Introduction
Bone is a solid yet dynamic organ, which is in a
constant state of remodelling. These remodelling activities occur
throughout the entire lifespan of an individual and are tightly
regulated by the cooperation of osteoblast-mediated disruption and
the osteoclast-mediated facilitation of bone resorption. These two
cell types are normally in balance to maintain homeostasis and
warrant a constant amount of healthy bone (1). However, the imbalance between bone
formation and resorption may lead to severe skeletal diseases,
including osteoporosis and osteopetrosis (2,3).
Osteoclasts are members of the monocyte/macrophage
hematopoietic lineage with specific morphological characteristics,
including multiple nuclei and ruffled borders (4,5).
Their number and resorptive function are usually increased in
osteoporosis. Since osteoclasts are unique in their ability to
resorb bone (6), the formation of
mature multi-nucleated osteoclasts is a critical event in the
development of osteoporosis (7,8).
Furthermore, osteoclasts have become one of the key targets for the
treatment of osteoporosis. The formation of osteoclasts includes
two critical steps, namely a commitment of the mono-nuclear cell
lineages to become pre-osteoclasts and cell-cell fusion to generate
multinucleated giant osteoclasts (9). Each step may serve as a potential
target for therapeutic intervention on osteoporosis. However, it
has been verified that intervention on the first step may have
severe adverse effects on the hematopoietic system (10). The strategy of interfering with
the second step has been confirmed to attenuate the efficiency of
bone resorption by decreasing the actin-rich structure of podosomes
(10). Fusion failure may lead to
an obvious reduction of bone-resorbing activity and an increase in
bone mass, as observed in osteopetrosis (11). However, excessive bone resorption
by osteoclasts is also involved in the pathogenesis of
bone-associated disorders, including osteoporosis.
Pre-osteoclastic RAW264.7 cells may be induced into
osteoclasts by receptor activator of nuclear factor-κB ligand
(RANKL). RANKL is a member of the tumour necrosis factor (TNF)
family, and it is the most significant for the process of
osteoclast formation and activation (12,13). After binding to its receptor,
RANK, RANKL stimulates the osteoclastic differentiation of monocyte
macrophages and the maturation of osteoclasts (14,15). In detail, RANKL binding to RANK
recruits TNF receptor-associated factor 6 (TRAF6) and sequentially
activates the transcription factors, NF-κB, and several
inflammation-associated mitogen-activated protein kinase (MAPK)
pathways, including extracellular signal-regulated kinase (ERK)1/2,
c-Jun N-terminal kinase (JNK) and p38 pathways (16-18). These pathways, in turn, stimulate
the key transcription factors nuclear factor of activated T cells 1
(NFATc1) and c-Fos (19,20). Stimulated NFATc1 translocates into
the nucleus and activates the expression of osteoclast marker
genes, including RANK, calcitonin receptor (CTR),
tartrate-resistant acid phosphatase (TRAP) and dendritic
cell-specific transmembrane protein (DC-STAMP), which enable
osteoclastogenesis and bone resorption by osteoclasts (Fig. 1) (6,21).
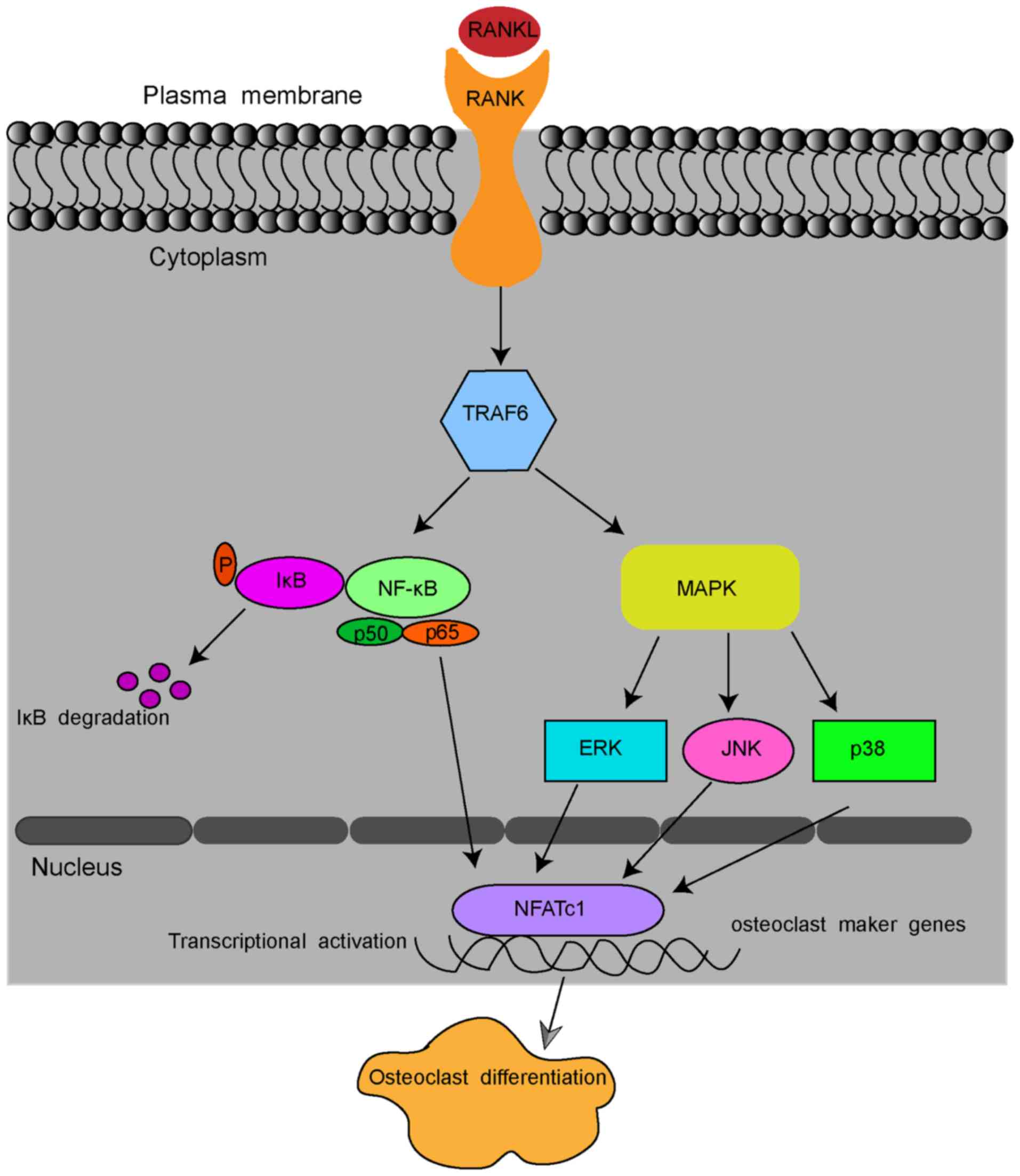 | Figure 1Schematic presentation of
RANKL-induced osteoclast formation. By binding to RANK, RANKL
recruits TRAF6 and sequentially activates transcription factors
NF-κB, ERK1/2, JNK and p38 pathways. The signal is then transmitted
to NFATc1 and c-Fos. Stimulated NFATc1 translocates into the
nucleus and activates the expression of osteoclast marker genes,
including RANK, CTR, TRAP and DC-STAMP.
RANKL, receptor activator of nuclear κB ligand; CTR, calcitonin
receptor; TRAP, tartrate-resistant acid phosphatase; DC-STAMP,
dendritic cell-specific transmembrane protein; NFATc1, nuclear
factor of activated T cells 1; TRAF6, tumour necrosis
factor-associated factor 6. NF-κB, nuclear factor-κB; ERK1/2,
extracellular regulated protein kinases; JNK, c-Jun N-terminal
kinase. |
Zoledronic acid (ZOL), a third-generation,
nitrogen-containing, long-acting bisphosphonate, has been
identified to significantly increase bone mineral density. It is
used for the treatment of osteoporosis and decreases the incidence
of osteoporotic fractures in patients with post-menopausal
conditions when applied systemically by intravenous infusion once a
year (22,23). Such agents are known to act by
inhibiting osteoclast proliferation and inducing the apoptosis of
osteoclasts (24,25). There are pioneer studies on the
effect of nitrogen-containing bisphosphonates, e.g., minodronate
and alendronate, on osteoclastogenesis (26,27). However, the mechanisms through
which ZOL inhibits osteoclastogenesis remain to be fully elucidated
(26,28). The present study aimed to further
explore the effects of ZOL on RANKL-induced osteoclast
differentiation and bone resorption activity in vitro, and
to further explore the underlying mechanisms.
Materials and methods
Cells, reagents and antibodies
The RAW264.7 mouse macrophage (osteoclast precursor)
cell line was obtained from the American Type Culture Collection.
Foetal bovine serum (FBS) and the alpha modification of Eagle's
medium (α-MEM) were purchased from Gibco-BRL (Thermo Fisher
Scientific, Inc.). The cell counting kit (CCK-8) was purchased from
Dojindo Laboratories. ZOL (dissolved in purified water) and a TRAP
staining kit (387A, Sigma-Aldrich) were obtained from Sigma-Aldrich
(Merck KGaA). DAPI and TRITC phalloidin were purchased from Beijing
Solarbio. The recombinant human soluble RANK ligand (sRANKL) was
purchased from R&D Systems. Polyvinylidene difluoride (PVDF)
membranes were obtained from EMD Millipore. Specific antibodies
against p38 (#8690), phospho-p38 (p-p38, #4511) (Thr180/Tyr182),
IκBα (#4812), phospho-IκBα (p-IkBa, #2859) (Ser32), extracellular
signal-regulated kinase 1/2 (ERK1/2, #9102), phospho-ERK (p-ERK,
#4370) (Thr202/Tyr204), c-Jun N-terminal kinase (JNK, #9252),
phospho-JNK (p-JNK, #4668) (Thr183/Tyr185), p65(#8242), phospho-p65
(p-p65, #3033) were obtained from Cell Signaling Technology.
HRP-conjugated goat- anti-rabbit IgG secondary antibody (#014-090P)
was purchased from Bioprimacy.
Cell culture
The RAW264.7 cells were cultured in α-MEM
supplemented with antibiotics (100 units of penicillin and 100
µg/ml streptomycin, purchased from HyClone) and 10%
heat-inactivated FBS at 37°C in a humidified atmosphere of 95% air
and 5% CO2. The cells were used after 3-5 passages in
α-MEM.
Cell viability assay
The effects of various concentrations of ZOL on the
growth and viability of RAW264.7 cells in the presence or absence
of RANKL were evaluated using a CCK-8 kit. In brief, the RAW264.7
cells were seeded into 3 96-well plates, at a density of
3×103 cells/well and cultured in α-MEM supplemented with
10% FBS and 1% penicillin and streptomycin for 24 h. The medium was
discarded and serially diluted ZOL (0, 0.1, 1, 5, 15, 30 and 50
µM) with or without 100 ng/ml RANKL were added to the cells
at the same time, followed by further incubation at 37°C for 24, 48
or 72 h, respectively. A total of 10 µl CCK-8 reagent was
added to each well, and the cells were incubated for an additional
2 h at 37°C with 5% CO2. The optical density at 450 nm
was read on an ELX800 microplate reader (BioTek Instruments), and
the background reading (medium) was subtracted. Six replicates were
used for each condition, and the experiments were repeated at least
3 times. The cell growth curves of the ZOL-treated cells were
generated using GraphPad Prism 6.0 (GraphPad Software, Inc).
In vitro osteoclastogenesis assays
RAW264.7 cells differentiate into osteoclast-like
cells in the presence of RANKL. The cells were seeded in 96-well
tissue culture plates at a density of 1.5×103 cells/well
with α-MEM (supplemented with 10% FBS and 1%
penicillin-streptomycin) and incubated at 37°C overnight to allow
the cells attach to the inner surface of a 6-well plate, and the
cell culture was then supplemented with (RANKL group) or without
(RANKL-free and ZOL-free, denoted vehicle group) 100 ng/ml RANKL
and various concentrations of ZOL (0, 0.1, 1 or 5 µM) for 5
days at 37°C, and the cell culture medium were replaced with fresh
complete medium every 2 days until a large number of mature
osteoclasts formed in the group treated with RANKL only. In
addition, RAW264.7 cells treated with or without 1 µM ZOL
and 100 ng/ml RANKL were cultured for 3, 5 or 7 days at 37°C. To
determine osteoclast differentiation at the end of each incubation,
the cells were washed twice and fixed with 4% paraformaldehyde for
20 min. The TRAP staining kit was then used to stain for TRAP, an
osteoclast marker, according to the manufacturer's instructions.
TRAP-positive multinucleated cells with >3 nuclei identified
under an inverted microscope (Olympus IX 51) were considered as
osteoclast-like cells.
Immunofluorescence
RAW264.7 cells, cultured on glass coverslips, were
treated with or without 100 ng/ml RANKL and 0, 0.1 or 5 µM
ZOL until mature osteoclasts appeared in the control wells. To
detect the formation of F-actin rings and nuclei, the cells were
stained with TRITC phalloidin and DAPI, and analysed according to
the manufacturer's instructions. In brief, the osteoclasts were
fixed with 4.0% paraformaldehyde in PBS for 20 min. After washing
with PBS 3 times, the cells were permeabilized using 0.25% (v/v)
Triton X-100 for 5 min, followed by blocking in blocking buffer (3%
bovine serum albumin in PBS, Thermo Fisher Scientific) for 1 h and
then washed 3 times with PBS again. F-actin rings were stained with
TRITC rhodamine-conjugated TRITC phalloidin and the nuclei with
DAPI at room temperature for 30 min or 30 sec, respectively.
Finally, the cells were washed with PBS and observed under a
fluorescence microscope (BX51; Olympus) and the fluorescence images
were obtained using Zeiss ZEN software (Zen 2.6, Zeiss AG).
Resorption pit assay
The resorptive function of the mature osteoclasts
derived from the RANKL-differentiated RAW264.7 cells was analysed
on sterile bovine bone slices (IDS Nordic), which were placed in
96-well plates with 3 replicates for each condition. The RAW264.7
cells were plated at a density of 1.5×103 cells/well
onto the bovine bone slices. The cells were treated with 100 ng/ml
RANKL and 0, 0.1 or 5 µM ZOL to induce osteoclast
differentiation. After 10 days of culture (medium was changed every
48 h), all cells were removed from the bone slices, and the
resorption pits were then visualised under a scanning electron
microscope (Hitachi E-1010). The total number and area of
resorption pits was quantified and compared using Image J software
6.0 (National Institutes of Health).
RNA extraction and reverse
transcription-quantitative (RT-qPCR)
The RAW264.7 cells were seeded onto 6-well plates at
a density of 1×105 cells per well and cultured in
complete α-MEM in the presence or absence of 100 ng/ml RANKL. These
cells were then incubated with 0, 0.1, 1 or 5 µM ZOL at 37°C
for 3 days until mature osteoclasts formed. The cells were
transferred into a tube containing TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and total RNA was isolated
according to the manufacturer's instructions. Complementary DNA was
synthesised from 1 mg total RNA using PrimeScript™ reverse
transcriptase (2690A, Takara Bio Inc.) and stored at −70°C until
further use. RT-qPCR was performed to verify the differential
expression of the specific genes during osteoclast formation or of
GAPDH using the SYBR® Premix Ex Taq™ kit (Takara
Bio Inc.). For the analysis of mRNAs encoding osteoclastogenic
proteins and osteoclast-specific markers, TRAP, CTR,
RANK, NFATc1, c-Fos, DC-STAMP and
GAPDH were amplified. The specific primer sequences are
listed in Table I.
 | Table ISequences of primers used in
RT-qPCR. |
Table I
Sequences of primers used in
RT-qPCR.
| Primer | Gene sequence |
|---|
| Mouse CTR
forward |
5′-GTCCAGAGTGAAAAGGCGGA-3′ |
| Mouse CTR
reverse |
5′-AGGGCAACTGATGAATCCGG-3′ |
| Mouse TRAP
forward |
5′-AAGAGATCGCCAGAACCGTG-3′ |
| Mouse TRAP
reverse |
5′-TTCCAGCCAGCACATACCAG-3′ |
| Mouse RANK
forward |
5′-TTCGACTGGTTCACTGCTCC-3′ |
| Mouse RANK
reverse |
5′-TCAGGTGCTTTTCAGGGGAC-3′ |
| Mouse
DC-STAMP forward |
5′-CCCTTGGGCTGTTCTTCCTT-3′ |
| Mouse
DC-STAMP reverse |
5′-AGGAATGCAGCTCGGTTCAA-3′ |
| Mouse NFATc1
forward |
5′-GACCGAAGATACCTGGCTCG-3′ |
| Mouse NFATc1
reverse |
5′-GTCAGAAGTGGGTGGAGTGG-3′ |
| Mouse c-Fos
forward |
5′-CCGGTTCCTTCTATGCAGCA-3′ |
| Mouse c-Fos
reverse |
5′-GCTTGGGAAGGAGTCAGCTT-3′ |
| Mouse GAPDH
forward |
5′-GGTTGTCTCCTGCGACTTCA-3′ |
| Mouse GAPDH
reverse |
5'-TGGTCCAGGGTTTCTTACTCC-3′ |
The thermocycling conditions for PCR were as
follows: Initial denaturation for 1 min at 95°C, followed by 40
cycles of 95°C for 15 sec and extension at 60°C for 1 min. The
2-∆∆Cq method (29)
was used to calculate relative mRNA expression as described
previously, and each sample was run and analysed in triplicate. The
expression levels of each gene in all experimental groups were
normalised to the endogenous reference gene (GAPDH) and
indicated as relative fold changes of the control.
Protein preparation and western blot
analysis
The RAW264.7 cells were seeded in 6-well plates at a
density of 1×106 cells/well, and following incubation at
37°C overnight, the cells were pre-treated with or without 5
µM ZOL for 4 h, and then cultured with 100 ng/ml RANKL for a
further 0, 5, 10, 20, 30 or 60 min. Whole-cell lysates were
prepared from harvested cells using radioimmunoprecipitation assay
buffer consisting of 150 mM NaCl, 50 mM Tris-HCl, 5 mM EDTA, 1%
Triton X-100, 1 mM sodium vanadate, 1 mM sodium fluoride, 1%
deoxycholate and protease inhibitors. Cell debris was removed by
centrifugation at 12,000 × g at 4°C for 10 min. The lysates were
boiled in the loading buffer for 10 min and the protein
concentrations in the whole-cell extracts were quantified using the
bicinchoninic acid method (Beijing Solarbio). Total protein (30
µg per lane) was then separated by 10% SDS-PAGE and
transferred onto PVDF membranes. After blocking in 5% non-fat milk
in Tris-buffered saline containing Tween-20 at room temperature for
2 h, the membranes were incubated with a 1:1,000 dilution of the
indicated primary antibodies at 4°C overnight, followed by
horseradish-peroxidase-conjugated secondary antibodies diluted at
1:10,000 in the blocking buffer at room temperature for 1 h. After
washing, the membranes were soaked in enhanced chemiluminescence
solution (ECL, Millipore) for 1 min, and the bands were detected
using the Gene Gnome Imaging System (Syngene). The band intensities
were quantified using ImageJ software 1.48 q (NIH). Phosphorylated
proteins (e.g., p-p65, p-IkBa, p-ERK1/2, p-p38 and p-JNK) were
visualized by its specific primary antibody and corresponding
secondary antibody. To detect total protein in the same membrane,
antibodies, detecting each phosphorylated protein, were stripped
from the membranes by using Stripping Buffer purchased from
Solarbio® LIFE SCIENCE (SW3020, Beijing). Antibody-free
membranes were then re-incubated with antibodies binding to total
proteins (e.g., p65, IκBa, p38, JNK). The GAPDH protein was used as
an internal reference.
Statistical analysis
All experiments were performed 3 times and values
are expressed as the means ± standard deviation. The groups were
compared using one-way or two-way ANOVA analysis of variance
followed by Tukey's post-hoc test for multiple comparisons. All
data analyses were performed with GraphPad Prism 6.0 (GraphPad
Software, Inc) and differences between means were considered
statistically significant at P<0.05.
Results
Cytotoxic effects of ZOL on RAW264.7
cells and osteoclasts
The effect of ZOL on the viability of RAW264.7 cells
in the presence or absence of RANKL was assessed using a CCK-8
assay. First, the cytotoxicity of ZOL (0, 0.1, 1, 5, 15, 30 and 50
µM) on RAW264.7 cells without RANKL was analysed. The
results indicated that the cell growth was suppressed by ZOL at
concentrations of 15, 30 and 50 µM (Fig. 2A). Subsequently, the cytotoxicity
of ZOL (at the same concentrations) in the presence of 100 ng/ml
RANKL was assessed. As expected, the result was similar to that
observed in the RANKL-free group (Fig. 2B).
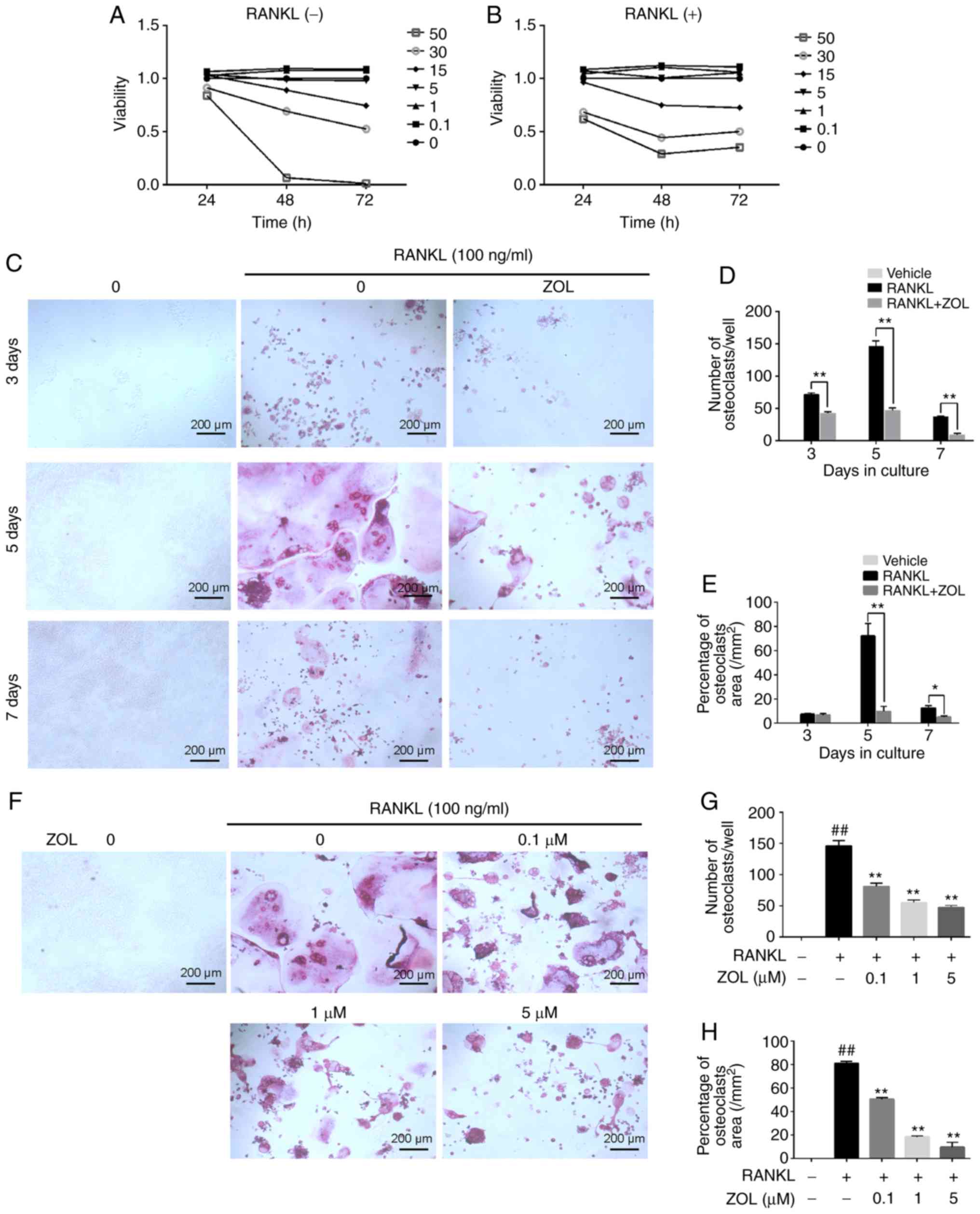 | Figure 2ZOL inhibits RANKL-induced osteoclast
differentiation without cytotoxicity. RAW264.7 cells were cultured
with ZOL (0, 0.1, 1, 5, 15, 30 or 50 µM). (A and B) A Cell
Counting Kit-8 assay was performed to determine cell viability at
various time-points (24, 48 and 72 h). The results (A) without or
(B) with 100 ng/ml RANKL were plotted as cellular growth curves.
(C) RAW264.7 cells were incubated with different concentrations of
ZOL (0, 0.1, 1 and 5 µM) in the presence or absence of 100
ng/ml RANKL for 5 days, and then stained using a TRAP staining kit.
(D and E) The numbers and percentages of osteoclasts were
determined. (F) RAW264.7 cells were cultured with or without 1
µM ZOL, and then stained for TRAP at 3, 5 and 7 days,
respectively. (G and H) Osteoclast numbers and area percentage were
counted at 3, 5 and 7 days, respectively. ##P<0.01
vs. the vehicle group; *P<0.05,
**P<0.01 vs. the RANKL-only group. ZOL, zoledronic
acid; RANKL, receptor activator of nuclear-κB ligand; TRAP,
tartrate-resistant acid phosphatase. |
ZOL suppresses the RANKL-induced
osteoclastic differentiation of RAW264.7 cells
After excluding the possibility that the inhibitory
effects of ZOL on TRAP activity were due to cytotoxicity at
concentrations of up to 5 µM, the effects of ZOL on
RANKL-induced osteoclastogenesis were assessed. In the RANKL group,
the RAW264.7 cells exhibited characteristic morphological changes
toward osteoclasts at day 3 of RANKL-induced differentiation, with
increasing cell-cell fusion into large and multinucleate
TRAP-positive osteoclast cells, reaching completion at day 5
(Fig. 2C). However, the number of
osteoclasts (Fig. 2D) and
percentage of the osteoclast area (Fig. 2E) was significantly suppressed by
incubation with 1 µM ZOL for different periods of time (3, 5
and 7 days). Furthermore, ZOL suppressed osteoclastogenesis in a
dose-dependent manner (Fig.
2F-H). Taken together, these results suggest that ZOL inhibits
osteoclast formation.
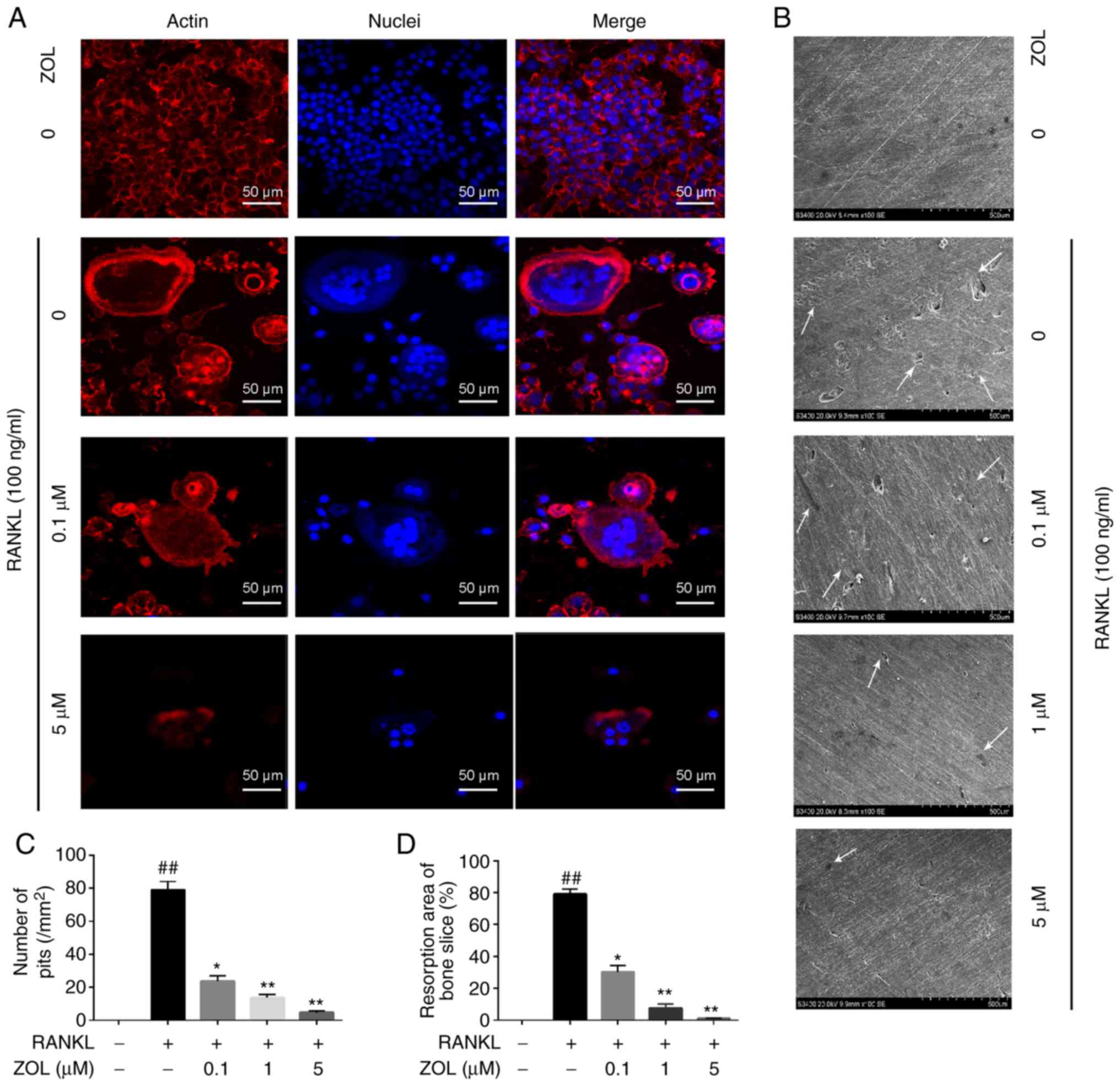
ZOL inhibits the formation of F-actin
rings and multiple nuclei
Mature osteoclasts contain actin ring structures
that create sealing zones between the cells and bone matrix, and it
is a prerequisite for osteoclast bone resorption (30,31). Thus, immunofluorescence analysis
was performed to examine the effects of ZOL on F-actin rings and
cell nuclei. Well-structured F-actin rings were observed by
confocal fluorescence microscopy in the sealing zones of RANKL
induced osteoclasts (Fig. 3A).
However, the formation of the F-actin ring and the gathering of
nuclei was markedly inhibited by ZOL in a concentration-dependent
manner. Therefore, osteoclast morphology appeared abnormal or
immature (Fig. 3A).
Effects of ZOL on bone resorption in
RANKL-induced RAW264.7 cells
We then investigated whether ZOL modulates mature
osteoclast activity by performing a resorption pit assay. RAW264.7
cells were plated on bone slices, which were treated with various
concentrations of ZOL in the presence or absence of 100 ng/ml
RANKL. The results indicated that the area of osteoclast bone
resorption pits was markedly decreased by ZOL in a dose-dependent
manner compared with the ZOL-free group. Furthermore, almost no
resorption pits were observed in the groups treated with 5
µM ZOL (Fig. 3B-D). These
results suggested that treatment with ZOL markedly attenuates the
bone-resorption activity of osteoclasts. This may, at least
partially, be explained by the effect of ZOL to impair
osteoclastogenesis.
Effects of ZOL on the mRNA expression of
osteoclast differentiation-specific genes in RAW264.7 cells
To further elucidate the effects of ZOL on
osteoclast formation and resorptive function, the expression of
specific osteoclast differentiation-associated genes in ZOL-treated
cells was assessed by RT-qPCR. It was indicated that treatment with
RANKL markedly increased the expression levels of CTR,
RANK, TRAP, DC-STAMP, NFATc1 and
c-Fos. However, this upregulation was significantly
suppressed by ZOL in a dose-dependent manner during
osteoclastogenesis compared with that in the ZOL-free group
(Fig. 4). These results suggest
that ZOL inhibits the expression of RANKL-induced genes involved in
osteoclast differentiation and function.
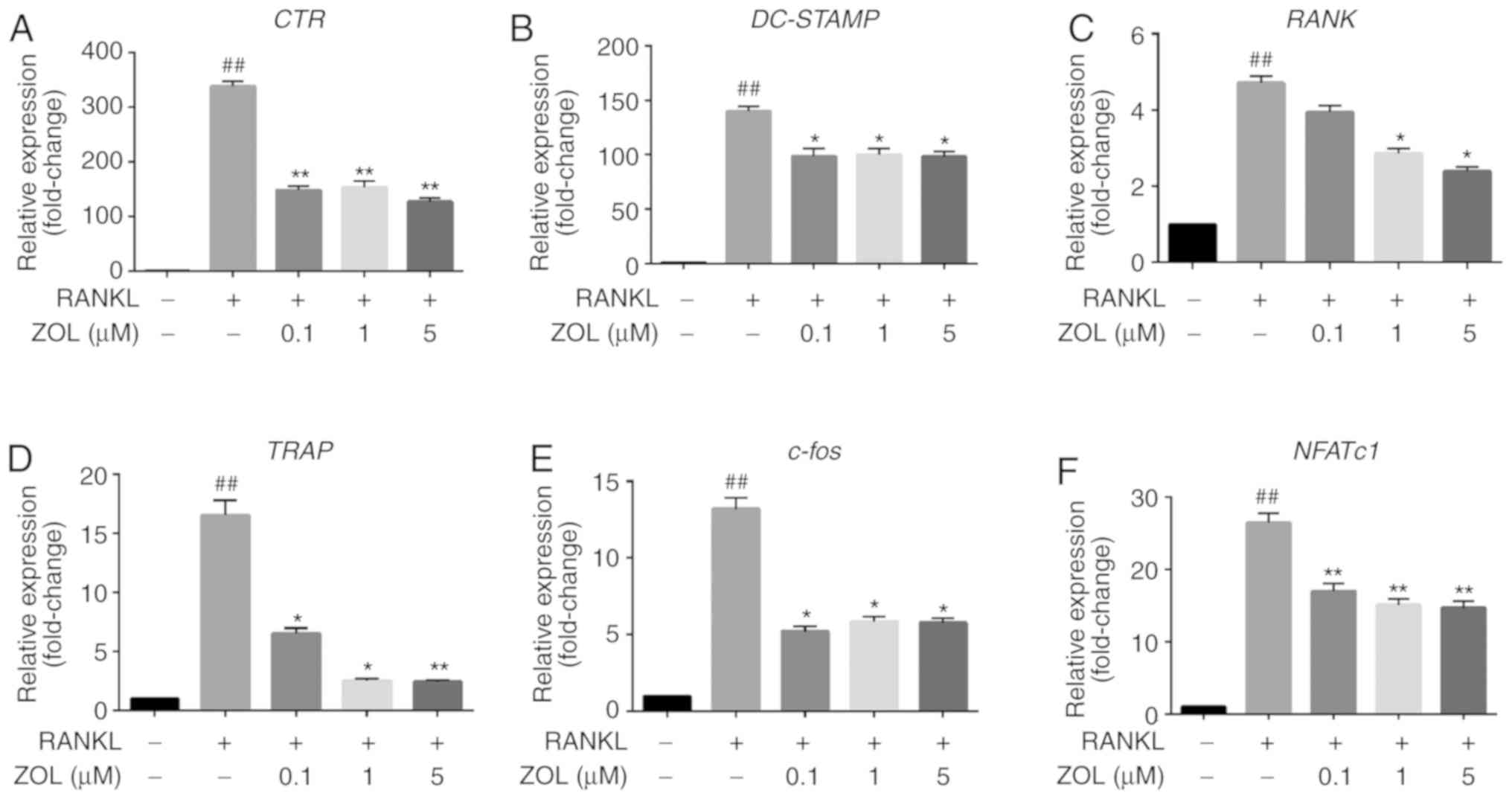 | Figure 4ZOL inhibits RANKL-induced
osteoclast-specific gene expression. RAW264.7 cells were cultured
with or without 100 ng/ml RANKL and treated with 0, 0.1, 1 or 5
µM ZOL. (A-F) The expression of osteoclast-specific genes
(CTR, DC-STAMP, RANK, TRAP, c-Fos and
NFATc1) was detected by RT-qPCR. Results were normalized to
the expression of the GAPDH gene. ##P<0.01 vs. the
vehicle group; *P<0.05, **P<0.01 vs.
the RANKL-only group. CTR, calcitonin receptor; TRAP,
tartrate-resistant acid phosphatase; DC-STAMP, dendritic
cell-specific transmembrane protein; ZOL, zoledronic acid; RANKL,
receptor activator of nuclear-κB ligand; NFATc1, nuclear factor of
activated T cells 1. |
ZOL inhibits NF-κB and JNK
signalling
Previous studies have revealed that NF-κB, p38,
ERK1/2 and JNK play critical roles in osteoclast differentiation
(Fig. 1) (32-34). To explore the pathways through
which ZOL regulates osteoclastogenesis, the protein levels of
RANKL-induced signalling pathways were investigated by western blot
analysis. As presented in Fig.
5A, the rapid activation of NF-κB was detected by the
phosphorylation of IκBα, the inhibitor of NF-κB, at 5 min following
RANKL exposure. As expected, RANKL treatment induced a significant
increase in the RANKL-induced phosphorylation of p65. In addition,
induction with RANKL markedly increased the phosphorylation levels
of p38, ERK and JNK, which exhibited a maximum increase at 10 or 20
min (Fig. 5A).
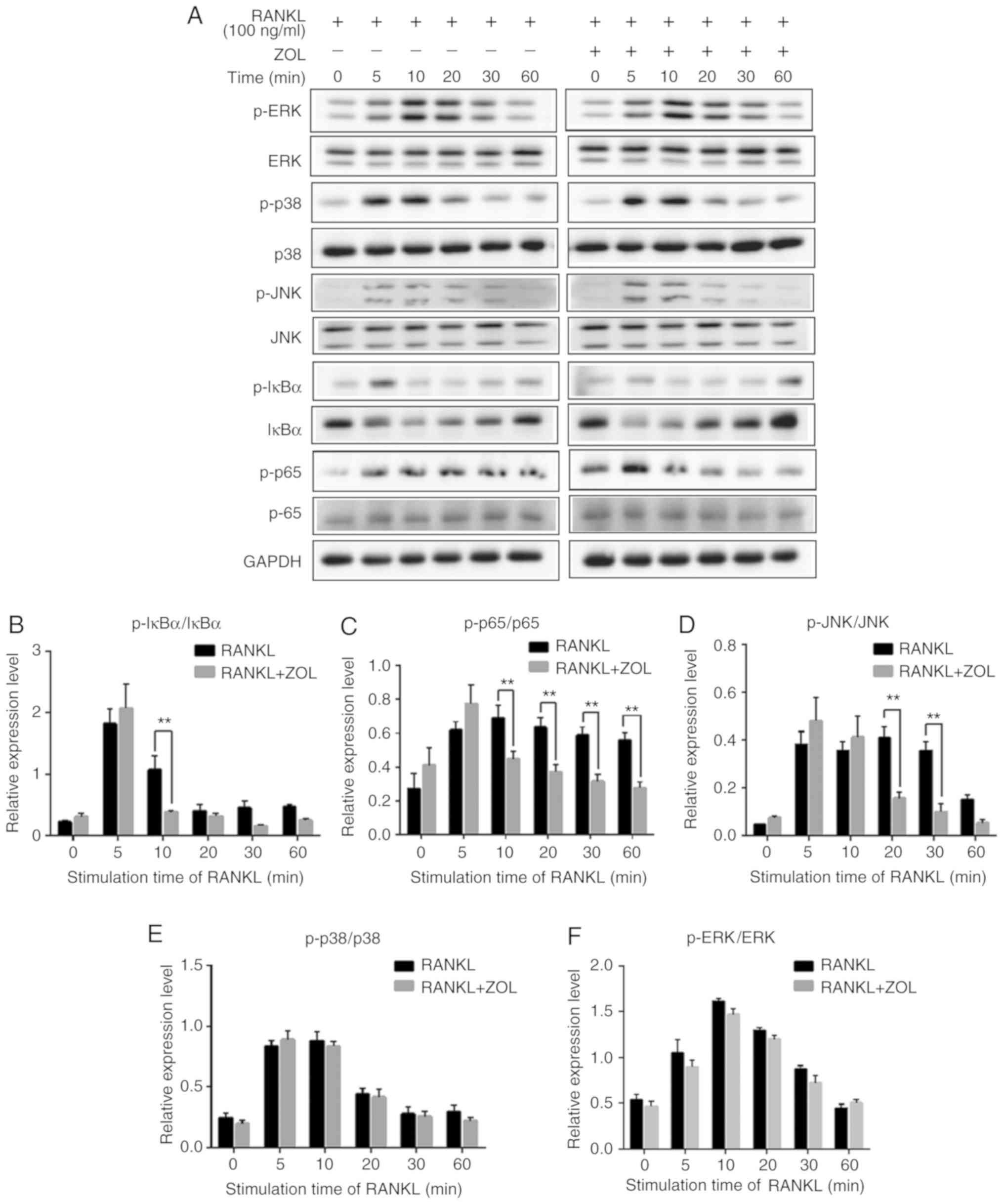 | Figure 5ZOL specifically attenuates
RANKL-induced NF-κB and JNK signalling. (A) RAW264.7 cells were
induced with RANKL for the indicated times following pre-treatment
with ZOL (5 µM) for 4 h. Western blot analysis was performed
using specific antibodies. The band intensities were quantified
using Image J software. The ratios of band intensity of (B)
p-IκBα/IκBα, (C) p-p65/p65, (D) p-JNK/JNK, (E) p-p38/p38 and (F)
p-ERK/ERK are shown. (G) ZOL inhibited osteoclast differentiation
at an early stage. RAW264.7 cells were treated with or without 5
µM ZOL at days 0, 1, 3 and 4. They were subsequently treated
with 100 ng/ml RANKL for 5 days, and then subjected to TRAP
staining. (H) The number and (I) area of osteoclasts were
quantified. *P<0.05, **P<0.01 vs. the
RANKL-only group. TRAP, tartrate-resistant acid phosphatase; ZOL,
zoledronic acid; RANKL, receptor activator of nuclear-κB
ligand. |
To further examine the influence of ZOL on NF-κB and
MAPK mediated osteoclast differentiation, the intensity of each
phosphorylated protein was divided by the intensity of
corresponding total protein in both RANKL and RANKL+ZOL treated
groups. The results revealed that ZOL significant inhibited
RANKL-dependent phosphorylation of IκBα (Fig. 5B) and p65 (Fig. 5C). Among the MAPK family proteins,
the phosphorylation of JNK (Fig.
5D) was significantly inhibited by ZOL. However, the
phosphorylation of p38 and ERK proteins were not significantly
affected by ZOL (Fig. 5E and F).
Thus, these results indicate that ZOL may inhibit NF-κB and JNK
signalling by reducing the levels of p-IκBα, p-p65 and p-JNK. In
other words, ZOL may reduce the formation of osteoclasts by
suppressing the RANKL-induced activation of the NF-κB and JNK
signalling pathways.
As reported previously, JNK and NF-κB signalling
play a vital role at the early stage of osteoclast differentiation
(35-37). Thus, it was further explored
whether ZOL also suppresses early-stage osteoclast formation.
Following the addition of RANKL, the RAW264.7 cells were treated
with ZOL on days 0, 1, 2, 3 and 4. It was observed that treatment
with ZOL at the early stage resulted in a prominent decrease in
osteoclastogenesis in the RAW264.7 cells (Fig. 5G). However, the extent of
osteoclastogenesis was comparable to that in the control group if
ZOL was added at a later stage (Fig.
5H and I). These results thus indicate that ZOL suppresses the
early stage of osteoclast formation, which is consistent with the
findings of western blot analysis, according to which ZOL
suppressed NF-κB and JNK signalling at the early stage of
osteoclast differentiation.
Discussion
Osteoporosis is a silent disease and remains a major
health concern; it is characterized by low bone mineral density and
quality, as well as an abnormal microarchitecture of bone tissue
(38). A previous study indicated
that nitrogen-containing bisphosphonates, e.g., minodronate and
alendronate, inhibit osteoclastogenesis (26,27). ZOL belongs to the class of
nitrogen-containing bisphosphonates and is widely used to prevent
bone loss. Kimachi et al (26) indicated that ZOL inhibited RANK
expression and the migration of osteoclast precursors during
osteoclastogenesis, and that the inhibitory effects on RANK
expression were likely to be associated with the suppression of the
NF-κB pathway. However, the mechanisms of the inhibitory effects of
ZOL on osteoclastogenesis remain to be fully elucidated (26,28). In the present study, the effects
of ZOL on osteoclastogenesis were explored by using RANKL-induced
RAW264.7 cells as a model. The results indicated that ZOL inhibited
osteoclast formation in dose-dependent manner at the early stage.
It was also demonstrated to impair the formation of the actin
cytoskeleton and the bone resorption ability of RANKL-induced
Raw264.7 cells. Furthermore, it was revealed that ZOL inhibited
osteoclastogenesis through the NF-κB and JNK pathways, as indicated
by the inhibition of the RANKL-induced expression CTR,
RANK, TRAP, DC-STAMP, NFATc1 and
c-Fos genes by ZOL.
The NF-κB signalling pathway may be activated by the
binding of RANKL to RANK (39,40). RANKL/RANK/TRAF6 signalling may
activate IKK and subsequently, IκB-α becomes phosphorylated and is
degraded (20). As a result,
NF-κB is released and translocated to the nucleus to increase the
expression of NFATc1 and c-Fos, which have been identified as two
important transcription factors that regulate osteoclast formation
via initiating the transcription of certain downstream targets that
are osteoclastogenesis-associated genes (41-43). Previous studies using gene
knock-out experiments have indicated that mice lacking NF-κB
dimmers may not form osteoclasts normally and present with serious
osteopetrosis (44,45). The present study suggested that
ZOL exerts an inhibitory effect on the RANKL-induced degradation of
IκB-α and phosphorylation. These results suggest that ZOL may
attenuate RANKL-induced osteoclastogenesis by blocking the NF-κB
signalling pathway.
Another group of essential signalling pathways,
namely MAPKs, are downstream pathways of RANKL/RANK/TRAF6
signalling. The RANKL-RANK interaction results in the
phosphorylation of MAPKs, including JNK, p38 and ERK, promoting the
activation of c-Fos and facilitating the translocation of activator
protein-1, an essential translation factor for osteoclast
formation. Previous studies have confirmed that inhibitors of p38,
JNK or ERK inhibit osteoclast formation (46,47). In the present study, ZOL
diminished the phosphorylation of JNK induced by RANKL. It may be
speculated that the blockade or downregulation of NF-κB and JNK
signalling pathways by ZOL may result in decreased expression of
downstream molecules required for osteoclast differentiation. Thus,
it may be suggested that ZOL exerts a marked inhibitory activity on
osteoclast differentiation through the inhibition of NF-κB and JNK
signalling. The present results not only testified the conclusion
drawn in the study by Kimachi et al (26), but also further indicated that the
JNK pathway was inhibited by ZOL. However, further studies are
required to determine the biological efficacy of ZOL in in
vitro or in vivo models and selective inhibitors of
NF-κB or JNK should also be administrated to investigate the
expression of associated proteins, so as to further verify the
present results.
In recent years, bisphosphonate therapy has been
prescribed for an increasing number of patients with osteoporosis
and bone cancer metastasis. Despite these significant advances, the
evidence for bisphosphonate-related osteonecrosis of the jaw
(BRONJ), first noted in 2003 and now widely recognised as a
complication of bisphosphonate therapy, has been increasingly
regarded as a limitation (26).
The majority of reported cases of bisphosphonate osteonecrosis were
caused by dental extractions, intraoral surgical intervention or
mucosal trauma (12). Previous
studies have also reported on the development of BRONJ along with
bacterial infection (48-50). However, the pathogenic mechanisms
of BRONJ remain elusive and successful treatments are currently
unavailable. In the present study, the cytotoxic effects of ZOL on
RAW264.7 cells and osteoclasts were first explored. It was revealed
that low concentrations of ZOL (0.1-5 µM) were non-toxic,
but suppressed osteoclast formation in a dose- and time-dependent
manner during osteoclast precursor differentiation. Based on this
finding, it can by hypothesized that exposure to ZOL at appropriate
dosages and for suitable durations may provide a benefit in the
treatment of osteoporosis. However, beyond this, it may be expected
to have severe side-effects, e.g., BRONJ. Thus, it is suggested
that avoiding overexposure to ZOL may be an effective way to avoid
BRONJ. In addition, clinical investigations are still required to
develop novel therapeutic agents that do not cause these
side-effects.
In dental implantation, osteoporosis may result in
poor primary stability and subsequent prosthetic loosening, as well
as severe inflammatory bone loss, e.g., peri-implantitis.
Therefore, it is widely discussed whether patients who have
osteoporosis are suitable for tooth implantation. Numerous attempts
have been made to identify a reliable therapeutic strategy to
prevent osteoporosis (51-53).
Since the RANKL/RANK interaction is mechanistically involved in the
pathological processes of bone loss, it has received a large amount
of attention. RANKL targeted therapy has been a valid target for
the modulation of bone formation and resorption as an approach for
the development of anti-osteoporotic and anti-resorptive drugs. For
instance, the Food and Drug Administration of the USA has approved
the anti-RANKL monoclonal antibody denosumab, which acts by
decreasing bone resorption, for the treatment of post-menopausal
women with osteoporosis (43,54,55). Furthermore, a large international
clinical trial demonstrated that osteoporotic patients treated with
ZOL exhibited significant improvements in bone mineral density and
bone metabolism markers. Treatment with ZOL reduces the risk of
vertebral fracture by 70% and hip fracture by 41% over 3 years
relative to placebo (56,57). Therefore, discovering the
underlying mechanisms of the effects of ZOL to prevent bone loss
may further promote the development of drugs for the treatment of
osteoporosis.
In conclusion, the present results may shed light on
the mechanisms of action of ZOL and the pathology of BRONJ. The
optimal dosage and timing of ZOL administration should be further
determined to enhance the prospects of this drug as a candidate for
the treatment of osteoporosis.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81660179, 31560318 and
U1812403) and the Science and Technology Foundation of Guizhou
Province [grant no. (2016)1124].
Availability of data and materials
All data generated or analysed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
JL, WH and ZZG conceived and designed the research.
XLH, LYH, YTC, FL, QZ, CW and QHS performed the experiments. WH,
XLH and LJ wrote the manuscript. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim HS, Suh KS, Sul D, Kim BJ, Lee SK and
Jung WW: The inhibitory effect and the molecular mechanism of
glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells.
Int J Mol Med. 29:169–177. 2012.
|
|
2
|
Villa A, Guerrini MM, Cassani B, Pangrazio
A and Sobacchi C: Infantile malignant, autosomal recessive
osteopetrosis: The rich and the poor. Calcif Tissue Int. 84:1–12.
2009. View Article : Google Scholar
|
|
3
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nijweide PJ, Burger EH and Feyen JH: Cells
of bone: Proliferation, differentiation, and hormonal regulation.
Physiol Rev. 66:855–886. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soysa NS, Alles N, Aoki K and Ohya K:
Osteoclast formation and differentiation: An overview. J Med Dent
Sci. 59:65–74. 2012.PubMed/NCBI
|
|
7
|
Zeng Z, Zhang C and Chen J:
Lentivirus-mediated RNA interference of DC-STAMP expression
inhibits the fusion and resorptive activity of human osteoclasts. J
Bone Miner Metab. 31:409–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng XZ, He LG, Wang S, Wang K, Zhang YY,
Tao L, Li XJ and Liu SW: Aconine inhibits RANKL-induced osteoclast
differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1
activation and DC-STAMP expression. Acta Pharmacol Sin. 37:255–263.
2016. View Article : Google Scholar
|
|
9
|
Oikawa T, Kuroda Y and Matsuo K:
Regulation of osteoclasts by membrane-derived lipid mediators. Cell
Mol Life Sci. 70:3341–3353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liou YM, Chan CL, Huang R and Wang CA:
Effect of l-caldesmon on osteoclastogenesis in RANKL-induced
RAW264.7 cells. J Cell Physiol. 233:6888–6901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Islam R, Bae HS, Yoon WJ, Woo KM, Baek JH,
Kim HH, Uchida T and Ryoo HM: Pin1 regulates osteoclast fusion
through suppression of the master regulator of cell fusion
DC-STAMP. J Cell Physiol. 229:2166–2174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abe K, Yoshimura Y, Deyama Y, Kikuiri T,
Hasegawa T, Tei K, Shinoda H, Suzuki K and Kitagawa Y: Effects of
bisphosphonates on osteoclastogenesis in RAW264.7 cells. Int J Mol
Med. 29:1007–1015. 2012.PubMed/NCBI
|
|
13
|
Mediero A, Perez-Aso M and Cronstein BN:
Activation of adenosine A(2A) receptor reduces osteoclast formation
via PKA- and ERK1/2-mediated suppression of NFκB nuclear
trans-location. Br J Pharmacol. 169:1372–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan M, Chen J and Zeng Z: Knockdown of
macrophage inhibitory cytokine-1 in RPMI-8226 human multiple
myeloma cells inhibits osteoclastic differentiation through
inhibiting the RANKL-Erk1/2 signaling pathway. Mol Med Rep.
14:5199–5204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
17
|
Huang P, Han J and Hui L: MAPK signaling
in inflammation- associated cancer development. Protein Cell.
1:218–226. 2010. View Article : Google Scholar
|
|
18
|
Ihn HJ, Lee D, Lee T, Shin HI, Bae YC, Kim
SH and Park EK: The 1,2,3-triazole derivative KP-A021 suppresses
osteoclast differentiation and function by inhibiting
RANKL-mediated MEK-ERK signaling pathway. Exp Biol Med (Maywood).
240:1690–1697. 2015. View Article : Google Scholar
|
|
19
|
Negishi-Koga T and Takayanagi H:
Ca2+-NFATc1 signaling is an essential axis of osteoclast
differentiation. Immunol Rev. 231:241–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Wang Z, Xie X, Wang J, Wang Y,
Peng QS, Zhang M, Wu D, Liu N, Wang HB and Sun WC: Tatarinan N
inhibits osteoclast differentiation through attenuating NF-κB,
MAPKs and Ca2+-dependent signaling. Int Immunopharmacol.
65:199–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakashima T and Takayanagi H:
Osteoimmunology: Crosstalk between the immune and bone systems. J
Clin Immunol. 29:555–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lambrinoudaki I, Vlachou S, Galapi F,
Papadimitriou D and Papadias K: Once-yearly zoledronic acid in the
prevention of osteoporotic bone fractures in postmenopausal women.
Clin Interv Aging. 3:445–451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dalle Carbonare L, Zanatta M, Gasparetto A
and Valenti MT: Safety and tolerability of zoledronic acid and
other bisphosphonates in osteoporosis management. Drug Healthc
Patient Saf. 2:121–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benford HL, McGowan NW, Helfrich MH,
Nuttall ME and Rogers MJ: Visualization of bisphosphonate-induced
caspase-3 activity in apoptotic osteoclasts in vitro. Bone.
28:465–473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yasen M, Li X, Jiang L, Yuan W, Che W and
Dong J: Effect of zoledronic acid on spinal fusion outcomes in an
ovariectomized rat model of osteoporosis. J Orthop Res.
33:1297–1304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimachi K, Kajiya H, Nakayama S, Ikebe T
and Okabe K: Zoledronic acid inhibits RANK expression and migration
of osteoclast precursors during osteoclastogenesis. Naunyn
Schmiedebergs Arch Pharmacol. 383:297–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsubaki M, Komai M, Itoh T, Imano M,
Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, Fujiwara D,
et al: Nitrogen-containing bisphosphonates inhibit RANKL- and
M-CSF-induced osteoclast formation through the inhibition of ERK1/2
and Akt activation. J Biomed Sci. 21:102014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tai TW, Su FC, Chen CY, Jou IM and Lin CF:
Activation of p38 MAPK-regulated Bcl-xL signaling increases
survival against zoledronic acid-induced apoptosis in osteoclast
precursors. Bone. 67:166–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Teitelbaum SL: Osteoclasts: What do they
do and how do they do it? Am J Pathol. 170:427–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jurdic P, Saltel F, Chabadel A and
Destaing O: Podosome and sealing zone: Specificity of the
osteoclast model. Eur J Cell Biol. 85:195–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stevenson DA, Schwarz EL, Carey JC,
Viskochil DH, Hanson H, Bauer S, Weng HY, Greene T, Reinker K,
Swensen J, et al: Bone resorption in syndromes of the Ras/MAPK
pathway. Clin Genet. 80:566–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suda T, Kobayashi K, Jimi E, Udagawa N and
Takahashi N: The molecular basis of osteoclast differentiation and
activation. Novartis Found Symp. 232:235–247; discussion 247-250.
2001.PubMed/NCBI
|
|
34
|
Li DZ, Zhang QX, Dong XX, Li HD and Ma X:
Treatment with hydrogen molecules prevents RANKL-induced osteoclast
differentiation associated with inhibition of ROS formation and
inactivation of MAPK, AKT and NF-kappa B pathways in murine
RAW264.7 cells. J Bone Miner Metab. 32:494–504. 2014. View Article : Google Scholar
|
|
35
|
Ikeda F, Nishimura R, Matsubara T, Tanaka
S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, et
al: Critical roles of c-Jun signaling in regulation of NFAT family
and RANKL-regulated osteoclast differentiation. J Clin Invest.
114:475–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ikeda F, Matsubara T, Tsurukai T, Hata K,
Nishimura R and Yoneda T: JNK/c-Jun signaling mediates an
anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res.
23:907–914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siddiqi MH, Siddiqi MZ, Kang S, Noh HY,
Ahn S, Simu SY, Aziz MA, Sathishkumar N, Jiménez Pérez ZE and Yang
DC: Inhibition of osteoclast differentiation by ginsenoside rg3 in
RAW264.7 cells via RANKL, JNK and p38 MAPK pathways through a
modulation of cathepsin k: An in silico and in vitro study.
Phytother Res. 29:1286–1294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng X and McDonald JM: Disorders of bone
remodeling. Annu Rev Pathol. 6:121–145. 2011. View Article : Google Scholar
|
|
39
|
Wu K, Lin TH, Liou HC, Lu DH, Chen YR, Fu
WM and Yang RS: Dextromethorphan inhibits osteoclast
differentiation by suppressing RANKL-induced nuclear factor-κB
activation. Osteoporos Int. 24:2201–2214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang MR, Jo SA, Yoon YD, Park KH, Oh SJ,
Yun J, Lee CW, Nam KH, Kim Y, Han SB, et al: Agelasine D suppresses
RANKL-induced osteoclastogenesis via down-regulation of c-Fos,
NFATc1 and NF-κB. Mar Drugs. 12:5643–5656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee CC, Liu FL, Chen CL, Chen TC, Chang DM
and Huang HS: Discovery of 5-(2′,4′-difluorophenyl)-salicylanilides
as new inhibitors of receptor activator of NF-κB ligand
(RANKL)-induced osteoclastogenesis. Eur J Med Chem. 98:115–126.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu W and Zhang X: Receptor activator of
nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in
bone and other tissues (review). Mol Med Rep. 11:3212–3218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao XL, Chen JJ, Si SY, Chen LF and Wang
Z: T63 inhibits osteoclast differentiation through regulating MAPKs
and Akt signaling pathways. Eur J Pharmacol. 834:30–35. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim HJ, Yoon KA, Lee MK, Kim SH, Lee IK
and Kim SY: A novel small molecule, NecroX-7, inhibits osteoclast
differentiation by suppressing NF-κB activity and c-Fos expression.
Life Sci. 91:928–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Leotoing L, Wauquier F, Guicheux J,
Miot-Noirault E, Wittrant Y and Coxam V: The polyphenol fisetin
protects bone by repressing NF-κB and MKP-1-dependent signaling
pathways in osteoclasts. PLoS One. 8:e683882013. View Article : Google Scholar
|
|
46
|
Kong X, Wu W, Yang Y, Wan H, Li X, Zhong
M, Zhao H, Su X, Jia S, Ju D and Lin N: Total saponin from Anemone
flaccida Fr. Schmidt abrogates osteoclast differentiation and bone
resorption via the inhibition of RANKL-induced NF-κB, JNK and p38
MAPKs activation. J Transl Med. 13:912015. View Article : Google Scholar
|
|
47
|
Xu X, Liu N, Wang Y, Pan LC, Wu D, Peng Q,
Zhang M, Wang HB and Sun WC: Tatarinan O, a lignin-like compound
from the roots of Acorus tatarinowii Schott inhibits osteoclast
differentiation through suppressing the expression of c-Fos and
NFATc1. Int Immunopharmacol. 34:212–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fliefel R, Troltzsch M, Kuhnisch J,
Ehrenfeld M and Otto S: Treatment strategies and outcomes of
bisphosphonate-related osteonecrosis of the jaw (BRONJ) with
characterization of patients: A systematic review. Int J Oral
Maxillofac Surg. 44:568–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sakaguchi O, Kokuryo S, Tsurushima H,
Tanaka J, Habu M, Uehara M, Nishihara T and Tominaga K:
Lipopolysaccharide aggravates bisphosphonate-induced osteonecrosis
in rats. Int J Oral Maxillofac Surg. 44:528–534. 2015. View Article : Google Scholar
|
|
50
|
Wachi T, Shuto T, Shinohara Y, Matono Y
and Makihira S: Release of titanium ions from an implant surface
and their effect on cytokine production related to alveolar bone
resorption. Toxicology. 327:1–9. 2015. View Article : Google Scholar
|
|
51
|
Baek JM, Kim JY, Lee CH, Yoon KH and Lee
MS: Methyl gallate inhibits osteoclast formation and function by
suppressing Akt and Btk-PLCgamma2-Ca(2+) signaling and prevents
lipo-polysaccharide-induced bone loss. Int J Mol Sci. 18:E5812017.
View Article : Google Scholar
|
|
52
|
Sun X, Wei J, Lyu J, Bian T, Liu Z, Huang
J, Pi F, Li C and Zhong Z: Bone-targeting drug delivery system of
biomineral-binding liposomes loaded with icariin enhances the
treatment for osteoporosis. J Nanobiotechnology. 17:102019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yasuda H: The mechanism of anti-RANKL
antibody in the treatment of metabolic bone diseases including
osteoporosis-possible applications of anti-RANKL antibody to the
treatment of cancer patients. Nihon Yakurigaku Zasshi. 153:11–15.
2019.In Japanese. View Article : Google Scholar
|
|
54
|
Moen MD and Keam SJ: Denosumab: A review
of its use in the treatment of postmenopausal osteoporosis. Drugs
Aging. 28:63–82. 2011. View Article : Google Scholar
|
|
55
|
Sidlauskas KM, Sutton EE and Biddle MA:
Osteoporosis in men: Epidemiology and treatment with denosumab.
Clin Interv Aging. 9:593–601. 2014.PubMed/NCBI
|
|
56
|
Tai TW, Chen CY, Su FC, Tu YK, Tsai TT,
Lin CF and Jou IM: Reactive oxygen species are required for
zoledronic acid-induced apoptosis in osteoclast precursors and
mature osteoclast-like cells. Sci Re. 7:442452017.
|
|
57
|
Lyles KW, Colon-Emeric CS, Magaziner JS,
Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C,
Nordsletten L, Moore KA, et al: Zoledronic acid and clinical
fractures and mortality after hip fracture. N Engl J Med.
357:1799–1809. 2007. View Article : Google Scholar : PubMed/NCBI
|