Introduction
Pulmonary hypertension (PH), a progressive disease
of various origins, is associated with pulmonary vascular
remodeling and results in right heart dysfunction (1). According to the classification of
the World Health Organization (2), pulmonary arterial hypertension (PAH)
is a major type of PH. The main histopathological manifestations of
PAH include vasoconstriction, endothelial cell proliferation and
fibrosis, smooth muscle cell proliferation and thrombosis in small
pulmonary arteries, which lead to the elevation of pulmonary
vascular resistance and, consequently, result in PAH (3,4).
Despite the development and application of new types of anti-PAH
drugs, the clinical management of PAH patients represents a
challenge, and this condition is associated with high mortality
rates and markedly poor prognosis (5). Long-term PAH induces vascular
remodeling, thus resulting in pulmonary vascular complications,
which are a direct cause of death.
Cell apoptosis is a type of cell death process,
which is induced by the prestored cell death program and is
triggered by factors in vivo and in vitro (6); it is also referred to as programmed
cell death. Apoptosis is characterized by relatively intact cell
membrane and organelles, cell shrinkage and karyopyknosis in terms
of morphology (7). This
phenomenon is completely different from the comprehensive cell
structural dissolution and destruction that occur in necrosis. The
early changes caused by apoptosis occur on the cell membrane
surface. The balance between cell proliferation and apoptosis is an
important mechanism for maintaining the functional integrity of the
organs (8).
MicroRNAs (miRNAs) are a class of small, endogenous,
non-coding RNA molecules, 21-22 nucleotides in length. These small
miRNAs target one or more mRNAs, and they regulate gene expression
through inhibiting target mRNAs or suppressing their translation
(9). miRNAs are derived from
non-coding RNAs. They transform from pri-miRNAs to pre-miRNAs
(9). In addition, they produce
functionally mature miRNAs through the Dicer complex. The mature
miRNA can bind with the RNA-induced silencing complex (10) and exhibits complementary binding
with target mRNAs; therefore, it can inhibit or prevent their
translation. Several studies have indicated that miRNAs are
involved in almost all pathophysiological processes, including
embryonic development, cell differentiation, angiogenesis, tumor
formation and damage repair (11).
Vascular endothelial growth factor (VEGF) is the
most important factor for pulmonary vascular development (12). VEGF is a cytokine that remains
multifunctional during the entire embryonic, fetal and postnatal
periods. In addition, VEGF is the most potent and critical
regulatory factor of pulmonary vascular growth and maintenance, and
it is involved in the development of hyperoxia-induced lung injury
in newborns. VEGF mainly acts during the early stage of pulmonary
angiogenesis (13); it can
promote the formation of an original vascular network. The
expression levels of VEGF and its receptors (VEGFRs) are increased
in the lung tissue under chronic hypoxic conditions and in lung
tissue samples following monocrotaline (MCT)-induced lung injury
(14). The upregulated expression
of VEGF and VEGFR1-2 has been associated with pulmonary arteriolar
endothelial and smooth muscle cell growth, thus contributing to
pulmonary angiogenesis (15).
MicroRNA-15a-5p (miR-15a-5p) can target VEGF to regulate
inflammation, fibrosis, viability and matrix degradation, and it
participates in several pathophysiological processes (16,17).
The aim of the present study was to investigate the
role of miR-15a-5p in MCT-induced PAH and the underlying
pro-apoptotic mechanism by establishing an animal model of PAH. It
was also investigated whether the effects of miR-15a-5p on cell
apoptosis and modulation of p38 mitogen-activated kinase (MAPK;
p38)/matrix metalloproteinase (MMP)-2 levels in pulmonary artery
smooth muscle cells (PASMCs) were mediated by targeting VEGF.
Materials and methods
Animal model
Male Wistar rats (8-10 weeks old, 220-250 g) were
purchased from the Experimental Animal Center of Capital Medical
University (Beijing, China). The rats (n=12) were maintained under
standard conditions, including temperature 23±1°C, humidity 55-60%
and a 12-h light/dark cycle, and were given access to food and
water ad libitum. The rats were randomly assigned to the
control and PAH groups. PAH was induced in the rats by subcutaneous
injection of 60 mg/kg MCT (Sigma-Aldrich; EMD Millipore) under
anesthesia with 35 mg/kg pentobarbital sodium by intraperitoneal
injection. After 21 days, lung tissues were collected and
immediately frozen at -70°C for enzyme analysis.
Hematoxylin and eosin staining
Lung tissues were washed with PBS and fixed with 4%
paraformaldehyde for 24 h at room temperature. Lung tissues were
then cut into 5-µm sections and stained with hematoxylin and
eosin for 15 min at room temperature. Lung tissues were observed
using confocal microscopy (magnification, ×100; Leica SP5, Argon
laser; Leica Microsystems, Inc.).
Specimens and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from the lung tissue samples
or transfected pulmonary artery smooth muscle cells (PASMCs; see
below) using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. cDNA was
synthesized from total RNA using the miRNA reverse transcription
kit (Takara Biotechnology Co., Ltd.). The expression of miR-15a-5p
was measured in triplicate on a Prism 7300 real-time PCR machine
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using SYBR
Premix Ex Taq (Takara Biotechnology Co., Ltd.) using the following
thermo-cycling conditions: Pre-denaturation at 95°C for 10 min and
amplification at 95°C for 30 sec and at 60°C for 30 sec for 40
cycles. The sequences of primer pairs used to screen the transgenic
mice were as follows: miR-15a, forward 5′-GTC CTC ATC GCA TAC CAT
ACA-3′ and reverse 5′-GCT GAA GTA AGG TTG GCA ATA-3′; U6 forward
5′-GCT TCG GCA GCA CAT ATA CTA AAA T-3′ and reverse 5′-CGC TTC ACG
AAT TTG CGT GTC AT-3′. The relative expression levels were
calculated using the 2−ΔΔCq method (18).
Gene microarray hybridization
Total RNA was hybridized using the G4471A-021828
platform (Agilent Technologies, Inc.) and data were quantified with
Feature Extraction Software A.10.7.3.1 (Agilent Technologies,
Inc.).
Cell culture
Pulmonary artery tissue samples were incubated in
Hanks' solution containing collagenase (1.5 mg/ml, Invitrogen;
Thermo Fisher Scientific, Inc.) for 30 min at 4°C. The adventitia
was carefully stripped off using fine forceps, and the endothelium
was removed. The remaining smooth muscle was digested with
collagenase and elastase for 50 min at 37°C. The PASMCs were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in
a 5% CO2 incubator. After growing to 70-80% confluence,
2×105 cells were transfected with miR-15a-5p (5′-TAA GGC
ACG CGG TGA ATG CC-3′) and negative mimics (5′-CCC CCC CCC CCC CCC
C-3′) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following transfection for 4 h, a VEGF inhibitor
(regorafenib, 4 nM, MedChemExpress) was added to the cells for 44 h
at 37°C. Next, the cells (2×105 cells) were transfected
with miR-15a-5p and VEGF plasmid for 48 h.
Cell proliferation assays
For the CCK-8 assay, the PASMCs were transfected
with miR-15a-5p, and 10 µl of CCK-8 solution was added to
each well for 2 h at 37°C. The absorbance of each well was measured
with a microplate reader (Bio-Tek Instruments, Inc.) set at 570
nM.
Apoptosis assay
At 48 h post-transfection, the PASMCs
(1×106) were harvested, and 5 µl Annexin V-FITC
(Key GEN Biotech Co., Ltd.) and 5 µl PI solution (KeyGEN
Biotech Co., Ltd.) were added and stained for 15 min in the dark.
The relative percentage of apoptosis was analyzed by flow cytometry
(EPICS XL-MCL; Beckman Coulter, Inc.).
Caspase-3 and caspase-9 activity
assays
Caspase-3 and caspase-9 activity was measured by
using caspase-3 (cat. no. C1115, Beyotime Institute of
Biotechnology) and caspase-9 activity assay kits (cat. no. C1158,
Beyotime Institute of Biotechnology). At 48 h post-transfection,
the PASMCs (1×106) were harvested and lysed at 4°C for
30 min using radioimmuno-precipitation assay (RIPA) buffer (cat.
no. P0013K, Beyotime Institute of Biotechnology). Total protein was
quantified using a bicinchoninic acid (BCA) assay, followed by
incubation with 10 µl Ac-DEVD-pNA and 10 µl
Ac-LEHD-pNA at 37°C for 2 h. The absorbance of each well was
measured with a micro-plate reader (Bio-Tek Instruments, Inc.) set
at 405 nM.
Western blot analysis
The PASMCs were lysed at 4°C for 30 min using RIPA
buffer and total protein was quantified using a BCA assay. Total
protein (50 µg) was separated by electrophoresis on 8-10%
sodium dodecyl sulfate-poly-acrylamide gels and then transferred
onto nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked with 5% skimmed milk in 1X Tris-buffered
saline with 0.1% Tween-20 (TBST) followed by incubation overnight
at 4°C with the following primary antibodies: Anti-B-cell lymphoma
2 (Bcl-2; cat. no. sc-509; 1:1,000, Santa Cruz Biotechnology,
Inc.), anti-Bcl-2-associated X protein (Bax; cat. no. sc-20067;
1:1,000, Santa Cruz Biotechnology, Inc.), anti-VEGF (cat. no.
sc-81670; 1:500, Santa Cruz Biotechnology, Inc.),
anti-phosphorylated (p)-p38 MAPK (cat. no. sc-17852-R; 1:1,000,
Santa Cruz Biotechnology, Inc.), anti-p38 MAPK (cat. no. 8690S;
1:1,000, Cell Signaling Technology, Inc.), anti-MMP-2 (cat. no.
sc-53630; 1:1,000, Santa Cruz Biotechnology, Inc.) and anti-GAPDH
(cat. no. sc-51631; 1:5,000, Santa Cruz Biotechnology, Inc.). The
membranes were washed with TBST and then incubated with horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2004,
1:5,000, Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The membranes were detected by enhanced
chemiluminescence (Bio-Rad Laboratories, Inc.) using a ChemiDoc MP
system (Bio-Rad Laboratories, Inc.).
ELISA
At 48 h post-transfection, the supernatant was
collected following centrifugation at 1,000 × g and 4°C for 10 min
and used to measure the levels of tumor necrosis factor (TNF)-α
(cat. no. RAB0477), interleukin (IL)-1β (cat. no. RAB0274), IL-6
(cat. no. RAB0308) and IL-18 (cat. no. RAB0268) using ELISA kits
(Sigma-Aldrich; EMD Millipore). The absorbance of each well was
measured with a microplate reader (Bio-Tek Instruments, Inc.) set
at 450 nM.
Immunofluorescence
At 48 h post-transfection, the PASMCs were washed
with PBS and fixed with 4% paraformaldehyde for 20 min. The cells
were incubated with 5% bovine serum albumin (KeyGEN Biotech Co.,
Ltd.) and 0.1% Tris-X100 in PBS for 1 h and incubated with VEGF
(1:100, Santa Cruz Biotechnology, Inc.) at 4°C overnight. The cells
were then washed with PBS and incubated with goat anti-rabbit
IgG-CFL 555 (sc-362272; 1:100, Santa Cruz Biotechnology, Inc.) for
1 h at 37°C and stained with a DAPI assay for 15 min in the dark.
The cells were observed using confocal microscopy (Leica SP5, Argon
laser; Leica Microsystems, Inc.).
Statistical analysis
The data are expressed as the mean ± standard
deviation using SPSS 17.0 (SPSS, Inc.). Student's t-test and
one-way analysis of variance followed by a Bonferroni-Dunn test
were used for statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-15a-5p in rats with
MCT-induced PAH
The expression of miR-15a-5p was examined in PAH
rats using RT-qPCR analysis and a gene chip microarray. As shown in
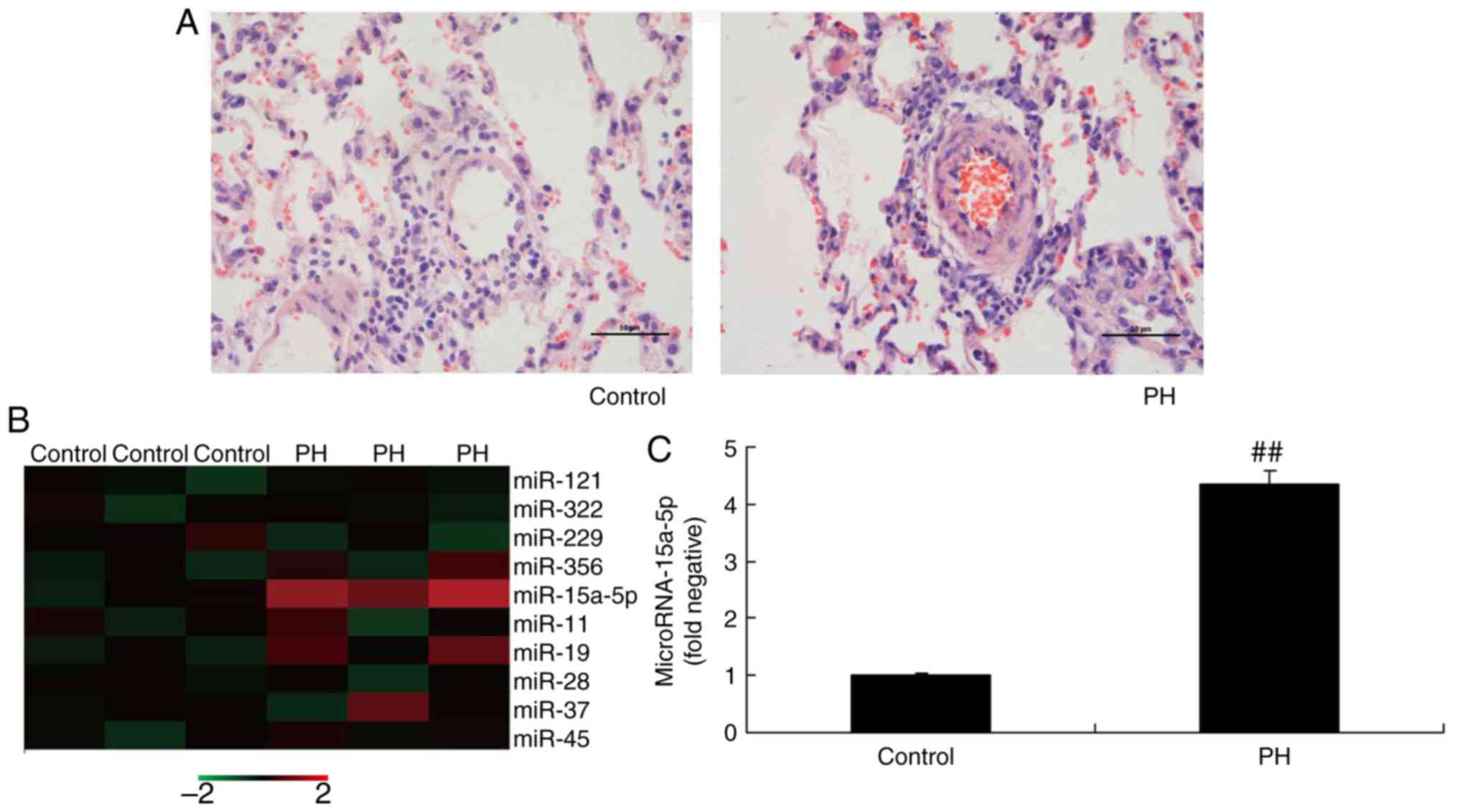
Fig. 1A, H&E staining
revealed that, compared with the control group, vascular lesions in
PAH rats were observed in the arteries present in bronchovascular
bundles, and they were typically characterized by fibrocellular
endothelial proliferation in the intimal layer and medial muscular
hypertrophy. The expression of several miRNAs was downregulated in
PAH rats, compared with that in the control group (Fig. 1B). However, the expression of
miR-15a-5p was significantly increased in PAH rats (Fig. 1C). Taken together, these results
indicate that the expression of miR-15a-5p is upregulated in PAH,
and it may represent an important regulatory factor.
Overexpression of miR-15a-5p inhibits the
proliferation and promotes the apoptosis of PASMCs in rats with
MCT-induced PAH
The expression of miR-15a-5p was upregulated in the
PASMCs by using miR-15a-5p mimics. As shown in Fig. 2A, the miR-15a-5p mimics increased
the expression of miR-15a-5p in the cells, compared with that in
the cells in the negative control group. As shown in Fig. 2B-K, the overexpression of
miR-15a-5p led to significantly reduced cell proliferation and
significantly increased the levels of lactate dehydrogenase (LDH)
and the apoptosis of PASMCs. The overexpression of miR-15a-5p
significantly increased the activity of caspase-3/9 and the protein
expression of Bax, and it decreased the expression of Bcl-2 in the
PASMCs of rats with MCT-induced PAH.
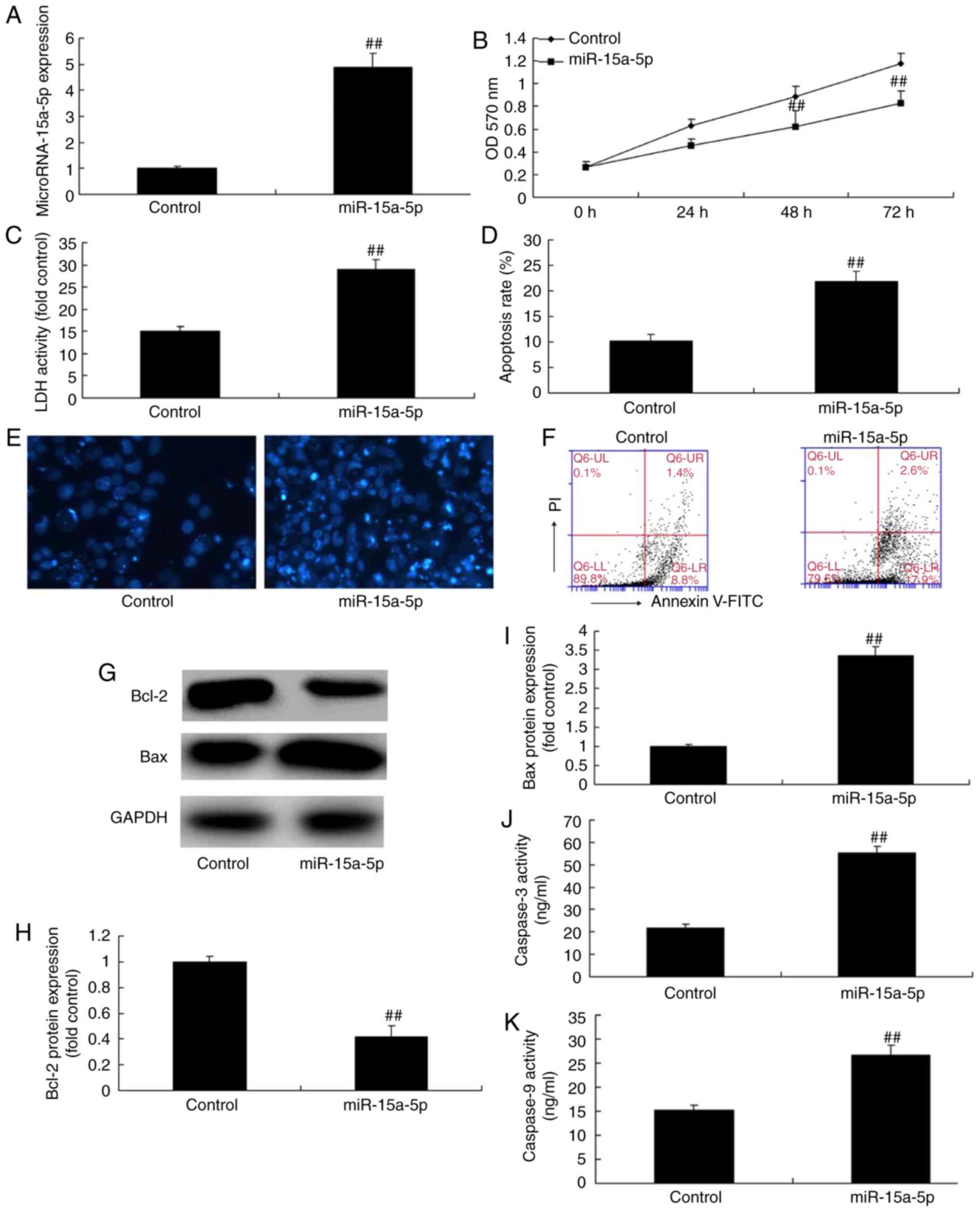 | Figure 2Overexpression of miR-15a-5p
inhibited the proliferation and promoted the apoptosis of PASMCs
from rats with monocrotaline-induced pulmonary arterial
hypertension. (A) Expression of miR-15a-5p; (B) cell proliferation;
(C) LDH levels; (D) apoptosis rate; (E) DAPI assay (original
magnification, ×100); (F) detection of cell apoptosis by flow
cytometry; (G) western blotting and (H and I) quantification of
Bcl-2 and Bax protein expression, respectively; (J and K) caspase-3
and caspase-9 activity, respectively. ##P<0.01 vs.
control group. miR, microRNA; control, negative control group;
miR-15a-5p, overexpression of miR-15a-5p; PASMCs, pulmonary artery
smooth muscle cells; LDH, lactate dehydrogenase; Bax, B-cell
lymphoma 2-associated X protein; Bcl-2, B-cell lymphoma 2; OD,
optical density. |
Downregulation of miR-15a-5p promotes the
proliferation and inhibits the apoptosis of PASMCs in rats with
MCT-induced PAH
To examine the mechanism of the apoptosis induced by
anti-miR-15a-5p, the PASMCs were transfected with anti-miR-15a-5p
mimics. As shown in Fig. 3A, the
expression of miR-15a-5p was downregulated in the cells compared
with that in cells of the negative control group. As expected, cell
proliferation was increased, whereas the level of LDH and apoptosis
rate were suppressed in the PASMCs following transfection (Fig. 3B-F). In addition, the reduced
expression of miR-15a-5p led to significant inhibition of
caspase-3/9 activity and protein expression of Bax, and it promoted
the expression of Bcl-2 in the PASMCs of rats with MCT-induced PAH
(Fig. 3G-K).
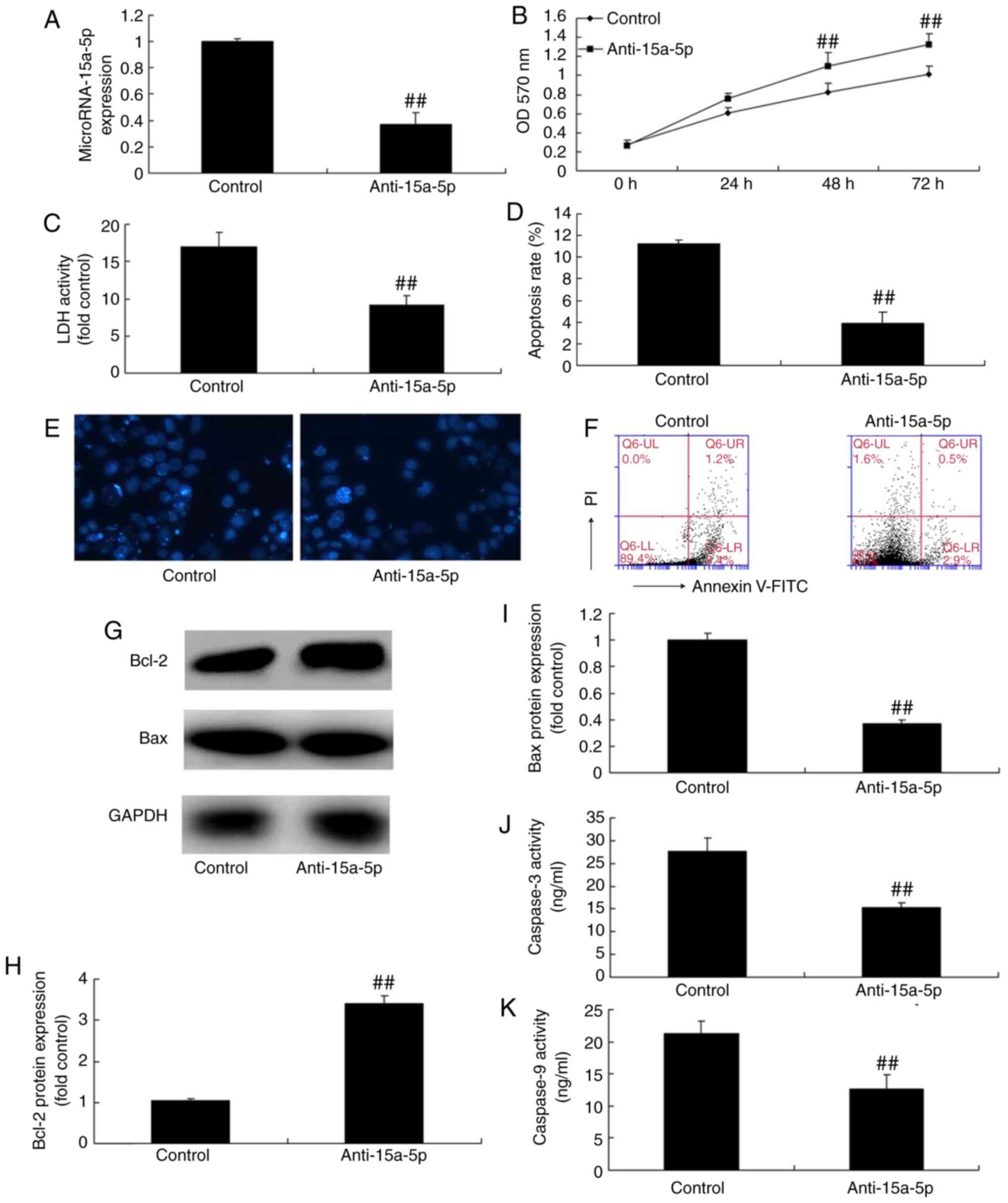 | Figure 3Downregulation of miR-15a-5p
increased proliferation and decreased apoptosis of PASMCs from rats
with monocrotaline-induced pulmonary arterial hypertension. Cell
proliferation and apoptosis in monocrotaline-induced pulmonary
hypertension by downregulation of miR-15a-5p. (A) Expression of
miR-15a-5; (B) cell proliferation; (C) LDH levels; (D) apoptosis
rate; (E) DAPI assay (original magnification, ×100); (F) detection
of cell apoptosis by flow cytometry; (G) western blotting and (H
and I) quantification of Bcl-2 and Bax protein expression,
respectively; (J and K) caspase-3 and caspase-9 activity,
respectively. ##P<0.01 vs. control group. miR,
microRNA; control, negative control group; anti-15a-5p, suppression
of miR-15a-5p; PASMCs, pulmonary artery smooth muscle cells; LDH,
lactate dehydrogenase; Bax, B-cell lymphoma 2-associated X protein;
Bcl-2, B-cell lymphoma 2; OD, optical density. |
Effects of miR-15a-5p on inflammation in
the PASMCs of rats with MCT-induced PAH
Subsequently, the potential role of miR-15a-5p in
inflammation in PASMCs was examined. As shown in Fig. 4A-D, the levels of TNF-α, IL-1β,
IL-18 and IL-6 were significantly increased in the PASMCs
overexpressing miR-15a-5p. The role of miR-15a-5p in inflammation
in the PASMCs was further examined. As shown in Fig. 4E-H, the levels of TNF-α, IL-1β,
IL-18 and IL-6 in the PASMCs were lower when the miR-15a-5p
expression was downregulated, as compared with those in cells of
the negative control group.
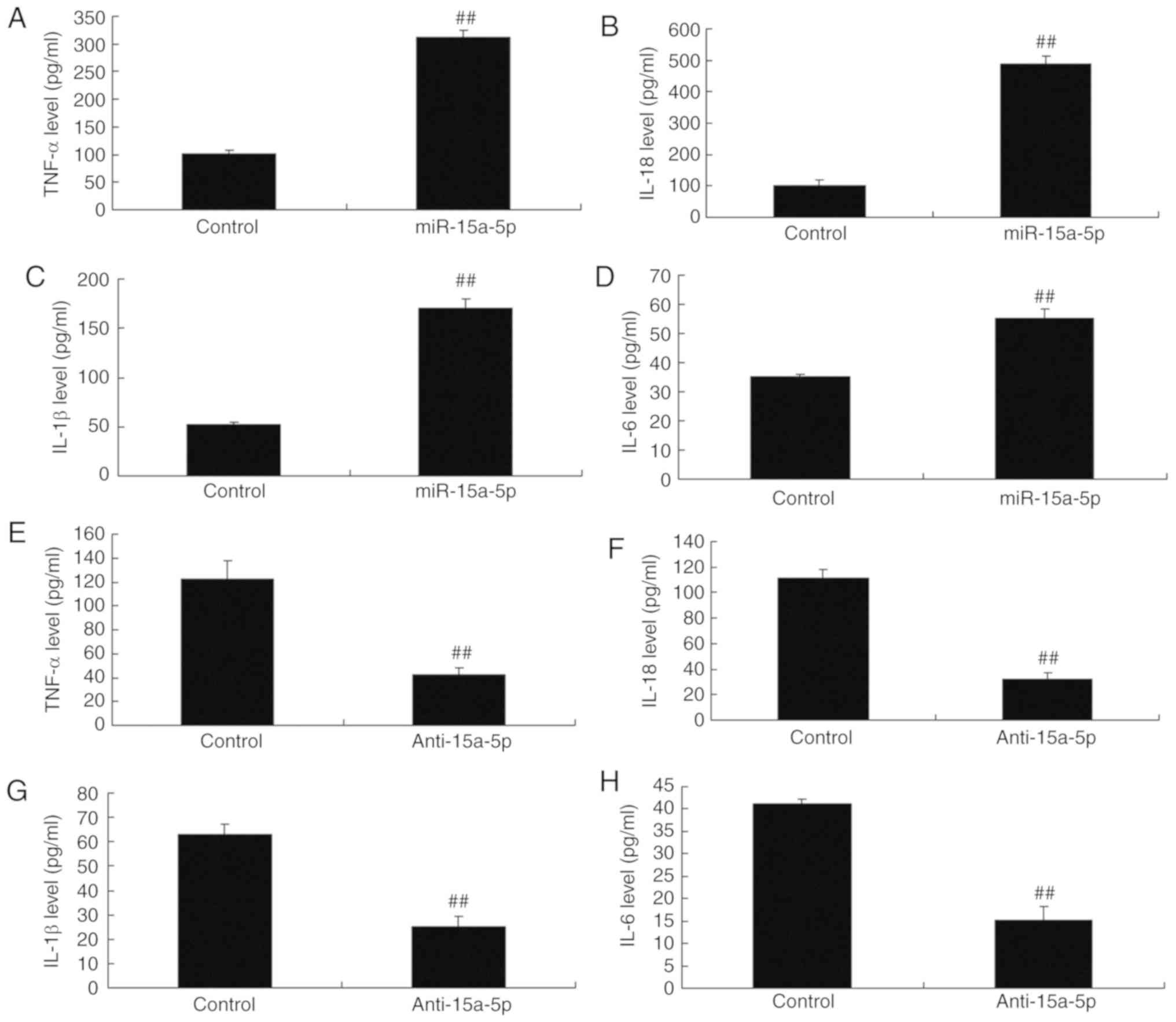 | Figure 4Changes of Inflammation in PASMCs
from rats with monocrotaline-induced pulmonary arterial
hypertension by miR-15a-5p. Levels of (A) TNF-α, (B) IL-18, (C)
IL-1β and (D) IL-6 with overexpression of miR-15a-5p. Levels of (E)
TNF-α, (F) IL-18, (G) IL-1β and (H) IL-6 with downregulation of
miR-15a-5p. ##P<0.01 vs. control group. miR,
microRNA; control, negative control group; miR-15a-5p,
overexpression of miR-15a-5p; anti-15a-5p, suppression of
miA-15a-5p; PASMCs, pulmonary artery smooth muscle cells; TNF,
tumor necrosis factor; IL, interleukin. |
miR-15a-5p targets the VEGF/p38/MMP-2
signaling pathway
A gene chip was used to examine the changes in
regulatory proteins, and it revealed that the protein expression of
VEGF differed in the PASMCs of rats with MCT-induced PAH with
varied expression of miR-15a-5p (Fig.
5A). The RT-qPCR analysis demonstrated that overexpression of
miR-15a-5p suppressed the mRNA expression of VEGF compared with
that in the negative control group (Fig. 5B). Subsequently, it was inferred
that miR-15a-5p targeted VEGF (Fig.
5C). Immunofluorescence demonstrated that the overexpression of
miR-15a-5p suppressed the protein expression of VEGF (Fig. 5D and E). Western blotting was then
performed to analyze the protein expression, and it demonstrated
that the overexpression of miR-15a-5p significantly suppressed the
protein expression of VEGF and enhanced the protein expression of
p-p38 and MMP-2 in the PASMCs (Fig.
6A-D). By contrast, following suppression of miR-15a-5p, the
protein expression of VEGF was enhanced and the protein expression
of p-p38 and MMP-2 was significantly suppressed in the PASMCs
(Fig. 6E-H). Taken together,
these results indicate that miR-15a-5p may exert a protective
effect against MCT-induced PAH via the VEGF/p38/MMP-2 signaling
pathway.
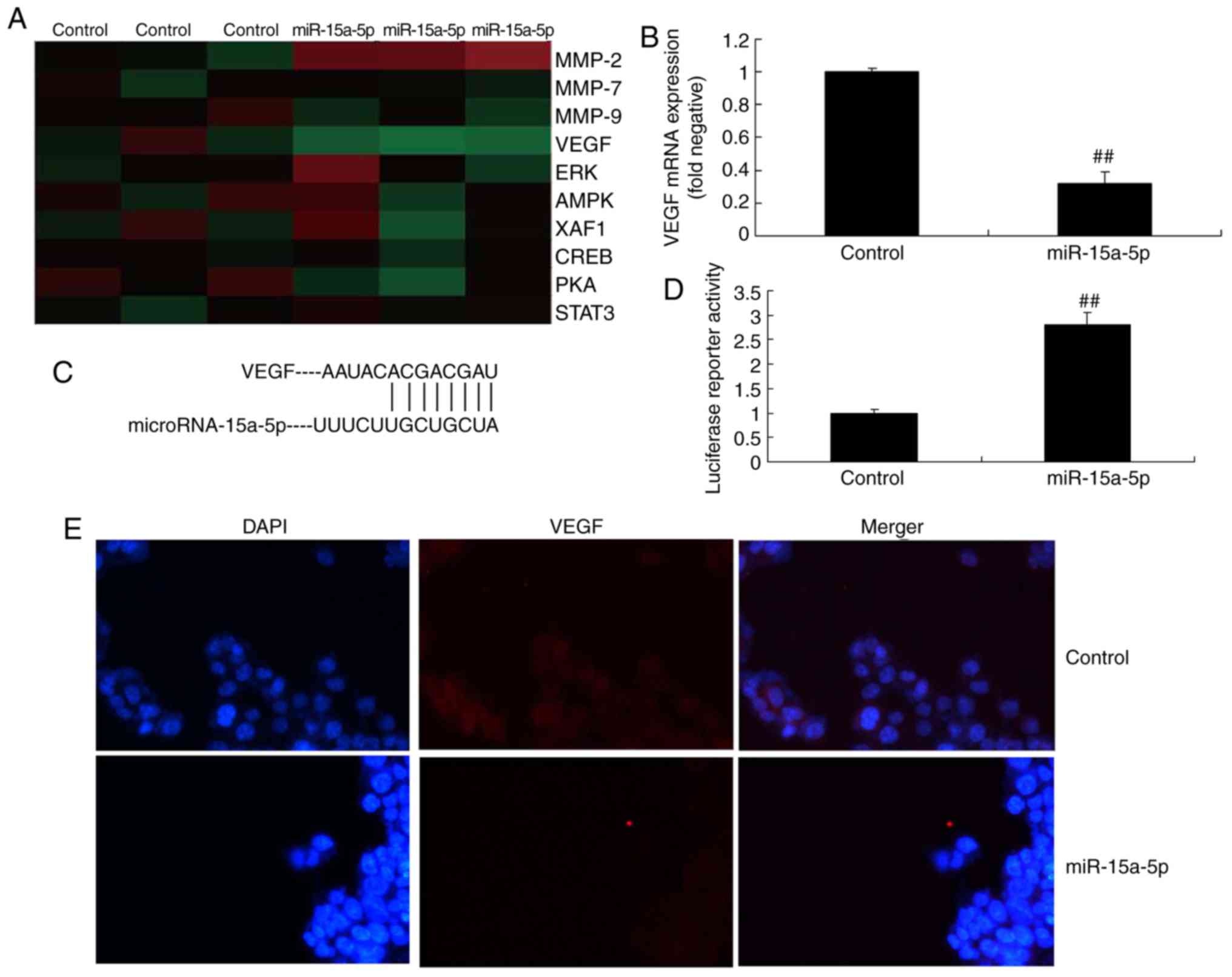 | Figure 5miR-15a-5p targeted the VEGF
signaling pathway in PASMCs from rats with monocrotaline-induced
pulmonary arterial hypertension. (A) Signaling pathway gene chip
analysis. (B) mRNA expression of VEGF. (C) miR-15a-5p targets
VEGFA. (D) Immunofluorescence results and (E) images showing the
protein expression of VEGF (original magnification, ×100).
##P<0.01 vs. control group. miR, microRNA; Control,
negative control group; miR-15a-5p, over-expression of miR-15a-5p;
VEGF, vascular endothelial growth factor; MMP, matrix
metalloproteinase; ERK, extracellular signal-regulated kinase;
AMPK, AMP-activated protein kinase; XAF1, X-linked inhibitor of
apoptosis protein-associated factor 1; CREB, cAMP response
element-binding protein; PKA, protein kinase A; STAT3, signal
transducer and activator of transcription 3; PASMCs, pulmonary
artery smooth muscle cells. |
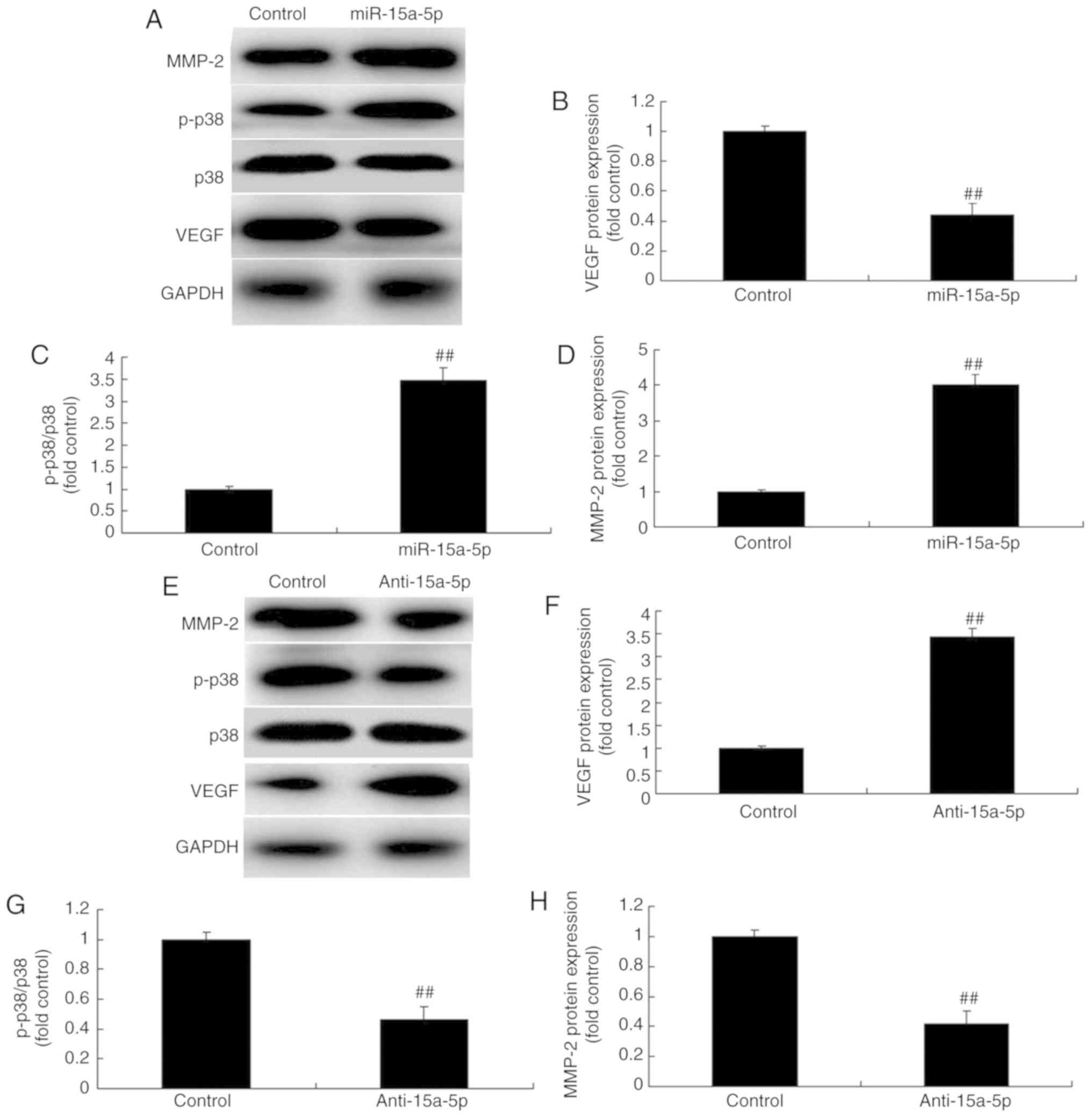 | Figure 6miR-15a-5p regulated the
VEGF/p38/MMP-2 signaling pathway in PASMCs from rats with
monocrotaline-induced pulmonary arterial hypertension. (A) Protein
expression of VEGF, p-p38, p38 and MMP-2 was detected using western
blot analysis. Quantification of (B) VEGF, (C) p-p38 and (D) MMP-2
protein expression with overexpression of miRNA-15a-5p. (E) Protein
expression of VEGF, p-p38, p38 and MMP-2 detected using western
blot analysis. Quantification of (F) VEGF, (G) p-p38 and (H) MMP-2
protein expression with downregulation of miR-15a-5p.
##P<0.01 vs. control group. miR, microRNA; control,
negative control group; miR-15a-5p, miR-15a-5p overexpression;
anti-15a-5p, downregulation of miR-15a-5p; VEGF, vascular
endothelial growth factor; MMP, matrix metalloproteinase; PASMCs,
pulmonary artery smooth muscle cells; p-p38, phosphorylated
p38. |
Inhibition of VEGF attenuates the effects
of anti-miR-15a-5p on the PASMCs of rats with MCT-induced PAH
As expected, western blot analysis demonstrated that
the inhibition of VEGF by 4 nM regorafenib for 48 h suppressed the
protein expression of VEGF and Bcl-2 and induced the protein
expression of p-p38, MMP-2 and Bax in PASMCs, which were
downregulated by miR-15a-5p inhibition (Fig. 7A-F). The molecular mechanism by
which VEGF reverses the effects of miR-15a-5p inhibition on
MCT-induced PAH was then investigated. As shown in Fig. 8A-E, the inhibition of VEGF
attenuated the effects of anti-miR-15a-5p on cell proliferation and
increased the apoptotic rate and activity of LDH in the PASMCs. In
addition, the inhibition of VEGF significantly increased the
activity of caspase-3/9 in MCT-induced PAH, which was decreased by
inhibition of miR-15a-5p (Fig. 8F and
G). Furthermore, the inhibition of VEGF significantly increased
the levels of TNF-α, IL-1β, IL-18, and IL-6 in MCT-induced PAH,
which were inhibited by miR-15a-5p downregulation (Fig. 8H-K).
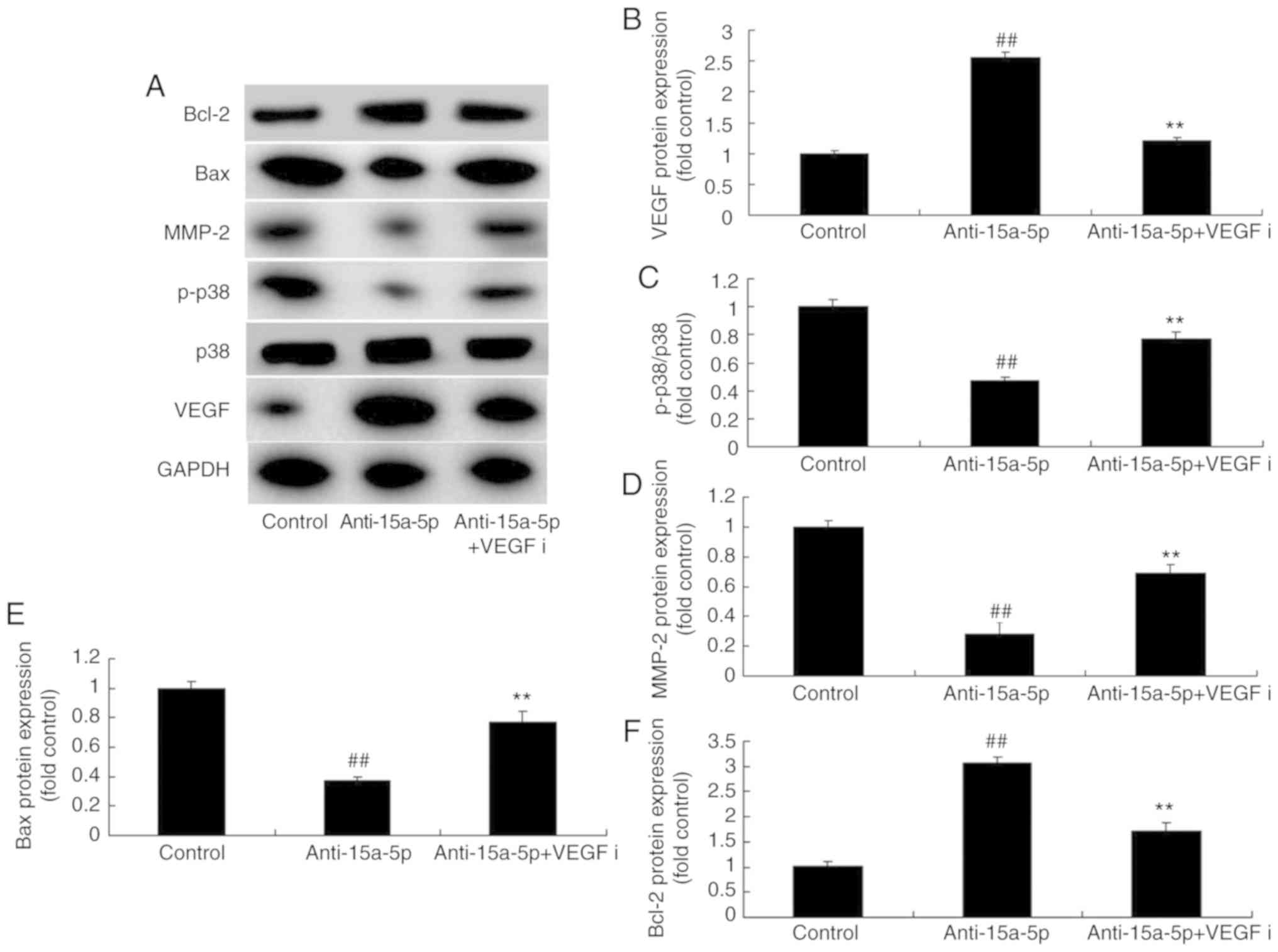 | Figure 7Inhibition of VEGF affected the
VEGF/p38/MMP-2 signaling pathway in PASMCs from rats with
monocrotaline-induced pulmonary arterial hypertension by
attenuating the effects of miR-15a-5p inhibition. (A) Expression of
VEGF, p-p38, MMP-2, Bax and Bcl-2 proteins detected using western
blot analysis. Quantification of (B) VEGF, (C) p-p38, (D) MMP-2,
(E) Bax and (F) Bcl-2 protein expression. ##P<0.01
vs. control group, **P<0.01 vs. anti-15a-5p group.
VEGF, vascular endothelial growth factor; MMP, matrix
metalloproteinase; PASMCs, pulmonary artery smooth muscle cells;
p-p38, phosphorylated p38; Bax, B-cell lymphoma 2-associated X
protein; Bcl-2, B-cell lymphoma 2; control, negative control group;
miR, microRNA; anti-15a-5p, downregulation of miR-15a-5p; VEGFi,
VEGF inhibitor. |
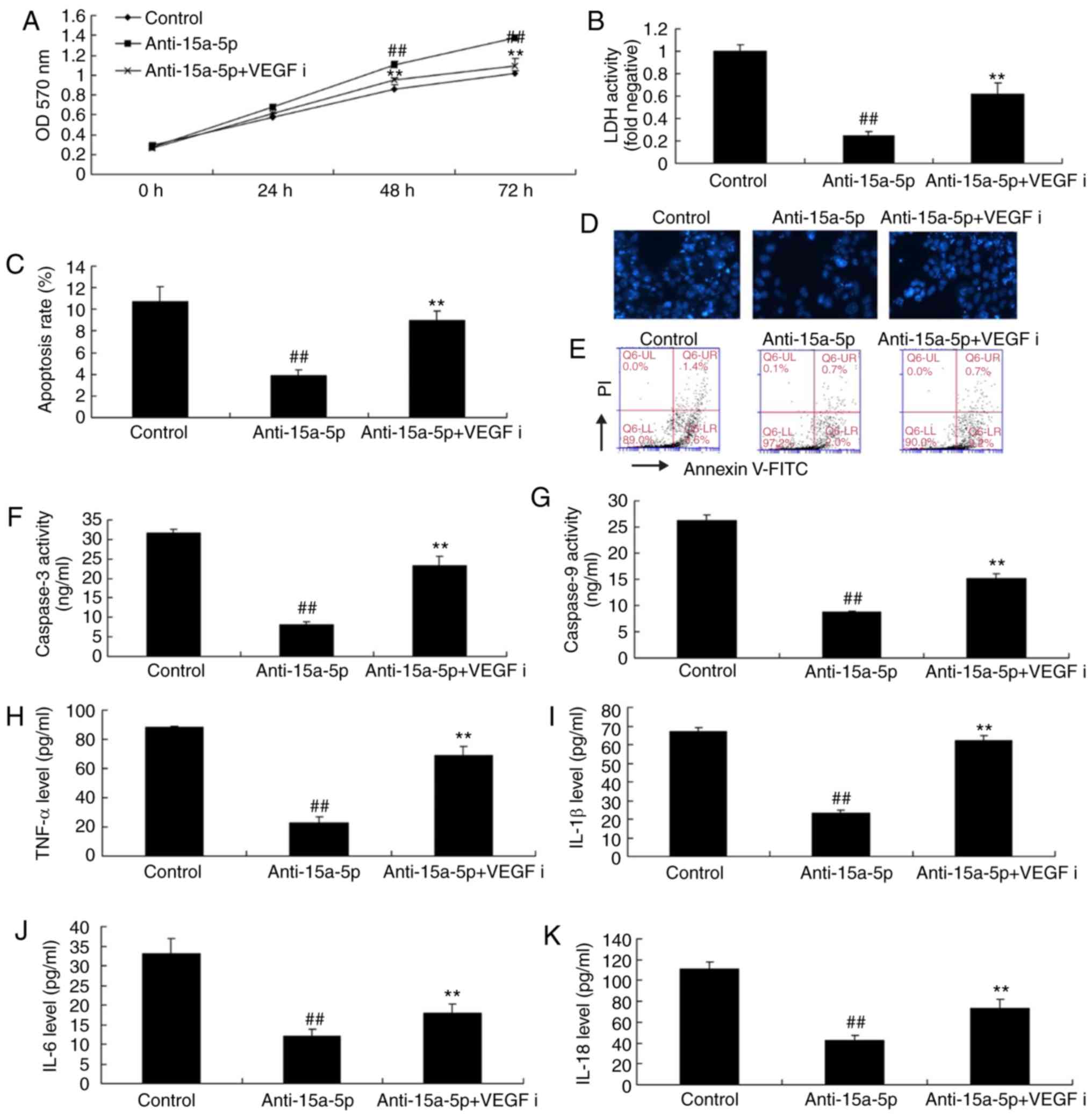 | Figure 8Inhibition of VEGF reduced the
effects of anti-miR-15a-5p on PASMCs from rats with
monocrotaline-induced pulmonary arterial hypertension. (A) Cell
proliferation; (B) LDH levels; (C) apoptosis rate; (D) DAPI assay
(original magnification, ×100); (E) cell apoptosis determined by
flow cytometry; activity of (F) caspase-3 and (G) caspase-9. Levels
of (H) TNF-α, (I) IL-1β, (J) IL-6 and (K) IL-18.
##P<0.01 vs. control group, **P<0.01
vs. anti-15a-5p group. miR, microRNA; VEGF, vascular endothelial
growth factor; PASMCs, pulmonary artery smooth muscle cells; LDH,
lactate dehydrogenase; control, negative control group;
anti-15a-5p, downregulation of miR-15a-5p; VEGFi, VEGF inhibitor
group. |
Upregulation of VEGF attenuates the
effects of miR-15a-5p on PASMCs in rats with MCT-induced PAH
To determine the effect of VEGF on the function of
miR-15a-5p in PAH, a VEGF plasmid was used. This plasmid reduced
the protein expression levels of p-p38, MMP-2, and Bax, and
increased the protein expression level of Bcl-2 in PASMCs
overexpressing miR-15a-5p, compared with that in the miR-15a-5p
overexpression group without the plasmid (Fig. 9A-F). As shown in Fig. 10A-G, the upregulation of VEGF
increased cell proliferation, reduced LDH activity and apoptotic
rate, and inhibited the activity of caspase-3/9 in PASMCs with
miR-15a-5p overexpression, compared with cells with miR-15a-5p
overexpression alone (Fig. 10F and
G). Therefore, VEGF upregulation significantly suppressed the
levels of TNF-α, IL-1β, IL-18 and IL-6 in the PASMCs overexpressing
miR-15a-5p (Fig. 10H-K).
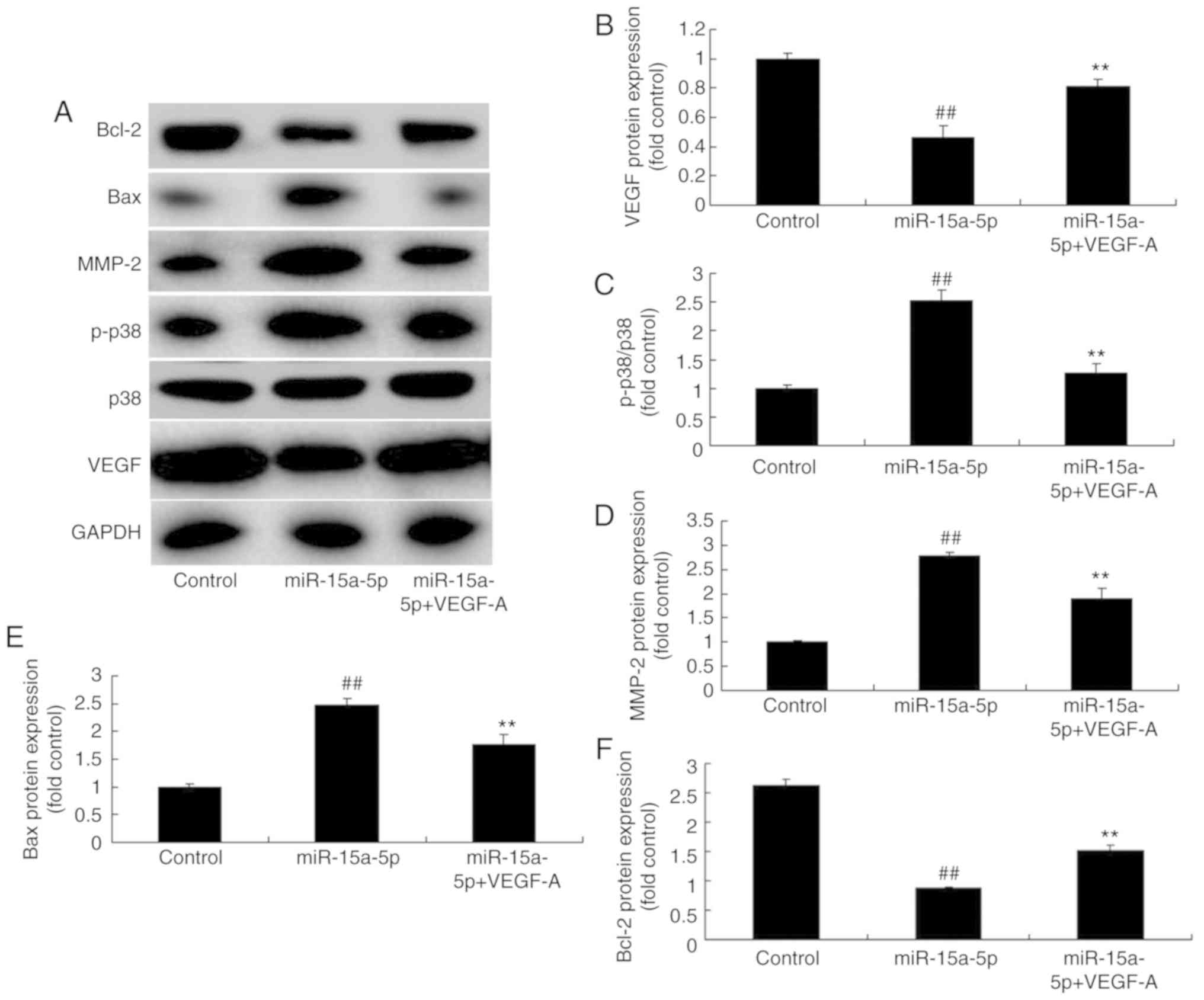 | Figure 9Upregulation of VEGF affected the
VEGF/p38/MMP-2 signaling pathway in PASMCs from rats with
monocrotaline-induced pulmonary arterial hypertension by the
effects of miR-15a-5p. (A) Results of the protein expression of
VEGF, p38, MMP-2, Bax and Bcl-2 measured using western blot
analysis. Protein expression of (B) VEGF, (C) p-p38, (D) MMP-2, (E)
Bax and (F) Bcl-2. ##P<0.01 vs. control,
**P<0.01 vs. miR-15a-5p group. Control, negative
control group; miR, microRNA; miR-15a-5p, miR-15a-5p overexpression
group; miR-15a-5p-VEGF-A, miRNA-15a-5p and VEGF plasmid group;
VEGF, vascular endothelial growth factor; p-p38,
phosphorylated-p38; MMP, matrix metalloproteinase; PASMCs,
pulmonary artery smooth muscle cells; Bax, B cell
lymphoma-2-associated X protein; Bcl-2, B-cell lymphoma 2. |
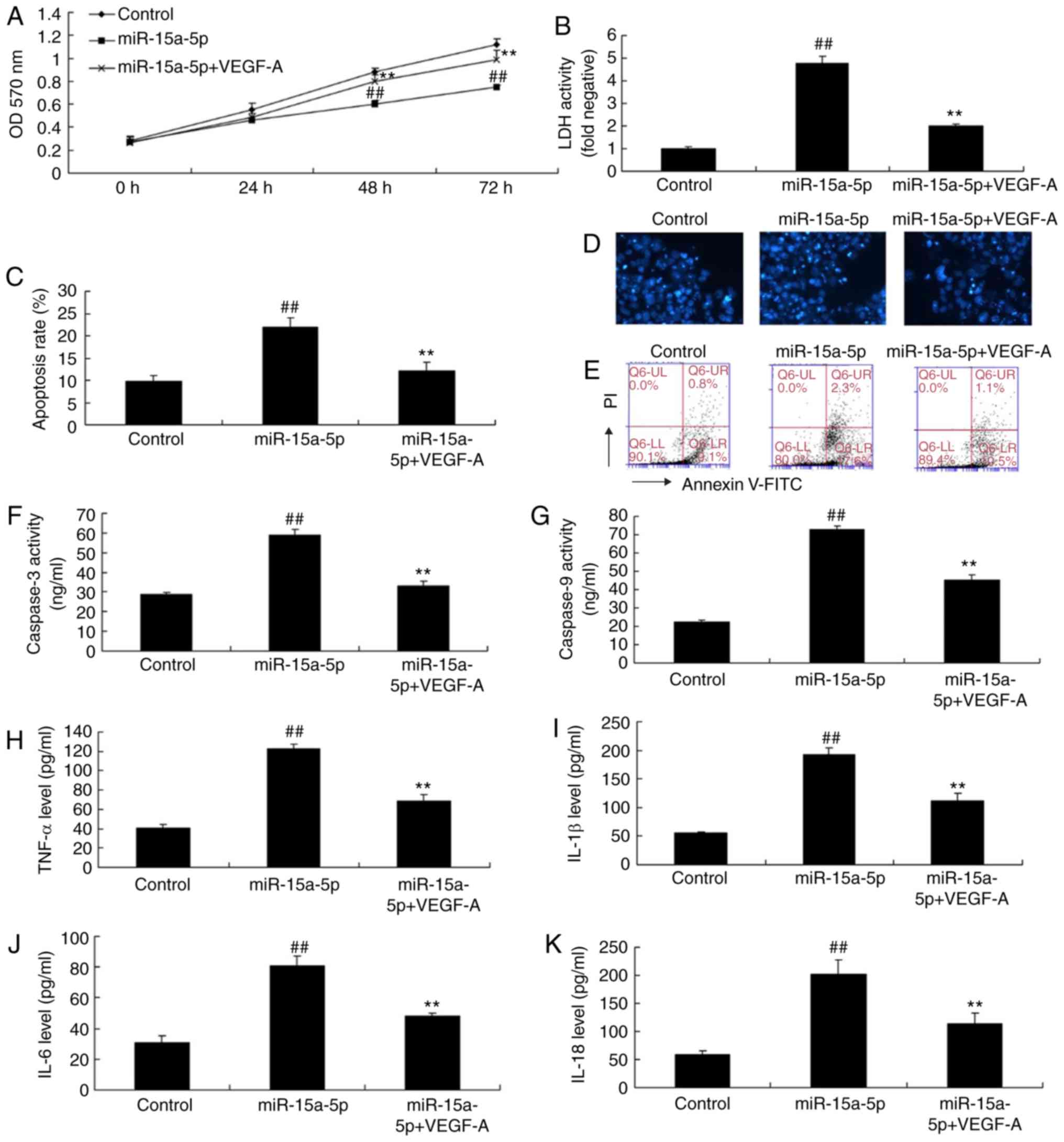 | Figure 10Upregulation of VEGF reduced the
effects of miR-15a-5p on the PASMCs of rats with
monocrotaline-induced pulmonary arterial hypertension. (A) Cell
proliferation, (B) LDH levels, (C) apoptosis rate, (D) DAPI assay
(original magnification, ×100), (E) cell apoptosis analysis by flow
cytometry, activity of (F) caspase-3 and (G) caspase-9, levels of
(H) TNF-α, (I) IL-18, (J) IL-1β and (K) IL-6.
##P<0.01 vs. control; **P<0.01 vs.
miR-15a-5p group. Control, negative control group; miR, microRNA;
miR-15a-5p, miR-15-5p overexpression group; miR-15a-5p-VEGF-A,
miR-15a-5p and VEGF plasmid group; VEGF, vascular endothelial
growth factor; PASMCs, pulmonary artery smooth muscle cells; LDH,
lactate dehydrogenase; TNF, tumor necrosis factor; IL, interleukin;
OD, optical density. |
Discussion
Pulmonary vascular development includes vascular
genesis and formation (19).
Vascular genesis marks the process of differentiation of stromal
cells to form blood vessels (19). By contrast, vascular formation is
the process during which existing vessels form new vessels through
germination (3). It has been
suggested that multiple factors regulate pulmonary vascular
development (3), and VEGF is one
of the most important factors. It has been reported that the
regulatory effects of premature birth, partial oxygen pressure,
inflammatory cytokines, and other signal systems on the expression
of normal growth factors may affect lung and pulmonary vascular
growth. However, most studies to date have mainly focused on VEGF.
VEGF is a multi-functional cytokine that regulates cell
proliferation, migration, survival and differentiation in several
physiological and pathological processes (20). VEGF is involved in endothelial PAS
domain-containing protein 1 mutation-induced congenital heart
disease (21). In addition, it is
well known that chronic hypoxia increases VEGF expression in lung
tissues and that VEGF is likely to be a modulator of chronic
hypoxia-induced pulmonary vascular remodeling (14). It has also been reported that VEGF
is increased in rats with hypoxia and MCT-induced PH and vascular
remodeling (14). Serum VEGF
levels are associated with the presence of PAH in systemic
sclerosis (22). Being an
angiogenic biomarker, the plasma concentration of VEGF is higher in
PH patients (23). Moreover, the
VEGF receptor inhibitor Su5416 may induce endothelial cell
apoptosis, but Su5416 combined with chronic hypoxia induced severe
PH in a rat model (24,25). The results of the present study
demonstrated that the expression of miR-15a-5p in lung tissue was
increased in the PAH group compared with that in the control group.
In addition, the overexpression of miR-15a-5p inhibited the
proliferation and promoted the apoptosis of PASMCs in rats with
MCT-induced PAH through the suppression of VEGF expression. Ye
et al reported that miR-15a reduced inflammation in the
retinal endothelial cell barrier by reducing transforming growth
factor-b3/VEGF signaling (26).
However, the results were obtained from one primary cell type only,
which is a limitation of the present study.
Vascular smooth muscle cells (VSMCs) are an
important component of the tunica media. Smooth muscle cell
proliferation and apoptosis resistance are the major reasons for
tunica media thickening in vascular modeling (27) and are important factors that
induce vascular remodeling. Inhibiting SMC proliferation and
promoting apoptosis are the focus of investigation on vascular
remodeling (8). It has been
demonstrated that multiple bioactive substances can regulate the
function of VSMCs (8). These
include nitric oxide, angiotensin II, platelet-derived growth
factor and VEGF. The present study indicated that miR-15a-5p
induced apoptosis and decreased proliferation of PASMCs by
inhibiting VEGF expression. Upregulation of VEGF attenuated the
effects of miR-15a-5p on PASMCs. Inflammation contributes to
exaggerated contractility and proliferation of vascular cells
(28). In the present study, it
was observed that overexpression of miR-15a-5p significantly
promoted cell apoptosis and was associated with the increase in
TNF-α, IL-1β, IL-18, and IL-6 expression in PASMCs. However, a
number of studies have reported that miR-15a-5p inhibited
inflammation and alleviated several pathological processes. For
example, Liu et al reported that miR-15a-5p alleviated the
atherosclerotic inflammatory response and arterial injury in
diabetic atherosclerotic rats (29). In addition, it was reported that
miR-15a deficiency facilitated inflammation by directing M1
macrophage polarization in the tumor (30). These conclusions were not
consistent with the findings of the present study. This may be
attributed to PASMC apoptosis. Apoptotic cells may become
pro-inflammatory through the release of pro-inflammatory cytokines
and DAMPs (31). The present
study only focused on the inflammation in PASMCs, but the role of
miR-15a-5p in inflammation in MCT-induced PAH in vivo
remains to be elucidated.
MAPKs are a family of serine-threonine kinases that
can be activated by various stimuli. MAPK is most closely
associated with the regulation of cell proliferation and it is a
type of biological signal transduction protein kinase in eukaryotic
cells. MAPK is a common pathway for information transduction
between extracellular signals and cell nuclei. This pathway uses
the conserved three-level kinase cascade reaction for signal
transduction. The MAPK pathway is activated by diverse
extracellular and intracellular stimuli, including growth factors,
cytokines and transcription factors; therefore, it can mediate
numerous intracellular biological effects (32). Ferrari et al reported that
VEGF signaling regulated TGF-β1-induced endothelial cell apoptosis
by shifting p38 from a pro-survival to a pro-apoptotic isoform
(33). In the present study,
miR-15a-5p increased p-p38 levels by targeting VEGF and promoted
PASMC apoptosis in the MCT-induced PAH model. Shi et al
demonstrated that miR-15a-5p regulates MAPK in human adipocyte
differentiation and obesity (34).
Extracellular matrix (ECM) remodeling is important
in pulmonary vascular remodeling. The ECM mainly includes collagen,
elastic fibers, proteoglycans and glycoproteins (35), among which collagen and elastic
fibers are the most abundant structural components in the ECM.
These substances form the complicated structural network of the
vascular walls (35). ECM exists
in a dynamic balance between continuous production and degradation
under physiological conditions. MMPs are a group of zinc
ion-dependent proteolytic enzymes. In addition, ECM fragments
degraded by MMP-9 exert chemo-tactic effects on inflammatory cells.
Elastic fiber-derived chemokines in the respiratory tract of
long-term smokers can induce aggregation of monocytes/macrophages
(36), thereby aggravating the
pulmonary inflammatory response and leading to destruction of the
alveolar wall. In addition to the ECM degradation, MMPs also
exhibit pro- and anti-apoptotic activity and affect cell apoptosis
(37). Their anti-apoptotic
action includes cleavage of the Fas ligand and activation of the
serine/threonine kinase AKT, also referred to as protein kinase B.
The pro-apoptotic activity of MMPs is usually associated with
changes in ECM composition. MMPs cause apoptosis by cleaving
adhesion molecules. MMP-3 induces apoptosis when overexpressed in
epithelial cells, possibly by digestion of laminin (38). A pentanoic acid derivative
targeting MMP-2 was shown to induce apoptosis in a chronic myeloid
leukemia cell line (39). Yu
et al reported that microRNA-2861 targeted MMP-2 to regulate
the proliferation and apoptosis of ectopic endometrial cells in
women with endometriosis (40).
The results of the present study demonstrated that the
over-expression of miR-15a-5p promoted the protein expression of
MMP-2 and increased PASMC apoptosis in the MCT-induced PAH
model.
The Bcl-2 gene family is closely associated with
mitochondrial regulation of cell apoptosis. Bcl-2 and Bax are the
main members of the Bcl-2 family, and Bcl-2 inhibits whereas Bax
promotes cell apoptosis (41). It
has been reported that Bcl-2 is located in the mitochondrial
permeability transition pore (PT pore) in the outer mitochondrial
membrane. The pro-apoptotic Bcl-2 gene family can promote the
opening of the PT pore when exogenous or endogenous stimulating
factors act on the mitochondrion; it releases cytochrome c,
activates the caspase cascade reaction, and binds with apoptotic
protease-activating factor 1 (Apaf-1). It also initiates the
Apaf-1-caspase-9-caspase-3 cell apoptin cascade reaction and
induces cell apoptosis (41). In
the present study, the overexpression of miR-15a-5p reduced cell
proliferation, induced apoptosis, and promoted the activity of
caspase-3/9 and the protein expression of Bax in the PASMCs of rats
with MCT-induced PAH. Chen et al reported that miR-15a-5p
suppressed cell survival and metastasis and induced apoptosis in
chronic myeloid leukemia (42).
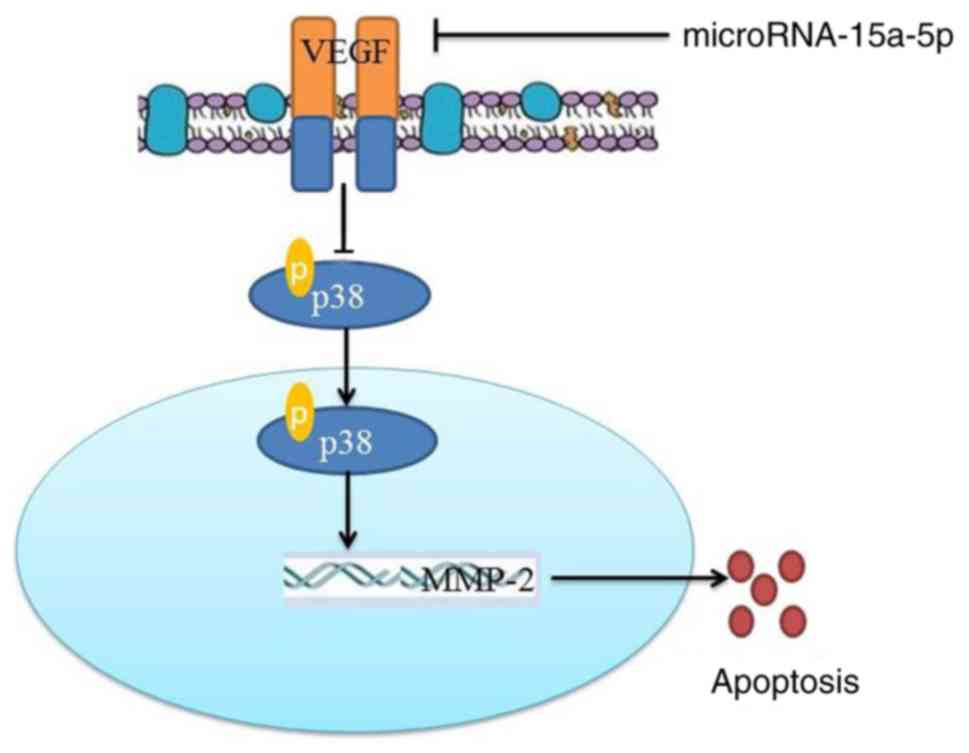
In conclusion, the present study demonstrated that
miR-15a-5p induced apoptosis of PASMCs in rats with MCT-induced PAH
through targeting the VEGF/p38/MMP-2 signaling pathway (Fig. 11). The results indicated that
miR-15a-5p may be involved in the pathogenesis of PAH, and it may
be a potential therapeutic target for PAH.
Acknowledgments
Not applicable.
Funding
The present study was partly supported by the
National Natural Science Foundation of China (grant. nos. 81800222
and 81500037).
Availability of data and materials
The analysed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ designed the experiments; WZ, YL, XX, SW, YL and
MS performed the experiments. WZ and GZ analysed the data; GZ wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Beijing Anzhen Hospital, Capital Medical University
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Veeraraghavan S, Koss MN and Sharma OP:
Pulmonary veno-occlusive disease. Curr Opin Pulm Med. 5:310–313.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prins KW and Thenappan T: World health
organization group I pulmonary hypertension: epidemiology and
pathophysiology. Cardiol Clin. 34:363–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dorfmuller P, Perros F, Balabanian K and
Humbert M: Inflammation in pulmonary arterial hypertension. Eur
Respir J. 22:358–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humbert M, Morrell NW, Archer SL, Stenmark
KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O,
Voelkel NF and Rabinovitch M: Cellular and molecular pathobiology
of pulmonary arterial hypertension. J Am Coll Cardiol. 43(12 Suppl
S): pp. 13S–24S. 2004, View Article : Google Scholar
|
|
5
|
Akagi S, Nakamura K, Akagi T, Nakagawa K,
Takaya Y, Sarashina T, Ejiri K and Ito H: Feasibility of repairing
defects followed by treatment with pulmonary hypertension-specific
drugs (Repair and Treat) in patients with pulmonary hypertension
associated with atrial septal defect: Study protocol for
interventional trial. Acta Med Okayama. 70:397–400. 2016.PubMed/NCBI
|
|
6
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–359. 2011. View Article : Google Scholar
|
|
8
|
Courboulin A, Barrier M, Perreault T,
Bonnet P, Tremblay VL, Paulin R, Tremblay E, Lambert C, Jacob MH,
Bonnet SN, et al: Plumbagin reverses proliferation and resistance
to apoptosis in experimental PAH. Eur Respir J. 40:618–629. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fischer SEJ: RNA interference and
MicroRNA-mediated silencing. Curr Protoc Mol Biol.
112:26.1.1–26.1.5. 2015. View Article : Google Scholar
|
|
11
|
Naveed A, Ur-Rahman S, Abdullah S and
Naveed MA: A concise review of MicroRNA exploring the insights of
MicroRNA regulations in bacterial, viral and metabolic diseases.
Mol Biotechnol. 59:518–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yun EJ, Lorizio W, Seedorf G, Abman SH and
Vu TH: VEGF and endothelium-derived retinoic acid regulate lung
vascular and alveolar development. Am J Physiol Lung Cell Mol
Physiol. 310:L287–L298. 2016. View Article : Google Scholar :
|
|
13
|
Muratore CS, Nguyen HT, Ziegler MM and
Wilson JM: Stretch-induced upregulation of VEGF gene expression in
murine pulmonary culture: A role for angiogenesis in lung
development. J Pediatr Surg. 35:906–912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Partovian C, Adnot S, Eddahibi S, Teiger
E, Levame M, Dreyfus P, Raffestin B and Frelin C: Heart and lung
VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced
pulmonary hypertension. Am J Physiol. 275:H1948–H1956.
1998.PubMed/NCBI
|
|
15
|
Tuder RM, Flook BE and Voelkel NF:
Increased gene expression for VEGF and the VEGF receptors KDR/Flk
and Flt in lungs exposed to acute or to chronic hypoxia. Modulation
of gene expression by nitric oxide. J Clin Invest. 95:1798–1807.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen H and Tian Y: MiR-15a-5p regulates
viability and matrix degradation of human osteoarthritis
chondrocytes via targeting VEGFA. Biosci Trends. 10:482–488. 2017.
View Article : Google Scholar
|
|
17
|
Shang J, He Q, Chen Y, Yu D, Sun L, Cheng
G, Liu D, Xiao J and Zhao Z: miR-15a-5p suppresses inflammation and
fibrosis of peritoneal mesothelial cells induced by peritoneal
dialysis via targeting VEGFA. J Cell Physiol. 234:9746–9755. 2019.
View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-timequantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Woik N and Kroll J: Regulation of lung
development and regeneration by the vascular system. Cell Mol Life
Sci. 72:2709–2718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Voelkel NF and Gomez-Arroyo J: The role of
vascular endothelial growth factor in pulmonary arterial
hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol.
51:474–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sergi C: EPAS 1, congenital heart disease,
and high altitude: Disclosures by genetics, bioinformatics, and
experimental embryology. Biosci Rep. 39:pii: BSR20182197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Papaioannou AI, Zakynthinos E, Kostikas K,
Kiropoulos T, Koutsokera A, Ziogas A, Koutroumpas A, Sakkas L,
Gourgoulianis KI and Daniil ZD: Serum VEGF levels are related to
the presence of pulmonary arterial hypertension in systemic
sclerosis. BMC Pulm Med. 9:182009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saleby J, Bouzina H, Lundgren J and
Radegran G: Angiogenic and inflammatory biomarkers in the
differentiation of pulmonary hypertension. Scand Cardiovasc J.
51:261–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kasahara Y, Tuder RM,
Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK,
Waltenberger J and Voelkel NF: Inhibition of VEGF receptors causes
lung cell apoptosis and emphysema. J Clin Invest. 106:1311–1319.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaudhary KR, Deng Y, Suen CM, Taha M,
Petersen TH, Mei SHJ and Stewart DJ: Efficacy of treprostinil in
the SU5416-hypoxia model of severe pulmonary arterial hypertension:
Haemodynamic benefits are not associated with improvements in
arterial remodelling. Br J Pharmacol. 175:3976–3989. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye EA, Liu L and Steinle JJ: miR-15a/16
inhibits TGF-beta3/VEGF signaling and increases retinal endothelial
cell barrier proteins. Vision Res. 139:23–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schermuly RT, Ghofrani HA, Wilkins MR and
Grimminger F: Mechanisms of disease: Pulmonary arterial
hypertension. Nat Rev Cardiol. 8:443–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rabinovitch M, Guignabert C, Humbert M and
Nicolls MR: Inflammation and immunity in the pathogenesis of
pulmonary arterial hypertension. Circ Res. 115:165–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Liu LY, Jia Y, Sun YY and Ma FZ:
Role of microRNA-15a-5p in the atherosclerotic inflammatory
response and arterial injury improvement of diabetic by targeting
FASN. Biosci Rep. 39:pii: BSR2018. 18522019. View Article : Google Scholar
|
|
30
|
Jia X, Hu X, Han S, Miao X, Liu H, Li X,
Lin Z, Wang Z and Gong W: Increased M1 macrophages in young
miR-15a/16−/− mice with tumour grafts or dextran
sulphate sodium-induced colitis. Scand J Immunol. 8:e127032018.
View Article : Google Scholar
|
|
31
|
Davidovich P, Kearney CJ and Martin SJ:
Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol
Chem. 395:1163–1171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferrari G, Terushkin V, Wolff MJ, Zhang X,
Valacca C, Poggio P, Pintucci G and Mignatti P: TGF-β1 induces
endothelial cell apoptosis by shifting VEGF activation of p38(MAPK)
from the prosurvival p38β to proapoptotic p38α. Mol Cancer Res.
10:605–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi C, Huang F, Gu X, Zhang M, Wen J, Wang
X, You L, Cui X, Ji C and Guo X: Adipogenic miRNA and
meta-signature miRNAs involved in human adipocyte differentiation
and obesity. Oncotarget. 7:40830–40845. 2016.PubMed/NCBI
|
|
35
|
Thenappan T, Chan SY and Weir EK: Role of
extracellular matrix in the pathogenesis of pulmonary arterial
hypertension. Am J Physiol Heart Circ Physiol. 315:H1322–H1331.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bihlet AR, Karsdal MA, Sand JM, Leeming
DJ, Roberts M, White W and Bowler R: Biomarkers of extracellular
matrix turnover are associated with emphysema and
eosinophilic-bronchitis in COPD. Respir Res. 18:222017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jablonska-Trypuc A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31(Sup 1):
S177–S183. 2016. View Article : Google Scholar
|
|
38
|
Si-Tayeb K, Monvoisin A, Mazzocco C,
Lepreux S, Decossas M, Cubel G, Taras D, Blanc JF, Robinson DR and
Rosenbaum J: Matrix metalloproteinase 3 is present in the cell
nucleus and is involved in apoptosis. Am J Pathol. 169:1390–1401.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mukherjee A, Adhikari N and Jha T: A
pentanoic acid derivative targeting matrix metalloproteinase-2
(MMP-2) induces apoptosis in a chronic myeloid leukemia cell line.
Eur J Med Chem. 141:37–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu H, Zhong Q, Xia Y, Li E, Wang S and Ren
R: MicroRNA-2861 targets STAT3 and MMP2 to regulate the
proliferation and apoptosis of ectopic endometrial cells in
endometriosis. Pharmazie. 74:243–249. 2019.PubMed/NCBI
|
|
41
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen D, Wu D, Shao K, Ye B, Huang J and
Gao Y: MiR-15a-5p negatively regulates cell survival and metastasis
by targeting CXCL10 in chronic myeloid leukemia. Am J Transl Res.
9:4308–4316. 2017.PubMed/NCBI
|