Introduction
Cerebral venous sinus thrombosis (CVST) accounts for
0.5-1.0% of strokes and is relatively more frequent in young adults
(1,2). The clinical manifestations of CVST
lack specificity, with headaches as the only symptom in most
patients. In severe cases, patients may experience focal
neurological symptoms, seizures and even coma (3). At present, the pathogenesis of CVST
remains poorly understood. In addition, the low rate of incidence
and its atypical symptoms and signs often lead to misdiagnosis,
which delays the initiation of treatment.
The key to successful CVST treatment is rapid
recanalization of the venous sinus. Mechanical thrombectomy can
rapidly restore blood flow in the sinus, which is an efficacious
treatment for patients with progressive deterioration of nerve
function. However, even with active venous-sinus mechanical
thrombectomy, the prognosis of some patients with CVST is not
improved (4). The reason for this
may be similar to the pathophysiological mechanism of acute
arterial occlusion clinically. Although stent thrombectomy can
rapidly restore blood flow in a short time, blood-flow reperfusion
further aggravates the inflammatory reaction and apoptosis caused
by ischemia. These pathological changes comprise the primary
mechanism of neuronal damage (5).
Studies have found that HMGB1 serves an important role in brain
ischemia/reperfusion (I/R) injury (6). Therefore, it was hypothesized that,
due to the high rate of venous-sinus flow, rapid recanalization of
an occluded sinus may occur similar to that in I/R injury following
arterial occlusion, and that HMGB1 may serve an important role in
this process.
Glycyrrhizin (GL) is a natural glycosyltriterpenoid
compound with anti-inflammatory and antitumor effects. Stronger
Neo-Minophagen C (SNMC), with GL as its main component, has been
used in the clinical treatment of chronic hepatitis (7). SNMC inhibits the release of HMGB1
from damaged cells by inhibiting the phosphorylation of HMGB1, and
also inhibits the expression of HMGB1 (8,9).
Studies have shown that GL can improve cerebral infarction and
cerebral edema following brain I/R injury by inhibiting HMGB1 and
its downstream inflammatory signaling pathway (10,11).
In the present study, based on successful
establishment of a rat CVST model, the mechanical thrombectomy
technique commonly used in clinical practice was simulated. The
study examined possible neuronal damage and its associated
molecular mechanisms subsequent to venous-sinus recanalization
following mechanical thrombectomy, and examined the neuroprotective
role of GL in CVO recanalization.
Materials and methods
Animals
The present study was approved by the Ethics
Committee of Fuzhou General Hospital (Fuzhou, China). All animal
experiments were conducted in accordance with the guidelines for
the care and use of laboratory animals. A total of 132 male
Sprague-Dawley rats (body weight 240-260 g, 6 weeks old) were
provided by Shanghai SLAC Laboratory Animals Co., Ltd. (Shanghai,
China). The rats were housed in a standard environment at 26-28°C
under a 10-h light/14-h dark light-dark cycle, and were provided
with sufficient food and water.
Experimental animal grouping
In order to investigate the occurrence of brain
injury following mechanical thrombectomy for CVO, 36 rats were
randomly divided into three groups, with 12 rats in each of the
following groups: Sham, CVST, and mechanical thrombectomy. To
examine the protective effect of GL, 60 rats were randomly divided
into five groups, with 12 rats in each of the following groups:
Mechanical thrombectomy + normal saline group (NS), mechanical
thrombectomy + 2 mg/kg GL group (2 mg/kg), mechanical thrombectomy
+ 4 mg/kg GL group (4 mg/kg), mechanical thrombectomy + 10 mg/kg GL
group (10 mg/kg), and mechanical thrombectomy + optimal-dose GL +
recombinant (r)HMGB1 (100 mg per rat) group (GL + rHMGB1). In these
groups, NS, GL or rHMGB1 (dissolved in saline; Asahi Kasei Pharma,
Tokyo, Japan) were injected intraperitoneally 1 h before mechanical
thrombectomy. To determine the effect of the time of administration
on GL protection, 36 rats were randomly divided into three groups,
with 12 rats in each of the following groups: 1 h before mechanical
thrombectomy (0 h), 6 h after mechanical thrombectomy (6 h), and 12
h after mechanical thrombectomy (12 h). In these groups, GL was
injected in equal doses for each group.
Animal modeling
Rat sagittal-sinus thrombosis was induced using a
40% FeCl3 solution (12). The rats were anesthetized with an
intraperitoneal injection of sodium pentobarbital (40 mg/kg). The
animals were placed on the operating table in the prone position to
maintain horizontal position of the calvaria. The skin on the head
was sterilized. Subsequently, 2% lidocaine (0.1 ml) was used to
pre-anesthetize the surgical incision. A cut at a length of ~1.5 cm
along the midline was made to carefully separate the muscles and
periosteum, and to reveal the skull. A dental electric grinder was
then used to drill between the bregma and lambda sutures under an
operating microscope, and a bone window of ~10 mm along the
sagittal suture was opened. A filter strip of 40% FeCl3
was placed on the superior sagittal sinus (SSS) for 5 min,
following which the residual FeCl3 was washed away with
normal saline. Subsequently, the skin was sutured (size 0) and
disinfected using iodophor. The animals were kept warm until
awaking and were then placed in a single cage with access to food
and water. At 6 h post-CVST, anesthesia was successfully performed.
The rats were fixed at the prone position, the suture was cut, the
bone window was washed with saline, and a blood-collection needle
was used to gently puncture the sinus wall of the sagittal sinus at
the leading edge of the bone window. A 0.014-inch
nerve-intervention microfilament (Aashi Intecc, Nagoya, Japan) was
inserted slowly into the venous sinus to perform thrombectomy. The
guide wire was gently pushed ~1 cm into the SSS and was then slowly
pulled out, for a total of three times, to observe the venous flow
in the venous sinus (Fig. 1A-C).
Gelatin-sponge compression was used to stop bleeding, and normal
saline was used to wash the wound. On confirming that there was no
active bleeding, a size-0 suture thread was used for suturing and
the wound was disinfected using iodophor. The animals were kept
warm until awake and were then placed in a single cage with access
to food and water.
Neurological evaluation
Neurological evaluation was performed in a
blinded-manner. Neurological evaluation of the rats was performed
prior to surgery and 24 h following CVST with reference to a
modified neurological severity score (mNSS) (13). All actions were scored separately
by two researchers and the average scores were used for analysis.
The mNSS mainly included the evaluation of movement, sensation,
balance and reflexes of the rats. Normal rats were given a score of
0. A higher score reflected more severe injury, and the maximum
score was 18 points.
Staining with 2,3,5-triphenyltetrazolium
chloride (TTC) and determination of cerebral infarction volume
The rats were sacrificed by intraperitoneal
injection of an excess of sodium pentobarbital 24 h after CVST, and
brain death (ceasing of self-breathing) was confirmed prior to
decapitation and brain harvesting. The brain tissue was coronally
sectioned at intervals of 2 mm and was then stained in a 1% TTC
phosphate-buffer solution (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) at 37°C for 30 min in the dark.
Following successful staining, the cells were fixed with a 10%
paraformaldehyde solution for 10 min. The sections were analyzed
using the image-analysis software ImageJ version 1.44 (National
Institutes of Health, Bethesda, MD, USA), and the infarct area,
ipsilateral-hemisphere area and contralateral-hemisphere area of
each section were measured. Each area was summed and multiplied by
the section thickness (2 mm) to obtain the corresponding volume. In
order to correct for the influence of ipsilateral-hemispheric edema
on cerebral infarction, the relative infarct volume was calculated
as follows (14): CIV
(%)=[CHV-(IHV-IV)] ×100/CHV. CIV represents corrected infarct
volume, CHV represents contralateral hemisphere volume, IHV
represents ipsilateral hemisphere volume, and IV represents infarct
volume.
Brain water content
Brain water content was determined using a dry- and
wet-weight method (15). The rats
were sacrificed by intraperitoneal injection of an overdose of
sodium pentobarbital at 24 h after CVST. Following death, each rat
was decapitated and ~0.5 g of the cerebral cortical tissue of the
sagittal sinus was removed and then weighed. The brain tissue was
then placed in a clean, dry culture dish and heated in a hot oven
at 110°C for 24 h, following which the dry weight was weighed. The
brain water content was calculated using the following formula:
Brain water content=[(wet weight-dry weight)/wet weight] ×100%.
Enzyme-linked immunosorbent assay
(ELISA)
The rats were sacrificed at the specified time
points according to the experimental design, and blood samples (5
ml) were collected from the abdominal aorta. The supernatant was
extracted by centrifugation at 1,000 × g for 10 min at 4°C. Serum
was stored in at −20°C until ELISA analysis. Serum inflammatory
factors, including HMGB1, RAGE, tumor necrosis factor-α (TNF-α),
interleukin-6 (IL-6) and IL-1β, in addition to superoxide dismutase
(SOD), nitric oxide synthase (NOS) and malondialdehyde (MDA), were
quantified using specific ELISA kits according to the
manufacturer's instructions (Nanjing Jiancheng Bioengineering
Institute).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Coronal sectioning was performed ~2 mm to the left
and right of the SSS. Subsequently, a cut was made along the upper
edge of the hippocampus. The outer cortex was the penumbra and the
medial cortex was the infarction core region. Total RNAs were
isolated from the penumbra of the infarct hemisphere using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific Inc. Waltham, MA,
USA) according to the manufacturer's instructions, and total RNAs
were then reverse transcribed into cDNA using the High-Capacity
cDNA RT kit (Roche Diagnostics GmbH, Mannheim, Germany) at 37°C for
15 min. The mRNA expression levels of HMGB1 and RAGE were analyzed
by qPCR using the SYBR Green PCR Master Mix kit (Applied
Biosystems; Thermo Fisher Scientific Inc.) and the ABI-Prism 7300
system (Applied Biosystems; Thermo Fisher Scientific Inc.). The
thermal cycling parameters were as follows: Heat-activated enzyme
was activated by heating at 95°C for 10 min, followed by 40 cycles
of heating at 95°C for 15 sec to denature, heating to 60°C for 30
sec to anneal, and final elongation at 72°C for 30 sec. The
concentration of the target genes was calculated by comparing the
quantitative cycle (Cq) value in each sample with the Cq value of
the internal standard curve using the 2−ΔΔCq method
(16). Melting-curve analysis and
gel-electrophoresis evaluation of the RT-qPCR products were
routinely performed to determine reaction specificity. GAPDH mRNA
was set as an internal reference to quantify the level of targeted
mRNA. The following primers were used: GAPDH, forward 5′-AGC AGG
CTG ACA GTG GAG TT-3′, and reverse 5′-AGC AGG CTG ACA GTG GAG
TT-3′; RAGE, forward 5′-CGA GTC CGT GTC TAC CAG ATT-3′ and reverse
5′-GCG GCT GGA ATG GAA ACT GAA-3′; and HMGB1, forward 5′-GCT CCA
TAG AGA CAG CGC CGG G-3′ and reverse 5′-CCT CAG CGA GGC ACA GAG TCG
C-3′.
Western blotting
The dissected brain tissues from the areas of
infarction were placed in RIPA buffer (Beyotime Institute of
Biotechnology, Shanghai, China). The protein concentration was
determined using a Bradford protein quantification kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The protein-loading
quantity was 40 µg on an 8-15% SDS-PAGE gel. The protein
bands were separated by electrophoresis and were electroporated
onto a PVDF membrane. Following blocking with 5% skim milk in a
Tris-buffered saline solution (with 1% Tween-20), the primary
antibody was added and allowed to incubate overnight at 4°C. The
antibodies used were monoclonal rabbit anti-HMGB1 (1:1,000; cat.
no. ab79823; Abcam, Cambridge, MA, USA) and monoclonal rabbit
anti-RAGE (1:500; cat. no. ab3611; Abcam). The membranes were
washed three times with PBS-Tween 20 and then incubated with
horseradish peroxidase-labeled goat anti-rabbit IgG secondary
antibody (1:500; cat. no. ab7090; Abcam) for 1 h at room
temperature. Development was performed using the SuperSignal™ West
Pico chemiluminescent substrate (Invitrogen; Thermo Fisher
Scientific, Inc.). The internal reference used was β-actin (1:500;
cat. no. ab8227; Abcam), and optical-density analysis was performed
using ImageJ version 1.44 (National Institutes of Health).
Immunofluorescence
The rats were anesthetized with an intraperitoneal
injection of sodium pentobarbital 24 h after CVST. Following
successful anesthesia, 4% paraformaldehyde was used for fixation
via cardiac perfusion. The signs of upper-extremity twitching and
stiffness in the rats indicated adequate perfusion. Following
perfusion using 100 ml of the fixative, the rats were sacrificed
and brain tissue was obtained and fixed in 4% paraformaldehyde for
24 h. Coronal brain sections (5-µm) from the ischemic core
region were immunostained. The rabbit anti-HMGB1 antibody was used
at a dilution of 1:200 (cat. no. ab79823; Abcam) overnight at 4°C.
488 AffiniPure Fab Fragment Goat Anti-Rabbit IgG (1:200; cat. no.
111-547-003, Jackson ImmunoResearch Laboratories, West Grove, PA,
USA) was used as a secondary antibody against HMGB1 for 1 h at 4°C.
The rabbit anti-RAGE antibody was used at a dilution of 1:200 (cat.
no. ab3611; Abcam) overnight at 4°C, and 594 AffiniPure Goat
Anti-Rabbit IgG (1:500; cat. no. 111-585-003; Jackson
ImmunoResearch Laboratories) was used as a secondary antibody
against RAGE for 1 h at 4°C. The sections were counterstained with
20 ml DAPI (cat. no. 236275; Roche Diagnostics GmbH) for 30 min at
4°C to stain the cell nucleus. The slides were coverslipped using
glycerin and images were captured under a fluorescent microscope.
Quantitative data were derived from the immunofluorescent images by
pixel analyses using Image-Pro Plus 6.0 software (Media
Cybernetics, Rockville, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed with SPSS 19.0 (IBM Corp.,
Armonk, NY, USA). Intergroup differences were analyzed using
Student's t-test or one-way analysis of variance. Tukey's post hoc
test was used for multiple comparisons among various groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Brain injury following recanalization
caused by mechanical thrombectomy for CVO
FeCl3 was used to induce SSS occlusion in
rats. To verify the stability of the model, TTC staining of a
coronal section of the brain was performed. No injury was observed
in the sham group, whereas a sinus cortex infarction area was
observed in the CVST group. Mechanical thrombectomy was performed 6
h after CVST in the recanalization group. The infarct size was
reduced compared with that in the CVST group, although it remained
significantly different from that in the sham group (Fig. 2A). In addition, in terms of the
neurological function scores and brain water content measurements
of each group, mechanical thrombectomy reduced neurological
deficits and cerebral edema, but did not restore them to the levels
in the sham group (Fig. 2B and
C). To determine whether HMGB1 and RAGE were involved in brain
damage following recanalization for CVO, mRNA and protein
expression in serum and brain tissues were measured. As mRNA is
more susceptible to degradation than protein, the detection of mRNA
was selected in the penumbra, whereas the detection of protein was
selected in the infarct area. The serum concentrations of HMGB1 and
RAGE were lower than those in the CVST group following
recanalization, but remained significantly higher than those in the
sham operation group (Fig. 2D).
Changes in the mRNA (Fig. 2E) and
protein (Fig. 2F and G)
expression of HMGB1 and RAGE in brain tissues showed the same
trend. These results confirmed that recanalization-induced brain
damage was present following CVO mechanical thrombectomy, and that
HMGB1 and RAGE may be involved in this process.
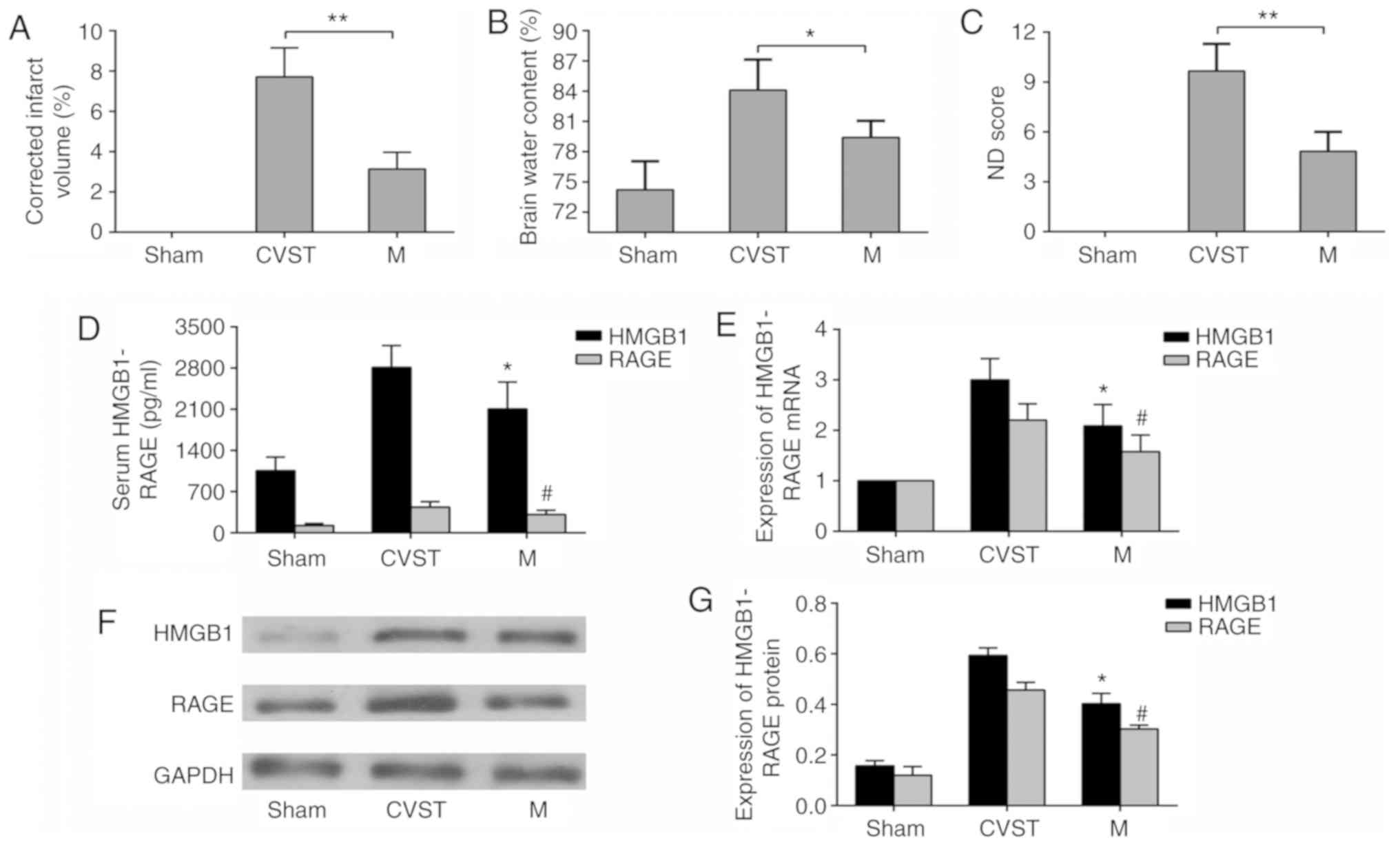 | Figure 2Changes in infarct volume, brain water
content and ND scores following mechanical thrombectomy, and
changes in mRNA and protein expression levels if HMGB1 and RAGE.
(A) Quantification of 2,3,5-triphenyltetrazolium chloride staining
results, (B) brain water content and (C) neurological function
scores, showing that brain parenchymal damage improved following
mechanical thrombectomy but was not completely reversed.
*P<0.05, **P<0.01. (D) ELISA results
showing that serum concentrations of HMGB1 and RAGE decreased
following mechanical thrombectomy. (E) Reverse
transcription-quantitative polymerase chain reaction results
showing that the mRNA expression levels of HMGB1 and RAGE in
paranasal sinus brain tissues were downregulated following
mechanical thrombectomy, (F) Western blot analysis and (G)
quantitative analysis showing that HMGB1 and RAGE proteins were
downregulated in paranasal sinus brain tissues following mechanical
thrombectomy. *P<0.05 and #P<0.05 vs.
CVST group. Data are expressed as the mean ± standard deviation.
Intergroup differences were analyzed using Student's t-test or
one-way analysis of variance. Tukey's post hoc test was used for
multiple comparisons among various groups. HMGB1, high-mobility
group box 1; RAGE, receptor of advanced glycation end products;
CVST, cerebral venous sinus thrombosis; M, mechanical thrombectomy;
ND, neurological deficit. |
GL protection against venous I/R
injury
The CVST rats with mechanical thrombectomy were
treated with intraperitoneal injection of different concentrations
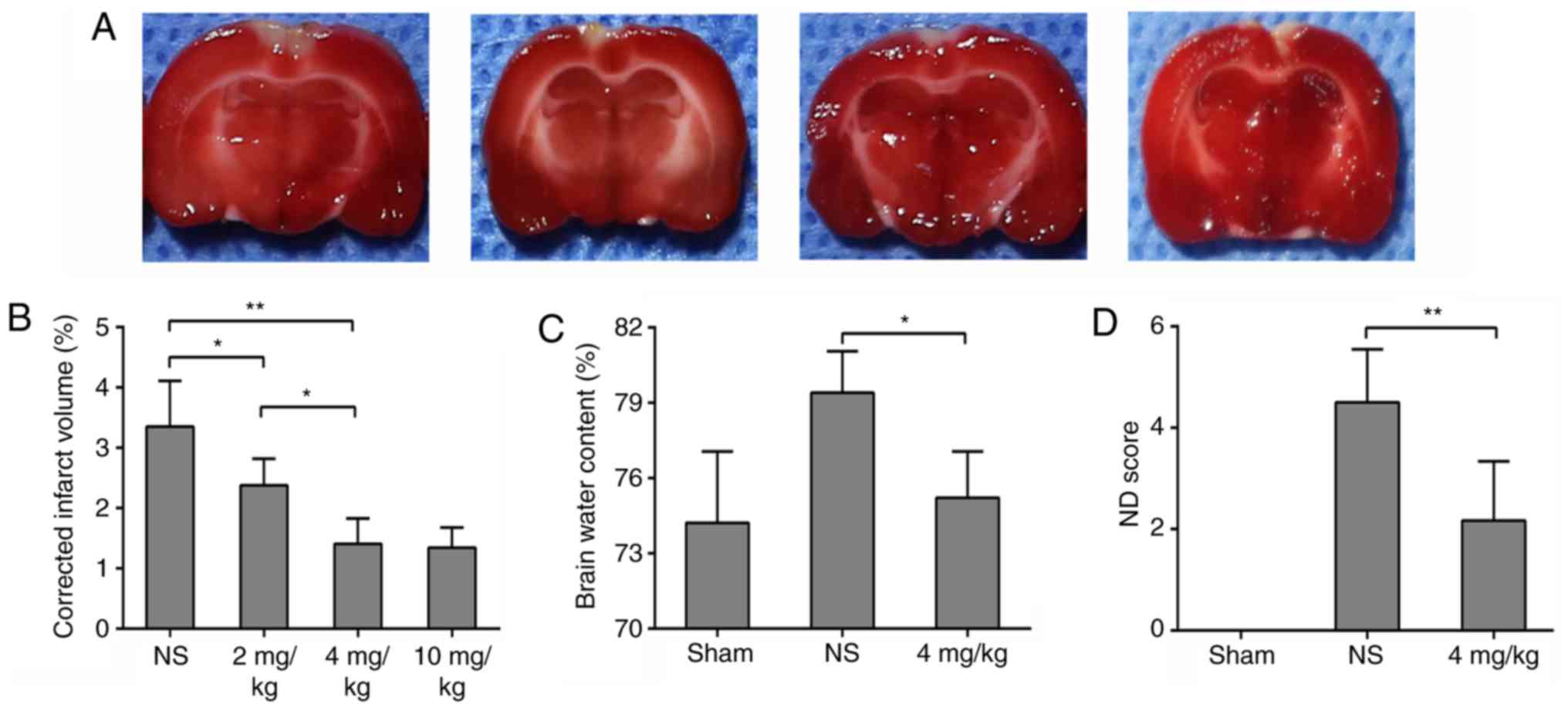
of GL. GL reduced infarct volume in a dose-dependent manner. The
volumes of cerebral infarction in rats treated with 4 and 10 mg/kg
GL were significantly decreased, and there was no significant
difference between the two groups (Fig. 3A and B). To reduce the side
effects of the drug, 4 mg/kg GL was selected for further
experiments. In addition, the brain water content and neurological
function scores of the 4 mg/kg GL-treated rats were significantly
improved, to levels similar to those of the sham group (Fig. 3C and D). These data indicate that
GL has a neuroprotective effect on brain damage following sinus
recanalization.
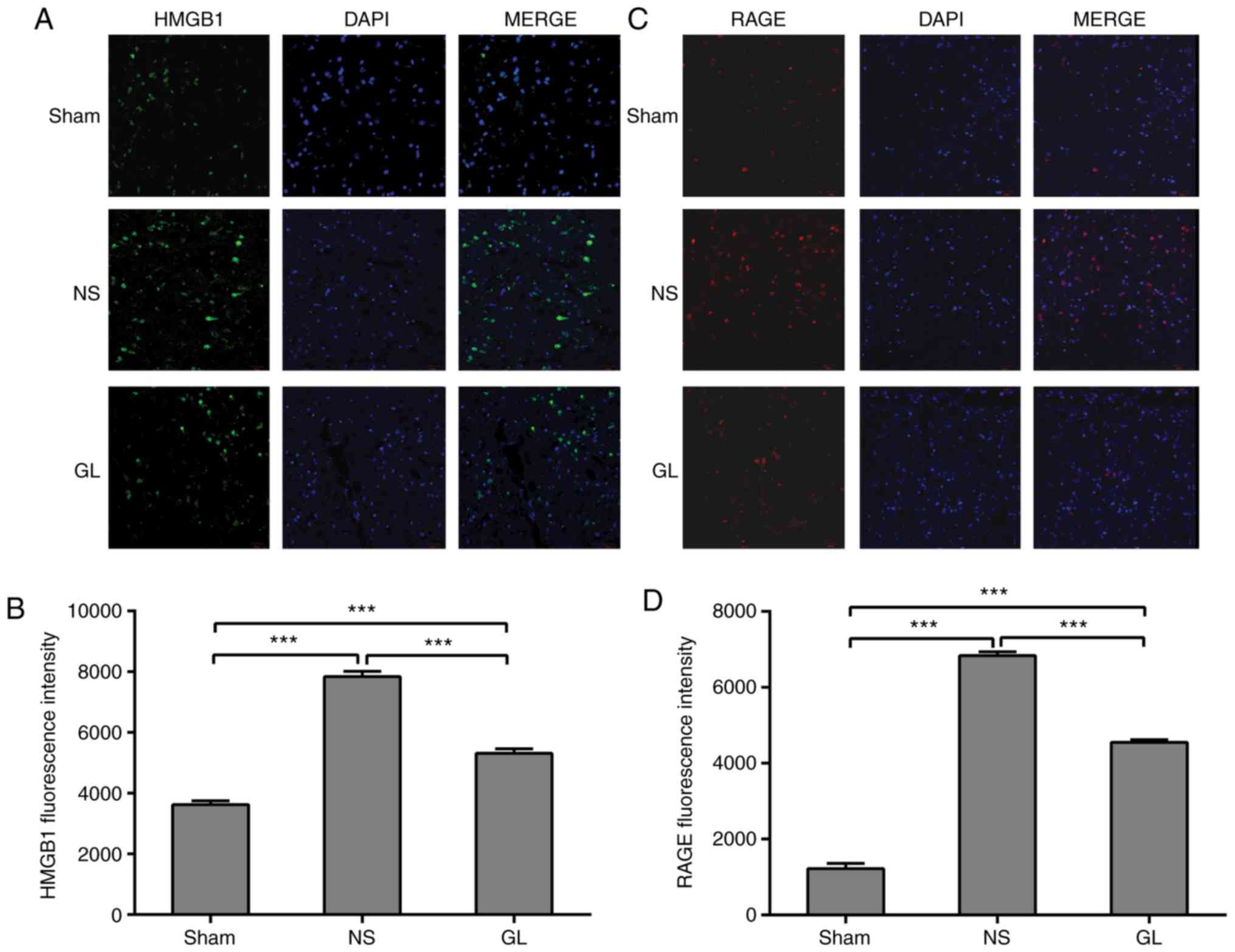
GL inhibits the transfer of HMGB1 and the
expression of RAGE following recanalization
HMGB1 is located in the nucleus under physiological
conditions (17). Using
fluorescent staining, HMGB1 was observed in the nucleus of the
sinus paracortex in the sham group. The expression of cytoplasmic
and extracellular HMGB1 was significantly increased following
mechanical thrombectomy, although some HMGB1 remained in the
nucleus. Following GL treatment, it was identified that the level
of cytosolic HMGB1 was significantly reduced compared with that in
the simple mechanical thrombectomy group, and HMGB1 was increased
in the nucleus (Fig. 4A and B).
RAGE is an HMGB1 receptor located on the plasma membrane (18). A small amount of RAGE was
expressed on the plasma membrane in the sham group. The expression
of RAGE was significantly increased following CVST, and its
expression was significantly decreased following further treatment
with GL (Fig. 4C and D).
GL inhibits the expression of HMGB1 and
its downstream inflammatory factors following recanalization
Intervention with equal doses of GL was performed at
different time points following mechanical thrombectomy in the
rats. It was observed that GL injection 1 h before mechanical
thrombectomy significantly reduced the concentrations of HMGB1 and
RAGE in the serum compared with levels at 6 and 12 h
post-thrombectomy (Fig. 5A). In
order to investigate the source of HMGB1 and RAGE in the serum, the
mRNA and protein expression levels of HMGB1 and RAGE in paranasal
sinus tissues were measured by RT-qPCR and western blot analyses,
respectively. It was found that GL injection 1 h before mechanical
thrombectomy significantly inhibited the mRNA and protein
expression of HMGB1 and RAGE. Injections at different time points
led to a similar trend of change in serum concentrations (Fig. 5B-D). To investigate the effect of
GL on the HMGB1-RAGE pathway, serum concentrations of TNF-α, IL-1β
and IL-6 were measured by ELISA. The concentrations of TNF-α, IL-1β
and IL-6 were significantly decreased in the 0 h group (Fig. 5E). The concentrations of MDA, SOD
and NOS in the serum were measured using the same method, and the
results followed a similar trend (Fig. 5F-H).
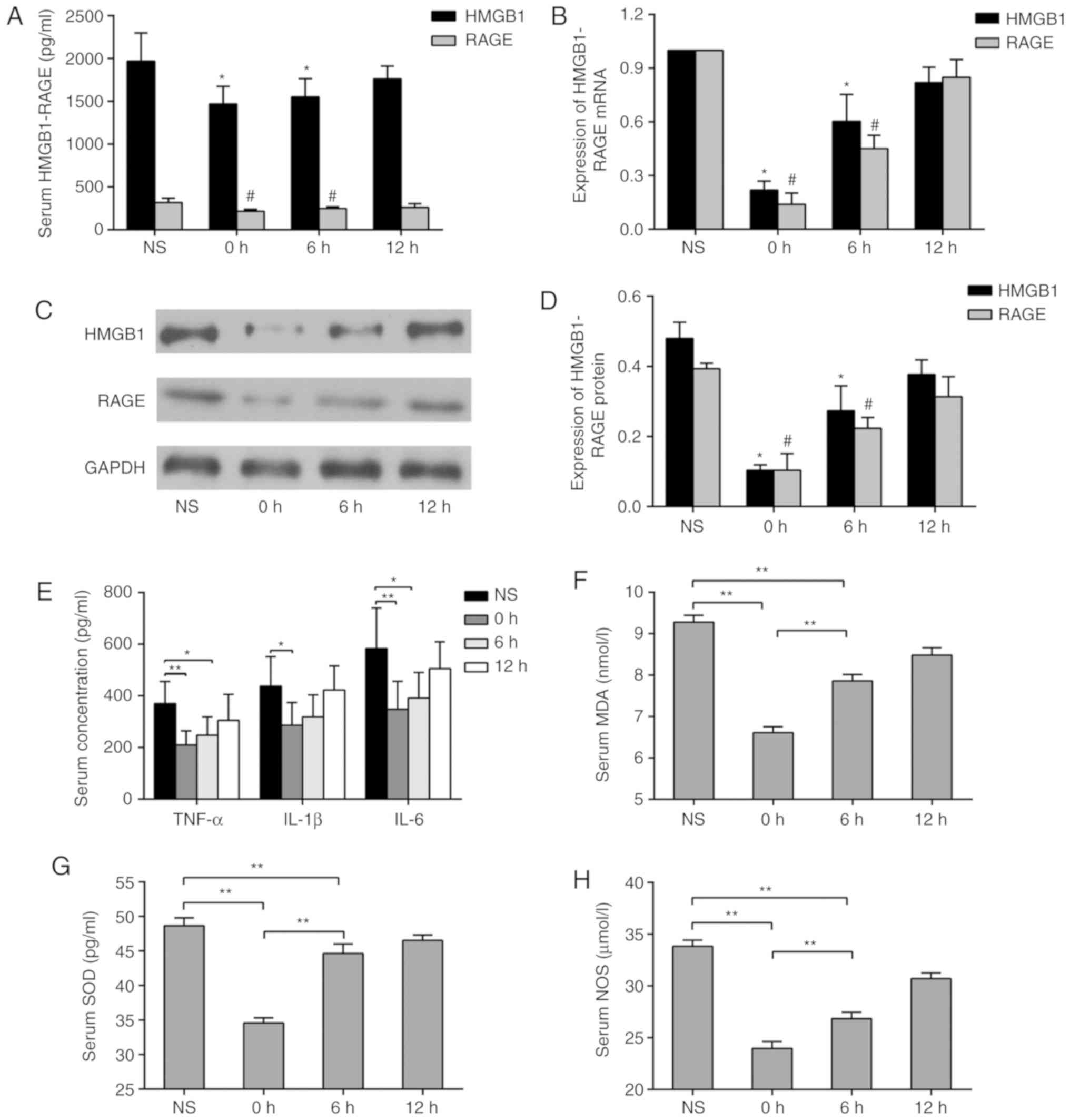 | Figure 5Expression of HMGB1 and its downstream
inflammatory factors following GL inhibition. (A) Changes in serum
concentrations of HMGB1 and RAGE at different administration time
points. (B) Changes in mRNA expression levels of HMGB1 and RAGE in
paranasal sinus brain tissues at different administration time
points; (C) Changes in protein expression levels of HMGB1 and RAGE
in sinus paraventricular tissues at different administration time
points; (D) quantification of protein expression levels.
*P<0.05 and #P<0.05 vs. NS groups.
Changes in serum concentrations of (E) TNF-α, IL-1β, IL-6, (F) MDA,
(G) SOD and (H) NOS at different doses. *P<0.05,
**P<0.01. Data are expressed as the mean ± standard
deviation. Intergroup differences were analyzed using one-way
analysis of variance Tukey's post hoc test was used for multiple
comparisons among various groups. GL, glycyrrhizin; NS, normal
saline; HMGB1, high-mobility group box 1; RAGE, receptor of
advanced glycation end products; TNF-α, tumor necrosis factor-α;
IL, interleukin; MDA, malondialdehyde; SOD, superoxide dismutase;
NOS, nitric oxide synthase. |
GL protects against brain damage
following CVO recanalization by antagonizing HMGB1
In order to further confirm that GL protected brain
damage following CVO recanalization by antagonizing HMGB1, GL was
combined with rHMGB1. The resulting infarct volume was
significantly increased compared with that of GL alone, with no
significant difference compared with that of the NS group
(P>0.05; Fig. 6A).
Furthermore, changes in the concentration of HMGB1 and its
downstream inflammatory factors were examined in the serum. Of
note, the inflammatory index of the GL + rHMGB1 group was
significantly increased compared with that of the GL-treated group
(Fig. 6B and C). The same trend
was found for oxidative stress-related indicators (Fig. 6D-F). These results further
demonstrated that GL protects against brain damage following
recanalization by inhibiting the HMGB1-RAGE pathway.
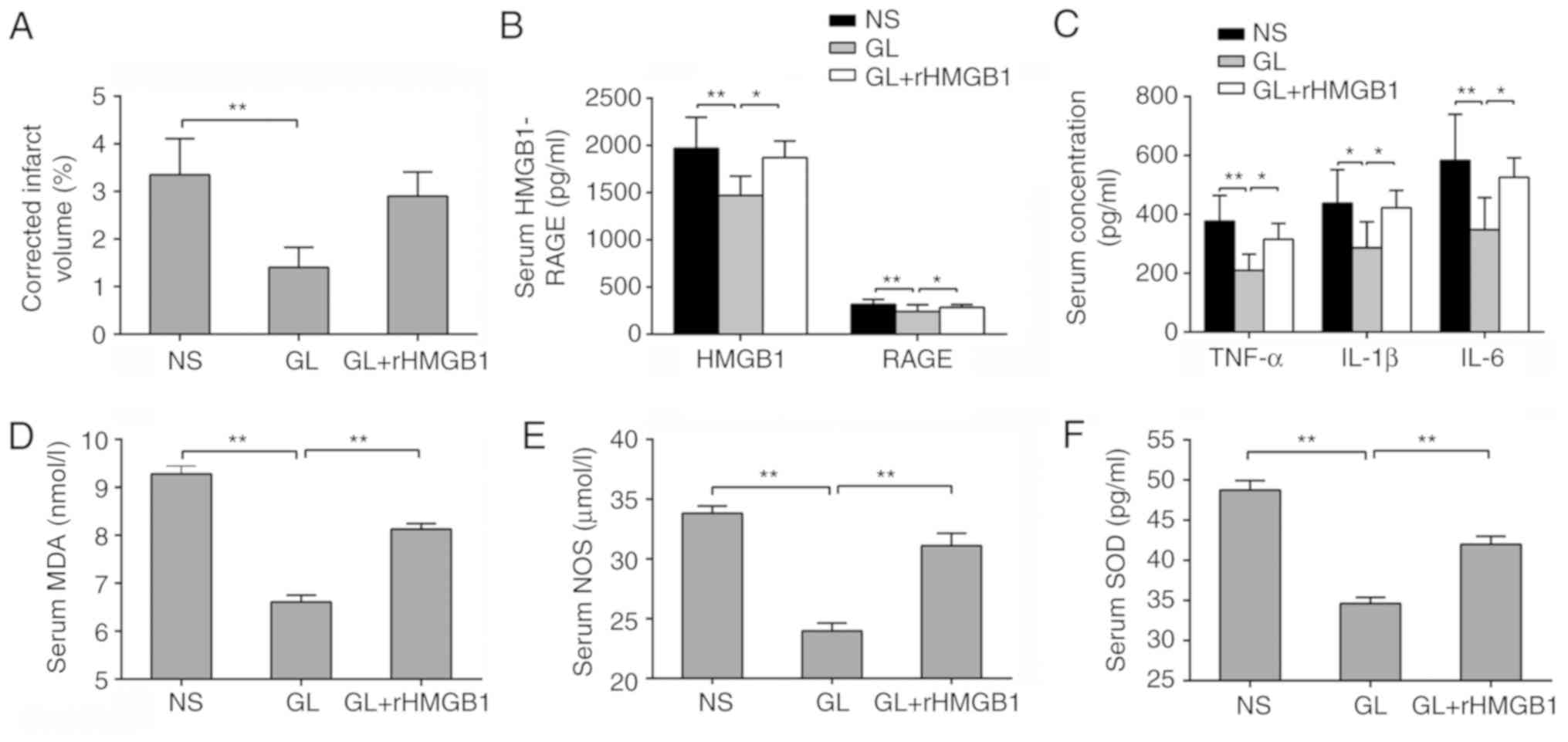 | Figure 6GL protects against brain damage
following CVO recanalization by antagonizing HMGB1. (A) Changes in
cerebral infarction volume in rHMGB1-treated or untreated rats.
Changes in (B) HMGB1 and RAGE, (C) TNF-α, IL-1β, IL-6, (D) MDA, (E)
NOS and (F) SOD in rHMGB1-treated or untreated rats. Data are
expressed as the mean ± standard deviation. Intergroup differences
were analyzed using one-way analysis of variance. Tukey's post hoc
test was used for multiple comparisons among various groups.
*P<0.05, **P<0.01. rHMGB1, recombinant
high mobility group box 1; RAGE, receptor for advanced glycation
end products; NS, normal saline; GL, glycyrrhizin; TNF-α, tumor
necrosis factor-α; IL, interleukin; MDA, malondialdehyde; SOD,
superoxide dismutase; NOS, nitric oxide synthase. |
Discussion
In the present study, FeCl3 was used to
induce CVST in rats, and mechanical thrombectomy was performed 6 h
after thrombosis to simulate the clinical recanalization observed
in human patients with CVST. The results demonstrated that CVST
mechanical thrombectomy reduced the volume of cerebral infarction
and reduced neurological deficits to a certain extent. However,
this protective effect on cerebral infarction volume reduction and
neurological function was limited; postoperative neurological
function remained significantly different from that of the
sham-operation group. In order to further improve neurological
function, GL treatment was administered following mechanical
thrombectomy. This combinatorial treatment was able to inhibit the
extracellular transport of HMGB1, and inhibited the cascade of
amplification of the HMGB1-RAGE inflammatory pathway and its
downstream inflammatory factors.
Clinically, the main goal of treating acute ischemic
stroke is to restore blood flow to ischemic brain tissue as soon as
possible. Although this treatment can restore cerebral blood flow
in a short time, improvements in cerebral infarction and nerve
function are limited. This treatment may lead to more widespread
damage of brain tissue at the ischemic penumbra, which is a
phenomenon known as brain I/R injury (19). In this process, reperfusion of
oxygen-carrying blood may further aggravate the inflammatory
reaction and the oxidative stress response of cerebrovascular
endothelial cells caused by ischemia and hypoxia, which may further
lead to destruction via cerebral infarction, damage to the
blood-brain barrier and worsening of neural function (20). An increasing number of clinical
cases indicates that this treatment has limitations (5,21,22). As the SSS is the main reflux
pathway of venous blood, long-term hypoxia and blood stasis can
lead to cerebral edema and even infarction following SSS occlusion.
Our previous studies and the results of the present study
demonstrate that FeCl3-induced CVST in rats can cause
parasitic brain tissue infarction, brain edema and other
substantial damage (16).
Therefore, a mechanism similar to that of arterial I/R was
suggested, in which brain damage following recanalization occurs
when the venous sinus is opened in brain tissues with substantial
damage. However, in the present study, rat cerebral infarction and
cerebral edema were marginally improved following mechanical
thrombectomy, which differs from arterial I/R. The reason for this
may be that the venous blood oxygen content is lower than that of
the artery. Correspondingly, endothelial cells are also less
susceptible to inflammatory and oxidative stress responses.
However, the results of these experiments suggest that mechanical
thrombectomy has limited improvement on brain damage and
neurological function, and I/R injury may still occur. According to
a recent meta-analysis, 40.2% of patients with CVST who underwent
endovascular treatment developed brain injury or led to a coma, and
the mortality rate was 14.3% at a later follow-up (4). Therefore, mechanical thrombectomy
can lead to certain defects, which further confirms our
hypothesis.
HMGB1 is a member of the damage-associated molecular
pattern molecular family and is involved in the inflammatory
cascade-amplification reaction in the pathophysiology of sepsis,
tumors and brain trauma. Under normal physiological conditions,
HMGB1 is expressed almost entirely in the nucleus of all cells and
is encoded by the 13q12.3 gene (17). HMGB1 is essential for the
maintenance of chromosomal structure and the physiological
activities of DNA (23).
Intracellular HMGB1 has the following two modes of release:
Immediate release upon cell death, and active release under the
action of stimulating factors (24). Under the action of inflammatory
stimuli, HMGB1 released extracellularly can provide information to
alert adjacent cells and activate the innate immune response. In
addition, extracellular HMGB1 can induce endocytosis in combination
with RAGE and carry other extracellular pro-inflammatory factors to
lysosomes to transmit hazardous information in a timely manner
(24). Inflammation is known to
be important in arterial I/R injury (25). Kim et al showed that
necrotic neurons in ischemic brain tissue can release HMGB1 in
large quantities and serve a role in the formation of brain I/R
injury (6). In our previous
studies, the mRNA and protein expression levels of HMGB1 and RAGE
were significantly upregulated in CVST rats with cerebral
infarction (16). The inhibition
of HMGB1 and RAGE by drugs significantly reduces nerve damage and
reduces cerebral infarction volume.
GL has natural anti-inflammatory properties and has
an antagonistic effect on HMGB1 (26). Ieong et al found that the
use of GL significantly reduced HMGB1-positive cells, downregulated
the mRNA and protein levels of HMGB1, and reduced brain-parenchymal
damage following subarachnoid hemorrhage (27). The present study found that,
following mechanical thrombectomy, a large amount of HMGB1 was
transferred from the nucleus to the cytoplasm and was released to
the outside of the cell, which may be the main reason for the
increase of serum HMGB1 concentrations. The application of GL
significantly suppressed the above-mentioned process. MDA, SOD and
NOS are sensitive indicators for evaluating oxidative stress. These
factors are activated following acute ischemic or hypoxic injury in
the brain, which triggers inflammation and apoptosis and induces a
series of brain injuries, including blood-brain-barrier destruction
(28-30). In the present study, it was
demonstrated that GL inhibited the expression of HMGB1 and RAGE and
their downstream inflammatory factors following thrombectomy, and
inhibited the expression of MDA, SOD and NOS. Following the
combined administration of exogenous rHMGB1, the factors
downregulated by GL protection were reversed to varying degrees. It
may be that microglia are activated immediately following cerebral
ischemia, and that HMGB1 activates microglia through the RAGE
receptor on the plasma membrane, releasing pro-inflammatory factors
including TNF-α, IL-1β and IL-6 and activating oxidative stress
responses. Therefore, GL may have a neuroprotective effect on the
activation of microglia by inhibiting HMGB1 during CVO
thrombectomy.
Combined anti-inflammatory medications and
intravascular management have gradually gained attention in
clinical practice. The present study primarily confirmed
recanalization-induced brain injury following CVO recanalization,
and showed that HMGB1 and RAGE induced an inflammatory response in
this process, whereas GL inhibited this signaling pathway by
exerting neuroprotective effects in CVO recanalization. Considering
inter-species differences between rats and humans, it is unclear
whether the results of the present study can be extended to humans.
Therefore, further translational research is required. In
conclusion, the present study provides a solid basis for the
clinical use of CVO mechanical thrombectomy in combination with
neuroprotective agents to improve therapeutic outcomes.
Funding
This study was supported by the Natural Science
Foundation of Fujian Province Grant (grant no. 2017J01323) to
Professor Jian-Jun Gu.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
SWM, YD, JJG and SSW were responsible for the study
concept and design. SWM, YD, YCF and HZ performed the experiments.
SWM, YD, JHZ and WW were responsible for data analyses and
interpretations. SWM and JJG drafted the manuscript. All authors
critically reviewed the manuscript and approved the final
version.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Fuzhou General Hospital. All animal experiments were
conducted in accordance with the guidelines for the care and use of
laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors sincerely thank Dr Xianhua Liu from
Fuzhou Provincial Hospital for her capable technical
assistance.
References
|
1
|
Bousser MG and Ferro JM: Cerebral venous
thrombosis: An update. Lancet Neurol. 6:162–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stam J: Thrombosis of the cerebral veins
and sinuses. N Engl J Med. 352:1791–1798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Einhäupl K, Stam J, Bousser MG, De Bruijn
SF, Ferro JM, Martinelli I and Masuhr F; European Federation of
Neurological Societies: EFNS guideline on the treatment of cerebral
venous and sinus thrombosis in adult patients. Eur J Neurol.
17:1229–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ilyas A, Chen CJ, Raper DM, Ding D, Buell
T, Mastorakos P and Liu KC: Endovascular mechanical thrombectomy
for cerebral venous sinus thrombosis: A systematic review. J
Neurointerv Surg. 9:1086–1092. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van den Berg LA, Dijkgraaf MG, Berkhemer
OA, Fransen PS, Beumer D, Lingsma HF, Majoie CB, Dippel DW, van der
Lugt A, van Oostenbrugge RJ, et al: Two-year outcome after
endovascular treatment for acute ischemic stroke. N Engl J Med.
376:1341–1349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim ID, Shin JH, Kim SW, Choi S, Ahn J,
Han PL, Park JS and Lee JK: Intranasal delivery of HMGB1 siRNA
confers target gene knockdown and robust neuroprotection in the
postischemic brain. Mol Ther. 20:829–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manns MP, Wedemeyer H, Singer A,
Khomutjanskaja N, Dienes HP, Roskams T, Goldin R and Hehnke U:
Glycyrrhizin in patients who failed previous interferon alpha-based
therapies: Biochemical and histological effects after 52 weeks. J
Viral Hepat. 19:537–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SW, Jin Y, Shin JH, Kim ID, Lee HK,
Park S, Han PL and Lee JK: Glycyrrhizic acid affords robust
neuroprotection in the postischemic brain via anti-inflammatory
effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol
Dis. 46:147–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu CX, He LX, Guo H, Tian XX, Liu Q and
Sun H: Inhibition effect of glycyrrhizin in
lipopolysaccharide-induced high-mobility group box 1 releasing and
expression from RAW264.7 cells. Shock. 43:412–421. 2015. View Article : Google Scholar
|
|
10
|
Zhang J, Wu Y, Weng Z, Zhou T, Feng T and
Lin Y: Glycyrrhizin protects brain against ischemia-reperfusion
injury in mice through HMGB1 TLR4-IL-17A signaling pathway. Brain
Res. 1582:176–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai
L, Yin L and Dong H: Protective effect of glycyrrhizin, a direct
HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced
inflammation, oxidative stress, and apoptosis in rats. PLoS One.
9:pp. e894502014, View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Srivastava AK, Gupta RK, Haris M, Ray M,
Kalita J and Misra UK: Cerebral venous sinus thrombosis: Developing
an experimental model. J Neurosci Methods. 161:220–222. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ansari S, Azari H, McConnell DJ, Afzal A
and Mocco J: Intraluminal middle cerebral artery occlusion (MCAO)
model for ischemic stroke with laser doppler flowmetry guidance in
mice. J Vis Exp: pii. 2879:2011.
|
|
15
|
Chen C, Wang Q, Gao Y, Lu Z, Cui X, Zheng
T, Liu Y, Li X, He X, Zhang X, et al: Photothrombosis combined with
thrombin injection establishes a rat model of cerebral venous sinus
thrombosis. Neuroscience. 306:39–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu JJ, Chen JB, Zhang JH, Zhang H and Wang
SS: Recombinant human soluble thrombomodulin protects against brain
injury in a CVST rat model, via downregulation of the HMGB1-RAGE
axis. Mol Med Rep. 14:5217–5222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sohun M and Shen H: The implication and
potential applications of high-mobility group box1 protein in
breast cancer. Ann Transl Med. 4:2172016. View Article : Google Scholar
|
|
18
|
Anggayasti WL, Mancera RL, Bottomley S and
Helmerhorst E: The self-association of HMGB1 and its possible role
in the binding to DNA and cell membrane receptors. FEBS Lett.
591:282–294. 2017. View Article : Google Scholar
|
|
19
|
Schaller B and Graf R: Cerebral ischemia
and reperfusion: The pathophysiologic concept as a basis for
clinical therapy. J Cereb Blood Flow Metab. 24:351–371. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goyal M, Menon BK, van Zwam WH, Dippel DW,
Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de
Miquel MA, et al: Endovascular thrombectomy after large-vessel
ischaemic stroke: A meta-analysis of individual patient data from
five randomised trials. Lancet. 387:1723–1731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alegiani AC, Dorn F, Herzberg M,
Wollenweber FA, Kellert L, Siebert E, Nolte CH, von Rennenberg R,
Hattingen E, Petzold GC, et al: Systematic evaluation of stroke
thrombectomy in clinical practice: The German stroke registry
endovascular treatment. Int J Stroke. Oct 22–2018, Epub ahead of
print. PubMed/NCBI
|
|
22
|
Mizuma A and Yenari MA: Anti-inflammatory
targets for the treatment of reperfusion injury in stroke. Front
Neurol. 8:4672017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bertheloot D and Latz E: HMGB1, IL-1alpha,
IL-33 and S100 proteins: Dual-function alarmins. Cell Mol Immunol.
14:43–64. 2017. View Article : Google Scholar
|
|
24
|
Andersson U, Yang H and Harris H:
High-mobility group box1 protein (HMGB1) operates as an alarmin
outside as well as inside cells. Semin Immunol. 38:40–48. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai J and Lyden PD: Revisiting cerebral
postischemic reperfusion injury: New insights in understanding
reperfusion failure, hemorrhage, and edema. Int J Stroke.
10:143–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mollica L, De Marchis F, Spitaleri A,
Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L,
Musco G and Bianchi ME: Glycyrrhizin binds to high-mobility group
box1 protein and inhibits its cytokine activities. Chem Biol.
14:431–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ieong C, Sun H, Wang Q and Ma J:
Glycyrrhizin suppresses the expressions of HMGB1 and ameliorates
inflammative effect after acute subarachnoid hemorrhage in rat
model. J Clin Neurosci. 47:278–284. 2018. View Article : Google Scholar
|
|
28
|
Jadhav RS, Ahmed L, Swamy PL and Sanaullah
S: Neuroprotective effects of polyhydroxy pregnane glycoside
isolated from Wattakaka volubilis (L.f.) Stapf. after middle
cerebral artery occlusion and reperfusion in rats Brain Res.
1515:78–87. 2013.
|
|
29
|
Zheng YQ, Liu JX, Wang JN and Xu L:
Effects of crocin on reperfusion-induced oxidative/nitrative injury
to cerebral microvessels after global cerebral ischemia. Brain Res.
1138:86–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Li J, Zhao F, Wang H, Qu Y and Mu
D: Nitric oxide synthase in hypoxic or ischemic brain injury. Rev
Neurosci. 26:105–117. 2015. View Article : Google Scholar : PubMed/NCBI
|