Introduction
Acute kidney injury (AKI) is a complex
pathophysiological process that is associated with the progression
of chronic kidney disease. Previous studies have highlighted the
role of the innate immune system and inflammatory mechanisms in the
progression of AKI, particularly the activation of
pattern-recognition receptors (PRRs) (1,2).
Nucleotide-binding and oligomerization domain (NOD)-like receptors
(NLRs), which are members of the family of PRRs, are known to be
involved in kidney ischemia/reperfusion (I/R) injury (3,4),
and may represent a potential therapeutic target to abrogate the
pathogenesis of AKI. Among the known NLRs, nucleotide-binding
oligomerization domain-containing 2 (NOD2) and nucleotide-binding
domain and leucine-rich repeat pyrin 3 domain (NLRP3) act as
sensors of 'cellular danger' (5),
and belong to two different subfamilies based on the nature of
their N-terminal domains. These proteins play a key role in the
control of inflammatory and immune responses through the modulation
of different signaling pathways, including those dependent on
nuclear factor (NF)-κB and the caspase-1-mediated cleavage of
interleukin (IL)-1β and IL-18, respectively (6,7).
Moreover, NOD2 and NLRP3 are extensively involved in the
progression of renal diseases (8-12).
Suppression of NOD2-mediated immune responses was found to
attenuate hypoxia-induced inflammatory effects and apoptosis in
proximal tubule epithelial cells (13), and knockout of NLRP3 was shown to
inhibit the activation of bone marrow-derived cells and T cells in
a mouse IgA nephropathy model (10). Therefore, preventing inflammation
mediated by the activation of NLRs may be considered as a potential
therapeutic option for AKI.
Ligustrazine is a bioactive alkaloid that is
extracted from the Chinese herb Ligusticum wallichii
Franchat, which has long been used for the treatment of cardiac and
cerebral diseases (14). As a
calcium antagonist and reactive oxygen species scavenger,
ligustrazine can significantly improve cardiac and cerebral blood
flow (15,16). It may also be used to alleviate
clinical renal injury following AKI. However, the mechanisms
underlying its protective effects remain poorly understood. The
anti-inflammatory effect of ligustrazine was recently demonstrated
in patients with rheumatic heart disease, an allergic asthma mouse
model, and a rat model of spinal cord I/R injury (17-19), suggesting that this effect may
represent the mechanism through which this compound confers renal
protection. The aim of the present study was to investigate whether
ligustrazine can inhibit NOD2-mediated inflammation.
Materials and methods
Animal studies
A total of 27 male Sprague-Dawley rats, aged 8 weeks
and weighing 280-300 g, were purchased from the Laboratory Animals
Center of Shandong University. The animals were housed in standard
cages and maintained under standard conditions at a constant room
temperature of 20-25°C, a humidity of 40-70% and a 12/12 h
light/dark cycle, with unrestricted access to food and water. The
methods for generating a kidney I/R injury model were as follows:
The rats were anesthetized via intraperitoneal injection of
pentobarbital sodium (50 mg/kg body weight). Subsequently, the left
renal artery and vein were exposed via an abdominal midline
incision and separated. Ischemia of the left kidney was induced by
occluding the artery with non-traumatic microvascular clamps. The
right renal artery was immediately separated from the branch
originating from the abdominal aorta and occluded by non-traumatic
microvascular clamps. The kidney color then changed from red to
black-red on visual inspection, which indicated that the cessation
of blood flow was successful. At 50 min after induction of
ischemia, the clamps were removed and the color of the kidneys
returned to red, indicating reperfusion. The incisions were
sutured, followed by the injection of penicillin and saline (30
µl/g body weight) to replenish fluid loss. Reperfusion
lasted for 24 h. The rats were allowed to recover from anesthesia
between I/R and the endpoint of the experiment 24 h later.
Ligustrazine-treated rats were administered ligustrazine
hydrochloride (40 mg/kg body weight; Harbin Medisan Pharmaceutical,
Co., Ltd.) via intraperitoneal injection once every 6 h during the
reperfusion period (20-22). During surgery, all rats were
placed on a homeothermic pad to maintain body temperature at 37°C,
and wet warm gauze was used to cover the incision to keep the
tissue moist. After 24 h of reperfusion, the rats were anesthetized
by intraperitoneal injection of 50 mg/kg pentobarbital sodium, and
then rapidly sacrificed using CO2 asphyxiation, with a
fill rate of 20% of the chamber volume/min. Death was confirmed
using a combination of criteria, including lack of pulse,
breathing, corneal reflex, response to a firm toe pinch and graying
of the mucous membranes, which conformed to the AVMA Guidelines for
the Euthanasia of Animals: 2013 Edition (23). Plasma and tissue samples were
collected and stored at −80°C or fixed in 4% formaldehyde at 4°C
for 24 h for analysis.
Assessment of renal function
Blood from the heart was collected into homemade
anticoagulant tubes with 3.8% sodium citrate, and serum was
isolated by centrifugation at 3,000 × g for 10 min at 4°C. Serum
creatinine (SCr) and blood urea nitrogen (BUN) were measured using
creatinine assay kits and urea assay kits in accordance with the
manufacturer's instructions (Nanjing Jiancheng Bioengineering
Institute).
Histological assessment
Formalin-fixed kidneys were embedded in paraffin,
and 4-µm sections were stained with hematoxylin for 5 min
and eosin for 1-3 min at room temperature (25°C). Histological
scoring was conducted in a blinded manner. The percent injury in
tubules of the outer medulla based on cell blebbing or
vacuolization and/or necrosis was scored as follows: 0 (none); 1
(0-10%); 2 (11-25%); 3 (26-45%); 4 (46-75%); or 5 (>75%). A
total of 10 high-power fields (magnification, ×200) per section
were examined by Leica DM 6000 B light microscope (Leica
Microsystems GmbH).
Immunohistochemical examination
Paraffin-embedded renal tissue sections were used
for immunohistochemistry and the samples were examined using a
Leica DM 6000 B light microscope (Leica Microsystems GmbH). The
sections were incubated with primary polyclonal antibodies against
NOD2 (cat. no. ab197030, 1:200, Abcam) and caspase 3/cleaved
caspase 3 (cat. no. WL02117, 1:100, Wanleibio Co., Ltd.) at 4°C
overnight. Subsequently, the sections were incubated with the
secondary HRP-goat anti-rabbit IgG antibodies for 30 min at room
temperature (25°C) according to the rabbit polymer detection system
(PV-6001; ZSGB-BIO).
RNA extraction and reverse transcription
quantitative PCR (RT-qPCR) analysis
Total RNA was isolated from the kidney using TRNzol
reagent (Tiangen Biotech Co., Ltd.) and converted to complementary
DNA by RT kit (Tiangen Biotech Co., Ltd.) at 70°C for 5 min, 37°C
for 5 min, 42°C for 60 min and 70°C for 10 min. qPCR reactions (20
µl) were performed with SYBR® Premix Ex Taq™
(Takara Bio Inc.), and the thermocycling conditions were as
follows: 94°C for 5 min, 95°C for 30 sec, followed by 40 cycles at
59°C for 30 sec, 72°C for 30 sec, 72°C for 10 min and 65-95°C for
15 min. Bio-Rad iCycler system software, version 3.1 (Bio-Rad
Laboratories, Inc.) was used for quantitative data analysis. The
specific primers were as follows: Forward, 5′-TAC CTG AGA AAG CAC
CAC CG-3′ and reverse, 5′-GCA CTG ACA GCC AAG TAG AAC G-3′ for
NOD2; and forward, 5′-TGC ATC CTG CAC CAC CAA CTG C-3′ and reverse,
5′-ACA GCC TTG GCA GCA CCA G TG G-3′ for the housekeeping gene
GAPDH. The 2−∆∆Cq method was used to calculate the
relative mRNA expression (24).
Cell culture and treatments
Rat proximal tubule epithelial cells (NRK-52E cells,
Falcon, BD Biosciences) were cultured in serum-free Dulbecco's
modified Eagle's medium (HyClone; GE Healthcare Life Sciences) at
pH 7.4 and 37°C with 5% CO2. The medium was changed 2 h
before all the experiments were performed.
In vitro experiments were performed using two
models to mimic hypoxic conditions. The first model included
incubating NRK-52E cells with different concentrations of
CoCl2 (0, 100, 250 and 500 µM) for 12 h (anoxia)
(13). The second model involved
oxygen and glucose deprivation (OGD) followed by reoxygenation, in
which NRK-52E cells were incubated in a hypoxic environment for 2 h
(1%O2 and glucose-free buffer), which was followed by
reoxygenation for 24 h. Subsequently, in vitro reperfusion
was achieved by incubating cells in normal medium for 24 h
(recovery); ligustrazine (30 and 50 µM) was added
immediately after reperfusion in routine culture medium for 24 h.
Chloroquine (CQ; Sigma-Aldrich; Merck KGaA), an autophagy
inhibitor, was also added at a concentration of 50 µM
immediately after reperfusion in routine culture medium for 24
h.
Western blot analysis
Renal cortical tissues and cultured cells were
homogenized in ice-cold RIPA lysis buffer with 1 mM
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology). Protein quantification was determined by Enhanced
BCA Protein Assay kit (P0006, Beyotime Institute of Biotechnology).
Equal amounts of protein extract (40 µg) were loaded per
lane and separated by 8-12% sodium dodecyl sulfate polyacrylamide
gel electrophoresis and transferred to polyvinylidene fluoride
membranes (EMD Millipore). Non-specific binding was blocked by
incubation with 5% skimmed milk for 15 min at room temperature. The
membranes were incubated with the indicated primary antibodies at
4°C overnight and subsequently hybridized with horseradish
peroxidase-conjugated secondary antibodies (ProteinTech Group,
Inc.) for 1 h at room temperature. The bands were visualized using
Millipore Immobilon ECL (EMD Millipore). Primary antibodies
included those against NOD2 (cat. no. ab197030, 1:1,000, Abcam),
LC3A/B (cat. no. 4108, 1:1,000, Cell Signaling Technology, Inc.),
CD68 (cat. no. wl01218, 1:100, Wanleibio Co., Ltd.) and β-actin
(cat. no. 60008-1-Ig, 1:5,000, ProteinTech Group, Inc.). The second
antibodies included HRP-conjugated Affinipure Goat Anti-Mouse IgG
(H + L) (cat. no. SA00001-1, 1:3,000, ProteinTech Group, Inc.) and
HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H + L) (cat. no.
SA00001-2, 1:3,000, ProteinTech Group, Inc.).
Detection of cytokines and
chemokines
Chemokines and cytokines in the kidney and cells
were measured using rat TNF-α, IL-6 and MCP-1 ELISA kits (RTA00,
R6000B and DY3144-05, respectively; R&D Systems, Inc.)
according to the procedure recommended by the manufacturer. The
samples were read at 450 nm within 30 min by Spectramax Microplate
Reader (Molecular Devices, LLC).
TUNEL assays
TUNEL assays were performed according to the
manufacturer's instructions (Roche Diagnostics) to detect cell
death in the kidney following I/R injury and ligustrazine
administration, and in NRK-52E cells in response to different
treatments. Samples were visualized using the Leica TCS SPE
confocal system (Leica Microsystems GmbH).
Cell viability
Cell Counting Kit-8 (CCK-8) assays (Beyotime
Institute of Biotechnology) were performed according to the
manufacturer's instructions. NRK-52E cells were plated in 96-well
plates and subjected to different treatments. CCK-8 reagents were
added and the cells were incubated in a cell incubator at 37°C for
4 h. The absorbance was measured at 450 nm using the
Infinite® 200 PRO multimode microplate reader (M200
Pro/F200 Pro, Tecan Group, Ltd.).
Statistical analyses
Data are expressed as means ± standard error of the
mean. The significance of the differences in mean values among
groups was examined by two-way ANOVA followed by Bonferroni post
hoc tests when >1 variables were compared, and others were
performed by one-way ANOVA followed by Duncan's multiple range
tests. SPSS software, version 17.0 (SPSS, Inc.) and GraphPad Prism
5 software (GraphPad Software, Inc.) were used for statistical
analyses. P<0.05 was considered to indicate statistically
significant differences.
Results
Ligustrazine protects against AKI by
suppressing tubular damage and inflammatory response following I/R
in a rat model
Compared with the sham-operated group, I/R resulted
in higher levels of SCr and BUN in rats, which was reduced by
ligustrazine treatment (Table I).
Histological examination following H&E staining revealed severe
morphological kidney injury, with scattered single cell necrosis or
desquamation of proximal tubular cells with intact basement
membranes, loss of the brush border and tubule dilatation
subsequent to I/R injury, which were alleviated in the ligustrazine
treatment group (Fig. 1A and B).
Ligustrazine was also found to reduce the levels of
pro-inflammatory mediators, including tumor necrosis factor
(TNF)-α, IL-6 and monocyte chemoattractant protein (MCP)-1, in
renal tissue after I/R injury, based on RT-qPCR analysis (Fig. 1C and D). Furthermore, western blot
analysis demonstrated that the protein levels of CD68 were also
suppressed by ligustrazine (Fig.
1E), and the number of infiltrating CD68+
macrophages was also decreased in the ligustrazine treatment groups
(Fig. 1F). Therefore, the
downregulation of the aforementioned cytokines and CD68+
macrophages indicated that ligustrazine may protect against AKI by
suppressing tubular damage and inflammatory response after I/R in a
rat model.
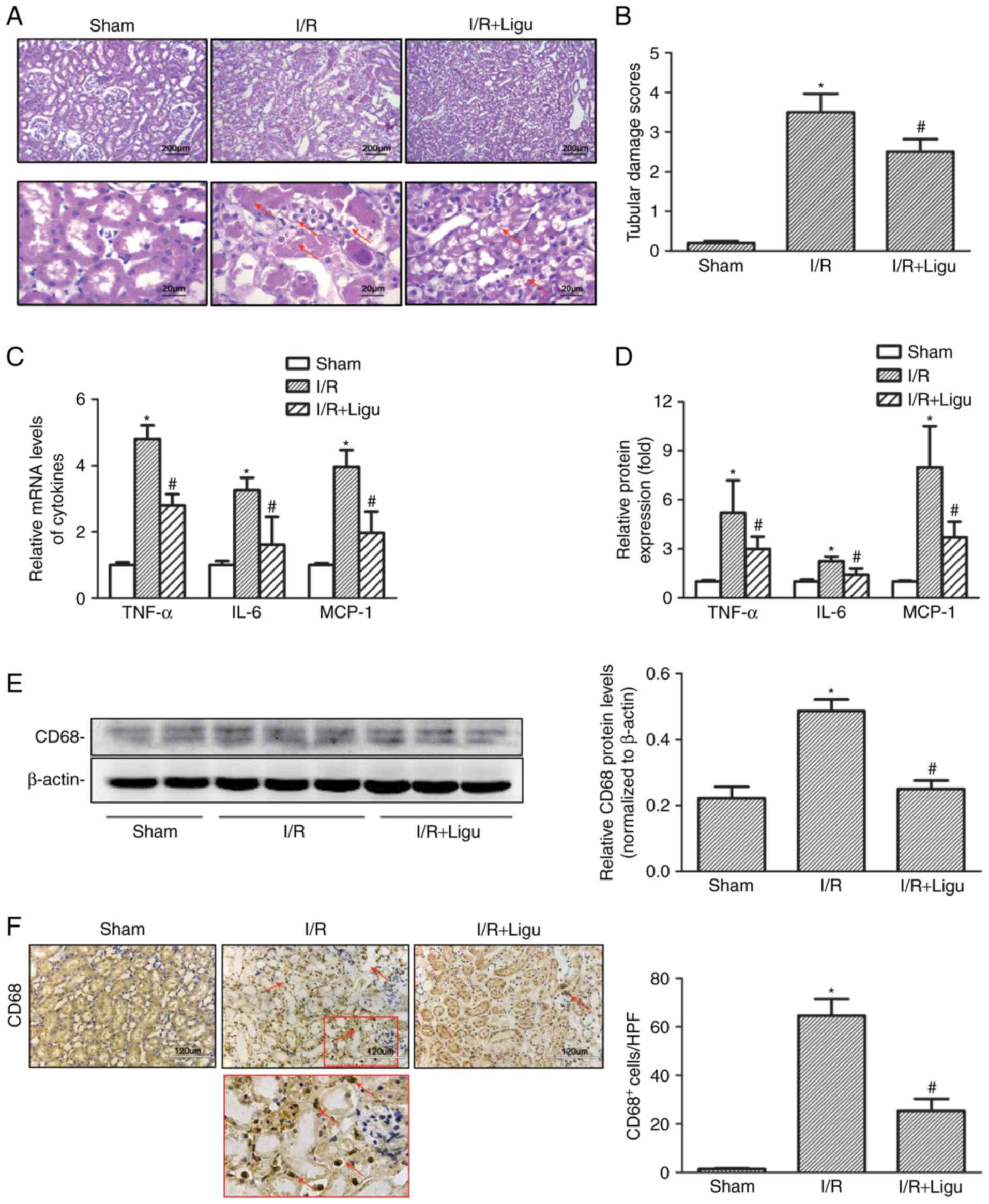 | Figure 1Ligustrazine protects against AKI by
suppressing tubular damage and inflammatory responses following I/R
in a rat model. (A) Representative photomicrographs showing the
morphological changes of kidneys from different groups of rats. Red
arrows indicated morphological kidney injury with scattered single
cell necrosis or desquamation of proximal tubular cells with intact
basement membranes, loss of the brush border and tubule dilation.
Bars, 200 µm at lower magnification (×40). Bars, 20
µm at higher magnification (×400). (B) Quantitative
assessment of tubular damage from different groups of rats. (C)
RT-qPCR analysis showing the levels of proinflammatory mediators
(TNF-α, IL-6 and MCP-1) in different groups of rats. (D) ELISA was
used to evaluate the levels of proinflammatory mediators in
different groups of rats. (E) Representative western blots and
summarized data showing the protein levels of CD68 in kidneys from
different groups. (F) Representative photomicrographs of CD68 IHC
staining in kidneys from different groups. Bars, 120 µm
(magnification, ×200). *P<0.05 vs. sham-operated rats
(n=9), #P<0.05 vs. I/R rats (n=9). Ligu,
ligustrazine; AKI, acute kidney injury; I/R, ischemia/reperfusion;
RT-qPCR, reverse transcription-quantitative PCR; TNF, tumor
necrosis factor; IL, interleukin; MCP, monocyte chemoattractant
protein. |
 | Table IPhysical and biochemical parameters
of experimental animals. |
Table I
Physical and biochemical parameters
of experimental animals.
| Variables | Sham | I/R | I/R + Ligu |
|---|
| Body weight
(g) | 222±18 | 220±20 | 223±15 |
| Blood urea nitrogen
(mmol/l) | 8.9±0.93 | 42.1±4. 57a | 22.7±0.85b |
| Serum creatinine
(µmol/l) | 69.8±10.26 | 206.1±25.73a | 145.6±15.49b |
| N | 9 | 9 | 9 |
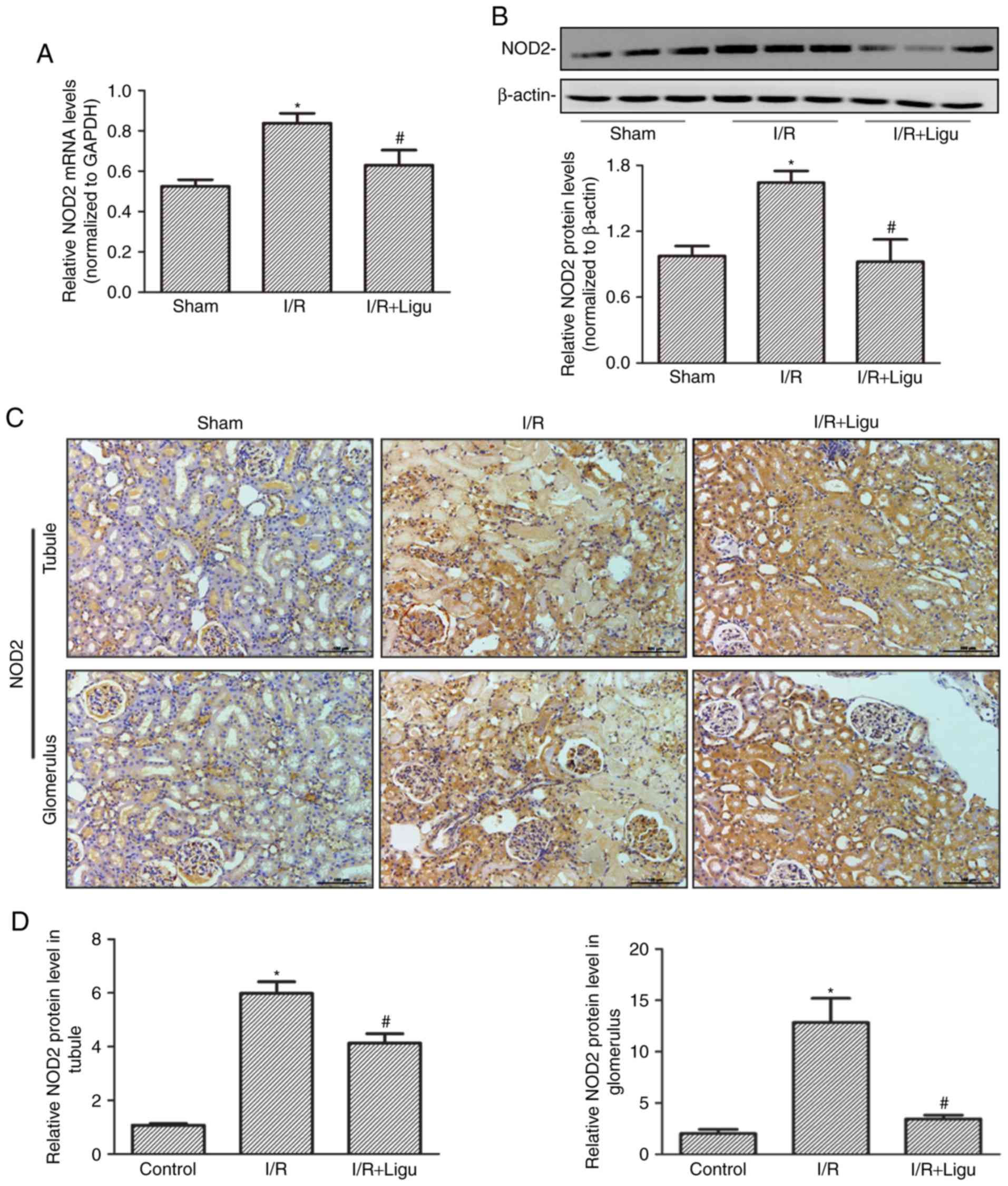
Ligustrazine inhibits the upregulation of
NOD2 following I/R
RT-qPCR (Fig. 2A)
and western blot (Fig. 2B)
analyses demonstrated that the upregulation of NOD2 expression
following I/R injury was inhibited by ligustrazine treatment at
both the mRNA and protein levels, respectively. Immunohistochemical
staining also identified high expression of NOD2 in the tubules and
glomerulus following I/R, which was inhibited by ligustrazine
treatment (Fig. 2C and D).
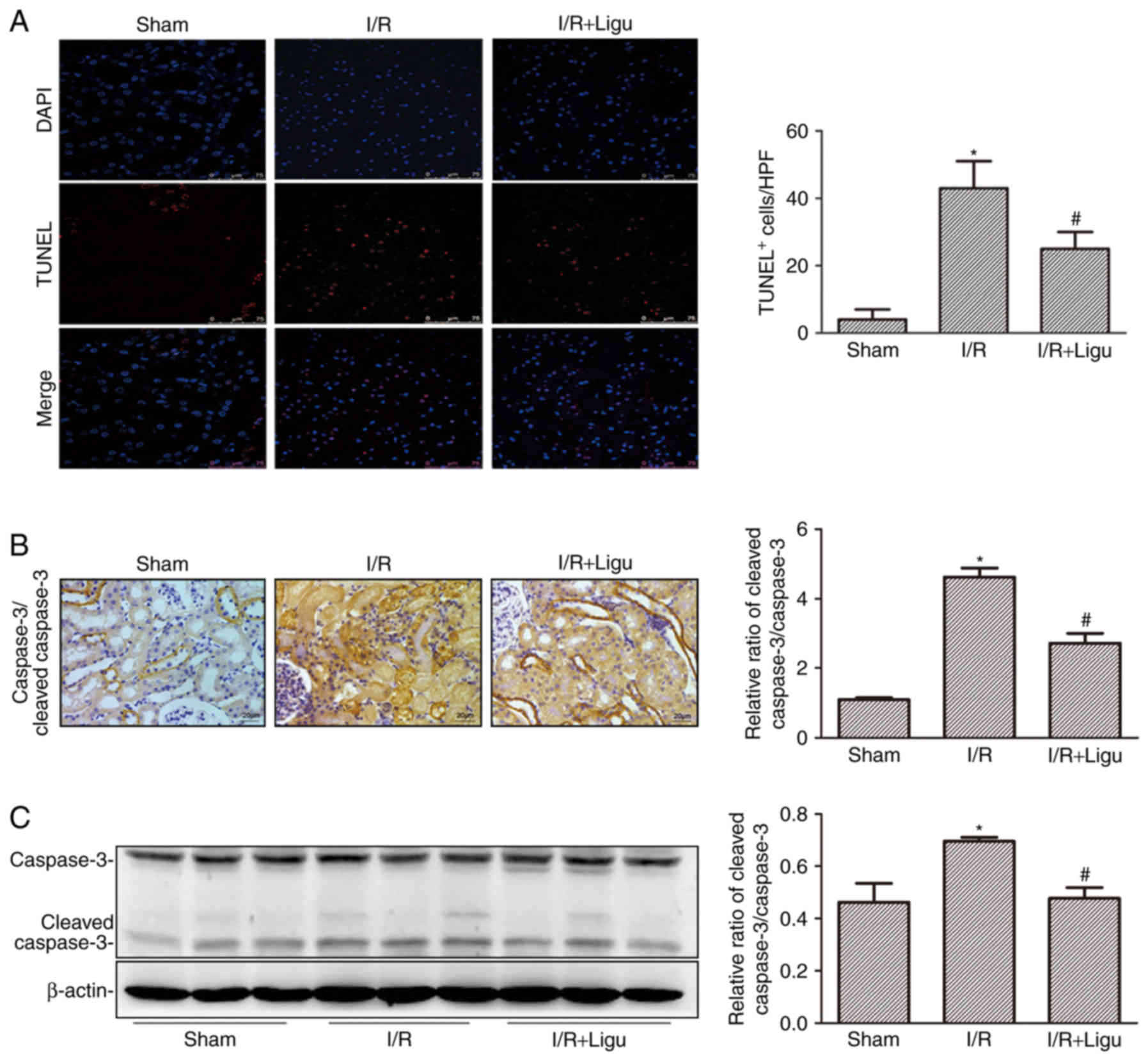
Ligustrazine inhibits apoptosis of kidney
cells following I/R
Ligustrazine reduced cell death, as demonstrated by
TUNEL staining and analysis (Fig.
3A). The inhibition of apoptosis by ligustrazine was further
confirmed by caspase 3/cleaved caspase 3 immunohistochemistry
(Fig. 3B) and western blotting
(Fig. 3C). These results
collectively indicated that ligustrazine exerted a renoprotective
effect by reducing apoptosis of kidney cells and promoting renal
function following I/R injury.
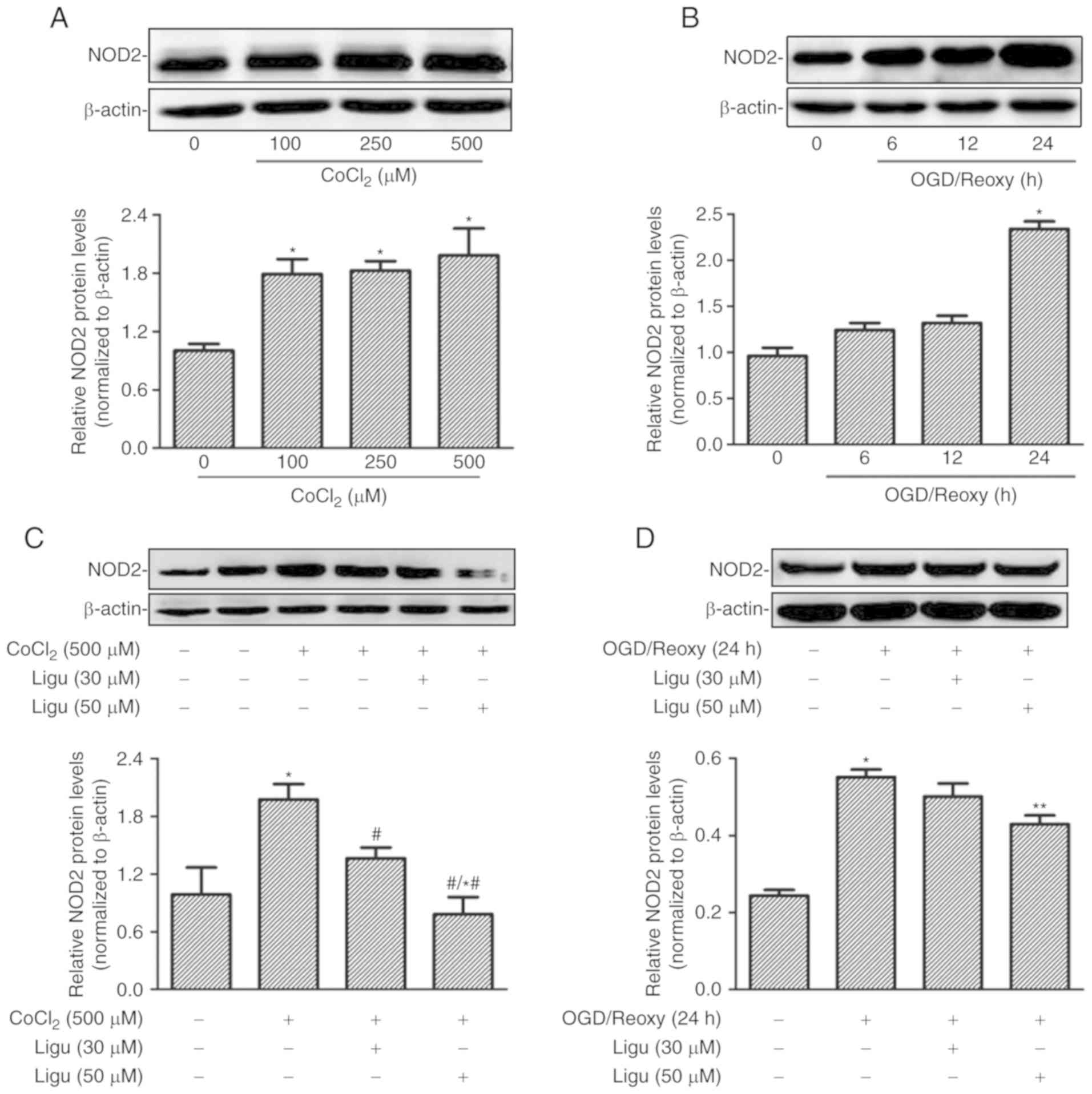
Ligustrazine suppresses NOD2 expression
induced by CoCl2 or hypoxia in rat NRK-52E cells
Proximal tubule injury is prominent during AKI;
therefore, rat proximal tubule epithelial cells (NRK-52E cells)
were treated with CoCl2 and OGD to model the effect of
AKI induced by hypoxia in vitro. The upregulation of NOD2
expression with different concentrations of CoCl2 was
demonstrated by western blot analysis (Fig. 4A). The expression of NOD2 was
shown to increase after OGD treatment followed by reoxygenation for
24 h (Fig. 4B). The effect of
ligustrazine on the upregulation of NOD2 expression was then
detected in both in vitro models in NRK-52E cells. Western
blot analysis revealed that ligustrazine at 50 µM was able
to significantly inhibit the upregulation of NOD2 expression
induced by CoCl2 (500 µM) and OGD followed by
reoxygenation for 24 h (Fig. 4C and
D).
Ligustrazine-mediated NOD2 downregulation
is blocked by inhibiting autophagy in NRK-52E cells
Inflammation and autophagy are two inextricably
linked and important pathophysiological processes. Since autophagy
was previously shown to protect the proximal tubule from
degeneration and acute ischemic injury (25), it was inferred that the
anti-inflammatory effect of ligustrazine was mediated by autophagy.
Consequently, this was tested in in vitro studies.
The differential autophagy response to
CoCl2-induced hypoxia in rat NRK-52E cells was
demonstrated by western blotting of LC3A/B-II/I. The ratio of
LC3A/B-II/I increased after treatment with a lower concentration of
CoCl2, indicating that this treatment could induce
autophagy, whereas at a concentration of 500 µM
CoCl2, the LC3A/B-II/I ratio was significantly decreased
compared with that with 100 and 250 µM, indicating that the
induction of autophagy was impaired with CoCl2 treatment
at higher concentration (Fig.
5A). Moreover, the ratio of LC3A/B-II/I following
CoCl2 treatment at a concentration of 500 µM
increased significantly with 50 µM ligustrazine (Fig. 5B), suggesting that the impaired
autophagy recovered following ligustrazine treatment.
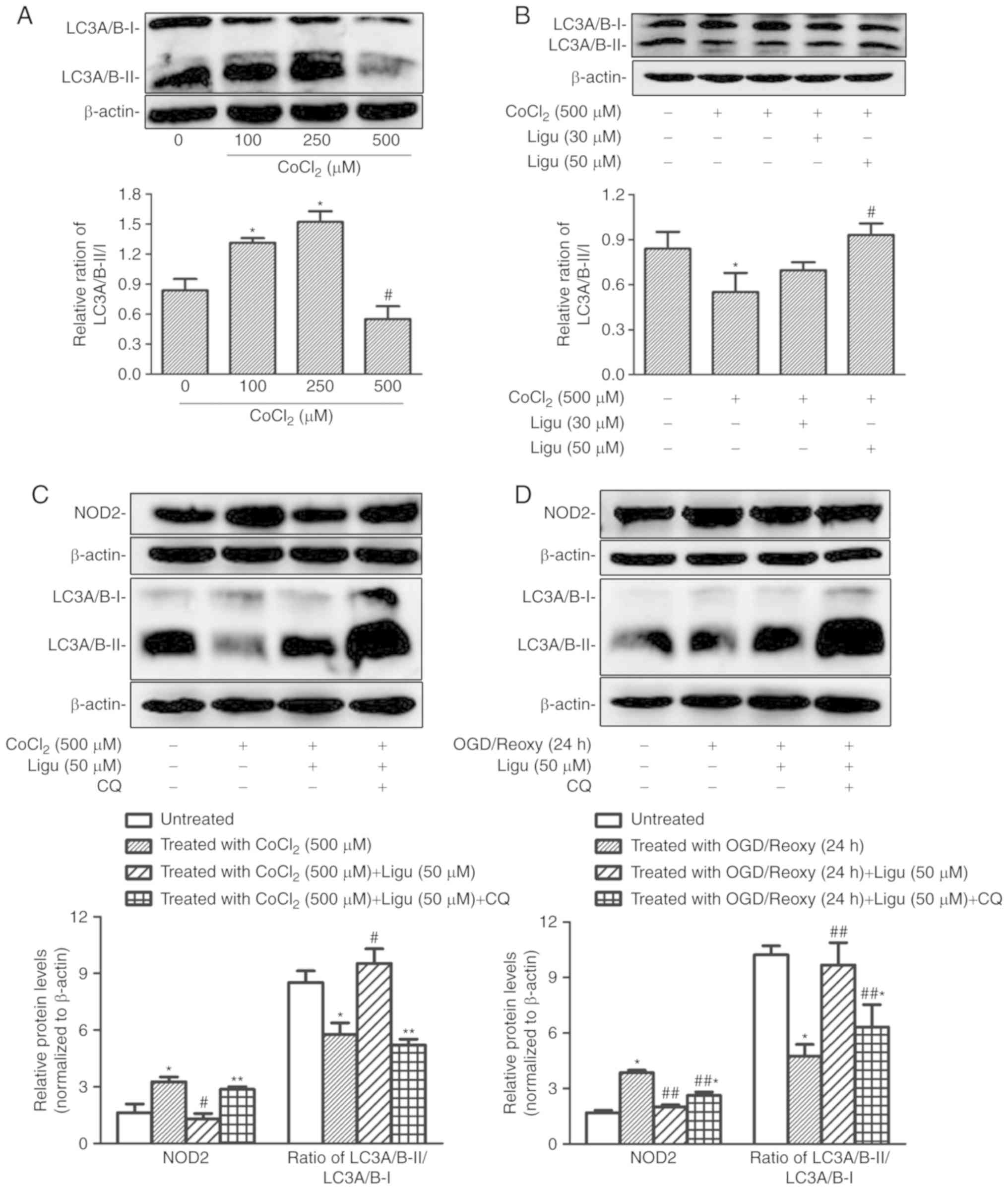 | Figure 5Ligustrazine-mediated NOD2
downregulation is blocked by inhibiting autophagy in NRK-52E cells
in vitro. (A) Representative western blots and summarized
data showing the relative ratio of LC3A/B-II/I in response to
CoCl2 treatment at different concentrations. The
treatment duration of CoCl2 was 12 h. (B) Representative
western blots and summarized data showing the relative ratio of
LC3A/B-II/I in response to ligustrazine (30 and 50 µM) after
CoCl2 treatment. The duration of CoCl2
treatment was 12 h and the duration of ligustrazine treatment was
24 h. (C) Representative western blots and summarized data showing
the protein levels of NOD2 in response to ligustrazine (50
µM) after CoCl2 treatment when the autophagy was
inhibited by CQ. (D) Representative western blots and summarized
data showing the protein levels of NOD2 in response to ligustrazine
after OGD treatment followed by reoxygenation (24 h) when the
autophagy was inhibited by CQ. *P<0.05 vs. control,
#P<0.05 vs. CoCl2 treatment group,
**P<0.05 vs. ligustrazine (50 µM) after
CoCl2 treatment group, ##P<0.05 vs. OGD
treatment followed by reoxygenation (24 h) group.
##,*P<0.05 vs. ligustrazine (50 µM) after OGD
treatment followed by reoxygenation (24 h) group. All the
experiments were performed in triplicate. Ligu, ligustrazine; I/R,
ischemia/reperfusion; reoxy, reoxygenation; NOD2,
nucleotide-binding oligomerization domain-containing 2; OGD, oxygen
and glucose deprivation; CQ, chloroquine. |
Next, the association between the inhibition of
inflammation and the induction of autophagy was addressed.
Autophagy was inhibited with CQ. As an inhibitor of autophagy, CQ
raises the lysosomal pH and ultimately inhibits the fusion between
autophagosomes and lysosomes, thus preventing the maturation of
autophagosomes into autolysosomes, and blocking a late step of
macroautophagy. Thus, an increase in LC3-І and LC3-II is expected
in the presence of CQ (26,27). Following CoCl2
treatment (500 µM), the inhibitory effect of ligustrazine on
the protein levels of NOD2 was diminished when autophagy was
inhibited, as determined by western blot analysis (Fig. 5C); moreover, western blot analysis
demonstrated that, after OGD treatment followed by reoxygenation
(24 h), the inhibitory effect of ligustrazine on NOD2 levels was
suppressed when autophagy was inhibited (Fig. 5D).
Ligustrazine-mediated downregulation of
inflammation and apoptosis is blocked by inhibiting autophagy in
NRK-52E cells in vitro
Furthermore, RT-qPCR analysis demonstrated that
ligustrazine treatment inhibited the production of pro-inflammatory
mediators, including TNF-α, IL-6, and MCP-1, and this effect was
blocked when autophagy was inhibited, after OGD treatment followed
by reoxygenation for 12 h (Fig.
6A). These results suggest that the anti-inflammatory effect of
ligustrazine depends to a certain extent on the induction of
autophagy. CCK-8 assays demonstrated that cell viability in
response to ligustrazine decreased after autophagy was inhibited by
CQ (Fig. 6B). TUNEL assays were
also performed to assess the effect of ligustrazine on cell death
induced by CoCl2 treatment. Following inhibition of
autophagy, the effect of ligustrazine on reducing cell death was
attenuated (Fig. 6C).
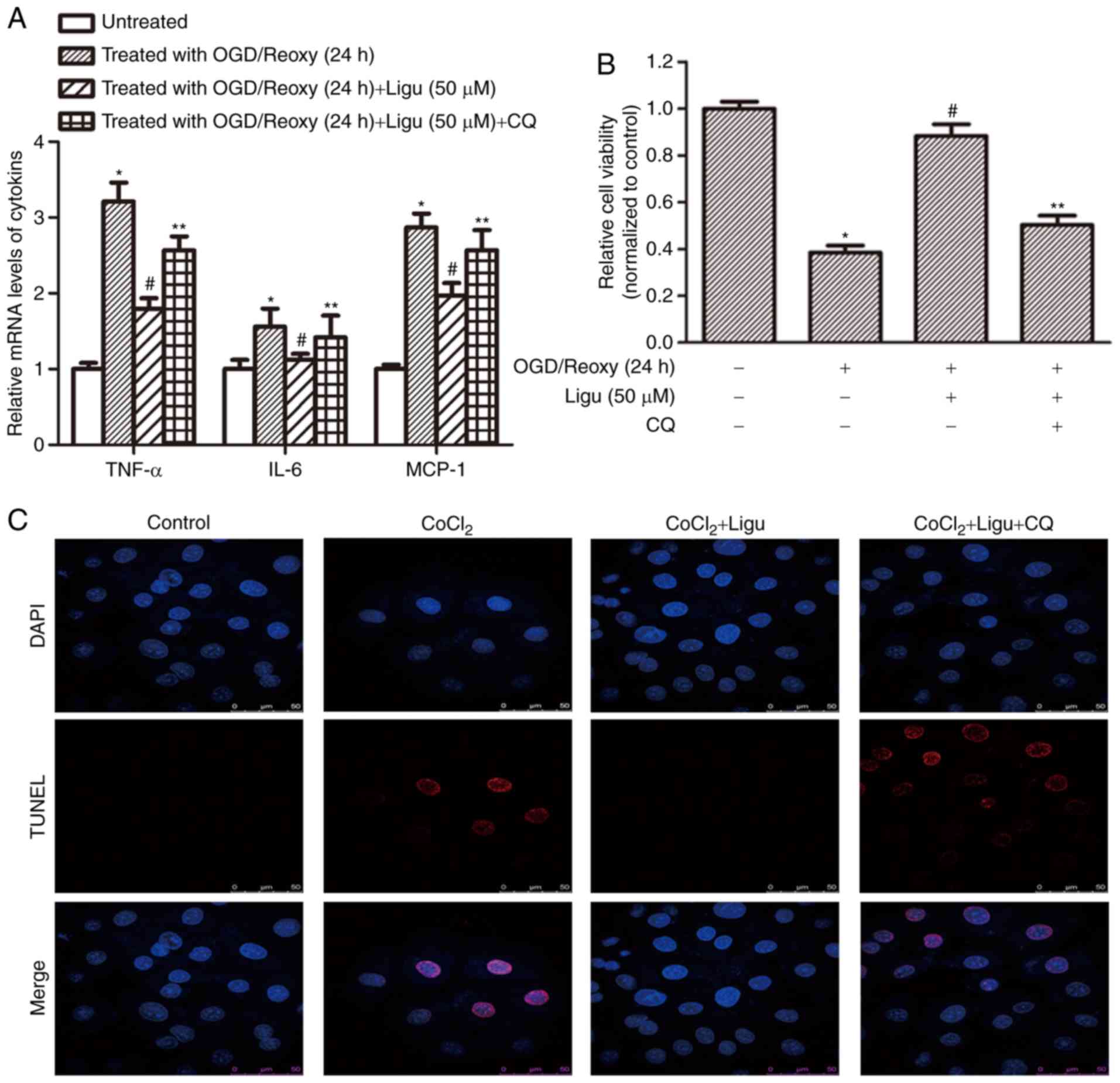 | Figure 6Ligustrazine-mediated downregulation
of inflammation and apoptosis is blocked by inhibiting autophagy in
NRK-52E cells in vitro. (A) RT-qPCR analysis demonstrated
that ligustrazine treatment suppressed the levels of
proinflammatory mediators, including TNF-α, IL-6 and MCP-1, and
this effect was attenuated when autophagy was inhibited by CQ. The
duration of reoxygenation was 24 h. (B) CCK-8 assays demonstrated
that cell viability in response to ligustrazine after OGD followed
by reoxygenation for 24 h was blocked when the autophagy was
inhibited by CQ. (C) TUNEL assays showing the changes of cell death
in response to ligustrazine after CoCl2 treatment when
autophagy was inhibited by CQ. The treatment duration of
CoCl2 was 12 h, and that of ligustrazine and CQ was 24
h. *P<0.05 vs. control, #P<0.05 vs. OGD
treatment, **P<0.05 vs. ligustrazine treatment group.
All the experiments were performed in triplicate. Ligu,
ligustrazine; I/R, ischemia/reperfusion; reoxy, reoxygenation; OGD,
oxygen and glucose deprivation; CQ, chloroquine; TNF, tumor
necrosis factor; IL, interleukin; MCP, monocyte chemoattractant
protein. |
Discussion
The present study demonstrated that ligustrazine
downregulates autophagy-induced NOD2 expression, attenuates cell
death and improves renal function following I/R injury in a rat
model. Furthermore, to the best of our knowledge, the present study
was the first to identify that the inhibitory effect of
ligustrazine on NOD2 depends on the induction of autophagy in
NRK-52E cells treated with CoCl2 in vitro.
I/R injury is a common clinical problem that has
been attracting increasing attention in an attempt to delineate
putative triggers of renal injury and design novel therapeutic
strategies. Ligustrazine is a natural small-molecule compound that
was previously characterized and found to be associated with few
side effects and favorable bioavailability in vivo (28), and determining its targets and
mechanism of action may be beneficial for its clinical use.
Recently, the anti-inflammatory role of ligustrazine was
demonstrated in patients with rheumatic heart disease, a mouse
model of allergic asthma and after spinal cord I/R injury in rats
(17,18,29), suggesting that these
anti-inflammatory effects may underlie its renoprotective
properties. The reduced level of pro-inflammatory mediators and
infiltration of CD68+ macrophages in renal tissue by
ligustrazine following I/R indicated that ligustrazine protects
against AKI by suppressing inflammatory response. Furthermore,
ligustrazine was found to suppress the expression of NOD2 and
enhance autophagy in the injured kidney cortex following I/R injury
in rats.
PRRs have been suggested to be important triggers of
ischemic injury (30,31). NOD2 is a well-characterized member
of the NLR family, which mediates the activation of NF-κB and
mitogen-activated protein kinases in response to muramyl dipeptide,
a peptidoglycan motif that is present in all gram-positive and
gram-negative bacteria (32). The
activation of NOD2 mainly leads to the production of
pro-inflammatory cytokines and the expression of co-stimulatory and
adhesion molecules, which are dependent on NF-κB activation
(33). NOD2 was suggested to not
only promote renal injury by exacerbating inflammation and podocyte
insulin resistance during diabetic nephropathy, but also to
participate in renal I/R, which is negatively regulated by
progranulin, a protective autocrine growth factor involved in AKI
(11,13). In the present study, it was
confirmed that the expression of NOD2 increases in the injured
kidney cortex following renal I/R injury and in NRK-52E cells
treated with CoCl2, a chemical reagent that promotes a
cellular anaerobic state in vitro. Therefore, it was
hypothesized that NOD2 may serve as a therapeutic target for AKI.
The present study was the first to demonstrate that ligustrazine is
associated with the innate immune response via NOD2.
To address the mechanism through which ligustrazine
suppresses NOD2 expression, autophagy, an important physiological
process that occurs during AKI, was investigated. In view of the
current data, autophagy induction in response to multiple stresses
induced by AKI is cytoprotective (34). In vitro, different levels
of autophagy were demonstrated in NRK-52E cells treated with
different concentrations of CoCl2. With a high
concentration of CoCl2, the ratio of LC3A/B-II/I was
significantly lower compared with that with low concentrations of
CoCl2, suggesting that a high concentration of
CoCl2 (500 µM) can cause serious cell damage
through the induction of hypoxia and subsequent reoxygenation.
Thus, autophagy was impaired to the extent that its protective
effect against cell injury was lost. Therefore, a high
concentration of CoCl2 was employed to test the effect
of ligustrazine on the reactivation of autophagy. The results
demonstrated that autophagy inhibition was significantly reversed
by ligustrazine; in addition, the expression of NOD2 was also
inhibited. We hypothesized that there is a functional association
between NOD2 and autophagy. To confirm this hypothesis, autophagy
was inhibited using an autophagy inhibitor, and the suppression of
NOD2 by ligustrazine was partially abrogated. This indicated that
the anti-inflammatory effect of ligustrazine depends on the
induction of autophagy, at least to some extent. These results also
provided more evidence supporting that NOD2 is a key mediator
through which autophagy regulates the innate immune response and
inflammation (35). Mammalian
target of rapamycin (mTOR) is a key negative regulator of
autophagy. Ligustrazine was previously shown to disrupt
phosphoinositide 3-kinase/AKT/mTOR signaling in angiotensin
II-activated hepatic stellate cells (36). Ligustrazine was also reported to
prevent apoptosis by promoting autophagy in an AMP-activated
protein kinase- and mTOR pathway-dependent manner in bone marrow
mesenchymal stem cells (37).
Autophagy consists of an intracellular degradation
system that is required for cellular homeostasis, and basal
autophagy is important for proximal tubule homeostasis. Cellular
stress during AKI, including hypoxia, oxidative injury and nutrient
deprivation, contributes to the induction of autophagy. Conditional
kidney proximal tubule-specific Atg5- or Atg7-knockout mice were
used to demonstrate the renoprotective effect of autophagy
following renal I/R injury (25,38). However, the precise mechanisms
underlying autophagy during AKI are unclear. In addition to the
fact that the effect of ligustrazine on NOD2 downregulation was
partially blocked by inhibiting autophagy, other studies also
demonstrated the association of autophagy with NLRs in immune
cells, which included the identification of cross-talk-related
processes. The autophagy-related protein ATG16L1 was found to
suppress inflammation selectively induced by the activation of NOD2
(35,39,40), and autophagy was also found to
play an important role as a macrophage-intrinsic negative regulator
of the NLRP3 inflammasome (32,33). Thus, the autophagic machinery
comprises a key cellular monitoring system that prevents excessive
NLRP3 inflammasome activation. Moreover, NOD2 and NLRP4 can
regulate autophagic processes by associating with the
autophagy-related protein Beclin 1 (41). However, the detailed underlying
mechanisms remain elusive.
Hypoxia-induced tubular epithelial damage is
prominent during renal I/R injury, whereas post-reperfusion
inflammation is a pathognomonic characteristic, involving multiple
cell types and cell signals (8).
Furthermore, glomerular injury and the excessive expression of
adhesion molecules followed by leukocyte infiltration must be taken
into consideration. A limitation of the present study was that the
effect of ligustrazine on glomerular injury was not extensively
addressed; in addition, whether ligustrazine regulates the
association between NOD2 and autophagy in renal immune cells was
not discerned. In the future, the effect of ligustrazine on the
activation of NOD2 in glomerular cells such as podocytes,
endothelial cells, or mesangial cells should be addressed. Another
limitation was the intervention time for ligustrazine. In the
pre-experiment, we constructed models of three time points, namely
24, 48 and 72 h, according to different reperfusion time. H&E
staining revealed that kidney injury in rats was the most severe at
24 h after I/R; 48 h later, the kidney injury in rats did not
become worse, and gradually recovered with the prolongation of
reperfusion time. This was in accordance with previous findings
(42). Therefore, in terms of
timing, we focused on 24 h after reperfusion. Pre-treatment with
drugs prior to ischemia was previously found to promote renal
function and attenuate inflammation after renal I/R injury
(13). In the present study, we
only investigated the effect of ligustrazine treatment after
ischemia, whereas the effect of ligustrazine pre-treatment was not
assessed. Additional studies must be conducted to explore the
regulation of inflammation by autophagy following pre-treatment
with ligustrazine.
Apoptosis is a major pathological process in AKI.
There is cross-talk between autophagy and apoptosis in addition to
inflammation (34,43). In the present study, ligustrazine
helped to preserve renal function in a model of I/R injury in part
by reducing apoptosis. In vitro, the effect of ligustrazine
on inhibiting cell death was diminished after autophagy was
suppressed. Thus, ligustrazine may be implicated in homeostasis and
renal pathophysiology via an interaction between autophagy and
apoptosis. Of note, although it was demonstrated that the
inhibition of inflammation and apoptosis by ligustrazine partly
depended on the induction of autophagy, other researchers have
demonstrated that ligustrazine pre-treatment may reverse the
increase in autophagy observed in an arsenic-induced nephrotoxicity
cell model, which is considered to induce necrosis (44). These opposite results suggest that
autophagy is a complex process, and its role in the protection
against diseases requires comprehensive consideration. Thus,
interfering with the regulation of inflammation by autophagy may be
of great value in the treatment for kidney injury (45).
In conclusion, the present study was, to the best of
our knowledge, the first to provide evidence that ligustrazine can
protect against renal injury after AKI by suppressing NOD2-mediated
inflammation, which is partly dependent on the induction of
autophagy. Ligustrazine was investigated for its potential
anti-inflammatory effects and its ability to induce autophagy,
which may broaden its clinical application spectrum for immune- and
autophagy-related diseases.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81500557 and
81601049); the Distinguished Middle-Aged and Young Scientist
Encourage and Reward Foundation of Shandong Province (grant no.
BS2014YY018); and the Natural Science Foundation of Shandong
Province (grant nos. ZR2016HP05, ZR2016HL11 and ZR2017BH055).
Authors' contributions
GJ, RX, WY and PD were responsible for study design,
data collection, statistical analysis and manuscript preparation;
LZ, WS, XM, HH, YH and LW were responsible for partial data
collection, statistical analysis and literature search; PD
supervised the entire project, study design, data analysis and
writing of the manuscript. All authors have reviewed and approved
the final manuscript.
Availability of materials and data
The data that support the findings of the present
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Binzhou Medical University
(protocol no. 2015-008).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Abbreviations:
|
AKI
|
acute kidney injury
|
|
NLRs
|
nucleotide-binding and oligomerization
domain (NOD)-like receptors
|
|
I/R
|
ischemia/reperfusion
|
|
PRRs
|
pattern-recognition receptors
|
|
NOD2
|
nucleotide-binding oligomerization
domain-containing 2
|
|
NLRP3
|
nucleotide-binding domain and
leucine-rich repeat pyrin 3 domain
|
|
BDMCs
|
bone marrow-derived cells
|
|
SCr
|
serum creatinine
|
|
BUN
|
blood urea nitrogen
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-1β
|
interleukin-1β
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
CQ
|
chloroquine
|
|
CCK-8
|
Cell Counting Kit-8
|
|
H&E
|
hematoxylin and eosin
|
Acknowledgments
Not applicable.
References
|
1
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rabb H, Griffin MD, McKay DB, Swaminathan
S, Pickkers P, Rosner MH, Kellum JA and Ronco C; Acute Dialysis
Quality Initiative Consensus XIII Work Group: Inflammation in AKI:
Current understanding, key questions, and knowledge gaps. J Am Soc
Nephrol. 27:371–379. 2016. View Article : Google Scholar :
|
|
3
|
Shigeoka AA, Kambo A, Mathison JC, King
AJ, Hall WF, da Silva Correia J, Ulevitch RJ and McKay DB: Nod1 and
nod2 are expressed in human and murine renal tubular epithelial
cells and participate in renal ischemia reperfusion injury. J
Immunol. 184:2297–2304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bakker PJ, Butter LM, Claessen N, Teske
GJ, Sutterwala FS, Florquin S and Leemans JC: A tissue-specific
role for Nlrp3 in tubular epithelial repair after renal
ischemia/reperfusion. Am J Pathol. 184:2013–2022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrilli V, Dostert C, Muruve DA and
Tschopp J: The inflammasome: A danger sensing complex triggering
innate immunity. Curr Opin Immunol. 19:615–622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kersse K, Bertrand MJ, Lamkanfi M and
Vandenabeele P: NOD-like receptors and the innate immune system:
Coping with danger, damage and death. Cytokine Growth Factor Rev.
22:257–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anders HJ and Lech M: NOD-like and
Toll-like receptors or inflammasomes contribute to kidney disease
in a canonical and a non-canonical manner. Kidney Int. 84:225–228.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shigeoka AA, Mueller JL, Kambo A, Mathison
JC, King AJ, Hall WF, Correia Jda S, Ulevitch RJ, Hoffman HM and
McKay DB: An inflammasome-independent role for epithelial-expressed
Nlrp3 in renal ischemia-reperfusion injury. J Immunol.
185:6277–6285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szeto HH, Liu S, Soong Y, Seshan SV,
Cohen-Gould L, Manichev V, Feldman LC and Gustafsson T:
Mitochondria protection after acute ischemia prevents prolonged
upregulation of IL-1β and IL-18 and arrests CKD. J Am Soc Nephrol.
28:1437–1449. 2017. View Article : Google Scholar
|
|
10
|
Chun J, Chung H, Wang X, Barry R, Taheri
ZM, Platnich JM, Ahmed SB, Trpkov K, Hemmelgarn B, Benediktsson H,
et al: NLRP3 localizes to the tubular epithelium in human kidney
and correlates with outcome in IgA nephropathy. Sci Rep.
6:246672016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du P, Fan B, Han H, Zhen J, Shang J, Wang
X, Li X, Shi W, Tang W, Bao C, et al: NOD2 promotes renal injury by
exacerbating inflammation and podocyte insulin resistance in
diabetic nephropathy. Kidney Int. 84:265–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xin R, Sun X, Wang Z, Yuan W, Jiang W,
Wang L, Xiang Y, Zhang H, Li X, Hou Y, et al: Apocynin inhibited
NLRP3/XIAP signalling to alleviate renal fibrotic injury in rat
diabetic nephropathy. Biomed Pharmacothe. 106:1325–1331. 2018.
View Article : Google Scholar
|
|
13
|
Zhou M, Tang W, Fu Y, Xu X, Wang Z, Lu Y,
Liu F, Yang X, Wei X, Zhang Y, et al: Progranulin protects against
renal ischemia/reperfusion injury in mice. Kidney Int. 87:918–929.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang GJ: The change in the nailfold
microcirculation in patients with acute cerebral thrombosis treated
with ligustrazine. Zhonghua Shen Jing Jing Shen Ke Za Zhi.
17:121–124. 1984.In Chinese. PubMed/NCBI
|
|
15
|
Pang PK, Shan JJ and Chiu KW:
Tetramethylpyrazine, a calcium antagonist. Planta Med. 62:431–435.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Wei T, Hou J, Li G, Yu S and Xin
W: Tetramethylpyrazine scavenges superoxide anion and decreases
nitric oxide production in human polymorphonuclear leukocytes. Life
Sci. 72:2465–2472. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YJ, Huang CS, Wang F, Gong JY and Pan
ZH: Effect of ligustrazine hydrochloride on coagulation reaction
and inflammation reaction in single valve replacement patients with
rheumatic heart disease undergoing cardiopulmonary bypass. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 34:531–535. 2014.In Chinese. PubMed/NCBI
|
|
18
|
Luo YC, Xiang QW and Wang Q: Effect of
ligustrazine on expression of RhoA mRNA, ROCK-II protein in the
lung and airway inflammation of allergic asthma model mice.
Zhonghua Er Ke Za Zhi. 46:868–869. 2008.In Chinese. PubMed/NCBI
|
|
19
|
Fan LP, Shen JZ, Fu HY, Zhou HR, Shen SF
and Yu AF: Effect of epigallocatechin-3-galate on human acute
monocytic leukemia cell line U937 and its relevant mechanism.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:286–290. 2010.In Chinese.
PubMed/NCBI
|
|
20
|
Bai XY, Wang XF, Zhang LS, Du PC, Cao Z
and Hou Y: Tetramethylpyrazine ameliorates experimental autoimmune
encephalomyelitis by modulating the inflammatory response. Biochem
Biophys Res Commun. 503:1968–1972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan Z: Neural protection by naturopathic
compounds-an example of tetramethylpyrazine from retina to brain. J
Ocul Biol Dis Infor. 2:57–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen S, Xiong L, Wang Q, Sang H, Zhu Z,
Dong H and Lu Z: Tetramethylpyrazine attenuates spinal cord
ischemic injury due to aortic cross-clamping in rabbits. BMC
Neurol. 2:12002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
AVMA Guidelines for the Euthanasia of
Animals: 2013 edition. https://www.avma.org/sites/default/files/resources/euthanasia.pdf
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Kimura T, Takabatake Y, Takahashi A,
Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T,
Soga T, et al: Autophagy protects the proximal tubule from
degeneration and acute ischemic injury. J Am Soc Nephrol.
22:902–913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fedorko M: Effect of chloroquine on
morphology of cytoplasmic granules in maturing human leukocytes-an
ultrastructural study. J Clin Invest. 46:1932–1942. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen T, Xu H, Weng W and Zhang J: Single-
and multiple-dose pharmacokinetics of a novel tetramethylpyrazine
reservoir-type transdermal patch versus tetramethylpyrazine
phosphate oral tablets in healthy normal volunteers, and in
vitro/in vivo correlation. Biol Pharm Bull. 36:931–937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan L, Wang K, Shi Z, Die J, Wang C and
Dang X: Tetramethylpyrazine protects spinal cord and reduces
inflammation in a rat model of spinal cord ischemia-reperfusion
injury. J Vasc Surg. 54:192–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goncalves GM, Castoldi A, Braga TT and
Camara NO: New roles for innate immune response in acute and
chronic kidney injuries. Scand J Immunol. 73:428–435. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X and Yi F: Implication of
pattern-recognition receptors in cardiovascular diseases. Antioxid
Redox Signal. 22:1130–1145. 2015. View Article : Google Scholar
|
|
32
|
Inohara N, Ogura Y, Fontalba A, Gutierrez
O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M,
et al: Host recognition of bacterial muramyl dipeptide mediated
through NOD2. Implications for Crohn's disease. J Biol Chem.
278:5509–5512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lipinski S, Till A, Sina C, Arlt A,
Grasberger H, Schreiber S and Rosenstiel P: DUOX2-derived reactive
oxygen species are effectors of NOD2-mediated antibacterial
responses. J Cell Sci. 122:3522–3530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaushal GP and Shah SV: Autophagy in acute
kidney injury. Kidney Int. 89:779–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Plantinga TS, Crisan TO, Oosting M, van de
Veerdonk FL, de Jong DJ, Philpott DJ, van der Meer JW, Girardin SE,
Joosten LA and Netea MG: Crohn's disease-associated ATG16L1
polymorphism modulates pro-inflammatory cytokine responses
selectively upon activation of NOD2. Gut. 60:1229–1235. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X, Zhang F, Kong D, Wu X, Lian N,
Chen L, Lu Y and Zheng S: Tetramethylpyrazine inhibits angiotensin
II-induced activation of hepatic stellate cells associated with
interference of platelet-derived growth factor β receptor pathways.
FEBS J. 281:2754–2768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Zhang HY, Gao B, Shi J, Huang Q,
Han YH, Hu YQ, Lu WG, Zhao ZJ, Liu BH, et al: Tetramethylpyrazine
protects against glucocorticoid-induced apoptosis by promoting
autophagy in mesenchymal stem cells and improves bone mass in
glucocorticoid-induced osteoporosis rats. Stem Cells Dev.
26:419–430. 2017. View Article : Google Scholar
|
|
38
|
Jiang M, Liu K, Luo J and Dong Z:
Autophagy is a renoprotective mechanism during in vitro hypoxia and
in vivo ischemia-reperfusion injury. Am J Pathol. 176:1181–1192.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorbara MT, Ellison LK, Ramjeet M,
Travassos LH, Jones NL, Girardin SE and Philpott DJ: The protein
ATG16L1 suppresses inflammatory cytokines induced by the
intracellular sensors Nod1 and Nod2 in an autophagy-independent
manner. Immunity. 39:858–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saitoh T, Fujita N, Jang MH, Uematsu S,
Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al:
Loss of the autophagy protein Atg16L1 enhances endotoxin-induced
IL-1beta production. Nature. 456:264–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jounai N, Kobiyama K, Shiina M, Ogata K,
Ishii KJ and Takeshita F: NLRP4 negatively regulates autophagic
processes through an association with beclin1. J Immunol.
186:1646–1655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pulskens WP, Teske GJ, Butter LM, Roelofs
JJ, van der Poll T, Florquin S and Leemans JC: Toll-like receptor-4
coordinates the innate immune response of the kidney to renal
ischemia/reperfusion injury. PLoS One. 3:e35692008. View Article : Google Scholar
|
|
43
|
Yang C, Kaushal V, Shah SV and Kaushal GP:
Autophagy is associated with apoptosis in cisplatin injury to renal
tubular epithelial cells. Am J Physiol Renal Physiol.
294:F777–F787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gong X, Ivanov VN, Davidson MM and Hei TK:
Tetramethylpyrazine (TMP) protects against sodium arsenite-induced
nephrotoxicity by suppressing ROS production, mitochondrial
dysfunction, pro-inflammatory signaling pathways and programed cell
death. Arch Toxicol. 89:1057–1070. 2015. View Article : Google Scholar :
|
|
45
|
Kimura T, Isaka Y and Yoshimori T:
Autophagy and kidney inflammation. Autophagy. 13:997–1003. 2017.
View Article : Google Scholar : PubMed/NCBI
|