Introduction
Diabetes and obesity are both associated with
lipotoxic cardiomyopathy exclusive of coronary artery disease and
hypertension (1-3). Lipotoxicities have become a public
health concern and are responsible for a significant portion of
clinical cardiac disease (4-7).
These abnormalities may be the result of a toxic metabolic shift to
more fatty acid and less glucose oxidation with concomitant
accumulation of toxic lipids (5).
Lipids can directly alter cellular structures and activate
downstream pathways, leading to toxicity (5). Previous data have implicated fatty
acids, fatty acyl coenzyme A, diacylglycerol, and ceramide in
cellular lipotoxicity, which may be caused by apoptosis, defective
insulin signaling, endoplasmic reticulum stress, activation of
protein kinase C, mitogen associated protein kinase activation, or
modulation of peroxisome proliferator-activated receptors (5). Free fatty acids (FFAs), a key of
type fat originating from the adipose tissue in the human body, can
function as an essential energy provider at their normal level and
are able to provide ~70% of adenosine triphosphate to
cardiomyocytes (8). A number of
studies have revealed that lipid deposition in cardiac tissue would
increase provided that the level of FFAs is significantly increased
compared with the normal value (9-11).
This type of increase in lipid deposition could cause the rise of
intracellular active oxygen species (ROS) in cardiomyocytes,
commonly called as lipotoxicity and such excessive levels of ROS
will in turn result in apoptosis in cardiomyocytes and related
cardiac complications (11-14). Therefore, inhibiting the rise of
intracellular ROS in cardiomyocytes would be a viable approach for
protecting the myocardium against lipotoxic injury. A previous
study on FFAs-induced myocardial injury indicated that
AMP-activated protein kinase (AMPK) was able to effectively inhibit
the ROS increase in cardiomyocytes by attenuating oxidative stress
and concomitantly, prevented the cardiomyocytes from apoptosis
(15). With this in mind, it can
be envisioned that the lipotoxic myocardial injury could be avoided
if certain antioxidants can be used for suppressing the
intracellular ROS increase by attenuating oxidative stress and
meanwhile, for activating the AMPK pathway in a safe, and effective
manner.
Curcumin (CUR) is a hydrophobic polyphenol
derivative that is usually extracted from natural herbs and it has
diverse pharmacological properties (16-18). In particular, CUR is relatively
safe to normal tissues in its therapeutic dose range. CUR has been
used as anti-inflammatory, antioxidant and anticancer agents over a
long period of time for the treatments of a number of types of
diseases, including varied types of cancers, cardiovascular
diseases and autoimmune diseases (19-24). A new study reveals that curcumin
has the ability to attenuate oxidative stress in the myocardium and
can play a protective and therapeutic role in the protection of the
myocardium against lipotoxic injury owing to its lipid-lowering
properties (25). In another
study involving diabetic rats, CUR shows the ability to reduce
cardiomyocyte remodeling and relieve cardiac insufficiency
(26). Nevertheless, satisfactory
pharmaceutical efficacy of CUR is difficult to achieve even though
a high dosage is applied because CUR is very hydrophobic, which
will lead to its rapid elimination in vivo; and in addition,
CUR is unstable in an aqueous medium and can be quickly degraded
in vivo (27,28). Therefore, it is necessary to use
suitable carriers to deliver CUR in order to prevent its
degradation, enhance its intracellular aggregation and increase its
bioavailability.
Nowadays, biocompatible nanoparticles (NPs) with
hydrophilic surface properties are frequently used for delivering
hydrophobic drugs because these NPs can prolong the in vivo
circulation of the loaded drugs and thus, increase their efficiency
and bioavailability (27,28). In this study, an attempt was made
to fabricate a type of CUR-loaded NPs using an amphiphilic
copolymer, monomethoxy poly (ethylene glycol)-b-poly (DL-lactide;
PEG-PDLLA), as a vehicle material to protect CUR from degradation
while enhancing its intracellular accumulation. A model based on
palmitate-induced cardiomyocyte injury was used to evaluate the
performance of CUR-loaded NPs and it was also examined whether
these CUR-loaded NPs had an ability to suppress the intracellular
ROS increase by attenuating oxidative stress in cardiomyocytes
through an unexplored AMPK pathway.
Materials and methods
Materials
H9C2 cardiomyocytes were procured from the Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS)
and type-II collagenase were purchased from Gibco; Thermo Fisher
Scientific, Inc. A malondialdehyde (MDA) assay kit was purchased
from Nanjing Jiancheng Bioengineering Institute. The superoxide
dismutase (SOD) assay kit was purchased from Dojindo Molecular
Technologies, Inc. An ROS assay kit was obtained from Applygen
Technologies, Inc. Terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL) detection kit was purchased from Roche
Diagnostics. Rabbit polyclonal antibodies against Bcl-2, Bcl-2
associated X Protein (Bax), phosphorlyated AMPK (p-AMPK), total
AMPK, p-mammalian target of rapamycin complex-1 (p-mTORC1), total
mTORC1, p-p70 ribosomal protein S6 kinase (p-p70S6K), total p70S6K
and β-actin were purchased from Cell Signaling Technology, Inc.
Hybond C membranes and ECL western blot detection kit were
purchased from Pierce; Thermo Fisher Scientific, Inc. The MTT assay
kit and dorsomorphin (compound C) were purchased from
Sigma-Aldrich; Merck KGaA. PEG-PDLLA (PEG, 5 kDa, PDLLA, 10 kDa)
was bought from Advanced Polymer Materials Inc. Palmitate and all
other reagents were purchased from Sigma-Aldrich; Merck KGaA.
Preparation and characterization of
CUR-loaded NPs
CUR-loaded NPs were prepared following a method
described elsewhere (29). In
brief, CUR (1 mg) and PEG-PDLLA (9 mg) were dissolved into
tetrahydrofuran (4 ml). This solution was added dropwise into 10 ml
distilled water with stirring. The mixture was then dialyzed
against water at ambient temperature for 3 days to form NPs and the
resulting CUR-loaded NPs were lyophilized for further use. For the
sake of simplicity, these CUR-loaded NPs are abbreviated as CUR NPs
in the following text.
CUR NPs were dispersed in methanol with ultrasonic
treatment and the amount of the extracted CUR was measured using
high performance liquid chromatography (Shimadzu LC-20AD) under the
following running conditions (Eclipse XDB-C18 column; 150×4.6 mm; 5
µm; Agilent Technologies, Inc.): Dexamethasone acetate was
used as internal standard; mobile phase, methanol containing 3 mM
mono potassium phosphate and acetic acid (methanol/mono potassium
phosphate/acetic acid/water=230/20/2/748, v/v); flow rate, 1.0
ml/min; injection volume, 20 µl; column temperature, 25°C;
and detection wavelength, 227 nm. Drug loading (DL) and loading
efficiency (LE) of CUR NPs were calculated using the following
formulas:
DL(%)=(M0/M)×100%
LE(%)=(M0/M1)×100%
where M0 is the mass of CUR encapsulated in NPs,
M1 is the mass of fed CUR and M is the
mass of NPs.
The morphology of NPs was viewed using a
transmission electron microscope (TEM; Tecnai G2-20). Size
distribution of NPs was determined using a dynamic light scattering
instrument equipped with a vertically polarized He-Ne laser (Wyatt
Technology, Ltd.). zeta potential (ζ) of NPs was measured using a
Nano-ZS instrument.
In vitro release
CUR NPs were suspended in 2 ml PBS (pH 7.4)
containing Tween-80 (1.0 wt%) and the solution was added to the
dialysis bag (MW cutoff: 3.5 kDa) for releasing testing. Briefly,
the sealed dialysis bag was introduced into a vial and immersed in
8 ml PBS. The vial was shaken on a shaking table at a frequency of
1 Hz at 37°C. At intervals of 2 h, 1 ml of medium was withdrawn and
the vial was replenished with the same volume of fresh buffer. The
released amount of CUR was determined via UV-vis analysis at a
detecting wavelength of 427 nm.
MTT assay
H9C2 cells were expended in DMEM, containing 2.25
g/l glucose and supplementing with 10% FBS, 100 U/ml penicillin,
and 100 mg/ml streptomycin in a humidified atmosphere of 5%
CO2 at 37°C. The expended H9C2 cells were suspended in
PBS for further use. H9C2 cells were seeded in 96-well plates at a
density of 1×105 cells/well. After incubation overnight,
cells were co-cultured with 0-100 µM of CUR or CUR NPs for a
period up to 48 h. At the end of the prescribed incubation periods,
cells in each well were thoroughly rinsed with PBS and additionally
incubated with the MTT agent (0.5 mg/ml) for 4 h at 37°C. After
that, the media were fully discarded and DMSO was added to each
well prior to spectro-photometric measurements (570 nm).
Cellular uptake
H9C2 cells were seeded in 6-well culture plates
(2×104 cells/well) in which each well was preset with a
cell culture slide and these cells were cultured with complete
medium for 24 h. After that, cells were treated with CUR or CUR NPs
(CUR equivalent: 100 µM in both cases) for 1 h or 24 h at
37°C. After removal of the supernatant, cells were washed with PBS
for 3 times and fixed in 800 µl formaldehyde solution (4%)
at ambient temperature for 20 min. These cells were subsequently
stained with DAPI for 10 min at room temperature and imaged by
using a confocal laser scanning microscope (CLSM; Olympus
Corporation).
Measurement of ROS levels
Dihydroethidium (DHE; Vigorous Biotechnology, Co.,
Ltd.), a ROS-level indicative fluorescence probe
(λex=535 nm, λem=610 nm), was used to detect
intracellular superoxide anions. Briefly, H9C2 cells were seeded in
6-well plates (2×104 cells/well) and cultured with
complete medium for 24 h. Cells were then divided into different
groups and exposed to palmitate (0.2 mM) or palmitate (0.2 mM)+CUR
NPs (CUR equivalent: 100 µM), respectively, for 24 h at
37°C. Afterwards, cells were washed with cold PBS and further
incubated with fresh medium containing 10 µM DHE at 37°C in
the dark for 20 min to perform nuclei staining. The harvested cells
were resuspended in PBS at a density of 2×107 cells/ml,
transferred to a light-shielded 96-well plate (100 µl cell
suspension per well), followed by determination of DHE intensity
using a fluorescence microplate reader (Bio-Rad Laboratories,
Inc.). Fluorescence microscopy (Olympus Corporation) was also used
to observe the intensity of the fluorescent signals.
Determination of major biochemical
parameters
MDA and SOD were measured using an MDA assay kit and
a SOD activity kit, respectively. MDA acted as an index for
indicating the intracellular oxidative stress levels and SOD was
served as an indicator for reflecting the extent of attenuation of
oxidative stress levels. Briefly, H9C2 cells that were cultured
with complete medium for 24 h were divided into different groups
and these groups were treated with palmitate (0.2 mM) or palmitate
(0.2 mM)+CUR NPs (prescribed CUR equivalent: 40, 80 and 100
µM) for 24 h at 37°C. The harvested cells were lysed with
RIPA lysis buffer (Cell Signaling Technology, Inc.) and the
supernatant was collected by centrifugation at 12,000 × g for 10
min at 4°C. Protein content in the supernatant was
detected using a bicinchoninic acid (BCA) kit. In addition, 100
µl supernatant was introduced into a centrifuge tube and an
MDA testing solution (200 µl) was added. After being mixed,
the mixture was boiled for 15 min and cooled to room temperature,
followed by centrifugation at 1,000 × g for 10 min at 4°C. A total
of 200 µl prepared supernatant was added to 96-well plate
and the absorbance was measured at 532 nm using a microplate reader
(Bio-Rad Laboratories, Inc.).
SOD activity was determined based on its ability to
inhibit the oxidation of oxymine by O2-• that was
produced from the xanthine/xanthine-oxidase system. The harvested
cells were homogenized and the obtained homogenate was centrifuged
at 12,000 × g for 10 min at 4°C. The protein content in supernatant
was determined using a BCA kit. To a centrifuge tube, 20 µl
of supernatant and 160 µl NBT/enzymatic working solution
were added, and mixed at 4°C for 5 min. The mixture was then added
with 20 µl of reaction-initiating working solution,
incubated at 37°C for 30 min. Such produced mixture was detected
for its absorbance at 560 nm using the same microplate reader
mentioned above.
TUNEL testing
An in situ cell death detection kit (Roche
Diagnostics) was employed for identifying apoptotic cells in
situ. H9C2 cells were seeded in 6-well plates and incubated
with complete medium for 24 h. They were divided into different
groups and treated with palmitate (0.2 mM) or palmitate (0.2
mM)+CUR NPs (CUR equivalent: 100 µM) for 24 h at 37°C,
respectively. The washed cells were subjected to the TUNEL testing
following the methods provided by the assay kit supplier. After
that, DAPI nucleus staining was performed using the same method
described above. Fluorescence intensity (red, TUNEL; and blue,
DAPI) was measured with a fluorescence microscope equipped with
microscope image analysis software (Olympus Stream v. 1.7; Olympus
Corporation) and images were taken using an electronic camera.
Apoptosis rate of cells was determined with flow cytometry (Beckman
Coulter, Inc.) following a method described in the literature
(30).
Western blot analysis
The abrogation effect of CUR NPs on
palmitate-induced cardiomyocyte apoptosis was examined via western
blot analysis. H9C2 cells cultured with complete medium for 24 h
were assigned into different groups and they were treated with
palmitate (0.2 mM) or palmitate (0.2 mM)+CUR NPs (CUR equivalent:
100 µM) for 24 h at 37°C. After that, the washed cells were
lysed with RIPA lysis buffer (Cell Signaling Technology, Inc.) for
30 min and cell lysates were collected by centrifugation at 12,000
× g and 4°C for 15 min. Protein concentrations in cell extracts
were determined using the bicinchoninic acid protein assay.
Approximately 30-50 µg of protein per sample was separated
by SDS-PAGE (8-12%) and transferred to a PVDF membrane. After
blocking the membrane with 5% nonfat milk for 1 h at room
temperature, the following primary antibodies were used for
blotting (all at 1:1,000 dilution): Bcl-2 (cat. no. 15071), Bax
(cat. no. 5023), p-AMPK (cat. no. 2535), AMPK (cat. no. 5831),
p-mTORC1 (cat. no. 5536), mTORC1 (cat. no. 2972), p-p70S6K (cat.
no. 9209), p70S6K (cat. no. 9202) and β-actin (cat. no. 4970).
Primary antibodies were incubated with membranes overnight at 4°C.
The membrane was then incubated with horseradish peroxidase-linked
rabbit anti-mouse IgG (cat. no. ab6728; 1:2,000; Abcam) for 2 h at
room temperature. Finally, the blots were visualized using a
chemiluminescence system and quantified using an image analysis
software (GeneTools; SynGene).
To examine the effect of CUR NPs on the regulation
of AMPK pathway in cardiomyocytes, compound C, a type of commonly
used inhibitor for AMPK pathway, was employed as a competitive
inhibitor when CUR NPs were cultured with cardiomyocytes. H9C2
cells cultured with complete medium for 24 h were divided into
different groups and these groups were respectively exposed to
palmitate (0.2 mM), palmitate (0.2 mM)+CUR NPs (CUR equivalent: 100
µM), palmitate (0.2 mM)+CUR NPs (CUR equivalent: 100
µM)+compound C (10 µM) for 24 h at 37°C. In the case
of compound C involved group, compound C was applied to cells for 1
h prior to palmi-tate treatment or the treatment of palmitate+CUR
NPs. The expression levels of several specific proteins, including
Bcl-2, Bax, AMPK, p-AMPK, mTORC1, p-mTORC1, p70S6K and p-p70S6K,
were measured using western blot analysis. In all above mentioned
H9C2 cell experiments, the untreated cells were used as
controls.
Statistical analysis
All experiments were performed a minimum of three
times, and the data were analyzed using GraphPad Prism v5 software
(GraphPad Software, Inc.). Data are presented as the means ±
standard deviation, and were analyzed using one-way analysis of
variance followed by Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Basic parameters of CUR NPs
In this study, an attempt was made to prepare a type
of CUR NPs that have small sizes and high DL as well as rational
LE. A preparation of NPs was optimized by mainly focusing on three
factors such as size, DL and LE of NPs based on an orthogonal test,
and optimal conditions are summarized in the experimental section.
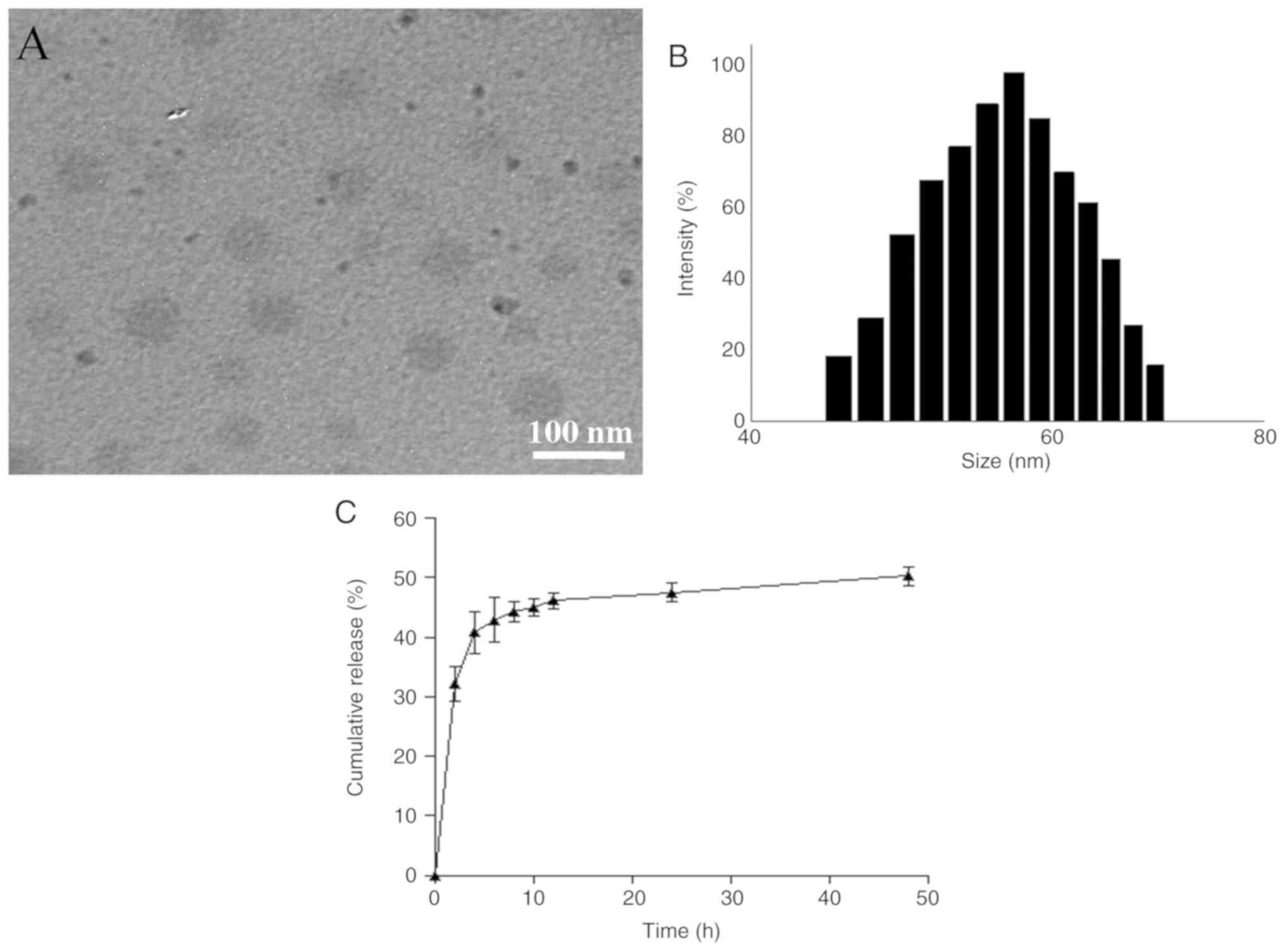
Fig. 1A shows a representative
TEM image for the optimally prepared CUR NPs, indicating that these
NPs exhibited a size <100 nm with good size uniformity. Fig. 1B presents the size of CUR NPs that
had an approximate Gaussian distribution charac ter. Average values
for several parameters such as mean size, polydispersity index,
zeta potential (ζ), DL and LE were calculated and data are listed
in Table I. It can be seen from
Table I that these CUR NPs had
small sizes, nearly neutral surface charge nature and high DL,
suggesting that they have potential for practical applications.
 | Table IParameters of CUR NPs. |
Table I
Parameters of CUR NPs.
| Sample name | Mean size (nm) | Polydispersity
index | ζ (mV) | Drug loading
(wt%) | Loading efficiency
(%) |
|---|
| CUR NPs | 57.09±4.52 | 0.19 | 0.44±0.018 | 8.81±0.92 | 82.3±3.71 |
Release profile of CUR NPs
Fig. 1C shows the
release profile for CUR NPs. The curve exhibits that CUR was
released from CUR NPs at fast rates during the first few hours and
cumulative amount of the released CUR reached ~40% within 4 h.
After that, the release rate of CUR NPs slowed down and entered a
plateau region after 12 h release.
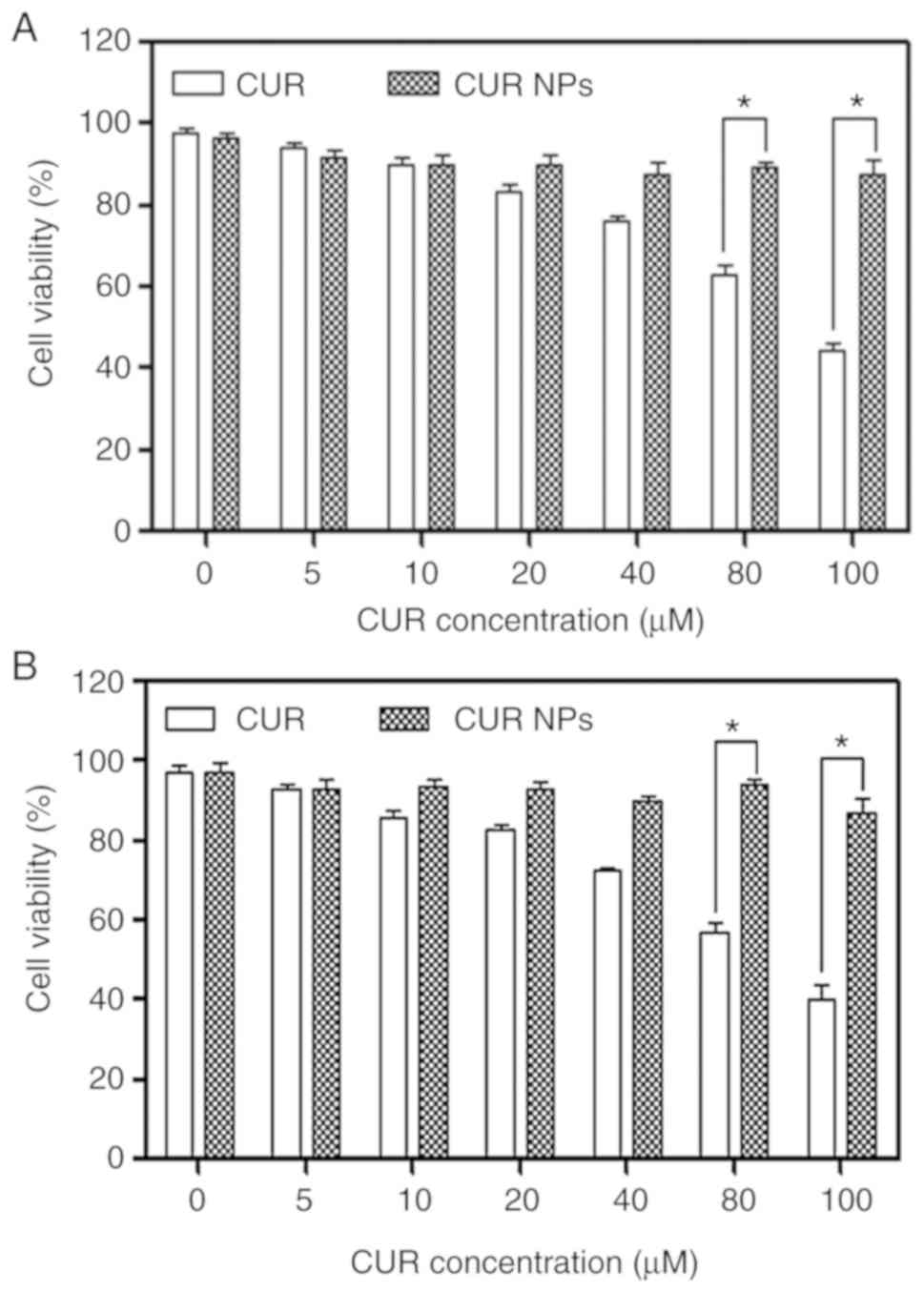
Effect of CUR NPs on viability of H9C2
cells
H9C2 cells were treated with varied amounts of CUR
NPs for a given period in order to figure out the safe dosage of
applicable CUR NPs and the obtained data are presented in Fig. 2. In cases of CUR treatment, the
viability of the treated cells was visibly dependent on the applied
CUR dose and the treatment time interval. After 24 h CUR treatment,
the viability of cells became ~80% or less when the applied CUR
dose reached 40 µM or higher; and with respect to 48 h CUR
treatment, the CUR dose had to be limited to <20 µM if
the cell viability needs to be maintained at ~80% or higher. As for
CUR NPs, the treated cells had a viability >90% even though the
CUR equivalent was 100 µM and the treatment time period
reached 48 h. These results verify that a much higher CUR amount
can be applied to H9C2 cells in comparison to the free CUR when NPs
are employed as a vehicle.
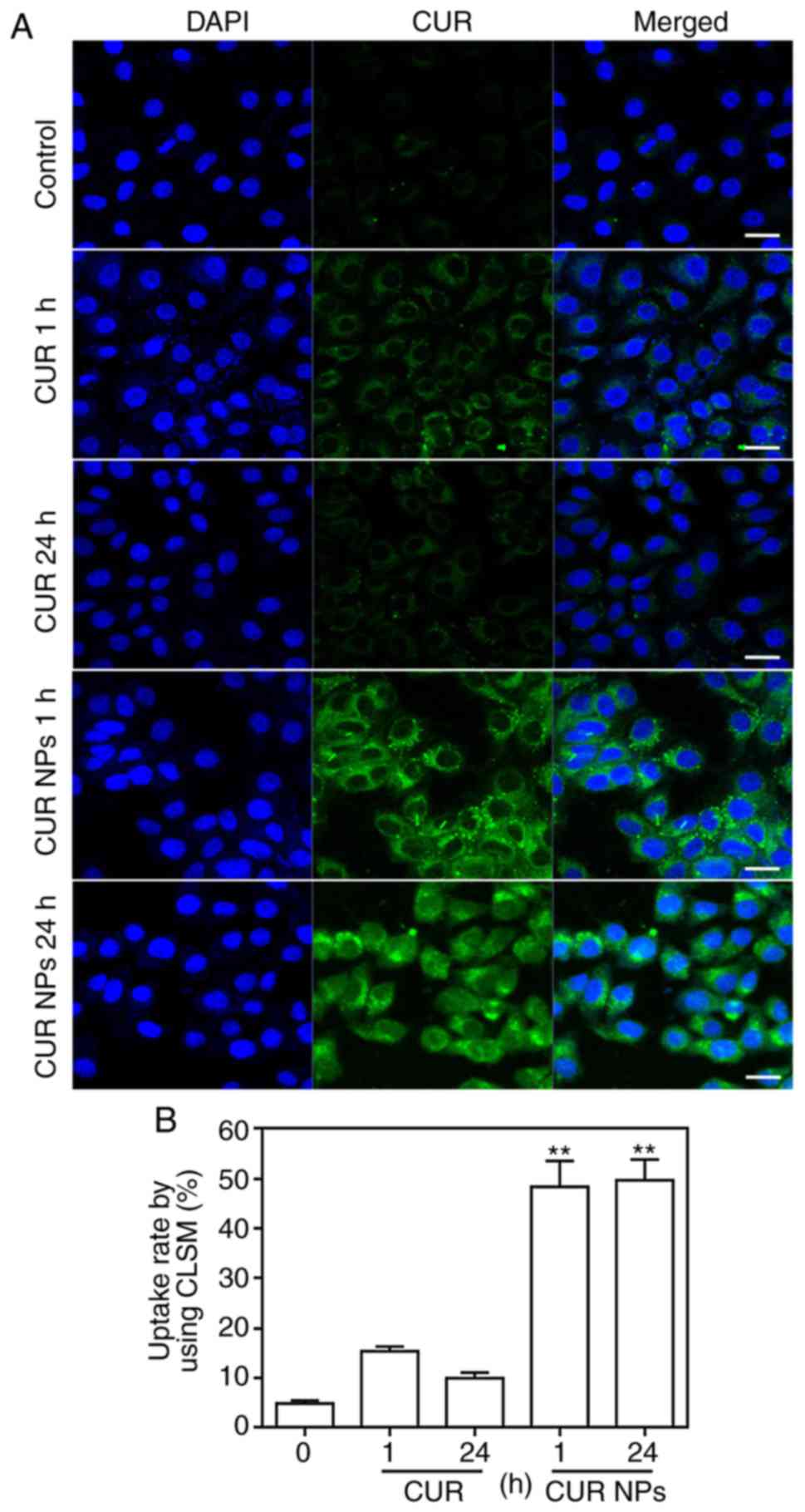
Cellular uptake assessment
To view the cellular uptake of CUR NPs, H9C2 cells
were incubated with CUR or CUR NPs for 1 h or 24 h, respectively
and imaged by CLSM for comparison. As shown in Fig. 3, in both cases of 1 and 24 h, the
fluorescence intensity of CUR-NPs group was stronger compared with
the CUR group. Furthermore, it was found that as incubation time
increased, the fluorescence intensity of free molecule CUR was
notably weakened, but the fluorescence intensity of intracellular
CUR-NPs was not altered significantly. The reason for these results
is that the high concentration of free molecule CUR has toxic
effects on cardiomyocytes and hence, cells undergo certain
apoptosis-related morphological changes such as cell membrane
rupture, and free molecule CUR gradually overflows from the cells,
resulting in a decrease in intracellular fluorescence intensity.
However, CUR-NPs with the same equivalent CUR concentration would
not cause any toxicity to cardiomyocytes (Fig. 2) and thus, the intracellular
fluorescence intensity corresponding to CUR-NPs is remained strong
after 24 h of incubation. These images confirm that the presently
developed CUR NPs can greatly enhance the intracellular CUR
accumulation.
Resistance effect of CUR NPs on
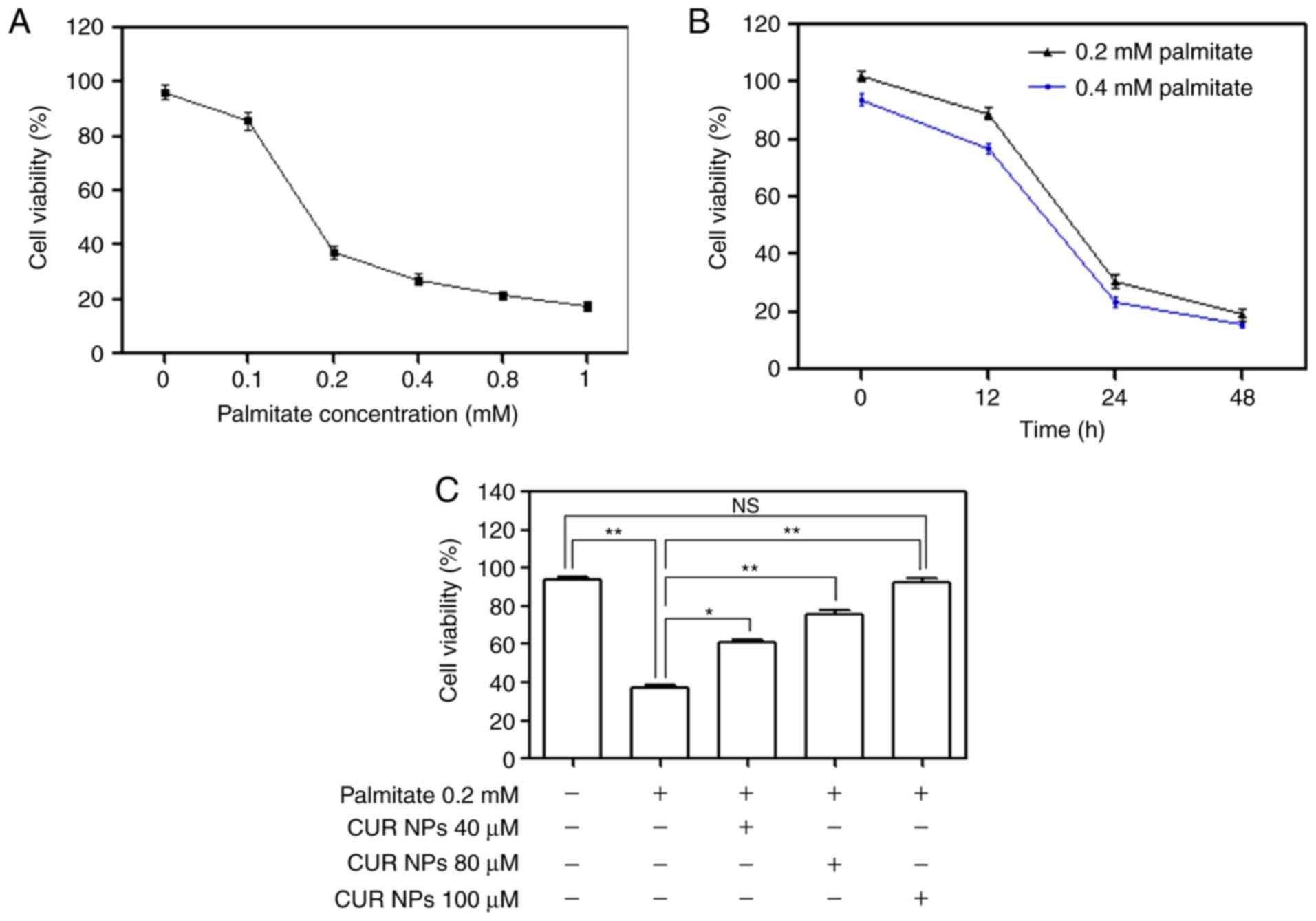
palmitate-induced lipotoxic cell damage
Various amounts of palmitate were incubated with
H9C2 cells to examine the palmitate-induced cell damage by
measuring cell viability and relevant results are provided in
Fig. 4A and B. Under conditions
of fixed incubation time, cell viability was found to sharply
decrease when the applied palmitate amount was >0.1 mM; and
alternatively, the treated cells also had decreased viability after
exposure to 0.2 or 0.4 mM palmitate for a period >12 h. These
data reveal that palmitate will induce cardiomyocyte damage when
the applied palmitate amount or the treatment time period exceeds
certain thresholds. Bar-graphs shown in Fig. 4C illustrate that CUR NPs can
effectively resist the palmitate-induced cell damage because the
cells treated with 0.2 mM palmitate together with various amounts
of CUR NPs for 24 h showed ascending cell viability with
significant differences when compared with those cells treated with
0.2 mM palmitate only (P<0.01). Based on these results, the
applied palmitate amount was selected as 0.2 mM for establishing
the palmitate-induced cell damage model for the following
experiments unless otherwise stated.
Effects of CUR NPs on palmitate-induced
ROS level, MDA content and SOD activity in cells
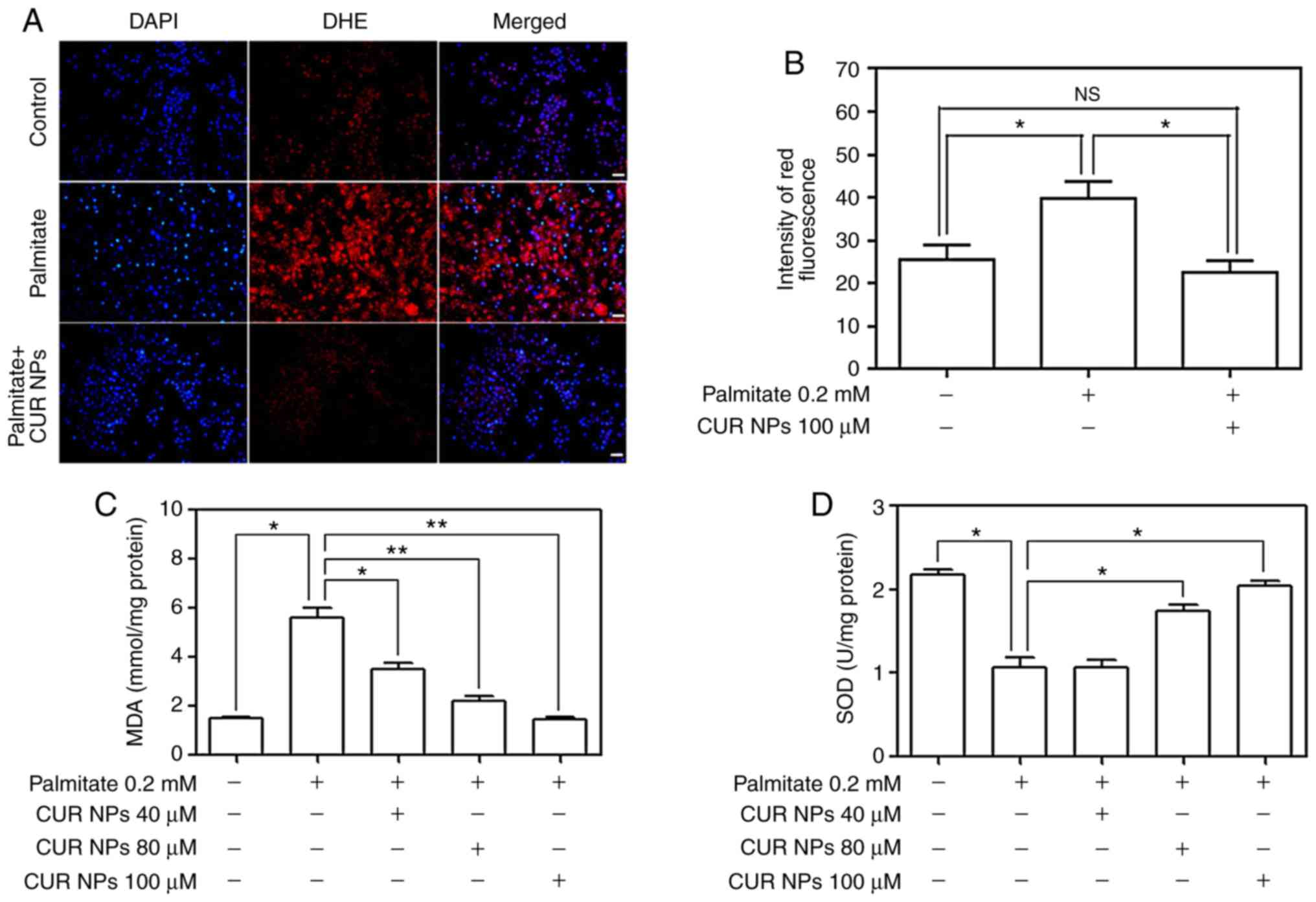
Palmitate-induced ROS levels were first detected by
using DHE as fluorescent probe and results are represented Fig. 5. Images in Fig. 5A denote that the cells treated
with palmitate alone show greater red fluorescence compared with
the matching control, signifying that the palmitate treatment can
lead to a clear increase in intracellular ROS levels. On the other
hand, the red fluorescence corresponding to the cells treated with
the combination of palmitate and CUR NPs exhibited largely reduced
brightness when compared with those cells treated by palmitate
only, demonstrating that the use of CUR NPs can prevent
intracellular ROS production. Data for DHE fluorescence intensity
are depicted in Fig. 5B and the
bar-graphs provide quantitative evidence that CUR NPs can
significantly inhibit the palmitate-induced increase in ROS levels
(P<0.05). Fig. 5C and D
demonstrate that the palmitate treatment resulted in a big increase
in the amount of MDA substance while leading to a significant
decrease in SOD activity (P<0.05) and such changes are strongly
correlated to the rise of intracellular oxidative stress levels. In
contrast to these observations, CUR NPs containing a CUR equivalent
of 100 µM can inhibit the increase in MDA amount and
regulate the SOD activity, allowing these two typical indexes to be
back to the normal cellular levels.
Abrogation effect of CUR NPs on
palmitate-induced cell apoptosis
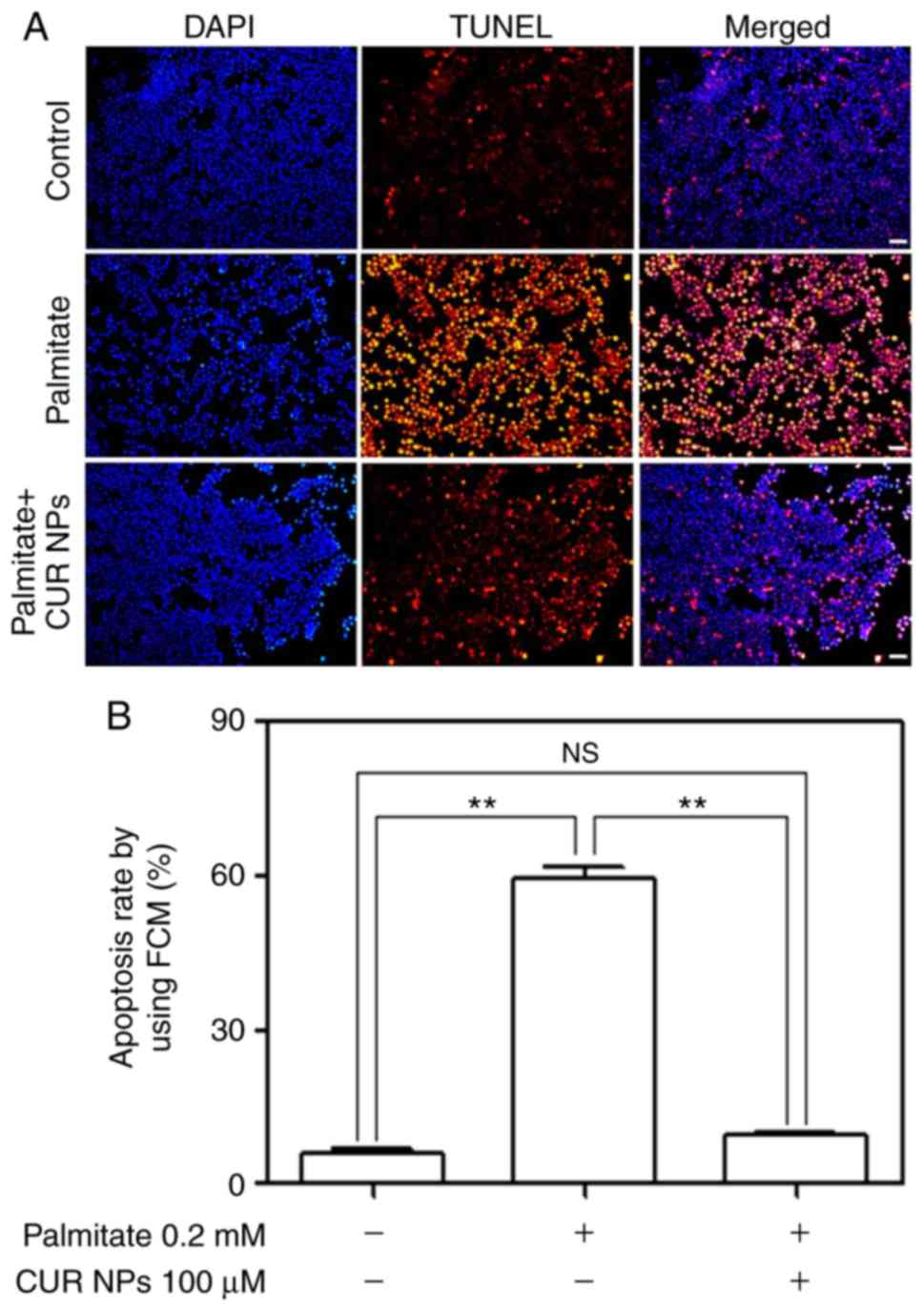
Fig. 6A shows that
the palmitate-treated cells exhibited greater fluorescence compared
with the matching with control (see middle column) and the
fluorescence brightness associated with the cells treated with the
combination of palmitate, and CUR NPs was largely reduced. The
results were consistent with TUNEL positive/negative image data
(Fig. S1). The fluorescence
intensity of the PA group was close to that of positive control
group and that of PA+CUR NPs group was close to that of the
negative control group. The quantitative results presented in
Fig. 6B demonstrate that CUR NPs
were able to completely inhibit the palmitate-induced increase in
apoptosis levels.
Possible mechanism for CUR NPs to protect
cells from palmitate-induced damage
The AMPK pathway plays an important role in
regulating cellular survival, oxidative stress and apoptosis
(15). The amount of AMPK and
p-AMPK as well as the key downstream proteins (p-mTORC1 and
p-p70S6K) was respectively measured for exploring their responses
to different treatments involving palmitate, the combination of
palmitate and CUR NPs and an AMPK inhibitor (compound C), and the
results are presented in Fig. 7.
In principle, AMPK is a metabolic fuel gauge and the activation of
AMPK acts to maintain cellular energy stores by switching on
catabolic pathways that initiate ATP production (15). p-AMPK at its normal level can
inhibit the activity of downstream p-mTORC1 and p-p70S6K, thereby
triggering the ATP production process to maintain the dynamic
balance between supply and demand for cellular energy. However,
under pathological conditions such as hyper-lipidemia, p-AMPK
appears to decrease and elevates the levels of p-mTORC1 and
p-p70S6K, which will in turn cause a cellular energy imbalance and
result in cell damage or apoptosis. Bands matching the
palmitate-treated group, as shown in Fig. 7A, registered a clear decrease in
p-AMPK, accompanied by increases in levels of both p-mTORC1 and
p-p70S6K, although the AMPK, mTORC1 and p70S6K expression remained
nearly unchanged in comparison with the control; and on the other
hand, the bands for the group treated with the combination of
palmitate and CUR NPs demonstrated that p-AMPK, p-mTORC1 and
p-p70S6K returned to their respective normal levels. Data presented
in Fig. 7B-D indicated that CUR
NPs were able to resist the abnormal changes in the levels of
p-AMPK, p-mTORC1 and p-p70S6K. In particular when compound C was
applied, the resistant effects of CUR NPs on the abnormal changes
of these three proteins would be eliminated. These results suggest
that CUR NPs can protect cardiomyocytes from lipotoxic injury
through the AMPK pathway.
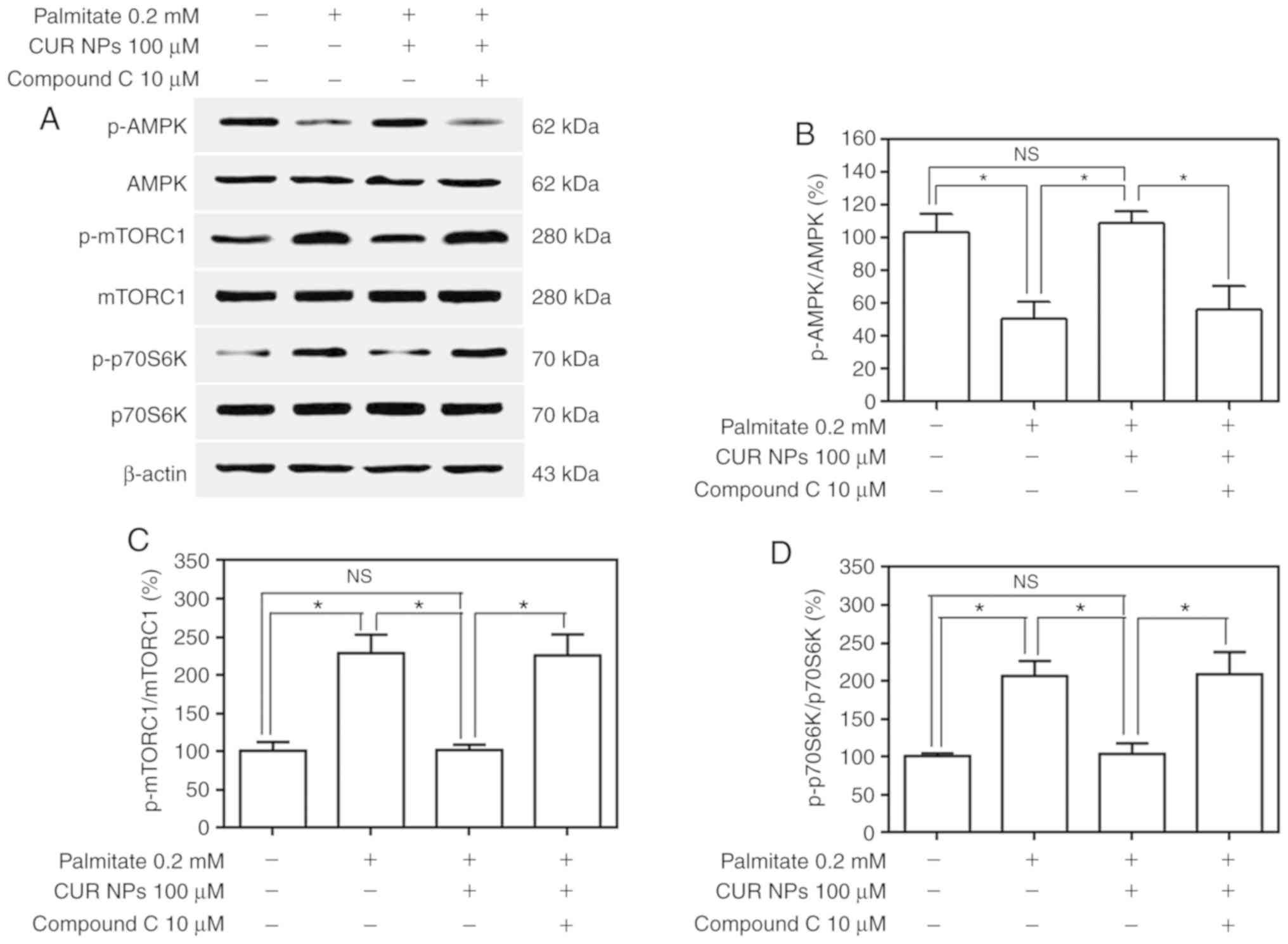 | Figure 7Effect of CUR NPs on regulation of
the AMPK pathway in H9C2 cells. (A) Representative bands of p-AMPK,
AMPK, p-mTORC1, mTORC1, p-p70S6K and p70S6K (inner reference:
β-actin). (B) Quantitative determination of p-AMPK/AMPK ratio. (C)
Quantitative determination of p-mTORC1/mTORC1 ratio. (D)
Quantitative determination of p-p70S6K/p70S6K ratio.
*P<0.05; NS, no significance; CUR, curcumin; NPs,
nanoparticles; AMPK, AMP-activated protein kinase; p-AMPK,
phosphorylated AMPK; p-mTORC1, phosphorylated mammalian target of
rapamycin complex-1C1; p-p70S6K, phosphorylated p70 ribosomal
protein S6 kinase. |
Resistant effect of CUR NPs on
palmitate-induced cell apoptosis via AMPK pathway
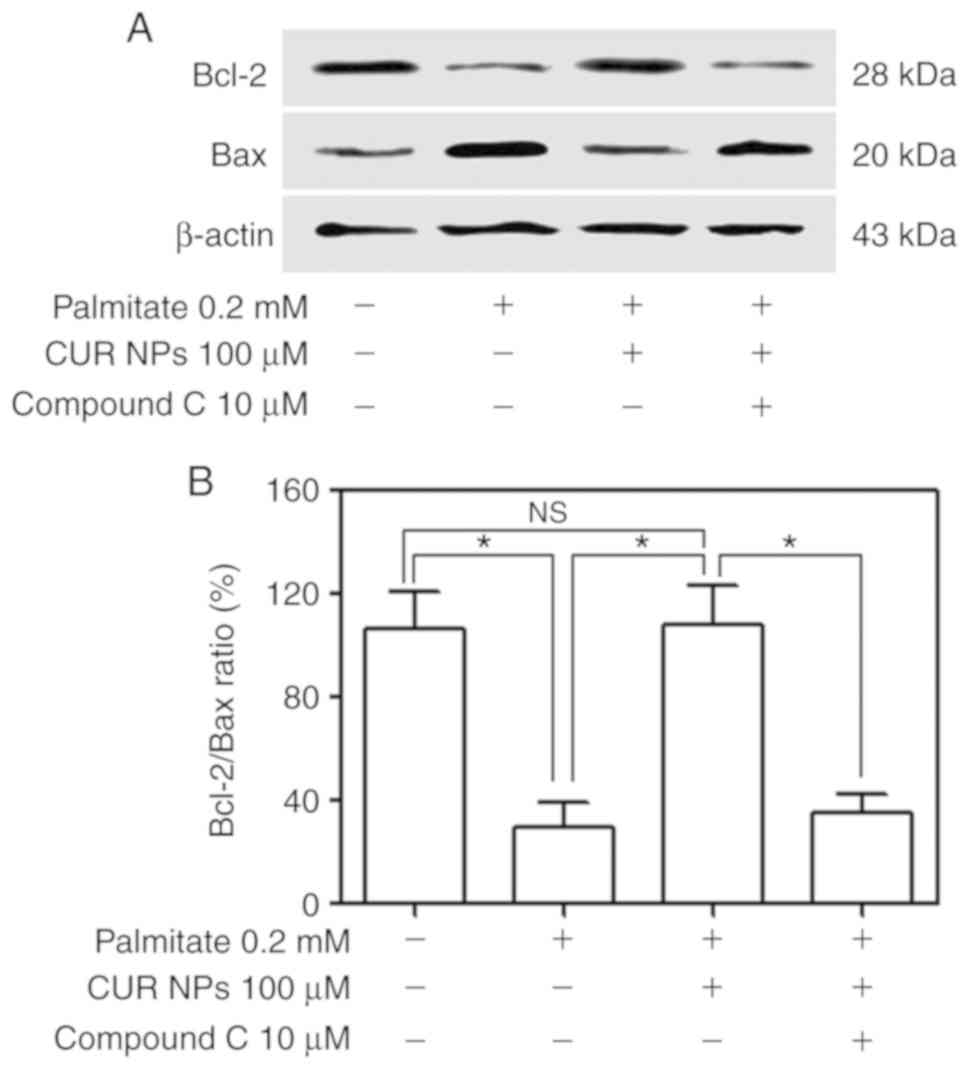
A total of two key regulatory proteins, Bax and
Bcl-2, were further measured using western blotting to examine how
they respond to the treatment of CUR NPs and results are presented
Fig. 8. Bands for Bax and Bcl-2
in Fig. 8A indicate that the
palmitate-treated cells had lower Bcl-2 levels but increased Bax
levels compared with the control and the cells treated with the
combination of palmitate, and CUR NPs exhibited Bcl-2 and Bax
levels similar to the control. Considering the fact that the Bcl-2
protein is indicative of the resistance to cell apoptosis while the
Bax protein pro-apoptotic, the correct ratio between Bcl-2 and Bax
is a crucial factor for cell survival. It can be seen from Fig. 8B that the Bcl-2/Bax ratio for the
palmitate-treated cells was ~3-fold lower compared with the
control, implying that these cells underwent apoptosis. On the
other hand, the cells treated with the combination of palmitate and
CUR NPs exhibited a Bcl-2/Bax ratio similar to that of the control
(P>0.005), demonstrating that the use of CUR NPs can completely
inhibit the palmitate-induced apoptosis. However, the Bcl-2/Bax
ratio for the group treated with the combination of palmitate, CUR
NPs and compound C was greatly reduced to a level similar to
palmitate-treated group.
Discussion
Cardiovascular disease is one of the leading causes
of death in the world (31).
Myocardial infarction, a kind of acute and fatal heart disease, is
found to be directly and strongly connected hyperlipidemia that
causes atherosclerosis, and in turn, results in a variety of blood
vessel related diseases (31).
Several studies indicate that hyperlipidemia directly participates
in the pathogenesis of high fat-induced cardiac injury in both the
human body and experimental animals by promoting the formation of
excessive oxidative stress in the heart, and in turn, resulting in
cardiomyocyte apoptosis (31-33). In the case of hyperlipidemia, a
typical indicator of the rise of oxidative stress in the heart, it
is closely linked to the overproduction of ROS and a damaged
antioxidant defense system (34),
and the disrupted balance between ROS generation and the ROS
scavenging system will result in intracellular formation and
accumulation of superoxide ions, leading to dysfunction and cell
damage (35). Considering that
the elevated ROS levels cause strong injurious effects on
hyperlipidemia patients, nowadays, various types of antioxidant
agents have been investigated for their potential in the treatment
of hyperlipidemia (36).
CUR has been used a natural cardioprotective agent
because it can eradicate the excessive amounts of ROS and enhance
antioxidant defense due to its demonstrated antioxidant properties
(37). In the present study, CUR
was loaded into NPs in order to protect it from degradation while
improving its therapeutic efficiency. Results presented above
confirm that the CUR NPs containing a CUR equivalent of 100
µM are able to attenuate oxidative stress in cardiomyocytes
and fully resist palmitate-induced cardiomyocyte apoptosis.
The occurrence of hyperlipidemia can be correlated
to multiple factors that can act individually or in combination.
These factors include superoxide generation from NADPH oxidases
(38), oxidative phosphorylation
(39), abnormal level of protein
kinase C (40) and the activation
of polyol, and hexosamine pathways (41). Recent studies reveal that the AMPK
pathway can play a critical role in regulating the
pathophysiological development of hyperlipidemia and co-morbidities
(42,43).
AMPK is known to be a major intracellular protein
kinase and it can regulate not only cellular metabolism involving
fatty acids and glucose but also anti-apoptotic processes (44). In the case of cardiomyocytes,
impaired intracellular metabolism will cause an inadequate energy
supply, which would disturb cellular homeostasis and result in
irregular contraction of myocardium (45). For this reason, stimulation of
AMPK, a key member of well-known cell survival pathways, is
believed to be feasible for protecting the myocardium through
improved energy utilization, enhanced mitochondrial biogenesis and
inhibiting cell apoptosis (46).
A recent study suggests that pharmacological activation of AMPK can
prevent palmitate-induced mitochondrial fragmentation and
dysfunction in endothelial cells (47). Based on these reported results,
the present study intends to discover whether CUR NPs can also
stimulate AMPK to protect myocytes from lipotoxic injury and also,
examine the prospective relationship between AMPK activation and
lipotoxic injury of cardiomyocytes.
Although several studies have been performed to
activate the AMPK pathway using CUR and the relevant effects on the
protection of adipocytes, endothelial cells and cardiomyocytes have
been examined (48-50), the general mechanism for CUR to
activate the AMPK pathway is still unclear. In the case of
endothelial cells, an alternative mechanism is that CUR regulates
uncoupling protein 2 and activates the AMPK pathway by inhibiting
excessive ATP production in endothelial cells (49). In the present study, besides the
achieved improvements on greatly enhanced intracellular
accumulation of CUR NPs and strong resistance effect of CUR NPs on
the palmitate-induced cardiomyocyte apoptosis, the
AMPK/mTORC1/p70S6K pathway in CUR NP treated cardiomyocytes, which
is different from other pathways mentioned in the literature and
has not been explored so far to the best of our knowledge, was
explored in order to find out an alternative mechanism. Under the
present experimental conditions, CUR NPs are able to suppress
p-mTORC1 and p-p70S6K through promoting the rise of p-AMPK back to
its normal level, and therefore, protect cardiomyocytes from
palmitate-induced lipotoxicity.
It is known that the Bax protein plays a crucial
role in mitochondrion-mediated apoptosis and on the other hand,
Bcl-2, a kind of antiapoptotic protein, can effectively prevent Bax
oligomerization (51). A
relatively stable balance between Bax and Bcl-2 is a key factor for
maintaining the normal state of cardiomyocytes (51). This effect of CUR NPs is blocked
when compound C, a typical inhibitor for AMPK pathway, is applied.
Moreover, the regulatory effects of CUR NPs on the expression of
Bax and Bcl-2 will also be blocked when AMPK pathway is inhibited
by compound C. Based on all observations mentioned in this study,
it can be deduced that CUR NPs may activate the AMPK/mTORC1/p70S6K
signaling pathway, regulate the expression of downstream proteins
and resist the palmitate-induced cardiomyocyte injury.
In conclusion, a type of CUR-loaded NPs with
necessitated drug-loading and suitable sizes was successfully
prepared, and these NPs showed definite ability to administrate the
CUR release in a sustainable manner over a period of ~12 h. These
NPs had much higher safety in comparison to free CUR and in
addition, were capable of greatly enhancing the intracellular CUR
accumulation in cardiomyocytes. They were found to be able to
inhibit the rise of intracellular ROS levels and resist
palmitate-induced cardiomyocyte apoptosis. A possible mechanism for
these NPs to play their roles in cardiomyocyte protection is that
the sustained release of CUR can attenuate palmitate-induced
oxidative stress in cardiomyocytes and concomitantly, activate the
AMPK pathway by regulating the expression of several specific
proteins to their respective normal levels. Results suggest that
the presently developed CUR-loaded NPs have potential in preventing
myocardium from lipotoxic injury. The present studies are focused
on the protective effects of CUR NPs against the palmitate-induced
injuries in cardiomyocytes in vitro. Some investigations
based on the animal model are now in progress and they may be used
to further demonstrate the protective effects of CUR NPs on
cardiomyocytes in vivo.
Supplementary Data
Acknowledgments
The authors would like to thank Miss Wei Zhang of
Changchun Institute of Applied Chemistry, Chinese Academy of
Sciences, for providing technical guidance for the synthesis of
nanoparticles.
Funding
The present study was supported by the National
Natural Science Foundation of China (project no. 51703055), the
Jilin Province Science and Technology Development Plan Project
(project no. 20170204011YY), the Hubei Natural Science Foundation
(grant no. 2017CFB699), the Hubei Science and Technology College
Diabetes Special Fund (grant no. 2016-18XZ09) and the Hubei
University of Science and Technology School Development Project
(grant no. 2016-18X042).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ and JL conceived and designed the study. JZ,
YWang, CB, TL, SL, JH and JL performed the experiments. JZ, YWan
and JL wrote the paper. JL reviewed and edited the manuscript. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim J, Joo S, Eom GH, Lee SH, Lee MA, Lee
M, Kim KW, Kim DH, Kook H, Kwak TH and Park WJ: CCN5 knockout mice
exhibit lipotoxic cardiomyopathy with mild obesity and diabetes.
PLoS One. 13:e02072282018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pulinilkunnil T, Kienesberger PC,
Nagendran J, Waller TJ, Young ME, Kershaw EE, Korbutt G, Haemmerle
G, Zechner R and Dyck JR: Myocardial adipose triglyceride lipase
overexpression protects diabetic mice from the development of
lipotoxic cardiomyopathy. Diabetes. 62:1464–1477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeong MH, Tran NK, Kwak TH, Park BK, Lee
CS, Park TS, Lee YH, Park WJ and Yang DK: β-Lapachone ameliorates
lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. PLoS
One. 9:e910392014. View Article : Google Scholar
|
|
4
|
Walls SM, Cammarato A, Chatfield DA, Ocorr
K, Harris GL and Bodmer R: Ceramide-protein interactions modulate
ceramide-associated lipotoxic cardiomyopathy. Cell Rep.
22:2702–2715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drosatos K and Schulze PC: Cardiac
lipotoxicity: Molecular pathways and therapeutic implications. Curr
Heart Fail Rep. 10:109–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Law BA, Liao X, Moore KS, Southard A,
Roddy P, Ji R, Szulc Z, Bielawska A, Schulze PC and Cowart LA:
Lipotoxic very-long-chain ceramides cause mitochondrial
dysfunction, oxidative stress, and cell death in cardiomyocytes.
FASEB J. 32:1403–1416. 2018. View Article : Google Scholar :
|
|
7
|
Pillutla P, Hwang YC, Augustus A, Yokoyama
M, Yagyu H, Johnston TP, Kaneko M, Ramasamy R and Goldberg IJ:
Perfusion of hearts with triglyceride-rich particles reproduces the
metabolic abnormalities in lipotoxic cardiomyopathy. Am J Physiol
Endocrinol Metab. 288:E1229–E1235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malfitano C, de Souza Junior AL, Carbonaro
M, Bolsoni-Lopes A, Figueroa D, de Souza LE, Silva KA,
Consolim-Colombo F, Curi R and Irigoyen MC: Glucose and fatty acid
metabolism in infarcted heart from streptozotocin-induced diabetic
rats after 2 weeks of tissue remodeling. Cardiovasc Diabetol.
14:1492015. View Article : Google Scholar
|
|
9
|
Carpentier AC: Abnormal myocardial dietary
fatty acid metabolism and diabetic cardiomyopathy. Can J Cardiol.
34:605–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mangolim AS, Brito LAR and Nunes-Nogueira
VS: Effectiveness of testosterone therapy in obese men with low
testosterone levels, for losing weight, controlling obesity
complications, and preventing cardiovascular events: Protocol of a
systematic review of randomized controlled trials. Medicine
(Baltimore). 97:e04822018. View Article : Google Scholar
|
|
11
|
Son NH, Yu S, Tuinei J, Arai K, Hamai H,
Homma S, Shulman GI, Abel ED and Goldberg IJ: PPARγ-induced
cardiolipotoxicity in mice is ameliorated by PPARα deficiency
despite increases in fatty acid oxidation. J Clin Invest.
120:3443–3454. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura H, Matoba S, Iwai-Kanai E, Kimata
M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y,
et al: p53 promotes cardiac dysfunction in diabetic mellitus caused
by excessive mitochondrial respiration-mediated reactive oxygen
species generation and lipid accumulation. Circ Heart Fail.
5:106–115. 2012. View Article : Google Scholar
|
|
13
|
Finck BN, Han X, Courtois M, Aimond F,
Nerbonne JM, Kovacs A, Gross RW and Kelly DP: A critical role for
PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic
cardiomyopathy: Modulation by dietary fat content. Proc Natl Acad
Sci USA. 100:1226–1231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Britto RM, Silva-Neto JAD, Mesquita TRR,
Vasconcelos CML, de Almeida GKM, Jesus ICG, Santos PHD, Souza DS,
Miguel-Dos-Santos R, de Sá LA, et al: Myrtenol protects against
myocardial ischemia-reperfusion injury through antioxidant and
anti-apoptotic dependent mechanisms. Food Chem Toxicol.
111:557–566. 2018. View Article : Google Scholar
|
|
15
|
Guo S, Yao Q, Ke Z, Chen H, Wu J and Liu
C: Resveratrol attenuates high glucose-induced oxidative stress and
cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol.
412:85–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu CW, Hao JL, Yao L, Li HJ and Zhou DD:
Efficacy of curcumin in inducing apoptosis and inhibiting the
expression of VEGF in human pterygium fibroblasts. Int J Mol Med.
39:1149–1154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mujtaba T, Kanwar J, Wan SB, Chan TH and
Dou QP: Sensitizing human multiple myeloma cells to the proteasome
inhibitor bortezomib by novel curcumin analogs. Int J Mol Med.
29:102–106. 2012.
|
|
18
|
Chen Z, Xue J, Shen T, Mu S and Fu Q:
Curcumin alleviates glucocorticoid-induced osteoporosis through the
regulation of the Wnt signaling pathway. Int J Mol Med. 37:329–338.
2016. View Article : Google Scholar :
|
|
19
|
Mohajeri M and Sahebkar A: Protective
effects of curcumin against doxorubicin-induced toxicity and
resistance: A review. Crit Rev Oncol Hematol. 122:30–51. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santezi C, Reina BD and Dovigo LN:
Curcumin-mediated photo-dynamic therapy for the treatment of oral
infections-a review. Photodiagnosis Photodyn Ther. 21:409–415.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hosseini A and Hosseinzadeh H: Antidotal
or protective effects of curcuma longa (turmeric) and its active
ingredient, curcumin, against natural and chemical toxicities: A
review. Biomed Pharmacother. 99:411–421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gawde KA, Sau S, Tatiparti K, Kashaw SK,
Mehrmohammadi M, Azmi AS and Iyer AK: Paclitaxel and di-fluorinated
curcumin loaded in albumin nanoparticles for targeted synergistic
combination therapy of ovarian and cervical cancers. Colloid Surf B
Biointerfaces. 167:8–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang S, Han J, Li T, Xin Z, Ma Z, Di W,
Hu W, Gong B, Di S, Wang D and Yang Y: Curcumin as a potential
protective compound against cardiac diseases. Pharmacol Res.
119:373–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao G, Liu Y, Yi X, Wang Y, Qiao S, Li Z,
Ni J and Song Z: Curcumin inhibiting Th17 cell differentiation by
regulating the metabotropic glutamate receptor-4 expression on
dendritic cells. Int Immunopharmacol. 46:80–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi Z, Wu M, Fu Y, Huang T, Wang T, Sun Y,
Feng Z and Li C: Palmitic acid curcumin ester facilitates
protection of neuroblastoma against oligomeric aβ40 insult. Cell
Physiol Biochem. 44:618–633. 2017. View Article : Google Scholar
|
|
26
|
Ren J and Sowers JR: Application of a
novel curcumin analog in the management of diabetic cardiomyopathy.
Diabetes. 63:3166–3168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li K, Liu Y, Zhang S, Xu Y, Jiang J, Yin
F, Hu Y, Han B, Ge S, Zhang L and Wang Y: Folate receptor-targeted
ultrasonic PFOB nanoparticles: Synthesis, characterization and
application in tumor-targeted imaging. Int J Mol Med. 39:1505–1515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang X, Zhong Y, Zheng L and Zhao J:
Nano-hydroxyapatite/collagen film as a favorable substrate to
maintain the phenotype and promote the growth of chondrocytes
cultured in vitro. Int J Mol Med. 41:2150–2158. 2018.PubMed/NCBI
|
|
29
|
Jiang C, Wang H, Zhang X, Sun Z, Wang F,
Cheng J, Xie H, Yu B and Zhou L: Deoxycholic acid-modified
chitooligosaccharide/mPEG-PDLLA mixed micelles loaded with
paclitaxel for enhanced antitumor efficacy. Int J Pharm. 475:60–68.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Q, Pang MH, Ye XH, Yang G and Lin C:
The toxoplasma gondii ME-49 strain upregulates levels of A20 that
inhibit NF-κB activation and promotes apoptosis in human leukaemia
T-cell lines. Parasite Vector. 11:3052018. View Article : Google Scholar
|
|
31
|
Shiomi M, Ishida T, Kobayashi T, Nitta N,
Sonoda A, Yamada S, Koike T, Kuniyoshi N, Murata K, Hirata K, et
al: Vasospasm of atherosclerotic coronary arteries precipitates
acute ischemic myocardial damage in myocardial infarction-prone
strain of the watanabe heritable hyperlipidemic rabbits.
Arterioscler Thromb Vasc Biol. 33:2518–2523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li TB, Zhang YZ, Liu WQ, Zhang JJ, Peng J,
Luo XJ and Ma QL: Correlation between NADPH oxidase-mediated
oxidative stress and dysfunction of endothelial progenitor cell in
hyperlipidemic patients. Korean J Intern Med. 33:313–322. 2018.
View Article : Google Scholar :
|
|
33
|
Yang SM, Liu J and Li CX: Intermedin
protects against myocardial ischemia-reperfusion injury in
hyperlipidemia rats. Genet Mol Res. 13:8309–8319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vendrov AE, Vendrov KC, Smith A, Yuan J,
Sumida A, Robidoux J, Runge MS and Madamanchi NR: NOX4 NADPH
oxidase-dependent mitochondrial oxidative stress in
aging-associated cardiovascular disease. Antioxid Redox Signal.
23:1389–1409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lucas ML, Carraro CC, Bello-Klein A, Kalil
AN, Aerts NR, Carvalho FB, Fernandes MC and Zettler CG: Oxidative
stress in aortas of patients with advanced occlusive and aneurysmal
diseases. Ann Vasc Surg. 52:216–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Murugesu K, Murugaiyah V, Saghir SAM,
Asmawi MZ and Sadikun A: Caffeoylquinic acids rich versus poor
fractions of gynura procumbens: Their comparative
antihyperlipidemic and antioxidant potential. Curr Pharm
Biotechnol. 18:1132–1140. 2017. View Article : Google Scholar
|
|
37
|
Hadzi-Petrushev N, Bogdanov J, Krajoska J,
Ilievska J, Bogdanova-Popov B, Gjorgievska E, Mitrokhin V, Sopi R,
Gagov H, Kamkin A and Mladenov M: Comparative study of the
antioxidant properties of monocarbonyl curcumin analogues C66 and
B2BrBC in isoproteranol induced cardiac damage. Life Sci.
197:10–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li TB, Zhang JJ, Liu B, Luo XJ, Ma QL and
Peng J: Dysfunction of endothelial progenitor cells in
hyperlipidemic rats involves the increase of NADPH oxidase derived
reactive oxygen species production. Can J Physiol Pharmacol.
95:474–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei S, Sun RZ, Wang D, Gong MZ, Su XP, Yi
F and Peng ZW: Increased hepatic fatty acids uptake and oxidation
by LRPPRC-driven oxidative phosphorylation reduces blood lipid
levels. Front Physiol. 7:2702016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan HC, Fernandez-Hernando C and Lai JH:
Protein kinase C isoforms in atherosclerosis: Pro- or
anti-inflammatory? Biochem Pharmacol. 88:139–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chiu CJ and Taylor A: Dietary
hyperglycemia, glycemic index and metabolic retinal diseases. Prog
Retin Eye Res. 30:18–53. 2011. View Article : Google Scholar
|
|
42
|
Yang R, Chu X, Sun L, Kang Z, Ji M, Yu Y,
Liu Y, He Z and Gao N: Hypolipidemic activity and mechanisms of the
total phenylpropanoid glycosides from ligustrum robustum (Roxb.)
Blume by AMPK-SREBP-1c pathway in hamsters fed a high-fat diet.
Phytother Res. 32:715–722. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin CH, Kuo YH and Shih CC: Effects of
bofutsusho-san on diabetes and hyperlipidemia associated with
AMP-activated protein kinase and glucosetransporter 4 in
high-fat-fed mice. Int J Mol Sci. 15:20022–20044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vinayagam R, Jayachandran M, Chung SSM and
Xu B: Guava leaf inhibits hepatic gluconeogenesis and increases
glycogen synthesis via AMPK/ACC signaling pathways in
streptozotocin-induced diabetic rats. Biomed Pharmacother.
103:1012–1017. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yeung PK, Kolathuru SS, Mohammadizadeh S,
Akhoundi F and Linderfield B: Adenosine 5′-triphosphate metabolism
in red blood cells as a potential biomarker for post-exercise
hypotension and a drug target for cardiovascular protection.
Metabolites. 8:E302018. View Article : Google Scholar
|
|
46
|
Mollica MP, Mattace Raso G, Cavaliere G,
Trinchese G, De Filippo C, Aceto S, Prisco M, Pirozzi C, Di Guida
F, Lama A, et al: Butyrate regulates liver mitochondrial function,
efficiency, and dynamics in insulin-resistant obese mice. Diabetes.
66:1405–1418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Y, Zhou ZH, Chen MH, Yang J, Leng J,
Cao GS, Xin GZ, Liu LF, Kou JP, Liu BL, et al: Inhibition of
mitochondrial fission and NOX2 expression prevent NLRP3
inflammasome activation in the endothelium: The role of corosolic
acid action in the amelioration of endothelial dysfunction.
Antioxid Redox Signal. 24:893–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lone J, Choi JH, Kim SW and Yun JW:
Curcumin induces brown fat-like phenotype in 3T3-L1 and primary
white adipocytes. J Nutr Biochem. 27:193–202. 2016. View Article : Google Scholar
|
|
49
|
Pu Y, Zhang H, Wang P, Zhao Y, Li Q, Wei
X, Cui Y, Sun J, Shang Q, Liu D and Zhu Z: Dietary curcumin
ameliorates aging-related cerebrovascular dysfunction through the
AMPK/uncoupling protein 2 pathway. Cell Physiol Biochem.
32:1167–1177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang K, Xu C, Li X and Jiang H:
Combination of D942 with curcumin protects cardiomyocytes from
ischemic damage through promoting autophagy. J Cardiovasc Pharmacol
Ther. 18:570–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mikhailov V, Mikhailova M, Pulkrabek DJ,
Dong Z, Venkatachalam MA and Saikumar P: Bcl-2 prevents Bax
oligomerization in the mitochondrial outer membrane. J Biol Chem.
276:18361–18374. 2001. View Article : Google Scholar : PubMed/NCBI
|