Introduction
Rheumatoid arthritis (RA), a chronic progressive
autoimmune disease, occurs primarily in the elderly and women. Its
pathological features include long-term inflammation of synovial
cells and chondrocytes leading to the destruction of bone and
joints (1). In the pathogenesis
of RA, tumor necrosis factor (TNF)-α and interleukin (IL)-6 are the
central pro-inflammatory factors able to induce the production of
matrix metalloproteinases (MMPs) (2,3).
Studies have indicated that RA is associated with increased protein
modification. Typical representatives are the production and
accumulation of advanced oxidation protein products (AOPPs) in
chondrocytes (4).
AOPPs are a class of protein cross-linking products
containing a double tyrosine, which arise mainly from activated
neutrophils during oxidative stress in vivo. Hypoxic acid
(HCLO) generated by myeloperoxidase is formed by active proteins
(mainly albumin), which is an oxidative stress protein marker and a
general term for end products produced by protein oxidation
(5). It has been found that the
levels of AOPPs in plasma increase with age (6).
AOPPs have been recognized as a hallmark of
oxidative stress and function as inflammatory mediators associated
with the pathophysiology of several disorders, including
accumulation in patients with arthritis (7,8).
Our previous studies confirmed that plasma AOPP levels were
inversely correlated with age-related changes in bone mass in
patients with RA and osteoporosis (9,10).
In addition, it was confirmed that AOPPs can accelerate cartilage
degradation in rabbit arthritis models and apoptosis in murine
chondrocytes (4,11). NADPH oxidase (NOX), a
membrane-bound enzyme composite, serves an essential function in
the production of reactive oxygen species (ROS). AOPPs have been
shown to induce apoptosis and inflammation of chondrocytes via
NOX-dependent ROS production (4,12).
In general, AOPPs may represent a novel pathogenic factor
accelerating the progression of RA (4,12-14).
Hydroxytyrosol (HT), a polyphenol that mainly exists
in extra virgin olive oil and red wine, possesses anti-inflammatory
and antioxidant effects and is a suitable candidate for therapeutic
interventions for arthritis (15,16). Silent information regulator 1
(SIRT1), as a nicotinamide adenine dinucleotide (NAD+)-dependent
histone deacetylase, regulates various physiological processes,
including cell degeneration, cell survival and cell growth
(17). Experiments have shown
that HT can induce autophagy via the SIRT1 signaling pathway in
primary chondrocytes of patients with osteoarthritis, and in human
C-28/I2 chondrocytes for anti-oxidant and anti-inflammatory effects
when challenged with H2O2 (18). However, whether and how HT
regulates the autophagy of chondrocytes against AOPPs has not yet
been investigated. Therefore, the present study aimed to
investigate the role of HT in protection against AOPP-related RA by
examining whether pharmacologically enhanced autophagy can prevent
oxidative stress and inflammation-induced damage to
chondrocytes.
Materials and methods
AOPP-rat serum albumin (RSA) preparation
and determination
According to the method described previously,
AOPP-RSA was prepared (10). The
specific procedure was as follows: RSA solution (20 mg/ml,
Sigma-Aldrich; Merck KGaA) was exposed to 200 mmol/l HOCL for 30
min at room temperature, following which the free HOCL was dialyzed
off with phosphate-buffered saline (PBS) at 4°C. Simultaneously,
the control was incubated with only RSA dissolved in PBS.
Endotoxins in all preparations were removed using a Detox-Gel
column (Thermo Fisher Scientific, Inc.). The levels of endotoxin in
the AOPP-RSA and unmodified RSA groups were assayed using an
Amoebic Cell Lysate Assay kit (Sigma, Merck KGaA). All reagents
prepared had an endotoxin concentration of <0.025 EU/ml.
Finally, the determination of AOPP content was conducted as
described in our previous study (19). The specific procedure was as
follows: 200 µl sample or chloramine-T was placed in each
well of a 96-well plate, to which 20 µl acetic acid was then
added. The absorbance at 340 nm was immediately measured using a
microplate reader. Finally, the AOPP content was determined to be
50.09±3.68 and 0.33±0.79 mmol/g protein in the AOPP-RSA and
unmodified RSA groups, respectively.
Cell culture
Under sterile conditions, fresh cartilage from the
knee joints of three newborn female Sprague-Dawley rats (weight,
8-10 g; Southern Medical University Animal Experient Center,
Guangzhou, China) was isolated and washed with PBS. Animals were
housed under standard conditions (temperature, 26-28°C; relative
humidity, 40-70%), with a 12 h light/dark cycle and ad
libitum access to food and water. The minced cartilage tissue
was then digested with 0.25% Trypsin (Gibco, Thermo Fisher
Scientific, Inc.) for 30 min, followed by 0.2% collagenase II
(Sigma, Merck KGaA) in DMEM//F12 (Gibco; Thermo Fisher Scientific,
Inc.) for further digestion of the cartilage tissue (3 h at 37°C).
The digested chondrocytes were centrifuged at 1,000 × g at room
temperature for 5 min, and the resulting articular chondrocytes
were resuspended in DMEM/F12 containing 10% FBS (Gibco, Thermo
Fisher Scientific, Inc.) and antibiotics (100 IU/ml penicillin and
100 IU/ml streptomycin, Gibco, Thermo Fisher Scientific, Inc.). The
cells were then seeded onto a culture dish at 37°C in a humidified
atmosphere (5% CO2, 95% air). The chondrocytes were
passaged at a ratio of 1:3, on reaching ~80-90% confluency.
Inverted phase contrast microscopy, Alcian blue staining and
immunohistochemical staining with collagen II were used to identify
the first passage chondrocytes. Only first passage chondrocytes
were used in the present study, in order to avoid phenotype loss.
The study was approved by the Institutional Animal Care and Use
Committee of Southern Medical University (Guangzhou, China).
CCK-8 assay
The chondrocytes were seeded in a 96-well plate at
37°C (10,000 cells/well) and cultured overnight. Prior to
treatment, the cells were extensively washed with PBS and cultured
in DMEM/F12 without serum for 12 h, and then incubated in the
absence or presence of 200 µg/ml AOPPs at 37°C; 75 µM
HT (Cayman Chemical) was added for 30 min prior to stimulation with
AOPPs. The autophagy inhibitor, chloroquine (50 µm; Macklin
Biochemical Co., Ltd.) was added to the cell medium for 30 min
prior to other treatments. Control cells received an equal volume
of DMEM/F12 + 10% FBS. Subsequently, CCK-8 (5 mg/ml) in a volume
equal to 10% of the culture volume was added to the cells and
incubated for 2 h at 37°C. The optical density (OD) was then
measured at 450 nm using a microplate reader (Tecan Group,
Ltd.).
Determination of ROS generation
The oxidation-sensitive fluorescent probe
2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma, Merck KGaA)
was used to assess the levels of intracellular ROS generation.
First, the chondrocytes were seeded at 70% (1×104 per
well) confluence into a 96-well plate and then labeled with 10
µM probe diluted in serum-free DMEM/F12 for 30 min in the
dark. To induce the production of ROS, the chondrocytes were
pre-treated with, or without, 75 µM HT for 30 min prior to
stimulation with AOPPs for 2 h. Subsequently, PBS was used to
replace the medium, and fluorescence was measured using a
SpectraMaxM5 Multimode Microplate Reader at excitation and emission
wavelengths of 488 and 525 nm, respectively. The experiment was
performed in triplicate for each condition.
Western blotting
The cultured chondrocytes were harvested and lysed
in radioimmunoprecipitation assay lysis buffer (Beyotime Institute
of Biotechnology). The protein concentration was determined using a
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). Total
protein (35 µg) was separated via SDS-polyacrylamide gel
electrophoresis using 10-12% acrylamide gels and then
electrotransferred onto PVDF membranes (Pall Life Science). The
membranes were then pre-incubated in blocking buffer (5% RSA) for
60 min at room temperature and further incubated with primary
antibodies overnight at 4°C. Membranes were washed three times with
TBST (TBS with Tween) and subsequently incubated with secondary
antibodies for 1 h at room temperature. Samples were then washed
three times with TBST prior to detection with chemiluminescence
detection reagents (Merck KGaA). The following antibodies (Abs)
were used: Rabbit anti-NOX4 mAb (1:1,000; cat. no. ab133303),
rabbit anti-NOX2 mAb (1:5,000; cat. no. ab129068) and anti-TNFα
(1:2,000; cat. no. ab66579) from Abcam; rabbit anti-LC3B mAb
(1:1,000; cat. no. #3868), rabbit anti-P62 mAb (1:1,000; cat. no.
#39786), rabbit anti-Sirt1 mAb (1:1,000; cat. no. #9475) rabbit
anti-ATG5 mAb (1:1,000; cat. no. #12994) and rabbit anti-ATG7 mAb
(1:1,000, #8558) from Cell Signaling Technology, Inc.; and goat
anti-mouse (1:2,000; cat. no. BA1050) and goat anti-rabbit
(1:2,000; cat. no. BA1056) IgG-horseradish peroxidase (HRP) from
Boster Biological Technology Co, Ltd. A rabbit anti-GAPDH pAb
(1:2,500; Abcam; cat. no. ab9485) was used as a loading control.
The integrated density of all protein bands were analyzed with
ImageJ software V1.47 (National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
According to the manufacturer's protocol, the
treated and control chondrocytes were collected for the extraction
of total RNA using TRIzol reagent (Takara Biotechnology Co., Ltd.).
The RNA samples (260/280 ratio >2.0) were quantified using a
NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Inc.).
The total RNA (1 µg) was used for RT using the
PrimeScript® RT Reagent kit with gDNA Eraser (Takara
Biotechnology Co., Ltd.). The resulting cDNA was PCR amplified
using an SYBR® Premix Ex Taq™ II kit (Takara
Biotechnology Co., Ltd.) with each specific primer. The sequences
for the primers used are listed in Table I. The PCR procedure were performed
on the Roche LightCycler 480 PCR system (Roche Diagnostics). The
steps, according to the manufacturer's instructions were as
follows: Initial enzyme activation at 95°C for 10 min; followed by
45 cycles of 95°C for 10 sec, 60°C for 20 sec and 65°C for 30 sec.
Following the amplification phase, a cooling step was performed at
40°C for 10 sec (ramp rate of 1.5°C/sec). The housekeeping gene
glyc-eraldehyde-3-phosphate dehydrogenase (GAPDH) was used for
normalization. The relative expression (fold-change from control)
was calculated using the 2−ΔΔCq method as previously
described (20).
 | Table ISequences of primers used for reverse
transcription-quantitative PCR analysis. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR analysis.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| SIRT1 | Forward |
GCTCGCCTTGCTGTGGACTTC |
| Reverse |
GTGACACAGAGATGGCTGGAACTG |
| NOX2 | Forward |
GCCATTCACACCATTGCACATCTG |
| Reverse |
CCAACCGAGTCACAGCCACATAC |
| NOX4 | Forward |
ACAGTCCTGGCTTACCTTCG |
| Reverse |
TTCTGGGATCCTCATTCTGG |
| IL-6 | Forward |
TGCTCTGGTCTTCTGGAGTTCCG |
| Reverse |
GTTGGATGGTCTTGGTCCTTAGCC |
| TNF-α | Forward |
GTAGCAAACCACCAAGCGGA |
| Reverse |
ATGGGCTCATACCAGGGCTT |
| MMP-13 | Forward |
CAAGATGTGGAGTGCCTGATGTGG |
| Reverse |
GCGTGTGCCAGAAGACCAGAAG |
| GAPDH | Forward |
GGCACAGTCAAGGCTGAGAATG |
| Reverse |
ATGGTGGTGAAGACGCCAGTA |
RNA interference
Rat small interfering RNA (CCT CAA GCC ATG TTC GAT
A), negative control siRNA (NC siRNA; cat. no. siN05815122147) and
the ribo FECT™CP Transfection kit were chemically
synthesized/obtained by/from Guangzhou Ribobio Co., Ltd. The
chondrocytes in antibiotic-free growth medium were seeded into
6-well plates at a density of 20,000/cm. When the cells reached 70%
confluency, they were transfected with a stock concentration of 20
µM synthesized siRNA targeting rat SIRT1 (forward, 5'-CCU
CAA GCC AUG UUC GAU A-3'; reverse, 5'-UAU CGA ACA UGG CUU GAG
G-3'), according to the manufacturer's instructions. The cells were
incubated for 24 h and then used in subsequent experiments. mRNA
and protein expression were evaluated following stimulation.
Immunocytochemical detection of
autophagosomes
Following stimulation, autophagy was detected in
viable cells using an Autophagy Detection kit (cat. no. ab139484,
Abcam) according to the manufacturer's protocol. The nuclei were
stained with DAPI (blue) and autophagic vesicles were stained with
FITC (green). For analysis, the cells were fixed by incubation with
methanol for 20 min at -20°С. Staining was visualized using a laser
scanning microscope (LSM 880 with Airyscan, Zeiss GmbH) at
excitation/emission wavelengths of 520/560 nm (FITC), and 360/460
nm (DAPI). The stained cells were counted in at least eight
different fields of view at ×400 magnification by two independent
observers.
Enzyme-linked immunosorbent assay
(ELISA)
The chondrocytes were seeded into 6-well culture
plates in DMEM/F12 + 10% FBS and incubated for 24 h. Following
pre-treatment with HT, the cells were exposed to AOPPs for 24 h.
Following this, the cell supernatants were collected and
centrifuged at 12,000 × g for 15 min at 4°C. The TNF-α ELISA kit
(MEIMIAN) and IL-6 ELISA kit (MEIMIAN) were then used to detect
cytokine secretion into the supernatant. The OD values were
determined at 450 nm using a spectrophotometric plate reader
(Molecular Devices, LLC). All experiments were performed in
triplicate.
Statistical analysis
Data are presented as the mean ± SEM of three
independent experiments. One-way ANOVA followed by Tukey's post hoc
test was used to determine the differences between groups.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using GraphPad
Prism version 6.0 (GraphPad Software, Inc.).
Results
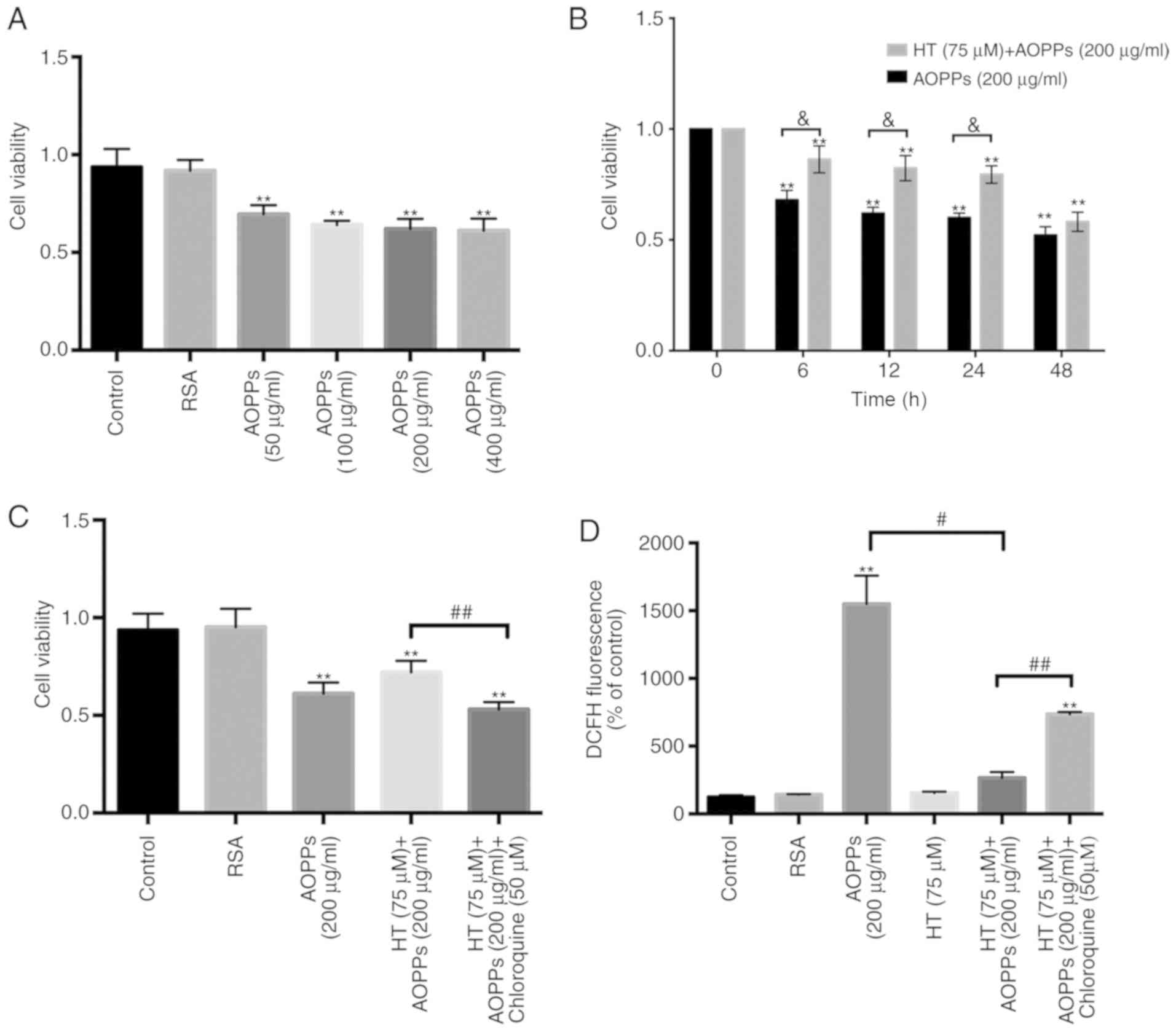
Protective effect of HT on chondrocyte
viability
The first aim of the present study was to evaluate
the protective effect of HT on the viability of rat chondrocytes in
the presence of AOPPs using a CCK-8 assay. The chondrocyte cultures
were exposed to different concentrations of AOPPs for 24 h and the
results revealed that AOPPs had a concentration-dependent adverse
effect on cells; as the concentration of AOPPs increased, cell
viability decreased (Fig. 1A).
Furthermore, the chondrocytes were pre-treated with HT (75
µm), which reversed the adverse effects of AOPPs on cell
viability in a time-dependent manner (Fig. 1B). In addition, when the
chondrocytes were incubated with the autophagy inhibitor
chloroquine (50 µm) for 30 min and then stimulated with HT
(75 µm) and AOPPs (200 µg/ml), the cell viability
decreased (Fig. 1C).
HT inhibits intracellular ROS generation
stimulated by AOPPs
In our previous studies, it was demonstrated that
NOX-dependent ROS generation was triggered by AOPPs. The
consequences of these oxidative products include mitochondrial
dysfunction and endoplasmic reticulum stress in chondrocytes. To
determine whether HT can inhibit AOPP-induced ROS accumulation,
intracellular ROS levels were evaluated following HT treatment. ROS
production decreased in the HT-AOPP group compared with that in the
AOPP only treatment group. To further verify the role of HT in ROS
generation, intracellular ROS levels were also measured following
the addition of chloroquine (an autophagy inhibitor). There was a
significant increase in the generation of ROS in this group
(Fig. 1D).
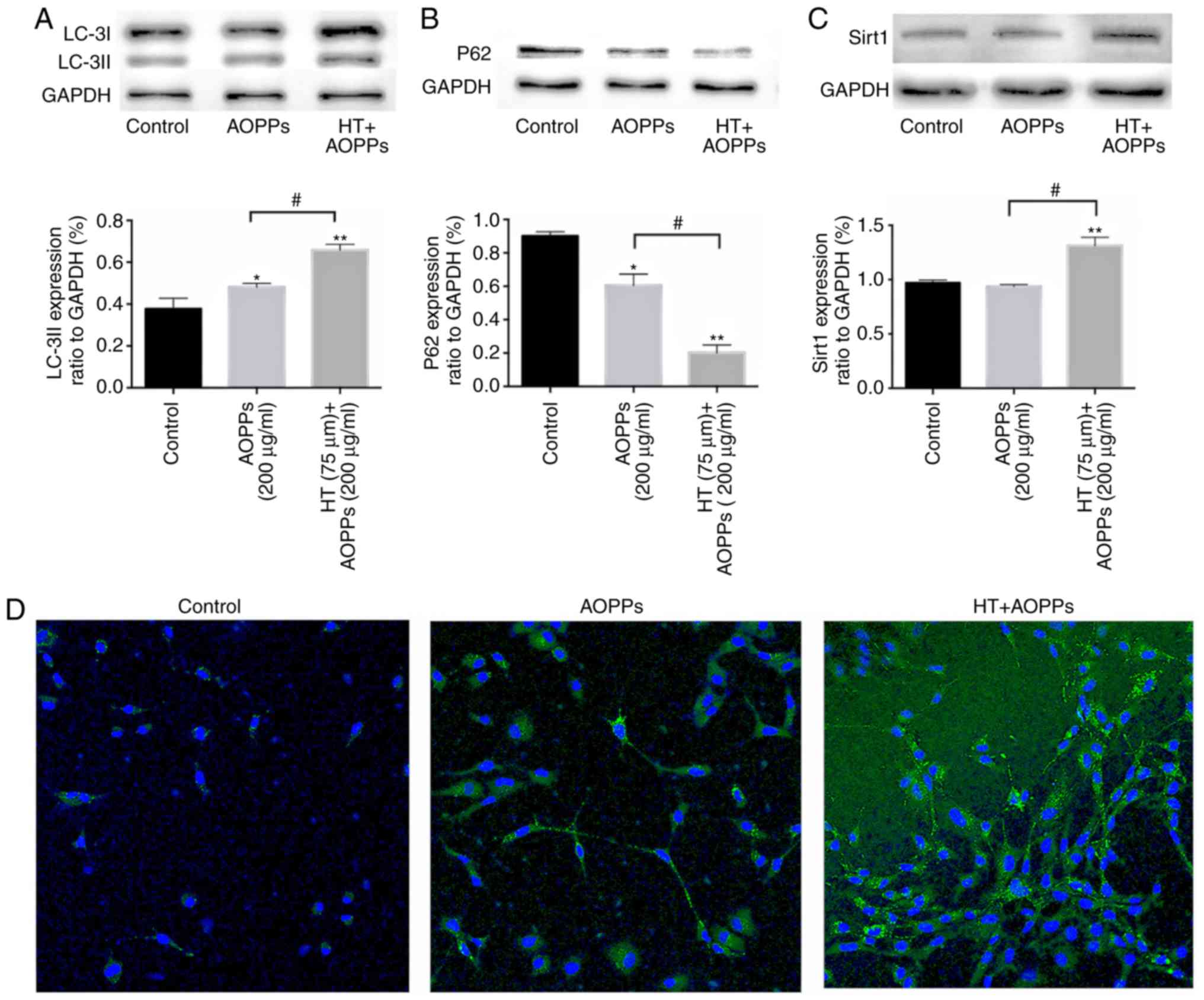
HT promotes autophagy in chondrocytes
stimulated with AOPPs and upregulates the expression of SIRT1
When observing the autophagic process, LC3-II and
P62 proteins are broadly utilized as markers of autophagy; reduced
levels of P62 in combination with increased LC3-II are typically
linked with autophagy (21). The
bulk degradation of subcellular components leads to the production
of autophagosomes/autolysosomes, marking the process of
macroautophagy. Atg5 and Atg7 are considered essential genes in
mammalian macroautophagy (22).
To determine the optimal stimulation concentration and time
required for HT, the present study evaluated different doses (10,
50 and 75 µm) and times (1, 2, 6, 12 and 24 h). Exposure to
75 µm HT caused the highest enhancement of autophagy
proteins: LC3II increased from 1 h and reached a peak at 2 h; P62
decreased, reaching the lowest level at 6 h (Fig. 2A and B). The chondrocytes were
exposed to AOPPs (200 µg/ml) for 2 h, in the presence or
absence of HT (75 µm). It was found that HT induced the
protein expression of LC3-II and SIRT1 considerably. Furthermore,
HT treatment decreased the protein level of P62 (Fig. 3A-C). Autophagosomes were assessed
using an autophagy kit from Abcam. Cells actively undergoing
autophagy exhibited a green fluorescent signal in the cytoplasm.
Increased MDC fluorescence was observed in the presence of HT under
AOPP stimulation, whereas the opposite effect was observed
following treatment with AOPPs alone (Fig. 3D).
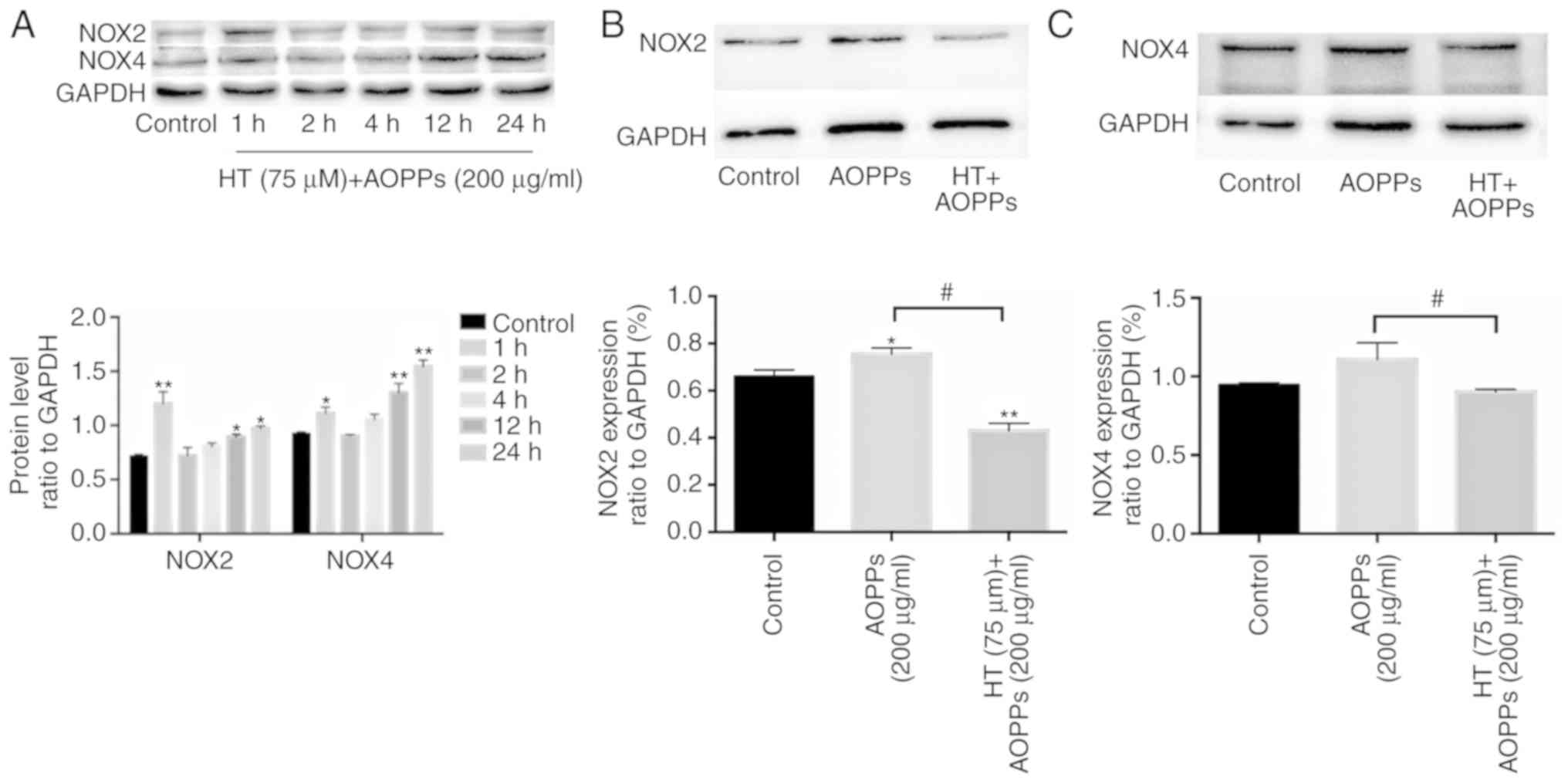
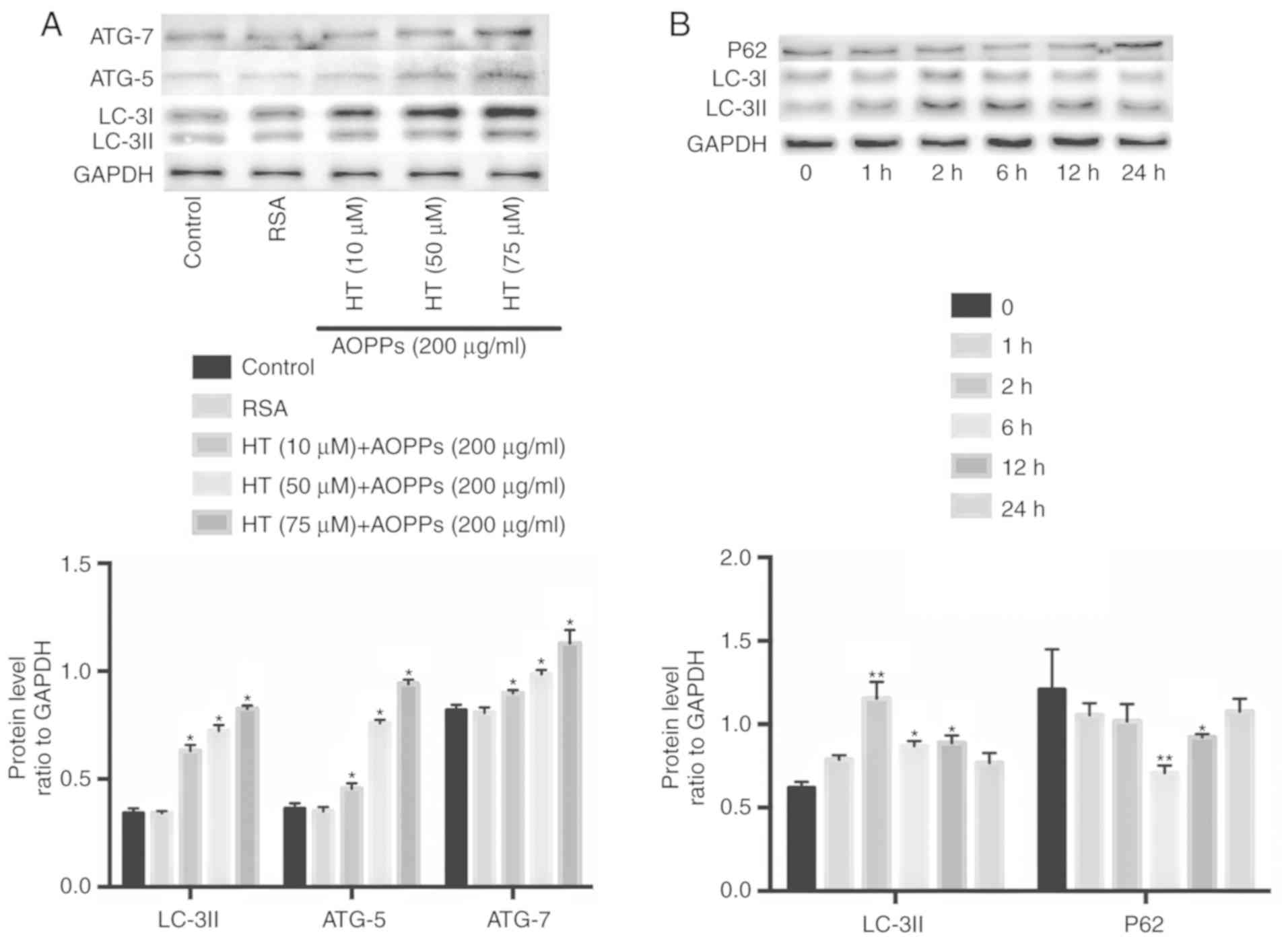 | Figure 2Autophagy-related cellular events
following HT treatment. Autophagy-related proteins were detected by
western blotting. (A) Autophagy proteins were enhanced by HT at
different concentrations (10, 50 and 75 µm) after 2 h. (B)
Chondrocytes were pretreated with 75 µm HT for 30 min and
then stimulated with 200 µg/ml AOPPs for different durations
(1, 2, 6, 12 and 24 h). Data are expressed as the mean ± SEM (n=3).
*P<0.05 and **P<0.01 vs. control group.
HT, hydroxytyrosol; RSA, rat serum albumin; AOPPs, advanced
oxidation protein products; LC-3II, microtubule-associated protein
1 light chain 3; ATG, autophagy related; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
HT inhibits AOPP-induced NOX in
chondrocytes
The protein expression of NOX was observed at
various time points (1, 2, 4, 12 and 24 h). The chondrocytes were
exposed to AOPPs (200 µg/ml) in the presence or absence of
HT (75 µm). The results showed that the levels of NOX2 and
NOX4 rose sharply by 1 h, but decreased by 2 h (Fig. 4A). The possible effects of HT on
NOX expression were also investigated. The protein expression
levels of NOX2 and NOX4 were markedly increased following AOPP
stimulation. However, HT pretreatment led to a significant
downregulation in the expression of NOX2 and NOX4 (Fig. 4B and C).
HT activates autophagy and inhibits
AOPP-induced NOX in chondrocytes through the SIRT1 pathway
As our previous experiments showed, HT increases
cell autophagy and upregulates SIRT1 pathway protein expression in
chondrocytes under AOPP conditions. Subsequent experiments aimed to
verify whether the function of HT in autophagy is influenced by the
SIRT1 pathway. Chondrocytes transfected with SIRT1 siRNA were used
for further experiments. Following stimulation subsequent to
previous HT treatment, the expression levels of SIRT1 and LC3II
were detected by western blotting and RT-qPCR analysis. The results
indicated that the knockdown of SIRT1 decreased the protein
expression of LC3II induced by HT (Fig. 5A and B). These findings suggest
that HT increases autophagy under AOPP conditions by activating the
SIRT1 pathway in chondrocytes.
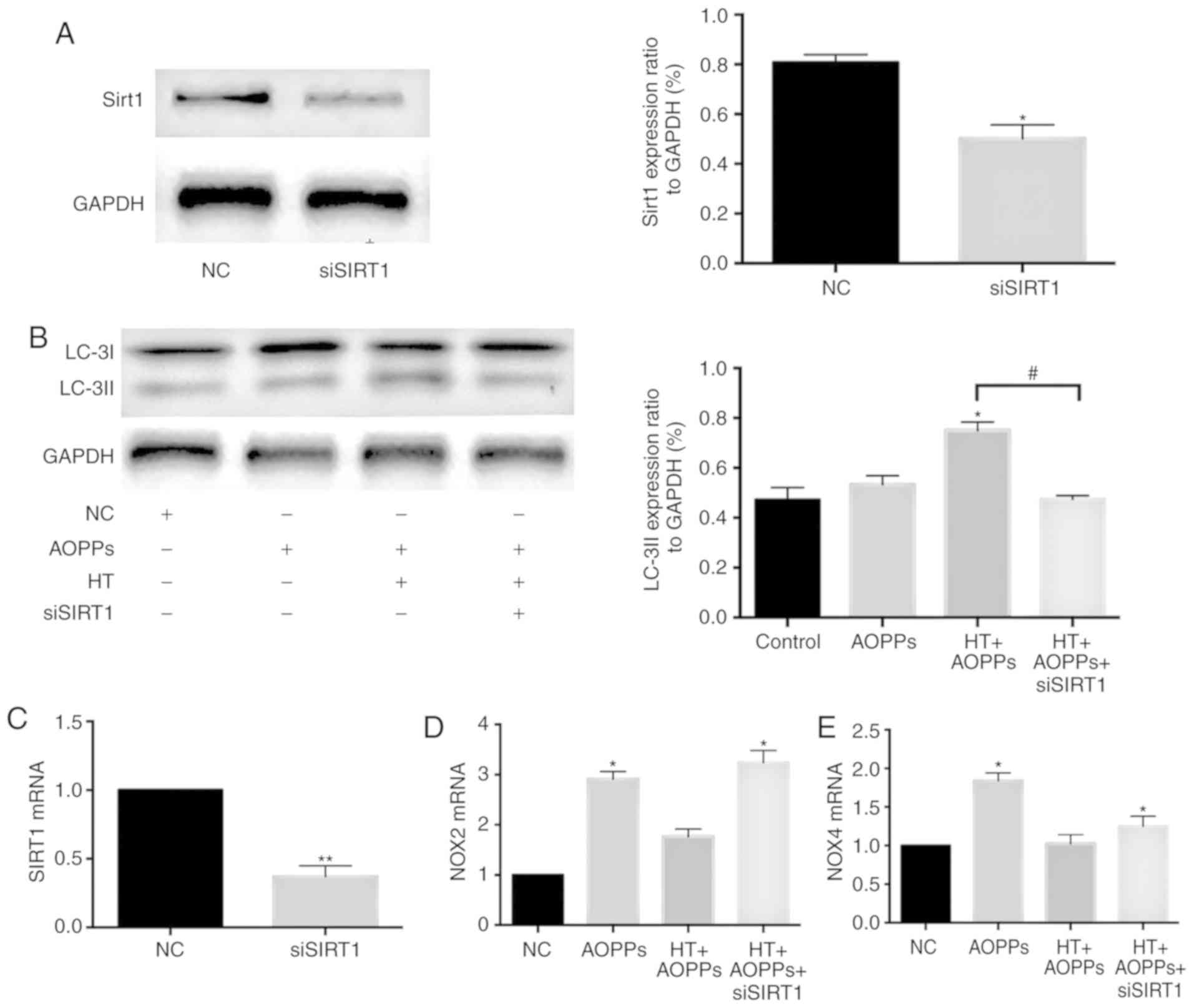 | Figure 5HT inhibits AOPP-induced NOX in
chondrocytes through the SIRT1 pathway. Chondrocytes transfected
with SIRT1 siRNA were pretreated with HT (75 µM), and AOPPs
were added for 2 h. Protein expression levels of (A) SIRT1 and (B)
LC3II were assessed by western blotting. Expression levels of (C)
SIRT1, (D) NOX2 and (E) NOX4 were detected by reverse
transcription-quantitative PCR analysis. The results are expressed
as the mean ± SEM (n=3). *P<0.05 and
**P<0.01 vs. control group. #P<0.05 HT
+ AOPPs group vs. HT + AOPPs + siSIRT1 group. HT, hydroxytyrosol;
AOPPs, advanced oxidation protein products; LC-3II,
microtubule-associated protein 1 light chain 3; NOX, NADPH oxidase;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SIRT1, silent
information regulator 1; siSIRT1, small interfering RNA targeting
SIRT1; NC, negative control. |
To further clarify the roles of NOX2, NOX4 and SIRT1
in the protective effects of HT, chondrocytes transfected with
SIRT1siRNA were used prior to HT-AOPP stimulation. The knockdown
efficiency of SIRT1 was measured according to the mRNA expression
of SIRT1 (Fig. 5C). The mRNA
expression levels of NOX2 and NOX4 were assessed by RT-qPCR
analysis. The results revealed that SIRT1 knockdown enhanced the
mRNA expression of NOX2 and NOX4 in chondrocytes (Fig. 5D and E) These findings suggest
that HT suppresses AOPP-induced NOX in chondrocytes by affecting
the SIRT1 pathway.
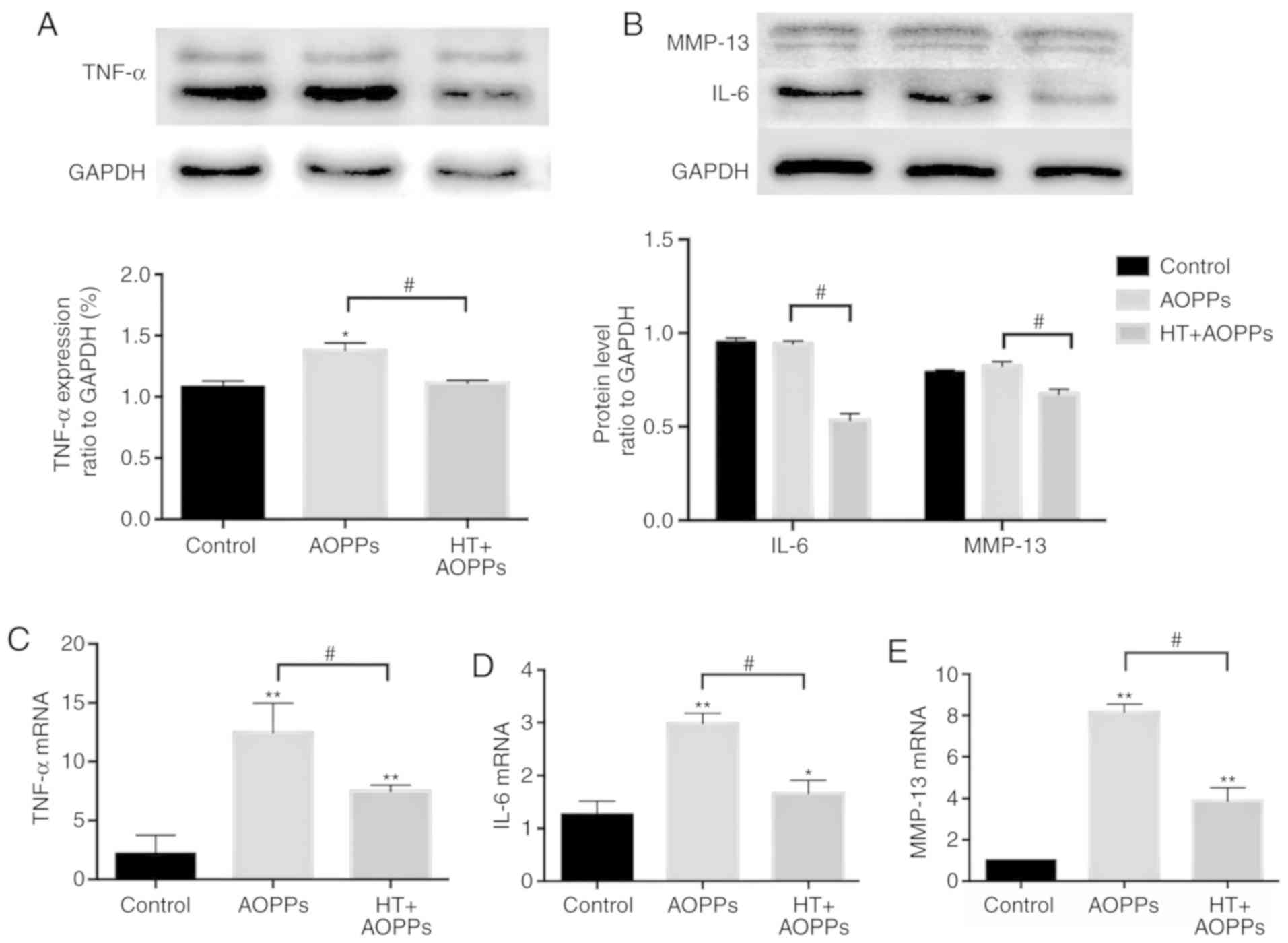
HT decreases the secretion of IL-6, TNF-α
and MMP-13 in chondrocytes stimulated with AOPPs
Finally, the present study assessed the ability of
HT to protect rat chondrocytes against inflammation under AOPP
conditions. Initially, the effects of HT on AOPP-induced IL-6,
TNF-α and MMP-13 were examined using western blot analysis. The
results revealed that the AOPP-driven release of IL-6, TNF-α and
MMP-13 was decreased by HT (Fig. 6A
and B). Consistently, the protective effects of HT on the
expression of IL-6, TNF-α and MMP-13 were quantified by RT-qPCR
analysis. As shown in Fig. 6C-E,
the mRNA levels of IL-6, TNF-α and MMP-13 in the HT treatment group
were also reduced compared with those in the AOPP treatment
group.
To further clarify the role of SIRT1 in HT cell
protection, chondrocytes transfected with SIRT1 siRNA were used
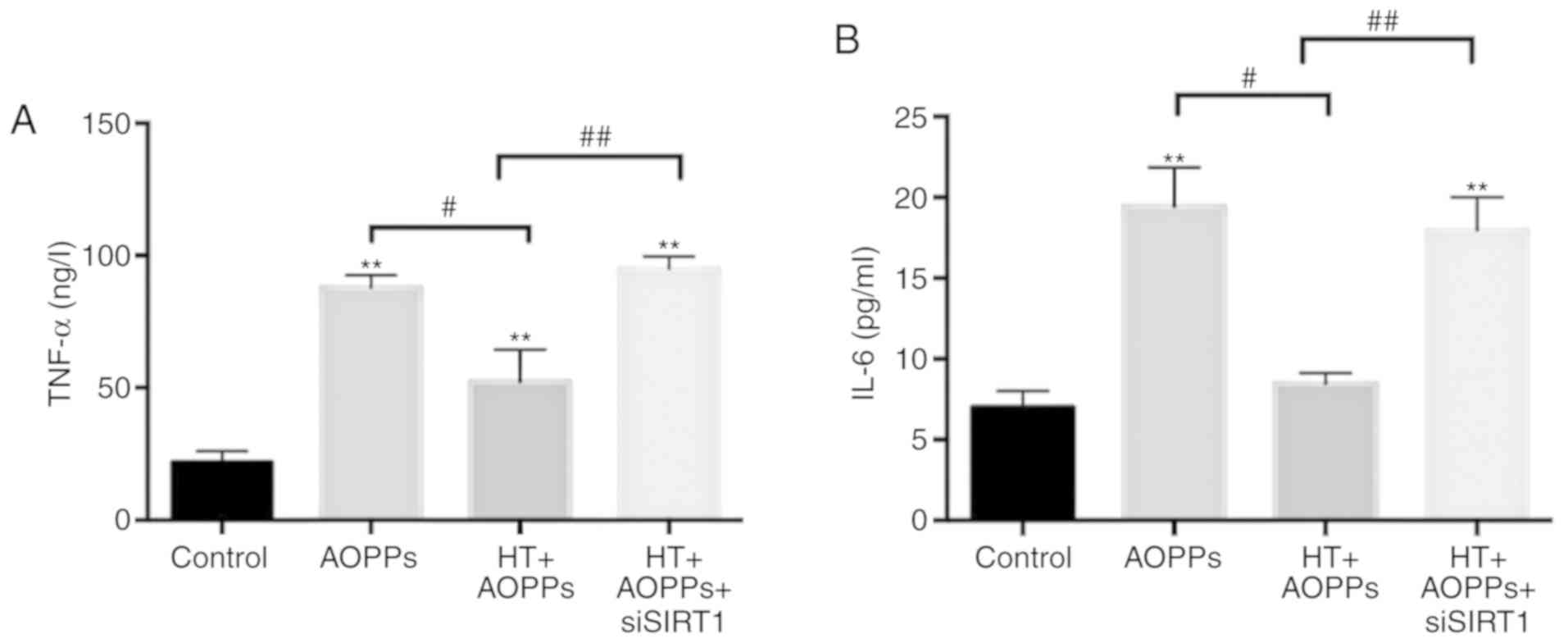
prior to HT-AOPP stimulation. The inflammatory cytokines IL-6 and
TNF-α were detected via ELISAs. The results revealed that the
knockdown of SIRT1 enhanced the levels of IL-6 and TNF-α
inflammatory cytokines in chondrocytes prior to AOPP stimulation
and HT co-treatment (Fig. 7A and
B). Overall, the results indicated that HT protects
chondrocytes from AOPP-induced inflammatory damage through the
SIRT1 pathway.
Discussion
In the present study, the cellular and molecular
events involved in the protective effect of HT on chondrocytes
stimulated with AOPPs were investigated. The principal findings
were as follows: i) HT protected chondrocytes against inflammation
under AOPP conditions; ii) HT inhibited AOPP-induced NOX in
chondrocytes stimulated with AOPPs through the SIRT1 pathway; iii)
HT exerted protective effects on chondrocytes stimulated with AOPPs
by promoting autophagy through the SIRT1 pathway.
The Mediterranean diet serves a significant role in
complimentary pharmacotherapy for individuals living with RA and
also contributes to reducing the risk of RA (23,24). HT is a polyphenol, found mainly in
extra virgin olive oil but also in red wine. It has a dominant
antioxidant effect correlated with hydrogen donation and promotes
radical stability. Furthermore, HT has other advantageous benefits
to human health, specifically anti-inflammatory and anticancer
effects, autophagy and mitochondrial function (25). MMPs can contribute to tissue
remodeling during morphogenesis and inflammation; MMP secretion by
chondrocytes has been observed in RA. Our previous study
demonstrated that AOPPs can upregulate the mRNA and protein
expression of TNF-α, IL-1β, MMP-3 and MMP-13 in a
concentration-dependent manner (11). However, the role of HT under AOPP
conditions has not been investigated, to the best of our knowledge.
The present study found that the treatment of chondrocytes with HT
encouraged vital functional recovery; the expression levels of
inflammatory TNF-α and IL-6, and the levels of MMP-13 were
substantially inhibited, as shown by western blot and RT-qPCR
analyses. Overall, these findings indicated that HT has a
significant effect against AOPP-induced inflammatory damage in
chondrocytes.
Autophagy is a self-degrading process that is
important for balancing energy sources at critical moments of
development and nutritional stress responses (26). An important hallmark of RA is
chondrocyte inflammation (27).
In early research, it was reported that autophagy has a substantial
influence on RA within the modulation of ROS, which promotes
oxidative stress and results in the pathological processes of
inflammation and apoptosis (28).
As shown in Fig. 2, it was found
in the present study that HT increased the autophagy in
chondrocytes under AOPP conditions. The autophagy was
concentration-dependent and reached a peak at 2 h. However, when
the autophagy inhibitor chloroquine was added, cell viability
decreased and ROS generation increased (Fig. 1C and D). Therefore, it appears
that HT activates autophagy to alleviate oxidative stress damage in
chondrocytes. In preliminary experiments, treatments were performed
with HT and chloroquine alone. However, no statistically
significant differences with the control group were found.
Therefore, the present study did not include HT and chloroquine
groups, separately.
In mammals, NOX is the main source of ROS generation
(29). Our previous
pre-laboratory experiments demonstrated that AOPPs induced a
time-dependent activation of NOX2 and NOX4 (13). Cetrullo et al (30) showed that HT prevented chondrocyte
death and lowered ROS levels under oxidative stress by inducing
autophagy. However, there is currently no information on the
association between NOX and HT. In the present study, when the
chondrocytes were pre-treated for 30 min with HT and then
co-cultured with AOPPs, it was found that the levels of NOX2 and
NOX4 increased sharply by 1 h, but decreased by 2 h (Fig. 4A). Therefore, it was hypothesized
that this phenomenon is associated with autophagy. The level of
autophagy was insufficient to inhibit the intracellular ROS
generation stimulated by AOPPs before 2 h, so the levels of NOX2
and NOX4 increased. As shown in Fig.
2B, it was found that HT and AOPP co-culture increased
autophagy in chondrocytes at 2-6 h, which in turn reduced the
expression of NOX2 and NOX4. After 6 h, the level of HT-activated
autophagy gradually decreased over time, and the antioxidant
capacity gradually decreased, which caused the increasing
expression levels of NOX2 and NOX4. Subsequently, further
experiments were designed and the observation point at 2 h was
selected. Following pre-treatment of the chondrocytes with HT for
30 min, AOPPs were added for 2 h. It was found that the levels of
NOX4 and NOX2 were decreased in the HT pre-treatment group,
indicating that HT can activate autophagy and reduce the NOX
induced by AOPPs in chondrocytes.
SIRT1, as a protein deacetylase, is essential for
chondrocyte survival and cartilage homeostasis (31). The SIRT1 pathway is also
considered to be involved with therapeutic reagents for RA; for
example, resveratrol is effective for the treatment of RA due to
the overexpression of SIRT1 (32,33). It has been reported that the
activation of SIRT1 can increase the expression of superoxide
dismutase 2 and regulate autophagy (34). Consistently, as shown in Fig. 3A-D, HT induced autophagy and
enhanced the expression of SIRT1 in the present study. These
findings confirm that HT enhances autophagy under AOPP conditions
by activating the SIRT1 pathway in chondrocytes. SIRT1 is also
involved in reducing the production of NOX, and it has been
confirmed that the inhibition of SIRT1 is involved in the
upregulation of NOX subunits (35). However, the functional role of
SIRT1 in the associations among HT, AOPPs and autophagy have not
been investigated. Chondrocytes transfected with SIRT1 siRNA were
used for verification. The knockdown of SIRT1 reduced the autophagy
protein LC3II (Fig. 5A-C) and
increased the expression of NOX2 and NOX4 (Fig. 5D and E) in chondrocytes following
HT pretreatment. In addition, higher levels of TNF-α and IL-6 were
observed in the SIRT1-knockdown group (Fig. 7A and B). These findings suggest
that the SIRT1 pathway serves a notable role in enhancing autophagy
and protecting chondrocytes against AOPP damage.
However, the present study only investigated at the
in vitro level and not the in vivo level. A possible
mechanism underlying the link between HT and AOPPs in chondrocytes
is thus tentatively proposed. The present study provides further
evidence demonstrating the role of HT in lowering ROS and NOX
levels against AOPP-induced inflammatory responses.
In conclusion, the present study confirmed that HT
enhances autophagy to protect against AOPP-induced inflammatory
damage in chondrocytes through the SIRT1 signaling pathway. These
findings provide novel insights into the links between HT,
autophagy and NOX in chondrocytes. These results suggest a novel
molecular mechanism for the protective role of HT and a possible
clinical application of HT in RA.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81772395).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TS and QC designed the study and conducted all
experiments. SYZ assisted with western blotting. QW and CRL
assisted with primary cell culture. XHW assisted with
immunocytochemical imaging. HTW and ZW revised the manuscript and
also helped with the extraction of primary cells. JTC supervised
all experiments. All authors were involved in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Animal
Care and Use Committee of Southern Medical University (Guangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheung PP, Dougados M, Andre V, Balandraud
N, Chales G, Chary-Valckenaere I, Chatelus E, Dernis E, Gill G,
Gilson M, et al: Improving agreement in assessment of synovitis in
rheumatoid arthritis. Joint Bone Spine. 80:155–159. 2013.
View Article : Google Scholar
|
|
2
|
Lavocat F, Osta B and Miossec P: Increased
sensitivity of rheumatoid synoviocytes to Schnurri-3 expression in
TNF-α and IL-17A induced osteoblastic differentiation. Bone.
87:89–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi YJ, Lee WS, Lee EG, Sung MS and Yoo
WH: Sulforaphane inhibits IL-1β-induced proliferation of rheumatoid
arthritis synovial fibroblasts and the production of MMPs, COX-2,
and PGE2. Inflammation. 37:1496–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Q, Zhong ZM, Zhu SY, Liao CR, Pan Y,
Zeng JH, Zheng S, Ding RT, Lin QS, Ye Q, et al: Advanced oxidation
protein products induce chondrocyte apoptosis via receptor for
advanced glycation end products-mediated, redox-dependent intrinsic
apoptosis pathway. Apoptosis. 21:36–50. 2016. View Article : Google Scholar
|
|
5
|
Witko-Sarsat V, Friedlander M,
Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers
P and Descamps-Latscha B: Advanced oxidation protein products as a
novel marker of oxidative stress in uremia. Kidney Int.
49:1304–1313. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Komosinska-Vassev K, Olczyk P,
Winsz-Szczotka K, Kuznik- Trocha K, Klimek K and Olczyk K: Age- and
gender-related alteration in plasma advanced oxidation protein
products (AOPP) and glycosaminoglycan (GAG) concentrations in
physiological ageing. Clin Chem Lab Med. 50:557–563. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalousová M, Skrha J and Zima T: Advanced
glycation end-products and advanced oxidation protein products in
patients with diabetes mellitus. Physiol Res. 51:597–604. 2002.
|
|
8
|
García-González A, Gaxiola-Robles R and
Zenteno-Savin T: Oxidative stress in patients with rheumatoid
arthritis. Rev Invest Clin. 67:46–53. 2015.PubMed/NCBI
|
|
9
|
Zhang YB, Zhong ZM, Hou G, Jiang H and
Chen JT: Involvement of oxidative stress in age-related bone loss.
J Surg Res. 169:e37–e42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie F, Sun S, Xu A, Zheng S, Xue M, Wu P,
Zeng JH and Bai L: Advanced oxidation protein products induce
intestine epithelial cell death through a redox-dependent, c-jun
N-terminal kinase and poly (ADP-ribose) polymerase-1-mediated
pathway. Cell Death Dis. 5:e10062014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Ye WB, Zhong ZM, Ding RT and Chen
JT: Effect of advanced oxidation protein products on articular
cartilage and synovium in a rabbit osteoarthritis model. Orthop
Surg. 7:161–167. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng S, Zhong ZM, Qin S, Chen GX, Wu Q,
Zeng JH, Ye WB, Li W, Yuan K, Yao L and Chen JT: Advanced oxidation
protein products induce inflammatory response in fibroblast-like
synoviocytes through NADPH oxidase-dependent activation of NF-κB.
Cell Physiol Biochem. 32:972–985. 2013. View Article : Google Scholar
|
|
13
|
Ye W, Zhong Z, Zhu S, Zheng S, Xiao J,
Song S, Yu H, Wu Q, Lin Z and Chen J: Advanced oxidation protein
products induce catabolic effect through oxidant-dependent
activation of NF-κB pathway in human chondrocyte. Int
Immunopharmacol. 39:149–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye W, Zhu S, Liao C, Xiao J, Wu Q, Lin Z
and Chen J: Advanced oxidation protein products induce apoptosis of
human chondrocyte through reactive oxygen species-mediated
mitochondrial dysfunction and endoplasmic reticulum stress
pathways. Fundam Clin Pharmacol. 31:64–74. 2017. View Article : Google Scholar
|
|
15
|
Ghanbari R, Anwar F, Alkharfy KM, Gilani
AH and Saari N: Valuable nutrients and functional bioactives in
different parts of olive (Olea europaea L.)-a review. Int J Mol
Sci. 13:3291–3340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chin KY and Pang KL: Therapeutic effects
of olive and its derivatives on osteoarthritis: From bench to
bedside. Nutrients. 9:2017. View Article : Google Scholar
|
|
17
|
Cerutti R, Pirinen E, Lamperti C, Marchet
S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, et al:
NAD(+)-dependent activation of Sirt1 corrects the phenotype in a
mouse model of mitochondrial disease. Cell Metab. 19:1042–1049.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Adamo S, Cetrullo S, Guidotti S, Borzi
RM and Flamigni F: Hydroxytyrosol modulates the levels of
microRNA-9 and its target sirtuin-1 thereby counteracting oxidative
stress-induced chondrocyte death. Osteoarthritis Cartilage.
25:600–610. 2017. View Article : Google Scholar
|
|
19
|
Wu Q, Zhong ZM, Pan Y, Zeng JH, Zheng S,
Zhu SY and Chen JT: Advanced oxidation protein products as a novel
marker of oxidative stress in postmenopausal osteoporosis. Med Sci
Monit. 21:2428–2432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Komatsu M and Ichimura Y: Physiological
significance of selective degradation of p62 by autophagy. FEBS
Lett. 584:1374–1378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishida Y, Arakawa S, Fujitani K,
Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y
and Shimizu S: Corrigendum: Discovery of Atg5/Atg7-independent
alternative macroautophagy. Nature. 533:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Forsyth C, Kouvari M, D'Cunha NM,
Georgousopoulou EN, Panagiotakos DB, Mellor DD, Kellett J and
Naumovski N: The effects of the Mediterranean diet on rheumatoid
arthritis prevention and treatment: A systematic review of human
prospective studies. Rheumatol Int. 38:737–747. 2018. View Article : Google Scholar
|
|
24
|
Philippou E and Nikiphorou E: Are we
really what we eat? Nutrition and its role in the onset of
rheumatoid arthritis. Autoimmun Rev. 17:1074–1077. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Echeverría F, Ortiz M, Valenzuela R and
Videla LA: Hydroxytyrosol and cytoprotection: A projection for
clinical interventions. Int J Mol Sci. 18:2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harre U and Schett G: Cellular and
molecular pathways of structural damage in rheumatoid arthritis.
Semin Immunopathol. 39:355–363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vomero M, Barbati C, Colasanti T,
Perricone C, Novelli L, Ceccarelli F, Spinelli FR, Di Franco M,
Conti F, Valesini G and Alessandri C: Autophagy and rheumatoid
arthritis: Current knowledges and future perspectives. Front
Immunol. 9:15772018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cetrullo S, D'Adamo S, Guidotti S, Borzi
RM and Flamigni F: Hydroxytyrosol prevents chondrocyte death under
oxidative stress by inducing autophagy through sirtuin 1-dependent
and -independent mechanisms. Biochim Biophys Acta. 1860:1181–1191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gabay O and Sanchez C: Epigenetics,
sirtuins and osteoarthritis. Joint Bone Spine. 79:570–573. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kong S, Yeung P and Fang D: The class III
histone deacetylase sirtuin 1 in immune suppression and its
therapeutic potential in rheumatoid arthritis. J Genet Genomics.
40:347–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakayama H, Yaguchi T, Yoshiya S and
Nishizaki T: Resveratrol induces apoptosis MH7A human rheumatoid
arthritis synovial cells in a sirtuin 1-dependent manner. Rheumatol
Int. 32:151–157. 2012. View Article : Google Scholar :
|
|
34
|
Lee IH, Cao L, Mostoslavsky R, Lombard DB,
Liu J, Bruns NE, Tsokos M, Alt FW and Finkel T: A role for the
NAD-dependent deacetylase Sirt1 in the regulation of autophagy.
Proc Natl Acad Sci USA. 105:3374–3379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wosniak J Jr, Santos CX, Kowaltowski AJ
and Laurindo FR: Cross-talk between mitochondria and NADPH oxidase:
Effects of mild mitochondrial dysfunction on angiotensin
II-mediated increase in Nox isoform expression and activity in
vascular smooth muscle cells. Antioxid Redox Signal. 11:1265–1278.
2009. View Article : Google Scholar : PubMed/NCBI
|