Introduction
It is well known that brain injury and ischemic
insults cause neuronal damage/death by increasing intracellular
calcium concentration, glutamate-mediated excitotoxicity and
oxidative stress following ischemic events (1-4).
In addition, overactivation of neuroinflammatory responses
including microglial activation and increases of proinflammatory
cytokines can lead to brain injury including neuronal death/loss
(5-8).
Chemokines, as regulators of brain inflammation, are
known to be detrimental factors following brain ischemic insults
because their overexpression can increase ischemia-induced brain
injuries and recruitment of inflammatory cells (9-11).
Among various chemokines, chemokine C-X3-C motif ligand 1 (CX3CL1,
also called as fractalkine) as one of CX3C chemokines is
constitutively expressed in the central nervous system. It is
especially localized in neurons (12). CX3C chemokine receptor 1 (CX3CR1),
a G-protein-coupled receptor, is the sole receptor for CX3CL1.
Certain previous studies have demonstrated that it is expressed in
microglia (12-14). It has widely been accepted that
the CX3CL1/CX3CR1 signaling pathway plays important roles in
regulating cellular interactions between neurons and microglia in
the brain (12,15). In addition, CX3CL1/CX3CR1 pathway
has been thought to be associated with the activation and
recruitment of microglia (15-17). CX3CL1 is known to suppress
microglial activation and CX3CR1 plays an important role in
modulating normal microglial activity by inhibiting microglial
activity (18-20).
Previous studies have reported ischemia-induced
changes of CX3CL1 and/or CX3CR1 expression in rodent brains
following focal cerebral ischemia (17,21-23). However, the expression and roles
of CX3CL1 and CX3CR1 in the brain following transient global
cerebral ischemia (tgCI) in rodents have not been fully elucidated
yet. It is known that tgCI in the brain is caused by impaired blood
flow, leading to deprivation of oxygen and glucose and resulting in
selective neuronal death/damage in vulnerable brain regions
including the hippocampus (24,25). In the hippocampus, pyramidal cells
in the CA1 field are known to be vulnerable to tgCI. Death of CA1
pyramidal cells induced by tgCI is called 'delayed neuronal death
(DND)' because CA1 pyramidal cells will die a few days after tgCI
for 5 min (24). Therefore, the
objective of the present study was to investigate time-dependent
changes of CX3CL1 and CX3CR1 expression in neuronal and/or glial
cells of the gerbil hippocampal CA1 field following 5 min of
tgCI.
Materials and methods
Experimental animals
Male Mongolian gerbils (Meriones
unguiculatus), aged 6 months (body weight ~68-73 g; n=84), were
obtained from the Experimental Animal Center, Kangwon National
University, (Chuncheon, Republic of Korea). The animals were housed
in a conventional state under adequate temperature (23˚C) and
humidity (60%) control, with a 12-h light/12-h dark cycle and were
provided with free access to food and water. Experimental
procedures for this study were approved by the Institutional Animal
Care and Use Committee at Kangwon National University (approval
number: KW-180124-1). In this study, numbers of animals used and
the suffering caused by the procedures used in this experiment were
minimized.
tgCI induction
According to the method of the authors' previous
studies (7,26,27), the induction of tgCI was
performed. In brief, the gerbils were anesthetized with a mixture
of isoflurane (2.5%) in oxygen (30%) and nitrous oxide (70%), with
a modification of methods of previous studies (28-30) and level of anesthesia was
confirmed by pedal reflex (firm toe pinch). Bilateral common
carotid arteries were occluded for 5 min using aneurysm clips. The
complete interruption of blood flow was confirmed by observing the
central artery in the retina using an ophthalmoscope (Heine
Optotechnik). Their rectal temperature (37±0.5˚C) was kept using a
thermometric blanket during and after tgCI. Sham operated gerbils
were subjected to the same procedure without bilateral common
carotid artery occlusion.
Western blotting
CX3CL1 and CX3CR1 protein levels in the CA1 field
were analyzed at designated times (6 h, 1, 2, 5 and 10 days after
tgCI) using western blot method. According to the authors'
published method (7), in short, 7
animals at each point in time were anaesthetized with sodium
pentobarbital (60 mg/kg, i.p.) and their brains were removed. Their
brains were serially and transversely cut into 400-µm
thickness using a vibratome (Leica Camera AG; Leica Microsystems,
Inc.). CA1 fields were dissected with a surgical blade and
homogenized in 50 mM phosphate-buffered saline (PBS; pH 7.4)
containing 0.1 mM ethylene glycol bis (2-aminoethyl
ether)-N,N,N0,N0 tetraacetic acid (pH 8.0), 0.2% Nonidet P-40, 10
mM ethylendiamine tetraacetic acid (pH 8.0), 15 mM sodium
pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2
mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 1
mM dithiothreitol (DTT). The homogenized tissues were centrifugated
at 16,000 x g for 20 min at 4˚C and protein levels of CX3CL1 and
CX3CR1 were determined using a Micro BCA protein assay kit (Pierce
Chemical, Co.). Aliquot containing total protein (20 mg) was boiled
in loading buffer containing 150 mM Tris-HCI (pH 6.8), 6% SDS, 3 mM
DTT, 0.3% bromophenol blue and 30% glycerol and loaded onto 10%
polyacrylamide gel. The gel was transferred to nitrocellulose
transfer membranes (Pall Corporation) after electrophoresis. The
background of the membrane was reduced with 5% nonfat dry milk in
PBS containing 0.1% Tween-20 for 40 min at room temperature and the
membrane was reacted with rabbit anti-CX3CL1 (cat. no. NBP1-49539;
1:1,000; Novus Biologicals, LLC) or mouse anti-CX3CR1 (cat. no.
824001; 1:1,000; BioLegend Inc.) overnight at 4˚C. Followed by
incubation with peroxidase-conjugated goat anti-rabbit or mouse IgG
(cat nos. ab6721 and ab205719; 1:5,000; Abcam) for 1 h at room
temperature and visualization with a Pierce ECL western blotting
substrate (cat. no. 32106; Thermo Fisher Scientific, Inc.). The
loading control was carried out using mouse anti-β-actin antibody
(cat. no. A5316; 1:5,000; Sigma-Aldrich; Merck KGaA). Western blot
analysis using homogenates at all experimental time-points was
performed simultaneously. Results of the western blotting were
scanned and the quantification of the bands was densitometrically
analyzed using ImageJ 1.46 software (National Institutes of
Health). The quantification was represented by relative optical
density (ROD). A ratio of the ROD was calibrated as %: The sham
operated gerbil was designated as 100%.
Preparation of histological sections
For cresyl violet (CV) staining, fluorojade B (FJB)
histofluorescence, immunohistochemical and double
immunofluorescence stainings, brain sections containing the
hippocampus were prepared from the sham and tgCI operated gerbils
(n=7 at each point in time) at designated times (6 h, 1, 2, 5 and
10 days after tgCI). According to the authors' published method
(7,26,27), the gerbils were anaesthetized with
sodium pentobarbital and perfused transcardially with 0.1 M PBS
followed by 4% paraformaldehyde. And then, their brains were
removed and postfixed with the same fixative for 8 h at room
temperature and cryoprotected by infiltration with 30% sucrose for
10 h. The tissues were serially sectioned into 30-µm frontal
sections in a cryostat (Leica Microsystems).
In addition, sham operated tissue was presented only
at 5 days after tgCI, because there were no significant differences
between the sham control samples at each designated time (data not
shown).
CV staining
CV histochemical staining was performed to
investigate cellular distribution and morphology. In brief,
according to the authors' published method (31), CV acetate (Sigma-Aldrich; Merck
KGaA) was dissolved (1%) in distilled water (DW) and glacial acetic
acid was added to this solution. Sections of each group were
stained with CV solution and dehydrated with serial ethanol. The
sections were examined using an AxioM1 light microscope (Carl Zeiss
AG).
FJB histofluorescence staining
FJB [a marker for neurodegeneration (32)] histofluorescence staining was
performed to examine tgCI-induced DND in the CA1 field. As
previously described (7,26,27), in brief, the sections were
serially stained with a 1% sodium hydroxide solution, a 0.06%
potassium permanganate solution and a 0.0004% FJB (cat. no. AG301;
Merck KGaA) solution at room temperature. The reacted sections were
examined using an epifluorescent microscope (Carl Zeiss AG)
equipped with a blue excitation light (450-490 nm) and a barrier
filter.
Immunohistochemistry
Immunohistochemical staining was carried out for
ionized calcium-binding adapter molecule 1 (Iba1; a marker for
microglia), CX3CL1 and CX3CR1. According to the authors' previous
studies (7,26,27), in brief, the sections were
incubated with rabbit anti-Iba1 (cat. no. 019-19741; 1:800; Wako
Pure Chemical Industries, Ltd.) for microglia, rabbit
anti-CX3CL1/Fractalkine (cat. no. NBP1-49539; 1:250; Novus
Biologicals, LLC), or mouse anti-CX3CR1 (cat. no. 824001; 1:100;
BioLegend Inc.) as primary antibodies overnight at 4˚C. The reacted
sections were exposed to biotinylated goat anti-rabbit IgG or horse
anti-mouse IgG (cat. nos. BA-1000 and BA-2000; 1:250; Vector
Laboratories) for 1 h at room temperature and streptavidin
peroxidase complex. Finally, the reacted sections were visualized
with 3,3′-diaminobenzidine at room temperature.
In order to confirm the specificity of each
immunoreaction, each negative control test was done using
pre-immune serum (Vector Laboratories) instead of each primary
antibody. Each negative control test showed no immunoreactivity in
each immunostained tissue. In addition, immunohistochemical
staining was performed simultaneously at all experimental
time-points.
Double immunofluorescence staining
To examine cell types containing CX3CR1
immunoreactivity, double immunofluorescence staining was performed
according to the authors' published protocol (27). In brief, mouse anti-CX3CR1 (cat.
no. 824001; 1:50; BioLegend Inc.)/rabbit anti-Iba1 (cat. no.
019-19741; 1:400; Wako Pure Chemical Industries, Ltd.) were used
for microglia. The sections were incubated in the mixture of the
antisera overnight at 4˚C and the incubated sections were reacted
in a mixture of both goat anti-mouse IgG, Alexa Fluor488 (cat. no.
A-11001; 1:500; Invitrogen; Thermo Fisher Scientific, Inc.) and
donkey anti-rabbit IgG, Alexa Fluor546 (cat. no. A10040; 1:500;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. The immunoreaction was observed under a confocal
microscope (LSM510 META NLO; Carl Zeiss AG) in the Korea Basic
Science Institute Chuncheon Center.
Data analyses
Data analyses were performed by two or three
investigators, who were blind to the experimental conditions.
First, the numbers of CV and FJB positive cells were analyzed
according to the authors' published method (27). In brief, 8 sections were selected
from each animal with 120-µm interval according to
anteroposterior -1.4 to -2.2 mm of the gerbil brain atlas. Images
of CV and FJB positive cells were captured with an AxioM1 light
microscope (Carl Zeiss AG) equipped with a digital camera (Axiocam;
Carl Zeiss AG) connected to a PC monitor. Cells were obtained in a
250×250 µm square and the cell counts were obtained by
averaging the total number of CV and FJB positive cells from each
animal per group using an image analyzing system (software: Optimas
6.5; CyberMetrics Corporation).
For quantitative analyses of Iba1, CX3CL1 and CX3CR1
immunoreactivities, 8 sections were selected with 120-µm
interval in each gerbil. Each immunoreactive image in the CA1 field
was captured with an AxioM1 light microscope (Carl Zeiss AG)
equipped with a camera (Axiocam; Carl Zeiss AG) connected to a PC
monitor. According to the authors' published method (7,26,27), each image was captured in a
corresponding area (250×250 µm) of the CA1 field at 40X
primary magnification and the image was calibrated into an array of
512×512 pixels. The densities of Iba1, CX3CL1 and CX3CR1
immunoreactive structures were evaluated on the basis of optical
density (OD): The OD was obtained after the transformation of the
mean gray level using the formula: OD = log (256/mean gray level).
After the background density was subtracted, a ratio of the OD was
calibrated as % [relative optical density (ROD)] and analyzed using
NIH ImageJ 1.59 software (National Institute of Health). A ratio of
the ROD was calibrated as %, with sham tgCI operated gerbils
designated as 100%.
Statistical analysis
The data shown in the present study represent the
mean ± standard error of the mean. The normality test was performed
using a Kolmogorov and Smirnov test for testing normal
distributions, and Bartlett test for testing identical standard
distributions. All data passed normality tests. Differences of the
means among the groups were statistically analyzed by analysis of
variance with Duncan's post hoc test in order to elucidate
ischemia-related differences among experimental groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
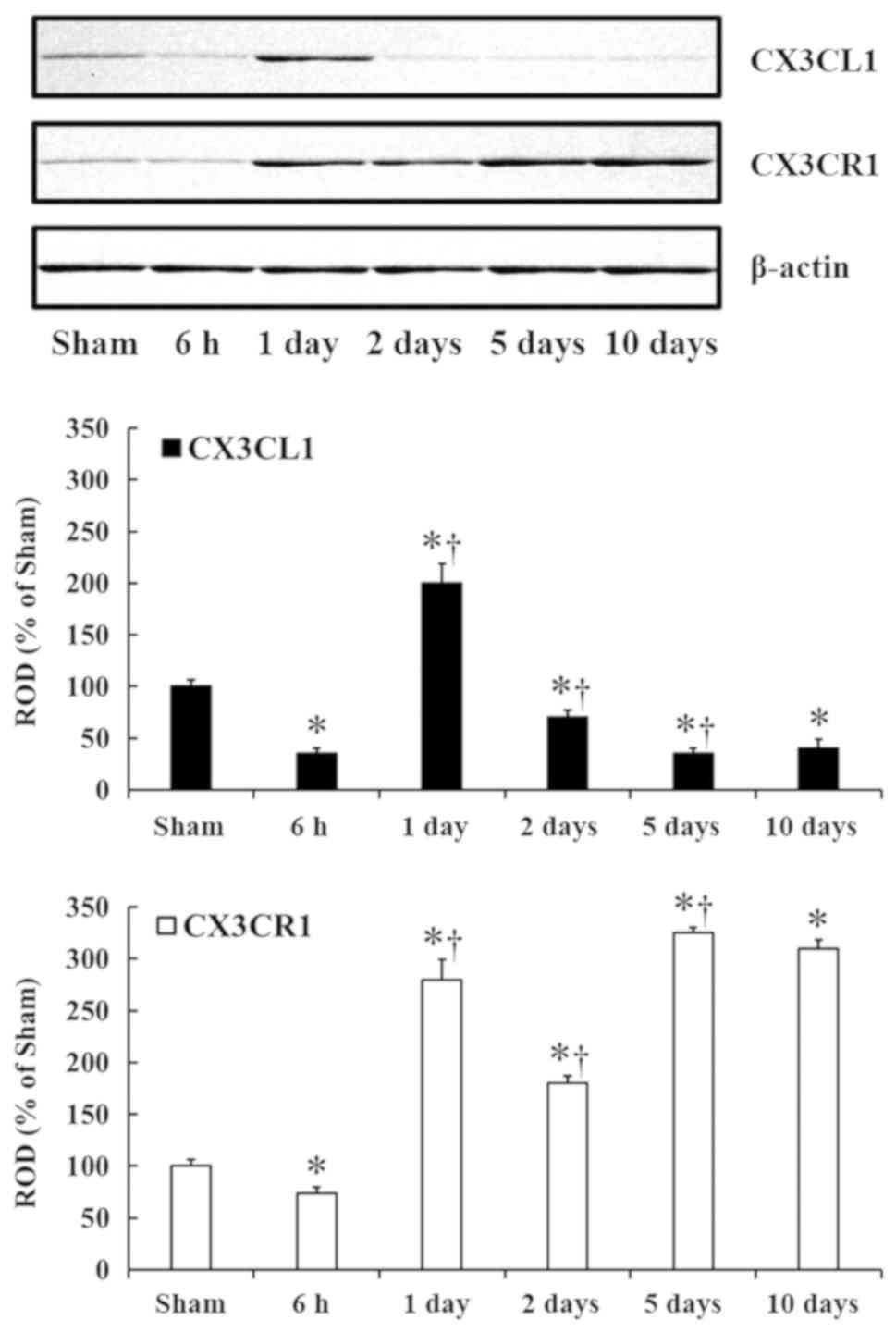
tgCI-induced changes in CX3CL1 and CX3CR1
protein levels
CX3CL1 and CX3CR1 protein levels in the CA1 field
were significantly changed with time after tgCI (Fig. 1). CX3CL1 and CX3CR1 protein levels
were significantly decreased by ~65% (P<0.05) and 26%
(P<0.05) at 6 h after tgCI compared with the sham operated
group. Additionally, compared with the 6 h group, CX3CL1 and CX3CR1
protein levels were significantly increased by ~165% (P<0.05)
and ~206% (P<0.05), respectively at 1 day after tgCI, and
compared with the 1 day group, CX3CL1 and CX3CR1 protein levels
were significantly decreased by ~130% (P<0.05) and 100%
(P<0.05), respectively, at 2 days after tgCI. At 5 days after
tgCI, CX3CL1 protein level was significantly decreased (P<0.05)
by ~35%; however, CX3CR1 protein level was significantly increased
(P<0.05) by ~145%, compared with those at 2 days after tgCI. At
10 days after tgCI, CX3CL1 and CX3CR1 protein levels were not
significantly different from those at 5 days post-tgCI.
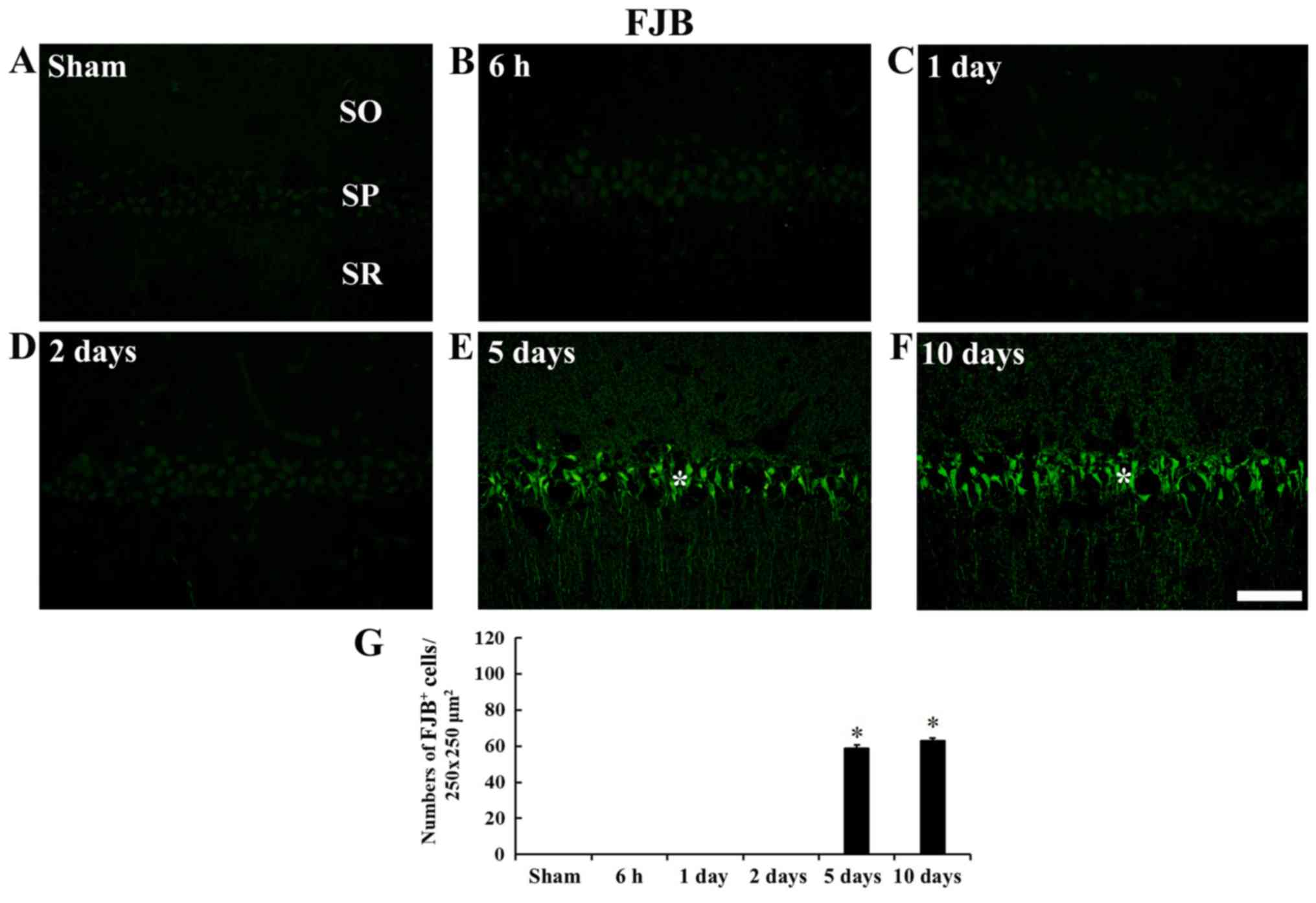
tgCI-induces DND and microglia
activation
tgCI-induced DND was examined in the CA1 field at 5
days after tgCI using CV staining and FJB histofluorescence
staining (Figs. 2 and 3). CV staining was shown in cells of the
stratum pyramidale, which are called pyramidal neurons, in the
CA1-3 field of the sham operated group (Fig. 2A and G). In the ischemia operated
group, pyramidal neurons were also well stained with CV until 2
days after tgCI (Fig. 2B, D, H and
J); however, numbers of CV positive pyramidal neurons were
significantly decreased (P<0.05) in the CA1 field from 5 days
after tgCI (Fig. 2E, F and
K-M).
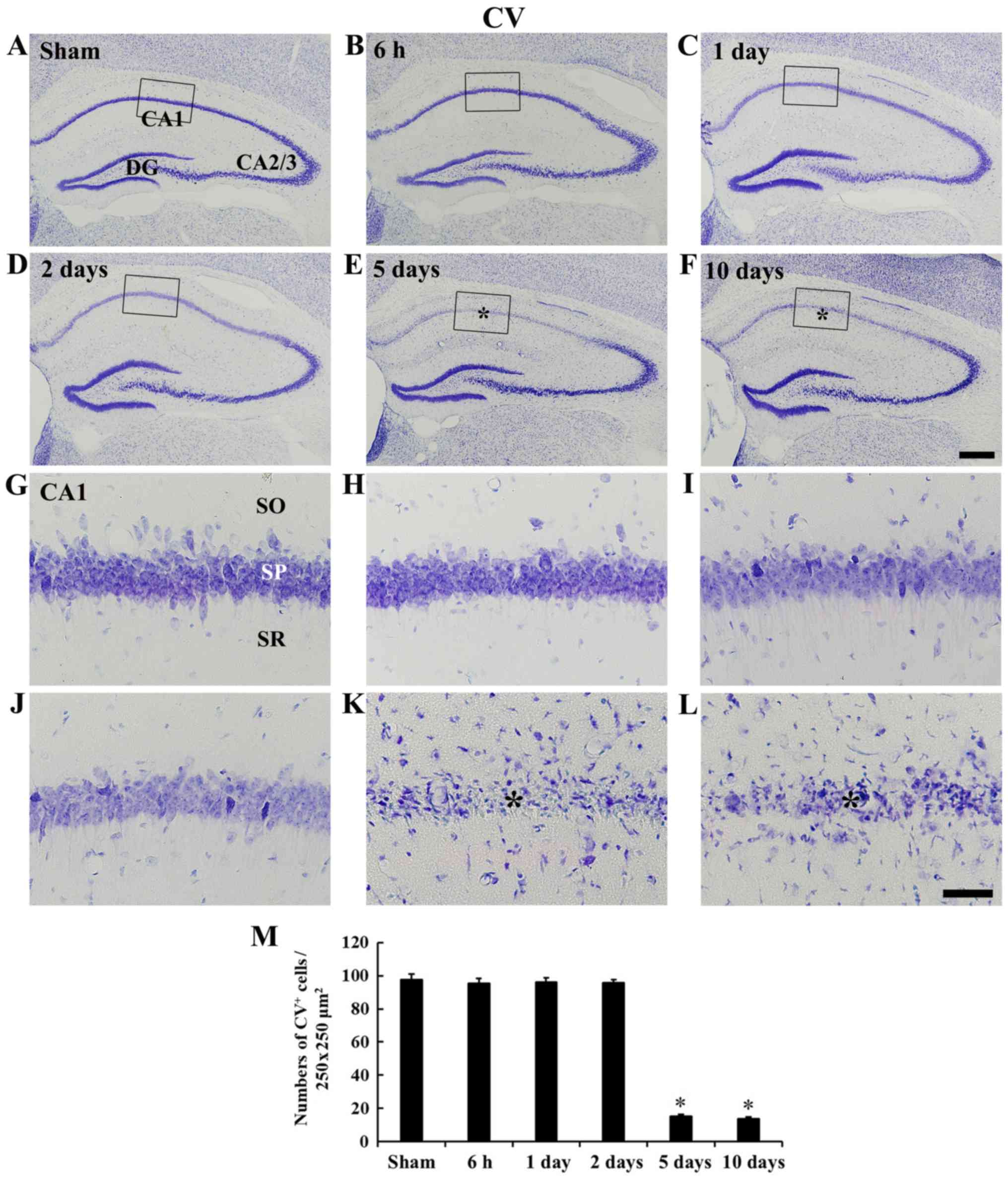 | Figure 2CV staining. Low magnification of the
hippocampus of the (A) sham group and ischemia operated groups at
(B) 6 h, (C) 1 day, (D) 2 days, (E) 5 days and (F) 10 days after
tgCI. High magnification of the CA1 field of the (G) sham group and
ischemia operated groups at (H) 6 h, (I) 1 day, (J) 2 days, (K) 5
days, and (L) 10 days after tgCI. CV positive cells are well
observed in the SP in the CA1-3 field in the sham operated group.
In the ischemia operated group, CV positive cells are significantly
decreased only in the SP (asterisks) of the CA1 field at 5 and 10
days after tgCI. Scale bars=400 µm (A-F) and 50 µm
(G-L). (M) Numbers of CV positive cells in the CA1 field (n=7 at
each point in time). *P<0.05 vs. the sham operated
group. The bars indicate the mean ± standard error of the mean. CV,
cresyl violet; SP, stratum pyramidale; SO, stratum oriens; SR,
stratum radiatum; tgCI, transient global cerebral ischemia. |
In the sham operated gerbils, no FJB positive cells
were found in any layer (Fig.
3A). In addition, FJB positive cells were not observed until 2
days post-tgCI (Fig. 3B-D).
However, at 5 and 10 days after tGCI, a number of FJB positive
cells were observed in the stratum pyramidale of the CA1 field, not
CA2/3 field, and the number of FJB positive cells was significantly
increased (P<0.05) compared with the sham group (Fig. 3E-G).
Significant changes of microglia were found, which
are mononuclear phagocytic cells, in the CA1 field after tgCI
(Fig. 4). Iba1 immunoreactive
microglia were observed as a resting form in the sham operated
group and they were scattered in all layers of the CA1 field
(Fig. 4A). In the ischemia
operated group, Iba1 immunoreactivity was gradually increased in a
time-dependent manner after tgCI and Iba1 immunoreactivity in all
ischemia operated groups was significantly increased (P<0.05)
compared with the sham group. In addition, the microglia were
morphologically activated in the ischemia operated group and they
were hypertrophied in the cytoplasm and thickened in processes
(Fig. 4B-G). Especially, at 5 and
10 days after tgCI, a number of activated Iba1 immunoreactive
microglia were aggregated in the stratum pyramidale, where
tgCI-induced DND of CA1 pyramidal cells occurred (Fig. 4E and F).
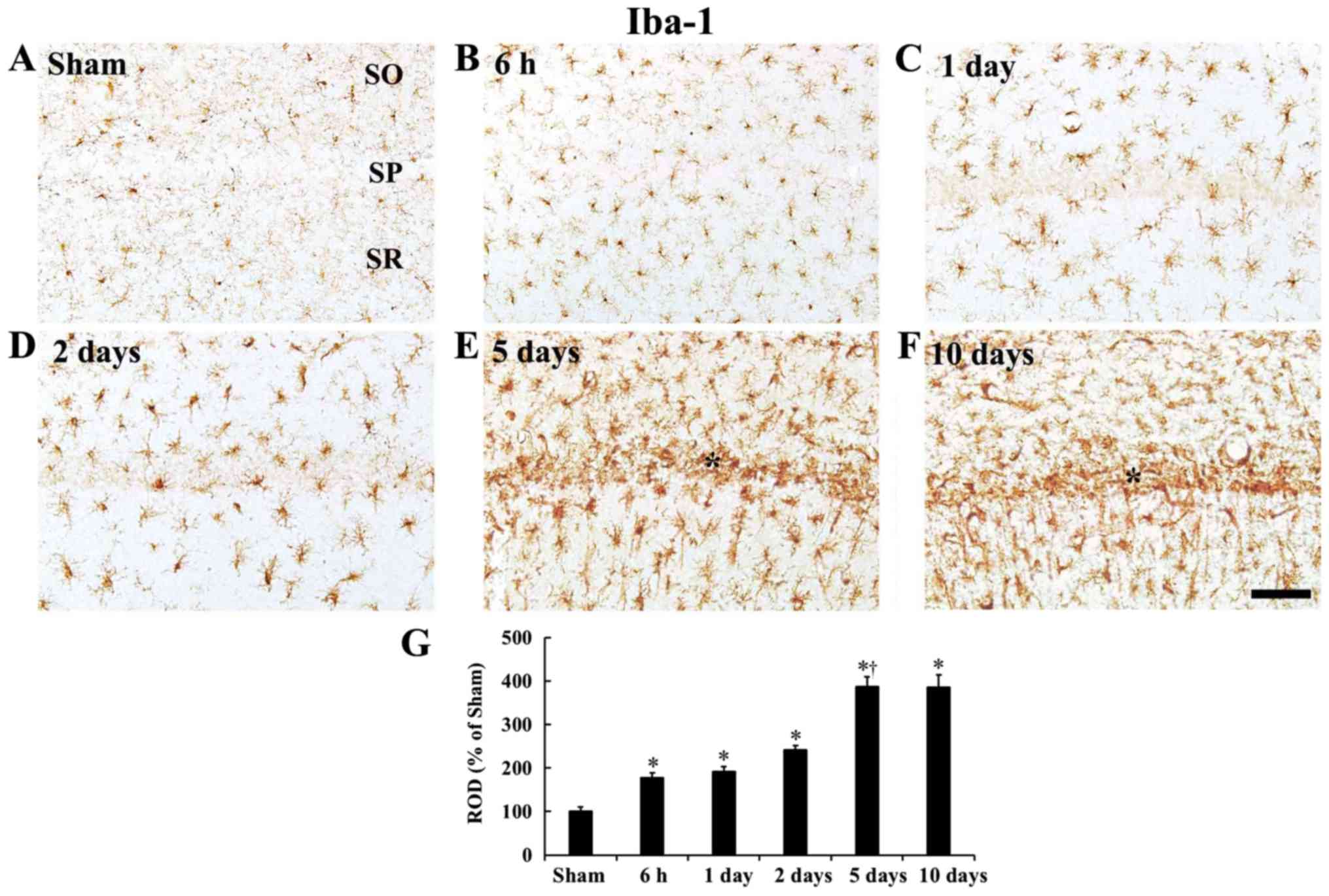 | Figure 4Iba-1 immunohistochemistry. (A-F)
Iba1 immunohistochemistry in the CA1 field of the (A) sham group
and ischemia operated groups at (B) 6 h, (C) 1 day, (D) 2 days, (E)
5 days and (F) 10 days after tgCI. In the ischemia operated
gerbils, Iba1 immunoreactivity is gradually increased after tgCI.
In particular, Iba1 immunoreactive microglia are aggregated in the
SP (asterisks) at 5 and 10 days after tgCI. Scale bar, 50
µm. (G) ROD as % of Iba1 immunoreactive structures after
tgCI (n=7 at each point in time). *P<0.05 vs. the
sham operated gerbils; †P<0.05 vs. the pre-time-point
gerbils. Bars indicate the mean ± standard error of the mean. SP,
stratum pyramidale; tgCI, transient global cerebral ischemia; ROD,
relative optical density; Iba1, ionized calcium-binding adapter
molecule 1. |
tgCI-induces change in CX3CL1
immunoreactivity
In the sham operated gerbils, CX3CL1
immunoreactivity was mainly observed in CA1 pyramidal cells
(Fig. 5A). However, in the
ischemia operated gerbils, CX3CL1 immunoreactivity in CA1 pyramidal
cells was very weak and significantly decreased (P<0.05) at 6 h
after tgCI (Fig. 5B and G). At 1
day after tgCI, CX3CL1 immunoreactivity was very strong in CA1
pyramidal cells, showing that the CX3CL1 immunoreactivity was
significantly increased (P<0.05, 172.2% of the sham operated
gerbils) compared with the sham operated gerbils (Fig. 5C and G). Thereafter, CX3CL1
immunoreactivity in CA1 pyramidal cells began to significantly
decrease from 2 days after tgCI (P<0.05, 54.7% of the sham
operated gerbils) and CX3CL1 immunoreactivity in the CA1 pyramidal
cells was weak at 5 and 10 days after tgCI (P<0.05, 21.2% and
P<0.05, 28.6% of the sham operated gerbils, respectively),
because death of CA1 pyramidal cells occurred after tgCI (Fig. 5D-G).
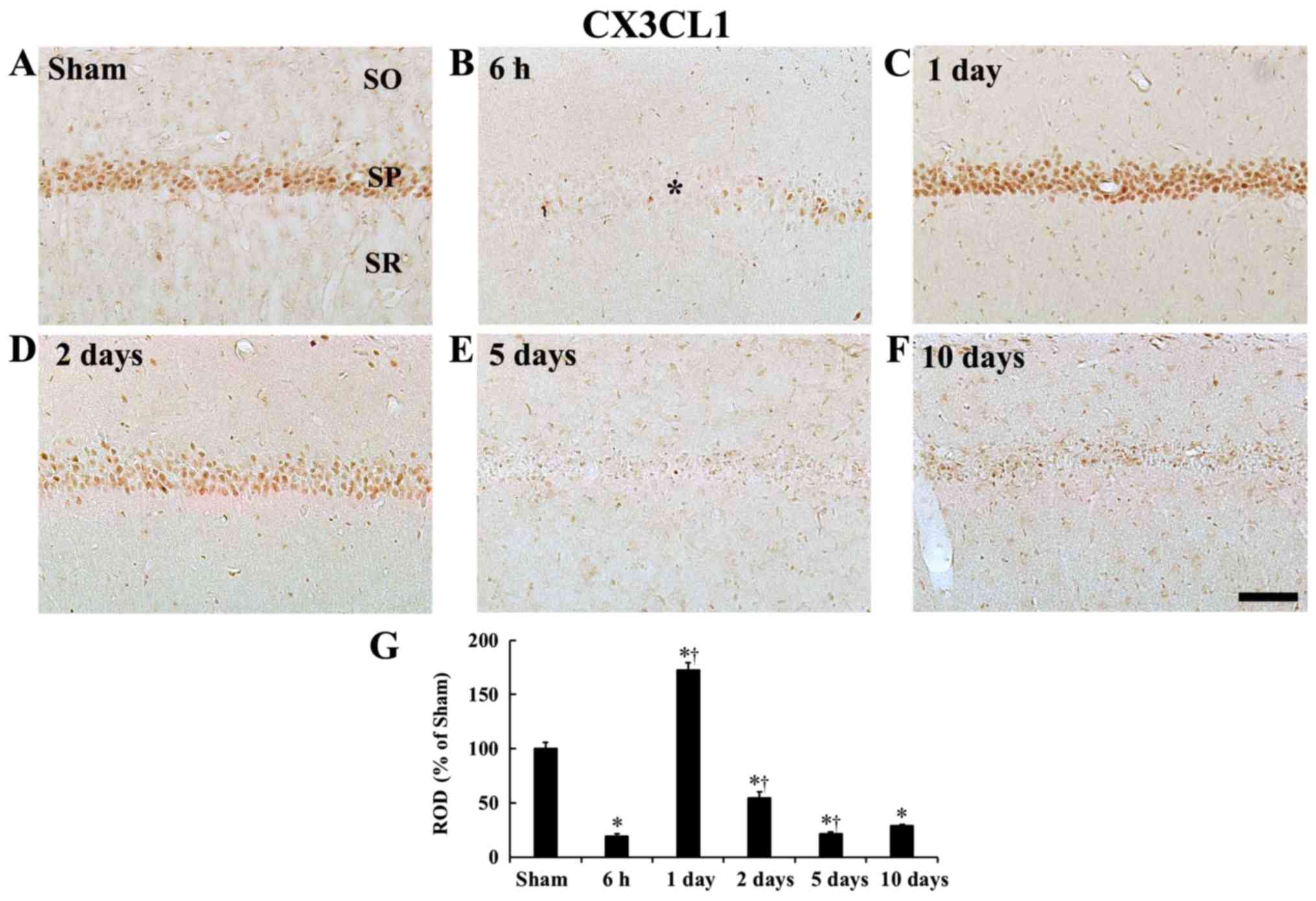 | Figure 5CX3CL1 immunohistochemistry. (A-F)
CX3CL1 immunohistochemistry in the CA1 field of the (A) sham group
and ischemia operated groups at (B) 6 h, (C) 1 day, (D) 2 days, (E)
5 days and (F) 10 days after tgCI. Strong CX3CL1 immunoreactivity
is shown in CA1 pyramidal cells of the SP at sham and 1 day after
tgCI. CX3CL1 immunoreactivity is very weak in the CA1 field at 6 h
(asterisk), 5 days and 10 days after tgCI. Scale bar, 50 µm.
(G) ROD as % of CX3CL1 immunoreactive structures in the CA1 field
after tgCI (n=7 at each point in time) *P<0.05 vs.
the sham operated gerbils; †P<0.05 vs. the
pre-time-point group. Bars indicate the means ± standard error of
the mean. SP, stratum pyramidale; ROD, relative optical density;
tgCI, transient global cerebral ischemia; CX3CL1, C-X3-C motif
ligand 1; CX3CR1, CX3C chemokine receptor 1. |
tgCI-induces change in CX3CR1
immunoreactivity
In the sham operated gerbils, weak CX3CR1
immunoreactivity was observed in CA1 pyramidal cells and their
processes (Fig. 6A). In the
ischemia operated gerbils, CX3CR1 immunoreactivity in the CA1 field
at 6 h after tgCI was significantly decreased (P<0.05) by 36.5%
compared with the sham operated gerbils (Fig. 6B and G). At 1 day after tgCI,
CX3CR1 immunoreactivity in the CA1 field was significantly
increased (P<0.05) by 103% compared with the sham operated
gerbils, showing that the increased CX3CR1 immunoreactivity was
shown in CA1 pyramidal cells (Figs.
6C and 5G). At 2 days after
tgCI, CX3CR1 immunoreactivity was significantly decreased
(P<0.05) by 25.4% compared with 1 day after tgCI (Fig. 6D and G). At 5 and 10 days after
tgCI, CX3CR1 immunoreactivity in the CA1 field was significantly
increased by 203.8% (P<0.05) and 189.4% (P<0.05),
respectively, compared with the sham operated gerbils. At these
points in time, strong CX3CR1 immunoreactivity was shown in cells
in strata oriens and radiatum (Fig.
6E-G).
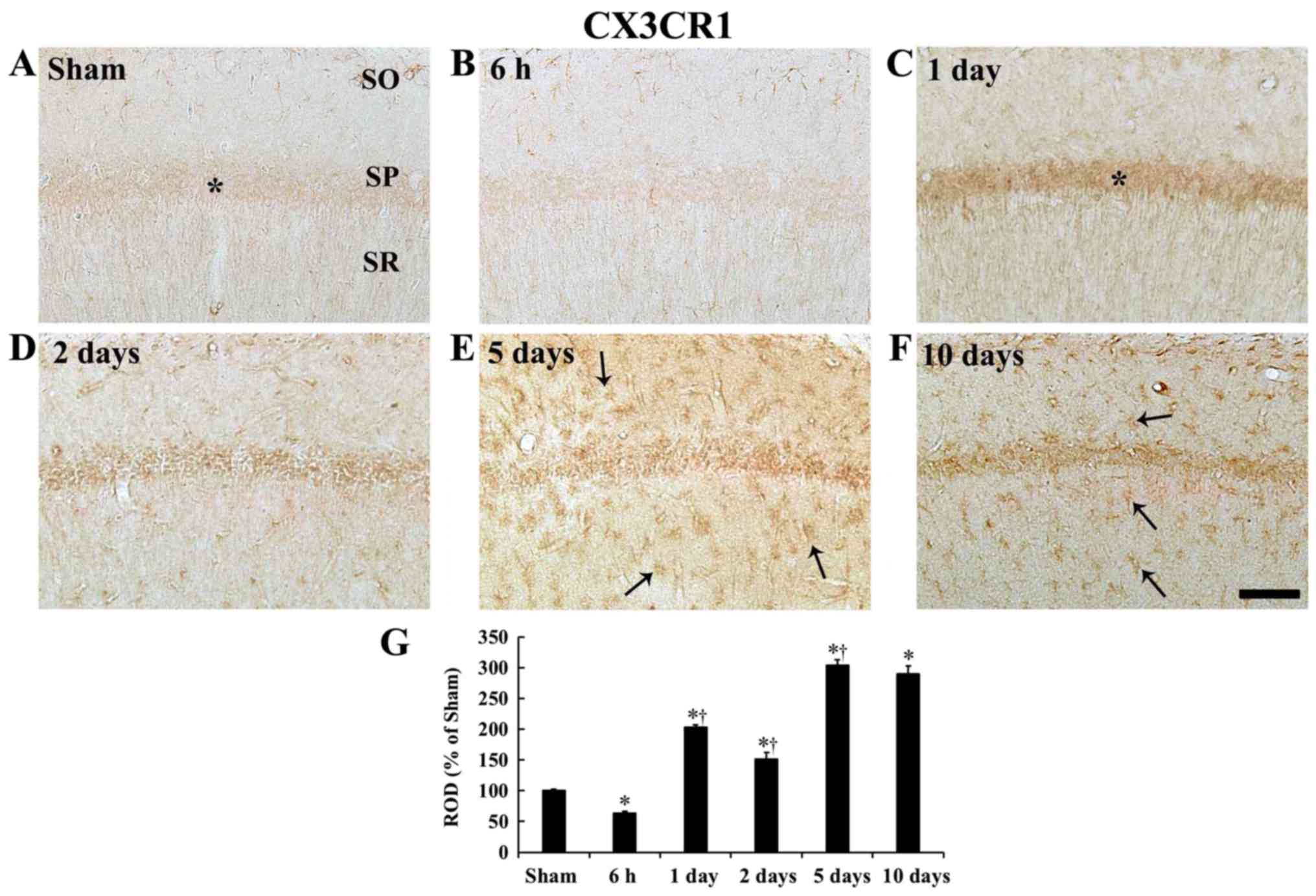 | Figure 6CX3CR1 immunohistochemistry. (A-F)
CX3CR1 immunohistochemistry in the CA1 field of the (A) sham group
and ischemia operated groups at (B) 6 h, (C) 1 day, (D) 2 days, (E)
5 days and (F) 10 days after tgCI. Weak CX3CR1 immunoreactivity is
detected in the SP (asterisk) of the sham operated gerbils and
CX3CR1 immunoreactivity in the SP (asterisk) at 1 day after tgCI is
strong. At 5 and 10 days after tgCI, strong CX3CR1 immunoreactivity
is shown in cells (arrows) in the SO and SR. Scale bar, 50
µm. (G) ROD as % of CX3CR1-immunoreactive structures in the
CA1 field after tgCI (n=7 at each point in time).
*P<0.05 vs. the sham operated gerbils;
†P<0.05 vs. the pre-time-point group. Bars indicate
the mean ± standard error of the mean. SO, stratum oriens; SP,
stratum pyramidale; SR, stratum radiatum; tgCI, transient global
cerebral ischemia; CX3CL1, C-X3-C motif ligand 1; CX3CR1, CX3C
chemokine receptor 1; ROD, relative optical density. |
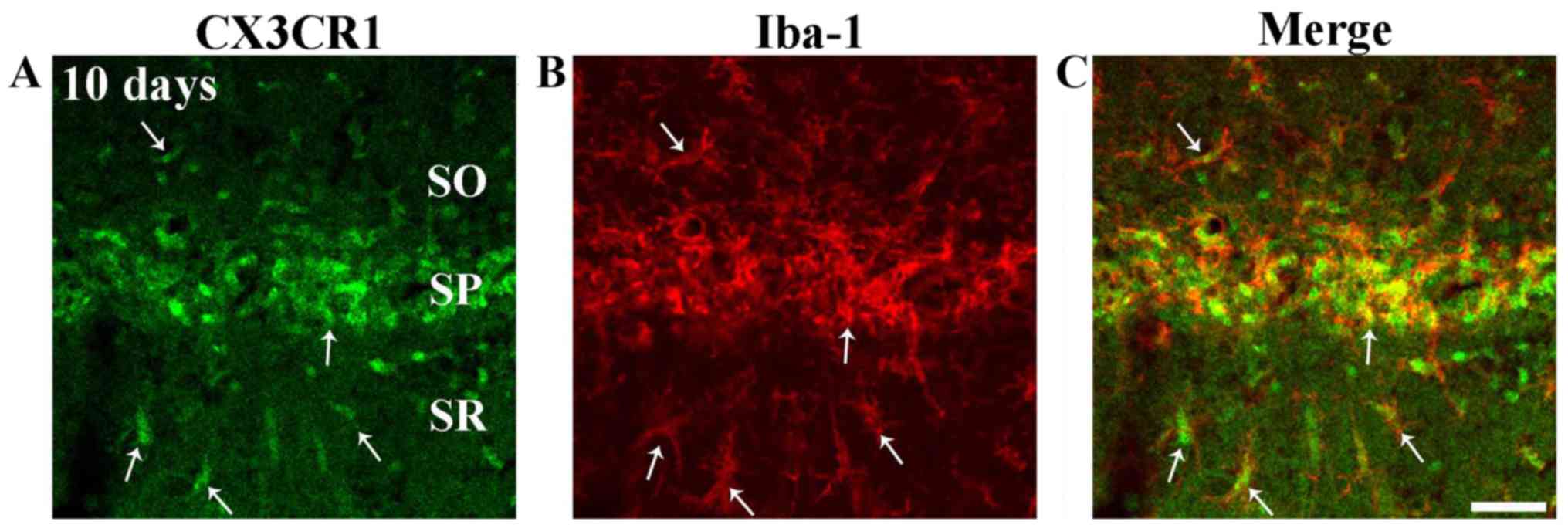
tgCI-induces CX3CR1 immunoreactivity in
microglia
Strong CX3CR1 immunoreactivity was shown in cells in
strata oriens and radiatum at 5 and 10 days after tgCI (Fig. 6E and F). Their cell type was
examined using double immunofluorescence staining and found that
cells expressing CX3CR1 in strata oriens and radiatum were
identified as Iba1 immunoreactive microglia (Fig. 7A-C).
Discussion
In the present study, strong CX3CL1 immunoreactivity
was found mainly in CA1 pyramidal cells in the sham operated
gerbils, consistent with a previous study showing that CX3CL1 was
constitutively expressed in neurons (12). In addition, weak CX3CR1
immunoreactivity was found in CA1 pyramidal cells in the sham
operated gerbils. Meucci et al (33) reported that CX3CR1 is expressed in
hippocampal neurons. They suggested that neuronal CX3CR1 might have
mediated neurotrophic effects of fractalkine and that both
fractalkine and CX3CR1 might be involved in cellular communications
between neurons and glia.
In this study, CX3CL1 and CX3CR1 immunoreactivities
were observed in CA1 pyramidal cells that were transiently and
reduced at 6 h after tgCI and then increased at 1 day after tgCI.
This result is somewhat consistent with a previous study showing
that CX3CL1/CX3CR1 expression is downregulated within 24 after
ischemic injury and upregulated at 48 h to 7 days following focal
cerebral ischemia in rats (17).
A number of studies have reported roles of
CX3CL1/CX3CR1 signaling pathway in the brain following ischemic
insults. However, relevant findings have been controversial.
Certain studies have demonstrated beneficial effects of CX3CL1
against cerebral ischemic damage and shown that administration of
CX3CL1 can decrease ischemia-induced infarct size, neuronal death
and neurologic deficits in the rat brain following focal cerebral
ischemia (34,35). On the other hand, it has been
reported that transient focal cerebral ischemia-induced cerebral
infarction is significantly lower in CX3CL1-deficient mice compared
with their wild-type littermates (36). Regarding CX3CR1, its deficiency
provides a neuroprotective effect against ischemia-induced brain
injury in mice following focal cerebral ischemia (18,22,37). However, a recent study has shown
that CX3CR1 deficiency does not affect lesion size after transient
focal cerebral ischemia in mice (38).
Liu et al (21) have demonstrated the detrimental
effects of CX3CL1/CX3CR1-mediated microglial activation in the
ischemic mouse brain. They reported that reduced expression of
CX3CR1 by CX3CR1 small interfering RNA could remarkably decrease
expression levels of proinflammatory cytokines, such as tumor
necrosis factor-α, interleukin (IL)-1β and IL-6, in a mouse brain
after bilateral common carotid artery stenosis as well as in BV2
microglia with oxygen-glucose deprivation. In addition, certain
studies have suggested detrimental roles of CX3CL1/CX3CR1 pathway
in ischemic brain injury. For example, CX3CL1- and CX3CR1-knockout
mice show less severe brain damage after focal cerebral ischemia
(34,39). In the present study, it was found
that microglia began to be activated and increased in number from 6
h after tgCI. In addition, it was found that CX3CR1
immunoreactivity was increased in microglia at 5 and 10 days after
tgCI. It is known that CX3CL1 signals to microglia by binding to
CX3CR1 which functions as an inhibitor of microglial activity
(15,20). Therefore, a transient reduction of
CX3CL1 and CX3CR1 immunoreactivities in the CA1 field at 6 h after
tgCI might be related to the beginning of ischemia-induced
microglial activation and recruitment while increases of CX3CL1 and
CX3CR1 immunoreactivities at 1 day after tgCI might be a
compensatory response to regulate microglial activation.
Thereafter, a gradual decrease of CX3CL1 and CX3CR1
immunoreactivities in the CA1 field at 2 days after tgCI might be
associated with a failure of a compensatory response, which led to
considerable microglial activation. However, there might be other
mechanisms to explain elevations of CX3CL1 and CX3CR1
immunoreactivities in CA1 pyramidal cells at 1 day after tgCI. Yeo
et al (40) have reported
that CX3CR1 immunoreactivity is transiently increased in
hippocampal pyramidal cells following pilocarpine-induced status
epilepticus and suggested that a transient upregulation of neuronal
CX3CR1 expression might play a role in neurodegeneration following
status epilepticus. In addition, a recent study has shown that
CX3CR1 is upregulated in ischemic hippocampal neurons under
oxygen-glucose deprivation as well as in ischemic neurons of mice
subjected to focal cerebral ischemia (22). That study has suggested that
neuronal CX3CR1 elevation might be associated with ischemia-induced
apoptotic neuronal death (22).
Based on the results of previous studies, it can be postulated that
transient decreases and subsequent increases of CX3CL1 and CX3CR1
immunoreactivities in the hippocampal CA1 field at early time after
tgCI might be closely associated with tgCI-induced microglial
activation as well as tgCI-induced delayed neuronal death.
In this study, weak CX3CL1 immunoreactivity in CA1
pyramidal cells was detected from 5 days after tgCI. The reduction
of CX3CL1 immunoreactivity in the CA1 pyramidal cells might be
associated with the process of delayed neuronal death of CA1
pyramidal cells following tgCI. On the other hand, CX3CR1
immunoreactivity was increased in microglial cells from 5 days
after tgCI. It is known that cerebral ischemia leads to microglial
activation including an increase of CX3CR1 expression in microglia,
which causes an increase in the generation of proinflammatory
cytokines in the activated microglia (21). In addition, other studies and the
authors' previous study have shown marked microglial activation in
the hippocampal CA1 field when tgCI-induced delayed neuronal death
occurs, suggesting that activated microglia participate in
phagocytic action (7,41-43). Thus, sustained and increased
CX3CR1 expression in activated microglia might participate in the
neuroinflammatory response and phagocytosis of activated microglia
in ischemic regions.
However, there are still some important limitations
of this study. As described above, a number of previous studies
have reported various effects of CX3CL1- and/or CX3CR1-deficiency
against ischemia-induced brain injury following focal cerebral
ischemia, not tgCI, using CX3CL1 and CX3CR1 mutant mice. Therefore,
a further study needs to investigate functional effects of CX3CL1
and/or CX3CR1 following tgCI using CX3CL1 and CX3CR1 mutant mice.
In addition, possible upstream regulators of CX3CL1 and CX3CR1 in
rodent brains following tgCI have not been fully elucidated yet.
Therefore, the possible upstream regulators, which are related to
the tgCI-induced changes of CX3CR1 and CX3CL1 in the hippocampus,
should be also investigated in a further study.
In conclusion, CX3CL1 and CX3CR1 immunoreactivities
were markedly changed in CA1 pyramidal cells and microglia in the
hippocampal CA1 field after tgCI, indicating that tgCI-induced
changes in CX3CL1 and CX3CR1 expression might be closely associated
with tgCI-induced delayed neuronal death and microglial activation
in ischemic brain regions.
Funding
This study was supported by the Bio & Medical
Technology Development Program of the NRF funded by the Korean
government, MSIP (grant no. NRF-2015M3A9B6066835) and by Basic
Science Research Program through the National Research Foundation
of Korea funded by the Ministry of Education (grant no.
NRF-2017R1D1A1B03029311).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JHA, MHW and CHL were responsible for experimental
design, data collection, data analysis and manuscript writing. JHP
and TKL performed the experiments, and DWK and HAL performed data
analysis and provided critical comments on the whole process of
this study.
Ethics approval and consent to
participate
Experimental procedures for this study were approved
by the Institutional Animal Care and Use Committee at Kangwon
National University (approval number: KW-180124-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Arundine M and Tymianski M: Molecular
mechanisms of glutamate-dependent neurodegeneration in ischemia and
traumatic brain injury. Cell Mol Life Sci. 61:657–668. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JC, Kim IH, Park JH, Ahn JH, Cho JH,
Cho GS, Tae HJ, Chen BH, Yan BC, Yoo KY, et al: Ischemic
preconditioning protects hippocampal pyramidal neurons from
transient ischemic injury via the attenuation of oxidative damage
through upregulating heme oxygenase-1. Free Radic Biol Med.
79:78–90. 2015. View Article : Google Scholar
|
|
3
|
Park JH, Kim YH, Ahn JH, Choi SY, Hong S,
Kim SK, Kang IJ, Kim YM, Lee TK, Won MH and Lee CH: Atomoxetine
protects against NMDA receptor-mediated hippocampal neuronal death
following transient global cerebral ischemia. Curr Neurovasc Res.
14:158–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rastogi L, Godbole MM, Ray M, Rathore P,
Rathore P, Pradhan S, Gupta SK and Pandey CM: Reduction in
oxidative stress and cell death explains hypothyroidism induced
neuroprotection subsequent to ischemia/reperfusion insult. Exp
Neurol. 200:290–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barone FC and Feuerstein GZ: Inflammatory
mediators and stroke: New opportunities for novel therapeutics. J
Cereb Blood Flow Metab. 19:819–834. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujiwara N, Som AT, Pham LD, Lee BJ,
Mandeville ET, Lo EH and Arai K: A free radical scavenger edaravone
suppresses systemic inflammatory responses in a rat transient focal
ischemia model. Neurosci Lett. 633:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee CH, Yoo KY, Choi JH, Park OK, Hwang
IK, Kim SK, Kang IJ, Kim YM and Won MH: Neuronal damage is much
delayed and microgliosis is more severe in the aged hippocampus
induced by transient cerebral ischemia compared to the adult
hippocampus. J Neurol Sci. 294:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito K, Suyama K, Nishida K, Sei Y and
Basile AS: Early increases in TNF-alpha, IL-6 and IL-1 beta levels
following transient cerebral ischemia in gerbil brain. Neurosci
Lett. 206:149–152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Hallenbeck JM, Ruetzler C, Bol D,
Thomas K, Berman NE and Vogel SN: Overexpression of monocyte
chemoattractant protein 1 in the brain exacerbates ischemic brain
injury and is associated with recruitment of inflammatory cells. J
Cereb Blood Flow Metab. 23:748–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garau A, Bertini R, Colotta F, Casilli F,
Bigini P, Cagnotto A, Mennini T, Ghezzi P and Villa P:
Neuroprotection with the CXCL8 inhibitor repertaxin in transient
brain ischemia. Cytokine. 30:125–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schilling M, Strecker JK, Ringelstein EB,
Schäbitz WR and Kiefer R: The role of CC chemokine receptor 2 on
microglia activation and blood-borne cell recruitment after
transient focal cerebral ischemia in mice. Brain Res. 1289:79–84.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishiyori A, Minami M, Ohtani Y, Takami S,
Yamamoto J, Kawaguchi N, Kume T, Akaike A and Satoh M: Localization
of fractalkine and CX3CR1 mRNAs in rat brain: Does fractalkine play
a role in signaling from neuron to microglia? FEBS Lett.
429:167–172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al Mamun A, Yu H, Romana S and Liu F:
Inflammatory responses are sex specific in chronic hypoxic-ischemic
encephalopathy. Cell Transplant. 27:1328–1339. 2018. View Article : Google Scholar
|
|
14
|
Jiang T, Zhang L, Pan X, Zheng H, Chen X,
Li L, Luo J and Hu X: Physical exercise improves cognitive function
together with microglia phenotype modulation and remyelination in
chronic cerebral hypoperfusion. Front Cell Neurosci. 11:4042017.
View Article : Google Scholar
|
|
15
|
Harrison JK, Jiang Y, Chen S, Xia Y,
Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S,
Thompson DA, et al: Role for neuronally derived fractalkine in
mediating interactions between neurons and CX3CR1-expressing
microglia. Proc Natl Acad Sci USA. 95:10896–10901. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chapman GA, Moores K, Harrison D, Campbell
CA, Stewart BR and Strijbos PJ: Fractalkine cleavage from neuronal
membranes represents an acute event in the inflammatory response to
excitotoxic brain damage. J Neurosci. 20:RC872000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tarozzo G, Campanella M, Ghiani M, Bulfone
A and Beltramo M: Expression of fractalkine and its receptor,
CX3CR1, in response to ischaemia-reperfusion brain injury in the
rat. Eur J Neurosci. 15:1663–1668. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dénes A, Ferenczi S, Halász J, Környei Z
and Kovács KJ: Role of CX3CR1 (fractalkine receptor) in brain
damage and inflammation induced by focal cerebral ischemia in
mouse. J Cereb Blood Flow Metab. 28:1707–1721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pabon MM, Bachstetter AD, Hudson CE, Gemma
C and Bickford PC: CX3CL1 reduces neurotoxicity and microglial
activation in a rat model of Parkinson's disease. J
Neuroinflammation. 8:92011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ransohoff RM, Liu L and Cardona AE:
Chemokines and chemokine receptors: Multipurpose players in
neuroinflammation. Int Rev Neurobiol. 82:187–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wu XM, Luo QQ, Huang S, Yang QW,
Wang FX, Ke Y and Qian ZM: CX3CL1/CX3CR1-mediated microglia
activation plays a detrimental role in ischemic mice brain via
p38MAPK/PKC pathway. J Cereb Blood Flow Metab. 35:1623–1631. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Gan Y, Han P, Yin J, Liu Q,
Ghanian S, Gao F, Gong G and Tang Z: Ischemia-induced neuronal cell
death is mediated by chemokine receptor CX3CR1. Sci Rep. 8:5562018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu J, Zhou Z, Liu Y and Zheng J:
Fractalkine and CX3CR1 are involved in the migration of
intravenously grafted human bone marrow stromal cells toward
ischemic brain lesion in rats. Brain Res. 1287:173–183. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onken M, Berger S and Kristian T: Simple
model of forebrain ischemia in mouse. J Neurosci Methods.
204:254–261. 2012. View Article : Google Scholar :
|
|
26
|
Park JH, Park O, Cho JH, Chen BH, Kim IH,
Ahn JH, Lee JC, Yan BC, Yoo KY, Lee CH, et al: Anti-inflammatory
effect of tanshinone I in neuroprotection against cerebral
ischemia-reperfusion injury in the gerbil hippocampus. Neurochem
Res. 39:1300–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JH, Shin BN, Ahn JH, Cho JH, Kim IH,
Kim DW, Won MH, Hong S, Cho JH and Lee CH: Ischemia-induced changes
of PRAS40 and p-PRAS40 immunoreactivities in the gerbil hippocampal
CA1 region after transient cerebral ischemia. Cell Mol Neurobiol.
36:821–828. 2016. View Article : Google Scholar
|
|
28
|
Kaufman GD, Shinder ME and Perachio AA:
Correlation of fos expression and circling asymmetry during gerbil
vestibular compensation. Brain Res. 817:246–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du X, Wang D, Li Y, Huo X, Li C, Lu J,
Wang Y, Guo M and Chen Z: Newly breeding an inbred strain of
ischemia-prone mongolian gerbils and its reproduction and genetic
characteristics. Exp Anim. 67:83–90. 2018. View Article : Google Scholar :
|
|
30
|
Zhu XL, Yan BC, Tang C, Qiu GW, Wu Y, Wang
J and Bo P: Neuroprotective effect of paeoniae radix rubra on
hippocampal CA1 region of mice induced by transient focal cerebral
ischemia via anti-gliosis and anti-oxidant activity. Chin Herb
Medicines. 11:86–91. 2019. View Article : Google Scholar
|
|
31
|
Ahn JH, Shin BN, Park JH, Kim IH, Cho JH,
Chen B, Lee TK, Tae HJ, Lee JC, Cho JH, et al: Long-term
observation of neuronal degeneration and microgliosis in the gerbil
dentate gyrus after transient cerebral ischemia. J Neurol Sci.
363:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmued LC and Hopkins KJ: Fluoro-Jade B:
A high affinity fluorescent marker for the localization of neuronal
degeneration. Brain Res. 874:123–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meucci O, Fatatis A, Simen AA and Miller
RJ: Expression of CX3CR1 chemokine receptors on neurons and their
role in neuronal survival. Proc Natl Acad Sci USA. 97:8075–8080.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cipriani R, Villa P, Chece G, Lauro C,
Paladini A, Micotti E, Perego C, De Simoni MG, Fredholm BB, Eusebi
F and Limatola C: CX3CL1 is neuroprotective in permanent focal
cerebral ischemia in rodents. J Neurosci. 31:16327–16335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin W, Li Z, Luo S, Wu R, Pei Z and Huang
R: Exogenous fractalkine enhances proliferation of endothelial
cells, promotes migration of endothelial progenitor cells and
improves neurological deficits in a rat model of ischemic stroke.
Neurosci Lett. 569:80–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soriano SG, Amaravadi LS, Wang YF, Zhou H,
Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D
and Pan Y: Mice deficient in fractalkine are less susceptible to
cerebral ischemia-reperfusion injury. J Neuroimmunol. 125:59–65.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang Z, Gan Y, Liu Q, Yin JX, Liu Q, Shi J
and Shi FD: CX3CR1 deficiency suppresses activation and
neurotoxicity of microglia/macrophage in experimental ischemic
stroke. J Neuroinflammation. 11:262014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van der Maten G, Henck V, Wieloch T and
Ruscher K: CX3C chemokine receptor 1 deficiency modulates microglia
morphology but does not affect lesion size and short-term deficits
after experimental stroke. BMC Neurosci. 18:112017. View Article : Google Scholar :
|
|
39
|
Fumagalli S, Perego C, Ortolano F and De
Simoni MG: CX3CR1 deficiency induces an early protective
inflammatory environment in ischemic mice. Glia. 61:827–842. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yeo SI, Kim JE, Ryu HJ, Seo CH, Lee BC,
Choi IG, Kim DS and Kang TC: The roles of fractalkine/CX3CR1 system
in neuronal death following pilocarpine-induced status epilepticus.
J Neuroimmunol. 234:93–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nitatori T, Sato N, Waguri S, Karasawa Y,
Araki H, Shibanai K, Kominami E and Uchiyama Y: Delayed neuronal
death in the CA1 pyramidal cell layer of the gerbil hippocampus
following transient ischemia is apoptosis. J Neurosci.
15:1001–1011. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan BC, Ohk TG, Ahn JH, Park JH, Chen BH,
Lee JC, Lee CH, Shin MC, Hwang IK, Moon SM, et al: Differences in
neuronal damage and gliosis in the hippocampus between young and
adult gerbils induced by long duration of transient cerebral
ischemia. J Neurol Sci. 337:129–136. 2014. View Article : Google Scholar
|
|
43
|
Sugawara T, Lewén A, Noshita N, Gasche Y
and Chan PH: Effects of global ischemia duration on neuronal,
astroglial, oligodendroglial, and microglial reactions in the
vulnerable hippocampal CA1 subregion in rats. J Neurotrauma.
19:85–98. 2002. View Article : Google Scholar : PubMed/NCBI
|