Introduction
Idiopathic pulmonary fibrosis (IPF) is a prevalent
and progressive fatal fibrotic lung disease with few available
effective therapies (1-4). It mainly occurs in elderly adults
with a median survival time of 2-3 years (5,6),
and the etiology of IPF remains unclear. A prevailing hypothesis
for IPF pathogenesis is that abnormal wound healing in response to
ongoing alveolar epithelial microinjuries causes fibroblast
activation and excess extracellular matrix deposition, ultimately
resulting in lung damage (7-9).
Alveolar epithelial cells (AECs) serve a crucial
role in IPF pathogenesis (10).
Persistent microinjuries to AECs are thought to be a trigger of
lung fibrosis. The origins of lung injury are often varied and
complex. Exposure to smoke, various types of dust, gastroesophageal
reflux and viral infection can induce AEC injury (11-15). Rebuilding AECs is a key component
of normal wound healing following injury. This requires a carefully
programmed response, including the proliferation and migration of
type II AECs. However, type II AECs isolated from the lungs of
patients with IPF are aberrantly activated with increased collagen,
α-smooth muscle actin (α-SMA) and fibronectin, and decreased
expression of E-cadherin (16).
The role of epithelial-mesenchymal transition (EMT) in lung
fibrosis remains controversial (17,18). Furthermore, AEC senescence,
endoplasmic reticulum (ER) stress, fibroblast resistance to
apoptosis, insufficient autophagy, ubiquitination dysfuction,
abnormal macrophage activation, gene mutation and epigenetic
changes are involved in IPF development (19-25).
The Na,K-ATPase β1 subunit has been reported to be
involved in organ fibrosis. Rajasekaran et al (26) demonstrated that Na,K-ATPase β1
subunit expression is significantly decreased in renal fibrotic
tissues. The knockdown of this subunit in porcine kidney LLC-PK1
cells induced EMT, as did its downregulation in retinal pigment
epithelial cells (27).
Na,K-ATPase, also known as a sodium pump, transports 3
Na+ and 2 K+ ions in opposite directions
across the cell membrane to maintain osmotic equilibrium. This
protein pump is composed of 3 subunits, α, β and γ. The functional
α subunit has 4 isoforms (α1, α2, α3 and α4), whereas the β (β1, β2
and β3) and γ (isoforms 1-7) subunits are regulatory (28). Additional functions of Na,K-ATPase
have been identified in the regulation of cell proliferation, cell
motility, and apoptosis (29,30).
In the present study, the expression of Na,K-ATPase
β1 subunit was revealed to be decreased in AECs of patients with
IPF and in a bleomycin-induced pulmonary fibrosis mouse model.
Based on this observation, the role of the downregulation of the
Na,K-ATPase β1 subunit in AECs during lung fibrosis was
investigated.
Materials and methods
Tissue samples form patients
Lung tissue samples from 13 patients with IPF and 5
healthy donors (all male; age 51.08±10.03 years) were obtained from
the China-Japan Friendship Hospital (Beijing, China) during
surgical lung biopsy and lung transplantation for inclusion in the
present study. The diagnosis of IPF was based on the 2011 American
Thoracic Society/European Respiratory Society/Japanese Respiratory
Society/Latin American Thoracic Association Guidelines for
Diagnosis and Management (5). All
patients provided signed consent and the study was approved by the
Ethics Committee of the China-Japan Friendship Hospital (approval
no. 2017-25-1).
Animal model
C57BL/6N mice (50 mice; male; 7-8 weeks old; 22-24
g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The animals were maintained
at a controlled temperature of 24±1°C with a 12/12 h light-dark
cycle, and were fed a standard diet. Water was freely available.
The mice were randomly divided into 2 groups: For the model of
pulmonary fibrosis, a dose of 2 mg/kg bleomycin (Nippon Kayaku Co.,
Ltd., Tokyo, Japan) was intratracheally administered, and the
control mice were injected intratracheally with the same volume of
saline. The bleomycin and saline were adminstrated only once. The
mice were euthanized on day 21 with an intraperitoneal injection of
1% pentobarbital sodium (100 mg/kg animal weight). This study was
approved by the Animal Ethics Committee of China-Japan Friendship
Hospital (Beijing, China).
Immunohistochemistry and
immunofluorescence
The preparation of the human and mouse lung
specimens for histology was performed as previously described
(23,31). Briefly, the samples were
dehydrated, paraffin-embedded, and cut into 4-μm sections.
The tissue sections were deparaffinized and rehydrated. Following a
microwave treatment for 20 min in EDTA buffer and subsequent
cooling, the endogenous peroxidase activity was blocked with 0.3%
hydrogen peroxide in methanol for 15 min in the dark. Following
blocking in 5% goat serum (OriGene Technologies, Inc., Beijing,
China) for 20 min, the sections were incubated with antibodies
against the Na,K-ATPase β1 subunit (cat. no. ab193669; 1:600
dilution) and fibronectin (cat. no. ab2413; 1:500 dilution) (both
Abcam, Cambridge, UK) overnight at 4°C as described previously
(32), the samples were observed
using an optical microscope (magnification, ×100) and were analyzed
using Aperio Imagescope version 12.0 software (Leica Microsystems,
Ltd., Milton Keynes, UK).
For immunofluorescence, the mouse lung tissue
sections (4 μm) were de-paraffinized, hydrated using xylene,
100, 95, 85 and 70% ethanol, and PBS solution. The non-specific
binding was blocked with 10% goat serum, and the samples were
incubated overnight at 4°C with the desired primary antibodies
against the Na,K-ATPase β1 subunit (cat. no. ab2873; 1:500
dilution) and prosurfactant protein C (cat. no. ab90716; 1:4,000
dilution) (both Abcam), and then incubated with a specific
fluorescence-conjugated secondary IgG (fluorescein
isothiocyanate-conjugated, cat. no. ZF-0311; rhodamine B
isothiocyanate-conjugated, cat. no. ZF-0313; both 1:100 dilution;
OriGene Technologies, Inc.) for 1 h in a light-protected chamber at
room temperature. Subsequently, the sections were counterstained
with DAPI (cat. no. P0131; Beyotime Institute of Biotechnology,
Haimen, China) at room temperature and immunofluorescence signals
were detected immediately using fluorescence microscopy
(magnification, ×100).
Cell culture and small interfering
(si)RNA transfection
Human lung carcinoma epithelial A549 cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA). A549 is a human AEC line with similar characteristics to type
II AECs. It has been used as a stable AEC line in a number of
studies (33,34). The cells were maintained in
RPMI-1640 medium with 10% fetal bovine serum (both Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and
100 mg/ml streptomycin (both Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA). Transforming growth factor β-1 (TGF-β1; 10 ng/ml;
R&D Systems, Inc., Minneapolis, MN, USA) was added to
subconfluent cultures and the same volume of citric acid was added
to the control cells. The cells were maintained in a humidified
incubator at 37°C in 95% air (21% O2) and 5%
CO2.
The A549 cells were seeded in 6-well plates and
incubated overnight. Na,K-ATPase β1 subunit siRNA (5 μM;
sequence, 5′-AAU GUU CUC ACC GUA CGC Ctt-3′) and negative control
siRNA (5 μM; cat. no. 4390843; Silencer® Select Negative
Control; Thermo Fisher Scientific, Inc.) were separately mixed with
Lipofectamine® 3000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and Opti-MEM medium (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The cells were
incubated for 24 h for the measurement of RNA levels and 48 h for
cell morphology observation under an optical microscope and protein
detection experiments.
RNA purification and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
As previously described (35), total RNA was isolated from the
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. RT was
performed on 1 μg total RNA with oligo(dT) primers in
25-μl reactions using the Omniscript RT kit (Tiangen Biotech
Co., Ltd., Beijing, China) at 37°C for 60 min according to the
manufacturer's instructions. The qPCR was performed on an ABI 7500
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using SYBR-Green PCR reagents (Tiangen Biotech Co., Ltd.). The
thermocycling conditions used were as follows: Initial denaturation
at 94°C for 2 min and 40 cycles of denaturation at 94°C for 15 sec,
annealing at 55°C for 20 sec and extension at 69°C for 35 sec. The
primers used were: β-actin forward, 5′-AGGCCAACCGTGAAAAGATG-3′; and
reverse, 5′-AGAGCATAGCCCTCGTAGATGG-3′; and Na,K-ATPase β1 subunit
forward, 5′-ATGTGCCCAGTGAACCGAAA-3′; and reverse,
5′-TCCAGAGCAATTTCCCAGCC-3′. The relative expression of the target
gene was calculated using the 2−ΔΔCq method (36), normalized to the levels of
β-actin.
Protein extraction and western blot
analysis
Total cell lysates were obtained using
radioimmunoprecipitation assay buffer (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) containing 1:100
phenylmethylsulfonyl fluoride, phosphatase inhibitors and protease
inhibitor. The cell lysates were resuspended in protein loading
buffer containing 5% mercaptoethanol. The protein concentration was
determined using a bicinchoninic acid assay kit. Western blotting
was performed as previously described (23). The denatured proteins (20
μg per lane) were separated by 10% SDS-PAGE using a
Mini-Protein electrophoresis module assembly (both Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 80 mV and transferred to
nitrocellulose membranes (Merck KGaA, Darmstadt, Germany) for
100-120 min using the Mini Trans-Blot electrophoresis transfer cell
(Bio-Rad Laboratories, Inc.) at 300 mA, according to the molecular
weight. The primary antibodies used were anti-human and mouse α-SMA
(cat. no. ab124964; 1:5,000 dilution), fibronectin (cat. no.
ab2413; 1:2,000 dilution), β-actin (cat. no. ab6267; 1:3,000
dilution), Na,K-ATPase β1 subunit (cat. no. ab2873; 1:600 dilution)
(all Abcam), cleaved Notch1 (cat. no. 4147T), extracellular
signal-regulated kinase (ERK)1/2 (cat. no. 9101), phosphorylated
ERK1/2 (cat. no. 8544), and immunoglobulin heavy chain-binding
protein (BiP, cat. no. 3177T) (all 1:1,000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA). The membranes were incubated
with the primary antibodies overnight at 4°C and treated with
IRDyeCW800 (green)- or IRDyeCW800 (red)-conjugated affinity
purified anti-rabbit (cat. no. 925-32211) or anti-mouse (cat. no.
925-32210) IgG (both 1:15,000 dilution; LI-COR Biosciences,
Lincoln, NE, USA). The intensity of the bands was evaluated using a
LI-COR Odyssey infrared double-fluorescence imaging system (LI-COR
Image Studio Software version 4.0; LI-COR Biosciences).
Statistical analysis
Data are expressed as mean ± standard error of the
mean. Two-tailed Student's t-test was performed for the comparison
of mRNA and protein expression levels. The statistical analyses
were performed using the Prism software version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate statistically significant differences.
Results
Na,K-ATPase β1 subunit expression is
downregulated in lung fibrosis
To examine the Na,K-ATPase β1 subunit expression
level in lung fibrosis, immunohistochemistry was performed on lung
tissue sections from patients with IPF. The Na,K-ATPase β1 subunit
was mainly expressed in the cytoplasm of AECs. The staining of this
subunit was visibly diminished in the fibrotic and lesion-adjacent
areas compared with that in the healthy lung tissue (Fig. 1A). Na,K-ATPase β1 subunit
expression was also investigated in tissue from a bleomycin-induced
pulmonary fibrosis mouse model. The results of the western blot and
immunohistochemistry analyses demonstrated that Na,K-ATPase β1
subunit expression was markedly decreased in the lung tissue of the
bleomycin group compared with that of control (Fig. 1B and C).
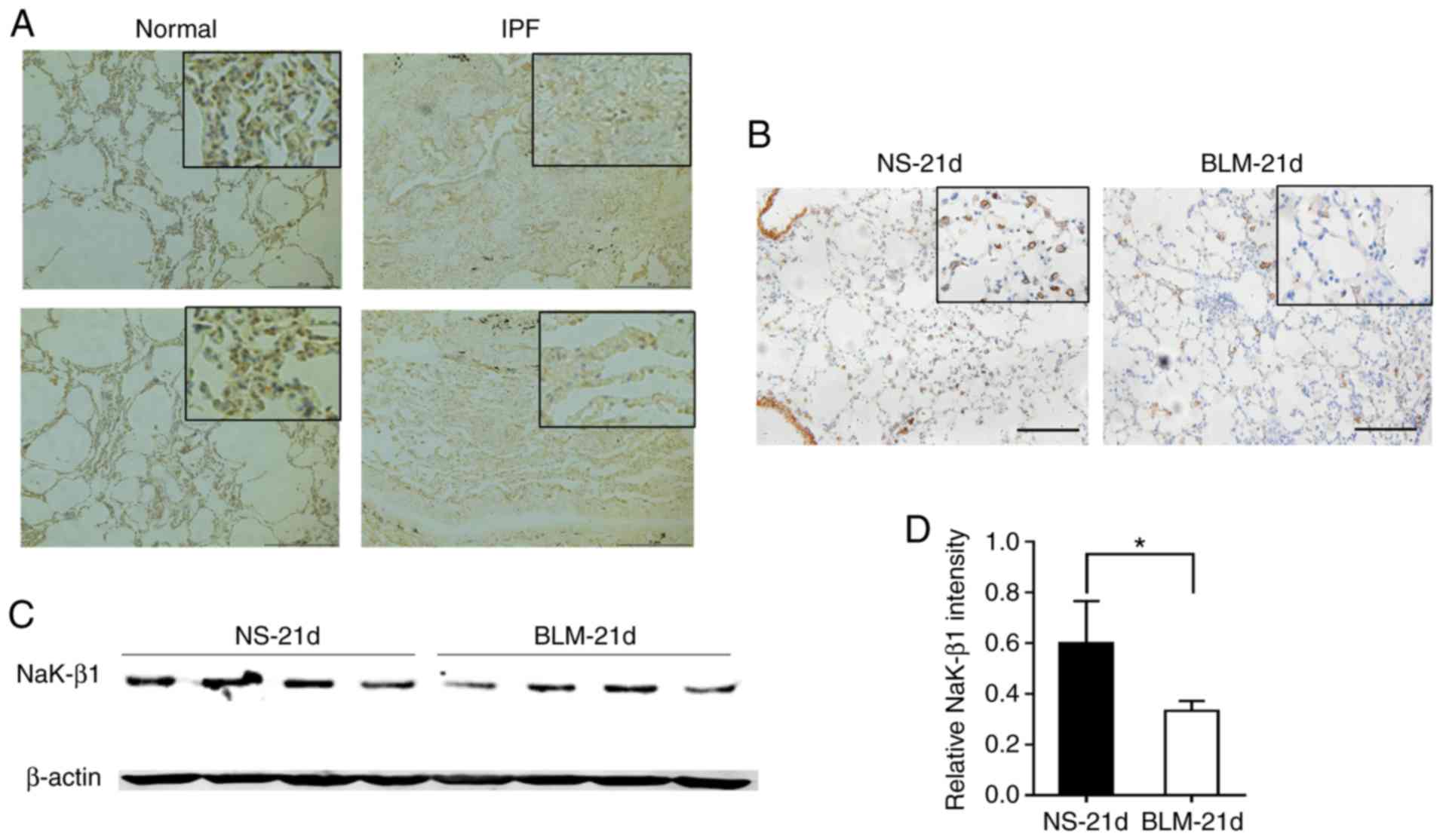 | Figure 1NaK-β1 expression in lung fibrosis.
(A) Representative immunohistochemistry images of NaK-β1 staining
in lung tissue sections from patients with IPF. Original
magnification, ×100; scale bar, 20 μm. (B) Representative
immunohistochemistry of NaK-β1 staining in lung tissue samples from
a bleomycin-induced pulmonary fibrosis mouse model. Original
magnification, 100×; scale bar, 10 μm; upper right panel
scale bar, 1 μm. (C) Western blot analysis of protein
extracted from lung tissue of a BLM-induced pulmonary fibrosis
mouse model. (D) Relative quantification of the western blot band
intensities. Data are expressed as the mean ± standard error of the
mean (n=4 mice per group). *P<0.05. IPF, idiopathic
pulmonary fibrosis; NaK-β1, Na,K-ATPase β1 subunit; NS,
saline-administered control group; BLM, bleomycin. |
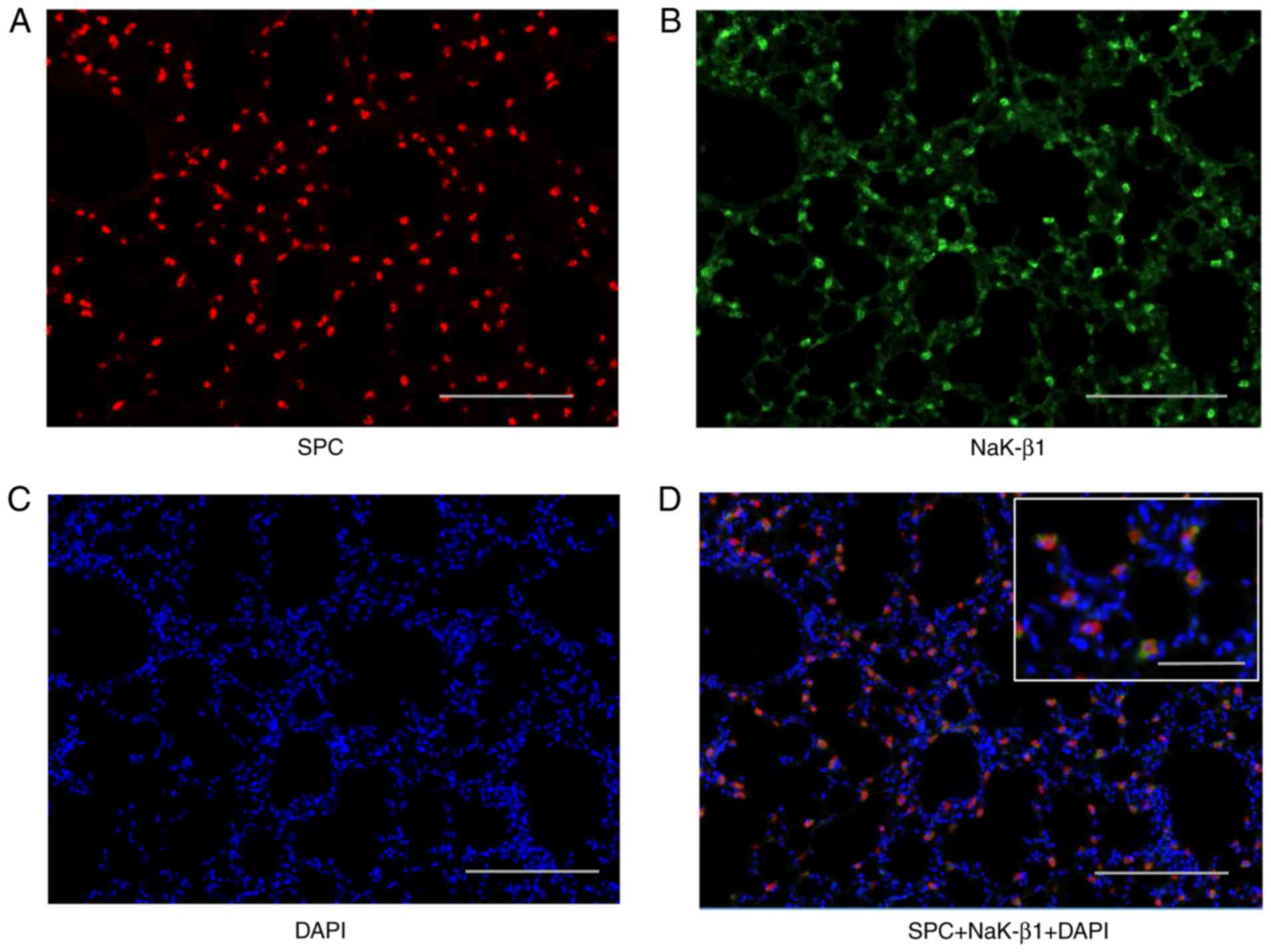
Na,K-ATPase β1 subunit expression in type
II AECs in mouse lung tissue
In contrast to the human lung tissue, the
immunohistochemistry of the mouse lung tissue revealed that not all
AECs express the Na,K-ATPase β1 subunit (Fig. 1B). Therefore, immunofluorescence
was used to identify which type of AECs express Na,K-ATPase β1
subunit in mouse lung. As displayed in Fig. 2, the Na,K-ATPase β1 subunit was
primarily expressed in the cell membrane of type II AECs.
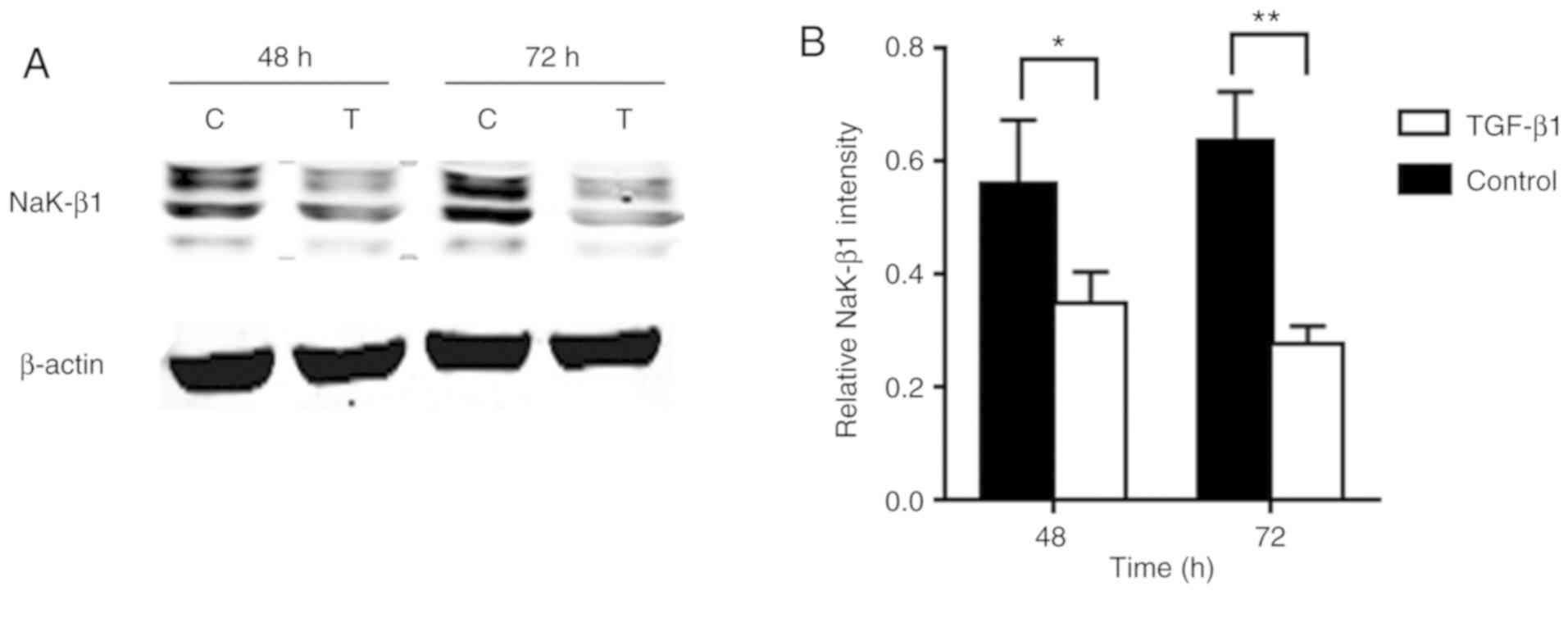
TGF-β1 treatment decreases Na,K-ATPase β1
subunit expression in A549 cells
TGF-β1 is an important profibrotic cytokine. To
assess the level of Na,K-ATPase β1 subunit in TGF-β1-stimulated
AECs, total protein was extracted from the cells following
treatment with 10 ng/ml TGF-β1 for 48 and 72 h. The results
indicated that the treatment led to a significant decrease in the
protein expression of Na,K-ATPase β1 subunit at the two tested time
points (Fig. 3).
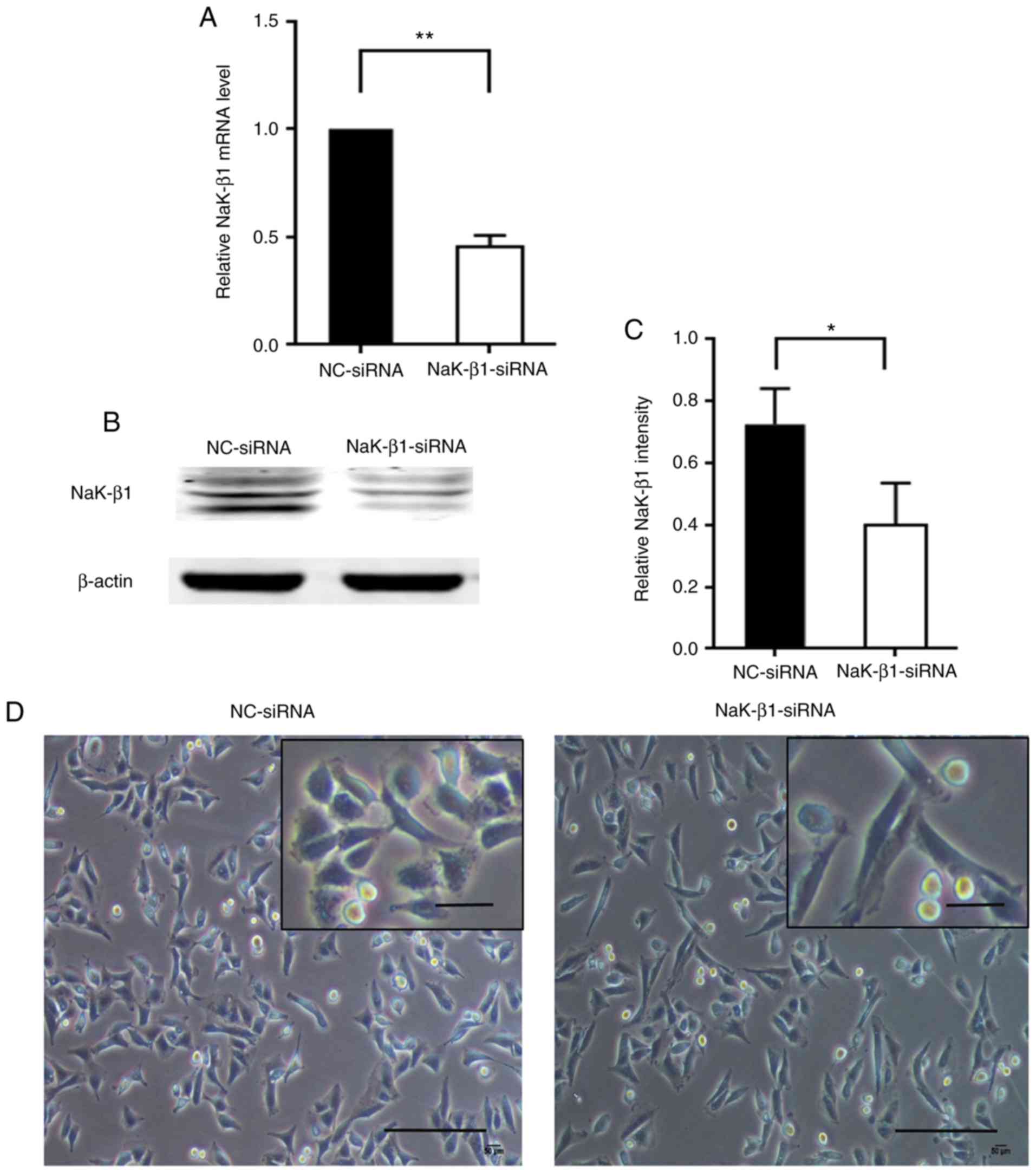
Knockdown of Na,K-ATPase β1 subunit
mediates changes in the cell morphology of A549 cells
Due to the observed significant difference between
the expression of Na,K-ATPase β1 subunit in lung fibrosis and
normal lung samples, the effects of the downregulation of the
Na,K-ATPase β1 subunit on AECs was explored. The Na,K-ATPase β1
subunit expression in A549 cells was knocked down using siRNA
interference. As demonstrated in Fig.
4A, Na,K-ATPase β1 subunit mRNA was significantly decreased 24
h post-transfection, as were the protein expression levels at 48 h
(Fig. 4B and C). In addition, the
knockdown of Na,K-ATPase β1 subunit resulted in an altered spindle
morphology in the A549 cells (Fig.
4D).
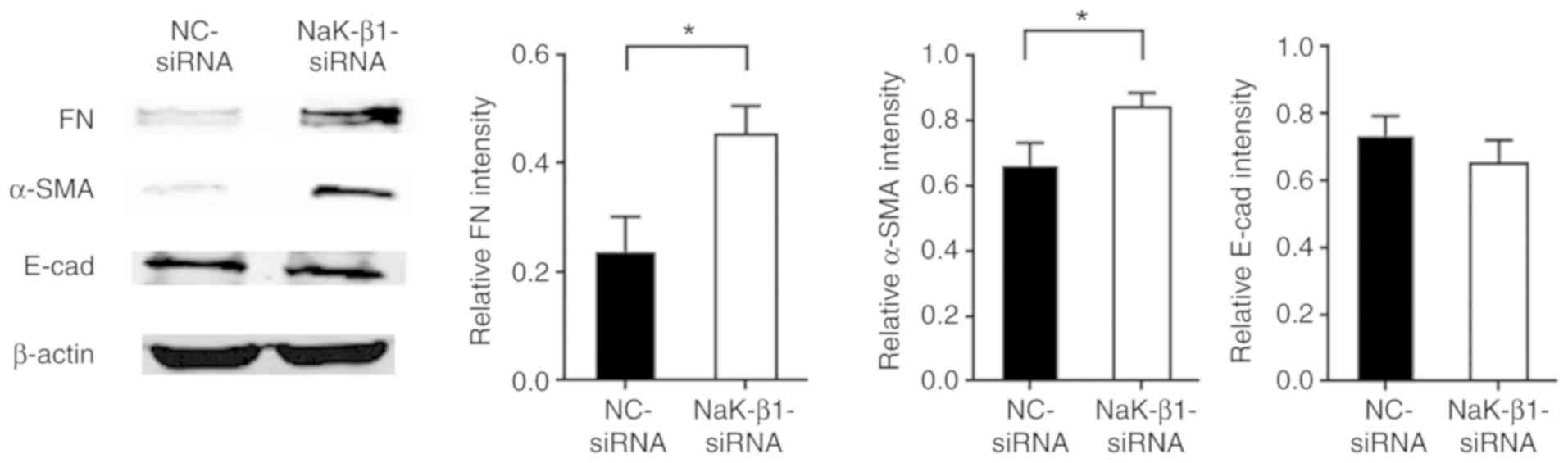
Knockdown of the Na,K-ATPase β1 subunit
promotes the upregulation of profibrotic proteins in A549
cells
To further investigate the role of the Na,K-ATPase
β1 subunit in AECs during lung fibrosis, the expression of
fibrosis-associated proteins fibronectin, α-SMA and E-cadherin was
examined in A549 cells following siRNA silencing of the Na,K-ATPase
β1 subunit. The results revealed that the fibronectin and α-SMA
levels were increased, but E-cadherin expression was not
significantly altered, compared with that in the cells transfected
with NC-siRNA (Fig. 5).
Knockdown of the Na,K-ATPase β1 subunit
activates ERK1/2 and neurogenic locus notch homolog protein 1
(Notch1) signaling and induces ER stress
The downstream signaling pathway of the Na,K-ATPase
β1 subunit was investigated. As observed in Fig. 6A, phosphorylated ERK1/2 and
cleaved Notch1 were significantly upregulated in cells with
Na,K-ATPase β1 subunit silencing, suggesting that a deficiency of
this protein may lead to the activation of the ERK1/2 and Notch1
signaling pathways, contributing to lung fibrosis. In addition, the
knockdown of Na,K-ATPase β1 subunit resulted in a significant
increase in the expression of BiP, an ER-stress protein marker
(Fig. 6B), suggesting that
downregulation of this ion pump causes ER stress in A549 cells.
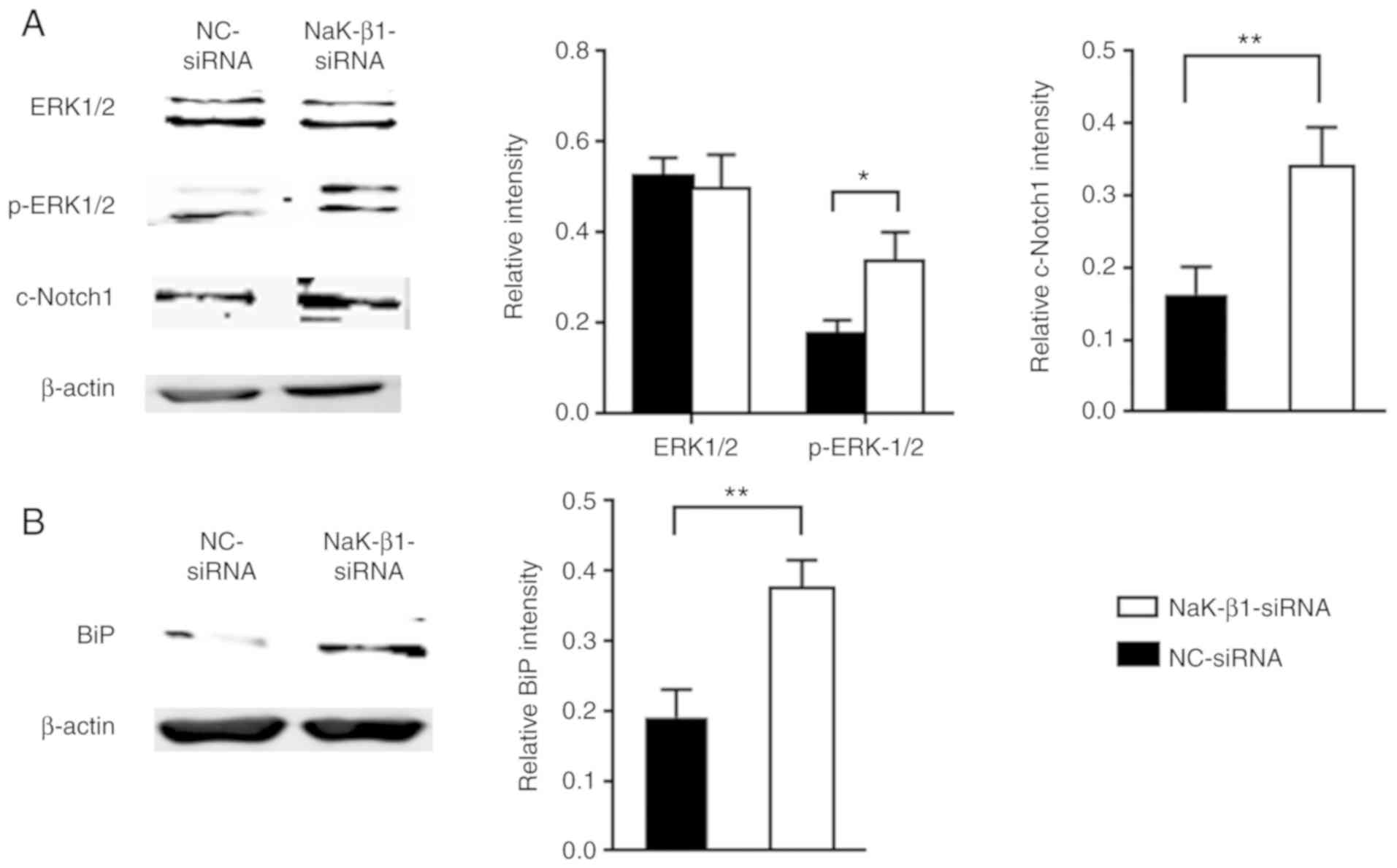 | Figure 6NaK-β1 knockdown activates ERK1/2 and
Notch1 signaling, inducing ER stress in A549 cells. Western blot
analysis and relative quantification of (A) ERK1/2, p-ERK1/2 and
cleaved Notch1 levels and (B) BiP levels in A549 cells transfected
with NaK-β1- and NC-siRNA. The data are expressed as the mean ±
standard error of the mean. *P<0.05;
**P<0.01. NaK-β1, Na,K-ATPase β1 subunit; ERK,
extracellular signal-regulated kinase; p-ERK1/2, phosphorylated
ERK1/2; Notch1, neurogenic locus notch homolog protein 1; c-Notch1,
cleaved Notch1; BiP, immunoglobulin heavy chain-binding protein;
NC, negative control; siRNA, small interfering RNA. |
Discussion
IPF is a chronic and lethal interstitial lung
disease. It is generally accepted that the initial progression of
IPF is stimulated by the aberrant activation of AECs in response to
repetitive microinjury. In the present study, the Na,K-ATPase β1
subunit protein expression was revealed to be downregu-lated in
lung fibrosis, mainly in AECs, enhancing profibrotic protein
expression, activating the ERK1/2 and Notch1 signaling pathways,
and inducing ER stress, consequently leading to lung fibrosis.
The present study has demonstrated that the
expression of the Na,K-ATPase β1 subunit is different in human and
mouse lung tissues. It is expressed in type I and II AECs, and
located in the cytoplasm of AECs in human lungs. However, in mouse
lungs, the Na,K-ATPase β1 subunit is mainly expressed in type II
AECs and is located in the cell membrane. The human Na,K-ATPase β1
subunit exhibits two bands in immunoblot analyses, where the lower
40-kDa band represents the intracellular immature fraction of the
subunit and the higher molecular weight band represents the mature
plasma membrane form. However, the mouse Na,K-ATPase β1 subunit
results in only a single 42-kDa band (37).
The Na,K-ATPase β1 subunit belongs to the N-linked
glycoproteins, and as a regulatory subunit, its main fuction is to
assist the folding of the α subunit and its transport from the ER
to the plasma membrance (38).
Furthermore, the Na,K-ATPase β1 subunit is a molecular partner of
Wolframin, an ER protein involved in ER stress (39). The results of the present study
indicated that the knockdown of this subunit led to the
upregulation of BiP, whereas the level of DNA damage-inducible
transcript 3 protein was not altered (data not shown). Over the
past decades, accumulating evidence has suggested that ER stress
serves an important role in the pathogenesis of lung fibrosis, as
ER stress markers are highly expressed in AECs in IPF. ER stress in
lung fibrosis induces AEC injury and apotosis, causing inflammation
and cell phenotype alteration (20,40-43).
The present data revealed that the knockdown of
Na,K-ATPase β1 subunit led to the enhanced expression of
profibrotic proteins fibronectin and α-SMA, but no changes in
epithelial marker E-cadherin were observed, suggesting that AECs
undergo incomplete activation and partly maintain epithelial
characteristics (44). Treatment
of A549 cells with TGF-β1 resulted in a decrease in Na,K-ATPase β1
subunit expression, which may affect electrolyte metabolism in
AECs. Further investigation is required to clarify the role of
Na,K-ATPase α1 subunit-mediated electrolyte metabolism dysfuction
in the pathegenesis of lung fibrosis.
Attempts to use plasmids to overexpress Na,K-ATPase
β1 subunit in A549 cells proved unsuccessful in the present study.
Ouabain, an inhibitor of Na,K-ATPase, leads to the upregulation of
Na,K-ATPase β1 subunit expression and suppresses EMT (26,45). In addition, a previous study of
the present group revealed that ouabain ameliorates
bleomycin-induced pulmonary fibrosis (46). Therefore, it can be inferred that
this inhibitor suppresses EMT due to the upregulation of of
Na,K-ATPase β1 subunit expression, providing direction of
subsequent studies.
In conclusion, Na,K-ATPase β1 subunit expression is
down-regulated in clinical human IPF samples, in the lung tissue of
a bleomycin-induced pulmonary fibrosis mouse model, and in
TGF-β1-stimulated lung carcinoma A549 cells. Additionally,
Na,K-ATPase β1 subunit deficiency in A549 cells upregulates
profibrotic protein expression, activates ERK1/2 and Notch1
signaling pathways and induces ER stress. Therefore, the results of
the present study suggest that decreased expression of Na,K-ATPase
β1 subunit in AECs serves a crucial role in the progression of lung
fibrosis.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81430001 and
81470258).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL, WN, HD and CW designed the experiments. BL
performed the experiments and drafted the manuscript. BL, XX and XH
analyzed the data. XX, WN, HD and CW revised the manuscript. All
authors have read and approved the final version for
publication.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee
(approval no. 2017-25-1) and the Animal Ethics Committee (approval
no. 2017-18-2) of China-Japan Friendship Hospital, Beijing,
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
This abstract was presented at the ERS International
Congress, September 15-19, 2018 in Paris, France and was published
as Abstract no. PA3720 in the European Respiratory Journal 52
(Suppl 62) 2018. The authors thank Professor Ying Li, Department of
Medical Research, Beijing Chao-Yang Hospital, Capital Medical
University, Beijing, China, and Professor Yunchao Su, Department of
Pharmacology and Toxicology, Charlie Norwood Veterans Affairs
Medical Center, Augusta, GA, USA, for their excellent technical
assistance.
References
|
1
|
Coultas DB, Zumwalt RE, Black WC and
Sobonya RE: The epidemiology of interstitial lung diseases. Am J
Respir Crit Care Med. 150:967–972. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mannino DM, Etzel RA and Parrish RG:
Pulmonary fibrosis deaths in the United States, 1979-1991. An
analysis of multiple-cause mortality data. Am J Respir Crit Care
Med. 153:1548–1552. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noble PW: Idiopathic pulmonary fibrosis:
Natural history and prognosis. Clin Chest Med. 27(1 Suppl 1):
S11–S16. v2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raghu G, Weycker D, Edelsberg J, Bradford
WZ and Oster G: Incidence and prevalence of idiopathic pulmonary
fibrosis. Am J Respir Crit Care Med. 174:810–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai M, Zhu M, Ban C, Su J, Ye Q, Liu Y,
Zhao W, Wang C and Dai H: Clinical features and outcomes of 210
patients with idiopathic pulmonary fibrosis. Chin Med J (Engl).
127:1868–1873. 2014.
|
|
7
|
Selman M, King TE and Pardo A; American
Thoracic Society; European Respiratory Society; American College of
Chest Physicians: Idiopathic pulmonary fibrosis: Prevailing and
evolving hypotheses about its pathogenesis and implications for
therapy. Ann Intern Med. 134:136–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thannickal VJ, Toews GB, White ES, Lynch
JP III and Martinez FJ: Mechanisms of pulmonary fibrosis. Annu Rev
Med. 55:395–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zoz DF, Lawson WE and Blackwell TS:
Idiopathic pulmonary fibrosis: A disorder of epithelial cell
dysfunction. Am J Med Sci. 341:435–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Selman M and Pardo A: Role of epithelial
cells in idiopathic pulmonary fibrosis: From innocent targets to
serial killers. Proc Am Thorac Soc. 3:364–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baumgartner KB, Samet JM, Stidley CA,
Colby TV and Waldron JA: Cigarette smoking: A risk factor for
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med.
155:242–248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taskar VS and Coultas DB: Is idiopathic
pulmonary fibrosis an environmental disease? Proc Am Thorac Soc.
3:293–298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raghu G, Freudenberger TD, Yang S, Curtis
JR, Spada C, Hayes J, Sillery JK, Pope CE II and Pellegrini CA:
High prevalence of abnormal acid gastro-oesophageal reflux in
idiopathic pulmonary fibrosis. Eur Respir J. 27:136–142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hubbard R, Lewis S, Richards K, Johnston I
and Britton J: Occupational exposure to metal or wood dust and
aetiology of cryptogenic fibrosing alveolitis. Lancet. 347:284–289.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly BG, Lok SS, Hasleton PS, Egan JJ and
Stewart JP: A rearranged form of Epstein-Barr virus DNA is
associated with idiopathic pulmonary fibrosis. Am J Respir Crit
Care Med. 166:510–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marmai C, Sutherland RE, Kim KK, Dolganov
GM, Fang X, Kim SS, Jiang S, Golden JA, Hoopes CW, Matthay MA, et
al: Alveolar epithelial cells express mesenchymal proteins in
patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell
Mol Physiol. 301:L71–L78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rock JR, Barkauskas CE, Cronce MJ, Xue Y,
Harris JR, Liang J, Noble PW and Hogan BL: Multiple stromal
populations contribute to pulmonary fibrosis without evidence for
epithelial to mesenchymal transition. Proc Natl Acad Sci USA.
108:E1475–E1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang C, Liu G, Luckhardt T, Antony V,
Zhou Y, Carter AB, Thannickal VJ and Liu RM: Serpine 1 induces
alveolar type II cell senescence through activating p53-p21-Rb
pathway in fibrotic lung disease. Aging Cell. 16:1114–1124. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korfei M, Ruppert C, Mahavadi P, Henneke
I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, et al:
Epithelial endoplasmic reticulum stress and apoptosis in sporadic
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med.
178:838–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Im J, Kim K, Hergert P and Nho RS:
Idiopathic pulmonary fibrosis fibroblasts become resistant to Fas
ligand-dependent apoptosis via the alteration of decoy receptor 3.
J Pathol. 240:25–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Araya J, Kojima J, Takasaka N, Ito S,
Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi
M, et al: Insufficient autophagy in idiopathic pulmonary fibrosis.
Am J Physiol Lung Cell Mol Physiol. 304:L56–L69. 2013. View Article : Google Scholar
|
|
23
|
Geng J, Huang X, Li Y, Xu X, Li S, Jiang
D, Liang J, Wang C and Dai H: Down-regulation of USP13 mediates
phenotype transformation of fibroblasts in idiopathic pulmonary
fibrosis. Respir Res. 16:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nie Y, Sun L, Wu Y, Yang Y, Wang J, He H,
Hu Y, Chang Y, Liang Q, Zhu J, et al: AKT2 regulates pulmonary
inflammation and fibrosis via modulating macrophage activation. J
Immunol. 198:4470–4480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peljto AL, Zhang Y, Fingerlin TE, Ma SF,
Garcia JG, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd
JE, et al: Association between the MUC5B promoter polymorphism and
survival in patients with idiopathic pulmonary fibrosis. JAMA.
309:2232–2239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rajasekaran SA, Huynh TP, Wolle DG,
Espineda CE, Inge LJ, Skay A, Lassman C, Nicholas SB, Harper JF,
Reeves AE, et al: Na,K-ATPase subunits as markers for
epithelial-mesenchymal transition in cancer and fibrosis. Mol
Cancer Ther. 9:1515–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mony S, Lee SJ, Harper JF, Barwe SP and
Langhans SA: Regulation of Na,K-ATPase beta1-subunit in
TGF-β2-mediated epithelial-to-mesenchymal transition in human
retinal pigmented epithelial cells. Exp Eye Res. 115:113–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pierre SV and Xie Z: The Na,K-ATPase
receptor complex: Its organization and membership. Cell Biochem
Biophys. 46:303–316. 2006. View Article : Google Scholar
|
|
29
|
Xie Z and Askari A: Na(+)/K(+)-ATPase as a
signal transducer. Eur J Biochem. 269:2434–2439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barwe SP, Anilkumar G, Moon SY, Zheng Y,
Whitelegge JP, Rajasekaran SA and Rajasekaran AK: Novel role for
Na,K-ATPase in phosphatidylinositol 3-kinase signaling and
suppression of cell motility. Mol Biol Cell. 16:1082–1094. 2005.
View Article : Google Scholar :
|
|
31
|
Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y,
Zheng X, Dong S, Liu X, Yang X, et al: Blocking follistatin-like 1
attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med.
212:235–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yogo Y, Fujishima S, Inoue T, Saito F,
Shiomi T, Yamaguchi K and Ishizaka A: Macrophage derived chemokine
(CCL22), thymus and activation-regulated chemokine (CCL17), and
CCR4 in idiopathic pulmonary fibrosis. Respir Res. 10:802009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang YC, Liu JS, Tang HK, Nie J, Zhu JX,
Wen LL and Guo QL: miR-221 targets HMGA2 to inhibit
bleomycin-induced pulmonary fibrosis by regulating
TGFβ1/Smad3-induced EMT. Int J Mol Med. 38:1208–1216. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng Q, Tong M, Ou B, Liu C, Hu C and
Yang Y: Isorhamnetin protects against bleomycin-induced pulmonary
fibrosis by inhibiting endoplasmic reticulum stress and
epithelial-mesenchymal transition. Int J Mol Med. 43:117–126.
2019.
|
|
35
|
Xu X, Wan X, Geng J, Li F, Yang T and Dai
H: Rapamycin regulates connective tissue growth factor expression
of lung epithelial cells via phosphoinositide 3-kinase. Exp Biol
Med (Maywood). 238:1082–1094. 2013. View Article : Google Scholar
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Tokhtaeva E, Sachs G and Vagin O: Assembly
with the Na, K-ATPase alpha(1) subunit is required for export of
beta(1) and beta(2) subunits from the endoplasmic reticulum.
Biochemistry. 48:11421–11431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lemas MV, Hamrick M, Takeyasu K and
Fambrough DM: 26 amino acids of an extracellular domain of the
Na,K-ATPase alpha-subunit are sufficient for assembly with the
Na,K-ATPase beta-subunit. J Biol Chem. 269:8255–8259.
1994.PubMed/NCBI
|
|
39
|
Zatyka M, Ricketts C, da Silva Xavier G,
Minton J, Fenton S, Hofmann-Thiel S, Rutter GA and Barrett TG:
Sodium-potassium ATPase 1 subunit is a molecular partner of
Wolframin, an endoplasmic reticulum protein involved in ER stress.
Hum Mol Genet. 17:190–200. 2008. View Article : Google Scholar
|
|
40
|
Lawson WE, Crossno PF, Polosukhin VV,
Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB,
et al: Endoplasmic reticulum stress in alveolar epithelial cells is
prominent in IPF: Association with altered surfactant protein
processing and herpesvirus infection. Am J Physiol Lung Cell Mol
Physiol. 294:L1119–L1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mulugeta S, Nguyen V, Russo SJ, Muniswamy
M and Beers MF: A surfactant protein C precursor protein BRICHOS
domain mutation causes endoplasmic reticulum stress, proteasome
dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol.
32:521–530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maguire JA, Mulugeta S and Beers MF:
Endoplasmic reticulum stress induced by surfactant protein C
BRICHOS mutants promotes proinflammatory signaling by epithelial
cells. Am J Respir Cell Mol Biol. 44:404–414. 2011. View Article : Google Scholar :
|
|
43
|
Ulianich L, Garbi C, Treglia AS, Punzi D,
Miele C, Raciti GA, Beguinot F, Consiglio E and Di Jeso B: ER
stress is associated with dedifferentiation and an
epithelial-to-mesenchymal transition-like phenotype in PC Cl3
thyroid cells. J Cell Sci. 121:477–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morbini P, Inghilleri S, Campo I, Oggionni
T, Zorzetto M and Luisetti M: Incomplete expression of
epithelial-mesenchymal transition markers in idiopathic pulmonary
fibrosis. Pathol Res Pract. 207:559–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
La J, Reed E, Chan L, Smolyaninova LV,
Akomova OA, Mutlu GM, Orlov SN and Dulin NO: Downregulation of
TGF-β Receptor-2 Expression and Signaling through Inhibition of
Na/K-ATPase. PLoS One. 11:e01683632016. View Article : Google Scholar
|
|
46
|
Li B, Huang X, Liu Z, Xu X, Xiao H, Zhang
X, Dai H and Wang C: Ouabain ameliorates bleomycin induced
pulmonary fibrosis by inhibiting proliferation and promoting
apoptosis of lung fibroblasts. Am J Transl Res. 10:2967–2974.
2018.PubMed/NCBI
|