Introduction
Vitiligo is the most common depigmentary disorder of
the skin, affecting 0.5-2% of the population worldwide. Vitiligo is
predominantly characterized by white patches of the skin, due to
the chronic and progressive loss of melanocytes from the epidermis
and the follicular reservoir, which may cause significant social
and psychological impacts on patients, especially teenagers
(1-3). Although autoimmune-mediated cell
death is currently accepted as the leading cause for the
disappearance of melanocytes in most cases of vitiligo, the
molecular mechanism by which a melanocyte-specific immune response
is exclusively triggered has never been clearly determined
(4). Within the skin, mature
melanocytes reside in the basal layer of the epidermis where they
form epidermal melanin units comprised of tight anatomical and
functional relationships between individual melanocytes and ~30-40
neighboring keratinocytes (5).
Unlike keratinocytes, which possess hemidesmosomes and desmosomes
that anchor them to the underlying basement membrane and adjacent
basal keratinocytes, epidermal melanocytes are simply anchored by
the cell-matrix and/or cell-cell adhesion molecules such as
integrin, E-cadherin and discoidin domain receptor 1 (6,7).
The reservoirs of melanocytes at the cellular and molecular levels
after their selective disappearance from vitiliginous skin remain
to be investigated. Vitiligo keratinocytes must re-establish
cell-cell contacts after melanocytes disappear, speculatively
forming a defective cell complex with an impaired ability to
photoprotect the skin against damage from harmful ultraviolet (UV)
radiation (8).
Current therapeutic modalities for vitiligo are
aimed at arresting progression of the disease and stimulating skin
repigmentation (a process that recruits existing melanocytes and
their precursors or stem cells back to the depigmented skin
lesions) (5,9). UVB-based phototherapy has been
clinically proven to be efficient for inducing repigmentation
(5,10). Different skin repigmentation
patterns, such as perifollicular, marginal, diffuse and combined
patterns, have been observed in vitiligo patients who have
undergone UVB-based phototherapy, suggesting that the replenishment
of melanocytes probably comes from diverse sources in the epidermis
and/or in hair follicles (11).
Over the past decades, effort has been devoted to investigating
whether dormant melanocyte stem cells that reside in the bulge
region of hair follicles are awakened to participate in the
perifollicular repigmentation of the skin (5,12-14). In contrast, much less is
understood about the induction of marginal repigmentation of the
skin, which possibly relies on the activation and migration of
melanocytes existing at the border of the depigmented vitiligo
macules.
The migration of melanocytes released from the
microenvironment of basal and suprabasal layers in pigmented human
epidermis in response to UVB phototherapy raises intriguing
questions. The critical issues that remain to be resolved include:
i) Which cells are preferentially mobilized by UVB irradiation, ii)
whether the migrating cells arise from a single population of
immature and/or mature melanocytes, and iii) whether the migrating
cells transiently secrete one or more proteolytic enzymes that
enable melanocytes to move out by dissolving intercellular adhesive
structures. Several studies have demonstrated that the expression
of matrix metallopro-teases (MMPs) in melanocytes appears to be
correlated with tissue remodeling or replasticity (15-17), little is known about whether MMP9
affects melanocyte migration in vitiligo repig-mentation. The
present study provides unequivocal evidence that the
p53-TRPM1/microRNA (miRNA/miR)-211-MMP9 axis is critical for
melanocyte migration induced by UVB exposure, which has potential
implications for understanding the molecular mechanisms underlying
UVB-induced repigmentation in vitiligo.
Materials and methods
Cell culture and UVB radiation
The primary human epidermal melanocytes were
isolated from healthy juvenile foreskin tissues, a total of 60
specimens, collected after circumcision between September 2017 and
May 2019 (donor age range, 13-19 years). After removing the
subcutaneous tissue, the samples were cut into small pieces and
incubated with 0.25% dispase at 4°C overnight to separate the
epidermis and dermis (18). The
present study was approved by the Ethical Committee of the Renmin
Hospital of Wuhan University. Written informed consent was obtained
from each participant before enrollment. Melanocytes were cultured
with complete Medium 254 supplemented with Human Melanocyte Growth
Supplement and 100 U/ml penicillin/streptomycin (all from Cascade
Biologics) in a humidified atmosphere at 37°C and 5%
CO2. Cells used in the present experiments were from
passages 2 to 6. Cells were seeded in 6-well plates at a population
density of 5×105. Before irradiation, the medium was
removed and warm PBS was used to wash the cells. The cells were
treated with single or repeated exposures to UVB using a high dose
targeted phototherapy system (The Daavlin Company) with a maximum
wavelength at 311 nm. The cumulative dose of UVB irradiation is
indicated in the figure legends. Cells of the UVB-unexposed control
were treated in same way but without UVB exposure.
Semi-quantitative reverse transcription
(RT)-PCR and quantitative PCR (qPCR)
Total RNAs were purified using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
protocol of the manufacturer. cDNAs were synthesized from total
RNAs (1.5 µg) using a Moloney murine leukemia virus reverse
transcriptase first strand kit (Invitrogen; Thermo Fisher
Scientific, Inc.). RT-PCR was performed under the following cycle
parameters: Predenaturation at 95°C for 2 min, followed by 35
cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec. PCR
was performed in triplicate with SYBR-Green PCR core reagents
(Applied Biosystems; Thermo Fisher Scientific, Inc.), 50 ng cDNA
and 1 µM forward and reverse primers for the following human
genes: p53 (forward: 5′-GTC TAC CTC CCG CCA TAA-3′; reverse: 5′-GCA
AGC AAG GGT TCA AAG-3′), TRPM1 (forward: 5′-TAC ACG CTA TTT CCC CGA
TGA-3′; reverse: 5′-GCG CGT GAT CTT TTG AAC TTG-3′), MMP9 (forward:
5′-GCT ACC ACC TCG AAC TTT GAC-3′; reverse: 5′-TCA GTG AAG CGG TAC
ATA GGG-3′) and the housekeeping gene β-actin (ACTB; forward:
5′-AGC GAG CAT CCC CCA AAG TT-3′; reverse: 5′-GGG CAC GAA GGC TCA
TCA TT-3′) was used as an endogenous internal control for the
normalization of p53, TRPM1, and MMP9 mRNA expression. For qPCR
analysis of miR-211, 10 ng total RNA was used in a TaqMan miRNA
assay according to the manufacturer′s protocol. Analysis of miRNA
was carried out using a CFX96 Detection System (Bio-Rad
Laboratories, Inc.) in triplicate and the SYBR-Green Supermix.
miR-211 specific primers: (Forward: 5′-TGC GCT TCC CTT TGT CAT CCT
T-3′; reverse: 5′-CTC AAG TGT CGT GGA GTC GG C AA-3′) loop primer:
5′-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACA GGC
GAA G-3′). The expression of miR-211 was normalized to the U6 small
nuclear RNA (forward: 5′-CTC GCT TCG GCA GCA CAT-3′; reverse:
5′-AAC GCT TCA CGA ATT TGC GT-3′). All primers used in this study
were synthesized and supplied by Invitrogen; Thermo Fisher
Scientific, Inc. qPCR was performed using an ABI 7500 system with
the following cycle parameters: Denaturation at 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec, 60°C for 4 sec and 72°C
for 30 sec. The purity of each PCR product was checked by
dissociation curve analysis, as well as by running each sample on
1% agarose gels with Goldview (cat. no. 110214; SBSGene).
Fold-change values were calculated using the formula of
2−ΔΔCq (19).
Western blotting analysis
Cells were harvested, washed in PBS and lysed in
extraction buffer containing 1% Nonidet P-40, 0.01% SDS and a
protease inhibitor cocktail (Roche Diagnostics). Protein contents
were determined using a bicinchoninic acid assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts of each protein
extract (20 µg per lane) were resolved using 10% SDS-PAGE.
Following transblotting onto Immobilon-P membranes (EMD Millipore)
and blocking with 5% nonfat milk in saline buffer for 1 h at room
temperature, the membranes were incubated with antibodies to p53
(cat. no. ab179477; Abcam; 1:1,000), TRPM1 (cat. no. ab72154;
Abcam; 1:1,500), MMP9 (cat. no. ab76003; Abcam; 1:1,000) and GAPDH
(cat. no. ab37168; Abcam; 1:10,000) for 1 h at room temperature.
The membranes were then washed and incubated with HRP-conjugated
goat anti-rabbit IgG (AS1107; Aspen Biological; 1:10,000) for 1 h
at room temperature. Each membrane was then washed again and
specific immunoreactive bands were visualized using an enhanced
chemiluminescent reaction (ECL kit; Amersham; GE Healthcare).
Signal intensities were quantified using ImageJ software version
1.47 (National Institutes of Health) and were normalized to GAPDH.
All data were obtained from more than two independent experiments
carried out in triplicate.
Immunofluorescent assay
Primary melanocytes (5×105) were seeded
in 6-well culture plates containing coverslips. After attachment,
the cells were treated with multiple UVB exposures for a cumulative
dose of 70 mJ/cm2 (8.75 mJ/cm2 per exposure
in 5 h intervals for a total of 8 times). Subsequently, the cells
were immediately fixed in 4% paraformaldehyde in PBS for 30 min at
room temperature, permeabilized with 0.3% Triton X- 100 in PBS for
15 min and then blocked for 1 h at 37°C using a blocking buffer
containing 10% normal goat serum (cat. no. C0265; Beyotime
Institute of Biotechnology). The anti-MMP9 (cat. no. ab76003;
Abcam; 1:200) antibody was diluted in blocking buffer and placed on
the cells at 4°C overnight. After the incubation, cells on
coverslips were washed three times in PBS and then incubated with
goat anti-rabbit IgG (AS1109; Aspen Biological; 1:50) for 1 h at
37°C. Nuclei were stained using 4′6′-diamidino-2-phenylin-dole
(DAPI) solution for 10 min at room temperature. Imaging was
performed using an FV1200 (Olympus Corporation) confocal
microscope.
Cell migration assay
Cell migration was assessed using Transwell cell
culture chambers as previously described (20). Polyvinylpyrrolidone-free
polycarbonate filters with an 8.0-µm pore size were
pre-coated with 10 mg/ml collagen IV (C6745; Sigma-Aldrich; Merck
KGaA) and placed on the lower surface of Transwell chambers
(Costar, 3422; Corning, Inc.). The filters were dried overnight at
room temperature, washed extensively in PBS and then dried
immediately before use. Melanocytes (1×105) were seeded
into the upper chambers, while the lower chambers were filled with
fresh complete medium supplemented with or without 0.2 nM GM6001
(HY-15768; MedChemExpress). Cell culture inserts were treated with
repeated exposure to UVB as detailed above. A total of 48 h later,
the filters were fixed in methanol for 15 min at room temperature
and stained with crystal violet for 20 min at room temperature.
Cells that had migrated into the lower surface of the membrane were
counted in five microscopic fields at ×200 magnification using an
automatic microscope (Olympus Corporation) at least three
independent experiments.
miRNA transfection
Primary melanocytes were seeded in 6-well culture
plates at a population density of 5×105. miR-211-mimic
and miR-negative control (miR-NC; cat. no. miR01101) were purchased
from Guangzhou RiboBio Co., Ltd. After 24 h of culture, the
transfection of 50 nM miR-211-mimic (cat. no. miR10022694;
sequence, 5′-GCA GGG ACA GCA AAG GGG UGC-3′) was performed with
RiboFECT™ CP transfection reagent (Guangzhou RiboBio Co., Ltd.)
according to the manufacturer's protocol. miR-NC, which contains a
sequence that lacks homology with other miRNAs, was transfected
under identical conditions into melanocytes as the NC. After 48 h
of transfection, the cells were harvested and assayed using qPCR
and western blotting.
Recombinant lentiviral vector
transfection
In order to produce a p53 expression construct, the
protein coding sequence region of the human p53 gene was
synthesized and inserted into BamH I/Age I restriction sites
of the lentiviral expression vector GV358 that contains the EGFP
gene (Shanghai GeneChem Co., Ltd.). 293T cells (Shanghai GeneChem
Co., Ltd.) were transfected with a mixture of plasmids, including
viral packaging plasmids and the p53 expression plasmid (GV359-p53)
or the control plasmid (GV358) via Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) based on the manufacturer's
protocol. The viral supernatant was collected at 48 h after
transfection and was used to infect melanocytes. Evaluation of p53
expression was conducted using fluorescence microscopy at 48 h
after viral infection by harvesting the cells and characterizing
their levels of mRNA and protein expression by qPCR and western
blotting, respectively.
Statistical analysis
Data are expressed as the mean ± standard deviation
from three independent experiments. SPSS version 19.0 (IBM Corps.)
and GraphPad Prism 8 (GraphPad Software, Inc.) software were used
for analysis of all data. Statistical differences were determined
by Student's t-test or one-way analysis of variance with Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Activation of the p53-TRPM1/miR-211-MMP9
axis in melanocytes treated with single or repeated exposures to
UVB
First, the expression profiles of the
p53-TRPM1/miR-211-MMP9 axis in melanocytes treated with single or
repeated exposures to UVB was examined. For the single UVB
exposures, cultured human melanocytes were exposed to a single dose
of UVB (0, 8.75, 17.5, 35 and 70 mJ/cm2). After 8 h, the
mRNA and protein expression levels of p53, TRPM1 and MMP9 were
measured using RT-qPCR and western blotting, respectively. The
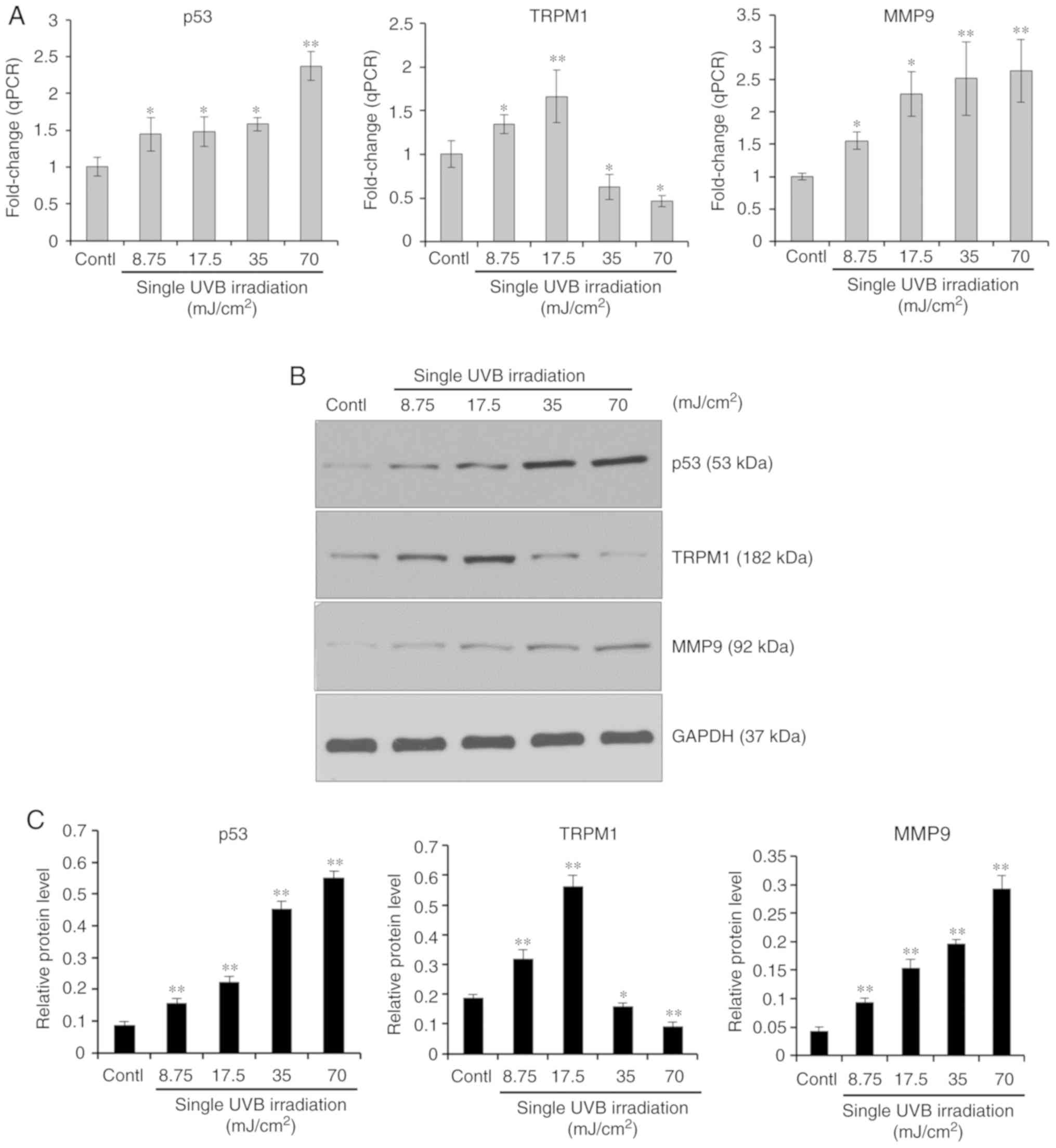
results (Fig. 1) showed that the
expression levels of p53 and MMP9 mRNAs and proteins were
upregulated in a UVB dose-dependent manner (8.75-70
mJ/cm2). Interestingly, TRPM1 mRNA and protein levels
were increased at lower doses (8.75 and 17.5 mJ/cm2) but
were reduced at higher doses (35 and 70 mJ/cm2) of UVB.
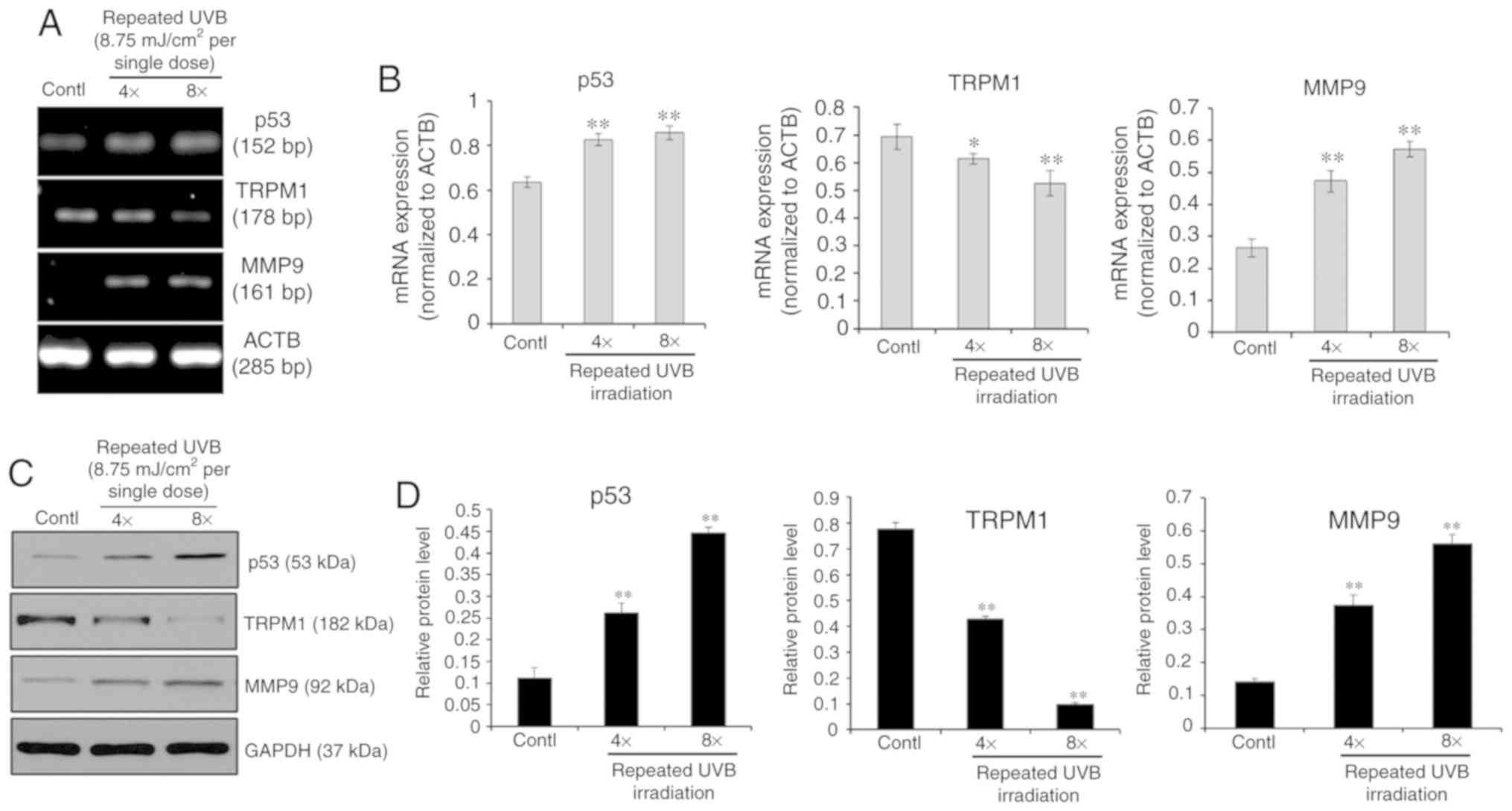
To simulate clinical settings with multiple exposures to UVB, the
cells were treated with repeated small UVB exposures for cumulative
doses of 35 or 70 mJ/cm2 (8.75 mJ/cm2 per
exposure in 5 h intervals for a total of 4 or 8 times), as shown in
Fig. 2. The upregulation of p53
and MMP9 mRNA and protein levels was seen in melanocytes treated
with 4 or 8 exposures to UVB, whereas mRNA and protein levels of
TRPM1 were significantly decreased in cells repeatedly exposed to
UVB compared with the UVB-unexposed control cells. These results
demonstrated that the p53-TRPM1/miR-211-MMP9 axis is significantly
activated in melanocytes by single and by repeated exposures to
UVB.
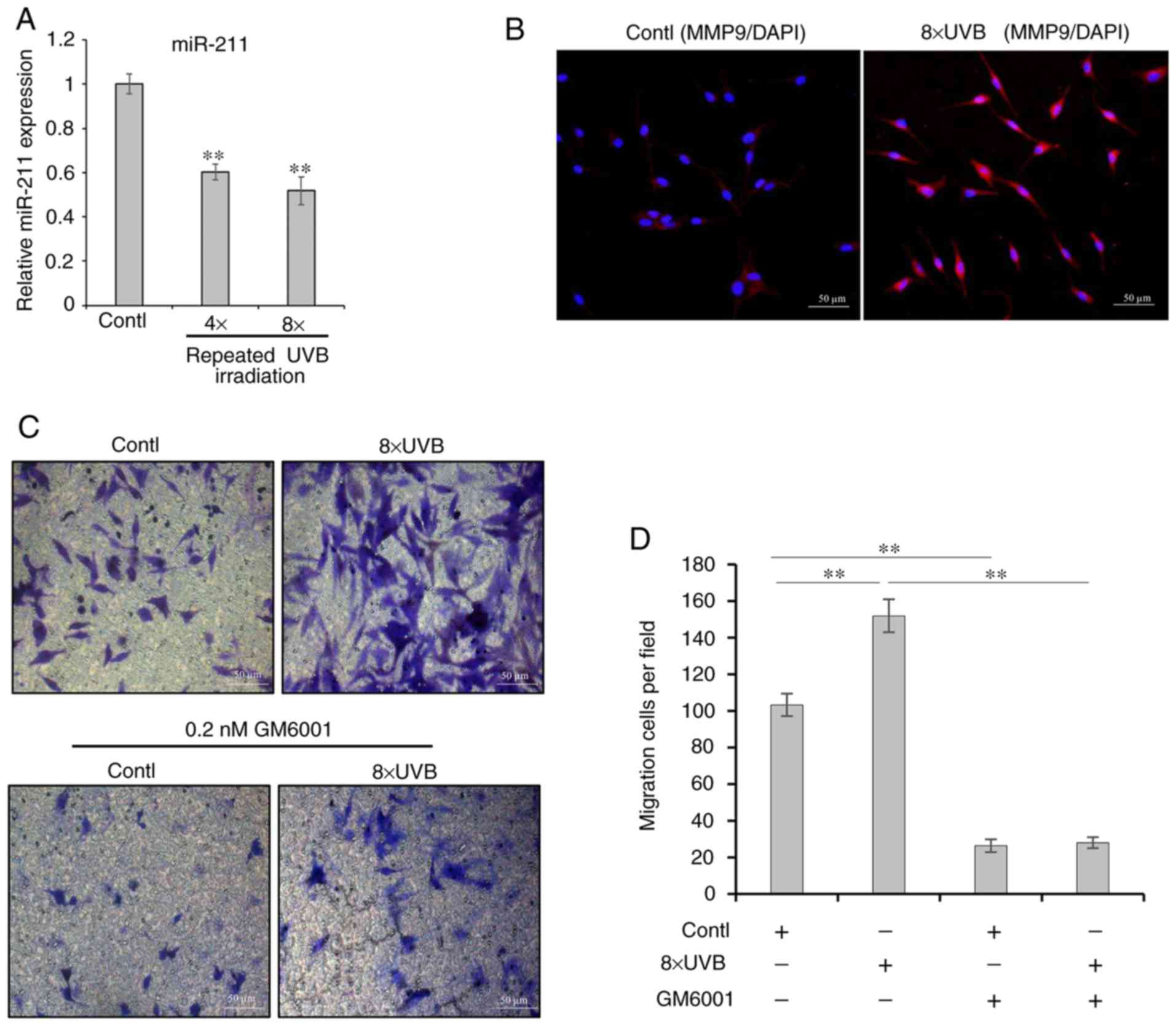
UVB-induced migration of melanocytes
involves the down- regulation of MMP9 by miR-211
To investigate whether miR-211 is involved in the
regulation of MMP9 and if this plays a central role in the
regulation of melanocyte migration, the capacity of UVB-exposed
melanocytes to migrate on collagen IV substrate was determined. The
results (Fig. 3) showed that
repeated UVB exposure significantly stimulated melanocyte migration
and that there was a highly significant inverse association between
miR-211 expression and MMP9 protein levels in the cells following
repeated exposure to UVB. The mRNA expression of miR-211 was
decreased and MMP9 was upregulated. To further characterize the
role of MMP9 in melanocyte migration, GM6001, a broad-spectrum MMP
inhibitor, was used to block MMP9-mediated cell migration. As
expected, the number of migrating cells in Transwell inserts with
collagen IV-coated membranes was much lower in the presence of 0.2
nM GM6001 compared with in the untreated control and even following
exposure to UVB (Fig. 3C and D).
Indeed, these findings strongly suggested that the
post-transcriptional regulation of the MMP9 gene by miR-211
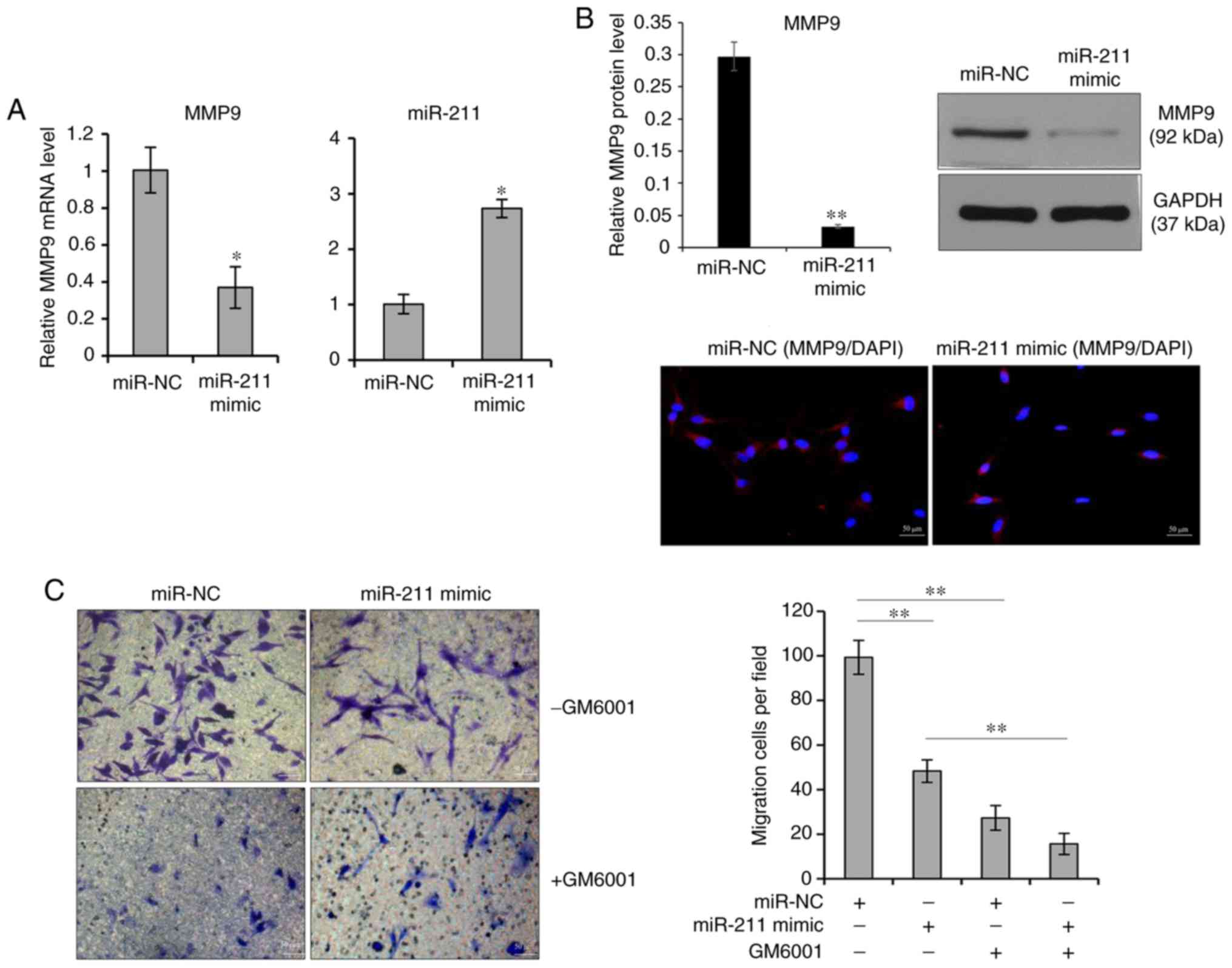
directly affects melanocyte migration. Furthermore, to determine
whether miR-211 has an effect on MMP9 expression and/or melanocyte
migration, a chemically synthesized miRNA mimic was transiently
transfected into melanocytes to determine the effects of miR-211.
After transfection with the miR-211 mimic, the level of miR-211 was
significantly increased in cells transfected with the miR-211 mimic
compared with the miR-NC (Fig.
4A). Next, qPCR and western blotting were performed to assess
the expression of MMP9 and found that the mRNA and protein levels
of MMP9 were significantly suppressed after transfection with the
miR-211 mimic (Fig. 4A and B).
The Transwell assays showed that melanocyte migration was
significantly reduced in the miR-211 mimic transfected cells
compared with the miR-NC transfected cells (Fig. 4C). Likewise, the inhibition of the
miR-211 mimic on melanocyte migration was neutralized by treatment
with 0.2 nM GM6001 (Fig. 4C).
Therefore, the present findings suggested that the
post-transcriptional control of the MMP9 gene by miR-211 might play
a critical role in the UVB-induced melanocyte migration.
UVB-induced melanocyte migration involves
the upregulation of MMP9 by p53
In this study, the expression of endogenous p53
protein was induced by UVB irradiation (Figs. 1 and 2). To address the question of whether
exogenous p53 overexpression would have a similar role in
melanocyte migration, the effect of a lentiviral vector-mediated
overexpression of p53 on the migration of cultured human
melanocytes was examined. As shown in Fig. 5, melanocytes were transfected with
p53-GFP lentiviral vectors and the transfection efficiency was
monitored by GFP detection. qPCR analysis demonstrated that the
relative levels of p53 and MMP9 were significantly increased in
cells transfected with the p53-GFP lentiviral vector compared with
those levels in the GFP vector control group, which was concomitant
with decreases in TRPM1 and miR-211 levels (Fig. 5A). The effectiveness of the
p53-GFP lentiviral vector was also confirmed by examining levels of
the p53, TRPM1 and MMP9 proteins by western blotting analysis. The
protein expression levels of p53 and MMP9 were significantly
increased in cells transfected with the p53-GFP lentiviral vector
compared with the control groups. Similar to the repression of
miR-211 after transfection of the p53-lentiviral vector, the
protein level of TRPM1 was also reduced compared with the control
group. The results of immunofluorescence staining also revealed
that the expression of MMP9 protein in transfected cells was
increased (Fig. 5B). The
Transwell assays showed that melanocyte migration was significantly
increased in cells that had a lentiviral vector-mediated
overexpression of p53 compared with the control group. Moreover,
the over-expression of p53 induced a higher migration of
melanocytes, which could be neutralized by GM6001 treatment
(Fig. 5C). These data suggest
that miR-211 exerts an anti-migration effect via the suppression of
MMP9, whereas the overexpression of p53 in melanocytes directly
reverses the inhibitory effect of miR-211 on cell migration.
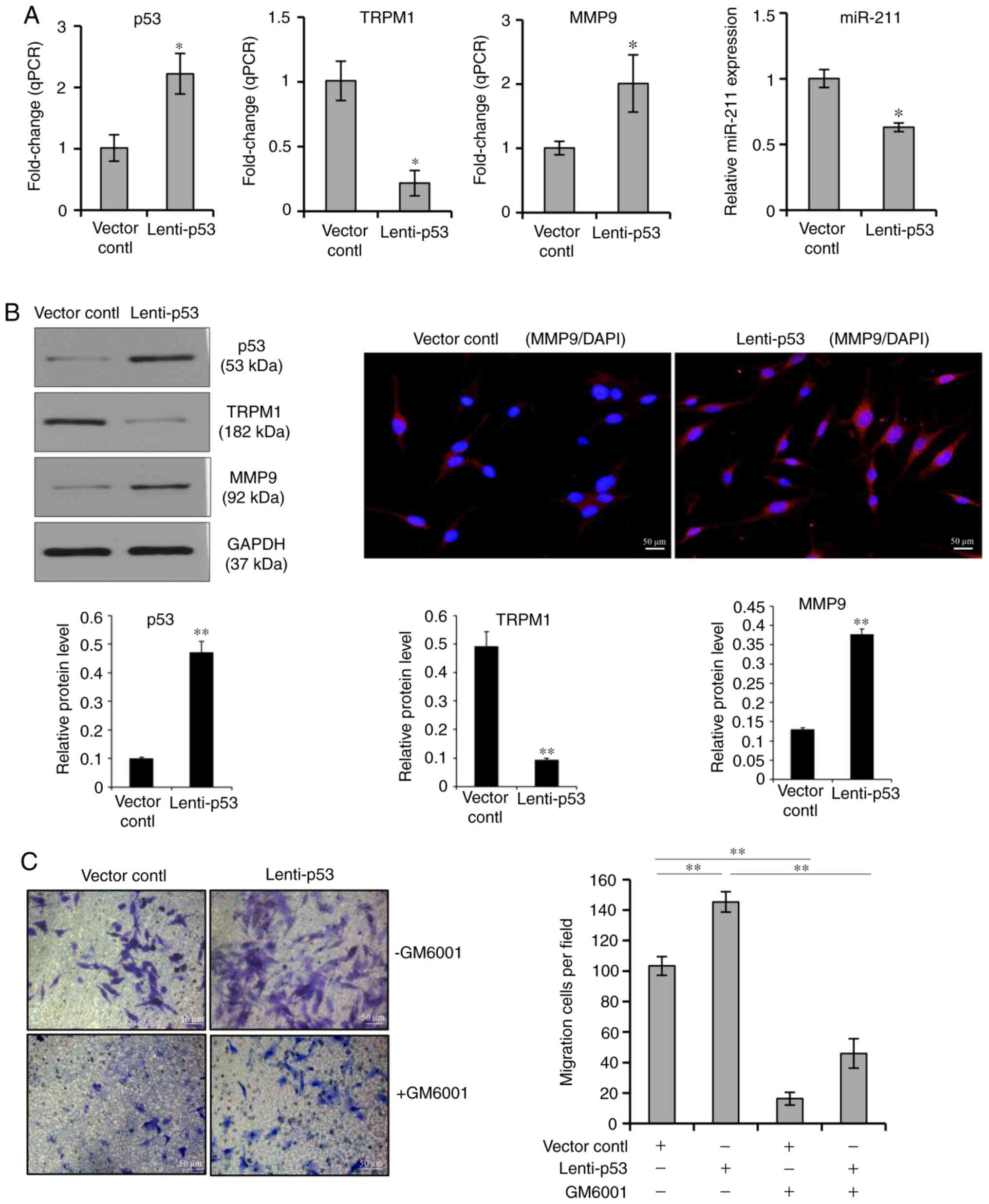 | Figure 5Effect of p53 overexpression on
MMP9-mediated cell migration. (A) Melanocytes were transfected with
p53-GFP lentiviral vectors, the transfection efficiency of the
cells was monitored by GFP detection. Quantitative PCR analysis was
performed to measure the p53, TRPM1, MMP9 and miR-211 level in
melanocytes transfected with the p53-GFP lentiviral vector or with
the GFP-vector control. Data represent the mean ± standard
deviation of three independent experiments. *P<0.05
vs. vector control. (B) Western blot analysis was carried out to
examine the protein levels of p53, TRPM1 and MMP9 in melanocytes
transfected with the p53-GFP lentiviral vector or with the
GFP-vector control. Immunofluorescence staining was carried out to
examine changes of the MMP9 protein in transfected cells. (C)
Transwell cell culture chambers were used for cell migration
assays. Cells were transfected with the p53-GFP lentiviral vector
or with the GFP-vector control. A total of 48 h later, migrating
cells on the bottom surface of each insert were stained with
crystal violet and counted in five microscopic fields using a
microscope. Scale bars, 50 mm. **P<0.01 vs. vector
control or lenti-p53. MMP, matrix metalloproteinase; TRPM1,
p53-transient receptor potential cation channel subfamily M member
1; GFP, green fluorescent protein; miR, microRNA. |
Discussion
Although UVB-based phototherapy has been clinically
proven to be an effective therapeutic option that improves
repigmentation outcomes in vitiligo patients, the precise mechanism
by which UVB activates and stimulates melanocyte migration in the
skin is still unclear. The present study showed that p53 regulates
MMP9 expression at the transcriptional level via a novel
miR-211-mediated mechanism. Previous studies have revealed that the
exons within the TRPM1 gene encode a Ca2+-permeable
cation channel protein (melastatin) that is involved in regulating
calcium homeostasis (21) and
triggering melanosome transfer (22). However, the 6th intron of the
TRPM1 gene also encodes miR-211, which acts as a critical modifier
of melanin synthesis by targeting transforming growth factor-β
receptor 2 (23) and of cell
migration by targeting MMP9 (24). MiR-211 has been predicted to
target MMP9, which is abundantly expressed in normal melanocytes
(25,26). Emerging evidence also indicates
that p53 itself serves as a sensor of UVB-damage to control cell
cycle arrest, DNA repair and apoptosis (27), constituting an extremely sensitive
adaptive photoprotective response of the skin against UVB damage
(28). There is a putative
p53-binding motif that has been identified 25 kb upstream of the
TRPM1 transcription start site (29). p53 is a transcription factor that
binds to this site, leading to a downregulation in TRPM1 gene
expression and a simultaneous decline in miR-211 expression, which
as a result upregulates MMP9. In this study, it was found that in
human melanocytes treated with a single dose or with repeated small
doses of UVB, p53 expression levels were upregulated in a UVB
dose-dependent manner. However, TRPM1 mRNA and protein levels were
upregulated at lower UVB doses (8.75 or 17.5 mJ/cm2) and
were reduced at higher UVB doses (35 or 70 mJ/cm2) of a
single UVB radiation. In agreement with this observation, Devi
et al (21) reported that
endogenous p53 could be induced in melanocytes by UVB irradiation,
whereas exposure to 35 mJ/cm2 UVB also led to the
downregulation of TRPM1 expression. Possible explanations for this
observation are that: i) In response to small doses of UVB,
melanocytes express higher protein levels of
microphthalmia-associated transcription factor (MITF) in
UVB-stressed melanocytes (30),
the increased level of MITF would bind to the M-box in the upstream
promoter region of the TRPM1 gene and activate its transcription
(23,31); ii) in response to repeated large
doses of UVB, increased levels of p53 in UVB-damaged melanocytes
inhibit TRPM1 gene expression. Therefore, the finely-tuned
expression level of p53 and MITF in UVB-exposed melanocytes is
critical for triggering MMP9-mediated cell migration.
The source of melanocytes that replenish the
unpigmented skin in lesions vitiligo patients who have undergone
marginal repigmentation may come from melanocytes existing at the
border of the white macules (2,5).
It is, however, difficult to conceive how melanocytes can easily
exit from a tightly interconnected epidermal microenvironment and
enter a different location of the skin to re-establish a functional
network with neighboring keratinocytes there. The present
observations show that the MMP9 expression level is increased in a
UVB dose-dependent manner as a result of the activation of the
p53-TRPM1/miR-211-MMP9 axis. It is well documented that MMP9 is
involved in the breakdown of the extracellular matrix and plays an
important role in tissue remodeling during skin wound healing
(32). In response to UVB
irradiation, the UVB-stressed melanocytes activate p53, the
activated p53 then binds DNA to downregulate TRPM1/miR-211
expression, which would lead to a transient increase of MMP9 and
endow the stressed melanocytes with the ability to move out.
Consistent with recent observations (33), melanocytes derived from vitiligo
skin showed a very low level of MMP9, suggesting that the impaired
expression of MMP9 exists in the perilesional skin of patients with
vitiligo, which correlates with a poor response to UVB-based
phototherapy. Future studies are necessary to examine the changes
of all members of this axis in the three-dimensional epidermal
models reconstructed from vitiligo melanocytes or/ normal
melanocytes to further confirm the role of this axis in melanocyte
migration induced by UVB-based phototherapy.
Taken together, this is the first study that reveals
the regulation of MMP9-mediated melanocyte migration via a novel
mechanism driven by the p53-TRPM1/miR-211-MMP9 axis. Activation of
this axis represents an attractive therapeutic target for improving
repigmentation outcomes in vitiligo patients.
Funding
The present study was supported by the National
Natural Science Foundation of China (NSFC Grants 81573028).
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MS carried out the cell culture, cell transfection
and wrote the paper. FM determined mRNA and protein levels by
RT-PCR and western blotting. SJ, YS and LL carried out cell culture
and migration assay. XH and JW collected skin samples and carried
out the cell cultures. SX discussed the results, commented on the
manuscript and analyzed the data. TCL designed the studies,
analyzed the data and wrote the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the Renmin Hospital of Wuhan University. Written
informed consent was obtained from each participant before
enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Gauthier Y, Cario Andre M and Taieb A: A
critical appraisal of vitiligo etiologic theories. Is melanocyte
loss a melanocytorrhagy? Pigment Cell Res. 16:322–332. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldstein NB, Koster MI, Hoaglin LG,
Spoelstra NS, Kechris KJ, Robinson SE, Robinson WA, Roop DR, Norris
DA and Birlea SA: Narrow band ultraviolet B treatment for human
vitiligo is associated with proliferation, migration, and
differentiation of melanocyte precursors. J Invest Dermatol.
135:2068–2076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boniface K, Seneschal J, Picardo M and
Taieb A: Vitiligo: Focus on clinical aspects, immunopathogenesis,
and therapy. Clin Rev Allerg Immunol. 54:52–67. 2018. View Article : Google Scholar
|
|
4
|
Rashighi M and Harris JE: Vitiligo
pathogenesis and emerging treatments. Dermatol Clin. 35:257–265.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Birlea SA, Costin GE, Roop DR and Norris
DA: Trends in regenerative medicine: Repigmentation in vitiligo
through melanocyte stem cell mobilization. Med Res Rev. 37:907–935.
2017. View Article : Google Scholar
|
|
6
|
Cario M: DDR1 and DDR2 in skin. Cell Adh
Migr. 12:386–393. 2018.PubMed/NCBI
|
|
7
|
Wang JX, Fukunaga-Kalabis M and Herlyn M:
Crosstalk in skin: Melanocytes, keratinocytes, stem cells, and
melanoma. J Cell Commun Signal. 10:191–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiong XX, Ding GZ, Zhao WE, Li X, Ling YT,
Sun L, Gong QL and Lu Y: Differences in the melanosome distribution
within the epidermal melanin units and its association with the
impairing background of leukoderma in vitiligo and halo nevi: A
retrospective study. Arch Dermatol Res. 309:323–333. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Reilly-Pol T and Johnson SL: Melanocyte
regeneration reveals mechanisms of adult stem cell regulation.
Semin Cell Dev Biol. 20:117–124. 2009. View Article : Google Scholar :
|
|
10
|
Esmat S, Mostafa W, Hegazy RA, Shalaby S,
Sheth V, Youssef R and El-Mofty M: Phototherapy: The vitiligo
management pillar. Clin Dermatol. 34:594–602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirobe T and Enami H: Activation of
melanoblasts and melanocytes after treatment with monochromatic
excimer light and narrowband-ultraviolet B of skin of vitiligo
patients. Int J Dermatol. 58:210–217. 2019. View Article : Google Scholar
|
|
12
|
Liu N, Matsumura H, Kato T, Ichinose S,
Takada A, Namiki T, Asakawa K, Morinaga H, Mohri Y, De Arcangelis
A, et al: Stem cell competition orchestrates skin homeostasis and
ageing. Nature. 568:344–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei TC, Virador V, Yasumoto K, Vieira WD,
Toyofuku K and Hearing VJ: Stimulation of melanoblast pigmentation
by 8-methoxypsoralen: The involvement of microphthalmia-associated
transcription factor, the protein kinase A signal pathway, and
proteasome-mediated degradation. J Invest Dermatol. 119:1341–1349.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou WC, Takeo M, Rabbani P, Hu H, Lee W,
Chung YR, Carucci J, Overbeek P and Ito M: Direct migration of
follicular melanocyte stem cells to the epidermis after wounding or
UVB irradiation is dependent on Mc1r signaling. Nat Med.
19:924–929. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coelho SG, Valencia JC, Yin L, Smuda C,
Mahns A, Kolbe L, Miller SA, Beer JZ, Zhang G, Tuma PL and Hearing
VJ: UV exposure modulates hemidesmosome plasticity, contributing to
long-term pigmentation in human skin. J Pathol. 236:17–29. 2015.
View Article : Google Scholar :
|
|
16
|
Tariq H, Bella J, Jowitt TA, Holmes DF,
Rouhi M, Nie Z, Baldock C, Garrod D and Tabernero L: Cadherin
flexibility provides a key difference between desmosomes and
adherens junctions. Proc Natl Acad Sci USA. 112:5395–5400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei TC, Vieira WD and Hearing VJ: In vitro
migration of melanoblasts requires matrix metalloproteinase-2:
Implications to vitiligo therapy by photochemotherapy. Pigment Cell
Res. 15:426–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshimura K, Tsukamoto K, Okazaki M,
Virador VM, Lei TC, Suzuki Y, Uchida G, Kitano Y and Harii K:
Effects of all- trans retinoic acid on melanogenesis in pigmented
skin equivalents and monolayer culture of melanocytes. J Dermatol
Sci. 27(Suppl 1): S68–S75. 2001. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Jiang G, Yang CS, Xu D, Sun C, Zheng JN,
Lei TC and Liu YQ: Potent anti-tumour activity of a novel
conditionally replicating adenovirus for melanoma via inhibition of
migration and invasion. Br J Cancer. 110:2496–2505. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devi S, Kedlaya R, Maddodi N, Bhat KM,
Weber CS, Valdivia H and Setaluri V: Calcium homeostasis in human
melanocytes: Role of transient receptor potential melastatin 1
(TRPM1) and its regulation by ultraviolet light. Am J Physiol Cell
Physiol. 297:C679–C687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu QM, Yi WJ, Su MY, Jiang S, Xu SZ and
Lei TC: Induction of retinal-dependent calcium influx in human
melanocytes by UVA or UVB radiation contributes to the stimulation
of melanosome transfer. Cell Prolif. 50:2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai XD, Rao CB, Li HR, Chen Y, Fan L, Geng
HQ, Li S, Qu J and Hou L: Regulation of pigmentation by microRNAs:
MITF-dependent microRNA-211 targets TGF-β receptor 2. Pigmemt Cell
Melanoma Res. 28:217–222. 2015. View Article : Google Scholar
|
|
24
|
Margue C, Philippidou D, Reinsbach SE,
Schmitt M, Behrmann I and Kreis S: New target genes of MITF-Induced
microRNA-211 contribute to melanoma cell invasion. PLoS One.
8:e734732013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sahoo A, Lee B, Boniface K, Seneschal J,
Sahoo SK, Seki T, Wang C, Das S, Han X, Steppie M, et al:
MicroRNA-211 regulates oxidative phosphorylation and energy
metabolism in human vitiligo. J Invest Dermatol. 137:1965–1974.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asuthkar S, Velpula KK, Chetty C, Gorantla
B and Rao JS: Epigenetic regulation of miRNA-211 by MMP-9 governs
glioma cell apoptosis, chemosensitivity and radiosensitivity.
Oncotarget. 3:1439–1454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui R, Widlund HR, Feige E, Lin JY,
Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter
SR and Fisher DE: Central role of p53 in the suntan response and
patho-logic hyperpigmentation. Cell. 128:853–864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verschooten L, Declercq L and Garmyn M:
Adaptive response of the skin to UVB damage: Role of the p53
protein. Int J Cosmet Sci. 28:1–7. 2006. View Article : Google Scholar
|
|
29
|
Wei CL, Wu Q, Vega VB, Chiu KP, Ng P,
Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al: A global map of
p53 transcription-factor binding sites in the human genome. Cell.
124:207–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terazawa S and Imokawa G: Signaling
cascades activated by UVB in human melanocytes lead to the
increased expression of melanocyte receptors, endothelin B receptor
and c-KIT. Photochem Photobiol. 94:421–431. 2018. View Article : Google Scholar
|
|
31
|
Zhiqi S, Soltani MH, Bhat KM, Sangha N,
Fang D, Hunter JJ and Setaluri V: Human melastatin 1 (TRPM1) is
regulated by MITF and produces multiple polypeptide isoforms in
melanocytes and melanoma. Melanoma Res. 14:509–516. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baron J, Marquardt Y, Steiner T, Holzle F,
Skazik-Voogt C, Heise R and Amann P: Effects of non-ablative
fractional erbium glass laser treatment on gene regulation and skin
physiology in human three-dimensional skin models. Laser Surg Med.
48:441. 2016.
|
|
33
|
Kumar R, Parsad D, Kanwar AJ and Kaul D:
Altered levels of Ets-1 transcription factor and matrix
metalloproteinases in melanocytes from patients with vitiligo. Br J
Dermatol. 165:285–291. 2011. View Article : Google Scholar : PubMed/NCBI
|