Introduction
Bone marrow-derived autologous stem cell therapy has
been proposed as a promising treatment for a variety of incurable
degenerative diseases such as graft versus host disease (1), spinal cord injury (2), myocardial infarction (3), autoimmune disease and osteoarthritis
(4,5). Currently, stem cell therapy is
mainly conducted in one of two ways. One approach is the use of
minimally manipulated cells from bone marrow aspirates (BMAs), in
which mononuclear cells (MNCs) are isolated by Ficoll-gradient
centrifugation and infused without in vitro expansion. BMAs
are used immediately after cell isolation from patients and bypass
the very expensive and elaborate cell culture methods. The other
approach is local or systemic transplant of ex vivo
long-term cultured stem cells, which have the homogenous cell types
including mesenchymal stem cells or endothelial progenitor cells
and show efficacy in specific diseases.
To date, numerous clinical trials using stem cells
have been attempted and the therapeutic effect of stem cell therapy
has been demonstrated (6,7). However, when considering that older
patients are primarily subjected to stem cell therapy, an
insufficient number of stem cells in these patients and the poor
viability of transplanted stem cells in vivo have been major
obstacles in the progression of stem cell therapy to successful
therapeutic outcomes (8,9).
In order to enhance stem cell activity and survival
in the clinical setting, the preconditioning strategies of
minimally manipulated cells from BMA or ex vivo cultured
stem cells from older patients should be accomplished. Currently,
the preconditioning of stem cells has been attempted through gene
modification, physical training such as cell straining, or
treatment with biological/chemical factors, which is expected to
contribute to improving the efficacy of stem cells in
vivo.
The transcranial application of repetitive
electromagnetic stimulation (rEMS) is a non-invasive technique that
repeatedly introduces electromagnetic currents in order to
stimulate a small area of the brain. Previously, rEMS has been
utilized in experimental neurophysiology to explore its therapeutic
implications (10) and has been
clinically applied to treat several psychiatric and neurological
diseases such as depression, stroke, Parkinson's disease, tinnitus,
epilepsy, and pain (11-16). Despite the wide clinical use of
rEMS, its effect on stem cells has not yet been fully investigated,
with only a few studies reporting the release of second messenger
production or cell signaling induced by rEMS (17-19). rEMS was found to increase the
affinity of brain-derived neurotrophic factors for tyrosine
receptor kinase B (TrkB), which promoted recruitment of
phospholipase Cγ1 and Shc/N-Shc to phosphorylated TrkB. This
activated downstream extracellular signal-regulated kinase 2 (Erk2)
and phosphoinositide 3-kinase in the prefrontal cortex and in
lymphocytes (17). An extremely
low frequency electromagnetic stimulus was able to activate
phosphorylated (p)-ERK and p-SAPK/JNK pathways in human melanocytes
(19). rEMS have been shown to
activate the cyclic adenosine monophosphate (cAMP)/cAMP response
element-binding protein (CREB) pathway in human-derived SH-SY5Y
neuroblastoma cells by generating cAMP and the subsequent
phosphorylation of CREB (18).
Diniz et al (20) revealed
that electromagnetic field stimulates osteoblast proliferation and
differentiation through nitric oxide. These studies described the
molecular action of electromagnetic stimulation, suggesting the
potential of electromagnetic preconditioning as a treatment.
The approach of the present study is based on the
hypothesis that transient rEMS preconditioning of adult stem cells
before cell transplantation can enhance their activity, which may
be a versatile tool for stem cell therapeutics. In this study, the
effects of rEMS treatment on bone marrow mesenchymal stem cells
(BM-MSCs) derived from BMA was explored by examining colony-forming
efficiency, Erk activation, nitric oxide (NO) production,
proliferation and survival. Additionally, the effect of rEMS on
cells derived from patients of different ages was comparatively
analyzed.
Materials and methods
Cell preparation and culture
Donors comprised young (mean age of 40±10.09 years,
n=21) and old (mean age of 69.6±7.4 years, n=30) patients. Patient
characteristics are shown in Table
SI. Human BMAs (10 ml) were obtained with the written consent
of patients and the protocol was approved by the St. Peter's
Hospital Institutional Review Board (KPH IRB 2009-001).
Briefly, the aspirates (10 ml) were harvested from
the posterior iliac crest of patients with a 20-gauge spinal
needle. The samples were centrifuged twice at 363 × g at 4°C for 10
min to separate MNCs at the interface. Total MNCs were harvested as
minimally manipulated BMA cells. For BM-MSC culture,
2×106 of MNCs were cultured in MSC growth medium (MSCGM;
Lonza Group, Ltd.) at 37°C in a humidified atmosphere of 5%
CO2. The growth medium was replaced every other day. The
cells were cultured until passage 4 for 1 month. The cells between
passages 0 and 3 were used in the experiments. BM-MSCs were
characterized by cell surface marker expression and
multi-differentiation assays (Table
SI; Figs. S1 and S2).
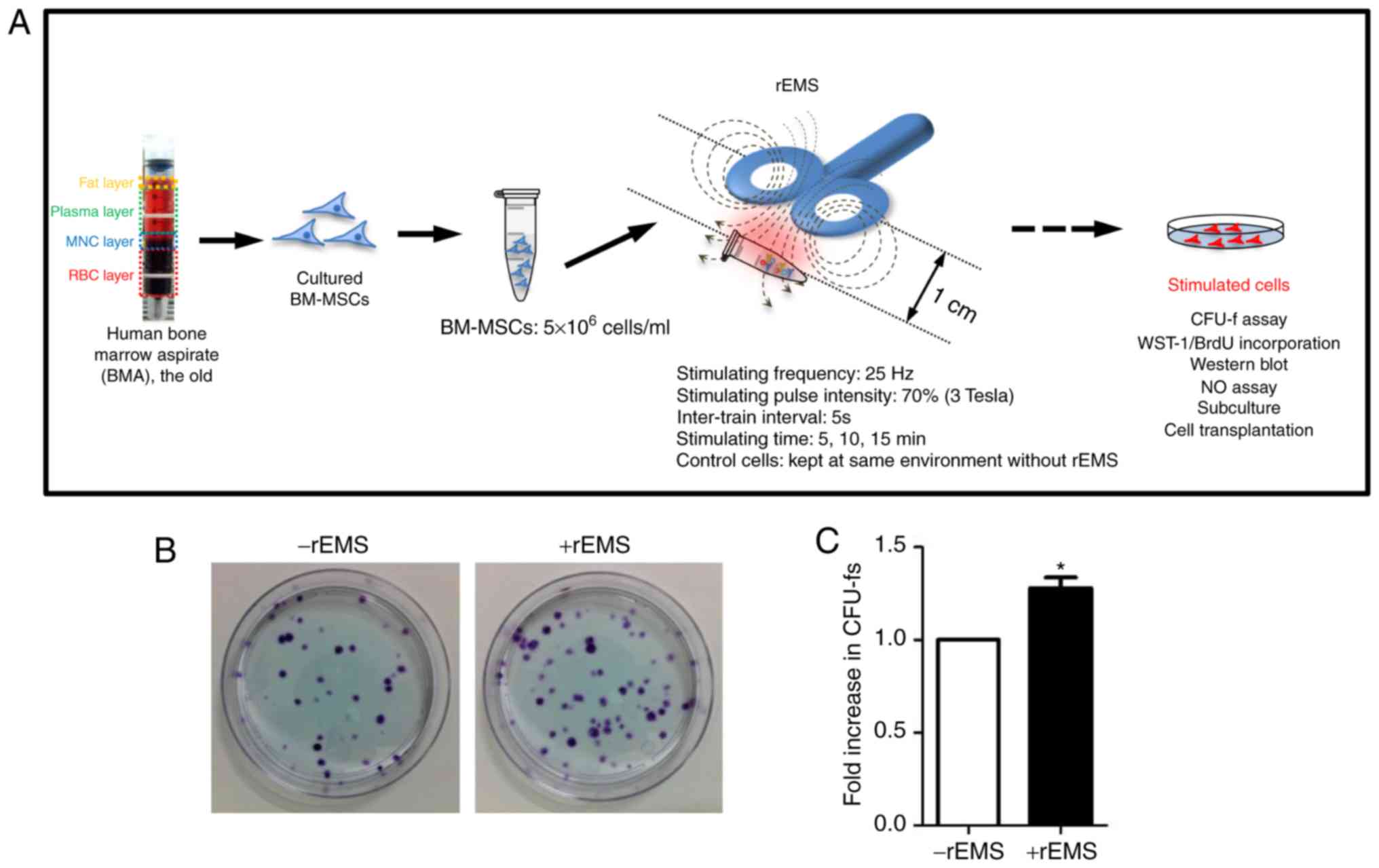
rEMS treatment
Harvested MNCs or primary cultured BM-MSCs
(5×106 cell) were placed at 1 cm from the magnetic coil
of an electromagnetic stimulator (TAMAS; CR Technology, Co. Ltd.).
The rEMS parameters were as follows: Stimulating frequency of 25
Hz, stimulating pulse intensity at 70% of the maximum power (3
Tesla), inter-train interval of 5 sec and a stimulation time of 5,
10, or 15 min. The untreated control cells were treated in the same
manner for the same time period but without rEMS. All stimulations
were performed on single-cell suspensions. Immediately after
stimulation, the cells were analyzed for Erk activation or plated
at a density of 2×104 cells per ml for cell
proliferation assays.
Fibroblastic colony-forming unit (CFU-f)
assay
MNCs (2×106; 36,300 cells/cm2
area, P0) or BM-MSCs (2×103, 36.3 cells/cm2
area) were stimulated with rEMS and then incubated in 100 mm at
37°C in 5% CO2. After 24 h, the medium was changed to
remove non-adherent cells. For the CFU-f assays, the cells were
cultured for 10 days and then fixed with 3.7% formaldehyde
(Sigma-Aldrich; Merck KGaA) for 20 min at room temperature. The
fixed colonies were stained with crystal violet (Sigma-Aldrich;
Merck KGaA) for 20 min at room temperature. Colonies were observed
by light microscopy (magnification, ×40; Olympus Corporation) and a
fibroblastic cluster consisting of >50 cells was counted as a
colony.
Bromo-2′-deoxyuridine (BrdU)
incorporation assay
At 20 h after plating, the cells were incubated with
10 µM BrdU (Sigma-Aldrich; Merck KGaA) for 4 h and fixed
with methanol (Merck KGaA) for 10 min at room temperature. Next,
the cells were incubated with anti-BrdU antibody (1:50; cat. no.
11170376001, Roche Molecular Diagnostics) for 1 h at room
temperature, then with Alexa 488-conjugated goat anti-mouse
antibody (1:100; cat. no. 115-545-205 Jackson ImmunoResearch
Laboratories, Inc.) for 1 h at room temperature and counterstained
with propidium iodide (PI; Sigma-Aldrich; Merck KGaA) for 5 min at
room temperature. The cells were mounted with Vectashield mounting
medium (Vector Laboratories, Inc.) and observed using an Eclipse Ti
fluorescence microscope (Nikon Corporation).
Cell viability assay
Cells were plated at a density of 2×105
cells per ml (2×104/well) immediately after rEMS
treatment. After 24 h, each well was treated with water-soluble
tetrazolium salt-1 (WST-1; Takara Bio, Inc.) and incubated for 30
min at 37°C in 5% CO2. The optical density was measured
at 490 nm using a microplate spectrophotometer (Promega
Corporation). Viability was calculated as a relative to the
control.
Western blot analysis
BM-MSCs were pretreated with 50 µM PD98059
[mitogen-activated protein kinase/Erk kinase (MEK), inhibitor, Cell
Signaling Technology, Inc.], 300 µM
(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (NO
scavenger, carboxy-PTIO), or 1 mM L-NG-monomethyl
arginine monoacetate (L-NMMA; NOS inhibitor; Sigma-Aldrich; Merck
KGaA) for 1 h before stimulation with rEMS. Immediately after rEMS,
the cells were prepared with cell lysis buffer (cat. no. 9803; Cell
Signaling Technology, Inc.) and the protein quantification was
performed using BCA reagents. Proteins (10 µg) were resolved
with 10% SDS-PAGE and transferred onto a nitrocellulose membrane.
To avoid non-specific binding, the membrane was incubated with 1%
skim milk for 40 min at room temperature. The membrane was
incubated with antibodies specific to p-Erk (1:2,000; cat. no.
9106; Cell Signaling Technology, Inc.) or pan-Erk (1:1,000; cat.
no. 9102; Cell Signaling Technology, Inc.) for 16 h at 4°C,
followed by incubation with horseradish peroxidase (HRP)-conjugated
secondary antibody (1:5,000; cat. no. 0300-0108P; Bio-Rad
Laboratories, Inc.) for 1 h at room temperature. The membrane was
developed using the Immobilon Western Chemiluminescent HRP
Substrate (cat. no. WBKLS0100; EMD Millipore) and visualized by
exposure to X-ray film (AGFA, Mortsel).
Measurement of NO production
BM-MSCs at 5×104 cells per ml were plated
immediately after 5, 10, or 15 min rEMS treatment and then
incubated at 37°C in 5% CO2 for 24 h in order to measure
NO. Griess reagent kit (Invitrogen; Thermo Fisher Scientific, Inc.)
was used to quantify nitrite concentration in the culture
supernatant. Absorbance was determined using a microplate
spectrophotometer at a wavelength of 550 nm.
In vivo transplantation
Six-week-old balb/c nude mice (22-23 g, male, n=6)
were purchased from Daehan Bio Link, Co., Ltd. Animals were
maintained under a 12-h light/12-h dark illumination cycle in an
animal holding room and allowed to acclimatize to the new
environment for a period of 1 week before initiation of the
experiments. All animals received a standard chow diet and water
ad libitum. This study was approved by the Ethical Committee
for Experimental Animals of Kyung Hee University (approval no.
KHMC-IACUC-14-010). All mice were anesthetized intraoperatively
using intraperitoneal injections of ketamine (100 mg/kg; Yuhan) and
Rumpun (1.2 mg/kg; Bayer).
BM-MSCs (2×106 cells per mouse) with or
without rEMS treatment were labeled with PKH26 red fluorescent cell
linker (Sigma-Aldrich; Merck KGaA), mixed with Matrigel (BD
Bioscience; Becton, Dickinson and Company), and implanted into the
subcutaneous tissue in the dorsal region of mice (Daehan Bio Link,
Co., Ltd.). A total of 8 weeks after transplantation, Mouse was
sacrificed by CO2 inhalation
Samples (transplanted cells and Matrigel) were
harvested, fixed in 3.7% formaldehyde for 24 h at room temperature
and embedded in paraffin or optical cutting temperature compound
(Sakura Finetek UK, Ltd.). Paraffin sectioned samples (5 µm)
were analyzed after hematoxylin & eosin (Sigma-Aldrich; Merck
KGaA) staining (5 min, room temperature). To track the transplanted
cells labeled with PKH26, cryosectioned samples were mounted with
Vectashield mounting medium with 4′,6-diamidino-2-phenylindole
(Vector Laboratories, Inc.) and observed using an Eclipse Ti
fluorescence microscope.
Statistical analysis
Experiments were repeated three times and data are
presented as the mean ± standard error of the mean. Comparisons
among three groups were performed using one way analysis of
variance followed by Tukey's post hoc test. All data were analyzed
using GraphPad Prism software (7.0; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Age impairs cellular activity and
proliferation of stem cells in the BM
In order to examine the effect of age on stem cell
activity, BMAs were prepared from young or old patients and then
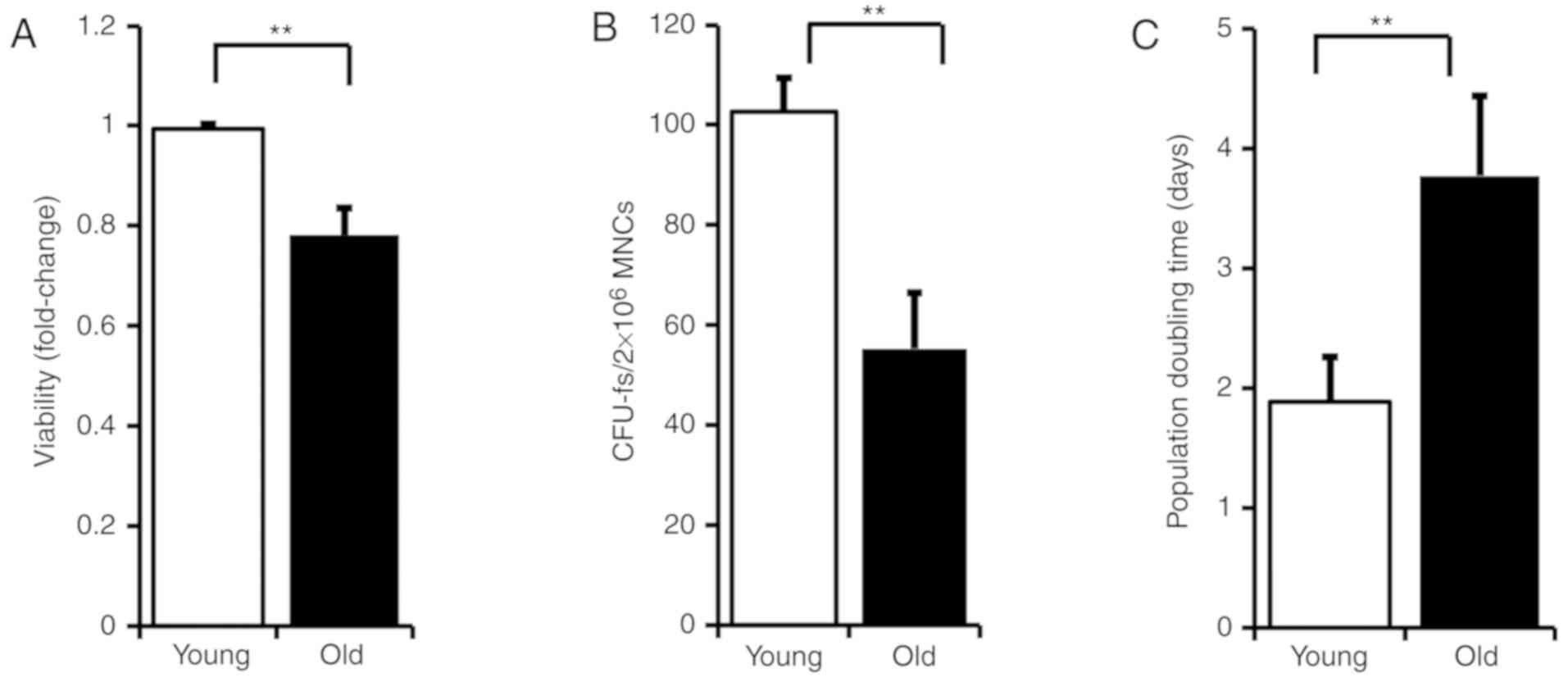
cellular activity was assessed by WST-1 assay (P<0.01; Fig. 1A). The activity of total BM-MNCs
was decreased in the old patients compared with young patients.
When BM-MNCs from the old patients were cultured in MSCGM, CFU-f
was found to be decreased and proliferation doubling time was
increased, compared with BM-MSCs from the young patients
(P<0.01; Fig. 1B: Young:
102.71±6.63 days, old: 55.29±11.15 days, Young vs. Old, P<0.01;
Fig. 1C: Young: 1.88±0.37 days,
old: 3.77±0.67 days, Young vs. Old).
This data suggests that as people get older, the
stem cell pool and its activity in the bone marrow may be weakened.
Also, due to increased population doubling time, the total
cumulative cell number to be collected after ex vivo culture
is less in old compared with young patients. Above all, when taking
into consideration that cell transplantation is chiefly required
for older patients, the establishment of modulations that enhance
stem cell activity in older patients are more important.
Effect of rEMS preconditioning on stem
cells is more prominent in aged BMAs
To explore the effect of rEMS on bone marrow cells,
MNCs derived from either the BMA of young or old patients were
subjected to rEMS treatment for 5 min and their CFU-f values were
analyzed and compared (Fig. 2).
In the absence of rEMS treatment, the CFU-f of BMAs from young
patients was increased compared with BMAs from the old patients.
rEMS treatment increased the CFU-f of the BM-MSC pools in both
groups. The effect was greater in the BMAs from the old patients,
with an ~53.8% increase, compared with a 21.2% increase seen in
BMAs from the young patients [The young, −rEMS group:
87.3+12.9/2×106 MNCs, +rEMS
group:105.8+14.6/2×106 MNCs, P<0.01 vs. −rEMS group;
The Old, rEMS treatment (−): 56.5+11.5/2×106 MNCs, rEMS
treatment (+): 86.9+21.52×106 MNCs, P<0.05 vs. rEMS
treatment (−)].
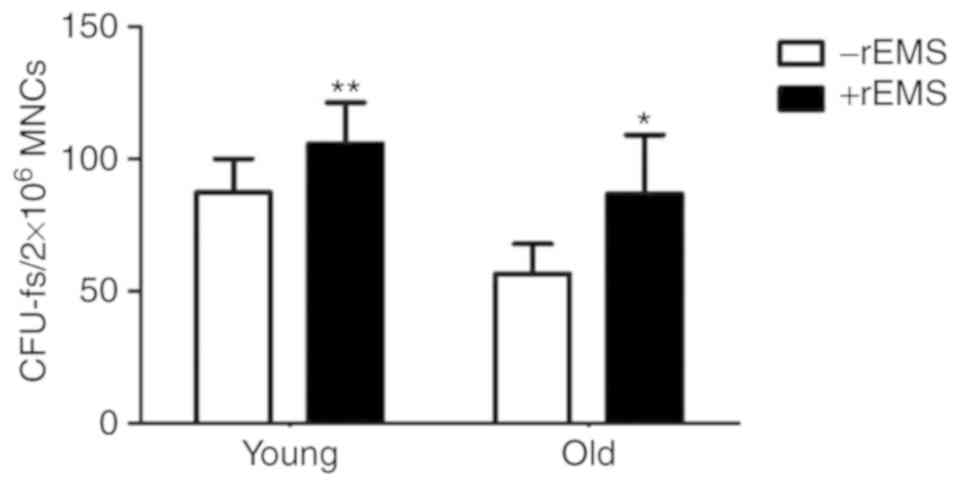 | Figure 2Effect of rEMS treatment on the
colony-forming efficiency of MNCs from young and old patients. To
evaluate the rEMS-mediated effect on the CFU-f, MNCs derived from
the BMAs of the two groups (mean age of young and old patients was
44±10.09 and 68.6±5.97 y, respectively) were stimulated with rEMS
for 5 min, and cultured in vitro. Their CFU-f efficiencies
were compared. The young: P<0.01 vs. -rEMS group. The old:
P<0.05 vs. -rEMS group (n=21 in young, n=30 in old). Data
represents the mean ± standard deviation; one-way analysis of
variance with Tukey's post hoc test. *P<0.05 and
**P<0.01 vs. respective -rEMS group. Young, MNC from
the young patients; Old, MNC from the old patients; BMA, bone
marrow aspirate; rEMS, repetitive electromagnetic stimulation;
MNCs, mononuclear cells; CFU-f, colony forming units. |
This finding indicates that rEMS preconditioning is
more efficient in BM stem cells from old patients (−rEMS group vs.
+rEMS group).
Transient application of rEMS enhances
the CFU-f of the BM-MSCs
To explore whether rEMS application affects the cell
proliferation and self-renewal capacity of cultured BM-MSCs from
the old, rEMS was applied to BM-MSCs (Fig. 3A). The effect of rEMS on BM-MSCs
was evaluated using CFU-f (Fig. 3B
and C). Transient application of rEMS increased the CFU-f by
27.8±4.8% compared with the untreated control (P<0.05; -rEMS
group vs. +rEMS group). Additionally, rEMS treatment did not
influence specific marker expression of MSCs from the old patients
(Fig. S3). In other words, these
data support that rEMS preconditioning can increase the
self-renewal capacity of stem cells cultured ex vivo. Thus,
rEMS treatment before stem cell transplantation is expected to
enhance the therapeutic gain of BM-MSCs without affecting the
characteristics of stem cells.
rEMS increases cell proliferation of
BM-MSCs, possibly through transient NO production and subsequent
Erk1/2 activation
The increase in CFU-f due to transient rEMS
treatment led the present study to explore the effect of rEMS on
BM-MSC proliferation. rEMS was used to treat BM-MSCs from the old
patients at different time lengths. rEMS treatment for 5 or 10 min
increased both cell viability (Fig.
4A) and the number of BrdU-incorporating cells in BM-MSCs
(Fig. 4B), compared with the
untreated controls. However, the effects of rEMS were not observed
after 15 min treatment. Moreover, rEMS treatment for 5 min resulted
in higher cell viability and higher cell proliferation relative to
the 10 min treatment. These results indicate that rEMS treatment
for 5 min may be optimal for BM-MSC viability and proliferation,
and longer treatment may be dispensable.
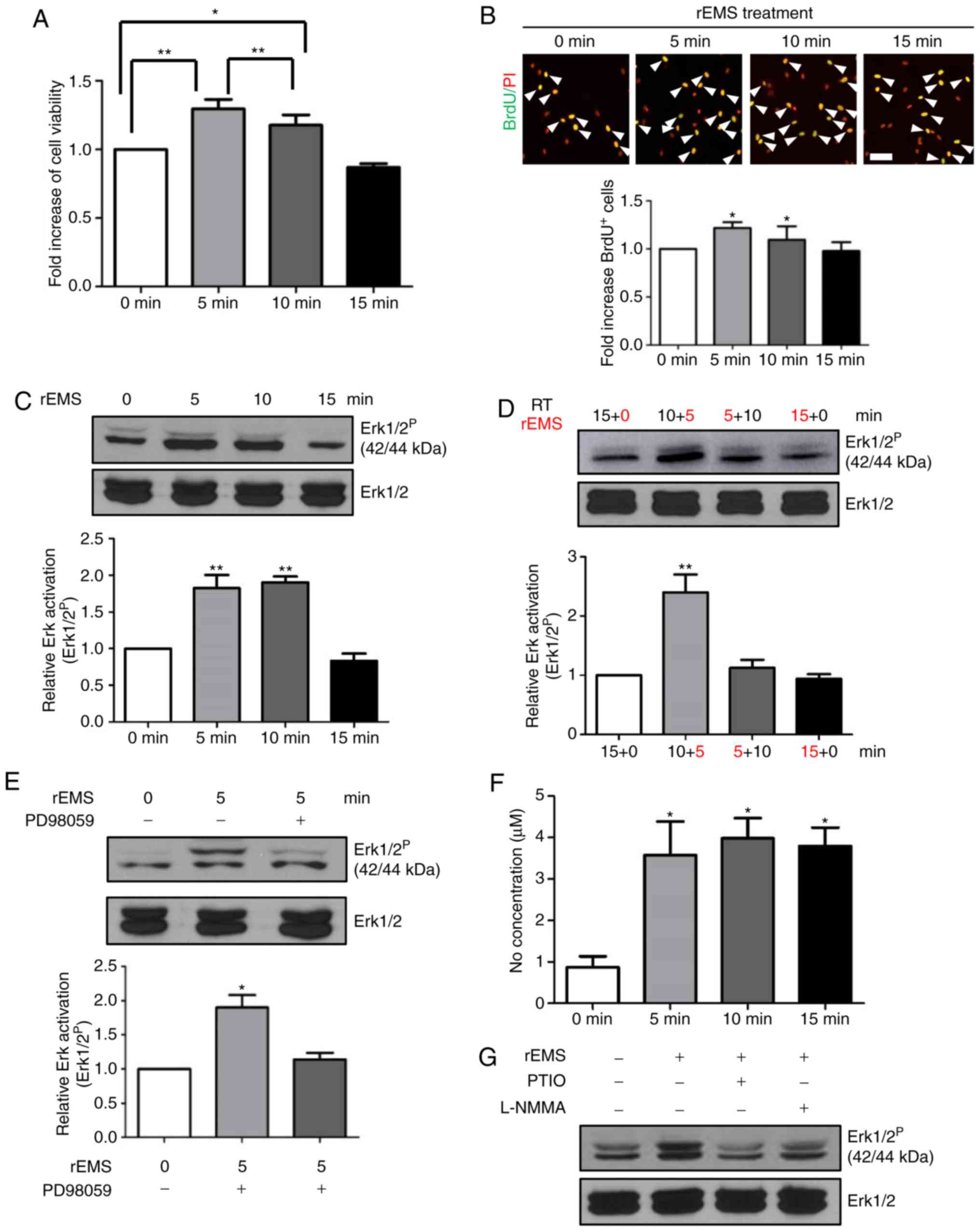 | Figure 4Effect of rEMS on BM-MSC cellular
activity and intracellular signaling pathways. MSCs from the old
patients were stimulated with rEMS and incubated at 37°C in 5%
CO2. (A) At 24 h after plating, BM-MSCs were incubated
with water-soluble tetrazolium salt-1 for 30 min and optical
density was measured. *P<0.05 and
**P<0.01 vs. 0 min. (B) BM-MSCs were incubated with
BrdU for 4 h and proliferating cells were measured. Representative
images of BrdU-incorporating cells (green) and PI staining for
nuclei (red) of the BM-MSCs are shown. *P<0.05 vs. 0
min, n=10. White arrows: BrdU-incorporated cells. Scale bar:
100-µm. (C) rEMS-induced Erk1/2 activation was analyzed by
western blotting and the quantitative analysis is shown.
**P<0.01 vs. 0 min n=10. (D) Decay of rEMS-induced
Erk1/2 activation is shown by western blot analysis. Duration of
rEMS treatment is noted in red. **P<0.01 vs. 0 min,
n=10. (E) Inhibition of rEMS-stimulated Erk1/2 activation by the
mitogen-activated protein kinase/Erk kinase inhibitor PD98059.
*P<0.05 vs. 0 min, n=10. (F) rEMS-stimulated NO
production. *P<0.05 vs. 0 min. (G) Inhibition of
rEMS-induced NO production by carboxy-PTIO NO scavenger or the
L-NMMA NOS inhibitor. Data represents the mean ± standard
deviation; one-way analysis of variance with Tukey's post hoc test.
BM-MSCs, bone marrow-mesenchymal stem cells; CFU-f, colony forming
units; rEMS, repetitive electromagnetic stimulation; L-NMMA NOS,
L-NG-monomethyl arginine monoacetate nitric oxide synthase; PI,
propidium iodide; Erk, extracellular signal regulated kinase; BrdU,
Bromo-2′-deoxyuridine. |
In order to investigate the early signaling
molecules involved in rEMS-mediated cell proliferation, BM-MSCs
were exposed to rEMS for 5, 10, or 15 min and cell lysates were
prepared for western blot analysis of Erk1/2 activation.
rEMS-mediated phosphorylation of Erk1/2 was detected in cells
exposed to rEMS for 5 and 10 min (Fig. 4C); however, continuous stimulation
for 15 min did not affect Erk1/2 phosphorylation, consistent with
the effects on cell viability and proliferation.
Next, the present study tested the sustainability of
rEMS-mediated phosphorylation of Erk1/2. When the cells were
subjected to rEMS for 5 min and then allowed to sit untreated for
10 min, Erk1/2 phosphorylation returned to basal levels, similar to
that of continuous 15 min rEMS treatment (Fig. 4D). Accordingly, rEMS-mediated
Erk1/2 activation peaked at 5 min of treatment and spontaneously
regressed to the basal level at 15 min, even when rEMS continued
for 15 min. This indicates that rEMS-induced Erk1/2 activation
occurred within 5 min after the initiation of rEMS treatment.
To explore the upstream signaling of rEMS-induced
Erk1/2 activation, PD98059, an inhibitor of MEK, was applied before
rEMS treatment (Fig. 4E). The
addition of PD98059 blocked rEMS-induced phosphorylation of Erk1/2,
suggesting that phosphorylation of Erk1/2 due to rEMS treatment is
mediated by the MEK-Erk1/2 signaling pathway.
Previously, it was reported that rEMS treatment
increased endothelial nitric oxide synthase (NOS) expression
(2,11) and NO is known to induce cell
proliferation through diverse signaling pathways including
activation of Erk1/2 or protein kinase C (6,14).
To explore the early signals mediated by rEMS treatment, whether
rEMS stimulated NO production in BM-MSCs or not was investigated.
As shown in Fig. 4F, rEMS
treatment promoted NO production within 5 min after rEMS treatment,
which was not further increased with increasing rEMS treatment
times. To investigate the role of rEMS-induced NO production in Erk
activation, either the carboxy-PTIO (NO scavenger) or the L-NMMA
(NOS inhibitor) was used to pretreat the BM-MSCs before rEMS
treatment (Fig. 4G). Blockage of
NO production through carboxy-PTIO or L-NMMA treatment completely
inhibited the rEMS-induced phosphorylation of Erk1/2.
Taken together, these results suggest that rEMS
treatment induced NO production in BM-MSCs, which then stimulates
Erk1/2 activation. Both NO production and Erk1/2 activation seem to
be promptly induced within 5 min after rEMS treatment, which most
likely leads to rEMS-induced cell proliferation.
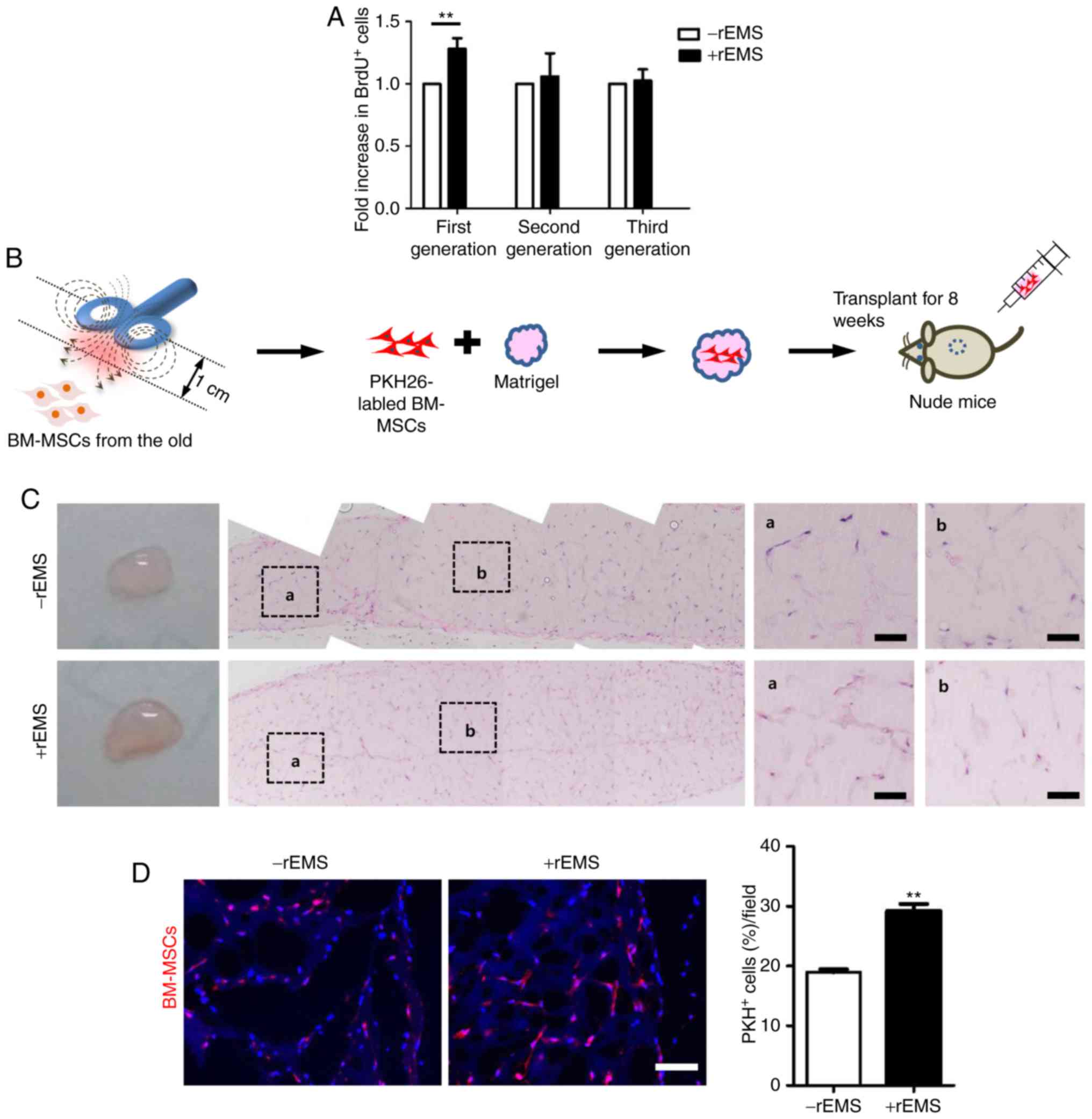
rEMS-induced cell proliferation does not
continue after cell passage
While the rEMS-induced proliferation of BM-MSCs may
be beneficial as a preconditioning step for stem cell therapeutics,
its sustained effects may evoke safety issues in vivo. To
examine the duration of rEMS effects, BM-MSCs were subjected to
rEMS treatment for 5 min, followed by BrdU-incorporating assays to
directly analyze cells after rEMS treatment and after an additional
cell passage (subculture; Fig.
5A). rEMS-mediated increase in BrdU-incorporating cells were
observed only in the first cell generation (P<0.01 vs. -rEMS
group; +rEMS group: 28.1±3.0%) and were not found in the cells
after subculture, suggesting that rEMS-accelerated cell
proliferation occurred transiently and was not sustained during
ex vivo culture. Thus, application of rEMS preconditioning
shortly before cell transplantation may exert positive effects on
stem cell activity, which might not arouse any safety issues
post-transplantation.
rEMS augments cell survival in vivo
Considering the stimulatory effect of rEMS on
cellular activity, rEMS preconditioning was anticipated to augment
survival or engraftment of transplanted cells in vivo. To
track transplanted cells in vivo, BM-MSCs that were treated
with rEMS for 5 min and labeled with PKH26 red fluorescence dye
were subcutaneously transplanted into BALB/c nude mice, along with
Matrigel (Fig. 5B). At eight
weeks post-transplantation, numerous host cells that were not
labeled with PKH26 were infiltrated into the Matrigel. Among them,
the number of rEMS-treated PKH26-labeled cells was ~53% increased
compared with the untreated cells (Fig. 5C and D).
This data indicates that rEMS preconditioning can
improve sustainability of transplanted cells in vivo. As the
rEMS-induced effect on cell proliferation was not sustained in the
subsequent passage of cells, a high level of PKH26-labeled cells
with rEMS is expected to be shown due to increased survival of
BM-MSCs and not proliferation in vivo.
Discussion
Stem cell therapy has potential to exert many
beneficial effects against critical disease. However, the very low
incidence of BM-MSCs in the BMA pool and their poor cell viability
is associated with the weak efficacy in vivo. Thus, the
age-related decrease in the stem cell pool and its activity has
been a critical issue and an important deterrent for the use of
stem cell therapy, especially in older patients (21-28). The present study was designed to
develop a cell preconditioning strategy to improve the activity of
stem cells from BMs using the transient application of rEMS.
The present comparative study of stem cell activity
from young and old patients revealed an age-related reduction in
stem cell activity with a lower yield of cells. Although MSCs from
the young patients were also treated with rEMS, the current goal is
to enhance the activity of MSCs from the old, not the young.
Therefore, after confirmation of the difference in activity of ADSC
between the young and old patients, the present study performed all
experiments with MSCs from the old patients.
This study demonstrated that rEMS treatment can
increase the CFU-f and promote cell proliferation of BM-MSCs from
the old patients. rEMS treatment did not influence cell-specific
maker expression of BM-MSCs. This supports the hypothesis that
improvement of the ability of repopulation or activity of stem
cells by rEMS treatment did not alter the characteristics of
BM-MSC. Moreover, considering the beneficial effects of rEMS
treatment on stem cells, the differentiation potential of stem cell
may be improved, which should be determined in further study.
Interestingly, rEMS treatment enhanced
sustainability of transplanted BM-MSCs from the old patients
without any safety issues. The intensity of PKH 26 is decreased as
the cell divides. However, transplanted cells rarely proliferate
in vivo. Thus, 50% more PKH26-labeled cells with rEMS
treatment may be not due to proliferation in vivo. This
phenomenon is inferred to be related to survival rate. PKH-26 cells
with rEMS might undergo apoptosis at a reduced rate compared with
PKH-26 cells without rEMS in vivo. rEMS treatment may be
involved in cell survival though controlling cellular activity or
paracrine action in vivo and the mechanism of cell survival
(rEMS-treated cells) will be explored in the future.
Under the current experimental conditions, the
rEMS-mediated positive effect seems to have peaked and was
saturated within 5 min of treatment. In multiple experiments,
longer rEMS treatment did not further increase NO production,
Erk1/2 activation, or cell proliferation. Furthermore, continuous
rEMS treatment for 15 min resulted in lower Erk activation compared
with that observed at 5 min, which is most likely due to the
spontaneous deactivation of Erk achieved by the 5 min of rEMS
treatment. The present study confirmed that rEMS treatment for 5
min is sufficient for BM-MSC preconditioning.
More importantly, rEMS treatment was effective in
stem cells from old rather than young patients. Stem cells are
chiefly transplanted to old patients with critical diseases and
these patients might have a stem cell pool of low activity.
Currently, allogenic transplantations of stem cells are performed
in the clinic; however, depending on background disease, the use of
allogenic cells is impracticable due to toxicity. Thus, the
restoration of the activity of stem cells from old patients could
have great potential in therapeutics and may become a safe
treatment approach used clinically. The present study investigated
the effect of rEMS on the stem cell pool of old patients and
confirmed the possibility for application of rEMS to stem cell
therapy in aged patients suffering from chronic diseases including
diabetes.
Moreover, current commercial rEMS instruments, which
are designed with two overlapping loops of wire in a figure-eight
arrangement to focus narrowly in deep areas of the brain, are not
easily modified for the electromagnetic stimulation of cell
cultures. The present configuration could be a useful tool for
other clinical applications, but diverse parameters such as
stimulation intensity, frequency and distribution of the electric
current by magnetic coils may be another important consideration in
the analysis of the exact series of signaling cascades and their
interplay, which needs to be further explored (29,30).
Supplementary Data
Funding
The present study was supported by a Korean Health
Technology R&D Project grant from the Ministry of Health and
Welfare, Republic of Korea (grant no. HI18C1492) and by Basic
Science Research Program through the National Research Foundation
of Korea funded by the Ministry of Education (grant no.
NRF-2018R1D1A1B07041048).
Availability of data and materials
The datasets used in the present study are available
from the corresponding authors upon reasonable request.
Authors' contributions
SN, SK, KY, YS and HH performed all experiments and
interpreted data. HH and YS drafted and finalized the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee for Experimental Animals of Kyung Hee University
(approval no. KHMC-IACUC-14-010).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ringdén O, Uzunel M, Rasmusson I,
Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos
A, Hassan Z, et al: Mesenchymal stem cells for treatment of
therapy-resistant graft-versus-host disease. Transplantation.
81:1390–1397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoon SH, Shim YS, Park YH, Chung JK, Nam
JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, et al: Complete
spinal cord injury treatment using autologous bone marrow cell
transplantation and bone marrow stimulation with granulocyte
macrophage-colony stimulating factor: Phase I/II clinical trial.
Stem Cells. 25:2066–2073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schächinger V, Erbs S, Elsässer A,
Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey
DG, Hamm CW, et al: Intracoronary bone marrow-derived progenitor
cells in acute myocardial infarction. N Engl J Med. 355:1210–1221.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Kleer IM, Brinkman DM, Ferster A,
Abinun M, Quartier P, Van Der Net J, Ten Cate R, Wedderburn LR,
Horneff G, Oppermann J, et al: Autologous stem cell transplantation
for refractory juvenile idiopathic arthritis: Analysis of clinical
effects, mortality, and transplant related morbidity. Ann Rheum
Dis. 63:1318–1326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu X, Wang X, Nian H, Yang D and Wei R:
Mesenchymal stem cells for treating autoimmune dacryoadenitis. Stem
Cell Res Ther. 8:1262017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghannam S, Bouffi C, Djouad F, Jorgensen C
and Noël D: Immunosuppression by mesenchymal stem cells: Mechanisms
and clinical applications. Stem Cell Res Ther. 1:22010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar
|
|
8
|
Jin Y, Hong HS and Son Y: Substance P
enhances mesenchymal stem cells-mediated immune modulation.
Cytokine. 71:145–153. 2015. View Article : Google Scholar
|
|
9
|
Liu H, Lu K, MacAry PA, Wong KL, Heng A,
Cao T and Kemeny DM: Soluble molecules are key in maintaining the
immnunomodulatory activity of murine mesenchymal stromal cells. J
Cell Sci. 125:200–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Platz T and Rothwell JC: Brain stimulation
and brain repair-rTMS: From animal experiment to clinical
trials-what do we know? Restor Neurol Neurosci. 28:387–398.
2010.
|
|
11
|
Khedr EM, Ahmed MA, Fathy N and Rothwell
JC: Therapeutic trial of repetitive transcranial magnetic
stimulation after acute ischemic stroke. Neurology. 65:466–468.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khedr EM, Kotb H, Kamel NF, Ahmed MA,
Sadek R and Rothwell JC: Longlasting antalgic effects of daily
sessions of repetitive transcranial magnetic stimulation in central
and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry.
76:833–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kleinjung T, Eichhammer P, Langguth B,
Jacob P, Marienhagen J, Hajak G, Wolf SR and Strutz J: Long-term
effects of repetitive transcranial magnetic stimulation (rTMS) in
patients with chronic tinnitus. Otolaryngol Head Neck Surg.
132:566–569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ridding MC and Rothwell JC: Is there a
future for therapeutic use of transcranial magnetic stimulation?
Nat Rev Neurosci. 8:559–567. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Talelli P, Greenwood RJ and Rothwell JC:
Exploring theta burst stimulation as an intervention to improve
motor recovery in chronic stroke. Clin Neurophysiol. 118:333–342.
2007. View Article : Google Scholar
|
|
16
|
Shirota Y, Ohtsu H, Hamada M, Enomoto H
and Ugawa Y; Research Committee on rTMS Treatment of Parkinson's
Disease: Supplementary motor area stimulation for parkinson
disease: A randomized controlled study. Neurology. 80:1400–1405.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HY, Crupi D, Liu J, Stucky A,
Cruciata G, Di Rocco A, Friedman E, Quartarone A and Ghilardi MF:
Repetitive transcranial magnetic stimulation enhances BDNF-TrkB
signaling in both brain and lymphocyte. J Neurosci. 31:11044–11054.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hellmann J, Jüttner R, Roth C, Bajbouj M,
Kirste I, Heuser I, Gertz K, Endres M and Kronenberg G: Repetitive
magnetic stimulation of human-derived neuron-like cells activates
cAMP-CREB pathway. Eur Arch Psychiatry Clin Neurosci. 262:87–91.
2012. View Article : Google Scholar
|
|
19
|
Kim YM, Cho SE, Kim SC, Jang HJ and Seo
YK: Effects of extremely low frequency electromagnetic fields on
melano-genesis through p-ERK and p-SAPK/JNK pathways in human
melanocytes. Int J Mol Sci. 18:E21202017. View Article : Google Scholar
|
|
20
|
Diniz P, Shomura K, Soejima K and Ito G:
Effects of pulsed electromagnetic field (PEMF) stimulation on bone
tissue like formation are dependent on the maturation stages of the
osteoblasts. Bioelectromagnetics. 23:398–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shibata KR, Aoyama T, Shima Y, Fukiage K,
Otsuka S, Furu M, Kohno Y, Ito K, Fujibayashi S, Neo M, et al:
Expression of the p16INK4A gene is associated closely with
senescence of human mesenchymal stem cells and is potentially
silenced by DNA methylation during in vitro expansion. Stem Cells.
25:2371–2382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stolzing A, Jones E, McGonagle D and Scutt
A: Age-related changes in human bone marrow-derived mesenchymal
stem cells: Consequences for cell therapies. Mech Ageing Dev.
129:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou S, Greenberger JS, Epperly MW, Goff
JP, Adler C, Leboff MS and Glowacki J: Age-related intrinsic
changes in human bone-marrow-derived mesenchymal stem cells and
their differentiation to osteoblasts. Aging Cell. 7:335–343. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wagner W, Bork S, Horn P, Krunic D,
Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, et
al: Aging and replicative senescence have related effects on human
stem and progenitor cells. PLoS One. 4:e58462009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kosan C, Heidel FH, Godmann M and Bierhoff
H: Epigenetic erosion in adult stem cells: Drivers and passengers
of aging. Cells. 7:E2372018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Neves J, Sousa-Victor P and Jasper H:
Rejuvenating strategies for stem cell-based therapies in aging.
Cell Stem Cell. 20:161–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baker N, Boyette LB and Tuan RS:
Characterization of bone marrow-derived mesenchymal stem cells in
aging. Bone. 70:37–47. 2015. View Article : Google Scholar
|
|
28
|
Turinetto V, Vitale E and Giachino C:
Senescence in human mesenchymal stem cells: Functional changes and
implications in stem cell-based therapy. Int J Mol Sci.
19:E11642016. View Article : Google Scholar
|
|
29
|
Post A, Müller MB, Engelmann M and Keck
ME: Repetitive transcranial magnetic stimulation in rats: Evidence
for a neuroprotective effect in vitro and in vivo. Eur J Neurosci.
11:3247–3254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueyama E, Ukai S, Ogawa A, Yamamoto M,
Kawaguchi S, Ishii R and Shinosaki K: Chronic repetitive
transcranial magnetic stimulation increases hippocampal
neurogenesis in rats. Psychiatry Clin Neurosci. 65:77–81. 2011.
View Article : Google Scholar : PubMed/NCBI
|