Introduction
In certain renal surgeries, including partial
nephrectomy and renal transplantation, the renal vasculature is
temporarily clamped followed by vascular re-perfusion to preserve
the kidney. The procedure might cause renal ischemia reperfusion
injury (IRI), which is one of the major causes of postoperative
acute renal dysfunction (1).
Postoperative renal prognosis is a particularly important factor
following such surgeries (2,3).
IRI can also occur following cardiovascular surgeries that require
cardiac arrest during cardiopulmonary bypass, because of the
associated non-physiological circulation (4,5).
IRI reportedly consists of numerous stages,
including transcriptional reprogramming during ischemia, followed
by an autoimmune inflammatory reaction, vascular leakage and
promotion of cell death, including apoptosis during reperfusion
(6). Alleviation of IRI improves
the prognosis of the preserved kidney (7). Previous animal studies reported that
ischemic preconditioning (IPC) alleviates renal IRI (8,9)
and numerous clinical studies have elucidated the IPC effects on
the kidney. Furthermore, these surgeries are performed under
general anesthesia. Administration of certain anesthetics, which is
considered anesthetic preconditioning (APC), also reportedly
alleviates renal IRI in rats (10-12). Specifically, sevoflurane APC was
reported to be effective in alleviating renal IRI both clinically
and in a mouse model (13,14).
A microRNA (miR) is a small sequence of ~22 bases
that binds to messenger RNA with a complementary sequence and
suppresses it by inhibiting translation or by promoting
degradation. miR mainly recognize seven bases on the target
messenger RNA sequence, namely the 2nd-8th base on the 3′ end
(15). The preconditioning
effects of anesthesia and ischemia on IRI are reportedly mediated
via changes in miR (16,17). However, the associations between
miRs and preconditioning effects on the kidney have not been fully
clarified.
The present study compared sevoflurane APC and IPC
in a rat renal IRI model, verified the dynamics of miRs and
researched target cell-survival pathways. A pathway analysis system
was used to estimate the connection between the related genes
affected by APC or IPC with canonical pathways from a database
including a large number of molecular interactions and
gene-to-phenotype studies. Rat IRI models have also been previously
used to elucidate the known cell-death or cell-survival substances
common to humans. The present study aimed to clarify the
cell-survival pathways that are affected by sevoflurane APC and
IPC, and to find commonalities and differences in these
preconditioning effects using a rat IRI model.
Materials and methods
Ethics statement
The experimental protocols were approved by the
Animal Research Committee at Nippon Medical School (Approval
number: 29-008, Approval date: April 01, 2017). In addition, all
the experimental protocols were performed in accordance with the
ARRIVE guidelines.
Surgical preparation
A total of 28 male Wistar rats (age 10-11 weeks,
weighing 336±24 g, obtained from Tokyo Laboratory Animals Science
Co., Ltd.) were kept in cages at 26°C under a 14:10 h light:dark
cycle in a specific pathogen-free facility, with free access to
food and water. Before the surgical procedure, anesthesia was
induced with 2 mg/kg midazolam, 2.5 mg/kg butorphanol and 0.15
mg/kg medetomidine hydrochloride injected intraperitoneally,
followed by injection of 0.6 mg/kg/h midazolam, 0.8 mg/kg/h
butorphanol and 0.05 mg/kg/h medetomidine hydrochloride to maintain
sedation and analgesia. Additional 0.6 mg/kg midazolam, 0.8 mg/kg
butorphanol and 0.05 mg/kg medetomidine hydrochloride were
administered if the rats moved their limbs in response to pain.
They were ventilated through a tracheal tube to maintain the
partial pressure of carbon dioxide at 35-45 mmHg. Rectal
temperature was maintained at 36.5-37.5°C. Mean arterial pressure
was monitored via the right femoral artery. To maintain fluid
volume, a normal saline solution (3 ml/h) was continuously infused
via the caudal vein. Under general anesthesia, the right kidney was
resected via a median abdominal incision (18). The time from initiation of general
anesthesia to resection of the right kidney was 40 min. Next, the
effects of IRI of the left kidney and the ameliorative effects of
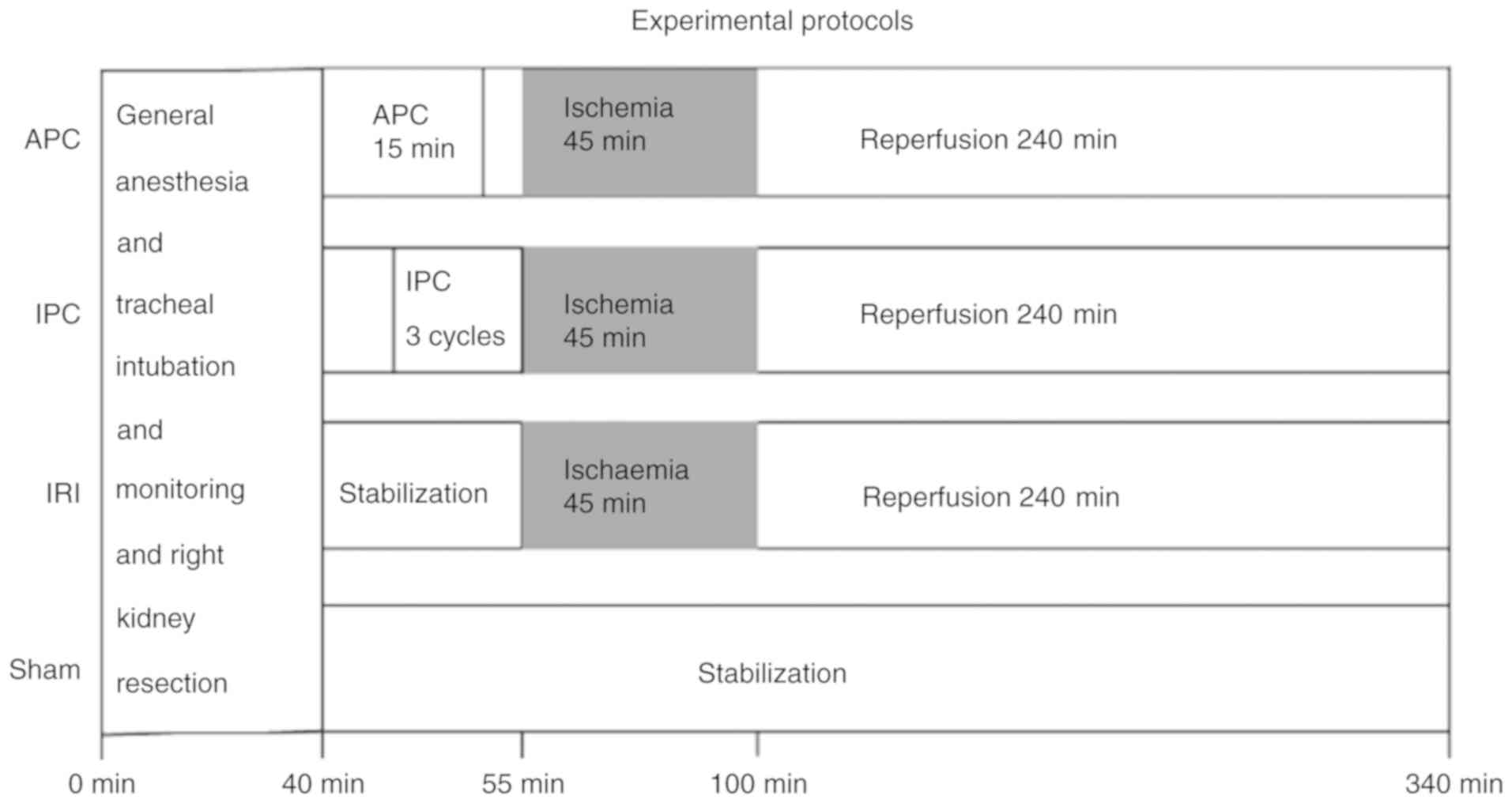
APC and IPC on IRI were evaluated. For this, the 28 rats were
randomly allocated to four groups: i) Sham group (no left renal
arteriovenous clamping); ii) IRI group (45 min of left renal
arteriovenous clamping and 4 h of reperfusion); iii) APC group
(exposure to one minimum alveolar concentration sevoflurane 2.2%
for 15 min, 10 min before the IRI procedure) and iv) IPC group
(three cycles of renal arteriovenous clamping for 2 min and
reperfusion for 5 min immediately before the IRI procedure)
(Fig. 1). To minimize technical
noise, four randomly chosen rats, one from each group, underwent
the surgical procedure at the same time. Accurate performance of
the IRI procedure was confirmed by observation of loss and return
of redness of the kidney. Reperfusion was considered successful
when redness in the entire kidney returned immediately after
arteriovenous release. At the end of the procedure, the left kidney
tissue was sampled for analysis of extracted miR expression at the
same time as blood was collected for measurement of serum
create-nine as an indicator of renal IRI. The rats were
subsequently sacrificed by exsanguination under general
anesthesia.
Serum creatinine assay
Blood samples (3 ml) were injected into a separating
agent, centrifuged at 4°C and 3,000 × g for 15 min, and the
resultant supernatant was collected as serum (19). Serum creatinine levels were
measured in a clinical laboratory using a clinical chemistry
analyzer (JCA-BM6070; JEOL Ltd.) and the results were reported in
mg/dl.
miR screening test
The left kidney tissue sample was permeated with RNA
later solution™ (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and stored at −80°C. The tissue samples were sufficiently
lysed in liquid nitrogen and total RNA was extracted using the
mirVana miRNA Isolation Kit™ (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and assessed with the NanoDrop ND-1000™
spectrophotometer (Thermo Fisher Scientific, Inc.) (17).
Quantitative measurement of miR
expression
The rat renal cyclic DNA was reverse transcribed
using a TaqMan™ MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and Megaplex™ RT
primers A and B (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the following temperature protocol: 40 cycles
of 16°C for 2 min, 42°C for 1 min and 50°C for 1 sec, followed by
85°C for 5 min, and storage at 4°C. Subsequently, 373 specific
kinds of well-known miRs derived from rats were amplified and
measured with the TaqMan Low Density Array™ (Applied Biosystems;
Thermo Fisher Scientific, Inc.) in the 7900 HT Fast Real-Time
polymerase chain reaction System™ (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using a comparative quantification
method.
The results of reverse transcription-quantitative
(RT-q)PCR were analyzed with Data Assist software version 2.0
(Applied Biosystems; Thermo Fisher Scientific, Inc.) as follows: i)
the control gene was set as Y1, which has the highest stable value
calculated among the candidates U6, U87 and Y1; ii) the ΔCq value
of each miR was obtained by comparing the number of amplifications
until it exceeded the automatic threshold value with that of Y1;
iii) the ΔΔCq value of each miR in each group was obtained by
subtracting the mean ΔCq value in the sham group from that in each
group and the miR expression value, as the Fold-Change
(F.C.=2−ΔΔCq) value, was obtained; and iv) the adjusted
F.C. value of each miR in each group was calculated by dividing its
F.C. value by the mean F.C. value of the sham group (20).
Search for the target pathway using
Ingenuity Pathway Analysis™
The list of differentially expressed miRs was
analyzed using Ingenuity Pathway Analysis™ (IPA; Qiagen, Inc.) to
estimate related genes and canonical pathways using the 'core
analysis' function, and mapped in the predicted network based on a
database including molecular interactions and gene-to-phenotype
studies from over 200,000 peer-reviewed scientific articles. This
analysis was performed to narrow down the canonical pathways
affected by the miRs assessed in this experiment.
Measurement of activation of the target
pathway using western blot analysis
Western blot analysis was performed to verify
activation of the target substances in cell-survival pathways that
were predicted by IPA™ to be related to the experimentally changed
miRs. Total renal proteins were extracted using T-PER Tissue
Protein Extraction Reagent™ (Pierce; Thermo Fisher Scientific,
Inc.) with Halt Protease Inhibitor Cocktail EDTA-Free™ (Pierce;
Thermo Fisher Scientific, Inc.), and quantified with Quick Start™
Bradford Reagent (Biorad Laboratories, Inc.). A total of 40
µg protein per lane were applied into Mini-PROTEAN TGX
Stain-Free Gels™ (4-15%; Bio-rad Laboratories, Inc.; cat. no.
456-8084) soaked in Tris/Glycine/SDS Buffer (Bio-Rad Laboratories,
Inc.) and transferred onto a polyvinylidene fluoride membrane.
Followed by incubation overnight at 4°C in TBS containing 0.1%
Tween-20 (Bio-Rad Laboratories, Inc.) with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) and several antibodies, including
anti-phosphatase and tensin homologue deleted from chromosome 10
(PTEN) (A2B1; Mouse monoclonal antibody; 1:200; Santa Cruz
Biotechnology, Inc.; cat. no. SC-7974), anti-protein kinase B (Akt)
(pan) (C67E7; Rabbit monoclonal antibody; 1:1,000; Cell Signaling
Technology, Inc.; cat. no. 4691S), anti-phosphorylated Akt (pAkt)
(Ser473) (XP Rabbit monoclonal antibody; 1:1,000; Cell Signaling
Technology, Inc.; cat. no. 4060S) and anti-β-actin (C4) (Mouse
monoclonal antibody; 1:200; Santa Cruz Biotechnology, Inc.; cat.
no. SC-47778), respectively after suppressing non-singular bands
using 5% Amersham ECL Blocking Agent™ (GE Healthcare UK Ltd.). The
protein bands of the four different groups were arranged in a row
on the same membrane (n=7 for each antibody used). Protein bands
were visualized with chemiluminescence using a goat poly-clonal
second antibody to rabbit IgG HRP (1:20,000; Abcam; cat. no.
643005) or goat polyclonal second antibody to mouse IgG1 HRP
(1:20,000; Cosmo Bio Co., Ltd.; cat. no. A90-105P), followed by
Amersham ECL Prime Western blotting detection reagent™ (GE
Healthcare UK Ltd.) in an ImageQuant LAS 4000 mini biomolecular
imager (GE Healthcare). The intensity of protein fragments was
quantified with ImageQuant TL ver. 8.1 (GE Healthcare). Since the
quantified signal values involved background differences between
the seven membranes used, the F.C. value of each target protein was
calculated as the ratio to β-actin. The adjusted F.C. value was
obtained by correction using a ratio of β-actin signal value in the
sham group on the same membrane to the average of that on all the
membranes used.
Statistical analysis
All the 28 kidneys sampled were analyzed. Vital
signs, serum creatinine values and adjusted F.C. values of the miRs
and the target proteins are expressed as mean ± standard deviation.
Sample size was calculated for the serum creatinine value and
adjusted F.C. values of the target proteins and miRs. A sample size
of seven per group has an effect size of 0.8 with the power one-way
analysis of variance (ANOVA) test using a significance value of
0.05 and a power of 0.9. All the ΔΔCq values of miRs and vital
signs and serum creatinine values were normally distributed and
inter-group comparisons were made using Tukey's test. To
sufficiently narrow down the miRs to be analyzed, a one way ANOVA
was performed on the apparently differentially expressed miRs
following APC or IPC, of which the adjusted F.C. value in APC or
IPC satisfied the following conditions: i) adjusted F.C. ≥1.2 and
not less than four times that in the IRI group; and ii) adjusted
F.C. ≤0.8, and not more than quarter that in the IRI group.
Multiple tests were corrected with the Storey's method for turning
the list of P-values into q-values, which measures the false
discovery rate. Then, the final intergroup difference determination
was performed by multiple comparisons with Tukey's tests, which
were performed on the miRs having significant inter-group
differences (P<0.05, q<0.5).
Since the expression values of target proteins were
not normally distributed by the Shapiro-Wilk test (P=0.0002), the
Kruskal Wallis test followed by Bonferroni's multiple comparison
test was performed for each group. R version 3.4.2 was used in all
statistical tests, which is a language and environment for
statistical computing (http://cran.r-project.org/).
Results
Hemodynamics during surgery
All the 28 rats allocated to the four groups (APC,
IPC, IRI and Sham group) survived during the surgical procedures.
There were no significant differences in heart rate and mean atrial
pressure at the four time points of the beginning of the
experimental protocol (baseline), immediately before reperfusion
(ischemia for 45 min), 3 min after ischemia release (reperfusion
for 3 min), and just before removal of the left kidney 4 h after
reperfusion (finish) between the four groups (Table I).
 | Table IVital signs of the rats in each group
during the experimental protocol. |
Table I
Vital signs of the rats in each group
during the experimental protocol.
A, Heart rate of
the rats in each group during the experimental protocol
|
|---|
| Group | Baseline,
min−1 | Ischemia for 45
min, min−1 | Reperfusion for 3
min, min−1 | Reperfusion for 4
h, min−1 |
|---|
| Anesthetic
preconditioning | 215±14 | 225±19 | 227±25 | 218±24 |
| Ischemic
preconditioning | 252±65 | 274±41 | 284±45 | 259±31 |
| Ischemia | 209±16 | 222±20 | 207±9 | 238±31 |
| Sham | 219±9 | 211±10 | 214±9 | 205±9 |
|
B, Mean atrial
pressure of the rats in each group during the experimental protocol
|
| Group | Baseline,
min−1 | Ischemia for 45
min, min−1 | Reperfusion for 3
min, min−1 | Reperfusion for 4
h, min−1 |
| Anesthetic
preconditioning | 86±18 | 87±24 | 81±14 | 96±26 |
| Ischemic
preconditioning | 100±18 | 104±17 | 105±12 | 93±15 |
| Ischemia | 103±20 | 91±16 | 100±26 | 81±17 |
| Sham | 94±14 | 88±11 | 87±10 | 75±9 |
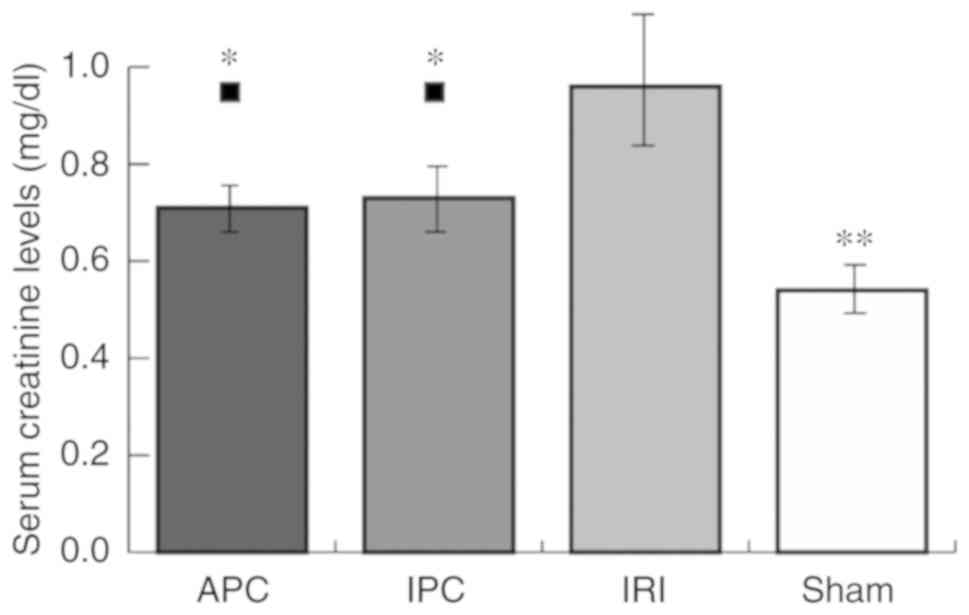
Serum creatinine levels
Serum creatinine values, as an indicator of IRI,
were significantly higher in the IRI group (0.96±0.13 mg/dl) than
the sham group (0.54±0.06 mg/dl; P<0.001). Serum creatinine
values in APC (0.71±0.08 mg/dl) and IPC (0.73±0.10 mg/dl) groups
were higher than those in the sham group (APC, P=0.029; IPC,
P=0.011), although they were significantly lower than those in the
IRI group (APC, P<0.001; IPC, P=0.002). There were no
significant differences between the APC and IPC groups (Fig. 2).
miR screening tests and analyses
Out of the 373 types of well-known miRs derived from
rats, one-way ANOVA was performed for 125 kinds of miRs that
fulfilled either condition 1 or 2, as previously described. Tukey's
test was performed on 10 miRs identified by one-way ANOVA corrected
with Storey's method as having significant differences in adjusted
F.C. values among the four groups (P<0.05, q<0.5).
As a result, significant differences in seven miRs
were noted between the APC and IRI group or between the IPC and IRI
group (P<0.05; Table II). The
dataset of the results of RT-qPCR are available in a data
repository (doi: 10.17632/dmngjht9sg.1).
 | Table IISignificant changes in miRs following
preconditioning procedures. |
Table II
Significant changes in miRs following
preconditioning procedures.
A, Significantly
different miRs between anesthetic preconditioning and ischemia
groups
|
|---|
| miR | Adjusted
fold-change
| APC/IRI | P-value |
|---|
| APC | IRI |
|---|
| miR-17-3p | 2.14±1.12 | 0.20±0.076 | 10.9 | 0.016
(<0.05) |
| miR-27a | 0.74±0.22 | 4.56±3.15 | 0.16 | 0.005
(<0.01) |
|
B, Significantly
different miRs between ischemic preconditioning and ischemia groups
|
| miR | Adjusted
fold-change
| IPC/IRI | P-value |
| IPC | IRI |
|
| miR-125a | 3.70±2.59 | 0.72±0.65 | 5.11 | 0.034
(<0.05) |
| miR-19a | 1.97±0.77 | 0.48±0.36 | 4.14 | 0.003
(<0.005) |
| miR-34a | 1.95±1.47 | 0.11±0.025 | 17.4 | 0.040
(<0.05) |
| miR-872 | 2.35±0.42 | 0.33±0.21 | 7.17 | <0.001 |
| miR-450a | 9.02±8.77 | 0.32±0.19 | 28.2 | 0.035
(<0.05) |
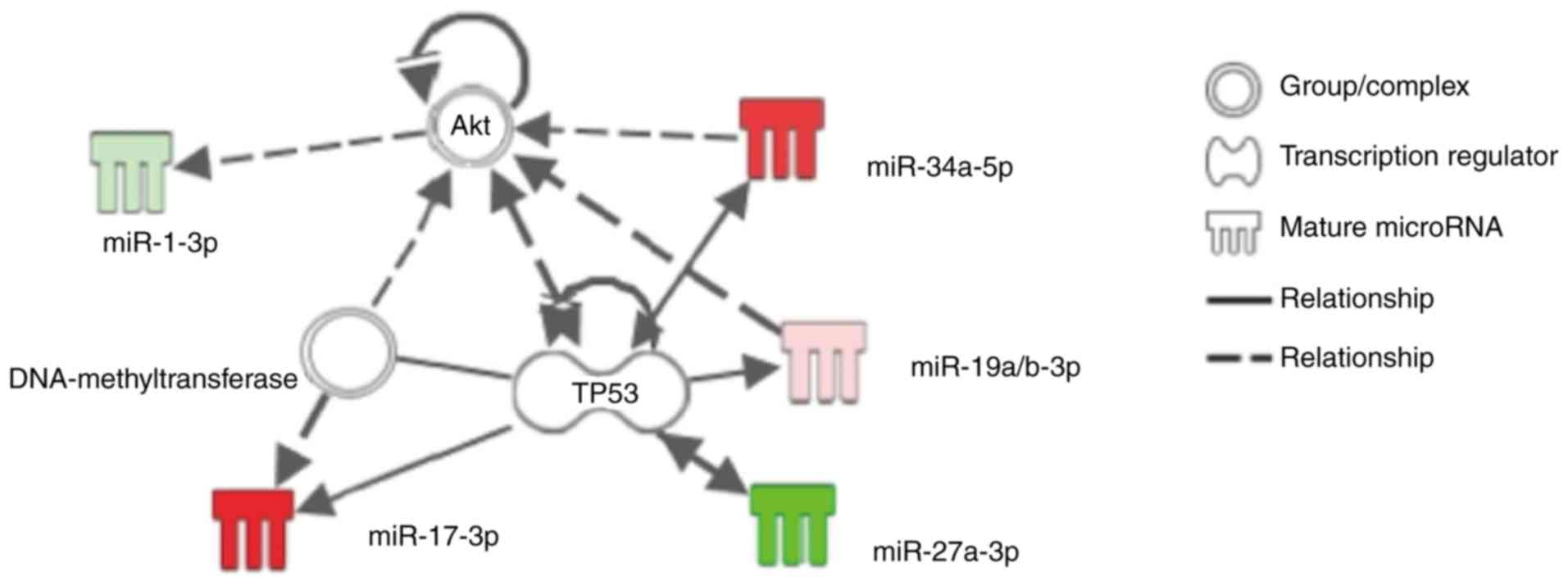
Search for the predicted networks between
specific miRs and the cell-survival pathway
IPA analysis of the seven miRs, which were
differentially expressed following APC or IPC procedures as
previously described, showed that miR-17-3p, miR-19a/b, miR-34a and
miR-27a might be involved in the cell-survival pathway associated
with the specific substance 'Akt' or 'TP53' (Fig. 3). RT-qPCR in the current study
showed that sevoflurane APC promoted miR-17-3p, but suppressed
miR-27a, and that IPC promoted miR-19a and miR-34a (Table II). Although IPC also promoted
miR-125a, miR-872 and miR-450a in the current study, IPA analysis
did not identify any association between the cell-survival pathway
and these miRs.
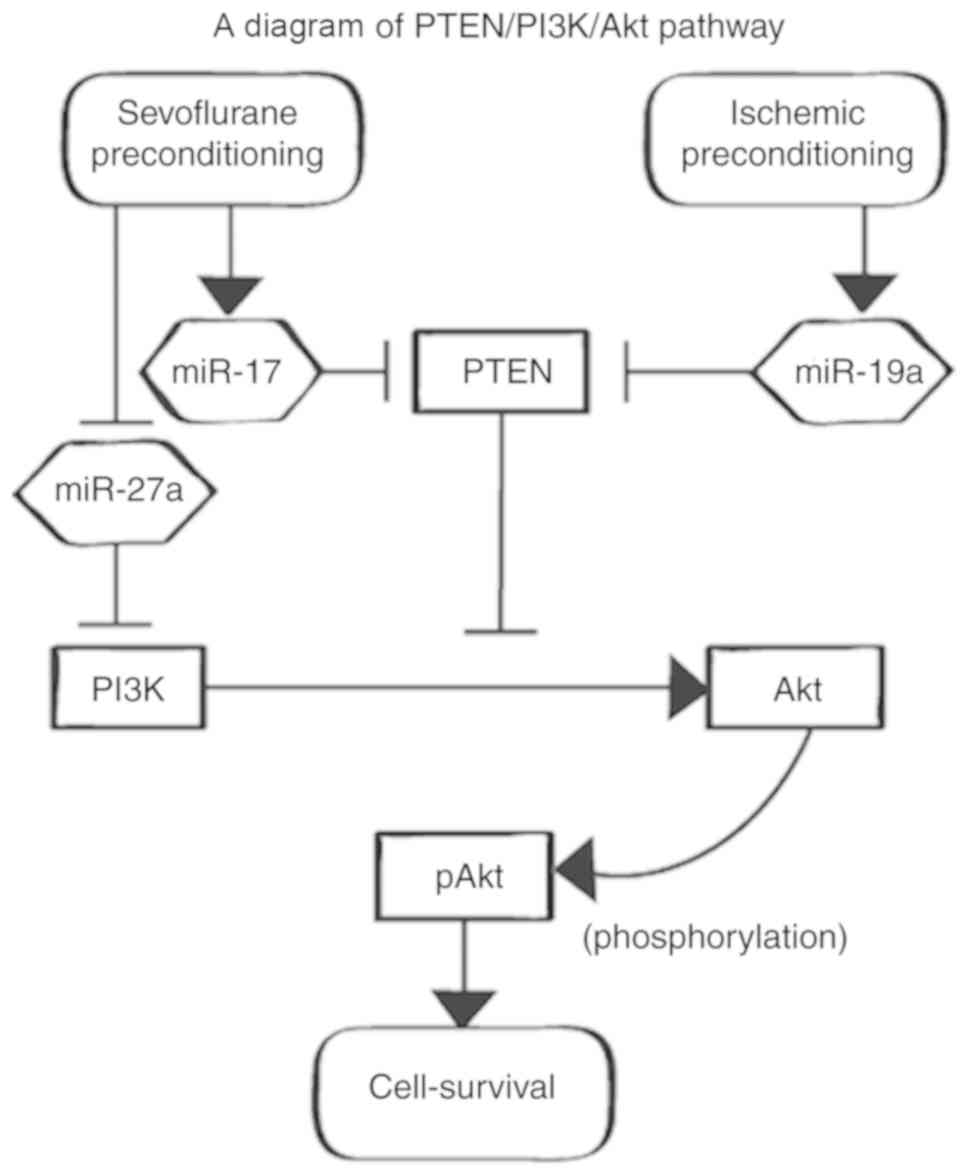
The predicted functional network presented by IPA™
and a further literature search suggested that the sevoflurane APC
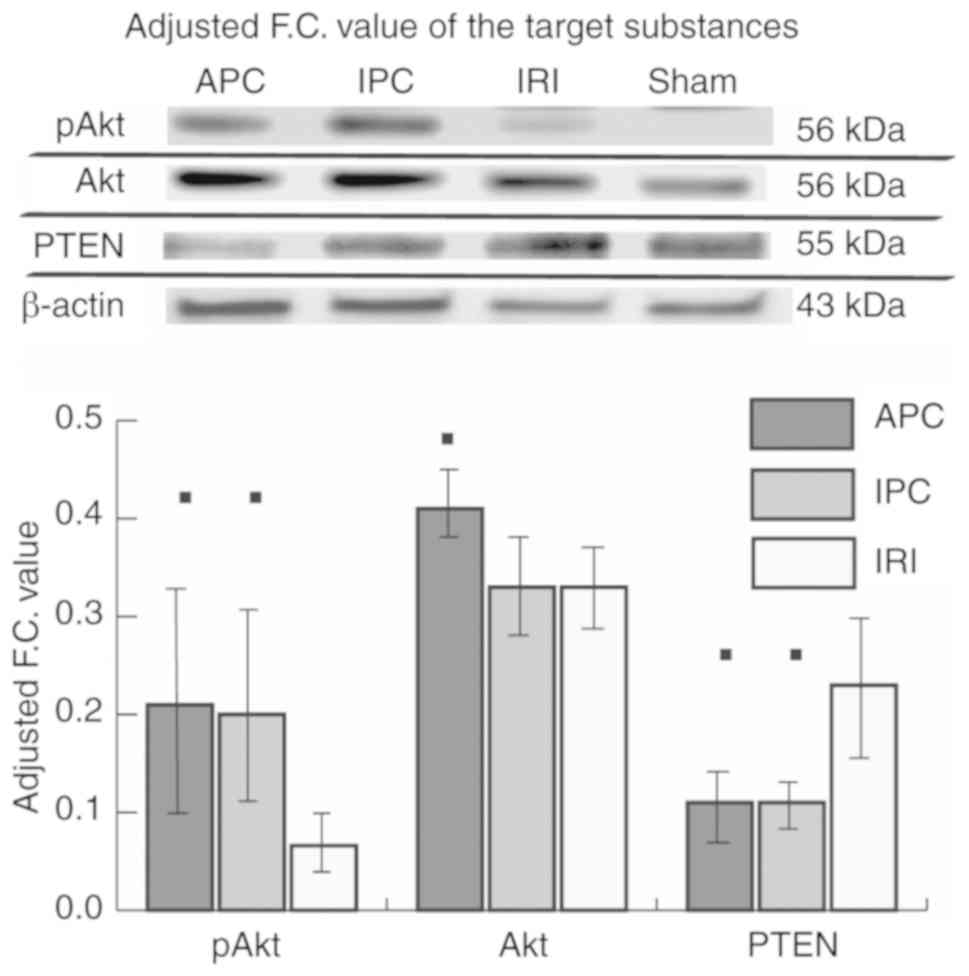
and IPC models might block PTEN and increase pAkt (Fig. 4). Western blot analysis indicated
decreased PTEN and increased pAkt in the APC and IPC groups.
IPA analysis suggested that sevoflurane APC and IPC
affect Akt phosphorylation. Western blot analysis also showed that
the adjusted F.C. value of pAkt was increased in both the APC and
IPC groups compared with the IRI group (P=0.013, P=0.013).
Furthermore, the adjusted F.C. value of PTEN was decreased in both
APC and IPC groups compared with the IRI group (P=0.013, P=0.013)
(Fig. 5; the full-length blots in
the main paper are displayed in Fig.
S1).
Discussion
The present study confirmed that sevoflurane APC and
IPC have reno-protective effects and affect specific miRs that were
predicted by IPA in the current study to regulate reno-protection.
However, there are few comparative reports of sevoflurane APC and
IPC in terms of the magnitude of reno-protective effects and the
dynamics of miRs regulating the reno-protection. This study
revealed that sevoflurane APC and IPC equally ameliorated renal
IRI, as indicated by serum creatinine values, equally suppressed
PTEN, and equally increased pAkt, although the effect was brought
about via different miRs.
The current study predicted that PTEN and pAkt are
important signaling agents involved in cell-survival, that are
regulated by the miRs differentially expressed after both
sevoflurane APC and IPC, using IPA and a further detailed
literature search. As revealed by the literature search, miR-17-3p
that increases with APC and miR-19a that increases with IPC,
respectively, block PTEN directly and increase pAkt via the
PTEN/phosphoinositide 3-kinase (PI3K)/Akt pathway, whereas miR-27a,
which decreases with APC, blocks PI3K directly and decreases pAkt
(21-23), along with suppression of PTEN,
although via different microRNAs, followed by equal increases in
pAkt.
Preconditioning effects have been reported in
clinical and animal research. In studies on sevoflurane APC during
cardiac surgery, acute renal IRI caused by cardiac arrest could be
ameliorated by administering sevoflurane immediately before cardiac
arrest (14). The sevoflurane APC
effect on mice kidneys was also reported in vivo (13). As far as is known, although the
efficacy of renal IPC has not yet been shown to be clinically
significant, unlike remote IPC for the kidney, renal IPC in mice
reportedly suppresses the cell-death caused by subsequent IRI
(8,9).
Multiple cell-survival pathways triggered by IRI
have been previously reported, based on the background of IRI and
the effects of preconditioning (24-26). The PTEN/PI3K/Akt pathway is an
important intracellular signaling pathway in the regulation of the
cell cycle. PTEN directly blocks downstream activity of the
cell-survival PI3K/Akt pathway (27). Akt is known to have three
isoforms, with each isoform playing several specific roles in the
signaling pathways, such as cell survival, cell proliferation,
neovascularization and glucose metabolism (28). Akt promotes cell-survival by
promoting several anti-apoptotic pathways (21,29). Activated PI3K alters the
conformation of Akt, to allow phosphoinositide-dependent kinase-1
to phosphorylate Akt, reportedly (30). Activation of the Akt pathway
reportedly reduces hypoxia-induced cell apoptosis, such that
overexpression of PTEN causes cell-death, while suppression of PTEN
has a cell-survival effect (27,29)
IPC reportedly increases pAkt via the PTEN/PI3K/Akt
pathway, resulting in suppression of rat myocardial cell apoptosis
(24,31). In addition, IPC of mice kidneys
suppressed the cell-death caused by subsequent IRI, increasing the
anti-apoptotic activity of pAkt (9). Sevoflurane APC reportedly
ameliorates rat myocardial IRI by regulating the PTEN/PI3K/Akt
pathway, similar to the rat IPC model (32). In the current study, it was also
found that sevoflurane APC and IPC ameliorate renal IRI in rats,
probably via the PTEN/PI3K/Akt pathway.
Genetic changes, including specific miR changes,
were reported to suppress IRI by affecting specific cell-survival
pathways (33-35). The PTEN/PI3K/Akt pathway is also
regulated by multiple miRs (23,36,37). The present study found that
sevoflurane APC promoted miR-17-3p expression, whereas IPC promoted
miR-19a expression. There was an important commonality between
sevoflurane APC and IPC effects. The miR-17/92 cluster consists of
seven miRs (miR-17-3p, miR-17-5p, miR-18, miR-19a, miR-20a,
miR-19b-1 and miR-92a-1) located close to the chromosome 13
(13q31.3) region and produced from a common host RNA (34). Reportedly, both miR-17 and miR-19a
directly block PTEN, because multiple binding sites for such miRs
as miR-19a/b, miR-17 and miR-20a are conserved in the
3′-untranslated region of PTEN messenger RNA (36,37). Other studies also reported that
miR-17-3p directly blocks PTEN and increases pAkt via the
PTEN/PI3K/Akt pathway, similar to miR-19a that blocks PTEN directly
and increases pAkt (21,22,38). Thus, sevoflurane APC and IPC might
equally suppress PTEN because of the similarity in their affected
miRs.
The effect of PTEN inhibition produced by both types
of preconditioning might affect a wide range of pathways involving
various protein kinases that regulate cell-survival and not only
the PI3K/Akt pathway. PTEN reportedly regulates apoptosis via
phosphorylated mitogen-activated protein kinase-1/2/extracellular
signal-regulated kinase-1/2 signaling and caspase-3/B-cell
lymphoma-2 signaling (39).
Although sevoflurane APC and IPC might affect the
common PTEN/PI3K/Akt signaling pathway, there are differences in
the directly affected miRs. The current study found that
sevoflurane APC suppresses miR-27a expression. In nucleus pulposus
cells, miR-27a directly blocks PI3K, but not PTEN (23). The authors anticipated that
sevoflurane APC might affect multiple signaling stages in the
PTEN/PI3K/Akt pathway, resulting in the increase in pAkt in renal
cells. Directly promoting PI3K might also activate other
anti-apoptotic pathways dependent on PI3K. Reportedly, a model of
reperfusion 3 days after permanent middle cerebral artery occlusion
resulted in activation of the fibroblast growth factor
21/fibroblast growth factor receptor 1/PI3K/caspase-3 pathway and
reduced neuronal apoptosis (40).
Sevoflurane APC might thus reduce caspase-3 by its close
involvement in the apoptotic process via the PI3K/Caspase-3
pathway.
The present study found that IPC promotes miR-34a
expression. It was previously reported that miR-34a expression is
promoted in renal cells with overexpression of the protein cMYC,
which directly binds and activates the miR-17/92 cluster (41). Therefore, the authors of the
current study anticipated that IPC might increase cMYC, activating
the miR-17/92 cluster and resulting in miR-34a overexpression. IPC
might thus increase pAkt not only by suppressing PTEN, but also by
activating the cMYC/miR-17/92 cluster/PTEN/PI3K/Akt pathway. In the
future, further research is necessary to elucidate detailed
differences between sevoflurane APC and IPC effects.
The present study showed that the sevoflurane APC
effect reported so far is also found in the rat kidney.
Furthermore, the current study found that sevoflurane APC has
equivalent reno-protective effects compared with IPC, as indicated
by both serum creatinine levels and those of the organ protective
pAkt, and partially compared with its genetic mechanism in renal
cells for the first time.
The present study has some limitations. The first
limitation is that the reperfusion time after renal ischemia was
set as 4 h. It is necessary to evaluate the optimal time at which
miR changes become most noticeable for accurate evaluation of the
preconditioning effects. The second limitation is that serum
creatinine values were not measured before surgery. Since the
volume of blood required for measurement is more than 1 ml,
preoperative blood sampling might cause organ ischemia. However,
since the weights of all the rats randomly allocated to the four
groups were similar and the environmental factors were also the
same, baseline serum creatinine values are assumed to have been
minimally different. The third limitation is that, although the
main outcome in the present study was to prove a decrease in acute
renal dysfunction with APC and IPC, it is necessary to prove the
anti-apoptotic effect of APC and IPC histologically using renal
tissue sampling in the future. Finally, although the current
protocol suggested that the reno-protective effects of APC and IPC
were related to regulation of specific miRs, as seen with RT-qPCR,
experiments to identify the pathways involved using miR inhibitors,
PTEN inhibitor and PI3K inhibitor are necessary in the future.
The present study concluded that sevoflurane APC and
IPC had equivalent reno-protective effects in rats, although by
different miR dynamics. However, it was estimated that both the
preconditioning procedures resulted in an increase in pAkt and a
decrease in PTEN. The current study estimated that these
preconditioning effects might occur via the common cell-survival
PTEN/PI3K/Akt pathway using IPA™ analysis and further literature
search. The current study results might be useful for examining
methods for alleviating renal IRI using general anesthesia and
surgical procedures. Further study will be necessary to elucidate
the differences in reno-protective effects induced by changing the
type or amount of general anesthetic drugs used.
Supplementary Data
Funding
The present study was supported in part by grants
from the Japanese Ministry of Education, named MEXT KAKENHI (grant
nos. JP15K10525 and JP18K08870).
Availability of data and materials
The dataset of quantitative PCR of the current study
was published and is available in the Mendeley Data repository
(doi: 10.17632/dmngjht9sg.1).
Authors' contributions
MY contributed to conceptualizing and designing the
study, data collection, analysis and interpretation of the data,
and drafting of the manuscript. TM contributed to conceptualizing
and designing the study, data collection, and critical revision of
the manuscript. MI participated in designing the study, analysis of
data and critical revision of the manuscript. AS contributed to
conceptualizing and designing the study, critical revision of the
manuscript, and was the principal investigator and had overall
responsibility for the trial. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Animal Research Committee at Nippon Medical School, Tokyo, Japan
(Approval number: 29-008, Approval date: April 01, 2017). In
addition, all the experimental protocols were performed in
accordance with ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
Akt
|
protein kinase B
|
|
APC
|
anesthetic preconditioning
|
|
IPA
|
Ingenuity Pathway Analysis
|
|
IPC
|
ischemic preconditioning
|
|
IRI
|
ischemia reperfusion injury
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
PTEN
|
phosphatase and tensin homologue
deleted from chromosome 10
|
Acknowledgments
The authors wish to thank Ms. Miyuki Takatori and
Ms. Kiyomi Kikukawa (Collaborative Research Center Laboratory for
Clinical Research, Nippon Medical School, Tokyo, Japan) for their
help as scientific advisers. The authors thank the laboratory
technicians at the Department of Inspection, Nippon Medical School,
for their help in testing the blood samples.
References
|
1
|
Debout A, Foucher Y, Trébern-Launay K,
Legendre C, Kreis H, Mourad G, Garrigue V, Morelon E, Buron F,
Rostaing L, et al: Each additional hour of cold ischemia time
significantly increases the risk of graft failure and mortality
following renal transplantation. Kidney Int. 87:343–349. 2015.
View Article : Google Scholar
|
|
2
|
Girerd S, Frimat L, Ducloux D, Le Meur Y,
Mariat C, Moulin B, Mousson C, Reiu P, Dali-Youcef N, Merckle L, et
al: EPURE transplant (eplerenone in patients undergoing renal
transplant) study: Study protocol for a randomized controlled
trial. Trials. 19:5952018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tonelli M, Wiebe N, Knoll G, Bello A,
Browne S, Jadhav D, Klarenbach S and Gill J: Systematic review:
Kidney transplantation compared with dialysis in clinically
relevant outcomes. Am J Transplant. 11:2093–2109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lannemyr L, Bragadottir G, Hjärpe A,
Redfors B and Ricksten SE: Impact of cardiopulmonary bypass flow on
renal oxygenation in patients undergoing cardiac surgery. Ann
Thorac Surg. 107:505–511. 2019. View Article : Google Scholar
|
|
5
|
Nadim MK, Forni LG, Bihorac A, Hobson C,
Koyner JL, Shaw A, Arnaoutakis GJ, Ding X, Engelman DT, Gasparovic
H, et al: Cardiac and vascular surgery-associated acute kidney
injury: The 20th international consensus conference of the ADQI
(acute disease quality initiative) group. J Am Heart Assoc.
7:e0088342018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion- from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eltzschig HK: Targeting hypoxia-induced
inflammation. Anesthesiology. 114:239–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi HS, Hwang JK, Kim JG, Hwang HS, Lee
SJ, Chang YK, Kim JI and Moon IS: The optimal duration of ischemic
preconditioning for renal ischemia-reperfusion injury in mice. Ann
Surg Treat Res. 93:209–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang HS, Kim J, Kim KY, Kim JI, Cho MH and
Park KM: Previous ischemia and reperfusion injury results in
resistance of the kidney against subsequent ischemia and
reperfusion insult in mice; a role for the Akt signal pathway.
Nephrol Dial Transplant. 27:3762–3770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang Y, Li Z, Mo N, Li M, Zhuang Z, Wang
J, Wang Y and Guo X: Isoflurane preconditioning ameliorates renal
ischemia-reperfusion injury through antiinflammatory and
anti-apoptotic actions in rats. Biol Pharm Bull. 37:1599–1605.
2014. View Article : Google Scholar
|
|
11
|
Su MW, Chang SS, Chen CH, Hung CC, Chang
SW, Tsai YC and Lam CF: Preconditioning renoprotective effect of
isoflurane in a rat model of virtual renal transplant. J Surg Res.
189:135–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lempiäinen J, Finckenberg P, Mervaala EE,
Storvik M, Lindstedt K, Levijoki J and Mervaala EM: Dexmedetomidine
preconditioning ameliorates kidney ischemia-reperfusion injury.
Pharmacol Res Perspect. 2:e000452014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HT, Chen SW, Doetschman TC, Deng C,
D'Agati VD and Kim M: Sevoflurane protects against renal ischemia
and reperfusion injury in mice via the transforming growth
factor-beta1 pathway. Am J Physiol Renal Physiol. 295:F128–F136.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Julier K, da Silva R, Garcia C, Bestmann
L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von
Segesser LK, et al: Preconditioning by sevoflurane decreases
biochemical markers for myocardial and renal dysfunction in
coronary artery bypass graft surgery: A double-blinded,
placebo-controlled, multicenter study. Anesthesiology.
98:1315–1327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Godwin JG, Ge X, Stephan K, Jurisch A,
Tullius SG and Iacomini J: Identification of a microRNA signature
of renal ischemia reperfusion injury. Proc Natl Acad Sci USA.
107:14339–14344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morita T, Ishikawa M and Sakamoto A:
Identical microRNAs regulate liver protection during anaesthetic
and ischemic preconditioning in rats: An animal study. PLoS One.
10:e01258662015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takayama J, Takaoka M and Matsumura Y:
Acute and chronic renal failure model in rats and mice. Nihon
Yakurigaku Zasshi. 131:37–42. 2008.In Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Pang XL, Shang WJ, Xie HC, Wang JX
and Feng GW: Over-expressed microRNA-181a reduces glomerular
sclerosis and renal tubular epithelial injury in rats with chronic
kidney disease via down-regulation of the TLR/NF-κB pathway by
binding to CRY1. Mol Med. 24:492018. View Article : Google Scholar
|
|
20
|
Ishikawa M, Tanaka S, Arai M, Genda Y and
Sakamoto A: Differences in microRNA changes of healthy rat liver
between sevoflurane and propofol anesthesia. Anesthesiology.
117:1245–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luan Y, Chen M and Zhou L: miR-17 targets
PTEN and facilitates glial scar formation after spinal cord
injuries via the PI3K/Akt/mTOR pathway. Brain Res Bull. 128:68–75.
2017. View Article : Google Scholar
|
|
22
|
Sun G, Lu Y, Li Y, Mao J, Zhang J, Jin Y,
Sun Y, Liu L and Li L: miR-19a protects cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis via PTEN/PI3K/p-Akt
pathway. Biosci Rep. 37:BSR201708992017. View Article : Google Scholar :
|
|
23
|
Liu G, Cao P, Chen H, Yuan W, Wang J and
Tang X: miR-27a regulates apoptosis in nucleus pulposus cells by
targeting PI3K. PLoS One. 8:e752512013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han J, Xuan JL, Hu HR and Chen ZW:
Protective effect against myocardial ischemia reperfusion injuries
induced by hyperoside preconditioning and its relationship with
PI3K/Akt signaling pathway in rats. Zhongguo Zhong Yao Za Zhi.
40:118–123. 2015.In Chinese. PubMed/NCBI
|
|
25
|
Wu J, Yu J, Xie P, Maimaitili Y, Wang J,
Yang L, Ma H, Zhang X, Yang Y and Zheng H: Sevoflurane
postconditioning protects the myocardium against
ischemia/reperfusion injury via activation of the JAK2-STAT3
pathway. PeerJ. 5:e31962017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu C, Liu Y, Shen Z, Mial L, Zhang K,
Wang F and Li Y: Sevoflurane preconditioning reduces intestinal
ischemia-reper-fusion injury: Role of protein kinase C and
mitochondrial ATP-sensitive potassium channel. PLoS One.
10:e01414262015. View Article : Google Scholar
|
|
27
|
Sun G, Zhou Y, Li H, Guo Y, Shan J, Xia M,
Li Y, Li S, Long D and Feng L: Over-expression of microRNA-494
up-regulates hypoxia-inducible factor-1 alpha expression via
PI3K/Akt pathway and protects against hypoxia-induced apop-tosis. J
Biomed Sci. 20:1002013. View Article : Google Scholar
|
|
28
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
29
|
Satake A, Takaoka M, Nishikawa M, Yuba M,
Shibata Y, Okumura K, Kitano K, Tsutsui H, Fujii K, Kobuchi S, et
al: Protective effect of 17beta-estradiol on ischemic acute renal
failure through the PI3K/Akt/eNOS pathway. Kidney Int. 73:308–317.
2008. View Article : Google Scholar
|
|
30
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Liu XB, Cheng C, Xu DL, Lu QH and
Ji XP: Rho-kinase inhibition is involved in the activation of
PI3-kinase/Akt during ischemic-preconditioning-induced
cardiomyocyte apoptosis. Int J Clin Exp Med. 7:4107–4114. 2014.
|
|
32
|
Zhang SB, Liu TJ, Pu GH, Li BY, Gao XZ and
Han XL: MicroRNA-374 exerts protective effects by inhibiting SP1
through activating the PI3K/Akt pathway in rat models of myocardial
ischemia-reperfusion after sevoflurane preconditioning. Cell
Physiol Biochem. 46:1455–1470. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Hong Q, Wang Z, Yu Y, Zou X and Xu
L: miR-21 inhibits autophagy by targeting Rab11a in renal
ischemia/reperfusion. Exp Cell Res. 338:64–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou M, Cai J, Tang Y and Zhao Q:
miR-17-92 cluster is a novel regulatory gene of cardiac
ischemic/reperfusion injury. Med Hypotheses. 81:108–110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu Q, Luo W, Huang L, Huang R and Chen R:
Apoptosis-related microRNA changes in the right atrium induced by
remote ischemic perconditioning during valve replacement surgery.
Sci Rep. 6:189592016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Huang ZP, Seok HY, Ding J, Kataoka
M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, et al: mir-17-92 cluster
is required for and sufficient to induce cardiomyocyte
proliferation in postnatal and adult hearts. Circ Res.
112:1557–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Benhamou D, Labi V, Novak R, Dai I,
Shafir-Alon S, Weiss A, Gaujoux R, Arnold R, Shen-Orr SS, Rajewsky
K and Melamed D: A c-Myc/miR17-92/Pten axis controls PI3K-mediated
positive and negative selection in B cell development and
reconstitutes CD19 deficiency. Cell Rep. 16:419–431. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Q, Peng Z, Wang L, Li Y, Wang K, Zheng
J, Liang Z and Liu T: miR-19a correlates with poor prognosis of
clear cell renal cell carcinoma patients via promoting cell
proliferation and suppressing PTEN/SMAD4 expression. Int J Oncol.
49:2589–2599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang Z, Pan Q, Zhang Z, Huang C, Yan Z,
Zhang Y and Li J: MicroRNA-125a-5p controls the proliferation,
apoptosis, migration and PTEN/MEK1/2/ERK1/2 signaling pathway in
MCF-7 breast cancer cells. Mol Med Rep. 20:4507–4514.
2019.PubMed/NCBI
|
|
40
|
Zheng W, Matei N, Pang J, Luo X, Song Z,
Tang J and Zhang JH: Delayed recanalization at 3 days after
permanent MCAO attenuates neuronal apoptosis through
FGF21/FGFR1/PI3K/Caspase-3 pathway in rats. Exp Neurol.
320:1130072019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamamura S, Saini S, Majid S, Hirata H,
Ueno K, Chang I, Tanaka Y, Gupta A and Dahiya R: MicroRNA-34a
suppresses malignant transformation by targeting c-Myc
transcriptional complexes in human renal cell carcinoma.
Carcinogenesis. 33:294–300. 2012. View Article : Google Scholar :
|