Introduction
Mycoplasma pneumoniae pneumonia (MPP), a
common acute respiratory tract infectious disease in children that
has a variety of clinical manifestations, is caused by
Mycoplasma pneumoniae (MP) infection, and can lead to
multi-organ and multi-system damage (1). In recent years, the cases of
refractory MPP (RMPP) are increasing annually. Additionally, the
risk of extra-pulmonary complications is elevated, which can
severely affect the health of patients (1). MPP affects the quality of life of
children and their families; therefore clinical investigations into
this disease is required (2,3).
MicroRNA (miRNA/miR) can regulate gene expression by
activating or suppressing gene transcription, serving a key role in
physiological development, as well as the development of disease
(4). Additionally, miRNA not only
exerts a crucial part in cell differentiation and organ development
(5), but can also serve as a
molecular marker for various physiological and pathological states
(5). Numerous studies indicated
that miRNAs are closely associated with the genesis, development
and prognosis of pulmonary infection (6,7);
however, no intensive studies have been performed, yet further
investigation is required (6). On
the contrary, research into miRNAs have provided notable insight
into the molecular mechanism, clinical diagnosis and treatment for
pulmonary infectious disease (6).
Osei et al (8) revealed
that decreased levels of miR-146a-5p in chronic obstructive
pulmonary disease-associated fibroblasts may induce a more
pronounced pro-inflammatory phenotype. Pradhan et al
reported that miRNAs interfere with translation of their target
gene and regulate a variety of biological actions exerted by these
target genes (9).
Member 1 of human transporter subfamily (ABCA1) and
ATP-binding cassette subfamily G member 1 (ABCG1) belong to the ATP
binding cassette transporter (ABC) superfamily, whose main function
is to promote the outflow of intracellular free cholesterol
(10). The ABCA1- and
ABCG1-regulated cholesterol outflow from macrophages is key step in
preventing and reversing foam cell formation (10).
The ABCA1- and ABCG1-regulated cholesterol outflow
from macrophages serves a key role in scavenging excessive
cholesterol in tissues, including vascular walls (11,12). Therefore, dysfunctions in ABCA1
and ABCG1 may lead to excessive cholesterol accumulation in
macrophages, forming foam cells, which subsequently invade the
vascular wall and promote MPP genesis and development (11,12).
Interleukin (IL)-1 receptor-associated kinases
(IRAKs) are the members with similar composition and structure of
serine-threonine; four members have been reported as of yet,
including IRAK-1, IRAK-2, IRAK-M and IRAK-4 (13). Among them, only IRAK-1 and IRAK-4
possess kinase activity, while IRAK-4 is considered as an essential
factor required to activate the Toll-IL receptor and myeloid
differentiation primary response 88 (MyD88)-dependent pathway
(13,14). Following the phosphorylation
processes in the aforementioned pathways, IRAKs dissociate from
MyD88, and bind with tumor necrosis factor receptor (TNF)
associated factor 6 (TRAF6) to form an IRAK1-TRAF6 complex
(14). Subsequently, nuclear
factor-κB (NF-κB) and transcription factor activated activator
protein-1 are activated, while the release of pro-inflammatory
cytokines, including IL-6, IL-1 and TNF-α is promoted, inducing the
downstream cascade of inflammatory reactions, resulting in tissue
inflammatory injury (15). Li
et al (16) revealed that
miR-146a-5p antagonized advanced glycation end products (AGEs)- and
Porphyromonas gingivalis (P.g)-LPS-induced ABCA1 and ABCG1
dysregulation in macrophages via IRAK-1 downregulation (16). In the present study, the function
of miR-146a-5p in patients with refractory MPP was
investigated.
Materials and methods
Patients with MPP
Children diagnosed (male, n=10; female, n=10) with
MPP were enrolled from Renmin Hospital. The age range was 1 month
to 12 years. The peripheral blood of all patients was collected,
and patients underwent chest radiography and M. pneumonia
tests, including specific IgM in by ELISA. The exclusion criteria
for the enrollment of patients were: i) Those with congenital heart
diseases, hereditary metabolic diseases, neurological disorders,
bronchopulmonary dysplasia, and immunodeficiency; and ii) patients
co-infected with other pathogens. The present study was approved by
the Ethics Committee of Renmin Hospital, Hubei University of
Medicine. Written informed consent obtained from pthe family of
patients.
Quantification of miRNA level. Total RNA was
extracted from lung tissue or cell samples using TRIzol reagent
(Thermo Fisher Scientific, Inc.) and cDNA was obtained using a
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) at 42°C for 60 min and 82°C for 10 sec. 500 ng
RNA was labeled and using an equimolar concentration of
Cyanine-3-CTP-labelled universal mouse reference (Stratagene).
miRNA analysis was performed on an ABI 7500 real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc) using TaqMan
Universal PCR Master Mix (Thermo Fisher Scientific, Inc.). miRNA
expression levels were calculated using an endogenous control with
the 2−ΔΔCq method (17). PCR amplification conditions were
as follows: Initial denaturation at 95°C for 10 min, 40 cycles of
95°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec. Sequence
information: miR-146a-5p: Forward, 5′-GGG GTG AGA ACT GAA TTC
CAT-3′ and reverse, 5′-CAG TGC GTG TCG TGG AGT-3′; U6: Forward,
5′-GCT TCG GCA GCA CAT ATA CTA AAA T-3′ and reverse, 5′-CGC TTC ACG
AAT TTG CGT GTC AT-3′. Data were examined using Genespring 12.6.1
(Agilent Technologies) and Agilent Feature Extraction Software
(version A.10.7.3.1).
Gene chip analysis
The total RNA was extracted using mirVana™ miRNA
Isolation kit (Ambion; Thermo Fisher Scientific, Inc.). The Agilent
Human miRNA array (8*60K, V16.0, Agilent Technologies, Inc.) and
total RNA was labeled by Cy-3 using miRNA Complete Labeling and Hyb
kit (5190-0456, Agilent Technologies, Inc.). Data was performed by
Agilent Microarray Scanner (G2565CA, Agilent Technologies, Inc.)
and normalized by Quantile algorithm with Gene Spring Software 12.6
(Agilent Technologies, Inc.).
MPP model, and hematoxylin and eosin
staining
Ethical approval was obtained from Renmin Hospital,
Hubei University of Medicine. Male C57BL/6 mice (4-5 g, 1 week old,
n=20) were purchased from the Animal laboratory of Renmin Hospital.
C57BL/6 mice of model group were injected with 2 mg/kg of
lipopolysaccharide (LPS, intraperitoneally) for 12 h under
anesthesia with 50 mg/kg sodium pentobarbital as reported
previously by Fang et al (18). C57BL/6 mice of sham group were
injected with 50 mg/kg sodium pentobarbital. Then, mice were
sacrificed via decollation whilst anesthetized. Lung tissue samples
were collected and fixed with 4% paraformaldehyde for 24 h at room
temperature or stored at -80°C.
Lung tissue samples fixed with paraformaldehyde were
paraffin-embedded. The samples were cut into 10-µm sections
using a paraffin slicing machine (RM2235; Leica Microsystems GmbH),
stained with hematoxylin and eosin for 10 min at room temperature,
and observed under a light microscope (magnification, ×100,
BH3-MJL; Olympus Corporation).
Cell culture
A549 cells was maintained in Dulbecco's Modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.). 100 ng/ml miR-146a-5p (5′-CCG ATA TAT ATC CTC ACT T-3′),
anti-miR-146a-5p (5′-ATG GGC TAT ATA GGA GTG AAC C-3′) and negative
mimics (5′-TTC TCC GAA CGT GTC ACG T-3′), small interfering RNA
(si)-IRAK-1 (sc-35704, Santa Cruz Biotechnology, Inc.) or si-RXR
(sc-36447, Santa Cruz Biotechnology, Inc.) were transfected into
cells using Lipofectamine® 2000 for 48 h. After 48 h,
cells were treated with 100 ng/ml of LPS for 6 h at 37°C as
reported by Guo and Cheng (19).
ELISA kits
Serum samples were centrifuged at 2,000 × g for 20
min at 4°C and cell samples were centrifuged at 1,000 × g for 10
min at 4°C. Serum samples were used to measure the levels of TNF-α
(cat. no. H052), IL-1β (cat. no. H002), IL-6 (cat. no. H007) and
IL-18 (H015) using ELISA kits (Nanjing Jiancheng Institute of
Bioengineering). Cell samples were lysed with
radioimmunoprecipitation assay (RIPA; (Beyotime Institute of
Biotechnology) and used to measure TNF-α, IL-1β, IL-6 and IL-18
levels using ELISA kits.
Luciferase reporter assay
The network of signaling pathway components revealed
that IRAK-1 may targets of miR-146a-5p be important using
http://www.targetscan.org/vert_71/.IRAK-1-expressing
pc-DNA-3.1 plasmids and anti-miR-146a-5p were co-transfected into
cells using Lipofectamine 2000 for 48 h. Reporter activity levels
were quantified 48 h after transfection using a Dual-Luciferase
Reporter Assay kit (Beyotime Institute of Biotechnology).
Measurements were obtained using a Lumat LB 9507 instrument
(Berthold Technologies). Normalization was performed via
comparisons with Renilla luciferase activity.
Western blotting analysis
Cells were harvested and lysed in RIPA buffer.
Protein content was measured using a BCA assay (Beyotime Institute
of Biotechnology). Protein (50 µg) was separated via 10%
SDS-PAGE and transferred to poly-vinylidene difluoride membranes,
which were washed in Tris-buffered saline with Tween-20 (TBST) for
three times and blocked with 5% non-fat in TBST for 1 h at room
temperature. Subsequently, the membranes were incubated with
antibodies against ABCG1 (ab218528, 1:1,000, Abcam), IRAK-1
(sc-515512, 1:1,000, Santa Cruz Biotechnology, Inc.), retinoid X
receptor (RXR, sc-46659, 1:1,000, Santa Cruz Biotechnology, Inc.),
liver X receptor (LXR, sc-271064, 1:1,000, Santa Cruz
Biotechnology, Inc.) and GAPDH (sc-69778, 1:5,000, Santa Cruz
Biotechnology, Inc.) at 4°C overnight. The membrane was washed
three times with TBST for 15 min and incubated with a horseradish
peroxidase-conjugated secondary antibody (sc-2004, 1:5,000, Santa
Cruz Biotechnology, Inc.) at 37°C for 1 h. Proteins were detected
by Super Signal chemiluminescent reagent (Thermo Fisher Scientific,
Inc.) and measured using Flowjo 7.6.1 (FlowJo, LLC).
Immunofluorescence (IF)
Cells were washed with PBS and fixed with 4%
paraformaldehyde for 20 min at room temperature. Cells were blocked
with 5% bovine serum albumin (Beyotime Institute of Biotechnology)
and 0.1% Triton X-100 for 1 h and incubated with anti-IRAK-1 (Santa
Cruz Biotechnology, Inc.) at 4°C overnight. Cells were washed with
PBS and incubated with goat anti-rabbit IgG-CFL 555 (Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Cells were washed
with PBS and stained with DAPI for 15 min at room temperature.
Then, cells were washed with PBS and observed under a light
microscope (magnification, ×100, BH3-MJL; Olympus Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance between groups was determined by a
Student's t-test or one-way analysis of variance followed by a
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-146a-5p in patients or
mice with refractory MPP
To investigate the effects of miR-146a-5p in a mouse
model with refractory MPP, we first analyzed changes in the
expression of miR-146a-5p in a mouse model with refractory MPP. The
results revealed that the expression serum levels of TNF-α, IL-1β,
IL-6 and IL-18 were significantly increased in MPP mice, compared
with sham mice (Fig. 1A-D).
H&E staining showed that pulmonary alveoli were smaller in the
MPP mouse model, compared with sham mice (Fig. 1E). In addition, the expression of
miR-146a-5p was significantly reduced in mice with refractory MPP,
compared with the sham group (Fig. 1F
and G). Consistently, miR-146a-5p expression was significantly
downregulated in patients with refractory MPP compared with the
control normal group (Fig. 1H).
Therefore, miR-146a-5p may serve a role in the inflammation
associated with refractory MPP.
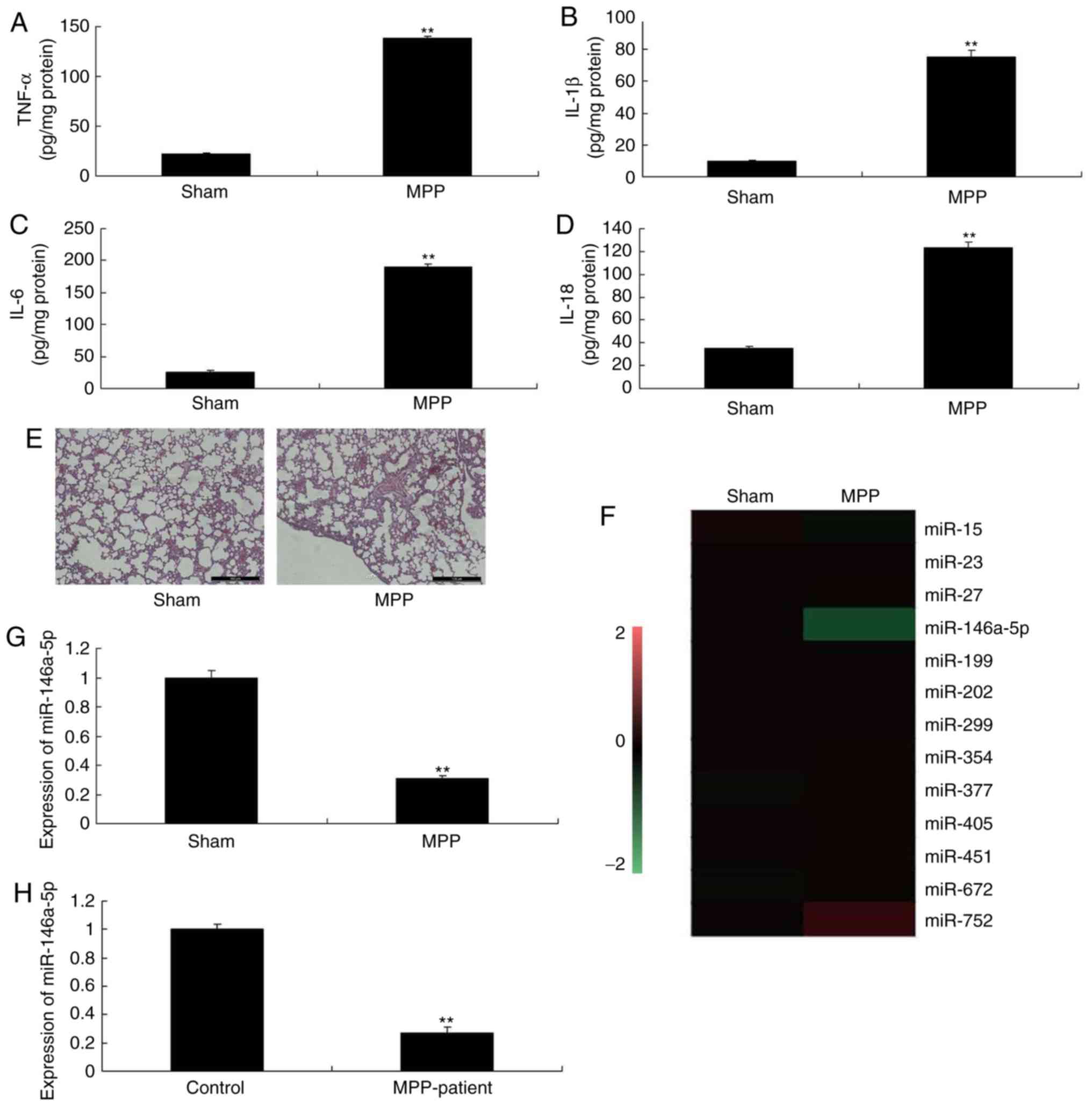 | Figure 1Expression of miR-146a-5p in patients
or mice with refractory MPP. (A) TNF-α, (B) IL-1β, (C) IL-6 and (D)
IL-18 levels. Lung tissues from mice were analyzed using (E)
H&E staining (magnification, ×100), (F) gene chip for miRNA
expression, and (G and H) reverse transcription-quantitative
polymerase chain reaction for miR-146a-5p expression in mice with
refractory MPP and patients with refractory MPP.
**P<0.01 vs. sham group. Control, control normal
group; MPP, Mycoplasma pneumoniae pneumonia; miR, microRNA;
IL, interleukin; Sham, sham control group; TNF-α, tumor necrosis
factor-α. |
miR-146a-5p regulates inflammation in an
in vitro model of refractory MPP
Then, downregulation of miR-146a-5p significantly
increased the levels of TNF-α, IL-1β, IL-6 and IL-18 in an in
vitro model of refractory MPP, compared with negative control
group (Fig. 2). However,
overexpression of miR-146a-5p significantly reduced the levels of
TNF-α, IL-1β, IL-6 and IL-18 in an in vitro model of
refractory MPP compared with the negative control group (Fig. 3). These results suggested that
miR-146a-5p was involved in the inflammation associated with MPP
in vitro.
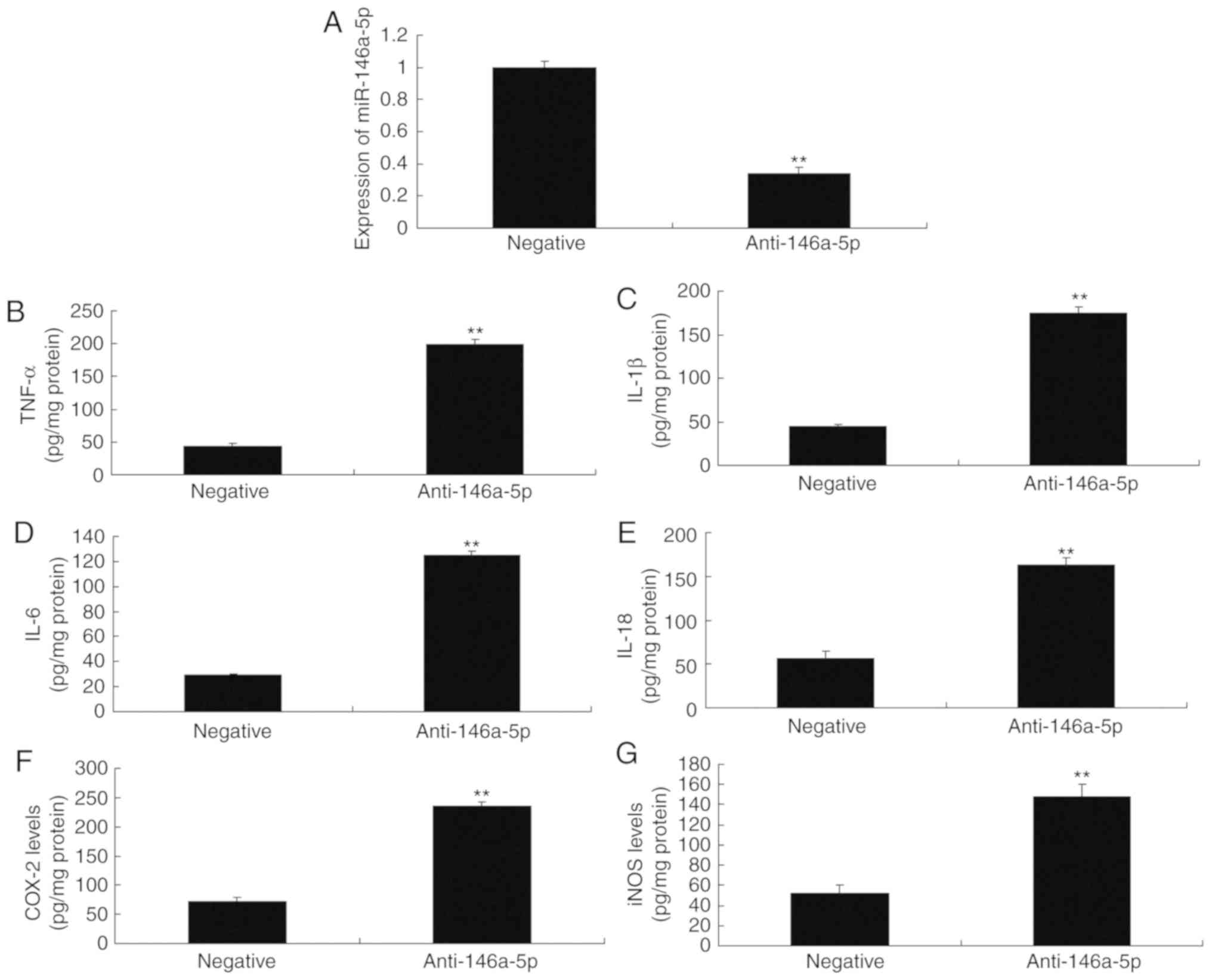 | Figure 2Anti-miR-146a-5p regulates
inflammation in an in vitro model of refractory
Mycoplasma pneumoniae pneumonia. Reverse
transcription-quantitative polymerase chain reaction for (A)
miR-146a-5p, (B) TNF-α, (C) IL-1β, (D) IL-6, (E) IL-18, (F) COX-2
and (G) iNOS levels. **P<0.01 vs. negative group.
anti-146a-5p, downregulation of miR-146a-5p expression group;
COX-2, cyclooxygenase-2; IL, interleukin; iNOS, inducible nitric
oxide synthase; miR, microRNA; negative, negative mimics group;
TNF-α, tumor necrosis factor-α. |
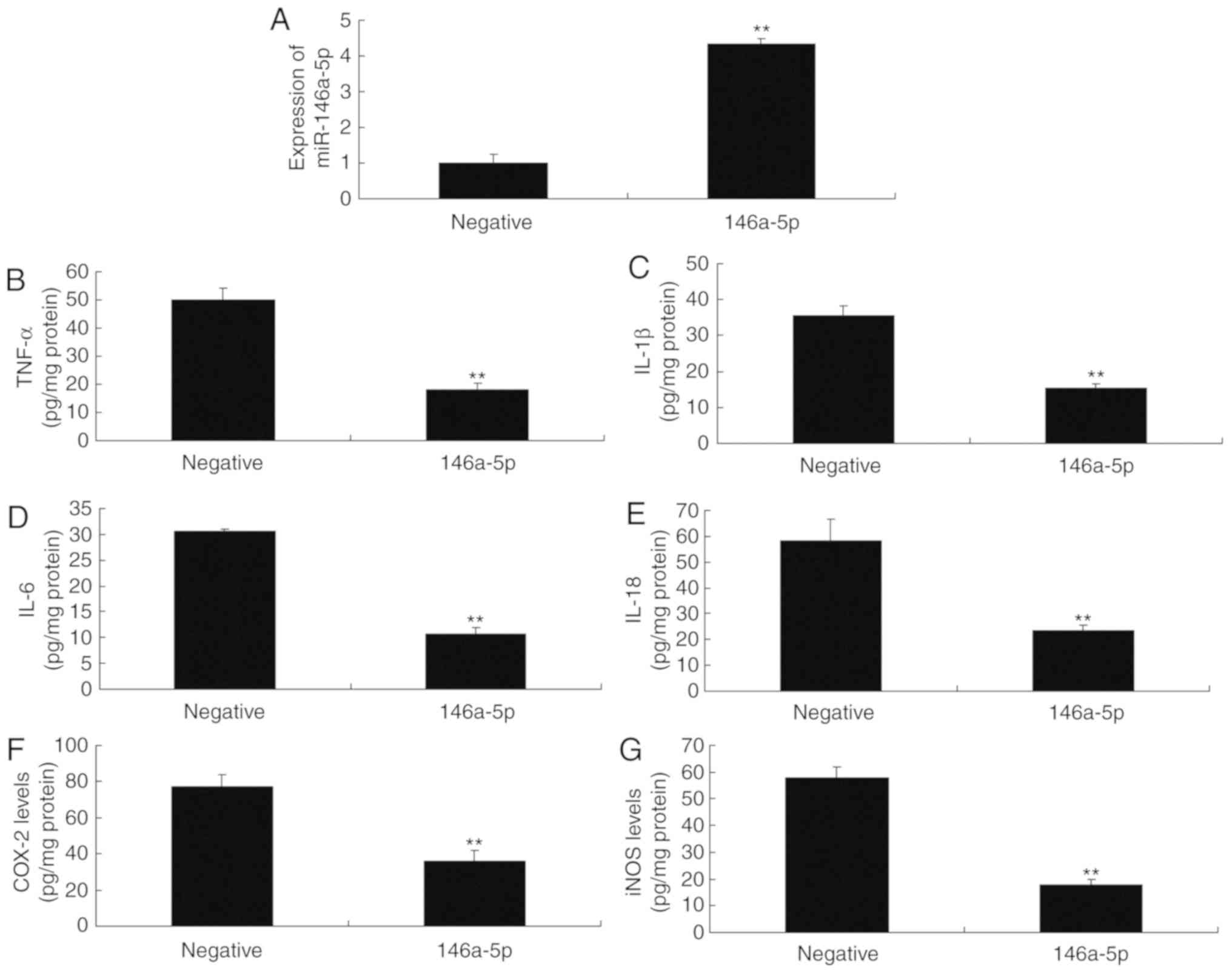 | Figure 3miR-146a-5p regulates inflammation in
an in vitro model of refractory Mycoplasma pneumoniae
pneumonia. Reverse transcription-quantitative polymerase chain
reaction for (A) miR-146a-5p expression. ELISA of (B) TNF-α, (C)
IL-1β, (D) IL-6, (E) IL-18, (F) COX-2 and (G) iNOS levels.
**P<0.01 vs. negative group. COX-2, cyclooxygenase-2;
IL, interleukin; iNOS, inducible nitric oxide synthase; miR,
microRNA; negative, negative mimics group; TNF-α, tumor necrosis
factor-α. |
miR-146a-5p regulates the protein
expression of ABCG1/IRAK-1 in an in vitro model of refractory
MPP
To confirm the mechanism of miR-146a-5p in
inflammation in an in vitro model of refractory MPP,
miR-146a-5p and anti-miR-146a-5p mimics were respectively
transfected into cells. Gene chip analysis revealed that the
protein expression levels of ABCG1 and IRAK-1 were increased in an
in vitro model of refractory MPP following down-regulation
of miR-146a-5p, compared with the negative group (Fig. 4A). The 3-untranslated region of
IRAK-1 is complementary to the seed sequence of anti-miR-146a-5p;
the luciferase activity levels were significantly increased in an
in vitro model of refractory MPP following down-regulation
of miR-146a-5p, compared with the negative control group (Fig. 4B and C). The network of signaling
pathway components revealed that ABCG1 and IRAK-1 may be important
in the development of MPP (Fig.
4D) using http://www.targetscan.org/vert_71/. These results of
IF analysis revealed that IRAK-1 protein expression was induced in
an in vitro model of refractory MPP following downregulation
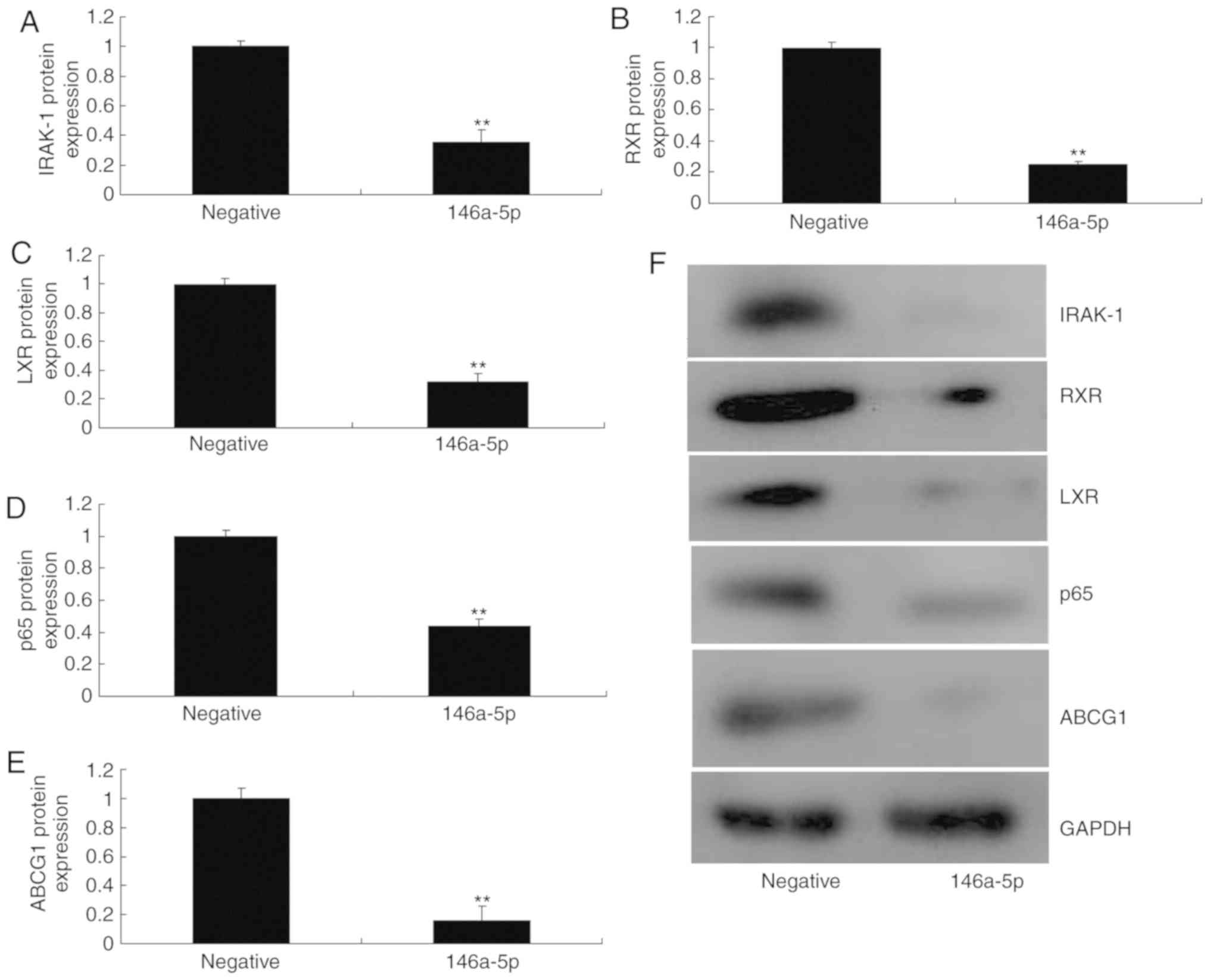
of miR-146-5p compared with the negative control group (Fig. 4E). Upregulation of miR-146a-5p
significantly reduced the protein expression of IRAK-1, RXR, LXR,
p65 and ABCG1 in an in vitro model of refractory MPP in
comparison with the negative control group (Fig. 5). However, downregulation of
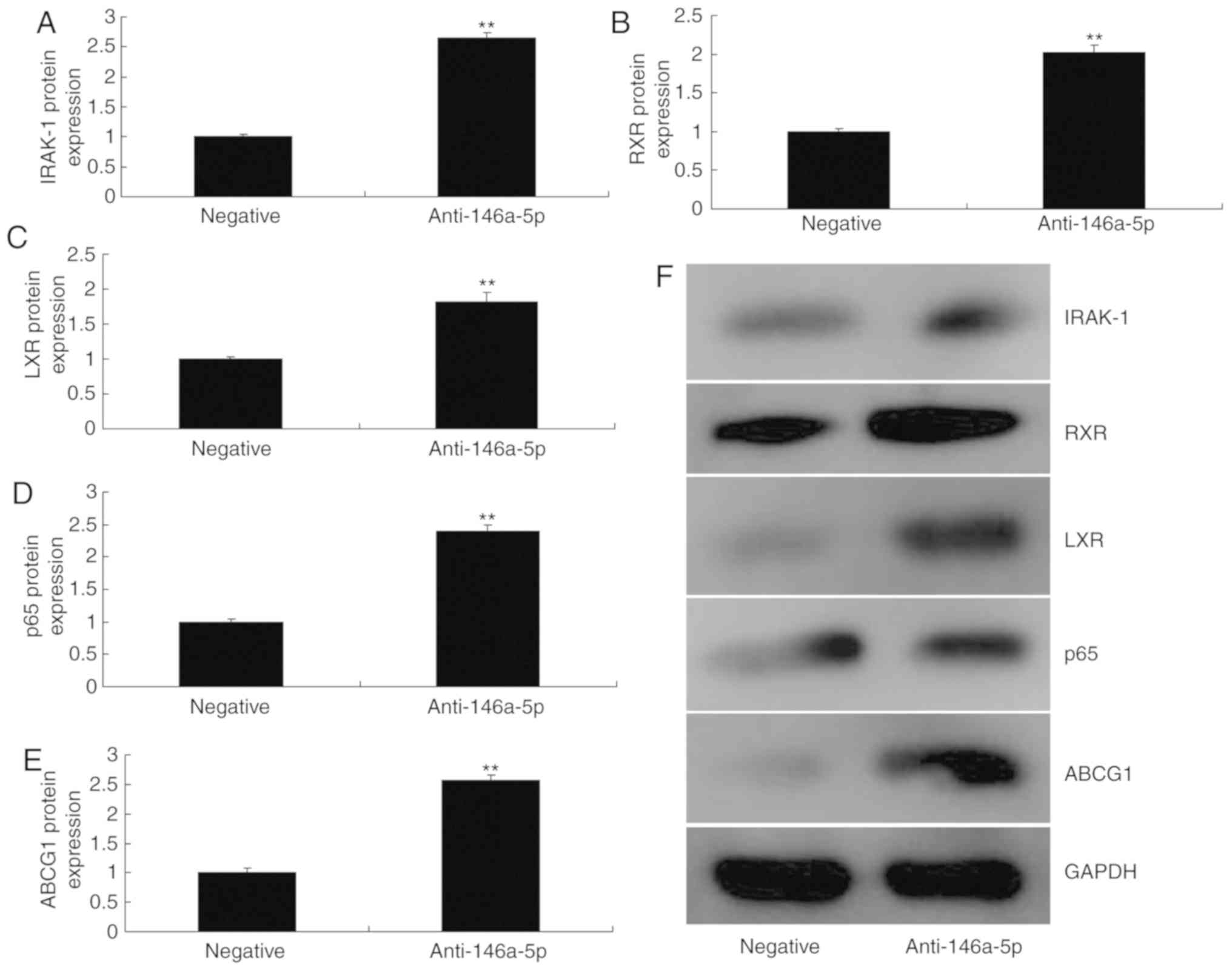
miR-146a-5p significantly increased the protein expression of
IRAK-1, RXR, LXR, p65 and ABCG1 in an in vitro model of
refractory MPP, compared with the negative control group (Fig. 6).
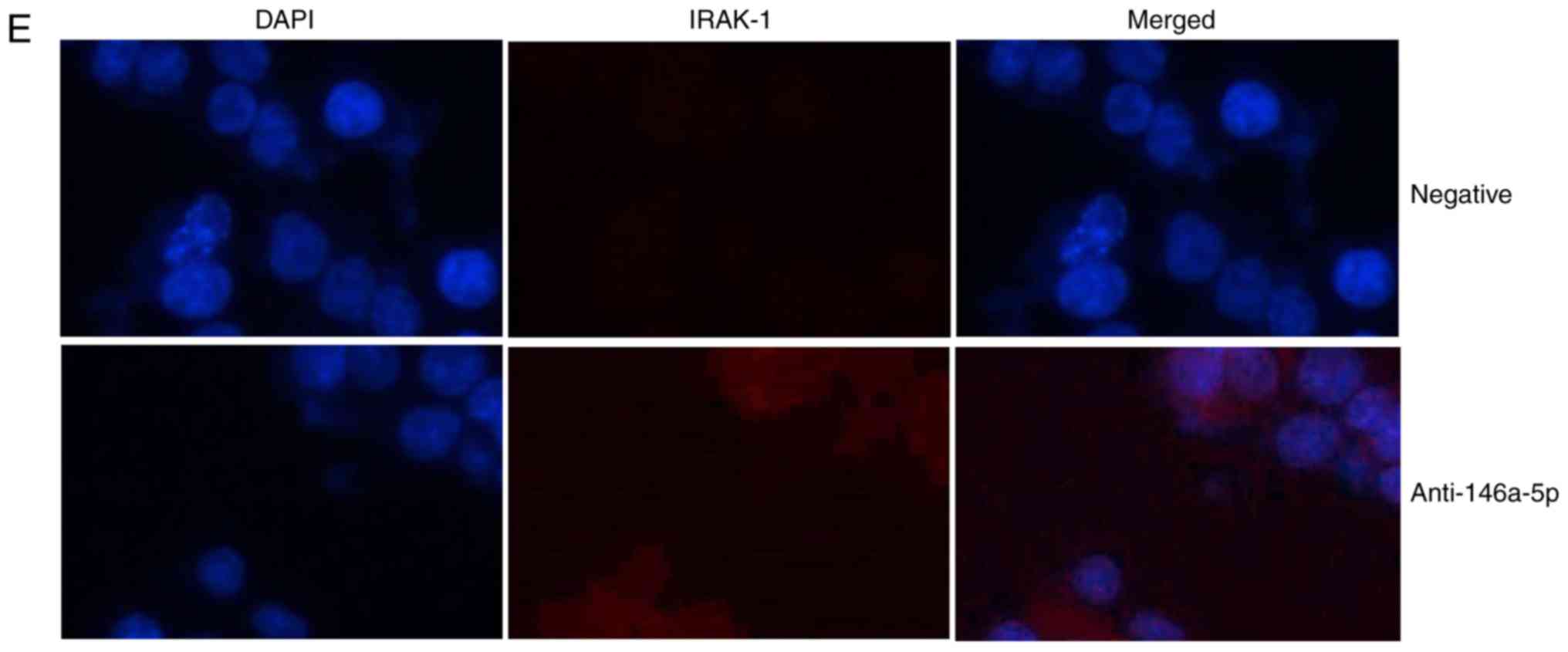 | Figure 4miR-146a-5p regulates ABCG1 and
IRAK-1 protein expression in an in vitro model of refractory
Mycoplasma pneumoniae pneumonia. (A) Gene chip analysis to
investigate the signaling pathway. (B) miR-146a-5p seed sequence in
the 3-UTR of IRAK-1; (C) dual-luciferase reporter assay. (D)
Network analysis of components associated with the signaling
pathway. **P<0.01 vs. negative group. (E)
Immunofluorescence for IRAK-1 protein expression (magnification,
×200). miR, microRNA; negative, negative mimics group;
anti-146a-5p, downregulation of miR-146a-5p expression group;
ABCG1, ATP-binding cassette subfamily G member 1; CHK1, checkpoint
kinase 1; hsa, homo sapiens; IRAK-1, interleukin 1
receptor-associated kinase 1; NEDD1, neural precursor cell
expressed, developmentally downregulated 1; PDK1,
phosphoinositide-dependent kinase 1; PI3K, phosphoinositide
3-kinase; TRAF6, tumor necrosis factor associated factor 6; Tpl2,
mitogen-activated protein kinase kinase kinase 8; UTR, untranslated
region; ZEB1, zinc finger E-box-binding homeobox 1. |
Small interfering RNA (si)-IRAK-1
attenuates the effects of miR-146a-5p on inflammation and ABCG1 in
an in vitro model of refractory MPP
To evaluate the function of IRAK-1 in the effects of
miRNA-146a-5p on inflammation in an in vitro model of
infantile pneumonia, si-IRAK-1 was used to downregulate the protein
expression of IRAK-1; the expression levels of RXR, LXR, p65 and
ABCG1 were significantly downregulated in an in vitro model
of infantile pneumonia, compared with the group transfected with
anti-miR-146a-5p (Fig. 7A-F). In
addition, the increased levels of TNF-α, IL-1β, IL-6 and IL-18 due
to downregulated miR-146a-5p were reduced in an in vitro
model of infantile pneumonia following silencing of IRAK-1
expression (Fig. 7G-L).
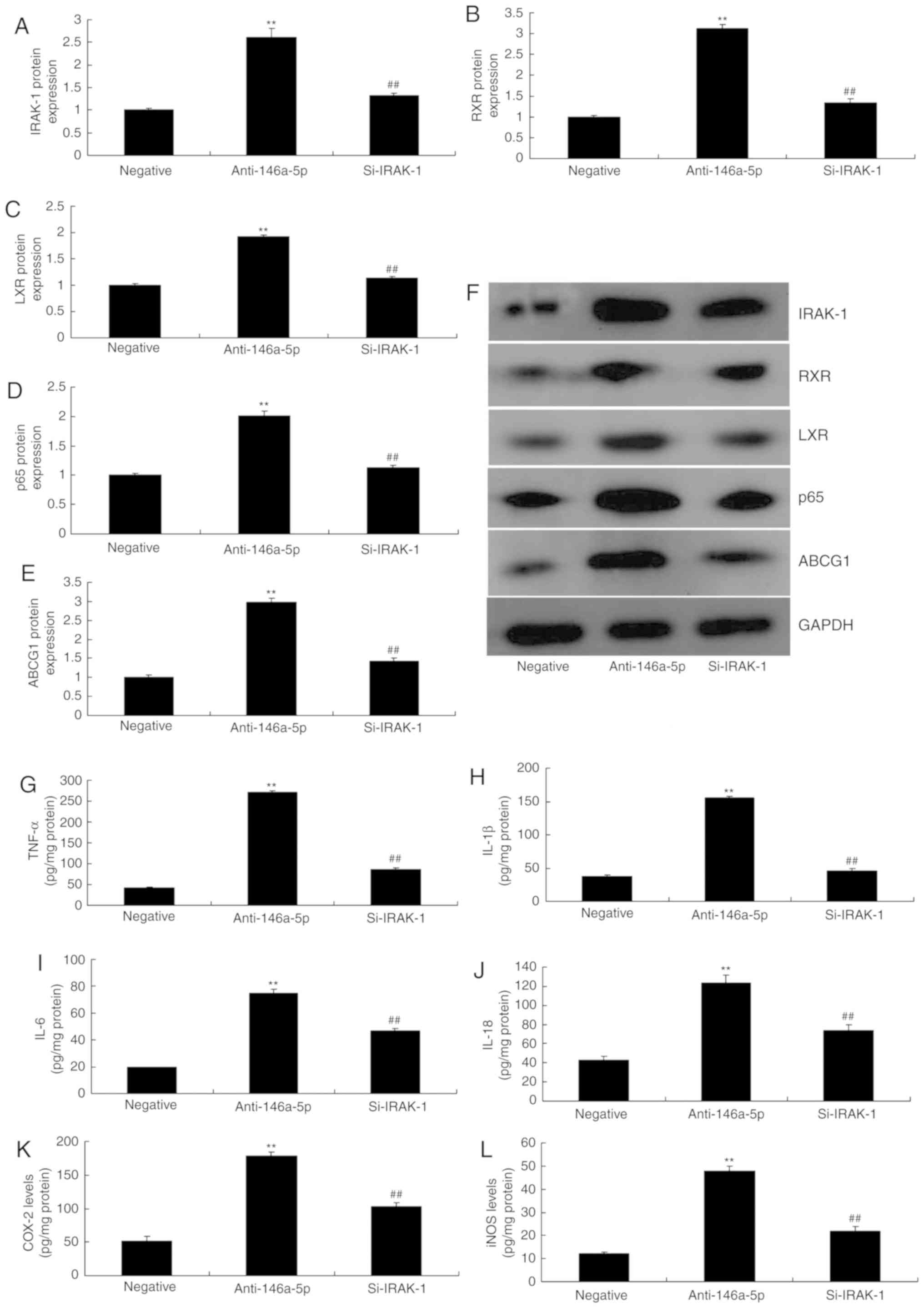 | Figure 7Si-IRAK-1 reverses the effects of
anti-miR-146a-5p on inflammation and ABCG1 in an in vitro
model of refractory Mycoplasma pneumoniae pneumonia. (A-E)
Densitometry analysis of IRAK-1, RXR, LXR, p65 and ABCG1 protein
expression following western blotting. (F) Western blot gel. (G)
TNF-α, (H) IL-1β, (I) IL-6, (J) IL-18, (K) COX-2 and (L) iNOS
levels. **P<0.01 vs. negative group;
##P<0.01 vs. anti-146a-5p group. ABCG1, ATP-binding
cassette subfamily G member 1; COX-2, cyclooxygenase-2; IL,
interleukin; iNOS, inducible nitric oxide synthase; IRAK-1,
interleukin 1 receptor-associated kinase 1; LXR, liver X receptor;
RXR, retinoid X receptor; negative, negative mimics group;
anti-146a-5p, downregulation of microRNA-146a-5p expression group;
Si, small interfering RNA; TNF-α, tumor necrosis factor-α. |
si-RXR attenuates the effects of
miR-146a-5p on inflammation and ABCG1 in an in vitro model of
refractory Mycoplasma Pneumoniae pneumonia
Finally, si-RXR was used to suppress the protein
expression of RXR, LXR, p65 and ABCG1 in an in vitro model
of refractory MPP following downregulation of miR-146a-5p. Compared
with the anti-miR-146a-5p transfected group, silencing of RXR
expression significantly suppressed the expression of the
aforementioned proteins (Fig.
8A-E). In addition, down-regulation of RXR significantly
attenuated the effects of anti-miR-146a-5p on the levels of TNF-α,
IL-1β, IL-6 and IL-18 in an in vitro model of refractory MPP
(Fig. 8F-K). Overall, the results
demonstrated that miR-146a-5p may be critical for inflammation and
the expression of ABCG1 in an in vitro model of refractory
MPP.
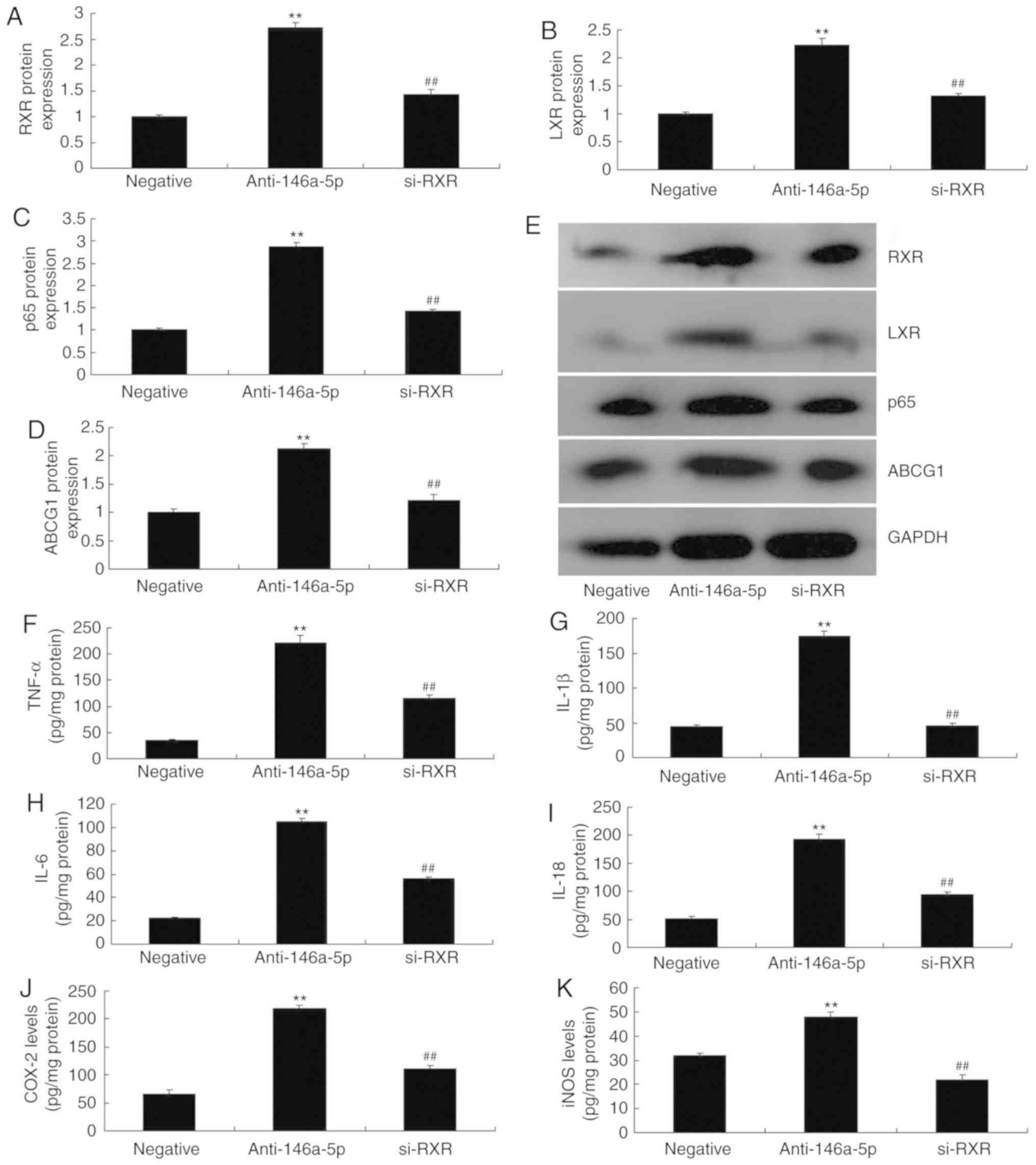 | Figure 8Small interfering-RXR reverses the
effects of miR-146a-5p on inflammation and ABCG1 in an in
vitro model of refractory Mycoplasma pneu- moniae
pneumonia. (A-D) Densitometry analysis of RXR, LXR, p65 and ABCG1
protein expression following western blotting. (E) Western blot
gel. (F) TNF-α, (G) IL-1β, (H) IL-6, (I) IL-18, (J) COX-2 and (K)
iNOS levels. **P<0.01 vs. negative group;
##P<0.01 vs. anti-146a-5p group. ABCG1, ATP-binding
cassette subfamily G member 1; COX-2, cyclooxygenase-2; IL,
interleukin; iNOS, inducible nitric oxide synthase; miR, microRNA;
IRAK-1, interleukin 1 receptor-associated kinase 1; LXR, liver X
receptor; RXR, retinoid X receptor; negative, negative mimics
group; anti-146a-5p, downregulation of miR-146a-5p expression
group; TNF-α, tumor necrosis factor-α. |
Discussion
RMPP is a type of MPP, which cannot be alleviated
with macrolide antibiotics; instead, RMPP rapidly develops into a
form of intra-pulmonary disease, thereby potentially inducing
various extra-pulmonary complications as a life-threatening
condition (19). Therefore, early
diagnosis and treatment are problems to overcome, yet the precise
pathogenesis and regulatory factors of MP infection remain unclear
(20). Previously, the role of
pattern recognition receptors in MP infection has attracted wide
attention (20). Our findings
demonstrated that miR-146a-5p expression was inhibited in patients
with refractory MPP. Osei et al (8) showed that decreased levels of
miR-146a-5p in chronic obstructive pulmonary disease-associated
fibroblasts may induce a more pronounced pro-inflammatory
phenotype. Ramkaran et al (21) reported miR-146a as a target for
lowering inflammation in coronary artery disease patients. These
results indicated miR-146a as a potential target for regulating
inflammation in MPP. Additionally, we used LPS to induce refractory
MPP in a mouse model. LPS can exhibit a broad range of effects and
may not recapitulate the conditions of mycoplasma infection as a
whole; thus, this poses as a the limitation of the present study.
We aim to generate a more precise model in the future to validate
our findings.
miRNAs serve a key role in regulating gene
expression, and its role has attracted increasing attention
(22). Numerous miRNAs are
involved in inducing an intra-pulmonary inflammatory response,
which is closely associated with tumors (22). Epidemiological analysis suggests
that, ~1/4 of tumors are induced by chronic infection and other
chronic inflammatory responses, while active measures to control
the inflammatory response may suppress tumor genesis (23). However, the relationship between
miRNAs and the intra-pulmonary inflammatory response, and its role
require further investigation (24), which may provide novel insight
into the differential expression of miRNAs in the blood to diagnose
and treat lung disease (22). Our
findings demonstrated that downregulation of miR-146a-5p increased
the levels of TNF-α, IL-1β, IL-6 and IL-18 in an in vitro
model of refractory MPP. Wu et al (25) reported that miR-146a-5p inhibits
TNF-α-induced adipogenesis via targeting insulin receptors in
primary porcine adipocytes. In the present study, A549 cells were
treated with LPS to generate an in vitro model of
Mycoplasma pneumoniae infection; however, as of the
aforementioned limitations, additional models are required to
validate our findings.
Accumulating evidence has revealed that, IRAK-1/4
serves critical roles in regulating the Toll-like receptor (TLR)
pathway (26). IRAK1
phosphorylation by IRAK4 is the earliest activation step in the TLR
and MyD88-dependent pathway, which is closely related to the
formation of an early receptor complex and activation of downstream
signaling molecules (26). Of
note, the expression of downstream cytokines associated with the
TLR signaling pathway is severely suppressed in IRAK4−/−
knockout mice (11). In addition,
research on IRAKs, which play key regulatory roles in the TLR
signaling pathway, has focused n peripheral cells and
IRAK-1/4-induced inflammation (27). Our findings revealed that
downregulation of miR-146a-5p induced IRAK-1, RXR, LXR, p65 and
ABCG1 protein expression in an in vitro model of refractory
MPP. Lo et al (28)
reported that miR-146a-5p mediates high glucose-induced endothelial
inflammation via targeting IRAK-1 expression.
ABCG1 belongs to the adenosine triphosphate binding
cassette subfamily G family of proteins, which can regulate outflow
of the intracellular free cholesterol and prevent the formation of
foam cells (29). ABCG1 is also
highly expressed in endothelial cells, and its effect on promoting
cholesterol outflow from endothelial cells serves a key role in
protecting the normal endothelial cell function (30). Upregulation of ABCG1 expression
can reduce TNF-α-induced vascular endothelial cell injury, the
mechanism of action of which may be related to its suppression on
TNF-α-induced vascular endothelial cell inflammation (10). Our results indicate that si-IRAK-1
and si-RXR reduced the effects of miR-146a-5p on inflammation and
ABCG1 in an in vitro model of refractory MPP. Li et
al (16) showed that
miR-146a-5p antagonized AGEs- and P.g-LPS-induced ABCA1 and ABCG1
dysregulation in macrophages via IRAK-1 downregulation (16).
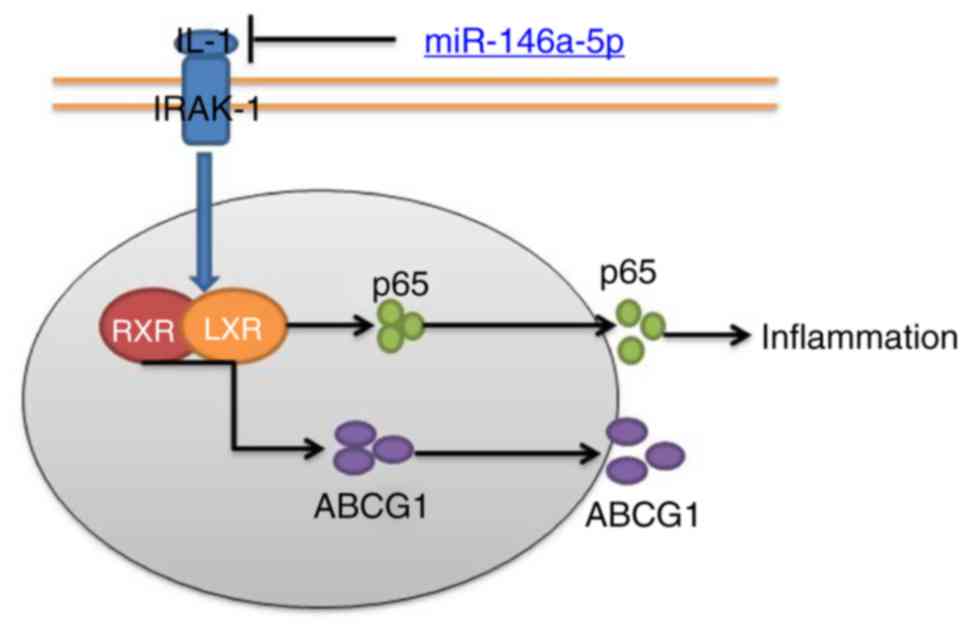
In conclusion, the present study demonstrated that
miR-146a-5p expression in refractory MPP was reduced. miR-146a-5p
was proposed to attenuate inflammation and ABCG1 expression in
refractory MPP via the IRAK-1/RXR/LXR signaling pathway (Fig. 9). We proposed a novel
anti-inflammatory role of miR-146a-5p in refractory MPP, suggesting
that miR-146a-5p may be a potential therapeutic target for the
treatment of refractory MPP.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
LS made substantial contributions to the design of
the study; HNL, XZ, YJZ, FD and JL performed the experiments. LS
and HNL analyzed the data, and LS wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Renmin Hospital,
Hubei University of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that have no competing
interests.
References
|
1
|
Jeong J, Kang I, Kim S, Park KH, Park C
and Chae C: Comparison of 3 vaccination strategies against porcine
reproductive and respiratory syndrome virus, mycoplasma
hyopneumoniae, and porcine circovirus type 2 on a 3 pathogen
challenge model. Can J Vet Res. 82:39–47. 2018.PubMed/NCBI
|
|
2
|
Zhang Y, Zhou Y, Li S, Yang D, Wu X and
Chen Z: The clinical characteristics and predictors of refractory
mycoplasma pneu-moniae pneumonia in children. PLoS One.
11:e01564652016. View Article : Google Scholar
|
|
3
|
Marrie TJ, Beecroft M, Herman-Gnjidic Z
and Poulin- Costello M: Symptom resolution in patients with
mycoplasma pneumoniae pneumonia. Can Respir J. 11:573–577. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao J, Chen C, Guo M, Tao Y, Cui P, Zhou
Y, Qin N, Zheng J, Zhang J and Xu L: MicroRNA-7 deficiency
ameliorates the pathologies of acute lung injury through elevating
KLF4. Front Immunol. 7:3892016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alipoor SD, Adcock IM, Garssen J, Mortaz
E, Varahram M, Mirsaeidi M and Velayati A: The roles of miRNAs as
potential biomarkers in lung diseases. Eur J Pharmacol.
791:395–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jadideslam G, Ansarin K, Sakhinia E,
Alipour S, Pouremamali F and Khabbazi A: The microRNA-326:
Autoimmune diseases, diagnostic biomarker, and therapeutic target.
J Cell Physiol. 233:9209–9222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan X, Li W, Yang L, Dong W, Chen W, Mao
Y, Xu P, Li D, Yuan H and Li YH: MiR-135a protects vascular
endothelial cells against ventilator-induced lung injury by
inhibiting PHLPP2 to activate PI3K/Akt pathway. Cell Physiol
Biochem. 48:1245–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osei ET, Florez-Sampedro L, Tasena H, Faiz
A, Noordhoek JA, Timens W, Postma DS, Hackett TL, Heijink IH and
Brandsma CA: miR-146a-5p plays an essential role in the aberrant
epithelial-fibroblast cross-talk in COPD. Eur Respir J.
49:16025382017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pradhan AK, Emdad L, Das SK, Sarkar D and
Fisher PB: The enigma of miRNA regulation in cancer. Adv Cancer
Res. 135:25–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao XJ, Zhang MJ, Zhang LL, Yu K, Xiang Y,
Ding X, Fan J, Li JC and Wang QS: TLR4 mediates high-fat diet
induced physiological changes in mice via attenuating
PPARgamma/ABCG1 signaling pathway. Biochem Biophys Res Commun.
503:1356–1363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo L, Chen CH, Zhang LL, Cao XJ, Ma QL,
Deng P, Zhu G, Gao CY, Li BH, Pi Y, et al: IRAK1 mediates
TLR4-induced ABCA1 downregulation and lipid accumulation in VSMCs.
Cell Death Dis. 6:e19492015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma G, Huang X, Bi Y, Ren D, Xu F, Sun Q,
Zhang R, Hu J, Niu W, Guo Z, et al: Association study between
ABCB1, ABCB6 and ABCG1 polymorphisms and major depressive disorder
in the Chinese han population. Psychiatry Res. 270:1170–1171. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li YW, Zhao F, Mo ZQ, Luo XC, Li AX and
Dan XM: Characterization, expression, and functional study of
IRAK-1 from grouper, epinephelus coioides. Fish Shellfish Immunol.
56:374–381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng KW, Zhang T, Fu H, Liu GX and Wang
XM: Schisandrin B exerts anti-neuroinflammatory activity by
inhibiting the toll-like receptor 4-dependent MyD88/IKK/NF-κB
signaling pathway in lipopolysaccharide-induced microglia. Eur J
Pharmacol. 692:29–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KH, Jeong J, Woo J, Lee CH and Yoo CG:
Globular adiponectin exerts a pro-inflammatory effect via IκB/NF-κB
pathway activation and anti-inflammatory effect by IRAK-1
downregulation. Mol Cells. 41:762–770. 2018.PubMed/NCBI
|
|
16
|
Li X, Ji Z, Li S, Sun YN, Liu J, Liu Y,
Tian W, Zhou YT and Shang XM: miR-146a-5p antagonized AGEs- and
P.g-LPS-induced ABCA1 and ABCG1 dysregulation in macrophages via
IRAK-1 downregulation. Inflammation. 38:1761–1768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Fang X, Liu X, Meng C, Fu Y, Wang X, Li B,
Tu F, Zhao F and Ren S: Breed-linked polymorphisms of porcine
toll-like receptor 2 (TLR2) and TLR4 and the primary investigation
on their relationship with prevention against mycoplasma pneumoniae
and bacterial LPS challenge. Immunogenetics. 65:829–834. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo J and Cheng Y: MicroRNA-1247 inhibits
lipopolysaccha-rides-induced acute pneumonia in A549 cells via
targeting CC chemokine ligand 16. Biomed Pharmacother. 104:60–68.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vranckx K, Maes D, Marchioro SB,
Villarreal I, Chiers K, Pasmans F and Haesebrouck F: Vaccination
reduces macrophage infiltration in bronchus-associated lymphoid
tissue in pigs infected with a highly virulent mycoplasma
hyopneumoniae strain. BMC Vet Res. 8:242012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramkaran P, Khan S, Phulukdaree A, Moodley
D and Chuturgoon AA: miR-146a polymorphism influences levels of
miR-146a, IRAK-1, and TRAF-6 in young patients with coronary artery
disease. Cell Biochem Biophys. 68:259–266. 2014. View Article : Google Scholar
|
|
22
|
Chen X, Cheng JY and Yin J: Predicting
microRNA-disease associations using bipartite local models and
hubness-aware regression. RNA Biol. 15:1192–1205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Q, Zhang D and Li Y and Li Y and Li
Y: Paclitaxel alleviated liver injury of septic mice by alleviating
inflammatory response via microRNA-27a/TAB3/NF-κB signaling
pathway. Biomed Pharmacother. 97:1424–1433. 2018. View Article : Google Scholar
|
|
24
|
Liu H, He Y, Jiang Z, Shen S, Mei J and
Tang M: Prodigiosin alleviates pulmonary fibrosis through
inhibiting miRNA-410 and TGF-β1/ADAMTS-1 signaling pathway. Cell
Physiol Biochem. 49:501–511. 2018. View Article : Google Scholar
|
|
25
|
Wu D, Xi QY, Cheng X, Dong T, Zhu XT, Shu
G, Wang LN, Jiang QY and Zhang YL: miR-146a-5p inhibits
TNF-α-induced adipogenesis via targeting insulin receptor in
primary porcine adipocytes. J Lipid Res. 57:1360–1372. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vollmer S, Strickson S, Zhang T, Gray N,
Lee KL, Rao VR and Cohen P: The mechanism of activation of IRAK1
and IRAK4 by interleukin-1 and toll-like receptor agonists. Biochem
J. 474:2027–2038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma H, Zhang S, Xu Y, Zhang R and Zhang X:
Analysis of differentially expressed microRNA of TNF-α-stimulated
mesenchymal stem cells and exosomes from their culture supernatant.
Arch Med Sci. 14:1102–1111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lo WY, Peng CT and Wang HJ:
MicroRNA-146a-5p mediates high glucose-induced endothelial
inflammation via targeting Interleukin-1 receptor-associated kinase
1 expression. Front Physiol. 8:5512017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, Villeneuve L, Jonker D, Couture F,
Laverdière I, Cecchin E, Innocenti F, Toffoli G, Lévesque E and
Guillemette C: ABCC5 and ABCG1 polymorphisms predict
irinotecan-induced severe toxicity in metastatic colorectal cancer
patients. Pharmacogenet Genomics. 25:573–583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aleidi SM, Howe V, Sharpe LJ, Yang A, Rao
G, Brown AJ and Gelissen IC: The E3 ubiquitin ligases, HUWE1 and
NEDD41 are involved in the post-translational regulation of the
ABCG1 and ABCG4 lipid transporters. J Biol Chem. 290:24604–24613.
2015. View Article : Google Scholar : PubMed/NCBI
|