Introduction
Bladder cancer (BC) is one of the most commonly
diagnosed urological malignancies and originates from uncontrolled
proliferation of abnormal bladder cells. Based on statistics from
2018, there are nearly 550,000 estimated newly diagnosed BC cases
globally each year and BC causes approximately 200,000 deaths
annually worldwide (1). Based on
pathology, BC can be divided into two major categories, non-muscle
invasive and muscle invasive (2).
Low-grade non-muscle invasive BC is characterized by a weak
invasive ability and is suitable for localized therapy (3). However, disease in patients with
low-grade non-invasive BC progresses rapidly to become muscle
invasive BC (4). Despite advances
in therapeutic strategy the prognosis of patients with BC remains
poor, particularly in those with muscle invasive BC (1). Therefore, unveiling the molecular
mechanism of bladder cancer cells remains pivotal to facilitating
the development of new therapeutic approach for patients with
BC.
Long non-coding RNAs (lncRNAs) are defined as
single-stranded transcripts of more than 200 nucleotides in length
with no protein coding potential (5). Previously thought of as 'junk RNAs',
previous findings have demonstrated that lncRNAs can regulate gene
expression through a variety of mechanisms (6). One well-characterized mechanism of
lncRNA action is that they can serve as competing endogenous RNAs,
blocking miRNA binding sites in order to positively regulate gene
expression (7). Several lncRNAs
have been identified as oncogenes or tumor suppressors in BC. These
lncRNAs act via regulation of the expression of key genes that
control cell proliferation, migration, cell cycle progression and
stemness (8). A high level of
retrotransposon-derived lncRNA PEG10 (PEG10) expression was
observed in BC tissues and experiments based on BC cell lines also
suggested that PEG10 prevented miR-134 from inhibiting Wnt and
JAK/STAT signaling pathways, leading to sustained cell
proliferation and strong metastatic ability (9). A previous RNA sequencing-based
profiling study of non-coding RNAs identified several lncRNAs that
were differentially expressed between healthy and BC tissues
(10). Among them, lncRNA
maternally expressed 3 (MEG3) was indicated to act as a tumor
suppressor or oncogene in BC cells (11,12). Also shown to be differentially
expressed was lncRNA breast cancer anti-estrogen receptor 4
(BCAR4), a recognized oncogene in several cancer types (13,14). However, the role of BCAR4 in BC is
unknown.
The Wnt signaling pathway is involved in BC cell
proliferation, survival, metastasis and maintenance of stemness
(15). Core components of the Wnt
signaling pathway include the extracellular factor Wnt and the
transmembrane receptor β-catenin (16). As a member of the Wnt family, upon
activation, Wnt7a triggers signaling transduction and
dephosphorylation of β-catenin, resulting in the translocation of
β-catenin and the activation of target gene expression (15,17). In cancer cells, Wnt7a has been
suggested to be a target gene of miRNAs such as miR-15b, miR-127
and miR-370-3p (15,18,19). It is unclear how miRNAs and Wnt7a
are involved in BC.
The focus of the present study was to identify the
role of BCAR4 in BC. RT-qPCR indicated an elevation of BCAR4 mRNA
in BC tissues compared with matched healthy tissues. Downregulation
of BCAR4 also inhibited cell proliferation and induced cell
apoptosis in two BC cell lines. Mechanistically, the downregulation
of BCAR4 led to inactivation of Wnt signaling via prevention of the
binding of tumor suppressor miR-370-3p and subsequent elevation of
Wnt7a expression. There was also a strong association between
BCAR4, miR-370-3p and Wnt7a expression in tumors from patients with
BC. Taken together, these data identified a BCAR4/miR-370-3p/Wnt7a
axis in the promotion of the proliferation and survival of BC
cells. Therefore, lncRNA BCAR4 is an lncRNA with oncogenic
potential in BC.
Materials and methods
Collection of normal and tumor
tissues
Tumor tissues and paired healthy tissues were
collected from 40 patients diagnosed with BC at The First Hospital
of Jilin University (Jilin, China) between January 2016 and May
2017 (patients were aged 40-75 years, with a median age of 61
years). The tumor tissues were histopathologically examined after
surgical removal. None of the patients had received chemotherapy or
radiotherapy prior to surgery. Written informed consent was
obtained from all the participants. All the experiments were
performed under the supervision of the Ethics Committee of The
First Hospital of Jilin University. Tissues were stored at −80°C
immediately after harvesting before RNA extraction.
Cell culture
Human BC cell lines 5637, T24 and SW780 and human
immortalized ureter epithelial cell line SV-HUC-1 were purchased
from the American Type Culture Collection (Manassas) and used
within 6 months. These cell lines were cultured in RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) in an incubator with 5%
CO2 at 37°C.
Silencing of BCAR4
BCAR4 siRNA (5′-GCU GCG AGG GUA GAC AUC UCU GUU
U-3′) and control siRNA (5′-UAA GGC UAU GAA GAG AUA C-3′) were
synthesized by and purchased from GenePharma Co. Ltd. The silencing
of BCAR4 was achieved by the transfection of BCAR4 siRNA into 5637
and T24 cells using Lipofectamine RNAiMax (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 200 pM BCAR4 siRNA was incubated with 9 µl
Lipofectamine RNAiMax in 500 µl Opti-MEM (Invitrogen; Thermo
Fisher Scientific) for 5 mins. This mixture was then added into
each well of 6-well plates seeded with cells. After 72 h, the cells
were harvested for the subsequent experiments.
Cell viability assay
The viability of 5637 and T24 cells were determined
using a cell counting kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc.) following the manufacturer's protocol. The
cells were seeded at a density of 1×105 cells per well
in each well of a 96-well plate. The following day, the cells were
transfected with BRCA4 siRNA. CCK-8 solution was added after 0, 24,
48 or 72 h. CCK-8 solution (10 µl) was added into each well
and the cells were incubated for a further 2 h. The medium
containing the CCK-8 solution was then moved to another 96-well
plate and the absorbance at 450 nM was measured by iMark Microplate
Reader (Bio-Rad) to determine cell number.
RNA extraction and reverse
transcription-quantitative PCR
Total RNA was extracted from tissues and cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's protocol. The isolated RNA was
quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Inc.).
RNA was reverse transcribed to DNA using PrimeScript™ RT Master Mix
(Takara Bio., Inc.) following the manufacturer's protocol. The qPCR
was performed with TB Green® Premix Ex Taq™ II (Takara
Bio., Inc.) on a CFX-96 RT-qPCR System (Bio-Rad Technologies,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 95°C for 30 sec followed by 40 cycles of 95°C for 5
sec and 60°C for 30 sec. U6 and β-actin were used as internal
controls for miRNA and lncRNA/mRNA respectively. The relative
expression of genes was calculated with the 2−ΔΔCq
method (20). The BCAR4 primers
used were: Forward: 5′-ACA GCA GCT TGT TGC TCA TCT-3′; reverse:
5′-TTG CCT TGG GGA CAG TTC AC-3′. The Wnt7a primers used were:
forward: 5′-CTC CGG ATC GGT GGC T-3′; reverse: 5′-CCC ATT TGT GAG
CCT TCT CCT-3′. The β-actin primers used were: Forward: 5′-TCT GGC
TGA GGC TGG TTG AC-3′; reverse: 5′-CTC CTT AAT GTC ACG CAC GAT-3′.
The Stemloop primer used was: 5′-CTC AAC TGG TGT CGT GGA GTC GGC
AAT TCA GTT GAG ACC AGG TT-3′. The miR-370-3p primers used were:
Forward: 5′-ACA CTC CAG CTG GGG CCT GCT GGG GTG GAA-3′; reverse:
5′-CTC AAC TGG TGT CGT GGA-3′. The U6 primers used were: Forward:
5′-CTC GCT TCG GCA GCA CA-3′; reverse: 5′-CTC GCT TCG GCA GCA
CA-3′.
Flow cytometric analysis
The apoptotic cells were detected by flow cytometry
with a Dead Cell Apoptosis kit with Annexin V Alexa Fluor™ 488 and
Propidium Iodide (PI) kit (Invitrogen; Thermo Fisher Scientific)
following manufacturer's protocol. Apoptotic cells were Annexin V
positive with or without PI positive.
Protein extraction and western
blotting
Antibodies against Wnt7a (rabbit polyclonal
antibody, ab100792, 1:2,000), E-cadherin (mouse monoclonal
antibody, ab1416, 1:2,000), Vimentin (mouse monoclonal antibody,
ab8978, 1:1,000) and β-actin (mouse monoclonal antibody, ab8224,
1:5,000) were purchased from Abcam. Phospho-β-catenin (rabbit
polyclonal antibody, no. 2009, 1:2,000) antibody was purchased from
Cell Signaling Technology. Secondary antibodies against mouse
(A3682, 1:50,000) and rabbit (A8275, 1:50,000) were obtained from
Sigma-Aldrich. Proteins were extracted from cells using RIPA lysis
buffer (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The protein concentration was determined
with a Pierce BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.). Lysate containing 20 µg proteins was loaded into each
lane of an 8% SDS-PAGE gel (GenScript). After electrophoresis,
proteins on the gel were transferred to a PVDF membrane. The
membrane was blocked with 5% non-fat milk for 1 h at room
temperature. The membrane was then incubated with primary
antibodies overnight at 4°C. On the following day the membrane was
incubated with secondary antibodies for 1 h at room temperature.
The blots were developed using SuperSignal Western Blot Enhancer
(Thermo Fisher Scientific, Inc.). The relative protein expression
was determined with Image J software V 1.8.0 (National Institutes
of Health) and β-actin served as the internal control.
Dual luciferase reporter assay
The BCAR4 sequences were amplified from the cDNA of
T24 cells and ligated into a pGL3-basic luciferase reporter plasmid
(Promega Corporation). Primer sequences used were: BCAR4-forward:
5′-CTC TAGA AGT TAG TGC TGG GAA ACAG-3′; reverse: 5′-CTC TAG ACA
GAT TTT ATT TCTA TTTA-3′. Three site mutations were then introduced
into the pGL3-BCAR4-wild type (WT) plasmid at the putative binding
sites to construct pGL3-BCAR4-mutant (Mut). Then, 2 µg
plasmids were transfected into cells using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). The luciferase
activity was analyzed using the Dual-Glo Luciferase Reporter Assay
kit (Promega Corporation) according to the manufacturer's protocol.
The relative luciferase activity was determined 48 h after
transfection. The Firefly luciferase was normalized to
Renilla luciferase.
Overexpression of Wnt7a
The full length of the Wnt7a open reading frame was
amplified from T24 cDNA and ligated into pcDNA3.1 (Shaanxi Yuanbang
Biotech., Co., Ltd.). Primer sequences used were: Wnt7a-forward:
5′-CAA GCT TAT GAA CCG GAA AGC GCG GC-3′; reverse: 5′-GGA ATT CTC
AGT TGC ACG TGT ACA TC-3′. For overexpression of Wnt7a, 2 µg
pcDNA3.1-Wnt7a or pcDNA3.1 plasmids were transfected into cells
with Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.). The cells were harvested 72 h later for subsequent
experiments.
Bioinformatics analysis
The expression of BCAR4 in bladder tumors and normal
tissues was analyzed on Starbase V3 (http://starbase.sysu.edu.cn/). The potential binding
sites of miRNAs to BCAR4 were predicted in miRDB (http://mirdb.org/).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6.0 software (GraphPad Software, Inc.) and are presented as
the mean ± SD. All the experiments were repeated three times.
Correlations were analyzed using Pearson's correlation analysis.
For comparisons, two-tailed Student's t-tests were performed where
there were two groups and one-way ANOVA was applied to three groups
followed by Newman Keuls post-hoc test. P<0.05 was considered to
be statistically significant.
Results
High expression levels of BCAR4 in
BC
Previous lncRNA expression profiling identified that
lncRNA-BCAR4 was one of several significantly upregulated lncRNAs
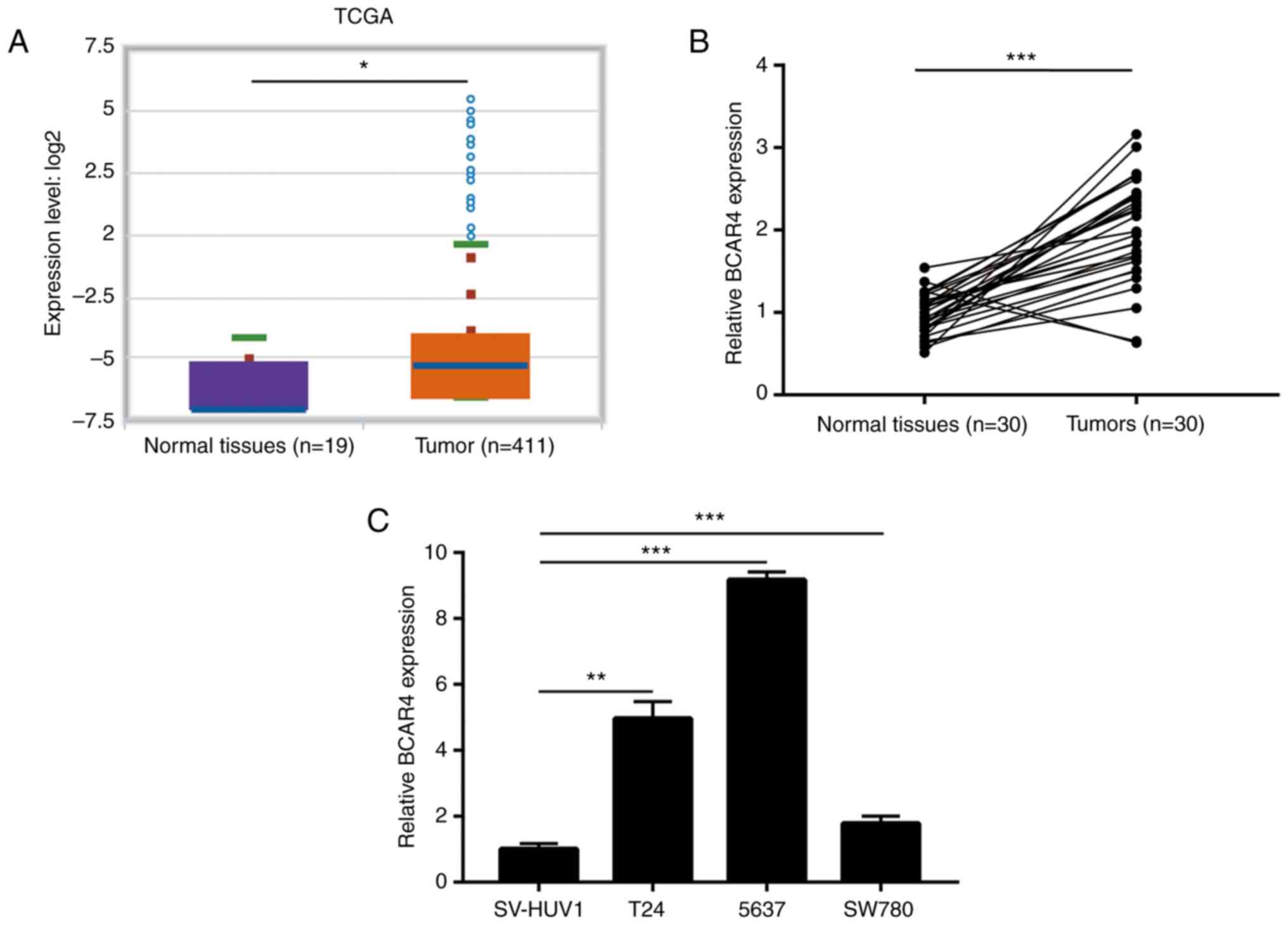
in bladder tumors, when compared with normal tissues (10). To confirm this observation,
StarBase V3 was used to compare the expression of BCAR4 in bladder
tumors and normal tissues using data derived from The Cancer Genome
Atlas. The result suggested that BCAR4 was overexpressed in 411
bladder tumors compared with 19 healthy tissues (Fig. 1A). For validation, 30 pairs of
tumors and matched normal tissues from patients with BC were
collected. RT-qPCR data showed that BCAR4 was increased in the
majority of patients with BC (Fig.
1B). In addition, in a panel of BC cell lines (T24, 5637 and
SW780), the expression of BCAR4 was significantly increased
compared to the immortalized bladder cell line SV-HUV1 (Fig. 1C). These data indicated that BCAR4
was highly expressed in BC.
Knockdown of BCAR4 inhibits cell
proliferation and induces cell apoptosis in BC cells
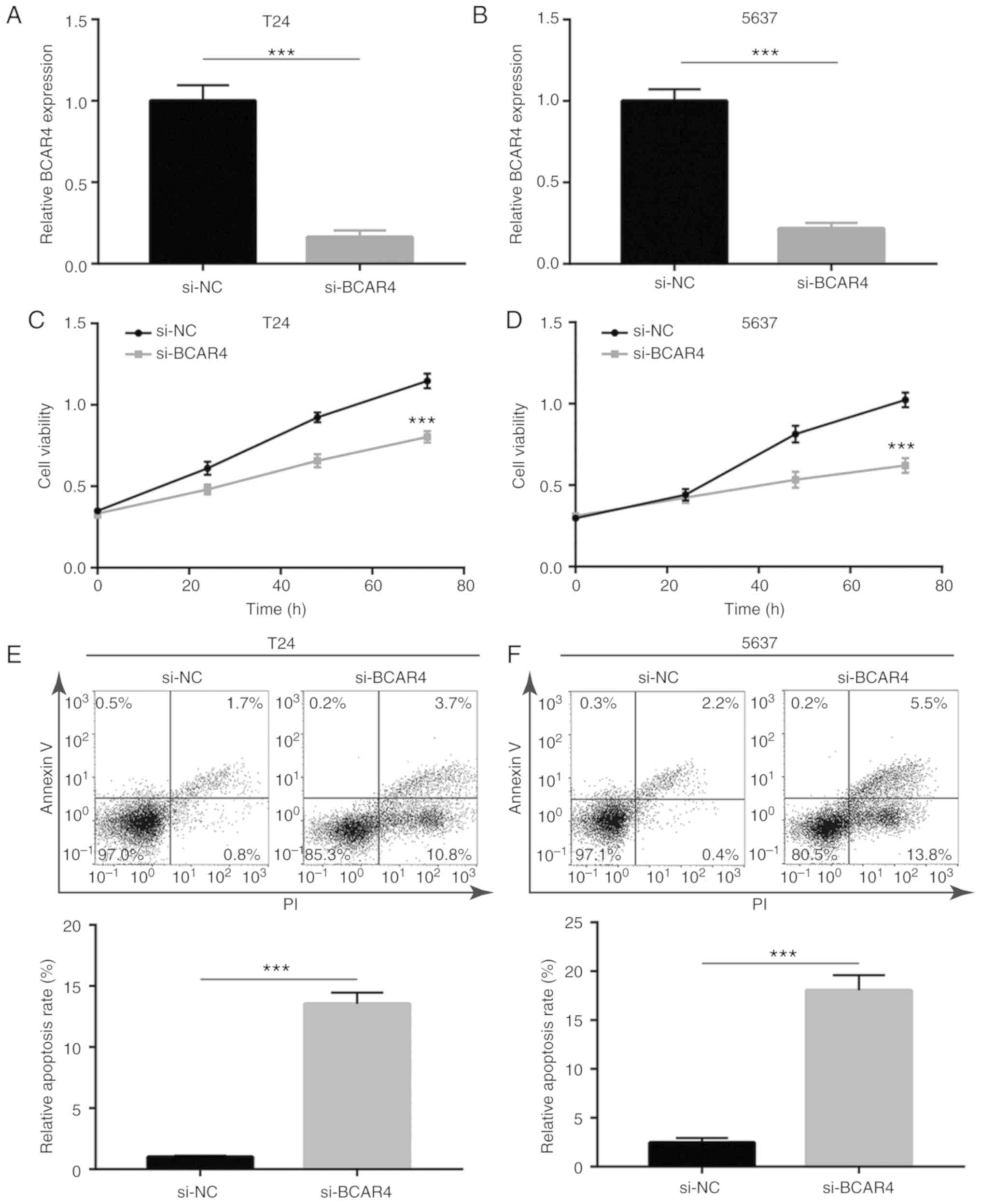
BCAR4 siRNA was transfected into BC cell lines to
investigate the activity of BCAR4 in BC cells. Transfection of
BCAR4 siRNA decreased BCAR4 expression in both T24 and 5637 cells
(Fig. 2A and B). Knockdown of
BCAR4 resulted in a reduction in cell viability in T24 cells
(Fig. 2C). Similarly, the cell
viability of 5637 cells was also greatly inhibited after BCAR4
knockdown (Fig. 2D). To clarify
whether the reduction in cell viability caused by BCAR4 knockdown
was associated with cell death, flow cytometric analysis was used
to determine the cell apoptotic rate in T24 cells transfected with
BCAR4 siRNA. A significant elevation of the percentage of cells in
early [PI (propidium iodide)+/
AnnexinV-FITC−] and late
(PI+/AnnexinV-FITC+) apoptosis was observed
in BCAR4-silenced T24 cells (Fig.
2E). Similar to T24 cells, BCAR4 knockdown also induced cell
apoptosis in 5637 cells (Fig.
2F). The data suggested that BCAR4 may promote BC cell
proliferation and survival.
Silencing of BCAR4 inactivates
Wnt/β-catenin signaling in BC cells
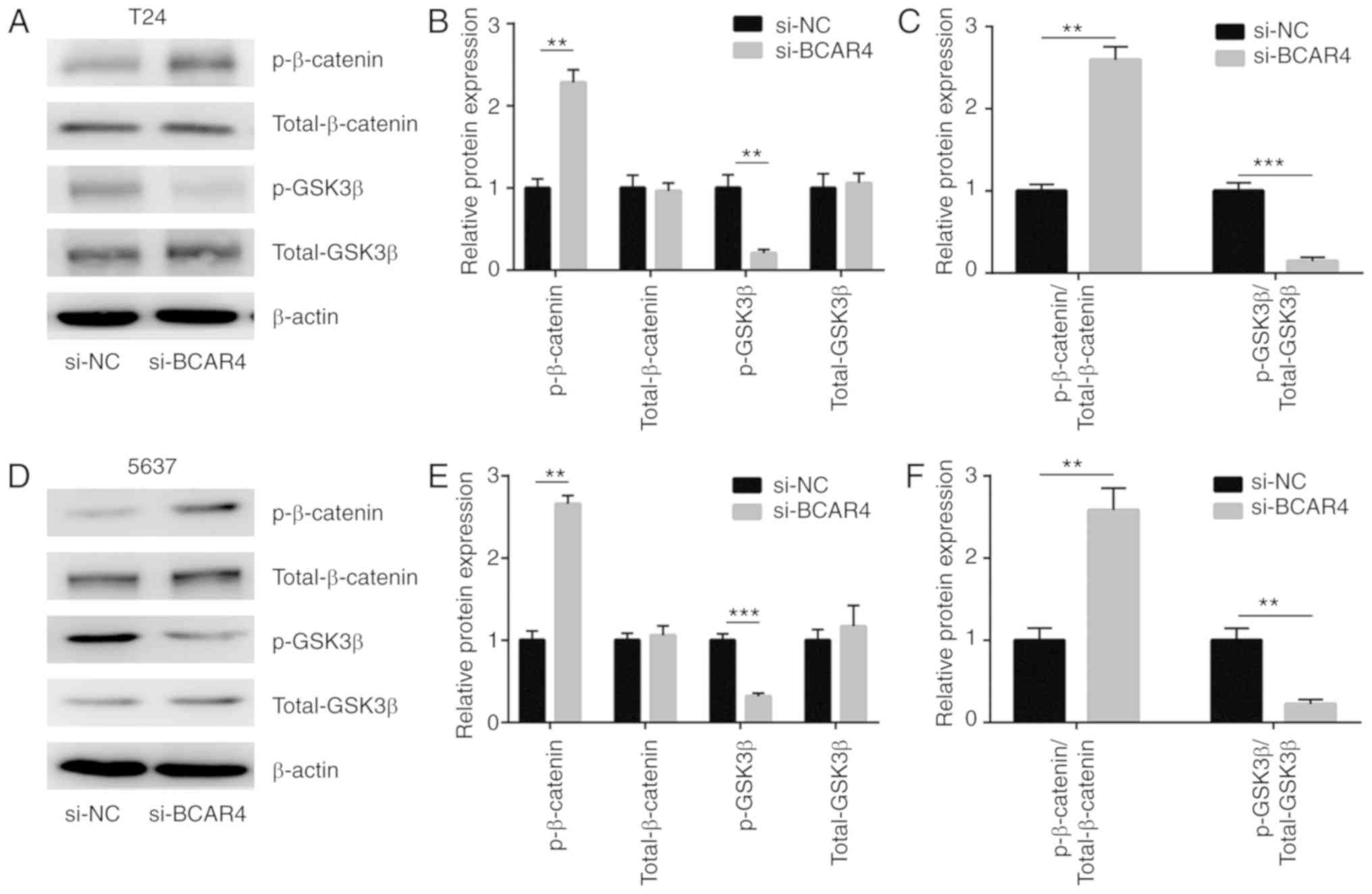
Wnt/β-catenin signaling is a well-characterized
oncogenic pathway in BC. Sustained activation of Wnt/β-catenin
signaling is critical for the survival of BC cells. To determine
whether Wnt/β-catenin signaling was involved in BCAR4-mediated BC
cell survival, the expression levels of the inactive form of
β-catenin [phosphorylated (p)-β-catenin] and the active form of
GSK3β (p-GSK3β) were assessed in cells transfected with BCAR4
siRNA. Western blot analysis revealed that p-β-catenin levels were
increased and p-GSK3β levels were decreased in T24 cells after
BCAR4 silencing (Fig. 3A and B).
The ratio of p-β-catenin to total β-catenin was increased, while
the ratio of p-GSK3β to GSK3β was decreased, suggesting the
shutdown of Wnt/β-catenin signaling (Fig. 3C). As in T24 cells, the level of
p-β-catenin was increased and the p-GSK3β level was decreased in
5637 cells after BCAR4 silencing (Fig. 3D and E). Wnt/β-catenin signaling
was inactivated after BCAR4 silencing, as the ratio of p-β-catenin
to total β-catenin was increased and the ratio of p-GSK3β to GSK3β
was decreased (Fig. 3F). BCAR4
positively regulated Wnt7a expression via the repression of
miR-370-3p in BC cells.
lncRNAs regulate gene expression via
sponging miRNAs in cancer cells
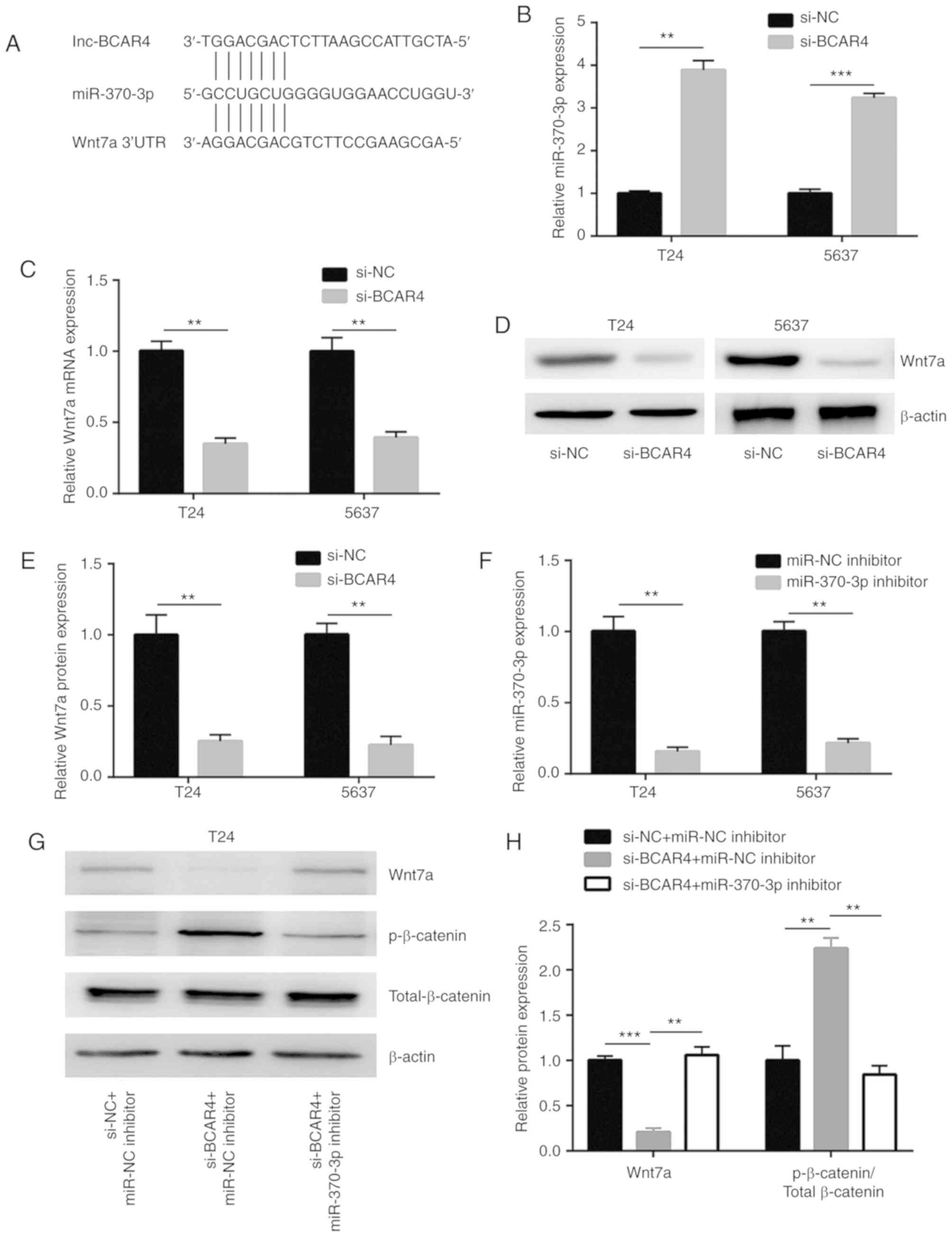
The potential binding sites of miRNAs to BCAR4 were
predicted in miRDB. There were 22 miRNAs predicted to have binding
sites on BCAR4. Through searching the literature, miR-370-3p was
identified as a tumor suppressor in BC via targeting Wnt7a, a
positive regulator of Wnt/β-catenin signaling (Fig. 4A). Silencing of BCAR4 led to a
significant upregulation of the miR-370-3p level in T24 and 5637
cells (Fig. 4B). In addition, the
downregulation of BCAR4 decreased the Wnt7a mRNA level in these
cells (Fig. 4C). A reduction in
Wnt7a protein expression was also found in T24 and 5637 cells after
BCAR4 silencing (Fig. 4D and E).
To explore whether BCAR4 regulated Wnt7a and downstream targets via
control of miR-370-3p expression, miR-370-3p inhibitor was
transfected into T24 and 5637 cells to downregulate miR-370-3p
expression (Fig. 4F). As
hypothesized, the downregulation of miR-370-3p reversed the
reduction in Wnt7a protein expression observed in T24 cells treated
with BCAR4 siRNA (Fig. 4G and H).
In addition, upregulation of p-β-catenin/Total β-catenin induced by
BCAR4 silencing was also reversed following miR-370-3p inhibition
(Fig. 4G and H). As observed in
T24 cells, the downregulation of miR-370-3p also reversed reduction
of the Wnt7a protein expression and upregulation of
p-β-catenin/Total β-catenin in 5637 cells treated with BCAR4 siRNA
(Fig. 4I and J).
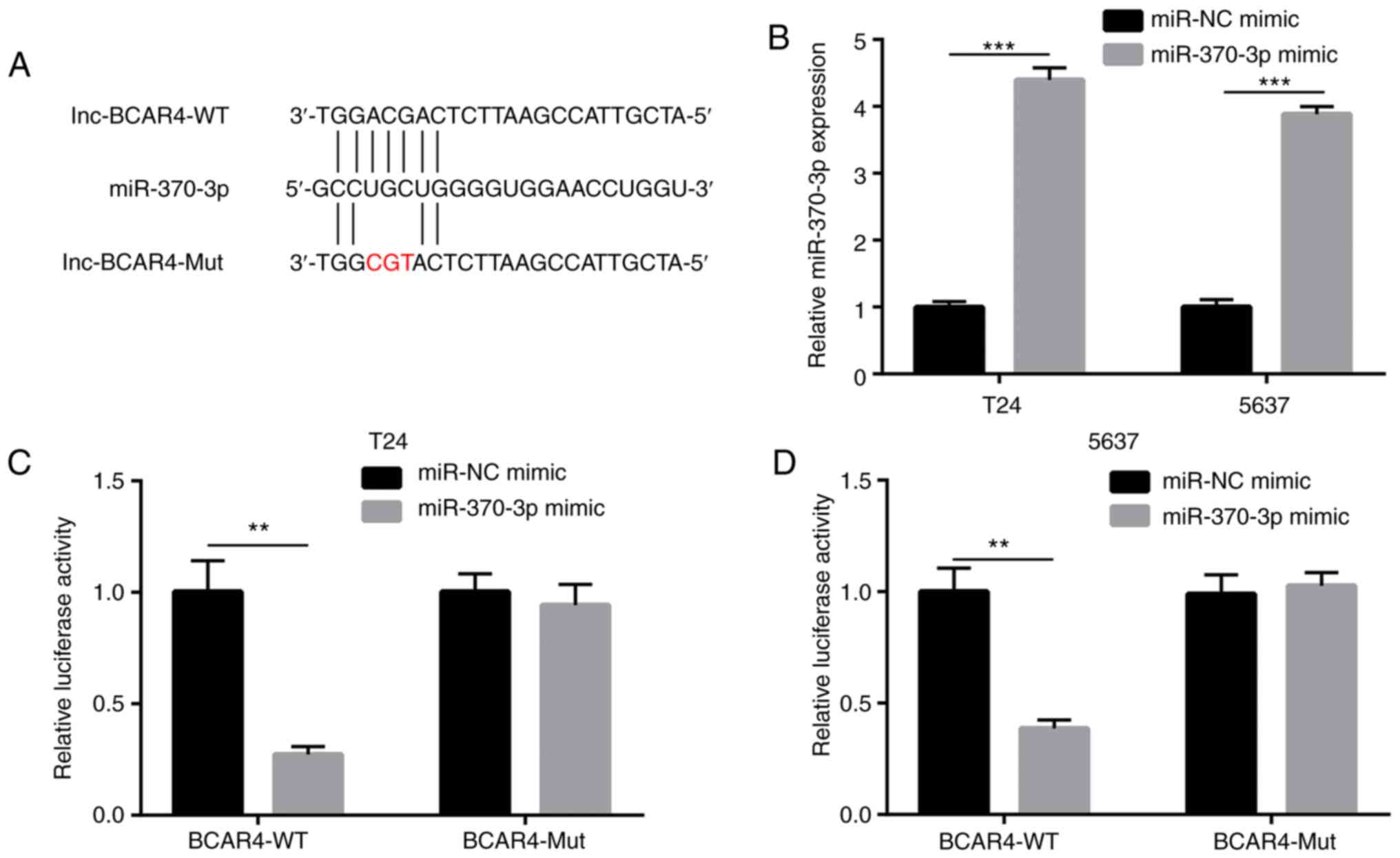
BCAR4 represses miR-370-3p activity in BC
cells
To determine whether BCAR4 directly regulates
miR-370-3p expression, luciferase plasmids containing BCAR4-WT
BCAR4-Mut were constructed with a mutation at the putative binding
sites of miR-370-3p (Fig. 5A).
miR-370-3p mimic was transfected into T24 and 5637 cells to
overexpress miR-370-3p (Fig. 5B).
Overexpression of miR-370-3p reduced relative luciferase activity
of T24 cells with BCAR4-WT, but not BCAR4-Mut (Fig. 5C). Similarly, miR-370-3p mimic
also reduced relative luciferase activity of 5637 cells with
BCAR4-WT, but not BCAR4-Mut (Fig.
5D). Collectively, the data demonstrated that BCAR4 directly
repressed miR-370-3p expression via the prevention of miR-370-3p
binding in BC cells.
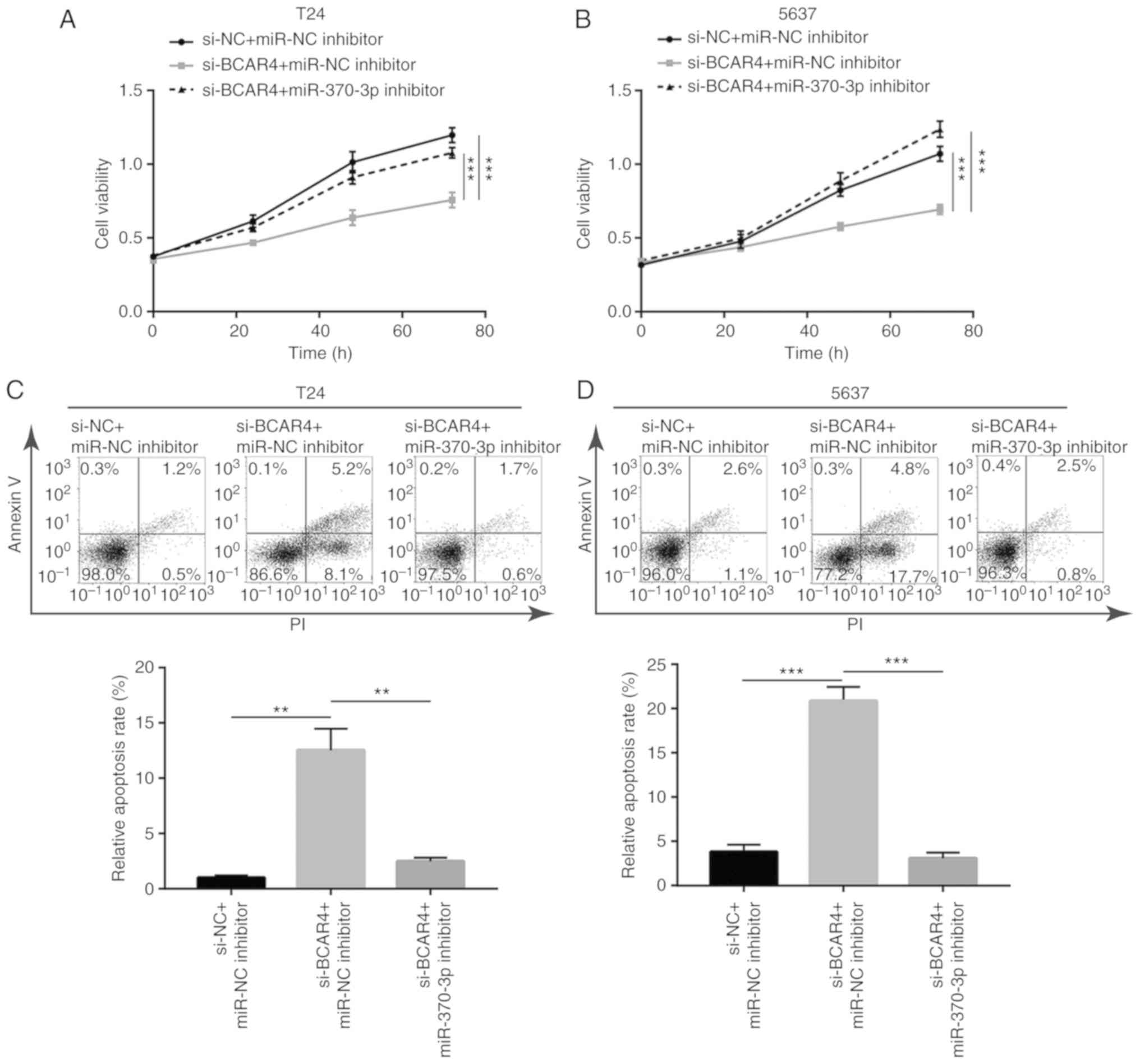
BCAR4 controls cell proliferation and
survival via sponging miR-370-3p in BC cells
To examine the role of miR-370-3p in the function of
BCAR4 in BC, T24 cells were transfected with BCAR4 siRNA with or
without miR-370-3p inhibitor. The inhibitory effect of BCAR4
silencing on cell viability was reversed after miR-370-3p
inhibition in T24 cells (Fig.
6A). Additionally, the cell viability reduction led by BCAR4
silencing was reversed following miR-370-3p inhibition in 5637
cells (Fig. 6B). Additionally,
the cell apoptosis induced by BCAR4 silencing was reversed after
miR-370-3p inhibition in T24 cells (Fig. 6C), which was also observed in 5637
cells (Fig. 6D).
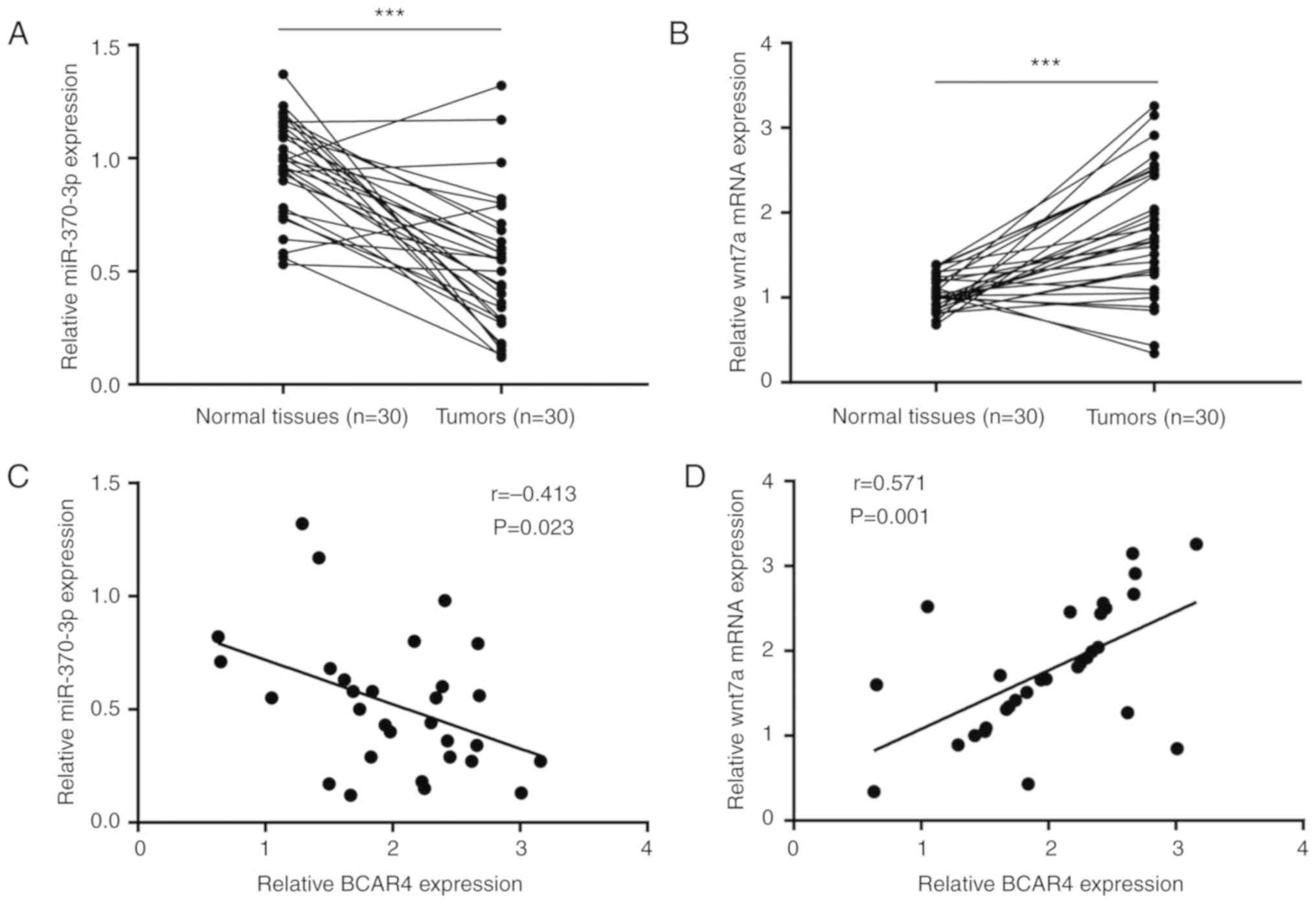
BCAR4 expression is associated with
miR-370-3p and Wnt7a expression in bladder tumors
The clinical association between BCAR4, miR-370-3p
and Wnt7a was then examined. RT-qPCR results showed that miR-370-3p
was reduced in bladder tumors compared with matched healthy tissues
(Fig. 7A). By contrast, Wnt7a
mRNA expression was significantly increased in bladder tumors
(Fig. 7B). Pearson's correlation
analysis suggested that BCAR4 expression negatively correlated with
miR-370-3p expression (Fig. 7C)
and positively correlated with Wnt7a mRNA expression (Fig. 7D).
Discussion
lncRNAs have been demonstrated to act as oncogenes
or tumor suppressors in BC and have been indicated to control cell
proliferation, migration, cell cycle progression and stemness
(8). lncRNA BCAR4 has been
identified to be an oncogene in numerous types of cancer. BCAR4 was
shown to induce proliferation, invasion and metastasis of non-small
cell lung cancer cells by regulating epithelial-mesenchymal
transition (13). Additionally,
elevation of BCAR4 was demonstrated to induce the proliferation and
migration of cervical cancer cells (14). BCAR4 was found to induce cisplatin
resistance and predicts poor prognosis in patients with gastric
cancer via activating Wnt/β-catenin signaling pathway (21). BCAR4 also promoted the progression
of colon cancer through activation of the Wnt/β-catenin signaling
pathway as well as castration resistant prostate cancer by
activation of GLI2 signaling (22,23). Nevertheless, its function in BC is
unknown.
The present study identified an elevation of BCAR4
in BC compared with matched healthy tissues. Thus, the data also
supported elevation of BCAR4 in various types of cancer as observed
in non-small cell lung cancer, cervical cancer and gastric cancer
(13,14,21). In two BC cell lines, our data
showed that downregulation of BCAR4 inhibited cell proliferation,
which was also reported in several cancer types such as glioma and
non-small cell lung cancer (24,25). In addition, downregulation of
BCAR4 induced cell apoptosis in BC cells. The involvement of BCAR4
in cell apoptosis was previously identified in glioma and non-small
cell lung cancer (13,25). These findings suggested the
pivotal role of BCAR4 in the progression of BC.
There is a regulatory relationship between lncRNAs
and miRNAs (26). lncRNAs
regulate gene methylation, transcription/translation and other
processes by conjugation with mRNAs and miRNAs (27). miRNAs are small non-coding RNAs
(20-22 nucleotides in length), which target the 3′-UTR of target
mRNAs by sequence complementarity (28). miRNAs function as oncogenes and
tumor suppressors in carcinogenesis (29). miRNAs are also involved in the
apoptosis, proliferation, and metastasis of cancer cells (30). BCAR4 was reported to sponge
miR-665 to upregulate expression of STAT3 in colorectal cancer
cells (31). Thus, whether
miRNAs/ mRNAs could be regulated by BCAR4 in BC has become a topic
of interest.
BCAR4 regulates the activation of the Wnt/β-catenin
signaling pathway in multiple cancer types; for instance, BCAR4
promotes the progression of colon cancer through activation of the
Wnt/β-catenin signaling pathway (22). BCAR4 is elevated in osteosarcoma
and promotes the development of osteosarcoma through the
Wnt/β-catenin signaling pathway (32). In addition, elevation of BCAR4 in
gastric cancer induced the expression of tumor stem cell-related
biomarkers through the Wnt signaling pathway (21). However, although those studies
only mentioned that BCAR4 controls the activity of the
Wnt/β-catenin signaling pathway in cancer cells, the molecular
mechanism by which BCAR4 regulated Wnt/β-catenin signaling pathway
was not previously reported. As a member of the Wnt family, Wnt7a,
which regulates the dephosphorylation and translocation of
β-catenin, is targeted by miR-370-3p to promote BC cell
proliferation, survival, metastasis and maintenance of stemness
(15). Furthermore, an increasing
number of studies have shown that miR-370 is involved in the
progression of BC. miR-370 is expressed at reduced levels in BC
clinical specimens when compared with controls and miR-370
activates p21 expression by targeting the p21 promoter, thereby
suppressing the proliferation and metastasis of human BC cells
(33-35). miR-370 also inhibits cell growth
of BC by targeting DNA replication complex GINS protein SLD5
(36). By contrast, it is unclear
whether BCAR4 regulates miR-370-3p and Wnt7a expression in BC.
Bioinformatic analysis revealed miR-370-3p as a potential target
miRNA of BCAR4, which was further validated by RT-qPCR and dual
luciferase reporter assay. Downregulation of miR-370-3p attenuated
BCAR4 siRNA-mediated cell proliferation inhibition and cell
apoptosis in BC cells. These data indicated that a high expression
of miR-370-3p was due to overexpression of BCAR4 in BC. Moreover,
via sponging miR-370-3p, BCAR4 upregulated Wnt7a and activated Wnt
signaling. Thus, our results indicated that BCAR4 activated Wnt
signaling by directly sponging miR-370-3p and elevated Wnt7a
expression in BC cells. Furthermore, there was a strong association
between BCAR4, miR-370-3p and Wnt7a expression in tumors from
patients with BC, indicating a BCAR4/ miR-370-3p/Wnt7a axis in BC
tumors.
In summary, the results of the present study
indicate that the BCAR4/miR-370-3p/Wnt7a axis has a role in
promoting the proliferation and survival of BC cells. Therefore,
lncRNA BCAR4 was a lncRNA with oncogenic potential in BC.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of materials and data
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CC conceived and designed the study. CC, RZ, JW and
EJ performed the experiments. JZ, NL and RZ analyzed the data, and
wrote the manuscript, CC revised the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
This current study was approved by the Ethics
Committee of The First Hospital of Jilin University. Written
informed consent was obtained from all the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Bohle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar
|
|
3
|
Anastasiadis A and de Reijke TM: Best
practice in the treatment of nonmuscle invasive bladder cancer.
Ther Adv Urol. 4:13–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah JB, McConkey DJ and Dinney CP: New
strategies in muscle-invasive bladder cancer: On the road to
personalized medicine. Clin Cancer Res. 17:2608–2612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarroux J, Morillon A and Pinskaya M:
History, discovery, and classification of lncRNAs. Adv Exp Med
Biol. 1008:1–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adelman K and Egan E: Non-coding RNA: More
uses for genomic junk. Nature. 543:183–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martens-Uzunova ES, Bottcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang F, Qi W, Wang Y, Wang W and Fan L:
lncRNA PEG10 promotes cell survival, invasion and migration by
sponging miR-134 in human bladder cancer. Biomed Pharmacother.
114:1088142019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seitz AK, Christensen LL, Christensen E,
Faarkrog K, Ostenfeld MS, Hedegaard J, Nordentoft I, Nielsen MM,
Palmfeldt J, Thomson M, et al: Profiling of long non-coding RNAs
identifies LINC00958 and LINC01296 as candidate oncogenes in
bladder cancer. Sci Rep. 7:3952017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng SQ, Zhang XY, Fan HT, Sun QJ and
Zhang M: Up-regulation of LncRNA MEG3 inhibits cell migration and
invasion and enhances cisplatin chemosensitivity in bladder cancer
cells. Neoplasma. 65:925–932. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan J, Li X, Wu W, Xue M, Hou H, Zhai W
and Chen W: Long non-coding RNA UCA1 promotes cisplatin/gemcitabine
resistance through CREB modulating miR-196a-5p in bladder cancer
cells. Cancer Lett. 382:64–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N, Gao WJ and Liu NS: LncRNA BCAR4
promotes proliferation, invasion and metastasis of non-small cell
lung cancer cells by affecting epithelial-mesenchymal transition.
Eur Rev Med Pharmacol Sci. 21:2075–2086. 2017.PubMed/NCBI
|
|
14
|
Zou R, Chen X, Jin X, Li S, Ou R, Xue J,
Yan X, Chen L, Hu Y and Zhu H: Up-regulated BCAR4 contributes to
proliferation and migration of cervical cancer cells. Surg Oncol.
27:306–313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang X, Zhu H, Gao Z, Li J, Zhuang J,
Dong Y, Shen B, Li M, Zhou H, Guo H, et al: Wnt7a activates
canonical Wnt signaling, promotes bladder cancer cell invasion, and
is suppressed by miR-370-3p. J Biol Chem. 293:6693–6706. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacLean JA II, King ML, Okuda H and
Hayashi K: WNT7A Regulation by miR-15b in ovarian cancer. PLoS One.
11:e01561092016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Wang X and Jiang X: miR-127
suppresses gastric cancer cell migration and invasion via targeting
Wnt7a. Oncol Lett. 17:3219–3226. 2019.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Wang L, Chunyan Q, Zhou Y, He Q, Ma Y, Ga
Y and Wang X: BCAR4 increase cisplatin resistance and predicted
poor survival in gastric cancer patients. Eur Rev Med Pharmacol
Sci. 21:4064–4070. 2017.PubMed/NCBI
|
|
22
|
Ouyang S, Zheng X, Zhou X, Chen Z, Yang X
and Xie M: LncRNA BCAR4 promotes colon cancer progression via
activating Wnt/β-catenin signaling. Oncotarget. 8:92815–92826.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai Z, Wu Y, Li Y, Ren J and Wang L: BCAR4
activates GLI2 signaling in prostate cancer to contribute to
castration resistance. Aging (Albany NY). 10:3702–3712. 2018.
View Article : Google Scholar
|
|
24
|
Wei L, Yi Z, Guo K and Long X: Long
noncoding RNA BCAR4 promotes glioma cell proliferation via
EGFR/PI3K/AKT signaling pathway. J Cell Physiol. 234:23608–23617.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang H, Yan L, Sun K, Sun X, Zhang X, Cai
K and Song T: lncRNA BCAR4 increases viability, invasion, and
migration of non-small cell lung cancer cells by targeting
glioma-associated oncogene 2 (GLI2). Oncol Res. 27:359–369. 2019.
View Article : Google Scholar
|
|
26
|
Cao MX, Jiang YP, Tang YL and Liang XH:
The crosstalk between lncRNA and microRNA in cancer metastasis:
Orchestrating the epithelial-mesenchymal plasticity. Oncotarget.
8:12472–12483. 2017.
|
|
27
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JQ, Papp G, Szodoray P and Zeher M:
The role of microRNAs in the pathogenesis of autoimmune diseases.
Autoimmun Rev. 15:1171–1180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lawrie CH: MicroRNAs and haematology:
Small molecules, big function. Br J Haematol. 137:503–512. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ouyang S, Zhou X, Chen Z, Wang M, Zheng X
and Xie M: LncRNA BCAR4, targeting to miR-665/STAT3 signaling,
maintains cancer stem cells stemness and promotes tumorigenicity in
colorectal cancer. Cancer Cell Int. 19:722019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Dou P, Liu T and He S: Application
of long noncoding RNAs in osteosarcoma: Biomarkers and therapeutic
targets. Cell Physiol Biochem. 42:1407–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshino H, Chiyomaru T, Enokida H,
Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N and Nakagawa
M: The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C, Chen Z, Ge Q, Hu J, Li F, Hu J, Xu
H, Ye Z and Li LC: Up-regulation of p21(WAF1/CIP1) by miRNAs and
its implications in bladder cancer cells. FEBS Lett. 588:4654–4664.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Ge Q, Chen Z, Hu J, Li F and Ye Z:
Promoter-associated endogenous and exogenous small RNAs suppress
human bladder cancer cell metastasis by activating p21 (CIP1/WAF1)
expression. Tumour Biol. 37:6589–6598. 2016. View Article : Google Scholar
|
|
36
|
Yamane K, Naito H, Wakabayashi T, Yoshida
H, Muramatsu F, Iba T, Kidoya H and Takakura N: Regulation of SLD5
gene expression by miR-370 during acute growth of cancer cells. Sci
Rep. 6:309412016. View Article : Google Scholar : PubMed/NCBI
|