Introduction
Lung cancer is the most common type of malignant
tumor, with the highest morbidity and mortality rates globally,
resulting in >100,000 deaths annually (1). Pathologically, lung cancer is
classified into two broad subgroups, namely small-cell lung cancer
and non-small-cell lung cancer (NSCLC), with NSCLC accounting for
85% of all cases (2,3). Patients with early-stage lung cancer
are often asymptomatic and, thus, are often first diagnosed with
advanced-stage lung cancer, at which point resection of the tumor
may not be possible; therefore, patients with advanced-stage lung
cancer are most frequently treated with chemotherapy or
radiotherapy (4-6). Cisplatin (DDP) is a first-line
chemotherapy for lung cancer (7).
DDP-DNA cross-linking prevents DNA replication, resulting in
apoptosis of lung cancer cells (8,9).
However, patients frequently develop resistance to chemotherapy
(10-12). Therefore, identifying therapeutics
that can reverse drug resistance by enhancing the sensitivity of
tumor cells to drugs, thereby reducing the concentration of drugs
used, may improve the outcomes of patients.
Herbal/botanical-based medicines have been
intensively studied for several decades, as some exert beneficial
effects when used to treat several different diseases (13,14), including various types of cancer
(15,16). Hesperidin and the hesperidin
derivative hesperetin possess various beneficial biological
properties (17). Hesperidin
inhibits proliferation and induces apoptosis in lung cancer cells,
without notable toxic effects on normal lung epithelial cells
(18). Furthermore, hesperidin
inhibits the migration and invasion of lung cancer cells by
regulating the SDF1/CXCR4 axis (19). In vivo, hesperidin
pretreatment protects against the development of carcinogen-induced
lung cancer from multiple carcinogens (20-23).
Hesperetin, the glycoside ligand derivative of
hesperetin, exhibits good bioavailability (24). It has been demonstrated that
hesperetin prevented 1,2-dimethylhydrazine-induced colorectal
cancer (25,26) and induced apoptosis of colorectal
cancer cells in a dose-dependent manner (27). The aim of the present study was to
investigate the effects of hesperetin treatment on the sensitivity
of A549/DDP cells to certain drugs. Understanding the molecular
mechanism of action of hesperetin in the drug resistance of tumor
cells may provide the basis for the use of hesperetin as an
adjuvant to prevent multidrug resistance (MDR) in the clinical
setting.
Materials and methods
Cell culture
Human lung cancer A549 and A549/DDP cells were
obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and cultured with RPMI-1640 medium
containing 10% FBS (both from HyClone; GE Healthcare) and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
with a 5% CO2 atmosphere at 37°C.
Preparation of hesperetin, DDP and JSH-23
solutions
Hesperetin powder (Sigma-Aldrich; Merck KGaA) was
dissolved in DMSO and diluted to 0.6, 1.25, 2.5, 5, 10, 20, 40, 80
and 160 µM using RPMI-1640 medium. DDP was dissolved in
sterile PBS to 1 mg/ml, and subsequently diluted to 0.6, 1.25, 2.5,
5, 10, 20, 40, 80 and 160 µg/ml using culture medium. The
nuclear factor (NF)-κB signaling pathway inhibitor JSH-23
(Sigma-Aldrich; Merck KGaA) was dissolved in DMSO and diluted to 1
µM.
Cell counting kit-8 (CCK-8) assay
A549 and A549/DDP cells were seeded into 96-well
plates at a density of 1×104 cells/well and treated with
hesperetin, alone or in combination with DDP, for 72 h. The medium
was removed, and the cells were incubated with 10% CCK-8 reagent
for 2 h at 37°C. Absorbance values were measured at 450 nm using an
enzyme-labeling instrument (iMARK, Bio-Rad Laboratories, Inc.).
Experiments were performed in triplicates.
Flow cytometry
A549/DDP cells were seeded into 6-well plates at a
density of 8×105 cells/well and treated with 1.25, 2.5,
5 and 10 µM hesperetin for 72 h. Subsequently, 50
µg/ml DDP was added and thecells were further incubated for
48 h. Cells treated with 50 µg/ml DDP were used as a
positive control. Untreated cells served as the negative control.
Subsequently, cells were collected and stained using an Annexin V-
FITC/PI kit (cat. no. CA1020; Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's instructions.
The proportion of apoptotic cells was analyzed using flow cytometry
(BD Biosciences). Analysis of apoptosis was performed by FlowJo 7.6
software (Becton, Dickinson and Company). Experiments were
performed in triplicates.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
A549/DDP cells were seeded in 6-well plates at a
density of 8×105 cells/well and treated with hesperetin
or DDP for 72 h, as described above. Cells were harvested and total
RNA was extracted using TRIzol® reagent, according to
the manufacturer's protocol (Thermo Fisher Scientific, Inc.). The
purity and concentration of the extracted total RNA were measured
using an ultraviolet spectrophotometer, and an A260/A280 value of
1.8-2 was considered acceptable. A total of 1 µg RNA was
reverse-transcribed into cDNA according to the manufacturer's
protocol. The reverse transcription conditions were 37°C for 15
min; 85°C for 5 sec; and held at 4°C. Reverse transcription and
SYBR-Green qPCR kits were obtained from Beijing Transgen Biotech
Co., Ltd. qPCR primers were purchased from Sangon Biotech Co., Ltd.
Subsequently, using cDNA as a template and β-actin as an internal
reference, the relative expression was determined using an ABI7500
Real Time PCR system (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Pre-denaturation at 95°C
for 5 min, followed by 40 cycles of denaturation at 95°C for 15 sec
and annealing at 60°C for 15 sec. qPCR was performed in
triplicates, and the relative expression levels of the target genes
were calculated using the 2−ΔΔCq method (28). All reactions were performed in
triplicate. The sequences of the primers used were as follows:
P-glycoprotein (P-gp) forward, TTG CTG CTT ACA TTC AGG TTT CA and
reverse, AGC CTA TCT CCT GTC GCA TTA; epidermal growth factor
receptor-2 (c-erbB-2) forward, TGT GAC TGC CTG TCC CTA CAA and
reverse, CCA GAC CAT AGC ACA CTC GG; glutathione s-transferase
(GST-π) forward, TTG GGC TCT ATG GGA AGG AC and reverse, GGG AGA
TGT ATT TGC AGC GGA; and β-actin forward, CCT CGC CTT TGC CGA TCC
and reverse, GGA TCT TCA TGA GGT AGT CAG TC.
Western blotting
After A549/DDP cells were treated with hesperetin
for 72 h, and total protein was extracted using ice-cold RIPA lysis
buffer (Beyotime Institute of Biotechnology). The protein
concentration of each group was determined using a bicinchoninic
acid assay. Proteins (30 µg per lane) were loaded on a 10%
SDS-gel, resolved using SDS-PAGE, transferred to PVDF membranes
(EMD Millipore) and subsequently blocked with 5% skimmed milk for 2
h at room temperature. Membranes were probed with one of the
following primary antibodies: Rabbit anti-P-gp (1:1,000; cat. no.
ab129450; Abcam), mouse anti-IκB (1:1,000; cat. no. sc-1643; Santa
Cruz Biotechnology, Inc.), mouse anti-phosphorylated (p-)IκB
(1:1,000; cat. no. sc-8404; Santa Cruz Biotechnology, Inc.), rabbit
anti-NF-κB p65 (1:1,000; cat. no. ab16502; Abcam), rabbit
anti-NF-κB p65 (p-S536) (1:1,000; cat. no. ab28856; Abcam), rabbit
anti-histone H3 antibody (1:2,000; cat. no. ab201456; Abcam) or
rabbit anti-human β-actin primary antibody (1:4,000; cat. no.
ab179467; Abcam), overnight at 4°C. Subsequently, the membranes
were incubated with a horseradish peroxidase-conjugated goat
anti-rabbit antibody (cat. no. ab6721; Abcam) or horseradish
peroxidase-conjugated goat anti-mouse antibody (cat. no. ab6789;
Abcam) both at 1:5,000 at room temperature for 3 h. Signals were
visualized with enhanced chemiluminescence solution (EMD Millipore)
and developed using chemiluminescence apparatus (GE Healthcare).
Densitometry analysis was performed using Quantity One, version
4.6.7 (Bio-Rad Laboratories, Inc.). Experiments were repeated three
times.
Preparation of nuclear and cytosolic
extracts
A nucleo-protein separation kit (NE-PER™ Nuclear and
Cytoplasmic Extraction Reagents) was purchased from Thermo Fisher
Scientific, Inc. (cat. no. 78833). Nuclear and cytosolic extracts
were prepared according to manufacturer's protocol. All steps were
performed on ice or at 4°C. Briefly, A549/DDP cells were seeded in
6-well plates at a density of 8×105 cells per well and
treated with hesperetin for 72 h as described above. After
digesting, re-suspending and centrifuging at 500 × g for 4 min at
4°C, the cells were incubated in CER I on ice for 10 min and
pre-cooled CER II was added for 1 min. The supernatant (cytosolic
extract) was collected by centrifugation at 15,000 × g for 10 min
at 4°C. Subsequently, the insoluble compounds were immersed in NER
on ice for 40 min, and centrifuged at 15,000 × g for 10 min at 4°C.
The supernatant was the nuclear extract and was analyzed by western
blotting. Experiments were repeated three times independently.
Immunofluorescence
A549/DDP cells were seeded at a density of
1×105 cells/well in a 6-well plate preloaded with
sterile glass coverslips. After treatment with hesperetin for 72 h,
cell slides were removed and fixed with 4% paraformaldehyde for 15
min at room temperature, and permeabilized with 0.25% Triton for 10
min and blocked with 5% BSA for 1 h. Subsequently, cells were
incubated with 5% BSA-diluted rabbit anti-NF-κB p65 antibody
(1:300; cat. no. ab16502; Abcam) or mouse anti-P-gp antibody
(1:300; cat. no. ab80594; Abcam) overnight at 4°C, followed by
incubation with 5% BSA-diluted goat anti-mouse IgG H&L (Alexa
Fluor® 555; 1:300; cat. no. ab150078; Abcam) or goat
anti-rabbit IgG H&L (Alexa Fluor® 488; 1:300; cat.
no. ab150077; Abcam) for 2 h at room temperature. Finally, nuclei
were stained with DAPI (1:10,000; Beyotime Institute of
Biotechnology, Inc.) for 5 min at room temperature and imaged
immediately using a fluorescence microscope (magnification, ×200;
Olympus Corporation). Experiments were repeated three times.
Rhodamine 123 efflux assay to assess P-gp
function
A549/DDP cells were plated at a density of
8×105 cells/well in 6-well plates and treated with
hesperetin for 72 h. Untreated cells were used as the control.
Subsequently, the cells were stained with 5 µg/ml rhodamine
123 (Santa Cruz Biotechnology, Inc.), followed by incubation at
37°C in 5% CO2 for 1 h. The cells were centrifuged at
400 × g for 5 min at room temperature, washed twice with medium and
re-suspended. The fluorescence value was analyzed by flow cytometry
at an excitation/emission wavelength of 488/530 nm. Experiments
were performed in triplicates.
Xenograft experiments
Five-week-old nude mice were maintained and handled
according to the instructions approved by the Animal Care Committee
of 900 Hospital of the Joint Log istics Team. A549/DDP cells at the
logarithmic growth phase were collected and adjusted to a density
of 5×107 cells/ml. Nude mice were randomly divided into
three groups: Control, DDP-treated and co-treated with DDP and
hesperetin, with 6 mice in each group. The cells (5×106
cells in 0.1 ml) were subcutaneously injected in the right armpit
of the nude mice. Mice in the co-treatment group were
intragastrically administered 2 mg/kg hesperetin every 2 days. Mice
in the control and DDP treatment groups were administered PBS. The
diameter of the subcutaneous tumor was measured every 4 days. After
3 weeks, when the mean tumor diameter reached 5 mm, the DDP-treated
and the co-treatment groups were administered 2 mg/kg DDP every 2
days intraperitoneally. The control group mice were injected with
an equivalent volume of PBS. When the maximum tumor diameter
exceeded 12 mm (52 days after subcutaneous injection), the nude
mice were sacrificed by cervical dislocation, and tumor volume was
measured. All mouse experiments were approved by the Animal Care
and Use Committee of 900 Hospital of the Joint Logistics Team and
carried out in accordance with the Guide for the Care and Use of
Laboratory Animals.
Statistical analysis
Data were analyzed and graphing was performed using
SPSS, version 21.0 (IBM Corp.) and GraphPad Prism, version 6.0
(GraphPad Software, Inc.), respectively. Statistical results are
presented as the mean ± standard deviation. Differences between
multiple groups were compared using one-way ANOVA with a post-hoc
Dunnett's test (when all groups are compared with the control
group) or Bonferroni test (when all groups are compared).
Differences between two groups were analyzed using Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Toxic effects of different concentrations
of hesperetin on parental A549 cells and DDP-resistant (A549/DDP)
cells
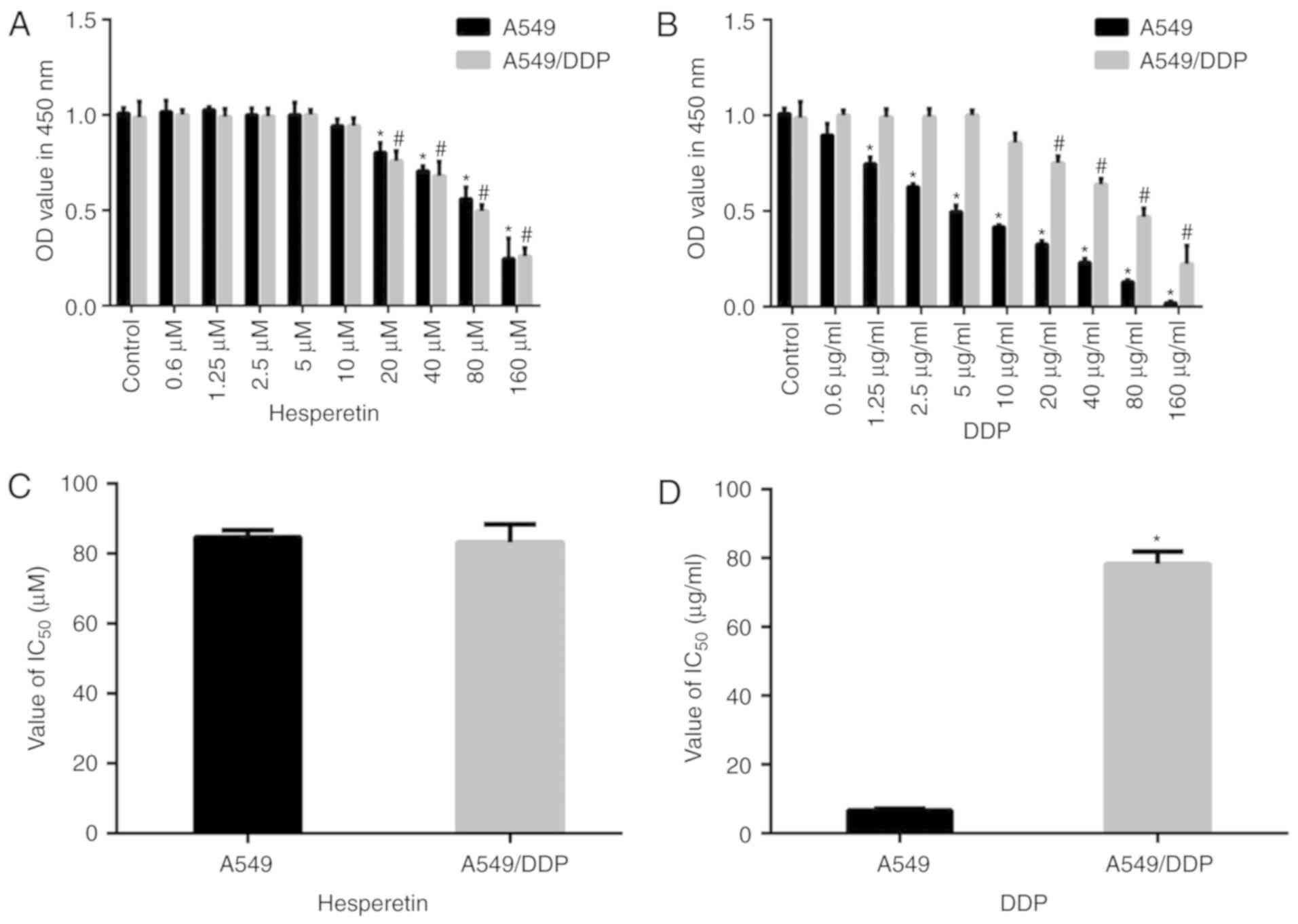
As shown in Fig.
1, compared with the matched control group, low concentrations
of hesperetin (<5 µM) exerted no effect on A549 and
A549/DDP cells (P>0.05). In addition, low concentrations of DDP
(<5 µM) had no effect on A549/DDP cells (P>0.05).
Higher concentrations (>20 µM) of hesperetin and DDP
significantly reduced the proliferation of both types of cells in a
dose-dependent manner (P<0.05). Furthermore, there was no
significant difference in the IC50 values between these
two types of cells treated with hesperetin (P>0.05), whereas the
IC50 values differed significantly between DDP-treated
A549 and A549/DDP cells (6.28±1.39 vs. 78.3±4.31 µg/ml,
respectively; P<0.05).
Hesperetin pretreatment exerts a
synergistic effect on A549/DDP cells
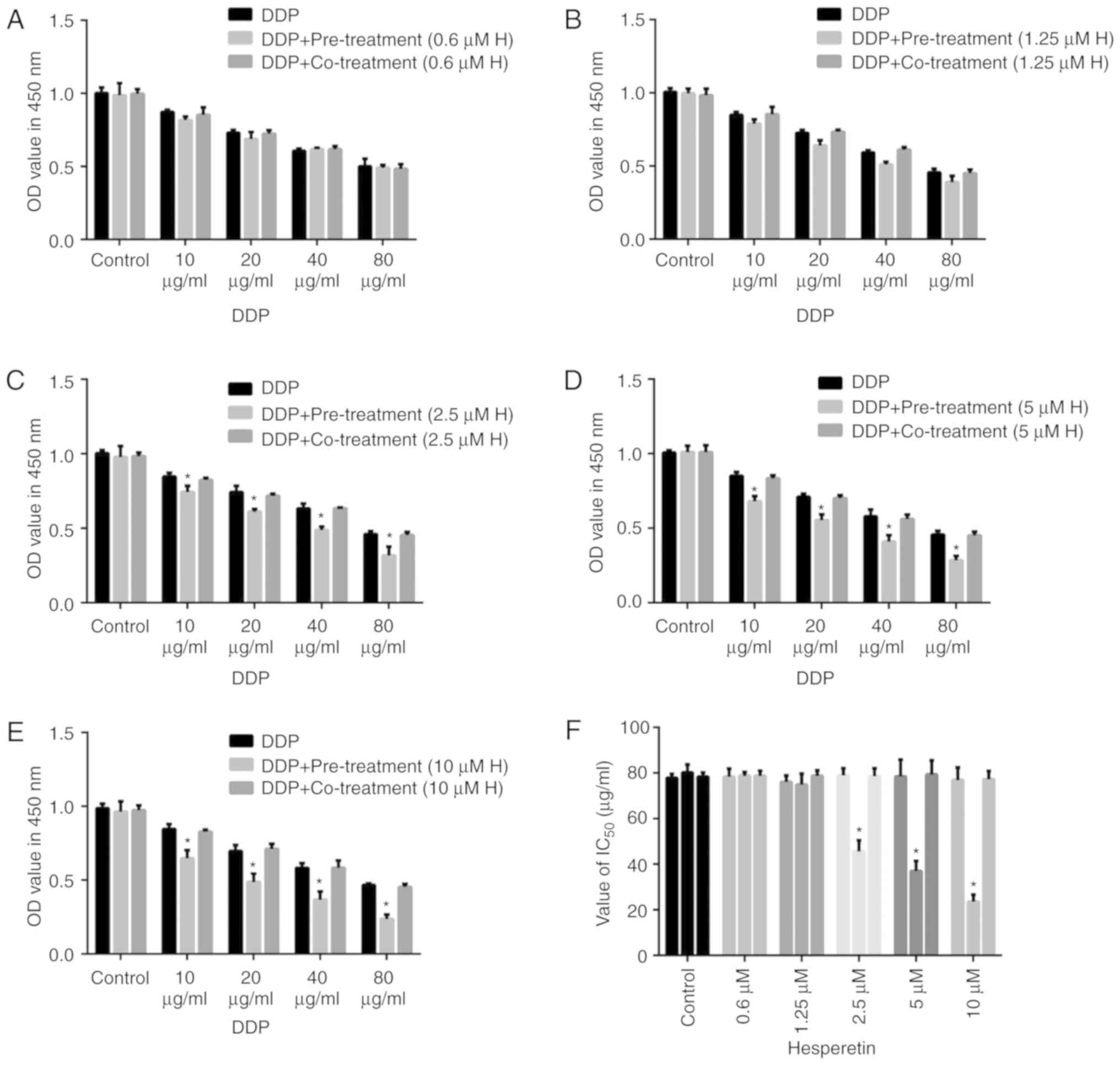
To determine whether hesperetin improved the
sensitivity of A549/DDP cells to DDP, cells were treated with
hesperetin either alone or combined with DDP. Cells were treated
with different concentrations of hesperetin (0.6, 1.25, 2.5, 5 or
10 µM) for 72 h, and subsequently incubated with different
concentrations of DDP (10, 20, 40 or 80 µg/ml) for 48 h, or
treated with hesperetin and DDP together. Cells treated with DDP
alone were used as the control group. Cell viability was measured
using CCK-8 assays. When the cells were treated with 0.6 or 1.25
µM hesperetin followed by treatment with various
concentrations of DDP, no significant difference was observed among
the different groups (Fig. 2).
The IC50 values of DDP in A549/DDP cells did not differ
significantly (P>0.05). However, when the concentration of
hesperetin was increased to 2.5, 5 or 10 µM, the effect of
DDP on cells was significantly increased. Additionally, the
IC50 value was significantly decreased compared with the
control cells (P<0.05). In the xenograft mouse model, all nude
mice received subcutaneous injection of A549/DDP cells, followed by
administration of hesperetin. Treatment of mice with hesperetin had
no effect on tumor growth; however, hesperetin treatment followed
by administration of DDP resulted in a significant reduction in
tumor growth in the nude mice compared with DDP treatment alone.
The tumor volume was measured 52 days after inoculation, and it was
demonstrated that the DDP-treated group exhibited significantly
reduced cell proliferation compared with the control group.
Furthermore, compared with the DDP-treated group, the tumor volume
in the hesperetin and DDP co-treatment group was significantly
reduced.
Hesperetin pretreatment increases the
proportion of apoptotic cells in DDP-treated A549/DDP cells
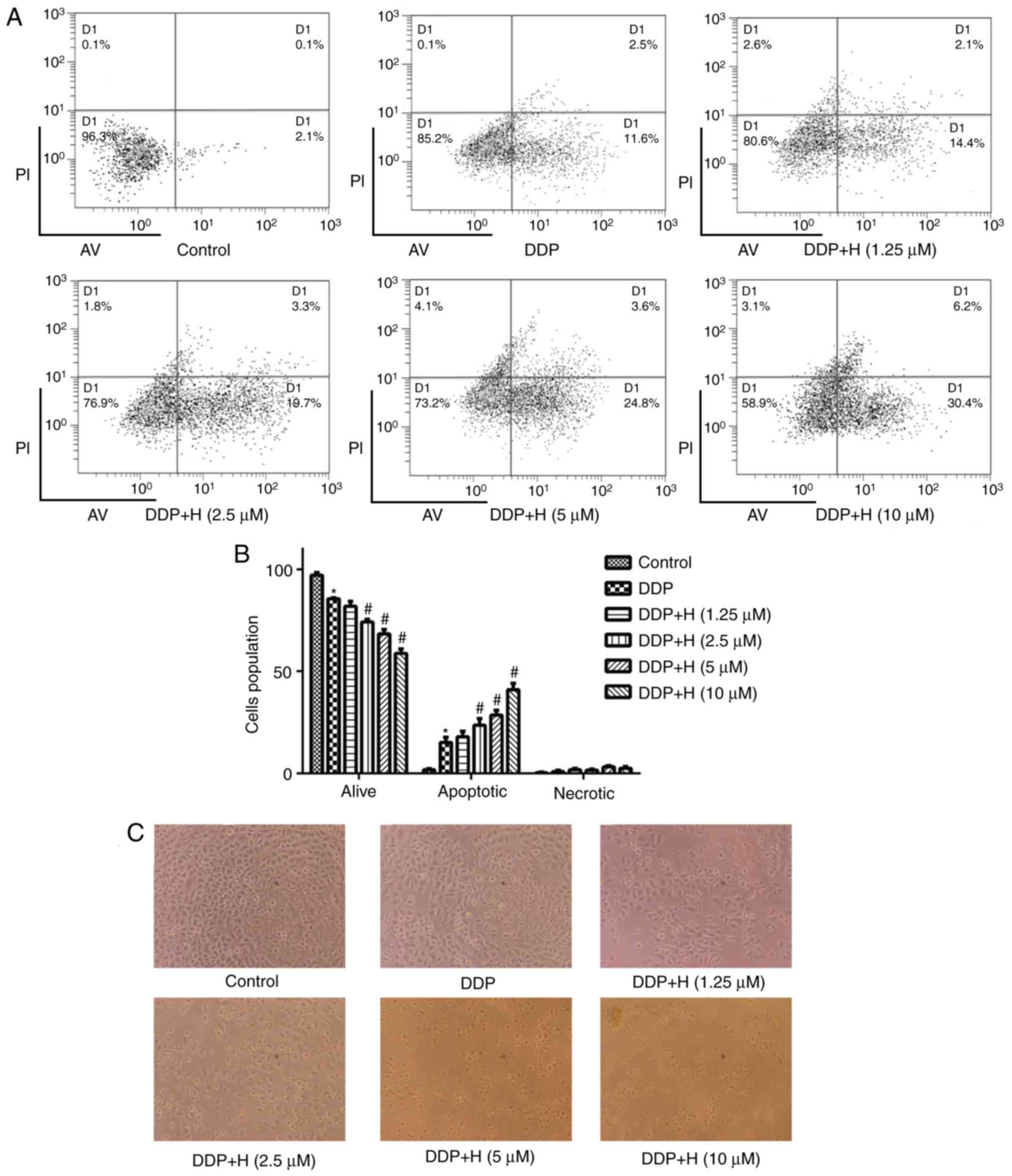
To validate the mechanism by which hesperetin
treatment enhances the sensitivity of A549/DDP cells to DDP, cells
were treated with different concentrations of hesperetin and
subsequently treated with DDP. Cell apoptosis was measured by flow
cytometry. Compared with the positive control group, the proportion
of apoptotic A549/DDP cells following hesperetin pretreatment was
significantly increased (Fig. 3;
P<0.05).
Hesperetin decreases the expression of
P-gp
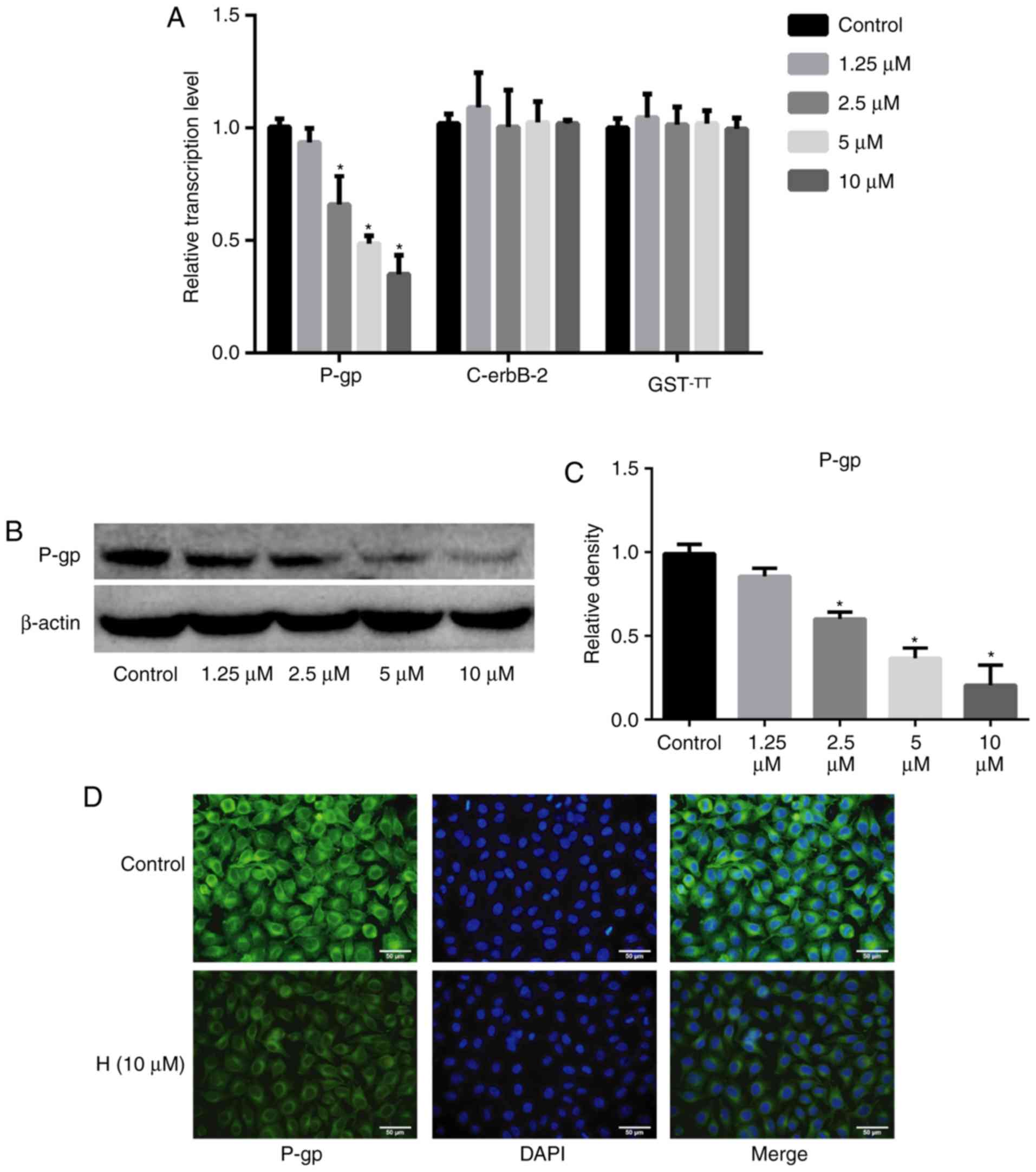
To determine the mechanism by which hesperetin
enhances the sensitivity of A549/DDP cells to DDP, the expression
of P-gp and the drug resistance-associated genes, c-erbB-2 and
GST-π, was assessed using RT-qPCR, western blotting and
immunofluorescence assays. Hesperetin downregulated the mRNA levels
of P-gp (P<0.05), whereas it exerted no effect on the mRNA
levels of c-erbB-2 and GST-π (P>0.05; Fig. 4). Western blotting and
immunofluorescence analysis also demonstrated that hesperetin
significantly decreased the protein expression levels of P-gp
(P<0.05).
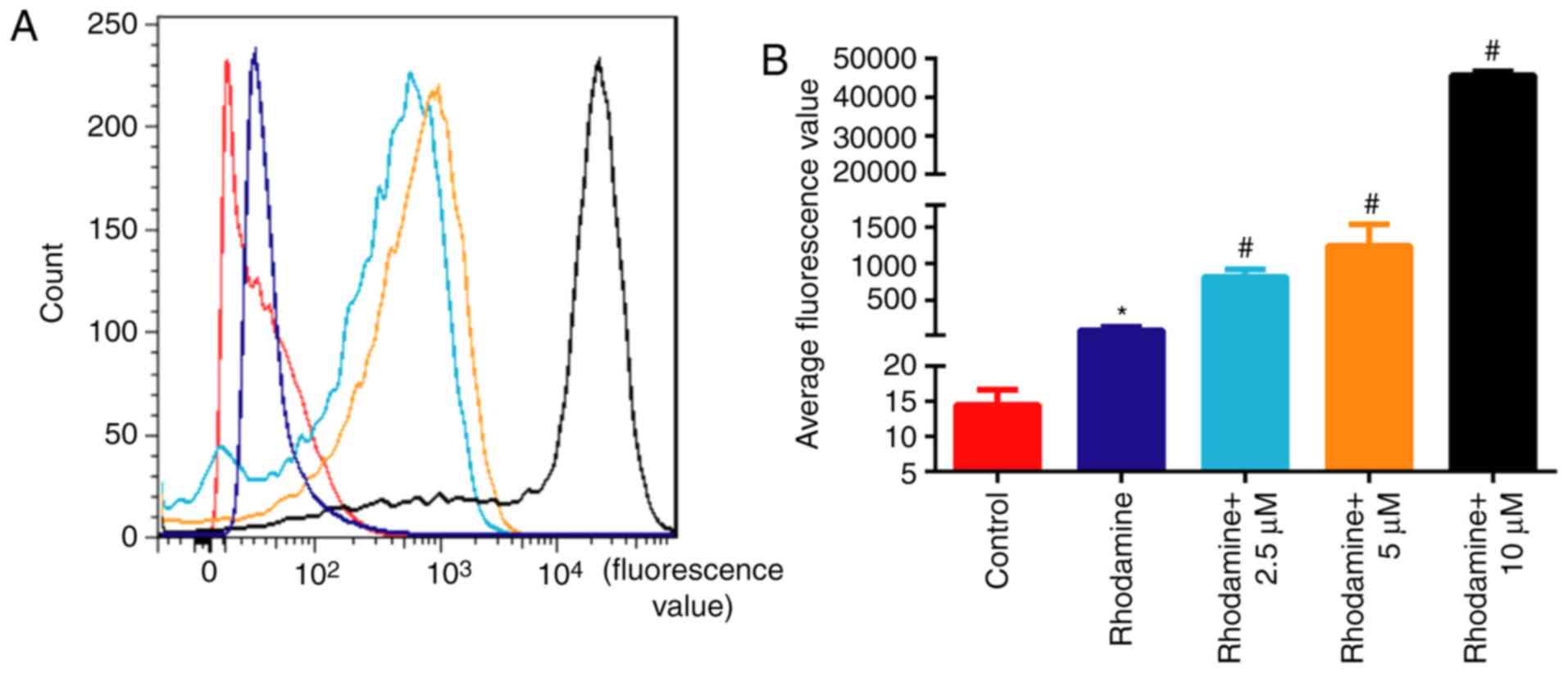
Hesperetin treatment promotes the
accumulation of rhodamine 123 in A549/DDP cells
To elucidate the mechanism by which hesperetin
sensitizes A549/DDP cells to DDP, cells were treated with 10
µM hesperetin and stained with rhodamine 123. The
fluorescence values of cells incubated with rhodamine alone were
significantly higher compared with those of untreated cells
(P<0.05; Fig. 5). The
fluorescence values of A549/DDP cells treated with hesperetin were
significantly higher compared with those of cells incubated with
rhodamine alone (P<0.05), suggesting that hesperetin treatment
resulted in an accumulation of rhodamine 123 in A549/DDP cells.
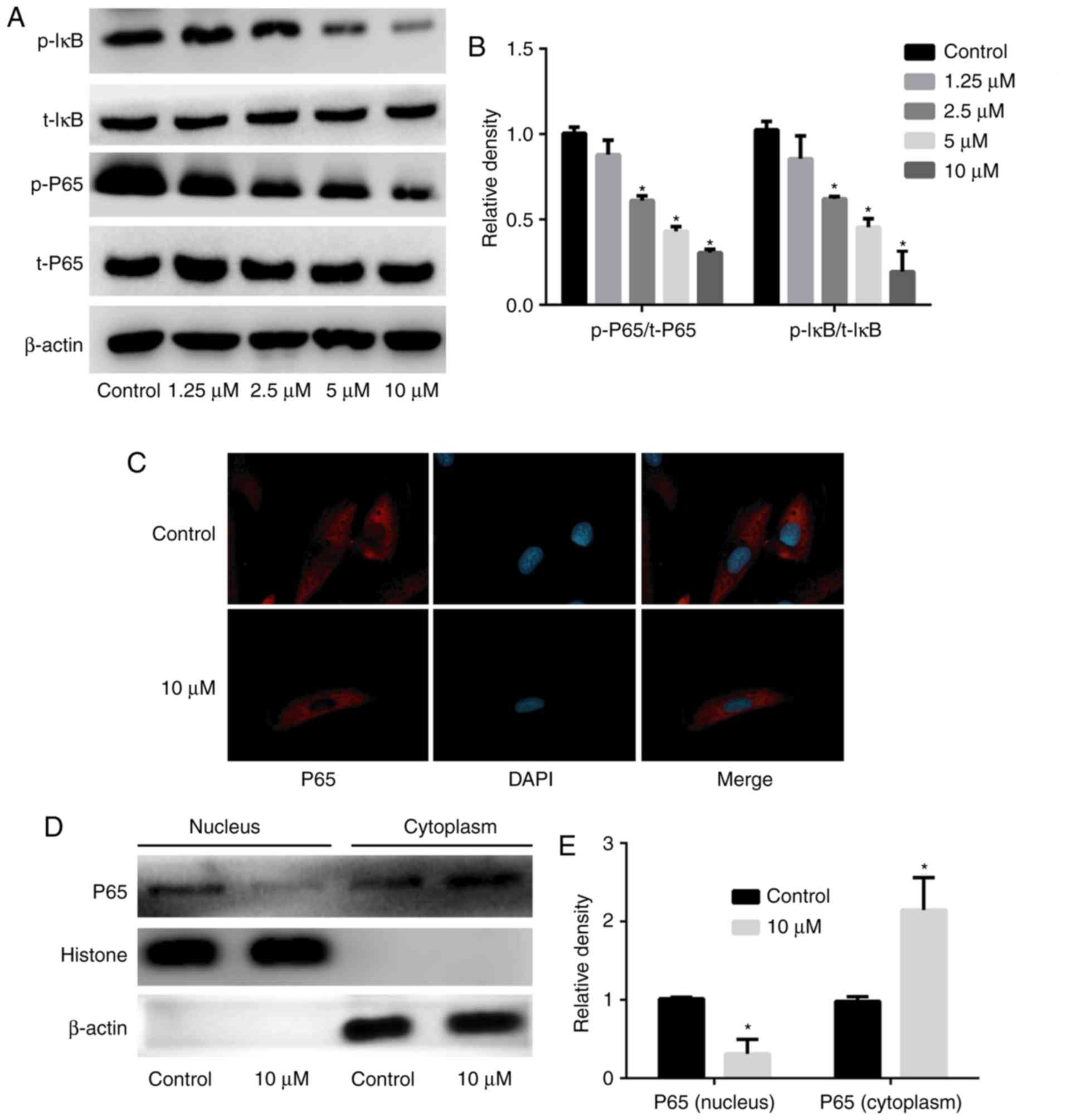
Hesperetin treatment inhibits the
activation of the NF-κB signaling pathway
To verify the mechanism by which hesperetin
increases the sensitivity of A549/DDP cells to DDP through
downregulation of P-gp expression, A549/DDP cells were treated with
various concentrations of hesperetin (1.25, 2.5, 5 or 10
µM), total protein was extracted, and the expression and
activation of NF-κB signaling pathway-associated proteins were
assessed. The intracellular localization of p65 was determined by
immunofluorescence. Hesperetin downregulated the phosphorylation of
IκB in a dose-dependent manner (P<0.05), and attenuated the
expression of p-p65 (P<0.05); however, it had no significant
effect on the expression of total IκB and total p65 (P>0.05)
compared with the control group (Fig.
6). Immunofluorescence revealed that 10 µM hesperetin
could inhibit p65 entry into the nucleus. Extracted cytoplasmic and
nuclear proteins were assessed by western blotting and, compared
with the control group, the levels of p65 in the cytoplasm were
significantly increased (P<0.05), whereas the levels in the
nucleus were significantly decreased (P<0.05) when A549/DDP
cells were treated with 10 µM hesperetin.
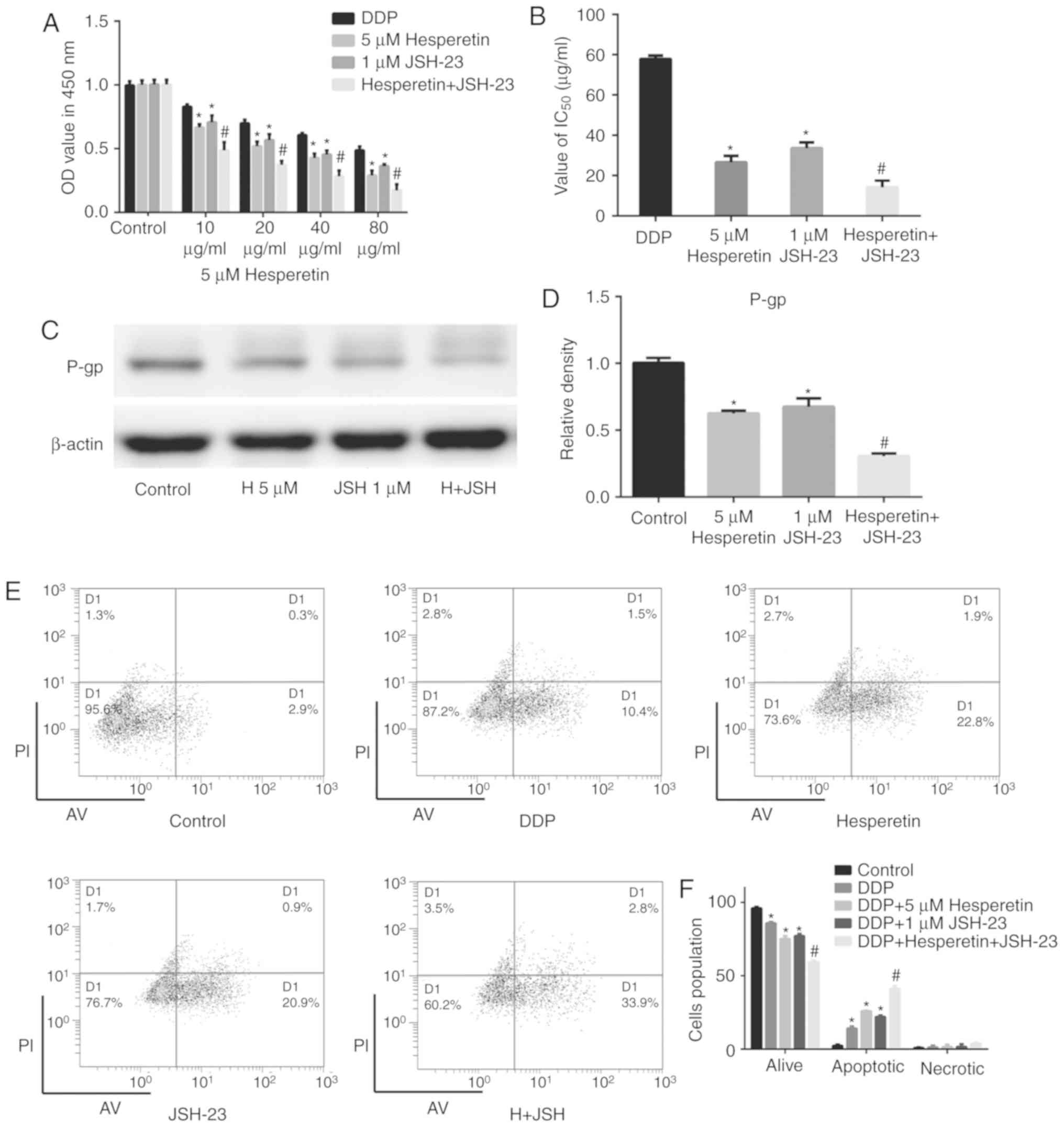
Combination treatment with hesperetin and
the NF-κB signaling pathway inhibitor JSH-23 significantly enhances
the sensitivity of A549/DDP cells to DDP
The results mentioned above demonstrated that
hesperetin attenuated the expression of P-gp by inhibiting the
activation of the NF-κB signaling pathway, thereby increasing the
sensitivity of A549/DDP cells to DDP. To investigate the
therapeutic value of the combination of hesperetin with other
therapeutic drugs for the treatment of lung cancer, A549/DDP cells
were treated with hesperetin alone or in combination with JSH-23.
DDP-treated cells were used as the positive control and untreated
cells were used as the negative control. Compared with the negative
control group, hesperetin or JSH-23 treatment alone significantly
enhanced the effect of DDP on A549/DDP cells (P<0.05; Fig. 7). Furthermore, compared with cells
treated with hesperetin or JSH-23 alone, the combination of
hesperetin and JSH-23 synergistically improved the effect of DDP on
A549/DDP cells (P<0.05), and the IC50 values were
also notably decreased (P<0.05; Fig. 7). The results of flow cytometry
were consistent with those of the CCK-8 assay. Western blotting
demonstrated that the combination of hesperetin and JSH-23
significantly attenuated the expression of P-gp (P<0.05;
Fig. 7).
Discussion
Despite the rapid development of novel strategies
for cancer treatment, DDP remains the most frequently used
first-line treatment for patients with lung cancer (7,29).
Patients treated with DDP frequently develop resistance, which
represents a major clinical challenge. Therefore, compounds that
can sensitize patients to chemotherapy or reverse drug resistance
may improve patient outcomes. Chinese herbs and their natural
extracts have exhibited beneficial anticancer properties by
mediating the expression of epithelial-to-mesenchymal
transition-associated markers and the expression of genes
associated with drug resistance, apoptosis and cell cycle
progression (30,31). Tangerine peel is a common Chinese
herbal medicine containing a variety of natural compounds, of which
hesperidin and its derivative, hesperetin, have exhibited antitumor
properties in vitro and in vivo (19,24,32,33). Hesperetin derived from the
catabolism of hesperidin in the intestine has been widely used and
investigated (34-36). Previous studies suggested that
hesperetin exhibits numerous beneficial biological functions,
including anti-inflammatory and antioxidant properties, and induces
apoptosis of tumor cells (37,38). In the present study, hesperetin
pretreatment affected the sensitivity of A549/DDP lung cancer cells
to DDP; thus, it was hypothesized that hesperetin may sensitize
cells to chemotherapy and may be used to reverse drug resistance in
patients with lung cancer.
In the present study, A549 and A549/DDP lung cancer
cells were treated with various concentrations of hesperetin to
determine its toxicity using a proliferation assay, and it was
demonstrated that it did not exert any toxic effects on cells when
used at <10 µM; therefore, <10 µM hesperetin
was used for all subsequent experiments to avoid its effects on
cell proliferation and apoptosis. When hesperetin was used at 0.6
and 1.25 µM, it did not result in increased cell death when
combined with DDP in A549/DDP cells. When increasing the
concentration of hesperetin to 2.5, 5 or 10 µM, the effects
were significantly improved. In vivo, tumor growth in
xenograft mouse models treated with hesperetin resulted in
significantly smaller tumors. Thus, it was preliminarily suggested
that hesperetin pretreatment increased the sensitivity of A549/DDP
cells to DDP.
The mechanism of drug resistance is a complex
adaptive process (39,40), and one of the methods by which it
manifests is by reducing the accumulation and toxicity of
chemotherapeutic drugs in cells by upregulating the expression
levels of the proteins that pump these drugs out of the cell or
detoxify the drugs, such as P-gp and GST-π (41,42). Mechanistically, hesperetin
treatment resulted in the downregulation of the MDR-associated
protein P-gp, whereas the expression levels of c-erbB-2 and GST-π
did not differ significantly. Additionally, previous studies
demonstrated that, when the NF-κB signaling pathway was activated,
p65 was phosphorylated and trans-located into the nucleus,
initiating the transcription of P-gp. Conversely, inhibition of p65
expression or its phosphorylation reduces the transcription levels
of P-gp (43,44). In the present study, the
downregulation of P-gp expression induced by hesperetin resulted in
inhibition of the phosphorylation of p65, thus preventing its
translocation to the nucleus to exert its transcription factor
effects. The effect of hesperetin on rhodamine accumulation in
A549/DDP cells was determined using a rhodamine efflux assay, a
suitable research model for studying intracellular drug
accumulation (45,46). Rhodamine 123 accumulation was
found to be lower in A549/DDP cells (lower fluorescence values) in
the absence of hesperetin, whereas hesperetin pretreatment
significantly increased the accumulation of rhodamine 123,
suggesting that hesperetin enhanced the sensitivity of A549/DDP
cells to DDP.
The results of the present study demonstrated that
hesperetin downregulated the expression of P-gp by inhibiting the
activation of the NF-κB signaling pathway, thereby increasing the
accumulation of chemotherapeutic drugs in tumor cells and enhancing
the toxic effects on cancer cells. Therefore, cells were treated
with the NF-κB signaling pathway inhibitor JSH-23, which
specifically inhibits translocation of p65 into the nucleus
(47,48). The results demonstrated that
JSH-23 treatment significantly enhanced the toxic effects of DDP on
A549/DDP cells by decreasing its IC50 concentration.
When the cells were pretreated with JSH-23 and hesperetin in
combination, the toxic effects of DDP on A549/DDP cells were
significantly increased compared with those in cells treated with
JSH-23 or hesperetin alone. Furthermore, compared with the group
pretreated with JSH-23 or hesperetin alone, co-treatment of cells
with JSH-23 and hesperetin significantly decreased the expression
of P-gp and significantly increased apoptosis, suggesting that
hesperetin enhanced the chemosensitivity of drug-resistant cells
when used in combination with other drugs.
Taken together, the results suggested that
hesperetin increases the sensitivity of lung cancer A549/DDP cells
to DDP through downregulation of the phosphorylation of IκB, thus
inhibiting the phosphorylation of p65 and its translocation to the
nucleus and reducing the transcription and translation of P-gp.
Hesperetin sensitized tumor cells to chemotherapeutic drugs,
providing a theoretical basis for its application as an adjuvant
treatment in the clinical setting.
Funding
The present study was supported in part by Joint
Funds for the Innovation of Science and Technology from Fujian
Province (grant no. 2017Y9127).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WK and ZY wrote the manuscript; ZY and GL designed
and supervised the study; WK, XL, YC, XW, ZZ, WW and SW performed
the experiments; WK, XL and YC analyzed and interpreted the
experimental data; all the authors discussed the results and
commented on the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All mouse experiments were approved by the Animal
Care and Use Committee of 900 Hospital of the Joint Logistics Team
and carried out in accordance with the Guide for the Care and Use
of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Girard L, Rodriguez-Canales J, Behrens C,
Thompson DM, Botros IW, Tang H, Xie Y, Rekhtman N, Travis WD,
Wistuba II, et al: An expression signature as an aid to the
histologic classification of non-small cell lung cancer. Clin
Cancer Res. 22:4880–4889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nizzoli R, Tiseo M, Gelsomino F,
Bartolotti M, Majori M, Ferrari L, De Filippo M, Rindi G, Silini
EM, Guazzi A and Ardizzoni A: Accuracy of fine needle aspiration
cytology in the pathological typing of non-small cell lung cancer.
J Thorac Oncol. 6:489–493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar
|
|
5
|
Watanabe SI, Nakagawa K, Suzuki K,
Takamochi K, Ito H, Okami J, Aokage K, Saji H, Yoshioka H, Zenke Y,
et al: Neoadjuvant and adjuvant therapy for Stage III non-small
cell lung cancer. Jpn J Clin Oncol. 47:1112–1118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pöttgen C, Eberhardt W, Stamatis G and
Stuschke M: Definitive radiochemotherapy versus surgery within
multimodality treatment in stage III non-small cell lung cancer
(NSCLC)-a cumulative meta-analysis of the randomized evidence.
Oncotarget. 8:41670–41678. 2017. View Article : Google Scholar
|
|
7
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, et al: Systemic therapy for stage IV non-small-cell lung
cancer: American society of clinical oncology clinical practice
guideline update. J Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rancoule C, Guy JB, Vallard A, Ben Mrad M,
Rehailia A and Magné N: 50th anniversary of cisplatin. Bull Cancer.
104:167–176. 2017.In French. View Article : Google Scholar
|
|
9
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang K, Wang X and Wang H: Effect and
mechanism of Src tyrosine kinase inhibitor sunitinib on the
drug-resistance reversal of human A549/DDP cisplatin-resistant lung
cancer cell line. Mol Med Rep. 10:2065–2072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao C, Jiang J, Tu Y, Ye S, Du H and Zhang
Y: β-elemene reverses the drug resistance of A549/DDP lung cancer
cells by activating intracellular redox system, decreasing
mitochondrial membrane potential and P-glycoprotein expression, and
inducing apoptosis. Thorac Cancer. 5:304–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv J and Tian Y: Effect of Src tyrosine
kinase inhibition on the drug-resistance as well as MDR1 and LRP
expression of the human cis-platinum-resistant lung cancer cell
line A549/DDP. Zhongguo Fei Ai Za Zhi. 15:501–506. 2012.In Chinese.
PubMed/NCBI
|
|
13
|
Zhong Y, Lee K, Deng Y, Ma Y, Chen Y, Li
X, Wei C, Yang S, Wang T, Wong NJ, et al: Arctigenin attenuates
diabetic kidney disease through the activation of PP2A in
podocytes. Nat Commun. 10:45232019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner L, Cramer H, Klose P, Lauche R,
Gass F, Dobos G and Langhorst J: Herbal medicine for cough: A
systematic review and meta-analysis. Forsch Komplementmed.
22:359–368. 2015.
|
|
15
|
Wong YK, Xu C, Kalesh KA, He Y, Lin Q,
Wong WSF, Shen HM and Wang J: Artemisinin as an anticancer drug:
Recent advances in target profiling and mechanisms of action. Med
Res Rev. 37:1492–1517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Safarzadeh E, Sandoghchian Shotorbani S
and Baradaran B: Herbal medicine as inducers of apoptosis in cancer
treatment. Adv Pharm Bull. 4(Suppl 1): 421–427. 2014.PubMed/NCBI
|
|
17
|
Roohbakhsh A, Parhiz H, Soltani F, Rezaee
R and Iranshahi M: Molecular mechanisms behind the biological
effects of hesperidin and hesperetin for the prevention of cancer
and cardiovascular diseases. Life Sci. 124:64–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia R, Sheng X, Xu X, Yu C and Lu H:
Hesperidin induces apoptosis and G0/G1 arrest in human non-small
cell lung cancer A549 cells. Int J Mol Med. 41:464–472. 2018.
|
|
19
|
Xia R, Xu G, Huang Y, Sheng X, Xu X and Lu
H: Hesperidin suppresses the migration and invasion of non-small
cell lung cancer cells by inhibiting the SDF-1/CXCR-4 pathway. Life
Sci. 201:111–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bodduluru LN, Kasala ER, Barua CC, Karnam
KC, Dahiya V and Ellutla M: Antiproliferative and antioxidant
potential of hesperetin against benzo(a)pyrene-induced lung
carcinogenesis in Swiss albino mice. Chem Biol Interact.
242:345–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamaraj S, Anandakumar P, Jagan S,
Ramakrishnan G and Devaki T: Modulatory effect of hesperidin on
benzo(a)pyrene induced experimental lung carcinogenesis with
reference to COX-2, MMP-2 and MMP-9. Eur J Pharmacol. 649:320–327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamaraj S, Anandakumar P, Jagan S,
Ramakrishnan G and Devaki T: Hesperidin attenuates mitochondrial
dysfunction during benzo(a)pyrene-induced lung carcinogenesis in
mice. Fundam Clin Pharmacol. 25:91–98. 2011. View Article : Google Scholar
|
|
23
|
Kamaraj S, Ramakrishnan G, Anandakumar P,
Jagan S and Devaki T: Antioxidant and anticancer efficacy of
hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice.
Invest New Drugs. 27:214–222. 2009. View Article : Google Scholar
|
|
24
|
Wolfram J, Scott B, Boom K, Shen J, Borsoi
C, Suri K, Grande R, Fresta M, Celia C, Zhao Y, et al: Hesperetin
liposomes for cancer therapy. Curr Drug Deliv. 13:711–719. 2016.
View Article : Google Scholar
|
|
25
|
El Daibani AA, Xi Y, Luo L, Mei X, Zhou C,
Yasuda S and Liu MC: Sulfation of hesperetin, naringenin and
apigenin by the human cytosolic sulfotransferases: A comprehensive
analysis. Nat Prod Res. 6:1–7. 2018. View Article : Google Scholar
|
|
26
|
Aranganathan S and Nalini N: Efficacy of
the potential chemopreventive agent, hesperetin (citrus flavanone),
on 1,2-dimethylhydrazine induced colon carcinogenesis. Food Chem
Toxicol. 47:2594–2600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sivagami G, Vinothkumar R, Bernini R,
Preethy CP, Riyasdeen A, Akbarsha MA, Menon VP and Nalini N: Role
of hesperetin (a natural flavonoid) and its analogue on apoptosis
in HT-29 human colon adenocarcinoma cell line-a comparative study.
Food Chem Toxicol. 50:660–671. 2012. View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Heist RS: First-line systemic therapy for
non-small cell lung cancer. Hematol Oncol Clin North Am. 31:59–70.
2017. View Article : Google Scholar
|
|
30
|
Zhang XW, Liu W, Jiang HL and Mao B:
Chinese herbal medicine for advanced non-small-cell lung cancer: A
systematic review and meta-analysis. Am J Chin Med. 46:923–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li TM, Yu YH, Tsai FJ, Cheng CF, Wu YC, Ho
TJ, Liu X, Tsang H, Lin TH, Liao CC, et al: Characteristics of
Chinese herbal medicine usage and its effect on survival of lung
cancer patients in Taiwan. J Ethnopharmacol. 213:92–100. 2018.
View Article : Google Scholar
|
|
32
|
Byun EB, Kim HM, Song HY and Kim WS:
Hesperidin structurally modified by gamma irradiation induces
apoptosis in murine melanoma B16BL6 cells and inhibits both
subcutaneous tumor growth and metastasis in C57BL/6 mice. Food Chem
Toxicol. 127:19–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elango R, Athinarayanan J, Subbarayan VP,
Lei DKY and Alshatwi AA: Hesperetin induces an apoptosis-triggered
extrinsic pathway and a p53- independent pathway in human lung
cancer H522 cells. J Asian Nat Prod Res. 20:559–569. 2018.
View Article : Google Scholar
|
|
34
|
Chen X, Wei W, Li Y, Huang J and Ci X:
Hesperetin relieves cisplatin-induced acute kidney injury by
mitigating oxidative stress, inflammation and apoptosis. Chem Biol
Interact. 308:269–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Q, Miao Z, Wang R, Yang J and Zhang D:
Hesperetin induces apoptosis in human glioblastoma cells via p38
MAPK activation. Nutr Cancer. Jul 11–2019.Epub ahead of print.
|
|
36
|
Shokri Afra H, Zangooei M, Meshkani R,
Ghahremani MH, Ilbeigi D, Khedri A, Shahmohamadnejad S, Khaghani S
and Nourbakhsh M: Hesperetin is a potent bioactivator that
activates SIRT1-AMPK signaling pathway in HepG2 cells. J Physiol
Biochem. 75:125–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mary Lazer L, Sadhasivam B, Palaniyandi K,
Muthuswamy T, Ramachandran I, Balakrishnan A, Pathak S, Narayan S
and Ramalingam S: Chitosan-based nano-formulation enhances the
anticancer efficacy of hesperetin. Int J Biol Macromol.
107:1988–1998. 2018. View Article : Google Scholar
|
|
38
|
Li WX, Chen X, Yang Y, Huang HM, Li HD,
Huang C, Meng XM and Li J: Hesperitin derivative-11 suppress
hepatic stellate cell activation and proliferation by targeting
PTEN/AKT pathway. Toxicology. 381:75–86. 2017. View Article : Google Scholar
|
|
39
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964.
2017.PubMed/NCBI
|
|
40
|
Senthebane DA, Rowe A, Thomford NE,
Shipanga H, Munro D, Mazeedi MAMA, Almazyadi HAM, Kallmeyer K,
Dandara C, Pepper MS, et al: The role of tumor microenvironment in
chemo-resistance: To survive, keep your enemies closer. Int J Mol
Sci. 18:E15862017. View Article : Google Scholar
|
|
41
|
Cavaco MC, Pereira C, Kreutzer B, Gouveia
LF, Silva-Lima B, Brito AM and Videira M: Evading P-glycoprotein
mediated-efflux chemoresistance using solid lipid nanoparticles.
Eur J Pharm Biopharm. 110:76–84. 2017. View Article : Google Scholar
|
|
42
|
Hermawan A, Wagner E and Roidl A:
Consecutive salinomycin treatment reduces doxorubicin resistance of
breast tumor cells by diminishing drug efflux pump expression and
activity. Oncol Rep. 35:1732–1740. 2016. View Article : Google Scholar
|
|
43
|
Feng Q, Yang W, Gao Z, Ruan X and Zhang Y:
Up-regulation of P-gp via NF-kB activation confers protection
against oxidative damage in the retinal pigment epithelium cells.
Exp Eye Res. 181:367–373. 2019. View Article : Google Scholar
|
|
44
|
Shi Y, Wang SY, Yao M, Sai WL, Wu W, Yang
JL, Cai Y, Zheng WJ and Yao DF: Chemosensitization of HepG2 cells
by suppression of NF-kappaB/p65 gene transcription with
specific-siRNA. World J Gastroenterol. 21:12814–12821. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim M, Cooper DD, Hayes SF and Spangrude
GJ: Rhodamine-123 staining in hematopoietic stem cells of young
mice indicates mitochondrial activation rather than dye efflux.
Blood. 91:4106–4117. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jancis EM, Chen HX, Carbone R, Hochberg RB
and Dannies PS: Rapid stimulation of rhodamine 123 efflux from
multidrug-resistant KB cells by progesterone. Biochem Pharmacol.
46:1613–1619. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Q, Dong X, Li N, Wang Y, Guan X, Lin
Y, Kang J, Zhang X, Zhang Y, Li X and Xu T: JSH-23 prevents
depressive-like behaviors in mice subjected to chronic mild stress:
Effects on inflammation and antioxidant defense in the hippocampus.
Pharmacol Biochem Behav. 169:59–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kumar A, Negi G and Sharma SS: JSH-23
targets nuclear factor-kappa B and reverses various deficits in
experimental diabetic neuropathy: Effect on neuroinflammation and
antioxidant defence. Diabetes Obes Metab. 13:750–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|