Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy that
occurs in nasopharyngeal epithelial tissues and has a specific
ethnic and geographic distribution, with the highest incidence in
Southeast Asia and North Africa (1). Despite great improvements in
diagnosis and surgical treatment, the 5-year survival rate for
advanced NPC is approximately 70%, which remains unsatisfactory
(2,3). Currently, the main therapeutic
methods for NPC are irradiation therapy, alone or combined with
chemotherapy (4,5). However, the physical and
psychological side effects of the treatments are severe. Therefore,
an investigation of the molecular mechanisms of this disease is
required to develop novel treatment strategies for patients with
NPC.
Circular RNAs (circRNAs) are a class of non-coding
transcripts that contain a ring structure (6). Increasing evidence indicates that
the aberrant expression of circRNAs is associated with a variety of
diseases, including cancer (7-9).
Additionally, some circRNAs play vital roles in the development and
progression of NPC. For example, circRNA ZNF609 (10), circRNA_0008450 (11) and circRNA_000543 (12) accelerate NPC tumorigenesis and
metastasis. Circular RNA CTDP1 (circCTDP1) was reported to function
as a competitive endogenous RNA (ceRNA) for microRNA
(miRNA/miR)-29a-3p to regulate the expression of hyaluronan
synthase 3, integrin subunit β1, DNA methyltransferase 3β and
vascular endothelial growth factor A (VEGFA), and subsequently
promote the growth and metastasis of bladder cancer (13). However, the molecular mechanisms
of circCTDP1 action in the tumorigenesis of NPC are unclear.
miRNAs are a family of small non-coding RNAs of
approximately 22 nucleotides in length, which regulate gene
expression by complementary binding or complex mechanisms (14). In multiple studies, several
miRNAs, such as miR-101 (15),
miR-184 (16), miR-543 (17) and miR-449 (18), have been demonstrated to suppress
proliferation, migration and invasion of NPC. miR-320b was also
reported to act as a tumor suppressor during occurrence and
progression in different types of cancer, including NPC. In a study
by Li et al (19), the
overexpression of miR-320b inhibited NPC cell proliferation and
promoted apoptosis, while knockdown of miR-320b accelerated tumor
growth and inhibited apoptosis. circRNAs can act as ceRNAs to
regulate the development and progression of various cancers
(20-22), which led to the hypothesis that
circCTDP1 may promote NPC progression via miR-320b.
Homeobox A10 (HOXA10), a member of the
homeobox gene family, plays a critical role in embryonic
development (23). Abnormal
expression of HOXA10 has been observed in several types of
cancer, including endometrial carcinoma, ovarian cancer and breast
cancer (24-26). Shen et al (27) also reported that HOXA10 is
upregulated in NPC tissues compared to normal tissues, and promotes
NPC progression by binding to the promoter of Zic family member 2.
However, the precise mechanism of the regulation of HOXA10
in NPC remains unclear.
In the present study, the molecular mechanism of the
circCTDP1/miR-320b/HOXA10/transforming growth factor β2
(TGFβ2) axis was investigated with regard to tumorigenesis
and the progression of NPC. The aim of the study was to provide a
better understanding of NPC initiation and progression, which may
help in the future development of diagnostic and therapeutic
targets for NPC.
Materials and methods
Clinical specimens
A total of 32 paired NPC tissues and paracarcinoma
tissues were obtained from 32 NPC patients (22 males and 10
females) with a median age of 47 years (range, 27-79 years) between
August, 2016 and May, 2018. The patients all provided written
informed consent for the use of their samples. All experimental
protocols were approved by the Ethical and Scientific Committee of
the Third Affiliated Hospital of Soochow University (Jiangsu,
China).
Cell culture
A normal human nasopharyngeal epithelial cell line
(NP69), 3 NPC cell lines (SUNE1, SUNE2 and 6-10B) and 293T cells
were obtained from the American Type Culture Collection (ATCC;
Manassas). The cell lines were cultured in RPMI-1640 medium, and
supplemented with 10% fetal bovine serum. The 293T cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) with 10% FBS and 1%
penicillin-streptomycin. All the cell lines were maintained at 37°C
in a humidified atmosphere with 5% CO2.
Cell transfection
The short hairpin RNA (shRNAs) targeting circCTDP1
(shcircCTDP1; 5′-UCA AGA AUG CAG GCU CAA C-3′) with negative
control (shNC; 5′-UCUCCG AUGCAG GCU CAA C-3′), miR-320b mimics
(5′-AAA GCU GGG UUG AGA GGG CAA-3′) with negative control (miR-NC;
5′-AAU UCU CCG AAC GUG UCA CUU-3′) and miR-320b inhibitor (5′-UUG
CCC UCU CAA CCC AGC UUU U-3′) with negative control (inh-miR-NC;
5′-CAG UAC UUU UGU GUA GUA CAA-3′) were synthesized by GenePharma
(Shanghai, China). The full length of HOXA10 was subcloned
into pcDNA3.1 to overexpress HOXA10 levels with empty
pcDNA3.1 serving as control. The pcDNA3.1 vector was bought from
GenePharma (Shanghai). Transfection of the cells with shcircCTDP1
(10 nM) or shNC (10 nM) and the miR-320b mimics (10 nM) or miR-NC
(10 nM) and miR-320b inhibitor (10 nM) or inh-miR-NC (10 nM) was
conducted with Lipofectamine 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The efficiency of transfection was determined in each
experiment using RT-qPCR 24 h post-transfection. All functional
experiments were carried out 48 h post-transfection.
RT-qPCR
According to the manufacturer's instruction, total
RNA was extracted from tissues and cell lines using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA was reverse
transcribed to cDNA by using a Reverse Transcription Kit (Takara).
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95°C for 3 min; 40 cycles of 95°C for 5 sec
and 60°C for 30 sec. A melt curve step from 65-95°C was performed
in increments of 0.5°C per 5 sec. The relative expression levels
were calculated by comparing to the expression of GAPDH or U6 using
the 2-ΔΔCq method (28). The primer sequences used for
amplification were: circCTDP1 forward, 5′-TAA GAA CGG GAA GCA GCA
GG3′ and reverse, 5′-TCC AAG TCC ACC ATG AGC AC3′; miR-320b
forward, 5′-TCC GAA ACG GGA GAG TTG G-3′ and reverse, 5′-GTG CAG
GGT CCG AGG T-3′; HOXA10 forward, 5′-GGG TAA GCG GAA TAA
ACT-3′ and reverse, 5′-GCA CAG CAG CAA TAC AAT A-3′; GAPDH forward,
5′-TGC ACC ACC AAC TGC TTA GC-3′ and reverse, 5′-GGC ATG CAC TGT
GGT CAT GAG-3′; and U6 forward, 5′-GCT TCG GCA GCA CAT ATA CTA AAA
T-3′ and reverse, 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′.
Wound healing assay
The migration ability of cells was evaluated by
wound healing assay. Transfected SUNE2 and 6-10B cells were
cultured in RPMI-1640 supplemented with 10% FBS at a density of
8×104 cells/ml in a humidified atmosphere of 5%
CO2 at 37°C and grown to a fully confluent monolayer.
After 6 h, culture medium was replaced with serum-free medium, and
a sterile tip was employed to generate single-line scratch and then
washed twice with phosphate-buffered saline (PBS) to remove
detached cells from the plates. After 24 h, the medium was replaced
with PBS, and the wound gap was observed. Images of cell migration
were captured with an inverted microscope (magnification, ×200;
Olympus Corporation). The gap distance of each monolayer was
quantitatively evaluated using ImageJ.
Cell invasion assay
Cell invasion was determined by Transwell chambers
(8 µm pore size; Millipore) precoated with 100 µl of
Matrigel (BD Biosciences). Transfected SUNE2 and 6-10B cells
(8×104 cells) were added to the upper chamber containing
150 µl RPMI-1640 without FBS. Extra 550 µl RPMI-1640
medium was added to the lower chamber. After 24 h of incubation at
37°C with 5% CO2, the cells that did not pass through
the membrane were cleared using cotton swabs and 4%
paraformaldehyde was added to fix the cells at room temperature for
20 min, followed by staining the cells with 0.1% crystal violet
(Sigma-Aldrich; Merck KGaA) for 20 min at room temperature. The
number of cells invading through the Matrigel was counted in 3
randomly selected visual fields from the central and peripheral
portion of the filter using an inverted microscope (magnification,
×200; Olympus Corporation).
Flow cytometric analysis
Flow cytometric analysis was performed using a FITC
Annexin V Apoptosis kit (BD Biosciences) according to the
manufacturer's instructions. Briefly, transfected SUNE2 and 6-10B
cells were washed twice with PBS and resuspended in 1X Annexin V
binding buffer containing 10 mM HEPES/NaOH (pH 7.4)
(1×106 cells/ml). Then the cells were mixed with
FITC-Annexin V (5 µl) and propidium iodide for at 37°C for
20 min, and analyzed using a flow cytometer (BD Biosciences).
Bioinformatic prediction and luciferase
reporter assay
StarBase (http://starbase.sysu.edu.cn) and TargetScan databases
(http://www.targetscan.org) were used to
predict the potential miRNAs that can bind to circCTDP1. A
luciferase reporter assay was employed to investigate the
regulatory relationship between circCDTP1 and miR-320b. circCTDP1
that contained the miR-320b binding site was cloned into the
psiCHECK-2 vector (Promega Corporation) to construct wild-type
circCTDP1. Mutant circCTDP1 that included a mutated version of the
miR-320b binding site was also cloned into psiCHECK-2.
Subsequently, miR-320b mimic or miR-NC and miR-320b inhibitor or
miR-NC inhibitor were transfected into 293T cells that were
transfected with wild-type circCTDP1 or mutant circCTDP1. The
relationship between miR-320b and HOXA10 was confirmed using
the same method. Luciferase activity was evaluated by
Dual-Luciferase Reporter Analysis system (Promega Corporation).
Firefly lucif-erase activity was normalized to Renilla
(Promega Corporation) luciferase gene activity.
Western blot analysis
Following transfection for 48 h, proteins were
extracted from transfected SUNE2 and 6-10B cells using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Protein concentration was measured with the
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Following denaturation, 10 µg protein/lane was separated by
10% SDS-PAGE. Proteins were transferred onto polyvinylidene
difluoride (PVDF) membranes and blocked in 5% non-fat milk for 2 h
at room temperature. The membranes were incubated with primary
antibodies against HOXA10 (1:1,000; mouse monoclonal
antibody, sc-271139; Santa Cruz Biotechnology) and GAPDH (1:1,000;
mouse monoclonal antibody, sc-47724; Santa Cruz Biotechnology)
overnight at 4°C. Following primary incubation, membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:1,000; goat anti-mouse IgG, ab205719 and goat
anti-rabbit IgG, ab205718; Abcam) for 2 h at room temperature.
Protein bands were visualized using the Pierce ECL Western Blotting
kit (Pierce; Thermo Fisher Scientific, Inc.). Protein expression
was quantified using Image-Pro® Plus software (version
6.0; Media Cybernetics, Inc.). GAPDH was used as endogenous
control for data normalization.
Xenograft experiment
Six male BALB/c nude mice (6 weeks old) were
maintained under specific pathogen-free conditions and randomly
divided into two groups, and were housed individually in
microisolator ventilated cages (temperature, 26-28°C; 40-60%
humidity and ventilation 10-15 times/h) with free access to water
and food. Cells transfected with shNC or shcircCTDP1 were suspended
in 100 µl PBS and injected subcutaneously into the mice.
Tumors were examined every 5 days. On 30th day, the mice were
sacrificed by cervical dislocation after deep anesthesia with 2%
isoflurane (Baxter Healthcare Corporation) to obtain the tumors.
The tumors were photographed and tumor weights were measured. Tumor
length (L) and width (W) were measured. Tumor volume was calculated
using the formula: V = 1/2 × L × W2. The animal
experiments were approved by the Ethics Committee of the Third
Affiliated Hospital of Soochow University.
Statistical analysis
Statistical analyses were performed with SPSS 18.0
software (SPSS, Inc.). Data were presented as mean ± SD.
Comparisons of parameters between two groups were analyzed by a
paired Student's t-test. Comparisons among multiple groups were
performed using one-way ANOVA followed by Tukey's test. The
correlation between gene expression levels was analyzed by
Pearson's correlation coefficient. Kaplan-Meier analysis and the
log-rank test were used to estimate survival curves. Cut-off values
were determined using Youden's index. P<0.05 was considered to
indicate a statistically significant difference.
Results
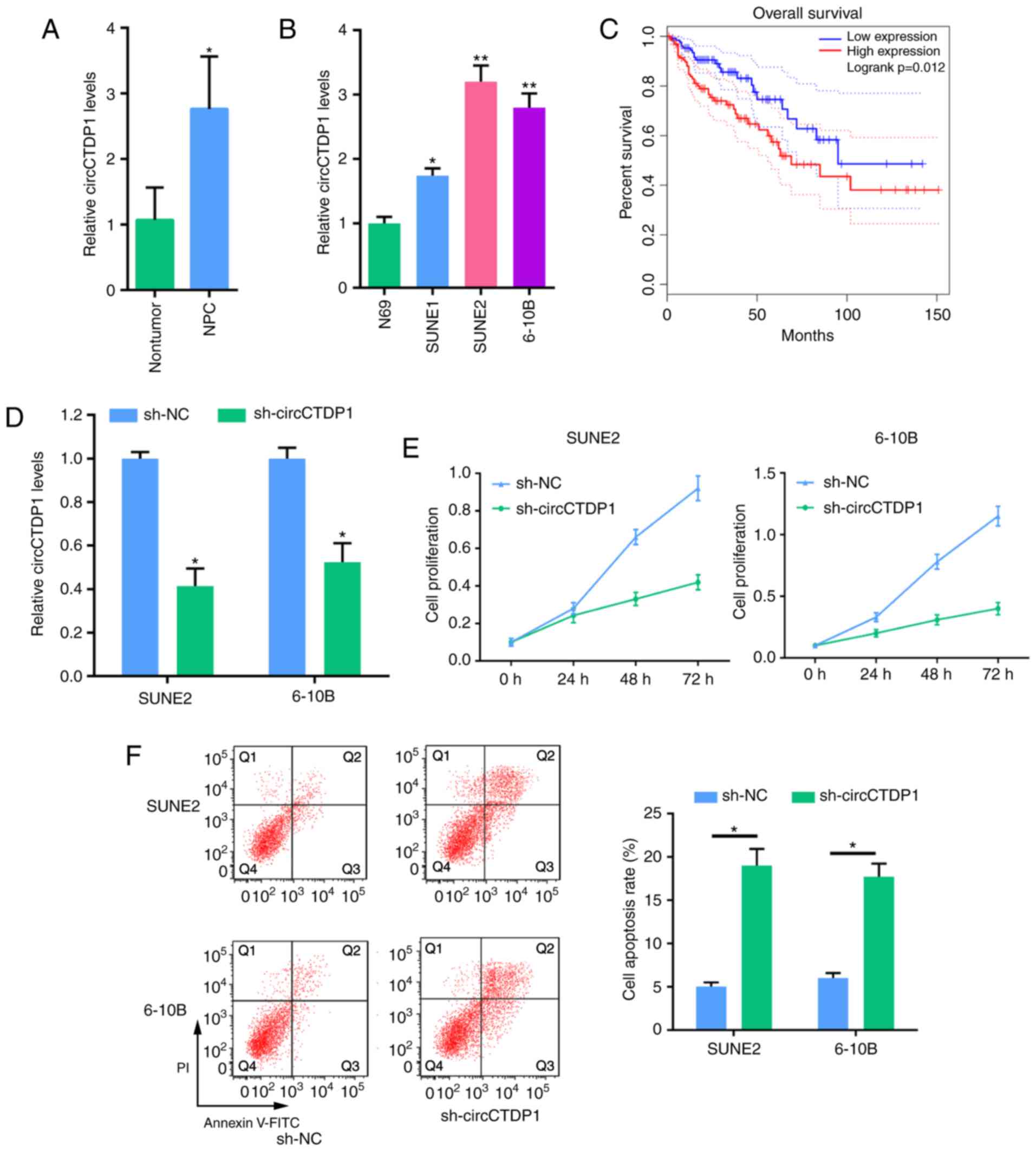
CircCTDP1 is upregulated in NPC tissues
and cell lines
Using RT-qPCR, the expression levels of circCTDP1 in
clinical tissues (NPC specimens and paracarcinoma tissues) was
examined. As presented in Fig.
1A, circCTDP1 was significantly upregulated in NPC tissues
compared with normal tissues. Similarly, circCTDP1 expression in 3
NPC cell lines (SUNE1, SUNE2 and 6-10B) was markedly upregulated
compared with expression in NP69 cells, a normal nasopharyngeal
epithelial cell line (Fig. 1B).
Moreover, Kaplan-Meier analysis showed that patients with high
circCTDP1 expression exhibited a worse prognosis compared with
patients with low circCTDP1 expression (Fig. 1C). These results suggest circCTDP1
may play a pivotal role in the progression of NPC.
Knockdown of circCTDP1 suppresses NPC
progression
To investigate the involvement of circCTDP1 in NPC,
shRNAs targeting circCTDP1 were designed. RT-qPCR indicated that
the expression of circCTDP1 in SUNE2 and 6-10B cells was decreased
after cells transfected with shcircCTDP1 (Fig. 1D). Subsequently, an MTT assay
revealed that cell viability was markedly reduced when NPC cells
were transfected with shcircCTDP1 (Fig. 1E). Moreover, flow cytometric
analysis revealed that circCTDP1 knockdown significantly promoted
apoptosis in SUNE2 and 6-10B cell lines (Fig. 1F). To further explore the effect
of circCTDP1 on the migration and invasion of NPC cells, wound
healing and cell invasion assays were performed. As presented in
Fig. 1G and H, the migration and
invasion abilities of the shcircCTDP1 cells were reduced relative
to shNC cells. Moreover, an in vivo xenograft experiment
revealed that depletion of circCTDP1 reduced the tumor growth rate
in mice (Fig. 1I-K). A
statistically significant difference in tumor volume was observed
between the two groups and the maximum tumor volume was 1,153
mm3. These data indicate that circCTDP1 knockdown
suppresses proliferation, mobility and promotes apoptosis of NPC
cells.
miR-320b inhibitor rescues the effects of
circCTDP1 knock- down on NPC cells
Kaplan-Meier survival analysis showed that NPC
patients with low miR-320b expression had a shorter OS time
compared with those patients with high miR-320b expression
(Fig. 2A). Using starBase
bioinformatic analysis software, circCTDP1 was suggested to bind to
miR-320b via complementary base pairing (Fig. 2B). A dual luciferase reporter
assay showed that a marked decrease in the luciferase activities
following co-transfection of the cells with miR-320b mimics and
wild-type circCTDP1 expression vector, but not with the mutant-type
circCTDP1 vector (Fig. 2C). To
investigate whether the effects of circCTDP1 on NPC were mediated
by miR-320b, the miR-320b inhibitor was transfected into
shcircCTDP1-expressing SUNE2 and 6-10B cells. RT-qPCR analysis
demonstrated that the introduction of miR-320b inhibitor reduced
miR-320b expression in NPC cells transfected with shcircCTDP1
(Fig. 2D). The proliferation of
shcircCTDP1-transfected SUNE2 and 6-10B cells was increased when
transfected with miR-320b inhibitor, as revealed by MTT assay
(Fig. 2E). Furthermore, flow
cytometry showed that knockdown of circCTDP1 promoted apoptosis,
whereas the cell apoptotic rate was reduced by co-transfection with
miR-320b inhibitor (Fig. 2F).
Similarly, transfection with miR-320b inhibitor partly reversed the
shcircCTDP1-mediated reduction of the migration and invasion
abilities of NPC cells (Fig. 2G and
H). Therefore, it was concluded that miR-320b antagonizes
circCTDP1-regulated NPC progression in vitro.
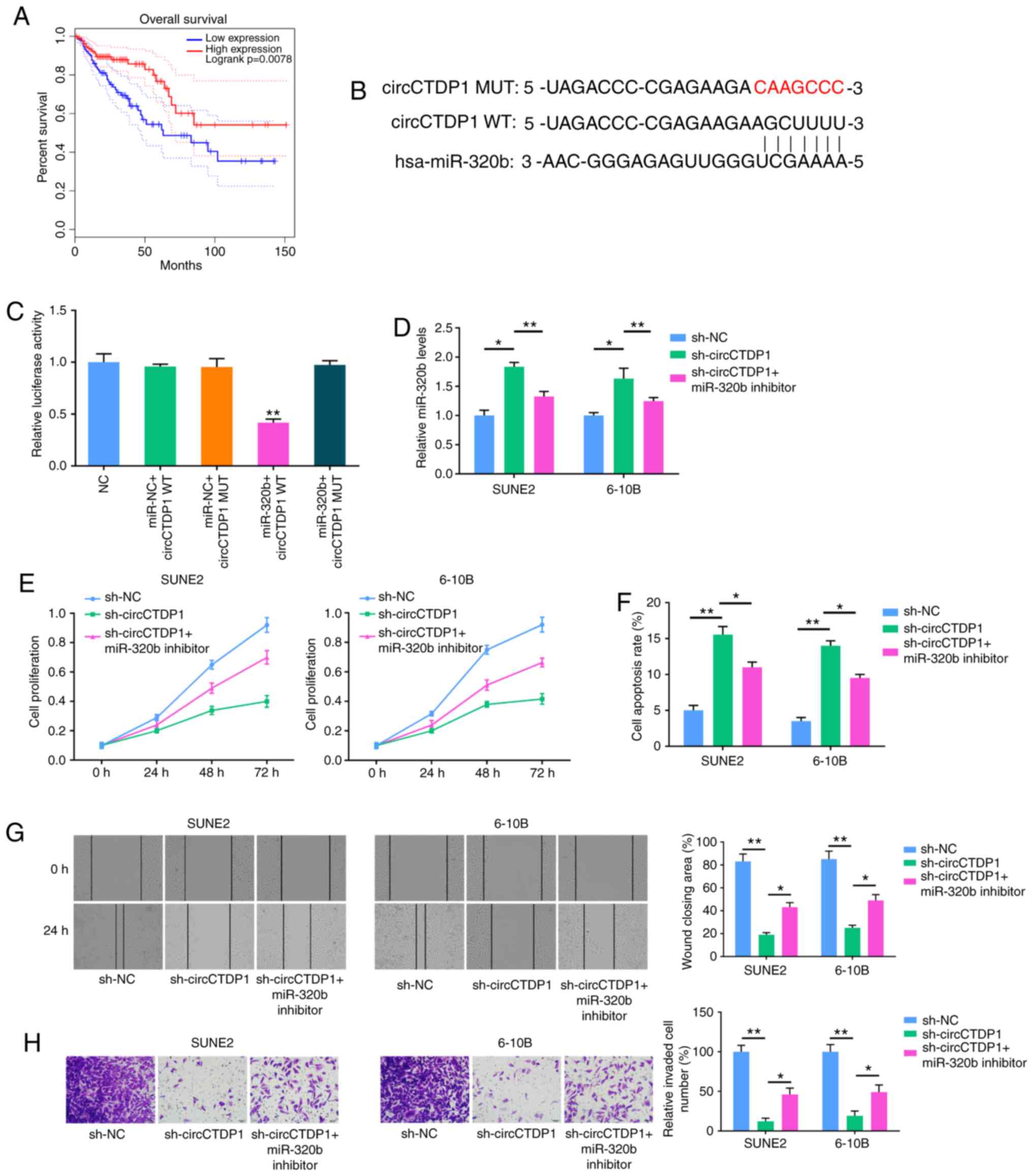 | Figure 2MiR-320b inhibitor restores the
attenuated progression of shcircCTDP1-transfected NPC cell lines.
(A) Kaplan-Meier survival analysis shows correlation between
miR-320b expression and prognosis of NPC patients. (B)
Bioinformatic prediction of binding site of miR-320b by circCTDP1.
(C) Dual luciferase reporter assay shows fluorescence intensity in
293T cells transfected with NC, miR-NC+circCTDP1 WT,
miR-NC+circCTDP1, MUT miR-320b+circCTDP1 WT and miR-320b+circCTDP1
MUT. (D) RT-qPCR analysis shows the relative miR-320b expression of
SUNE2 and 6-10B cell lines transfected with shNC, shcircCTDP1 and
shcircCTDP1 plus miR-320b inhibitor. (E) MTT assay shows cell
growth rate of SUNE2 and 6-10B cell lines transfected with shNC,
shcircCTDP1 and shcircCTDP1 plus miR-320b inhibitor at different
time points of 0, 24, 48, and 72 h. (F) Flow cytometry assay shows
the relative cell apoptosis rate of SUNE2 and 6-10B cell lines
transfected with shNC, shcircCTDP1 and shcircCTDP1 plus miR-320b
inhibitor. (G) Wound healing assay of SUNE2 and 6-10B cell lines
transfected with shNC, shcircCTDP1 and shcircCTDP1 plus miR-320b
inhibitor. (H) Cell invasion assay of SUNE2 and 6-10B cell lines
transfected with shNC, shcircCTDP1 and shcircCTDP1 plus miR-320b
inhibitor. The data were presented as mean ± SD
(*P<0.05; **P<0.01). |
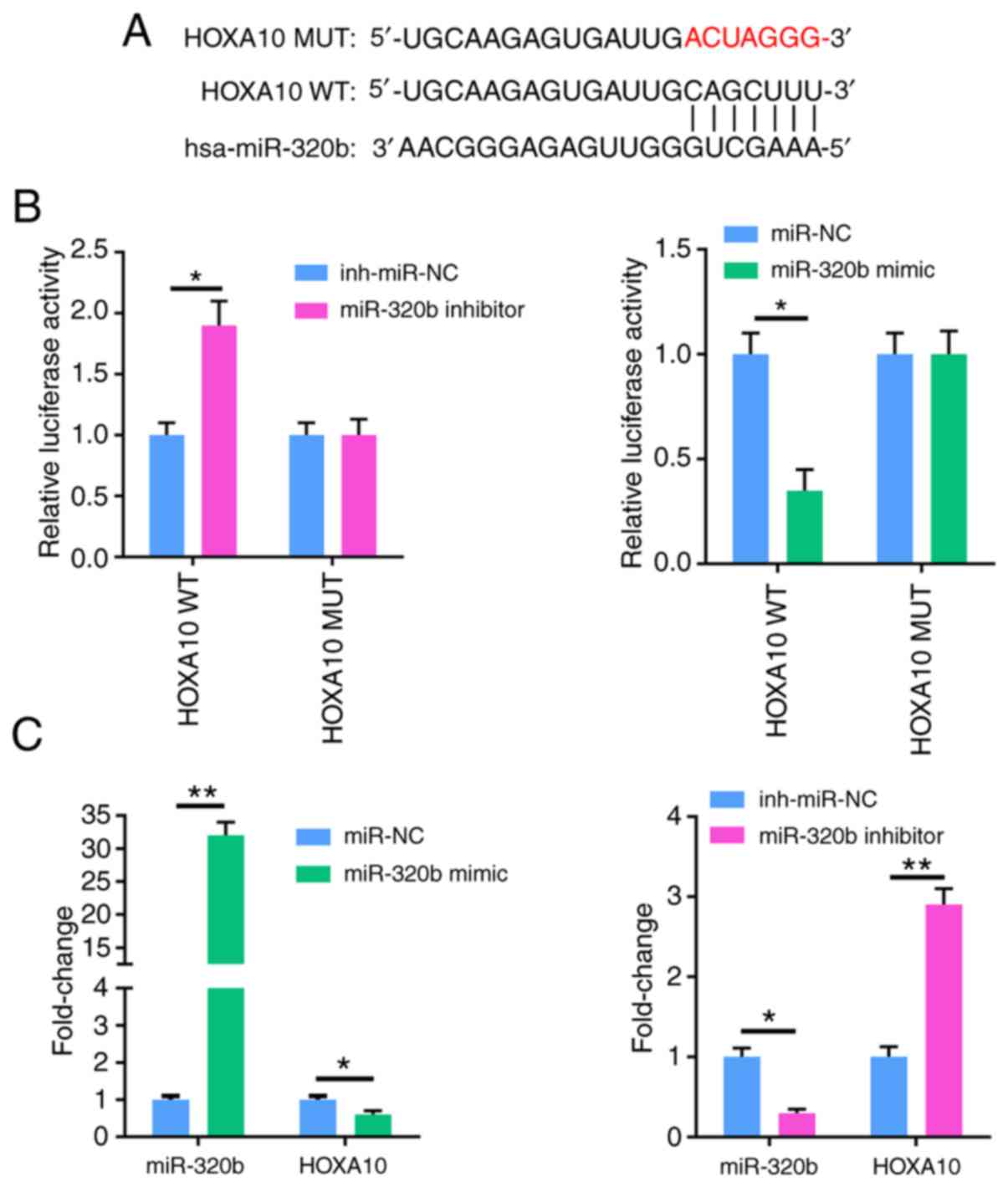
miR-320b directly targets HOXA10
Using the TargetScan database, it was found that
miR-320b could target HOXA10 as they have complementary DNA
regions (Fig. 3A). To further
examine the binding between miR-320b and HOXA10, a dual
luciferase reporter assay was performed. Compared with NC cells,
the results showed that the miR-320b mimic significantly reduced
the luciferase activity of the wild-type HOXA10 3′UTR,
whereas the miR-320b inhibitor increased luciferase activity. On
the other hand, there was no effect on cells transfected with a
mutant 3′-UTR of HOXA10 (Fig.
3B). Furthermore, transfection with miR-320b mimic
significantly suppressed the expression of HOXA10, while
miR-320b inhibitor markedly enhanced the HOXA10 expression
(Fig. 3C). Taken together, these
data indicate that miR-320b directly targets HOXA10 and
negatively regulates its expression.
circCTDP1/miR-320b regulates the
progression of NPC via HOXA10
Based on the aforementioned results, miR-320b was
indicated to directly target HOXA10. Therefore, it was
hypothesized that HOXA10 may act as a critical factor in the
circCTDP1/miR-320b axis in the development and progression of NPC.
Firstly, Kaplan-Meier survival analysis showed that patients with
NPC who exhibited a high HOXA10 expression had a shorter OS
time compared with patients with a low HOXA10 expression
(Fig. 4A). RT-qPCR analysis
further demonstrated that the expression of HOXA10 was
markedly reduced in NPC cells transfected with shHOXA10, but
was notably increased in NPC cells transfected with HOXA10
overexpression plasmid (Fig. 4B).
In addition, HOXA10 was overexpressed in shcircCTDP1- and
miR-320b mimic-transfected cell lines. As presented in Fig. 4C and D, the expression level of
HOXA10 was significantly increased in
shcircCTDP1+HOXA10 and miR-320b+HOXA10 groups.
Subsequently, an MTT assay was performed to examine cell
proliferation. As shown in Fig.
4E, HOXA10 could restore the attenuated cell
proliferation of shcircCTDP1- or miR-320b-trans-fected SUNE2 and
6-10B cells. Furthermore, it was found that the overexpression of
HOXA10 inhibited apoptosis and enhanced cell mobility in
shcircCTDP1- or miR-320b mimic-transfected SUNE2 and 6-10B cells
(Fig. 4F-H). Moreover, there was
a positive correlation between the expression of circCTDP1 and
HOXA10 in NPC tissues, whereas there was a negative
correlation between the expression of miR-320b and HOXA10
(Fig. 4I). Based on these
results, the effects of shcircCTDP1 and miR-320b mimic on NPC cells
were neutralized by overexpression of HOXA10, therefore
suggesting that circCTDP1/mirR-320b/HOXA10 may be an
important signaling pathway involved in the progression of NPC.
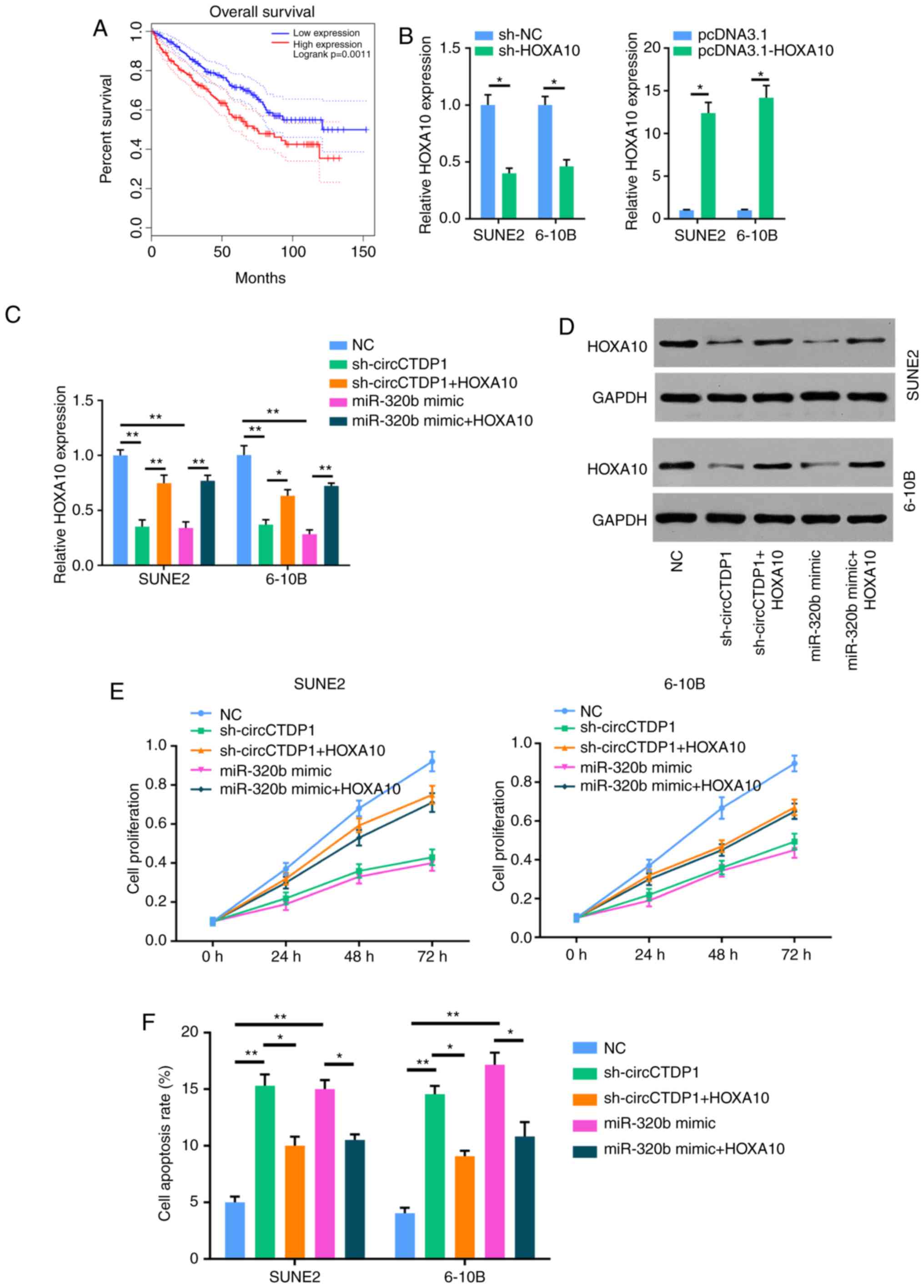 | Figure 4CircCTDP1 upregulates the expression
of HOXA10 via targeting miR-320b. (A) Kaplan-Meier survival
analysis shows correlation between HOXA10 expression and
prognosis of NPC patients. (B) RT-qPCR analysis shows relative
HOXA10 expression of SUNE2 and 6-10B cell lines (shNC or
shHOXA10 and pcDNA3.1 or pcDNA3.1-HOXA10). (C)
RT-qPCR analysis shows relative HOXA10 expression of SUNE2
and 6-10B cell lines (NC, shcircCTDP1, shcircCTDP1 plus
HOXA10, miR-320b mimic, and miR-320b mimic plus
HOXA10). (D) Western blot analysis shows the relative
HOXA10 protein level of SUNE2 and CINE1 cell lines (NC,
shcircCTDP1, shcircCTDP1 plus HOXA10, miR-320b mimic, and
miR-320b mimic plus HOXA10). (E) MTT assay shows the cell
growth rate of SUNE2 and 6-10B cell lines (NC, shcircCTDP1,
shcircCTDP1 plus HOXA10, miR-320b mimic, and miR-320b mimic
plus HOXA10) at different time points of 0, 24, 48, and 72
h. (F) Flow cytometry assay shows the relative cell apoptosis rate
of SUNE2 and 6-10B cell lines (NC, shcircCTDP1, shcircCTDP1 plus
HOXA10, miR-320b mimic, and miR-320b mimic plus
HOXA10). The data were presented as mean ± SD
(*P<0.05; **P<0.01). CircCTDP1
upregulates the expression of HOXA10 via targeting miR-320b.
(G) Cell invasion assay of SUNE2 and 6-10B cell lines (NC,
shcircCTDP1, shcircCTDP1 plus HOXA10, miR-320b mimic, and
miR-320b mimic plus HOXA10). (H) Wound healing assay of
SUNE2 and 6-10B cell lines (NC, shcircCTDP1, shcircCTDP1 plus
HOXA10, miR-320b mimic, and miR-320b mimic plus
HOXA10). (I) The circCTDP1 expressions were positively
correlated with HOXA10 expressions within included NPC
tissues. The miR-320b expressions were negatively correlated with
HOXA10 expressions within included NPC tissues. The data
were presented as mean ± SD (*P<0.05;
**P<0.01). |
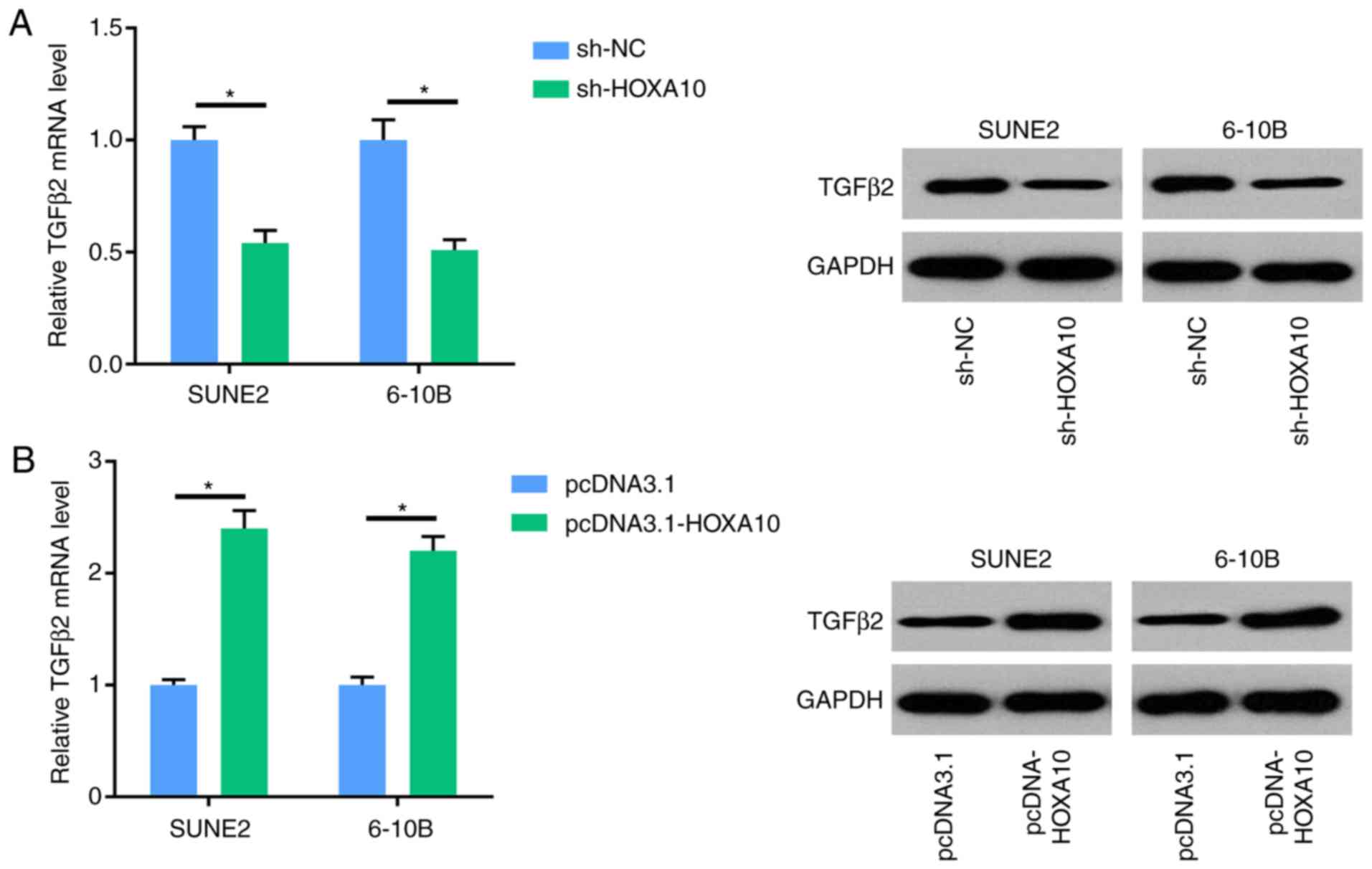
HOXA10 regulates the expression of TGFβ2
in NPC cells
TGFβ2 is a vital modulator of tumor invasion
and motility. RT-qPCR and western blot analysis showed that
knockdown of HOXA10 decreased the expression of TGFβ2
in SUNE2 and 6-10B cells, whereas overexpression of HOXA10
increased the expression of TGFβ2. These results
demonstrated that HOXA10 could regulate the expression of
TGFβ2 in NPC cells (Fig. 5A
and B).
Discussion
NPC is one of the most life-threatening tumors
worldwide which is mainly ascribed to tumor recurrence and distant
metastasis (29,30). Thus, developing novel prognostic
markers for NPC is crucial. To the best of our knowledge, in the
present study, it was demonstrated for the first time that the
circCTDP1/miR-320b/HOXA10 axis may contribute to the
development and progression of NPC.
Previous findings have demonstrated that aberrant
expression of circRNA is involved in various human cancers
(31-33). For instance, circ_0067934
overexpression is associated with poor prognosis and facilitates
the development and progression of thyroid carcinoma (34). Shuai et al (35) reported that circRNA_0000285 serves
as a prognostic biomarker for nasopharyngeal carcinoma. However,
the biological role and potential molecular mechanism of circRNAs
in NPC remain to be elucidated. In the current study, the
association between the expression level of circCTDP1 and the
prognosis of patients with NPC was analyzed. circCTDP1 expression
was also determined in in NPC tissues and cell lines, and found to
be upregulated. Knockdown of circCTDP1 suppressed the
proliferation, migration and invasion of NPC cells, suggesting for
the first time that circCTDP1 is associated with the progression of
NPC.
Previous studies have demonstrated that circRNAs
interact with miRNAs by acting as ceRNA to regulate the
proliferation and migration of tumor cells (36-38). Recently, Cao et al
(39) reported that circ0001429
promoted the progression of bladder cancer through sponging
miR-205-3p and upregulating expression of VEGFA. Regarding NPC, Ke
et al reported that circHIPK3 acts as a ceRNA to upregulate
E74 like ETS transcription factor 3 by sponging miR-4288 (40). In the present study, circCTDP1 was
found to directly interact with miR-320b, and to inhibit its
expression through bioinformatic analysis and luciferase reporter
assay. Furthermore, miR-320b inhibitor could significantly abolish
the inhibitory effect of shcircCTDP1 on NPC phenotypes.
HOX genes are divided into 4 groups (HOXA,
HOXB, HOXC and HOXD) encode transcription
factors involved in the control of cell growth (41,42). It has been reported that
HOXA10 is associated with cell migration, proliferation and
survival in various types of cancer (43,44). In the current study, it was
demonstrated that HOXA10 was a direct downstream target of
miR-320b through bioinformatic prediction and in vitro
experiments, and overexpression of HOXA10 was found to
promote the progression and development of NPC cells. Moreover,
expression of HOXA10 was positively correlated with that of
circCTDP1, but negatively correlated with miR-320b expression.
These results indicated that miR-320b may inhibit the
proliferation, migration and invasion of NPC cells by
downregulating HOXA10 expression.
HOXA10 has been reported to increase the
levels of TGFβ2 in pancreatic cancer cells (45). Therefore, it was hypothesized that
HOXA10 may exert its role in NPC by regulating the
expression of TGFβ2. The findings of the present study
demonstrated that upregulation of HOXA10 increased the
expression of TGFβ2 and downregulation of HOXA10
decreased the expression of TGFβ2.
In the current study, it was demonstrated that
circCTDP1 promotes proliferation, migration and invasion of NPC
cells through a miR-320b/HOXA10 axis. This study offers an
improved understanding of the pathogenesis of NPC, and the
circCTDP1, miR-320b, HOXA10 and TGFβ2 may have
potential as therapeutic targets for NPC.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HL, CC and XT designed the study. JY and HX
performed experiments. HL and CC analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Third Affiliated Hospital of Soochow University.
Written informed consent was obtained from all patients prior to
the study start. All animal experiments were conducted with the
approval of the Third Affiliated Hospital of Soochow
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan F, Ruan Z, Li J, Pang X, Zhang Y, Zou
L and Liang H: Radiotherapy combined docetaxel and oxaliplatin
chemotherapy is effective in patients with locally advanced
nasopharyngeal carcinoma. Med Oncol. 32:2522015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colaco RJ, Betts G, Donne A, Swindell R,
Yap BK, Sykes AJ, Slevin NJ, Homer JJ and Lee LW: Nasopharyngeal
carcinoma: A retrospective review of demographics, treatment and
patient outcome in a single centre. Clin Oncol (R Coll Radiol).
25:171–177. 2013. View Article : Google Scholar
|
|
4
|
Ma DD, Yuan LL and Lin LQ: LncRNA HOTAIR
contributes to the tumorigenesis of nasopharyngeal carcinoma via
up-regulating FASN. Eur Rev Med Pharmacol Sci. 21:5143–5152.
2017.PubMed/NCBI
|
|
5
|
Zhuang M, Zhao M, Qiu H, Shi D, Wang J,
Tian Y, Lin L and Deng W: Effusanin E suppresses nasopharyngeal
carcinoma cell growth by inhibiting NF-κB and COX-2 signaling. PLoS
One. 9:e1099512014. View Article : Google Scholar
|
|
6
|
Pei W, Tao L, Zhang LW, Zhang S, Cao J,
Jiao Y, Tong J and Nie J: Circular RNA profiles in mouse lung
tissue induced by radon. Environ Health Prev Med. 22:362017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li R, Wu B, Xia J, Ye L and Yang X:
Circular RNA hsa_ circRNA_102958 promotes tumorigenesis of
colorectal cancer via miR-585/CDC25B axis. Cancer Manag Res.
11:6887–6893. 2019. View Article : Google Scholar :
|
|
8
|
Chen L, Nan A, Zhang N, Jia Y, Li X, Ling
Y, Dai J, Zhang S, Yang Q, Yi Y and Jiang Y: Circular RNA 100146
functions as an oncogene through direct binding to miR-361-3p and
miR-615-5p in non-small cell lung cancer. Mol Cancer. 18:132019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Xiao Y, Wu L and Ma D:
Comprehensive circular RNA profiling reveals the regulatory role of
the circRNA-000911/miR-449a pathway in breast carcinogenesis. Int J
Oncol. 52:743–754. 2018.PubMed/NCBI
|
|
10
|
Zhu L, Liu Y, Yang Y, Mao XM and Yin ZD:
CircRNA ZNF609 promotes growth and metastasis of nasopharyngeal
carcinoma by competing with microRNA-150-5p. Eur Rev Med Pharmacol
Sci. 23:2817–2826. 2019.PubMed/NCBI
|
|
11
|
Wei H, Liu D, Sun J, Mao Y, Zhao L, Zhu W,
Xu G and Gao Z: Circular RNA circ_0008450 upregulates CXCL9
expression by targeting miR-577 to regulate cell proliferation and
invasion in nasopharyngeal carcinoma. Exp Mol Pathol.
110:1042882019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Zhou H and Guan Z: CircRNA_000543
knockdown sensitizes nasopharyngeal carcinoma to irradiation by
targeting miR-9/platelet-derived growth factor receptor B axis.
Biochem Biophys Res Commun. 512:786–792. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang M, Zhong Z, Lv M, Shu J, Tian Q and
Chen J: Comprehensive analysis of differentially expressed profiles
of lncRNAs and circRNAs with associated co-expression and ceRNA
networks in bladder carcinoma. Oncotarget. 7:47186–47200.
2016.PubMed/NCBI
|
|
14
|
Sun Q, Liu T, Zhang T, Du S, Xie GX, Lin
X, Chen L and Yuan Y: MiR-101 sensitizes human nasopharyngeal
carcinoma cells to radiation by targeting stathmin 1. Mol Med Rep.
11:3330–3336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu RS, Qiu EH, Zhu JJ, Wang JR and Lin HL:
MiR-101 promotes nasopharyngeal carcinoma cell apoptosis through
inhibiting Ras/Raf/MEK/ERK signaling pathway. Eur Rev Med Pharmacol
Sci. 22:150–157. 2018.PubMed/NCBI
|
|
16
|
Zhu HM, Jiang XS, Li HZ, Qian LX, Du MY,
Lu ZW, Wu J, Tian XK, Fei Q, He X and Yin L: miR-184 inhibits tumor
invasion, migration and metastasis in nasopharyngeal carcinoma by
targeting Notch2. Cell Physiol Biochem. 49:1564–1576. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang X, Dai B and Feng L: miR-543
promoted the cell proliferation and invasion of nasopharyngeal
carcinoma by targeting the JAM-A. Hum Cell. 32:477–486. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin W, Shi L and Mao Y: MicroRNA-449b-5p
suppresses cell proliferation, migration and invasion by targeting
TPD52 in nasopharyngeal carcinoma. J Biochem. 166:433–440. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Tang X, He Q, Yang X, Ren X, Wen X,
Zhang J, Wang Y, Liu N and Ma J: Overexpression of mitochondria
mediator gene TRIAP1 by miR-320b loss is associated with
progression in nasopharyngeal carcinoma. PLoS Genet.
12:e10061832016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu J, Zhang PY, Li P, Xie JW, Wang JB, Lin
JX, Chen QY, Cao LL, Huang CM and Zheng CH: Circular RNA
hsa_circ_0001368 suppresses the progression of gastric cancer by
regulating miR-6506-5p/FOXO3 axis. Biochem Biophys Res Commun.
512:29–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
An J, Shi H, Zhang N and Song S: Elevation
of circular RNA circ_0003645 forecasts unfavorable prognosis and
facilitates cell progression via miR-1179/TMEM14A pathway in
non-small cell lung cancer. Biochem Biophys Res Commun.
511:921–925. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang
C, Liu D, Wang M, Wang L, Zeng F and Jiang G: CircHIPK3 sponges
miR-558 to suppress heparanase expression in bladder cancer cells.
EMBO Rep. 18:1646–1659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zanatta A, Rocha AM, Carvalho FM, Pereira
RM, Taylor HS, Motta EL, Baracat EC and Serafini PC: The role of
the Hoxa10/HOXA10 gene in the etiology of endometriosis and its
related infertility: A review. J Assist Reprod Genet. 27:701–710.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Wang DL and Yu P: LncRNA H19
regulates the expression of its target gene HOXA10 in endometrial
carcinoma through competing with miR-612. Eur Rev Med Pharmacol
Sci. 22:4820–4827. 2018.PubMed/NCBI
|
|
25
|
Liu J, Jiang Y, Wan Y, Zhou S, Thapa S and
Cheng W: MicroRNA-665 suppresses the growth and migration of
ovarian cancer cells by targeting HOXA10. Mol Med Rep.
18:2661–2668. 2018.PubMed/NCBI
|
|
26
|
Park SM, Choi EY, Bae M, Choi JK and Kim
YJ: A long-range interactive DNA methylation marker panel for the
promoters of HOXA9 and HOXA10 predicts survival in breast cancer
patients. Clin Epigenetics. 9:732017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen ZH, Zhao KM and Du T: HOXA10 promotes
nasopharyngeal carcinoma cell proliferation and invasion via
inducing the expression of ZIC2. Eur Rev Med Pharmacol Sci.
21:945–952. 2017.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Lee AW, Poon YF, Foo W, Law SC, Cheung FK,
Chan DK, Tung SY, Thaw M and Ho JH: Retrospective analysis of 5037
patients with nasopharyngeal carcinoma treated during 1976-1985:
Overall survival and patterns of failure. Int J Radiat Oncol Biol
Phys. 23:261–270. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vokes EE, Liebowitz DN and Weichselbaum
RR: Nasopharyngeal carcinoma. Lancet. 350:1087–1091. 1997.
View Article : Google Scholar
|
|
31
|
Sheng M, Wei N, Yang HY, Yan M, Zhao QX
and Jing LJ: CircRNA UBAP2 promotes the progression of ovarian
cancer by sponging microRNA-144. Eur Rev Med Pharmacol Sci.
23:7283–7294. 2019.PubMed/NCBI
|
|
32
|
Jin C, Shi L, Li Z, Liu W, Zhao B, Qiu Y,
Zhao Y, Li K, Li Y and Zhu Q: Circ_0039569 promotes renal cell
carcinoma growth and metastasis by regulating miR-34a-5p/CCL22. Am
J Transl Res. 11:4935–4945. 2019.PubMed/NCBI
|
|
33
|
Yu T, Wang Y, Fan Y, Fang N, Wang T, Xu T
and Shu Y: CircRNAs in cancer metabolism: A review. J Hematol
Oncol. 12:902019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Yan X, Zhang H and Zhan X: CircRNA
circ_0067934 overexpression correlates with poor prognosis and
promotes thyroid carcinoma progression. Med Sci Monit.
25:1342–1349. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shuai M, Hong J, Huang D, Zhang X and Tian
Y: Upregulation of circRNA_0000285 serves as a prognostic biomarker
for nasopharyngeal carcinoma and is involved in radiosensitivity.
Oncol Lett. 16:6495–6501. 2018.PubMed/NCBI
|
|
36
|
Liu L, Yang J, Zhu X, Li D, Lv Z and Zhang
X: Long noncoding RNA H19 competitively binds miR-17-5p to regulate
YES1 expression in thyroid cancer. FEBS J. 283:2326–2339. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hou Z, Xu X, Zhou L, Fu X, Tao S, Zhou J,
Tan D and Liu S: The long non-coding RNA MALAT1 promotes the
migration and invasion of hepatocellular carcinoma by sponging
miR-204 and releasing SIRT1. Tumour Biol. 39:10104283177181352017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang SH, Ma F, Tang ZH, Wu XC, Cai Q,
Zhang MD, Weng MZ, Zhou D, Wang JD and Quan ZW: Long non-coding RNA
H19 regulates FOXM1 expression by competitively binding endogenous
miR-342-3p in gallbladder cancer. J Exp Clin Cancer Res.
35:1602016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao W, Zhao Y, Wang L and Huang X:
Circ0001429 regulates progression of bladder cancer through binding
miR-205-5p and promoting VEGFA expression. Cancer Biomark.
25:101–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ke Z, Xie F, Zheng C and Chen D: CircHIPK3
promotes proliferation and invasion in nasopharyngeal carcinoma by
abrogating miR-4288-induced ELF3 inhibition. J Cell Physiol.
234:1699–1706. 2019. View Article : Google Scholar
|
|
41
|
Chen KN, Gu ZD, Ke Y, Li JY, Shi XT and Xu
GW: Expression of 11 HOX genes is deregulated in esophageal
squamous cell carcinoma. Clin Cancer Res. 11:1044–1049.
2005.PubMed/NCBI
|
|
42
|
Carrera M, Bitu CC, de Oliveira CE,
Cervigne NK, Graner E, Manninen A, Salo T and Coletta RD: HOXA10
controls proliferation, migration and invasion in oral squamous
cell carcinoma. Int J Clin Exp Pathol. 8:3613–3623. 2015.PubMed/NCBI
|
|
43
|
Shao L, Chen Z, Peng D, Soutto M, Zhu S,
Bates A, Zhang S and El-Rifai W: Methylation of the HOXA10 promoter
directs miR-196b-5p-dependent cell proliferation and invasion of
gastric cancer cells. Mol Cancer Res. 16:696–706. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu J, Li C, Jiang Y, Wan Y, Zhou S and
Cheng W: Tumor-suppressor role of miR-139-5p in endometrial cancer.
Cancer Cell Int. 18:512018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cui XP, Qin CK, Zhang ZH, Su ZX, Liu X,
Wang SK and Tian XS: HOXA10 promotes cell invasion and MMP-3
expression via TGFβ2-mediated activation of the p38 MAPK pathway in
pancreatic cancer cells. Dig Dis Sci. 59:1442–1451. 2014.
View Article : Google Scholar : PubMed/NCBI
|