Introduction
Population aging is one of the main problems facing
the 21st century. Aging is associated with an increased incidence
of age-related diseases such as Parkinson's, Alzheimer's,
cardiovascular and other diseases (1). Cardiovascular disease is a chronic
disease closely related to aging (2) and atherosclerosis is the primary
cause of cardiovascular disease. As we age, the structure and
function of artery vasculature changes, the vessel lumen expands,
vascular stiffness increases, eventually affecting other tissues
and organs (3). Noticeably, the
damage and functional changes of vascular endothelial cells play a
crucial role in the occurrence and development of atherosclerosis,
and penetrate into the whole pathological process of
atherosclerosis (4).
Vascular endothelial cells, which are monolayer
squamous epithelial cells located between vascular endothelium
subcutaneous tissues and blood, function as a physical barrier
between blood and tissues. Vascular endothelial cells play an
important role in maintaining normal vascular function and tissue
structure and in the regulation of cells proliferation (5,6).
Endothelial cell senescence, which is closely related to
atherosclerosis, is accompanied by the destruction of endothelial
cell integrity and functional damage of endothelial cells that in
turn cause vascular dysfunction, therefore creating conditions for
the occurrence of cardiovascular diseases (7,8).
Thus, it is highly necessary to understand the mechanism of
endothelial cell senescence, to provide patients with effective
clinical treatment and reduce the occurrence of cardiovascular
diseases.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
and have been regarded as therapeutic targets and biomarkers for
evaluating cardiovascular disease in recent years (9). Among miRNAs, miR-222-221 can inhibit
the proliferation and migration of endothelial cells and induce
apoptosis (10,11). miR-126-3p and miR-126-5p are
considered as potential biomarkers of atherosclerosis (12). A recent study reported that
miR-20b is up-regulated in insulin-resistant skeletal muscle and is
involved in glucose metabolism regulation (13). Moreover, miR-20b has been shown to
play a critical role in maintaining vascular integrity, as the
down-regulation of miR-20b was found to be able to down-regulate
the level of a cellular senescence marker (14). However, studies are still needed
to confirm the role and mechanism of miR-20b in endothelial cell
senescence. In this study, the occurrence of senescence of HUVECs
cells was induced by hydrogen peroxide (H2O2)
and the role of miR-20b in the process of cellular senescence and
its possible mechanism were investigated.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from Sciencell Research Laboratories, Inc., (cat. no.
8000). The cells were cultured in the endothelium cell medium
(Sciencell Research Laboratories, Inc.) containing 5% fetal bovine
serum (Sigma-Aldrich; Merck KGaA), 1% endothelial growth factor
(Sigma-Aldrich; Merck KGaA), 100 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc.) in an incubator with 95%
O2 and 5% CO2 at 37°C.
Induction of HUVECs cell senescence by
H2O2
H2O2 was used to stimulate and
induce cell senescence in HUVECs. The cells were randomly divided
into 5 groups and treated with different concentrations of
H2O2 (0, 10, 50, 100 and 500 µM). The
blank group (0 µM H2O2) was cultured
in normal medium, whereas the H2O2 group was
incubated with normal medium at different concentrations of
H2O2 for 24 h. The cell viability, proportion
of senescent cells and cell cycle in each group were determined by
Cell-Counting-Kit 8 (CCK-8; Beyotime Institute of Biotechnology),
SA-β-galactosidase (SA-β-gal; Beyotime Institute of Biotechnology)
and FACSCalibur Flow Cytometer (cat. no. 342973; BD Biosciences;
Becton, Dickinson and Company).
Transfection
The specific groups were: Scramble group, miR-20b
mimic group, miR-20b inhibitor group, scramble +
H2O2 group, miR-20b mimic +
H2O2 group and miR-20b inhibitor +
H2O2 group. The sequences required for
transfection are shown in Table
I. The cells were transfected with the miR-20b mimic (100
pmol), miR-20b inhibitor (100 pmol) or 100 pmol scramble
(Sigma-Aldrich; Merck KGaA) using Lipofectamine 2000 Transfection
Reagent (Invitrogen; Thermo fisher Scientific, Inc.). Next, the
Lipofectamine 2000 reagent was mixed with DMEM and incubated with
the cells for 24 h at 37̊C with 5% CO2. After that, the
medium was replaced and certain groups of cells were stimulated by
H2O2 (100 µM) for 24 h, while the rest
was left untreated. The Scramble served as a negative control and
whether the transfection was successful was confirmed by performing
reverse transcription-quantitative (RT-q)PCR.
 | Table IThe sequences required for
transfection. |
Table I
The sequences required for
transfection.
| Sequence | 5′-3′ |
|---|
| miR-20b mimics |
ACUGUAGUAUGGGCACUUCCAG |
| miR-20b
inhibitor |
UGCUCAUAGUGCAGGUAGUU |
| Scramble C |
AUACAUUACCCGAAGUCUA |
| siTXNIP | |
| Forward |
CUCCCUGCUAUAUGGAUGUTT |
| Reverse |
ACAUCCAUAUAGCAGGGAGTT |
| siTXNIP negative
control | |
| Forward |
UUCUCCGAACGUGUCACGUTT |
| Reverse |
ACGUGACACGUUCGGAGGAGAATT |
Knocking down thioredoxin interacting
protein (TXNIP) using small interfering RNA (siRNA)
TXNIP was knocked down by siRNA (Forward, RNA oligo:
5′-AGA GAA AAA GCC UUC U UUC CC-3′, and reverse, RNA oligo: 5′-GAA
AGA AGG CUU UUU CUC UGA-3′, GE Healthcare Dharmacon Inc.) and a
non-specific siRNA served as a negative control (NC sense RNA
oligo: 5′-AGA AUC ACU GUA CAU CAA CUC UA-3′, antisense RNA oligo:
5′-GAG UUG AUG UAC AGU GAU UCU GC-3′). The HUVECs cells were
transfected with siTXNIP (100 pmol) by Lipofectamine 2000
Transfection Reagent, at the same time, the cells incubated with
Lipofectamine 2000 only were used as blank control group. Whether
the transfection was successful was confirmed by performing
RT-qPCR.
Next, the cells were co-transfected with miR-20b
inhibitor (100 pmol) and siTXNIP (100 pmol) or NC, with NC group
served as the control group. Some of the cells were stimulated by
H2O2 (100 µM) for 24 h, while the rest
was left untreated. The specific groups were as follows: NC group,
siTXNIP miR-20b inhibitor + NC group, miR-20b inhibitor + siTXNIP
group, miR-20b inhibitor + H2O2 + NC group
and miR-20b inhibitor + H2O2 + siTXNIP
group.
CCK-8 assay
The experimental operation of determining cell
viability was performed according to the protocol of the CCK-8
(Beyotime Institute of Biotechnology). The cells were cultured at
37°C with 95% O2 and 5% CO2 in an incubator
for 24 h, and CCK-8 solution was then added to further culture the
cells for 2 h. The optical density (OD) value of each well was
measured at 450 nm by enzyme-labeling instrument (Muliskan MK3;
Thermo Fisher Scientific, Inc.).
SA-β-gal assay
The previously treated HUVECs were washed twice by
PBS (Gibco; Thermo Fisher Scientific, Inc.) and fixed by cell
fixative solution in β-gal staining kit (Beyotime Institute of
Biotechnology) for 20 min at 37°C. Next, the cells were washed
three times by PBS to remove the solution. β-gal dyeing liquid
prepared in following the instruction was mixed with the cells and
incubated together overnight in a regular incubator at 37̊C for 24
h without CO2. The cells with blue cytoplasm by β-gal
staining were positive under a light microscope (BX41; Olympus
Corporation). A total of 10 fields in each group were selected
under the microscope and the total cells and SA-β-gal positive
cells in each field were counted by ImageJ (version 5.0; Bio-Rad
Laboratories, Inc.). The proportion of positive cells was obtained
by calculating the ratio of average SA-β-gal positive cell number
to average total cell number in each group.
Cell cycle assay
The cells were fixed by precooled 70% ethanol at 4°C
overnight, then re-suspended in PBS and cultured with RNAase (10
mg/ml) at 4°C for 1 h. The cells were then incubated with propidium
iodide (PI; 10 µg/ml) solution in the dark at 4°C for 1 h.
The FACSCalibur Flow Cytometer (BD Biosciences; Becton, Dickinson
and Company) was used to detect cell cycle, and data were processed
by CellQuest software (version 5.1; BD Biosciences; Becton,
Dickinson and Company).
Target gene prediction of miR-20b
Potential target genes for miR-20b were predicted by
Targetscan (http://www.targetscan.org/). RT-qPCR was carried out
to determine the effect of miR-20b on the expression of target
genes and Targetscan was used to predict the binding sites between
the target genes and miR-20b.
Plasmid transfection and dual luciferase
reporter assay
The binding ability of miR-20b and target genes was
detected by dual luciferase reporter. The wild-type or mutant (mut)
3′-untranslated region (UTR) sequences of TXNIP, or NLRP3 targeting
miR-20b was amplified by RT-qPCR and then respectively inserted
into the pGL3 control vectors (Promega Corporation). The HUVECs
were randomly divided into eight groups, namely: Control +
TXNIP-3′-UTR group, miR-20b mimic + TXNIP-3′-UTR group, Control +
TXNIP-3′-UTR mut group, miR-20b mimic + TXNIP-3′-UTR mut group,
Control + NLRP3-3′-UTRgroup, miR-20b mimic + NLRP3-3′-UTR group,
Control + NLRP3-3′-UTR mut group and miR-20b mimic + NLRP3-3′-UTR
mut group and transfected by Lipofectamine 2000 Transfection
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
recombinant vectors or miR-20b mimic was respectively transfected
into the cells for 24 h. The luciferase activities were measured
using the Dual-Glo Luciferase assay kits (Promega Corporation) and
normalized using a Renilla luciferase reference plasmid.
RT-qPCR
Total RNA was extracted by using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and total RNA (2
µg) were reverse-transcribed into cDNA by using a Taqman
MicroRNA Reverse Transcription kit (Applied Biosystems, Inc.) at
37°C for 15 min, at 85°C for 5 sec and preserved at 4°C. A
SYBR-Green Master (Rox) kit (Thermo Fisher Scientific, Inc.) and
RT-qPCR Detection System (ABI 7500; Life Technology; Thermo Fisher
Scientific, Inc.) were used to perform RT-qPCR, the cycles were set
as follows: Pretreatment at 95°C for 2 min, followed by 40 cycles
at 95°C for 30 sec, at 60°C for 35 sec and at 95°C for 15 sec and
finally preserved at 4°C. The 2−ΔΔCq method was used to
calculate the data (15). U6 and
GAPDH expression served as the internal control. The primer
sequences for RT-qPCR are shown in Table II.
 | Table IIThe primer sequences for reverse
transcription-quantitative PCR. |
Table II
The primer sequences for reverse
transcription-quantitative PCR.
| Primer | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| miR-20b |
GCTCATAGTGCAGGTAGAA |
TGTCAACGATACGCTACG |
| STAT3 |
CCTTCCTCACCGTGTACTGG |
AGCGTAGGGTAAGGTTCTTGC |
| SMAD7 |
ACTCCAGATACCCGATGGATTT |
CCTCCCAGTATGCCACCAC |
| TXNIP |
CAGAAGCTCCTCCCTGCTATATG |
GATGCAGGGATCCACCTCAG |
| NLRP3 |
CTTCCTTTCCAGTTTGCTGC |
TCTCGCAGTCCACTTCCTTT |
| p16 |
CACGGGTCGGGTGAGAGT |
CCCAACGCACCGAATAGTTAC |
| p21 |
GCCTGGACTGTTTTCTCTCG |
ATTCAGCATTGTGGGAGGAG |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| GAPDH |
AGTCAGCTCTCTCCTTTCAGG |
TCCACCACCCTGTTGCTGTA |
Western blotting (WB)
The cells were lysed to obtain total proteins using
radio immunoprecipitation assay lysis buffer (RIPA; Sigma Aldrich;
Merck KGaA). The concentration of proteins was determined by Pierce
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) and equal
amounts of total proteins (2 µg) were added to 10% SDS-PAGE
and transferred to polyvinylidene difluoride membrane (PVDF;
Bio-Rad Laboratories, Inc.). The PVDF membrane was blocked by 5%
non-fat milk at room temperature for 30 min and first incubated
with primary antibodies for 4°C overnight and then with secondary
antibodies for 2 h. The antibodies used during WB were anti-TXNIP
antibody (Rabbit; cat. no. ab188865; 1:1,000; Abcam), anti-GAPDH
antibody (mouse; cat. no. ab8245; 1:1,000; Abcam), anti-NLRP3
antibody (Rabbit; cat. no. ab214185; 1:200; Abcam), anti-Cleaved
Caspase-1 antibody (Rabbit; cat. no. 4199; 1:1,000; Cell Signaling
Technology, Inc.), anti-Caspase-1 antibody (Rabbit; cat. no. 3866;
1:1,000; Cell Signaling Technology, Inc.), anti-β-catenin antibody
(Rabbit, cat. no. ab32572; 1:5,000; Abcam), goat anti-mouse IgG
H&L (HRP; 1:2,000; cat. no. ab205719; Abcam) and goat
anti-rabbit IgG H&L (HRP; 1:2,000; cat. no. ab205718; Abcam).
ECL Blotting Detection Reagents (Applygen Technologies, Inc.) was
used to analyze the protein bands. The gray values of the protein
bands were analyzed by ImageJ (version 5.0; Bio-Rad, Laboratories,
Inc.) and the relative expressions of the target proteins were
determined by the ratio of the gray scale of target protein to the
internal reference. GAPDH served as internal control.
Statistical analysis
Data were analyzed by SPSS 21.0 system (IBM, Corps.)
and shown as the mean ± standard deviation. Significant differences
among different groups were analyzed using analysis of variance
followed by the Tukey post hoc test. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were repeated three times.
Results
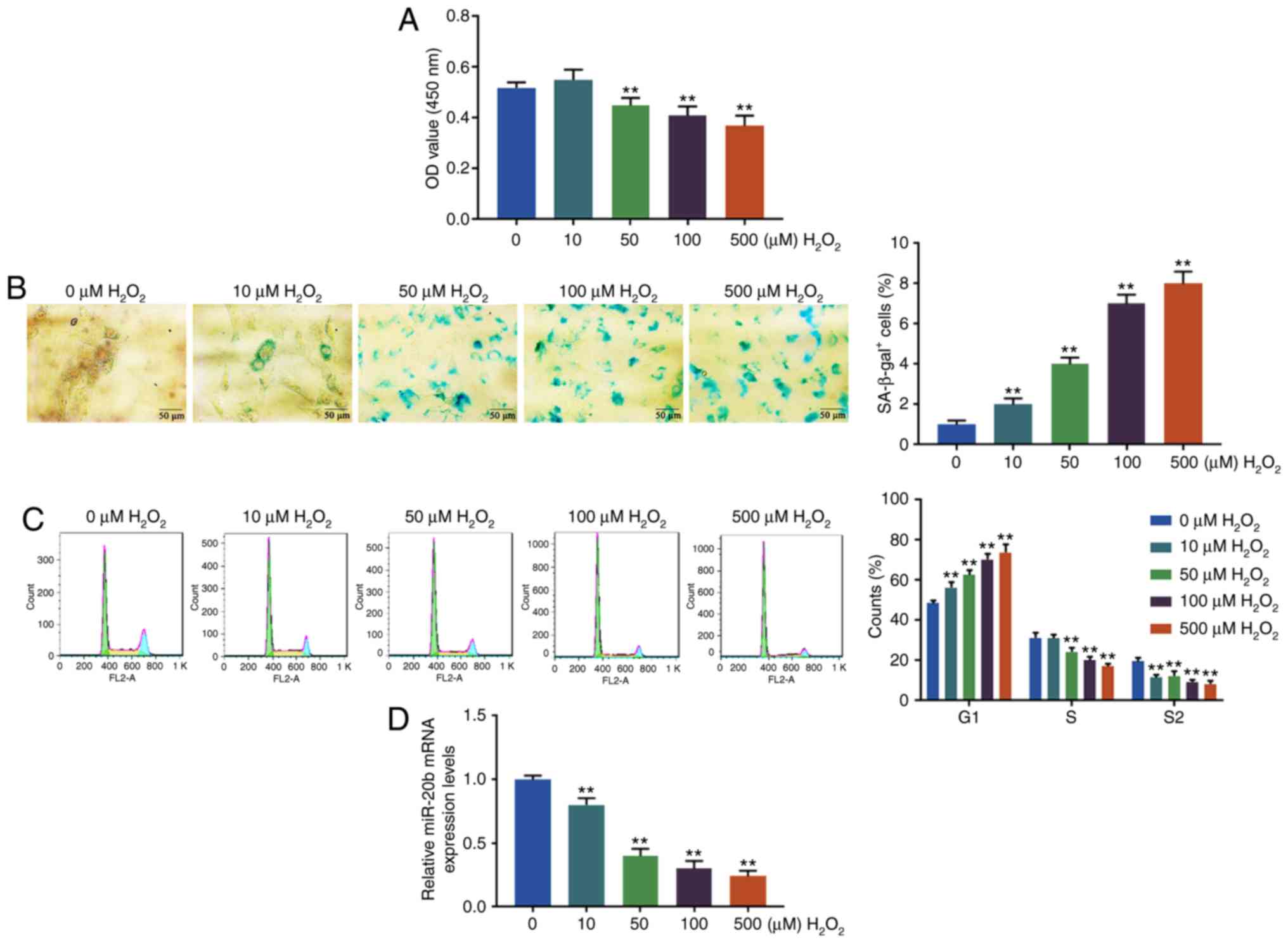
H2O2 induces cell
senescence and inhibits miR-20b expression
The cell viability, senescence and cycle of the
HUVECs were determined by CCK-8, SA-β-gal staining and flow
cytometry, respectively, after treating HUVECs at different
H2O2 concentrations (0, 10, 50, 100 and 500
µM). It was found that H2O2 at 50, 100
or 500 µM significantly reduced the cell viability of HUVECs
compared with 0 µM (P<0.01; Fig. 1A). As shown in Fig. 1B, the blue cytoplasm was positive
and the proportion of the positive cells significantly increased as
the concentration of H2O2 became higher
(P<0.01; Fig. 1B). At the same
time, the proportion of HUVECs in G1 phase significantly increased
with the increase of H2O2 concentration,
while the proportion of HUVECs in S and G2 phase significantly
decreased after H2O2 treatment (P<0.01;
Fig. 1C). The results suggested
that H2O2 inhibited cell viability, caused
cell cycle arrest in G1 phase and induced cell senescence.
Moreover, the expression level of miR-20b in HUVECs
was determined by RT-qPCR and the present study found that the
miR-20b level was significantly downregulated by
H2O2 treatment (P<0.01; Fig. 1D). The percentage of SA-β-gal+
cells in HUVECs exposed to H2O2 at 50
µM was only ~4%, while the percentage of SA-β-gal + cells in
HUVECs exposed to H2O2 at 100 µM was
7% (Fig. 1B). Based on the above
results and previous studies (16,17), to obtain more senescent cells and
avoid potentially excessive damage from H2O2
at higher concentration (500 µM), H2O2
at 100 µM was chosen for further experiments.
HUVECs cells are successfully transfected
with an miR-20b mimic or miR-20b inhibitor
In order to investigate the effect of miR-20b
expression on the senescence of HUVECs, the miR-20b mimic and
miR-20b inhibitor were respectively transfected into cells. RT-qPCR
was performed to confirm whether the transfection was successfully
conducted. The results demonstrated that compared with the scramble
group, the expression of the miR-20b in miR-20b mimic and miR-20b
mimic + H2O2 group were increased, while
those in miR-20b inhibitor, scramble + H2O2
and miR-20b inhibitor + H2O2 group were
significantly decreased (P<0.05; Fig. 2A). Compared with the scramble +
H2O2 group, the miR-20b expression was
significantly increased in miR-20b mimic +
H2O2 group, but significantly decreased in
miR-20b inhibitor + H2O2 group (P<0.05;
Fig. 2A). Therefore, the present
results showed that the expression of miR-20b in HUVECs was
successfully upregulated and downregulated.
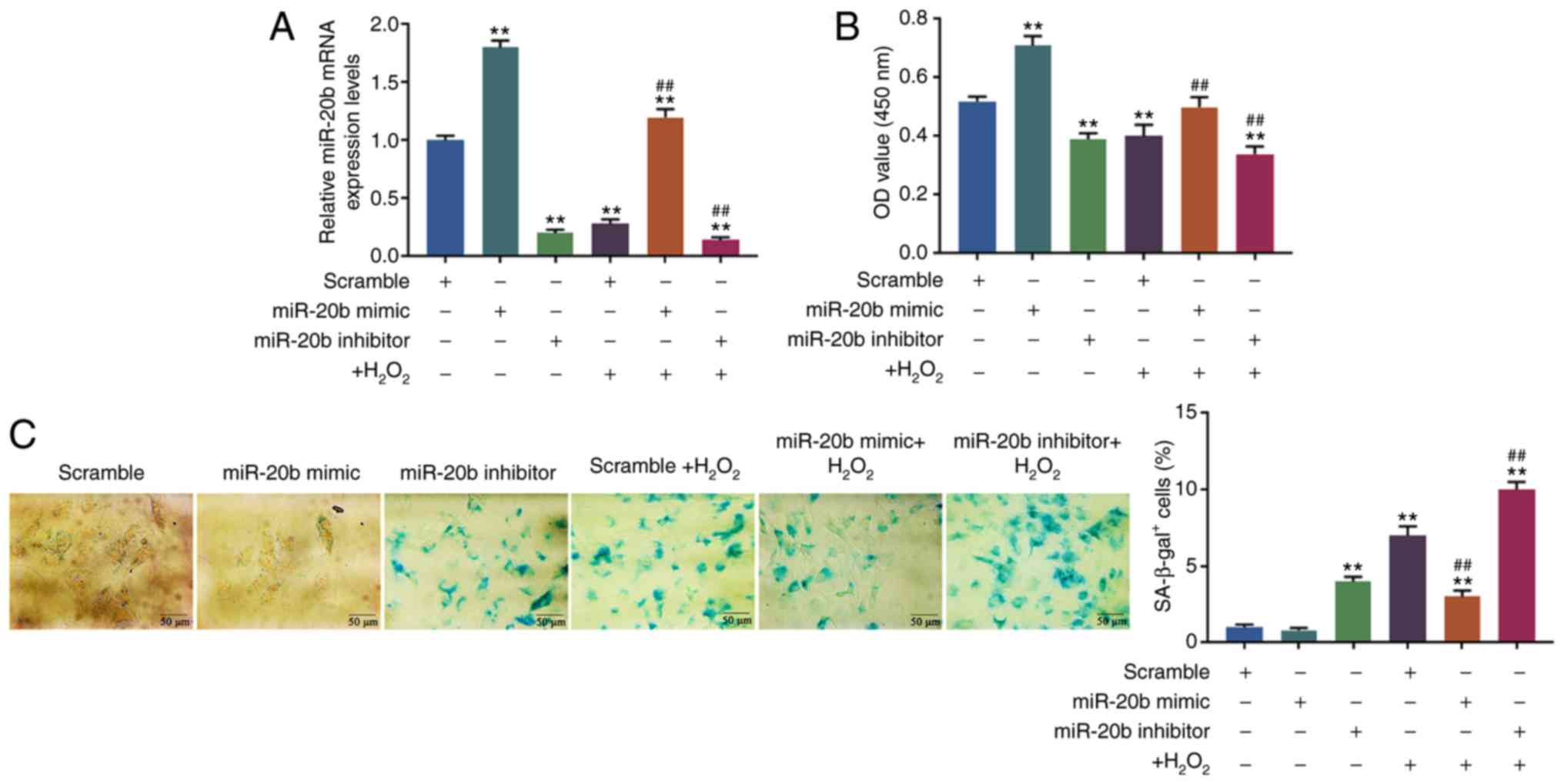 | Figure 2Effects of miR-20b expression level
on cell senescence. (A) The cells were transfected with the miR-20b
mimic, miR-20b inhibitor or scramble and the transfection rate was
detected by reverse transcription-quantitative PCR. (B) The effect
of miR-20b on cell viability was detected by Cell Counting Kit-8.
(C) The effect of miR-20b on cell senescence was detected by
SA-β-gal staining. The HUVECs were divided into 6 groups, namely
the scramble group, miR-20b mimic group, miR-20b inhibitor group,
scramble + H2O2 group, miR-20b mimic +
H2O2 group and miR-20b inhibitor +
H2O2 group. The cells were transfected with
the miR-20b mimic, miR-20b inhibitor or scramble and then
stimulated with H2O2 (100 µM), or left
untreated. **P vs. scramble; ##P<0.001 vs. scramble +
H2O2. HUVECs, human umbilical vein
endothelial cells; miR, microRNA; SA-β-gal, SA-β-galactosidase; OD,
optical density. |
High expression of miR-20b inhibits cell
senescence
The cell viability and senescence were determined by
CCK-8 and SA-β-gal staining, respectively, and it was found that
the cell viability was upregulated in miR-20b mimic group but
down-regulated in miR-20b inhibitor and miR-20b inhibitor +
H2O2 group compared with the scramble group
(P<0.05; Fig. 2B). In
comparison with the scramble + H2O2 group,
the cell viability was increased in the miR-20b mimic +
H2O2 group but decreased in the miR-20b
inhibitor + H2O2 group (P<0.05; Fig. 2B).
Moreover, the SA-β-gal staining results showed that
the proportion of β-gal positive cells significantly increased in
the miR-20b inhibitor, scramble + H2O2,
miR-20b mimic + H2O2 and miR-20b inhibitor +
H2O2 group (P<0.05; Fig. 2C). However, compared with scramble
+ H2O2 group, the proportion of β-gal
positive cells decreased in the miR-20b mimic +
H2O2 group but increased in miR-20b inhibitor
+ H2O2 group (P<0.05; Fig. 2C).
The results indicated that miR-20b could increase
cell viability and inhibit cell senescence, and that miR-20b
produced protective effect on HUVECs cells from
H2O2-induced cell senescence.
TXNIP is the target gene for miR-20b
Targetscan predicted that the possible target genes
for miR-20b were signal transducer and activator of transcription 3
(STAT3), mothers against decapentaplegic homolog 7 (SMAD7), TXNIP
and NLRP3. RT-qPCR was performed to detect the effect of miR-20b on
the possible target genes and it was found that in the scramble +
H2O2, miR-20b mimic +
H2O2, and miR-20b inhibitor +
H2O2 groups, the levels of STAT3 were
significantly downregulated, while that of SMAD7 was significantly
upregulated compared with the scramble group (P<0.05; Fig. 3A). The levels of TXNIP and NLRP3
were significantly decreased in the miR-20b mimic group compared
with those in the scramble group, but were still increased in the
miR-20b inhibitor, scramble + H2O2, miR-20b
mimic + H2O2 and miR-20b inhibitor +
H2O2 groups compared with those in the
scramble group (P<0.05; Fig.
3A). Compared with the scramble + H2O2
group, the levels of TXNIP and NLRP3 were significantly
downregulated in the miR-20b mimic + H2O2
group but were significantly upregulated in the miR-20b inhibitor +
H2O2 group (P<0.05; Fig. 3A). Thus, the data indicated that
the TXNIP and NLRP3 may be the target genes for miR-20b.
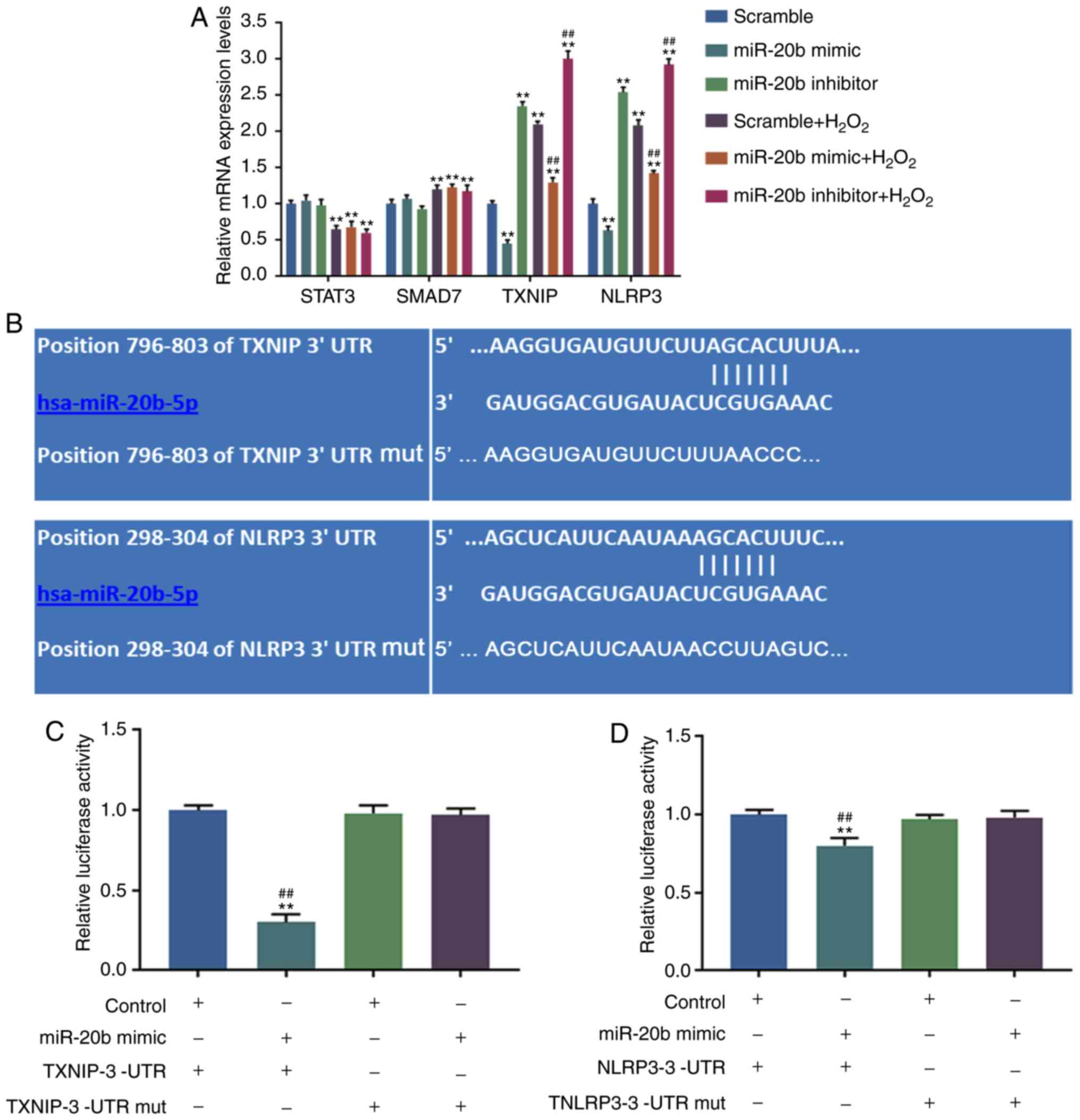 | Figure 3Target gene of miR-20b was predicted.
(A) The potential target genes of miR-20b were predicted by
Targetscan (http://www.targetscan.org/) and RT-qPCR was performed
for detection. **P<0.001 vs. scramble;
##P<0.001 vs. scramble + H2O2.
(B) The binding sites of the target gene and miR-20b were predicted
by Targetscan. (C) The binding abilities of miR-20b and TXNIP were
detected by dual luciferase reporter. **P<0.001 vs.
Control + TXNIP-3′-UTR; ##P<0.001 vs. miR-20b mimic +
TXNIP-3′-UTR mut. (D) The binding abilities of miR-20b and NLRP3
were detected by dual luciferase reporter. **P<0.001
vs. Control + TXNIP-3′-UTR; ##P<0.001 vs. miR-20b
mimic + TXNIP-3′-UTR mut. HUVECs were divided into 8 groups,
namely, Control + TXNIP-3′-UTR group, miR-20b mimic + TXNIP-3′-UTR
group, Control + TXNIP-3′-UTR mut group, miR-20b mimic +
TXNIP-3′-UTR mut group, Control + NLRP3-3′-UTRgroup, miR-20b mimic
+ NLRP3-3′-UTR group, Control + NLRP3-3′-UTR mut group and miR-20b
mimic + NLRP3-3′-UTR mut group. The pGL3 control vectors were used
to generate recombinant vectors with mut sequence or a 3′UTR
sequence of TXNIP or NLRP3 and the recombinant vectors and/or
miR-20b mimic were transfected into cells. Mut, mutant; UTR,
untranslated region; NLRP3, NLR family pyrin domain containing 3;
HUVECs, human umbilical vascular cell; TXNIP, thioredoxin
interacting protein. |
In order to further determine the direct target gene
for miR-20b, Targetscan website was used to predict the binding
sites between miR-20b and TXNIP, and NLRP3 (Fig. 3B), and the results were further
verified by dual luciferase reporter assay. The present study
observed that in the miR-20b mimic + TXNIP-3′-UTR and miR-20b mimic
+ NLRP3-3′-UTR groups, the relative luciferase activities were
significantly decreased, and the relative luciferase activity
changes of TXNIP was the most significant (P<0.05; Fig. 3C and D). Thus, TXNIP was
determined as the direct target gene for miR-20b.
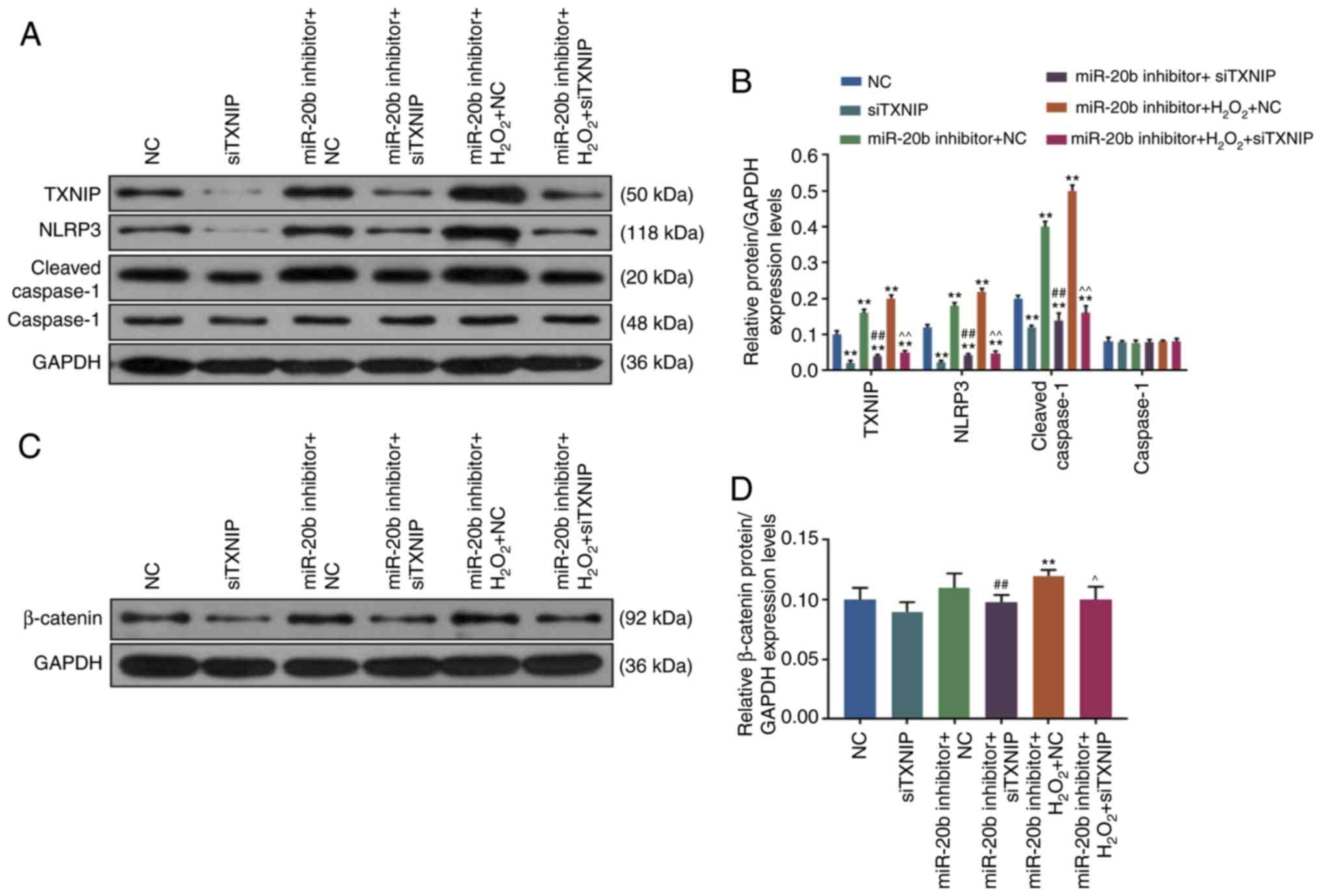
siTXNIP reverses the effects of miR-20b
inhibitor and H2O2
In order to investigate the mechanism of miR-20b in
the cell senescence of HUVECs, TXNIP siRNA was used to transform
HUVECs and the results were verified by RT-qPCR and WB. As shown in
Fig. 4B and C, the expression of
TXNIP decreased significantly in siTXNIP group (P<0.05),
suggesting that HUVECs cells with a low expression of TXNIP were
successfully constructed.
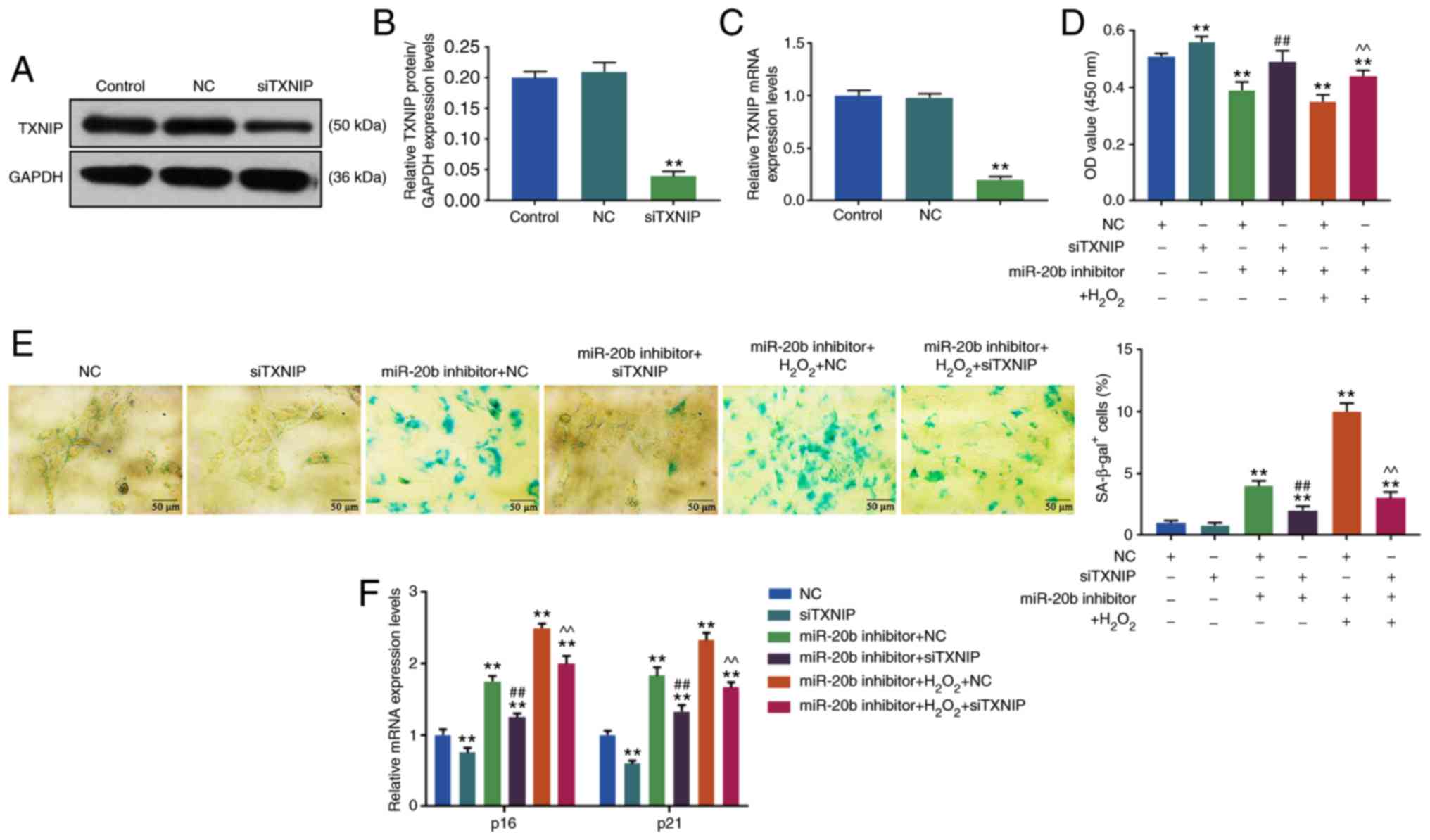 | Figure 4Effects of siTXNIP on cell
senescence. (A) Western blotting was used to determine the level of
TXNIP. The knockdown of TXNIP was achieved by siRNA, the HUVECs
cells were transfected with siTXNIP, the blank control group and NC
group were set at the same time (Control group, NC group, and
siTXNIP group). (B) The siTXNIP reduced the expression of TXNIP
protein. (C) RT-qPCR was used to determine the TXNIP mRNA
expression. (D) Cell viability was detected by Cell Counting Kit-8.
The HUVECs were divided into 6 groups, namely, NC group, siTXNIP
group, miR-20b inhibitor + NC group, miR-20b inhibitor + siTXNIP
group, miR-20b inhibitor + H2O2 + NC group
and miR-20b inhibitor + H2O2 + siTXNIP group.
The cells were co-transfected with miR-20b inhibitor and siTXNIP or
NC, and stimulated by H2O2 (100 µM),
or left untreated. (E) Cell senescence was detected by SA-β-gal
staining. (F) The expression of cell senescence-related genes were
detected by RT-qPCR. **P<0.001 vs. NC;
##P<0.001 vs. miR-20b inhibitor + NC;
^^P<0.001 vs. miR-20b inhibitor +
H2O2 + NC. RT-qPCR, reverse
transcription-quantitative; miR, microRNA; si, small interfering;
HHUVECs, human umbilical vascular endothelial cells; NC, negative
control; TXNIP, thioredoxin interacting protein. |
The cell viability and senescence of HUVECs were
determined by CCK-8 and SA-β-gal staining, respectively, and it was
found that the cell viability of HUVECs was increased in the
siTXNIP group compared with in the NC group, moreover, the miR-20b
inhibitor + NC group had a lower cell viability compared with in
the miR-20b inhibitor + siTXNIP group. Furthermore, the cell
viability of HUVECs was significantly increased in the miR-20b
inhibitor + H2O2 + siTXNIP group compared
with in miR-20b inhibitor + H2O2 + NC group
(P<0.05; Fig. 4D) and the cell
viability in miR-20b inhibitor + NC and miR-20b inhibitor +
H2O2 + NC groups decreased compared with NC
group (P<0.05; Fig. 4D).
SA-β-gal staining results showed that the
proportions of β-gal positive cells in miR-20b inhibitor + NC and
miR-20b inhibitor + H2O2 + NC groups were
significantlu increased compared with the NC group (P<0.05;
Fig. 4E), but decreased in the
miR-20b inhibitor + siTXNIP group compared with in miR-20b
inhibitor + NC group (P<0.05; Fig.
4E). Moreover, the number of β-gal positive cells was
significantly increased in miR-20b inhibitor +
H2O2 + siTXNIP group compared with that in
miR-20b inhibitor + H2O2 + NC group
(P<0.05; Fig. 4E).
The present data suggested that siTXNIP increased
cell viability and partially reversed the inhibitory effects of
miR-20b inhibitor and H2O2 on cell viability
and the promoting effects of miR-20b inhibitor and
H2O2 on cell senescence.
SiTXNIP reduces the expression of cell
senescence-related genes
The results of WB showed that compared with NC
group, the levels of p16 and p21 were decreased in siTXNIP group
but were significantly increased in the miR-20b inhibitor + NC and
miR-20b inhibitor + H2O2 + NC groups
(P<0.05; Fig. 4F). However,
the levels of p16 and p21 were decreased in the miR-20b inhibitor +
siTXNIP group compared with those in the miR-20b inhibitor + NC
group, and that the levels of p16 and p21 were lower in the miR-20b
inhibitor + H2O2 + siTXNIP group compared
with those in miR-20b inhibitor + H2O2 + NC
group (P<0.05; Fig. 4F).
Compared with NC group, the expression of TXNIP,
NLRP3 and cleaved Caspase-1 was significantly decreased in the
siTXNIP group but increased in the miR-20b inhibitor + NC and
miR-20b inhibitor + H2O2 + NC groups
(P<0.05; Fig. 5B). Moreover,
compared with the miR-20b inhibitor + NC group, the expression of
TXNIP, NLRP3 and cleaved Caspase-1 in miR-20b inhibitor + siTXNIP
group was decreased. However, the present study also found that the
expression of TXNIP, NLRP3 and cleaved Caspase-1 was significantly
decreased lower in the miR-20b inhibitor +
H2O2 + siTXNIP group compared with miR-20b
inhibitor + H2O2 + NC group, (P<0.05;
Fig. 5B). Thus, the data showed
that siTXNIP reduced the expression of senescence-related genes and
inhibited cell senescence.
SiTXNIP inhibits the Wnt/β-catenin
signaling pathway
The present study found that compared with NC group,
the expression level of β-catenin was downregulated in siTXNIP
group but was significantly upregulated in miR-20b inhibitor +
H2O2 + NC group (P<0.05; Fig. 5D), at the same time, the
expression of β-catenin in miR-20b inhibitor + siTXNIP and miR-20b
inhibitor + H2O2 + siTXNIP groups was
significantly decreased compared with in the miR-20b inhibitor + NC
and miR-20b inhibitor + H2O2 + NC groups
(P<0.05; Fig. 5D), suggesting
that siTXNIP decreased β-catenin expression and inhibited the
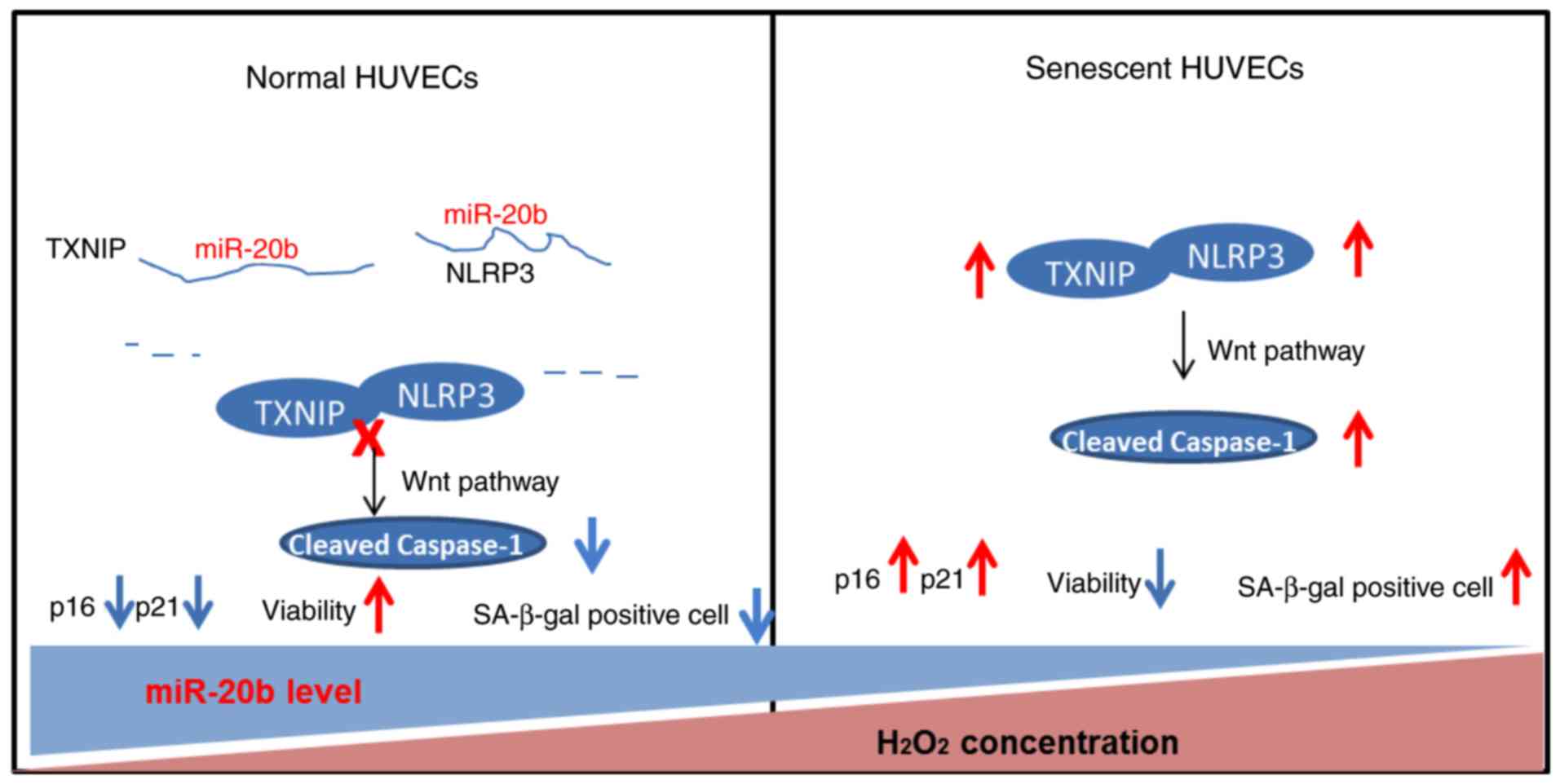
Wnt/β-catenin pathway. The working model of miR-20b is shown in
Fig. 6.
Discussion
Organismal aging, which refers to the progressive
decline of physiological functions, also involves a series of
inevitable physiological events (18-21). Manifested as growth stagnation and
changes in gene expression profiles, cell senescence is defined by
the irreversible detachment of cells from the cell cycle and the
loss of proliferative capacity (22,23). Vascular senescence refers to
cellular senescence in the vascular system and is closely related
to the occurrence of cardiovascular and metabolic disorders
(24). Endothelial cell
senescence, which can lead to vascular endothelial dysfunction and
aging of tissues and organs, is the major risk factor for
developing cardiovascular disease (6). A previous study demonstrated that
aging of endothelial cells is triggered by a variety of factors
such as oxidative stress, ionizing radiation and telomere
dysfunction (25). Oxidative
stress plays a crucial role in the occurrence of endothelial
dysfunction and is characterized by increased oxygen free radical
level and damage to organs and tissues (26,27).
A series of structural and functional changes, for
example, reduced cell division and proliferation, increased dyeing
rate of SA-β-gal, expression of senescence markers and
senescent-associated secretory phenotypes could also occur during
the process of endothelial cell senescence (28). It has been reported that different
concentrations of H2O2 treatment can induce
cell senescence in a variety of types of cells (29). In the current study, the HUVECs
were stimulated by H2O2 at different
concentrations and it was found that the cell viability was
decreased, while SA-β-gal positive cells were increased, and cell
cycle arrest occurred after H2O2 stimulation
intervention. Such results indicated that HUVECs cells treated by
H2O2 had typical characteristics of cell
senescence and that H2O2 can induce cell
senescence through oxidative stress. Meanwhile, the
H2O2 reduced miR-20b expression.
Interestingly, H2O2 at 10 M did not decrease
the cell viability, however, the level of miR-20b was
downregulated, suggesting that the miR-20b expression change was
more sensitive to H2O2 stimulation than cell
viability change and that the reduced expression in miR-20b at 10 M
H2O2 stimulation was not enough to cause
decreased cell viability.
miRNAs are directly involved in the pathological
process of numerous cardiovascular diseases (30). Lou et al (31) pointed out the unique role of
miR-20b in controlling tuberculosis progression. Wong et al
(32) showed that hsa-miR-20b is
downregulated in tumor necrosis factor (TNF)-α-induced senescent
microvascular endothelial cells. In addition, miR-20b is associated
with aging and tends to be highly-expressed in the thymus of young
mice (33) and upregulated in
UVB-induced senescent diploid fibroblasts (34). However, the exact mechanisms of
miR-20b in the regulation of endothelial cell senescence remains to
be further studied, for such a purpose, the present study
successfully constructed HUVECs cells with high and low expression
of miR-20b. The results showed that the high expression of miR-20b
increased cell viability and inhibited cell senescence, while the
low expression of miR-20b produced the opposite effects, suggesting
that a high level of miR-20b protected endothelial cells and
inhibited H2O2-mediated cell senescence.
These results indicated that loss of miR-20b expression might be
involved in promoting senescence of HUVECs. Additionally, it would
be better to perform cell cycle analysis on the miR-20b mimic or
miR-20b inhibitor transfected cells. However, the present study
focused on the cell senescence phenotype and cell viability, and
didn't have enough resources to perform the cell cycle assay in
each stage of this experiment. In addition, previous studies in
animal models indicate that miR-20b is positively involved in
hepatic ischaemia/reperfusion injury (35), breast cancer resistance (36), cardiac hypertrophy (37). However, whether it regulates the
cardiovascular senescence in animal model remains unknown.
To study the mechanism of miR-20b in endothelial
cell senescence, the potential target genes for miR-20b were
predicted by Targetscan and verified by RT-qPCR and dual luciferase
reporter. One recent report indicated that SMAD7 is a targeted gene
for miR-20b in insulin-resistant skeletal muscle (13). Another recent study also showed
that miR-20b is a circulating biomarker associated with type 2
diabetes and can target STAT3 (38). In the current study, SMAD7, STAT3,
TXNIP and NLRP3 were all predicted to be the targets for miR-20b by
Targetscan. However, RT-qPCR and dual lucif-erase reporter analyses
showed that TXNIP and NLRP3 were the main direct target genes for
miR-20b, while SMAD7, STAT3 could not be regulated by miR-20b.
Nevertheless, the expression of SMAD7 and STAT3 were reduced by
H2O2 stimulation. One study showed that
depletion of SMAD7 causes β cell aging (39). Another study also indicated that
the activation of STAT3 is necessary for TNFα-induced senescence
(40). Thus, the present study
inferred that SMAD7 and STAT3 may have a role in
H2O2 -induced cell senescence, although it
has not been confirmed in this study. Additionally, it seems that
the luciferase activity of cells transfected with TXNIP-3-UTR could
be more severely suppressed by the miR-20b mimic than cells
transfected with NLRP3-3-UTR, thus TXNIP was chosen for further
exploration. siRNA technology was applied to reduce the expression
of TXNIP and detect the role of TXNIP in endothelial cell
senescence. It was discovered that siTXNIP increased cell
viability, but decreased SA-β-gal positive cells and partially
reversed the effects of the miR-20b inhibitor and
H2O2 on endothelial cells. Senescent cells
are typically characterized by increased expression of cell
cell-cycle inhibitors (such as p21 and/or p16),
senescence-associated secretion phenotype, DNA damage and induced
SA-β-gal activity (41). The
senescence markers p16 and p21 play a regulatory role in the
process of cell senescence, and can mediate cell cycle arrest and
reduce the accumulation of damaged DNA (42,43). RT-qPCR data revealed that the
levels of p16 and p21 were downregulated by siTXNIP, and that low
level of TXNIP could partially reverse the senescence of
endothelial cell induced by miR-20b inhibitor and
H2O2.
Endothelial dysfunction is associated with the
activation of the TXNIP/NLRP3 inflammasome axis (44). TXNIP plays a critical role in
redox regulation, cell growth and other pathways, moreover, the
increased expression of TXNIP leads to cell senescence of
endothelial cells (45). The
NLRP3 inflammasome is involved in the mechanism of cells senescence
(46). TXNIP can bind to NLRP3
under oxidative stress, thereby activating the NLRP3 inflammasome
(47). The activated NLRP3
inflammasome can further activate Caspase-1, while the activated
Caspase-1 increases the expression of pro-inflammatory cytokines
and promotes inflammation response and oxidative stress, therefore
inducing endothelial cell senescence (48,49). In the current study, siTXNIP
reduced the expression of TXNIP, NLRP3 and cleaved Caspase-1. The
results reveal that siTXNIP inhibits the activation of the NLRP3
inflammasome and Caspase-1, reduces oxidative stress and reverses
the cell senescence induced by oxidative stress.
The Wnt/β-catenin pathway is an important pathway
that regulates the biological behavior of cells and plays different
roles in diverse tissues and diseases (50). Some studies revealed that the
excessive activation of the Wnt/β-catenin signaling pathway is
associated with cellular senescence and that the p53/p21 pathway is
the main mediator in cell senescence induced via Wnt/β-catenin
pathway (51,52). In this study, siTXNIP was found to
inhibit Wnt/β-catenin pathway and the present study speculated that
TXNIP mainly regulate cell senescence via regulating the
Wnt/β-catenin pathway. Moreover, a relatively slight decrease of
cleaved Caspase-1 was observed compared with the change in TXNIP
and NLRP3. A previous study indicated that the PI3K pathway is also
involved in the activation of cleaved Caspase-1 and the present
study inferred that TXNIP and NLRP3 regulate the activation of
cleaved-Caspase 1; however, it is not the only pathway involved in
activating cleaved-Caspase 1 in senescent HUVECs. In addition, as
the interleukin (IL)-1β precursor is cleaved by cleaved-Caspase 1,
further experiments should be performed to determine the level of
IL-1β in HUVECs. Additionally, experiments in animals should be
performed to confirm the present conclusion.
In conclusion, the present study demonstrated high
expression of miR-20b promotes cell viability and inhibits cell
senescence induced by oxidative stress. TXNIP is a direct target
gene for miR-20b, and silencing of TXNIP enhances cell viability,
reduces the expression of cell-senescence related genes and
inhibits the Wnt/β-catenin pathway. The high expression of miR-20b
inhibits endothelial cell senescence, which is possibly realized
through regulating the TXNIP/NLRP3 axis to inhibit the
Wnt/β-catenin pathway.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
Substantial contributions to conception and design:
FD and XZ, Data acquisition, data analysis and interpretation: SD,
YL, KW and YQ, Drafting the article or critically revising it for
important intellectual content: FD and XZ. Final approval of the
version to be published: All authors. Agreement to be accountable
for all aspects of the work in ensuring that questions related to
the accuracy or integrity of the work are appropriately
investigated and resolved: All authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Mitchell SJ, Scheibye-Knudsen M, Longo DL
and de Cabo R: Animal models of aging research: Implications for
human aging and age-related diseases. Annu Rev Anim Biosci.
3:283–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu IC, Lin CC and Hsiung CA: Emerging
roles of frailty and inflammaging in risk assessment of age-related
chronic diseases in older adults: The intersection between aging
biology and personalized medicine. Biomedicine (Taipei). 5:12015.
View Article : Google Scholar
|
|
3
|
Thijssen DH, Carter SE and Green DJ:
Arterial structure and function in vascular ageing: Are you as old
as your arteries? J Physiol. 594:2275–2284. 2016. View Article : Google Scholar :
|
|
4
|
Maeda M, Hayashi T, Mizuno N, Hattori Y
and Kuzuya M: Intermittent high glucose implements stress-induced
senescence in human vascular endothelial cells: Role of superoxide
production by NADPH oxidase. PLoS One. 10:e01231692015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su JB: Vascular endothelial dysfunction
and pharmacological treatment. World J Cardiol. 7:719–741. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donato AJ, Morgan RG, Walker AE and
Lesniewski LA: Cellular and molecular biology of aging endothelial
cells. J Mol Cell Cardiol. 89:122–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang S, Mi X, Chen Y, Feng C, Hou Z, Hui R
and Zhang W: MicroRNA-216a induces endothelial senescence and
inflammation via Smad3/IKBα pathway. J Cell Mol Med. 22:2739–2749.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao Q, Chen K, Gao L, Zheng Y and Yang YG:
Thrombospondin-1 signaling through CD47 inhibits cell cycle
progression and induces senescence in endothelial cells. Cell Death
Dis. 7:e23682016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dangwal S and Thum T: microRNA
therapeutics in cardiovascular disease models. Annu Rev Pharmacol
Toxicol. 54:185–203. 2014. View Article : Google Scholar
|
|
10
|
Xue Y, Wei Z, Ding H, Wang Q, Zhou Z,
Zheng S, Zhang Y, Hou D, Liu Y, Zen K, et al: MicroRNA-19b/221/222
induces endothelial cell dysfunction via suppression of PGC-1α in
the progression of atherosclerosis. Atherosclerosis. 241:671–681.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CW, Sung HC, Lin SR, Wu CW, Lee CW,
Lee IT, Yang YF, Yu IS, Lin SW, Chiang MH, et al: Resveratrol
attenuates ICAM-1 expression and monocyte adhesiveness to
TNF-α-treated endothelial cells: Evidence for an anti-inflammatory
cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci
Rep. 7:446892017. View Article : Google Scholar
|
|
12
|
Schober A, Nazari-Jahantigh M, Wei Y,
Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H,
Hristov M, et al: MicroRNA-126-5p promotes endothelial
proliferation and limits atherosclerosis by suppressing Dlk1. Nat
Med. 20:368–376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao D, Hu Y, Fu Y, Wang R, Zhang H, Li M,
Li Z, Zhang Y, Xuan L, Li X, et al: Emodin improves glucose
metabolism by targeting microRNA-20b in insulin-resistant skeletal
muscle. Phytomedicine. 59:1527582019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamakuchi M and Hashiguchi T: Endothelial
cell aging: How miRNAs contribute? J Clin Med. 7:pii: E170. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Lin YJ, Zhen YZ, Wei J, Liu B, Yu ZY and
Hu G: Effects of Rhein lysinate on H2O2-induced cellular senescence
of human umbilical vascular endothelial cells. Acta Pharmacol Sin.
32:1246–1252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitsumoto A, Takanezawa Y, Okawa K,
Iwamatsu A and Nakagawa Y: Variants of peroxiredoxins expression in
response to hydroperoxide stress. Free Radic Biol Med. 30:625–635.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bulterijs S, Hull RS, Björk VC and Roy AG:
It is time to classify biological aging as a disease. Front Genet.
6:2052015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gems D: The aging-disease false dichotomy:
Understanding senescence as pathology. Front Genet. 6:2122015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos
D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, et al:
Cellular senescence drives age-dependent hepatic steatosis. Nat
Commun. 8:156912017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Childs BG, Durik M, Baker DJ and van
Deursen JM: Cellular senescence in aging and age-related disease:
From mechanisms to therapy. Nat Med. 21:1424–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garrido AM and Bennett M: Assessment and
consequences of cell senescence in atherosclerosis. Curr Opin
Lipidol. 27:431–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan LM, Douglas G, Bendall JK, McNeill E,
Crabtree MJ, Hale AB, Mai A, Li JM, McAteer MA, Schneider JE, et
al: Endothelial cell-specific reactive oxygen species production
increases susceptibility to aortic dissection. Circulation.
129:2661–2672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katsuumi G, Shimizu I, Yoshida Y and
Minamino T: Vascular senescence in cardiovascular and metabolic
diseases. Front Cardiovasc Med. 5:182018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Bloom SI and Donato AJ: The role of
senescence, telomere dysfunction and shelterin in vascular aging.
Microcirculation. 26:e124872019. View Article : Google Scholar
|
|
26
|
Conti V, Corbi G, Simeon V, Russomanno G,
Manzo V, Ferrara N and Filippelli A: Aging-related changes in
oxidative stress response of human endothelial cells. Aging Clin
Exp Res. 27:547–553. 2015. View Article : Google Scholar
|
|
27
|
Liu R, Liu H, Ha Y, Tilton RG and Zhang W:
Oxidative stress induces endothelial cell senescence via
downregulation of Sirt6. Biomed Res Int. 2014:9028422014.
View Article : Google Scholar
|
|
28
|
Mistriotis P and Andreadis ST: Vascular
aging: Molecular mechanisms and potential treatments for vascular
rejuvenation. Ageing Res Rev. 37:94–116. 2017. View Article : Google Scholar
|
|
29
|
Crowe EP, Tuzer F, Gregory BD, Donahue G,
Gosai SJ, Cohen J, Leung YY, Yetkin E, Nativio R, Wang LS, et al:
Changes in the transcriptome of human astrocytes accompanying
oxidative stress-induced senescence. Front Aging Neurosci.
8:2082016. View Article : Google Scholar
|
|
30
|
Bu H, Wedel S, Cavinato M and Jansen-Dürr
P: MicroRNA regulation of oxidative stress-induced cellular
senescence. Oxid Med Cell Longev. 2017:23986962017. View Article : Google Scholar
|
|
31
|
Lou J, Wang Y, Zhang Z and Qiu W: MiR-20b
inhibits myco-bacterium tuberculosis induced inflammation in the
lung of mice through targeting NLRP3. Exp Cell Res. 358:120–128.
2017. View Article : Google Scholar
|
|
32
|
Wong PF, Jamal J, Tong KL, Khor ES, Yeap
CE, Jong HL, Lee ST, Mustafa MR and Abubakar S: Deregulation of
hsa-miR-20b expression in TNF-α-induced premature senescence of
human pulmonary microvascular endothelial cells. Microvasc Res.
114:26–33. 2017. View Article : Google Scholar
|
|
33
|
Guo D, Ye Y, Qi J, Tan X, Zhang Y, Ma Y
and Li Y: Age and sex differences in microRNAs expression during
the process of thymus aging. Acta Biochim Biophys Sin (Shanghai).
49:409–419. 2017. View Article : Google Scholar
|
|
34
|
Greussing R, Hackl M, Charoentong P, Pauck
A, Monteforte R, Cavinato M, Hofer E, Scheideler M, Neuhaus M,
Micutkova L, et al: Identification of microRNA-mRNA functional
interactions in UVB-induced senescence of human diploid
fibroblasts. BMC Genomic. 14:2242013. View Article : Google Scholar
|
|
35
|
Tang B, Bao N, He G and Wang J: Long
noncoding RNA HOTAIR regulates autophagy via the miR-20b-5p/ATG7
axis in hepatic ischemia/reperfusion injury. Gene. 686:56–62. 2019.
View Article : Google Scholar
|
|
36
|
Ao X, Nie P, Wu B, Xu W, Zhang T, Wang S,
Chang H and Zou Z: Decreased expression of microRNA-17 and
microRNA-20b promotes breast cancer resistance to taxol therapy by
upregulation of NCOA3. Cell Death Dis. 7:e24632016. View Article : Google Scholar
|
|
37
|
Zhang M, Jiang Y, Guo X, Zhang B, Wu J,
Sun J, Liang H, Shan H, Zhang Y, Liu J, et al: Long non-coding RNA
cardiac hypertrophy-associated regulator governs cardiac
hypertrophy via regulating miR-20b and the downstream PTEN/AKT
pathway. J Cell Mol Med. 23:7685–7698. 2019. View Article : Google Scholar
|
|
38
|
Katayama M, Wiklander OPB, Fritz T,
Caidahl K, El-Andaloussi S, Zierath JR and Krook A: Circulating
exosomal miR-20b-5p is elevated in type 2 diabetes and could impair
insulin action in human skeletal muscle. Diabetes. 68:515–526.
2019.
|
|
39
|
Xiao X, Chen C, Guo P, Zhang T, Fischbach
S, Fusco J, Shiota C, Prasadan K, Dong H and Gittes GK: Forkhead
box protein 1 (FoxO1) inhibits accelerated β cell aging in
pancreas-specific SMAD7 mutant mice. J Biol Chem. 292:3456–3465.
2017. View Article : Google Scholar
|
|
40
|
Kandhaya-Pillai R, Miro-Mur F,
Alijotas-Reig J, Tchkonia T, Kirkland JL and Schwartz S:
TNFα-senescence initiates a STAT-dependent positive feedback loop,
leading to a sustained interferon signature, DNA damage, and
cytokine secretion. Aging (Albany NY). 9:2411–2435. 2017.
View Article : Google Scholar
|
|
41
|
Wu Q, Jiang D, Matsuda JL, Ternyak K,
Zhang B and Chu HW: Cigarette smoke induces human airway epithelial
senescence via growth differentiation factor 15 production. Am J
Respir Cell Mol Biol. 55:429–438. 2016. View Article : Google Scholar
|
|
42
|
Jin H, Lian N, Bian M, Zhang C, Chen X,
Shao J, Wu L, Chen A, Guo Q, Zhang F and Zheng S: Oroxylin A
inhibits ethanol-induced hepatocyte senescence via YAP pathway.
Cell Prolif. 51:e124312018. View Article : Google Scholar
|
|
43
|
Khan SY, Awad EM, Oszwald A, Mayr M, Yin
X, Waltenberger B, Stuppner H, Lipovac M, Uhrin P and Breuss JM:
Premature senescence of endothelial cells upon chronic exposure to
TNFα can be prevented by N-acetyl cysteine and plumericin. Sci Rep.
7:395012017. View Article : Google Scholar
|
|
44
|
Li Y, Yang J, Chen MH, Wang Q, Qin MJ,
Zhang T, Chen XQ, Liu BL and Wen XD: Ilexgenin A inhibits
endoplasmic reticulum stress and ameliorates endothelial
dysfunction via suppression of TXNIP/NLRP3 inflammasome activation
in an AMPK dependent manner. Pharmacol Res. 99:101–115. 2015.
View Article : Google Scholar
|
|
45
|
Riahi Y, Kaiser N, Cohen G, Abd-Elrahman
I, Blum G, Shapira OM, Koler T, Simionescu M, Sima AV, Zarkovic N,
et al: Foam cell-derived 4-hydroxynonenal induces endothelial cell
senescence in a TXNIP-dependent manner. J Cell Mol Med.
19:1887–1899. 2015. View Article : Google Scholar :
|
|
46
|
Yin Y, Zhou Z, Liu W, Chang Q, Sun G and
Dai Y: Vascular endothelial cells senescence is associated with
NOD-like receptor family pyrin domain-containing 3 (NLRP3)
inflammasome activation via reactive oxygen species
(ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int J
Biochem Cell Biol. 84:22–34. 2017. View Article : Google Scholar
|
|
47
|
Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y
and Chen Y: Trimethylamine N-oxide induces inflammation and
endothelial dysfunction in human umbilical vein endothelial cells
via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res
Commun. 481:63–70. 2016. View Article : Google Scholar
|
|
48
|
Spadaro O, Goldberg EL, Camell CD, Youm
YH, Kopchick JJ, Nguyen KY, Bartke A, Sun LY and Dixit VD: Growth
hormone receptor deficiency protects against age-related NLRP3
inflam-masome activation and immune senescence. Cell Rep.
14:1571–1580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He B, Zhang B, Wu F, Wang L, Shi X, Qin W,
Lin Y, Ma S and Liang J: Homoplantaginin inhibits palmitic
acid-induced endothelial cells inflammation by suppressing TLR4 and
NLRP3 inflammasome. J Cardiovasc Pharmacol. 67:93–101. 2016.
View Article : Google Scholar
|
|
50
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang DY, Wang HJ and Tan YZ:
Wnt/β-catenin signaling induces the aging of mesenchymal stem cells
through the DNA damage response and the p53/p21 pathway. PLoS One.
6:e213972011. View Article : Google Scholar
|
|
52
|
Chen J, Jia YS, Liu GZ, Sun Q, Zhang F, Ma
S and Wang YJ: Role of LncRNA TUG1 in intervertebral disc
degeneration and nucleus pulp-osus cells via regulating
Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun.
491:668–674. 2017. View Article : Google Scholar : PubMed/NCBI
|