Introduction
Crohn's disease (CD) is a chronic relapsing form of
inflammatory bowel disease, which is typically characterized by
transmural inflammation, lymphangiectasia, and lymphatic and
fibrous tissue hyperplasia (1,2).
It is clinically characterized by segmental inflammatory injury of
the digestive tract, which can involve any part of the digestive
tract, and seriously affects the quality of life of patients
(3). It has been suggested that
dysfunctional regulation of the immune system of gastrointestinal
tract was closely associated with CD (4). It is widely known that the imbalance
of inflammatory mediators is an important mechanism underlying the
pathogenesis of CD (5).
Therefore, immune modulatory drugs have been widely utilized for
the treatment of CD (6); however,
maintaining the efficiency and the reducing severe side effects
should be addressed.
In CD, inflammatory cytokines, including interleukin
(IL)-6, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ),
are produced by infiltrating cells and macrophages, which serve an
important role in colonic tissue destruction (7-9).
In inflammatory cells, the inappropriate activation of nuclear
factor-κB (NF-κB), a key transcription factor, regulates the
expression of the inflammatory mediators, which has been associated
with the occurrence and development of CD (10). In addition, studies have
demonstrated that the mitogen-activated protein kinase (MAPK)
signaling pathway is critical in CD (11). MAPK signaling comprises p38, JNK
and ERK, and regulates important biological processes, such as cell
growth, cells apoptosis and inflammation (12-14). It was reported that the activity
of p38 was notably increased in patients with CD (15). Additionally, the inhibition of
stress-activated MAPKs could improve the clinical condition of
patients (16).
Abelmoschus manihot L. Medic is a traditional
herbal medicine has been used as a neuroprotective drug for
cerebral ischemic reperfusion injury (17). Total flavone of A. manihot
L. Medic (TFA) is the main active ingredient, which has been used
as an anti-inflammatory and myocardial ischemia protective drug
(18-20). It has been demonstrated that TFA
could decrease urinary albumin excretion in early-stage diabetic
nephropathy (21). In addition,
TFA has neuroprotective effects on neuronal damage, including
cerebral ischemia injury (22);
however, the role of TFA in CD and the underlying mechanisms remain
unknown. In the present study, we studied the effects of TFA on a
murine model of TNBS-induced colitis and a cells model induced by
LPS, and its underlying mechanism.
Our study demonstrated that TFA could ameliorate the
inflammatory response in mice with TNBS-induced colitis by
inhibiting the NF-κB and MAPK signaling pathways. Therefore, the
present study proposed that TFA may inhibit the pathogenesis of CD
via the anti-inflammatory properties of TFA. These findings may
provide insight into the function of TFA and its application in the
treatment of CD.
Materials and methods
Drugs
Flowers of A. manihot L. Medic were collected
from Jiangyan district of Jiangsu, by Professor Yu-Gen Chen. The
specimen was stored at the Herbarium of Nanjing University of
Chinese Medicine for future reference and verification. TFA was
extracted from the flowers of A. manihot L. Medic by Nanjing
University of Chinese Medicine. The extraction process of TFA was
as follows: Three extractions with 70% alcohol for 50 min each at
room temperature, and the yield was ~35%. The purity of TFA was
90%. TFA was suspended in 1% carboxymethyl cellulose solution at
different concentrations (125, 250 and 500 mg/kg).
High performance liquid chromatography
(HPLC) analysis of TFA
A total of eight standards (purity >98%) were
purchased from Shanghai Yuanye Bio-Technology Co., Ltd. TFA was
examined using a Waters 2694 series HPLC instrument (Waters
Corporation). The sample was separated on a C18 column
(4.6×250 mm, 5 μm) and the mobile phase gradient contained
acidified water with acetonitrile (solvent A) and phosphoric acid
(solvent B, 0.2%). The gradient program was performed as follows:
0-10 min, 86% B; 10-15 min, 92% B; 15-25 min, 92% B; 25-30 min, 81%
B; 30-65 min, 81% B; 65-70 min, 86% B. Chromatography was performed
at 30°C at a flow rate of 1.0 ml/min and aliquots of 10 μl
were analyzed.
Animals
A total of 60 female BALB/c mice (6-8 weeks old)
with a body weight of 18-22 g were provided by Nanjing Medical
University. All mice were housed in standard animal cages under
specific pathogen-free conditions. The housing conditions were
maintained at 22-23°C, with a 12-h light/dark cycle; mice had ad
libitum access to food and water. In addition, mice were given
1 week to acclimatize to the facility prior to the start of
experimentation. The present study was approved by the
Institutional Ethics Committee of Nanjing University of Chinese
Medicine.
Model establishment
For application of 2,4,6-trinitrobenzene sulfonic
acid (TNBS), colitis was induced via intracolonic administration of
TNBS. Briefly, 150 mg/kg TNBS in 48% ethanol was administered once
every 7 days for a total of four treatments, while the normal group
received sterile saline (n=10). A catheter was inserted into the
colonic cavity for 4 cm, in which the TNBS solution was discharged,
and the animal was held in the Trendelenburg position for 2 min to
ensure contact with the intestinal mucosa.
Treatment
Mice were randomly assigned to six treatment groups
(n=10), including the control (distilled sterile saline only),
TNBS, positive drug salazosulfapyridine (SASP), 125 mg/kg TFA, 250
mg/kg TFA and 500 mg/kg TFA treatment groups. Details of treatment
were presented in Table I; the
drug was intraperitoneally administered. SASP is the first-line
therapy for the induction and maintenance of remission in patients
with ulcerative colitis and those with CD, and is widely used in
China (23). Furthermore, SASP
was used as a positive control in ulcerative colitis and CD
research (24,25). In addition, the weight and common
symptoms of CD, including blood in the stool, abdominal pain and
constipation of mice in every group was analyzed weekly. However,
any mice that were scored 4 for bleeding (26) or had diarrhea were euthanized.
Furthermore, mice were euthanized at day 28; blood samples were
collected from the tail vein and centrifuged at 12,000 × g for 5
min at 4°C to obtain serum. The distal colon was carefully excised
and the colon was weighed and measured length.
 | Table ITreatment in different groups. |
Table I
Treatment in different groups.
| Groups | Treatment |
|---|
| Control | Sterile saline/day
for a total of 28 days |
| TNBS | TNBS enema/7 days
for a total of four treatments + sterile saline/days for a total of
28 days |
| SASP | TNBS enema/7 days
for a total of four treatments + SASP/d for a total of 28 days |
| 125 mg/kg TFA | TNBS enema/7 days
for a total of four treatments + 125 mg/kg TFA/day for a total of
28 days |
| 250 mg/kg TFA | TNBS enema/7 days
for a total of four treatments + 250 mg/kg TFA/day for a total of
28 days |
| 500 mg/kg TFA | TNBS enema/7 day
for a total of four treatments + 500 mg/kg TFA/day for a total of
28 days |
Assessment of disease activity
During the experiment, body weight, stool features,
and fecal occult blood were recorded daily. The disease activity
index (DAI) was calculated by scoring weight loss, stool features
and fecal occult blood based on a previously described scoring
system (Table II).
 | Table IIScoring of disease activity
index. |
Table II
Scoring of disease activity
index.
| Score | Body weight loss
(%) | Stool feature | Fecal occult
blood |
|---|
| 0 | 0 | Normal formed | Negative |
| 1 | 1-5 | | |
| 2 | 5-10 | Loose stool | Positive |
| 3 | 10-20 | | |
| 4 | >20 | Diarrhea | Gross bleeding |
H&E staining
Colonic segments were excised and washed in PBS,
fixed in 4% formaldehyde for 30 min at room temperature, embedded
in paraffin and sectioned (5 μm) and finally stained with
H&E for visual analysis. At least three different sections were
examined for each group using a light microscope to assess the
histopathological changes at ×200 magnification.
Myeloperoxidase (MPO) enzyme activity
assay
Colonic tissues were cut into small pieces and
homogenized on ice with normal saline. The levels of MPO were
determined using commercial assay kits (Alpha Diagnostic
International). Briefly, colon tissues were weighed, cut into fine
pieces, and mixed with 200 μl radioimmunoprecipitation assay
(RIPA) lysate per 20 mg tissue. The samples were homogenized using
a glass homogenizer. Following lysis, the samples were centrifuged
at 10,000 × g for 3 min at 4°C to obtain the supernatant. The
protein concentration was determined using a Bicinchoninic Acid
Protein Assay Kit (Beyotime Institute of Biotechnology).
Subsequently, MPO activity was investigated according to the
manufacturer's protocols of the kit employed. MPO activity of the
supernatants was determined and expressed as units per gram of
total protein (U/g).
Cell culture
The RAW264.7 cell line was purchased from the
Shanghai Cell Bank of Chinese Academy of Sciences. RAW264.7 cells
were cultured in Dulbecco's Modified Eagles medium (Gibco; Thermo
Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) in a humidified 5% CO2
atmosphere at 37°C.
Cell Counting Kit-8 (CCK-8) assay
RAW264.7 cells (1.0×104/well) were seeded
in 96-well plates and incubated for 24 h at 37°C. Then, LPS (Gibco;
Thermo Fisher Scientific, Inc.) in the presence or absence of
various doses of TFA (aforementioned) were added into cells. After
24 h of culture, 10 μl of CCK-8 solution (Dojindo Molecular
Technologies, Inc.) was then added to each well. After incubation
at 37°C in 5% CO2 for 1 h, cell viability was evaluated
with a microplate reader (Bio-Rad Laboratories, Inc.), and the
optical density at 450 nm was measured.
ELISA
Colon tissues were cut and weighed. The samples were
lysed and homogenized using a glass homogenizer. RAW264.7 cells
were treated with LPS in the presence or absence of various doses
of TFA. Following lysis, the samples were centrifuged at 10,000 × g
for 5 min at 4°C to obtain the supernatant. The supernatants of RAW
264.7 cells were collected and centrifuged (10,000 × g, 5 min) at
4°C. The concentration of cytokines in the colonic tissues and cell
supernatant were determined by ELISA for mouse TNF-α (ab208348,
Abcam), IFN-γ (ab100689, Abcam), IL-6 (ab100712, Abcam), IL-1β
(ab100704, Abcam), IL-12 (ab236717, Abcam), IL-17 (ab100702, Abcam)
and IL-10 (ab108870, Abcam) following the manufacturer's
instructions. Briefly, 100 μl of 2-fold diluted Standard, 80
μl of Assay Buffer (included in the kit) and 20 μl
sample was added to the sample well. A total of 50 μl of the
diluted corresponding antibodies were added to each well and
incubated at room temperature for 2 h on a microplate shaker set at
300 rpm. Then, 100 μl of diluted Streptavidin-horseradish
peroxidase was added to each well and incubated at room temperature
for 0.5 h on a microplate shaker set at 300 rpm. Subsequently, 100
μl of Substrate Solution was added to each well and
incubated at room temperature for 10 min on a microplate shaker set
at 300 rpm. Stop Solution (100 μl) was added to each well.
The 96-well microplates were analyzed using a PowerWave X340
microplate reader (BioTek China).
Western blotting
The total protein from the cell supernatant was
extracted using RIPA lysis buffer. Protein was extracted and
concentration was measured with a Bicinchoninic acid Protein Assay
kit (Beyotime Institute of Biotechnology, Haimen, China). Equal
amounts of protein (30 μg) from each sample was separated by
12% SDS-PAGE and then transferred to a polyvinylidene fluoride
membrane (EMD Millipore). Subsequently, the membrane was blocked in
5% non-fat milk for 2 h at room temperature and incubated with
primary antibodies overnight at 4°C, including phosphorylated
(p)-ERK1/2 (cat. no. 1150, 1:500; Cell Signaling Technology, Inc.),
ERK1/2 (cat. no. 9103, 1:1,000; Cell Signaling Technology, Inc.),
p-JNK (cat. no. 9250, 1:500; Cell Signaling Technology, Inc.), JNK
(cat. no. 9252, 1:500; Cell Signaling Technology, Inc.), p-p38
(cat. no. 7946S, 1:1,000; Cell Signaling Technology, Inc.), p38
(cat. no. 6279S, 1:1,000; Cell Signaling Technology, Inc.), p-IκBα
kinase (IKK)α/β/γ (cat. no. 1023, 1:500; Cell Signaling Technology,
Inc.), IκBα (cat. no. 1146S, 1:1,000; Cell Signaling Technology,
Inc.), p65 (cat. no. 8242, 1:1,000; Cell Signaling Technology,
Inc.), p-p65 (cat. no. 3033, 1:1,000; Cell Signaling Technology,
Inc.), p52 (cat. no. 4882, 1:1,000; Cell Signaling Technology,
Inc.) and p100 (cat. no. 3017, 1:1,000; Cell Signaling Technology,
Inc.). Then, the membrane was probed with a horseradish
peroxidase-conjugated secondary antibody (cat. no. 7076; 1:5,000;
Cell Signaling Technology, Inc.) for 1 h at room temperature. The
protein bands were visu-alized using ECL detection reagent (EMD
Millipore) and the results were measured using ImageJ software 1.48
(National Institutes of Health).
Statistical analysis
GraphPad Prism 5.0 software (GraphPad Software,
Inc.) was performed to analyze all data. The data were presented as
the mean ± standard deviation. One-way analysis of variance was
applied to compare difference between multiple groups followed by a
Tukey's post-hoc test. The differences between two groups were
statistically analyzed using a Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
TFA ameliorates weight loss and colon
length
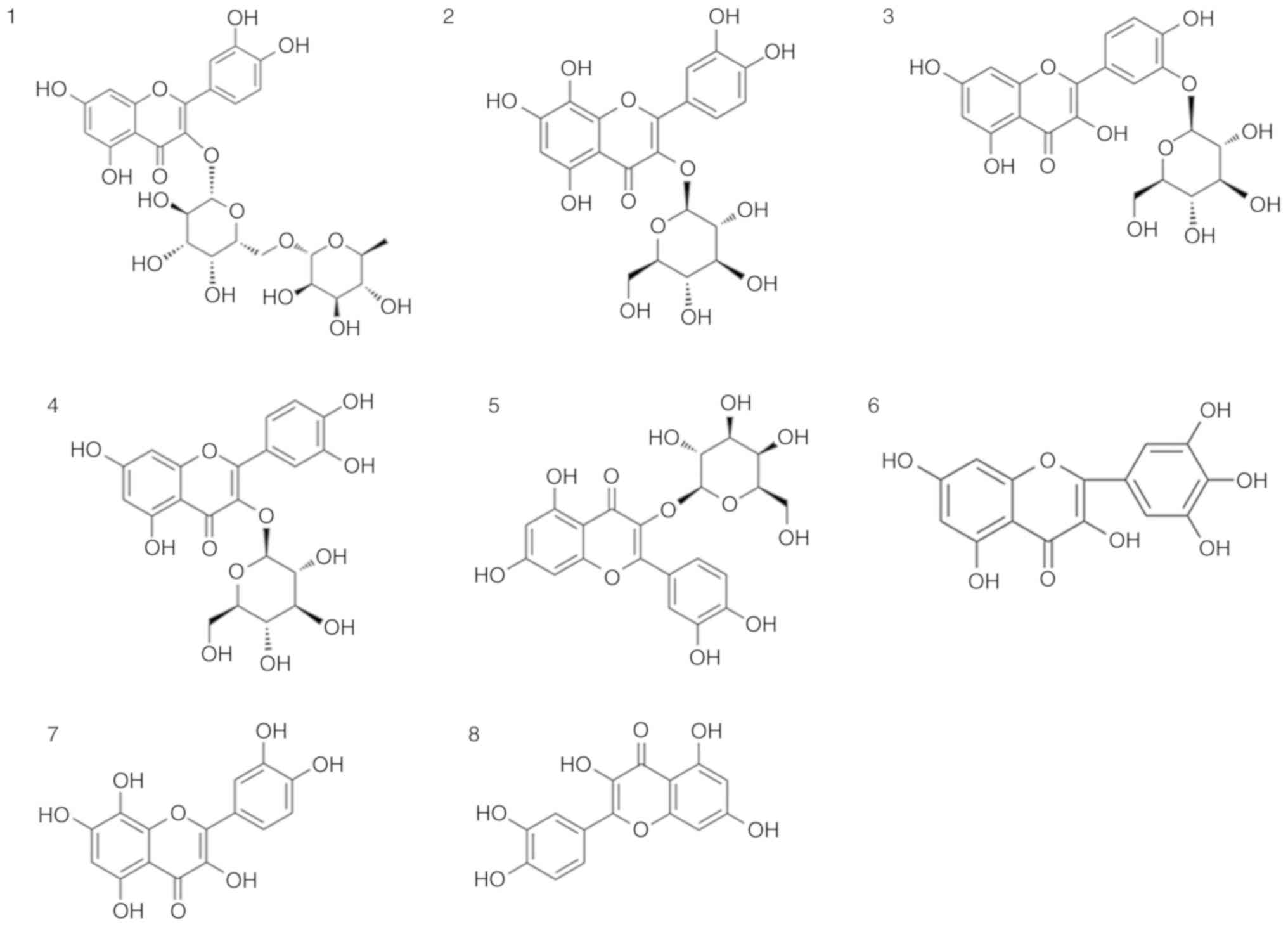
TFA mainly comprises eight flavone glycosides, which
were characterized by HPLC, including quercetin-3-O-robinobioside,
gossypetin-3-O-glucoside, quercetin-3′-O-glucoside,
isoquer-cetin, hyperoside, myricetin, gossypetin and quercetin
(Figs. 1 and 2).
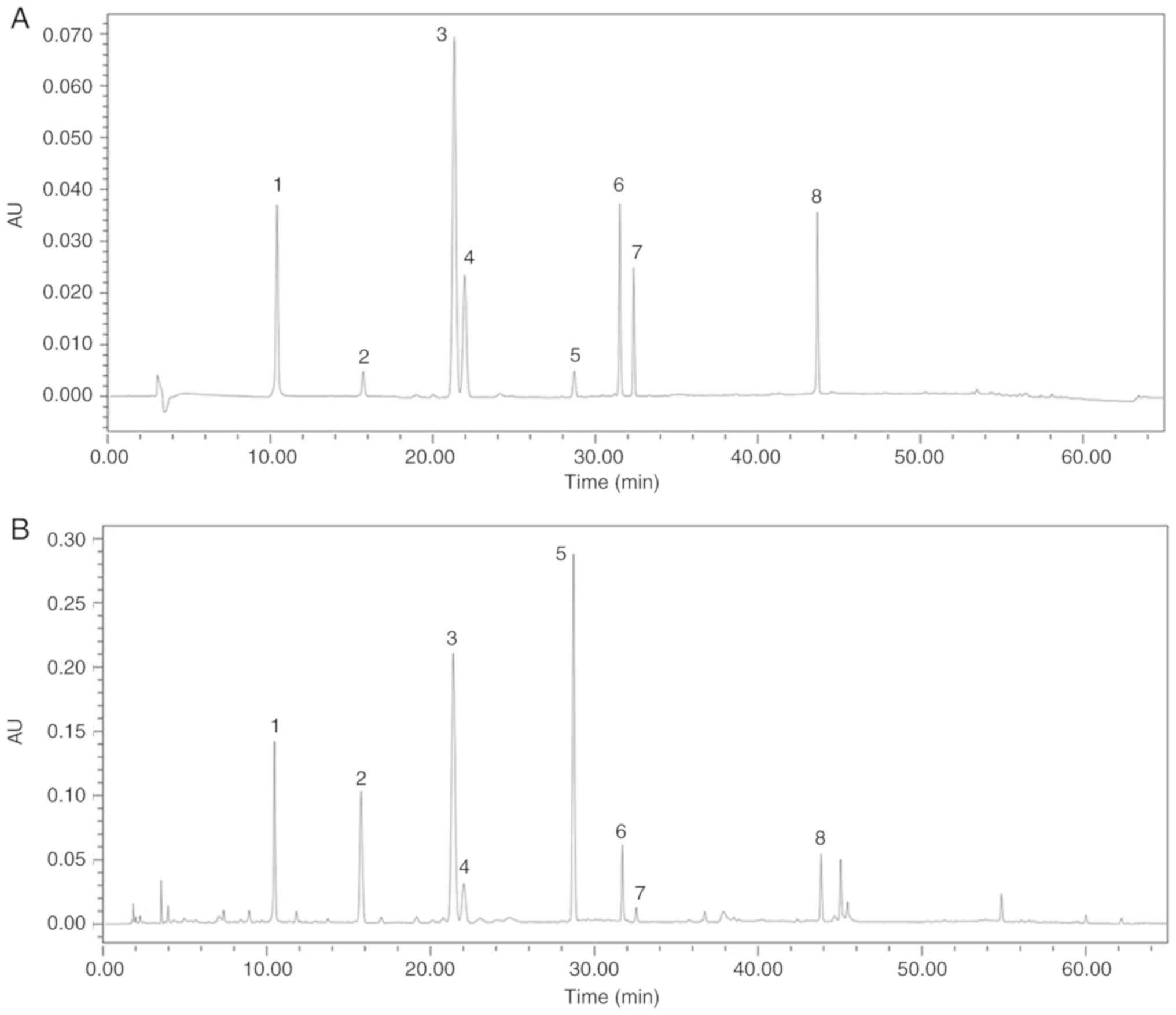 | Figure 1Chromatographic analyses of standards
materials to TFA by high-pressure liquid chromatography. (A) HPLC
chromatograms of standards (1, quercetin-3-O-robinobioside, 2,
gossypetin-3-O-glucoside, 3, quercetin-3′-O-glucoside, 4,
isoquercetin, 5, hyperoside, 6, myricetin, 7, gossypetin and 8,
quercetin) and (B) TFA. TFA, total flavone of Abelmoschus
manihot L. Medic. |
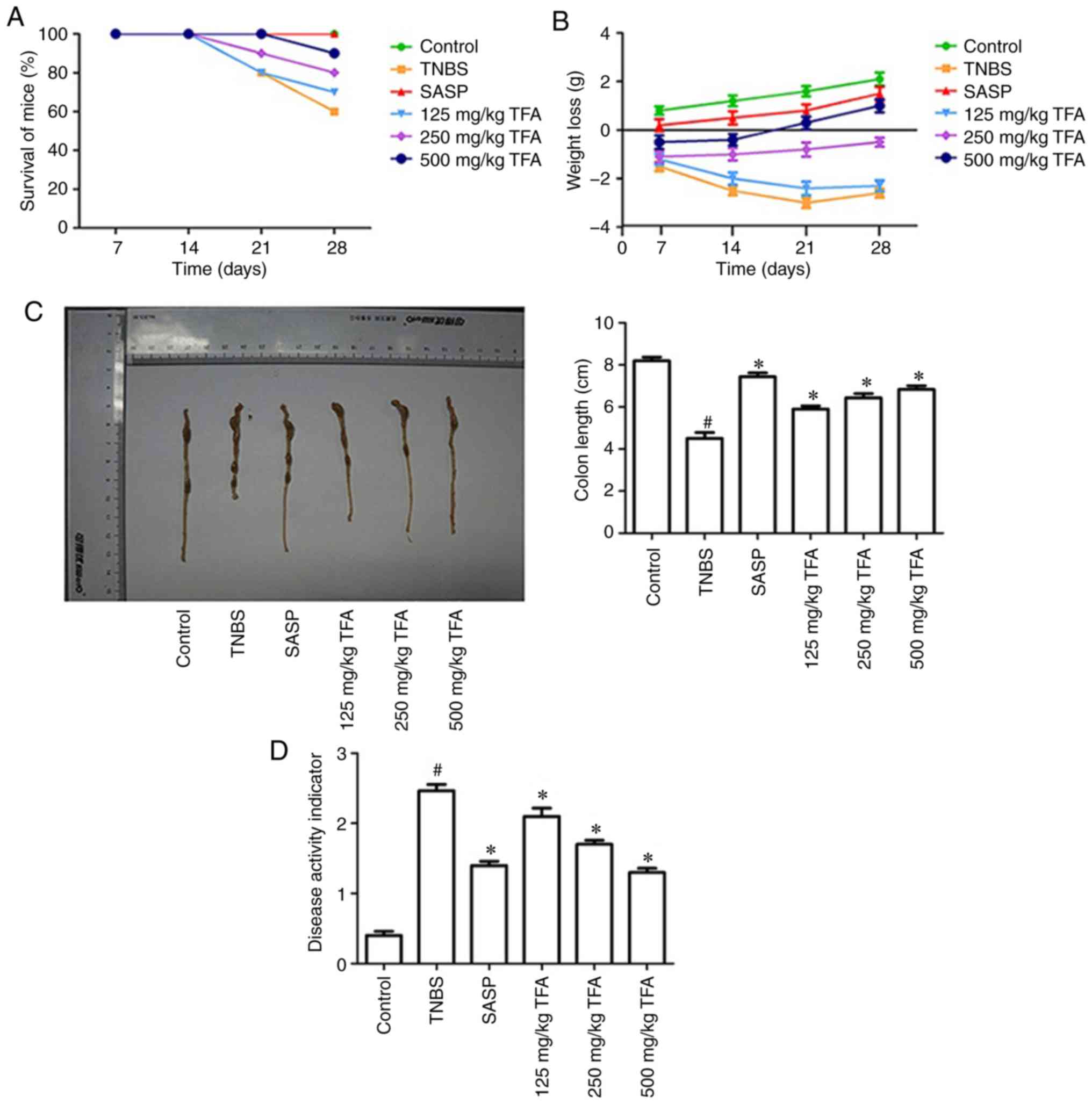
Initially, to determine whether TFA exhibits
protective effects against colitis, the survival of mice with
TNBS-induced colitis were investigated. The results demonstrated
that TNBS significantly promoted mouse mortality, while the mice of
the positive drug SASP or TFA groups notably promoted survival in
TNBS-induced colitis (Fig. 3A).
Subsequently, the effects of TFA on the body weight of mice were
analyzed. The results demonstrated that TNBS notably promoted body
weight loss, whereas treatment with TFA or SASP notably decreased
this loss (Fig. 3B). Colon
shortening is an indirect marker of inflammation (27). The result of the present study
revealed that the colon length in the TNBS-induced colitis group
significantly decreased compared with the control group, while
treatment with TFA or SASP increased colon length (Fig. 3C). In addition, the DAI score, an
indicator of the severity of colitis, is based on the results
including weight loss, stool features and fecal occult blood
(28). As presented in Fig. 3D, the DAI score for the TNBS group
was significantly increased compared with the control group, while
the DAI scores following treatment with TFA or SASP were
significantly reduced compared with the TNBS group. In particular,
there was no significant difference between the effects of 500
mg/kg TFA and the positive drug SASP.
TFA improves histopathological
abnormalities
The histological characteristics of the colon
samples were evaluated by histopathological staining. The results
indicated that mice maintained an integrated normal colonic
structure in the control group, but mice in the TNBS-induced
colitis group exhibited marked infiltration of inflammatory cells,
loss of crypts, destruction of the mucosal layer and edema. In
contrast, TNBS-induced colitis in mice pre-treated with TFA or SASP
exhibited mild inflammation (Fig.
4A).
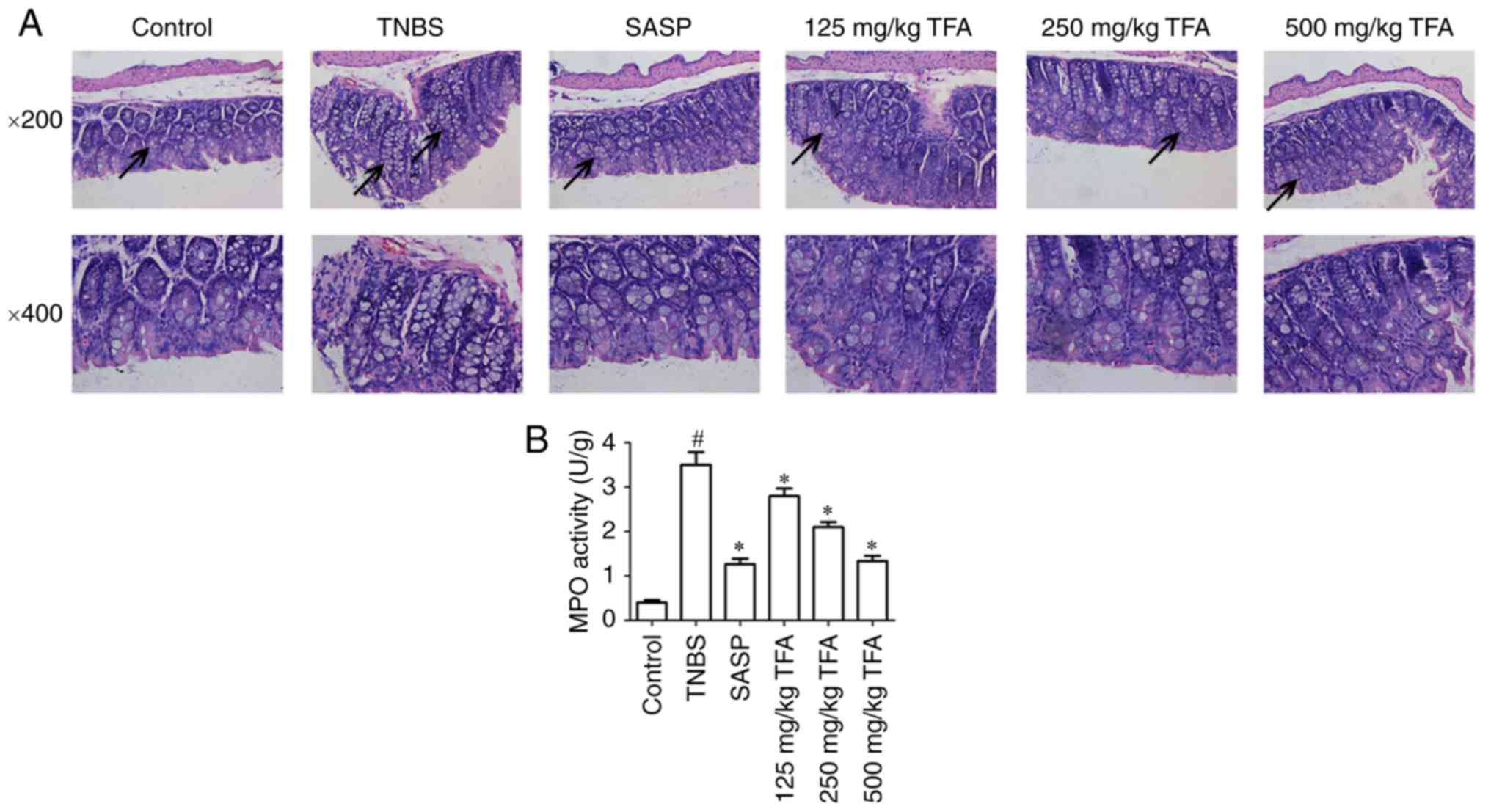 | Figure 4TFA improves histopathological
alterations in TNBS-induced colitis. (A) Paraffin embedded colon
sections were stained with hematoxylin and eosin the assessment of
epithelial damage of colitis mice. Images (magnification, ×200 and
400) of the colon of mice in different groups were collected. The
colons of mice in the control group exhibited a normal structure
without damage; however, in the TNBS model group, the colon
exhibited glandular defects, mucosal ulcerations and inflammatory
cell infiltration, but these alterations were attenuated to varying
degrees by treatment with 125, 250 and 500 mg/kg TFA or SASP.
Arrows indicate the aforementioned features observed in each group.
(B) The levels of MPO activity in colon tissues were evaluated.
#P<0.05 vs. control group, *P<0.05 vs.
TNBS group. Each experiment was performed in triplicate. TFA, total
flavone of Abelmoschus manihot L. Medic; MPO,
myeloperoxidase; SASP, salazosulfapyridine; TNBS,
2,4,6-trinitrobenzene sulfonic acid. |
TFA inhibits MPO activity in colon
tissues
MPO is an enzyme expressed by neutrophils and its
activity is linearly associated with the infiltration of
neutrophils in inflammatory tissues (29). In the present study, the activity
of MPO in the TNBS-induced colitis group was significantly
increased in colon tissues compared with the control group;
however, treatment with TFA or SASP led to a significant inhibition
of MPO activity in colon tissues; notably similar effects on MPO
activity were observed with 500 mg/kg TFA and SASP (Fig. 4B).
TFA suppresses the production of
inflammatory cytokines in mice with TNBS-induced colitis
In order to investigate the protective effects of
TFA in mice with TNBS-induced colitis, sera and colon tissues were
collected. The results demonstrated that TNBS significantly
elevated the production of cytokines, including TNF-α, IFN-γ, IL-6,
IL-1β, IL-12 and IL-17, in the sera and colon tissues compared with
the control group. This was consistent with a previous report in
which the levels of inflammatory cytokines, such as IFN-γ, IL-1,
IL-6 and TNF-α were increased in the colon tissues of patients with
CD (30). However, the
administration of TFA or SASP in mice with TNBS-induced colitis led
to a significant decrease in the production of TNF-α, IFN-γ, IL-6,
IL-1β, IL-12 and IL-17 in the sera and colon tissues compared with
TNBS treatment (Fig. 5). These
results indicated that TFA may serve a role in the modulation of
cytokine production under conditions of colonic inflammation.
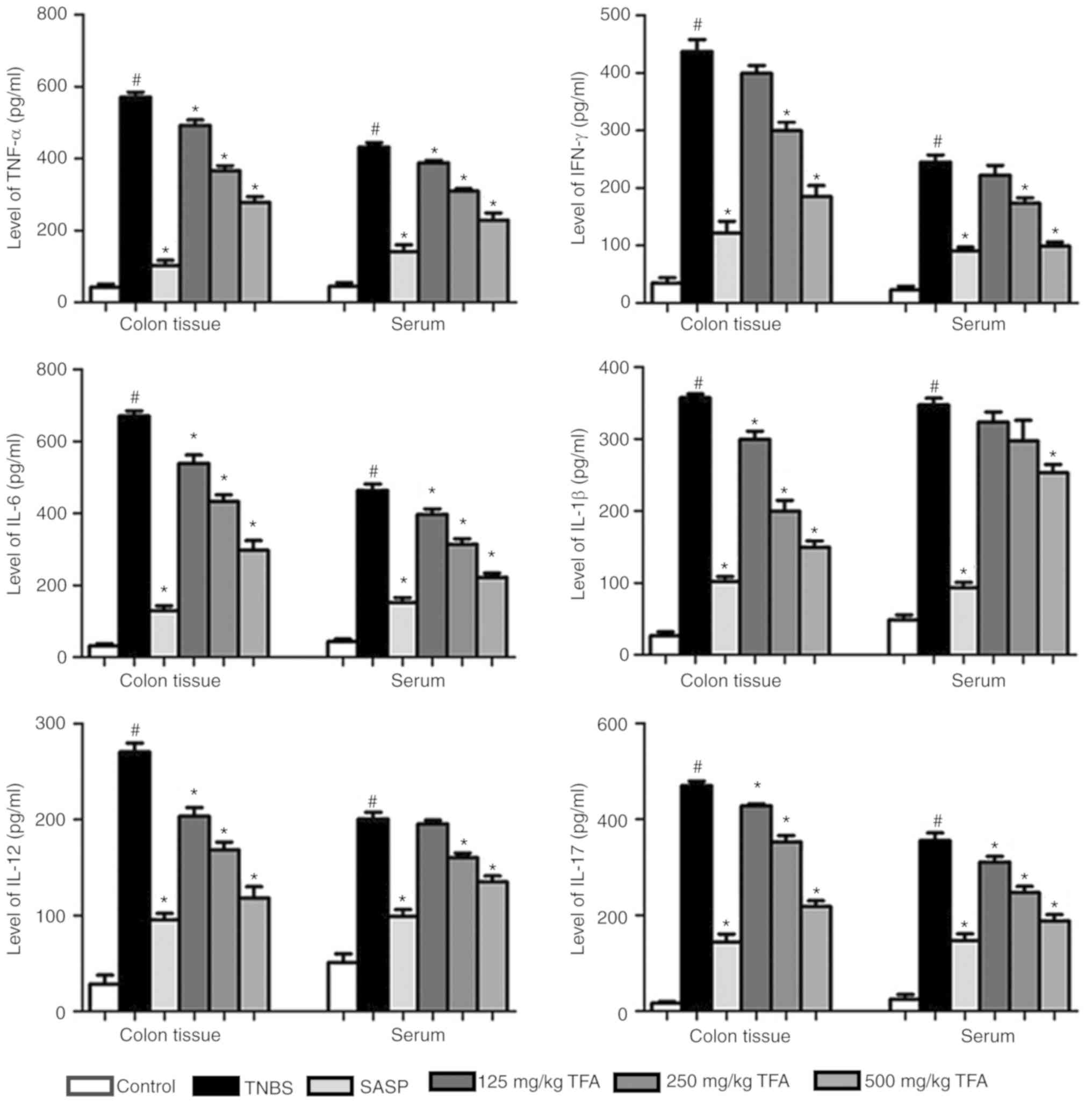 | Figure 5TFA suppresses the production of
inflammation cytokines in TNBS-induced colitis. ELISAs were
performed to detect the production of cytokines, including TNF-α,
IFN-γ, IL-6, IL-1β, IL-12 and IL-17, in the sera and colon tissues.
#P<0.05 vs. control group, *P<0.05 vs.
TNBS group. Each experiment was performed in triplicate. IL,
interleukin; SASP, salazosulfapyridine; TFA, total flavone of
Abelmoschus manihot L. Medic; TNF-α, tumor necrosis
factor-α; TNBS, 2,4,6-trinitrobenzene sulfonic acid. |
TFA inhibits the activation of the NF-κB
and MAPK signaling pathways
Activation of the NF-B and MAPK signaling pathways
has been associated with the pathogenesis of CD (31). We evaluated the effects of TFA on
the NF-κB and MAPK signaling pathways in mice with TNBS-induced
colitis. The results of western blotting showed that the expression
levels of IκBα and p100 in colon tissues were significantly
increased in colon tissues in the TNBS-induced colitis group
compared with the control group. Conversely, administration with
TFA significantly inhibited the expression of the aforementioned
reversed this effect, which indicated that TFA could block the
NF-κB signal pathway (Fig. 6A).
Additionally, the associated proteins of the MAPK signaling pathway
were analyzed. The results suggested that the expression levels of
p-ERK1/2, p-JNK and p-p38 were significantly increased in the colon
tissues in the TNBS-induced colitis group, while SASP or TFA
suppressed the expression of these proteins (Fig. 6B). These results indicated that
TFA could inhibit the activation of the NF-κB and MAPK signaling
pathways in TNBS-induced colitis.
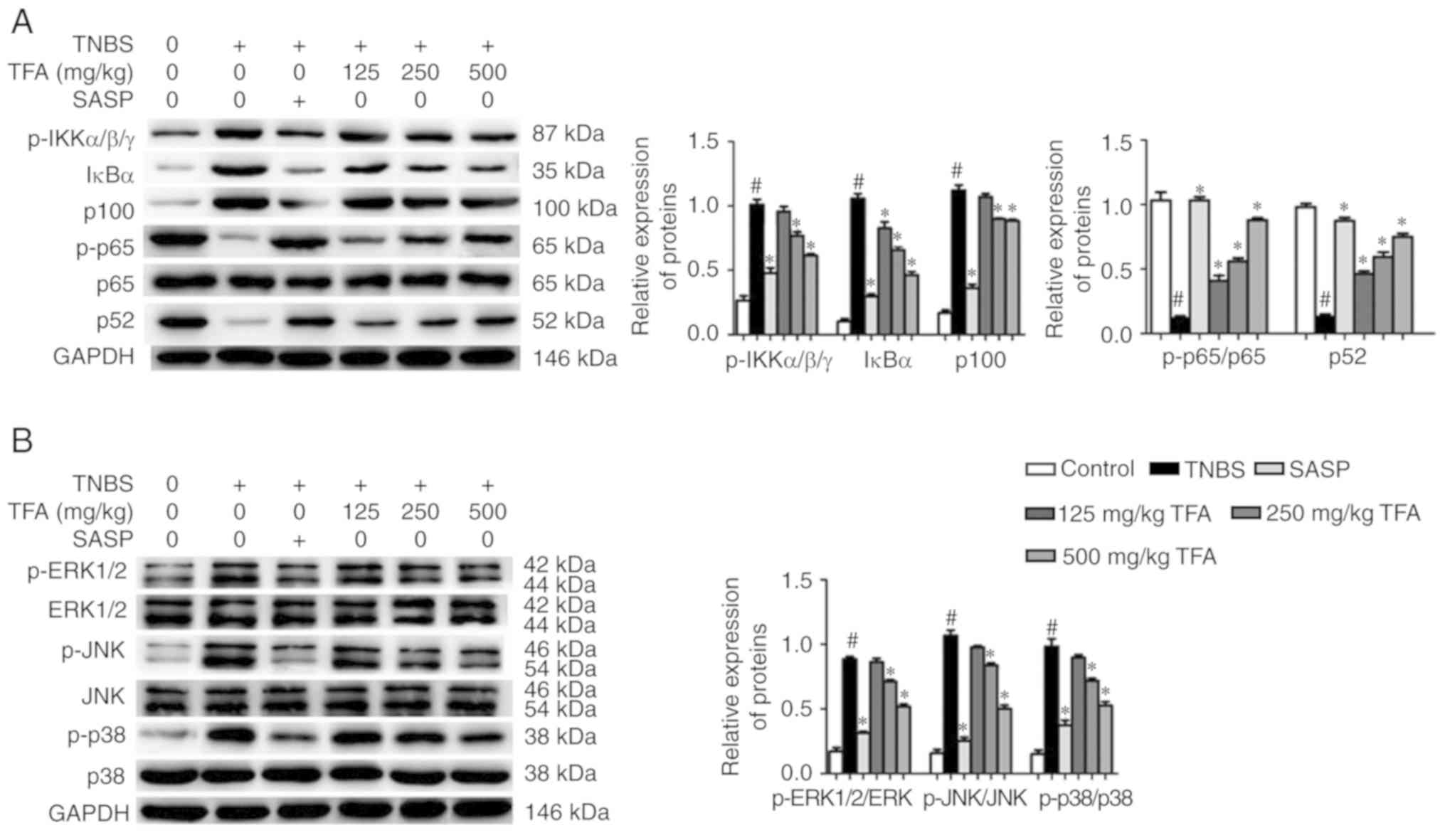 | Figure 6TFA inhibits the activation of the
NF-κB and MAPK signaling pathways in TNBS-induced colitis. (A)
Western blotting was conducted to determine the expression of
related-proteins of the NF-κB signaling pathway in colon tissues.
Quantification of protein expression in each group was presented.
(B) The expression of related-proteins of the MAPK signaling
pathway was evaluated by western blotting. Quantification of
protein expression in each group was presented.
#P<0.05 vs. control group, *P<0.05 vs.
TNBS group. ERK, extracellular signal-regulated kinase; IKK, IκBα
kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated
protein kinase; NF-κB, nuclear factor-κB; p, phosphorylated; SASP,
salazosulfapyridine; TFA, total flavone of Abelmoschus
manihot L. Medic; TNBS, 2,4,6-trinitrobenzene sulfonic
acid. |
Effects of TFA on RAW264.7 cell
cytotoxicity
The cytotoxicity of TFA was evaluated using a CCK-8
assay. RAW264.7 cells were incubated with TFA of various
concentrations (0, 5, 10, 25, 50, 75, 100, 150 and 200
μg/ml). The results reveled no significant changes in cell
viability, indicating that TFA was not cytotoxic at ≤200
μg/ml (Fig. 7A).
Therefore, 50, 100 and 200 μg/ml were selected for
subsequent in vitro analyses.
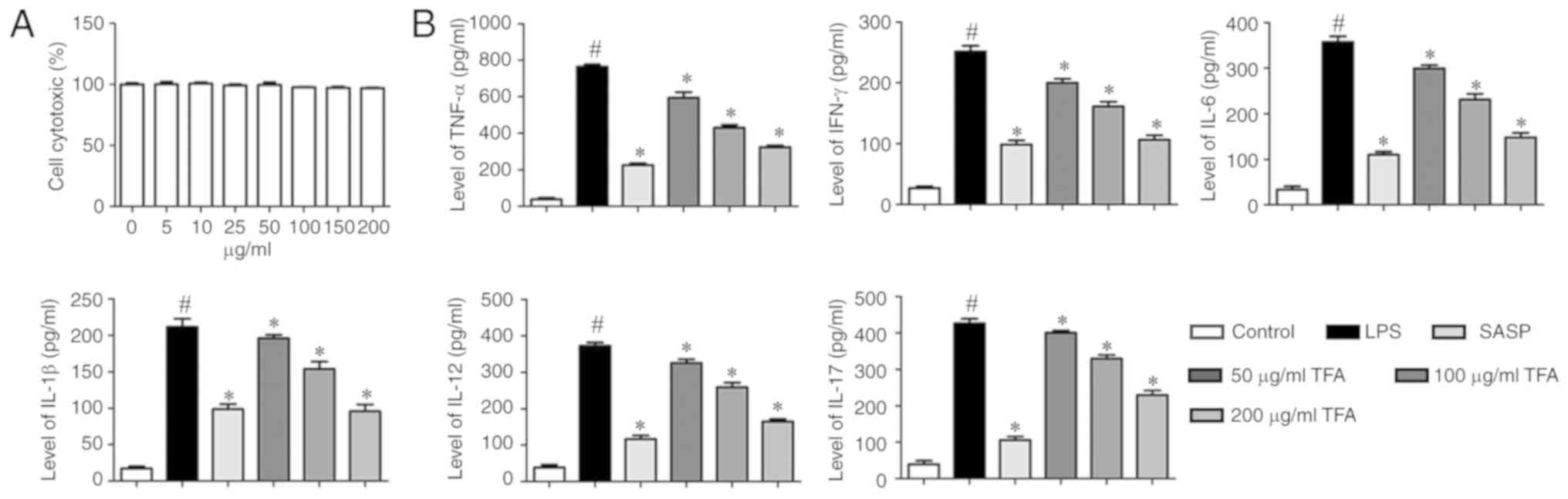 | Figure 7TFA decreases the production of
inflammatory cytokines in LPS-induced macrophage RAW264.7 cells.
(A) RAW264.7 cells were incubated with TFA of various
concentrations (0, 5, 10, 25, 50, 75, 100, 150 and 200
μg/ml). Then, cell viability was evaluated by a Cell
Counting Kit-8 assay. (B) RAW 264.7 cells were stimulated with LPS
(1 μg/ml) for 24 h in the presence or absence various TFA
concentrations or SASP. The supernatants were harvested and
analyzed by ELISA. The levels of inflammatory factors were
presented. #P<0.05 vs. control group,
*P<0.05 vs. TNBS group. Each experiment was performed
in triplicate. IL, interleukin; LPS, lipopolysaccharide; SASP,
salazosulfapyridine; TFA, total flavone of Abelmoschus
manihot L. Medic; TNBS, 2,4,6-trinitrobenzene sulfonic acid;
TNF-α, tumor necrosis factor-α. |
TFA decreases the production of
inflammatory cytokines in LPS-induced RAW264.7 cells
To further study the effects of TFA in vitro,
ELISA was performed to evaluate the production of inflammatory
cytokines in LPS-stimulated RAW264.7 cells. The results
demonstrated that LPS significantly promoted the levels of
inflammatory cytokines in RAW264.7 cells compared with the control,
including TNF-α, IFN-γ, IL-6, IL-1β, IL-12 and IL-17. However, SASP
or TFA treatment significantly suppressed cytokine production
compared with LPS treatment (Fig.
7B).
TFA inhibits the NF-κB and MAPK signaling
pathways in LPS-stimulated RAW264.7 cells
To provide further insight into the mechanisms of
TFA, the activation of the NF-κB and MAPK signaling pathways in
LPS-stimulated RAW264.7 macrophages was evaluated by western
blotting. The results showed that the expression levels of
p-IKKα/β/γ, IκBα and p100 were significantly increased by LPS
treatment, but were downregulated by TFA in a dose-dependent
manner. Additionally, SASP and TFA significantly promoted the
expression of p-p65 and p52 in RAW264.7 macrophages in a
dose-dependent manner, which was reversed by LPS (Fig. 8A). In addition, we investigated
the effects of TFA on the activation of MAPK signaling pathway. The
results indicated that LPS significantly upregulated
phosphorylation of ERK1/2, JNK and p38 compared with the control,
while SASP or TFA decreased the expression of these proteins. Total
protein expression levels of ERK1/2, JNK and p38 were markedly
altered (Fig. 8B).
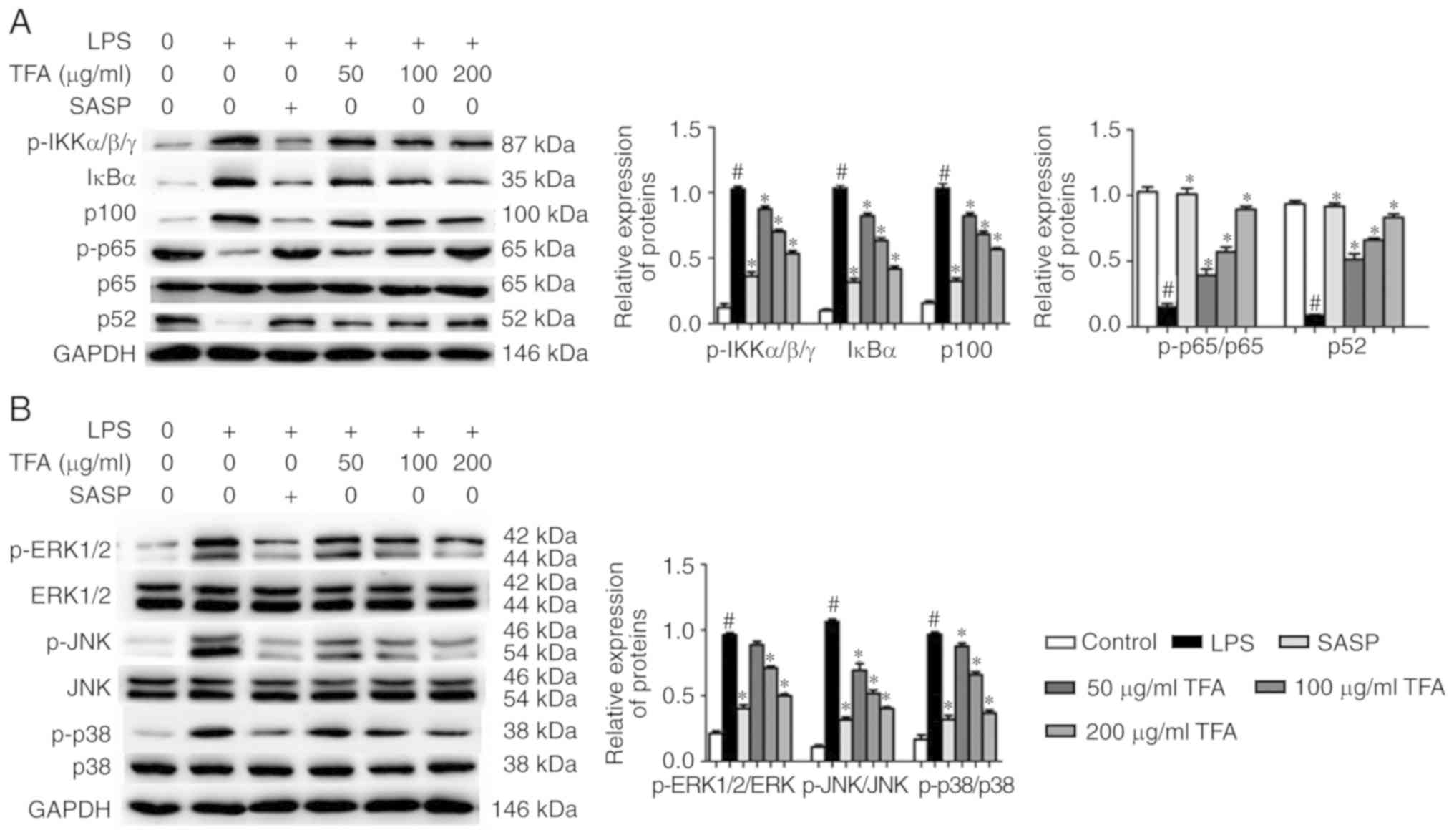 | Figure 8TFA suppresses the NF-κB and MAPK
signaling pathways in LPS-stimulated RAW264.7 cells. (A) Western
blotting was adopted to determine the expression of
related-proteins of the NF-κB signaling pathway in LPS-stimulated
RAW264.7 cells. Quantification of expression levels of proteins in
each group was presented. (B) The expression levels of
related-proteins of the MAPK signaling pathway in LPS-stimulated
RAW264.7 cells were evaluated by western blotting. Quantification
of expression levels of proteins in each group was presented.
#P<0.05 vs. control group, *P<0.05 vs.
TNBS group. ERK, extracellular signal-regulated kinase; IKK, IκBα
kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated
protein kinase; NF-κB, nuclear factor-κB; p, phosphorylated; SASP,
salazosulfapyridine; TFA, total flavone of Abelmoschus
manihot L. Medic; TNBS, 2,4,6-trinitrobenzene sulfonic
acid. |
Discussion
CD is an intestinal inflammatory disease, which can
occur in any region of the gastrointestinal tract, particularly the
terminal ileum and right colon (32). CD and chronic nonspecific
ulcerative colitis are collectively referred to as inflammatory
bowel disease. Its clinical manifestations include abdominal pain,
diarrhea, intestinal obstruction, and other enteral manifestations,
such as fever and malnutrition (33). This disease is also known as
localized enteritis, localized ileocolitis, segmental enteritis and
granulomatous enteritis (34).
The pathological features are granulomatous inflammation, fibrosis
and ulceration, ulcers, paving stone changes and intestinal
stenosis in the digestive tract (34). At present, the treatment of CD is
mainly comprises drugs, including glucocorticoids, salicylic acid
preparations, immunosuppressive agents, antibiotics, methotrexate
and biological agents (35-38). In addition, the long-term use of
western medicine in treating CD was proposed to be unsatisfactory
with high recurrence rates and side effects following treatment
(39).
Traditional Chinese medicine has a history of
thousands of years and has made notable contributions to human
health (40). It has markedly
improved the treatment of intestinal diseases. The intervention of
CD with traditional Chinese medicine has various targets and
mechanisms to inhibit intestinal inflammation, and restores the
intestinal mucosal immune balance (41). This field of research has gained
increasing attention and has become an important research direction
in the treatment of CD. A. manihot L. Medic is a traditional
herbal medicine, which has been used as a neuroprotective drug for
cerebral ischemic reperfusion injury (17). TFA is the main active ingredient,
which has been used as an anti-inflammatory and myocardial
ischemia-protective drug. In the present study, TFA as observed to
ameliorate TNBS-induced colitis weight loss and reductions in colon
length. Additionally, the colons of TNBS-induced in mice
pre-treated with TFA exhibited only mild inflammation.
CD is characterized by T cell activation and
inflammatory cell aggregation in the mucosa (42-44). In the process of occurrence and
development of CD, cytokines can aggravate inflammation through
various mechanisms, resulting in chronic intestinal tissue injury
(45). The present study reported
that the administration of TFA in mice with TNBS-induced colitis
led to a significant decrease in the production of TNF-α, IFN-γ,
IL-6, IL-1β, IL-12 and IL-17 in the sera and colon tissues. In
addition, we found that TFA treatment significantly inhibited the
expression of inflammatory factors in LPS-induced RAW264.7. These
results indicated that TFA may serve a role in the modulation of
cytokine production under conditions of colonic inflammation.
The NF-κB signaling pathway is a predominant pathway
involved in the regulation of immune and inflammatory responses
(46). NF-κB, which is markedly
upregulated in patients with CD, has been identified to serve an
important role in the regulation of mucosal inflammation (47). MAPK signaling, including ERK1/2,
JNKs, and p38 MAPK, can mediate cell growth, differentiation and
death via regulation of the expression of numerous genes (48). A previous study demonstrated that
MAPK signaling could mediate the LPS-stimulated expression of
inflammation mediators (49).
Additionally, inhibition of MAPK signaling pathway could reduce
inflammation (50). In the
present study, we reported that TFA inhibited the activation of the
NF-κB and MAPK signaling pathways.
In summary, TFA notably attenuated colon damage and
inflammation associated with TNBS-colitis. Our findings indicated
the protective effects of TFA on colon health, possibly via
inhibition of macrophages by suppression of the NF-κB and MAPK
signaling pathways. The results of the present study may provide a
basis for the development of novel therapeutic approaches with TFA
in treating patients CD.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81573978). This
study was also supported by the Priority Academic Program
Development of Jiangsu Higher Education Institutions and Jiangsu
Province Special Program of Medical Science (grant no. BL2014100)
and by the Peak Academic Talents plan (grant no. BRA2017536) of the
Jiangsu Province Hospital of Chinese Medicine.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DZ, PZ, YGC made substantial contributions to the
design of the present study. YL, YS, JYZ, FJ, TC and BLY performed
the experiments.. DZ and YGC wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Ethics Committee of Nanjing University of Chinese
Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Manning G, Whyte DB, Martinez R, Hunter T
and Sudarsanam S: The protein kinase complement of the human
genome. Science. 298:1912–1934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matricon J, Barnich N and Ardid D:
Immunopathogenesis of inflammatory bowel disease. Self Nonself.
1:299–309. 2010. View Article : Google Scholar
|
|
4
|
Strober W, Fuss I and Mannon P: The
fundamental basis of inflammatory bowel disease. J Clin Investig.
117:514–521. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cominelli F: Cytokine-based therapies for
Crohn's disease-New paradigms. N Engl J Med. 351:2045–2048. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv QK, Liu JX, Li SN, Gao YJ, Lv Y, Xu ZP,
Huang BX, Xu SY, Yang DX, Zeng YL, et al: Mycophenolate mofetil
modulates differentiation of Th1/Th2 and the secretion of cytokines
in an active crohn's disease mouse model. Int J Mol Sci.
16:26654–26666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orenstein R: Anti-interleukin-12 antibody
for active Crohn's disease. N Engl J Med. 352:627–628. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sartor RB: Mechanisms of disease:
Pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin
Pract Gastroenterol Hepatol. 3:390–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo JH, Zhang CY, Lu CY, Guo GH, Tian YP
and Li YL: Serum expression level of cytokine and chemokine
correlates with progression of human ovarian cancer. Eur J Gynaecol
Oncol. 38:33–39. 2017.PubMed/NCBI
|
|
10
|
Atreya I, Atreya R and Neurath MF:
NF-kappaB in inflammatory bowel disease. J Intern Med. 263:591–596.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mi Y, Zhang D, Jiang W, Weng J, Zhou C,
Huang K, Tang H, Yu Y, Liu X, Cui W, et al: miR-181a-5p promotes
the progression of gastric cancer via RASSF6-mediated MAPK
signalling activation. Cancer Lett. 389:11–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao T, Wen D, Ma B, Hu JQ, Qu N, Shi RL,
Liu L, Guan Q, Li DS and Ji QH: Yes-associated protein 1 promotes
papillary thyroid cancer cell proliferation by activating the
ERK/MAPK signaling pathway. Oncotarget. 8:11719–11728. 2017.
View Article : Google Scholar :
|
|
15
|
Docena G, Rovedatti L, Kruidenier L,
Fanning A, Leakey NAB, Knowles CH, Lee K, Shanahan F, Nally K,
Mclean PG, et al: Down-regulation of p38 mitogen-activated protein
kinase activation and proinflammatory cytokine production by
mitogen-activated protein kinase inhibitors in inflammatory bowel
disease. Clin Exp Immunol. 162:108–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hommes D, van den Blink B, Plasse T,
Bartelsman J, Xu C, Macpherson B, Tytqat G, Peppelenbosch M and Van
Deventer S: Inhibition of stress-activated MAP kinases induces
clinical improvement in moderate to severe Crohn's disease.
Gastroenterology. 122:7–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen JY and Chen ZW: Protective effect of
pharmacological preconditioning of total flavones of Abelmoschl
manihot on cerebral ischemic reperfusion injury in rats. Am J Chin
Med. 35:653–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai X, Liang H, Zhao Y and Wang B:
Simultaneous determination of seven active flavonols in the flowers
of Abelmoschus manihot by HPLC. J Chromatogr Sci. 47:206–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang XR, Zhou ZH, Du AQ and Huang ZM:
Studies on the flavonol constituents of Abelmoschus manihot L.
Medic Chin J Nat Med. 2:91–93. 2004.
|
|
20
|
Fan L, Dong LY, Chen ZW, Cen DY, Jiang Q
and Ma CG: Analgesic effect of total flavone of Abelmoschl manihot
L Medic. Pharmacol Clin Chin Mater Med. 19:12–14. 2003.
|
|
21
|
Zhou L, An XF, Teng SC, Liu JS, Shang WB,
Zhang AH, Yuan YG and Yu JY: Pretreatment with the total flavone
glycosides of flos Abelmoschus manihot and hyperoside prevents
glomerular podo-cyte apoptosis in streptozotocin-induced diabetic
nephropathy. J Med Food. 15:461–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao S, Fan L, Dong LY, Zhao WZ and Chen
ZW: Effect of TFA on cell apoptosis in MCAO rats. Chin Pharmacol
Bull. 19:704–707. 2003.
|
|
23
|
Gu P, Zhu L, Liu Y, Zhang L, Liu J and
Shen H: Protective effects of paeoniflorin on TNBS-induced
ulcerative colitis through inhibiting NF-kappaB pathway and
apoptosis in mice. Int Immunopharmacol. 50:152–160. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu L, Gu P and Shen H: Protective effects
of berberine hydrochloride on DSS-induced ulcerative colitis in
rats. Int Immunopharmacol. 68:242–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu L, Gu P and Shen H: Gallic acid
improved inflammation via NF-κB pathway in TNBS-induced ulcerative
colitis. Int Immunopharmacol. 67:129–137. 2019. View Article : Google Scholar
|
|
26
|
Fu K, Lv X, Li W, Wang Y, Li H, Tian W and
Cao R: Berberine hydrochloride attenuates
lipopolysaccharide-induced endome-tritis in mice by suppressing
activation of NF-κB signal pathway. Int Immunopharmacol.
24:128–132. 2015. View Article : Google Scholar
|
|
27
|
Lee JC, Biasci D, Roberts R, Gearry RB,
Mansfield JC, Ahmad T, Prescott NJ, Satsangi J, Wilson DC, Jostins
L, et al: Genome-wide association study identifies distinct genetic
contributions to prognosis and susceptibility in Crohn's disease.
Nat Genet. 49:262–268. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu F, Guo NJ, Tian H, Marohn M, Gearhart
S, Bayless TM, Brant SR and Kwon JH: Peripheral blood MicroRNAs
distinguish active ulcerative colitis and Crohn's disease. Inflamm
Bowel Dis. 17:241–250. 2011. View Article : Google Scholar
|
|
29
|
Gałecki P, Gałecka E, Maes M, Chamielec M,
Orzechowska A, Bobińska K, Lewiński A and Szemraj J: The expression
of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients
with recurrent depressive disorder. J Affect Disord. 138:360–366.
2012. View Article : Google Scholar
|
|
30
|
Nunberg MY, Werner L, Kopylov U, Haberman
Y, Lahad A, Weiss B and Shouval DS: Impaired IL-10 receptor
mediated suppression in monocyte from patients with Crohn's
disease. J Pediatr Gastroenterol Nutr. 66:779–784. 2018. View Article : Google Scholar
|
|
31
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan T, Guo HY, Zhang H, Liu AP, Wang XX
and Ren FZ: Oral administration of Lactobacillus paracasei
alleviates clinical symptoms of colitis induced by dextran sulphate
sodium salt in BALB/c mice. Benef Microbes. 5:315–322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dou W, Zhang J, Ren G, Ding L, Sun A, Deng
C, Wu X, Wei X, Mani S and Wang Z: Mangiferin attenuates the
symptoms of dextran sulfate sodium-induced colitis in mice via
NF-κB and MAPK signaling inactivation. Int Immunopharmacol.
23:170–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lian L, Huang Q, Zhang L, Qin H, He X, He
X, Ke J, Xie M and Lan P: Anti-fibrogenic potential of mesenchymal
stromal cells in treating fibrosis in Crohn's Disease. Dig Dis Sci.
63:1821–1834. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feagan BG, Rutgeerts P, Sands BE, Hanauer
S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese
S, et al: Vedolizumab as induction and maintenance therapy for
ulcerative colitis. N Engl J Med. 369:699–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Franke A, Balschun T, Karlsen TH,
Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M,
Nothnagel M, Ellinghaus D, et al: Sequence variants in IL10, ARPC2
and multiple other loci contribute to ulcerative colitis
susceptibility. Nat Genet. 40:1319–1323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cohen P, Pagnoux C, Mahr A, Arène JP,
Mouthon L, Le Guern V, André MH, Gayraud M, Jayne D, Blöckmans D,
et al: Churg-Strauss syndrome with poor-prognosis factors: A
prospective multicenter trial comparing glucocorticoids and six or
twelve cyclophosphamide pulses in forty-eight patients. Arthritis
Rheum. 57:686–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lichtenstein GR, Diamond RH, Wagner CL,
Fasanmade AA, Olson AD, Marano CW, Johanns J, Lang Y and Sandborn
WJ: Clinical trial: Benefits and risks of immunomodulators and
maintenance infliximab for IBD-subgroup analyses across four
randomized trials. Aliment Pharmacol Ther. 30. pp. 210–226. 2009,
View Article : Google Scholar
|
|
39
|
Vetter M and Neurath MF: Treatment
perspectives in Crohn's disease. Digestion. 98:135–142. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Geng CA, Yang TH, Huang XY, Yang J, Ma YB,
Li TZ, Zhang XM and Chen JJ: Anti-hepatitis B virus effects of the
traditional Chinese herb Artemisia capillaris and its active
enynes. J Ethnopharmacol. 224:283–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun J, Shen X, Dong J, Wang H, Zuo L, Zhao
J, Zhu W, Li Y, Gong J and Li J: Tripterygium wilfordii Hook F as
maintenance treatment for Crohn's disease. Am J Med Sci.
350:345–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Izutani R, Loh EY, Reinecker HC, Ohno Y,
Fusunyan RD, Lichtenstein GR, Rombeau JL and Macdermott RP:
Increased expression of interleukin-8 mRNA in ulcerative colitis
and Crohn's disease mucosa and epithelial cells. Inflamm Bowel Dis.
1:37–47. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fine SN: Adalimumab for the treatment of
fistulas in patients with Crohn's disease. Inflamm Bowel Dis.
17:667–668. 2011. View Article : Google Scholar
|
|
44
|
Peyrin-Biroulet L, Oussalah A, Williet N,
Pillot C, Bresler L and Bigard MA: Impact of azathioprine and
tumour necrosis factor antagonists on the need for surgery in newly
diagnosed Crohn's disease. Gut. 60:930–936. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bamias G and Cominelli F: Cytokines and
intestinal inflammation. Curr Opin Gastroenterol. 32:437–442. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lawrence T and Fong C: The resolution of
inflammation: Anti-inflammatory roles for NF-kappaB. Int J Biochem
Cell Biol. 42:519–523. 2010. View Article : Google Scholar
|
|
47
|
Han YM, Koh J, Kim JW, Lee C, Koh SJ, Kim
B, Lee KL, Im JP and Kim JS: NF-kappa B activation correlates with
disease phenotype in Crohn's disease. PLoS One. 12:e01820712017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Karin M: Mitogen activated protein kinases
as targets for development of novel anti-inflammatory drugs. Ann
Rheum Dis. 63(Suppl 2): ii62–ii64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signaling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peroval MY, Boyd AC, Young JR and Smith
AL: A critical role for MAPK signaling pathways in the
transcriptional regulation of toll like receptors. PLoS One.
8:e512432013. View Article : Google Scholar
|