Introduction
Human lens epithelial cells (HLECs) are vulnerable
to oxidative stress, which has deleterious effects on lens
transparency and ultimately leads to cataracts. Aberrant reactive
oxygen species (ROS) accumulation and scavenging are major
contributors to oxidative damage (1-3).
Alterations in the cellular microenvironment in response to
hydrogen peroxide (H2O2) manifest as
apoptosis and result in the production of pro-inflammatory
mediators in HLECs (4). The risk
of oxidative damage to the transparent lens may be compensated by
the presence of antioxidant enzymes. Notably, catalase (CAT) and
peroxiredoxins (PRDXs) are antioxidant enzymes that act as ROS
scavengers and potential antioxidant protectors against cataract
development (5,6). Forkhead box (Fox)O1 belongs to the
FoxO family of transcription factors and, when activated, serves a
protective role in antioxidative responses (7). Therefore, it is important to
establish an antioxidative approach for preventing the formation
and progression of cataracts.
Epithelial-mesenchymal transition (EMT) may be
initiated by oxidative stress and manifests in the loss of
epithelial characteristics and the acquisition of mesenchymal
properties (8,9). Aquaporins (AQPs) are intrinsic
plasma membrane proteins that possess H2O2
permeability properties. AQP1 protein expression is confined to the
lens epithelium, where it acts as an epithelial marker. AQP1 serves
a crucial role in the maintenance of ocular lens homeostasis, and
its insufficient function may cause cataracts (10). The relationship between EMT and
oxidative stress is an important factor in cataract
progression.
It is well known that the lens is an organ that
lacks nerves (11); therefore,
neurodegeneration is not involved in cataract formation.
Acetyl-L-carnitine (ALC) is widely used in neurodegenerative
diseases due to its neurobiological effects (12). Due to the effects of ALC on
neurodegeneration, which is not involved in cataract formation, the
present study aimed to explore the effects of the antioxidant
L-carnitine (LC) on cataract prevention, not ALC. LC is a
water-soluble, vitamin-like molecule that is naturally found in
meat; since its recognition, LC has garnered much attention. LC is
a pivotal agent involved in protecting the cell and DNA against
damage induced by oxidative stress (13,14). LC protects the ocular surface; for
example, LC protects against hyperosmotic stress in dry eye disease
(15). It has previously been
reported that perturbation of the carnitine shuttle by increased
plasma levels of long-chain acylcarnitines leads to a compromised
cellular capacity to prevent ROS generation in age-related macular
degeneration (16). Despite these
findings, the connection between LC and cataract prevention remains
unclear. The present study aimed to explore the effects of LC on
H2O2-induced oxidative damage in HLECs, and
to identify the molecular pathways involved in this protection.
Materials and methods
Cell culture
The HLE B-3 cell line was obtained from American
Type Culture Collection. The cells were cultured in Dulbecco's
modified Eagle's medium/F-12 (HyClone; GE Healthcare Life Sciences)
containing 20% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and 10 µg/l gentamicin at 37°C in a humidified
atmosphere containing 5% CO2. The cells were incubated
with 0, 100, 150, 200, 250, 300 and 350 µM
H2O2 alone for 24 h, or were pretreated with
LC (Sigma-Aldrich; Merck KGaA) at 0, 10, 100, 300, 500 and 700
µM for 16 h. All treatments were carried out at 37°C in a
cell culture incubator. In addition, cells were pretreated with LC
(500 µM), ERK inhibitor (FR180204, 1.25 µM) or p38
inhibitor (PD169316, 1.25 µM), or a combination of LC and
FR180204 or LC and PD169316, for 2 h prior to treatment with 250
µM H2O2 treatment for 24 h. FR180204
and PD169316 were purchased from Sigma-Aldrich; Merck KGaA.
Cell viability assay
The Cell Counting kit-8 assay was used to detect the
effects of different concentrations of H2O2
and LC on the viability of HLECs. The optical density (OD) was
measured using the CCK-8 method (Dojindo Molecular Technologies,
Inc.). Cells were placed in 96-well plates at 2×103
cells/well in 200 µl growth medium and were cultured at 37°C
in a humidified incubator with 5% CO2. Following
treatment with H2O2 or LC, 10 µl CCK-8
solution was added to each well. After 2 h at 37°C, the absorbance
was measured at 450 nm. Cell survival rate was calculated according
to the following formula: Cell viability (%)=(As-Ab)/(Ac-Ab), where
As is the average OD value of the experimental group, Ab is the
average OD value of the blank group, and Ac is the average OD value
of the control group.
Measurement of cellular ROS
production
Cellular ROS production was detected using a
Reactive Oxygen Species Assay kit (cat. no. CA1410; Beijing
Solarbio Science & Technology Co., Ltd.). The DCFH-DA ROS
probe, which permeates the cell membrane with no fluorescence, was
used according to the manufacturer's protocol. ROS induces the
production of fluorescent DCF through oxidizing DCFH. Subsequently,
ROS levels can be determined by detecting the fluorescence of DCF.
Images were captured using a fluorescence microscope (DM4000 B LED;
Leica Microsystems GmbH).
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from HLECs using the
RNAsimple Total RNA Extraction kit [cat. no. dp419; Tiangen Biotech
(Beijing) Co., Ltd.] in accordance with the manufacturer's
protocol. The extracted RNA was quantified using a NanoDrop 2000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.) and stored at −80°C prior to use. First-strand cDNA was
synthesized from 2.0 µg total RNA using a M-MLV Reverse
Transcriptase kit (Promega Corporation) according to the
manufacturer's protocol, and was then stored at -20°C before use.
qPCR was performed using the StepOnePlus™ Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.), and SYBR
Premix Ex Taq™ (Takara Biotechnology Co., Ltd.). The thermocycling
conditions were as follows: 95°C for 3 min, followed by 40 cycles
at 95°C for 12 sec and 62°C for 40 sec, and a final dissociation
stage at 95°C for 15 sec, 65°C for 1 min and 95°C for 15 sec. GAPDH
served as an internal control and was used to detect the expression
levels of genes in HLECs. Relative gene expression was calculated
using the 2−ΔΔCq fold change method (17). The primer sequences used for
RT-qPCR are listed in Table
I.
 | Table IPrimer sequences of all genes used in
quantitative PCR. |
Table I
Primer sequences of all genes used in
quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|
| H-GAPDH |
5′-TGCCCTCAACGACCACTTTG-3′ |
5′-CTGGTGGTCCAGGGGTCTTA-3′ |
| H-PRDX4 |
5′-GAAGGAACAGCTGTGATCGA-3′ |
5′-AGAGCATGCTACCACTTCAGT-3′ |
| H-FOXO1 |
5′-ATGGCTTGGTGTCTTTCTTTTCT-3′ |
5′-TGTGGCTGACAAGACTTAACTCAA-3′ |
| H-CAT |
5′-TCTGGAGAAGTGCGGAGATT-3′ |
5′-TCCAATCATCCGTCAAAACA-3′ |
| H-Caspase-3 |
5′-AGCGAATCAATGGACTCTGGA-3′ |
5′-GGTTTGCTGCATCGACATCT-3′ |
| H-PCNA |
5′-CACTCCACTCTCTTCAACGGT-3′ |
5′-ATCCTCGATCTTGGGAGCCA-3′ |
| H-COX2 |
5′-ATAACGTGAAGGGCTGTCCC-3′ |
5′-ATCATCTAGTCCGGAGCGGG-3′ |
| H-IL1 |
5′-TCATACCAAGGAGAAGTAATAAGCC-3′ |
5′-ACCAAAGAAGTACAGCGCCAT-3′ |
| H-IL6 |
5′-CAAACTTCCTGGAGTTCACC-3′ |
5′-TGTGTCCAATGGACAGGATG-3′ |
| H-IL8 |
5′-ACCTCACTGTGCAAATTCAG-3′ |
5′-TATGACTCTTGCTGCTCAGC-3′ |
| H-AQP1 |
5′-TAACCCTGCTCGGTCCTTTG-3′ |
5′-AGTCGTAGATGAGTACAGCCAG-3′ |
| H-CDK2 |
5′-AAATTCATGGATGCCTCTGC-3′ |
5′-CAGGGACTCCAAAAGCTCTG-3′ |
| H-CDK4 |
5′-GAACTGACCGGGAGATCAAG-3′ |
5′-TCAGATCTCGGTGAACGATG-3′ |
| H-α-SMA |
5′-TGACATTTGTGAAACTTCGGGT-3′ |
5′-TGAAGCAATGGTAGCTGGGT-3′ |
| H-Vimentin |
5′-AAATGGCTCGTCACCTTCGT-3′ |
5′-AGAAATCCTGCTCTCCTCGC-3′ |
Western blot analysis
Cells treated with H2O2 and LC
were homogenized using RIPA lysis buffer with protease and
phosphatase inhibitor cocktail (Beyotime Institute of
Biotechnology). Protein samples were quantified using the
bicinchoninic acid protein assay (Thermo Fisher Scientific, Inc.).
Equal quantities of proteins per lane (30-50 µg, according
to the thickness of the gel) were separated by 10-12% SDS-PAGE,
transferred onto PVDF membranes and were then incubated with 5%
blocking reagent (5% skim milk) for 1.5 h at room temperature.
Subsequently, the membranes were incubated with the following
antibodies overnight at 4°C: Anti-GAPDH (1:1,000; cat. no.
ab181602), anti-PRDX4 (1:1,000; cat. no. ab184167), anti-cleaved
caspase-3 (1:1,000; cat. no. ab2302), anti-interleukin (IL)-1β
(1:1,000; cat. no. ab45692), anti-vimentin (1:1,000; cat. no.
ab92547) and anti-cyclooxygenase-2 (COX2; 1:1,000; cat. no.
ab179800) (all from Abcam); anti-proliferating cell nuclear antigen
(PCNA; 1:1,000; cat. no. 13110), anti-ERK (1:1,000; cat. no. 4695),
anti-phosphorylated (P)-ERK (1:1,000; cat. no. 4370), anti-p38
(1:1,000; cat. no. 8690) and anti-p-p38 (1:1,000; cat. no. 4511)
(all from Cell Signaling Technology, Inc.). The membranes were
washed three times with phosphate buffered saline-0.1% Tween-20
(Beijing Solarbio Science & Technology Co., Ltd.), and were
then incubated with horseradish peroxidase-conjugated secondary
antibodies (1:10,000; cat. no. ab6721; Abcam) for 2 h at room
temperature. Blots were detected using enhanced chemiluminescence
reagents (EMD Millipore) and were exposed to chemiluminescent film
(Kodak) or using G:BOX F3 (Syngene). The images were analyzed using
ImageJ software (v.1.42q; National Institutes of Health).
Statistical analysis
Data are presented as the mean ± standard error of
the mean from three independent experiments. The significance of
differences between two groups was evaluated using two-tailed
Student's t-test, and one-way ANOVA (significant variation was
indicated as α=0.05) followed by Dunnett's multiple comparisons
test or Tukey's multiple comparisons test were used to evaluate the
significance of differences among multiple groups. GraphPad Prism
version 7.0 software (GraphPad Software, Inc.) was used to conduct
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
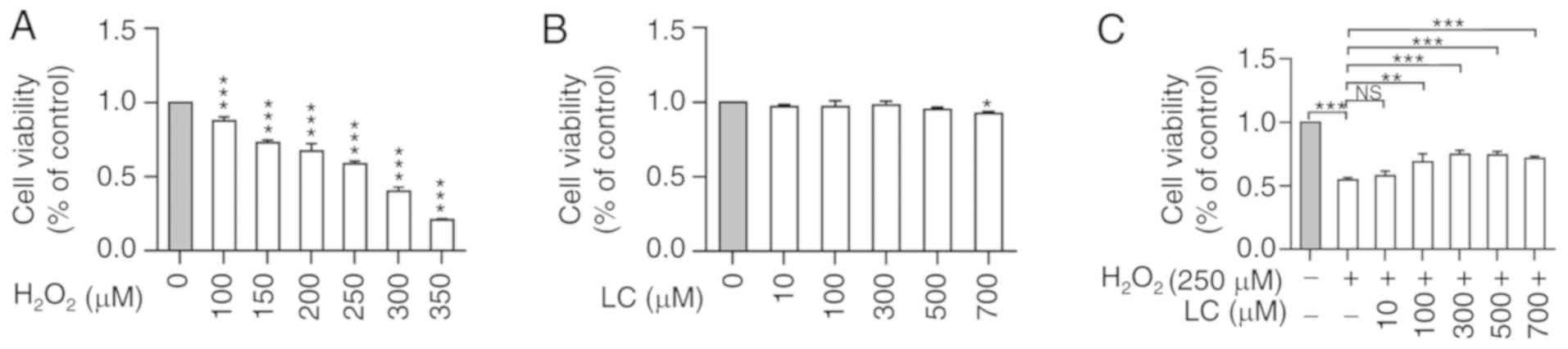
LC reverses the effects of
H2O2 on viability of HLE B-3 cells
H2O2 induced a significant
decrease in HLE B-3 cell viability in a dose-dependent manner
(Fig. 1A). Subsequently, 250
µM was chosen as the optimal concentration in the subsequent
experiments, because it was approximately equal to the
IC50 of H2O2. Conversely,
treatment with LC induced minor alterations in cell viability
(Fig. 1B), indicating the minimal
cytotoxicity of LC. In addition, LC exerted an ameliorating effect
on H2O2-induced suppression of cell
viability; however, this effect was not dose-dependent (Fig. 1C). Notably, cell viability was
reduced to some extent when exposed to 700 µM LC alone;
therefore, LC concentrations at 100, 300 and 500 µM were
chosen for subsequent experiments.
LC decreases the generation of ROS in
HLECs and promotes antioxidant production
To determine the role of LC in ROS-induced oxidative
damage, HLE B-3 cells were exposed to H2O2
with or without LC pretreatment. This study aimed to determine
whether exposure to H2O2 and LC could modify
ROS generation. A marked increase in DCF-positive cells was
observed by fluorescence microscopy in HLE B-3 cells exposed to
H2O2, as shown in Fig. 2A. DCF fluorescence was markedly
reduced by LC pretreatment, thus suggesting that LC partially
restrained H2O2-induced ROS generation in
cells induced by H2O2.
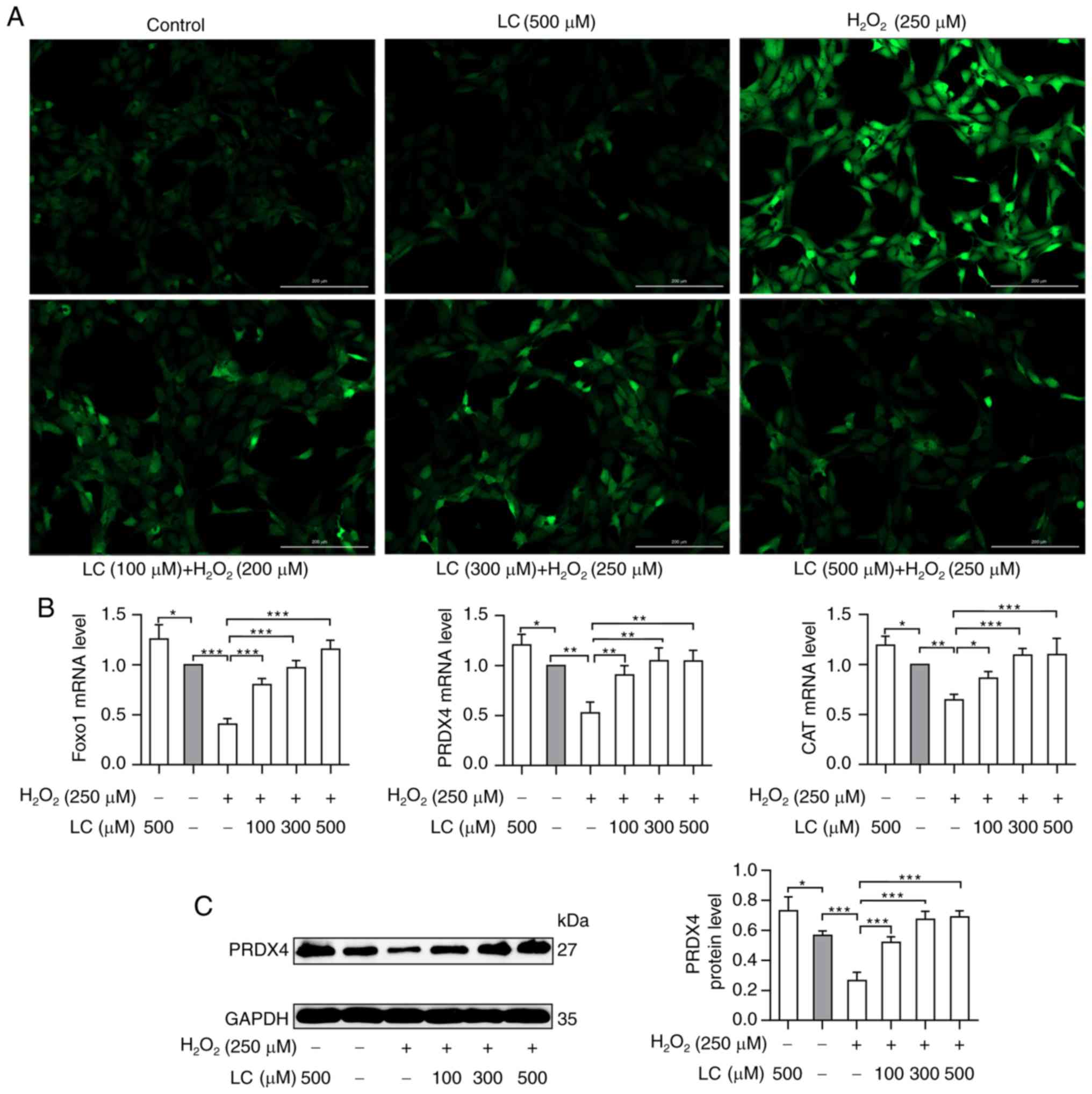 | Figure 2Effects of LC on ROS accumulation and
FoxO1, PRDX4 and CAT expression. (A) Increased ROS levels induced
by H2O2 were reversed by LC treatment in a
concentration-dependent manner. Scale bar, 200 µm. (B)
Reverse transcription-quantitative PCR analysis of the mRNA
expression levels of FoxO1, PRDX4 and CAT. Compared with the
H2O2 group, FoxO1, PRDX4 and CAT mRNA levels
were upregulated by the indicated LC treatment. (C) Western blot
analysis of PRDX4. PRDX4 protein levels were significantly elevated
in the presence of LC. Gray values were calculated for
semi-quantification. Data are expressed as the mean ± standard
error of the mean (n=3). *P<0.05,
**P<0.01, ***P<0.001. CAT, catalase;
FoxO1, forkhead box O1; H2O2, hydrogen
peroxide; LC, L-carnitine; PRDX4, peroxiredoxin 4; ROS, reactive
oxygen species. |
As shown in Fig.
2B, compared with in the control group, the mRNA expression
levels of FoxO1 exhibited a ~50% reduction in response to
H2O2 treatment, but were increased following
treatment with LC. Similarly, compared with in the
H2O2 group, the mRNA expression levels of the
antioxidant enzymes PRDX4 and CAT were increased upon exposure of
HLE B-3 cells to LC (P<0.05). Similar results were obtained by
western blotting to detect PRDX4 protein expression (Fig. 2C). These findings indicated that
LC may exert protective effects on cells suffering from oxidative
damage.
LC inhibits
H2O2-induced increase of apoptosis-associated
and inflammation-associated genes
Cleaved-caspase-3 was detected as a marker of
apoptosis; its expression was increased in HLECs exposed to
H2O2. Conversely, pretreatment with LC
partially reversed the increase in cleaved-caspase-3 mRNA and
protein expression (Fig. 3A and
B; P<0.05). Notably, compared with in the control group,
H2O2 exposure induced a ~1.6-fold increase in
cleaved-caspase-3 protein expression levels, as determined by
western blotting (Fig. 3B).
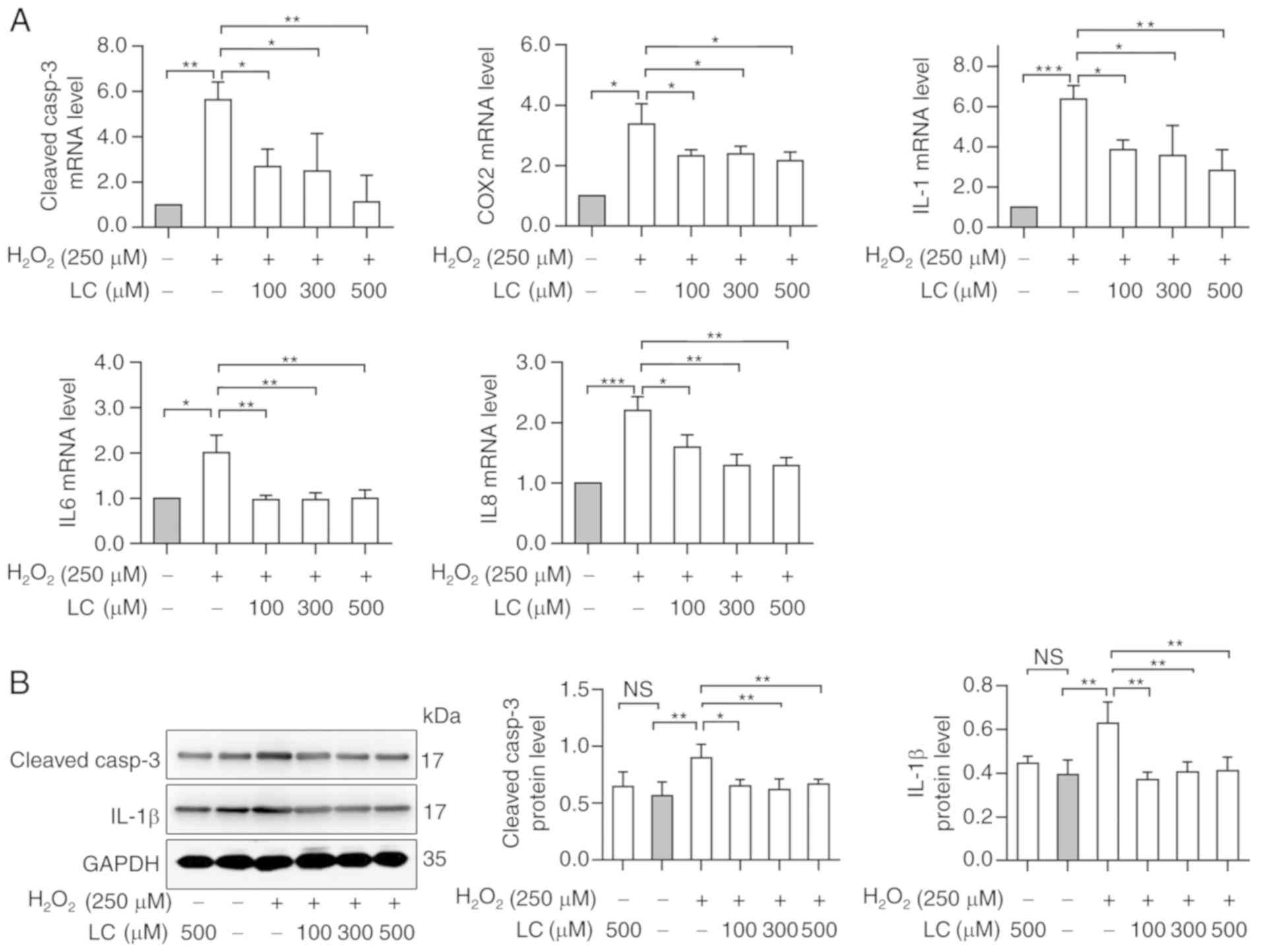 | Figure 3LC inhibits
H2O2-induced inflammation and apoptosis. (A)
Reverse transcription-quantitative PCR analysis revealed that
caspase-3, COX2, IL1, IL6 and IL8 levels were reduced by the
indicated LC treatment compared with in the
H2O2 group. (B) Western blot analysis
demonstrated that cleaved-caspase-3 and IL-1β levels were reduced
by the indicated LC treatment. Gray values were calculated for
semi-quantification. Data are expressed as the mean ± standard
error of the mean (n=3). *P<0.05,
**P<0.01, ***P<0.001; ns, no
significant differences (P≥0.05). COX2, cyclooxygenase-2;
H2O2, hydrogen peroxide; IL, interleukin; LC,
L-carnitine. |
The mRNA expression levels of inflammatory markers
COX2, IL1, IL6 and IL8 were increased with
H2O2 exposure (Fig. 3A), indicating the possible
involvement of inflammation during cataract progression. LC
reversed the inflammatory reaction induced by
H2O2 exposure; however, the effects were not
dose-dependent. Western blot analysis revealed that the protein
expression levels of IL-1β were increased following
H2O2 treatment, whereas these levels were
reduced by LC pretreatment (Fig.
3B). Taken together, these data indicated that LC may have a
role in reducing H2O2-induced apoptosis via
alleviating inflammatory responses.
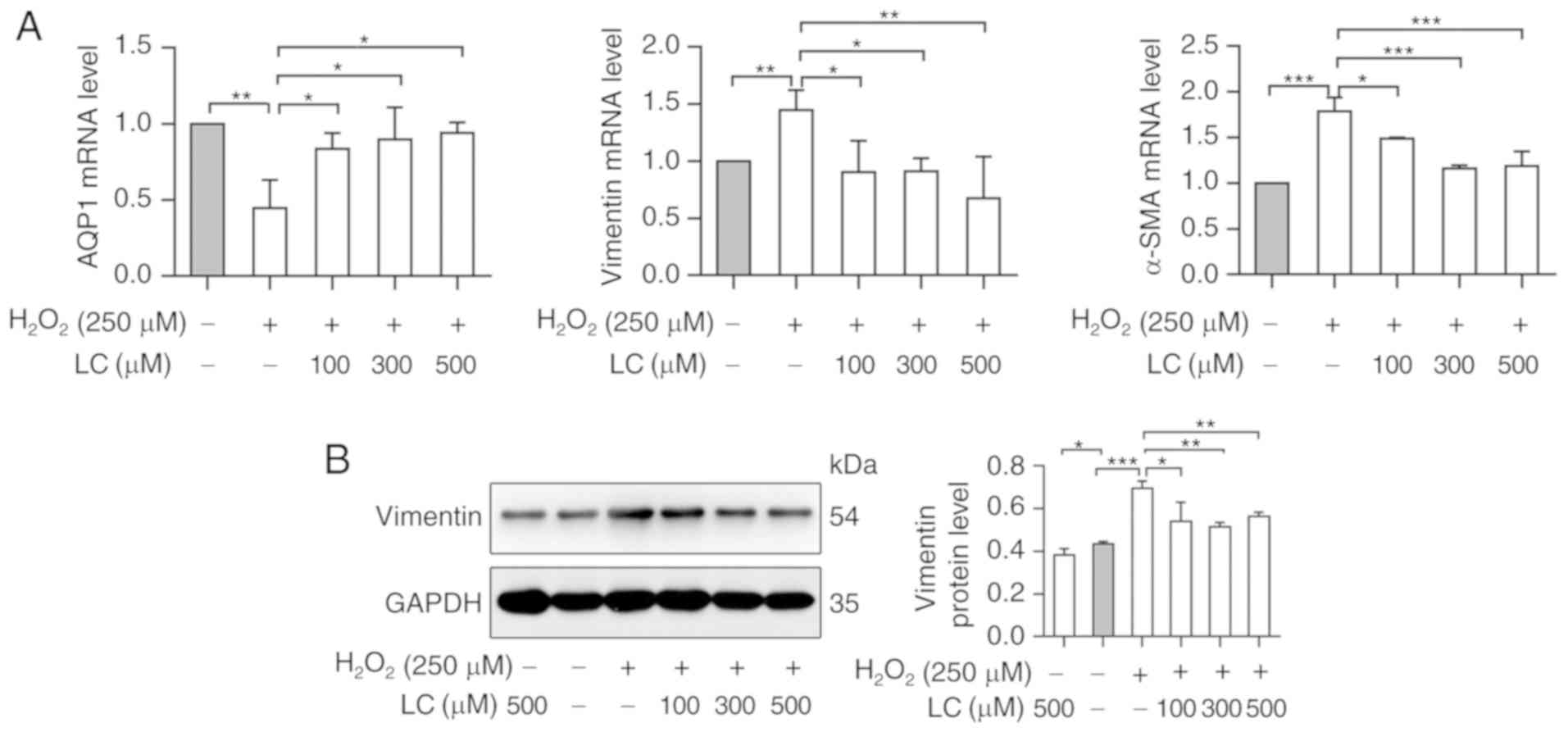
LC restores proliferation and suppresses
ROS-induced EMT in HLECs
The expression levels of EMT-associated genes were
detected in HLE B-3 cells exposed to H2O2.
The expression levels of AQP1, an epithelial marker, were reduced
by H2O2. Conversely, the expression levels of
the mesenchymal markers vimentin and α-smooth muscle actin (α-SMA)
were increased in the H2O2 group (P<0.05).
Conversely, LC pretreatment significantly reversed the expression
patterns of the aforementioned genes at the mRNA level (P<0.05;
Fig. 4A). Western blot analysis
further verified the effects of H2O2 and LC
on the protein expression levels of vimentin, thus indicating that
LC inhibited ROS-induced EMT (Fig.
4B).
Subsequently, the modulatory effects of LC on
proliferative markers were analyzed. PCNA expression was decreased
to ~70% of the level in the control group in response to
H2O2 exposure (P<0.05). LC pretreatment
increased PCNA expression at the mRNA and protein levels compared
with in the H2O2 group (Fig. 5A and B). CDK2 and CDK4 mRNA
expression was reduced upon H2O2 exposure,
whereas LC restored their expression (Fig. 5A; P<0.05).
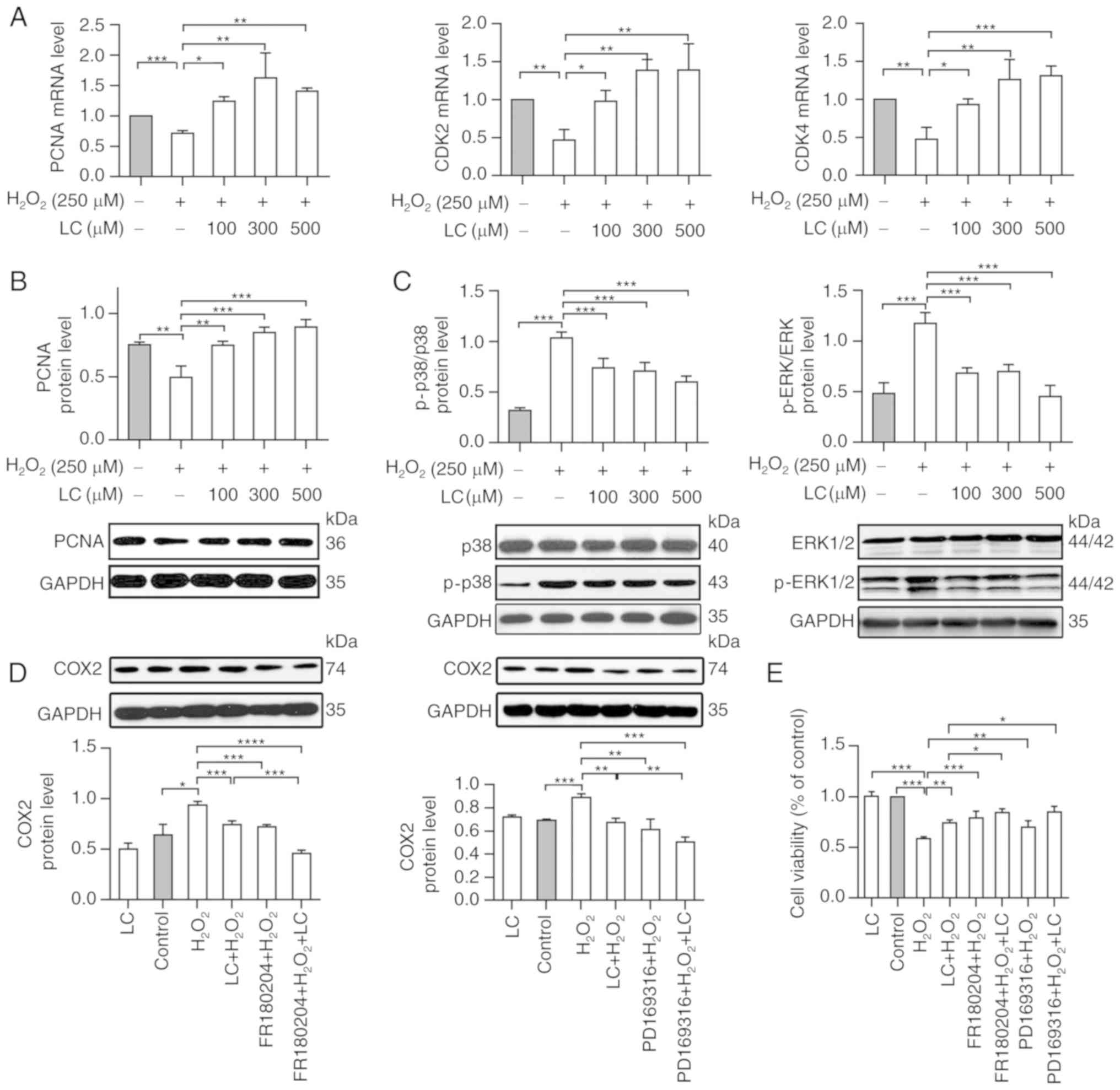 | Figure 5LC restores cell proliferation and
regulates cell damage through the MAPK pathway. (A) Relative mRNA
expression levels of PCNA, CDK2 and CDK4 were normalized to GAPDH.
Compared with in the H2O2 group, PCNA, CDK2
and CDK4 mRNA expression was upregulated by LC pretreatment. (B and
C) PCNA, ERK1 and ERK2, P-ERK1 and P-ERK2, p38 and p-p38 levels
were assessed by western blotting. PCNA was upregulated by the
indicated LC treatment, whereas p-p38, P-ERK1 and P-ERK2 were
downregulated by LC treatment compared with in the
H2O2 group. The ERK antibody detected
ERK1/ERK2, and the P-ERK antibody detected P-ERK1/P-ERK2. (D and E)
Human lens epithelial cells were pretreated with LC (500
µM), ERK inhibitor (FR180204, 1.25 µM) or p38
inhibitor (PD169316, 1.25 µM), or a combination of LC and
FR180204 or LC and PD169316, for 2 h prior to treatment with 250
µM H2O2 treatment for 24 h. (D) COX2
protein levels were measured using western blotting. Gray values
were calculated for quantification. (E) Cell viability was detected
by Cell Counting kit-8 assay. Data are expressed as the mean ±
standard error of the mean (n=3). *P<0.05,
**P<0.01, ***P<0.001. CDK,
cyclin-dependent kinase; COX2, cyclooxygenase-2;
H2O2, hydrogen peroxide; LC, L-carnitine; P-,
phosphorylated; PCNA, proliferating cell nuclear antigen. |
LC regulates oxidative damage through the
mitogen-activated protein kinase (MAPK) pathway
Intracellular ROS activates p38 MAPK, an oxidative
sensor that belongs to the MAPK family (18); therefore, to decipher the
potential cellular mechanism underlying the effects of LC on
oxidative damage, this study evaluated whether the MAPK signaling
pathway was involved. As determined by western blot analysis
(Fig. 5C), p-ERK1, p-ERK2 and
p-p38 were significantly enhanced by H2O2;
however, they were significantly reduced by LC in a dose-dependent
manner (P<0.05), thus suggesting the involvement of the MAPK
pathway in LC-modified oxidative stress.
To further evaluate the role of the MAPK signaling
pathway in mediating the protective effects of LC on
H2O2-induced cell inhibition and
inflammation, cell viability and COX-2 expression was assessed in
cells exposed to H2O2 and LC in the presence
of an ERK inhibitor (FR180204) or p38 inhibitor (PD169316). As
shown in Fig. 5D,
H2O2 markedly enhanced COX-2 expression,
whereas LC reversed COX-2 expression. Furthermore, pretreatment
with FR180204 or PD169316 abolished
H2O2-induced COX-2 expression. In addition,
HLE B-3 cells exposed to H2O2 and LC combined
with FR180204 or PD169316 exhibited considerably increased cell
viability (Fig. 5E). These
results indicated that LC may exert beneficial effects against
oxidative damage via MAPK signaling.
Discussion
Oxidative stress is a risk factor for cataracts
caused by the overproduction of ROS. H2O2 is
a main type of ROS that leads to oxidative damage in HLECs.
Antioxidants that scavenge excess ROS serve as a defense against
cell damage (19). In this study,
it was demonstrated that LC exhibited minimal cytotoxicity and
reversed H2O2-induced ROS production.
Exposure of HLE B-3 cells to
H2O2 triggered oxidative damage, which was
reflected in the destructed antioxidant defense mechanism.
Antioxidant substances, including FoxO1, PRDX4 and CAT, are
involved in ROS scavenging and serve as potential protectors.
H2O2-induced oxidative damage is associated
with decreased FoxO1, PRDX4 and CAT activities (20,21), indicating the probable mechanisms
underlying cataract formation. The present study focused on the
oxidative damage caused by ROS imbalance; therefore, FoxO1, PRDX4
and CAT were detected as antioxidative substances.
FoxO1 is highly expressed as a downstream
antioxidant when activated (22).
The present study revealed that FoxO1 is highly expressed in HLECs
treated with LC, suggesting that LC possesses antioxidative
potential. The present results indicated that LC may exert
beneficial effects on ROS scavenging by increasing the expression
levels of the antioxidative enzymes CAT and PRDX4. This finding is
consistent with previous findings, which suggested that LC may
protect retinal pigment epithelial cells from
H2O2-induced oxidative damage by increasing
antioxidant and antioxidant enzyme activity (23). This study hypothesized that LC may
act as a potential antioxidant protector against cataract
formation. Our future study aims to further explore alterations in
transcriptional regulation and the potential underlying mechanism.
In addition, further studies are required to determine the optimal
therapeutic delivery method of LC to the lens. Notably, widely used
topical inserts and colloidal drug delivery systems (24), such as nanowafers (25), may represent possible
pharmacological vehicles to enhance therapeutic efficacy.
PCNA is an auxiliary protein that facilitates cell
cycle progression. CDK2 and CDK4 are well-known stimulators and
promoters of the cell cycle from the G0/G1
into S phase (26,27). In this study, PCNA, CDK2 and CDK4
expression were enhanced by LC in the presence of
H2O2, demonstrating the role of LC in
protecting HLECs against oxidative damage. Given its antioxidant
properties, LC may promote the cell cycle and thereby increase cell
proliferation.
Exposure to H2O2 may promote
EMT in the transparent lens. In this study, marked decreases in the
expression of the epithelial marker AQP1, together with an increase
in mesenchymal markers (vimentin and α-SMA), were observed in HLECs
exposed to H2O2. Prevention of EMT was
demonstrated by elevated AQP1 expression, and attenuated vimentin
and α-SMA expression in the presence of LC. This result is
consistent with a previous study suggesting that LC prevents the
expression of EMT-associated biomarkers in renal fibrosis (28). It was hypothesized that LC may
become activated in response to ROS production and scavenging;
however, the exact mechanisms require further analysis.
Oxidative stress is closely associated with
inflammatory processes, which are important for the initiation and
progression of cataracts (29,30). The expression of proinflammatory
cytokines, including IL-1β, IL6 and IL8, was reduced by LC
pretreatment. COX2 is a major oxygenase, and its expression
increases along with oxidative stress-induced inflammation
(31). This study further
revealed that the production of COX2 was markedly induced by
H2O2, but significantly rescued by LC.
Inflammation triggers LECs to undergo an apoptotic response and
subsequently initiate cataract formation (32). Cleaved-caspase-3 expression was
decreased and inflammation was inhibited upon LC exposure. In the
present study, it was revealed that H2O2 may
act as a mediator of inflammation and apoptosis in HLECs, whereas
LC could significantly attenuate inflammation and reduce apoptosis
of HLECs.
The well-known MAPK signaling pathway, which
includes p38/MAPK, ERK and JNK, is involved in regulating oxidative
damage when cataracts occurs (33,34). The present results indicated that
ERK/MAPK and p38/MAPK were significantly activated upon
H2O2 exposure, whereas LC significantly
reduced the phosphorylation of ERK and p38 induced by
H2O2. Therefore, it was hypothesized that the
p38/MAPK and ERK/MAPK pathways are involved in the protective
mechanism underlying the effects of LC on oxidative damage in
HLECs. It has been reported that MAPK pathway inhibitors can
regulate apoptosis and inflammatory responses. The present results
revealed that ERK and p38 inhibitors significantly reduced
H2O2-induced cytotoxicity and inhibited the
expression of the inflammatory cytokine COX2 induced by exposure to
H2O2. These findings provide insight into how
oxidative modification of LC contributes to cataract
prevention.
In conclusion, the protective effects of LC against
oxidative stress may be attributed to its ROS-scavenging ability.
Oxidative damage in HLECs may be reversed by LC, which prevents the
induction of inflammation, apoptosis and EMT through the p38/MAPK
and ERK/MAPK pathways. The obtained results suggested that LC may
serve an important role in protecting HLECs from peroxidative
damage and may be a promising therapeutic modality for the
treatment of cataracts.
Acknowledgments
The authors would like to thank Ms. Ruifang Han, Mr.
Ming Ying and Mr. Peng Hao (Tianjin Key Laboratory of Ophthalmology
and Visual Science) for their technical assistance.
Funding
This study was supported in part by the National
Nature Science Foundation of China (grant no. 81670817); the
Tianjin Research Program of Application Foundation and Advanced
Technology (grant no. 17JCYBJC27200); the Science & Technology
Foundation for Selected Overseas Chinese Scholar, Bureau of
Personnel of China, Tianjin; Talent Innovation Group of 131, Bureau
of Personnel, Tianjin; Tianjin Science and Technology Project
(Popularization of Science grant no. 17KPHDSF00230), and China
Postdoctoral Science Foundation (grant no. 2018M641665).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and HL made substantial contributions to the
concept and design of the present study. XL, FM, XH and LW
performed the experiments. XL analyzed the data and wrote the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagai N, Ito Y and Takeuchi N: Correlation
between hyper-sensitivity to hydrogen peroxide and low defense
against Ca(2+) influx in cataractogenic lens of Ihara cataract
rats. Biol Pharm Bull. 34:1005–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuercher J, Neidhardt J, Magyar I, Labs S,
Moore AT, Tanner FC, Waseem N, Schorderet DF, Munier FL,
Bhattacharya S, et al: Alterations of the 5′ untranslated region of
SLC16A12 lead to age-related cataract. Invest Ophthalmol Vis Sci.
51:3354–3361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang D, Zhang X, Rong S, Sha Q, Liu P,
Han T and Pan H: Serum antioxidative enzymes levels and oxidative
stress products in age-related cataract patients. Oxid Med Cell
Longev. 2013:5878262013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mok JW, Chang DJ and Joo CK: Antiapoptotic
effects of anthocyanin from the seed coat of black soybean against
oxidative damage of human lens epithelial cell induced by
H2O2. Curr Eye Res. 39:1090–1098. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujii J, Ikeda Y, Kurahashi T and Homma T:
Physiological and pathological views of peroxiredoxin 4. Free Radic
Biol Med. 83:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamada S and Guo X: Peroxiredoxin 4
(PRDX4): Its critical in vivo roles in animal models of metabolic
syndrome ranging from atherosclerosis to nonalcoholic fatty liver
disease. Pathol Int. 68:91–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DH, Park CH, Park D, Choi YJ, Park MH,
Chung KW, Kim SR, Lee JS and Chung HY: Ginsenoside Rc modulates
Akt/FoxO1 pathways and suppresses oxidative stress. Arch Pharm Res.
37:813–820. 2014. View Article : Google Scholar
|
|
8
|
Kubo E, Shibata T, Singh DP and Sasaki H:
Roles of TGF β and FGF signals in the lens: Tropomyosin regulation
for posterior capsule opacity. Int J Mol Sci. 19:E30932018.
View Article : Google Scholar
|
|
9
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schey KL, Petrova RS, Gletten RB and
Donaldson PJ: The role of aquaporins in ocular lens homeostasis.
Int J Mol Sci. 18:E26932017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dahm R: Dying to see. Sci Am. 291:82–89.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Traina G: The neurobiology of
acetyl-L-carnitine. Front Biosci (Landmark Ed). 21:1314–1329. 2016.
View Article : Google Scholar
|
|
13
|
Nutrients Editorial Office: Erratum:
l-Carnitine supplementation in recovery after exercise; Nutrients
2018, 10, 349. Nutrients. 10:E5412018. View Article : Google Scholar
|
|
14
|
Mishra A, Reddy IJ, Gupta PS and Mondal S:
L-carnitine mediated reduction in oxidative stress and alteration
in transcript level of antioxidant enzymes in sheep embryos
produced in vitro. Reprod Domest Anim. 51:311–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng R, Su Z, Hua X, Zhang Z, Li DQ and
Pflugfelder SC: Osmoprotectants suppress the production and
activity of matrix metalloproteinases induced by hyperosmolarity in
primary human corneal epithelial cells. Mol Vis. 20:1243–1252.
2014.PubMed/NCBI
|
|
16
|
Mitchell SL, Uppal K, Williamson SM, Liu
K, Burgess LG, Tran V, Umfress AC, Jarrell KL, Cooke Bailey JN,
Agarwal A, et al: The carnitine shuttle pathway is altered in
patients with neovascular age-related macular degeneration. Invest
Ophthalmol Vis Sci. 59:4978–4985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Werner E, Wang H and Doetsch PW: Opposite
roles for p38MAPK-driven responses and reactive oxygen species in
the persistence and resolution of radiation-induced genomic
instability. PLoS One. 9:e1082342014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai J, Yang F, Dong L and Zheng Y: Ghrelin
protects human lens epithelial cells against oxidative
stress-induced damage. Oxid Med Cell Longev. 2017:19104502017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akasaki Y, Alvarez-Garcia O, Saito M,
Caramés B, Iwamoto Y and Lotz MK: FoxO transcription factors
support oxidative stress resistance in human chondrocytes.
Arthritis Rheumatol. 66:3349–3358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel H, Chen J and Kavdia M: Induced
peroxidase and cytoprotective enzyme expressions support adaptation
of HUVECs to sustain subsequent H2O2
exposure. Microvasc Res. 103:1–10. 2016. View Article : Google Scholar
|
|
22
|
Zhu L, Li J, Wu D and Li B: The protective
effect of beta-casomor-phin-7 via promoting Foxo1 activity and
nuclear translocation in human lens epithelial cells. Cutan Ocul
Toxicol. 37:267–274. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shamsi FA, Chaudhry IA, Boulton ME and
Al-Rajhi AA: L-carnitine protects human retinal pigment epithelial
cells from oxidative damage. Curr Eye Res. 32:575–584. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thrimawithana TR, Rupenthal ID, Räsch SS,
Lim JC, Morton JD and Bunt CR: Drug delivery to the lens for the
management of cataracts. Adv Drug Deliv Rev. 126:185–194. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan X, Marcano DC, Shin CS, Hua X,
Isenhart LC, Pflugfelder SC and Acharya G: Ocular drug delivery
nanowafer with enhanced therapeutic efficacy. ACS Nano.
9:1749–1758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao C, Fu MJ and Qiu LH: Molecular
cloning and functional characterization of cyclin E and CDK2 from
penaeus monodon. Genet Mol Res. 15:2016. View Article : Google Scholar
|
|
27
|
Kanska J, Zakhour M, Taylor-Harding B,
Karlan BY and Wiedemeyer WR: Cyclin E as a potential therapeutic
target in high grade serous ovarian cancer. Gynecol Oncol.
143:152–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chou HC, Wen LL, Chang CC, Lin CY, Jin L
and Juan SH: From the cover: l-Carnitine via PPARγ- and
Sirt1-dependent mechanisms attenuates epithelial-mesenchymal
transition and renal fibrosis caused by perfluorooctanesulfonate.
Toxicol Sci. 160:217–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baierle M, Nascimento SN, Moro AM, Brucker
N, Freitas F, Gauer B, Durgante J, Bordignon S, Zibetti M, Trentini
CM, et al: Relationship between inflammation and oxidative stress
and cognitive decline in the institutionalized elderly. Oxid Med
Cell Longev. 2015:8041982015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dogru M, Kojima T, Simsek C and Tsubota K:
Potential role of oxidative stress in ocular surface inflammation
and dry eye disease. Invest Ophthalmol Vis Sci. 59:DES163–DES168.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hua X, Chi W, Su L, Li J, Zhang Z and Yuan
X: ROS-induced oxidative injury involved in pathogenesis of fungal
keratitis via p38 MAPK activation. Sci Rep. 7:104212017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu D, Zhu H, Fu Q, Xu S, Sun W, Chen G and
Lv X: Ketamine delays progression of oxidative and damaged cataract
through regulating HMGB-1/NF-κB in lens epithelial cells.
Immunopharmacol Immunotoxicol. 40:303–308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia Z, Song Z, Zhao Y, Wang X and Liu P:
Grape seed proanthocyanidin extract protects human lens epithelial
cells from oxidative stress via reducing NF-κB and MAPK protein
expression. Mol Vis. 17:210–217. 2011.PubMed/NCBI
|
|
34
|
Yao K, Ye P, Zhang L, Tan J, Tang X and
Zhang Y: Epigallocatechin gallate protects against oxidative
stress-induced mitochondria-dependent apoptosis in human lens
epithelial cells. Mol Vis. 14:217–223. 2008.PubMed/NCBI
|