Introduction
Lung carcinoma is one of the most common malignant
tumors worldwide; its incidence is secondary to prostate cancer
among men and to breast cancer among women (1). A previous study released by the
International Agency for Research on Cancer reported that the
incidence rate of lung cancer was 23.1/100,000 and its mortality
rate was 19.7/100,000 in 2012 (2). A previous study also suggested that
lung cancer was the most common and fatal cancer in China in 2015
(3). The annual mortality rate of
lung cancer is higher than that caused by colon cancer, breast
cancer and prostate cancer in total (4). Lung cancer can be divided into two
main types: Small cell lung cancer and non-small cell lung cancer;
these types are characterized by cell size and type. Non-small cell
lung cancer accounts for ~80% of lung cancer cases, leading to
~900,000 deaths worldwide on an annual basis (5). Non-small cell lung cancer is
normally classified into three types: Squamous cell carcinoma,
adenocarcinoma and undifferentiated large cell carcinoma. Various
approaches have been adopted to treat lung cancer, including
surgery, radiation therapy, chemotherapy and molecular targeted
therapy (6). However, the exact
pathogenesis of and mechanisms underlying non-small cell lung
cancer remain unclear.
Molecular biology has revealed that the majority of
tumor tissues exhibit different degrees of hypoxic cells, and that
hypoxic cells are resistant to radiation, which may induce failure
of tumor radiotherapy and recurrence (7). Although scientists have applied
direct or indirect methods to increase oxygen content in tumors to
overcome hypoxic conditions, the therapeutic effect remains
unsatisfactory (8-10). Therefore, the development and
exploration of tumor hypoxic cell radiosensitizers has attracted
much attention from researchers in the field of tumor
radiotherapy.
Cyclocarya paliurus (CP), a member of the
Juglandaceae family, is a unique species and an endangered plant in
China (11). CP polysaccharide
(CPP) is a heteropolysaccharide and contains protein (8.44%), 17
amino acids and 18 mineral elements (12). CP has previously been reported to
possess anti-oxidant effect (12). Furthermore, CPP has garnered much
interest in fields of antihypertensive, hypoglycemic, antioxidant
and anticancer research (13).
Modern pharmacological studies have demonstrated that CPP possesses
significant hypolipemic, hypoglycemic (14) and antitumor activity (13). However, to the best of our
knowledge, only one study has been conducted regarding the
radiosensitizing effect of polysaccharides on lung cancer (15).
Hypoxia-inducible factor-1α (HIF-1α) is a
well-studied oxygen regulatory factor (16). Oxygen concentration is able to
regulate the expression levels of relevant genes (17), and low oxygen concentration
affects the malignant phenotype of tumors. Mammalian target of
rapamycin (mTOR) and survivin are highly expressed in malignant
tumors and are closely associated with tumor apoptosis (18). Furthermore, the expression levels
of mTOR and survivin are associated with low oxygen status in
tumors (19,20). Numerous studies have reported that
the growth of tumor cells is closely related to the
phosphati-dylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt/mTOR and
HIF-1α/survivin pathways (21-23). Therefore, mTOR, HIF-1α and
survivin are often considered as targets for tumor therapy.
However, the accurate mechanisms underlying the modulatory effects
of PI3K/Akt/mTOR and HIF-1α/survivin pathways on the proliferation
and apoptosis of non-small cell lung carcinoma cells remain
unknown.
This study assessed the association between CPP and
the radiosensitivity of hypoxic A549 and H520 non-small cell lung
carcinoma cells. Furthermore, the exact roles and mechanisms of CPP
in combination with radiation on the growth and apoptosis of
hypoxic A549 and H520 non-small lung carcinoma cells were
investigated.
Materials and methods
Cell culture and reagents
Human non-small cell lung carcinoma cell lines (A549
and H520) were acquired from the Cell Bank of Chinese Academy of
Sciences. A549 and H520 cells were incubated in RPMI 1640 medium
(cat. no. 61870044; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; cat. no. 10099141;
Gibco; Thermo Fisher Scientific, Inc.) in an environment containing
5% CO2 at 37°C. CP was obtained from Institute of
Materia Medica, CAMS & PUMC. CPP was extracted from CP as
previously described (24). The
proportion of CPP content was ~8.11% in the CP leaves, and CPP
accounted for 75.34% of the total sugar content.
GroupingControl
In the control group, normal A549 and H520 cells
were treated with PBS at 37°C for 48 h.
Hypoxia group
After being cultured under normoxic conditions for
24 h, cells in the hypoxia group were placed in a sterile and
closed hypoxia modular incubator chamber (Billups-Rothenberg,
Inc.). The hypoxic conditions were controlled using a high/low
oxygen control system (ProOx 110; BioSpherix, Ltd.), which can
automatically inject nitrogen to reduce oxygen concentration. The
cells were maintained in a humidified atmosphere containing 5%
CO2 and 1% O2 at 37°C for 30 min, and were
then transferred to normoxic conditions. After culturing for 24 h
at 37°C, the cells were collected and used in subsequent
experiments.
CPP/hypoxia group
Cells in the logarithmic growth phase were
inoculated into 6-well plates (~130 cells/well). RPMI-1640 medium
without serum was added to the cells, and 3 ml CPP solution was
then added to the cells (A549 cells, 100 nmol/l; H520, 60 nmol/l).
After 24 h, the cell medium was replaced with RPMI-1640 containing
10% FBS. The plates were placed in a hypoxic incubator and
maintained under hypoxic conditions for 30 min. Subsequently, cells
were incubated in a normoxic incubator. After culturing for 24 h at
37°C, the cells were collected and used in subsequent
experiments.
X-ray/hypoxia group
Cells in the logarithmic growth phase were
inoculated into 6-well plates (~130 cells/well). The plates were
then placed in a hypoxic incubator and maintained under hypoxic
conditions for 30 min at 37°C. Subsequently, cells were incubated
in a normoxic incubator. After culturing for 2 h at 37°C, the cells
were irradiated with 6 MV-X radiation at a dose rate of 400 cGy/min
using TrueBeam (Varian Medical Systems) for 1 h. The irradiation
field was 20×20 cm and the source skin distance (SSD) was 100 cm,
as previously described (25).
After culturing for 24 h at 37°C, the cells were collected and used
in subsequent experiments.
CPP/X-ray/hypoxia group
Cells in the logarithmic growth phase were
inoculated into 6-well plates (~130 cells/well). Subsequently, 3 ml
CPP solution was added to the cells (A549 cells, 100 nmol/l; H520,
60 nmol/l) and they were cultured in a normoxic incubator for 24 h
at 37°C, the cell medium was then replaced with RPMI-1640 that
contained 10% FBS. The plates were then maintained under hypoxic
conditions for 30 min at 37°C. Subsequently, cells were incubated
in a normoxic incubator. After culturing for 2 h at 37°C, the cells
were irradiated with 6 MV-X radiation at a dose rate of 400 cGy/min
for 1 h. The irradiation field was 20×20 cm and the SSD was 100 cm.
After culturing for 24 h at 37°C, the cells were collected and used
in subsequent experiments.
Cell viability analysis
Viability of hypoxic A549 and H520 cells was
analyzed using the Cell Counting kit-8 (CCK-8; cat. no. C0038;
Beyotime Institute of Biotechnology). Approximately
1×103 cells/well cultured hypoxic A549 and H520 cells in
the logarithmic phase were seeded into 96-well plates (cat. no.
FPT011; Beyotime Institute of Biotechnology) and were then
maintained in an environment containing 5% CO2 at 37°C
for 12 h. After being treated with 10, 20, 50, 100, 200 and 400
nmol/l CPP, A549 and H520 cells were then maintained at 37°C for 24
h. Subsequently, 10 µl CCK-8 reagent was added to the wells
of 96-well plates and the cells were incubated for 3 h at 37°C. A
microplate reader (Bio-Rad Laboratories, Inc.) was used to record
the absorbance at 450 nm. The inhibition ratio was calculated
according to the following equation: Inhibition ratio (%) =
(1-absorbance of experimental group/absorbance of blank control
group) × 100%.
Apoptosis analysis
The apoptosis of A549 and H520 cells was evaluated
using flow cytometry (FCM). After being washed with PBS, cultured
cells were trypsinized in 0.25% trypsin (cat. no. C0201; Beyotime
Institute of Biotechnology). Subsequently, the supernatant was
discarded and A549 and H520 cells prepared for detection were
suspended in 1X Annexin binding buffer (Thermo Fisher Scientific,
Inc.) at a final density of 1×106 cells/ml. The cells
were then incubated with 1X Annexin V-FITC and 100 µg/ml
propidium iodide (cat. nos. C1063, ST512; Beyotime Institute of
Biotechnology) in the dark for 15 min at room temperature. A
FACSCalibur flow cytometer (BD Biosciences) equipped with CellQuest
software (version 3.3; BD Biosciences) was used to detect cell
apoptosis.
Colony formation assay
Following irradiation, the cells in culture medium
were incubated in an incubator containing 5% CO2 at 37°C
for 14 days. Subsequently, the culture medium was discarded, and
the cells were washed with PBS and fixed with ethanol (cat. no.
A112719; Aladdin Shanghai Biochemical Technology Co., Ltd.) for 20
min at room temperature. The cells were then stained with 0.1%
crystal violet (cat. no. C0121; Beyotime Institute of
Biotechnology) for 20 min at room temperature, and washed three
times with PBS. The number of colonies containing ≥5 cells was
counted in a 6-well culture plate under a low magnification
confocal microscope, and cell colony formation rate was calculated
according to the formula: Colony formation rate = number of
colonies formed following treatment/number of cells inoculated ×
100%.
Western blot analysis
Proteins were extracted using NP40 lysis buffer
(Beyotime Institute of Biotechnology) and the bicinchoninic acid
assay was applied to determine protein concentration. Proteins (20
µg) were separated by 10% SDS-PAGE and then transferred onto
a PVDF membrane (cat. no. FFP28; Beyotime Institute of
Biotechnology). The membrane was blocked using 5% skimmed milk at
room temperature for 60 min and was then incubated with the
following rabbit anti-human primary antibodies at 4°C for 12 h:
Anti-HIF-1α (1:500; cat. no. ab51608; Abcam); anti-survivin
(1:5,000; cat. no. ab76424; Abcam); anti-cleaved caspase-3 (1:500;
cat. no. ab2302; Abcam); anti-phosphorylated (p)-mTOR (1:1,000;
cat. no. ab109268; Abcam); anti-mTOR (1:2,000; cat. no. ab2732;
Abcam); anti-p-Akt (1:500; cat. no. ab38449; Abcam); anti-Akt
(1:500; cat. no. ab8805; Abcam); anti-p-PI3K (1:1,000; cat. no.
ab182651; Abcam); anti-PI3K (1:1,000; cat. no. ab191606; Abcam);
and anti-GAPDH (1:2,500; cat. no. ab9485, Abcam). The membrane was
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (1:5,000; cat. no. ab205718;
Abcam) at room temperature for 1 h. The bands were visualized by
enhanced chemiluminescence (EMD Millipore), and densitometry was
performed using the Bio-Rad ChemiDoc system with Image Lab software
version 6.0 (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Subsequently, RNA was reverse transcribed
into cDNA using a RT kit (cat. no. D7168L; Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. qPCR
analysis was performed using SYBR Premix Ex Taq™ Real-Time PCR kit
(Takara Bio, Inc.) on an ABI 7500 Thermocycler (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The PCR thermocycling conditions
were as follows: 4 min pretreatment at 94°C, followed by 30 cycles
at 94°C for 30 sec and 65°C for 30 sec, and a final extension step
at 72°C for 10 min; the samples were then maintained at 4°C. The
primers were designed by Invitrogen; Thermo Fisher Scientific,
Inc., as follows: HIF-1α, forward 5′-CAGTCGACACAGCCTGGATA-3′,
reverse 5′-CCACCTCTTTTGGCAAGCAT-3′ (product: 210 bp); survivin,
forward 5′-TAGCTGCACACCTGACAAGA-3′, reverse
5′-CCGTCAGCTCAGTGAAGTCT-3′ (product: 200 bp); and GAPDH, forward
5′-CCATCTTCCAGGAGCGAGAT-3′ and reverse 5′-TGCTGATGATCTTGAGGCTG-3′
(product: 222 bp). GAPDH was used as a control of the input RNA
level. Gene expression was quantified using the 2−ΔΔCq
method (26).
Statistical analysis
All experimental results are presented as the mean ±
SD for at least three independent experiments. The CCK-8, FCM,
RT-qPCR and western blotting data were analyzed by one-way ANOVA
followed by Tukey's test using IBM SPSS statistical software
(version 19; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
CPP evidently inhibits the viability of
hypoxic A549 and H520 cells
The present study detected the viability of hypoxic
A549 and H520 cells treated with various concentrations of CPP, in
order to investigate the antitumor effect of CPP. The results of
the CCK-8 assay revealed that the inhibition rates of hypoxic A549
cells treated with 50, 100, 200 and 400 nmol/l CPP were
significantly higher than those in the control group; the
IC50 value was calculated as 109.4 nmol/l (Fig. 1A and B; P<0.01). Similarly,
based on CCK-8 data, the inhibition rates of hypoxic H520 cells
treated with 20, 50, 100, 200 and 400 nmol/l CPP were significantly
higher than those in the control group; the IC50 value
was calculated as 67.4 nmol/l (Fig.
1C and D; P<0.01). These findings indicated that CPP could
significantly reduce the viability of hypoxic A549 and H520 cells
in a dose-dependent manner. Furthermore, the optimal concentrations
of CPP for hypoxic A549 and H520 cells were 100 and 60 nmol/l,
respectively; these concentrations were used in subsequent
experiments.
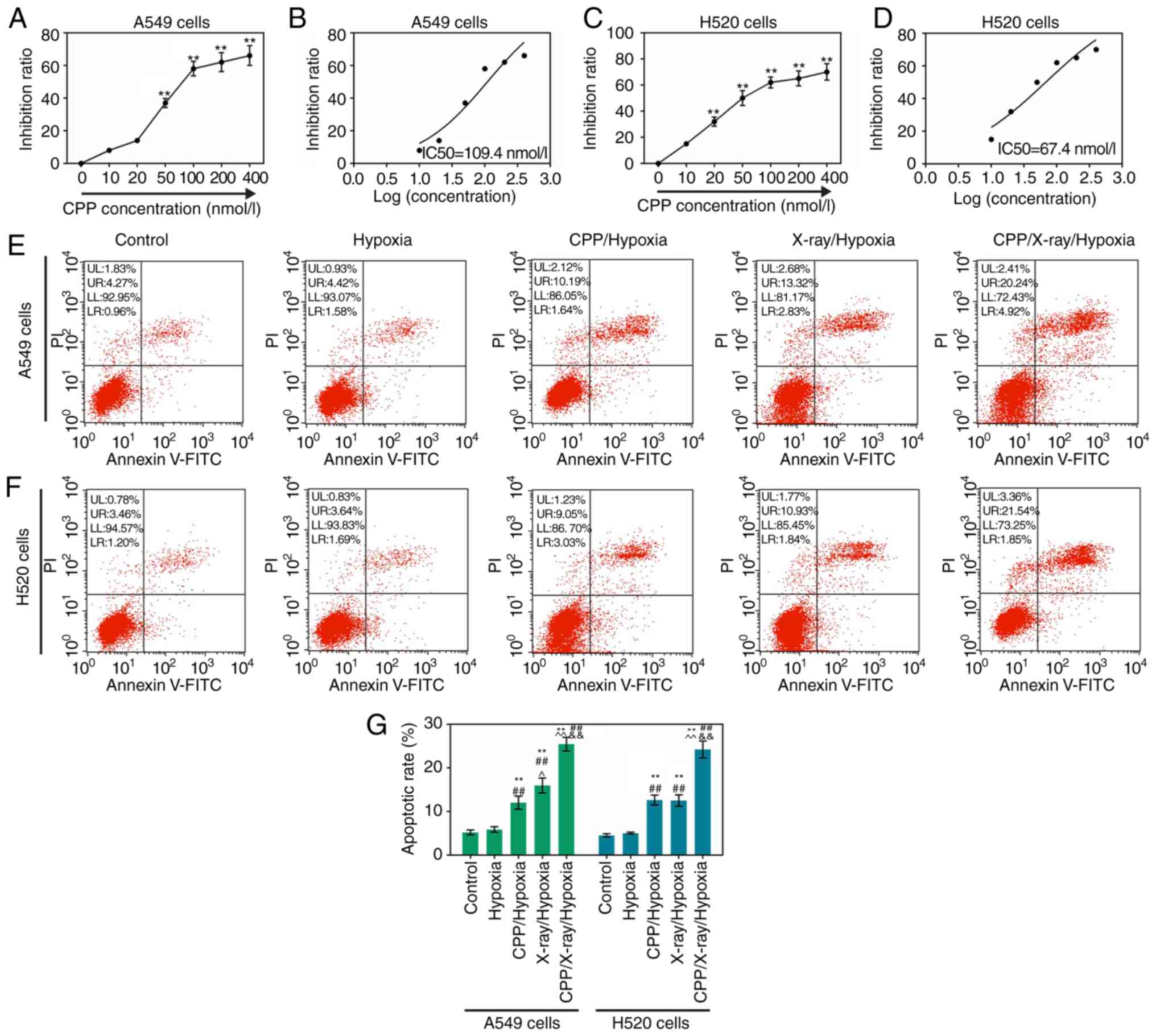 | Figure 1CPP inhibits the viability of hypoxic
A549 and H520 cells, and the apoptosis of hypoxic A549 and H520
cells is enhanced by CPP in combination with radiation. Cell
Counting kit-8 assays were performed on hypoxic (A and B) A549 and
(C and D) H520 cells treated with PBS, 10, 20, 50, 100, 200 and 400
nmol/l CPP. (E-G) Flow cytometry was performed to detect the
apoptosis of (E) A549 and (F) H520 cells in the Control, Hypoxia,
CPP/Hypoxia, X-ray/Hypoxia and CPP/X-ray/Hypoxia groups.
**P<0.01 vs. Control or 0 nmol/l;
##P<0.01 vs. Hypoxia; ^P<0.05 and
^^P<0.01 vs. CPP/Hypoxia;
&&P<0.01 vs. X-ray/Hypoxia. CPP,
Cyclocarya paliurus polysaccharide; PI, propidium
iodide. |
Apoptosis of hypoxic A549 and H520 cells
is enhanced by CPP in combination with radiation
Since CPP could obviously inhibit the viability of
hypoxic A549 and H520 cells, further studies were conducted to
determine whether CPP also affected the apoptosis of hypoxic A549
and H520 cells. The apoptosis of hypoxic A549 and H520 cells
treated with CPP and radiation was detected using FCM; the
apoptotic rate was determined as the sum of the right upper
quadrant and the right lower quadrant percentages. As shown in
Fig. 1E and G, the results
indicated that the proportion of apoptotic A549 cells in the
control group was 5.23%. Following treatment with hypoxia,
CPP/hypoxia, X-ray/hypoxia and CPP/X-ray/hypoxia, the apoptotic
rates of A549 cells were increased to 6.00, 11.83, 16.15 and
25.16%, respectively. In addition, the proportion of apoptotic H520
cells in the control group was 4.66%. Following treatment with
hypoxia, CPP/hypoxia, X-ray/hypoxia and CPP/X-ray/hypoxia, the
apoptotic rates of H520 cells were increased to 5.33, 12.08, 12.77
and 23.39%, respectively (Fig. 1F and
G). These findings indicated that, to some extent, CPP and
radiation could enhance the apoptotic capacities of hypoxic A549
and H520 cells. In addition, compared to CPP or radiation alone,
the combination of CPP and radiation significantly accelerated the
apoptosis of hypoxic A549 and H520 cells.
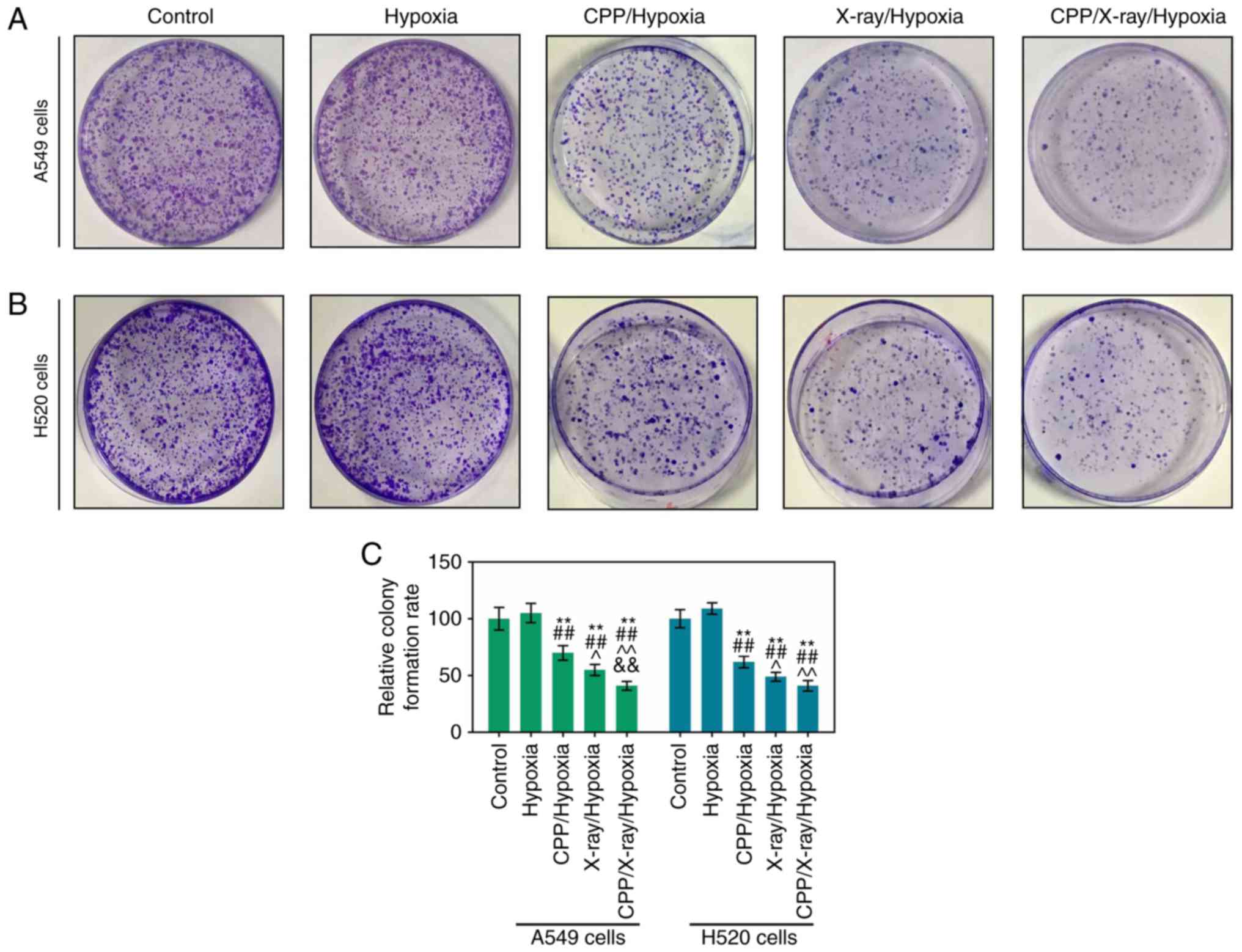
Proliferation of hypoxic A549 and H520
cells is suppressed by CPP in combination with radiation
This study demonstrated that the apoptotic
capacities of hypoxic A549 and H520 cells were markedly enhanced by
CPP in combination with radiation; therefore, the proliferative
abilities of hypoxic A549 and H520 cells treated with CPP and X-ray
were further detected. Colony formation assay results (Fig. 2A and B) revealed that CPP and
X-ray could, to some extent, reduce the proliferation of hypoxic
A549 and H520 cells. Furthermore, a noticeable reduction in the
proliferative abilities of hypoxic A549 and H520 cells was
determined in response to treatment with CPP and X-ray compared
with CPP or radiation alone (Fig.
2A-C; P<0.05).
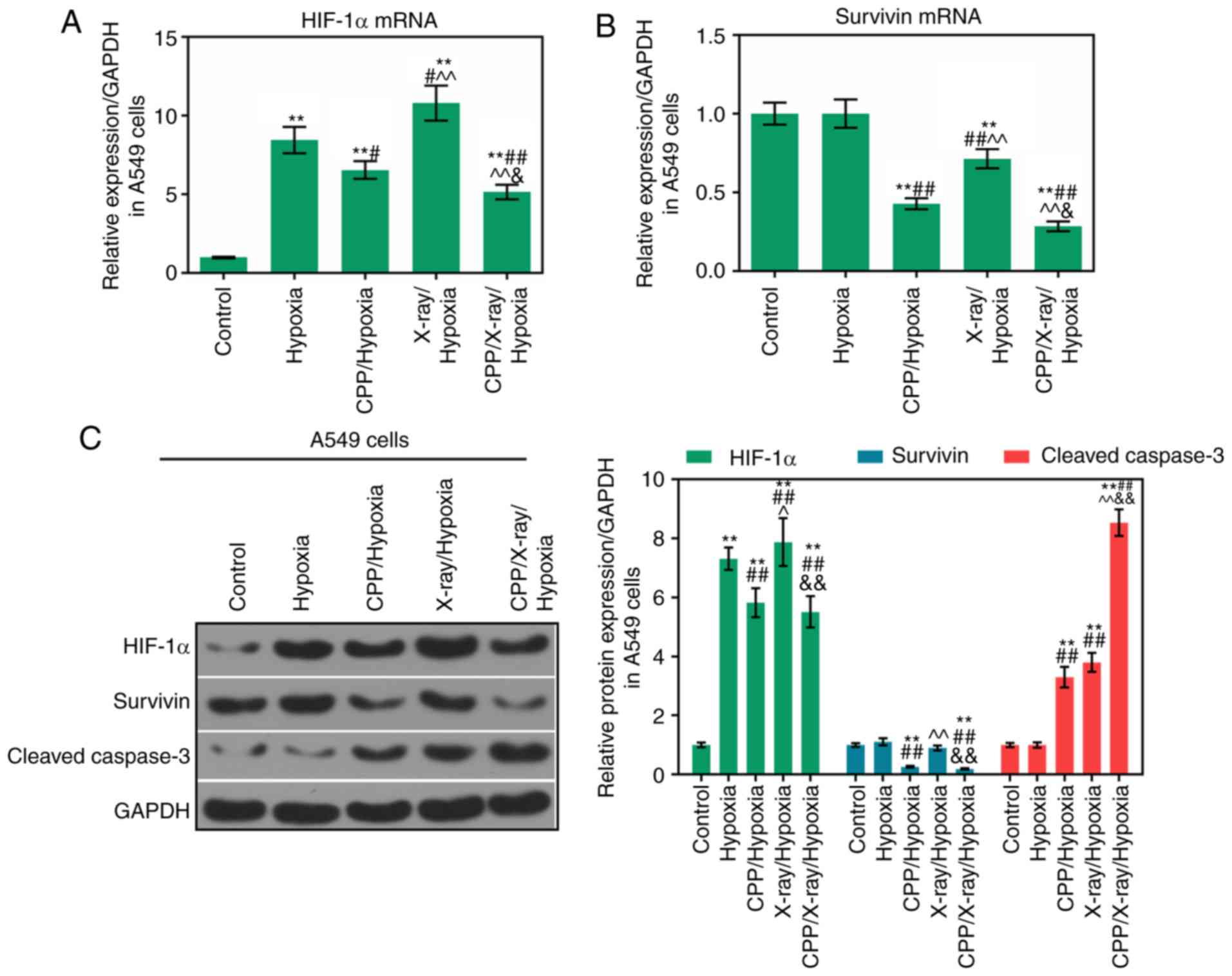
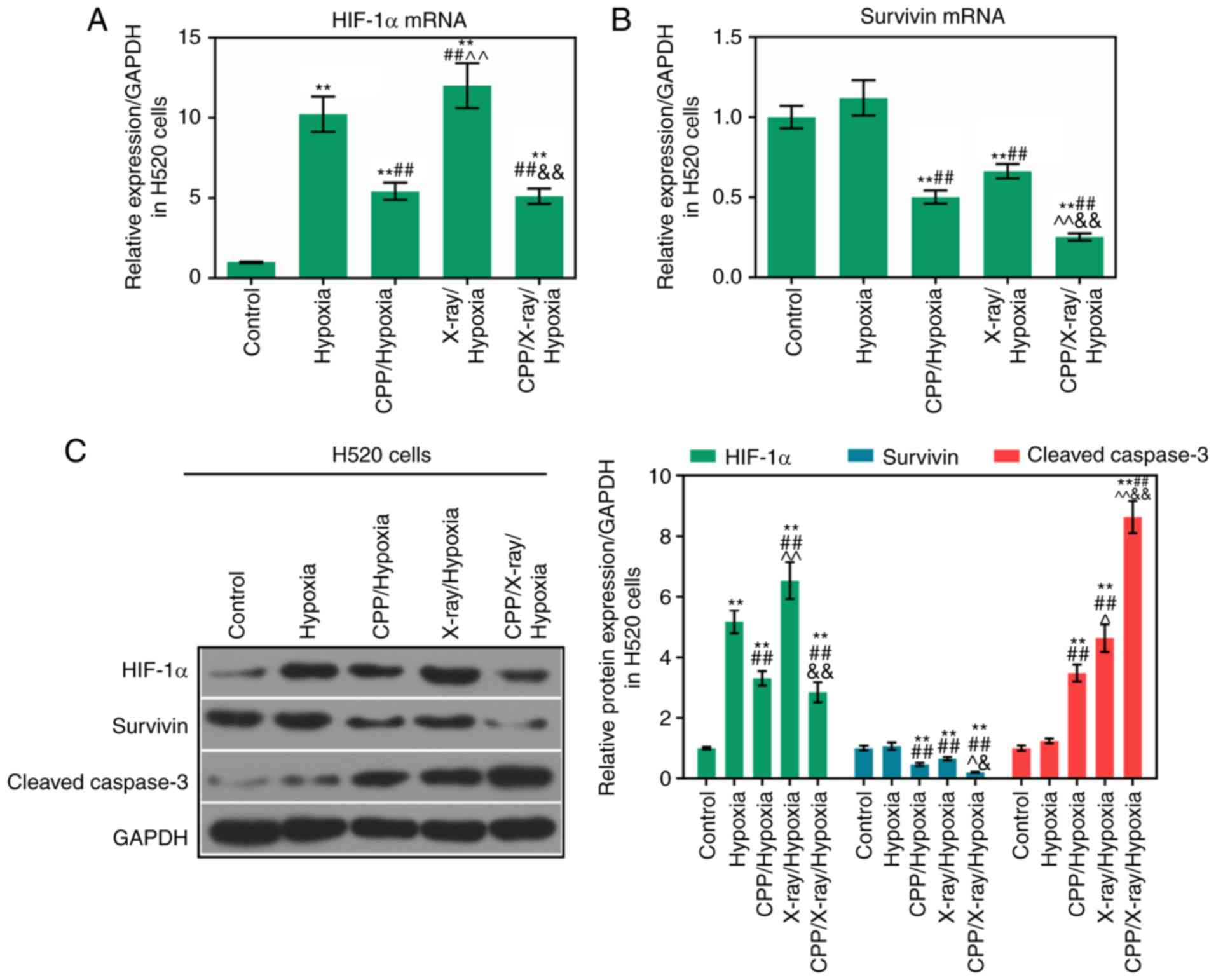
Expression levels of HIF-1α, survivin and
cleaved caspase-3 are modulated by CPP in combination with
radiation
To investigate the relevant mechanisms through which
CPP in combination with radiation affected the apoptosis and
proliferation of hypoxic A549 and H520 cells, the expression levels
of HIF-1α, survivin and cleaved caspase-3 were determined in A549
and H520 cells from each group. According to the RT-qPCR results,
compared to CPP or radiation alone, the mRNA expression levels of
HIF-1α and survivin were significantly decreased in hypoxic A549
and H520 cells following treatment with CPP in combination with
X-ray (Figs. 3A and B, and
4A and B; P<0.05). According
to western blot analysis, compared to CPP or radiation alone,
HIF-1α and survivin protein levels were significantly decreased in
hypoxic A549 and H520 cells following treatment with CPP in
combination with X-ray (Figs. 3C
and 4C; P<0.05). In addition,
compared to CPP or radiation alone, CPP in combination with X-ray
markedly promoted the activation of caspase-3 in hypoxic A549 and
H520 cells (Figs. 3C and 4C; P<0.05). These findings suggested
that CPP in combination with X-ray markedly downregulated the
expression levels of HIF-1α and survivin, and upregulated cleaved
caspase-3 expression in hypoxic A549 and H520 cells. Therefore, CPP
in combination with X-ray may affect the apoptosis and
proliferation of hypoxic A549 and H520 cells via modulating the
expression levels of HIF-1α, survivin and cleaved caspase-3.
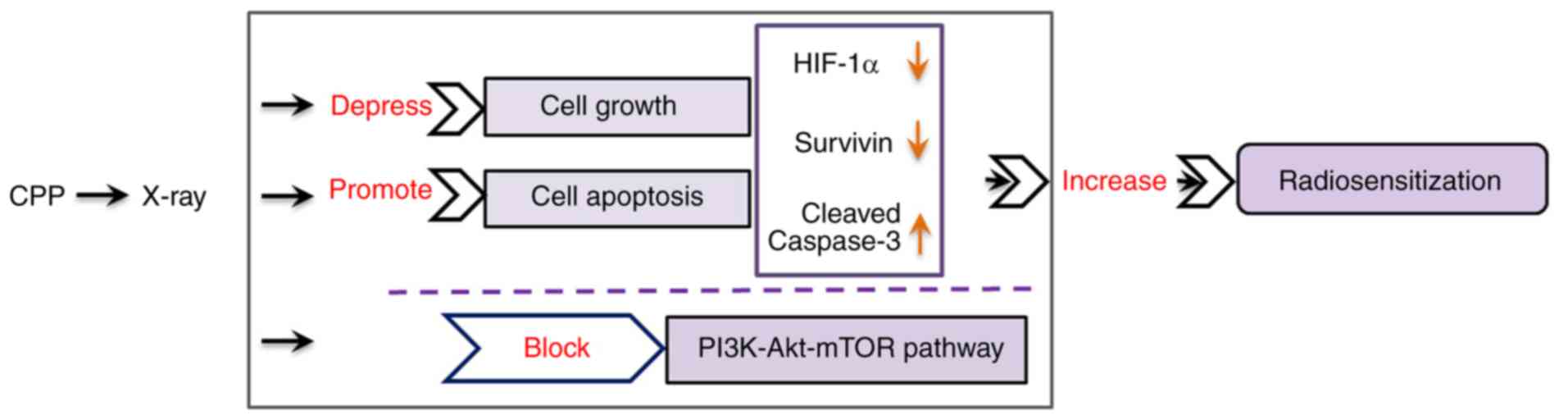
CPP in combination with radiation affects
the mTOR/Akt/PI3K pathway
To further investigate the mechanisms underlying the
effects of the combination of CPP and radiation on hypoxic A549 and
H520 cells, further studies were conducted on the mTOR/Akt/PI3K
signaling pathway. Western blotting revealed that the expression
levels of p-mTOR, p-Akt and p-PI3K were significantly lower in
hypoxic A549 cells treated with CPP in combination with X-ray
compared with in the X-ray/hypoxia group (Fig. 5A-D; P<0.05). Furthermore, the
expression levels of p-mTOR, p-Akt and p-PI3K were significantly
downregulated in hypoxic H520 cells following treatment with CPP in
combination with X-ray compared with in the X-ray/hypoxia group
(Fig. 5E-H; P<0.05). These
data indicated that CPP in combination with radiation could inhibit
the phosphorylation of mTOR, Akt and PI3K in hypoxic A549 and H520
cells. The schematic representation of the findings of this study
is presented in Fig. 6.
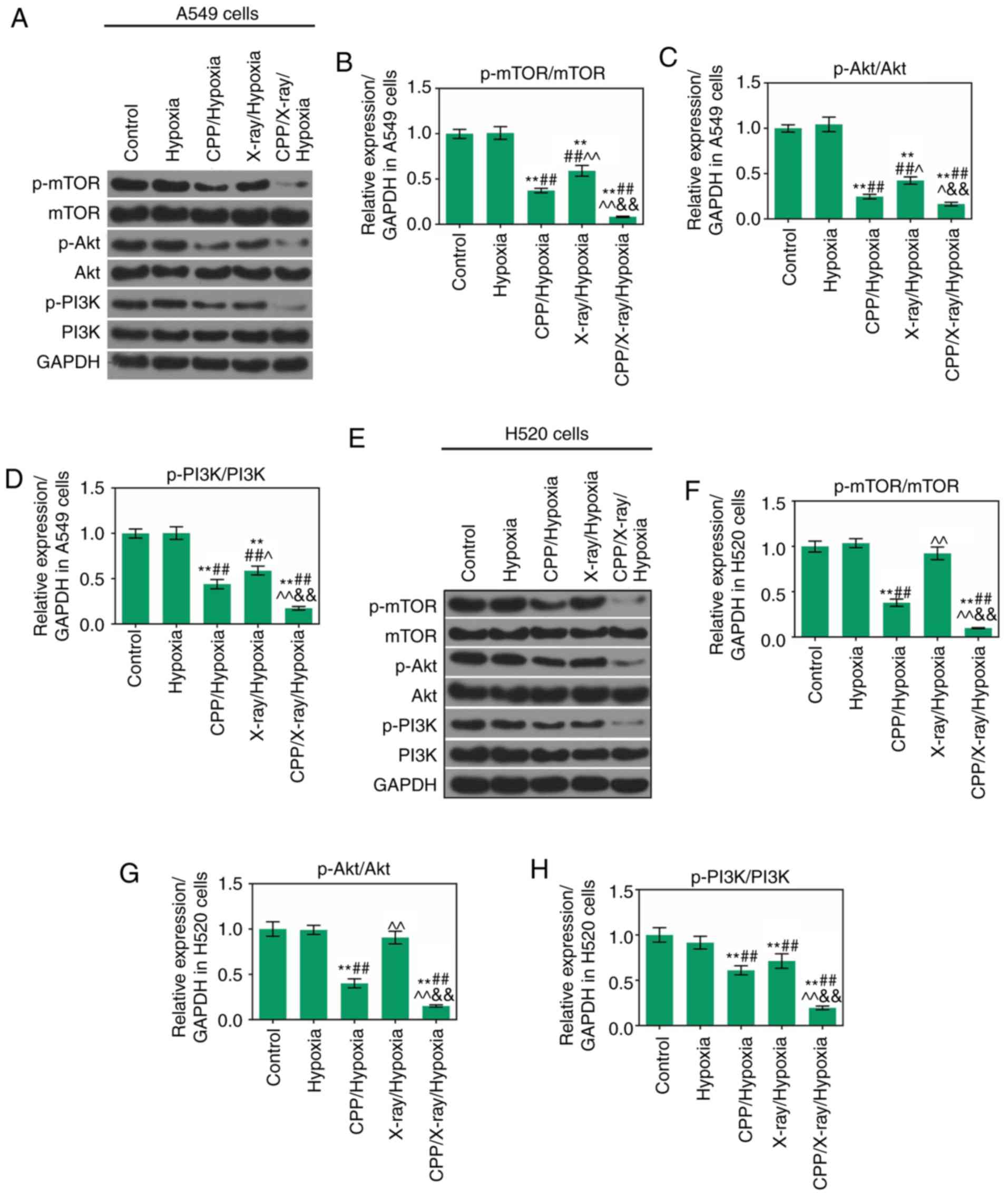 | Figure 5CPP in combination with radiation
affects the mTOR/Akt/PI3K pathway. Western blotting was carried out
to assess the expression levels of p-mTOR, mTOR, p-Akt, Akt, p-PI3K
and PI3K in (A-D) A549 and (E-H) H520 cells in the Control,
Hypoxia, CPP/Hypoxia, X-ray/Hypoxia and CPP/X-ray/Hypoxia groups.
**P<0.01 vs. Control; ##P<0.01 vs.
Hypoxia; ^P<0.05 and ^^P<0.01 vs.
CPP/Hypoxia; &&P<0.01 vs. X-ray/Hypoxia. CPP,
Cyclocarya paliurus polysaccharide; mTOR, mammalian target
of rapamycin; p-, phosphorylated; PI3K,
phosphatidylinositol-4,5-bisphosphate 3-kinase. |
Discussion
Radiotherapy is a widely adopted method used to
treat non-small cell lung cancer; however, due to the resistance of
tumor cells to radiation, locally recurrent or residual tumor cells
hinder the efficacy of radiotherapy. Increasing the sensitivity of
tumor cells to radiation has become the goal of radiotherapy
(27-29). Radiosensitizers have therefore
been developed and clinically applied; however, their application
is not as satisfactory as expected because they are largely toxic
and associated with side effects (30-32). It is of great clinical value to
develop highly effective sensitizing drugs with low toxicity.
Various traditional Chinese medicines and their extracts, for
example Lycium barbarum polysaccharide and irisquinone, have
been reported to possess antitumor and radiosensitizing effects
(15,33). CPP is polysaccharide contained in
CP, which has been shown to possess numerous bioactivities,
including antioxidant, hypolipemic, hypoglycemic, antitumor and
immunomodulatory effects (13).
However, the radiosensitizing effects of CPP on tumor cells remain
unclear. Therefore, CPP was selected as the research focus of this
investigation. The IC50 values of CPP on A549 and H520
cells after 24 h were determined by CCK-8 assay; the results of
which were 109.4 and 67.4 nmol/l, respectively. Therefore, 100 and
60 nmol/l CPP was used to treat hypoxic A549 and H520 cells in
subsequent experiments, respectively. In order to investigate the
radiosensitizing effects of CPP on hypoxic A549 and H520 cells, the
apoptotic capacities of hypoxic A549 and H520 cells treated with
CPP and X-ray were further assessed. The FCM results revealed that
in response to the same irradiation dose, the apoptotic rate of
cells treated with CPP in combination with radiation was higher
than that in the radiation only group. Furthermore, the
proliferative abilities of hypoxic A549 and H520 cells treated with
CPP and X-ray were determined. The results of a colony formation
assay demonstrated that CPP in combination with radiation markedly
suppressed the proliferation of hypoxic A549 and H520 cells. These
findings indicated that CPP could significantly enhance the
radiosensitization of hypoxic lung cancer cells.
Hypoxia is a critical feature of solid tumors and is
represented by decreased oxygen content caused by oxygen diffusion
disorders, poor blood flow and blockage (34,35). Molecular biology studies have
reported that the majority of tumor tissues possess hypoxic cells.
The resistance of hypoxic cells to radiation is also a direct cause
of the failure of tumor radiotherapy, which may result in
recurrence. HIF-1α is a well-studied gene in hypoxia. At low oxygen
levels, the oxygen-dependent degradation domain of HIF-1α is unable
to undergo hydroxylation and ubiquitination; therefore, the HIF-1α
protein is not degraded and is more stable (36-38). Previous studies have suggested
that hypoxia is associated with mTOR and survivin. Under short-term
and moderate hypoxic conditions, the mechanism of mTOR upregulation
may be associated with regulation of the 5′AMP-activated protein
kinase pathway (39). In the
present study, CPP combined with radiation treatment enhanced
radiosensitivity of hypoxic A549 and H520 by blocking the
PI3K/Akt/mTOR signaling pathway. In experiments conducted on human
breast cancer cells and pancreatic cancer cells in vitro,
researchers have demonstrated that the mRNA expression levels of
survivin are increased under hypoxic conditions, compared with in
normoxic group cells (40). In
summary, mTOR, HIF-1α and survivin are oxygen-regulating molecules,
and hypoxia can promote their expression. Therefore, this study
evaluated the expression levels of HIF-1α, survivin, cleaved
caspase-3 and the mTOR/Akt/PI3K pathway in hypoxic A549 and H520
cells treated with CPP and X-ray. RT-qPCR and western blotting
demonstrated that CPP in combination with radiation significantly
enhanced the expression levels of cleaved caspase-3, and reduced
HIF-1α and survivin expression in hypoxic A549 and H520 cells.
These outcomes suggested that CPP in combination with radiation
affected the apoptosis and proliferation of hypoxic A549 and H520
cells via modulating the expression levels of HIF-1α, survivin and
cleaved caspase-3. Furthermore, this study demonstrated that CPP in
combination with radiation markedly suppressed the phosphorylation
of mTOR, Akt and PI3K in hypoxic A549 and H520 cells. These results
indicated that CPP may enhance the radiosensitivity of A549 and
H520 cells via regulating the mTOR/Akt/PI3K pathway; however, the
detailed mechanisms underlying the involvement of the mTOR/Akt/PI3K
pathway in the radiosensitizing effect of CPP require a further
investigation.
Taken together, this study demonstrated that CPP
enhanced radiosensitization of hypoxic lung cancer cells through
accelerating apoptosis and suppressing proliferation of hypoxic
A549 and H520 cells. In addition, inactivation of the mTOR/Akt/PI3K
pathway may be a possible mechanism underlying the radiosensitizing
effect of CPP. Previous studies (13,41) have suggested that CPP may inhibit
MGC-803 cancer cell growth. Therefore, the present results were in
line with the results of previous studies. Enhanced
radiosensitizers in cancer cells (such as nitric oxide) may explain
the increased radiosensitization in lung cancer cells treated with
CPP. However, a previous study revealed that CPP is able to protect
against oxidative stress in RAW264.7 cells (42). It was hypothesized that the
differences may be related to the cell type and cell context. In
summary, the present study provided novel information, which may
improve understanding of the pathogenesis of non-small cell lung
carcinoma, and may identify a potential approach for treating
non-small cell lung carcinoma.
There are some limitations to this study; for
example, although the hypoxic microenvironment of solid tumors in
the body was simulated in tumor cells through maintenance in a
humidified incubator containing 5% CO2 and 1%
O2, whether the radiosensitizing effect of CPP can be
applied to solid non-small cell lung carcinoma tumors remains to be
determined. In addition, the effects of CPP on human non-small cell
lung cancer under normoxic conditions must be further
elucidated.
In conclusion, this study revealed that CPP enhanced
the radiosensitivity of hypoxic A549 and H520 cells, and indicated
that CPP may be an effective agent for the treatment of non-small
cell lung carcinoma.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ made substantial contributions to study
conception and design. LM and BF acquired, analyzed and interpreted
the data. All authors drafted the article, critically revised it
for important intellectual content and gave final approval of the
version to be published. BF and LM agree to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giroux Leprieur E, Dumenil C, Julie C,
Giraud V, Dumoulin J, Labrune S and Chinet T: Immunotherapy
revolutionises non-small-cell lung cancer therapy: Results,
perspectives and new challenges. Eur J Cancer. 78:16–23. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garrison GW: Lung cancer screening. Cancer
Cytopathol. 124:533–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Remon J, Besse B and Soria JC: Successes
and failures: What did we learn from recent first-line treatment
immunotherapy trials in non-small cell lung cancer? BMC Med.
15:552017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, et al: Systemic therapy for stage IV non-small-cell lung
cancer: American society of clinical oncology clinical practice
guideline update. J Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bjørnetrø T, Handeland KR, Meltzer S,
Samiappan R, Lyckander LG, Jegerschöld C, Sønstevold L, Thusyanthan
NS, Redalen KR and Ree AH: Low release of exosomal miR-663a from
hypoxic tumor cells and poor tumor response to neoadjuvant
radiotherapy in rectal cancer patients. Cancer Res.
77:45142017.
|
|
8
|
Horsman MR and Overgaard J: The impact of
hypoxia and its modification of the outcome of radiotherapy. J
Radiat Res. 57(Suppl 1): i90–i98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song G, Ji C, Liang C, Song X, Yi X, Dong
Z, Yang K and Liu Z: TaOx decorated perfluorocarbon nanodroplets as
oxygen reservoirs to overcome tumor hypoxia and enhance cancer
radiotherapy. Biomaterials. 112:257–263. 2017. View Article : Google Scholar
|
|
10
|
Bonnet M, Hong CR, Wong WW, Liew LP, Shome
A, Wang J, Gu Y, Stevenson RJ, Qi W, Anderson RF, et al:
Next-generation hypoxic cell radiosensitizers: Nitroimidazole
alkylsulfonamides. J Med Chem. 61:1241–1254. 2018. View Article : Google Scholar
|
|
11
|
Xiao HT, Wen B, Ning ZW, Zhai LX, Liao CH,
Lin CY, Mu HX and Bian ZX: Cyclocarya paliurus tea leaves enhances
pancreatic β cell preservation through inhibition of apoptosis. Sci
Rep. 7:91552017. View Article : Google Scholar
|
|
12
|
Xie JH, Xie MY, Nie SP, Shen MY, Wang YX
and Li C: Isolation, chemical composition and antioxidant
activities of a water-soluble polysaccharide from Cyclocarya
paliurus (Batal.) Iljinskaja. Food Chem. 119:1626–1632. 2010.
View Article : Google Scholar
|
|
13
|
Xie JH, Liu X, Shen MY, Nie SP, Zhang H,
Li C, Gong DM and Xie MY: Purification, physicochemical
characterisation and anticancer activity of a polysaccharide from
Cyclocarya paliurus leaves. Food Chem. 136:1453–1460. 2013.
View Article : Google Scholar
|
|
14
|
Li S, Li J, Guan XL, Li J, Deng SP, Li LQ,
Tang MT, Huang JG, Chen ZZ and Yang RY: Hypoglycemic effects and
constituents of the barks of Cyclocarya paliurus and their
inhibiting activities to glucosidase and glycogen phosphorylase.
Fitoterapia. 82:1081–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu CX and Cheng BQ: Radiosensitizing
effects of Lycium barbarum polysaccharide for Lewis lung cancer.
Zhong Xi Yi Jie He Za Zhi. 11:611–612. 5821991.In Chinese.
|
|
16
|
Meng X, Grötsch B, Luo Y, Knaup KX,
Wiesener MS, Chen XX, Jantsch J, Fillatreau S, Schett G and Bozec
A: Hypoxia-inducible factor-1α is a critical transcription factor
for IL-10-producing B cells in autoimmune disease. Nat Commun.
9:2512018. View Article : Google Scholar
|
|
17
|
Szalowska E, Stoopen G, Rijk JC, Wang S,
Hendriksen PJ, Groot MJ, Ossenkoppele J and Peijnenburg AA: Effect
of oxygen concentration and selected protocol factors on viability
and gene expression of mouse liver slices. Toxicol In Vitro.
27:1513–1524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou K, Tong E, Xu Y, Deng X and Zou L:
Down regulation of mammalian target of rapamycin decreases HIF-1α
and survivin expression in anoxic lung adenocarcinoma A549 cell to
elemene and/or irradiation. Tumour Biol. 35:9735–9741. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peery RC, Liu JY and Zhang JT: Targeting
survivin for therapeutic discovery: Past, present, and future
promises. Drug Discov Today. 22:1466–1477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakahara T, Morita A, Yagasaki R, Mori A
and Sakamoto K: Mammalian target of rapamycin (mTOR) as a potential
therapeutic target in pathological ocular angiogenesis. Biol Pharm
Bull. 40:2045–2049. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bahrami A, Hasanzadeh M, Hassanian SM,
ShahidSales S, Ghayour-Mobarhan M, Ferns GA and Avan A: The
potential value of the PI3K/Akt/mTOR signaling pathway for
assessing prognosis in cervical cancer and as a target for therapy.
J Cell Biochem. 118:4163–4169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao ZX, Yang YT, Yu S, Li YZ, Wang WW,
Huang J, Xie XF, Xiong L, Lei S and Peng C: Pogostone induces
autophagy and apoptosis involving PI3K/Akt/mTOR axis in human
colorectal carcinoma HCT116 cells. J Ethnopharmacol. 202:20–27.
2017. View Article : Google Scholar
|
|
23
|
Oh TI, Lee YM, Nam TJ, Ko YS, Mah S, Kim
J, Kim Y, Reddy RH, Kim YJ, Hong S and Lim JH: Fascaplysin exerts
anti-cancer effects through the downregulation of survivin and
HIF-1α and inhibition of VEGFR2 and TRKA. Int J Mol Sci. 18:pii:
E20742017. View Article : Google Scholar
|
|
24
|
Wang Z, Xie J, Yang Y, Zhang F, Wang S, Wu
T, Shen M and Xie M: Sulfated Cyclocarya paliurus polysaccharides
markedly attenuates inflammation and oxidative damage in
lipopolysaccharide-treated macrophage cells and mice. Sci Rep.
7:404022017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheki M, Shirazi A, Mahmoudzadeh A, Bazzaz
JT and Hosseinimehr SJ: The radioprotective effect of metformin
against cytotoxicity and genotoxicity induced by ionizing radiation
in cultured human blood lymphocytes. Mutat Res. 809:24–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Her S, Jaffray DA and Allen C: Gold
nanoparticles for applications in cancer radiotherapy: Mechanisms
and recent advancements. Adv Drug Deliv Rev. 109:84–101. 2017.
View Article : Google Scholar
|
|
28
|
Weichselbaum RR, Liang H, Deng L and Fu
YX: Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev
Clin Oncol. 14:365–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garcia-Aranda M, Téllez T, Muñoz M and
Redondo M: Clusterin inhibition mediates sensitivity to
chemotherapy and radiotherapy in human cancer. Anticancer Drugs.
28:702–716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Y, Sun W, Gong T, Chai Y, Wang J, Hui
B, Li Y, Song L and Gao Y: miR-30a radiosensitizes non-small cell
lung cancer by targeting ATF1 that is involved in the
phosphorylation of ATM. Oncol Rep. 37:1980–1988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Wang Y, Mei H, Yin Z, Geng Y,
Zhang T, Wu G and Lin Z: The BET bromodomain inhibitor JQ1
radiosensitizes non-small cell lung cancer cells by upregulating
p21. Cancer Lett. 391:141–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Mu X, He H and Zhang XD: Cancer
radiosensitizers. Trends Pharmacol Sci. 39:24–48. 2018. View Article : Google Scholar
|
|
33
|
Xu H, Sun G, Wang H, Yue Q, Tang H and Wu
Q: Dynamic observation of the radiosensitive effect of irisquinone
on rabbit VX2 lung transplant tumors by using
fluorine-18-deoxyglucose positron emission tomography/computed
tomography. Nucl Med Commun. 34:220–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brown JM and Le QT: Tumor hypoxia is
important in radiotherapy, but how should we measure it? Int J
Radiat Oncol Biol Phys. 54:1299–1301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toffoli S and Michiels C: Intermittent
hypoxia is a key regulator of cancer cell and endothelial cell
interplay in tumours. FEBS J. 275:2991–3002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bruick RK and Mcknight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berra E, Roux D, Richard DE and Pouysségur
J: Hypoxia-inducible factor-1 alpha (HIF-1 alpha) escapes
O(2)-driven proteasomal degradation irrespective of its subcellular
localization: Nucleus or cytoplasm. EMBO Rep. 2:615–620. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated
prolyl hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wouters BG and Koritzinsky M: Hypoxia
signalling through mTOR and the unfolded protein response in
cancer. Nat Rev Cancer. 8:851–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rödel F, Hoffmann J, Distel L, Herrmann M,
Noisternig T, Papadopoulos T, Sauer R and Rödel C: Survivin as a
radioresistance factor, and prognostic and therapeutic target for
radiotherapy in rectal cancer. Cancer Res. 65:4881–4887. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han C, Nie SP, Huang DF, Chen YQ, Xie JH
and Xie MY: Effects of polysaccharides from Cyclocarya paliurus
(Batal.) Iljinskjk on growth of MGC803 Cells. Nat Prod Res Dev.
21:952–955. 2009.
|
|
42
|
Xie JH, Wang ZJ, Shen MY, Nie SP, Gong B,
Li HS, Zhao Q, Li WJ and Yang G: Sulfated modification,
characterization and antioxidant activities of polysaccharide from
Cyclocarya paliurus. Food Hydrocoll. 53:7–15. 2016. View Article : Google Scholar
|