Introduction
Aging has become a research interest in the field of
medicine. Aging is an unavoidable process in the increase of
lifespan. In general, the functions of various organs of the body
exhibit gradual decreases in this process. The kidney, as an active
metabolic and major excretory organ, is one of the organs to
experience rapid tissue aging, which is frequently accompanied by
renal fibrosis (1,2). Studies have indicated that exercise
is an important means of rehabilitation, which may effectively
improve myocardial fibrosis (3).
Whether exercise is able to improve the renal fibrosis caused by
natural aging requires further investigation.
A large number of studies have suggested that aging
of the kidneys is accompanied by interstitial fibrosis (4), which mainly manifests as an
accumulation of extracellular matrix (ECM). The
epithelial-mesenchymal transition (EMT) of renal tubular epithelial
cells is considered to be one of the major reasons for the marked
accumulation of ECM (5,6). EMT occurs in renal tubular
epithelial cells and induces changes in epithelial surface markers,
including E-cadherin and α-smooth muscle actin (α-SMA), a
myofibroblast surface marker of the tubulointerstitium (7).
Autophagy is a catabolic degradation system used to
digest the unnecessary or damaged components of a cell. The
decrease of autophagic activity caused by aging is closely linked
to the occurrence and development of fibrosis (8). Previous studies have shown that
transforming growth factor (TGF)-β1 is the most critical factor in
promoting the occurrence of EMT (9,10).
In addition, TGF-β1 serves an important role in regulating
autophagy. Ding et al (11) found that the deletion of light
chain (LC)3B [LC3(−/−)] resulted in increased collagen deposition
and increased mature profibrotic factor TGF-β levels in the
obstructed kidneys of mice. Beclin 1 heterozygous (Beclin 1+/−)
mice also exhibited increased collagen deposition in the obstructed
kidneys after UUO. Signaling downstream of the TGF-β receptor
complexes is regulated by the Smads family, a canonical pathway
(12,13). TGF-β1 signaling via the non-Smads
pathways is also involved in the development of fibrosis. Previous
reports have demonstrated that TGF-β1-activated kinase 1 (TAK1), a
member of the mitogen-activating protein (MAP) kinase kinase (MKK)
kinase family, is involved in TGF-β signaling in the non-canonical
pathway (14,15). The decreased autophagic activity
of the ECM is closely linked to the occurrence and development of
fibrosis, and in a fibrosis model, the expression levels of
autophagic proteins Beclin 1 and LC3 were decreased (16). Furthermore, Kim et al
(17) indicated the involvement
of the TGF-β1/TAK1/MKK3/p38MAPK signaling pathway in the induction
of autophagy. Therefore, it may be hypothesized that TGF-β1
improves renal fibrosis by regulating the TAK1/MKK3/p38MAPK
signaling pathway and inducing autophagic activation.
A number of basic and clinical studies have shown
that exercise is able to delay the aging of skeletal muscle and
brain tissue (18,19) and improve cardiopulmonary exercise
function. Therefore, the purpose of the present study was to
subject aged mice to incremental load training, to compare and
observe whether such incremental load training leads to an
improvement of renal fibrosis in aged mice, and to further clarify
whether the underlying mechanisms include the
TGF-β1/TAK1/MMK3/p38AMPK signaling pathway and induction of
autophagic activation. The results may provide an experimental
basis for the development of novel interventions to prevent and
treat renal fibrosis.
Materials and methods
Experimental animals
A total of 36-healthy male C57/BL mice
(19-month-old, n=24; weight, 26-28 g; and 2-month-old, n=12;
weight, 14-16 g) were purchased from the Laboratory Animal Breeding
and Research Center, Army Medical University (Chongqing, China;
license no. SYXK-PLA-20170002). All surgical and care procedures
were approved by the Laboratory Animal Welfare and Ethics Committee
of the Third Military Medical University (Chongqing, China). All
mice were housed in cages in a constant environment with 55±10%
humidity, a temperature of 20±5°C and a 12-h light/dark cycle. Food
and water were provided ad libitum.
Animal grouping and exercise
training
The 36 healthy male C57/BL mice were divided into
three groups: Young control group (YC group, n=12), elderly control
group (OC group, n=12) and elderly exercise group (OY group, n=12).
The OC group received free food and water. Exercise training was
performed 5 days/week in the same room in which the animals were
housed, for a duration of 6 weeks in which the load was gradually
increased (low-intensity activity, referring to a movement speed
≤10 m/min, and exercise intensity of 30-40% VO2 max)
(20,21). At the end of the exercise training
period, the mice were sacrificed by cervical dislocation. Certain
kidney tissues were fixed with neutral formaldehyde and the
remaining tissues were rapidly frozen in liquid nitrogen. The
exercise protocol is shown in Table
I (22).
 | Table IExercise training protocol. |
Table I
Exercise training protocol.
| Week | Session time
(min) | Rotations/min | Length (m) |
|---|
| 1 | 15 | 16 | 48 |
| 2 | 30 | 16 | 96 |
| 3 | 30 | 20 | 120 |
| 4 | 45 | 20 | 180 |
| 5 | 60 | 20 | 240 |
| 6 | 60 | 24 | 288 |
Reverse transcription-semiquantitative
PCR (RT-sqPCR) analysis
The synthesis of primers was performed by Shanghai
Bioengineering Co, Ltd. The purity and concentration of RNA were
detected with a nucleic acid protein analyzer following extraction
with TRIzol reagent. The RT of RNA into complementary (c)DNA was
performed (cat. no. T2240; Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature. The total volume of the
PCR mixture was 25 μl, which consisted of 15 μl 2X GO
Tap Green Mix (Takara Bio, Inc.), 2 μl forward primer, 2
μl reverse primer, 1 μl cDNA and 5 μl DEPC.
The PCR amplification protocol was as follows: Pre-denaturation at
95°C for 5 min, denaturation at 95°C for 30 sec and renaturation at
55°C for 30 sec (35 cycles) (C1000 Touch™ Thermal Cycler; Bio Rach
Laboratories, Inc.). Amplification products were separated via
agarose gel electrophoresis (Sigma-Aldrich; Merck KGaA). Image-Pro
Plus 6.0 image analysis software (Media Cybernetics, Inc.) was used
to automatically analyze the images and calculate the gray scale
values. The sequences of the primers of the target genes were as
follows: Beclin 1, forward 5′-ATG GAG GGG TCT AAG GCG TC-3′ and
reverse 5′-TGG GCT GTG GTA AGT A AT GA-3′; LC3II forward 5′-GAC CGC
TGT AAG GAG GTG-3′ and reverse 5′-AGA AGC CGA AGG TTT CTT G-3′;
β-actin, forward primer 5′-GTG ACG TTG ACA TCC GTA-3′ and reverse
5′-GTA ACA GTC CGC CTA-3′.
Masson's staining
The 4-μm-thick kidney tissue slices were
dehydrated through a graded series of ethanol. According to the
protocol of the Masson's staining kit (cat. no. G1340; Beijing
Solarbio Science and Technology, Co., Ltd.), the procedure was as
follows: Tissues were stained with Weigert's hematoxylin in acid
solution for 5 min, replaced with Masson's blue solution and
incubated for 3 min, followed by rinsing in distilled water for 1
min, staining for 5 min with Ponceau acid fuchsin, differentiation
in phosphomolybdic acid solution for 2 min, rinsing in distilled
water for 1 min, immediately stained with aniline blue solution for
5 min and mounted with neutral gum sealant (all steps at room
temperature). Pathological changes in the kidney tissues and
collagen fibers in mice were observed under an inverted microscope.
The percentage of fibrosis was determined as the blue area divided
by the area of the entire field.
Immunohistochemical staining
The kidney tissue sections were deparaffinized and
antigen retrieval was performed using a microwave oven at high
temperature (300 W) for 30 min. Following the exhaustion of
endogenous peroxidase with methanol and hydrogen peroxide for 30
min at room temperature, the slides were blocked with 0.5% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) for 60 min at 37°C and
incubated with TAK1 antibody (1:200 dilution; cat. no. ab109526;
Abcam) overnight at 4°C. The SABC staining kit (cat. no. SA1026;
Boster Biological Technology) was used to perform the chromogenic
reaction. Following rinsing in PBS, the samples were incubated with
the mouse anti-rabbit antibody (cat. no. SA1026; Boster Biological
Technology) at 37°C for 30 min, followed by rinsing in PBS three
times for 5 min. The samples were covered in a drop of DAB
developing solution, cell nuclei were stained with hematoxylin at
room temperature for 5 min, following dehydration with an alcohol
gradient, the samples were cleared with dimethylbenzene xylene and
mounted with neutral gum sealant. Photomicrographs of different
fields of view were captured using an Olympus DSX100 optical
microscope (Olympus Corporation; magnification, ×400). and the
average optical density (OD) was calculated using Image-pro plus
6.0 analysis software (Media Cybernetics, Inc.).
Immunofluorescence staining
The immunohistochemical staining steps were followed
until incubation with the primary antibodies. The samples were
incubated with Beclin 1 (1:100 dilution; cat. no. 3738; Cell
Signaling Technology, Inc.) or LC3 (1:100 dilution; cat. no. 3868;
Cell Signaling Technology, Inc.) overnight at 4°C. Following
thorough washing with PBS, the samples were incubated with
fluorescently labeled mouse anti-rabbit (1:2,000 dilution; cat. no.
sc-516248; Santa Cruz Biotechnology, Inc.) at 37°C for 3 h
(protected from light) and then rinsed in PBS three times for 5
min. The cell nuclei were stained with Hoechst (cat. no. sc-200908;
Santa Cruz Biotechnology, Inc.) at room temperature for 5 min, and
following mounting with a drop of antifade solution, images were
captured with a laser scanning confocal microscope. Image-pro plus
6.0 image analysis software (Media Cybernetics, Inc.) was used to
automatically analyze the images and calculate the average optical
density.
Western blot analysis
The kidney tissues were obtained by homogenization
in a tissue protein extraction reagent supplemented with complete
protease and phosphatase inhibitors. The lysate was centrifuged
(12,000 × g) at 4°C for 5 min and the supernatant was used. The
total protein concentrations were determined using a bicinchoninic
acid protein assay kit (Thermo Fisher Scientific, Inc). Protein
buffer and 8% glycerin were added to degenerate the protein for 10
min, and equal quantities of protein (40 μg/lane) were
resolved by 10% SDS-PAGE, followed by transferred onto
polyvinylidene difluoride (PVDF) membranes with a transfer
apparatus (Bio-Rad Laboratories, Inc.). The PVDF membranes were
incubated in 5% nonfat milk containing Tris-buffered saline
containing Tween-20 (TBST) for 3 h at room temperature. The PVDF
membranes were sequentially incubated with primary antibodies
against MKK3 (cat. no. 8535; Cell Signaling Technology, Inc.),
phosphorylated (p)-MKK3 (cat. no. 12280; Cell Signaling Technology,
Inc.), p38 MAPK (cat. no. ab31828; Abcam), p-p38MAPK (cat. no.
ab4822; Abcam) and GAPDH (cat. no. AF0006; Shanghai Boyun Biotech
Co., Ltd.) at a dilution of 1:1,000 overnight at 4°C. Following
incubation with primary antibody, the membranes were washed in a
TBST four times for 10 min and incubated in mouse anti-rabbit IgG
(H&L)-horseradish peroxidase conjugate (1:1,500 dilution; cat.
no. sc2357; Santa Cruz Biotechnology, Inc.) for 3 h at room
temperature. The blots were visualized using Enhanced
Chemiluminescence Western Blotting Luminol Reagent (GE Healthcare)
and were semi-quantified using Image-Pro Plus 6.0 image analysis
software (Media Cybernetics, Inc.).
Transmission electron microscopy
The kidney tissue was evaluated by autophagosome
screening under a JEM-1010 transmission electron microscope
(Matsunaga Manufacturing, Co., Ltd., Gifu, Japan). The tissue
samples were fixed with 1% OsO4 in PBS (pH 7.0) for 2 h
and washed three times in PBS, and were then dehydrated with a
series of ethanol concentrations for 15-min intervals. The kidney
tissues were infiltrated with propylene oxide, and embedded in a
mixture of pure Araldite 502 resin and acetone at room temperature
for 24 h, 48°C for 48 h and 60°C for 48 h, and then cut into 50-60
nm sections. Finally, the sections were cut into ultrathin
sections, and observed with a transmission electron microscope.
Statistical analysis
Statistical analysis of experimental results was
performed using GraphPad Prism 5 (GraphPad Software, Inc.), ImageJ
1.8.0 (National Institutes of Health), Image-Pro Plus 6, and SPSS
22.0 (IBM Corp.) statistical software. All data are expressed as
the mean ± SD. Differences among groups were analyzed by one-way
analysis of variance followed by Tukey's post hoc test using SPSS
22.0. P<0.05 was considered to indicate a statistically
significant difference.
Results
Incremental load training ameliorates
pathological changes of kidney tissue and reduces collagen
deposition in aged mice
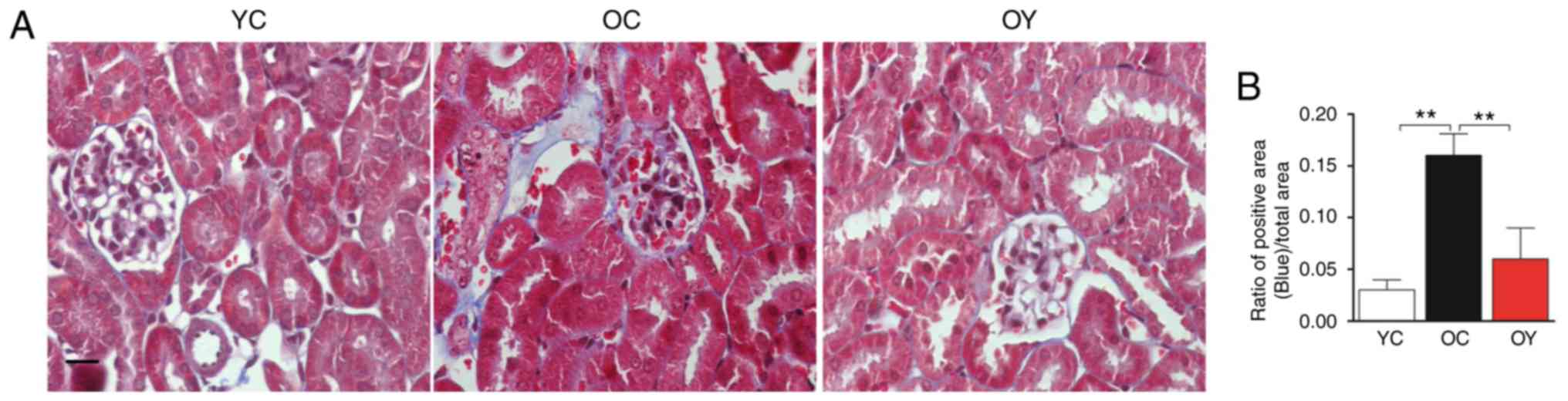
Masson's staining is the most classical method of
collagen fiber staining, with regions of blue staining representing
collagen fiber. Microscopic observation revealed normal glomerular
morphology and tubular structure in the YC group; furthermore,
almost no collagen fibers were produced. In the OC group,
glomerular atrophy, an increased level of cytolysis, decreased gaps
between the junctions of renal tubular epithelial cells, thickening
of the renal tubular wall and deposition of collagen fibers were
observed. In the OY group, glomerular atrophy was observed,
although the renal tubule walls were not thickened and there was no
obvious tubulointerstitial fibrosis (Fig. 1A). Collagen deposition was
significantly increased in the OC group compared with that in the
YC group (P<0.01) and, compared with that in the OC group
collagen deposition was significantly decreased following
incremental load training (P<0.01) (Fig. 1B). The above results demonstrated
that natural aging may significantly increase the deposition of
collagen fibers in kidney tissues and that incremental load
training may reduce the deposition of collagen fibers.
Incremental load training postpones EMT
changes in aged mice
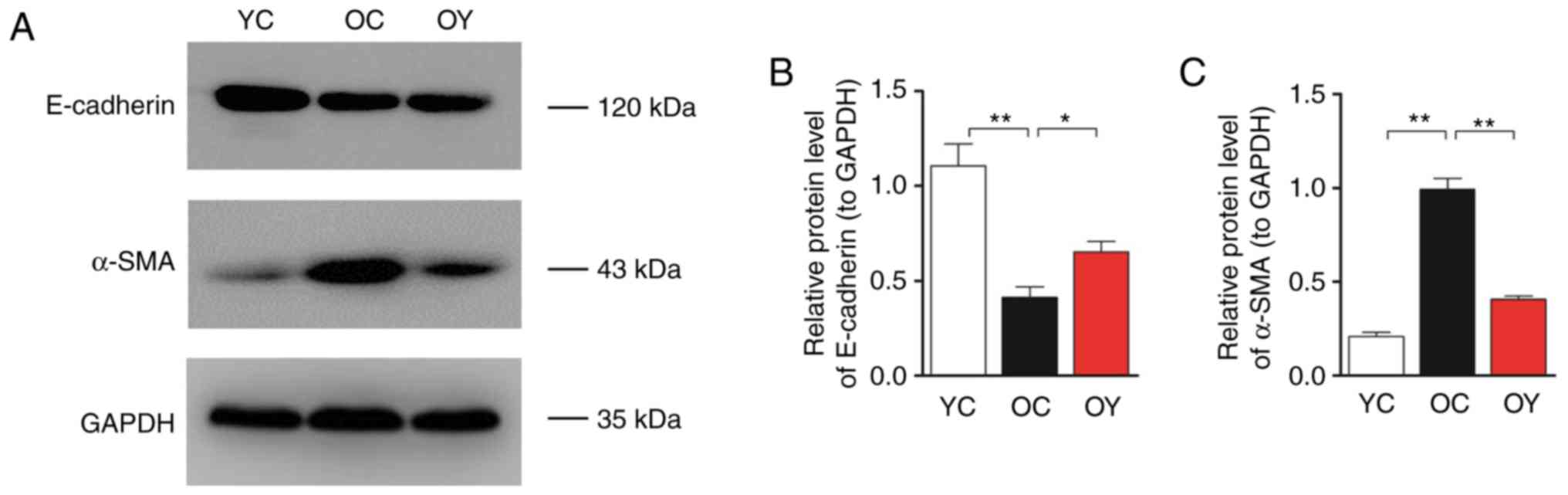
E-cadherin and α-SMA are markers of mesenchymal
changes in renal tubular epithelial cells, therefore, their
expression in renal tissue reflects the degree of fibrosis. Western
blot analysis was used to assess the protein levels of E-cadherin
and α-SMA (Fig. 2A). The bar
graph suggests that the expression levels of E-cadherin in the OC
group were lower than those in the YC group (P<0.01), whereas
the expression levels of α-SMA in the OC group were significantly
higher than those in the YC group (P<0.01). Following
incremental load training, the expression levels of E-cadherin in
the OY group were significantly higher (P<0.05; Fig. 2B) and the expression levels of
α-SMA were significantly lower than those in the OC group
(P<0.01; Fig. 2C).
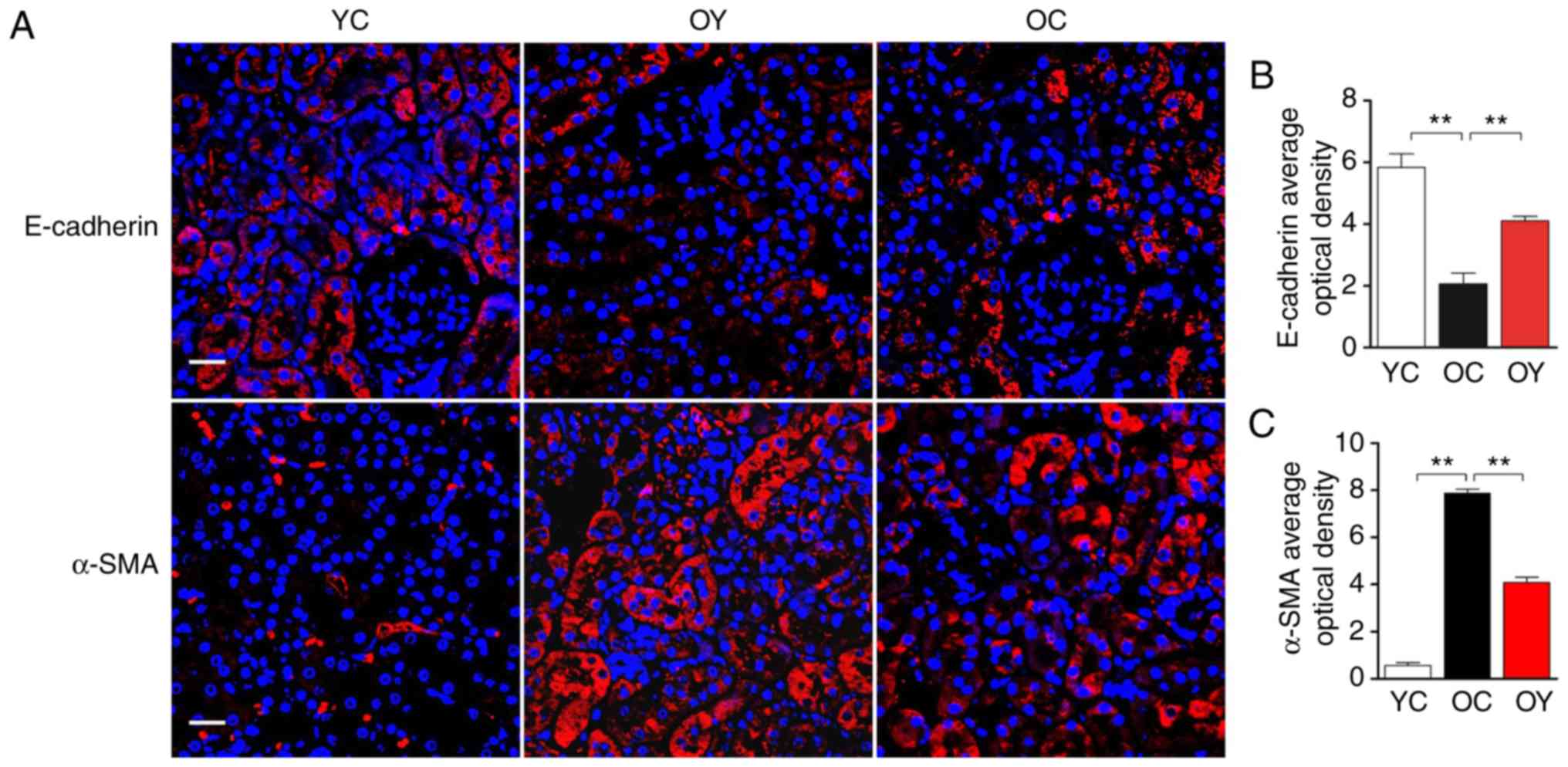
The analysis of immunofluorescence under confocal
laser scanning microscopy indicated that, in the YC group,
E-cadherin-positive signals were present in the renal tubular
epithelium and vascular wall, and a lower level of expression was
observed in the renal tubular junction; in the OC group, only a
slight E-cadherin-positive signal was present in the renal tubular
epithelium. In the OY group, the E-cadherin-positive signals were
weaker than those in the YC group but higher than those in the OC
group (Fig. 3A). The quantitative
analysis suggested that the OD value of the E-cadherin-positive
signal in the OC group was significantly lower than that in the YC
group (P<0.01), and incremental load training significantly
increased the OD value of the E-cadherin-positive signal, which
differed significantly compared with that in the OC group
(P<0.01; Fig. 3B), indicating
that incremental load training increased epithelial cell surface
markers. However, α-SMA exhibited an opposite trend, in the YC
group, α-SMA was only expressed at low levels in the renal tubular
epithelium and vascular wall. In the OC group, the α-SMA-positive
signal was high; there were uniform, strong positive signals in the
renal tubular epithelium and vascular wall, and α-SMA was also
expressed at low levels in the renal tubular junctions and the wall
of the renal capsule. In the OY group, the α-SMA-positive signals
in the above-mentioned regions were weaker than those in the OC
group, but stronger than those in the YC group (Fig. 3A). The quantitative analysis
suggested that the OD value of the α-SMA-positive signal was
significantly higher than that in the YC group (P<0.01), and
incremental load training significantly reduced the OD value of the
α-SMA-positive signal, which differed significantly from that in
the OC group (P<0.01), indicating that incremental load training
reduced fibroblast surface markers (Fig. 3C).
According to the above experimental results, it can
be concluded that incremental load training inhibits the EMT of
renal tubular epithelial cells by increasing the expression of
epithelial surface marker E-cadherin and decreasing the expression
of fibroblast surface marker α-SMA. Subsequently, the specific
molecular mechanisms were investigated. Previous studies have
suggested that the TGF-β1/TAK1/MKK3/p38MAPK pathway is closely
linked to the occurrence and development of EMT. Changes in the
expression of these signaling molecules reflect the development of
renal fibrosis.
Incremental load training decreases the
activity of the TGF-β1/TAK1/MKK3/p38 MAPK signaling pathway
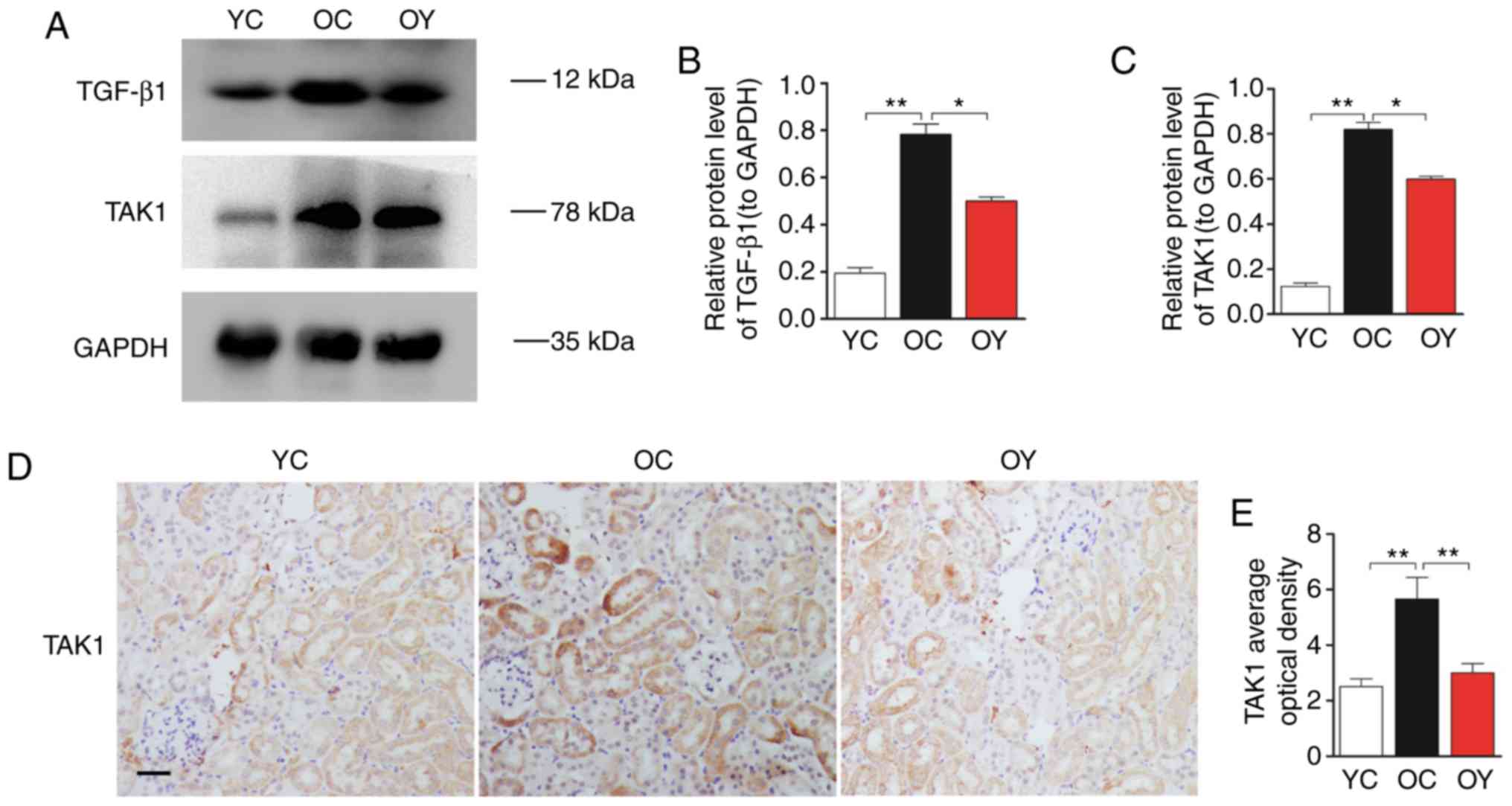
Western blot analysis was used to assess the protein
levels of TGF-β1 and TAK1 (Fig.
4A). The quantitative analysis suggested that the expression
levels of TGF-β1 and TAK1 in the OC group were higher than those in
the YC group (P<0.01). Following incremental load training, the
expression levels of TGF-β1 and TAK1 in the OY group were
significantly lower than those in the OC group (P<0.05; Fig. 4B and C). Immunohistochemical
staining indicated that the TAK1-positive signal was weak in the YC
group. In the OC group, the TAK1-positive signal was strong and was
located in the mesangial cells and renal tubular epithelial cells.
In the OY group, the TAK1-positive signals in the above-mentioned
regions were weaker than those in the OC group, but were stronger
than those in the YC group (Fig.
4D). The OD value of the TAK1-positive signal was significantly
higher than that in the YC group (P<0.01). Incremental load
training significantly reduced the OD value of the TAK1-positive
signal, which differed significantly compared with that in the OC
group (P<0.01; Fig. 4E).
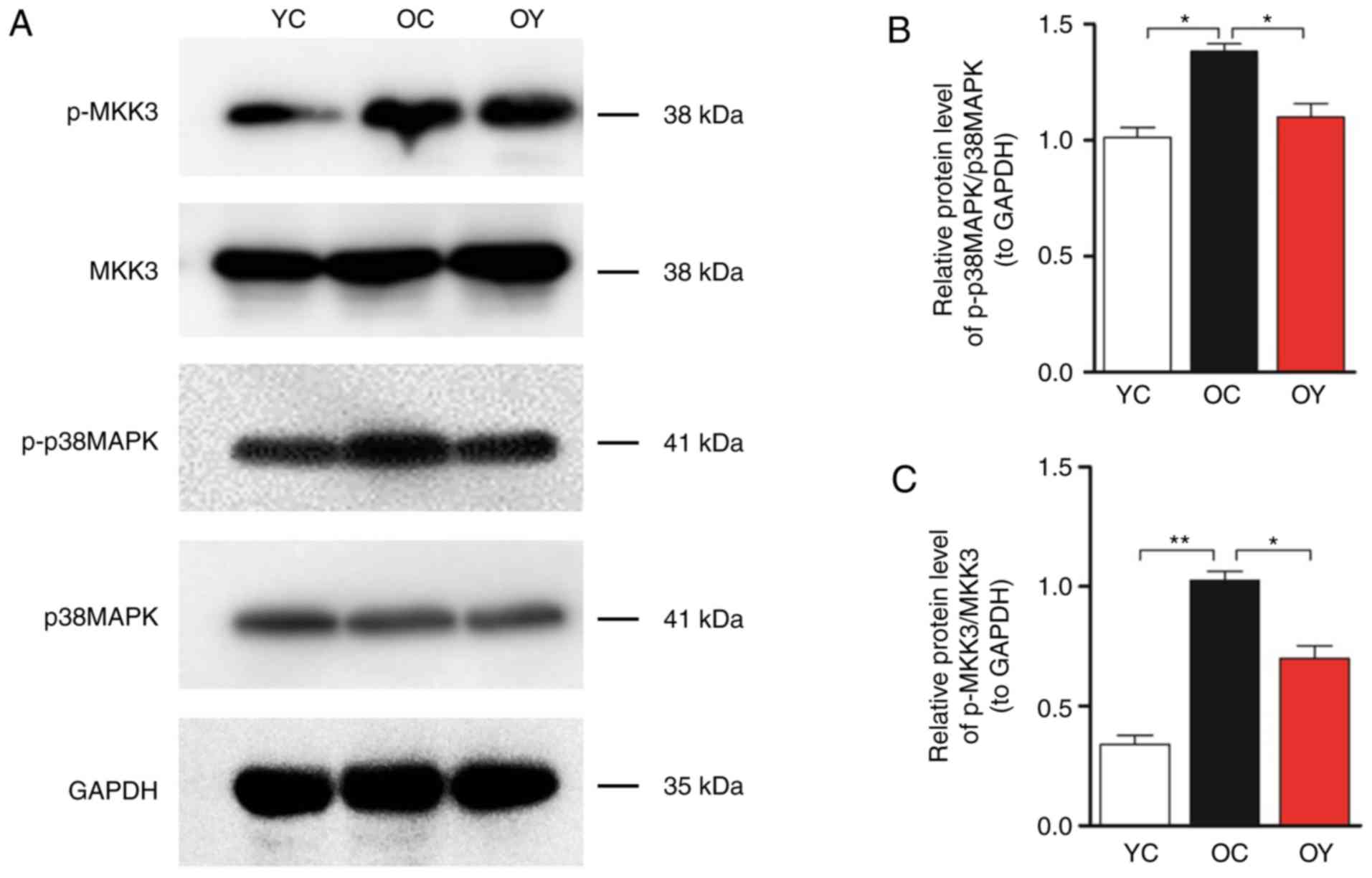
Western blot analysis of MKK3, p-MKK3, p38MAPK and
p-p38MAPK was then performed (Fig.
5A). The quantitative analysis indicated that the levels of
p-MKK3/MKK3 (P<0.01) and p-p38 MAPK/p38 MAPK (P<0.05) in the
OC group were significantly higher than those in the YC group,
whereas incremental load training significantly reduced the levels
of p-MKK3/MKK3 and p-p38MAPK/p38MAPK compared with those in the OC
group (P<0.05; Fig. 5B and
C).
Incremental load training enhances
autophagy markers Beclin 1 and LC3
The results of the RT-sqPCR analysis suggested that
the mRNA expression levels of Beclin 1 (P<0.01) and LC3
(P<0.05) in the OC group were lower than those in the YC group,
whereas incremental load training significantly increased the mRNA
levels of Beclin 1 (P<0.01) and LC3 (P<0.05) compared with
those in the OC group (Fig.
6A-C). The immunofluorescence staining indicated that, in the
YC group, the signal of Beclin 1 was high and uniform, and positive
signals were observed in the basal region of the renal tubular
epithelium in addition to the renal capsule wall and vascular wall.
In the OC group, the positive signal of Beclin 1 was weak, and
Beclin 1 was uniformly expressed at low levels in the renal tubular
epithelium. In the OY group, the overall expression in the
above-mentioned regions was higher than that in the OC group
(Fig. 6D). The quantitative
analysis indicated that the OD value of the Beclin 1-positive
signal in the OC group was significantly lower than that in the YC
group (P<0.01; Fig. 6E),
whereas incremental load training significantly increased the OD
value of the Beclin 1-positive signal compared with that in the OC
group (P<0.01; Fig. 6E). In
the YC group, the LC3-positive signals were observed in the renal
tubular epithelium and vascular wall, and a low level of positive
expression was present in the renal tubular junction; in the OC
group, LC3 was only expressed at low levels in the renal tubular
epithelium. In the OY group, the LC3-positive signals were weaker
than those in the YC group, but stronger than those in the OC group
(Fig. 6D). The quantitative
analysis suggested that the OD value of the LC3-positive signal in
the OC group was significantly lower than that in the YC group
(P<0.01), whereas incremental load training significantly
increased the OD value of the LC3-positive signal compared with
that in the OC group (P<0.01; Fig.
6F). In the YC group, an increased number of autophagosomes
with typical bilayer membrane morphology was observed by
transmission electron microscopy. The numbers of intracellular
autophagosomes in the OY group was increased compared with that in
the OC group, indicating that incremental load training increased
autophagic activity (Fig.
6G).
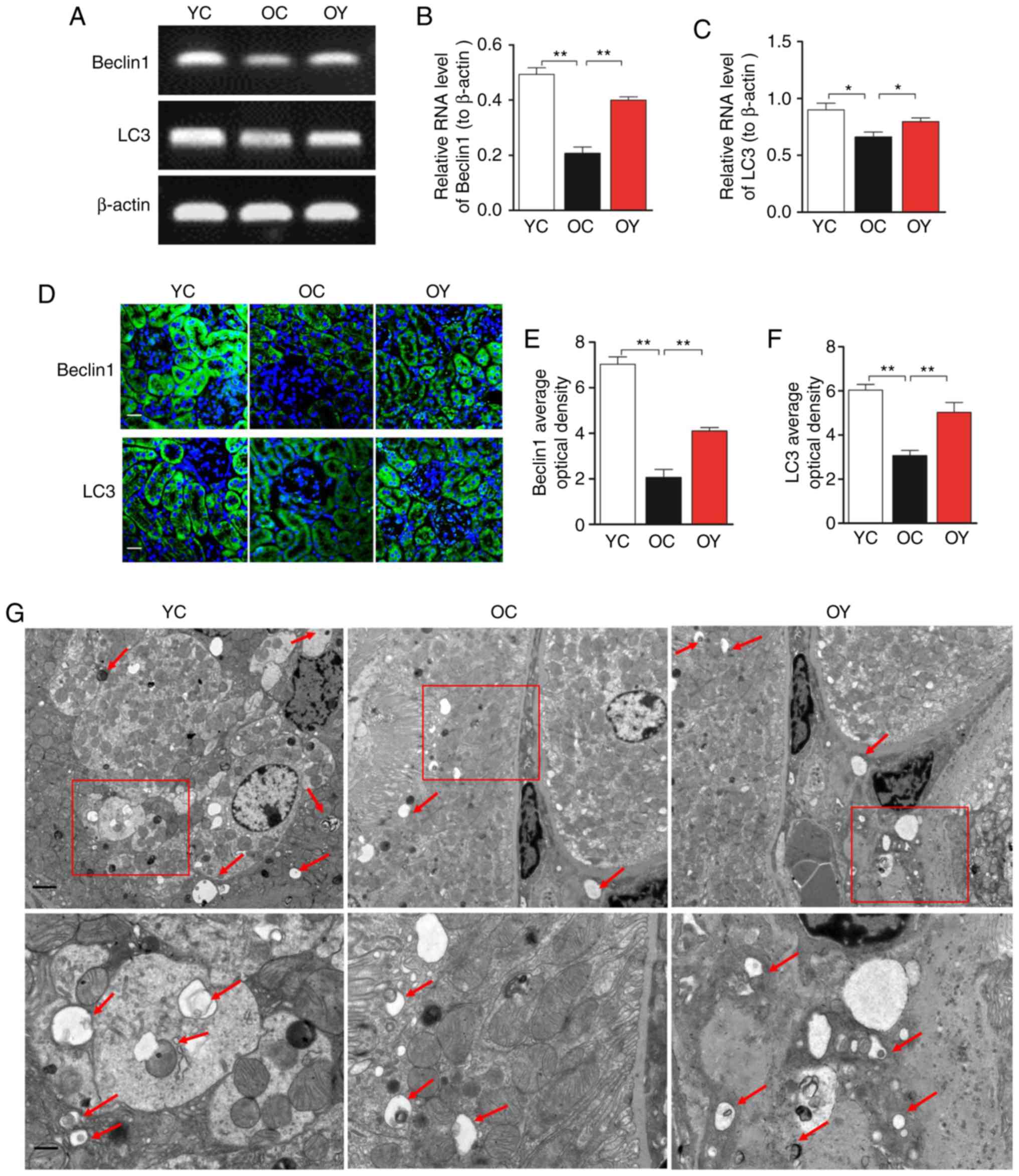 | Figure 6Effects of incremental load training
on autophagic markers Beclin 1 and LC3 in the kidney tissues of
aged mice. (A) Comparison of the effect of incremental load
training on the relative mRNA expression of Beclin 1 and LC3 in
kidney tissues. Bar graphs show the relative quantification of (B)
Beclin 1 and (C) LC3 mRNA. (D) Expression of Beclin 1 and LC3 was
assessed by immunofluorescence staining (magnification, ×200; scale
bar=20 μm). The green color indicates the positive signal;
cell nuclei were counterstained with DAPI (blue). Bar graphs show
the relative OD values of (E) Beclin1 and (F) LC3 staining. Values
are expressed as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01. (G) Assessment of
autophagic activity by transmission electron microscopy
(magnification, ×6,000 and ×15,000; scale bars, 2 and 1 μm).
The arrows indicate autophagic vacuoles. YC, young control group;
OC, elderly control group; OY, elderly exercise group; OD, optical
density; LC3, light chain 3. |
Discussion
The present study indicated the presence of slight
pathological structural changes and collagen deposition in
naturally aged kidney tissues. Decreases in the epithelial surface
marker E-cadherin and the autophagic markers Beclin 1 and LC3, and
an increase of the fibroblast surface marker α-SMA were also
observed. Incremental load training was shown to inhibit the
TGF-β1/TAK1/MKK3/P38MAPK signaling pathway and enhance autophagic
activity, to further reduce collagen deposition, and to indirectly
upregulate E-cadherin and downregulate α-SMA, thereby improving
renal fibrosis induced by collagen deposition in aged mice.
With the advent of an aging population, health
problems caused by aging and age-related diseases have become
increasingly prominent. The results of an epidemiological survey
indicated that diseases linked to renal aging have become a threat
to the health and life of middle-aged and elderly individuals
(23). A large number of studies
have shown that aged kidney tissues exhibit notable fibrosis
(24,25). Sangaralingham et al
(26) performed Sirius red
staining on the renal tissues of rats of different ages, and the
results indicated that, as age increased, the deposition of
collagen fibers in the cortex and medulla increased significantly.
Ning et al (27) performed
Masson's staining and PAS staining to reveal that, compared with a
young age group, the glomerular basement membrane and renal tubular
wall in an older age group were thickened and fibrosis was present.
In the present study, Masson's staining was used to observe
2-month-old and 19-month-old mice, and the results suggested the
presence of glomerular atrophy, increased cytolysis and marked
deposition of collagen fibers in the aged mice, indicating fibrosis
in the kidney tissues of the aged mice, which is consistent with
the results of previous studies.
Exercise is one of the effective rehabilitation
methods to delay aging, which may delay the aging of skeletal
muscle and brain tissue (28-30). Previous studies have indicated
that exercise may also improve glomerular filtration function and
glomerulosclerosis in obese individuals (31). Furthermore, the effect of exercise
on improving renal fibrosis caused by disease in models has been
demonstrated. Peng et al (32) subjected rats with chronic kidney
disease to swimming exercise and revealed that this exercise
reduced the expression of α-SMA, matrix metalloproteinase-2 and
CD34, inhibited the process of epithelial cell transdifferentiation
and reduced the deposition of ECM, thereby improving renal
fibrosis. However, whether exercise is able to improve the renal
fibrosis caused by natural aging has not been reported previously.
In the present study, 12 aged mice were trained using rotarod
treadmill incremental load training for 6 weeks, and the results
demonstrated that the level of cytolysis in the kidney tissues of
the exercise group was lower than that in the non-training group
and the level of collagen fiber deposition was significantly lower
than that in the control group, suggesting that incremental load
training is able to delay renal fibrosis in aged mice.
Renal fibrosis is mainly characterized by the
excessive deposition of ECM components, and EMT is an important
factor leading to renal fibrosis. In this process, epithelial cells
initiate the reprogramming of gene expression, which is manifested
by a decrease in epithelial surface marker E-cadherin and increase
in fibroblast surface marker α-SMA. In a rat model of renal
fibrosis, El-Wakeel et al (33) demonstrated that the expression of
E-cadherin decreased and the expression of α-SMA increased
significantly. Similar results were also obtained in rat models of
natural aging-associated renal fibrosis and of ureteral occlusion
renal fibrosis (34,35). The results of the
immunofluorescence analysis in the present study also indicated
that the positive signals of α-SMA in the YC group were weak, and
that α-SMA was uniformly expressed at low levels in the renal
tubular epithelium; furthermore, the positive signal of α-SMA in
the OC group was strong, and numerous positive signals were
observed in the renal tubular epithelium, also being expressed in
the wall of the renal capsule. However, the positive signal of
E-cadherin exhibited an opposite trend, suggesting that incremental
load training may delay EMT, reduce the deposition of collagen
fibers and improve renal fibrosis in aged mice.
Autophagy is a conserved process of cell component
degradation and cycling depending on lysosomes that eliminates
damaged organelles and abnormal proteins to maintain a stable
intracellular environmental and stress response (36). To date, numerous basic and
clinical studies have reported on the critical function of
autophagy in the occurrence and development of fibrosis (37,38). Ceylan-Isik et al (37) indicated that aging-induced cardiac
interstitial fibrosis is closely linked to the decrease of
autophagic activity. Histological examination revealed that
interstitial fibrosis in aged mice was significantly aggravated,
with a reduction of autophagic markers Beclin-1, autophagy related
(Atg)7, Atg5 and LC3. TGF-β1 is a multipotent cytokine that has
been established as a central mediator of kidney fibrosis (39). TGF-β1 is closely linked to the
occurrence and development of autophagy, which results in a
decrease of autophagic flux (40). TAK1 is one of the MKK kinases that
is involved in TGF-β signal transduction in TGF-β1-induced type I
collagen and fibronectin expression through the activation of
MKK3/p38 (41). Kim et al
(42) demonstrated that the
TAK1/MKK3/p38 signaling pathway mediated the induction of autophagy
by TGF-β1, controlling the level of type I collagen. In the present
study, the immunohistochemical analysis of TAK1 indicated that
numerous positive signals were present in the renal tubular
epithelium in the OC group, and TAK1 was also expressed in the wall
of the renal capsule; however, in the YC group, limited positive
signals were observed in the renal tubular epithelium. The results
of the western blot analysis indicated that the levels of TGF-β1,
TAK1, p-MKK3 and p-p38MAPK in the OC group were significantly
higher than those in the YC group. Furthermore, the levels were
decreased following incremental load training. The above results
suggest that incremental load training is able to improve renal
fibrosis in aged mice by regulating the TGF-β1/TAK1/MKK3/p38 MAPK
signaling pathway, enhancing autophagic activity and the expression
of Beclin 1 and LC3, increasing the expression of E-cadherin,
downregulating the expression of α-SMA and reducing collagen
deposition, thus improving aging-associated renal fibrosis.
In conclusion, the present study first indicated
that incremental load training, as an important rehabilitation
intervention, was able to delay renal fibrosis in aged mice, and
its mechanism is may linked to inhibition of the
TGF-β1/TAK1/MKK3/p38MAPK signaling pathway, increases in the
expression of Beclin 1 and LC3, the downregulation of α-SMA and the
upregulation of E-cadherin. However, the direct effects of
incremental load training on the TGF-β1/TAK1/MKK3/p38MAPK signaling
pathway in delaying renal fibrosis in aged mice remains uncertain.
In particular, the further use of TGF-β1 inhibitors is required to
determine the direct effect of the TGF-β1/TAK1/MKK3/p38MAPK
signaling pathway on renal fibrosis in aged mice.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National
Natural Science Foundation of China (grant nos. 31500969 to HL and
31471148 to ZY).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BS and HLL contributed equally to this study; HLL
conceived and designed the experiments; CCB performed the
experiments; QL analyzed the data; QYC prepared figures; ZY
collected the data, and edited and revised the manuscript; all
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All surgical and care procedures were approved by
the Laboratory Animal Welfare and Ethics Committee of the Third
Military Medical University (Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y: Renal fibrosis: New insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang HC and Fogo AB: Fibrosis and renal
aging. Kidney Int Suppl. 4:75–78. 2014. View Article : Google Scholar
|
|
3
|
Wang B, Xu M, Li W, Li X, Zheng Q and Niu
X: Aerobic exercise protects against pressure overload-induced
cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation.
PLoS One. 12:pp. e01796482017, View Article : Google Scholar
|
|
4
|
Zeina K and Jennifer T: Anatomic and
physiologic changes of the aging kidney. Clin Geriatr Med.
29:555–564. 2013. View Article : Google Scholar
|
|
5
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hinz B, Phan SH, Thannickal VJ, Galli A,
Bochaton-Piallat ML and Gabbiani G: The myofibroblast: One
function, multiple origins. Am J Pathol. 170:1807–1816. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harikrishna T, Xu XC, Polosukhin VV,
Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG,
Blackwell TS and Lawson WE: Contribution of epithelial-derived
fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit
Care Med. 180:657–665. 2009. View Article : Google Scholar
|
|
8
|
Fîlfan M, Sandu RE, Zăvăleanu AD, GreşiŢă
A, Glăvan DG, Olaru DG and Popa-Wagner A: Autophagy in aging and
disease. Rom J Morphol Embryol. 58:27–31. 2017.PubMed/NCBI
|
|
9
|
Samy L, Jian X and Rik D: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar
|
|
10
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding Y, Kim SL, Lee SY, Koo JK, Wang Z and
Choi ME: Autophagy regulates TGF-β expression and suppresses kidney
fibrosis induced by unilateral ureteral obstruction. J Am Soc
Nephrol. 25:2835–2846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Islam SS, Mokhtari RB, El Hout Y, Azadi
MA, Alauddin M, Yeger H and Farhat WA: TGF-β1 induces EMT
reprogramming of porcine bladder urothelial cells into collagen
producing fibroblasts-like cells in a Smad2/Smad3-dependent manner.
J Cell Commun Signal. 8:39–58. 2014. View Article : Google Scholar
|
|
13
|
Kaur A, Riaz M, Singh SK and Kishore U:
Human surfactant protein D suppresses epithelial-to-mesenchymal
transition in pancreatic cancer cells by downregulating TGF-β.
Front Immunol. 15:18442018. View Article : Google Scholar
|
|
14
|
Kajino T, Omori E, Ishii S, Matsumoto K
and Ninomiya-Tsuji J: TAK1 MAPK kinase kinase mediates transforming
growth factor-beta signaling by targeting SnoN oncoprotein for
degradation. J Biol Chem. 282:9475–9481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi ME, Ding Y and Kim SI: TGF-β
signaling via TAK1 pathway: Role in kidney fibrosis. Semin Nephrol.
32:244–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Chen K, Li S, Feng J, Liu T, Wang F,
Zhang R, Xu S, Zhou Y, Zhou S, et al: Protective effect of fucoidan
fromFucus vesiculosuson liver fibrosis via the TGF-β1/Smad
pathway-mediated inhibition of extracellular matrix and autophagy.
Drug Des Devel Ther. 10:619–630. 2016.
|
|
17
|
Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ and
Choi ME: Autophagy promotes intracellular degradation of type I
collagen induced by transforming growth factor (TGF)-β1. J Biol
Chem. 287:11677–11688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banks L, Buchan TA and Dizonno V: Aerobic
exercise attenuates ageing of the athletic heart. J Physiol.
594:3183–3184. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inoue A, Cheng XW, Huang Z, Hu L, Kikuchi
R, Jiang H, Piao L, Sasaki T, Itakura K, Wu H, et al: Exercise
restores muscle stem cell mobilization, regenerative capacity and
muscle metabolic alterations via adiponectin/AdipoR1 activation in
SAMP10 mice. J Cachexia Sarcopenia Muscle. 8:370–385. 2017.
View Article : Google Scholar :
|
|
20
|
Baker EJ and Gleeson TT: The effects of
intensity on the energetics of brief locomotor activity. J Exp
Biol. 202:3081–3087. 1999.PubMed/NCBI
|
|
21
|
Bedford TG, Tipton CM, Wilson NC, Oppliger
RA and Gisolfi CV: Maximum oxygen consumption of rats and its
changes with various experimental procedures. J Appl Physiol Respir
Environ Exerc Physiol. 47:1278–1283. 1979.PubMed/NCBI
|
|
22
|
Di Felice V, Macaluso F, Montalbano A,
Gammazza AM, Palumbo D, Angelone T, Bellafiore M and Farina F:
Effects of conjugated linoleic acid and endurance training on
peripheral blood and bone marrow of trained mice. J Strength Cond
Res. 21:193–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Sullivan ED, Hughes J and Ferenbach DA:
Renal aging: Causes and consequences. J Am Soc Nephrol. 28:407–420.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou CL, Wang MJ, Sun C, Huang Y, Jin S, Mu
XP, Chen Y and Zhu YC: Protective effects of hydrogen sulfide in
the ageing kidney. Oxid Med Cell Longev. 2016.7570489:2016.
|
|
25
|
Lin CH, Chen J, Ziman B, Marshall S,
Maizel J and Goligorsky MS: Endostatin and kidney fibrosis in
aging: A case for antagonistic pleiotropy? . Am J Physiol Heart
Circ Physiol. 306:H1692–H1699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sangaralingham SJ, Heublein DM, Grande JP,
Cataliotti A, Rule AD, McKie PM, Martin FL and Burnett JC Jr:
Urinary C-type natriuretic peptide excretion: A potential novel
biomarker for renal fibrosis during aging. Am J Physiol Renal
Physiol. 301:943–952. 2011. View Article : Google Scholar
|
|
27
|
Ning YC, Cai GY, Zhuo L, Gao JJ, Dong D,
Cui S, Feng Z, Shi SZ, Bai XY, Sun XF and Chen XM: Short-term
calorie restriction protects against renal senescence of aged rats
by increasing autophagic activity and reducing oxidative damage.
Mech Ageing Dev. 134:570–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reyes DRA, Gomes MJ, Rosa CM, Pagan LU,
Zanati SG, Damatto RL, Rodrigues EA, Carvalho RF, Fernandes AAH,
Martinez PF, et al: Exercise during transition from compensated
left ventricular hypertrophy to heart failure in aortic stenosis
rats. J Cell Mol Med. 23:1235–1245. 2019. View Article : Google Scholar :
|
|
29
|
Zhou CN, Chao FL, Zhang Y, Jiang L, Zhang
L, Luo YM, Xiao Q, Chen LM and Tang Y: Sex differences in the white
matter and myelinated fibers of APP/PS1 mice and the effects of
running exercise on the sex differences of AD mice. Front Aging
Neurosci. 10:2432018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kou X, Li J, Liu X, Chang J, Zhao Q, Jia
S, Fan J and Chen N: Swimming attenuates D-galactose-induced brain
aging via suppressing miR-34a-mediated autophagy impairment and
abnormal mitochondrial dynamics. J Appl Physiol.
1985.122:1462–1469. 2017. View Article : Google Scholar
|
|
31
|
Martínez R, Kapravelou G, López-Chaves C,
Cáceres E, Coll-Risco I, Sánchez-González C, Llopis J, Arrebola F,
Galisteo M, Aranda P, et al: Aerobic interval exercise improves
renal functionality and affects mineral metabolism in obese Zucker
rats. Am J Physiol Renal Physiol. 316:F90–F100. 2019. View Article : Google Scholar
|
|
32
|
Peng CC, Chen KC, Hsieh CL and Peng RY:
Swimming exercise prevents fibrogenesis in chronic kidney disease
by inhibiting the myofibroblast transdifferentiation. PLoS One.
7:pp. e373882012, View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Wakeel SA, Rahmao RM and EI-Abhar HS:
Anti-fibrotic impact of Carvedilol in a CCl-4 model of liver
fibrosis via serum microRNA-200a/SMAD7 enhancement to bridle
TGF-β1/EMT track. Sci Rep. 8:143272018. View Article : Google Scholar
|
|
34
|
Dong D, Cai GY, Ning YC, Wang JC, Lv Y,
Hong Q, Cui SY, Fu B, Guo YN and Chen XM: Alleviation of senescence
and epithelial-mesenchymal transition in aging kidney by short-term
caloric restriction and caloric restriction mimetics via modulation
of AMPK/mTOR signaling. Oncotarget. 8:16109–16121. 2017.PubMed/NCBI
|
|
35
|
Qi FH, Cai PP, Liu X and Si GM:
Adenovirus-mediated P311 ameliorates renal fibrosis through
inhibition of epithelial-mesen-chymal transition via
TGF-β1-Smad-ILK pathway in unilateral ureteral obstruction rats.
Int J Mol Med. 41:3015–3023. 2018.PubMed/NCBI
|
|
36
|
De Rechter S, Decuypere JP, Ivanova E, van
den Heuvel LP, De Smedt H, Levtchenko E and Mekahli D: Autophagy in
renal diseases. Pediatr Nephrol. 31:737–752. 2016. View Article : Google Scholar
|
|
37
|
Ceylan-Isik AF, Dong M, Zhang Y, Dong F,
Turdi S, Nair S, Yanagisawa M and Ren J: Cardiomyocyte-specific
deletion of endothelin receptor A rescues aging-associated cardiac
hypertrophy and contractile dysfunction: Role of autophagy. Basic
Res Cardiol. 108:3352013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang S, Abdulla R, Lu C and Zhang L:
Inhibition of microRNA-376b protects against renal interstitial
fibrosis via inducing macrophage autophagy by upregulating Atg5 in
mice with chronic kidney disease. Kidney Blood Press Res.
43:1749–1764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding Y and Choi ME: Regulation of
autophagy by TGF-β: Emerging role in kidney fibrosis. Semin
Nephrol. 34:62–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sosulski ML, Gongora R, Danchuk S, Dong C,
Luo F and Sanchez CG: Deregulation of selective autophagy during
aging and pulmonary fibrosis: The role of TGFβ1. Aging Cell.
14:774–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ono K, Ohtomo T, Ninomiya-Tsuji J and
Tsuchiya M: A dominant negative TAK1 inhibits cellular fibrotic
responses induced by TGF-β. Biochem Biophys Res Commun.
307:332–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim SI, Kwak JH, Zachariah M, He Y, Wang L
and Choi ME: TGF-beta-activated kinase 1 and TAK1-binding protein 1
cooperate to mediate TGF-beta1-induced MKK3-p38 MAPK activation and
stimulation of type I collagen. Am J Physiol Renal Physiol.
292:F1471–F1478. 2007. View Article : Google Scholar : PubMed/NCBI
|