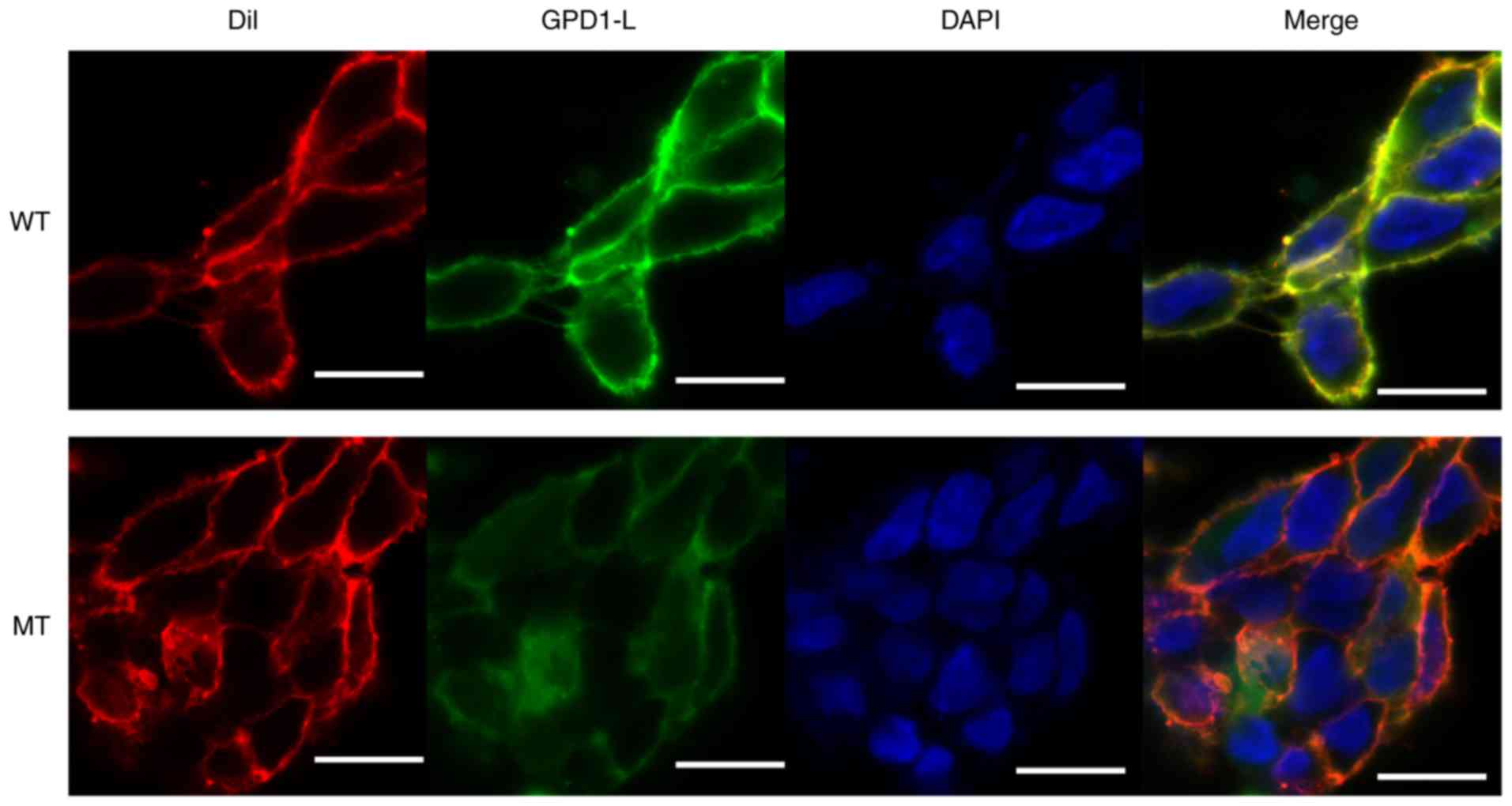
|
1
|
Tikkanen JT, Anttonen O, Junttila MJ, Aro
AL, Kerola T, Rissanen HA, Reunanen A and Huikuri HV: Long-term
outcome associated with early repolarization on
electrocardiography. N Engl J Med. 361:2529–2537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Junttila MJ, Tikkanen JT, Kenttä T,
Anttonen O, Aro AL, Porthan K, Kerola T, Rissanen HA, Knekt P and
Huikuri HV: Early repolarization as a predictor of arrhythmic and
nonarrhythmic cardiac events in middle-aged subjects. Heart Rhythm.
11:1701–1706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang Y, Stahl-Herz J and Sampson BA:
Molecular diagnostics of cardiovascular diseases in sudden
unexplained death. Cardiovasc Pathol. 23:1–4. 2014. View Article : Google Scholar
|
|
4
|
Gourraud JB, Le Scouarnec S, Sacher F,
Chatel S, Derval N, Portero V, Chavernac P, Sandoval JE, Mabo P,
Redon R, et al: Identification of large families in early
repolarization syndrome. J Am Coll Cardiol. 61:164–172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCorquodale A, Poulton R, Hendry J,
Norrish G, Field E, Mead-Regan S, Lowe M and Kaski JP: High
prevalence of early repolarization in the paediatric relatives of
sudden arrhythmic death syndrome victims and in normal controls.
Europace. 19:1385–1391. 2017. View Article : Google Scholar
|
|
6
|
Koncz I, Gurabi Z, Patocskai B, Panama BK,
Szél T, Hu D, Barajas-Martinez H and Antzelevitch C: Mechanisms
underlying the development of the electrocardiographic and
arrhythmic manifestations of early repolarization syndrome. J Mol
Cell Cardiol. 68:20–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chauveau S, Janin A, Till M, Morel E,
Chevalier P and Millat G: Early repolarization syndrome caused by
de novo duplication of KCND3 detected by next-generation
sequencing. Heart Rhythm Case Rep. 3:574–578. 2017.
|
|
8
|
Watanabe H, Ohkubo K, Watanabe I,
Matsuyama TA, Ishibashi-Ueda H, Yagihara N, Shimizu W, Horie M,
Minamino T and Makita N: SCN5A mutation associated with ventricular
fibrillation, early repolarization, and concealed myocardial
abnormalities. Int J Cardiol. 165:e21–e23. 2013. View Article : Google Scholar
|
|
9
|
Medeiros-Domingo A, Tan BH, Crotti L,
Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp
D, et al: Gain-of-function mutation S422L in the KCNJ8-encoded
cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave
syndromes. Heart Rhythm. 7:1466–1471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Shen Y, Xie J, Bao H, Cao Q, Wan R,
Xu X, Zhou H, Huang L, Xu Z, et al: A mutation in the CACNA1C gene
leads to early repolarization syndrome with incomplete penetrance:
A Chinese family study. PLoS One. 12:e1775322017.
|
|
11
|
Chen Y, Barajas-Martinez H, Zhu D, Wang X,
Chen C, Zhuang R, Shi J, Wu X, Tao Y, Jin W, et al: Novel trigenic
CACNA1C/DES/MYPN mutations in a family of hypertro-phic
cardiomyopathy with early repolarization and short QT syndrome. J
Transl Med. 15:782017. View Article : Google Scholar
|
|
12
|
Burashnikov E, Pfeiffer R,
Barajas-Martinez H, Delpón E, Hu D, Desai M, Borggrefe M,
Häissaguerre M, Kanter R, Pollevick GD, et al: Mutations in the
cardiac L-type calcium channel associated with inherited J-wave
syndromes and sudden cardiac death. Heart Rhythm. 7:1872–1882.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Stolfo G, Palumbo P, Castellana S,
Mastroianno S, Biagini T, Palumbo O, Leone MP, De Luca G, Potenza
DR, Mazza T, et al: Sudden cardiac death in J wave syndrome with
short QT associated to a novel mutation in Nav 1.8 coding gene
SCN10A: First case report for a possible pharmacogenomic role. J
Electrocardiol. 51:809–813. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delaney JT, Muhammad R, Blair MA, Kor K,
Fish FA, Roden DM and Darbar D: A KCNJ8 mutation associated with
early repolarization and atrial fibrillation. Europace.
14:1428–1432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu D, Barajas-Martinez H, Terzic A, Park
S, Pfeiffer R, Burashnikov E, Wu Y, Borggrefe M, Veltmann C,
Schimpf R, et al: ABCC9 is a novel Brugada and early repolarization
syndrome susceptibility gene. Int J Cardiol. 171:431–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valdivia CR, Ueda K, Ackerman MJ and
Makielski JC: GPD1L links redox state to cardiac excitability by
PKC-dependent phos-phorylation of the sodium channel SCN5A. Am J
Physiol Heart Circ Physiol. 297:H1446–H1452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang H, Chen YQ, Fan LL, Guo S, Li JJ,
Jin JY and Xiang R: Whole-exome sequencing identifies a novel
mutation of GPD1L (R189X) associated with familial conduction
disease and sudden death. J Cell Mol Med. 22:1350–1354. 2018.
|
|
18
|
Barbosa EC, Bomfim Ade S,
Benchimol-Barbosa PR and Ginefra P: Ionic mechanisms and vectorial
model of early repo-larization pattern in the surface
electrocardiogram of the athlete. Ann Noninvasive Electrocardiol.
13:301–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antzelevitch C: Genetic, molecular and
cellular mechanisms underlying the J wave syndromes. Circ J.
76:1054–1065. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo Q, Ren L, Chen X, Hou C, Chu J, Pu J
and Zhang S: A novel mutation in the SCN5A gene contributes to
arrhythmo-genic characteristics of early repolarization syndrome.
Int J Mol Med. 37:727–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Norstrand DW, Valdivia CR, Tester DJ,
Ueda K, London B, Makielski JC and Ackerman MJ: Molecular and
functional characterization of novel glycerol-3-phosphate
dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death
syndrome. Circulation. 116:2253–2259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makiyama T, Akao M, Haruna Y, Tsuji K, Doi
T, Ohno S, Nishio Y, Kita T and Horie M: Mutation analysis of the
glycerol-3 phosphate dehydrogenase-1 like (GPD1L) gene in Japanese
patients with brugada syndrome. Circ J. 72:1705–1706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abriel H and Kass RS: Regulation of the
voltage-gated cardiac sodium channel Nav1.5 by interacting
proteins. Trends Cardiovasc Med. 15:35–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hallaq H, Yang Z, Viswanathan PC, Fukuda
K, Shen W, Wang DW, Wells KS, Zhou J, Yi J and Murray KT:
Quantitation of protein kinase A-mediated trafficking of cardiac
sodium channels in living cells. Cardiovasc Res. 72:250–261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weiss R, Barmada MM, Nguyen T, Seibel JS,
Cavlovich D, Kornblit CA, Angelilli A, Villanueva F, McNamara DM
and London B: Clinical and molecular heterogeneity in the brugada
syndrome: A novel gene locus on chromosome 3. Circulation.
105:707–713. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
London B, Michalec M, Mehdi H, Zhu X,
Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL,
Madhusudanan M, et al: Mutation in glycerol-3-phosphate
dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current
and causes inherited arrhythmias. Circulation. 116:2260–2268. 2007.
View Article : Google Scholar : PubMed/NCBI
|