Introduction
Diabetes is one of the major risk factors of
cardiovascular disease. Diabetic cardiomyopathy (DCM) is a distinct
clinical entity of cardiovascular disease that cannot be explained
by coronary atherosclerotic heart disease, hypertensive heart
disease and heart disease caused by other factors (1). The disease is associated with
metabolic disorders and microvascular lesions, causing a wide range
of necrosis and subclinical cardiac dysfunction. This ultimately
promotes the occurrence of heart failure, arrhythmia and
cardiogenic shock, and even leads to sudden death in some severe
cases.
One of the most important characteristics of DCM is
myocardial fibrosis (2).
According to the traditional concept, the myocardial fibrosis is
caused by resident cardiac interstitial fibroblasts, simultaneously
involving the proinflammatory process and synthesis of
extracellular matrix (ECM) (3).
In 1994, Bucala et al (4)
found the novel leukocyte subpopulation that expressed COL-I, CD34
and vimentin. As these are obtained from the peripheral blood, they
are given the name of circulating fibrocytes.
Circulating fibrocytes are a type of novel and
unique cells that are derived from hematopoietic stem cells (HSCs).
They are also referred to as 'peripheral blood fibrocytes', 'bone
marrow-derived fibrocytes' and 'circulating fibroblast precursors'
(5-7). These aliases reflect some common
features with the circulating fibrocytes, such as their existence
in the peripheral blood, compromise of a minor component (<1%)
of peripheral blood mononuclear cells (PBMCs) and are considered as
bone marrow-derived precursor cells of fibroblasts, and
myofibroblasts. Circulating fibrocytes synthesize a variety of ECM
proteins including type I and III collagens, fibronectin, vimentin
and growth factors, playing an important role in the process of
multiple pathophysiological states. Chun Li et al (8) found that the circulating fibrocytes
were recruited from the peripheral blood to the lung through the
CXC chemokine receptor 4 (CXCR4)/stromal-derived factor-1 (SDF-1)
axis and played an important role in bronchopulmonary dysplasia,
which eventually led to pulmonary fibrosis. A recent study showed
that the blood concentration of fibrocytes expressing CXCR4 was
significantly correlated with Hermansky Pudlak syndrome (HPS),
which is a genetic disease caused by interstitial lung disease
(ILD). The concentration of CXCR4+ in circulating
fibrocytes may be used as a new biomarker for the outcome of ILD in
patients with HPS (9). Lin et
al (10) found that
activation of the CXCR4/SDF-1 axis caused expression of CTGF and
promoted cell differentiation, eventually leading to interstitial
lung fibrosis. These results suggested that fibrocytes might play a
crucial role in organ fibrosis through the CXCR4/SDF-1 axis.
Diabetic patients could develop severe organ
fibrosis and so a previous study hypothesized that there may be
more circulating fibrocytes in the peripheral blood of diabetic
patients (11). As expected, our
previous study (12) confirmed
that the ratio of cells co-expressing cluster of differentiation
(CD)45 and COL-I to PBMCs was significantly increased in diabetic
patients compared with normal glucose tolerance (NGTs; 1.93±1.01
vs. 0.52±0.35%; P<0.01) patients. In addition, high glucose
promoted the proliferation and the expression of CXCR4 and CTGF of
circulating fibrocytes. However, whether high glucose levels
regulated the circulating fibrocytes through the CXCR4/SDF-1 axis
has not been investigated yet. Therefore, in this study, the
effects of high glucose on the function of circulating fibrocytes
and its underlying mechanism were explored by investigating i) the
effects of high glucose concentration medium on the invasion and
migration ability of circulating fibrocytes; ii) the involvement of
the CXCR4/SDF-1 axis in the production of ECM COL-I and the
fibrogenic factor connective tissue growth factor (CTGF) of
circulating fibrocytes, and iii) whether AMD3100, the specific
blocker of CXCR4, could relieve the progression of fibrosis.
Combined with the authors previous study, it is hoped that the
present study will provide a more detailed explanation regarding
the effects of high glucose on circulating fibrocytes.
Materials and methods
Patients
A total of 15 NGT patients (11 males and 4 females)
and 15 type 2 diabetes mellitus (T2DM) patients (13
males and 2 females) from the department of Cardiology, Zhejiang
Hospital (Hangzhou, China) were recruited from June 2016 to June
2017. The patients' age ranged from 65-75 years old. Inclusion
criteria of NGT patients were as follows: The blood glucose levels
should be <7.8 mmol/l after 2 h of oral glucose tolerance test
experiment and the T2DM patients were confirmed to have
type 2 diabetes according to the 1999 WHO diagnostic criteria for
diabetes. The exclusion criteria of the patients were as follows:
Patients i) with severe heart and kidney dysfunction, such as
myocardial infarction (MI), hypertrophic cardiomyopathy; ii) with
severe pulmonary infection, chronic obstructive pulmonary disease
and interstitial disease; iii) with severe trauma and burns; iv)
with stage 2 and 3 hypertension; v) with malignant tumors; vi)
taking hormonal drugs recently; vii) with coagulopathy; and viii)
with connective tissue diseases. According to the previous
literature reports (13-15), these diseases might be associated
with an increase in the number of circulating fibrocytes. The
present study was approved by the Zhejiang Hospital Ethics
Committee and all subjects signed the informed consent form.
Isolation and cultivation of circulating
fibrocytes
A total of 25 ml of peripheral blood from NGT
patients and 5 ml from T2DM patients were obtained and
mixed with 1.6% heparinized normal saline solution. Density
gradient centrifugation was performed to obtain the PBMC layer
(16). The cell sediment was
washed twice with 5 ml PBS at 500 × g for 10 min at 20°C (Genom
Biotechnology). The cells were resuspended in 25 mmol/l glucose
DMEM containing (Thermo Fisher Scientific, Inc.) 20% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml;
Genom Biotechnology) and streptomycin (100 µg/ml; Genom
Biotechnology). After that, the cells at a density of
1×106/ml were seeded into 6-well plates and placed in a
humidified incubator at 37°C in 5% CO2. The medium was
changed every 3 days and the non-adherent cells were removed, and
the adherent cells were obtained after cultivation for 14 days. The
morphological characteristics of the cells were observed under an
inverted phase contrast microscope.
Identification of circulating fibrocytes
by immunofluorescence
COL-I and CD45 are considered as biomarkers to
identify the circulating fibrocytes as mentioned before. The
adherent cells were digested with Accutase solution (Yeasen). After
washing twice (450 × g, 10 min, 20°C), the cells were resuspended
with high glucose (25 mmol/l) DMEM containing 20% FBS. After
counting, the cells were diluted to 8×104/ml and placed
on coverslips (JingAn Biological) in 24-well plates. After 24 h,
the cells were washed twice and fixed with 4% paraformaldehyde at
20°C for 15 min. Then, 200 µl fixation and permeabilization
solution was added in each well and the coverslips were incubated
at 4°C for 20 min. The cells were washed with 1X wash buffer twice
and then 100 µl FBS was added for 30 min at 4°C. The primary
antibody, mouse anti-human CD45 (1:100; Ancell; cat. no. 196-030)
and rabbit anti-human COL-I (1:500; Rockland Immunochemicals, Inc.;
cat. no. 600-401-103S) were added to the cells and then the
coverslips were incubated overnight at 4°C. After washing twice,
the cells were incubated with Alexa Fluor 488 labeled goat
anti-mouse immunoglobulin (Ig) G (1:200; Yeasen; cat. no.
33206ES60) and Alexa Fluor 594 labeled goat anti-rabbit IgG
secondary antibody (1:200; Yeasen; cat. no. 33112ES60) for 30 min
at 4°C. After washing twice, DAPI (Abcam) was added and incubated
for 5 min at 4°C, washed with PBST (0.1% tween 20) twice and then
observed under a confocal microscope.
Measurement of proliferation of
circulating fibrocytes by Cell Counting Kit (CCK)-8
After 14 days of culture, the circulating fibrocytes
obtained from the NGT group were digested by Accutase solution,
diluted to 4×104/ml and seeded in 96-well plates. After
24 h incubation, the cells were synchronized with minimum Eagle's
medium (MEM; 5.5 mmol/l glucose; Gibco; Thermo Fisher Scientific,
Inc.) for 24 h. After that, 100 µl of 5.5 mmol/l glucose
DMEM, 5.5 mmol/l glucose+100 µmol/l AMD3100 DMEM (MedChem
Express) 30 mmol/l glucose DMEM and 30 mmol/l glucose+100
µmol/l AMD3100 DMEM, respectively were added. After 24, 48
and 72 h incubation, the intervention solution was aspirated and
stored at −80°C for subsequent experiments. A total of 100
µl MEM containing 10% CCK-8 (7sea biotech) was added to each
well and continued to culture for 1 h for measuring the absorbance
at 450 nm using a microplate reader (Thermo Fisher Scientific,
Inc.).
Measurement of cell apoptosis by
Hoechst33258 staining
The circulating fibrocytes from NGT patients were
diluted to 5×104/ml and then seeded in 24-well plates.
After 24 h incubation, the fibrocytes were synchronized with MEM
for 24 h. Then cells were treated with 5.5 mmol/l DMEM, 30 mmol/l
DMEM and 5.5 mmol/l DMEM +200 µmol/l
H2O2 (as positive control group) for 48 h.
After treatment with different concentrations of DMEM, cells were
fixed with 4% paraformaldehyde at 20°C for 15 min. After that, 0.5
µg/ml Hoechst33258 (R&D Systems, Inc.) was added for 15
min in the dark. Synchronized cells without any DMEM interventions
were stained at 20°C for 15 min with 0.5 µg/ml Hoechst33258
and served as a negative control. Finally, the morphology and
brightness of the nucleus of circulating fibrocytes were observed
under confocal microscopy (Leica Microsystems GmbH). The excitation
wavelength was 550 nm.
Detection of COL-I expression of
fibrocytes by flow cytometry
After 7 days of cultivation, the fibrocytes of NGT
patients were diluted to 6×104/ml and synchronized with
MEM for 24 h. After synchronization, 1 ml of 5.5 mmol/l DMEM, 5.5
mmol/l DMEM+100 µmol AMD3100, 30 mmol/l DMEM and 30 mmol/l
of DMEM+ 100 µmol AMD3100 were added, respectively. After 48
h, the fibrocytes were digested by Accutase solution. All the cells
were collected after centrifugation (450 × g for 10 min at 20°C)
and then were treated with cytofix/cytoperm solution (Abcam) for 20
min, followed by the addition of 100 µl FBS for 30 min at
4°C. The primary antibody, rabbit anti-human COL-I (1:2,000; Abcam;
cat. no. ab6577) was applied to the cells and incubated at 4°C
overnight. Then the goat anti-rabbit fluorescein isothiocyanate-IgG
(1:1,000; Abcam; cat. no. ab6717) was added for 20 min in the dark.
After staining, the expression of COL-I was detected by Cytomics™
FC 500 and the non-stained cells were used as isotype controls. The
results were analyzed using Flowjo software (Tree Star, Inc.;
version 7.6.1).
Invasion and chemotaxis assay of
fibrocytes
After 14 days of cultivation, the fibrocytes of NGTs
were diluted to 8×104/ml and synchronized with MEM for
24 h. After that, the fibrocytes were treated with 5.5 and 30
mmol/l of DMEM, respectively for 48 h. A Transwell chamber with an
8 µm pore PET membrane (Corning Inc.) was used for
evaluating the invasion and chemotaxis of fibrocytes. Matrigel was
diluted with serum-free MEM (1:8), coated on the upper chamber of
the bottom membrane of the Transwell chamber and then placed at
37°C for 30 min for solidification. Stimulated fibrocytes were
digested by Accutase solution, diluted with serum-free MEM to
5×104/ml and seeded onto the upper chambers (150
µl per well). The lower chambers were filled with different
concentrations of 25 mmol/l glucose DMEM containing 20% FBS [25
mmol/l glucose DMEM, 25 mmol/l glucose DMEM+50 µmol/l SDF-1
(R&D Systems, Inc.), 25 mmol/l glucose DMEM+100 µmol/l
AMD3100 and 25 mmol/l glucose DMEM+50 µmol/l and SDF-1+100
µmol/l AMD3100]. The whole culture system was placed into a
humidified incubator with 5% CO2. After 48 h, the
matrigel was removed. The cells were fixed with 4% paraformaldehyde
for 15 min at 20°C and then stained with 1% crystal violet solution
for 10 min at 20°C (Yantuo Shanghai Trade, Co., Ltd.). Finally, the
cells were observed under inverted phase contrast microscope and
the total cell number of five non-overlapping fields was
counted.
Measurement of SDF-1 secretion by
ELISA
A 5.5 mmol/l supernatant and 30 mmol/l glucose DMEM
were obtained as described in the above section. The DMEM was
centrifuged at 10,000 × g for 10 min at 4°C. The content of SDF-1
in the supernatants was quantified using a commercial SDF-1 ELISA
kit (PeproTech, Inc.; cat. no. 900-K92) according to the
manufacturer's protocol. The detection limitation was 100
pg/ml.
Measurement of CTGF by western
blotting
The identified circulating fibrocytes of NGT
patients were diluted to 8×104/ml. The cells were seeded
into 6-well plates. After 24 h of synchronization, each well was
added with 5.5 mmol/l glucose DMEM, 5.5 mmol/l glucose DMEM+100
µmol/l AMD3100, 30 mmol/l glucose DMEM and 30 mmol/l+100
µmol/l AMD3100 1 ml, respectively for 48 h. Total protein
was extracted after intervention and the protein concentration was
determined by the bicinchoninic acid method. A total of 20
µg of each sample was taken for electrophoresis and then
transferred onto the polyvinylidene difluoride (PVDF) membrane. The
PVDF membranes were incubated with 5% milk in TBST (0.1% tween 20)
buffer for 1 h at 20°C. Specific primary antibody [1:1,000; rabbit
anti-human CTGF IgG (Abcam; cat. no. ab227180) and mouse anti-human
β-actin IgG (Abcam; cat. no. ab6276)] were added and then the
membranes were incubated with 1:5,000 diluted goat anti-mouse
(Abcam; cat. no. ab205719) and goat anti-rabbit (Abcam; cat. no.
ab205718) horseradish peroxidase labeled secondary antibodies for 1
h at 20°C. Finally, 300 µl ECL operating fluid (Abcam) was
added per band. Quantitative data were obtained with the ChemiDoc
XRS gel imaging system and gray values were analyzed using Image J
software (National Institutes of Health; version 1.8.0).
Statistical analysis
Data was analyzed by SPSS 19.0 software (IBM Corp.)
and the results were expressed as the mean ± standard deviation.
Comparison between groups (>2) was performed using one-way
analysis of variance (ANOVA) and the between pair differences were
performed using least significant difference test. Prior to one-way
ANOVA, the normality was confirmed using the Shapiro-Wilk test
(P>0.05) and the homogeneity of variances using the F test
(P>0.05). P<0.05 was considered to indicate a statistically
significant difference.
Results
Basic patient information
The clinical information of all included subjects
was collected and the important sections are presented in Table I. The results showed no
differences in age between the NGT group and the T2DM
group (P>0.05), but showed statistically significant differences
between the two groups in the parameters of fasting blood glucose,
body mass index and hemoglobin A1c (P<0.05).
 | Table IBasic information of T2DM and
NGTs. |
Table I
Basic information of T2DM and
NGTs.
| Group | n | Age (year) | Male/female | BMI | HbA1c | Fasting blood
glucose |
|---|
| NGTs | 15 | 70.33±5.22 | 11/4 | 23.77±3.54 | 5.27±0.49 | 5.41±0.66 |
|
T2DM | 15 | 72.67±6.88 | 13/2 | 26.58±4.93 | 7.78±0.53 | 7.34±0.71 |
| P-value | | >0.05 | | <0.01 | <0.01 | <0.01 |
Identification of human circulation
fibrocytes
According to the previous studies,
CD45+COL-I+ or
CD34+COL-I+ was regarded as the gold standard
to identify human circulating fibrocytes (4,8,9).
Immunofluorescence technique was used to examine whether the
cultured-adherent cells were the target objects. The dyed cells
were observed by Olympus FV3000 confocal microscope after
immunofluorescence staining. As described in our previous study
(12), after 14 days of
cultivation, both CD45 (green fluorescence) and COL-I (red
fluorescence) showed significant expression in the authors previous
research. In addition, there were more spindle-shaped fibrocytes in
T2DM patients compared with NGT patients. However, after
21 days, the fibrocytes showed only COL-I expression and CD45
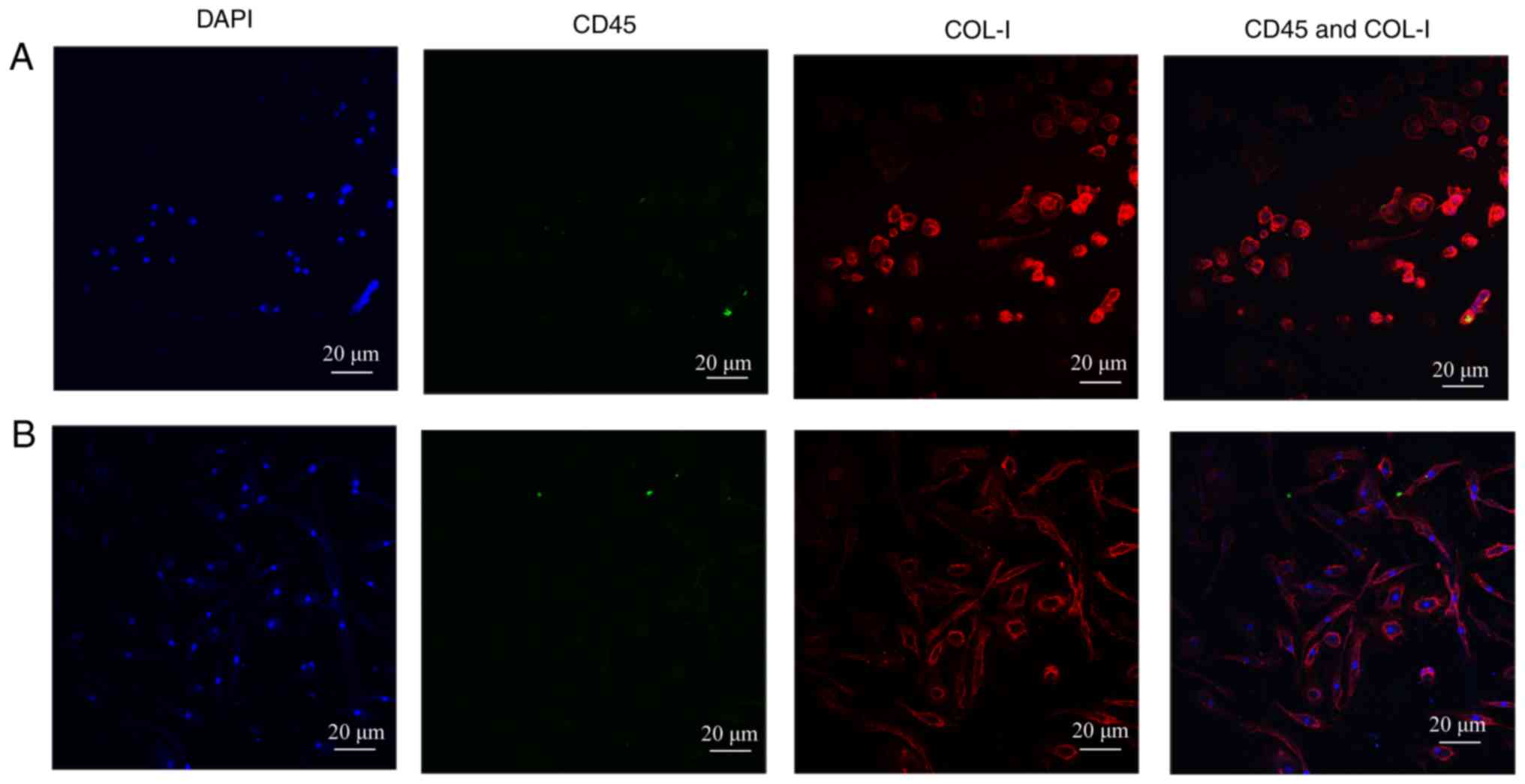
expression was hardly observed (Fig.
1A and B). The results were consistent with the differentiation
process of circulating fibrocytes as reported in the literatures
(17,18). As is known, the expression of
hematopoietic surface antigens, like CD34 and CD45, are decreased
during the differentiation process of circulating fibrocytes, and
at the same time, the secretion of ECM proteins, like COL-I are
increased. Finally, the appearance of α-smooth muscle actin (sma)
indicated that the circulating fibrocytes were completely
differentiated into myofibroblasts (5).
AMD3100 had no effect on high glucose
induced proliferation of circulating fibrocytes
The proliferation of circulating fibrocytes after
treatment with different glucose concentrations of DMEM (5.5, 25
and 30 mmol/l glucose DMEM and 5.5 mmol/l glucose+30 mmol/l
mannitol) stimulation was detected by CCK-8 reagent as done in the
our earlier study (12). As is
known, the osmotic pressure of the culture media had an effect on
the growth and proliferation of the cells (19,20). The osmotic pressure of 5.5 mmol/l
glucose DMEM was different from that of 30 mmol/l glucose DMEM.
Therefore, in the our previous experiment (12), the 5.5 mmol/l glucose +30 mmol/l
mannitol group was added as a negative control group to exclude the
influence of osmotic pressure to cell proliferation. In fact, there
are a number of similar experiments for measuring cell
proliferation that choose mannitol as a negative control (21,22). The results showed that the number
of cells was proportional to the value of absorbance. As expected,
the proliferation of circulating fibrocytes showed a significant
concentration-time correlation. Furthermore, when stimulated with
30 mmol/l glucose DMEM for 48 h, the number of fibrocytes reached
the maximum value. Mannitol intervention did not promote the
proliferation of fibrocytes when compared with the 5.5 mmol/l
glucose group (12).
Existing data demonstrated that CXCR4/SDF-1 axis
plays a key role in tumor cells proliferation. Marchesi et
al (23) found that human
pancreatic tumor cells showed higher expression levels of CXCR4
compared with normal and AMD3100 partially inhibited the
proliferation of tumor cells. Therefore, it was hypothesized that
high glucose stimulated cell proliferation through the CXCR4/SDF-1
axis. Whether AMD3100 could inhibit the proliferation of
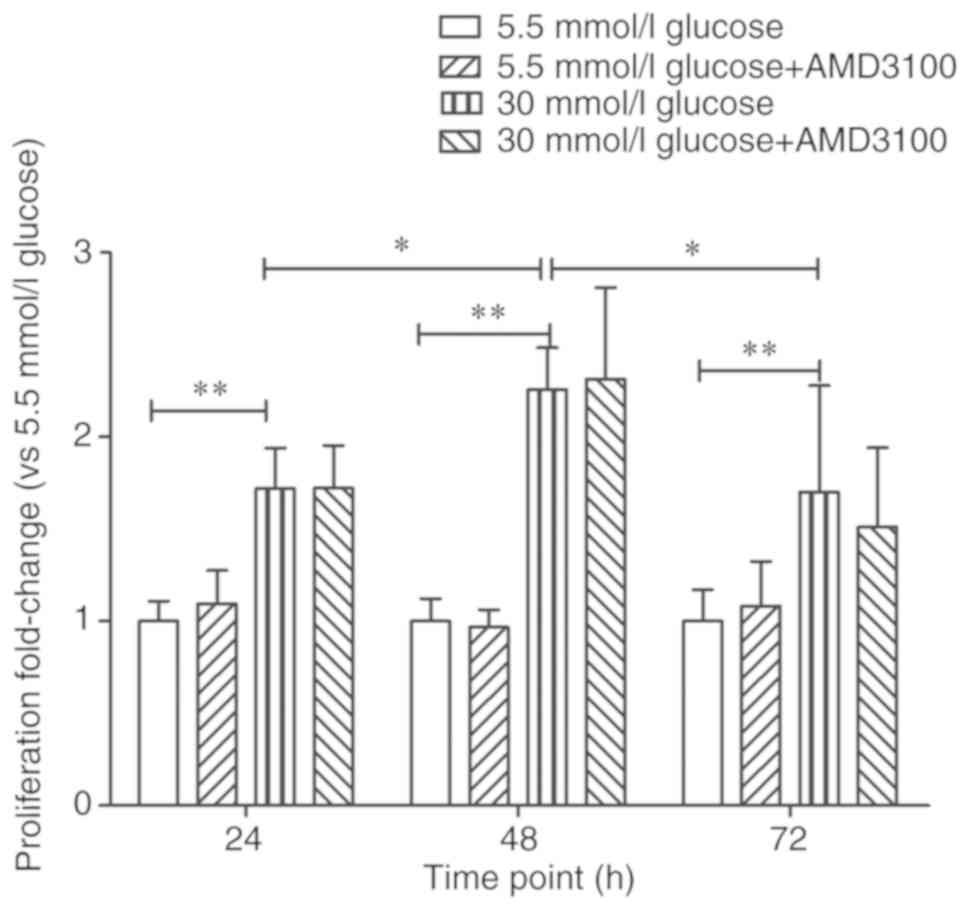
circulating fibrocytes was then investigated. The absorbance of 5.5
mmol/l glucose DMEM at 24 h was regarded as '1', the proliferation
fold-change of 5.5 mmol/l glucose+100 µmol/l AMD3100 DMEM
group, 30 mmol/l glucose DMEM group and 30 mmol/l glucose+100
µmol/l AMD3100 DMEM group at 24 h were 1.09±0.18
(P>0.05), 1.72±0.22 (P<0.01) and 1.72±0.23 (P<0.01)
respectively. However, there were no statistical differences
between 5.5 mmol/l glucose DMEM group and 5.5 mmol/l glucose+100
µmol/l AMD3100 DMEM group (P>0.05) or 30 mmol/l glucose
DMEM group and 30 mmol/l glucose+100 µmol/l AMD3100 DMEM
group (P>0.05). The absorbance of 5.5 mmol/l glucose DMEM at 48
h was regarded as '1' and the proliferation fold-change of
sequential intervention groups at 48 h were 0.97±0.10 (P>0.05),
2.26±0.23 (P<0.01) and 2.32±0.50 (P<0.01), respectively. The
absorbance of 5.5 mmol/l glucose DMEM at 72 h was regarded as '1'
and the proliferation fold-change of sequential intervention groups
at 72 h were 1.08±0.24 (P>0.05), 1.70±0.58 (P<0.01) and
1.55±0.43 (P<0.01), respectively. Similarly, AMD3100 showed no
effect on the proliferation of circulating fibrocytes at 48 and 72
h. At the same time, although the fold-change was slightly
different from the authors' study, the proliferation of cells
reached peak at 48 h (P<0.05). The results confirmed that high
glucose DMEM promoted the proliferation of circulating fibrocytes.
However, AMD3100 showed no effects on the proliferation of
circulating fibrocytes (Fig.
2).
High glucose had no effect on the
apoptosis of circulating fibrocytes
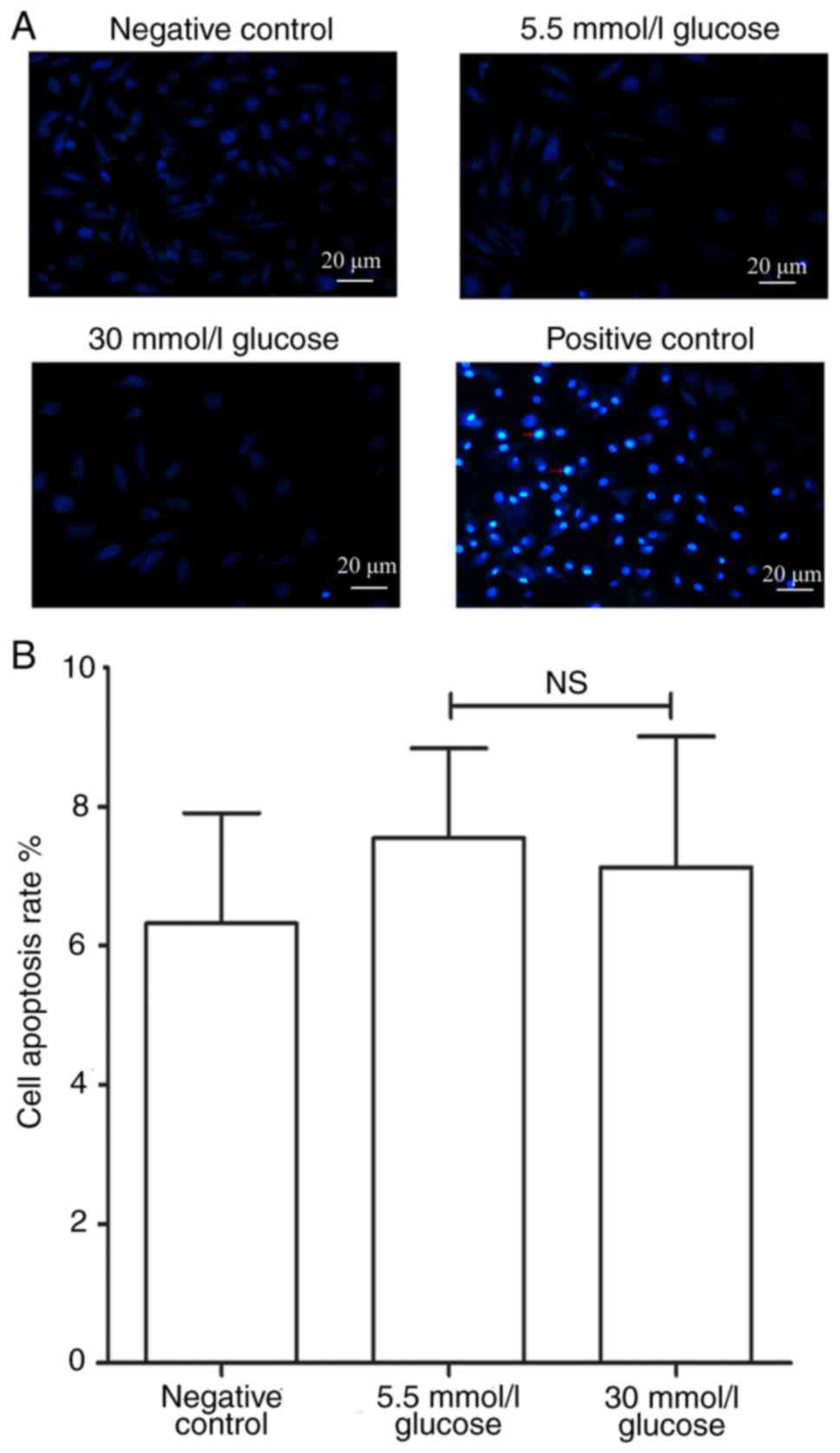
The effects of glucose concentration on apoptosis of
circulating fibrocytes was further studied. In order to make the
results more convincing, a negative control group and a
H2O2 induced positive control group were
established. Due to damage of the nuclear envelope occurring at
early apoptosis, Hoechst 33258 bound to nucleic acid. The nucleus
of the circulating fibrocytes of the positive control group
therefore showed a bright blue color after staining (Fig. 3A). The results of Hoechst33258
staining showed no statistically significant difference in the
apoptotic rate between 5.5 mmol/l glucose group and 30 mmol/l
glucose group (7.33±1.61 vs. 7.08±2.05%; P>0.05, Fig. 3B).
AMD3100 inhibits high glucose induced
promotion of COL-I expression on circulating fibrocytes
Deposition of ECM proteins are one of the most
important features of organ fibrosis. Rodríguez-Nieves et al
(24) found that the CXCR4/SDF-1
axis mediated myofibroblast phenoconversion and promoted the
expression of α-sma and COL-I via non-canonical epithelial growth
factor receptor/dual specificity mitogen-activated protein kinase
kinase 1/extracellular signal-regulated kinase (ERK) signaling.
Therefore, whether glucose concentration affected the expression of
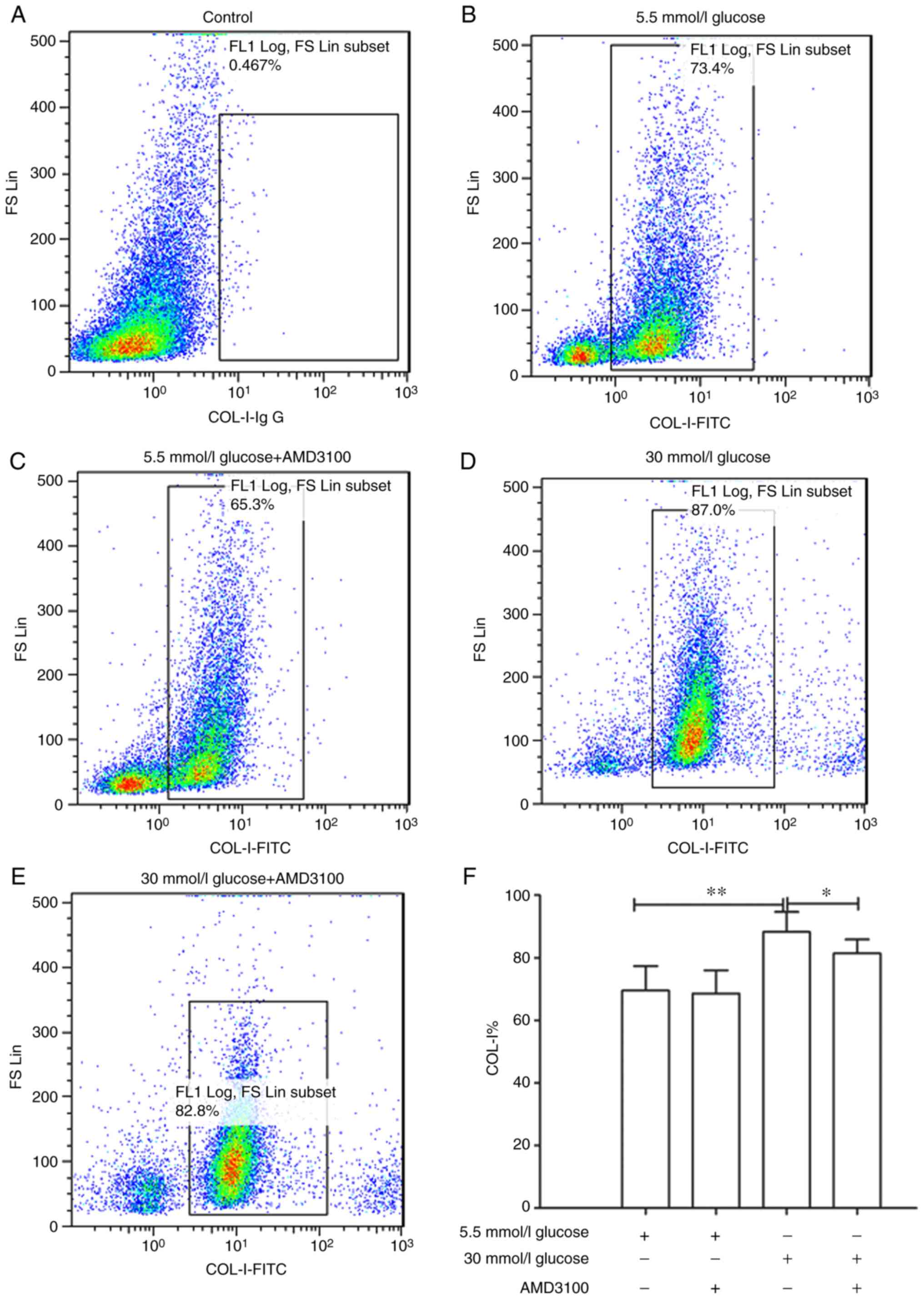
COL-I was investigated. The results showed that 30 mmol/l glucose
DMEM significantly promoted the expression of COL-I compared with
5.5 mmol/l group (88.26±6.40 vs. 69.57±7.79%; P<0.01). In
addition, AMD3100 could significantly inhibit the promotion of
COL-I expression caused by high glucose DMEM (88.26±6.40 vs.
80.43±4.42%; P<0.05). These results demonstrated that high
glucose DMEM may stimulate the expression of COL-I via the
CXCR4/SDF-1 axis (Fig. 4B-F).
AMD3100 inhibits high glucose induced
promotion of CTGF on circulating fibrocytes
CTGF belonged to a new class of cysteine-rich growth
factor family and promoted cell proliferation, migration and
differentiation (25,26). Recently, several studies have
shown a close relationship between CTGF and organ fibrosis: Wang
et al (27) found that
miR-30c played an important role in the initiation and progression
of diabetic nephropathy by targeting CTGF. A further study revealed
that Rapamycin upregulated the expression of CTGF in hepatic
progenitor cells via TGF-β-Smad2 dependent signaling, which serves
a critical role in liver fibrosis (28). Guan and Zhou (29) revealed that the knockdown of FZD7
inhibited the TGF-β1-induced expression of α-SMA, collagen I,
fibronectin and CTGF. Our previous study (12) confirmed that the expressions of
CXCR4 and CTGF were concentration dependent after treatment with
different glucose concentrations of DMEM when compared with the 5.5
mmol/l glucose DMEM group.
To further explore whether the CXCR4/SDF-1 axis was
involved in the fibrotic process caused by high glucose DMEM,
AMD3100 was used to block the CXCR4/SDF-1 axis. The expression of
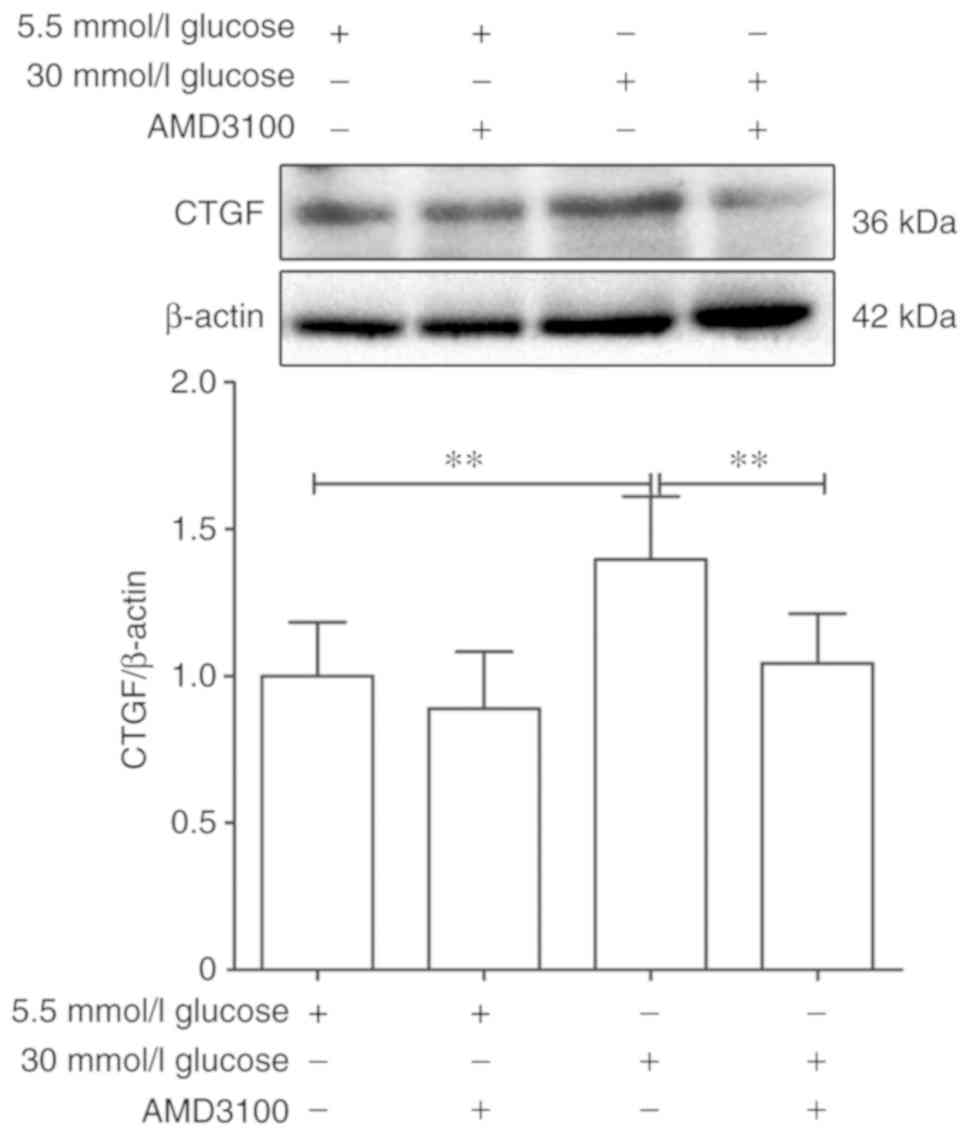
CTGF of 5.5 mmol/l glucose DMEM group was regarded as '1'. After
intervention for 48 h, the expression of CTGF was decreased
significantly by 30 mmol/l glucose+100 µmol/l AMD3100 DMEM
compared with 30 mmol/l glucose DEMM group (1.75±0.27 vs.
1.01±0.19; P<0.01), while showed no significant difference
between 5.5 mmol/l glucose group and 5.5 mmol/l glucose+100
µmol/l AMD3100 DMEM group (1±0.21 vs. 0.86±0.22, P>0.05;
Fig. 5). These results
demonstrated that AMD3100 could significantly inhibit the
expression of CTGF.
High glucose promotes transfer and
invasive ability of circulating fibrocytes
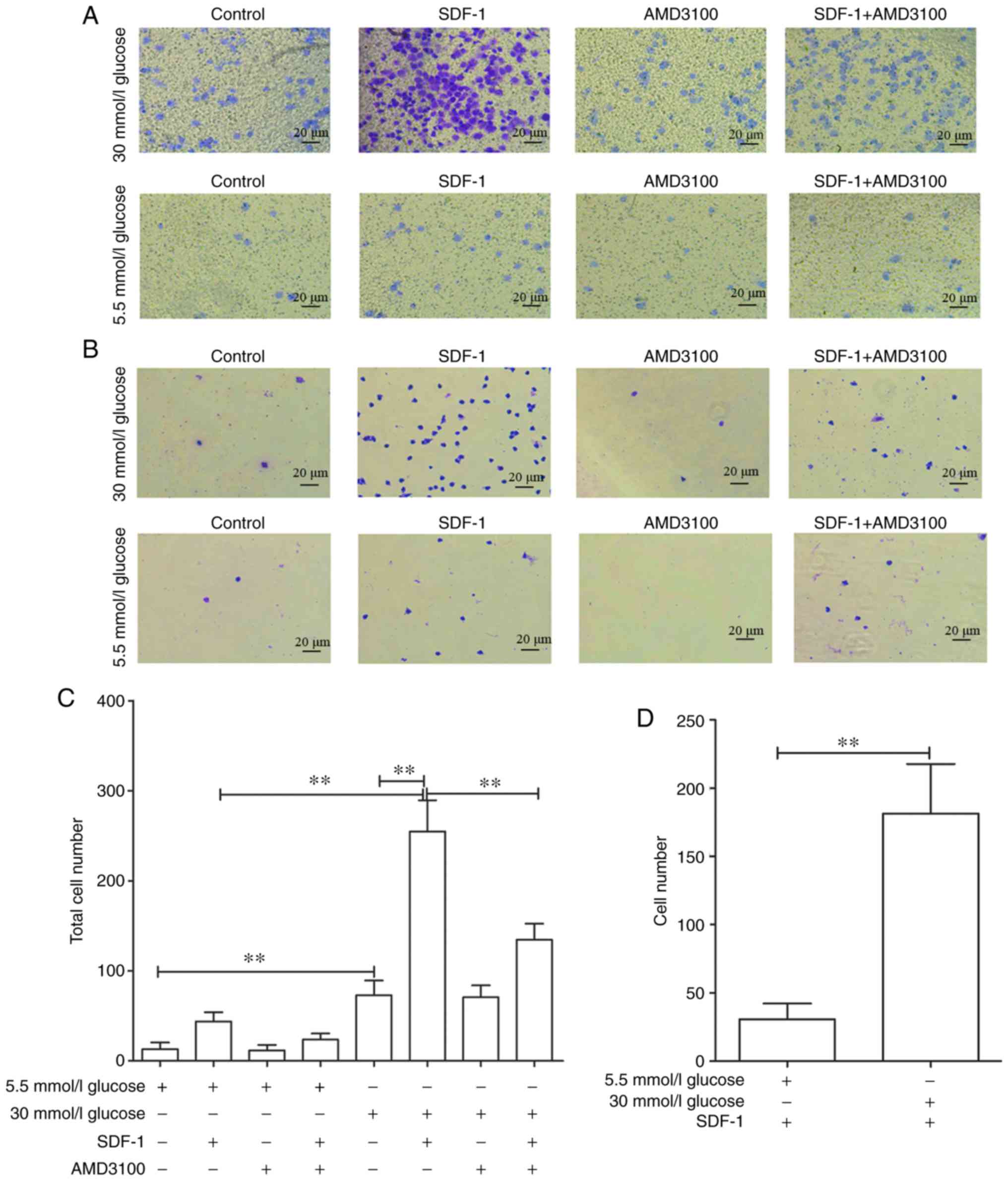
To explore the effects of high glucose on the
invasive and transfer abilities of circulating fibrocytes, a cell
chemotaxis assay was performed. As some cells pass through the
membrane and are adsorbed on the lower chamber, the total number of
cells was defined as the number of cells on the basement membrane
plus the number of cells in the lower chamber (Fig. 6A and B). As expected, 30 mmol/l
glucose DMEM significantly promoted the invasive ability of cells
[72.8±16.42 vs. 13.2±5.35 (P<0.01)], compared with 5.5 mmol/l
glucose DMEM group. Also, 30 mmol/l glucose DMEM+SDF-1 group
significantly increased the number of transmembrane fibrocytes
[254.8±34.2 vs. 72.4±16.42 (P<0.01)] compared with 30 mmol/l
glucose DMEM group. This promotion could also be blocked by AMD3100
(134.6±17.78 vs. 254.8±34.52; Fig.
6C). Similar results were obtained in 5.5 mmol/l glucose groups
(data not shown). On the other hand, the number of cells affected
by chemokines to migrate through membrane was defined as the total
number of 5.5/30 mmol/l glucose+SDF-1 DMEM group minus 5.5/30
mmol/l glucose DMEM group. The results revealed that high glucose
significantly promoted the migration of fibrocytes [181.4±36.26 vs.
30.6±11.64 (P<0.01; Fig.
6D)].
High glucose DMEM had no effect on the
secretion of SDF-1
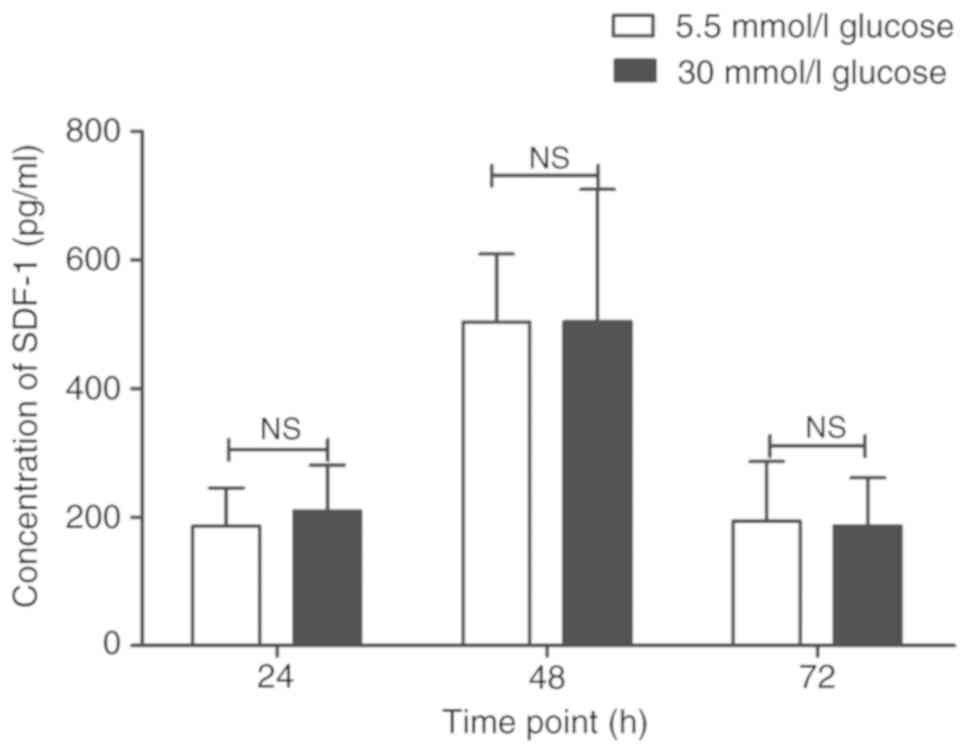
The effects of glucose concentration on the
secretion of SDF-1 of circulating fibrocytes at different time
points (24, 48 and 72 h) were further explored. The results showed
no significant differences between 5.5 mmol/l glucose group and 30
mmol/l glucose group at any time point (24 h: 186.02±59.05 pg/ml
vs. 209.36±71.42 pg/ml; P>0.05; 48 h: 502.98±106.1 pg/ml vs.
503.46±205.82 pg/ml; P>0.05; 72 h: 193.80±92.30 pg/ml vs.
186.20±74.87 pg/ml; P>0.05). However, the secretion of SDF-1
reached peak at 48 h when compared with 24 and 72 h (P<0.01;
Fig. 7).
Discussion
The present study demonstrated that high glucose
in vitro stimulated the proliferation, invasive and transfer
abilities of circulating fibrocytes and promoted the expression of
ECM proteins including COL-I and CTGF. Moreover, AMD3100, the
specific antagonist of CXCR4, could reduce a part of the changes
caused by high glucose. These results suggested that circulating
fibrocytes might be an important factor that leads to organs
fibrosis in patients with diabetic cardiomyopathy via the
CXCR4/SDF-1 axis. The present study may provide a different
direction for understanding diabetes-caused fibrosis.
As described previously, the circulating fibrocytes
derived from the bone marrow, differentiated from CD14+
monocytes and existed in the peripheral blood circulation, were
considered as precursors of myofibroblasts (30). Previously, the double-positive
expression of CD34 and COL-I or CD45 and COL-I was regarded as the
accepted standard to identify circulating fibrocytes in the
laboratory (5,30). CD34 was selectively expressed on
the surface of HSCs, reflecting that the fibrocytes are a type of
bone marrow-derived cells. CD45 was the common surface antigen of
leukocytes and the expression of both antigens (CD34 and CD45) were
decreased during the differentiation process of circulating
fibrocytes (31,32). COL-I, the product of mesenchymal
cells, is considered to be an important component of ECM and showed
increased expression during cell differentiation. In the present
study it was demonstrated that after 14 days of culture, the
adherent cells expressed CD45 (green) and COL-I (red). After 21
days, the adherent cells only expressed COL-I, while the
fluorescence of CD45 was hardly observed. In addition, as the
culture time prolongs, the proportion of spindle-shape cells
increasing in our pervious study. This was consistent with the
results reported in the current literature (5). The results demonstrated that the
obtained cells were indeed the circulating fibrocytes.
Beale et al (33) suggested that circulating
fibrocytes served an important role in myocardial fibrosis by
revealing that CD34, CD45 and collagen I expressed fibrocytes were
increased in patients with ischemia-reperfusion induced myocardial
fibrosis and post-MI scar formation. In addition, the secretion of
collagen such as laminin was also significantly increased.
Similarly, circulating fibrocytes were involved in the myocardial
tissue of Mst-1 transgenic mice model of dilated cardiomyopathy and
angiotensin II-induced cardiac hypertrophy model (34). Keeley et al (14) found that in patients with
hypertensive heart disease, the total number of circulating
fibrocytes in peripheral blood were significantly increased and
showed a positive correlation with left ventricular mass index.
Further studies confirmed that diabetes can cause
myocardial fibrosis and heart failure (35,36). However, it is still not clear
whether there was any relationship between diabetes, circulating
fibrocytes and myocardial fibrosis. Previous flow cytometry
experiments showed that the proportion of cells that co-express
CD45 and COL-I in PBMCs of diabetic patients was increased compared
with NGT patients (1.93±1.01 vs. 0.52±0.35%) (12). Furthermore, in vitro
experiments showed that high glucose DMEM stimulated the
proliferation of circulating fibrocytes in a concentration-time
dependent manner. When stimulated by 30 mmol/l glucose DMEM for 48
h, the cell proliferation reached its peak (12). In addition, high glucose did not
affect the apoptosis of circulating fibrocytes. Finally, high
glucose increased the number of fibrocytes. These findings
demonstrated that the increased circulating fibrocytes was probably
the first step in organ fibrosis.
Current studies discovered that the effects of
AMD3100 on cell proliferation are considered to be a double-edged
sword. It could promote the proliferation of Ewing sarcoma cell
lines in vitro (37), but
also inhibited cancer cell growth and invasion (38,39). AMD3100 showed no effect on cell
proliferation in the present experiment. It was hypothesized that
the CXCR4/SDF-1 axis may not have any effect on the proliferation
and apoptosis of circulating fibrocytes. However more evidence is
still warranted to confirm these results.
Several studies showed that circulating fibrocytes
could secrete a variety of cytokines, including CCR3, CCR5, CCR7
(40-42). SDF-1, also known as chemokine 12
(CXCL12), is a member of secreted CXC chemotactic protein
super-family and a specific ligand for CXCR4. The SDF-1/CXCR4 axis
played an important role in lymphatic homing, tumor metastasis and
embryonic development (43-45). CTGF, a member of the growth factor
family, played an important role in cell proliferation,
differentiation and migration, leading to fibrosis in multiple
diseases, and hence is considered as one of the indicators of
fibrosis. Previously, numerous studies have confirmed that
SDF-1/CXCR4 axis promoted the expression of CTGF, eventually
accelerating the process of fibrosis (10,46). Lin et al (10) found that CTGF expression induced
by CXCL12 showed a significant increase in patients with pulmonary
fibrosis. Song et al (46)
reported that AMD3100 could reduce bleomycin-induced lung fibrosis
in mice, directly illustrating the role of CXCR4/SDF-1 axis in
pulmonary fibrosis. The authors' previous western blotting results
showed that the expressions of CXCR4 and CTGF in circulating
fibrocytes were promoted by high glucose DMEM after 48 h
intervention in a concentration dependent manner. To further
explore whether the CXCR4/SDF-1 axis was the upstream signal of
CTGF, AMD3100 was used to investigate the role of the CXCR4/SDF-1
axis in the expression of CTGF. As demonstrated, compared with the
30 mmol/l glucose DMEM group, the expression of CTGF was inhibited
in the 30 mmol/l glucose+AMD3100 DMEM group. The results indicated
that CTGF could be the downstream effector molecule of the
CXCR4/SDF-1 axis. Furthermore, another important feature of
fibrosis process was the deposition of ECM components, like COL-I,
COL-III and so on (47). As shown
by flow cytometry results, high glucose DMEM promoted the rate of
cell expression of COL-I. Meanwhile, the application of AMD3100
inhibited the production of COL-I caused by high glucose. In
conclusion, CXCR4/SDF-1 axis may be involved in the deposition of
ECM proteins and played an indispensable role in the process of
fibrosis.
Invasion and migration of cells to specific parts
were prerequisites for numerous fibrotic pathophysiological
processes (48-50). Whether high glucose could affect
the invasion and migration abilities of fibrocytes was explored by
Transwell assay. As expected, the total number of trans-membrane
cells was increased in the 30 mmol/l glucose group compared with
the 5.5 mmol/l glucose group. These results may be associated with
increased expression of matrix metalloproteinases (MMPs) and for
the ability of circulating fibrocytes to secrete MMPs, including
MMP2, MMP5 and MMP9. These results have been confirmed by several
studies (51,52); however, it still needed further
experiments, like gelatin zymography, for verification. Compared
with the 30 mmol/l glucose group, the total number of transmembrane
cells was increased in the 30 mmol/l gluose+SDF-1 group. Similar
results were obtained in the 5.5 mmol/l glucose DMEM group (data
not shown). Furthermore, compared with 5.5 mmol/l glucose+SDF-1
group, the transfer ability of 30 mmol/l glucose+SDF-1 group was
improved. Meanwhile, AMD3100 treatment partially decreased the
number of trans-membrane cells. Combined with the results from
western blotting as described in the authors' previous experiments
(12), high glucose may affect
cell migration by regulating the expression of CXCR4. These results
indicated that CXCR4/SDF-1 axis may play an important role in the
migration of fibrocytes.
Interestingly, there were no significant differences
in SDF-1 secretion between 30 mmol/l glucose group and 30 mmol/l
glucose+100 µmmol/l AMD3100 group. In addition, AMD3100 did
not completely inhibit the migration promoted by SDF-1. This might
be due to the fact that the CXCR4/SDF-1 axis is not the only way to
induce the transfer of fibrocytes. Numerous studies have suggested
that other signaling pathways, such as the CX3CL1/CX3CR1 axis and
CCL2/CCR2, may also participate in the migration of fibrocytes
(41,53). Blocking the CXCR4/SDF-1 axis may
not be able to prevent the migration of circulating fibrocytes
completely.
Studies have reported that SDF-1 is significantly
elevated in the serum of patients with diabetes (54,55). Circulating fibrocytes showed
autocrine signaling of SDF-1, which in turn may also affect the
results of migration experiments. However, there was no significant
difference between 5.5 mmol/l glucose and 30 mmol/l glucose groups
and demonstrated that high glucose may not have an effect on the
secretion of SDF-1. Interestingly, the secretion of SDF-1 reached
peak at 48 h, which was similar to the result of CCK-8 assay in our
pervious experiment (12). The
authors of the present study suspected that the nutrient content of
the culture fluid and the density of the inoculated cells together
may affect the results of the experiment, but further
investigations were required.
The effects of high glucose on the
pathophysiological role of circulating fibrocytes in vitro
were explored. Nevertheless, the present study had some
limitations. Firstly, although the cells cultured for 14 days were
identified by immunofluorescence, the purity of the obtained cells
was not verified further. The purity of cells may have certain
effects on the results of the experiment. Secondly, the present
study on the mechanism of the CXCR4/SDF-1 was not in-depth.
Although a series of appearances of cell changes caused by high
glucose and applied AMD3100, the specific inhibitor of CXCR4, which
proved that CXCR4/SDF-1 did play a role in certain pathophysiology
process from the other aspect was elaborated on, what signal path
the CXCR4/SDF-1 axis regulates these functions through was not
further clarified. In addition a certain amount of literature about
the CXCR4/SDF-1 axis was consulted. Lin et al (10) found that CXCL12 could induced the
expression of CTGF of human lung fibroblasts according the
Rac1/ERK, JNK and AP-1 pathways. Kajiyama et al (56) found that expression of dipeptidyl
peptidase IV (DPPIV) and CXCR4 increased on human peritoneal
mesothelial cells (HPMCs) by transforming growth factor (TGF)-β1
treatment, TGF-β1, DPPIV and the CXCR4/SDF-1 axis played crucial
roles in regulating the migratory potential of HPMCs. In brief, the
CXC4/SDF-1 axis participated in numerous pathophysiological
processes. These existing studies provided a good starting point.
Due to a number of different reasons, like funds and time, the
present study had to stop here. The authors hope to do more
in-depth research in this area in the future.
In conclusion, the present study demonstrated that
high glucose DMEM stimulated the proliferation, invasion and
migration of circulating fibrocytes, promoted the expression of ECM
proteins including COL-I and CTGF. Application of specific blockers
of CXCR4/SDF-1 axis may help in reducing the ability of fibrosis
and providing a therapeutic strategy for the treatment of diabetic
fibrosis in the future.
Funding
The present study was supported by grants obtained
from the Scientific Technological Projects for Medicine and Health
of Zhejiang Province (grant no. 2015128660), the Natural Science
Foundation of Zhejiang province (Project for Young Scientists;
grant no. LQ13H020004), the National Natural Science Foundation of
China (grant no. 81500284) and the Major Research and Development
Project for the Zhejiang Science and Technology Agency (grant no.
2017C03034).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW and JL performed the experiments and wrote the
manuscript. XL and SL obtained venous blood samples. CX performed
statistical analysis. CD and LT were led the experiments of the
current study and provided technological assistance.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of Zhejiang Hospital prior to the start of the study. All
human procedures were performed in accordance with the declaration
of Helsinki. All patients signed informed consent.
Patient consent for publication
The patient, parent, guardian or next of kin (in
case of deceased patients) provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Hayat SA, Patel B, Khattar RS and Malik
RA: Diabetic cardio-myopathy: Mechanisms, diagnosis and treatment.
Clin Sci (Lond). 107:539–557. 2004. View Article : Google Scholar
|
|
2
|
Fang ZY, Prins JB and Marwick TH: Diabetic
cardiomyopathy: Evidence, mechanisms, and therapeutic implications.
Endocr Rev. 25:543–567. 2015. View Article : Google Scholar
|
|
3
|
Zhang L, Zhou LN, Shen ZZ, Zhang XY, Yin
M, Liu GZ, Guo P and Zhu XX: Effects of myocardial fibroblasts on
fibrosis in diabetic cardiomyopathy. Fudan Univ J Med Sci.
29:399–402. 2002.In Chinese.
|
|
4
|
Bucala R, Spiegel LA, Chesney J, Hogan M
and Cerami A: Circulating fibrocytes define a new leukocyte
subpopulation that mediates tissue repair. Mol Med. 1:71–81. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varcoe RL, Mikhail M, Guiffre AK, Pennings
G, Vicaretti M, Hawthorne WJ, Fletcher JP and Medbury HJ: The role
of the fibrocyte in intimal hyperplasia. J Thromb Haemost.
4:1125–1133. 2010. View Article : Google Scholar
|
|
6
|
Gomer R and Pilling D: Compositions and
methods for suppressing fibrocytes and for detecting fibrocyte
differentiation. Analyst. 2:217–220. 2010.
|
|
7
|
Laws DA, Kraft AS, Leddy LR and Larue AC:
Abstract A46: The role of circulating fibroblast precursors in
promoting metastatic sarcoma. Cancer Res. 75(Suppl 1): pp.
A462015
|
|
8
|
Li C, Li X, Deng C and Guo C: Circulating
fibrocytes are increased in neonates with bronchopulmonary
dysplasia. PLoS One. 11:pp. e01571812016, View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trimble A, Gochuico BR, Markello TC,
Fischer R, Gahl WA, Lee JK, Kim Y, Burdick MD, Strieter RM and
Mehrad B: Circulating fibrocytes as biomarker of prognosis in
Hermansky-Pudlak syndrome. Am J Respir Crit Care Med.
190:1395–1401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin CH, Shih CH, Tseng CC, Yu CC, Tsai YJ,
Bien MY and Chen BC: CXCL12 induces connective tissue growth factor
expression in human lung fibroblasts through the Rac1/ERK, JNK, and
AP-1 pathways. PLoS One. 9:pp. e1047462014, View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Behjati M and Hashemi M: Application of
fibrocytes in the treatment of diabetic foot: As a potential new
therapeutic approach. Diabetes Res Clin Pract. 86:152–153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weng Y, Qi J, Du C, Liu X, Xu C, Zeng G
and Tang L: Effect of high glucose on circulating fibrocytes
proliferation and the expression of CXCR4 and CTGF. Chin J
Diabetes. 26:227–233. 2018.In Chinese.
|
|
13
|
Murray LA: Commonalities between the
pro-fibrotic mechanisms in COPD and IPF. Pulm Pharmacol Ther.
25:276–280. 2012. View Article : Google Scholar
|
|
14
|
Keeley EC, Mehrad B, Janardhanan R,
Salerno M, Hunter JR, Burdick MM, Field JJ, Strieter RM and Kramer
CM: Elevated circulating fibrocyte levels in patients with
hypertensive heart disease. J Hypertens. 30:1856–1861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
White MJV, Galvis-Carvajal E and Gomer RH:
A brief exposure to tryptase or thrombin potentiates fibrocyte
differentiation in the presence of serum or SAP. J Immunol.
194:142–150. 2015. View Article : Google Scholar
|
|
16
|
Ulmer AJ, Scholz W, Ernst M, Brandt E and
Flad HD: Isolation and subfractionation of human peripheral blood
mononuclear cells (PBMC) by density gradient centrifugation on
percoll. Immunobiology. 166:238–250. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quan TE, Cowper S, Wu SP, Bockenstedt LK
and Bucala R: Circulating fibrocytes: Collagen-secreting cells of
the peripheral blood. Int J Biochem Cell Biol. 36:598–606. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt M, Sun G, Stacey MA, Mori L and
Mattoli S: Identification of circulating fibrocytes as precursors
of bronchial myofibroblasts in asthma. J Immunol. 171:380–389.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Zhou J, Wang H, Wu Y, Gao Q, Wang L,
Zhao Q, Liu P, Gao S, Wen W, et al: The activation of p38 MAPK
limits the abnormal proliferation of vascular smooth muscle cells
induced by high sodium concentrations. Int J Mol Med. 37:74–82.
2016. View Article : Google Scholar :
|
|
20
|
Zhou YK and Xue YM: High glucose
inhibiting proliferation of RSC96 schwann cells by high osmotic
pressure. J Kunming Univ Sci Technol. 38:60–64. 2013.In
Chinese.
|
|
21
|
Chen HT, Wang DY, Geng HZ, Chen HQ, Wu YX,
Deng SQ and Wang ZL: Adiponectin exerts antiproliferative effect on
high glucose-induced BeWo cell proliferation in vitro. Zhonghua Fu
Chan Ke Za Zhi. 51:204–208. 2016.In Chinese. PubMed/NCBI
|
|
22
|
Ho FM, Liu SH, Lin WW and Liau CS:
Opposite effects of high glucose on MMP-2 and TIMP-2 in human
endothelial cells. J Cell Biochem. 101:442–450. 2010. View Article : Google Scholar
|
|
23
|
Marchesi F, Monti P, Leone BE, Zerbi A,
Vecchi A, Piemonti L, Mantovani A and Allavena P: Increased
survival, proliferation, and migration in metastatic human
pancreatic tumor cells expressing functional CXCR4. Cancer Res.
64:8420–8427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodríguez-Nieves JA, Patalano SC, Almanza
D, Gharaee- Kermani M and Macoska JA: CXCL12/CXCR4 axis activation
mediates prostate myofibroblast phenoconversion through
non-canonical EGFR/MEK/ERK signaling. PLoS One. 11:pp.
e01594902016, View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pandey DP, Lappano R, Albanito L, Madeo A,
Maggiolini M and Picard D: Estrogenic GPR30 signalling induces
proliferation and migration of breast cancer cells through CTGF.
EMBO J. 28:523–532. 2014. View Article : Google Scholar
|
|
26
|
Li M, Xie Z, Wang P, Li J, Liu W, Tang S,
Liu Z, Wu X, Wu Y and Shen H: The long noncoding RNA GAS5
negatively regulates the adipogenic differentiation of MSCs by
modulating the miR-18a/CTGF axis as a ceRNA. Cell Death Dis.
9:5542018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Duan L, Guo T, Gao Y, Tian L, Liu
J, Wang S and Yang J: Downregulation of miR-30c promotes renal
fibrosis by target CTGF in diabetic nephropathy. J Diabetes
Complications. 30:406–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Wang W, Peng XM, He Y, Xiong YX,
Liang HF, Chu L, Zhang BX, Ding ZY and Chen XP: Rapamycin
upregulates connective tissue growth factor expression in hepatic
progenitor cells through TGF-β-Smad2 dependent signaling. Front
Pharmacol. 9:8772018. View Article : Google Scholar
|
|
29
|
Guan S and Zhou J: Frizzled-7 mediates
TGF-β-induced pulmonary fibrosis by transmitting non-canonical Wnt
signaling. Exp Cell Res. 359:226–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiang HY, Chu PH and Lee TH: R1R2 peptide
ameliorates pulmonary fibrosis in mice through fibrocyte migration
and differentiation. PLoS One. 12:pp. e01858112017, View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lassance L, Marino GK, Medeiros CS,
Thangavadivel S and Wilson SE: Fibrocyte migration, differentiation
and apoptosis during the corneal wound healing response to injury.
Exp Eye Res. 170:177–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamichi M, Akishimafukasawa Y, Fujisawa
C, Mikami T, Onishi K and Akasaka Y: Basic fibroblast growth factor
induces angiogenic properties of fibrocytes to stimulate vascular
formation during wound healing. Am J Pathol. 186:3203–3216. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beale A, Fang L, Ellims A, Ling L, Taylor
A, Chin-Dusting J and Dart A: Fibrocytes, a novel fibroblast-like
population, are increased in cardiac fibrosis. Heart Lung Circ.
21(Suppl 1): pp. S59. 2012, View Article : Google Scholar
|
|
34
|
Haudek SB, Cheng J, Du J, Wang Y,
Hermosillo-Rodriguez J, Trial J, Taffet GE and Entman ML: Monocytic
fibroblast precursors mediate fibrosis in angiotensin-II-induced
cardiac hypertrophy. J Mol Cell Cardiol. 49:3499–507. 2010.
View Article : Google Scholar
|
|
35
|
Russo I and Frangogiannis NG:
Diabetes-associated cardiac fibrosis: Cellular effectors, molecular
mechanisms and therapeutic opportunities. J Mol Cell Cardiol.
90:84–93. 2016. View Article : Google Scholar :
|
|
36
|
Feng B, Chen S, Gordon AD and Chakrabarti
S: miR-146a mediates inflammatory changes and fibrosis in the heart
in diabetes. J Mol Cell Cardiol. 105:70–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berning P, Schaefer C, Clemens D,
Korsching E, Dirksen U and Potratz J: The CXCR4 antagonist
plerixafor (AMD3100) promotes proliferation of Ewing sarcoma cell
lines in vitro and activates receptor tyrosine kinase signaling.
Cell Commun Signal. 16:1–13. 2018. View Article : Google Scholar
|
|
38
|
Reeves PM, Abbaslou MA, Kools FRW and
Poznansky MC: CXCR4 blockade with AMD3100 enhances Taxol
chemotherapy to limit ovarian cancer cell growth. Anticancer Drugs.
28:935–942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li JK, Yu L, Shen Y, Zhou LS, Wang YC and
Zhang JH: Inhibition of CXCR4 activity with AMD3100 decreases
invasion of human colorectal cancer cells in vitro. World J
Gastroenterol. 14:2308–2313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishida Y, Kimura A, Nosaka M, Kuninaka Y,
Hemmi H, Sasaki I, Kaisho T, Mukaida N and Kondo T: Essential
involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced
pulmonary fibrosis via regulation of fibrocyte and M2 macrophage
migration. Sci Rep. 7:168332017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Phillips RJ, Burdick MD, Hong K, Lutz MA,
Murray LA, Xue YY, Belperio JA, Keane MP and Strieter RM:
Circulating fibrocytes traffic to the lungs in response to CXCL12
and mediate fibrosis. J Clin Invest. 114:438–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ishida Y, Kimura A, Kondo T, Hayashi T,
Ueno M, Takakura N, Matsushima K and Mukaida N: Essential roles of
the CC chemokine ligand 3-CC chemokine receptor 5 axis in
bleomycin- induced pulmonary fibrosis through regulation of
macrophage and fibrocyte infiltration. Am J Pathol. 170:843–854.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luo X, Wang X, Xia Z, Chung SK and Cheung
CW: CXCL12/CXCR4 axis: An emerging neuromodulator in pathological
pain. Rev Neurosci. 27:83–92. 2016. View Article : Google Scholar
|
|
44
|
Katsura M, Shoji F, Okamoto T, Shimamatsu
S, Hirai F, Toyokawa G, Morodomi Y, Tagawa T, Oda Y and Maehara Y:
Correlation between CXCR4/CXCR7/CXCL12 chemokine axis expression
and prognosis in lymph-node-positive lung cancer patients. Cancer
Sci. 109:154–165. 2018. View Article : Google Scholar
|
|
45
|
Ho SY, Ling TY, Lin HY, Liou JT, Liu FC,
Chen IC, Lee SW, Hsu Y, Lai DM and Liou HH: SDF-1/CXCR4 signaling
maintains stemness signature in mouse neural stem/progenitor cells.
Stem Cells Int. 2017:24937522017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song JS, Kang CM, Kang HH, Yoon HK, Kim
YK, Kim KH, Moon HS and Park SH: Inhibitory effect of CXC chemokine
receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary
fibrosis. Exp Mol Med. 42:465–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chun TH: Peri-adipocyte ECM remodeling in
obesity and adipose tissue fibrosis. Adipocyte. 1:89–95. 2012.
View Article : Google Scholar
|
|
48
|
Friedl P and Gilmour D: Collective cell
migration in morphogenesis, regeneration and cancer. Nat Rev Mol
Cell Biol. 10:445–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wolf K, Mazo I, Leung H, Engelke K, von
Andrian UH, Deryugina EI, Strongin AY, Bröcker EB and Friedl P:
Compensation mechanism in tumor cell migration: Mesenchymal
amoeboid transition after blocking of pericellular proteolysis. J
Cell Biol. 160:267–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sandbothe M, Buurman R, Reich N, Greiwe L,
Vajen B, Gürlevik E, Schäffer V, Eilers M, Kühnel F, Vaquero A, et
al: The microRNA-449 family inhibits TGF-β-mediated liver cancer
cell migration by targeting SOX4. J Hepatol. 66:1012–1021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Galligan CL and Fish EN: Circulating
fibrocytes contribute to the pathogenesis of collagen
antibody-induced arthritis. Arthritis Rheum. 64:3583–3593. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Craig VJ, Zhang L, Hagood JS and Owen CA:
Matrix metal-loproteinases as therapeutic targets for idiopathic
pulmonary fibrosis. Am J Respir Cell Mol Biol. 53:585–600. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Singh SR, Sutcliffe A, Kaur D, Gupta S,
Desai D, Saunders R and Brightling CE: CCL2 release by airway
smooth muscle is increased in asthma and promotes fibrocyte
migration. Allergy. 69:1189–1197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Derakhshan R, Arababadi MK, Ahmadi Z,
Karimabad MN, Salehabadi VA, Abedinzadeh M, Khorramdelazad H,
Balaei P, Kennedy D and Hassanshahi G: Increased circulating levels
of SDF-1 (CXCL12) in type 2 diabetic patients are correlated to
disease state but are unrelated to polymorphism of the SDF-1β gene
in the Iranian population. Inflammation. 35:900–904. 2012.
View Article : Google Scholar
|
|
55
|
Lovshin JA, Rajasekeran H, Lytvyn Y,
Lovblom LE, Khan S, Alemu R, Locke A, Lai V, He H, Hittle L, et al:
Dipeptidyl peptidase-4 inhibition stimulates distal Tubular
natriuresis and increases in circulating SDF-1α (1-67) in patients
with type 2 diabetes. Diabetes Care. 40:1073–1081. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kajiyama H, Shibata K, Ino K, Nawa A,
Mizutani S and Kikkawa F: Possible involvement of
SDF-1alpha/CXCR4-DPPIV axis in TGF-beta1-induced enhancement of
migratory potential in human peritoneal mesothelial cells. Cell
Tissue Res. 330:221–229. 2007. View Article : Google Scholar : PubMed/NCBI
|