Introduction
Cancer is a principal public health problem globally
and breast cancer is one of the most frequently diagnosed types of
cancer. It is estimated that there will be 268,670 newly diagnosed
cases (2,550 male cases and 266,120 female cases) of breast cancer
in the United States in 2018 (1).
High-grade types of breast cancer have high aggression and are
associated with poor prognosis and shorter survival time (2). The intensive study of molecular
mechanisms underlying the progress of breast cancer could aid early
diagnosis and treatment. In this respect, the identification of
genetic/epigenetic mutations of oncogenes/anti-oncogenes is a
potential research direction. At present, a few of
immunohistochemistry (IHC) markers as well as clinicopathological
variables have become the basis of prognosis prediction and therapy
selection for breast cancer (3,4).
Human epidermal growth factor receptor 2 (HER2),
estrogen receptor and progesterone receptor are the most commonly
used IHC markers for breast cancer. HER2 is an important member of
epidermal growth factor receptor family (5,6).
In breast cancer clinics, cases associated with HER2
overexpression, which is defined as HER2 positive status, account
for ~20% of all patients (5). At
the cellular level of breast cancer, HER2 is mainly located in the
cell membrane and acts as an oncogene (7,8).
In ~90% of HER2 positive breast cancer cases, HER2
over-expression is caused by HER2 gene amplification (9). Within the 17q12-21 amplicon, HER2 is
located at 17q12 (10) and
multiple coamplified genes including growth factor receptor bound
protein 7 (GRB7), StAR related lipid transfer domain containing 3,
DNA topoisomerase II α, protein phosphatase 1 regulatory inhibitor
subunit 1B, thyroid hormone receptor α, and retinoic acid receptor
α have been identified on the 17q12-21 amplicon (11,12). Coamplified genes on the HER2
amplicon may activate cellular processes that are not directly
oncogenic, but have become necessary for the oncogenic state.
Therefore, multiple targeted therapies in HER2 positive breast
cancer are necessary.
Accumulating evidence has proven that microRNAs
(miRNAs/miR) serve important roles in cancer metastasis by reducing
the expression of their targets, including mRNA, long noncoding
RNA, circular RNA and pseudogenes (13-15). miR-193a-3p has been identified as
a key tumor suppressor in cancer (16,17), but little is known about the role
of miR-193a-3p in HER2 positive breast cancer.
GRB7 is part of the 17q12-21 amplicon, located close
to the HER2 gene (18).
Transcript analysis indicates that in breast cancer cells GRB7 RNA
expression is always high synchronously with HER2/neuraminidase 1
(Neu) (19). More and more
evidence has demonstrated that overexpression of GRB7 is correlated
with a metastatic phenotype and deceased survival in breast cancer
(18,20). Therefore, GRB7 may have the
potential to become a novel therapeutic target in breast
cancer.
In order to Figure out better therapies for breast
cancer, it is crucial to understand the pathogenesis more thorough.
In the present study, insights are provided into the potential
effects of miR-193a-3p in HER2 positive breast cancer. As the
increase of DNA methylation in the miRNA promoter could reduce the
transcription efficiency (21-23), it was identified that during HER2
positive cancer development and progression, miR-193a-3p was
silencing by DNA hypermethylation, and the epigenetic silencing of
miR-193a-3p made the expression of GRB7 higher and therefore
activated the extracellular signal-regulated kinase/forkhead box M1
(ERK/FOXM1) signaling pathway.
Materials and methods
Cell culture and human tissues
The human HER2 positive breast cancer cell lines
HCC-1954, 21MT1 and JimT1 and human normal breast cell line MCF-10A
were bought from the American Type Culture Collection (Manassas,
VA, USA). All of the 4 cell lines were cultured in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), which contained
10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and incubated at 37°C with 5% CO2. A
total of 35 pairs of the clinical HER2 positive breast cancer and
adjacent tissues were collected from 35 patients (age, 21 to 58
years old) who received resection surgery in The Third Affiliated
Hospital of Kunming Medical University (Kunming, China) from April
2015 to August 2017. All of the human tissues used in the present
study were obtained with written informed consent. The present
study was approved by the Ethics Committee of The Third Affiliated
Hospital of Kunming Medical University.
Plasmid and cell transfection
Synthetic pre-miR-193a-3p (Shanghai GenePharma Co.,
Ltd., Shanghai, China) was used to transfect cells to overexpress
miR-193a-3p as previously described (24). GRB7 overexpression plasmid was
constructed as previously described (25). HCC-1954, 21MT1 and JimT1 cells
were plated in 6-well plates (2.5×105 cells/well) and
were transfected using Lipofectamine 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the protocol.
Quantitative polymerase chain reaction
(qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from all the 3 cell
lines with or without treatment with 5-Aza-dc (5 µM) at room
temperature for 4 days and human tissues. Universal cDNA Synthesis
kit (Exiqon; Qiagen, Inc., Valencia, CA, USA) was used to
synthesize first-strand complementary DNA. miRCURY LNA™ Universal
RT microRNA PCR (Exiqon; Qiagen, Inc.) was used to conduct qPCR
determining miRNA. cDNA synthesis was conducted at 95°C for 12 min,
and the qPCR was conducted with the following thermocycling
conditions: 97°C for 5 min, followed by 35 cycles at 95°C for 30
sec, 65°C for 30 sec and 73°C for 1 min, and a final step at 73°C
for 10 min; samples were then kept at 4°C until use. U6 was used as
an endogenous control. Primers of hsa-miR-193a-3p (product no.
204591) were obtained from Exiqon. The forward primer of
miR-193a-3p was 5′-CTGAGGGCTGGGTCTTTGC-3′ and the reverse primer
was 5′-GCCGAGAACTGGGACTTTGT-3′. The forward primer and reverse
primer of U6 were 5′-CTCGCTTCGGCAGCACA-3′ and
5′-ACGCTTCACGAATTTGCGT-3′, respectively.
Western blotting
Western blotting was conducted as previously
described (26). Antibodies
against GRB7 (1:2,000; cat. no. sc-13954; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), ERK (1:1,500; cat. no. 4795; Cell Signaling
Technology, Inc., Danvers, MA, USA), phosphorylated ERK (1:2,000;
cat. no. 4795; Cell Signaling Technology, Inc.), FOXM1 (1:2,000;
cat. no. 5436; Cell Signaling Technology, Inc.) and β-actin
(1:10,000; cat. no. AC-74; Sigma-Aldrich; Merck KGaA) were used in
the present study. The secondary antibody used was horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:1,000;
cat. no. sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Semi-quantitative analysis was performed using ImageJ software
v1.8.0 (National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to measure cell
proliferation ability. HCC-1954, 21MT1 and JimT1 cells
(3×103 cells/well) were plated in 96-well culture plates
for 72 h. The CCK-8 reagent was added to each well and incubated at
37°C for 1 h. Cell viability was assessed by testing the absorbance
at 450 nm using Multiskan MS (Thermo Labsystems, Helsinki,
Finland).
Colony formation assay
HCC-1954, 21MT1 and JimT1 cells (500 cells/well)
were plated in 6-well plates and then cultured in complete media
for 10 days. After removing media and being washed by ice-cold PBS
2 times, the colonies were fixed with methanol for 15 min at 4°C
and stained with crystal violet for 30 min at room temperature.
Images were captured using a digital camera (Canon, Inc., Tokyo,
Japan).
Wound healing assay
The wound-healing assay was carried out to measure
the migration ability of HER2 positive breast cancer cells. All 3
cell lines (5×105 cells/well) were fused to form a
single layer in 6-well plates and a 200-µl sterile pipette
tip was used to scratch a single wound on the cell layer. Following
2 rinses with PBS, cells were incubated for another 24 h. The
scratch wounds were visualized under an inverted microscope (CKX41;
Olympus Corporation, Tokyo, Japan) and the scratch widths were
quantified with ImageJ software v1.8.0 (National Institutes of
Health).
Cell invasion assay
Transwell invasion assay was performed using
Transwell cell invasion assay kits (Corning, Inc., Corning, NY,
USA). A total of 3×104 cells were digested and put in
the serum-free medium in the upper chamber, with a 2 mg/ml
Matrigel-coated membrane containing 8-m pores. The lower Transwell
chamber contained Dulbecco's modified Eagle's medium supplemented
with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific,
Inc.). Following incubation for 72 h (37°C, 5% CO2), the
cells were removed from the upper part of the filters by wiping
with a cotton swab. Then, cells on the lower surface of the
membrane were fixed with 4% formaldehyde at room temperature for 10
min and stained with 0.5% crystal violet for 15 min at room
temperature. Finally, the number of invading cells was imaged and
counted at ×200 magnification using an inverted microscope (Nikon
Corporation, Tokyo, Japan).
Luciferase reporter assay
The sequence of the GRB7 3′-UTR which is the
potential target of miR-193a-3p was ligated into the pmirGLO
plasmid (Promega Corporation, Madison, WI, USA). HCC-1954 cells
were cotransfected with the pmirGLO-3′-UTR plasmid of the above
plasmids or a blank vector using Lipofectamine™ 2000(Invitrogen;
Thermo Fisher Scientific, Inc.). The Dual-Luciferase Assay kit
(Promega Corporation) was used to conduct luciferase activities,
and the transfection efficiency was normalized by co-transfecting
with Renilla-luciferase.
Pyrosequencing analysis
A pyrosequencing assay was conducted to detect the
percentage of methylation in miR-193a-3p in HER2 positive breast
cancer tissues. PSQ Assay Design Software (version 1.0.6; Biotage,
Uppsala, Sweden) was used to design the primers used in
pyrosequencing analysis.
RNA immunoprecipitation (RIP)
RIP experiments were performed using the Magna RIP™
RNA-Binding Protein Immunoprecipitation kit (EMD Millipore,
Billerica, MA, USA) according to the manufacturer's protocol. The
co-precipitated RNAs were detected by reverse transcription PCR, as
aforementioned. Total RNA (input controls) and normal mouse
immunoglobulin G (1:2,000; cat. no. SLM66-0100; Equitech-Bio, Inc.,
Kerrville, TX, USA) controls were assayed simultaneously to
demonstrate that the detected signals were from the RNA that was
specifically bound to GRB7 (n=3 for each experiment).
Statistical analysis
All experiments were repeated at least 3 times
independently. Data are presented as the mean ± standard deviation.
Two-tailed Student's t-test and one-way analysis of variance
followed by Dunnett's C were used to calculate statistically
significant differences. All statistical analyses were performed
using SPSS software (version 20.0; IBM, Corps., Chicago, IL, USA).
P<0.05 was considered to indicate a statically significant
difference.
Results
miR-193a-3p is downregulated in HER2
positive breast cancer depending on the malignant degree
In order to investigate the potential role of
miR-193a-3p in HER2 positive breast cancer, the expression of
miR-193a-3p in 35 pairs of tumor/adjacent HER2 positive breast
cancer tissues were determined and compared. As depicted in
Fig. 1A, the expression of
miR-193a-3p in tumor tissues was significantly decreased compared
with the normal tissues (P<0.001). The expression of miR-193a-3p
in HER2 positive breast cancer tissues of different stages and
grades was also detected. As presented in Fig. 1B and C, the level of miR-193a-3p
decreases significantly with the increase of tumor stage and grade
(P<0.001), which means the level of miR-193a-3p in HER2 positive
breast cancer is also associated with the malignant degree.
Furthermore, the expression of miR-193a-3p in HER2 positive breast
cancer cells (3 different cell lines) and normal breast cell was
tested by qPCR. The result demonstrated that compared with normal
breast cells, miR-193a-3p was significantly downregulated in HER2
positive breast cancer cells (P<0.01; Fig. 1D).
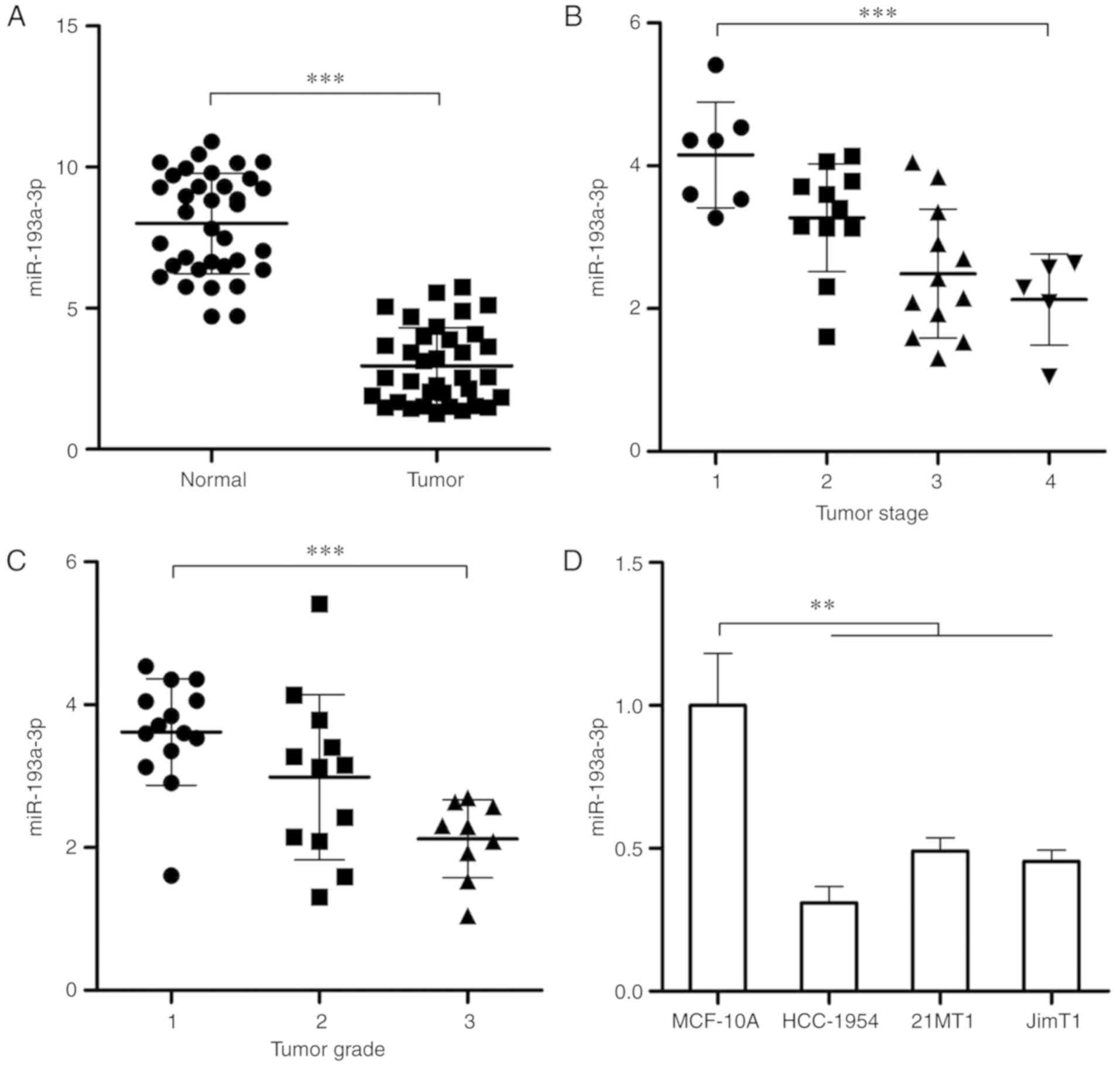 | Figure 1Expression of miR-193a-3p is
decreased in HER2 positive breast cancer and is associated with
tumor stage and grade. (A) RT-qPCR was conducted to test the
expression of miR-193a-3p in 35 pairs of HER2 positive breast
cancer tissues and adjacent tissues. (B) The expression of
miR-193a-3p in HER2 positive breast cancer tissues at different
stages (Stage 1, n=7; Stage 2, n=11; Stage 3, n=12; Stage 4, n=5).
(C) The expression of miR-193a-3p in HER2 positive breast cancer
tissues at different grades (Grade 1, n=14; Grade 2, n=12; Grade 3,
n=9). (D) RT-qPCR was conducted to determine the expression of
miR-193a-3p in normal human breast cells and HER2 positive breast
cancer cells. **P<0.01 and ***P<0.001,
as indicated. RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; HER2, human epidermal growth factor
receptor 2; miR, microRNA. |
DNA methylation causes the reduction of
miR-193a-3p in HER2 positive breast cancer cells
Then the molecular mechanisms underlying the
decrease of miR-193a-3p were investigated in HER2 positive breast
cancer. According to the usual regulatory mechanism of miRNA in
cancer, the alterations of miR-193a-3p expression depending on DNA
methylation in HER2 positive breast cancer cells were investigated.
After treating with a demethylating agent, 5-Aza-dc (5 µM)
for 4 days, qPCR was conducted to detect the expression of
miR-193a-3p, which was demonstrated to significantly increase in
the 2 tested HER2 positive breast cancer cell lines (P<0.01;
Fig. 2A). Subsequently,
pyrosequencing analysis demonstrated a significant increase of
miR-193a-3p DNA methylation in higher-stage and higher-grade tumors
(P<0.01 and P<0.05; Fig. 2B and
C, respectively). These results demonstrated that the loss of
miR-193a-3p in HER2 positive breast cancer may be caused by
DNA hypermethylation.
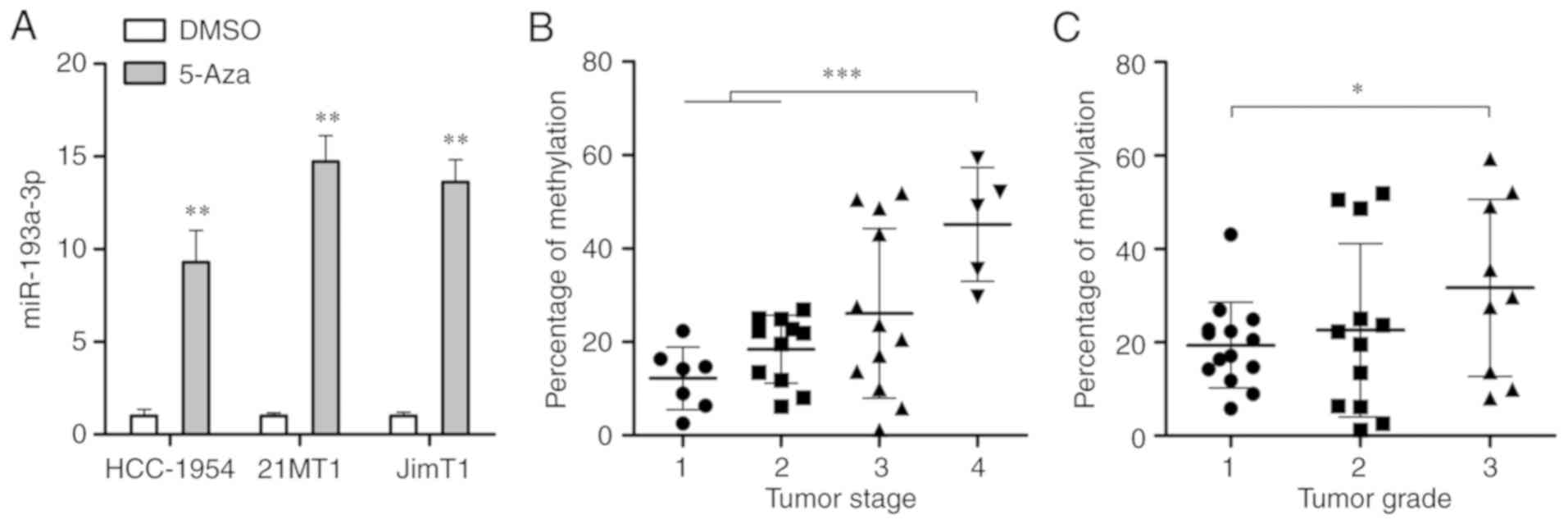 | Figure 2Identification of DNA methylation
leads to the downregulation of miR-193a-3p in breast cancer cells.
(A) Quantitative polymerase chain reaction was carried out to
determine the expression of miR-193a-3p in HER2 positive breast
cancer cells following 4-days treatment with 5-Aza-dc.
**P<0.01 vs. DMSO. (B) Pyrosequencing analysis was
conducted to analyze the percentage of methylation in the
miR-193-3p promoter in HER2 positive breast cancer tissues at
different stages (Stage 1, n=7; Stage 2, n=11; Stage 3, n=12; Stage
4, n=5). (C) Pyrosequencing analysis was conducted to analyze the
percentage of methylation in the miR-193-3p promoter in HER2
positive breast cancer tissues at different grades (Grade 1, n=14;
Grade 2, n=12; Grade 3, n=9). *P<0.05 and
***P<0.001, as indicated. HER2, human epidermal
growth factor receptor 2; miR, microRNA. |
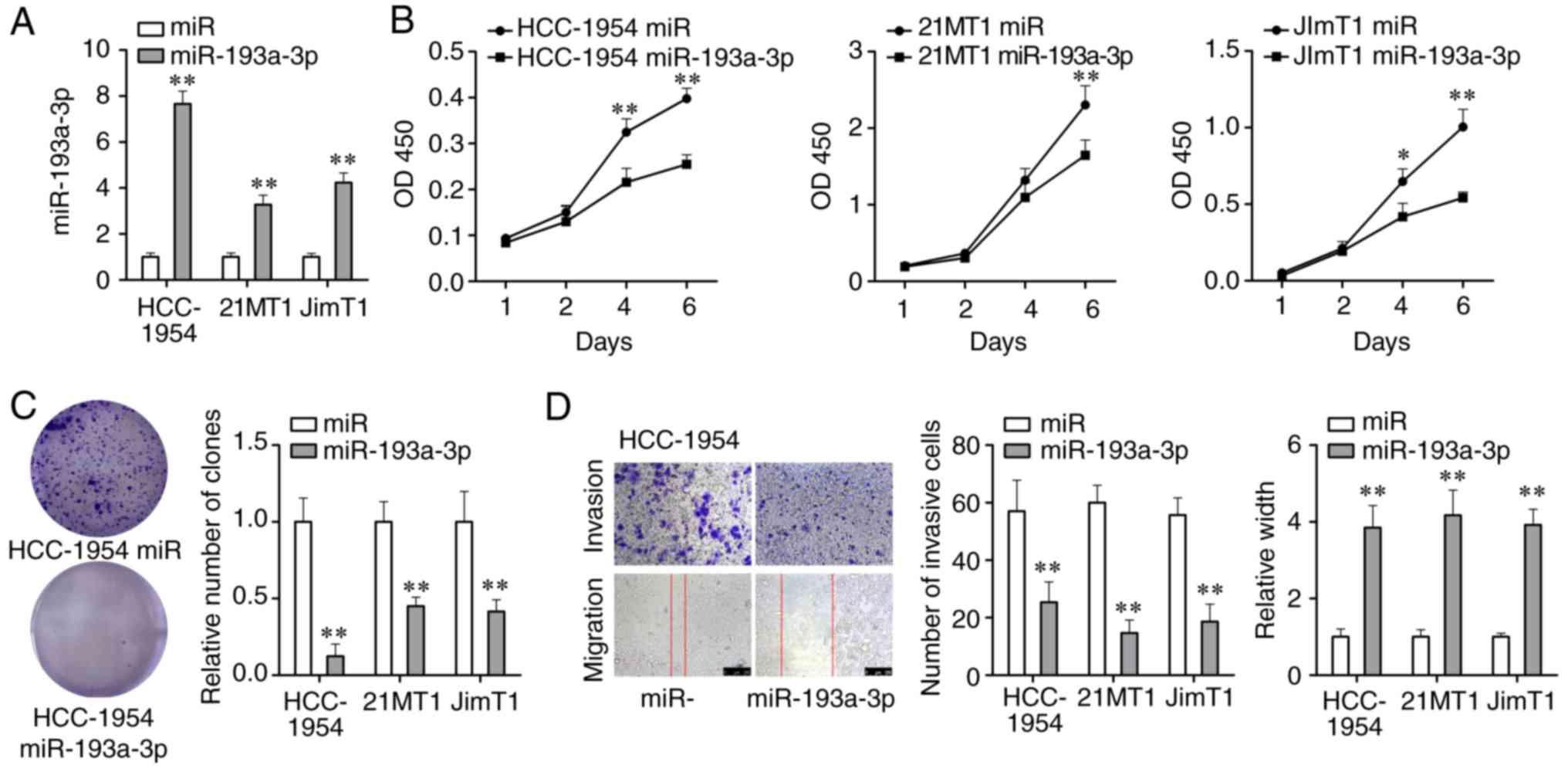
Overexpression of miR-193a-3p could
inhibit proliferation, migration and invasion of HER2 positive
breast cancer cells
In order to further investigate the role miR-193a-3p
serves during the development of HER2 positive breast cancer, the
changes of cell viability, colony formation ability, migration
ability and invasion ability of HER2 positive breast cancer cells
overexpressing miR-193a-3p were tested. miR-193a-3p mimics were
transfected into 3 HER2 positive breast cancer cell lines and its
expression was significantly upregulated (P<0.01; Fig. 3A). Through the CCK-8 assay, it was
demonstrated that cell proliferation was significantly weakened
following 4-6 days of the treatment with miR-193a-3p mimics
(P<0.01; Fig. 3B). Colony
formation assay demonstrated that miR-193a-3p mimics could
significantly reduce the number of cancer cell colonies formed
(P<0.01; Fig. 3C). The
wound-healing and Transwell assays were carried out to investigate
the effect of miR-193a-3p mimics on cell migration and invasion
abilities. As Fig. 3D displays,
cell migration and invasion abilities of HER2 positive breast
cancer cells were significantly suppressed by miR-193a-3p mimics
(P<0.01). Moreover, overexpression of miR-193a-3p could inhibit
proliferation, migration and invasion of another 2 HER2 positive
breast cancer cell lines 21MT1 and JimT1 (Fig. S1). In brief, these findings
indicated that the overexpression of miR-193a-3p could inhibit
proliferation, migration and invasion of HER2 positive breast
cancer cells and further inhibit the development of HER2 positive
breast cancer.
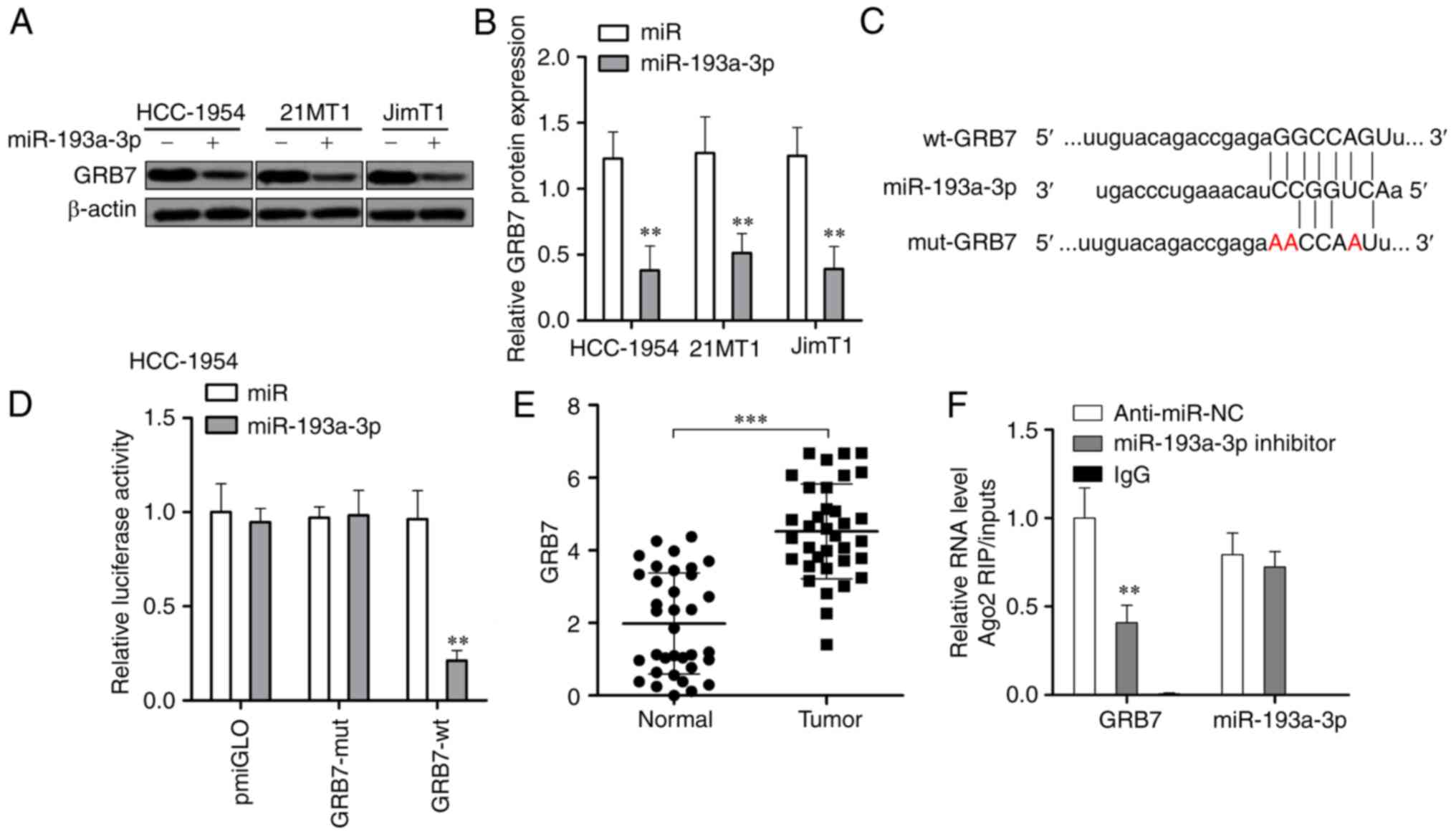
miR-193a-3p could directly repress the
expression of GRB7 through binding to its 3′-UTR
The upregulation of miR-193a-3p significantly
downregulated the expression of GRB7 at the protein level in all 3
tested HER2 positive breast cancer cells (P<0.01; Fig. 4A and B). Then, a luciferase
reporter assay was carried out to investigate if miR-193a-3p could
directly target GRB7. As presented in Fig. 4C and D, miR-193a-3p could
specifically downregulate wild-type GRB7 but could not affect the
expression of miR-193a-3p with a mutant 3′-UTR in HER2 positive
breast cancer cells. GRB7 was significantly upregulated in HER2
positive breast cancer tissues (Fig.
4E). The interaction between GRB7 and miR-193a-3p was further
confirmed via RIP assays (Fig.
4F). These results suggested that miR-193a-3p could reduce GRB7
through direct targeting its 3′-UTR.
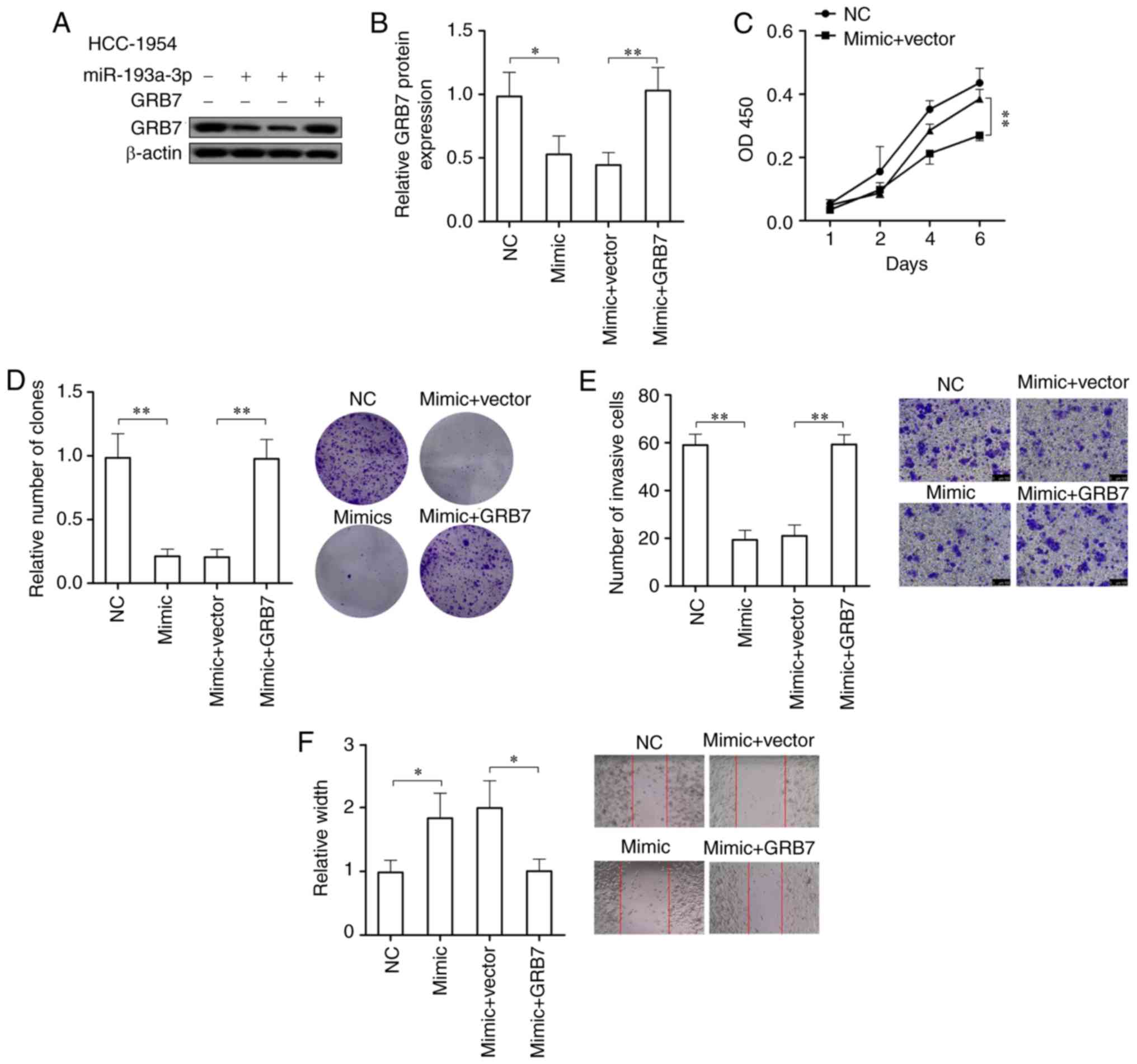
GRB7 overexpression could counteract the
inhibitory effect of miR-193a-3p on the oncogenic capacity of
breast cancer
In order to prove if miR-193a-3p suppresses the
development of HER2 positive breast cancer through targeting GRB7,
the cell viability, colony formation ability, migration ability and
invasive ability of HER2 positive breast cancer cells
overexpressing miR-193a-3p were tested following overexpressing
GRB7. After overexpressing miR-193a-3p, GRB7 in HCC-1954 cells was
significantly downregulated (P<0.05), while overexpression of
GRB7 could significantly abolish the reduction of GRB7 caused by
overexpression of miR-193a-3p (P<0.01; Fig. 5A and B). In HCC-1954 cells
overexpressing miR-193a-3p, the overexpression of GRB7
significantly promoted the cell viability, colony formation
ability, migration ability and invasive ability (P<0.05;
Fig. 5C-F). In conclusion, GRB7
overexpression could abolish the effects on HER2 positive breast
cancer cells caused by the overexpression of miR-193a-3p and
further promote the development of HER2 positive breast cancer.
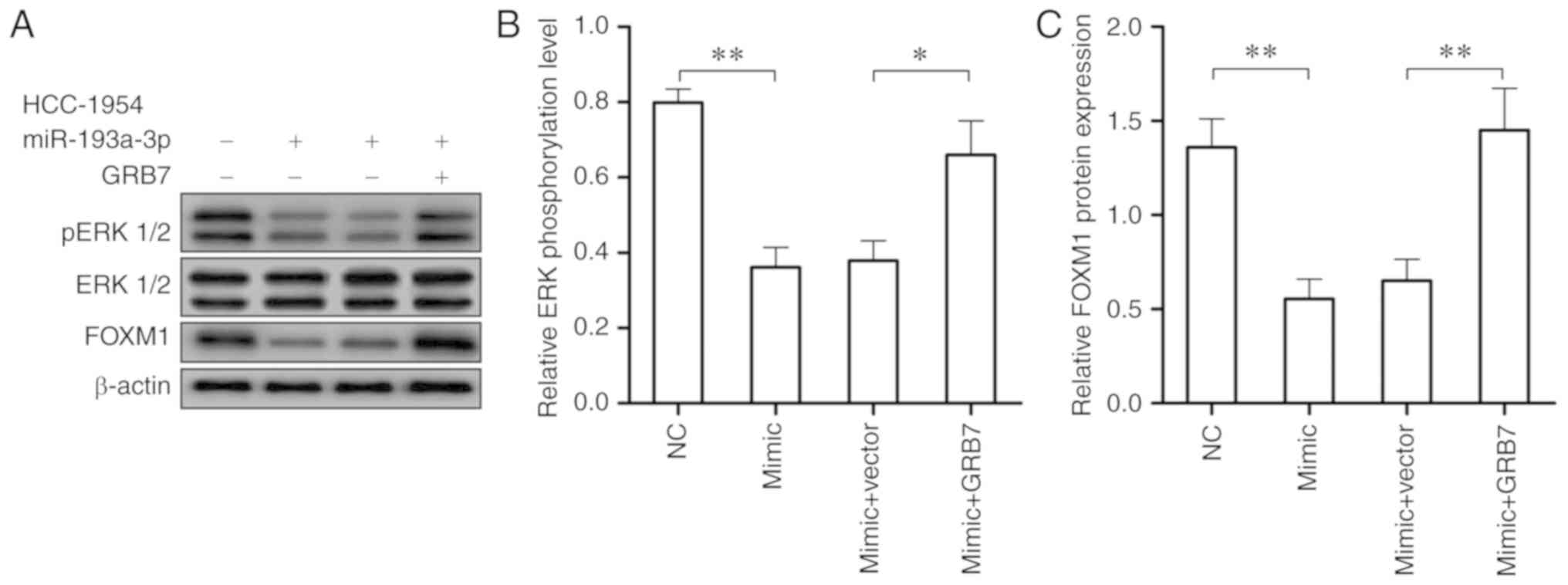
GRB7/ERK/FOXM1 signaling pathway may take
part in the effect that miR-193a-3p has on HER2 positive breast
cancer
According to the close association between the
activation of the ERK/FOXM1 signaling pathway and HER2 positive
breast cancer, western blotting was conducted to investigate if
miR-193a-3p also represses HER2 positive breast cancer through
ERK/FOXM1 signaling pathway. Following the over-expression of
miR-193a-3p in HCC-1954 cells, the expression of phosphorylated ERK
1/2 and FOXM1 were significantly reduced (P<0.01; Fig. 6A and B). While the overexpression
of GRB7 in HCC-1954 cells overexpressing miR-193a-3p could recover
the activity of ERK/FOXM1 signaling pathway, which could accelerate
HER2 positive breast cancer tumorigenesis (Fig. 6). These findings demonstrated
miR-193a-3p may inhibit HER2 positive breast cancer through
downregulating GRB7 and inactivating the ERK/FOXM1 signaling
pathway.
Discussion
The molecular variation within the 17q12-21 amplicon
is one of the major causes of heterogeneity of HER2 positive breast
cancer. In 1994, the coexpression of HER2 and GRB7 in human breast
cancer cells was first reported by Stein et al (27). Lamy et al (28) analyzed the amplification of 11
genes localized within the 17q12-21 amplicon and demonstrated the
frequency of coamplification with HER2 decreases as the distance of
the gene from HER2 increases. GRB7 coamplification with HER2
occurred at the greatest frequency, with GRB7 coamplification
occurring in 97.7% of HER2 positive breast cancer cases (84 of 86
cases). Several studies have demonstrated that GRB7 can facilitate
HER2/Neu-mediated signal transduction and tumor progression
(19,29). Therefore, these characteristics of
GRB7 make it an attractive therapeutic target for HER2 positive
breast cancer. In the present study, it was demonstrated that
miR-193a-3p could directly target GRB7 to suppress the tumor.
Furthermore, the evidence was also provided that miR-193a-3p could
target not only GRB7, but also ERK and FOXM1 signaling in HER2
positive breast cancer cell lines.
miR-193a-3p has been reported to be downregulated in
several types of cancer (30,31). The results revealed that
miR-193a-3p was decreased in HER2 positive breast cancer and the
expression was decreased as the malignant degree of the tumor
increased. The present study provides the first evidence to the
best of our knowledge, concerning dysregulation of miR-193a-3p in
HER2 positive breast cancer. Therefore, attention was focused on
understanding the mechanism leading to the downregulation of
miR-193a-3p.
In human cancer, epigenetic silencing of tumor
suppressors is frequently observed (32). Hypermethylation of the promoter is
a major cause of inactivation of tumor suppressors (33). In the present study,
hypermethylation of the promoter of miR-193a-3p was also observed
in HER2 positive breast cancer and the percentage of methylation of
miR-193a-3p was positively associated with the tumor stage and
grade. The results of the present study suggest that DNA
methylation serves an important role in regulating miR-193a-3p in
HER2 positive breast cancer.
In several types of cancer, miR-193a-3p has been
demonstrated to be a tumor suppressor (16,31). To further investigate the role
miR-193a-3p serves during the development of HER2 positive breast
cancer miR-193a-3p was overexpressed in HER2 positive breast cancer
cell lines and increased expression of miR-193a-3p could inhibit
tumor proliferation, invasion and metastasis.
Through an in silico study, Chen et al
(34) revealed that miR-193a-3p
was the main target of human GRB7 and miR-193a-3p was frequently
downregulated and was inversely correlated with the high expression
of GRB7 in ovarian cancer cell lines. In the present study,
downregulation of miR-193a-3p and upregulation of GRB7 were also
observed in three HER2 positive breast cancer cell lines. Moreover,
the result of luciferase reporter assay provided the direct
evidence that miR-193a-3p could target GRB7 through binding to its
3′-UTR. To further investigate the role that GRB7 served in the
suppressive effect of miR-193a-3p in HER2 positive breast cancer,
GRB7 was overexpressed in 3 HER2 positive breast cancer cell lines.
Indeed, GRB7 overexpression could counteract the inhibitory effect
that miR-193a-3p makes on the oncogenic capacity of breast cancer.
Therefore, it was hypothesized that miR-193a-3p suppressed HER2
positive breast cancer through targeting GRB7.
ERK signaling serves a critical role in controlling
cancer cell proliferation, survival, metastasis and drug
resistance, and abnormal activation of ERK signaling occurs in
>85% types of human cancer (35). It is reported that GRB7 can lead
to increased ERK1/2 phosphorylation through its interaction with
Ras (36). In the present study,
it was demonstrated that overexpression of miR-193a-3p could
inhibit the phosphorylation of ERK1/2 and overexpression of GRB7
would abolish this effect. FOXM1 is a key transcriptional regulator
of the cell cycle, which can be activated by cyclincyclin dependent
kinase and ERK mediated phosphorylation (37-39). The activation of FOXM1 can promote
nuclear localization to overexpress cell cycle regulators including
cell division cycle 25B, baculoviral IAP repeat containing 5 and
polo-like kinase 1 (37). In HER2
positive breast cancer, FOXM1 is overexpressed and serves a
critical role in tumourigenesis (40). The results of the present study
suggested that FOXM1 was a direct or indirect target of
miR-193a-3p. The expression of FOXM1 was decreased following
overexpression of miR-193a-3p and overexpressing GRB7 could rescue
the low expression of FOXM1.
In conclusion, it was determined that miR-193a-3p
was downregulated in HER2 positive breast cancer. miR-193a-3p could
affect cell proliferation, migration and invasion of HER2 positive
breast cancer through affecting different targets. These results
reveal the critical role of miR-193a-3p in the progress of HER2
positive breast cancer and implicate its potential application in
therapy.
Supplementary Materials
Funding
The present study was supported by the Yunnan
Scientific and Technology Committee and Kunming Medical University
[Kunming, China; grant no. 2017FE468(-074)].
Availability of data and materials
All data generated or analyzed during this study are
included in this manuscript.
Authors' contributions
YT and MW performed the experiments of the study and
were responsible for data acquisition. DL conceived and designed
the study. YL and SY were responsible for data analysis. YZ and QZ
were responsible for statistical analysis. YT and MW were involved
in drafting the manuscript. DL revised it critically for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All of the human tissues used in the present study
were obtained with written informed consent. This study was
approved by the Ethics Committee of The Third Affiliated Hospital
of Kunming Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spizzo G, Obrist P, Ensinger C, Theurl I,
Dünser M, Ramoni A, Gunsilius E, Eibl G, Mikuz G and Gastl G:
Prognostic significance of Ep-CAM AND Her-2/neu overexpression in
invasive breast cancer. Int J Cancer. 98:883–888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walker RA: Immunohistochemical markers as
predictive tools for breast cancer. J Clin Pathol. 61:689–696.
2008. View Article : Google Scholar
|
|
4
|
Dunnwald LK, Rossing MA and Li CI: Hormone
receptor status, tumor characteristics, and prognosis: A
prospective cohort of breast cancer patients. Breast Cancer Res.
9:R62007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iqbal N and Iqbal N: Human epidermal
growth factor receptor 2 (HER2) in cancers: Overexpression and
therapeutic implications. Mol Biol Int. 2014:8527482014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy AJ, Yankee RA, Brivkalns A and Fitch
M: Viability of granulocytes obtained by filtration leukapheresis.
Transfusion. 15:539–547. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh R, Gupta S, Pawar SB, Pawar RS,
Gandham SV and Prabhudesai S: Evaluation of ER, PR and HER-2
receptor expression in breast cancer patients presenting to a semi
urban cancer centre in Western India. J Cancer Res Ther. 10:26–28.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Jung WH and Koo JS: Expression of
autophagy-related proteins according to androgen receptor and HER-2
status in estrogen receptor-negative breast cancer. PLoS One.
9:e1056662014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J and Ullrich A:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson SK, Sullivan TR, Davies R and
Ruszkiewicz AR: Her-2/neu gene amplification in esophageal
adenocarcinoma and its influence on survival. Ann Surg Oncol.
18:2010–2017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sahlberg KK, Hongisto V, Edgren H, Mäkelä
R, Hellström K, Due EU, Moen Vollan HK, Sahlberg N, Wolf M,
Børresen- Dale AL, et al: The HER2 amplicon includes several genes
required for the growth and survival of HER2 positive breast cancer
cells. Mol Oncol. 7:392–401. 2013. View Article : Google Scholar
|
|
12
|
Kauraniemi P and Kallioniemi A: Activation
of multiple cancer-associated genes at the ERBB2 amplicon in breast
cancer. Endocr Relat Cancer. 13:39–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valastyan S: Roles of microRNAs and other
non-coding RNAs in breast cancer metastasis. J Mammary Gland Biol
Neoplasia. 17:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang N, Wang X, Huo Q, Sun M, Cai C, Liu
Z, Hu G and Yang Q: MicroRNA-30a suppresses breast tumor growth and
metastasis by targeting metadherin. Oncogene. 33:3119–3128. 2014.
View Article : Google Scholar
|
|
15
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pekow J, Meckel K, Dougherty U, Huang Y,
Chen X, Almoghrabi A, Mustafi R, Ayaloglu-Butun F, Deng Z, Haider
HI, et al: miR-193a-3p is a key tumor suppressor in ulcerative
colitis-associated colon cancer and promotes carcinogenesis through
upregulation of IL17RD. Clin Cancer Res. 23:5281–5291. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou NH, Lo YH, Wang KC, Kang CH, Tsai CY
and Tsai KW: MiR-193a-5p and -3p play a distinct role in gastric
cancer: miR-193a-3p suppresses gastric cancer cell growth by
targeting ETS1 and CCND1. Anticancer Res. 38:3309–3318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bivin WW, Yergiyev O, Bunker ML, Silverman
JF and Krishnamurti U: GRB7 expression and correlation with HER2
amplification in invasive breast carcinoma. Appl Immunohistochem
Mol Morphol. 25:553–558. 2017. View Article : Google Scholar
|
|
19
|
Nadler Y, González AM, Camp RL, Rimm DL,
Kluger HM and Kluger Y: Growth factor receptor-bound protein-7
(Grb7) as a prognostic marker and therapeutic target in breast
cancer. Ann Oncol. 21:466–473. 2010. View Article : Google Scholar :
|
|
20
|
Lesurf R, Griffith OL, Griffith M, Hundal
J, Trani L, Watson MA, Aft R, Ellis MJ, Ota D, Suman VJ, et al:
Genomic characterization of HER2-positive breast cancer and
response to neoadjuvant trastuzumab and chemotherapy-results from
the ACOSOG Z1041 (Alliance) trial. Ann Oncol. 28:1070–1077. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lujambio A, Ropero S, Ballestar E, Fraga
MF, Cerrato C, Setién F, Casado S, Suarez-Gauthier A,
Sanchez-Cespedes M, Git A, et al: Genetic unmasking of an
epigenetically silenced microRNA in human cancer cells. Cancer Res.
67:1424–1429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito Y, Liang G, Egger G, Friedman JM,
Chuang JC, Coetzee GA and Jones PA: Specific activation of
microRNA-127 with down-regulation of the proto-oncogene BCL6 by
chromatin-modifying drugs in human cancer cells. Cancer Cell.
9:435–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, et al:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targetin PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan Q, Hu X, Zhang H, Wang S, Zhang H, You
C, Zhang CY, Liang H, Chen X and Ba Y: MiR-193a-3p is an important
tumour suppressor in lung cancer and directly targets KRAS. Cell
Physiol Biochem. 44:1311–1324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Chan DW, Liu VW, Chiu P and Ngan
HY: Differential functions of growth factor receptor-bound protein
7 (GRB7) and its variant GRB7v in ovarian carcinogenesis. Clin
Cancer Res. 16:2529–2539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mak CS, Yung MM, Hui LM, Leung LL, Liang
R, Chen K, Liu SS, Qin Y, Leung TH, Lee KF, et al: MicroRNA-141
enhances anoikis resistance in metastatic progression of ovarian
cancer through targeting KLF12/Sp1/survivin axis. Mol Cancer.
16:112017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stein D, Wu J, Fuqua SA, Roonprapunt C,
Yajnik V, D'Eustachio P, Moskow JJ, Buchberg AM, Osborne CK and
Margolis B: The SH2 domain protein GRB-7 is co-amplified,
overexpressed and in a tight complex with HER2 in breast cancer.
EMBO J. 13:1331–1340. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamy PJ, Fina F, Bascoul-Mollevi C,
Laberenne AC, Martin PM, Ouafik L and Jacot W: Quantification and
clinical relevance of gene amplification at chromosome 17q12-q21 in
human epidermal growth factor receptor 2-amplified breast cancers.
Breast Cancer Res. 13:R152011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai T and Luoh SW: GRB-7 facilitates
HER-2/Neu-mediated signal transduction and tumor formation.
Carcinogenesis. 29:473–479. 2008. View Article : Google Scholar
|
|
30
|
Meng F, Qian L, Lv L, Ding B, Zhou G,
Cheng X, Niu S and Liang Y: miR-193a-3p regulation of
chemoradiation resistance in oesophageal cancer cells via the PSEN1
gene. Gene. 579:139–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao
X, Chen WS and Li B: LncRNA-UCA1 exerts oncogenic functions in
non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett.
371:99–106. 2016. View Article : Google Scholar
|
|
32
|
Cui X, Chen X, Wang W, Chang A, Yang L,
Liu C, Peng H, Wei Y, Liang W, Li S, et al: Epigenetic silencing of
miR-203 in Kazakh patients with esophageal squamous cell carcinoma
by MassARRAY spectrometry. Epigenetics. 12:698–707. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang G and Weisenberger DJ: DNA
methylation aberrancies as a guide for surveillance and treatment
of human cancers. Epigenetics. 12:416–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen K, Liu MX, Mak CS, Yung MM, Leung TH,
Xu D, Ngu SF, Chan KK, Yang H, Ngan HY and Chan DW:
Methylation-associated silencing of miR-193a-3p promotes ovarian
cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways.
Theranostics. 8:423–436. 2018. View Article : Google Scholar :
|
|
35
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16(Suppl 2):
S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu PY, Li TK, Ding ST, Lai IR and Shen
TL: EGF-induced Grb7 recruits and promotes Ras activity essential
for the tumorigenicity of Sk-Br3 breast cancer cells. J Biol Chem.
285:29279–29285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laoukili J, Stahl M and Medema RH: FoxM1:
At the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.
|
|
38
|
Lüscher-Firzlaff JM, Lilischkis R and
Lüscher B: Regulation of the transcription factor FOXM1c by Cyclin
E/CDK2. FEBS Lett. 580:1716–1722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Major ML, Lepe R and Costa RH: Forkhead
box M1B transcriptional activity requires binding of Cdk-cyclin
complexes for phosphorylation-dependent recruitment of p300/CBP
coactiva-tors. Mol Cell Biol. 24:2649–2661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Francis RE, Myatt SS, Krol J, Hartman J,
Peck B, McGovern UB, Wang J, Guest SK, Filipovic A, Gojis O, et al:
FoxM1 is a downstream target and marker of HER2 overexpression in
breast cancer. Int J Oncol. 35:57–68. 2009.PubMed/NCBI
|