Introduction
Skin photodamage is a specific type of damage to
skin tissue produced by ultraviolet (UV) irradiation. It is
characterized by erythema, edema, dyspigmentation, sallowness, fine
and coarse wrinkles, telangiectasia and roughness (1,2). A
number of studies have demonstrated that ~80% of exposed skin aging
is caused by UV-induced photo-damage (3,4).
The risk of skin aging in high-exposure populations is twice as
high as that in low-exposure populations, and the onset time of
aging could be 10 years earlier (5).
UV-induced oxidative stress and inflammatory damage
are considered to play important roles in the occurrence of
photodamage (6,7). The NF-κB signaling pathway,
activated by UV-induced reactive oxygen species (ROS), is an
important cell signaling pathway in the process of skin
inflammation and aging (8,9).
NF-κB exists in an inactive state in the cytoplasm due to NF-κB
inhibitors under non-inflammatory conditions. The activation of the
NF-κB is initiated by inhibitor of NF-κB (IκB) kinase (IKK), which
degrades cytoplasmic IκB protein, thereby triggering the rapid
release of NF-κB from IκB and intranuclear translocation (10). UV-induced excess ROS activate IKK,
which phosphorylates IκBα and activates NF-κB (11). Activated NF-κB subsequently
translocates from the cytosol to the nucleus, where it promotes the
release of various proinflammatory cytokines and chemokines,
including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and
interleukin-1β (IL-1β) (12). It
also upregulates key matrix metalloproteinases (MMPs) and degrades
extracellular matrix components, thus leading to photoaging
(13).
In addition, under oxidizing conditions, the
increased ROS also activate the nuclear factor E2-related factor 2
(Nrf2) signaling pathway (8).
Activation of the Nrf2 signaling pathway plays a pivotal role in
maintaining the cellular redox balance and protecting against
inflammation by activating antioxidant cascades (14). On the one hand, moderate levels of
ROS from solar radiation induce Nrf2 activation; when cells are
activated by ROS or some other nucleophilic agent, Nrf2 uncouples
from kelch-like ECH-associated protein 1 (keap1) (15). The activated Nrf2 is translocated
into the nucleus, then combines with antioxidant response element
(ARE), which results in a cytoprotective adaptive response
(16). The primary target genes
of Nrf2 include glutamate-cysteine ligase catalytic subunit (GCLC),
hemeoxygenase-1 and NAD(P)H quinone oxidoreductase 1 (NQO1)
(17). These antioxidant genes
help to eliminate excessive ROS to decrease oxidative stress and
the inflammatory response (18).
On the other hand, excessive ROS induced by high-dose UV inactivate
Nrf2 and block the Nrf2/ARE signal transduction pathway, which
results in decreased activity of antioxidant enzymes and a
disturbed antioxidant defense system; therefore, the damage is
exacerbated (19).
Andrographolide sodium bisulfate (ASB), a soluble
derivative composed of andrographolide and sodium bisulfate, has
excellent water solubility (20).
Intravenous infusion of ASB at 150 mg/kg body weight is considered
safe for rats (21). ASB has
anti-inflammatory, antipyretic and analgesic activities (22). ASB is associated with blockage of
oxidative damage and NF-κB-mediated inflammation in diabetic
cardiomyopathy (23), and ASB has
advantages over andrographolide for the treatment of LPS-induced
lesions (20). A previous study
demonstrated that ASB prevents UV-induced skin photodamage by
inhibiting oxidative stress and inflammation in vivo
(24); however, the associated
underlying mechanisms remain unclear. The aim of the present study
was to investigate the effects and underlying mechanisms of action
of ASB on oxidative stress and inflammation in UV-induced
photo-damage in HaCaT cells.
Materials and methods
Chemicals and reagents
ASB (cat. no. 111655-201503; purity >98%) was
purchased from the National Institutes for Food and Drug Control
(Fig. S1). Anti-Nrf2 rabbit
antibody (cat. no. ab62352), anti-keap1 rabbit antibody (cat. no.
ab218815), anti-IκBα rabbit antibody (cat. no. ab32518) and
anti-Lamin B1 rabbit antibody (cat. no. ab16048) were purchased
from Abcam. Anti-p65 rabbit antibody (cat. no. 8242S) and
anti-GAPDH rabbit antibody (cat. no. 14C10) were purchased from
Cell Signaling Technology, Inc. Goat anti-rabbit lgG H&L
[horseradish peroxidase (HRP)] (cat. no. ab6721) was purchased from
Abcam. DyLight 488-conjugated goat anti-rabbit lgG H&L was
purchased from Abbkine Scientific Co., Ltd. (cat. no. A23220).
DMEM, FBS and penicillin/streptomycin were purchased from Gibco;
Thermo Fisher Scientific, Inc. PBS was bought from HyClone; GE
Healthcare Life Sciences. MTT was purchased from BioFrox (cat. no.
3580MG250; http://www.saiguobio.com/info.aspx?id=230).
Cell culture
HaCaT cells were donated by the Guangdong Hospital
of Traditional Chinese Medicine (Guangzhou, China). Cells were
cultured in DMEM containing 10% FBS and 1% (v/v) antibodies (50
U/ml penicillin and 50 mg/ml streptomycin) in an atmosphere of 5%
CO2 at 37°C.
UV irradiation
Cells were pretreated with ASB (10, 30 and 100
µM) or DMEM for 24 h. The cells were then washed with PBS
twice and covered with a thin layer of PBS to avoid drying. The
cells were subsequently exposed to 300 µW/cm2•sec
UV for 300 sec (a total dose of 90 mJ/cm2) with a UVB
3.0 halogen lamp (UVA, 320-400 nm; UVB, 275-320 nm; UVA/UVB =97:3;
NPMOYPET®). Following UV irradiation, cells were
re-covered with fresh DMEM.
MTT assay
HaCaT cells (3.5×104/ml) were plated in
96-well plates, and then treated with different concentrations of
ASB (0, 0.01, 0.1, 1, 10, 100, 500, 1,000 or 2,000 µM) for
24 h. An MTT assay was used to measure the cytotoxicity of ASB. In
addition, in order to study the effect of ASB on UV-induced HaCaT
cells, cells were pretreated with ASB (10, 30 and 100 µM)
for 24 h. After UV irradiation (300 µW/cm2 sec ×
300 sec), cells were re-covered with fresh medium and cultured for
24 h. The MTT assay was then performed. HaCaT cells were stained
with MTT (5 mg/ml) at 37°C for 4 h. The medium was removed, and 150
µl DMSO was then added to each well. The optical density was
measured at 490 nm. The absorbance of the control cells was
considered equal to the viability.
Assessment of morphological changes using
fluorescence microscopy
Apoptotic and necrotic cells were determined with
Hoechst 33342 and propidium iodide (PI) double staining as
previously described (25). In
brief, HaCaT cells (3.5×104/ml) were pretreated with ASB
at concentrations of 10, 30 and 100 µM for 24 h and then
irradiated by UV (300 µW/cm2 sec × 300 sec).
Cells were re-covered with fresh medium and cultured for 12 h.
Cells were then incubated with 1 ml Hoechst 33342 solution (10
µg/ml) for 25 min and incubated with 1 ml PI (2.5
µg/ml) in the dark for 15 min. After staining, the
fluorescence intensity was observed under a fluorescence microscope
(original magnification, ×100; BX53; Olympus Corporation), and the
fluorescence was measured at 400-500 nm emission for Hoechst 33342
dye, and >630 nm emission for PI.
Measurement of intracellular production
of ROS
Intracellular ROS levels were determined by the
oxidative conversion of cell-permeable
2′,7′-dichlorodihydro-fluorescein diacetate (DCFH-DA) to
fluorescent DCF, as previously described (26). Cells (3.5×104/ml) were
seeded in a 96-well plate and pretreated with DMEM containing ASB
for 24 h. Then, the cells were irradiated with UV (300
µW/cm2 sec × 300 sec). The cells were then
cultured with fresh medium for 1 h. Subsequently, the cells were
washed twice with PBS and incubated with 1 ml DCFH-DA (6 µM)
solution for 30 min at 37°C. The cells were further washed twice
with PBS, and DCFH-DA fluorescence was measured using a
fluorescence microscope connected to an imaging system (original
magnification, ×200; BX53; Olympus Corporation). The fluorescence
was measured at 525 nm emission for DCFH-DA, and the fluorescence
intensity over the entire field of vision was measured using Image
J software (version 1.48; National Institutes of Health).
Measurement of inflammatory
cytokines
HaCaT cells (3.5×104/ml) were pretreated
with different concentrations of ASB for 24 h and then irradiated
with UV (300 µW/cm2 sec × 300 sec). Cells were
re-covered with fresh DMEM medium and cultured for 12 h.
Subsequently, the supernatant was collected for further
experiments. Levels of TNF-α (cat. no. CSB-E04740h), IL-6 (cat. no.
CSB-E04638h) and IL-1β (cat. no. CSB-E08053h) in the supernatant of
HaCaT cells were detected using commercially available ELISA kits
(Cusabio Technology LLC), according to the manufacturer's
protocol.
Immunofluorescence staining
The nuclear translocation of Nrf2 and p65 was
measured via immunofluorescence. HaCaT cells
(3.5×104/ml) grown in laser confocal petri dishes were
incubated with ASB for 24 h. The cells were then irradiated with UV
(300 µW/cm2 sec × 300 sec). Following UV
irradiation, the cells were washed three times with PBS (3 min
each), fixed with 4% paraformaldehyde for 15 min at room
temperature, permeabilized with Triton X (0.5% in PBS) for 20 min,
then washed and blocked with 5% goat serum (Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min at room temperature.
The cells were then stained with primary antibodies (Nrf2, 1:100;
Nrf2, 1:400) at 4°C overnight, washed with PBS with Tween-20 (PBST;
0.5% Tween-20 in PBS) and incubated with fluorochrome-conjugated
secondary antibodies (1:200) for 1 h at room temperature. The cells
were then incubated for 5 min with DAPI (5 µg/ml) in the
dark, washed with PBST four times and mounted on microscopic slides
with a drop of fluorescent mounting medium at room temperature. The
antibody localization was visualized using a fluorescence
microscope (original magnification, ×200; BX53; Olympus
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
HaCaT cells (3.5×104/ml) were solubilized
with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was extracted according to the
manufacturer's protocol. First-strand cDNA was synthesized from 500
ng total RNA using Bestar™ Moloney Murine Leukemia Virus reverse
transcriptase (DBI® Bioscience). The reaction conditions
were 37°C for 15 min, 98°C for 5 min, and holding at 4°C. Then, the
cDNA was amplified for individual PCR reactions using Bestar™
SybrGreen qPCR Mastermix (DBI® Bioscience). The primer
sequences used were as follows: GCLC sense, 5′-CTG GAG CAA CCT ACT
GTC TAA-3′ and antisense, 5′-TCA GGT CCC AGG TAG TCT TTA-3′; NQO1
sense, 5′-TCT CCT CAT CCT GTA CCT CTT T-3′ and antisense, 5′-CTG
GAG CAA CCT ACT GTC TAA G-3′; and GAPDH sense, 5′-GGA GTC AAC GGA
TTT GGT CG T-3′ and antisense, 5′-GCT TCC CGT TCT CAG CCT TGA-3′.
The reaction conditions were as follows: Initial denaturation at
95°C for 2 min; DNA amplification at 95°C for 10 sec, 55°C for 30
sec and 72°C for 30 sec (40 cycles); and a final extension step of
65-95°C (5 sec/cycle; 0.5°C/cycle) for 38 cycles. The level of mRNA
was normalized to the level of GAPDH, and compared with the normal
control (NC) group (treated with the same volume of complete DMEM)
using the 2−ΔΔCq method (27).
Western blot analysis
Proteins expression was measured by western blotting
as described previously (28).
Nuclear and cytoplasmic proteins were extracted from cultured cells
using a nuclear or cytoplasmic protein extraction kit (Beyotime
Institute of Biotechnology), respectively (Cells were seeded into
plates at a density of 3.5 × 104/ml). Protein
concentrations were measured using a BCA protein assay kit
(Beyotime Institute of Biotechnology). Then, cell lysates (10
µg/lane) were separated by SDS-PAGE (10%) and
electrophoretically transferred onto a polyvinylidene fluoride
membrane. The membrane was then washed with TBS with Tween-20
(TBST) three times, and blocked with 5% non-fat milk in TBST (1%
Tween-20) for 2 h at room temperature. The membrane was incubated
with Nrf2 (1:1,000), keap1 (1:300), p65 (1:3,000), IκBα (1:1,000),
GAPDH (1:1,000) and Lamin B (1:1,000) antibodies overnight at 4°C,
and incubated with HRP-coupled secondary antibodies (1:2,000) for 1
h at room temperature. Proteins were detected using enhanced
chemiluminescence (Fdbio Science). The protein intensity was
measured using Image J software (version 1.48; National Institutes
of Health). The relative protein levels were normalized to
GAPDH/Lamin B1 protein.
Statistical analysis
All data are expressed as the mean ± SD. Statistical
analyses were performed using SPSS 20 (IBM Corp.). Group
differences were assessed by one-way ANOVA followed by Tukey's test
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
ASB increases the cell viability of
UV-induced HaCaT cells
As presented in Fig.
1A, 0.01-2,000 µM ASB exerted no cytotoxic effect on
HaCaT cells. Compared with the NC group, the viability of
UV-induced HaCaT cells was significantly decreased (P<0.01).
However, the decreased cell viability was significantly increased
by ASB, and ASB at a concentration of 100 µM increased cell
viability by nearly 1-fold (P<0.01 vs. UV-alone group; Fig. 1B).
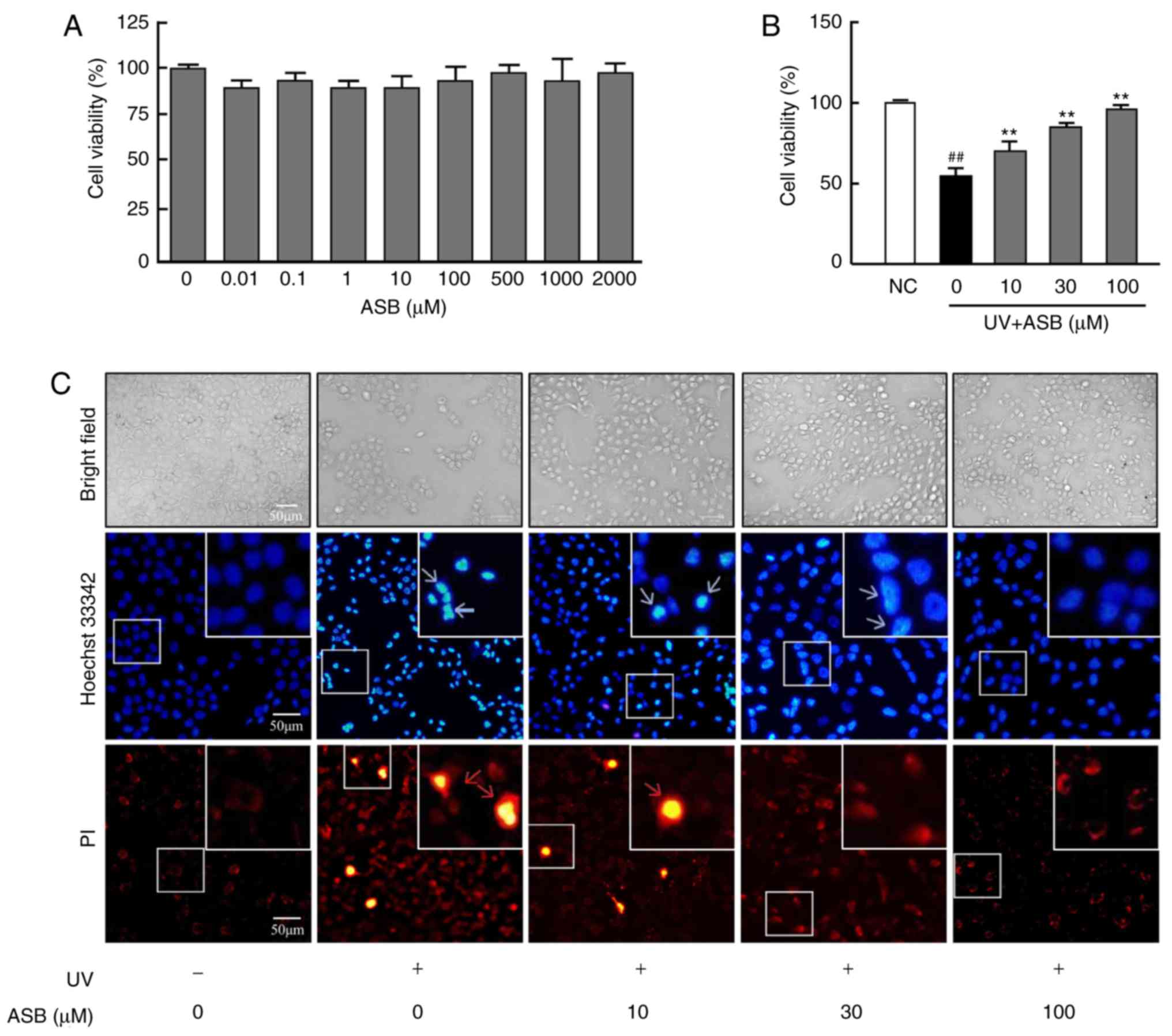 | Figure 1Protective effect of ASB on
UV-induced HaCaT cells. (A) The cells were treated with 0, 0.01,
0.1, 1, 10, 100, 500, 1,000 and 2,000 µM ASB for 24 h. The
cell viability was determined by MTT assay (n=5 for each group).
(B) The cells were preincubated with ASB (10, 30 and 100 µM)
and irradiated with UV (90 mJ/cm2). Cell viability was
measured by MTT assay (n=5 for each group). (C) The cells were
preincubated with ASB (10, 30 and 100 µM) and irradiated
with UV (90 mJ/cm2). Cell apoptosis and death were
measured by staining with Hoechst 33342 and PI. The blue arrowheads
indicate apoptotic cells, and the red arrowheads indicate dead
cells. The images were examined by bright field and fluorescence
microscopy. The results are expressed as the mean ± SD. ##P<0.01
vs. NC group; **P<0.01 vs. UV-alone group. UV,
ultraviolet; ASB, andrographolide sodium bisulfite; NC, normal
control; PI, propidium iodide. |
ASB inhibits the UV-induced apoptosis and
necrosis of HaCaT cells
As presented in Fig.
1C, under observation with ordinary light following UV
irradiation, the number of the HaCaT cells was decreased and the
cell refraction and adhesion abilities were weakened. ASB treatment
markedly increased cell number, restored cell morphology, and
enhanced cell refraction and adhesion abilities. Furthermore, the
observation with fluorescent light detected that, compared with the
NC group, the number of apoptotic cells (bright blue) in the UV
group was increased, which manifested as decreased cell volume and
nuclear fragmentation. At the same time, a number of necrotic cells
(bright red) appeared. However, compared with the UV group,
preincubation with ASB decreased the number of apoptotic and
necrotic cells.
ASB inhibits oxidative stress in
UV-induced HaCaT cells
In order to investigate whether ASB could decrease
the UV-induced oxidative stress, the ROS production was
investigated using the fluorescent ROS probe DCFH-DA, and the mRNA
expression levels of antioxidant genes were measured by RT-qPCR. As
presented in Fig. 2A, UV
irradiation (90 mJ/cm2) increased the production of ROS,
which was significantly higher than that in the NC group
(P<0.01; Fig. 2A and B).
Furthermore, the mRNA expression levels of GCLC and NQO1 in the UV
group were lower than those in the NC group (P<0.05; Fig. 2C and D). Notably, ASB pretreatment
inhibited the UV-induced ROS production (P<0.01; Fig. 2A and B), and high concentrations
of ASB significantly upregulated GCLC and NQO1 expression levels by
nearly 1-fold and 1.6-fold respectively (P<0.05 vs. UV group;
Fig. 2C and D).
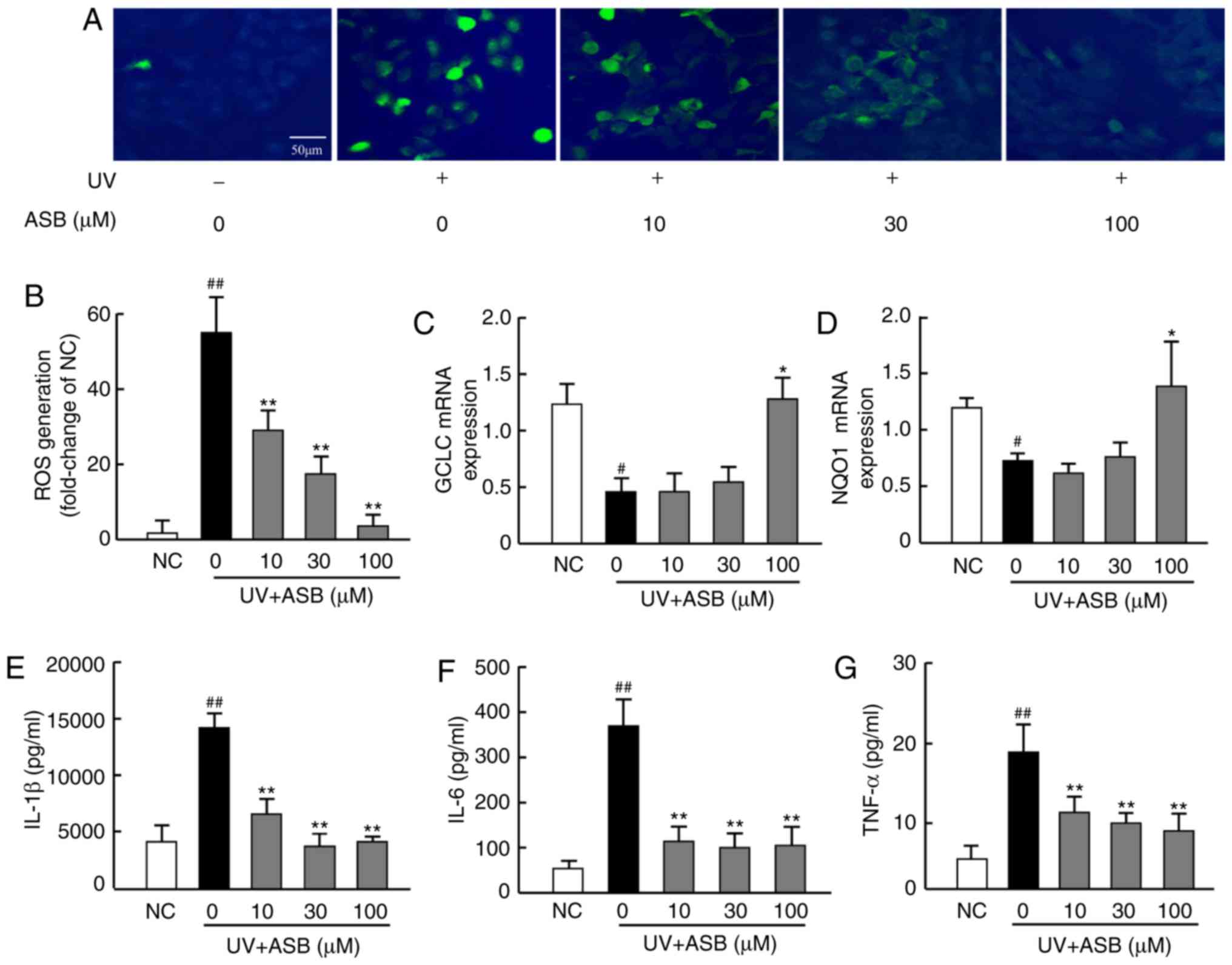 | Figure 2ASB decreases oxidative stress and
proinflammatory cytokines in UV-irradiated HaCaT cells. The cells
were preincubated with ASB (10, 30 and 100 µM) and
irradiated with UV (90 mJ/cm2). (A) The intracellular
ROS in HaCaT cells was assessed by DCFH-DA. The appearance of green
fluorescence represents the intensity of the generated ROS. (B) The
fluorescence intensity was quantified with Image J software. The
mRNA expression levels of (C) GCLC and (D) NQO1 in HaCaT cells were
measured by reverse transcription-quantitative PCR. The production
of (E) IL-1β, (F) IL-6 and (G) TNF-α was measured by ELISA (n=4 for
each group). The results are expressed as the mean ± SD.
#P<0.05, ##P<0.01 vs. NC group;
*P<0.05, **P<0.01 vs. UV-alone group.
UV, ultraviolet; ASB, andrographolide sodium bisulfite; ROS,
reactive oxygen species; GCLC, glutamate-cysteine ligase catalytic
subunit; NQO1, NAD(P)H quinone oxidoreductase 1; IL-1β,
interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α; NC, normal control. |
ASB alleviates inflammatory cytokine
production in UV-induced HaCaT cells
The results revealed that UV-induced HaCaT cells
exhibited excessive production of IL-1β, IL-6 and TNF-α.
Conversely, ASB pretreatment efficiently decreased the UV-induced
expression of IL-1β, IL-6 and TNF-α. ASB at 100 µM decreased
IL-1β, IL-6 and TNF-α levels by nearly 2-fold, 3-fold and 1.5-fold,
respectively (P<0.01 vs. UV group; Fig. 2E-G).
Effect of UV irradiation on the NF-κB and
Nrf2 signaling pathways
As presented in Fig.
3, UV induced the abnormal expression of p65, IκBα, Nrf2 and
keap1 in the cytosol and nucleus in a time-dependent manner.
Following UV irradiation, the protein expression levels of p65 and
IκBα in the cytosol increased between 0 and 6 h, while the nuclear
expression of p65 increased from 0.5 h, peaked at 2 h, and then
gradually decreased thereafter (Fig.
3). By contrast, the protein expression levels of Nrf2 in the
cytosol and nucleus and keap1 started to rise between 0 and 3 h,
peaked around 3 h, and then gradually decreased (Fig. 3).
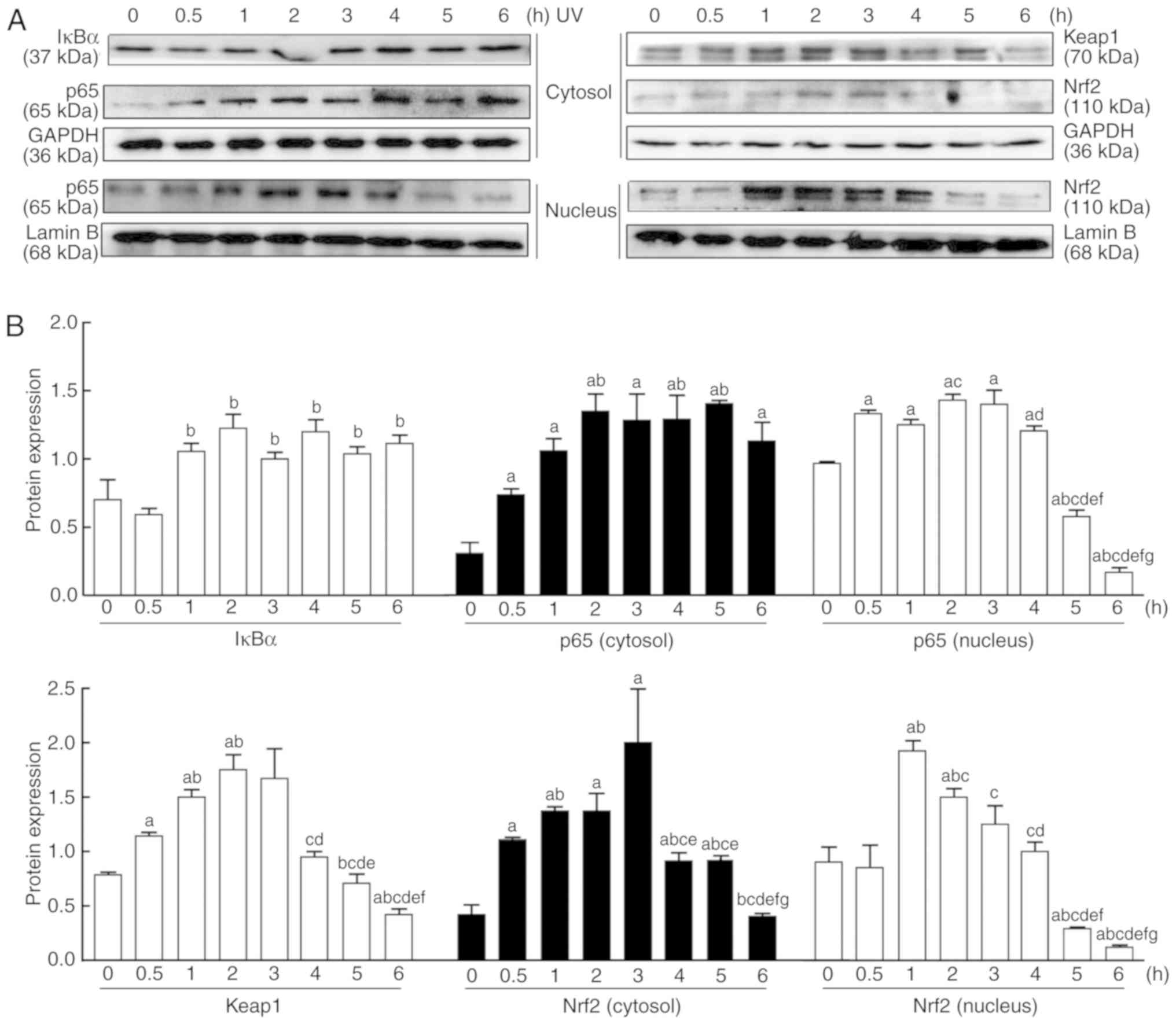 | Figure 3Changes in the Nrf2 and NF-κB
signaling pathways in HaCaT cells after UV irradiation. (A) Nuclear
and cytoplasmic proteins were extracted from the cultured cells at
0, 0.5, 1, 2, 3, 4, 5 and 6 h after UV irradiation (90
mJ/cm2). The protein expression levels of NF-κB-mediated
p65, and IκBα and Nrf2-mediated Nrf2 and keap1, were measured by
western blotting. (B) Relative changes in protein intensities were
quantified by densitometric analysis and are presented as bar
diagrams (n=3 for each group). The results are expressed as the
mean ± SD. aP<0.05 vs. the 0 h group;
bP<0.05 vs. the 0.5 h group; cP<0.05
vs. the 1 h group; dP<0.05 vs. the 2 h group;
eP<0.05 vs. the 3 h group; fP<0.05 vs.
the 4 h group; gP<0.05 vs. the 5 h group. UV,
ultraviolet; IκBα, NF-κB inhibitor-α; keap1, kelch-like
ECH-associated protein 1; Nrf2, nuclear factor E2-related factor
2. |
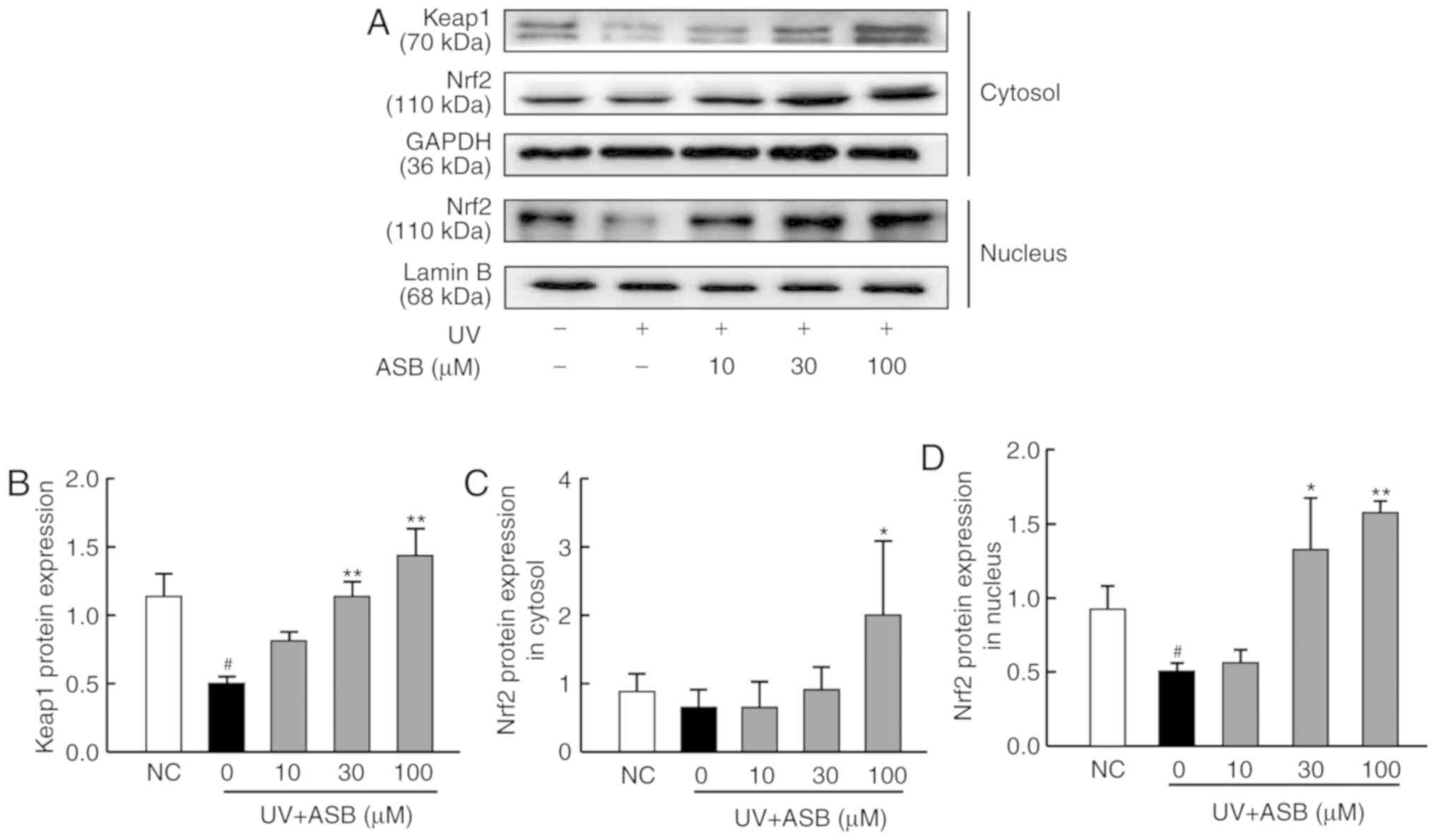
ASB activates the Nrf2 signaling pathway
in UV-induced HaCaT cells
As presented in Fig.
4, the expression levels of keap1 and nuclear Nrf2 were
significantly down-regulated in the UV-induced group (P<0.05 vs.
NC group; Fig. 4A, B and D).
Conversely, ASB pretreatment increased the protein expression
levels of keap1, cytoplasmic and nuclear Nrf2 in a dose-dependent
manner (P<0.05 or P<0.01 vs. UV group; Fig. 4). Furthermore, ASB at a high
concentration was able to increase the expression levels of keap1,
cytoplasmic Nrf2 and nuclear Nrf2 by nearly 1.9-fold, 1.9-fold and
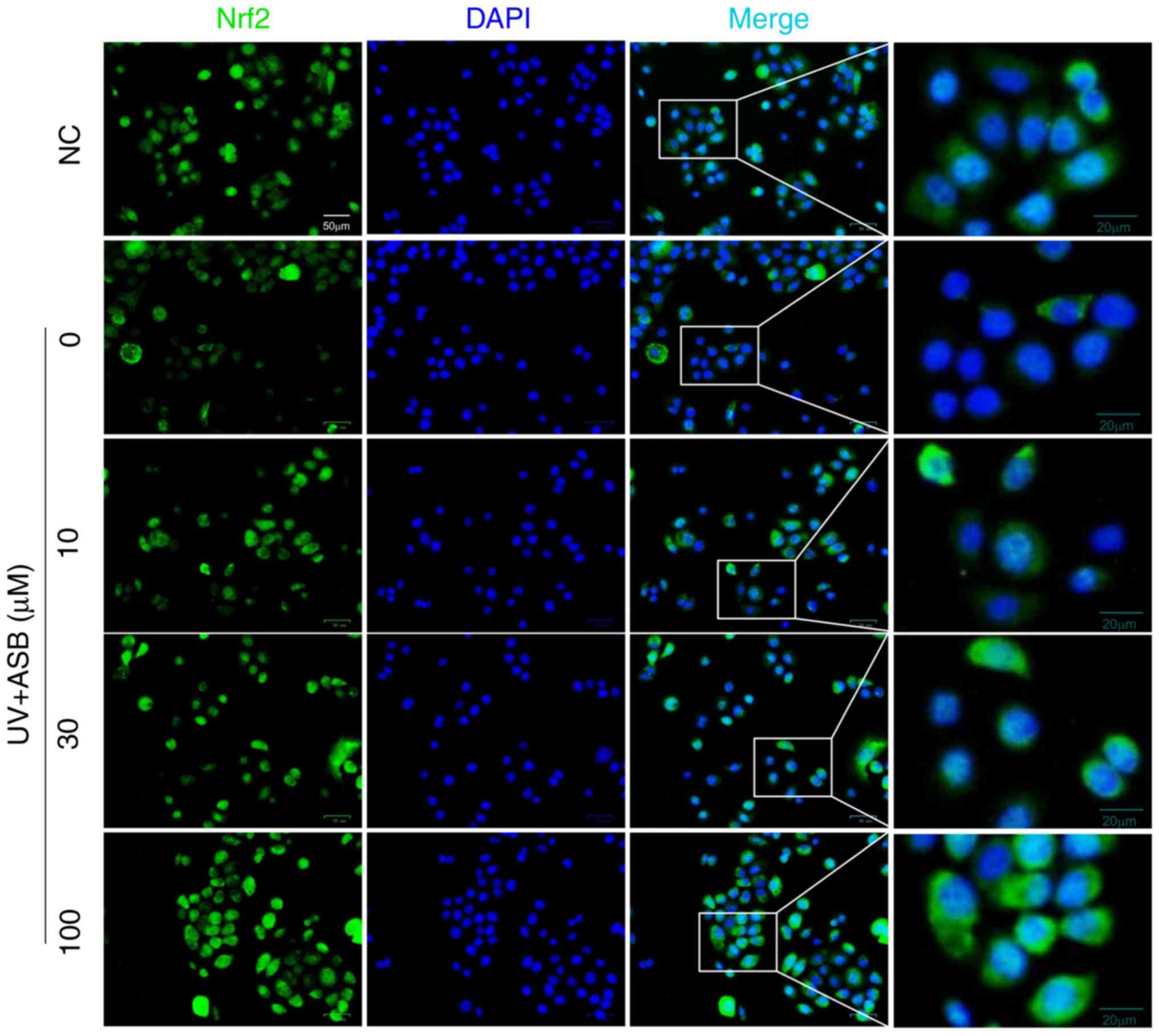
2.1-fold respectively. Notably, the fluorescence intensity of Nrf2
was in accordance with the western blotting results. The
immunofluorescence staining results indicated that the fluorescence
intensity of Nrf2 in the nucleus was decreased in UV-induced cells.
However, ASB pretreatment markedly reversed the abnormal nuclear
translocation of Nrf2 (Fig.
5).
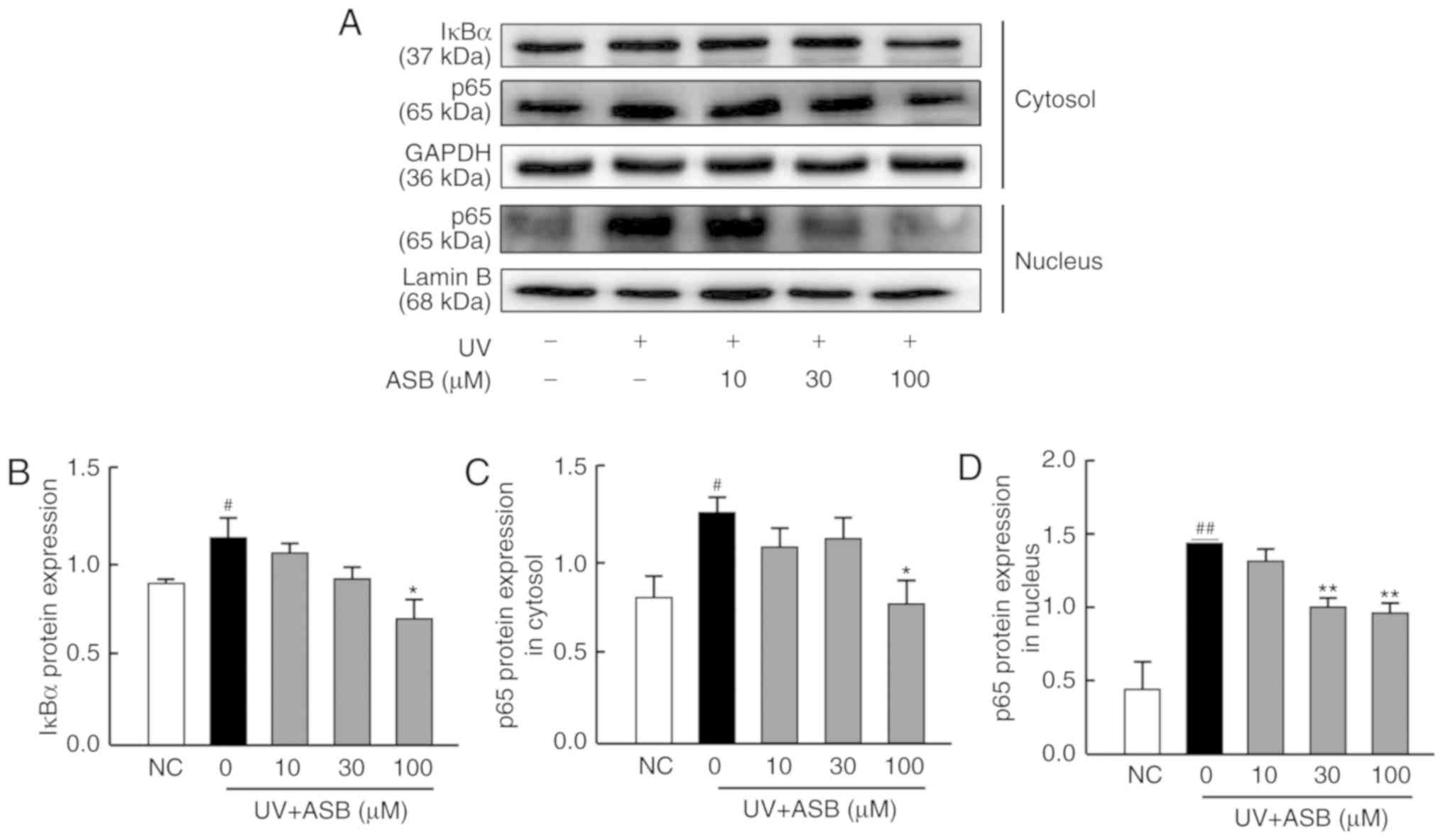
ASB inhibits the NF-κB signaling pathway
in UV-induced HaCaT cells
As presented in Fig.
6, the expression levels of IκBα, cytoplasmic p65 and nuclear
p65 were significantly upregulated in the UV-induced group
(P<0.05 or P<0.01 vs. NC group). The moderate concentration
of ASB significantly decreased the protein expression level of p65
in the nucleus, while high concentrations were able to downregulate
the expression levels of IκBα, cytoplasmic p65 and nuclear p65 by
61.7, 52.6 and 46.4%, respectively (P<0.05 or P<0.01 vs. UV
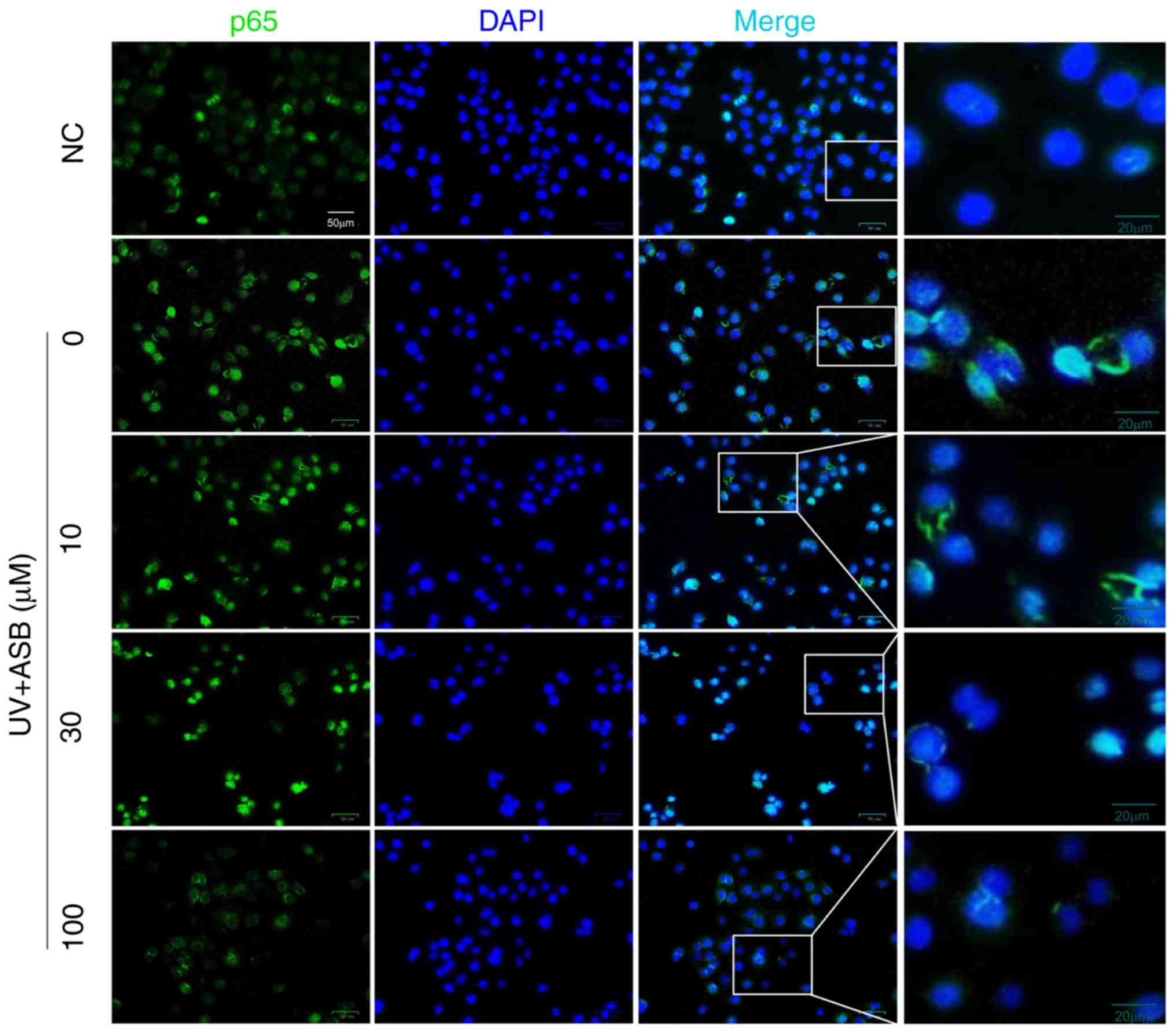
group; Fig. 6B-D). In particular,
the fluorescence intensity of p65 was in accordance with the
western blotting results. The immunofluorescence staining results
indicated that the fluorescence intensity of p65 in the nucleus was
increased in UV-induced cells. However, ASB pretreatment markedly
reversed the abnormal nuclear translocation of p65 (Fig. 7).
Discussion
UV irradiation is a predominant environmental factor
in the pathogenesis of photo-damage. Over time it is possible to
develop actinic keratosis and skin cancer (29). It has been reported that the
oxidative stress and inflammatory response mediated by UV are
critical factors in the pathogenesis of photo-aging (6,7).
Thus the development of effective anti-UV-induced photoaging drugs
will focus on the prevention of oxidative damage and inflammation
during photoaging. ASB has potential antioxidant and
anti-inflammatory activities, and a previous study reported that
ASB could prevent UV-induced skin photo-damage in vivo
(24).
Excessive UV exposure could accelerate the
accumulation of ROS in the skin, increasing oxidative stress in
cutaneous cells, thereby resulting in photodamage. UV-induced ROS
production activates the NF-κB signaling pathway, which further
induces inflammation and apoptosis in cells and causes skin aging
(8,30). In its inactive form, NF-κB is
sequestered in the cytoplasm and bound by members of the IκB family
of inhibitor proteins. Accumulation of ROS that activate NF-κB
causes the nuclear localization of p65 (8). In the nucleus, NF-κB binds to a
consensus sequence (5′GGGACTTTCC-3′) in various genes (such as
IL-1β, IL-6 and TNF-α), and thus activates their transcription.
Furthermore, proinflammatory cytokines subsequently stimulate the
signal transduction pathway to activate NF-κB, thus causing a
feedback loop (12). Such
inflammatory mediators further promote the expression levels of
MMPs (13). The results of the
present study demonstrated that UV irradiation could cause HaCaT
cell apoptosis via qualitative analysis, which will be confirmed
through quantitative analysis in a further study. The results also
showed that UV irradiation could upregulate ROS, p65 and IκBα
levels, as well as the production of IL-1β, IL-6 and TNF-α
cytokines in HaCaT cells. However, ASB pretreatment significantly
decreased the UV-induced accumulation of ROS, and downregulated the
protein expression of p65 in the nucleus, while subsequently
lessening the secretion of proinflammatory cytokines and reducing
the apoptosis of HaCaT cells.
The Nrf2 pathway is an important antioxidative and
anti-inflammatory pathway involved in UV-ROS-induced skin damage
(31). Under normal physiological
conditions, keap1 is associated with Nrf2. However, under oxidizing
conditions, the increased level of ROS promotes the dissociation of
Nrf2 and keap1, and dissociated Nrf2 translocates to the nucleus,
combines with Maf proteins and ARE, and subsequently regulates the
expression of downstream antioxidant genes, such as GCLC and NQO1
(32). Nrf2 transcriptional
activation and its antioxidant genes repair UV-induced skin
inflammatory damage, protect cells against UV insult and decrease
photo-oxidative damage (33). It
has been demonstrated that andrographolide markedly suppresses
oxidative stress injury by increasing Nrf2 activation and the
expression levels of Nrf2 downstream genes, both in vivo and
in vitro (34). In the
present study, increased Nrf2 nuclear localization was observed
after ASB treatment in HaCaT cells, which was followed by the
upregulation of GCLC and NQO1 mRNA levels. These results indicated
that ASB could activate Nrf2 signaling to suppress the UV-induced
oxidative stress present in photo-damaged cells.
Recent studies have suggested that there is a
potential dynamic balance system and possible crosstalk between the
Nrf2 and NF-κB pathways (35,36). Liu et al (37) demonstrated that NF-κB
competitively dissociates Nrf2 from CREB-binding protein and
facilitates the recruitment of histone deacetylase 3 to MafK,
resulting in the inactivation of Nrf2. Overexpression of p65
decreases Nrf2 protein levels by promoting Nrf2 ubiquitination
(38). By contrast, inhibition of
Nrf2 expression also accelerates the activation of NF-κB. For
instance, the absence of Nrf2 enhances NF-κB-dependent inflammation
following scratch injury (39).
In addition, phenethyl isothiocyanate and sulforaphane, as Nrf2
activators, inhibit NF-κB subunit p65 nuclear translocation,
consequently inactivating the NF-κB signaling pathway (40). In the present study, the timepoint
results represented a new phenomenon, in that the protein
expression levels of IκBα and cytoplasmic p65 were continuously
increased, while the protein expression levels of Nrf2 pathway
proteins were increased at first and then decreased, between 0 and
6 h after UV irradiation. This indicated the presence of a dynamic
balance between the NF-κB and Nrf2 signaling pathways in HaCaT
cells, and continuous UV stimulation rendered the negative
regulation of Nrf2 by NF-κB advantageous. In addition, the present
study indicated that the Nrf2 and NF-κB pathways share common
effectors and regulatory points, and that they can be activated by
ROS at the same time (41).
Therefore, there is a likely to be a dynamic balance between the
NF-κB and Nrf2 signaling pathways in HaCaT cells.
The present study revealed that ASB inhibited
UV-ROS-induced inflammation and oxidative stress following skin
cell damage by downregulating the NF-κB pathway and activating the
Nrf2 pathway (Fig. 8). Thus, ASB
may be considered to be a promising strategy for preventing skin
photo-damage. In UV-induced photoaging models, biological functions
and genetic variations are different between HaCaT cells and normal
human keratinocytes, which may impact the cellular responses to UV
irradiation. Thus, normal human keratinocytes should be used in
future research.
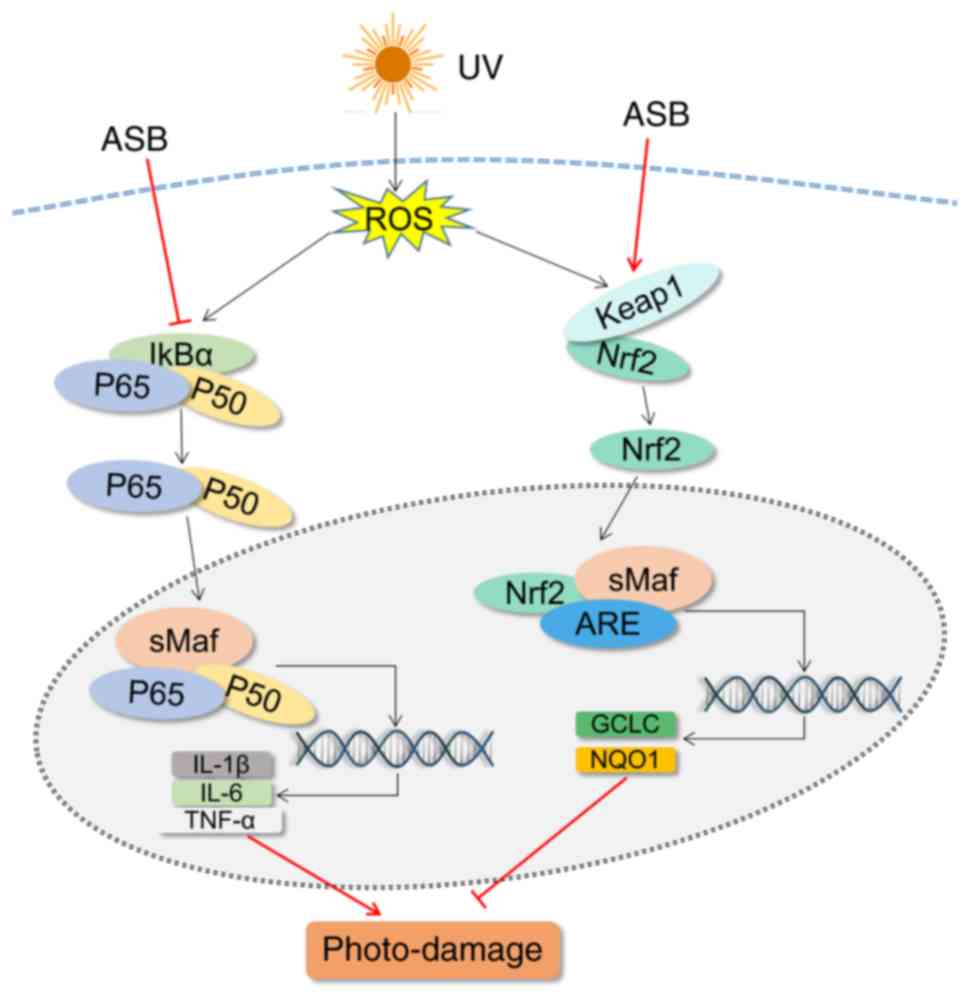 | Figure 8Schematic diagram of the mechanism of
action of ASB in UV-induced photo-damage in HaCaT cells. UV,
ultraviolet; ASB, androgra-pholide sodium bisulfite; ROS, reactive
oxygen species; keap1, kelch-like ECH-associated protein 1; Nrf2,
nuclear factor E2-related factor 2; IL, interleukin; TNF-α, tumor
necrosis factor-α; GCLC, glutamate-cysteine ligase catalytic
subunit; NQO1, NAD(P)H quinone oxidoreductase 1; IκBα, NF-κB
inhibitor-α; ARE, antioxidant response element. |
Supplementary Data
Acknowledgments
Not applicable.
Funding
This study was supported by National Natural Science
Foundation of China (grant no. 81503318), Guangzhou University of
Chinese Medicine Young Talent Project (grant no. QNYC20170106), and
Guangdong Public Welfare Research and Capacity Building Projects
(grant no. 2016A020217016).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZRS and JYXZ made substantial contributions to the
design of the work. BQL, YC and JY were involved in the study
conception and design. MLW and QYZ performed the experiments,
analyzed the data and wrote the paper. YHL and YFH were involved in
the experiments in the present study. BQL contributed to drafting
and revising the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Battie C and Verschoore M: Cutaneous solar
ultraviolet exposure and clinical aspects of photodamage. Indian J
Dermatol Venereol Leprol. 78(Suppl 1): S9–S14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogden S, Samuel M and Griffiths CE: A
review of tazarotene in the treatment of photodamaged skin. Clin
Interv Aging. 3:71–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kageyama H and Waditee-Sirisattha R:
Antioxidative, anti-inflammatory, and anti-aging properties of
mycosporine-like amino acids: Molecular and cellular mechanisms in
the protection of skin-aging. Mar Drugs. 17:E2222019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puizina-Ivić N: Skin aging. Acta
Dermatovenerol Alp Pannonica Adriat. 17:47–54. 2008.
|
|
5
|
Wu SL, Li H, Zhang XM and Li ZF: Optical
features for chronological aging and photoaging skin by optical
coherence tomography. Lasers Med Sci. 28:445–450. 2013. View Article : Google Scholar
|
|
6
|
Schuch AP, Moreno NC, Schuch NJ, Menck CFM
and Garcia CCM: Sunlight damage to cellular DNA: Focus on
oxidatively generated lesions. Free Radic Biol Med. 107:110–124.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HK: Protective effect of garlic on
cellular senescence in UVB-exposed HaCaT human keratinocytes.
Nutrients. 8:E4642016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang JX, Wang XL, Vikash V, Ye Q, Wu D,
Liu Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee B, Moon KM, Son S, Yun HY, Han YK, Ha
YM, Kim DH, Chung KW, Lee EK, An HJ, et al: (2R/S,4R)-2-
(2,4-Dihydroxyphenyl)thiazolidine-4-carboxylic acid prevents
UV-induced wrinkle formation through inhibiting NF-κB-mediated
inflammation. J Dermatol Sci. 79:313–316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pires BRB, Silva RCMC, Ferreira GM and
Abdelhay E: NF-kappaB: Two sides of the same coin. Genes (Basel).
9:E242018. View Article : Google Scholar
|
|
11
|
Kim M, Park YG, Lee HJ, Lim SJ and Nho CW:
Youngiasides A and C isolated from youngia denticulatum inhibit
UVB-induced MMP expression and promote type I procollagen
production via repression of MAPK/AP-1/NF-κB and activation of
AMPK/Nrf2 in HaCaT cells and human dermal fibroblasts. J Agric Food
Chem. 63:5428–5438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muzaffer U, Paul VI, Prasad NR,
Karthikeyan R and Agilan B: Protective effect of juglans regia L.
Against ultraviolet B radiation induced inflammatory responses in
human epidermal keratinocytes. Phytomedicine. 42:100–111. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim S and Chung JH: Berberine prevents
UV-induced MMP-1 and reduction of type I procollagen expression in
human dermal fibroblasts. Phytomedicine. 15:749–753. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thimmulappa RK, Lee H, Rangasamy T, Reddy
SP, Yamamoto M, Kensler TW and Biswal S: Nrf2 is a critical
regulator of the innate immune response and survival during
experimental sepsis. J Clin Invest. 116:984–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schäfer M and Werner S: Nrf2-A regulator
of keratinocyte redox signaling. Free Radic Biol Med. 88(Pt B):
243–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang DD and Hannink M: Distinct cysteine
residues in Keap1 are required for Keap1-dependent ubiquitination
of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and
oxidative stress. Mol Cell Biol. 23:8137–8151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Guan C, Sun X, Zhao Z, Li J, Fu X,
Qiu Y, Huang M, Jin J and Huang Z: Tanshinone IIA protects against
acetaminophen-induced hepatotoxicity via activating the Nrf2
pathway. Phytomedicine. 23:589–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hseu YC, Korivi M, Lin FY, Li ML, Lin RW,
Wu JJ and Yang HL: Trans-cinnamic acid attenuates UVA-induced
photoaging through inhibition of AP-1 activation and induction of
Nrf2-mediated antioxidant genes in human skin fibroblasts. J
Dermatol Sci. 90:123–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saw CL, Huang MT, Liu Y, Khor TO, Conney
AH and Kong AN: Impact of Nrf2 on UVB-induced skin
inflammation/photo-protection and photoprotective effect of
sulforaphane. Mol Carcinog. 50:479–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo W, Liu W, Chen G, Hong S, Qian C, Xie
N, Yang X, Sun Y and Xu Q: Water-soluble andrographolide sulfonate
exerts anti-sepsis action in mice through down-regulating p38 MAPK,
STAT3 and NF-κB pathways. Int Immunopharmacol. 14:613–619. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu H, Zhang XY, Zhou YQ, Wen X and Zhu LY:
Proteomic alterations in mouse kidney induced by andrographolide
sodium bisulfite. Acta Pharmacol Sin. 32:888–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu H, Zhang XY, Wang YQ, Zheng XL,
Yin-Zhao, Xing WM and Zhang Q: Andrographolide sodium
bisulfate-induced apoptosis and autophagy in human proximal tubular
endothelial cells is a ROS-mediated pathway. Environ Toxicol
Pharmacol. 37:718–728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang E, Liu X, Du Z, Yang R and Zhao Y:
Andrographolide ameliorates diabetic cardiomyopathy in mice by
blockage of oxidative damage and NF-κB-mediated inflammation. Oxid
Med Cell Longev. 2018:90867472018. View Article : Google Scholar
|
|
24
|
Zhan JYX, Wang XF, Liu YH, Zhang ZB, Wang
L, Chen JN, Huang S, Zeng HF and Lai XP: Andrographolide sodium
bisulfate prevents UV-induced skin photoaging through inhibiting
oxidative stress and inflammation. Mediat Inflamm.
2016:32714512016. View Article : Google Scholar
|
|
25
|
Sun S, Jiang P, Su W, Xiang Y, Li J, Zeng
L and Yang S: Wild chrysanthemum extract prevents UVB
radiation-induced acute cell death and photoaging. Cytotechnology.
68:229–240. 2016. View Article : Google Scholar :
|
|
26
|
Li H, Gao A, Jiang N, Liu Q, Liang B, Li
R, Zhang E, Li Z and Zhu H: Protective effect of curcumin against
acute ultraviolet B irradiation-induced photo-damage. Photochem
Photobiol. 92:808–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong M, Zhang P, Li C, Ma X and Yang D:
Protective mechanism of adipose-derived stem cells in remodelling
of the skin stem cell niche during photoaging. Cell Physiol
Biochem. 51:2456–2471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuadrado A, Martin-Moldes Z, Ye J and
Lastres-Becker I: Transcription factors NRF2 and NF-κB are
coordinated effectors of the Rho family, GTP-binding protein RAC1
during inflammation. J Biol Chem. 289:15244–15258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xian DH, Gao XQ, Xiong X, Xu J, Yang L,
Pan L and Zhong J: Photoprotection against UV-induced damage by
skin-derived precursors in hairless mice. J Photochem Photobiol B.
175:73–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng XX, Yu XT, Li WJ, Kong SZ, Liu YH,
Zhang X, Xian YF, Zhang XJ, Su ZR and Lin ZX: Effects of topical
application of patchouli alcohol on the UV-induced skin photoaging
in mice. Eur J Pharm Sci. 63:113–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pollet M, Shaik S, Mescher M, Frauenstein
K, Tigges J, Braun SA, Sondenheimer K, Kaveh M, Bruhs A, Meller S,
et al: The AHR represses nucleotide excision repair and apoptosis
and contributes to UV-induced skin carcinogenesis. Cell Death
Differ. 25:1823–1836. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katsuoka F, Motohashi H, Ishii T,
Aburatani H, Engel JD and Yamamoto M: Genetic evidence that small
Maf proteins are essential for the activation of antioxidant
response element-dependent genes. Mol Cell Biol. 25:8044–8051.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jastrzab A, Gegotek A and Skrzydlewska E:
Cannabidiol regulates the expression of keratinocyte proteins
involved in the inflammation process through transcriptional
regulation. Cells. 8:E8272019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan H, Huang Z, Bai Q, Sheng Y, Hao Z,
Wang Z and Ji L: Natural product andrographolide alleviated
APAP-induced liver fibrosis by activating Nrf2 antioxidant pathway.
Toxicology. 396-397:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herpers B, Wink S, Fredriksson L, Di Z,
Hendriks G, Vrieling H, de Bont H and van de Water B: Activation of
the Nrf2 response by intrinsic hepatotoxic drugs correlates with
suppression of NF-κB activation and sensitizes toward TNFα-induced
cytotoxicity. Arch Toxicol. 90:1163–1179. 2016. View Article : Google Scholar
|
|
36
|
Chiou YS, Huang QR, Ho CT, Wang YJ and Pan
MH: Directly interact with Keap1 and LPS is involved in the
anti-inflammatory mechanisms of (-)-epicatechin-3-gallate in
LPS-induced macrophages and endotoxemia. Free Radic Biol Med.
94:1–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu GH, Qu J and Shen X: NF-kappaB/p65
antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and
facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta.
1783:713–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu M, Li H, Liu Q, Liu F, Tang L, Li C,
Yuan Y, Zhan Y, Xu W, Li W, et al: Nuclear factor p65 interacts
with Keap1 to repress the Nrf2-ARE pathway. Cell Signal.
23:883–892. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan H, Wang H, Wang X, Zhu L and Mao L:
The absence of Nrf2 enhances NF-κB-dependent inflammation following
scratch injury in mouse primary cultured astrocytes. Mediators
Inflamm. 2012:2175802012. View Article : Google Scholar
|
|
40
|
Cheung KL and Kong AN: Molecular targets
of dietary phenethyl isothiocyanate and sulforaphane for cancer
chemoprevention. AAPS J. 12:87–97. 2010. View Article : Google Scholar :
|
|
41
|
Buelna-Chontal M and Zazueta C: Redox
activation of Nrf2 and NF-κB: A double end sword? Cell Signal.
25:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|