Introduction
Epidemiological studies have shown that exposure to
ethanol during pregnancy can result in fetal intrauterine growth
retardation (IUGR) and an increased risk for depression in
offspring (1-3). Adults with fetal alcohol effects who
were older than 18 years of age were more likely to suffer from a
substantial mental illness, and 11 of the 25 subjects were
diagnosed as having depression (1). The surveys suggest that offspring
exposed to alcohol in utero are more susceptible to
depression, and that some types of depression might have a fetal
origin. Currently, it is widely recognized that the pathogenesis of
depression is influenced by genetic factors and biochemical
abnormalities (4,5). However, the intrauterine programming
mechanisms that increase the susceptibility of prenatal ethanol
exposure (PPE) adult offspring to depression have not been
thoroughly investigated.
Brain-derived neurotrophic factor (BDNF), one of the
important neurotrophic factors in the brain, is widely expressed in
the central nervous system of both developmental stage and adult
mammals, with the highest expressions being found in the
hippocampus and cortex. BDNF promotes neuronal survival, as well as
the differentiation and plasticity of synapses. Some evidence
suggests that decreased BDNF in the prefrontal cortex or
hippocampus play an important role in depression. Moreover, a
growing number of studies have investigated the biological role of
BDNF, and a 'BDNF hypothesis' has been proposed to help explain the
pathogenic mechanism of depression (4,6).
The cAMP response element binding protein (CREB) is an important
transcription factor that facilitates the expression of genes that
encode for neurotrophic factor and its downstream effector. CREB
binds to its binding sites located in the promoter region of the
BDNF gene, and thereby promotes BDNF expression (7). Thus, both CREB and BDFN play
important roles in depression (8,9).
Tyrosine kinase receptor B (TrkB), a high-affinity membrane
receptor, binds BDNF, and then exerts multiple biological effects
by activating mitogen-activated protein kinase/extracellular
signal-regulated kinase (the multiple signaling MAPK/ERK cascade),
phosphatidylinositol 3-kinase, and phospholipase C (10). In addition to being a target of
CREB, BDNF can itself recruit this particular transcription factor
via BDNF/TrkB-mediated signaling enabled by the stimulation of ERK,
which subsequently leads to CREB activation. As a result, the
expression of BDNF and its related pathways are strongly associated
with the pathogenesis of depression. However, it remains unclear
whether BDNF is involved in the increased susceptibility to
depression found among adult offspring exposed to ethanol in fetus,
and if so, what the possible mechanism might be.
This study was designed to verify the enhanced
susceptibility to depression in PEE offspring rats by detecting
depressive behavior. We also explored possible intrauterine
programming mechanisms for increased susceptibility to depression
by examining the expression of genes related to the BDNF signaling
pathway in the hippocampus of PEE offspring both before and after
birth. Our results provide theoretical and experimental evidence
that can be used for the early prevention and treatment of
depression.
Materials and methods
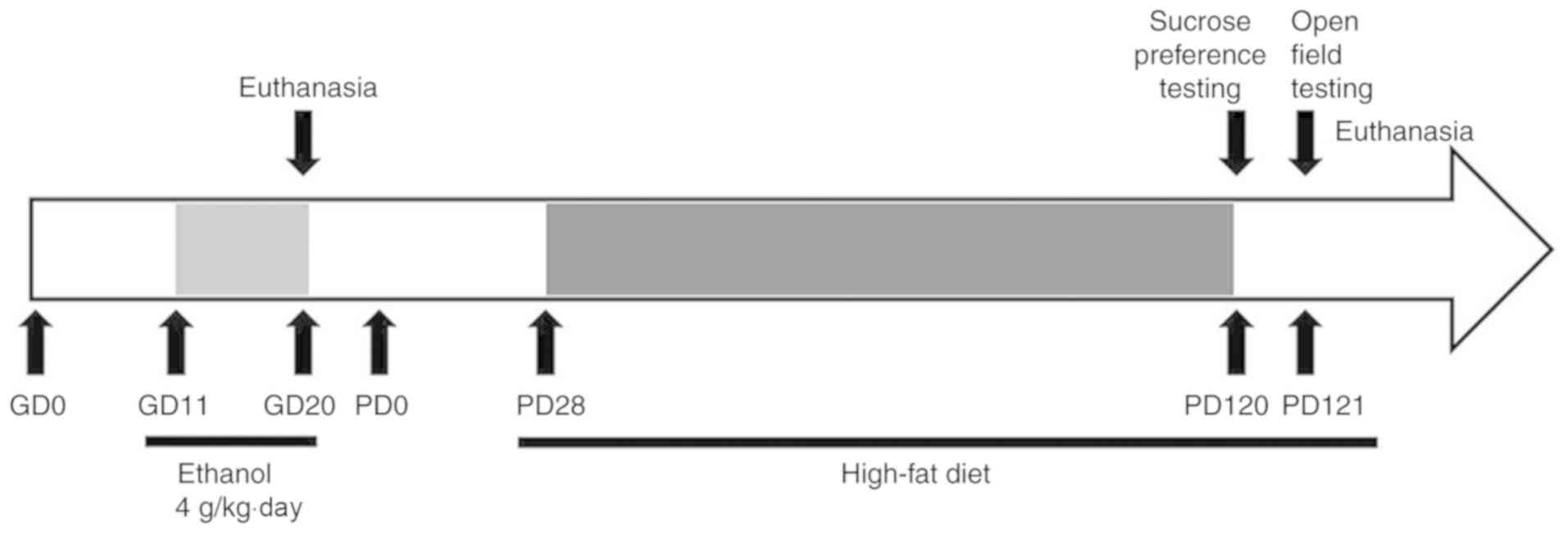
Animals and treatment
The study protocol was approved by the Committee on
the Ethics of Animal Experiments of the Wuhan University School of
Medicine (Hubei, China). Experimental procedures conducted on
animals were performed in accordance with the 'Guidelines for the
Care and Use of Laboratory Animals' published by the Chinese Animal
Welfare Committee, and guidelines created by the International
Council on Research Animal Care.
Wistar rats (weights of 200-240 g for females and
260-300 g for males) were acclimated and housed under controlled
conditions of 18-22°C, 40-60% humidity, and allowed to mate.
Gestational day (GD) 0 was defined as the day on which mating could
be confirmed by the appearance of sperm in a vaginal smear.
Pregnant rats were randomly assigned to an ethanol group and a
control group, separately being given ethanol (4 g/kg/day) and the
same volume of distilled water by gavage administration from GD11
to GD20. Eight randomly selected pregnant rats with 8-14 live
fetuses in each group were euthanized on GD20. In each group, five
whole fetal brains were randomly selected and routinely fixed for
histologic and ultra-structural examinations; while 8 fetal
hippocampus isolated under a dissecting microscope were collected
for gene expression analyses. The remaining pregnant rats were
maintained until achieving a normal delivery (GD21). On postnatal
day 1 (PD1) the number of pups in each group was normalized to 8
pups per litter to ensure that adequate and standardized nutrition
could be maintained until weaning at PD28. At PD28, one male and
one female offspring rat randomly selected from 22 liters (11
liters from control and 11 liters from PEE) were assigned to four
groups (control male/female groups versus PEE male/female groups,
n=11 for each group). All offspring rats were given a high-fat diet
composed of 0.5% (w/w) cholesterol, 11.5% (w/w) lard, and 88.0%
(w/w) cereal. After performing behavior tests at PD120 (sucrose
preference test) and PD121 (open field test), the offspring rats
was decapitated for histological examination and gene expression
analyses (Fig. 1).
Sucrose preference test and open field
test
The methods used for assessing sucrose preference
and open field tests were modified from those described by Briones
et al (11). For the
sucrose preference test, the rats were acclimated to making one of
a two-bottle choice of either drinking water or a 1% sucrose
solution for 2 days. On the day of testing, two pre-weighted
bottles, one containing 5% sucrose solution and the other
containing tap water, were presented. To prevent any possible
effects of side preference on drinking behavior, the positions of
the bottles were switched after 12 h of testing. The amount of
liquid consumed from each bottle, corrected for body weight, was
calculated at the end of a 24-h test period. Sucrose preference was
calculated using the equation: Sucrose preference (%)=sucrose
intake/(sucrose intake + water intake) ×100. The open field test
was conducted on the day after the sucrose preference test, and
using a black plexiglas square box (100×100×50 cm). The box was
divided into two areas consisting of a peripheral area and a center
area (60×60 cm each), both of which were cleaned with 70% ethanol
after each rat was tested. During testing, each rat was placed in
the center area, then allowed to freely explore for 10 min.
Activity in the open field was recorded with a video recorder.
Histological examination
The brain of each adult offspring rats was fixed
overnight in 4% paraformaldehyde solution, then processed using the
paraffin sectioning technique. The sections (5 µm) were
stained with hematoxylin and eosin (H&E) and photographed with
an Olympus AH-2 light microscope (Olympus). The total number of
neurons was estimated by applying the optical fractionator method
(12). The boundaries of the
hilus of the DG and of the CA3 and CA1 hippocampal fields were
consistently defined at all levels along the rostrocaudal axis of
the brain on the basis of cell morphology and cytoarchitectonic
criteria (13). The number of
cells/mm was counted from three sections (separated by
approximately 400 µm) for each animal, and the relative
percentage of neurons loss compared with the same region in control
group was calculated.
Ultrastructural analysis
The hippocampus was removed and force-fixed in 2.5%
glutaraldehyde, then post-fixed in 1% OsO4. The tissue specimens
were dehydrated and embedded. Ultrathin sections were stained with
4.7% uranyl acetate and lead citrate. Images were captured by a
Hitachi H600 transmission electron microscope (Hitachi).
RT-qPCR
Detail protocols for RT-qPCR were provided in our
previous study (14). Briefly,
total RNA was isolated from hippocampus tissue using TRIzol reagent
according to the manufacturer's protocol. Single-strand cDNA was
prepared from total RNA according to a protocol included with the
Exscript RT reagent kit (Takara Biotechnology). The primer
sequences are shown in Table SI.
Relative standard curves were constructed for specific rat target
genes, including mineralocorticoid (MR), glucocorticoid
(GR), CREB, BDNF, and TrkB, as well as
for the housekeeping gene glyceraldehyde phosphate dehydrogenase
(GAPDH). PCR assays were performed in 36-well optical
reaction plates using a RG-3000 Rotor-Gene 4 Channel Multiplexing
system (Qiagen). The mRNA level of the housekeeping gene GAPDH was
measured and used as a quantitative control. The expression levels
of the above genes were calculated using the 2−∆∆Cq
method (15). The PCR cycling
conditions are shown in Table
SI.
Multiplex analysis of gene
expression
A multiplex analysis of two housekeeping genes and
18 target genes was performed using a GenomeLab GeXP Genetic
Analysis system (Beckman-Coulter). A multiplex optimization
procedure (e.g., primer validation and attenuation) was completed
according to the manufacturer's instructions. The target genes and
primer pairs analyzed in the study are shown in Table SII. The genes include MR,
GR, CREB, BDNF, TrkB, NMDA glutamate
receptor subunit 1 (NR1), glutamate NMDA receptor subunit 2A
(NR2A), glutamate NMDA receptor subunit 2B (NR2B),
glutamate AMPA receptor subunit 2 (GluA 2), GAPDH,
synapsin I (Syn 1), synaptosomal-associated protein 25
(SNAP25), cell cycle protein A (Cyclin A), Ras
homolog gene family members A (RhoA), ras-related C3
botulinum toxin substrate 1 (Rac1), cell division cyclin 42
(Cdc42), cyclin-dependent kinase 2 (Cdk2), B cell
lymphoma/leukemia 2 gene (bcl-2), and rattus norvegicus
tubulin, beta 3 (Tubb3). The relative levels of RNA
expression were calculated relative to pooled RNA standard and were
normalized to the levels of GAPDH and Tubb3 RNA
expression.
Statistical analysis
The data are presented as the mean ± SEM.
Statistical analyses were performed with the software SPSS 17.0
(SPSS, Inc.). Comparisons between two groups were performed using
Student's t-test. Comparisons among more than two groups were
conducted by one-way analysis of variance followed by a post hoc
Dunnett's test. Statistical significance was designated at
P<0.05.
Results
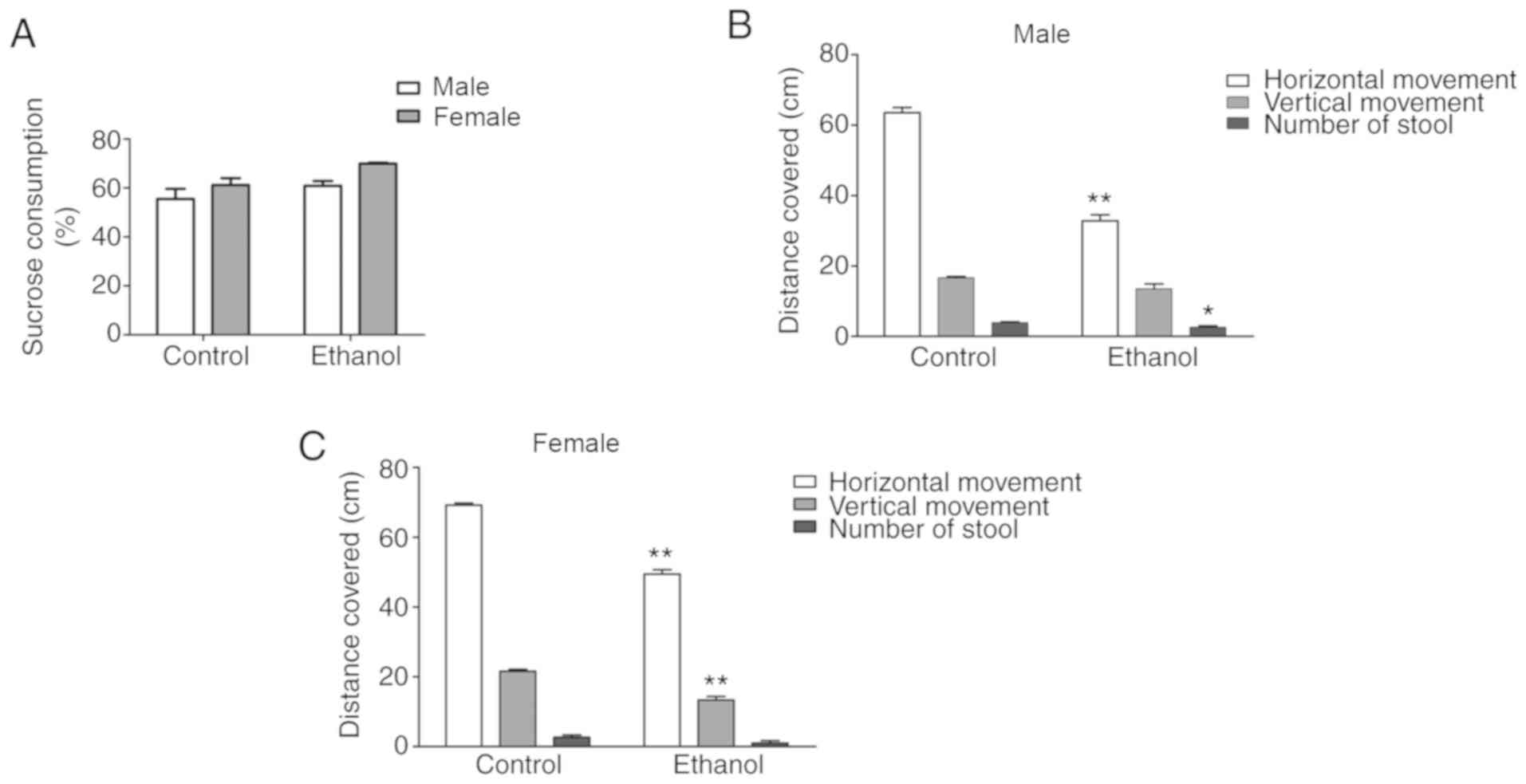
Behavioral changes in adult
offspring
To assess depression-like behavior, the adult
offspring rats were assessed using the sucrose preference test and
open-field test. When compared with the control group, the PEE
group had no significant reduction in sugar intake, and there was
no difference in sugar intake between sexes (Fig. 2A). In the open-field test, the
male PEE offspring displayed significantly reduced horizontal
movement (P<0.01), and the female PEE offspring displayed
significantly reduced horizontal and vertical movement when
compared with offspring in the control group (P<0.01) (Fig. 2B and C). The male PEE offspring
had significantly fewer stools than control group (P<0.05);
however, there was no difference in stool number between female PEE
offspring and control group (Fig. 2B
and C).
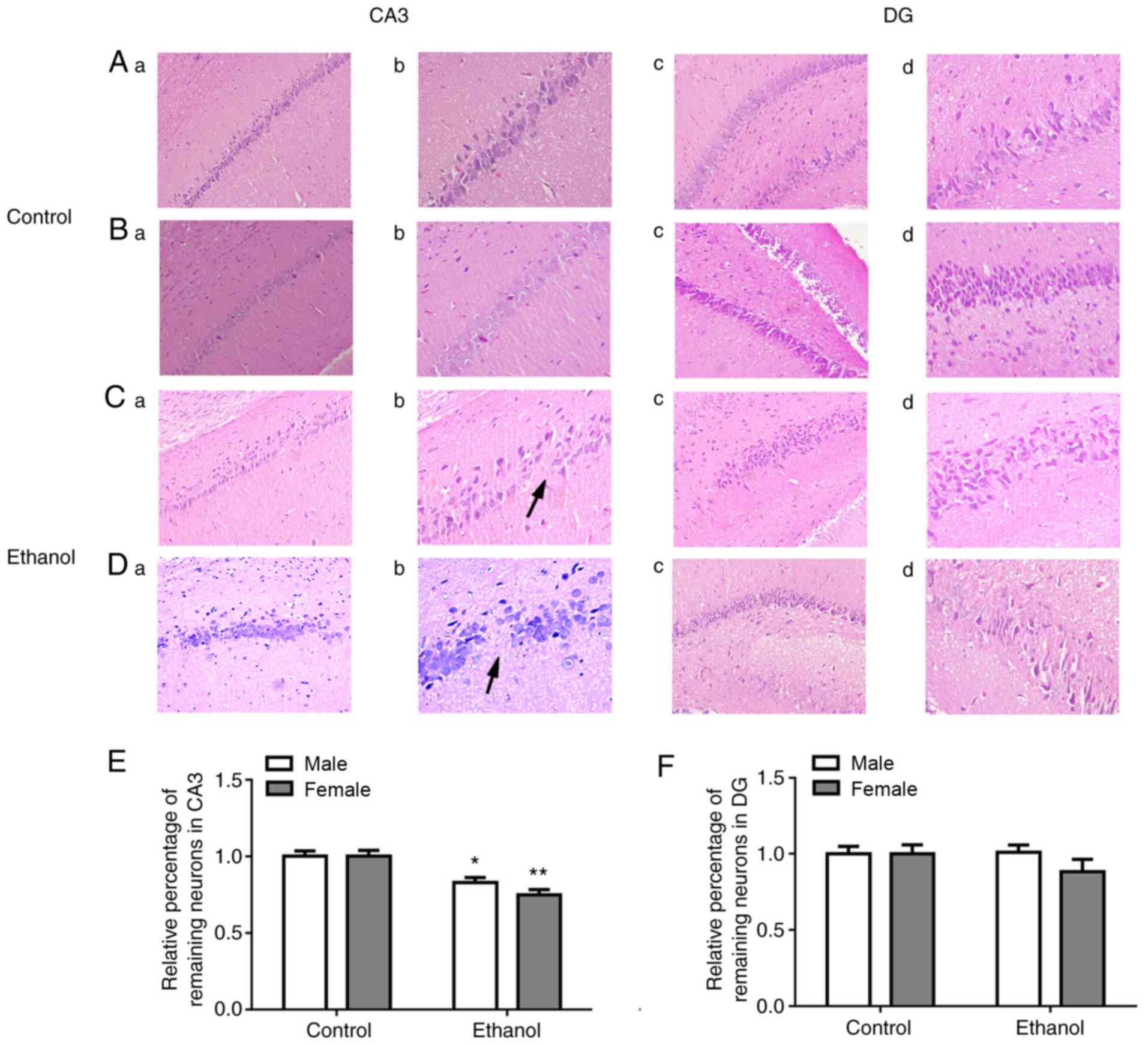
Histological changes in adult offspring
hippocampus
The sections stained by H&E showed that
hippocampal pyramidal cells in the adult offspring rats of control
group were thick, intact, had sharp edges, and displayed a neat,
tight arrangement, with no differences between sexes (Fig. 3A and B). By contrast, the
hippocampal pyramidal cells in PEE adult offspring rats were sparse
and thin, displayed a loose, disordered arrangement, showed
wrinkled nuclei, and were deeply stained. Additionally, fewer
pyramidal cells, especially in the Cornu Ammonis (CA) 3 region of
the hippocampus, and worse morphology of hippocampus in female rats
than male rats were identified (Fig.
3C and D). Based on the quantitative analysis of remaining
neurons in CA3 and DG region, the loss of neurons in CA3 has
significant differences in female and male offspring rats
(P<0.01 and P<0.05) compared with the control group (Fig. 3E). In addition, there was no
difference of remaining neurons in DG region between the two groups
(Fig. 3F).
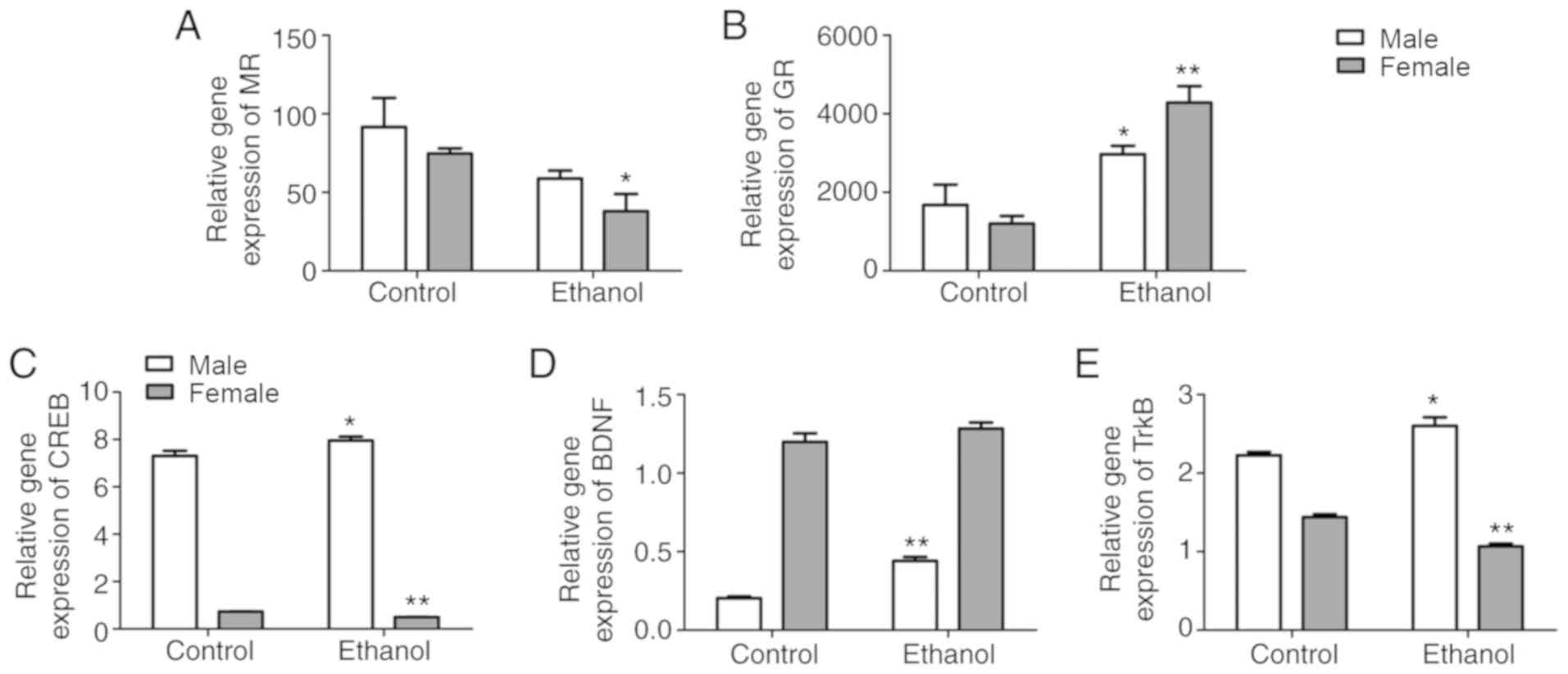
MR, GR, CREB, BDNF, and TrkB expression
in adult offspring hippocampus
When compared with the control group, mRNA
expression of MR in the hippocampus of PEE female offspring
rats was significantly decreased (P<0.05); however, no changes
were found in the PEE male offspring rats. mRNA expression of
GR in the hippocampus of both female and male PEE offspring
were significantly increased when compared to the control group
(P<0.05 and P<0.01, respectively) (Fig. 4A and B). Furthermore, mRNA
expression of hippocampal BDNF in the PEE female offspring
and control group were similar; however, the mRNA expressions of
CREB and TrkB in the hippocampus of PEE female
offspring were much lower than those of the control group
(P<0.05 and P<0.01, respectively). Moreover, the mRNA
expression of CREB, BDNF and TrkB in the
hippocampus of PEE male offspring was significantly increased when
compared with those in the control group (P<0.05 or P<0.01;
Fig. 4C-E).
Histological changes in fetal
hippocampus
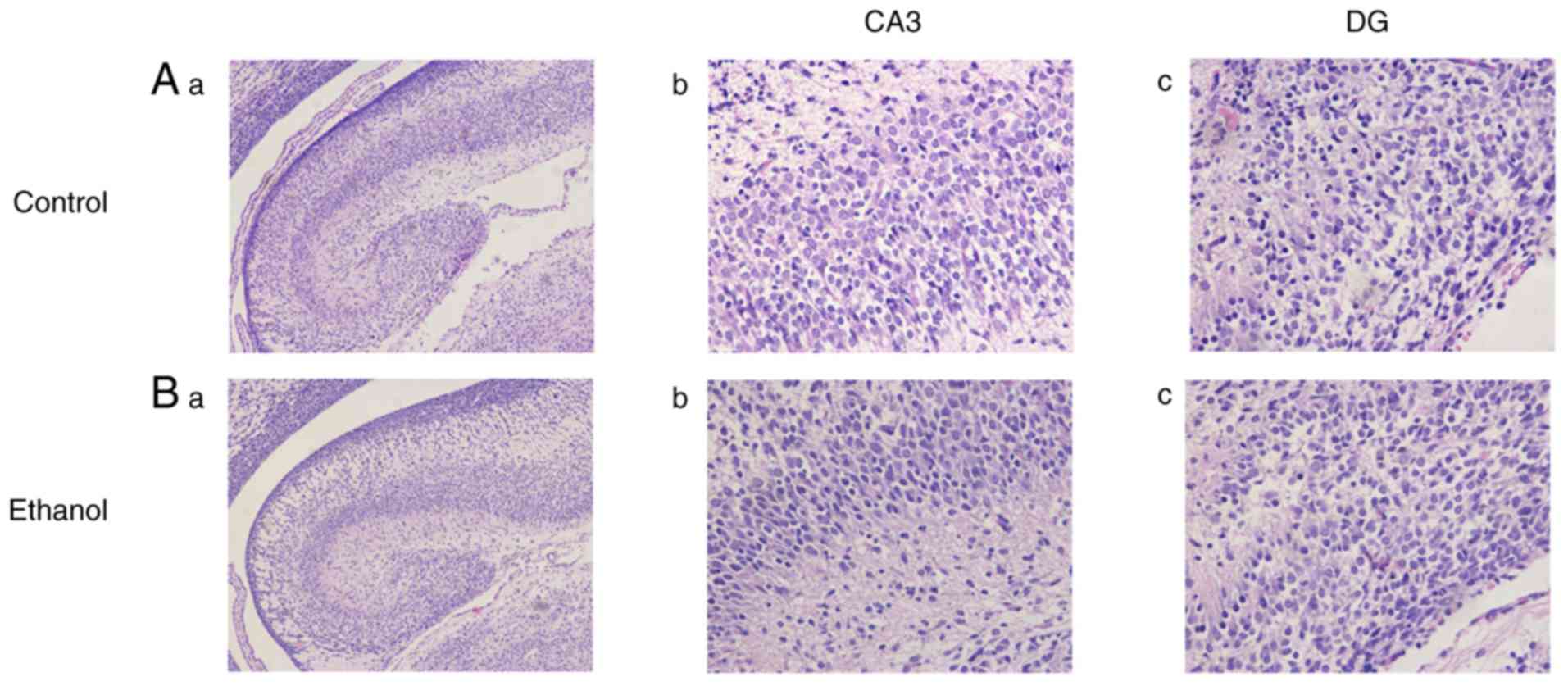
H&E staining revealed that the fetal hippocampus
of PEE group were similar with the control group in terms of their
overall shape, size, cellular structure, and morphology (Fig. 5).
Ultrastructural changes in fetal
hippocampal neurons
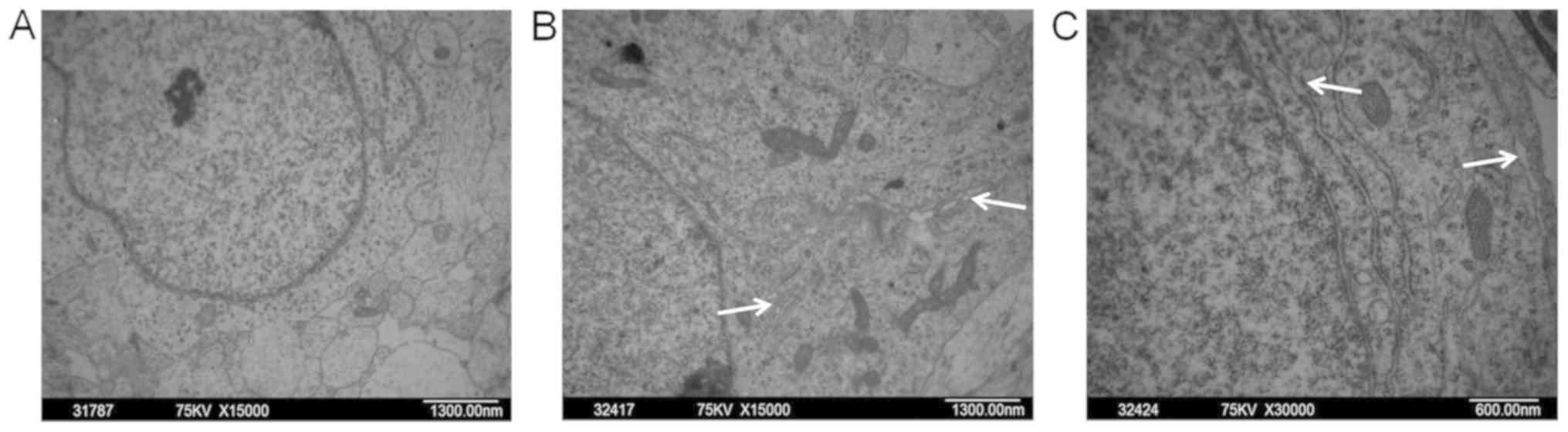
It was observed by transmission electron microscopy
that hippocampal neurons of fetal rats in the control group had an
intact nuclear membrane and contained abundant and normal
organelles (e.g., mitochondria and endoplasmic reticulum) in the
cytoplasm (Fig. 6A). However,
several hippocampal neurons in the PEE fetal rats displayed Golgi
hypertrophy (Fig. 6B) and
extensive rough endoplasmic reticulum cavities (Fig. 6C).
Multi-gene expression analysis of the
fetal hippocampus
An analysis was performed using the GenomeLab GexP
Genetic Analysis System to detect the expression of genes thought
to be closely associated with signal transduction and functional
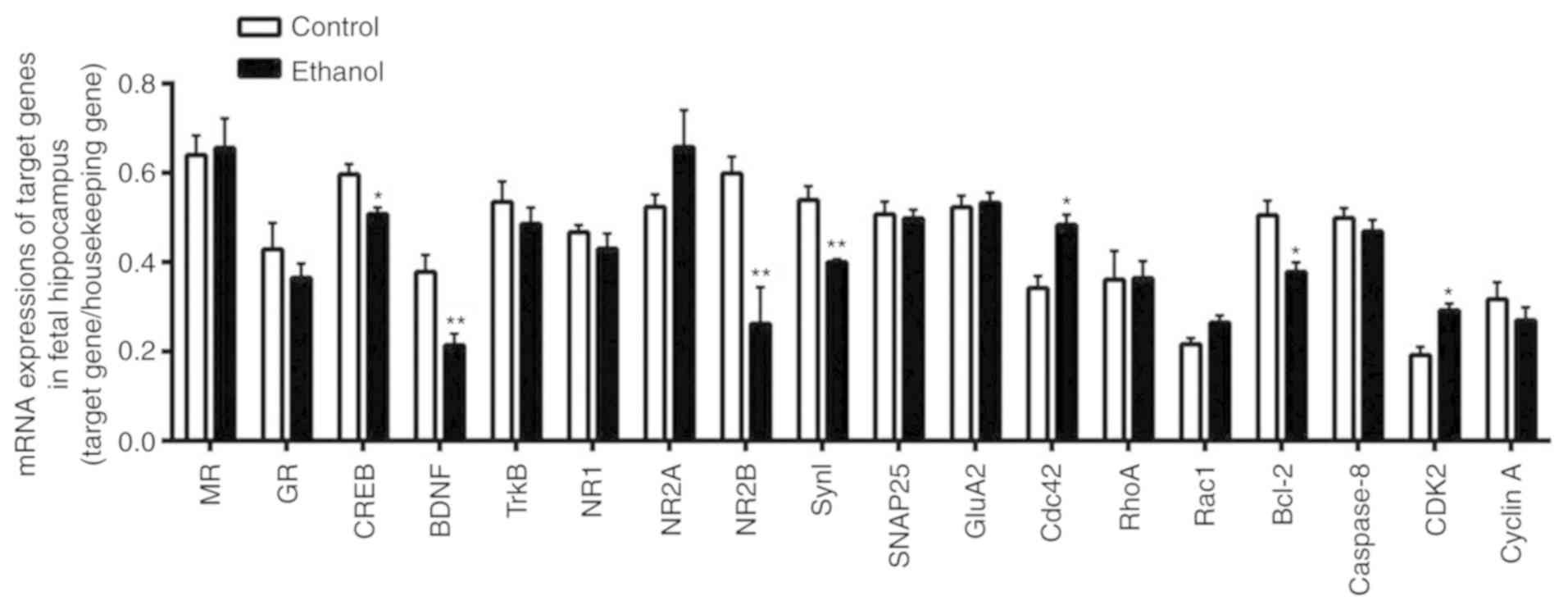
changes in fetal hippocampus. The mRNA expression levels of
BDNF and CREB in the fetal rat hippocampus were
significantly decreased in the PEE group when compared with those
in the control group (P<0.01 and P<0.05, respectively), while
TrkB mRNA expression was not significantly different between
the two groups. We also analyzed the mRNA expression levels of
synaptic protein in the hippocampus of fetal rats. The results
showed that the mRNA expression levels of NR2B and Syn
I were significantly decreased in the PEE group when compared
with those in the control group (P<0.01), while mRNA expression
for NR1, NR2A, SNAP25, and GluA2 was
not significantly different between the two groups. Furthermore,
the mRNA expression levels of MR and GR, which assist
in regulating the hypothalamic-pituitary-adrenal (HPA) axis, showed
no significant difference between the two groups (Fig. 7).
The GenomeLab GexP Genetic Analysis System was also
used to detect the expression of genes related to cellular
composition, cell cycling, and apoptosis. Compared with control
group, the expression of Cdc42 and CDK2 mRNA in the
fetal rat hippocampus were significantly increased in the PEE group
(P<0.05), while cyclin A mRNA expression showed no
significant difference between the two groups. Moreover, the mRNA
expression of Rac1 and RhoA, which assist in
cytoskeleton formation, showed no difference between the two
groups. Additionally, Bcl-2 mRNA expression, which inhibits
apoptosis in the fetal rat hippocampus, was significantly decreased
in the PEE group when compared with the control group (P<0.05),
while there was no difference of caspase 8 mRNA expression
between the two groups (Fig.
7).
Discussion
Depression is a heterogeneous clinical disorder, and
its cause remains unclear. The main symptoms displayed by patients
with depression are a depressed mood, impotence, anhedonia, and
cognition impairment (16). The
open-field and sucrose preference tests have been widely used to
detect abnormal animal behaviors, and to evaluate the main
characteristics of depression, such as lack of interest, anhedonia,
and despair behavior. Horizontal movement in the open-field test
reflects the activity of animal, and vertical movement reflects its
curiosity for the new environment. The sucrose preference test can
effectively detect the presence or absence of anhedonia (17,18). In the present study, we observed
that after being fed a high-fat diet, the independent activity of
PEE offspring gradually decreased, their dynamic performance and
exploratory behavior was reduced, and they appeared sluggish with
rigid behavior. The statistical analysis revealed that female PEE
adult offspring rats showed reduced horizontal and vertical
movement in the open-field test, suggesting that the reduced
mobility and interest in their environment reflected
depression-like behavior in the PEE offspring rats. However, the
male PEE adult offspring rats only showed reduced horizontal
movement in the open field test, and their depression-like behavior
was not obvious. These results suggest a sex difference in the
susceptibility of PEE offspring rats to depression. Moreover, we
observed that the hippocampal pyramidal cells of PEE adult
offspring rats were thin, loose, disordered, and sparse, and that a
few cells were even missing. These findings were especially
significant in the CA3 region of the hippocampus, where the female
offspring rats showed worse morphology than males, and to some
extent consistent with the sex difference of depression behavior in
PEE offspring rats.
Our previous findings showed that PEE inhibits the
development of fetal HPA axis, resulting in low activity levels and
high sensitivity to stress exerted on the HPA axis after birth in
offspring rats (19). Higher
activity and sensitivity of the HPA axis is thought to play an
important role in the occurrence of depression (20,21). It is also known that a high-fat
diet can increase the HPA axis activity (22,23). Thus we fed offspring rats with a
high-fat diet to increase the activity of their HPA axis. We
observed that the female PEE offspring rats have more obvious
depression-like behaviors when compared with their males, which is
consistent with the higher incidence of clinical depression found
among female human subjects. Our previous findings also showed that
the effect of a high-fat diet on glucose and lipid metabolism was
sex-specific, in terms of its ability to induce changes in insulin
and adiponectin signaling pathways (24). High-fat diet-induced HPA
axis-associated neuroendocrine dysfunction is more severe in female
rats than males, as it reduces the expression of GR in the
hypothalamus (25). This finding
suggests that the HPA axis in females is more sensitive to a
fat-induced nutritional imbalance. Moreover, Hill et al
suggested that maternal separation produces sex-specific long-term
effects on BDNF expression and signaling, and that alterations in
BDNF signaling might mediate the sex-specific effects of
developmental stress on anhedonic behavior (26). Therefore, we speculated that
PEE-induced changes in HPA axis programming and the BDNF signaling
pathway may both be involved in sex differences regarding
depression-like behavior among PEE adult offspring rats fed a
high-fat diet.
CREB, an important transcription factor, is involved
in the transcription of BDNF. Previous results showed that among
suicide victims, depression was accompanied by reduced expression
and activation of CREB (27,28). Inactivation of CREB reduces the
levels of BDNF expression, which can lead to depression-like
behavior and a weakened effect of antidepressant drugs (7,9,29).
Related clinical research has confirmed the presence of
significantly reduced BDNF levels in the blood of normal
individuals prone to depression and patients with depression.
Certain antidepressant treatments might significantly increase the
levels of BDNF in the blood of patients with depression (30). Previous study also revealed
decreased levels of BDNF expression in the hippocampus of rats
which displayed depression-like behavior (31). It is possible that an oriented
injection of BDNF into an animal's hippocampus might provide an
antidepressant effect (7).
Another study showed that oriented knockout of the BDNF gene
in the dentate gyrus resulted in decreased hippocampal neurogenesis
and led to obvious depressive behavior (32). Additionally, mice with a mutant
BDNF gene also showed obvious depressive behavior (33). TrkB is a BDNF receptor, and
studies have suggested that BDNF and TrkB mRNA and protein
expression are decreased in the hippocampus and other brain regions
of depressed individuals, as well as suicide victims (33-37). All of these studies suggest that
reduced levels of BDNF and BDNF signaling activity in the
hippocampus are closely associated with the occurrence of
depression.
Caldwell et al reported that adult offspring
mice exposed to ethanol throughout gestation displayed
depressive-like behaviors, which were associated with reduced
levels of BDNF protein and mRNA in the hippocampus and frontal
cortex (38). Our study found
that adult offspring rats that had been exposed to ethanol during
the middle-late stage of pregnancy (GD11-20) displayed
depressive-like behaviors, thereby confirming the observation of
Caldwell. Differently, we fed the PEE offspring a high-fat diet
after weaning, and then examined changes in the expression of
BDNF-related signaling in the hippocampus. We found that it has no
difference between the BDNF mRNA expression in the hippocampus of
female PEE offspring rats and those in control rats; however, the
expression of CREB and TrkB mRNA in the female PEE offspring were
significantly decreased. These results suggest that the BDNF
pathway in the hippocampus of adult female PEE offspring was in an
inactivated state, which might be the main cause for those rats
with depression-like behavior.
We used PEE fetal rats as a model in which to
identify a possible intrauterine mechanism for depression-like
behavior and a damaged hippocampus in adult PEE offspring rats. We
found that some fetal hippocampal neurons in the PEE group showed
Golgi hypertrophy and an extensive rough endoplasmic reticulum
cavity. Additionally, we examined the expression of BDNF pathways
in PEE offspring during their intrauterine period. Results of a
multi-gene expression analysis of the fetal hippocampus revealed
that the levels of BDNF and CREB mRNA expression, as well as the
expression levels of synaptic plasticity-related genes NR2B
and Syn I, were significantly lower in the PEE group when
compared with those in the control group. Our results suggest that
the depression-like behavior might be associated with weakened
expression of the BDNF pathway and impaired neural synaptic
plasticity in the hippocampus during the intrauterine period in PEE
adult offspring rats. We also found increased expression of genes
involved in cell proliferation and apoptosis in the fetal
hippo-campus, but did not find significant changes in the
expression of cytoskeleton-related genes. These findings suggest
that the rates of cell proliferation and apoptosis in PEE fetal rat
hippocampal neurons were higher than normal, possibly as a result
of ethanol exposure.
In a previous study, we found that PEE induced IUGR,
and led the fetus overexposure to elevated levels of maternal GC
(39). GC, an important stress
hormone, affects the function of neurons in the central nervous
system, and its presence at extra high levels might be related to
the pathogenesis of depression (40). When under the influence of high GC
levels, MR is quickly saturated and GR becomes activated;
furthermore, excessive activation of GR might damage hippocampal
neurons and inhibit BDNF expression (41). Both acute and chronic stimulation
can significantly reduce BDNF expression in the hippocampus
(31). This suggests that in our
study, high levels of GC induced increased GR expression in the
hippocampus. This increased GR expression blocked the BDNF-mediated
MAPK/ERK signaling pathway, resulting in damage to neurons and
reduced synaptic plasticity, which influenced the transmission of
information by 'neural circuits' and induced the occurrence of
depression. Based on results obtained, we speculate that under
conditions of intrauterine exposure to high levels of maternal GC
as a result of PEE, the hippocampus of the offspring fetus was not
damaged, and GR expression remained unchanged. However, at the same
time, CREB and BDNF expression were declined, and their functions
were restrained, causing neurogenesis and synaptic plasticity being
suppressed in fetal hippocampus. Additionally, being fed a high-fat
diet in adulthood further aggravated the toxic effects of high GC
levels in female offspring rats (26,27), and induced high levels of GR
expression, which restrained the BDNF pathway in adult hippocampus.
These changes resulted in impaired hippocampal morphology and
function, which directly and indirectly increased the
susceptibility to depression in offspring rats. In short, the
intrauterine mechanism for increased susceptibility to depression
among adult PEE offspring is most likely continues suppression of
the BDNF pathway from fetus to adult.
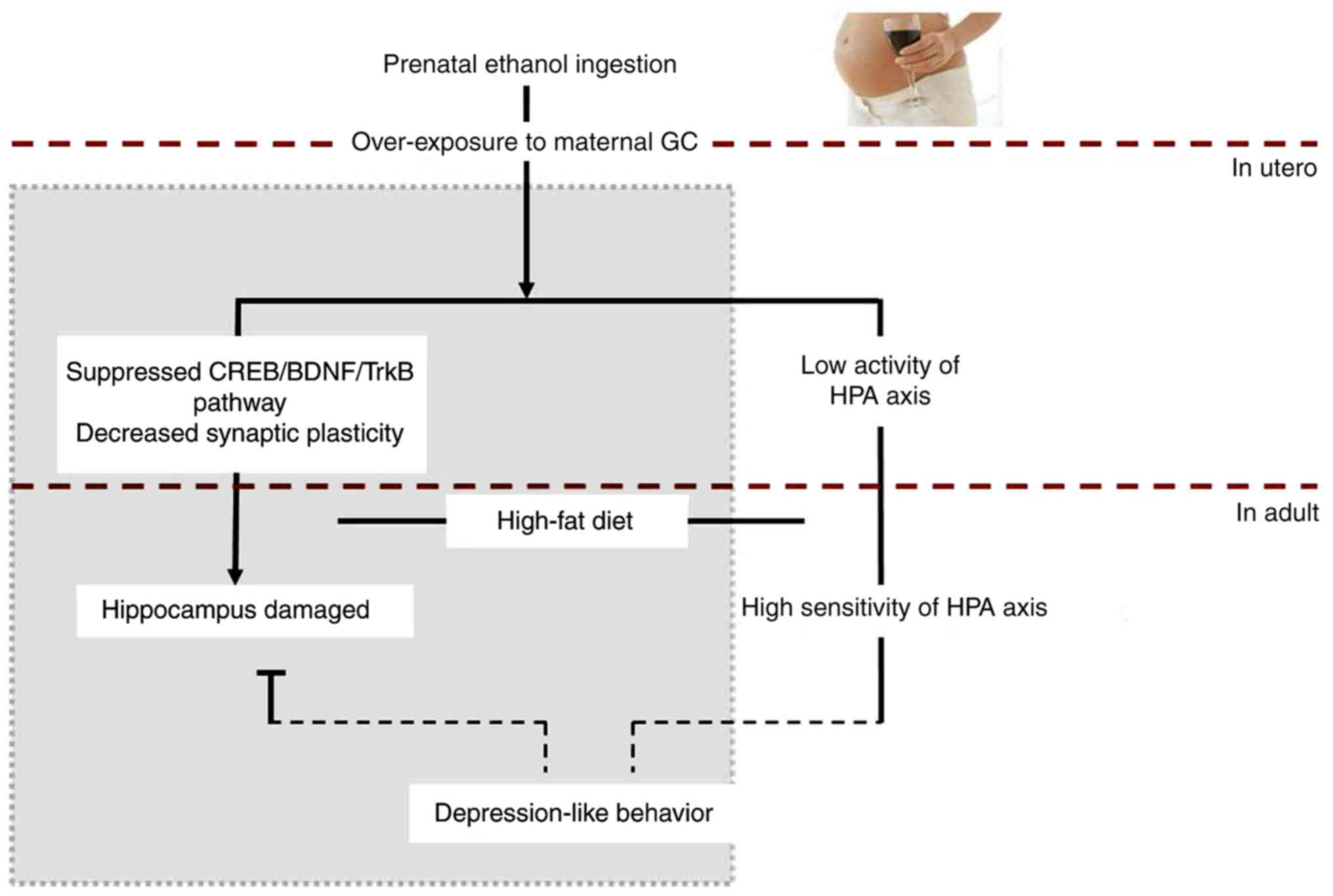
In conclusion, our data indicated that PEE is
capable of inducing depression-like behavior in adult offspring
rats fed a high-fat diet, and especially in female offspring
(Fig. 8). PEE resulted in
overexposure of the fetus to maternal GC, and inhibited the
expression of CREB and its target gene BDNF in the fetal
hippocampus. This inhibition led to an alteration of HPA axis
programming and suppressed function of the HPA axis in fetal rats.
These effects continued until birth, and even into adulthood. The
high-fat diet fed after birth and the high excitability of the HPA
axis caused elevated expression of hippocampal GR in female
offspring, and further inhibited the BDNF pathways in offspring
hippocampus. This could result in reduced levels of neurogenesis
and synaptic plasticity in hippocampus, which eventually caused
depression-like behavior in the female offspring rats.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National
Science and Technology Pillar Program of China (no. 2013BAI12B01-3)
and the National Natural Science Foundation of China (nos.
81300984, and 81430089).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YY and HW conceived and designed the study. YY, DX,
SC, LZ, ZS, JQ and ZZ performed the experiments, and acquired,
analyzed and interpreted the data. YY and HW drafted and edited the
manuscript. All authors have read and revised the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Committee on
the Ethics of Animal Experiments of the Wuhan University School of
Medicine. All experimental procedures conducted on animals were
performed in accordance with 'Guidelines for the Care and Use of
Laboratory Animals' published by the Chinese Animal Welfare
Committee, and guidelines created by the International Council on
Research Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Famy C, Streissguth AP and Unis AS: Mental
illness in adults with fetal alcohol syndrome or fetal alcohol
effects. Am J Psychiatry. 155:552–554. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sood B, Delaney-Black V, Covington C,
Nordstrom-Klee B, Ager J, Templin T, Janisse J, Martier S and Sokol
RJ: Prenatal alcohol exposure and childhood behavior at age 6 to 7
years: I. dose-response effect. Pediatrics. 108:E342001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Connor MJ, Shah B, Whaley S, Cronin P,
Gunderson B and Graham J: Psychiatric illness in a clinical sample
of children with prenatal alcohol exposure. Am J Drug Alcohol
Abuse. 28:743–754. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duman RS and Monteggia LM: A neurotrophic
model for stress-related mood disorders. Biol Psychiatry.
59:1116–1127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levinson DF: The genetics of depression: A
review. Biol Psychiatry. 60:84–92. 2006. View Article : Google Scholar
|
|
6
|
Nestler EJ, Barrot M, DiLeone RJ, Eisch
AJ, Gold SJ and Monteggia LM: Neurobiology of depression. Neuron.
34:13–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shirayama Y, Chen ACH, Nakagawa S, Russell
DS and Duman RS: Brain-derived neurotrophic factor produces
antidepressant effects in behavioral models of depression. J
Neurosci. 22:3251–3261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liebenberg N, Müller HK, Fischer CW,
Harvey BH, Brink CB, Elfving B and Wegener G: An inhibitor of
cAMP-dependent protein kinase induces behavioural and neurological
antidepressant-like effects in rats. Neurosci Lett. 498:158–161.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Réus GZ, Stringari RB, Ribeiro KF, Ferraro
AK, Vitto MF, Cesconetto P, Souza CT and Quevedo J: Ketamine plus
imipramine treatment induces antidepressant-like behavior and
increases CREB and BDNF protein levels and PKA and PKC
phosphorylation in rat brain. Behav Brain Res. 221:166–171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patapoutian A and Reichardt LF: Trk
receptors: Mediators of neurotrophin action. Curr Opin Neurobiol.
11:272–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Briones TL and Woods J: Chronic binge-like
alcohol consumption in adolescence causes depression-like symptoms
possibly mediated by the effects of BDNF on neurogenesis.
Neuroscience. 254:324–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
West MJ, Slomianka L and Gundersen HJ:
Unbiased stereological estimation of the total number of neurons in
thesubdivisions of the rat hippocampus using the optical
fractionator. Anat Rec. 231:482–497. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amaral DG and Witter MP: Hippocampal
formation. The rat nervous system. Paxinos C: 2nd edition. Academic
Press Ltd; London: pp. 443–493. 1995
|
|
14
|
Ao Y, Sun Z, Hu S, Zuo N, Li B, Yang S,
Xia L, Wu Y, Wang L, He Z and Wang H: Low functional programming of
renal AT2R mediates the developmental origin of glomerulosclerosis
in adult offspring induced by prenatal caffeine exposure. Toxicol
Appl Pharmacol. 287:128–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Kennedy SH: Core symptoms of major
depressive disorder: Relevance to diagnosis and treatment.
Dialogues Clin Neurosci. 10:271–277. 2008.PubMed/NCBI
|
|
17
|
Overstreet DH: Modeling depression in
animal models. Psychiatric Disorders Springer. 125–144. 2012.
View Article : Google Scholar
|
|
18
|
Yan HC, Cao X, Das M, Zhu XH and Gao TM:
Behavioral animal models of depression. Neurosci Bull. 26:327–337.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia LP, Shen L, Kou H, Zhang BJ, Zhang L,
Wu Y, Li XJ, Xiong J, Yu Y and Wang H: Prenatal ethanol exposure
enhances the susceptibility to metabolic syndrome in offspring rats
by HPA axis-associated neuroendocrine metabolic programming.
Toxicol Lett. 226:98–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guerry JD and Hastings PD: In search of
HPA axis dysregulation in child and adolescent depression. Clin
Child Fam Psychol Rev. 14:135–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Höhne N, Poidinger M, Merz F, Pfister H,
Brückl T, Zimmermann P, Uhr M, Holsboer F and Ising M: Increased
HPA axis response to psychosocial stress in remitted depression:
The influence of coping style. Biol Psychol. 103:267–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan O, Inouye K, Riddell MC, Vranic M and
Matthews SG: Diabetes and the hypothalamo-pituitary-adrenal (HPA)
axis. Minerva Endocrinol. 28:87–102. 2003.PubMed/NCBI
|
|
23
|
Shin AC, MohanKumar SM, Sirivelu MP,
Claycombe KJ, Haywood JR, Fink GD and MohanKumar PS: Chronic
exposure to a high-fat diet affects stress axis function
differentially in diet-induced obese and diet-resistant rats. Int J
Obes (Lond). 34:1218–1226. 2010. View Article : Google Scholar
|
|
24
|
Shen L, Liu Z, Gong J, Zhang L, Wang L,
Magdalou J, Chen L and Wang H: Prenatal ethanol exposure programs
an increased susceptibility of non-alcoholic fatty liver disease in
female adult offspring rats. Toxicol Appl Pharmacol. 274:263–273.
2014. View Article : Google Scholar
|
|
25
|
Zhang L, Xu D, Zhang B, Liu Y, Chu F, Guo
Y, Gong J, Zheng X, Chen L and Wang H: Prenatal food restriction
induces a hypothalamic-pituitary-adrenocortical axis-associated
neuroendocrine metabolic programmed alteration in adult offspring
rats. Arch Med Res. 44:335–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hill RA, Klug M, Kiss Von Soly S, Binder
MD, Hannan AJ and van den Buuse M: Sex-specific disruptions in
spatial memory and anhedonia in a 'two hit' rat model correspond
with alterations in hippocampal brain-derived neurotrophic factor
expression and signaling. Hippocampus. 24:1197–1211. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dwivedi Y, Conley RR, Roberts RC, Tamminga
CA and Pandey GN: [3H] cAMP binding sites and protein kinase a
activity in the prefrontal cortex of suicide victims. Am J
Psychiatry. 159:66–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pandey GN, Dwivedi Y, Ren X, Rizavi HS,
Roberts RC and Conley RR: Cyclic AMP response element-binding
protein in post-mortem brain of teenage suicide victims: Specific
decrease in the prefrontal cortex but not the hippocampus. Int J
Neuropsychopharmacol. 10:621–629. 2007. View Article : Google Scholar
|
|
29
|
Zhang LL, Wang JJ, Liu Y, Lu XB, Kuang Y,
Wan YH, Chen Y, Yan HM, Fei J and Wang ZG: GPR26-deficient mice
display increased anxiety-and depression-like behaviors accompanied
by reduced phosphorylated cyclic AMP responsive element-binding
protein level in central amygdala. Neuroscience. 196:203–214. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sen S, Duman R and Sanacora G: Serum
brain-derived neuro-trophic factor, depression, and antidepressant
medications: Meta-analyses and implications. Biol Psychiatry.
64:527–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murakami S, Imbe H, Morikawa Y, Kubo C and
Senba E: Chronic stress, as well as acute stress, reduces BDNF mRNA
expression in the rat hippocampus but less robustly. Neurosci Res.
53:129–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taliaz D, Stall N, Dar DE and Zangen A:
Knockdown of brain-derived neurotrophic factor in specific brain
sites precipitates behaviors associated with depression and reduces
neurogenesis. Mol Psychiatry. 15:80–92. 2010. View Article : Google Scholar :
|
|
33
|
Sakata K, Jin L and Jha S: Lack of
promoter IV-driven BDNF transcription results in depression-like
behavior. Genes Brain Behav. 9:712–721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pandey GN, Ren X, Rizavi HS, Conley RR,
Roberts RC and Dwivedi Y: Brain-derived neurotrophic factor and
tyrosine kinase B receptor signalling in post-mortem brain of
teenage suicide victims. Int J Neuropsychopharmacol. 11:1047–1061.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thompson Ray M, Weickert CS, Wyatt E and
Webster MJ: Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in
the hippocampus of individuals with schizophrenia and mood
disorders. J Psychiatry Neurosci. 36:195–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Banerjee R, Ghosh AK, Ghosh B,
Bhattacharyya S and Mondal AC: Decreased mRNA and protein
expression of BDNF, NGF, and their receptors in the hippocampus
from suicide: An analysis in human postmortem brain. Clin Med
Insights Pathol. 6:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ray MT, Shannon Weickert C and Webster MJ:
Decreased BDNF and TrkB mRNA expression in multiple cortical areas
of patients with schizophrenia and mood disorders. Transl
Psychiatry. 4:e3892014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Caldwell KK, Sheema S, Paz RD,
Samudio-Ruiz SL, Laughlin MH, Spence NE, Roehlk MJ, Alcon SN and
Allan AM: Fetal alcohol spectrum disorder-associated depression:
Evidence for reductions in the levels of brain-derived neurotrophic
factor in a mouse model. Pharmacol Biochem Behav. 90:614–624. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang G, Chen M, Pan XL, Zheng J and Wang
H: Ethanol-induced inhibition of fetal
hypothalamic-pituitary-adrenal axis due to prenatal overexposure to
maternal glucocorticoid in mice. Exp Toxicol Pathol. 63:607–611.
2011. View Article : Google Scholar
|
|
40
|
Lam VYY, Raineki C, Ellis L, Yu W and
Weinberg J: Interactive effects of prenatal alcohol exposure and
chronic stress in adulthood on anxiety-like behavior and central
stress-related receptor mRNA expression: Sex-and time-dependent
effects. Psychoneuroendocrinology. 97:8–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suri D and Vaidya VA: Glucocorticoid
regulation of brain-derived neurotrophic factor: Relevance to
hippocampal structural and functional plasticity. Neuroscience.
239:196–213. 2013. View Article : Google Scholar
|