(G4N2G4) (19) within the hypocretin upstream
promoter region.
Materials and methods
Ethics statement
All animal experiments were conducted in accordance
with the Guidelines for the Care and Use of Laboratory Animals of
the National Institute of Health (20), and were approved by the Ethics
committee on Animal Experiments of the Kansai Medical University
(Hirakata, Japan; approval ID: 17-050) and the Tokyo Metropolitan
Institute of Medical Science (Tokyo, Japan).
Animals
A total of 41 male C57 black/6J mice were obtained
from Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan) housed
under a 12-h light/dark cycle, with the lights on between 8:00 a.m.
and 8:00 p.m., corresponding to Zeitgeber time (ZT) 0-12. The mice
were maintained at 22-24°C, and were provided food and water ad
libitum. A total of 20 mice (weight, 28-30 g; age, 12 weeks)
were sacrificed at ZT6, ZT18, or ZT6 with 6 h of total sleep
deprivation (SD6h) for immunohistochemical analysis. SD6h
experiments were conducted using the Seesaw shaker (shaking angle,
10°; Wave-SI; Taitec Corporation, Koshigaya, Japan) for examination
of sleep/wakefulness organization. To examine the effects of
fasting, nine mice (weight, 28-30 g; age, 12 weeks) were deprived
of food at ZT6 and were sacrificed within the following 2 days at
ZT6. Another group of 12 mice were fed a high-fat diet (HFD;
D12451; Research Diets Inc., New Brunswick, NJ, USA) beginning at 8
weeks of age. A normal chow diet (MF; Oriental Yeast Co., Ltd.,
Tokyo, Japan) provided 3.6 kcal/g of energy (61% carbohydrate, 26%
protein and 13% fat), whereas the HFD provided 4.7 kcal/g of energy
(35% carbohydrate, 20% protein and 45% fat). After 4 weeks of the
HFD, the mice were sacrificed at ZT6. There was no difference in
food intake between the control and HFD groups (28.3±1.4 vs.
26.2±3.8 g at 8 weeks of age); however, a significant difference
was observed in body weight between the two groups following each
diet intake (28.0±2.4 vs. 35.3±2.1 g at 12 weeks of age;
P<0.01).
Double immunohistochemical staining
combined with RNAscope (in situ hybridization) to determine
specificity of the PLAGL1 antibody
To confirm the specificity of the PLAGL1 antibody
[ZAC1 antibody (C-7): cat. no. sc-166944, Santa Cruz Biotechnology,
Inc., Dallas, TX, USA], double immunohistochemical staining was
performed combined with in situ hybridization.
Mice were deeply anesthetized with pentobarbital (50
mg/kg, i.p.), and transcardially perfused with PBS, followed by 4%
formaldehyde in 0.1 M phosphate buffer (pH 7.4). The whole brains
were removed, immersed in 4% formaldehyde in 0.1 M phosphate buffer
(pH 7.4) for 12 h at 4°C, cryoprotected in 20% sucrose in 0.1 M
phosphate buffer (pH 7.4) for 6 h at 4°C, and cryosectioned into
25-µm coronal sections. Sections were stored in PBS/0.3% Triton
X-100 (PBST) with 60% glycerol at −20°C until use. The
free-floating sections were blocked with PBST/5% bovine serum
albumin (BSA; cat. no. A-4503; Sigma-Aldrich; Merck KGaA) at room
temperature for 2 h and incubated overnight at 4°C with a rabbit
anti-hypocretin-1 antibody (1:5,000; cat. no. H-003-30; Phoenix
Pharmaceuticals, Inc., Burlingame, CA, USA) and mouse anti-PLAGL1
antibody [1:1,000; ZAC1 antibody (C-7)] in PBST/5% BSA. The next
day, sections were incubated with a donkey Cy5-labeled anti-mouse
immunoglobulin (Ig)G (1:500; cat. no. 715-175-150, Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and a goat
Alexa Fluor® 488-labeled anti-rabbit IgG (1:3,000; cat.
no. A-11008, Molecular Probes; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 30 min at room temperature. After washing
with PBST, the stained sections were mounted on Superfrost Plus
slides (Thermo Fisher Scientific, Inc.) and air-dried. Once washed
with distilled water, slides were baked at 60°C for 30 min.
Subsequently, in situ hybridization was conducted with the
RNAscope® 2.5 HD Reagent kit-RED (cat. no. 322350;
Advanced Cell Diagnostics, Inc., Newark, CA, USA), and probe
Mm-Plagl1 (cat. no. 462941; Advanced Cell Diagnostics, Inc.),
positive control probe Mm-RNA polymerase II subunit A (cat. no.
312471; Advanced Cell Diagnostics, Inc.) or negative control probe
DapB (cat. no. 310043; Advanced Cell Diagnostics, Inc.), according
to the manufacturer's protocols. After final amplification, Fast
Red chromogenic detection was performed. The slides were
coverslipped with DAPI Fluoromount-G (cat. no. 0100-20; Southern
Biotech, Birmingham, AL, USA), and the resulting hypocretin, PLAGL1
protein and Plagl1 mRNA signals were visualized using confocal
laser microscopy (LMS700; Zeiss GmbH, Jena, Germany). Bright-field
images of Fast Red staining were captured using the Eclipse E-1000M
(Nikon Corporation, Tokyo, Japan) with a digital camera.
The antibody used to identify
hypocretin-immunoreactive neurons exhibits 100% cross-reactivity
with human, bovine, mouse and rat hypocretin-1, but no
cross-reactivity with human hypocretin-2 or other related peptides.
Control experiments included the omission of the primary antibody,
as well as extensive tests for cross-reactivity of the secondary
antibodies. All control experiments resulted in the absence of
staining.
Double immunohistochemical staining
The procedure of fixing, brain sectioning, blocking
and probing with primary antibodies of male C57 black/6J mice (age,
12 and 18 weeks), was the same as the aforementioned procedure.
After blocking, free-floating sections were incubated overnight at
4°C with rabbit anti-hypocretin-1 antibody (1:5,000) and mouse
anti-PLAGL1 antibody (1:1,000), as well as goat anti-PLAGL2
antibody (1:500; cat. no. sc-19907; Santa Cruz Biotechnology,
Inc.), goat anti-PLAG1 zinc finger antibody (1:500; cat. no.
sc-20320; Santa Cruz Biotechnology, Inc.), mouse anti-FEV antibody
(1:500; cat. no. H00054738-A01; Abnova, Taipei, Taiwan), goat
anti-PRRX1 antibody (1:500; cat. no. LS-B2380; LifeSpan
BioSciences, Inc., Seattle, WA, USA) and sheep anti-VSX2 antibody
(1:500; cat. no. PAB9843; Abnova) in PBST/5% BSA. The next day,
free-floating sections were incubated with goat, chicken or donkey
Alexa Fluor® 594-labeled anti-mouse (cat. no. A11005),
anti-goat (cat. no. A21468), or anti-sheep (cat. no. A11016) IgG,
and goat (cat. no. A11008) or donkey (cat. no. R37118) Alexa
Fluor® 488-labeled anti-rabbit IgG (1:3,000; Molecular
Probes) for 30 min at room temperature followed by mounting with
DAPI Fluoromount-G. The resulting immunopositive signals were
visualized under a fluorescence microscope mounted with a digital
camera (Olympus BX51 and DP70; Olympus Corporation, Tokyo,
Japan).
Expression vectors and reporter
plasmids
The CAG promoter derived from the pCA-pA expression
vector was cloned into the pcDNA3 vector (Invitrogen; Thermo Fisher
Scientific, Inc.), resulting in the development of the pCAGGS
vector as a control for in utero electroporation (mock).
High-fidelity PCR amplification of murine Plagl1 from the embryonic
brain at E10-E12 was performed using KOD plus DNA polymerase
(Toyobo Life Science, Osaka, Japan), betaine and either an
mPlagl1_546F primer with a KpnI site (5′-CGGGGTACCAAAGGCCATGGCTCCATTCCGCTGTCA-3′)
or an mPlagl1_2671R primer with a BamHI site
(5′-TAGATCCGGTGGATCCTTATCTAAATGCGTGATGGA-3′).
The KpnI and BamHI sites are underlined in the
sequences provided. Following PCR amplification, as follows: One
cycle at 98°C for 2 min, followed by 20 cycles of denaturation at
94°C for 30 sec, annealing at 60°C for 30 sec and extension at 68°C
for 2 min, and a final extension step at 68°C for 5 min, Plagl1 was
sub-cloned into the pCAGGS vector (pCAGGS-mPlagl1). The expression
vector pCAGGS-EGFP, which contains EGFP cDNA under the control of
the CAG promoter, was prepared to confirm the introduced locations
and check the introduction efficiencies for in utero
electroporation in the murine hypothalamus (8).
The reporter plasmids pGL3-basic and pGL4.74
[hRluc/TK] were purchased from Promega Corporation (Madison, WI).
The pGL4.74 [hRluc/TK], encoding Renilla luciferase, was
used as an internal control of transfection efficiency for the
reporter assay. The human prepro-hypocretin promoter sequence from
the position −3,278/+87 was subcloned into the upstream region of
the firefly luciferase gene in the pGL3-basic plasmid (3.2
kb-basic) (8). The murine
prepro-hypocretin promoter sequence from the position −382/+91
amplified with KOD plus DNA polymerase, mHcrt_+92_BglII
(5′-GGAAGATCTGGAACCTTTGTAGAAGGAAAG-3′;
the BglII site is underlined), and mHcrt_-385_XhoI
(5′-CCGCTCGAGAGGTACCCTCCCTACCTTCAA-3′;
the XhoI site is underlined) was subcloned into the
pGL3-basic plasmid (473 bp-basic). In the 473 bp-basic plasmid, the
PLAGL1 binding site was deleted from the prepro-hypocretin promoter
sequence.
All the constructs were confirmed to have no
mutation, insertion or deletion by sequencing analysis using the
BigDye Terminator Cycle Sequencing Reaction kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the ABI PRISM 3100
Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Both strands were read with sequence primers (Table SI).
Chromatin immunoprecipitation
(ChIP)-PCR
The brains of 12-week-old C57 black/6J mice were
rapidly removed at ZT6, and the hypothalamus was dissected
coronally from the optic chiasma to the mammillary bodies (−3.5 mm
from the bregma) using a brain slicer (Zivic Instruments,
Pittsburgh, PA, USA). The dorsal limit of the hypothalamus was the
roof of the third ventricle, while the lateral limit was the
amygdala (21). The hypothalamus
was finely minced with a razor blade and crosslinked with 1%
formaldehyde for 10 min at room temperature; a final concentration
of 0.125 M glycine was added to block further crosslinking. Tissues
were washed twice with ice-cold PBS, and a crude nuclear extract
was prepared according to a previously reported method (22). To obtain digested genomic DNA
ranging between 150 and 900 bp, the nuclear extract was incubated
with micrococcal nuclease (Cell Signaling Technology, Inc., Tokyo,
Japan) for 20 min at 37°C. The size of the DNA fragments was
confirmed by gel electrophoresis following treatment with RNase I
for 30 min at 37°C and Proteinase K for 2 h at 65°C. The lysate was
sonicated at 43 kHz three times (20 sec/sonication) at 4°C,
centrifuged and the obtained supernatant was diluted in ChIP buffer
(Cell Signaling Technology, Inc.) overnight at 4°C on a wheel
rotator with either 2 µg mouse anti-ZAC1 antibody (C-7)X (cat. no.
sc-166944 X; Santa Cruz Biotechnology, Inc.), which was used to
capture the PLAGL1 protein, or 2 µg normal rabbit IgG (cat. no.
2729; Cell Signaling Technology, Inc.), which was used as a
negative control. Samples were then incubated with Protein G
agarose beads for 2 h at 4°C. Beads were washed with low- and
high-salt wash buffers, and DNA templates for PCR were purified
from the resulting DNA-protein complexes. ChIP-PCR analysis was
performed using KOD plus DNA polymerase (Toyobo Life Sciences),
primers (Table SI) and a thermal
cycler, with the following settings: One cycle at 95°C for 5 min,
followed by 35 cycles of denaturation at 94°C for 30 sec, annealing
at 58°C for 30 sec and extension at 68°C for 30 sec.
Luciferase reporter assay
NIH3T3 (murine embryonic fibroblast; American Type
Culture Collection, Manassas, VA, USA) cells were grown in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing
5% CO2.
Cells were seeded at a density of 1x104
cells/well in 96-well cell culture plates. Two types of luciferase
plasmids and one expression vector were co-transfected into the
cells with Lipofectamine® 3000 Transfection Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The amounts of co-transfected plasmids and
vectors per well were as follows: 100 ng firefly
luciferase-encoding reporter plasmid (pGL3), 10 ng Renilla
luciferase-encoding internal control plasmid (pGL4.74), and 100 ng
expression vector pCAGGS (control plasmid as mock) or
pCAGGS-mPlagl1. Approximately 48 h post-transfection, luciferase
activities were measured sequentially in duplicate using Dual-Glo
Luciferase Reporter assay reagents (Promega Corporation) and an
EnSpire plate reader (PerkinElmer, Inc., Waltham, MA, USA),
according to the manufacturer's protocol. The activity of firefly
luciferase luminescence (FLU) from the pGL3 plasmid and the
activity of Renilla luciferase luminescence (RLU) from the
pGL4.74 plasmid were measured independently. The relative
luciferase activity per well was determined as FLU divided by RLU
values. Data for the relative activities were expressed as the mean
of at least eight independent experiments. Mouse Plagl1 mRNA
expression was confirmed after transfection by PCR with gene
specific primers (Table SI)
(data not shown).
In situ hybridization of embryos
In situ hybridization using
digoxigenin-labeled cRNA probes was performed on cryosections. The
procedure of fixing and sectioning brain samples at E13, E14, and
E18 were the same as aforementioned. Briefly, at E10-E12, embryos
were immersed in 4% formaldehyde in 0.1 M phosphate buffer (pH 7.4)
for 16 h at 4°C, cryoprotected in 20% sucrose in 0.1 M phosphate
buffer (pH 7.4) for 6 h at 4°C and cryosectioned into 25-µm coronal
sections. Riboprobes for murine Plagl1 and negative control
riboprobes for murine Plagl2 were generated by PCR with either the
mPlagl1_606F (5′-GAAGTTCACCATTCACAATTATTCC-3′) and mPlagl1_1992R
(5′-AATGGCTATATTCACAGCATCTACC-3′) primer pair, the mPlagl1_1968F
(5′-GGTAGATGCTGTGAATATAGCCATT-3′) and mPlagl1_3513R
(5′-TGAGTATCACAGTCCACAAAACACT-3′) primer pair, the mPlagl2_587F
(5′-GAGTGTGGTAAGAATTATAATACAAAGCTG-3′) and mPlagl2_1822R
(5′-TCCATATAGACACTAGAATTTTCTTTTTGA-3′) primer pair, or the
mPlagl2_3352F (5′-ACTAGGGTTTCAGGACAGACTACCT-3′) and mPlagl2_4857R
(5′-AACACCTACCTAAGGCAAAGTTTCT-3′) primer pair, with subsequent
sub-cloning of the PCR products into the pGEMT-easy vector (Promega
Corporation) and in vitro transcription with DIG-11-UTP
(cat. no. 3359247910; Sigma-Aldrich; Merck KGaA). Brain sections
were post-fixed in 4% formaldehyde for 10 min at 20-22°C,
acetylated with 0.5% acetic anhydride in 0.1 M triethanolamine (pH
8.0) for 10 min and rinsed in PBS. The slides were pre-hybridized
in a hybridization buffer without cRNA probe at room temperature
for 2 h and hybridized using a hybridization buffer (50% formamide,
5X SSPE, 1 mg/ml yeast tRNA, 0.2% sodium dodecyl sulfate)
containing 1 µg/ml cRNA probe at 60°C overnight. The next day,
slides were washed in 2X SSC containing 50% formamide at 60°C for 1
h. Hybridization was detected using an anti-digoxigenin Fab
(1:2,000; cat. no. 11093274910; Sigma-Aldrich; Merck KGaA) for 1 h
at 20-22°C coupled to alkaline phosphatase using NBT/BCIP. The
resulting digoxigenin signals with Plagl probes were captured using
a microscope mounted with a digital camera (Olympus BX51 and DP70;
Olympus Corporation).
In utero electroporation
In utero electroporation was performed
according to a previously reported method (8), with some modifications. Briefly, ~1
µl pCAGGS-mPlagl1 and pCAGGS-EGFP expression vectors (2 µg/µl) for
the PLAGL1 group, or 1 µl pCAGGS and pCAGGS-EGFP expression vectors
(2 µg/µl) for the mock group were mixed with 0.05% Fast Green as a
tracer and injected into the third ventricle of each C57 black/6J
mouse embryo (E12) using free-hand injections with a glass
capillary pipette through the uterine wall. Electrodes (3 mm round
shape; CUY650P3; Nepa Gene Co., Ltd., Ichikawa, Japan) were placed
on both sides of the embryo, near the head of the embryo, and
electro-poration was performed using a square-pulse electroporator
CUY21 EDIT in vivo electroporator (Nepa Gene Co., Ltd.)
(four pulses; amplitude, 39V; duration 50 msec; interval between
pulses, 950 msec), resulting in gene transfer into one side of the
hypothalamus. Control mice were electropor-ated with the same
vectors from the lateral ventricle into the cortex. Intact embryos
or gene electroporated-embryos were harvested at E14, E15, E18 or
postnatal day (P)0, and the embryos were transcardially perfused
with 4% formaldehyde in a 0.1 M phosphate buffer (pH 7.4). The
whole brains were removed, post-fixed, and cryosectioned into 30-µm
coronal sections. Hypocretin and PLAGL1 expression levels were
detected by immunofluorescence methods using either a rabbit
anti-hypocretin-1 or a mouse ZAC1 antibody, and a goat Alexa
Fluor® 594-labeled anti-rabbit or anti-mouse IgG. The
resulting hypocretin and PLAGL1 signals were visual-ized under a
fluorescence microscope mounted with a digital camera (Olympus BX51
and DP70; Olympus Corporation). To reduce the background signals in
embryo staining, the Mouse on Mouse Immunodetection kit (Vector
Laboratories, Inc., Burlingame, CA, USA) was used, according to the
manufacturer's protocols.
Quantitative PCR (qPCR)
Total RNA was isolated from the hypothalamus of each
of the control animals (n=8, 12 weeks old), HFD animals (n=8) at
ZT6, P0 embryos in the mock group (n=4) or P0 embryos in the PLAGL1
group (n=6) using Sepasol-RNA I Super G reagent (Nacalai Tesque,
Inc., Kyoto, Japan). Single-stranded cDNA was synthesized using the
PrimeScript RT reagent kit with gDNA Eraser (Takara Bio, Inc.,
Otsu, Japan), according to manufacturer's protocol. The expression
levels of each mRNA were determined by qPCR with the Rotor-Gene Q
system (Qiagen GmbH, Hilden, Germany) using the THUNDERBIRD qPCR
mix (Toyobo Life Science) and gene-specific primers (Table SI). PCR products were amplified
using the following thermocycling conditions: One cycle at 95°C for
1 min, followed by 40 cycles at 95°C for 10 sec and 60°C for 60
sec. The housekeeping gene with minimum diurnal variation in the
hypothalamus was identified in a previous study by checking gene
expression every 4 h over a 24-h period (23). Subsequently, the relative level of
target gene expression was evaluated using the 2-ΔΔCq
method (24) with hypoxanthine
phosphoribosyltransferase 1 as an internal control.
Statistical analyses
Statistical analyses were conducted using IBM SPSS
version 25 (IBM Corp., Armonk, NY, USA) or Microsoft Excel 2013
software (Microsoft Corporation, Redmond, WA, USA). Data are
presented as the means ± standard deviation. Normality was analyzed
by the Shapiro-Wilk normality test for all groups. F-test was used
to determine homoscedasticity for all comparisons. All groups were
normally distributed in the hypocretin neuron counts, PLAGL1 counts
and PLAGL1 immunopositivity experiments (P>0.05). One-way
analysis of variance (ANOVA), followed by Steel's post hoc test
against the control group (ZT6), was used to analyze the
differences; P<0.05 was considered to indicate a statistically
significant different (n=4-5). The differences in hypocretin and
Plagl1 expression due to HFD compared to the control group were
compared using two-tailed Student's t-tests. To evaluate
alterations in β-2-microglobulin (B2m) expression due to HFD, the
two-tailed Welch's t-test was used due to a lack of
homoscedasticity; P<0.05 was considered to indicate a
statistically significant difference (n=8). Alterations in promoter
activity for each reporter plasmid with/without the PLAGL1
expression vector, or from pGL3-basic, were compared using one-way
ANOVA followed by Tukey's post hoc test; P<0.05 was considered
to indicate a statistically significant difference (n=8). The qPCR
data of the in utero electroporation experiments were not
normally distributed (P<0.05); therefore, the Mann-Whitney
U-test was used to compare differences; P<0.05 compared to the
contralateral side (mock group, n=4; PLAGL1 group, n=6) was
considered to indicate a statistically significant difference.
Results
Localization of PLAGL1 in adult
hypothalamic hypocretin neurons
Plagl1 mRNA expression was clearly detected in the
lateral hypothalamic area (Fig.
S1), which has also been reported in the Allen Brain Atlas
database (25). FEV PRRX1, VSX2
and other PLAG family proteins, including PLAG1 and PLAGL2, were
not detected in the lateral hypothalamic area in this study (data
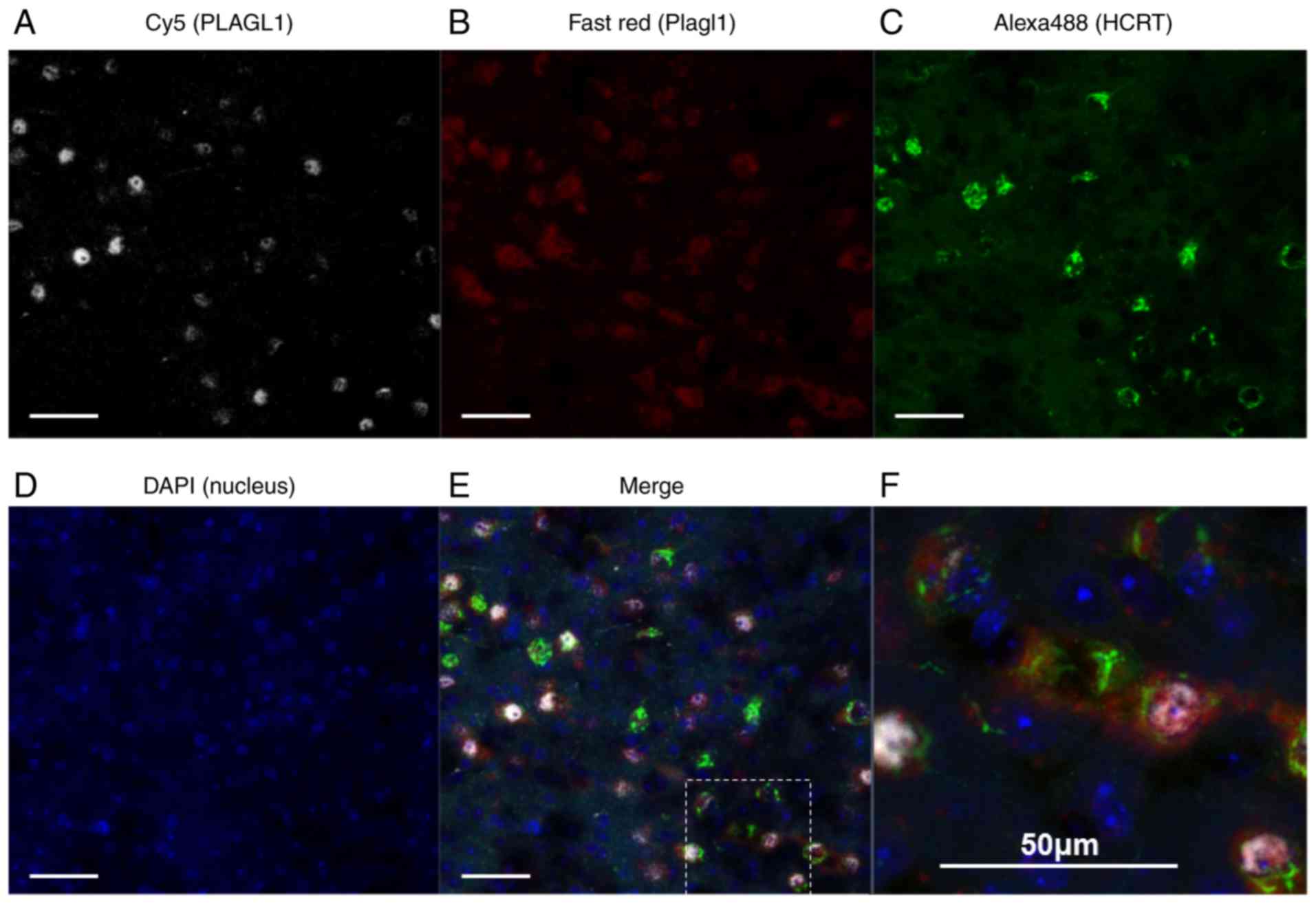
not shown). To confirm the specificity of the PLAGL1 antibody [ZAC1
antibody (C-7)], double immunohis-tochemical staining was performed
in combination with in situ hybridization. Neurons with
PLAGL1 signals (i.e., neurons immunopositive for the anti-PLAGL1
antibody) exhibited 100% merge with RNAscope Plagl1 signals in
three different fields. A total of 85 cells positive for PLAGL1
were detected in 85 cells with RNAscope Plagl1 signals. However,
neurons with RNAscope Plagl1 signals were not always merged with
the PLAGL1 signals stained by anti-PLAGL1 antibody. The results
demonstrated that 15% of RNAscope Plagl1-positive neurons were not
merged with the PLAGL1 signals stained by anti-PLAGL1 antibody
(Fig. S2). In the RNAscope
experiments, the hypothalamus sections were treated with Proteinase
K; therefore, it is possible that PLAGL1 proteins were partly
degraded, resulting in weak or no immunopositive signals. Notably,
hypocretin signals were weaker in Proteinase K-treated sections
(Figs. 1 and S3) than in non-treated sections
(Fig. 2).
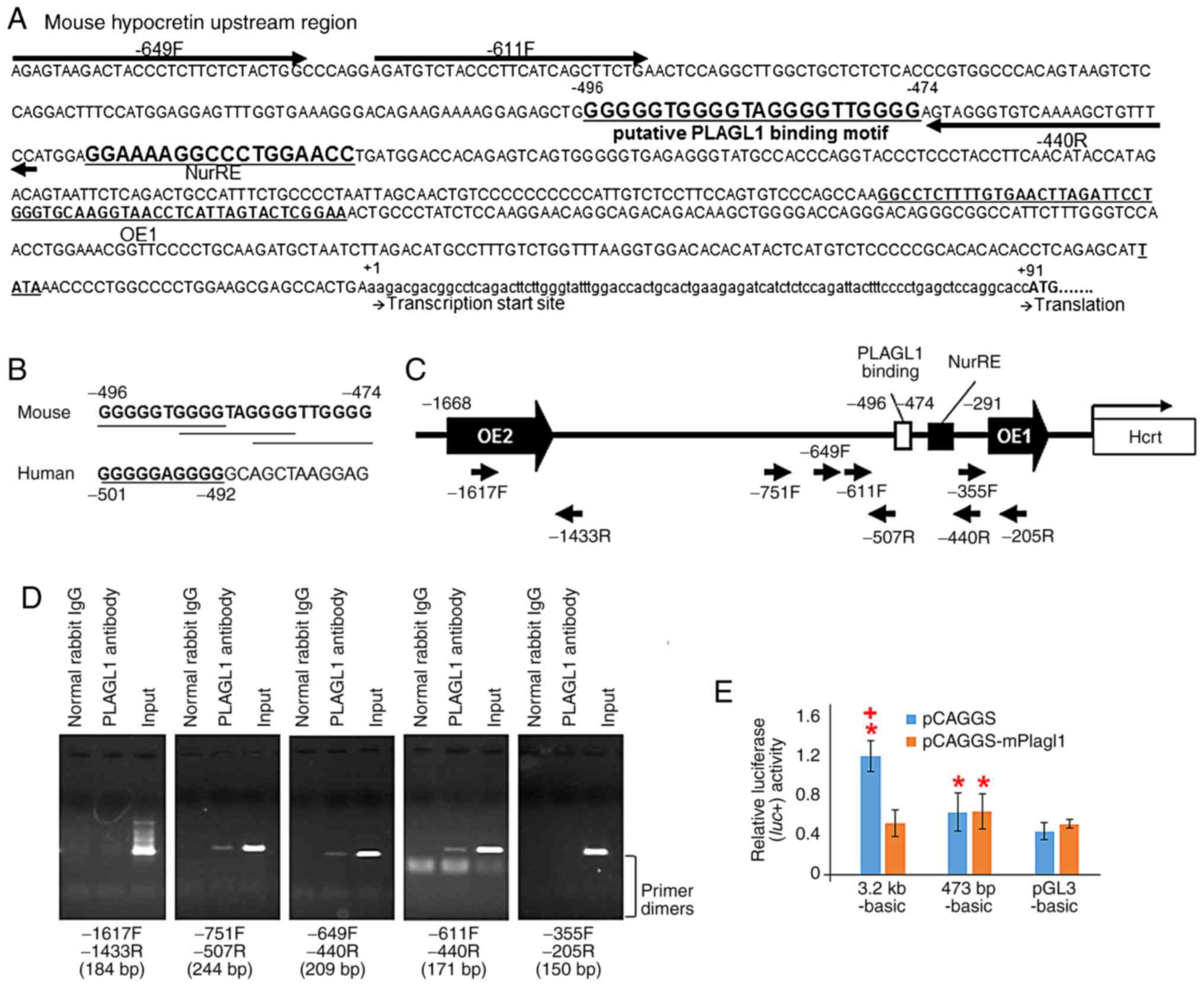
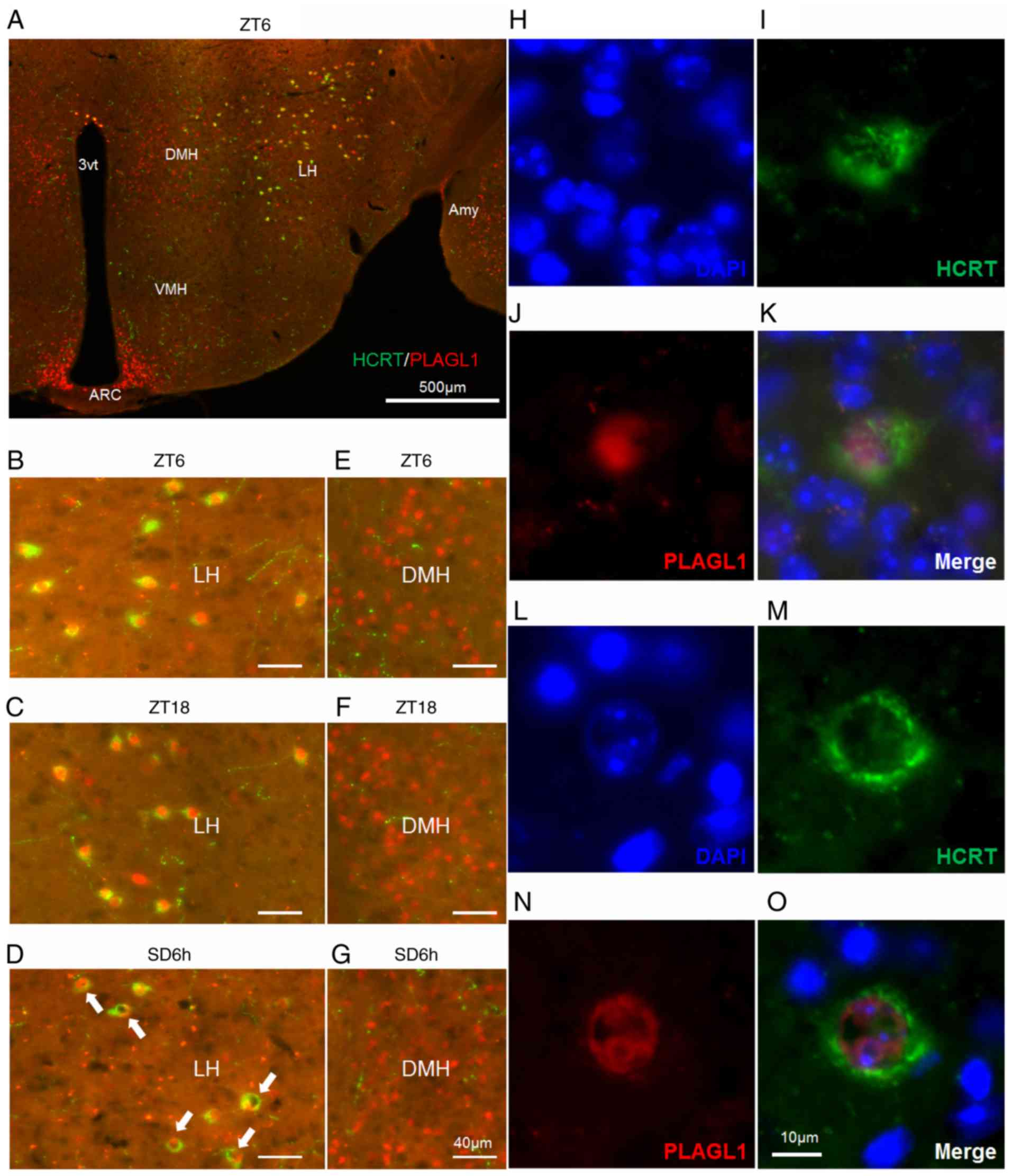 | Figure 2Localization of PLAGL1 in murine
hypocretin neurons under several conditions. (A-G) Murine HCRT was
visualized by staining with Alexa Fluor® 488 (green) and
murine PLAGL1 was visualized by staining with Alexa
Fluor® 594 (red). (A) Murine hypothalamic sections at
ZT6. The lateral hypothalamic area under (B) ZT6, (C) ZT18 and (D)
SD6h conditions, with a relatively specific and uneven distribution
of PLAGL1 (arrows). The dorsomedial hypothalamic nucleus under (E)
ZT6, (F) ZT18 and (G) SD6h conditions. (H) DAPI-labeled nuclei at
ZT6. (I) Alexa Fluor®488-labeled murine HCRT at ZT6. (J)
Alexa Fluor® 594-labeled murine PLAGL1 at ZT6. (K)
Merged image of (H, blue), (I, green) and (J, red)
immunofluorescence at ZT6. (L) DAPI-labeled nuclei at SD6h. (M)
Alexa Fluor®488-labeled murine HCRT at SD6h. (N) Alexa
Fluor®594-labeled murine PLAGL1 at SD6h. (O) Merged
image of (L, blue), (M, green), and (N, red) immunofluorescence at
SD6h. Scale bar, (A) 500 µm, (B-G) 40 µm, (O) 10 µm. (H-O) These
images are displayed at the same magnification. 3vt, third
ventricle; Amy, amygdala; ARC, arcuate nucleus; DMH, dorsomedial
hypothalamic nucleus; HCRT, hypocretin; LH, lateral hypothalamus;
PLAGL1, pleomorphic adenoma gene-like 1; SD6h, 6 h of sleep
deprivation; VMH, ventromedial nucleus of the hypothalamus; ZT,
Zeitgeber time. |
PLAGL1 is expressed in the lateral hypothalamic
area, dorsomedial hypothalamic nucleus and arcuate nucleus of the
adult male mouse hypothalamus at ZT6 (Fig. 2A) and is localized within
hypocretin neurons (Fig. 2A and
B). A relatively specific and uneven nuclear localization of
PLAGL1 was detected in the hypocretin neurons of the lateral
hypothalamic area in mice with SD6h (Fig. 2D, arrows) compared with ZT6
(Fig. 2B) and ZT18 (Fig. 2C) in the dorsomedial hypothalamic
nucleus at ZT6, ZT18 and SD6h (Fig.
2E-G), or the arcuate nucleus (data not shown). Subsequently,
DAPI staining revealed diffuse nuclear staining of PLAGL1 at ZT6
(Fig. 2B and H-K) and a
heterochromatin-like structure in the hypocretin neurons with SD6h.
The uneven nuclear localization of PLAGL1 was not merged with the
heterochromatin-like structure (Fig.
2L-O), suggesting that PLAGL1 was specifically related to the
euchromatin structure and transcription in hypocretin neurons
following SD6h. Furthermore, a significant increase was noted in
the number and frequency of PLAGL1-positive hypocretin neurons at
ZT18 compared with the control group (ZT6) (Fig. 3).
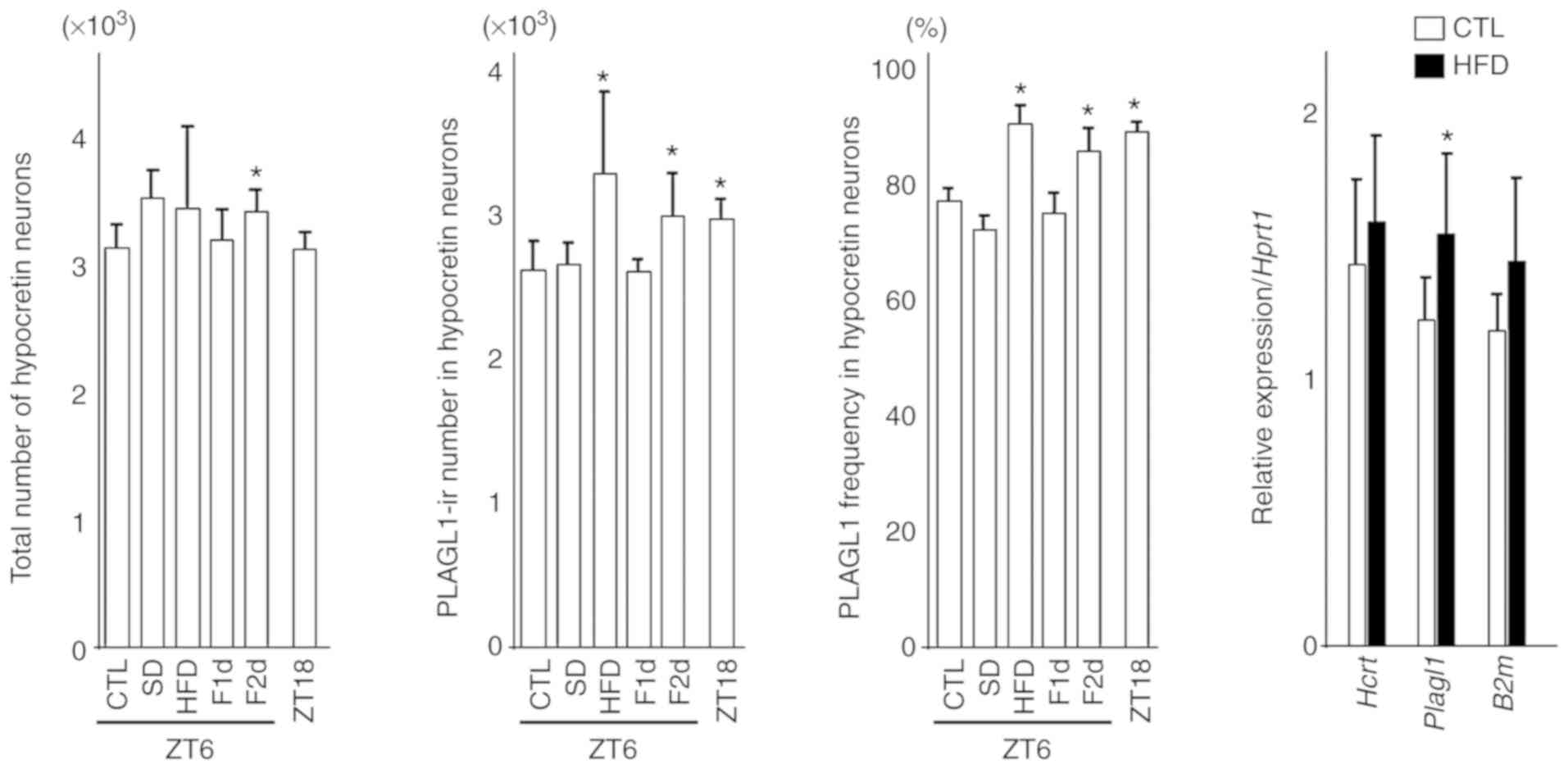 | Figure 3Number of hypocretin neurons,
PLAGL1-ir number in hypocretin neurons, frequency of PLAGL1 and
relative gene expression in hypocretin neurons. Differences in the
count of hypocretin neurons, PLAGL1 counts and PLAGL1
immunopositivity were compared using one-way analysis of variance
followed by Steel's post hoc test. *P<0.05 vs. the
control (CTL) group (ZT6). Four or five animals from each group
were used for each immunohistochemical analysis. The differences in
relative gene expression were compared using two-tailed Student's
t-tests for hypocretin and Plagl1 expression, and two-tailed
Welch's t-test for B2m. Eight animals from each group were used for
gene expression analysis. B2m, β-2-microglobulin; CTL, control
(mice sacrificed at ZT6); F1d, 1 day of fasting; F2d, 2 days of
fasting; Hcrt, hypocretin; HFD, high-fat diet; ir, immunoreactive;
Plagl1, pleomorphic adenoma gene-like 1; SD, 6 h of sleep
deprivation; ZT, Zeitgeber time; ZT18, mice sacrificed at ZT18.
Bars indicate means, and error bars represent standard
deviations. |
Notably, 1 month of a HFD slightly increased the
number of hypothalamic hypocretin neurons and induced a significant
increase in the number and frequency of PLAGL1-positive hypocretin
neurons compared with the control group (ZT6). In addition, 2 days
of fasting affected the number of hypothalamic hypocretin neurons,
and induced a significant increase in the number and frequency of
PLAGL1-positive hypocretin neurons compared with the control group
(ZT6) (Fig. 3).
There was a significant increase in Plagl1
expression in the HFD group (n=8) (mean ± standard deviation,
1.55±0.30; P<0.05) compared with the control group (n=8)
(1.22±0.15), although there was no significant difference in
hypocretin (HFD vs. control, 1.58±0.32 vs. 1.44±0.31) and B2m
(1.44±0.32 vs. 1.18±0.13) expression (Fig. 3). B2m was examined as a reference
gene.
PLAGL1 binds to the hypocretin upstream
region in vivo
The 'find' tool in Microsoft Word 2013 software
(Microsoft Corporation) was used to detect the known consensus
binding site for PLAGL1 (19,26) in the hypocretin gene upstream
region, which is highly conserved between humans and mice and
includes OE1 and OE2 (2,3). Newly identified PLAGL1-binding sites
(G4N2G4) (19) were found and are denoted as
GGGGGTGGGGTAGGGGTTGGGG at position −496/−474, relative to the
transcription start site, in the upstream region of the murine
hypocretin gene (Fig. 4A), and as
GGGGGAGGGG at position −501/−492 in the human sequence (Fig. 4B). Subsequently, a ChIP-PCR
analysis was performed using primers specific to the murine
hypocretin upstream region around the
G4N2G4 motifs (Fig. 4A and C) and negative control
primers (−1617F and −1433R pair, and −355F and −205R pair)
(Fig. 4C), with purified DNA
immu-noprecipitated with an anti-PLAGL1 antibody. ChIP-PCR analysis
with the mHcrt_-611F and mHcrt_-440R primer pair revealed a
positive reaction for the murine hypocretin upstream region around
the G4N2G4 motifs (Fig. 4C and D). An additional positive
reaction was found in the experiment using the mHcrt_-649F and
mHcrt_-440R primer pair and the mHcrt_-751F and mHcrt_-507R primer
pair (Fig. 4C and D). No
amplification was detected from the purified DNA incubated with
normal rabbit IgG (Fig. 4C and D)
or water, which were used as negative control templates, or
negative control experiments with the mHcrt_-1617F and mHcrt_-1433R
primer pair and the mHcrt_-355F and mHcrt_-205R primer pair
(Fig. 4D).
 | Figure 4Schematic representation of the
murine prepro-hypocretin gene regulatory region, putative PLAGL1
binding sites in mice and humans, ChIP-PCR and luciferase reporter
assay. (A) Sequence of the murine prepro-hypocretin gene regulatory
region. Highly conserved region orexin regulatory element 1, which
has been previously reported (2,3).
The first residues of the transcription start site and the
translation start site are marked as +1 and +91, respectively.
NurRE (8), the putative PLAGL1
binding site and primers for ChIP-PCR analysis are shown. (B)
Putative PLAGL1 binding sites in mouse and human promoter sequences
are shown in bold and are underlined. (C) Schematic representation
of the murine prepro-hypocretin gene regulatory region, and the
position of ChIP-PCR primers. (D) ChIP-PCR analysis of endogenous
PLAGL1 binding using ChIP-PCR primers. The length of each PCR
product is represented at the bottom of each image. (E)
Transcriptional activities of the human prepro-hypocretin promoter
and murine deletion mutants in NIH3T3 cells. *P<0.05
vs. pGL3-basic with pCAGGS vector. +P<0.05 vs. each
reporter with pCAGGS-mPlagl1. ChIP, chromatin immunoprecipitation;
IgG, immunoglobulin G; NurRE, nuclear receptor response element;
PLAGL1, pleomorphic adenoma gene-like 1; PCR, polymerase chain
reaction. |
PLAGL1 overexpression suppresses
hypocretin promoter activity in NIH3T3 cells
Co-transfection of the pGL3-basic plasmid with the
pCAGGS (mean ± standard deviation, 0.444±0.090) or pCAGGS-mPlagl1
(0.517±0.044) vectors exhibited similar luciferase expression in
NIH3T3 cells, indicating an absence of confounding endogenous
transcriptional regulatory elements in the pGL3-basic plasmid
(Fig. 4E). The luciferase
activity from 3.2 kb-basic with the pCAGGS vector (mock) exhibited
a significant three-fold increase compared with the pGL3-basic with
pCAGGS vector (mock) (1.205±0.161; P<0.001) in NIH3T3 cells
(Fig. 4E). The observed
activities in the 3.2 kb-basic group were suppressed by
co-transfection with the pCAGGS-mPlagl1 vector compared to mock in
NIH3T3 cells (0.521±0.135; P<0.05; Fig. 4E).
Subsequently a deletion mutant of the PLAGL1 binding
site was generated (473 bp-basic). This deletion mutant reporter
plasmid with mock led to significant increases in relative
lucif-erase activity compared to pGL3-basic with mock in NIH3T3
cells (0.637±0.198; P<0.05; Fig.
4E). No response to PLAGL1 was detected in the deletion mutant
473 bp-basic reporter plasmid with the pCAGGS-mPlagl1 vector
compared to the deletion mutant with mock (0.644±0.183; Fig. 4E), suggesting that the PLAGL1
binding ability to the preprohypocretin promoter region disappeared
in the 473 bp-basic reporter plasmid; however, the promoter
activities remained. Notably, the transcriptional activities of the
prepro-hypocretin promoter sequence may be suppressed by PLAGL1 via
the putative PLAGL1 binding site in NIH3T3 cells.
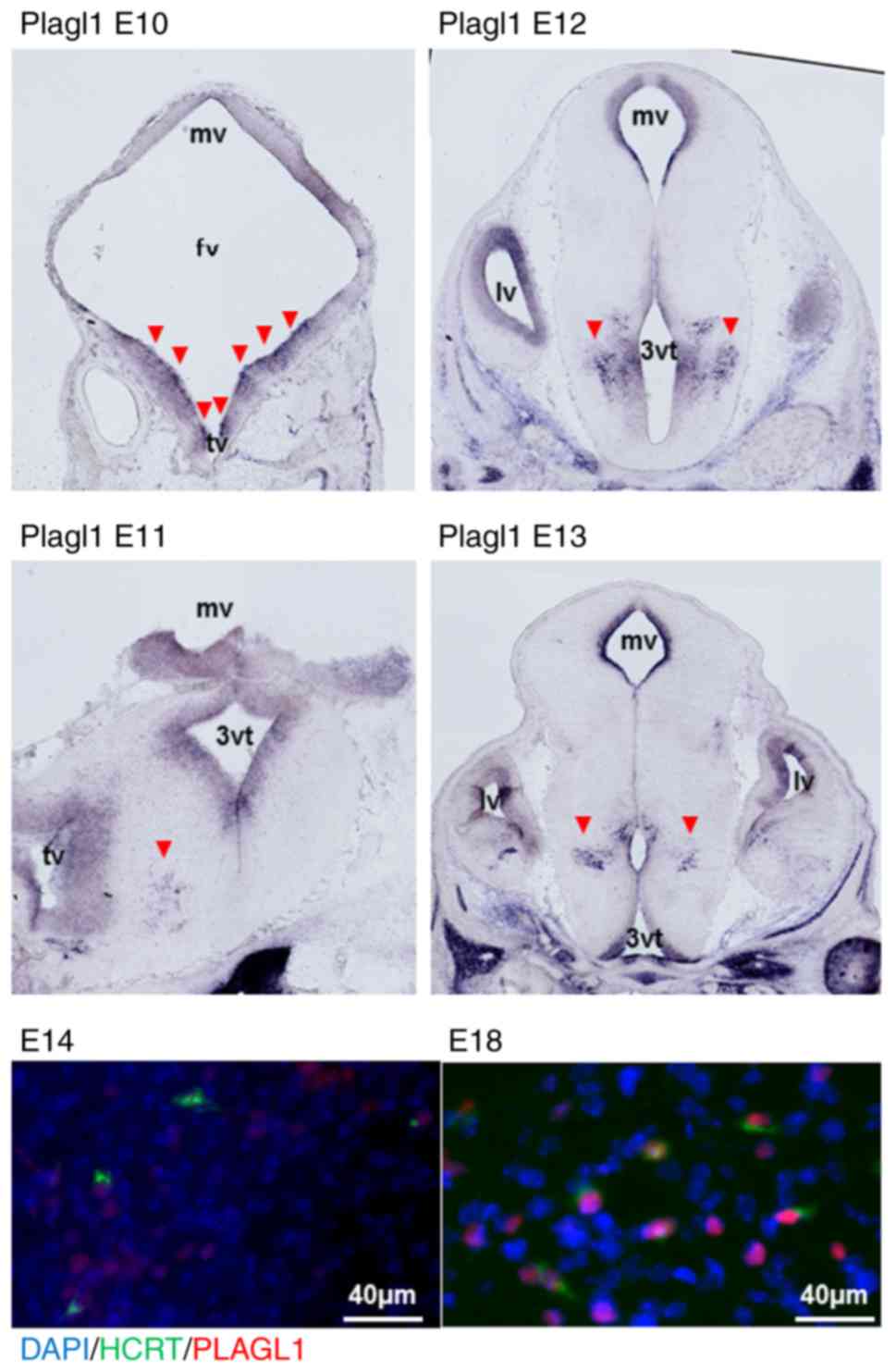
Localization of PLAGL1 within embryonic
hypocretin neurons
Murine Plagl1 was detected in the periventricular
zone of the mesencephalic, forebrain and telencephalic vesicle at
E10, using either the mouse Plagl1 cRNA 606F-1992R probe (Fig. 5) or the 1968F-3513R probe (data
not shown). Plagl1 signals were subsequently detected in
periventricular areas and the hypothalamic sulcus at E11-E13. The
co-expressed signals of hypocretin and PLAGL1 were also detected
after E14 using immunofluorescence detection. Hypocretin neurons
were positive for PLAGL1 in their nuclei at E18 (Fig. 5). There was no signal with Plagl2
probes (data not shown).
In utero electroporation
To confirm the physiological relevance of PLAGL1 in
hypothalamic hypocretin transcription, Plagl1 overexpression in the
murine hypothalamus was generated using in utero
electroporation. Electroporated EGFP expression was detected on one
side of the hypothalamus at E15 (Fig. S4, arrows). The present study
confirmed the co-expression of PLAGL1 and EGFP following
electroporation into the cortex using immunofluorescence detection
(Fig. S5); however, it was noted
that some background signals did not overlap with the EGFP signals
even when using the Mouse on Mouse Immunodetection kit, which could
be considered either endogenous neural expression, or the presence
of blood cells and vesicles in the cortex. Although most
EGFP-positive cells did not express hypocretin in the vicinity of
the third ventricle, some EGFP-positive cells that moved to the
cerebral parenchyma from the vicinity of the third ventricle were
hypocretin-positive (Fig. S6,
arrows). Hypocretin expression in the cortex could not be detected
after in utero electroporation of pCAGGS-mPlagl1 and
pCAGGS-EGFP into the lateral ventricle (Fig. S5), suggesting that PLAGL1
specifically affects hypothalamic hypocretin transcription. In some
cases, EGFP/Plagl1 might have been electroporated from the bottom
of the lateral ventricle or the outer portion of the dura matter,
inducing hypocretin expression in the subthalamic nucleus and
lateral hypothalamic area (Fig.
S7). These findings suggested that PLAGL1 may suppress
hypocretin expression in the vicinity of the third ventricle as no
hypocretin expression was detected in EGFP-positive cells in this
zone, whereas the effects of PLAGL1 on transcription may be
sufficient in some cells of the cerebral parenchyma, the lateral
hypothalamic area and the subthalamic nucleus.
In order to avoid false positives associated with
background signals in immunohistochemical staining, qPCR was
performed to confirm the transcriptional effects of PLAGL1 on
hypocretin. The EGFP expression levels were elevated on the
electroporated side as compared with the contralateral side
(contralateral side: mean ± standard deviation, 1,383.9±1,978.6 in
the mock group, 723.4±1,057.6 in the PLAGL1 group; electroporated
side: 42,006.1±75,966.2 in the mock group, 38,586.3±53,484.7 in the
PLAGL1 group) in both the mock and PLAGL1 groups (Fig. 6). There was no significant
difference in Plagl1 expression between the contralateral side
(2.8±2.4) and the electroporated side (2.6±1.8) in the mock group,
but there was a significant difference in Plagl1 expression
(P<0.005) between the contralateral side (3.7±1.4) and
electroporated side (7.9±1.5) in the PLAGL1 group. There was no
significant difference in hypocretin expression between the
contralateral side (27.6±20.1) and electroporated side (22.7±18.6)
in the mock group, but there was a significant difference
(P<0.01) in hypocretin expression between the contralateral side
(21.9±15.3) and electroporated side (83.5±25.9) in the PLAGL1
group. Therefore, these data suggested that PLAGL1 may upregulate
hypocretin transcription. PLAGL1 slightly affected neuronal
pentaxin 2 transcription and significantly affected prodynorphin
transcription. These two genes are concurrently expressed in
hypocretin neurons (27,28). There was no effect of PLAGL1 on
the housekeeping gene, B2m.
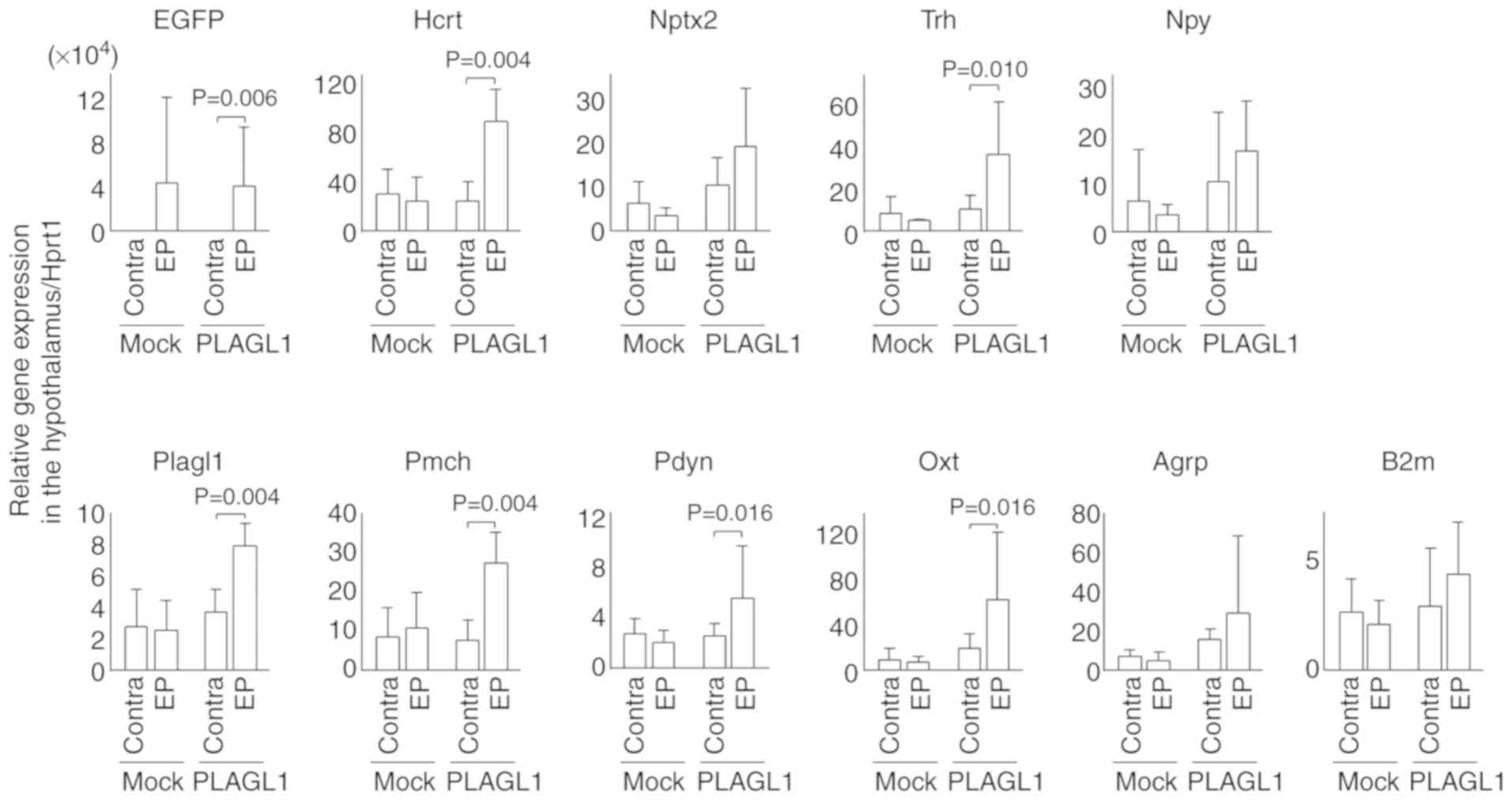 | Figure 6Relative expression levels of
hypothalamic genes following in utero electroporation. In
utero electroporation was performed with pCAGGS-EGFP and
pCAGGS-mPlagl1 (PLAGL1 group, n=6) to evaluate the effect of PLAGL1
on Hcrt transcription. Control mice were electroporated with
pCAGGS-EGFP and pCAGGS (mock group, n=4). Animals were harvested at
P0 stage. Following total RNA extraction from the hypothalamus and
cDNA synthesis, quantitative polymerase chain reaction was
performed. The relative expression levels of each gene were
normalized to the relative expression levels of Hprt1. Alterations
in transcription levels between the electroporated and contra sides
in each group were compared using the Mann-Whitney U-test. Data are
presented as the means ± standard deviation. Agrp, agouti related
neuropeptide; B2m, β-2-microglobulin; Contra, contralateral side
(no electroporation); EGFP, enhanced green fluorescent protein; EP,
electroporated side; Hcrt, hypocretin; Hprt1, hypoxanthine
phosphoribosyltransferase 1; Nptx2, neuronal pentaxin 2; Npy,
neuropeptide Y; Oxt, oxytocin; Pdyn, prodynorphin; Pmch,
pro-melanin-concentrating hormone; Trh, thyrotropin-releasing
hormone. |
Since PLAGL1 is expressed in the lateral
hypothalamic area, the dorsomedial hypothalamic nucleus and the
arcuate nucleus of the hypothalamus, this study examined whether
PLAGL1 affects transcription of other neuropeptides in hypothalamic
neurons that project to/from hypocretin neurons. PLAGL1
overexpression affected the expression of pro-melanin-concentrating
hormone, thyrotropin-releasing hormone (Trh) and oxytocin (Oxt),
but not that of neuro-peptide Y (Npy) and agouti related
neuropeptide (Agrp) (Fig. 6 and
Table SII).
Discussion
Hypocretin contributes to energy expenditure and
feeding behavior by regulating the activation of wakefulness and
the sympathetic system (29,30), as well as the promotion of
food-seeking behaviors (31-33). During fasting, hypocretin
expression is regulated by FOXA2, a downstream target of insulin
signaling (10). The present
study demonstrated that hypocretin-immunoreactive neurons increased
after fasting. It was hypothesized that PLAGL1, whose expression is
also altered in response to fasting, may contribute to food-seeking
behaviors after fasting. An increase in PLAGL1 frequency in the
hypocretin nucleus was detected in the HFD group. Since an
alteration in c-fos expression has been observed in 2-3-month-old
mice fed a HFD (34), PLAGL1 may
contribute to a change in hypocretin neuronal activity in response
to a HFD. In addition, hypocretin-immunoreactive neurons and
hypocretin mRNA were increased after a 1-month HFD. Therefore,
PLAGL1 may be associated with hypocretin transcription even under a
HFD condition. The role of hypo-cretin in the maintenance and
stability of normal sleep and wakefulness is well known (35). The present study revealed an
increase in PLAGL1 in the active phase (ZT18) of hypo-cretin
neurons, but no increase in hypocretin-immunoreactive cells in the
active phase (ZT18); these findings suggested that PLAGL1 may have
no effect on hypocretin transcription at night. Furthermore, the
changes in hypocretin neuronal activity and the fluctuation of
cerebrospinal fluid hypocretin-1 levels across the light-dark cycle
and sleep-wake activities have been well documented (36-38); therefore, PLAGL1 may be involved
in the modulation of hypocretin neuronal activity or the regulation
of the hypocretin secretion-related protein at night. Increases in
cerebrospinal fluid hypocretin levels following sleep deprivation
(39,40) are accompanied by an increase in
hypocretin-immunoreactive neurons (41), c-fos expression (42) and hypocretin mRNA expression
(43), thus suggesting that
hypocretin neurons may act to sustain vigilance during sleep
deprivation. However, the present study did not detect a
significant increase in hypocretin-immunoreactive neurons following
sleep deprivation. Instead, sleep deprivation induced an uneven
nuclear localization of PLAGL1. Because uneven nuclear localization
of PLAGL1 also existed in control conditions (ZT6) and in ZT18,
this change in distribution to an uneven position in the nucleus
may be associated with an unknown or undefined physiological
condition in hypocretin neurons. In the majority of interphase
cells, DAPI-bright heterochromatin is present in the peripheral
compartment, chromocenters and around the nucleoli. Heterochromatin
regions hold a packed conformation and the included genes are
silent. Euchromatin is a dim area in the inner compartment of the
nucleus (44-46). In this area, tissue-specific genes
are transcribed. PLAGL1 was localized in the euchromatin region of
hypocretin neuronal nuclei upon sleep deprivation. Since there was
no direct effect of PLAGL1 on hypocretin transcription in mice with
sleep deprivation, it may be suggested that PLAGL1 binds the
euchromatin region upon sleep deprivation in order to regulate
other mediators of hypocretin neuron activities.
The consensus binding site for PLAGL1 was identified
as the sequence G6C4 by the Systematic
Evolution of Ligands by EXponential (SELEX) enrichment method
(26); however, there were no
G6C4 sequences within the 0 to 2.0 kb
upstream of the transcription start site in the murine hypocretin
gene, as determined by the present manual search. Because SELEX
aptamers consist of randomly generated sequences, there is a
possibility that some genomic sequences were not examined using the
SELEX method. Novel PLAGL1 binding sites
(G4N2G4) have been identified by
ChIP and genome-wide transcriptomics in PLAGL1 vector-transfected
Neuro2a cells (19). However, no
hypocretin locus, including the ±20-kb region, was
immunoprecipitated in these experiments (19). The hypocretin gene locus might not
be in the active state, thus explaining transient expression of
PLAGL1 in Neuro2a cells. PLAGL1 cannot reach the hypocretin
upstream region and, therefore, the hypocretin locus would not
appear in the list of genes of Neuro2a cells (19). It has been established that
conversion from the inactive state to the active state by
epigenetic switching is critical to the transcriptional regulation
of hypocretin, but that requires time (47,48). Conversely, the present in
vivo ChIP assay using hypothalamic tissue revealed PLAGL1
binding to the hypocretin locus. PLAGL1 binding sites could be
present in a constant active state and activated by several
conditions. This G4N2G4 consensus
sequence is conserved even in the upstream region of the human
hypo-cretin gene; therefore, PLAGL1 might also be important in
human hypocretin transcription.
PLAGL1 can function as a transcription activator or
repressor (26,49,50). In this study, functional
differences in PLAGL1 were detected; specifically, PLAGL1 acted as
a repressor in NIH3T3 cells and as an activator in the embryonic
murine hypothalamus for hypocretin transcription. Under 2-day
fasting and HFD conditions, PLAGL1 frequency and
hypocretin-immunoreactive neurons were increased. One possible
explanation for this discrepancy is that PLAGL1 monomer binding to
G4N6G4 results in transcriptional
repression, while dimer binding to
G4N6G4 induces transactivation
(49,50). The same discrepancy might occur at
G4N2G4 sites. Since there are
three G4N2G4 binding sites in the
proximal region of the prepro-hypocretin gene, dimer binding of
PLAGL1 might occur after 2 days of fasting, a HFD and during
embryonic development. There might be different proteins or
small-RNAs that bind G4N2G4 sites
in NIH3T3 cells. As a result, PLAGL1 can only bind to one site of
G4N2G4 as a monomer in NIH3T3
cells.
In the present Plagl1 overexpression model using
in utero electroporation, it was revealed that PLAGL1 may
induce hypocretin expression, specifically in the hypothalamus. The
majority of EGFP signals co-localized with hypocretin were detected
in the cerebral parenchyma at a distance from the vicinity of the
third ventricle, suggesting that some progenitor cells of the third
ventricular zone need to migrate to the designated area from the
ependymal layer of the third ventricle. The differentiation of
these cells might also be affected by the gradient of some factors,
including the hypothalamic patterning factor sonic hedgehog
(9), the adult hypocretin
neurogenesis inducer fibroblast growth factor 2 (51), generators of hypocretin neurons
(47,48), or other unidentified factors at
the time of migration or arrival at the designated area.
Neurons expressing Trh and Oxt interact directly
with hypocretin neurons (52,53) and were shown to be modulated by
PLAGL1 overexpression in this study; however, no effect was
detected on Npy or Agrp expression. Neurons expressing Npy and Agrp
project to hypocretin neurons from the arcuate nucleus (52,53). It may be possible that there could
have been some indirect effects from other neurons affected by
PLAGL1 that project to hypocretin neurons.
In conclusion, the present study revealed the
involvement of PLAGL1 in hypocretin transcriptional regulation;
however, PLAGL1 is also expressed in other areas of the
hypothalamus. Therefore, further in vivo studies using
cross-hybridization with Plagl1 conditional knockout mice and
hypocretin promoter-Cre mice (54) are required to clarify whether
PLAGL1 directly or indirectly affects hypocretin expression.
Supplementary Materials
Funding
This study was supported by JSPS KAKENHI [grant nos.
15K08224 (ST) and 16K08533 (ST)] and the Takeda Science Foundation
(ST). The funding agency had no role in study design, data
collection and analysis, decision to publish or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SuT, ToK and HY were involved in study concept and
design. SuT, YoH, ShT, TaK, TY and NT acquired the data. SuT, YoH
and ShT processed the data. SuT, SO, YuH, YT, KK, ToK and HY
analyzed and interpreted the data. SuT drafted the manuscript and
ShT, TaK, SO, YuH, YT, KK, ToK and HY critically revised it for
important intellectual content. SuT conducted the statistical
analysis. SuT, YT, ToK and HY provided materials, and YuH, YT, KK,
ToK and HY supervised the study. All authors have approved the
final draft of the manuscript that was submitted.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the Guidelines for the Care and Use of Laboratory Animals of
the National Institute of Health, and were approved by the Ethics
committee on Animal Experiments of the Kansai Medical University
(approval ID: 17-050) and the Tokyo Metropolitan Institute of
Medical Science.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Hitomi Okabe
(Kansai Medical University) for her technical assistance.
References
|
1
|
Sakurai T: The role of orexin in motivated
behaviours. Nat Rev Neurosci. 15:719–731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakurai T, Moriguchi T, Furuya K, Kajiwara
N, Nakamura T, Yanagisawa M and Goto K: Structure and function of
human prepro-orexin gene. J Biol Chem. 274:17771–17776. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moriguchi T, Sakurai T, Takahashi S, Goto
K and Yamamoto M: The human prepro-orexin gene regulatory region
that activates gene expression in the lateral region and represses
it in the medial regions of the hypothalamus. J Biol Chem.
277:16985–16992. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amiot C, Brischoux F, Colard C, La Roche
A, Fellmann D and Risold PY: Hypocretin/orexin-containing neurons
are produced in one sharp peak in the developing ventral
diencephalon. Eur J Neurosci. 22:531–534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka S: Transcriptional regulation of
the hypocretin/orexin gene. Vitam Horm. 89:75–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogawa Y, Kanda T, Vogt K and Yanagisawa M:
Anatomical and electrophysiological development of the hypothalamic
orexin neurons from embryos to neonates. J Comp Neurol.
525:3809–3820. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zahr SK, Yang G, Kazan H, Borrett MJ,
Yuzwa SA, Voronova A, Kaplan DR and Miller FD: A translational
repression complex in developing mammalian neural stem cells that
regulates neuronal specification. Neuron. 97:520–537 e6. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka S, Kodama T, Nonaka T, Toyoda H,
Arai M, Fukazawa M, Honda Y, Honda M and Mignot E: Transcriptional
regulation of the hypocretin/orexin gene by NR6A1. Biochem Biophys
Res Commun. 403:178–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimogori T, Lee DA, Miranda-Angulo A,
Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H,
Avetisyan M, et al: A genomic atlas of mouse hypothalamic
development. Nature Neurosci. 13:767–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silva JP, von Meyenn F, Howell J, Thorens
B, Wolfrum C and Stoffel M: Regulation of adaptive behaviour during
fasting by hypothalamic Foxa2. Nature. 462:646–650. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dalal J, Roh JH, Maloney SE, Akuffo A,
Shah S, Yuan H, Wamsley B, Jones WB, de Guzman Strong C, Gray PA,
et al: Translational profiling of hypocretin neurons identifies
candidate molecules for sleep regulation. Genes Dev. 27:565–578.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Honda M, Eriksson KS, Zhang S, Tanaka S,
Lin L, Salehi A, Hesla PE, Maehlen J, Gaus SE, Yanagisawa M, et al:
IGFBP3 colocalizes with and regulates hypocretin (orexin). PLoS
One. 4:e42542009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De La Herrán-Arita AK, Zomosa-Signoret VC,
Millán-Aldaco DA, Palomero-Rivero M, Guerra-Crespo M, Drucker-Colín
R and Vidaltamayo R: Aspects of the narcolepsy-cataplexy syndrome
in O/E3-null mutant mice. Neuroscience. 183:134–143. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sánchez-García A, Cabral-Pacheco GA,
Zomosa-Signoret VC, Ortiz-López R, Camacho A, Tabera-Tarello PM,
Garnica-López JA and Vidaltamayo R: Modular organization of a
hypocretin gene minimal promoter. Mol Med Rep. 17:2263–2270.
2018.
|
|
15
|
Spengler D, Villalba M, Hoffmann A,
Pantaloni C, Houssami S, Bockaert J and Journot L: Regulation of
apoptosis and cell cycle arrest by Zac1, a novel zinc finger
protein expressed in the pituitary gland and the brain. EMBO J.
16:2814–2825. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kas K, Voz ML, Hensen K, Meyen E and Van
de Ven WJ: Transcriptional activation capacity of the novel PLAG
family of zinc finger proteins. J Biol Chem. 273:23026–23032. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamiya M, Judson H, Okazaki Y, Kusakabe M,
Muramatsu M, Takada S, Takagi N, Arima T, Wake N, Kamimura K, et
al: The cell cycle control gene ZAC/PLAGL1 is imprinted-a strong
candidate gene for transient neonatal diabetes. Hum Mol Genet.
9:453–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varrault A, Gueydan C, Delalbre A,
Bellmann A, Houssami S, Aknin C, Severac D, Chotard L, Kahli M, Le
Digarcher A, et al: Zac1 regulates an imprinted gene network
critically involved in the control of embryonic growth. Dev Cell.
11:711–722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varrault A, Dantec C, Le Digarcher A,
Chotard L, Bilanges B, Parrinello H, Dubois E, Rialle S, Severac D,
Bouschet T and Journot L: Identification of Plagl1/Zac1 binding
sites and target genes establishes its role in the regulation of
extracellular matrix genes and the imprinted gene network. Nucleic
Acids Res. 45:10466–10480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
21
|
Terao A, Wisor JP, Peyron C,
Apte-Deshpande A, Wurts SW, Edgar DM and Kilduff TS: Gene
expression in the rat brain during sleep deprivation and recovery
sleep: An Affymetrix GeneChip study. Neuroscience. 137:593–605.
2006. View Article : Google Scholar
|
|
22
|
Dignam JD, Lebovitz RM and Roeder RG:
Accurate transcription initiation by RNA polymerase II in a soluble
extract from isolated mammalian nuclei. Nucleic Acids Res.
11:1475–1489. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takizawa N, Tanaka S, Oe S, Koike T,
Matsuda T and Yamada H: Hypothalamo-hypophysial system in rats with
autotransplantation of the adrenal cortex. Mol Med Rep.
15:3215–3221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Lein ES, Hawrylycz MJ, Ao N, Ayres M,
Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ,
et al: Genome-wide atlas of gene expression in the adult mouse
brain. Nature. 445:168–176. 2007. View Article : Google Scholar
|
|
26
|
Varrault A, Ciani E, Apiou F, Bilanges B,
Hoffmann A, Pantaloni C, Bockaert J, Spengler D and Journot L: hZAC
encodes a zinc finger protein with antiproliferative properties and
maps to a chromosomal region frequently lost in cancer. Proc Natl
Acad Sci USA. 95:8835–8840. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou TC, Lee CE, Lu J, Elmquist JK, Hara
J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M,
et al: Orexin (hypocretin) neurons contain dynorphin. J Neurosci.
21:RC1682001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reti IM, Reddy R, Worley PF and Baraban
JM: Selective expression of Narp, a secreted neuronal pentraxin, in
orexin neurons. J Neurochem. 82:1561–1565. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuneki H, Wada T and Sasaoka T: Role of
orexin in the central regulation of glucose and energy homeostasis.
Endocr J. 59:365–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nattie E and Li A: Respiration and
autonomic regulation and orexin. Prog Brain Res. 198:25–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamanaka A, Beuckmann CT, Willie JT, Hara
J, Tsujino N, Mieda M, Tominaga M, Yagami Ki, Sugiyama F, Goto K,
et al: Hypothalamic orexin neurons regulate arousal according to
energy balance in mice. Neuron. 38:701–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burdakov D, Jensen LT, Alexopoulos H,
Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L and
Verkhratsky A: Tandem-pore K+ channels mediate
inhibition of orexin neurons by glucose. Neuron. 50:711–722. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
González JA, Jensen LT, Doyle SE,
Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA and Burdakov D:
Deletion of TASK1 and TASK3 channels disrupts intrinsic
excitability but does not abolish glucose or pH responses of
orexin/hypocretin neurons. Eur J Neurosci. 30:57–64. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Valdivia S, Patrone A, Reynaldo M and
Perello M: Acute high fat diet consumption activates the mesolimbic
circuit and requires orexin signaling in a mouse model. PLoS One.
9:e874782014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sakurai T: The neural circuit of orexin
(hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci.
8:171–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fujiki N, Yoshida Y, Ripley B, Honda K,
Mignot E and Nishino S: Changes in CSF hypocretin-1 (orexin A)
levels in rats across 24 hours and in response to food deprivation.
Neuroreport. 12:993–997. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee MG, Hassani OK and Jones BE: Discharge
of identified orexin/hypocretin neurons across the sleep-waking
cycle. J Neurosci. 25:6716–6720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshida Y, Fujiki N, Nakajima T, Ripley B,
Matsumura H, Yoneda H, Mignot E and Nishino S: Fluctuation of
extracellular hypocretin-1 (orexin A) levels in the rat in relation
to the light-dark cycle and sleep-wake activities. Eur J Neurosci.
14:1075–1081. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pedrazzoli M, D'Almeida V, Martins PJ,
Machado RB, Ling L, Nishino S, Tufik S and Mignot E: Increased
hypocretin-1 levels in cerebrospinal fluid after REM sleep
deprivation. Brain Res. 995:1–6. 2004. View Article : Google Scholar
|
|
40
|
Wu MF, John J, Maidment N, Lam HA and
Siegel JM: Hypocretin release in normal and narcoleptic dogs after
food and sleep deprivation, eating, and movement. Am J Physiol
Regul Integr Comp Physiol. 283:R1079–R1086. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Allard JS, Tizabi Y, Shaffery JP and
Manaye K: Effects of rapid eye movement sleep deprivation on
hypocretin neurons in the hypothalamus of a rat model of
depression. Neuropeptides. 41:329–337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Modirrousta M, Mainville L and Jones BE:
Orexin and MCH neurons express c-Fos differently after sleep
deprivation vs. recovery and bear different adrenergic receptors.
Eur J Neurosci. 21:2807–2816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martins PJ, Marques MS, Tufik S and
D'Almeida V: Orexin activation precedes increased NPY expression,
hyperphagia, and metabolic changes in response to sleep
deprivation. Am J Physiol Endocrinol Metab. 298:E726–E734. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Holmquist GP and Ashley T: Chromosome
organization and chromatin modification: Influence on genome
function and evolution. Cytogenet Genome Res. 114:96–125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sadoni N, Langer S, Fauth C, Bernardi G,
Cremer T, Turner BM and Zink D: Nuclear organization of mammalian
genomes. Polar chromosome territories build up functionally
distinct higher order compartments. J Cell Biol. 146:1211–1226.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Tomaso MV, Liddle P, Lafon-Hughes L,
Reyes-Ábalos A and Folle G: Chromatin damage patterns shift
according to Eu/Heterochromatin replication. The mechanisms of DNA
replication. D S: InTech. 2013.
|
|
47
|
Hayakawa K, Hirosawa M, Tabei Y, Arai D,
Tanaka S, Murakami N, Yagi S and Shiota K: Epigenetic switching by
the metabolism-sensing factors in the generation of orexin neurons
from mouse embryonic stem cells. J Biol Chem. 288:17099–17110.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hayakawa K, Sakamoto Y, Kanie O, Ohtake A,
Daikoku S, Ito Y and Shiota K: Reactivation of
hyperglycemia-induced hypocretin (HCRT) gene silencing by
N-acetyl-d-mannosamine in the orexin neurons derived from human iPS
cells. Epigenetics. 12:764–778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hoffmann A, Ciani E, Boeckardt J, Holsboer
F, Journot L and Spengler D: Transcriptional activities of the zinc
finger protein Zac are differentially controlled by DNA binding.
Mol Cell Biol. 23:988–1003. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yuasa S, Onizuka T, Shimoji K, Ohno Y,
Kageyama T, Yoon SH, Egashira T, Seki T, Hashimoto H, Nishiyama T,
et al: Zac1 is an essential transcription factor for cardiac
morphogenesis. Circ Res. 106:1083–1091. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu Y, Tamamaki N, Noda T, Kimura K,
Itokazu Y, Matsumoto N, Dezawa M and Ide C: Neurogenesis in the
ependymal layer of the adult rat 3rd ventricle. Exp Neurol.
192:251–264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Broberger C, De Lecea L, Sutcliffe JG and
Hökfelt T: Hypocretin/orexin- and melanin-concentrating
hormone-expressing cells form distinct populations in the rodent
lateral hypothalamus: Relationship to the neuropeptide Y and agouti
gene-related protein systems. J Comp Neurol. 402:460–474. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Elias CF, Saper CB, Maratos-Flier E,
Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh
GS, et al: Chemically defined projections linking the mediobasal
hypothalamus and the lateral hypothalamic area. J Comp Neurol.
402:442–459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matsuki T, Nomiyama M, Takahira H,
Hirashima N, Kunita S, Takahashi S, Yagami K, Kilduff TS, Bettler
B, Yanagisawa M and Sakurai T: Selective loss of GABA(B) receptors
in orexin-producing neurons results in disrupted sleep/wakefulness
architecture. Proc Natl Acad Sci USA. 106:4459–4464. 2009.
View Article : Google Scholar : PubMed/NCBI
|