Introduction
With improvements in therapeutic methodology methods
over the past 10 years, ischemic heart disease can be treated by
reperfusion, such as cardiac bypass surgery percutaneous coronary
intervention. However, in some patients, myocardial dysfunction and
tissue damage may be further aggravated by reperfusion. This is
called ischemia/reperfusion injury (1). Therefore, methods that may
prevent ischemia/reperfusion injury are being explored. Currently,
the prevention and treatment of myocardial ischemia/reperfusion
injury include ischemic pre-conditioning (2), post-conditioning
(3), and remote ischemic conditioning (4). These treatments promote
the release of endogenous protective substances, such as protein
kinase C (5), tilianin (6), penehyclidine hydrochloride (7), and
soluble receptor for advanced glycation end products (sRAGE) (8).
The protective mechanisms of these endogenous substances are
considered to have common properties, such as the inhibition of
apoptosis and of mitochondrial damage. Furthermore, sRAGE not only
showed a protective effect against cardiac reperfusion injury, but
also against a variety of inflammatory reactions.
sRAGE can act as a ‘decoy’ for RAGE ligands, just as
a ‘sponge’ that binds with ligands of the RAGE such as HMGB1, AGEs,
and S100 (9). sRAGE can be produced by proteolytic cleavage of
ADAM10 from the plasma membrane, and it consists of a coupled VC1
domain and an independent C2 domain (10-12). In addition to being a
competitive inhibitor of RAGE, sRAGE also promotes the secretion of
cytokines and exerts pro- and anti-inflammatory properties
(13–15).
Previous studies have shown that sRAGE may
significantly increase IFN-γ expression, which then inhibits the
I/R injury-induced apoptosis of cardiomyocytes (16). However, the
mechanism underlying sRAGE-promoted IFN-γ release in the reperfused
heart remains unclear. IFN-γ is a cytokine secreted by mononuclear
macrophages (17,18), lymphocytes and NK cells (19). A variety of
convergent signaling transduction pathways are involved in the
transcriptional regulation of IFN-γ (20). This raises a query
regarding the mechanisms by which sRAGE increases the release of
IFN-γ in the reperfused heart, the cellular source(s) of IFN-γ, and
the signaling pathways mediating IFN-γ synthesis and release. In
the present study, mice and macrophages were each used to simulate
ischemia/reperfusion, in order to evaluate the mechanisms
underlying the effect of sRAGE on IFN-γ expression in the I/R
heart.
Materials and methods
Experimental animal model
A total of 20 six-week-old (18-22 g), male C57BL/6J
mice were purchased from Vital River Laboratories Co., Ltd.
(Beijing, China). The relative humidity of the animal room was 45%
and the ambient temperature was 24°C, with a 12/12 h light/dark
cycle and ad libitum diet and drinking water. All animal
studies followed the Animal Care and Use Committee of Capital
Medical University (Beijing, China) (permit number: AEEI-2016-055).
Mice were anesthetized with isoflurane at a fraction of 2% in
volume. The left anterior descending coronary artery was ligated
for 30 min, and then reperfused for 24 h to create an
ischemia/reperfusion model (21). Mice in the sham group were
subjected to the same surgical procedure except for the ligation of
the blood vessels. sRAGE (00112-01-100; AVISCERA Bioscience, CA,
USA; 2 µg/mouse) or an equal volume of saline was
administered into the heart prior to surgery.
The mice subjected to I/R surgery were then randomly
divided into the I/R group (I/R group; n=6), injected with vehicle,
and the sRAGE treatment group (I/R+sRAGE group; n=6), injected with
sRAGE. The mice without ligated blood vessels were randomly divided
into the control group (sham group; n=6), injected with vehicle,
and the sRAGE treatment group (sRAGE group; n=6), injected with
sRAGE. After the mice were anesthetized by intraperitoneal
injection of tribromoethanol, the heart was removed, and fixed in
10% formalin or stored at −80°C for analysis.
Immunohistochemistry and hematoxylin and
eosin staining
Tissue sections (4-µm) were used to perform
immunostaining for CD68 and ly6g with a rabbit anti-CD68 antibody
(ab125212; Abcam, Cambridge, UK) and a goat anti-rat lgG
(horseradish peroxidase) (ab97057; Abcam). CD68 and ly6g in the
tissue were identified using the horseradish peroxidase-DAB
detection method. Hematoxylin and eosin staining was performed
following the manufacturer’s instructions. Sections were observed
using an Olympus BX63 microscope (magnification indicated in the
figures) (Olympus America, Inc., Center Valley, PA, USA).
I/R protocol in macrophages
The Raw264.7 cell line was purchased from Beina
Chuanglian Biotechnology Institute (BNCC100584; BBCI, Beijing,
China). After being synchronized for 6-8 h, the cells were
incubated with ‘Ischemia Buffer’ and 2% O2 for 2 h.
Then, the ‘Ischemia Buffer’ was replaced with a serum-free medium
for incubation at 37°C and 5% CO2 to replicate the
ischemia-reperfusion model. The ‘Ischemia Buffer’ comprised: 118
mmol/l NaCl, 24 mmol/l NaHCO3, 1.0 mmol/l
NaH2PO4, 2.5 mmol/l
CaCl2•2H2O, 1.2 mmol/l MgCl2, 20
mmol/l lactate, 16 mmol/l KCl, and 10 mmol/l deoxyglucose. The
cells in the control group were cultured in medium containing 10%
FBS at 37°C and 5% CO2. Bay117082 (ab141228: Abcam), an
inhibitor of NF-κB, and sRAGE were available and used during all
processes in the treated groups.
Cells treated with I/R were randomly divided into
the I/R group (I/R group, n=3), which was stimulated with PBS 30
min prior to I/R, the sRAGE group (I/R+sRAGE group, n=3), which was
stimulated with sRAGE (900 ng/ml) 30 min prior to I/R, and the
sRAGE and Bay117082 group (I/R+sRAGE+Bay117082 group, n=3), which
was stimulated with sRAGE (900 ng/ml) and Bay117082 (5 µM)
30 min prior to I/R. Control cells were randomly divided into three
different groups in the same manner as the I/R-treated cells (sham
group, sRAGE group, sRAGE+Bay117082 group, n=3 for each group).
Proliferation assay of macrophages
Macrophages were treated with recombinant sRAGE
proteins for 24 h prior to I/R treatment. 5-BrdU (10 µm;
B5002-1G; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used
during the process to label proliferating cells (22). Cells were
fixed in 4% paraformaldehyde in phosphate-buffered saline for 15
min at room temperature, followed by incubation with 2 N HCl for 1
h and 0.2% Triton-X solution in water for 45 min, then blocked with
1% FBS for 1 h. Next, cells were incubated with primary antibodies
for 5-BrdU (ab1893; Abcam) at 4°C overnight. Donkey Anti-Sheep IgG
H&L (Alexa Fluor® 647) and pre-adsorbed secondary
antibodies (ab150155; Abcam) were incubated for 1 h at room
temperature. Cell nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI; ZLI-9557; ZSGB-BIO, Beijing,
China). Slices were visualized via confocal microscopy (TCS SP8 X,
Leica Camera AG, Wetzlar, Germany). The number of 5-BrdU-positive
cells was calculated in at least 6 random fields in each dish per
group. Results were expressed as the percentage of 5-BrdU-positive
cells among total cells.
Analyses of macrophage
differentiation
After treatment, the cells were fixed in 4%
paraformaldehyde in PBS at room temperature for 15 min, followed by
0.2% Triton-X solution in PBS for 7 min. After blocking in 1% FBS
for 1 h, the cells were incubated with a primary anti-iNOS antibody
(ab15323; Abcam) and an Alexa Fluor 488-labeled goat anti-rabbit
antibody (ab150077; Abcam). Cell nuclei were stained with DAPI
(ZLI-9557; ZSGB-BIO). Slices were visualized using a fluorescence
microscope (930642; Nikon Corporation, Chiyoda-Ku, Tokyo, Japan).
The number of iNOS-positive cells in at least 6 random fields in
each dish per group was calculated. Results were expressed as the
percentage of iNOS-positive cells among total cells.
Detection of IFN-γ mRNA, IL-6, and IL-12
in macrophages
Total RNA was extracted from macrophages using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). cDNA was synthesized from 2 µg of total
RNA using a Prime Script RT Reagent Kit with a gDNA Eraser kit
(RR047A; TaKaRa Bio, Kusatsu, Japan) following the manufacturer’s
instructions. Expression of IFN-γ, IL-6 and IL-12 mRNA was analyzed
via q-PCR using SYBR-Green PCR Master Mix (RR420A; TaKaRa Bio) with
a 7500 Real-Time PCR System. The primers for IFN-γ were 5′-AGC AAG
GCG AAA AAG GAT GC-3′ (forward) and 5′-TCA TTG AAT GCT TGG CGC
TG-3′ (reverse). The primers for IL-6 were 5′-CTG GAG CCC ACC AAG
AAC GA-3′ (forward) and 5′-GCC TCC GAC TTG TGA AGT GGT-3′
(reverse). The primers for IL-12 were 5′-TCA TGG ACA TGA TGG GGC
TG-3′ (forward) and 5′-TCC CTC TGG GAA CGA TGT CT-3′ (reverse).
Western blot analysis
Proteins extracted from Raw264.7 macrophages were
separated by 8-15% SDS-PAGE followed by electro-blotting onto PVDF
membranes. After blocking in 5% milk for 1 h at room temperature,
the PVDF membrane was incubated overnight at 4°C with the following
primary antibodies: Mouse anti-IFN-γ (37895; Abcam), rabbit
anti-phospho-IKK (2078), rabbit anti-IKK (2370), rabbit
anti-phospho-IκB (2859), rabbit anti-IκB (4812), rabbit
anti-phospho-NF-κB (3033), rabbit anti-NF-κB (8242) GAPDH (5174;
all from Cell Signaling Technology, Inc., Danvers, MA, USA), and
anti-Histone H3 (ab1791; Abcam). Next, the PVDF membrane was washed
thrice with TBST (20 mM Tris, 150 mM NaCl, containing 0.05%
Tween-20, pH 7.4) followed incubation with a donkey anti-mouse
IgG-HRP (sc-2314; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
or an anti-rabbit HRP-linked secondary antibody (707; Cell
Signaling Technology, Inc.) for 1 h at room temperature. The
FluorChem™ FC3 system (Protein-Simple, Santa Clara, CA, USA) was
used for imaging of the strips on the PVDF membrane that were
developed using Immobilon™ Western Chemiluminescent HRP Substrate
(WBKLS0500; EMD Millipore, Billerica, MA, USA).
Extraction of cytoplasmic and nuclear
protein
In order to examine translocation of NF-κB,
cytoplasmic and nuclear proteins were extracted using Minute™
Cytoplasmic and Nuclear Extraction Kit (SC-003, Invent
Biotechnologies, Eden Prairie, Minnesota, USA) according to the
manufacturer’s protocol and analyzed with western blot. Briefly,
the cells were collected in 300 µl cytoplasmic extraction
buffer, incubated on ice for 5 min, and centrifuged at 21,130 × g
for 5 min at 4°C to extract cytoplasmic proteins. The precipitates
were resuspended with 150 µl nuclear extraction buffer and
centrifuged 21,130 x g for 5 min at 4°C to extract nuclear
proteins.
Chromatin immunoprecipitation (ChIP)
assay
A ChIP assay was performed according to the protocol
of the SimpleChIP® Enzymatic Chromatin IP Kit (9002;
Cell Signaling Technology, Inc.). Briefly, macrophages were
harvested 24 h after I/R or control treatment with or without sRAGE
(900 ng/ml) with ChIP buffer followed by sonication to obtain
200-1,000 bp DNA fragments. Immunoprecipitation was performed with
anti NF-κB (8242; Cell Signaling Technology, Inc.) at 4°C
overnight. The DNA fragments were eluted from antibody/protein G
microbeads followed by purification with DNA purification spin
columns (10010; Cell Signaling Technology, Inc.). The primers for
IFN-γ were designed for the -2,000 bp upstream region used for the
immunoprecipitation assay with qPCR, according to the instructions
of the kit. PCR was performed with the following primers: 5-CAT GGC
CAA AGG AAC TGC AC-3 (forward) and 5-TGG CTA TGG GTG CAG ACT TG-3
(reverse).
Statistical analysis
SPSS (version 16.0; IBM Corp., Armonk, NY, USA) was
used to process the experimental data. Experimental data are
expressed as the mean ± standard deviation (SD). Differences among
the groups were tested using one-way ANOVA. Comparisons between two
groups were evaluated using the least significant differences (LSD)
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
sRAGE improves heart function in mice
after I/R
To explore the effects of sRAGE in I/R-treated
heart, cardiac function was measured after I/R with or without
sRAGE. The results showed that cardiac output and stroke volume
were decreased in I/R-treated mice compared with in the sham group.
Cardiac output was increased from 12.68±3.57 to 17.71±1.42 ml/min,
and stroke volume was increased from 21.60±5.16 to 30.44±1.89
µl by sRAGE in I/R-treated mice (P<0.05; Fig. 1A-C).
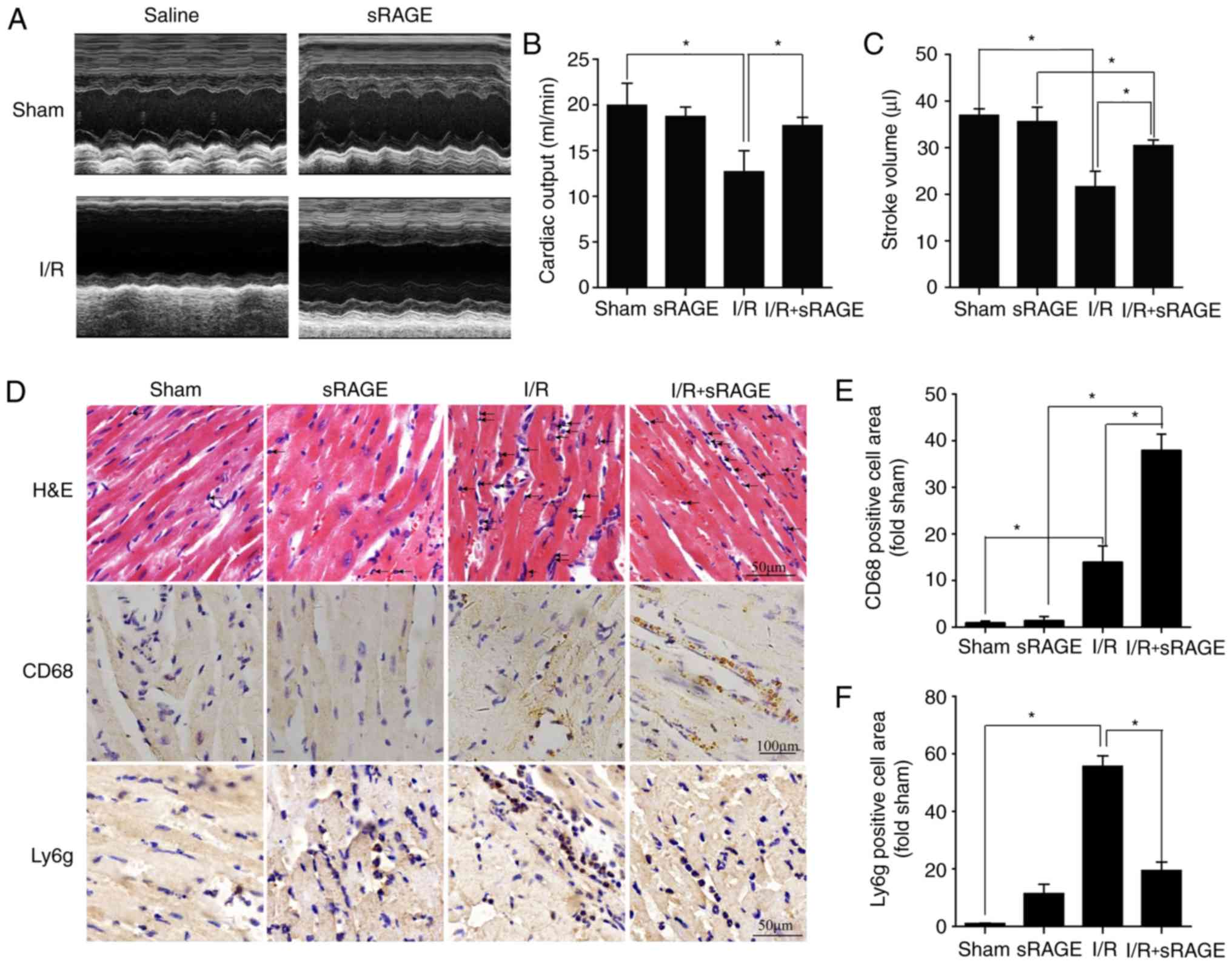 | Figure 1sRAGE increased macrophage
infiltration in myocardial tissue following I/R. (A) Representative
images of the M-mode of echocardiography for each group. (B) The
cardiac output was improved by sRAGE in I/R-treated heart. (C)
Stroke volume was improved by sRAGE in the I/R-treated heart. (D)
Representative images of hematoxylin and eosin staining,
immunohistochemistry for CD68 and Ly6g. (E) Macrophage infiltration
was expressed through the quantification of CD68-positive cells.
(F) Neutrophil infiltration was expressed through the
quantification of Ly6g-positive cells. The data are expressed as
the mean ± SD (n=6, *P<0.05). sRAGE, soluble receptor
for advanced glycation end-products; I/R, ischemia/reperfusion;
H&E, hematoxylin and eosin staining; CD68, cluster of
differentiation 68; Ly6g, lymphocyte antigen 6 complex, locus G;
SD, standard deviation; P, P-value. |
sRAGE increased macrophage infiltration
in myocardial tissue after I/R
The hearts were harvested 24 h after I/R or sham
treatment with or without sRAGE. The results from hematoxylin and
eosin and immunohistochemistry staining showed that the numbers of
inflammatory cells and neutrophils were decreased in I/R-treated
myocardial tissues with sRAGE compared with I/R-treated tissues
without sRAGE (Fig. 1D and F).
Results of immunohistochemistry tests showed that the proportion of
positive macrophages (CD68 positive) was increased in I/R
myocardial tissues treated with sRAGE (37.92±3.46, n=6) or without
sRAGE (13.97±3.46, n=6) compared with the sham group (0.99±0.35,
n=6) (P<0.05) (Fig. 1D and E).
Notably, macrophages recruited in sRAGE-treated I/R hearts were
1.9-3.8-fold that in I/R hearts (n=6; P<0.05) while no such
effects were observed in the sham group with or without sRAGE
treatment (0.99±0.35 in the sham group; 1.42±0.89 in the sRAGE
group; n=6; P>0.05). These results suggested that exogenous
administration of sRAGE attenuated the inflammatory response and
reduced neutrophils, but induced the recruitment of macrophages in
the I/R-treated heart.
sRAGE stimulated macrophage proliferation
and differentiation
To detect the proliferation of macrophages, a
5-BrdU-incorporation assay was used. The results indicated that
5-BrdU-positive macrophages in I/R-treated cells (2.29±0.284) (n=3;
P<0.05) increased compared with in control cells (1.00±0.191)
(n=3; P<0.05) (Fig. 2A and B).
Notably, sRAGE increased the number of 5-BrdU-positive macrophages
to 5.25±0.123 in I/R-treated cells and 2.73±0.451 in PBS treated
cells (n=3; P<0.05) (Fig. 2A and
B). These results suggested that exogenous administration of
sRAGE stimulates macrophage proliferation.
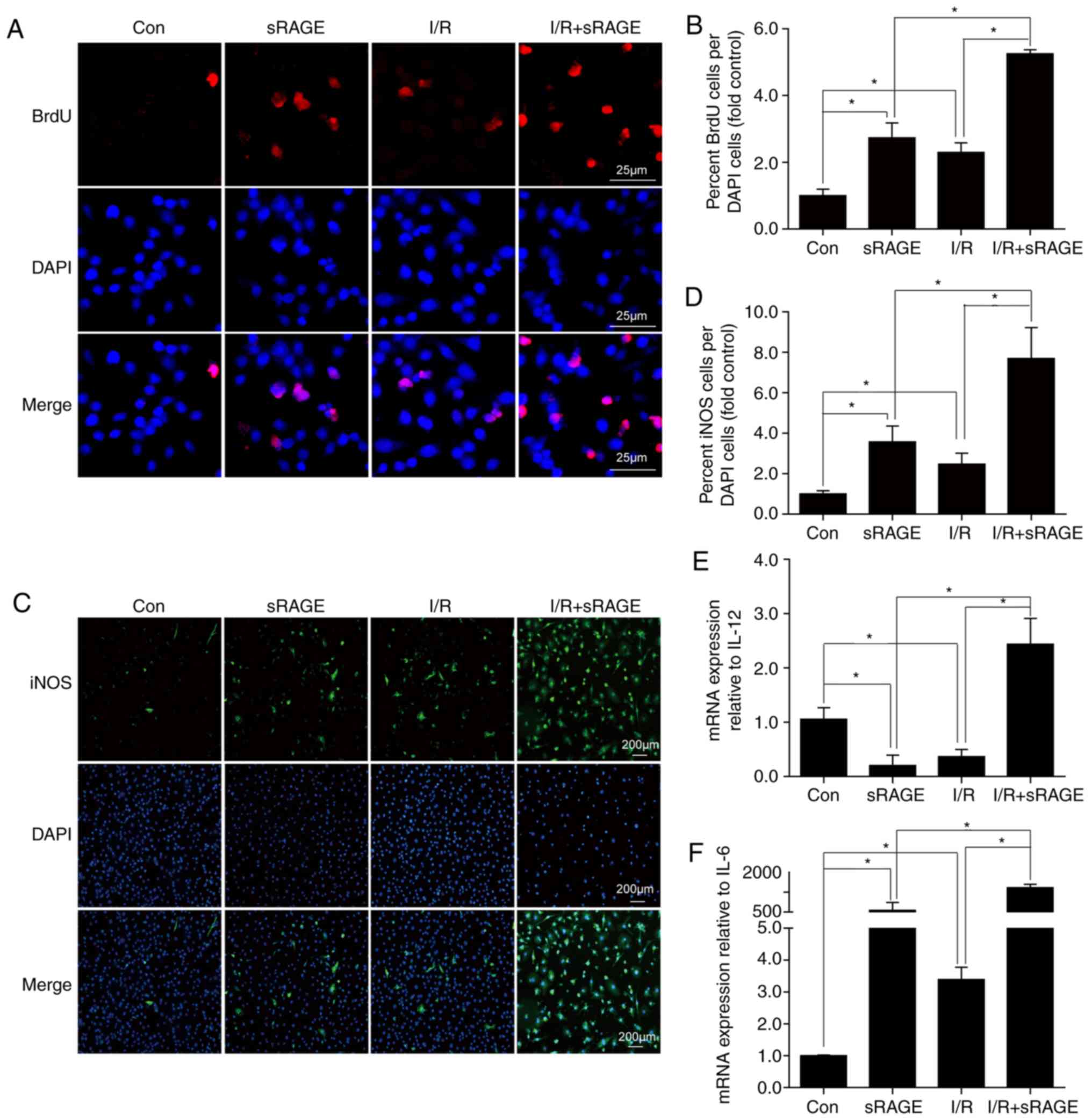 | Figure 2sRAGE stimulated macrophage
proliferation and differentiation. (A) Representative images of
immunofluorescence staining for 5-BrdU in macrophages. Red shows
5-BrdU-positive macrophages; blue shows the nucleus. Bar, 25
µm. (B) BrdU+ and BrdU- nuclei among the macrophages were
counted, and the percentages of BrdU+ nuclei were calculated (fold
sham). (C) Representative images of immunofluorescence staining for
iNOS in macrophages. Green shows iNOS-positive macrophages, blue
shows the nucleus. Bar, 200 µm. (D) The data for
iNOS-positive cells were expressed as a percentage of the total
nuclei. (E) Quantitative data of the mRNA levels of IL-12 in
macrophages. (F) Quantitative data of the mRNA levels of IL-6 in
macrophages. The data are expressed as the mean ± SD (n=3,
*P<0.05). sRAGE, soluble receptor for advanced
glycation end-products; I/R, ischemia/reperfusion; 5-BrdU,
5-Bromo-2′-Deoxyuridine; iNOS, inducible nitric oxide synthase; IL,
interleukin. |
To detect the differentiation of macrophages,
immunofluorescence analysis of the M1 macrophage marker, iNOS, was
used. The results showed that the percentage of iNOS-positive
macrophages was significantly increased from 0.99±0.154 in the
control group to 2.48±0.541 following I/R (n=3; P<0.05), and
further enhanced to 7.69±1.533 following sRAGE treatment (n=3;
P<0.05) (Fig. 2C and D).
Additionally, sRAGE enhanced the number of iNOS-positive
macrophages to 3.57±0.781 as well (n=3; P<0.05) (Fig. 2C and D). In order to further
confirm the effect of exogenous administration of sRAGE on Raw
264.7 cell polarization, levels of IL-6 and IL-12 (markers for M1)
were detected using a q-PCR assay. The results showed that the
levels of IL-6 and IL-12 were increased in I/R-treated macrophages
with sRAGE treatment (n=3; P<0.05) (Fig. 2E and F). These results
demonstrated that exogenous administration of sRAGE stimulated the
differentiation of macrophages to M1.
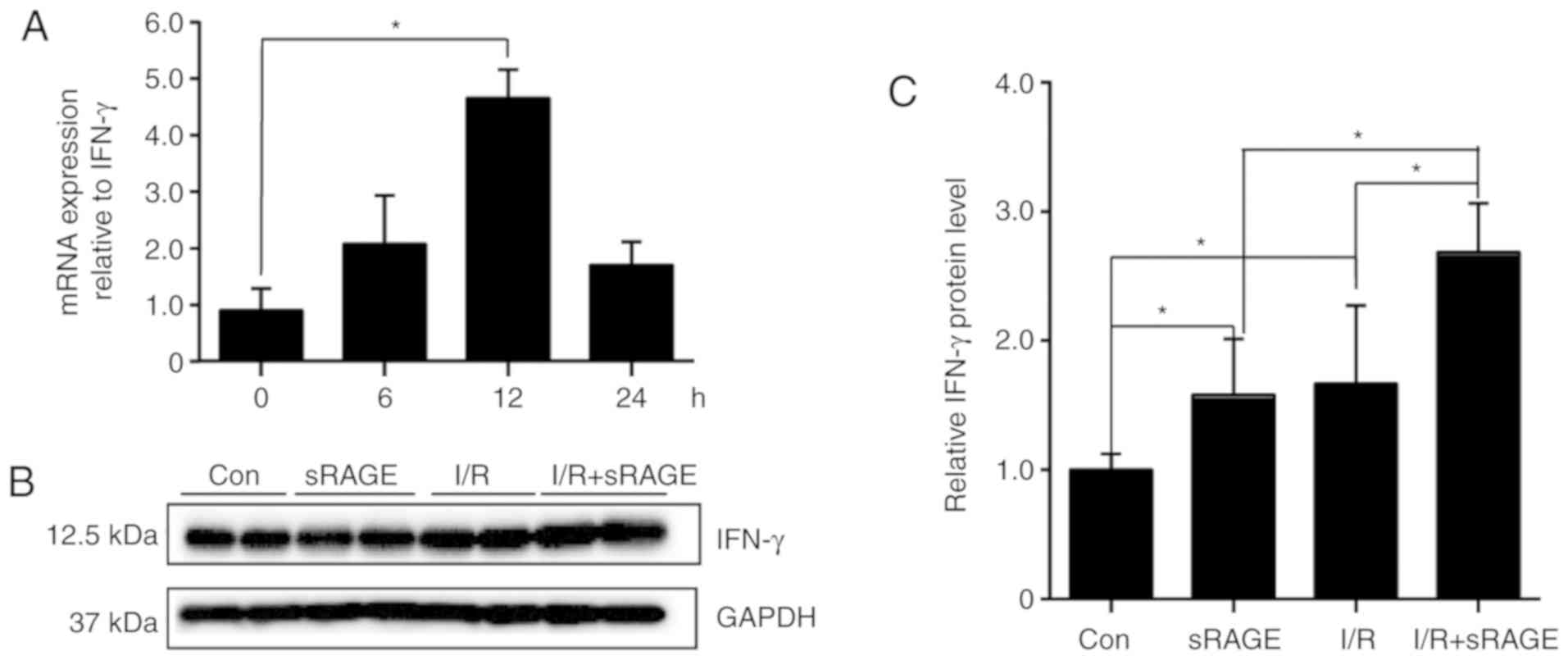
sRAGE augmented the expression of IFN-γ
in macrophages
Total RNA from Raw264.7 cells was used for IFN-γ
detection via qPCR. The results showed that the level of IFN-γ was
increased in a time dependent manner in I/R-treated macrophages
with sRAGE treatment, where the highest value was reached at 12 h
(n=3; P<0.05); (Fig. 3A). The
proteins from Raw264.7 cells were used for IFN-γ detection using
western blot. The results showed that the level of IFN-γ was
increased to 1.70±0.60 in I/R-treated cells compared with control
cells (1.00±0.124); (n=3; P<0.05); (Fig. 3B and C). Notably, sRAGE increased
the level of IFN-γ to 22.68±0.37 in I/R-treated cells and 1.58±0.43
in PBS treated cells (n=3; P<0.05); (Fig. 3B and C). These results suggested
that sRAGE augmented IFN-γ expression in macrophages with I/R
treatment.
sRAGE activated NF-κB signaling pathway
in macrophages
To detect the effect of the NF-κB signaling pathway
on IFN-γ expression in macrophages, the level of phosphorylated
IKK, IкB, NF-κB in cells treated with or without I/R, in the
presence or absence of sRAGE, was analyzed using western blotting.
Results indicated that p-NF-κB levels were increased to 1.87±0.23
in I/R-treated cells compared with control cells, and were further
increased to 3.15±0.23 by sRAGE in I/R-treated cells (n=3;
P<0.05); (Fig. 4A and B).
Additionally, sRAGE also increased the level of p-NF-κB to
2.29±0.44 in control macrophages (n=3; P<0.05) (Fig. 4A and B). The results also showed
that sRAGE increased p-IκB level to 1.47±0.13 in I/R-treated cells
and 1.34±0.05 in PBS treated cells (n=3; P<0.05) (Fig. 4A and C). At the same time, the
results showed that sRAGE increased p-IKK level to 1.74±0.109 in
I/R-treated cells (n=3; P<0.05) (Fig. 4A and D). These findings suggested
that sRAGE might activate the NF-κB signaling pathway in
macrophages with or without I/R treatment.
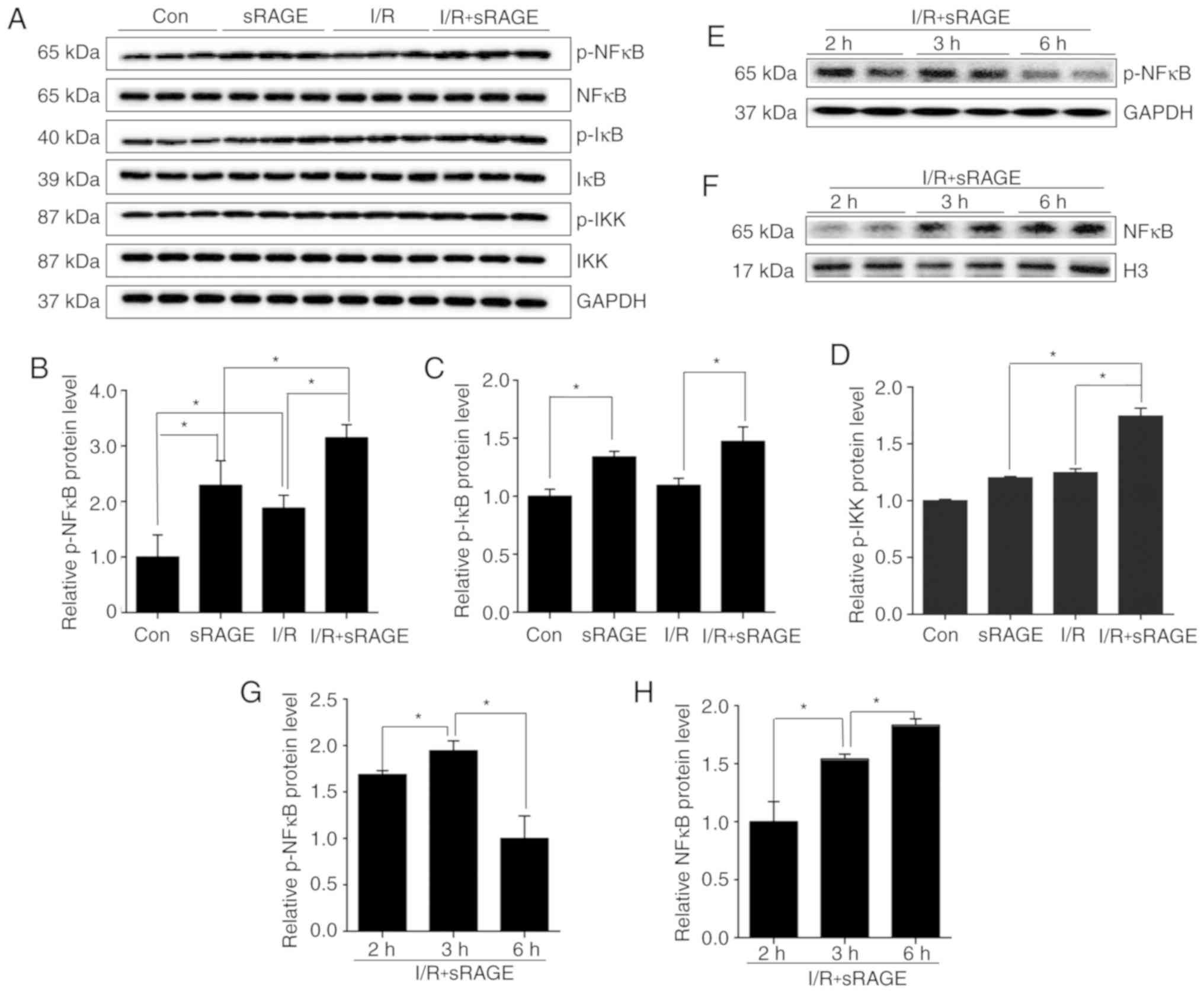 | Figure 4sRAGE activated the NF-κB pathway in
macrophages. (A) Representative images of western blotting for
p-NF-κB, NF-κB, p-IκB, IκB, p-IKK, IKK and GAPDH in macrophages.
(B) Quantification of relative protein levels of p-NF-κB/NF-κB in
macrophages. (C) Quantification of relative protein levels of
p-IκB/IκB in macrophages. (D) Quantification of relative protein
levels of p-IKK/IKK in macrophages. (E) Representative images of
western blot for p-NF-κB and GAPDH in the cytoplasm of macrophages.
(F) Representative images of western blotting for NF-κB and Histone
H3 in the nuclei of macrophages. (G) Quantification of relative
protein levels of p-NF-κB in the cytoplasm of macrophages. (H)
Quantification of relative protein levels of NF-κB in the nucleus
of macrophages. The data are expressed as the mean ± SD (n=3,
*P<0.05). NF-κB, nuclear factor-κB; p-NF-κB,
phosphorylated NF-κB; IκB, inhibitory κB; p-IκB, phosphorylated
IκB; IKK, inhibitory κB kinase complex; p-IKK, phosphorylated IKK;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
Activation of NF-κB was also associated with the
translocation of NF-κB into the nucleus. Western blotting was used
to detect NF-κB in the nucleus and phosphorylated (p)-NF-κB in the
cytoplasm in I/R- and sRAGE-treated macrophages. The results showed
that nuclear NF-κB was increased in a time-dependent manner, and
that the highest value was reached at 6 h (1.83±0.042) (n=3;
P<0.05) (Fig. 4F and H).
Additionally, the level of p-NF-κB in the cytoplasm was increased
from 1.68±0.033 at 2 h to 1.94±0.087 at 3 h. Notably, p-NF-κB
decreased to the lowest value at 6 h (n=3; P<0.05) (Fig. 4E and G). These findings indicated
that sRAGE significantly increased the nuclear translocation of
NF-κB.
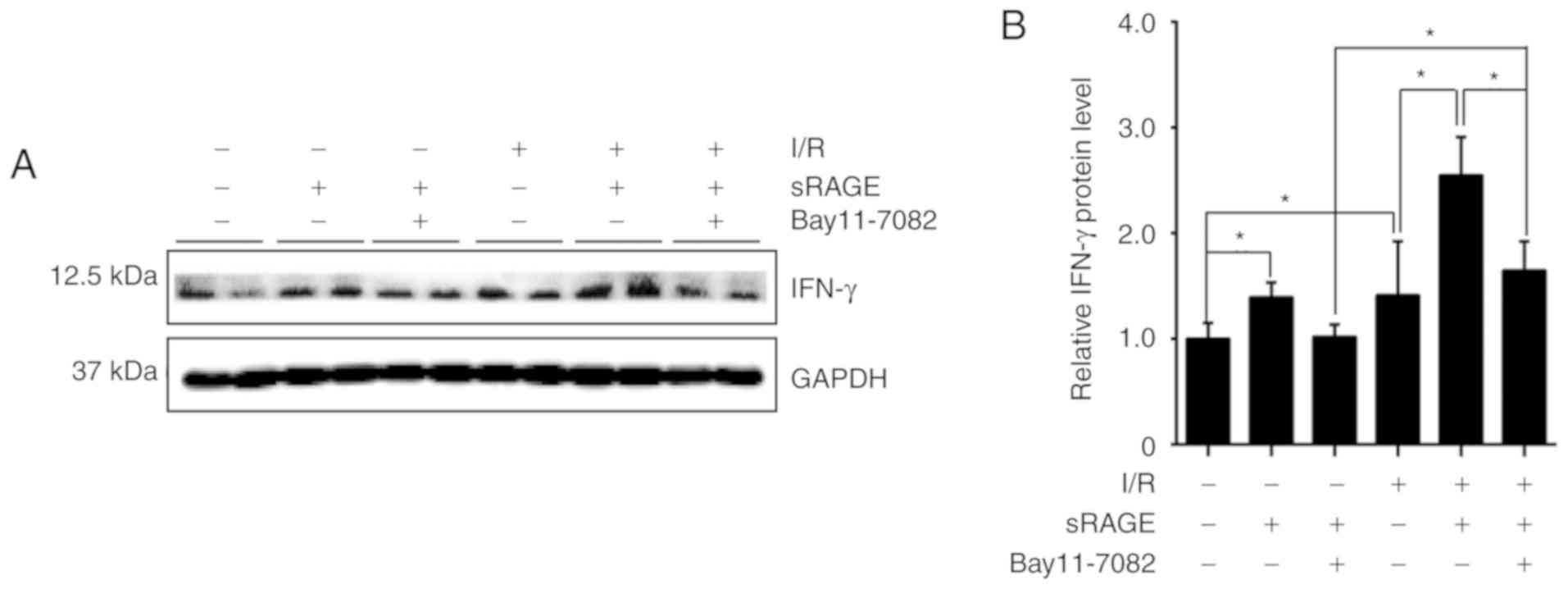
Suppression of NF-κB abolished the effect
of sRAGE on IFN-γ suppression
To detect the effect of NF-κB on IFN-γ expression,
an inhibitor of NF-κB, Bay117082, was simultaneously applied with
sRAGE. Western blotting was used to detect the expression of IFN-γ
in the cells. The results showed that the IFN-γ level was
significantly increased to 1.41±0.507 by I/R treatment compared
with the control cells, while sRAGE further increased the IFN-γ
level to 2.55±0.360 in I/R-treated cells, which was inhibited by
Bay117082 (1.64±0.276) (n=3; P<0.05) (Fig. 5A and B). Additionally, sRAGE alone
increased the level of IFN-γ to 1.39±0.139 in macrophages, which
was again inhibited by Bay117082 (1.02±0.117) (n=3; P<0.05)
(Fig. 5A and B). These findings
suggested that the NF-κB signaling pathway mediates the effects of
sRAGE on IFN-γ expression in macrophages.
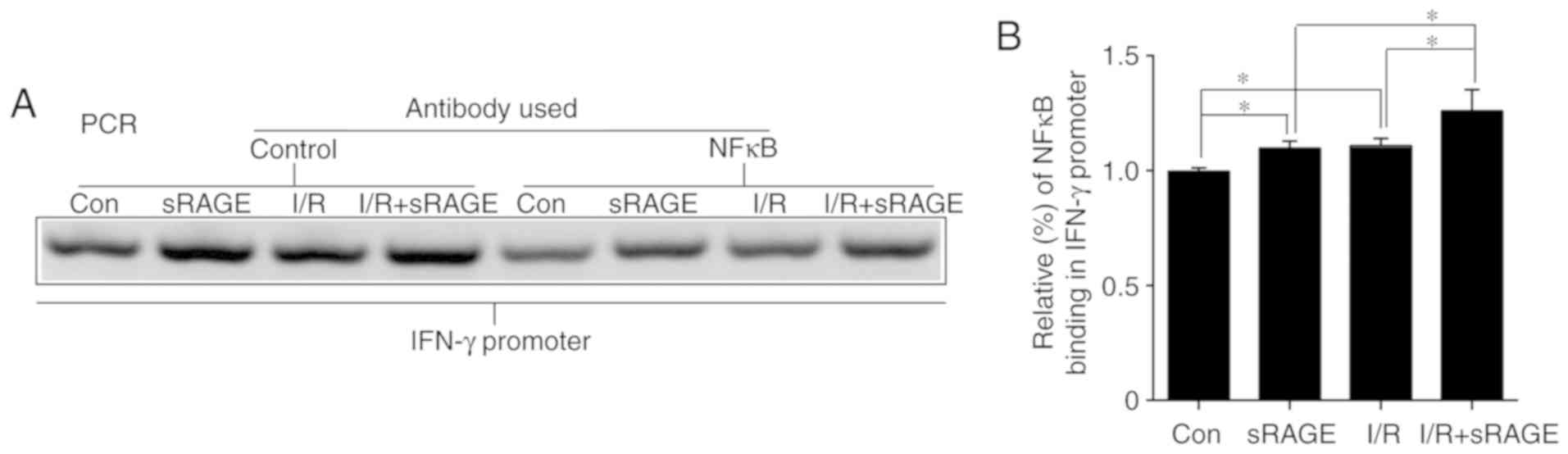
sRAGE induced an upregulation of IFN-γ
through the activation of NF-κB
Notably, p-NF-κB may regulate gene expression by
binding to the -2,000 bp promoter region of the cytokine. A ChIP
assay was used to identify the interaction between NF-κB and the
IFN-γ promoter. The results showed that binding between the IFN-γ
gene and NF-κB was increased to 1.108±0.033 (n=3; P<0.05) in
I/R-treated cells compared with in control cells, and further
increased to 1.259±0.094 by sRAGE in I/R-treated cells (n=3;
P<0.05) (Fig. 6).
Additionally, sRAGE increased the binding of the IFN-γ gene and
NF-κB to 1.097±0.031 in macrophages (n=3; P<0.05) (Fig. 6). Considered together, these
findings suggested that sRAGE promoted NF-κB binding to the
promoter of IFN-γ.
Discussion
The purpose of this study was to verify the
mechanisms of increased IFN-γ expression in I/R-treated hearts by
sRAGE, which protected the heart against I/R injury. The result
showed that sRAGE improved heart function in I/R-treated mice,
which was associated with the recruitment and differentiation of
macrophages in myocardial tissues following I/R. These effects
contributed to elevated IFN-γ expression via activating the NF-κB
pathway through sRAGE in differentiated M1 macrophage.
To explore the effects of sRAGE in the I/R-treated
heart, cardiac function was measured after I/R with or without
sRAGE in the present study. The results showed that the cardiac
output and stroke volume were improved by sRAGE in I/R-treated
hearts (Fig. 1A-C). These results
were consistent with published work from Dang et al
(16).
Previous studies have indicated that sRAGE protected
the heart against I/R injury via inducing the recruitment of
macrophages and increasing the expression of IFN-γ in hearts, as
demonstrated by higher numbers of IFN-γ+/CD68+ macrophages compared
with IFN-γ+/Ly6G+ neutrophils and IFN-γ+/CD3+ lymphocytes via
double-immunofluorescent staining in I/R-treated hearts (16,23).
The results of the present study also showed that sRAGE increased
the recruitment of macrophages in I/R hearts as well (Fig. 1D and E), whilst the myocardial
tissue inflammatory response was reduced by sRAGE (Fig. 1D). These results were consistent
with published work from Wang et al (24). Furthermore, we
also showed that sRAGE reduced neutrophil infiltration following
I/R (Fig. 1D and F), which might
be due to the dose-dependent action of sRAGE on the chemotactic
effects in neutrophils; the results showed that 10 ng/ml sRAGE
promoted the migration of neutrophils (13).
At present, activating phenotypes of macrophages are
broadly classified as M1 (classical macrophage) and M2 (alternative
macrophage) (25); these are two extreme states. Macrophages may
undergo dynamic switches in a variety of diseases; they play
diverse roles by producing cytokines to promote or inhibit tissue
injury or repair (26-28). Pro-inflammatory M1 macrophages release
cytokines such as IFN-γ in the early stages of myocardial ischemia,
while M2-macrophages release cytokines such as TGF-β, which promote
tumor growth and proliferation as well as tissue repair, and
protect the heart and kidneys from I/R injury (29). Strategies
targeting macrophage activation or infiltration may be helpful in
preventing various diseases following I/R. Pullerits et al
(13) demonstrated that sRAGE was an important chemotaxis molecule
that may directly interact with MHC-1 to trigger a pro-inflammatory
cytokine cascade. Additionally, Wang et al (24) proved that
sRAGE induced macrophage infiltration in the murine lung in
vivo. Therefore, the effects of sRAGE on the proliferation and
differentiation of macrophages were analyzed in the current study,
via a 5-BrdU incorporation assay and iNOS staining and the
detection of IL-6 and IL-12 mRNA. The results showed that
administration of sRAGE promoted the proliferation of macrophages
following I/R, and that M1 macrophages were dominant in the injured
area of I/R at 24 h (Fig. 2A-F).
This might be because of sRAGE, which promotes the differentiation
of macrophages into M1-macrophages, which, in turn, increases IFN-γ
expression in I/R-treated hearts.
Macrophages secrete various cytokines, such as
TGF-β, IFN-γ, TNF-α and IL-2 (30). Reports have indicated that
sRAGE inhibits apoptosis in I/R-induced myocardial tissue via
IFN-γ-induced immunoproteasome activity (16,31). The results of the
current study demonstrated that sRAGE enhanced IFN-γ expression in
macrophages with I/R treatment (Fig.
3). Therefore, it was concluded that sRAGE stimulated
macrophages to produce IFN-γ to protect the heart against I/R
injury.
Thus, there was a need to investigate the cell
signaling pathways mediating IFN-γ synthesis and release in
I/R-treated macrophages. NF-κB signaling has been reported to
regulate the transcription of IFN-γ by binding to the promoter
region. In this study, the results showed that sRAGE increased both
the translocation of NF-κB into the nucleus and the binding of
NF-κB to the promoter of IFN-γ. Meanwhile, sRAGE enhanced the
expression of IFN-γ, which was suppressed by the NF-κB inhibitor,
Bay117082, in macrophages with I/R treatment. However, the NF-κB
inhibitor did not inhibit the expression of IFN-γ completely,
suggesting that other signaling pathways may be involved in this
process. Thus, further studies are needed to clarify the effects of
sRAGE on the expression of IFN-γ.
Besides IFN-γ, IL-6 and IL-12 also were increased by
sRAGE in I/R-treated hearts, which was reported to be a potential
pathological mechanism in cardiac I/R injury, but no cardiac
injuries were observed after sRAGE infusion in I/R-treated hearts
in the present study. Therefore, it was supposed that IL-6, a
cytokine with pro-inflammatory properties, stimulated the growth
and differentiation of B-lymphocytes in the spleen, which initiated
a protective mechanism after I/R in mice as previously reported
(23,32). It has been reported that IL-35, a member of the IL-12
family (33), can significantly reduce infarct size after I/R and
become a protective immunomodulator in brain ischemic injury in
mice. This explained why sRAGE showed protective effects on the
I/R-treated heart, even though IL-6 and IL-12 were increased and
pro-inflammatory effects were observed in the sRAGE-treated I/R
heart. However, no experimental data supporting these hypotheses
were recorded, and further studies are needed to demonstrate
it.
In conclusion, sRAGE protected the heart from I/R
injury; this might be mediated via promoting the infiltration and
differentiation of macrophages to M1, which then synthesize and
secrete IFN-γ via activation of the NF-κB-activated pathway.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 30801217, 81570321,
81370313, and 81870265), the Beijing Nova Program (grant no.
2010B050), the Beijing Health System High Level Health Technical
Personnel Training Program (grant no. 2013-3-046), and the China
Young and Middle-aged Clinical Research Foundation (grant no.
2017CCA-VG045).
Availability of data and materials
All the data generated during this study are
included in this published article.
Authors’ contributions
XLZ, CXG, and XJZ designed the experiments. XXC and
MQD created the animal ischemia reperfusion model. XLZ performed
the experiments. HXW, BXC, FHD and HHL analyzed the experimental
results. XLZ and XJZ drafted and revised the article. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animals received humane care, and the
experimental protocol was approved by the Committee of Laboratory
Animals according to institutional guidelines of the Use Committee
of Capital Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
IFN-γ
|
interferon-γ
|
|
sRAGE
|
soluble receptor for advanced
glycation end-products
|
|
RAGE
|
receptor for advanced glycation
end-products
|
|
I/R
|
ischemia/reperfusion
|
|
NF-κB
|
nuclear factor-κB
|
|
p-NF-κB
|
phosphorylated NF-κB
|
|
IκB
|
inhibitory κB
|
|
p-IκB
|
phosphorylated IκB
|
|
IKK
|
IκB kinase complex
|
|
p-IKK
|
phosphorylated IKK
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
5-BrdU
|
5-Bromo-2′-Deoxyuridine
|
|
ChIP
|
chromatin immunoprecipitation
|
|
H&E
|
hematoxylin and eosin staining
|
|
CD68
|
cluster of differentiation 68
|
|
Ly6g
|
lymphocyte antigen 6 complex, locus
G
|
|
SD
|
standard deviation
|
|
P
|
P-value
|
|
iNOS
|
inducible nitric oxide synthase
|
|
IL-12
|
interleukin 12
|
|
IL-6
|
interleukin 6
|
|
PVDF
|
polyvinylidene fluoride
|
Acknowledgments
The authors would like to thank the other members of
the team for their assistance in the present study.
References
|
1
|
Maneechote C, Palee S, Chattipakorn SC and
Chattipakorn N: Roles of mitochondrial dynamics modulators in
cardiac ischaemia/reperfusion injury. J Cell Mol Med. 21:2643–2653.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kocman EA, Ozatik O, Sahin A, Guney T,
Kose AA, Dag I, Alatas O and Cetin C: Effects of ischemic
preconditioning protocols on skeletal muscle ischemia-reperfusion
injury. J Surg Res. 193:942–952. 2015. View Article : Google Scholar
|
|
3
|
Kunecki M, Plazak W, Podolec P and Golba
KS: Effects of endogenous cardioprotective mechanisms on
ischemia-reperfusion injury. Postepy Hig Med Dosw (Online).
71:20–31. 2017. View Article : Google Scholar
|
|
4
|
Hauerslev M, Mork SR, Pryds K, Contractor
H, Hansen J, Jespersen NR, Johnsen J, Heusch G, Kleinbongard P,
Kharbanda R, et al: Influence of long-term treatment with glyceryl
trinitrate on remote ischemic conditioning. Am J Physiol Heart Circ
Physiol. 315:H150–H158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang C, Li H, Wang S, Mao X, Yan D, Wong
SS, Xia Z and Irwin MG: Repeated non-invasive limb ischemic
preconditioning confers cardioprotection through PKC-/STAT3
signaling in diabetic rats. Cell Physiol Biochem. 45:2107–2121.
2018. View Article : Google Scholar
|
|
6
|
Yuan Y, Cao W, Hong Y, Guo X, Wang Y, Wang
Y, Wang X and Xu P: Tilianin pretreatment prevents myocardial
ischemia-reperfusion injury via preservation of mitochondrial
function in rat heart. Phytomedicine. 34:106–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Zhao L and Ma J: Penehyclidine
hydrochloride preconditioning provides cardiac protection in a rat
model of myocardial ischemia/reperfusion injury via the mechanism
of mitochondrial dynamics mechanism. Eur J Pharmacol. 813:130–139.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai XY, Lu L, Wang YN, Jin C, Zhang RY,
Zhang Q, Chen QJ and Shen WF: Association of increased S100B,
S100A6 and S100P in serum levels with acute coronary syndrome and
also with the severity of myocardial infarction in cardiac tissue
of rat models with ischemia-reperfusion injury. Atherosclerosis.
217:536–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wautier JL, Zoukourian C, Chappey O,
Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D and Schmidt AM:
Receptor-mediated endothelial cell dysfunction in diabetic
vasculopathy, Soluble receptor for advanced glycation end products
blocks hyperpermeability in diabetic rats. J Clin Invest.
97:238–243. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dattilo BM, Fritz G, Leclerc E, Kooi CW,
Heizmann CW and Chazin WJ: The extracellular region of the receptor
for advanced glycation end products is composed of two independent
structural units. Biochemistry. 46:6957–6970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raucci A, Cugusi S, Antonelli A, Barabino
SM, Monti L, Bierhaus A, Reiss K, Saftig P and Bianchi ME: A
soluble form of the receptor for advanced glycation endproducts
(RAGE) is produced by proteolytic cleavage of the membrane-bound
form by the sheddase a disintegrin and metalloprotease 10 (ADAM10).
FASEB. 22:3716–3727. 2008. View Article : Google Scholar
|
|
12
|
Yonchuk JG, Silverman EK, Bowler RP,
Agusti A, Lomas DA, Miller BE, Tal-Singer R and Mayer RJ:
Circulating soluble receptor for advanced glycation end products
(sRAGE) as a biomarker of emphysema and the RAGE axis in the lung.
Am J Respir Crit Care Med. 192:785–792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pullerits R, Brisslert M, Jonsson IM and
Tarkowski A: Soluble receptor for advanced glycation end products
triggers a proinflammatory cytokine cascade via beta2 integrin
Mac-1. Arthritis Rheum. 54:3898–3907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan SF, Yan SD, Herold K, Ramsamy R and
Schmidt AM: Receptor for advanced glycation end products and the
cardiovascular complications of diabetes and beyond: Lessons from
AGEing. Endocrinol Metab Clin North Am. 35:511–524. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt AM, Yan SD, Yan SF and Stern DM:
The multiligand receptor RAGE as a progression factor amplifying
immune and inflammatory responses. J Clin Invest. 108:949–955.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dang M, Zeng X, Chen B, Wang H, Li H, Du F
and Guo C: Interferon-gamma mediates the protective effects of
soluble receptor for advanced glycation end-product in myocardial
isch-emia/reperfusion. Lab Invest. 99:358–370. 2019. View Article : Google Scholar
|
|
17
|
Darwich L, Coma G, Pena R, Bellido R,
Blanco EJ, Este JA, Borras FE, Clotet B, Ruiz L, Rosell A, et al:
Secretion of interferon-gamma by human macrophages demonstrated at
the single-cell level after costimulation with interleukin (IL)-12
plus IL-18. Immunology. 126:386–393. 2009. View Article : Google Scholar :
|
|
18
|
Deng Y, Chu J, Ren Y, Fan Z, Ji X,
Mundy-Bosse B, Yuan S, Hughes T, Zhang J, Cheema B, et al: The
natural product phyllanthusmin C enhances IFN-gamma production by
human NK cells through upregulation of TLR-mediated NF-kappaB
signaling. J Immunol. 193:2994–3002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim BR, Chun S and Cho D: Association of
neutrophil-to-lymphocyte ratio and natural killer cell activity
revealed by measurement of interferon-gamma levels in a healthy
population. J Clin Lab Anal. 33:e226402018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang HB, Ahn KS, Oh SR and Kim JW:
Genkwadaphnin induces IFN-gamma via PKD1/NF-kappaB/STAT1 dependent
pathway in NK-92 cells. PLoS One. 9:e1151462014. View Article : Google Scholar
|
|
21
|
Pan Z, Sun X, Ren J, Li X, Gao X, Lu C,
Zhang Y, Sun H, Wang Y, Wang H, et al: miR-1 exacerbates cardiac
ischemia-reperfusion injury in mouse models. PLoS One.
7:e505152012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang BH, Wang W, Wang H, Yin J and Zeng
XJ: Promoting effects of the adipokine, apelin, on diabetic
nephropathy. PLoS One. 8:e604572013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brisslert M, Amu S and Pullerits R:
Intra-peritoneal sRAGE treatment induces alterations in cellular
distribution of CD19(+), CD3 (+) and Mac-1 (+) cells in lymphoid
organs and peritoneal cavity. Cell Tissue Res. 351:139–148. 2013.
View Article : Google Scholar
|
|
24
|
Wang Y, Wang H, Piper MG, McMaken S, Mo X,
Opalek J, Schmidt AM and Marsh CB: sRAGE induces human monocyte
survival and differentiation. J Immunol. 185:1822–1835. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ganz T: Macrophage function. New Horiz.
1:23–27. 1993.PubMed/NCBI
|
|
26
|
Ko GJ, Boo CS, Jo SK, Cho WY and Kim HK:
Macrophages contribute to the development of renal fibrosis
following ischaemia/reperfusion-induced acute kidney injury.
Nephrol Dial Transplant. 23:842–852. 2008. View Article : Google Scholar
|
|
27
|
Zhang L and Wang CC: Inflammatory response
of macrophages in infection. Hepatobiliary & pancreatic
diseases international. Hepatobiliary Pancreat Dis Int. 13:138–152.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou D, Huang C, Lin Z, Zhan S, Kong L,
Fang C and Li J: Macrophage polarization and function with emphasis
on the evolving roles of coordinated regulation of cellular
signaling pathways. Cell Signal. 26:192–197. 2014. View Article : Google Scholar
|
|
29
|
Khan MA, Assiri AM and Broering DC:
Complement and macrophage crosstalk during process of angiogenesis
in tumor progression. J Biomed Sci. 22:58–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saqib U, Sarkar S, Suk K, Mohammad O, Baig
MS and Savai R: Phytochemicals as modulators of M1-M2 macrophages
in inflammation. Oncotarget. 9:17937–17950. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo CX, Jiang X, Zeng XJ, Wang HX, Li HH,
Du FH and Chen BX: Soluble receptor for advanced glycation
end-products protects against ischemia/reperfusion-induced
myocardial apoptosis via regulating the ubiquitin proteasome
system. Free Radic Biol Med. 94:17–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Zhou Y, Yu Y, He K and Cheng LM:
Lipopolysaccharide preconditioning increased the level of
regulatory B cells in the spleen after acute ischaemia/reperfusion
in mice. Brain Res. 1701:46–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu C, Zhu H, Shen R, Feng Q, Zhou H and
Zhao Z: IL-35 is a protective immunomodulator in brain ischemic
injury in mice. Neurochem Res. 43:1454–1463. 2018. View Article : Google Scholar : PubMed/NCBI
|