Introduction
Small (SK1-3)- and intermediate (SK4)-conductance
Ca2+-activated potassium channels bind intracellular
Ca2+ to produce K+ outflow and transform
calcium signaling into changes in membrane potential. SK4
expression was found to be 9 times higher compared with SK1-3
expression during the development of the atrioventricular node
(AVN) (1). SK2 and SK4 are
expressed in the atrial and pulmonary vein regions in the mature
heart and regulate the late phase of cardiac repolarization
(2,3). These channels are also expressed in
the AVN and sinoatrial node (SAN) (4-6).
Previous studies have demonstrated that SK4 inhibitors can lower
the frequency of action potentials of the SAN (5-7),
whereas mathematical models have predicted that the upregulation of
SK4 would increase the automaticity of SAN cells (5,8).
It was recently reported that SK4 inhibitors exert a
suppressive effect on the pacemaker function of cardiomyocytes
derived from human embryonic stem cells (ESCs) (9), and the SK1-4 agonist EBIO, which
markedly slows the channel deactivation process (10), promoted the differentiation of
mouse and human ESCs and induced pluripotent stem cells (iPSCs)
into pacemaker cells (11,12).
In addition, downregulation of SK4 by RNA interference inhibits
this inducing effect and produces no spontaneously beating
cardiomyocytes, whereas SK1-3 knockdown does not alter EBIO
induction (11). It was
previously demonstrated that overexpression of SK4 plasmids in
mouse ESCs also enhanced the generation of cardiac and pacemaker
cells (13). Another recent study
reported that EBIO can modify the cardiac subtype of human ESCs and
iPSCs (14).
Adipose-derived stem cells (ADSCs) have the
advantages of convenient accessibility, low immunogenicity, and
autolo-gous and allogeneic transplantation (15). ADSCs differentiated into
pacemaker-like cells in a semisolid methylcellulose medium or by
transfecting transcription factor Tbx18 (16,17), which supports the use of ADSCs as
seed cells for biological pacemakers.
The aim of the present study was to examine whether
ADSCs are capable of differentiating into pacemaker-like cells
in vitro by overexpressing SK4 following transduction with
an adenovirus vector carrying the SK4 gene, and to investigate the
mechanisms underlying this differentiation.
Materials and methods
Ethical approval
All animal procedures were performed in agreement
with the Wuhan University institutional guidelines and in
compliance with suggestions from the panel of Euthanasia of the
American Veterinary Medical Association and the National Institutes
of Health Guide for the Care and Use of Laboratory Animals. The
study was approved by the Ethics Committee of Renmin Hospital of
Wuhan University (Wuhan, China).
Isolation and culture of ADSCs
Adult male Sprague Dawley (SD) rats (n=2; 4 weeks
old, weighing 80-100 g) were housed in an environmentally
controlled room at a temperature of 22±1°C and relative humidity
40-60% with a standard 12-h light/dark cycle. Food and water were
provided in the cages. The rats were anesthetized with 3% sodium
pentobarbital (30 mg/kg) by intraperitoneal injection. Following
cessation of pain reflexes, adipose tissue was obtained from the
inguen of the rats. The adipose tissue was cut into
1x1-mm3 pieces and digested with 1 mg/ml collagenase
type I (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C. The homogenate
was centrifuged at 300 x g for 10 min at 25°C, and the cells were
resuspended in Dulbecco's modified Eagle's medium/F12 supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.). Cells were cultured in an incubator at 37°C with a 5%
CO2 atmosphere, grown to 80-90% confluence and passaged
using 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.). Cell
passages 3-5 were used for subsequent experiments. Euthanasia was
conducted via sedation by CO2 followed by cervical
dislocation.
Adenovirus construction and
purification
CMV-MCS-EGFP (Genechem) was digested using
BamHI and AgeI. Polymerase chain reaction (PCR) was
used to amplify the open reading frame sequence of the mouse SK4
gene (GenScript), which was linearized and inserted into a vector
to construct CMV-MCS-EGFP-SK4. The recombinant plasmid was
transformed into DH5a-competent cells (Tiangen) to obtain positive
clones, which were identified using enzyme digestion and
sequencing. Large-scale preparations of recombinant plasmid were
created using the Plasmid Midi Preparation kit (Promega
Corporation). The concentration and purity of the plasmid DNA were
determined by UV absorption. The A260/A280 of the plasmid DNA was
between 1.8 and 2.0. When the density of the 293 cells (ATCC, cat.
no. CRL-1573) reached 50-60%, they were transfected with
CMV-MCS-EGFP-SK4 and the backbone vector pBHG using Lipofectamine
2000 (both from Genechem) for 6 h in an incubator at 37°C with a 5%
CO2 atmosphere. The supernatant was harvested after
virus amplification. Ad-GFP and Ad-SK4 were measured as
2x101 plaque-forming units/ml and preserved at
-80°C.
ADSCs transduced with Ad-SK4 and flow
cytometric analysis
Cells were grown to 70-80% confluence, and Ad-SK4 in
transduction enhancer Polybrene (Yeasen) was added to ADSCs at
different multiplicity of infection (MOI) values (0, 20, 50, 100,
150 and 300). Polybrene was added at the same concentration for 4 h
for different MOI values. The control group was transduced with
Ad-GFP. The medium was replaced after 4 h. Cells were observed
using light and fluorescence microscopy (BX51 systems, Olympus
Corporation). ADSCs with different MOI values were digested with
0.25% trypsin at 48 h after transduction. The cell suspension was
washed twice with phosphate-buffered saline (PBS). Non-transduced
cells served as a negative control. The percentage of GFP-positive
cells was detected using flow cytometric analysis (Becton,
Dickinson and Company).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
qPCR was performed to evaluate the mRNA expression
of mouse SK4, rat SK4, Oct-4, Sox-2, cardiac troponin I (cTnI),
hyperpolarization-activated cyclic nucleotide-gated potassium
channel 4 (HCN4) and transcription factors Tbx18 and Shox2. The
primers used were synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. (Table I). Total
RNA was extracted from the transduced ADSCs after 1 week using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and converted into cDNA using the First Strand cDNA Synthesis
kit (Toyobo Life Science). The PCR conditions were 45 cycles at
95°C for 15 sec and at 58°C for 1 min. RT-qPCR was performed using
the StepOne™ Real-Time PCR system (Thermo Fisher Scientific, Inc.).
Semilog amplification curves were analyzed using the
2-ΔΔCq comparative quantification method (18), and the expression of each gene was
normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
 | Table ISequences of primers for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Sequences of primers for reverse
transcription-quantitative polymerase chain reaction analysis.
| mRNA | Primers 5'-3' | Product size
(bp) |
|---|
| SK4 | Sense:
GTTCTGCACGCTGAGATGTTG | 126 |
| Antisense:
CTTGGCATGGAAGACCACAAT | |
| Oct-4 | Sense:
GTGTCGGAGTGGGGATTGA | 223 |
| Antisense:
AGAAACGGAGAACTACATAGGTCC | |
| Sox-2 | Sense:
GATGCACCGCTACGACGTC | 197 |
| Antisense:
TGGAGTGGGAGGAAGAGGTAAC | |
| Tbx18 | Sense:
GGAGACTTGGATGAGACAAGTGAT | 282 |
| Antisense:
TTGGCAAATGGATTCCTGTCT | |
| Shox2 | Sense:
ATCCAGACGCTTTTATGCGC | 214 |
| Antisense:
TCCTGCTGAAATGGCATCCT | |
| cTnI | Sense:
TTGGATGGGCTGGGCTT | 286 |
| Antisense:
CCTCCTTCTTCACCTGCTTGA | |
| HCN4 | Sense:
CACTAAGGGCAACAAGGAGACC | 281 |
| Antisense:
GGTAGTTGAAGACGCCTGAGTTG | |
| GAPDH | Sense:
CGCTAACATCAAATGGGGTG | 201 |
| Antisense:
TTGCTGACAATCTTGAGGGAG | |
Western blot analysis
The transduced ADSCs were plated in 6-well culture
dishes. Cells were harvested using radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology). The protein
concentration was detected by the BCA protein concentration assay
kit (AS1086, ASPEN). Equal amounts of protein (40 µg) were loaded
onto a 10% gel for sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane. The
membranes were incubated with primary antibodies against HCN4 (rat
monoclonal antibody, 1:1,000, ab32675, Abcam), SK4 (rabbit anti-rat
monoclonal antibody, 1:500, ab215990, Abcam), phosphorylated
extracellular signal-regulated kinase (p-ERK; rabbit anti-rat
monoclonal antibody, 1:1,000, cat. no. 4370, Cell Signaling
Technology, Inc.), phosphorylated c-jun N-terminal kinase (p-JNK;
rabbit anti-rat monoclonal antibody, 1:1,000, cat. no. 4668, Cell
Signaling Technology, Inc.) and p-p38 (rabbit anti-rat monoclonal
antibody, 1:1,000, cat. no. 4511, Absin) overnight at 4°C. Primary
antibodies were detected using horseradish peroxidase-conjugated
goat anti-rabbit secondary antibodies (1:10,000, AS1107, ASPEN) and
goat anti-rat secondary antibodies (1:10,000, AS1093, ASPEN).
Signal intensities were normalized to GAPDH levels. qPCR was
performed with the ABI Prism 7500 sequence detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
SYBR-Green real-time PCR Master Mix kit (Takara) was utilized in
subsequent PCR assays in accordance with the manufacturer's
instructions.
Immunostaining studies
Transduced ADSCs were cultured on gelatin-coated
coverslips in 6-well culture dishes, washed with PBS, and fixed
using 4% paraformaldehyde. Cells were incubated with primary
antibody against α-actinin (rabbit anti-rat monoclonal antibody,
1:200, ab13734, Abcam) overnight at 4°C. Secondary antibody
FITC-conjugated goat anti-rabbit (1:50, AS1110, ASPEN) was used to
detect α-actinin. The nuclei were visualized with
4',6-diamidino-2-phenylindole. The cells were observed under a
fluorescence microscope (Leica Microsystems GmbH). Three visual
fields in three different cell isolates were randomly selected to
observe positive cells.
Electrophysiological recordings
Transduced ADSCs were plated on gelatin-coated
coverslips in 24-well culture dishes. A whole-cell patch clamp was
used to record the If current 5-7 days after
transduction. The bath solution included the following components
(in mmol/l): 140 NaCl, 5.4 KCl, 1.0 MgCl2, 1.8
CaCl2, 1.0 BaCl2, 5.5 HEPES, and 5.0 glucose
(pH 7.3). The pipette solution contained the following components
(in mmol/l): 20 KCl, 125 K-gluconate, 1.0 MgCl2, 5.0
NaCl, 10 HEPES, and 5 K2ATP (pH 7.3). The impedance of
the fluid-filled electrode was 6-8 MΩ. Experiments were performed
using an Axon patch-clamp amplifier 700B (Molecular Devices, LLC).
A digital 700AD/DA converter and 6.0.4 pClamp (both from Axon
Instruments) were used for data recording and analysis. The
If current was recorded using a voltage clamp. The
holding potential was -40 mV, which was decreased to -140 mV with
each 10-mV sweep and returned to the resting potential. CsCl (2
mmol/l) was added to detect changes in If.
Transplantation of transduced ADSCs in
rats
Male SD rats (weight, 200-250 g) were randomly
divided into GFP and SK4 groups (n=8/group). Transduced ADSCs were
cultured for 5-7 days in vitro. The rats were anesthetized
with 3% sodium pentobarbital (30 mg/kg) by intraperitoneal
injection, and a ventilator and electrocardiograph were connected.
An injection site at the free wall of the left ventricle was
indicated using a suture. Each rat was injected subepicardially
around the suture with 106 transfected ADSCs in 0.1 ml
PBS using a micropipettor (30 G, Hamilton). Subsequently, the chest
was closed in layers. All animals were monitored carefully for 2
weeks post-injection.
Establishment of a complete
atrioventricular block (AVB) model in ex vivo rat hearts
The rats received an intraperi-toneal injection of
heparin 2 weeks after cell injection. The rats were anesthetized
with an intraperitoneal (IP) injection of 0.2 ml Telazol and
subjected to isoflurane inhalation. Following cessation of pain
reflexes, the heart was removed and connected to a Langendorff
cardiac perfusion device (AD Instruments) that contained Tyrode's
solution (in mmol/l): 135 NaCl, 5.4 KCl, 1.8 CaCl, 1
MgCl2, 0.3 Na2HP04, 10 HEPES, and
10 glucose (pH 7.4). The isolated hearts were perfused for 20 min
prior to further experimentation. The perfused heart was placed in
a Sylgard-coated plate filled with warm Tyrode's solution.
Electrocardiographic leads I and II were placed at appropriate
sites. After a 20-min equilibration period, an AVB model was
established in ex vivo hearts via injection of 70% ethanol
within the AVN region using a micropipettor (30 G, Hamilton). The
electrode pacing was performed at the site of the transgene
injection at 200-msec intervals. All measured signals were
amplified and filtered using a PowerLab system (AD
Instruments).
Statistical analysis
The reported data are expressed as means ± standard
deviation. The data on the effect of SK4 on cell numbers were
analyzed using a general linear model. Tukey's post hoc tests were
used to identify pairwise changes between groups on different days.
SK4 expression on different days was analyzed with two-way analysis
of variance (ANOVA) and Bonferroni's multiple comparison test. The
statistical significance of the differences between two groups was
examined using the unpaired and two-tailed t-test. One-way ANOVA
and Bonferroni's multiple comparison test were used to compare
differences among the three groups. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
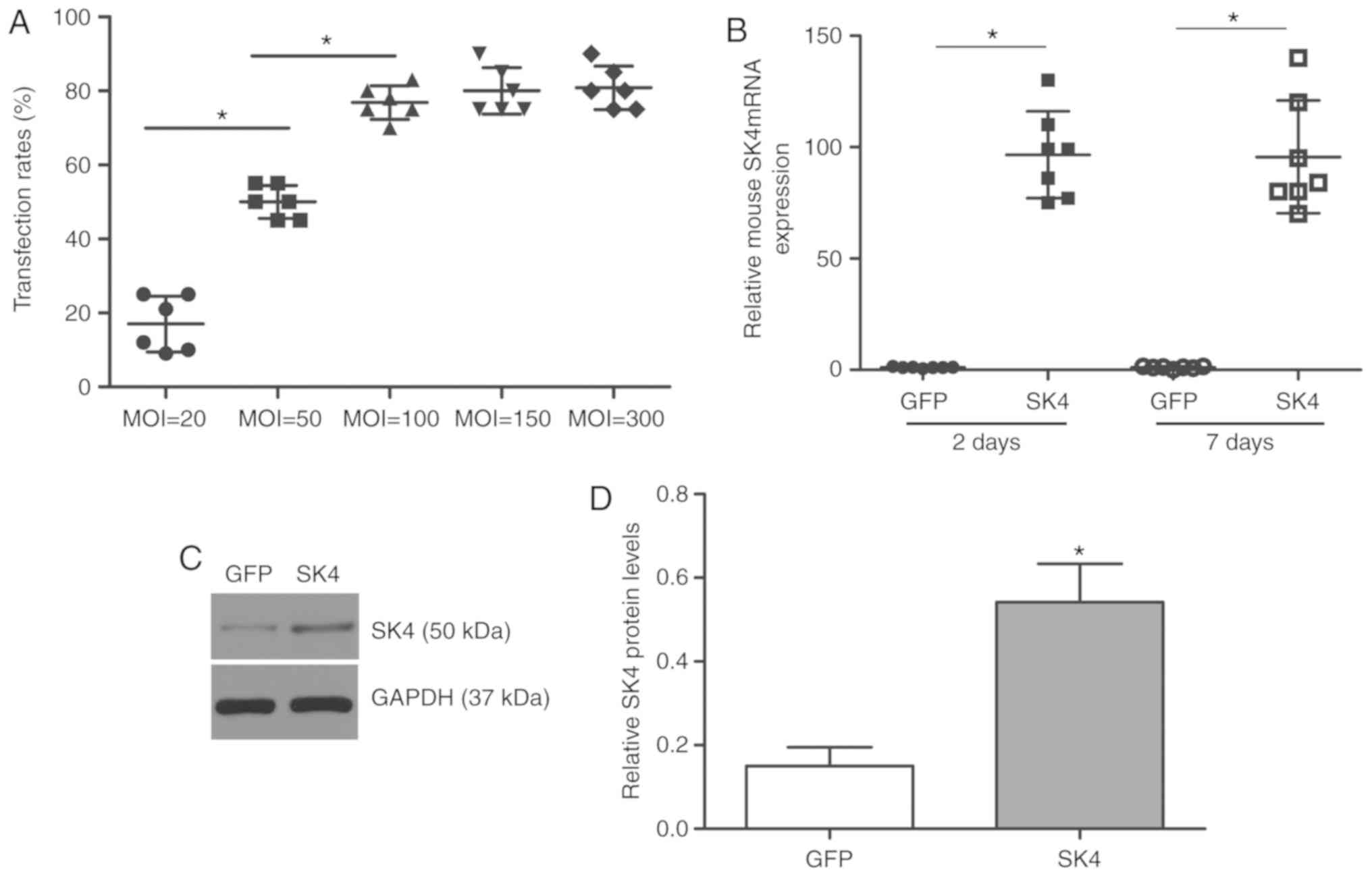
Transfection efficiency and SK4
expression after transfection
ADSCs were transfected with SK4 at different MOI
values (20, 50, 100, 150 and 300). The control group was
transfected with GFP at MOI=50. Flow cytometric analysis revealed
that the SK4 transfection efficiency was >70% at MOI≥100. The
transfection efficiencies were 76.8±4.5 and 80.0±6.3% at MOI=100
and MOI=150, respectively (P>0.05; Fig. 1A). Most cells appeared to float
and die at MOI=300. PCR analysis revealed that the level of mouse
SK4 was significantly elevated at 48 h and 7 days after
transfection (P<0.05; Fig.
1B). Western blotting also demonstrated increased SK4
expression 7 days after SK4 vector transduction (Fig. 1C and D), whereas the level of SK4
in the control group was low. These results confirmed that SK4 was
successfully and stably expressed in ADSCs.
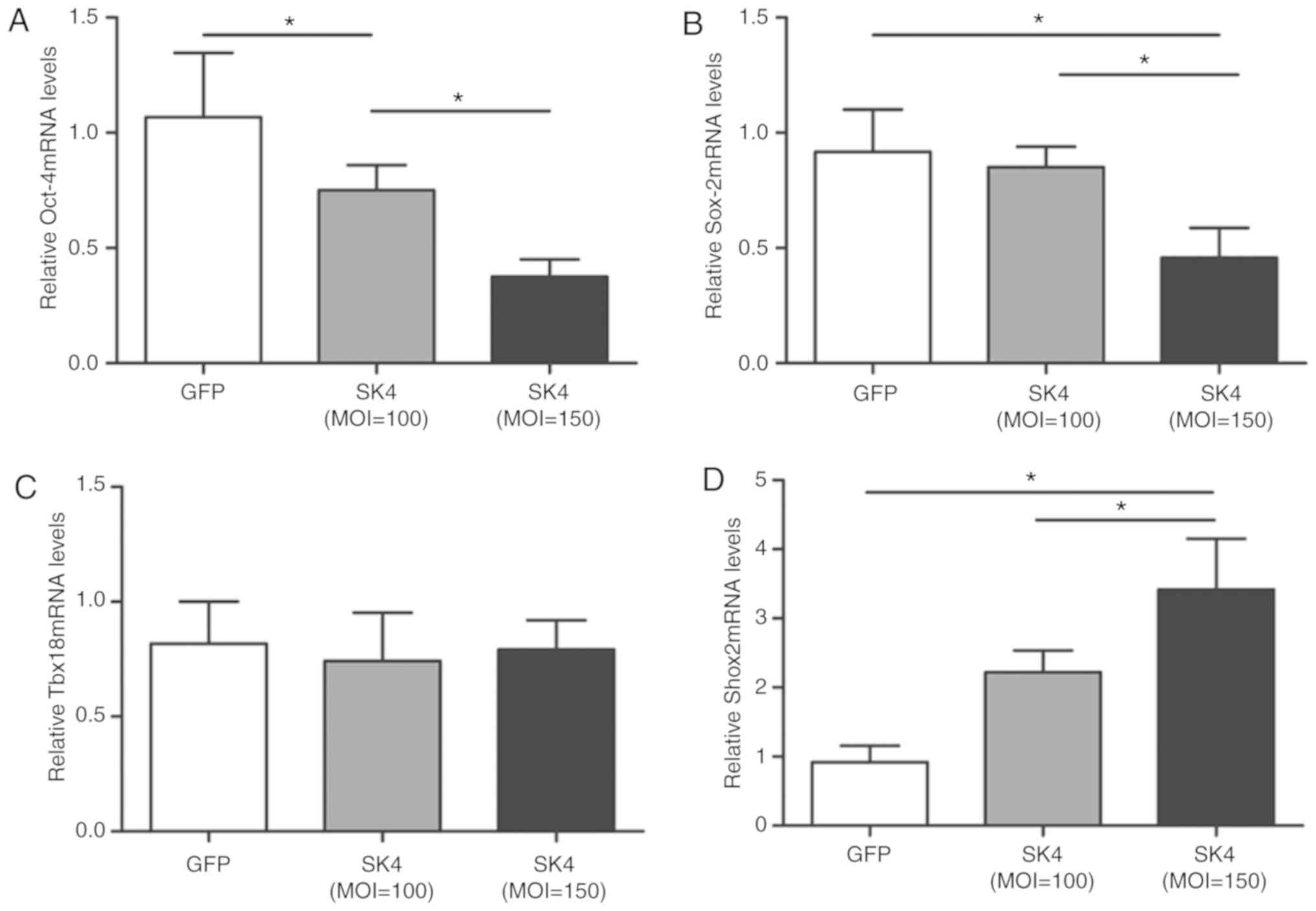
Effect of SK4 on the expression of
pluripotent markers Oct-4 and Sox-2 and transcription factors Tbx18
and Shox2 after SK4 vector transduction
Oct-4 and Sox-2 are embryonic SC markers that may
play important roles in the differentiation potential of ADSCs
(19,20). ADSCs express Oct-4 and Sox-2
(20,21). The expression of Oct-4 mRNA in the
SK4 group was significantly lower compared with that in the control
group and declined with increasing MOI values (P<0.05; Fig. 2A). Sox-2mRNA was significantly
downregulated in the SK4 group at MOI=150 (P<0.05; Fig. 2B). Therefore, increased expression
of SK4 appeared to promote the differentiation of ADSCs.
A number of transcription factors, including Tbx18
and Shox2, regulate the development of SAN (22). PCR analysis revealed that,
although no significant difference in Tbx18 mRNA expression was
observed between the two groups (Fig.
2C), Shox2 mRNA was significantly increased following SK4
transduction (P<0.05; Fig.
2D).
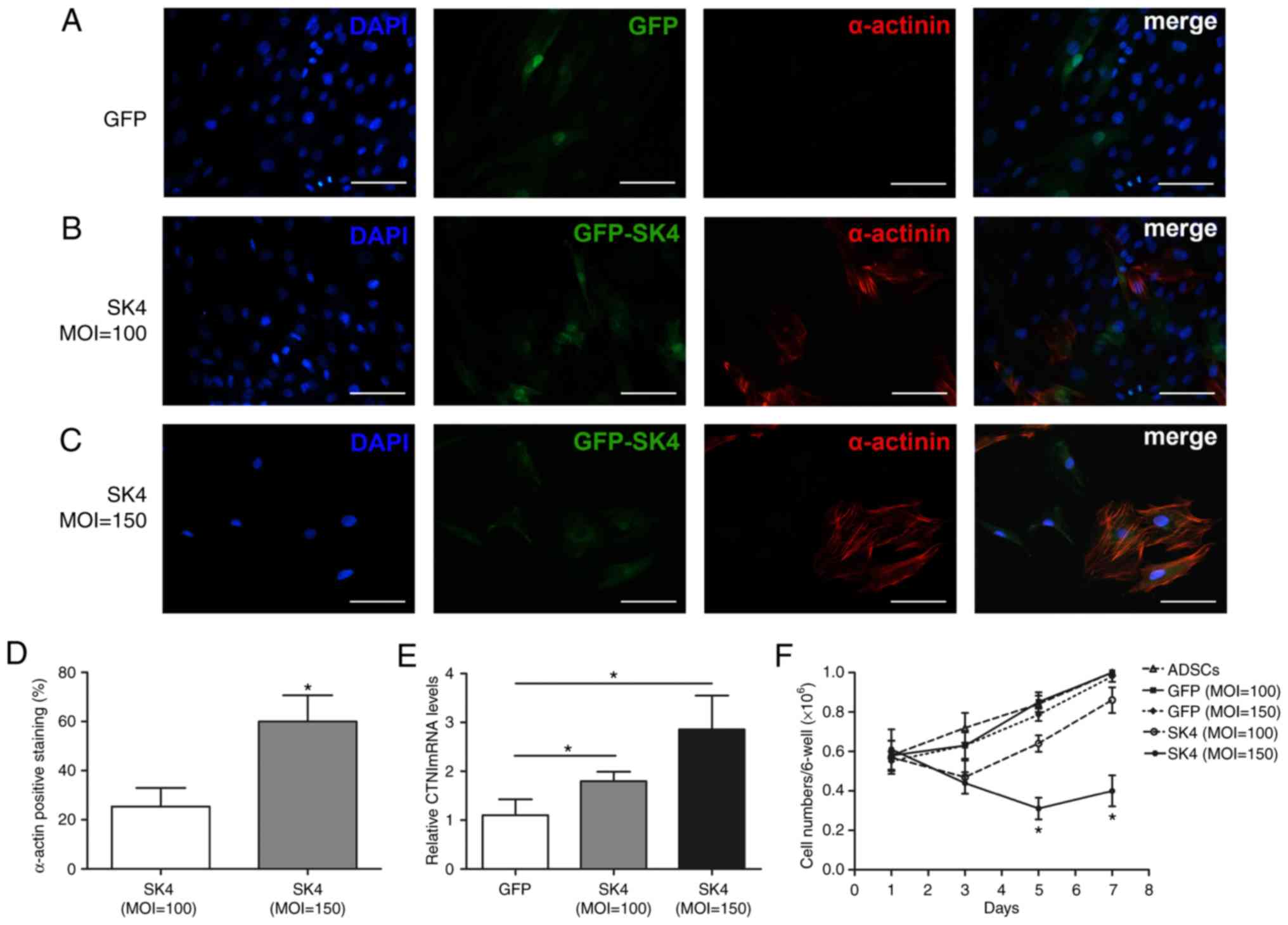
SK4 induces differentiation of ADSCs into
cardiomyocyte-like cells
The expressions of the myocardial-specific markers
α-actinin and cTnI were detected using PCR and immunofluorescence 7
days after transduction. α-Actinin expression was observed after
SK4 transduction at MOI=100 and MOI=150 using immunofluorescence.
The morphology of differentiated cardiomyocyte-like cells changed
to columnar or polygonal shapes compared with the fibroblast-like
shape of ADSCs transduced with GFP vector (Fig. 3A-C). The expression of α-actinin
was negative in the GFP group. Quantitative analyses demonstrated
that the α-actinin positivity rate in the SK4 group was
significantly higher at MOI=150 compared with MOI=100 (60.0±10.6%
vs. 25.4±7.6%, respectively; P<0.05; Fig. 3D). cTnI mRNA was significantly
upregulated after SK4 transduction, and its expression was greater
at MOI=150 compared with MOI=100 (P<0.05; Fig. 3E). The number of non-transduced
and GFP-transduced ADSCs gradually increased, which indicated that
GFP did not affect the proliferation of ADSCs. The cell number of
ADSCs transduced with SK4 at MOI=100 increased after 3 days and
reached 86±3.0% after 7 days. The cell number was reduced by nearly
~50% at 1-5 days after transduction with MOI=150 (61±4.6% vs.
31±2.4%, P<0.05), and did not change significantly at 5-7 days
(31±2.4% vs. 40±3.5%, P>0.05) (Fig. 3F). These results indicate that SK4
induces ADSC differentiation into cardiomyocyte-like cells, and the
proportion of cardiomyocyte-like cells increases with increasing
MOI values.
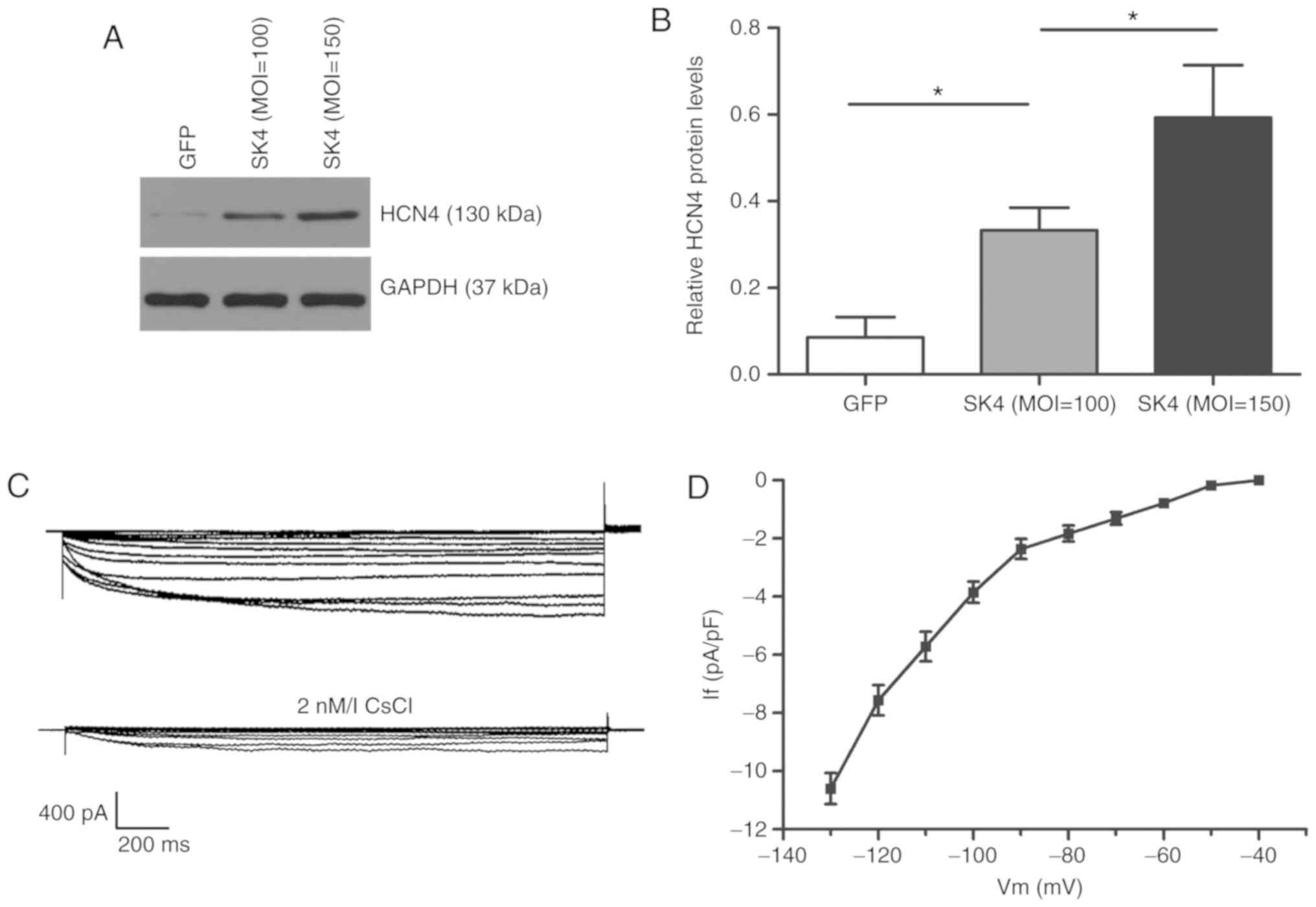
SK4 induces ADSCs to differentiate into
pacemaker-like cells
The expression of the pacemaker channel HCN4 was
significantly upregulated following SK4 transduction, and it was
positively associated with the MOI value using western blot
detection (Fig. 4A and B). The
hyperpolarizing activated pacemaker current If (8/20
cells) was detected in ADSCs transduced with SK4 after 5-7 days at
a of MOI=150, but not in the GFP group (Fig. 4C). CsCl (2 mmol/l) inhibited the
If current. The maximum current density of active
voltage was -10.6±0.5 pA/pF (n=8) (Fig. 4D).
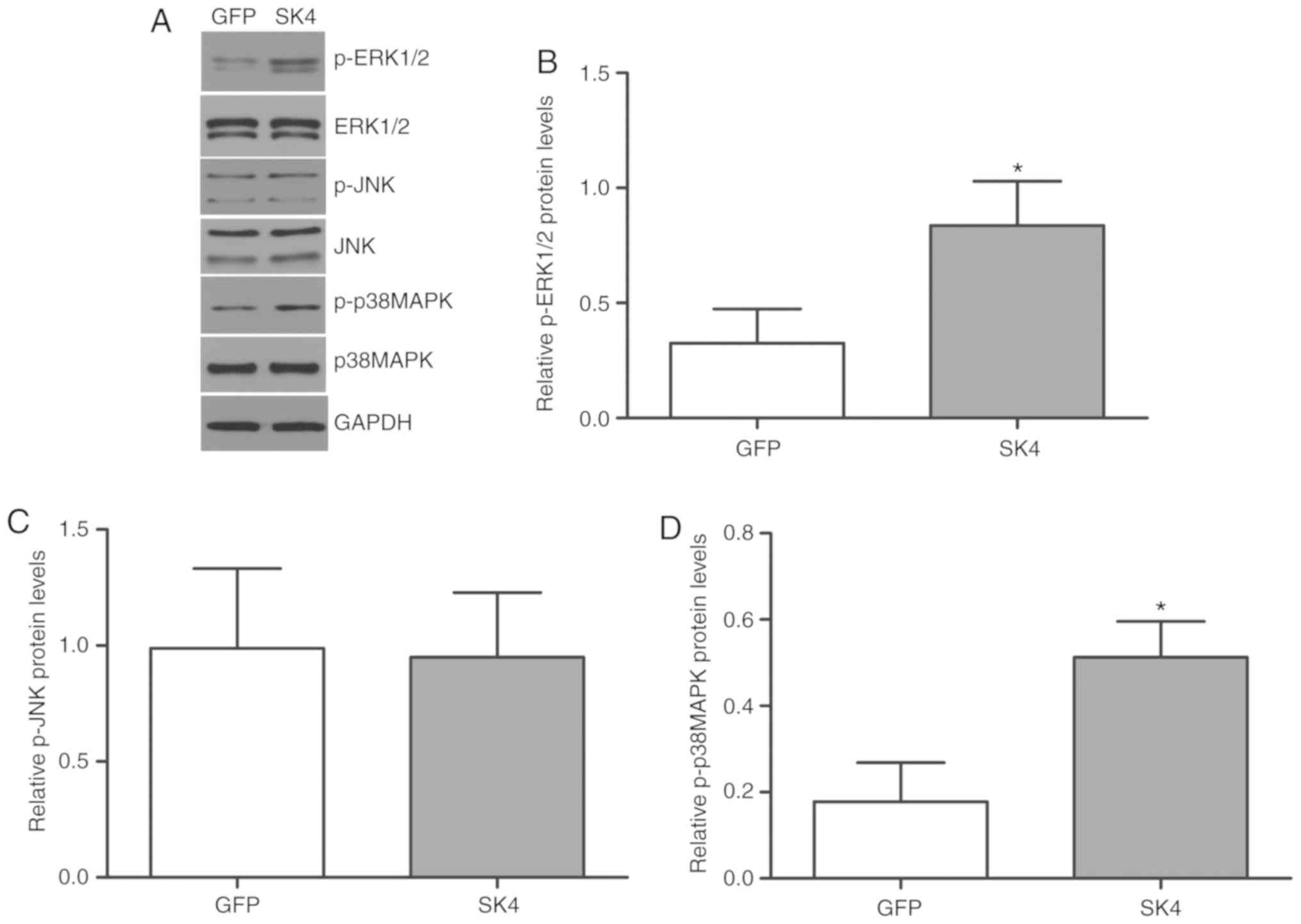
SK4 activates the ERK and p38 signaling
pathways
The mitogen-activated protein kinase (MAPK) family
plays an important role in cell differentiation and proliferation,
as well as organ development. ERK 1/2, c-jun N-terminal kinase
(JNK), and p38 MAPK are members of the MAPK family. Western blot
analysis demonstrated that p-ERK 1/2 and p-p38 MAPK expression
increased significantly in the SK4 group, but there was no
significant difference in p-JNK and total ERK 1/2, p-JNK or p38MAPK
proteins between the SK4 and GFP groups (Fig. 5A-D).
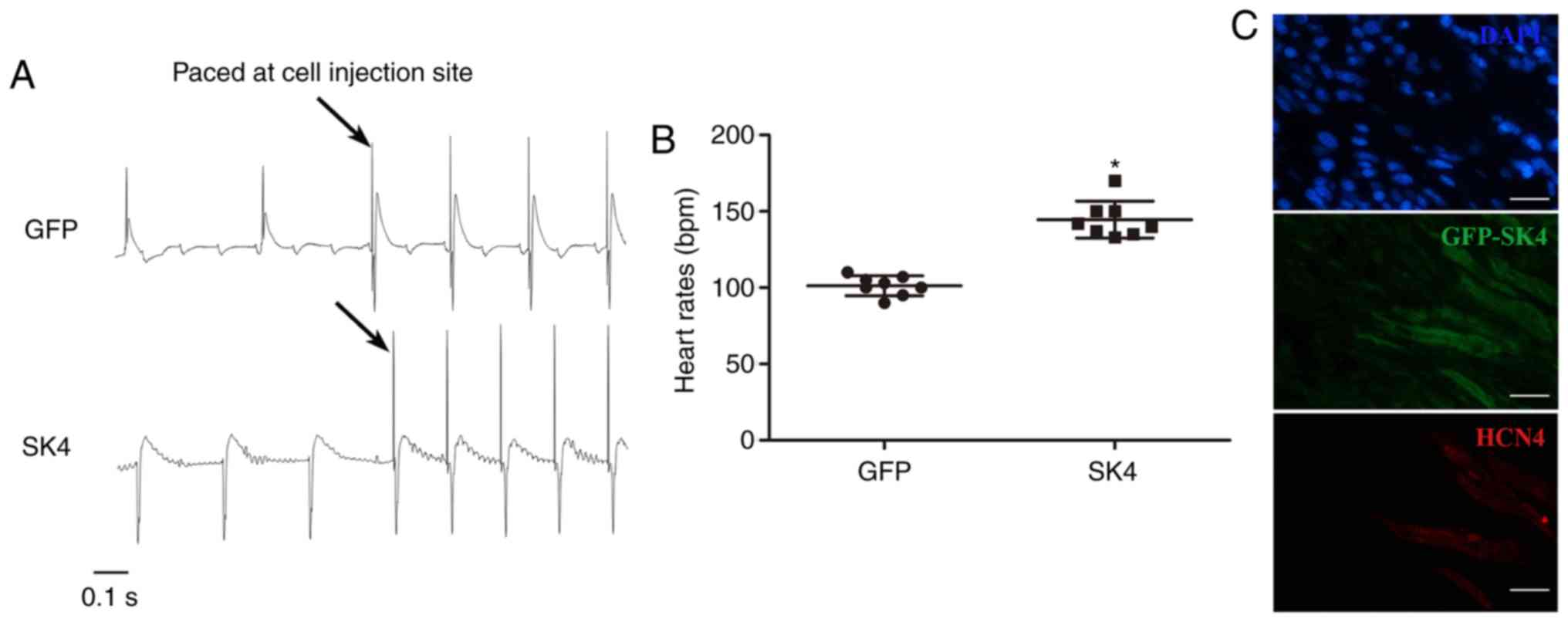
ADSCs transduced with SK4 generate
biological pacemakers in ex vivo rat hearts
The AVB model was successfully established, and
electrocardiograms revealed AV separation. The heart rate of the
SK4 group was significantly faster compared with that of the GFP
group (145±14 bpm vs. 103±5 bpm, respectively; P<0.05) (Fig. 6B). Stimulation of the cell
injection site revealed an electrocardiographic morphology that was
identical to the spontaneous rhythm in the SK4 group (Fig. 6A). These results indicated that
the ectopic pacing site was indeed the injection site. The GFP
group exhibited the opposite morphology following stimulation
(Fig. 6A). These results
demonstrated that transplantation of ADSCs transduced with SK4
produced an ectopic rhythm and assumed the function of a biological
pacemaker. Immunofluorescence confirmed that transduced ADSCs were
successfully implanted and expressed HCN4 in the SK4 group
(Fig. 6C).
Discussion
The present study combined gene-based and SC-based
therapies to create a biological pacemaker in ex vivo rat
hearts. The results demonstrated that recombinant adenoviral
vectors carrying the ion channel SK4 induced ADSCs to differentiate
into cardiomyocyte-like and pacemaker-like cells in a
dose-dependent manner in vitro. The underlying mechanism may
be associated with the upregulation of the ERK1/2 and p38 MAPK
signaling pathways. In addition, it was observed that SK4-induced
pacemaker-like cells generated a pacemaker function in ex
vivo rat hearts.
Biological pacemaker construction involves SCs and
gene therapy. Adult SCs primarily include ADSCs and bone marrow
(BM) SCs. Previous studies used BMSCs to construct biological
pacemakers via overexpression of the ion channels of the HCN family
(23-26). However, BMSCs were only used as
ion channel-carrying tools in those previous studies and did not
differentiate. The realization of the pacemaker function requires
the hyperpolarization of the adjacent host myocar-dium to activate
HCN (27). The present study
demonstrated that ADSCs overexpressing the ion channel SK4
differentiated into pacemaker-like cells. In addition, the induced
pacemaker-like cells expressed HCN4, which may interact with SK4 to
generate the pacemaker function.
Recent research has demonstrated that the ion
channel SKCa affects the differentiation of SCs
(11-13). SK4 agonists were shown to enhance
the differentiation of human ESCs and iPSCs into pacemaker-like
cells (11,12). Conversely, SK4 inhibitors
suppressed the pacemaker function of ESC-derived cardiomyocytes
(9). Another recent study
reported that EBIO can modify the cardiac subtype of human ESCs and
iPSCs (14); the study suggested
that the SK2/SK3 channel modulator exerted a similar EBIO-mediated
effect, but the effect of the SK4 activator was not the same. This
phenomenon may be attributed to the fact that SK4 current is not
recorded in hiPSCs (28), and the
expression of SK4 is low compared with that of SK2 and SK3 during
hESC differentiation (14). In
the present study, immunofluorescence and western blot analysis
detected low SK4 expression in undifferentiated ADSCs. The
expression of SK4 in ADSCs was previously investigated (29). Our results further confirmed that
an exogenous adenovirus vector carrying the SK4 gene was
successfully transduced into ADSCs to induce SK4
overexpression.
The present study demonstrated that SK4 reduced the
expression of pluripotent markers and increased the expression of
the myocardial markers cTnI and α-actinin, and the pacemaker
channel HCN4 in ADSCs. HCN4 plays an important role in phase 4 of
the automatic depolarization of SAN cells (30). Embryos cannot mature in HCN4
knockout mice due to pacemaker dysfunction (31). The results demonstrated that ADSCs
can differentiate into cardiomyocyte-like and pacemaker-like cells
via overexpression of SK4. The transcription factors Tbx18 and
Shox2, which regulate the development of the SAN, were also
investigated in the present study. Previous studies demonstrated
that Tbx18 and Shox2 increased HCN4 expression and induced ESCs,
ADSCs and BMSCs to differentiate into pacemaker-like cells
(17,32,33). The expression of Shox2 in the
conduction system is higher compared with that in the working
myocardium during the early stages of mouse embryonic development
(33). The present study
demonstrated increased expression of Shox2, but the expression of
Tbx18 did not significantly change following SK4 transduction. It
was hypothesized that upregulation of Shox2 after SK4 transduction
may be one of the mechanisms underlying increased HCN4 expression.
More importantly, induced pacemaker-like cells were further
confirmed by the pacemaker current If that was recorded
in SK4-transduced ADSCs, but not in GFP-transduced ADSCs, and the
maximum current density of the active voltage was greater than
ADSCs transduced with Tbx18 in a previous study (-10.6±0.5 pA/pF
vs. -5.43±1.36 pA/pF, respectively) (17). These findings suggest that SK4 and
Tbx18 induce cell differentiation via different mechanisms.
It was previously demonstrated that SK4 plays an
important role in the pacemaker function of SC-derived
cardiomyocytes (9,34). SK4 greatly activates HCN channels
in pacemaker cells, shortens the duration of the action potential,
and increases the slope of automatic depolarization (34). The opening of SK4 hyperpolarizes
the membrane potential and contributes to the activation of the
excitatory diastolic current If. These results suggest
that SK4 promotes the differentiation of ADSCs and increases the
current amplitude of the HCN channel in induced pacemaker-like
cells to strengthen the pacemaker function.
SCs differentiate toward the myocardial direction,
and these differentiated cells comprise cardiomyocytes and
non-cardiomyocytes. The former include the working myocar-dium and
pacemaker cells. Electrical heterogeneity affects the pacemaker
function and leads to arrhythmic potential (35,36). The SKCa agonist EBIO
reduces the number of non-cardiac progenitor cells and ventricular
myocytes, and increases the purity of pacemaker cells (14). The present study found that the
number of cells markedly decreased at MOI=150 in the SK4 group,
whereas the levels of cTnI, α-actinin and HCN4 were all upregulated
at MOI=100. These results suggest that the differentiation
efficiency of cardiomyocyte-like and pacemaker-like cells improved
with increasing MOI values. This phenomenon may be attributed to
the decrease in the number of differentiated non-cardiomyocytes, or
the increase in the amount of SK4, which directly promotes the
differentiation of pacemaker-like cells. These two activities may
jointly promote the increased differentiation efficiency.
Alleviation of cellular heterogeneity may facilitate the
realization of the pacemaker function in ex vivo hearts. The
transduced ADSCs were successfully implanted into the heart, and
the beating frequency markedly increased following implantation of
the SK4-transduced ADSCs. Accordingly, it may be inferred that SK4
is a potential target for biological pacemaker gene therapy.
Previous studies have demonstrated the role of
ERK1/2 and p38 MAPK in cell proliferation and differentiation
(37,38). p38 MAPK induced pluripotent SCs to
differentiate towards the cardiac lineage at an early stage of
differentiation, and inhibited differentiation towards the neural
lineage (39). It was also
demonstrated that p38 MAPK and ERK1/2 were activated in tandem to
promote the expression of relevant markers in cardiac progenitor
cells, such as MEF2c, GATA2 and ATF2 in P19CL6 cells (40). These studies demonstrated that
p38-MAPK and ERK1/2 play important roles in the differentiation of
pluripotent SCs towards the myocardial lineage. A recent study
demonstrated that EBIO induced ESCs to produce a cardiac lineage
via activation of the ERK signaling pathway (11). The present study demonstrated that
the levels of p-p38 MAPK and p-ERK1/2 were significantly increased
following SK4 transduction, which suggests that the effect of SK4
on differentiation is associated with the activation of the p38
MAPK and ERK1/2 signaling pathways.
There were several limitations to the present study.
First, patch clamps should be used to confirm that SK4 can
sufficiently activate the If current, and the potential
mechanism requires further study. Second, the duration of the
pacemaker function was not examined. Third, the pacemaker function
was only verified in ex vivo hearts. Further in vivo
studies are required to continuously monitor biological pacemaker
function and detect possible latent arrhythmias, and evaluate
safety and effectiveness.
In conclusion, the present study demonstrated that
SK4 induced ADSCs to differentiate into pacemaker-like cells via
upregulation of Shox2 and activation of the p38 MAPK and ERK1/2
pathways, which may provide a new approach to the construction of
adult SC-associated biological pacemaker.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fundamental
Research Funds for the Central Universities (grant no.
2018413000185) and the National Natural Science Foundation of China
(grant no. 81670303).
Availability of data and materials
All the datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
MY and CH contributed to the conception and design
of the study. MY, HZ, AY and FW performed the experiments. HZ., QZ
and MY analyzed data. XW, YT, HH and CH interpreted the results of
the experiments. MY, QZ and CH drafted and revised the manuscript.
All authors have approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal procedures were performed in agreement
with the Wuhan University institutional guidelines and in
compliance with suggestions from the panel of Euthanasia of the
American Veterinary Medical Association and the National Institutes
of Health Guide for the Care and Use of Laboratory Animals. The
study was approved by the Ethics Committee of Renmin Hospital of
Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Horsthuis T, Buermans HP, Brons JF,
Verkerk AO, Bakker ML, Wakker V, Clout DE, Moorman AF, 't Hoen PA
and Christoffels VM: Gene expression profiling of the forming
atrioventricular node using a novel tbx3-based node-specific
transgenic reporter. Circ Res. 105:61–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mangoni ME and Nargeot J: Properties of
the hyperpolarization-activated current (I(f)) in isolated mouse
sino-atrial cells. Cardiovasc Res. 52:51–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vinogradova TM, Zhou YY, Bogdanov KY, Yang
D, Kuschel M, Cheng H and Xiao RP: Sinoatrial node pacemaker
activity requires Ca(2+)/calmodulin-dependent protein kinase II
activation. Circ Res. 87:760–767. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Q, Timofeyev V, Lu L, Li N,
Singapuri A, Long MK, Bond CT, Adelman JP and Chiamvimonvat N:
Functional roles of a Ca2+-activated K+
channel in atrioventricular nodes. Circ Res. 102:465–471. 2008.
View Article : Google Scholar
|
|
5
|
Attali B, Weisbrod D, Bueno H, Behar J,
Haron-Khun S and Yadin D: SK4 Ca2+-activated
K+ channels regulate sinoatrial node firing rate and
cardiac pacing in vivo. Biophys J. 112:35a2017. View Article : Google Scholar
|
|
6
|
Haron-Khun S, Weisbrod D, Bueno H, Yadin
D, Behar J, Peretz A, Binah O, Hochhauser E, Eldar M, Yaniv Y, et
al: SK4 K+ channels are therapeutic targets for the
treatment of cardiac arrhythmias. EMBO Mol Med. 9:415–429. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oliván-Viguera A, Valero MS, Coleman N,
Brown BM, Laría C, Murillo MD, Gálvez JA, Díaz-de-Villegas MD,
Wulff H, Badorrey R and Köhler R: A novel pan-negative-gating
modulator of KCa2/3 channels, fluoro-di-benzoate, RA-2, inhibits
endothelium-derived hyperpolarization-type relaxation in coronary
artery and produces bradycardia in vivo. Mol Pharmacol. 87:338–348.
2015. View Article : Google Scholar
|
|
8
|
Kharche S, Yu J, Lei M and Zhang H: A
mathematical model of action potentials of mouse sinoatrial node
cells with molecular bases. Am J Physiol Heart Circ Physiol.
301:H945–H963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weisbrod D, Peretz A, Ziskind A, Menaker
N, Oz S, Barad L, Eliyahu S, Itskovitz-Eldor J, Dascal N,
Khananshvili D, et al: SK4 Ca2+ activated K+
channel is a critical player in cardiac pacemaker derived from
human embryonic stem cells. Proc Natl Acad Sci USA.
110:E1685–E1694. 2013. View Article : Google Scholar
|
|
10
|
Devor DC, Singh AK, Frizzell RA and
Bridges RJ: Modulation of Cl- secretion by benzimidazolones. I.
Direct activation of a Ca(2+)-dependent K+. channel Am J
Physiol. 271:L775–L784. 1996.
|
|
11
|
Kleger A, Seufferlein T, Malan D,
Tischendorf M, Storch A, Wolheim A, Latz S, Protze S, Porzner M,
Proepper C, et al: Modulation of calcium-activated potassium
channels induces cardiogenesis of pluripotent stem cells and
enrichment of pacemaker-like cells. Circulation. 122:1823–1836.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Müller M, Stockmann M, Malan D, Wolheim A,
Tischendorf M, Linta L, Katz SF, Lin Q, Latz S, Brunner C, et al:
Ca2+ activated K channels-new tools to induce cardiac
commitment from pluripotent stem cells in mice and men. Stem Cell
Rev Rep. 8:720–740. 2012. View Article : Google Scholar
|
|
13
|
Liebau S, Tischendorf M, Ansorge D, Linta
L, Stockmann M, Weidgang C, Iacovino M, Boeckers T, von Wichert G,
Kyba M and Kleger A: An inducible expression system of the
calcium-activated potassium channel 4 to study the differential
impact on embryonic stem cells. Stem Cells Int. 2011:4568152011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jara-Avaca M, Kempf H, Rückert M,
Robles-Diaz D, Franke A, la Roche J, Fischer M, Malan D, Sasse P,
Solodenko W, et al: EBIO does not induce cardiomyogenesis in human
pluripotent stem cells but modulates cardiac subtype enrichment by
lineage-selective survival. Stem Cell Reports. 8:305–317. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taha MF and Hedayati V: Isolation,
identification and multipotential differentiation of mouse adipose
tissue-derived stem cells. Tissue Cell. 42:211–216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Planat-Bénard V, Menard C, André M, Puceat
M, Perez A, Garcia-Verdugo JM, Pénicaud L and Casteilla L:
Spontaneous cardiomyocyte differentiation from adipose tissue
stroma cells. Circ Res. 94:223–229. 2004. View Article : Google Scholar
|
|
17
|
Yang M, Zhang GG, Wang T, Wang X, Tang YH,
Huang H, Barajas-Martinez H, Hu D and Huang CX: TBX18 gene induces
adipose-derived stem cells to differentiate into pacemaker-like
cells in the myocardial microenvironment. Int J Mol Med.
38:1403–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Reményi A, Lins K, Nissen LJ, Reinbold R,
Schöler HR and Wilmanns M: Crystal structure of a POU/HMG/DNA
ternary complex suggests differential assembly of Oct4 and Sox2 on
two enhancers. Genes Dev. 17:2048–2059. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Izadpanah R, Trygg C, Patel B, Kriedt C,
Dufour J, Gimble JM and Bunnell BA: Biologic properties of
mesenchymal stem cells derived from bone marrow and adipose tissue.
J Cell Biochem. 99:1285–1297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Liu T, Song K, Fan X, Ma X and Cui
Z: Adipose-derived stem cell: A better stem cell than BMSC. Cell
Biochem Funct. 26:664–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christoffels VM, Smits GJ, Kispert A and
Moorman AF: Development of the pacemaker tissues of the heart. Circ
Res. 106:240–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Potapova I, Plotnikov A, Lu Z, Danilo P
Jr, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J,
et al: Human mesenchymal stem cells as a gene delivery system to
create cardiac pacemakers. Circ Res. 94:952–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plotnikov AN, Shlapakova I, Szabolcs MJ,
Danilo P Jr, Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT,
Brink PR, et al: Xenografted adult human mesenchymal stem cells
provide a platform for sustained biological pacemaker function in
canine heart. Circulation. 116:706–713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Li S, Qu D, Li B, He B, Wang C
and Xu Z: Autologous biological pacing function with
adrenergic-responsiveness in porcine of complete heart block. Int J
Cardiol. 168:3747–3751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Li B, Li Z, Zhang J and Zeng M:
Adipose tissue-derived adult stem cells transfected with the gene
of hyperpolarization-activated cyclic nucleotide-gated ion channel
2 differentiated into pacemaker-like cells. Xi Bao Yu Fen Zi Mian
Yi Xue Za Zhi. 29:901–904. 9092013.In Chinese.
|
|
27
|
Chauveau S, Brink PR and Cohen IS: Stem
cell-based biological pacemakers from proof of principle to
therapy: A review. Cytotherapy. 16:873–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang P, Rushing SN, Kong CW, Fu J, Lieu
DK, Chan CW, Deng W and Li RA: Electrophysiological properties of
human induced pluripotent stem cells. Am J Physiol Cell Physiol.
298:C486–C495. 2010. View Article : Google Scholar :
|
|
29
|
Bai X, Ma J, Pan Z, Song YH, Freyberg S,
Yan Y, Vykoukal D and Alt E: Electrophysiological properties of
human adipose tissue-derived stem cells. Am J Physiol Cell Physiol.
293:C1539–C1550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi W, Wymore R, Yu H, Wu J, Wymore RT,
Pan Z, Robinson RB, Dixon JE, McKinnon D and Cohen IS: Distribution
and prevalence of hyperpolarization-activated cation channel (HCN)
mRNA expression in cardiac tissues. Circ Res. 85:e1–e6. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stieber J, Herrmann S, Feil S, Löster J,
Feil R, Biel M, Hofmann F and Ludwig A: The
hyperpolarization-activated channel HCN4 is required for the
generation of pacemaker action potentials in the embryonic heart.
Proc Natl Acad Sci USA. 100:15235–15240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Yang M, Zhang G, Li L, Ye B, Huang C
and Tang Y: Transcription factor TBX18 promotes adult rat bone
mesen-chymal stem cell differentiation to biological pacemaker
cells. Int J Mol Med. 41:845–851. 2018.
|
|
33
|
Feng Y, Luo S and Song Z: GW24-e3884
Canine bone marrow mesenchymal stromal cells modified with Shox2
gene rebuild biological pacemakers in vitro. Heart. 99:A462013.
|
|
34
|
Weisbrod D, Khun SH, Bueno H, Peretz A and
Attali B: Mechanisms underlying the cardiac pacemaker: The role of
SK4 calcium-activated potassium channels. Acta Pharmacol Sin.
37:82–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He JQ, Ma Y, Lee Y, Thomson JA and Kamp
TJ: Human embryonic stem cells develop into multiple types of
cardiac myocytes: Action potential characterization. Circ Res.
93:32–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang YM, Hartzell C, Narlow M and Dudley
SC Jr: Stem cell-derived cardiomyocytes demonstrate arrhythmic
potential. Circulation. 106:1294–1299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu J, Kubota J, Hirayama J, Nagai Y,
Nishina S, Yokoi T, Asaoka Y, Seo J, Shimizu N, Kajiho H, et al:
p38 Mitogen-activated protein kinase controls a switch between
cardiomyocyte and neuronal commitment of murine embryonic stem
cells by activating myocyte enhancer factor 2C-dependent bone
morphogenetic protein 2 transcription. Stem Cells Dev.
19:1723–1734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eriksson M and Leppä S: Mitogen-activated
protein kinases and activator protein 1 are required for
proliferation and cardiomyocyte differentiation of P19 embryonal
carcinoma cells. J Biol Chem. 277:15992–16001. 2002. View Article : Google Scholar : PubMed/NCBI
|