Introduction
Exendin-4 (EX-4) is widely used for the treatment of
type 2 diabetes worldwide (1).
There is an increasing evidence that EX-4 has multiple influences
on β-cells (2-4). EX-4 not only promotes the
proliferation of β-cells (5) and
insulin secretion (6), suppresses
appetite (7) and reduces body
weight (BW) (8), but also has
additional protective effects, such as inhibiting inflammation
(9,10), protecting cardiomyocytes via
improvement of mitochondrial function (11) and improving non-alcoholic
steatohepatitis by regulating hepatic fatty acid metabolism
(12). Therefore, it is important
to further explore the other protective effects of EX-4.
At present, metabolic inflammatory injury is
considered as an important mechanism in lipotoxicity-induced β-cell
apoptosis; however, no targeted interventions have yet been
developed. It is well known that toll-like receptor 4 (TLR4) is a
key factor in the innate immune activation of the inflammatory
signaling pathway, acting as an important mediator of
lipotoxicity-induced inflammation (13). To date, TLR4 has been confirmed to
be expressed in β-cells (14). A
previous study showed that overactivation of TLR4 induces oxidative
stress and leads to dysfunction in insulin secretion (15). Previous experiments demonstrated
that lipotoxicity directly activates the TLR4-JNK signaling pathway
and increases oxidative stress in islet β-cells, inducing insulin
secretion and apoptosis in β-cells (16). A previous study demonstrated that
inhibition of TLR4 could mitigate the damage caused by oxidative
stress in cardiomyocytes (17),
the liver (18) and the kidneys
(19). However, whether
inhibition of TLR4 activity in β-cells is beneficial for inhibiting
lipotoxicity-induced oxidative stress in β-cells requires further
exploration.
A number of scholars reported that EX-4 protects
β-cells from lipotoxicity (20)
and inhibits lipotoxicity-induced oxidative stress in β-cells
(21). However, whether the
protective effects of EX-4 on β-cells are related to the inhibition
of metabolic inflammation by TLR4 signaling has not yet been
elucidated. In addition, it has been confirmed that EX-4 has an
inhibitory effect on TLR4 and oxidative stress in other systems.
For example, Yang et al (22) reported the beneficial influences
of EX-4 on blood vessels, which were partially attributed to the
inhibition of oxidative stress and inflammation. Besides, it has
been suggested that the anti-inflammatory effect of EX-4 is related
to TLR4.
The present study aimed to explore the relationship
between the protective effects of EX-4 on lipotoxicity-induced
oxidative stress via the TLR4/NF-κB signaling pathway in β-cells,
by using βTC6 cells, high-fat diet-induced obese rats and TLR4
truncation rats as subjects. For the first time, to the best of our
knowledge, it was reported that EX-4 inhibited apoptosis,
dysfunction and lipotoxicity-induced oxidative stress in β-cells by
inhibiting metabolic and inflammatory pathways.
Materials and methods
Cell culture
The mouse islet cell line βTC6 (American Type
Culture Collection) was cultured in DMEM (4.5 g/l glucose; Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 2 mmol/l L-glutamine, 10 mmol/l
HEPES, 100 U/ml penicillin and 100 µg/ml streptomycin
(complete medium). Cells were incubated in the presence of 5%
CO2 at 37°C. According to the growth of the cells and
the pH value of the medium, the medium was changed once every 1-2
days. Cells at passages 15-18 were used for further experiments.
The cells were seeded at a density of 3×105
cells/ml.
Cell intervention
Preparation of medium for treatment
with palmitic acid (PA)
Referring to the method described by Ke et al
(23), the process was as
follows: 512 mg PA (Sigma-Aldrich; Merck KGaA; molecular mass,
256.42) was dissolved in 10 ml absolute ethanol, and then titrated
with 0.1 mol/l NaOH (10 ml) at 70°C. After that, 10 ml PA mixture
was added to 190 ml 10% BSA (Sigma-Aldrich; Merck KGaA) at 55°C to
form a complex at a concentration of 5 mmol/l. The stock solution
was filter-sterilized and stored at −20°C. A control solution,
containing 5% ethanol and 9.5% BSA, was similarly prepared.
PA or H2O2
intervention
βTC6 cells were cultured in 6-well plates for 24 h
after reaching 50% confluence. The cell culture medium was removed,
and the cells were washed twice with PBS and then treated with 0.5
mmol/l PA (Sigma-Aldrich; Merck KGaA) or 100 µmol/l
H2O2 with complete medium for 24 h. Cells
were treated with BSA/ethanol in complete medium without PA for 24
h as controls (Fig. S1).
EX-4 intervention
In the current study, βTC6 cells were treated with
0.5 mmol/l PA or 100 µmol/l H2O2 for
24 h, and then exposed to different concentrations (25, 50 and 100
nmol/l) of EX-4 with complete medium [dissolved in 0.1% DMSO (v/v);
ProSpec-Tany TechnoGene, Ltd.] for 72 h. PA-induced or
H2O2-induced cells without EX-4, and
non-induced cells exposed to EX-4, were used as controls.
Protein agonist intervention
In the present study, βTC6 cells were pretreated
with 10 mg/l TLR4 agonist [lipopolysaccharide (LPS), dissolved in
0.1% DMSO (v/v); Sigma-Aldrich; Merck KGaA) or 50 nmol/l NF-κB
agonist [tumor necrosis factor-α (TNF-α), dissolved in 0.1% DMSO
(v/v); Sigma-Aldrich; Merck KGaA] for 24 h before being exposed to
100 nmol/l EX-4 with complete medium for 72 h. Cells exposed to
LPS, TNF-α or complete medium alone were used as controls.
TLR4 small interfering (si)RNA and TLR4
cloning in βTC6 cells
Silencing with TLR4 siRNA was performed based on a
previously described method (16). The GV492 vector carrying mouse
TLR4 cDNA was supplied by Shanghai GenePharma Co., Ltd. The primers
used for the synthesis of TLR4 cDNA were as follows: TLR4
(24379-1)-P1 (5′-3′), AGG TCG ACT CTA GAG GAT CCC GCC ACC ATG ATG
CCT CCC TGG CTC CTG GCT AGG; and TLR4 (24379-1)-P2 (5′-3′), TCC TTG
TAG TCC ATA CCG GTC CAA GTT GCC GTT TCT TGT TC T TC (the PCR
results are shown in Fig. S2).
The cells were seeded at a density of 3×105 cells/ml.
The RNAi lentiviral vector or TLR4-expressing lentiviral vector was
diluted to 1×108 IU/ml, and then directly diluted in
DMEM + 10% FBS with 2 mmol/L L-glutamine at a multiplicity of
infection (MOI) of 50. After 10-12 h of incubation in the presence
of 5% CO2 at 37°C, the transduction medium (the 50 MOI
viral solution + DMEM + 10% FBS + 2 mmol/l L-glutamine) was
replaced with fresh complete medium (DMEM + 10% FBS + 2 mmol/l
L-glutamine), and then the cells were incubated for 72 h. The
expression level of TLR4 was detected by western blotting.
Glucose-stimulated insulin secretion
According to a previously described method (16), the treated βTC6 cells were washed
twice with glucose-free Krebs-Ringer bicarbonate buffer (KRBB, pH
7.4; KCl 4.8 mmol/l, KH2PO4 1.2 mmol/l, NaCl
129.0 mmol/l, NaHCO3 5.0 mmol/l, CaCl2 1.0
mmol/l, MgSO4 1.2 mmol/l, HEPES 10.0 mmol/l, BSA 0.1%)
and preincubated at 37°C for 30 min with glucose-free KRBB. Cells
were then washed once with glucose-free KRBB, and incubated for 60
min at 37°C in KRBB containing 5.6 or 20 mmol/l glucose. Aliquots
of supernatant were collected and assessed using an ELISA kit
(Cusabio Technology, LLC; cat. no. P01325).
Animal experiments
A total of 30 specific pathogen-free-grade male
Sprague-Dawley (SD) rats (5-6 weeks old; weighing 220±10 g; Animal
Experimental Center of Fujian Medical University, Fujian, China)
and 30 male TLR4 truncation SD rats (CRISPR/Cas9 gene targeting;
5-6 weeks old; weighing 220±10 g; TLR4trun/trun,
Shanghai GenePharma Co., Ltd.) were randomly selected. Construction
of the TLR4 truncation rats is described in Data S1. The rats had
free access to food and water during the whole experimental period.
The rats were housed in an intelligent artificial climate box
(RXZ-380C; Ningbo Jiangnan Instrument Factory) with the temperature
maintained at 20-25°C, a 12/12 h light/dark cycle, and a relative
humidity of 55±5%. SD rats and TLR4trun/trun rats were
randomly divided into a normal diet group (n=10) and a high-fat
diet (HFD) group (n=20). The HFD included 60% of the calories from
fat, 20% of the calories from protein and 20% of the calories from
carbohydrate (Research Diets, Inc.; cat. no. D12492; ingredients
listed in Table SI). The HFD group was randomly divided into two
subgroups after 16 weeks; one group was subcutaneously given
exenatide (EXE) at a dose of 5 µg/kg·day and another group
was subcutaneously given the same amount of normal saline for 16
weeks. Additionally, BW and body length (BL) were recorded weekly
during the study and Lee's index was calculated according to the
following formula: . The study was approved by the
Biomedical Research Ethics Committee of the First Affiliated
Hospital of Fujian Medical University.
Testing of biochemical indicators
The control and experimental rats were euthanized,
and the abdominal aorta blood was collected and centrifuged in
1,500 × g for 10 min at room temperature after being maintained at
room temperature for 30 min. The serum was then stored in a
refrigerator at −80°C for further analysis. The levels of insulin
and free fatty acid (FFA) were detected by ELISA (Cusabio
Technology, LLC; cat. no. PQ8BM4E6), and the levels of fasting
blood glucose (FBG), total cholesterol (TCHO), triglyceride (TG)
and low-density lipoprotein (LDL) were measured using an automatic
biochemical analyzer, according to a previously described method
(16). Homeostatic model
assessment of insulin resistance (HOMA-IR) was calculated as
follows: (FBG x fasting blood insulin)/22.5.
Analysis of apoptosis by TUNEL assay
Detection of apoptosis of βTC6
cells
The TUNEL assay was undertaken using a dead-End™
Fluorometric TUNEL System (Promega Corporation). Paraformaldehyde
solution (4%; pH 7.4) was used for fixing at 4°C for 30 min. In
addition, both apoptotic and non-apoptotic cells were stained red
with PI. Fluorescein-12-dUTP (10 µg/ml; incubated at 37°C
for 60 min) incorporation resulted in localized green fluorescence
within the nucleus of apoptotic cells only. PI was used at a
concentration of 1 µg/ml, and incubated for 30 min at room
temperature. Since the PI dye also binds to RNA at the same time,
the cells were digested with RNase (50 units of RNase for 15 min at
37°C or 30 min at room temperature) before PI staining. The
detection of apoptosis of islet β-cell by flow cytometry in rats
was performed according to previously described methods (24).
Detection of apoptosis of pancreatic
tissues
Pancreatic tail tissue sections were fixed in 4%
paraformaldehyde (pH 7.4) for 24 h at room temperature and
paraffin-embedded. Sections were dewaxed according to a
conventional method: Sections with a thickness of 4 µm were
immersed in xylene twice for 5 min, and then were soaked in 100,
95, 85 and 70% ethanol for 5 min, followed by PBS three times for 3
min. After permeabilization, the sections were washed with 3%
H2O2-methanol solution for 15 min.
Afterwards, prepared proteinase K (100 µl) was added into
each sample and reacted at 37°C for 30 min, followed by the
addition of 100 µl of fluorescein isothiocyanate
(FITC)-labeled streptavidin (FITC-SA) solution to each sample. The
reaction was incubated at 37°C for 1 h in the dark, followed by
washing, counterstaining with 100 µl (DAPI; 3 µg/ml)
and further washing at room temperature. Finally, the samples were
observed by fluorescence microscopy (10×40 magnification).
Protein extraction and western
blotting
Protein extraction
Whole-cell and nuclear extracts were prepared for
western blot analysis (25).
Islets were isolated from the pancreas of each SD rat (26). The cells and pancreatic tissues
were added to RIPA buffer (Beyotime Institute of Biotechnology),
containing 1 mmol/l PMSF, and lysed on ice for 30 min. The cells
and tissues were centrifuged at 12,900 × g for 10 min at 4°C, the
supernatant was transferred to a new EP tube, 15 µl
supernatant was left for the determination of protein
concentration, and the remainder was mixed with 5X SDS-PAGE sample
loading buffer. The protein was thoroughly denatured in a 95-100°C
water bath for 5 min and then stored at -20°C.
Western blotting
The protein concentration was detected by BCA assay.
SDS-PAGE analysis of proteins before and after treatment with 50 mM
DTT was performed on a 4-10% gradient gel containing 0.1% SDS
(Laemmli system). Preparations of the proteins (30 µg) were
incubated in a buffer containing 50 mM Tris-HCl (pH 6.8), 1% SDS,
10% glycerol, 0.001% bromophenol blue and 10 mM EDTA, with or
without 50 mM DTT, for 16 min at 100°C, and then applied to the
gel. Then the protein sample was transferred onto a PVDF membrane
(Invitrogen; Thermo Fisher Scientific, Inc.). TBS was transferred
into the upper membrane, and blocking solution (skimmed milk; 5%;
Beijing Dingguo Changsheng Biotechnology Co., Ltd.) was then added
into the wet PVDF membrane for 1 h at room temperature. After that,
anti-TLR4 (1:1,000; Abcam; cat. no. ab13556), anti-NF-κB p65
(1:1,000; Cell Signaling Technology, Inc.; cat. no. 8242), anti-P47
phox (1:200; Santa Cruz Biotechnology, Inc.; cat. no. NB100-790),
anti-GAPDH antibody (1:3,000; Abcam; cat. no. EPR1689) or
anti-β-actin (1:200; Santa Cruz Biotechnology, Inc.; cat. no.
932715) were added, and then incubated at 4°C overnight. The
membrane was incubated with horseradish peroxidase-labeled
secondary antibody (1:5,000; Beijing Dingguo Changsheng
Biotechnology Co., Ltd.; cat. no. IH-0011) for 1 h at room
temperature. The gel was visualized and analyzed using JESS
(ProteinSimple) and the images were then saved.
Detection of reactive oxygen species
(ROS)
βTC6 cells and frozen sections of pancreatic tissues
were assessed using a ROS assay kit (Beyotime Institute of
Biotechnology). The βTC6 cells or pancreatic tissues were incubated
in 10 µmol/l dichlorodihydro-fluorescein diacetate for 20
min at 37°C in 5% CO2. Confocal laser scanning
microscopy was used to directly observe the intracellular ROS
levels as described previously (27).
Immunof luorescence
Each group of pancreatic paraffin-embedded sections
was dewaxed according to a conventional method, hydrated, and then
the sections with a thickness of 4 µm were immersed in
xylene twice for 5 min. The sections were then soaked in 100, 95,
85 and 70% ethanol for 5 min, followed by PBS three times for 3
min, and heat-induced antigen retrieval (HIAR) was performed. HIAR
was carried out in citrate buffer (pH 6) at 120°C for 15 min in the
Rapid Microwave Histoprocessor Histos Pro (Milestone Medical). To
each section, 3% H2O2-methanol solution was
added for 10 min, and then the sections were blocked with 50-100
µl goat serum (Gibco; Thermo Fisher Scientific, Inc.; cat.
no. 16210064 for 20 min at room temperature. The sections were
incubated with 50-100 µl primary antibody (1:50) [anti-TLR4
(Abcam; cat. no. ab13556), anti-NF-κB p65 (Cell Signaling
Technology, Inc.; cat. no. 8242), anti-p47phox (Santa
Cruz Biotechnology, Inc.; cat. no. NB100-790), anti-GAPDH (Abcam;
cat. no. EPR1689) or anti-β-actin (Santa Cruz Biotechnology, Inc.;
cat. no. 932715)] for 2 h at 37°C in a wet box. This was followed
by incubation of the sections with 50-100 µl secondary
antibody conjugated to FITC/TRITC (1:200; Abcam; cat. no. ab6785.)
for 1 h at 37°C in the dark. Each slice was incubated in 50-100
µl DAPI solution for 5 min at room temperature in the dark,
and then sealed with anti-extraction sealant. The expression levels
in the cells were observed under a fluorescence microscope (×40
magnification), and the three expression regions were photographed
and stored (Olympus CKX53; Olympus Corporation).
RNA extraction and reverse
transcription-semiquantitative PCR (RT-sqPCR)
Total RNA was extracted from animal tissues using
the TRIzol® reagent (Thermo Fisher Scientific, Inc.)
method. The RNA was reverse transcribed into cDNA using a
PrimeScript™ RT reagent kit (Takara Bio, Inc.) in a 10-µl RT
reaction system, at 37°C for 15 min and 85°C for 5 sec. A total of
2 µl cDNA was taken for the PCR amplification in a
25-µl PCR reaction system (Invitrogen; Thermo Fisher
Scientific, Inc.). The reaction parameters were 95°C for 5 min,
95°C for 30 sec, 56.5°C for 30 sec and 72°C for 30 sec for 35
cycles, and finally extended to 72°C for 10 min. β-actin was used
as an internal reference for the PCR reaction. The sequences of
primers were as follows: TLR4 forward primer, 5′-AGT GCT TGT GTG
GTA GAG GCA A-3′; TLR4 reverse primer, 5′-CTG CCT ACC AAA TAG AAA
CCA GG-3′; β-actin forward primer, 5′-CAC CCG CGA GTA CAA CCT
TC-3′; and β-actin reverse primer, 5′-CCC ATA CCC ACC ATC ACA
CC-3′. Next, the PCR products (5 µl/lane) were analyzed by
electrophoresis on 1.5% agarose gels (with 2.5 µl ethidium
bromide) for 30 min at 160 mV. The electrophoresis results were
collected using a computer gel scanning imaging system and analyzed
by Image Lab software 3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp.). The results are expressed as the mean ± SEM
of three independent experiments. One-way ANOVA was used in a
completely randomized design. The Bonferroni test was undertaken to
make comparisons between each two groups. To control for the
effects of blood glucose, covariance analysis was used to
investigate the effect of EXE on animal experiments (Table SII).
P<0.05 was considered to indicate a statistically significant
difference.
Results
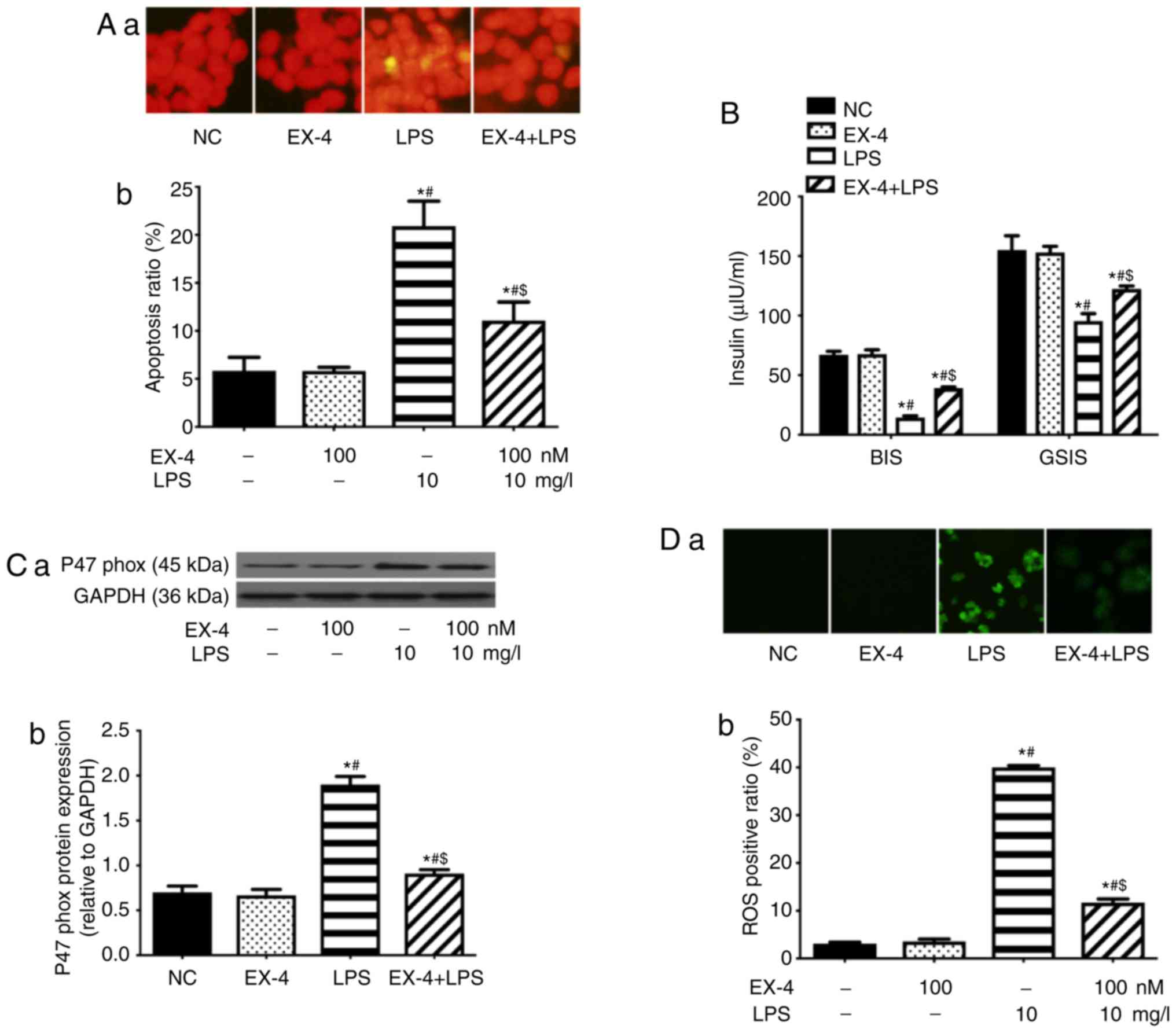
EX-4 inhibits oxidative stress in
PA-induced β-cells and the expression levels of TLR4 and NF-κB p65
subunit
A previous study showed that EX-4 improved
lipotoxicity-induced apoptosis and insulin secretion (28). According to a previously described
method, the present study observed the effects of EX-4 on
lipotoxicity-induced oxidative stress in β-cells, and the
expression levels of TLR4 and NF-κB p65 subunit. The results of the
present study revealed that EX-4 inhibited the expression levels of
TLR4 and NF-κB p65 subunit (Fig.
1A) in a concentration-dependent manner, inhibiting the
expression of p47phox (Fig. 1A) and decreasing the intracellular
level of ROS (Fig. 1B).
 | Figure 1EX-4 inhibits PA-induced oxidative
stress in β-cells and the expression levels of TLR4 and NF-κB p65
subunit in a dose-dependent manner. (A) Western blotting was
performed to detect the expression levels of p47phox,
TLR4 and NF-κB p65 subunit. (A-a) Representative western blot
images in each group. (A-b) The ratio of target protein to GAPDH.
(B) EX-4 reduced the PA-induced ROS positive ratio in β-cells.
(B-a) Representative images from fluorescent microscopy in each
group. (B-b) The collective analyses of all three independent
experiments. *P<0.05 vs. NC group (without PA and
EX-4), #P<0.05 vs. 0.5 mM PA group,
$P<0.05 vs. 0.5 mM PA + 25 nM EX-4 group,
^P<0.05 vs. 0.5 mM PA + 50 nM EX-4 group.
Magnification, ×40. EX-4, exendin-4; PA, palmitic acid; ROS,
reactive oxygen species; NC, negative control; TLR4, toll-like
receptor 4. |
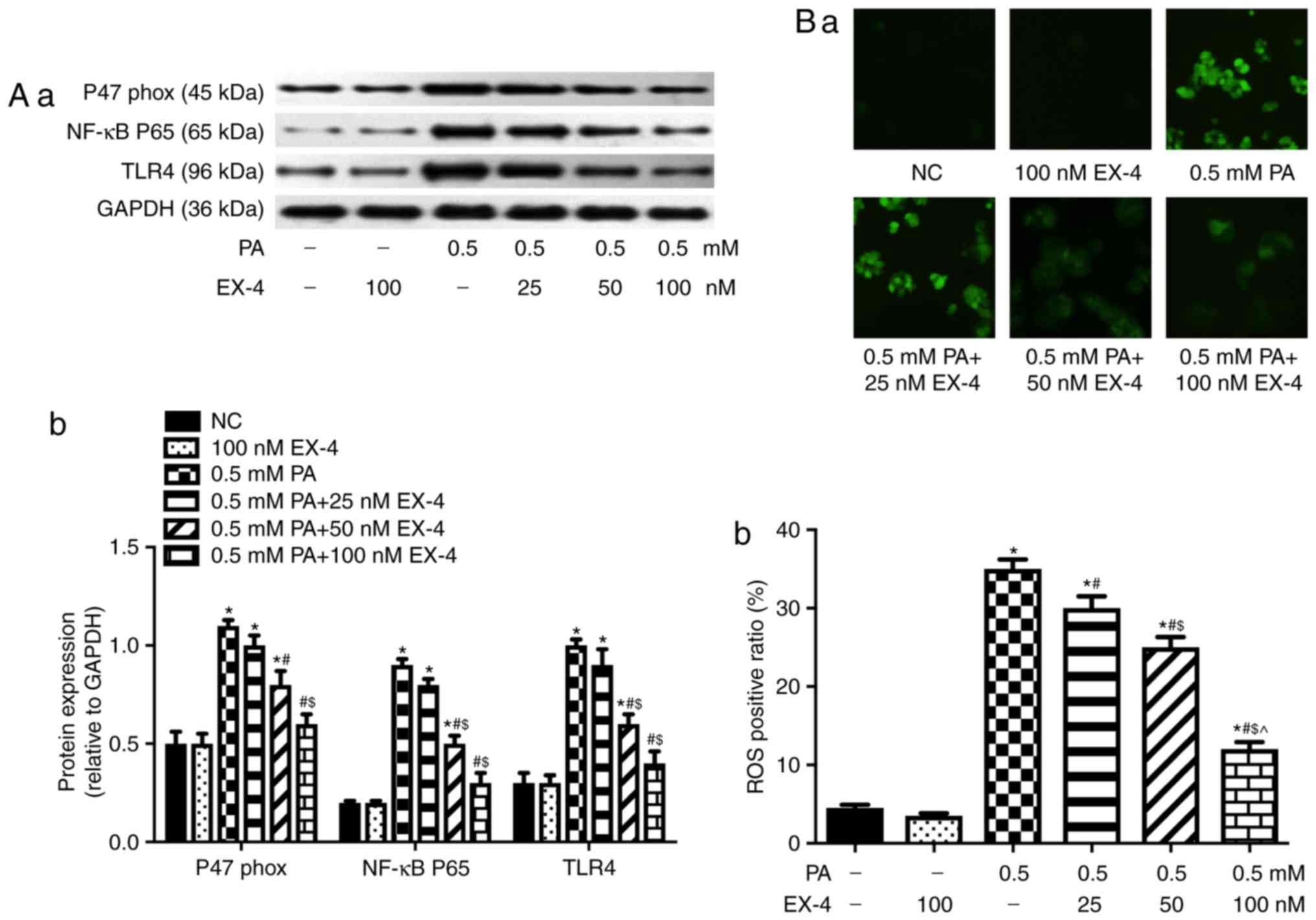
Regulation of TLR4 expression alters the
protective effects of EX-4 on lipotoxicity-induced oxidative stress
in β-cells
The aforementioned experiments showed that EX-4 can
inhibit oxidative stress in β-cells and decrease the expression
levels of TLR4 and NF-κB p65 subunit. Therefore, the present study
further assessed whether TLR4 could be involved in the protective
effects of EX-4 by lentivirus-mediated overexpression (Fig. 2A) or silencing (Fig. 2B) of TLR4 in β-cells. The results
demonstrated that upregulation of TLR4 attenuated the effect of
EX-4 on lipotoxicity-induced β-cell apoptosis (Fig. 2C), insulin secretion (Fig. 2D) and oxidative stress (Fig. 2E-G), and the opposite was observed
when the expression of TLR4 was inhibited (Fig. 2C-G).
 | Figure 2Regulation of TLR4 expression alters
the protective effects of EX-4 in PA-induced β-cells. Expression
levels of TLR4 were detected by western blotting in (A)
TLR4-overexpressing and (B) siRNA-transfected cells. (A-a and B-a)
Representative western blot images in each group. (A-b and B-b) The
ratio of target protein to GAPDH. *P<0.05 vs. NC
group, #P<0.05 vs. NC + Vector group. (C) Apoptosis
rate of β-cells. (C-a) Representative images from fluorescent
microscopy in each group. (C-b) The collective analyses of all
three independent experiments. (D) Insulin secretion.
*P<0.05 vs. NC group, #P<0.05 vs. 0.5
PA group, $P<0.05 vs. 0.5 PA + TLR4-OE group,
^P<0.05 vs. 0.5 PA + siRNA group,
&P<0.05 vs. PA + 100 EX-4 group,
§P<0.05 vs. PA + EX-4 + TLR4-OE group. Expression
level of p47phox in (E) TLR4-overexpressing cells. (E-a)
Representative western blot images in each group. (E-b) The ratio
of target protein to GAPDH. *P<0.05 vs. NC group,
#P<0.05 vs. 0.5 PA group, $P<0.05 vs.
PA + TLR4-OE group. Expression level of p47phox in (F)
siRNA-transfected cells. (F-a) Representative western blot images
in each group. (F-b) The ratio of target protein to GAPDH.
*P<0.05 vs. NC group, #P<0.05 vs. PA +
EX-4 group, $P<0.05 vs. PA + EX-4 + TLR4-OE group.
(G) ROS in β-cells was detected by immunofluorescence. (G-a)
Representative images from fluorescent microscopy in each group.
(G-b) The collective analyses of all three independent experiments.
*P<0.05 vs. NC group, #P<0.05 vs. 0.5
PA group, $P<0.05 vs. 0.5 PA + TLR4-OE group,
^P<0.05 vs. 0.5 PA + siRNA group,
&P<0.05 vs. PA + 100 EX-4 group,
§P<0.05 vs. PA + EX-4 + TLR4-OE group. Magnification,
×40. EX-4, exendin-4; PA, palmitic acid; OE, overexpression; NC,
negative control; siRNA, small interfering RNA; TLR4, toll-like
receptor 4; BIS, basal insulin secretion; GSIS, glucose-stimulated
insulin secretion. |
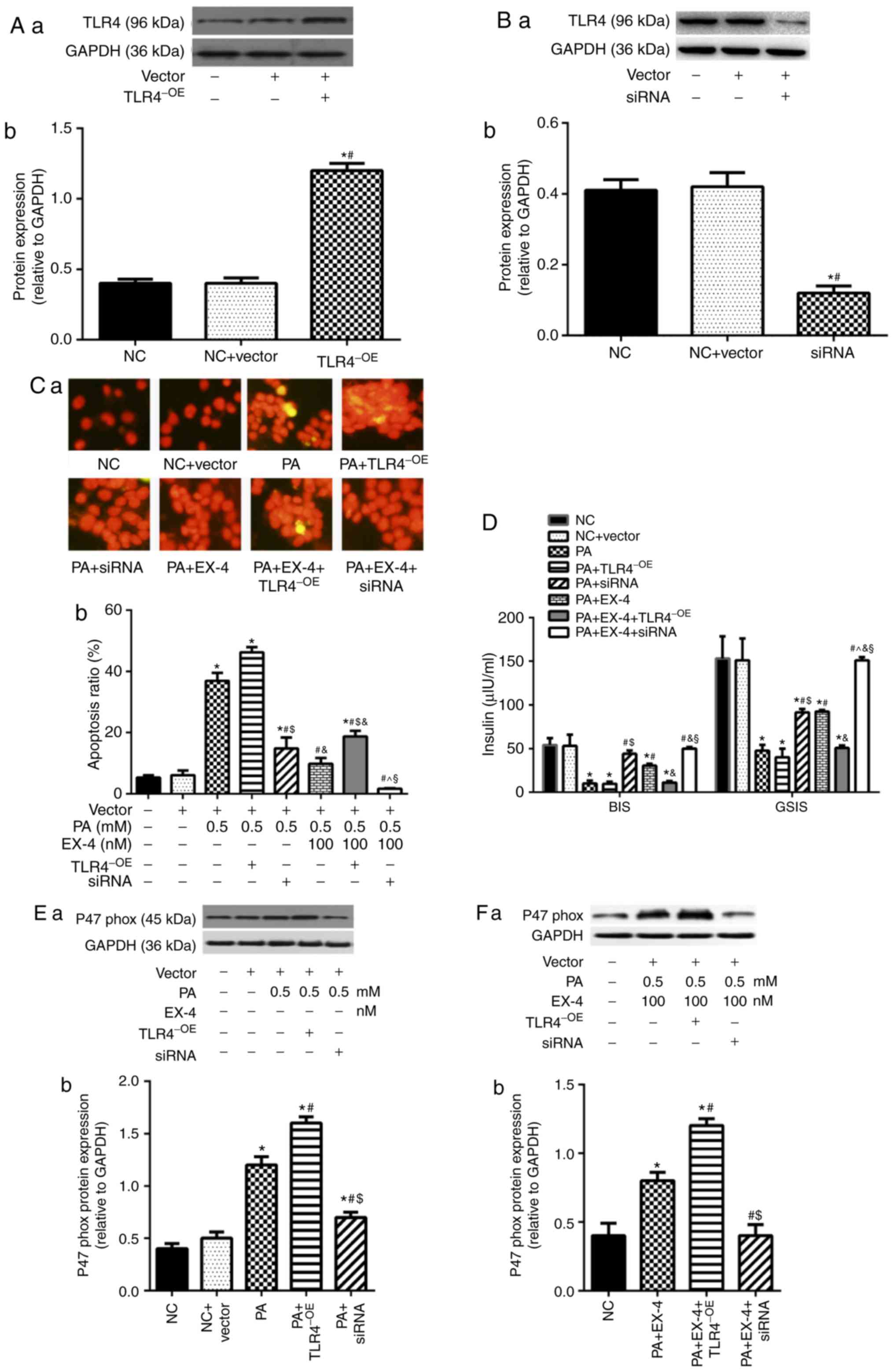
TLR4 is involved in the role of EX-4 in
attenuating H2O2-induced oxidative stress in
β-cells
The previous two parts of the study confirmed that
EX-4 was involved in the inhibition of oxidative stress in β-cells.
To further confirm the specificity of this process,
H2O2 was used to induce oxidative stress in
β-cells, followed by intervention with EX-4. The findings
demonstrated that EX-4 reduced H2O2-induced
apoptosis (Fig. 3A), increased
insulin secretion (Fig. 3B) and
inhibited oxidative stress (Fig. 3C
and D) in β-cells.
 | Figure 3TLR4 is involved in the role of EX-4
in improving H2O2-induced oxidative stress in
β-cells. (A) Apoptosis rate of β-cells. (A-a) Representative images
from fluorescent microscopy in each group. (A-b) The collective
analyses of all three independent experiments. (B) Insulin
secretion. (C) Expression level of p47phox. (C-a)
Representative western blot images in each group. (C-b) The ratio
of target protein to GAPDH. (D) Levels of ROS in β-cells. (D-a)
Representative images from fluorescent microscopy in each group.
(D-b) The collective analyses of all three independent experiments.
*P<0.05 vs. respective NC or vector group,
#P<0.05 vs. H2O2 group,
$P<0.05 vs. H2O2 + EX-4 group,
P<0.05 vs. H2O2 + EX-4 + siRNA group.
Magnification, ×40. NC, negative control; EX-4, exendin-4; siRNA,
small interfering RNA; TLR4, toll-like receptor 4; OE,
overexpression; BIS, basal insulin secretion; GSIS,
glucose-stimulated insulin secretion; ROS, reactive oxygen
species. |
Simultaneously, lentivirus-mediated overexpression
or silencing of TLR4 was used to verify the influences of TLR4
expression on the treatment with EX-4 in
H2O2-induced β-cells. The results showed that
up-regulation of TLR4 attenuated the inhibitory effect of EX-4 on
H2O2-induced apoptosis (Fig. 3A), insulin secretion (Fig. 3B), and oxidative stress (Fig. 3C and D) in β-cells. However,
silencing of TLR4 enhanced the effect of intervention with EX-4 in
H2O2-induced β-cells (Fig. 3).
EX-4 inhibits oxidative stress in β-cells
mediated by TLR4 or NF-κB
To confirm the effects of EX-4 on oxidative stress
in β-cells mediated by TLR4 or NF-κB, the efficacy of EX-4 on the
damage induced in β-cells using TLR4 agonists (LPS) or NF-κB
agonists (TNF-α) was investigated. The results demonstrated that
EX-4 decreased apoptosis (Fig. 4A and
E), restored insulin secretion (Fig. 4B and F), and inhibited oxidative
stress (Fig. 4C, D, G and H) in
β-cells, which were injured by LPS or TNF-α.
Effects of EX-4 on HFD-induced oxidative
stress in obese and TLR4trun/trun rats
The present study investigated the role of EX-4 in
lipotoxicity-induced oxidative stress on obese rats induced with an
HFD. The results uncovered that EX-4 showed no significant effect
on weight (Fig. 5A), the levels
of TCHO, TG, LDL (Fig. 5B and C)
or FFA (Fig. 5D), while it
reduced Lee's index (Fig. 5E),
HOMA-IR (Fig. 5F) and FBG
(Fig. 5G), and increased insulin
levels (Fig. 5H) in HFD rats.
Besides, EX-4 inhibited the expression levels of TLR4, NF-κB p65
subunit and p47phox (Fig.
5I), decreased cell apoptosis (Fig. 5J) and the level of ROS (Fig. 5K), and rebalanced the α- to β-cell
area ratio (α/β) in the pancreas (Fig. 5L).
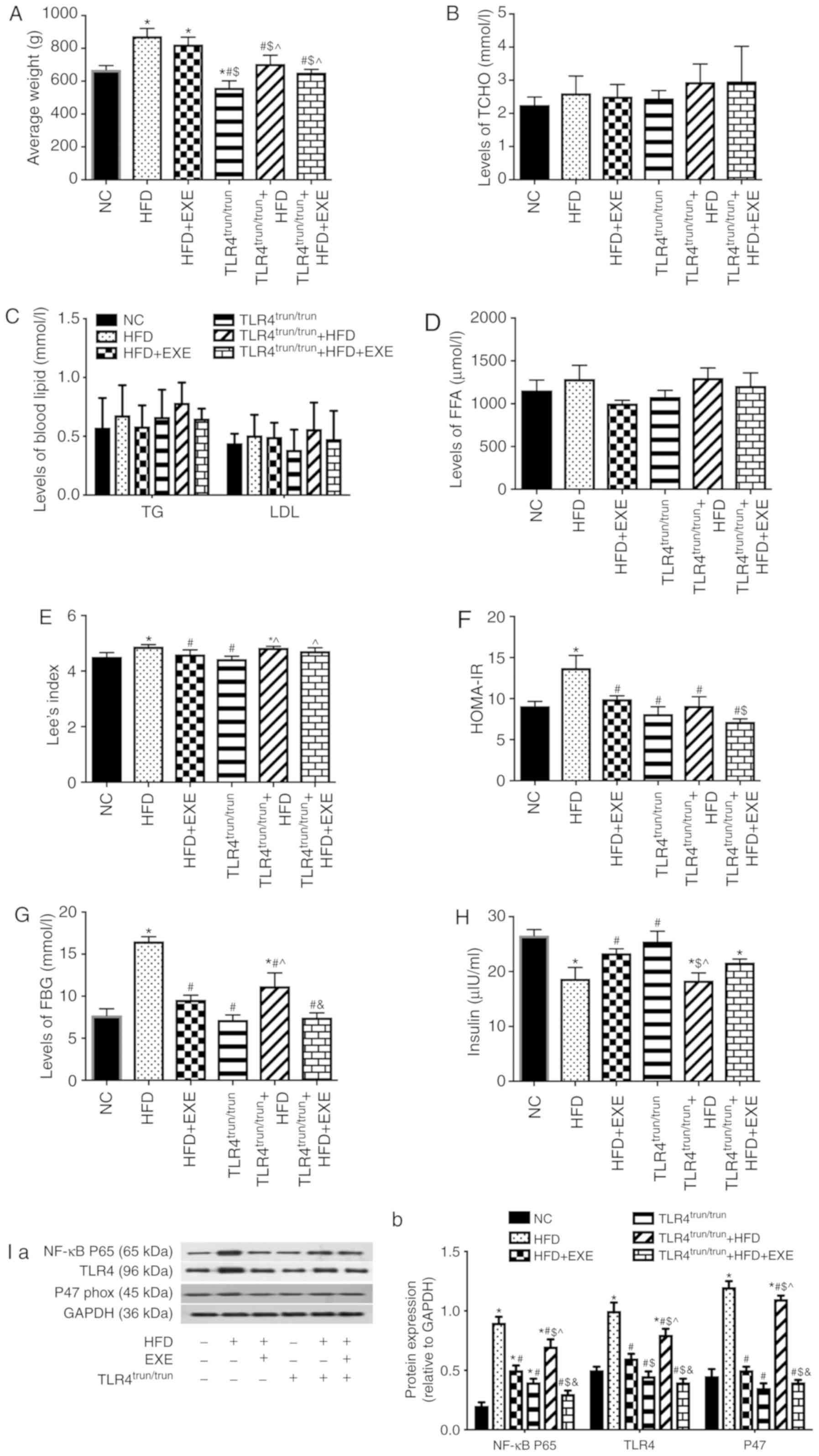 | Figure 5EXE alleviates islet oxidative stress
in obese rats, and truncation of TLR4 attenuates the damage caused
by a high-fat diet in synergy with EXE. (A) Average weight in each
group. Levels of blood lipids were measured in each group: (B)
TCHO, (C) TG and LDL, and (D) FFA. (E) Lee's index in each group.
(F) HOMA-IR. (G) Levels of FBG. (H) Insulin levels. (I) Expression
levels of NF-κB p65 subunit, TLR4 and p47phox in
pancreatic tissues. (I-a) Representative western blot images in
each group. (I-b) The ratio of target protein to GAPDH. (J)
Apoptosis in pancreatic tissue. (J-a) Representative images from
fluorescent microscopy in each group. (J-b) The collective analyses
of all three independent experiments. (K) The levels of ROS in
pancreatic tissue. (K-a) Representative images from fluorescent
microscopy in each group. (K-b) The collective analyses of all
three independent experiments. (L) Immunofluorescence images of α-
and β-cells in pancreatic tissues. (L-a) Representative images from
fluorescent microscopy in each group. (L-b) The collective analyses
of all three independent experiments.*P<0.05 vs. NC
group, #P<0.05 vs. HFD group, $P<0.05
vs. HFD + EXE group, ^P<0.05 vs.
TLR4trun/trun group, &P<0.05 vs.
TLR4trun/trun + HFD group. Magnification, ×40. EXE,
exenatide; TCHO, total cholesterol; FFA, free fatty acid; HOMA-IR,
homeostatic model assessment of insulin resistance; FBG, fasting
blood glucose; TG, triglyceride; LDL, low-density lipoprotein; HFD,
high-fat diet; TLR4, toll-like receptor 4; trun, truncated. |
To investigate the role of TLR4 in oxidative stress
induced by a HFD, the TLR4 gene was truncated in rats. Compared
with the wild-type with HFD group, truncation of TLR4 in the HFD
group led to a decrease in weight (Fig. 5A), HOMA-IR (Fig. 5F), FBG (Fig. 5G), the expression levels of TLR4,
NF-κB p65 subunit and p47phox (Fig. 5I), as well as the levels of cell
apoptosis (Fig. 5J) and ROS in
the pancreas (Fig. 5K), while
rebalanced the α/β in pancreas (Fig.
5L). Simultaneously, compared with EX-4-treated wild-type obese
rats, EX-4-treated TLR4 truncation of obese rats showed lower
levels of weight (Fig. 5A),
HOMA-IR (Fig. 5F), expression
levels of TLR4, NF-κB p65 subunit and p47phox (Fig. 5I), cell apoptosis (Fig. 5J), and the levels of ROS in the
pancreas (Fig. 5K), and
rebalanced the α/β in the pancreas (Fig. 5L).
Discussion
The present study used islet β-cells, obese SD rats
and a TLR4 truncation rat model to observe the role and mechanism
of EX-4 in lipotoxicity-induced oxidative stress in β-cells. The
results revealed that EX-4 restrained PA-induced oxidative stress
in β-cells and inhibited the expression levels of TLR4 and NF-κB
p65 subunit in a concentration-dependent manner. Simultaneously, it
was demonstrated that TLR4 was involved in the effects of EX-4 on
suppressing H2O2-induced oxidative stress in
β-cells. Moreover, EX-4 could inhibit TLR4- or NF-κB
agonist-induced oxidative stress in β-cells. Furthermore, it was
confirmed that in obese rats, EX-4 was able to reduce HOMA-IR,
rebalance the distribution of α- or β-cells, and inhibit islet cell
apoptosis, oxidative stress, and the expression levels of TLR4 and
NF-κB p65 subunit. It was also confirmed that truncation of the
TLR4 gene can delay the aforementioned damage induced by an
HFD.
The current research disclosed that EX-4 had a
direct impact on the suppression of oxidative stress in β-cells. To
date, animal and clinical trials have confirmed the intervention of
EX-4 on vascular oxidative stress (29-31). Animal experiments have also
demonstrated that liraglutide can increase the quality of β-cells
by inhibiting oxidative stress and endoplasmic stress (32). It is noteworthy that
p47phox is a major subunit of NADPH oxidase activity,
whose activity influences the function of β-cells in human
pancreatic islets (33). The
aforementioned experiments also supported the present findings.
However, the intervention of EX-4 on normal β-cells showed no
influence on NADPH oxidase and ROS. It was hypothesized that NADPH
oxidase and ROS are at very low levels under normal physiological
conditions, and that changes caused by intervention with EX-4 may
not be detectable.
At present, the pathway by which EX-4 inhibits
oxidative stress in β-cells needs further study. Wu et al
(34) reported that GLP-1
analogues can protect cardiac function by inhibiting the
ROCK/peroxisome proliferator-activated receptor-α pathway to
improve diabetic lipotoxic cardiomyopathy. A study showed that
GLP-1 analogues may exert an effect on metabolic syndrome through a
receptor-mediated mechanism (35). In the present study, it was found
that EX-4 inhibited lipotoxicity-induced oxidative stress, and
simultaneously the expression levels of TLR4 and NF-κB p65 subunit
during the experimental process. Therefore, it was hypothesized
that benign intervention with EX-4 was associated with inhibition
of the expression levels of TLR4 and NF-κB p65 subunit. There are
currently several clinical studies supporting this hypothesis.
Ceriello et al (36)
confirmed that GLP-1 analogues effectively improved endothelial
dysfunction, and inhibited inflammation and oxidative stress in
patients with type 2 diabetes. To further validate the hypothesis,
the expression of TLR4 was altered in order to observe whether TLR4
was involved in the protective effect of EX-4 in inhibiting
lipotoxicity-induced oxidative stress. The present results
demonstrated that overexpression of TLR4 attenuated the effects of
EX-4, while inhibition of TLR4 expression enhanced the influences
of EX-4. These results were also confirmed when EX-4 was
antagonized with H2O2-induced oxidative
stress in β-cells. Moreover, EX-4 was able to inhibit TLR4 or NF-κB
agonist-induced oxidative stress in β-cells. A previous study
demonstrated the ability of EX-4 to inhibit NF-κB, and thus
supported the present results (37). The results of the present study
suggested that EX-4 could improve lipotoxicity-induced oxidative
stress in β-cells by inhibiting the expression levels of TLR4 and
NF-κB p65 subunit.
However, other studies have shown that GLP-1
analogues can act as a benign intervention in cardiomyocytes
(38), the liver (39) and the kidney (40) by inhibiting inflammation or
oxidative stress. To date, the antagonistic effect of GLP-1
analogues on inflammation and oxidative stress was also confirmed
in β-cells (41). However, there
is no direct evidence that GLP-1 inhibits oxidative stress by
inhibiting the TLR4 signaling pathway. To further confirm the
present results in vitro, HFD-induced obese SD rats were
used, and it was confirmed that EX-4 can restrain
lipotoxicity-induced insulin resistance, oxidative stress, and the
expression levels of TLR4 and NF-κB p65 subunit. Furthermore, to
investigate the role of TLR4 in lipotoxicity-induced oxidative
stress and the effects of EX-4 on antagonizing the lipotoxicity,
the TLR4 gene was truncated in rats. It was found that truncation
of TLR4 amplified the effect of benign intervention with EX-4 on
obese rats, confirming that EX-4 improved hyperlipidemia-induced
oxidative stress by inhibiting the TLR4 pathway. Although the
protective effects of EX-4 in the TLR4trun/trun group
were significantly lower than those in wild-type group, there was
no significant difference between TLR4trun/trun HFD
group and TLR4trun/trun normal diet. This is due to the
substantial role of TLR4 in hyperlipidemia-induced oxidative
stress, and truncation of TLR4 can significantly inhibit the damage
induced by an HFD; besides, EX-4 showed a protective effect, mainly
by suppressing TLR4. Therefore, treatment with EX-4 led to no
significant differences in rats with truncation of the TLR4
gene.
The results of the present study revealed that EX-4
had no significant influence on weight; however, several previously
published studies showed the opposite effect (7,41-43). It is speculated that the
inconsistent results may be due to different intervention time
periods. The time recorded in the aforementioned research papers
was between 24 h and 3 weeks, and the intervention time in the
present study was 16 weeks. It was found that EX-4 can reduce the
BW of obese rats over the course of 4 weeks, while the weight was
regained in obese rats with a more prolonged duration of
intervention (Fig. S3). Thus, it
was hypothesized that obese rats would show resistance to EX-4 as
the intervention time was prolonged. To date, a study has supported
long-term intervention with EX-4 for reducing weight in obese
individuals (44).
In addition, the results of the present study
demonstrated that TLR4 protein is still expressed in truncation
rats, possibly due to the fact that the CRISPR/Cas9 system was used
for the TLR4 truncation (-178 bp +7 bp; exon -149 bp; Data S1),
without knocking out TLR4 DNA. The TLR4 protein was detected in
TLR4 truncation rats using western blot analysis, and the molecular
weight of TLR4 in TLR4 truncation rats was similar to that in
wild-type rats. It was speculated that this might be due to the
lack of specificity of the TLR4 antibody. Therefore, RT-sqPCR was
used to detect TLR4 mRNA expression, and it was found that TLR4 had
a length of 436 bp in truncation rats, demonstrating that the
truncation of the gene was successfully carried out (Fig. S4).
The present study showed that EX-4 decreased
lipotoxicity-induced insulin resistance, oxidative stress, and the
expression levels of TLR4 and NF-κB p65 subunit. Previous studies
have demonstrated that GLP-1 analogues can decrease the weight of
obese patients (45), reduce
insulin resistance (46) and
improve islet function (47).
However, obese patients are characterized by uncertain resistance
to GLP-1 analogues, which weakens the effectiveness of GLP-1
analogues in obese patients compared with those of average weight
(48). Therefore, the use of
GLP-1 as a drug for preventing diabetes in obese patients should be
further studied. Simultaneously, due to the benefits of TLR4 in
mediating metabolic inflammation and oxidative stress induced by
hyperlipidemia, TLR4 inhibitors could be used as potential
weight-loss drugs. Furthermore, the present results provide an
experimental basis for anti-inflammatory treatment in obese
patients to prevention of diabetes. However, the present study
contains certain limitations. Firstly, the mechanism of action EX-4
on TLR4 was not confirmed, and the direct or indirect interactions
between the two require further research. Secondly, the
relationship between the protective effects of EX-4 and GLP-1
analogues was not observed. Further research is therefore essential
to confirm the clinical data. Thirdly, the effects of blood glucose
fluctuations on the model animal in the present study were not
assessed. To control for the effects of blood glucose, covariance
analysis was used to investigate the effect of exenatide on the
model animals. It was found that exenatide inhibited HFD-induced
oxidative stress in β-cells, independent of blood glucose
fluctuations (Table SII). Lastly, βTC6 is a mouse islet cell line
and does not fully represent islet β cells.
In conclusion, it was found that EX-4 can inhibit
damage caused by lipotoxicity-induced oxidative stress in β-cells
through suppression of the TLR4/NF-κB signaling pathway. The
present study revealed a new mechanism by which EX-4 may inhibit
lipotoxicity-induced oxidative stress in β-cells, providing
experimental evidence for TLR4 as a therapeutic target in weight
loss and for treating oxidative stress in β-cells.
Supplementary Data
Funding
This study was supported by Science and Technology
Innovation Joint Fund Project Fujian Province (grant no.
2016Y9102), Grants from National Natural Science Foundation of
China (grant nos. 81500632 and 81870572), Natural Science
Foundation of Fujian Province (grant nos. 2019J01455 and
2015J01453), Fujian Province Higher Education Institute New Century
Research Project (grant no. 2018B049), and the Medical Innovation
Fund Project of Fujian Province (grant no. 2018-CX-23).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XS made substantial contributions to the conception
and design of the study, and was involved in drafting the
manuscript and revising it critically for important intellectual
content; LY designed the study and reviewed/edited the drafts, and
is guarantor; LL and MY were involved in the acquisition of data
and drafting the manuscript; YL and JL were involved in the
interpretation of data and drafting the manuscript. LY is the
guarantor of this work. All authors took responsibility for the
integrity of the data and the accuracy of the data analysis and
have given final approval of the version to be published and agreed
to be accountable for all aspects of the work.
Ethics approval and consent to
participate
The study was approved by the Biomedical Research
Ethics Committee of the First Affiliated Hospital of Fujian Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Hirota Y, Matsuda T, Nakajima S, Takabe M,
Hashimoto N, Nakamura T, Okada Y, Sakaguchi K and Ogawa W: Effects
of exenatide and liraglutide on postchallenge glucose disposal in
individuals with normal glucose tolerance. Endocrine. 64:43–47.
2019. View Article : Google Scholar
|
|
2
|
Gu J, Wei Q, Zheng H, Meng X, Zhang J and
Wang D: Exendin-4 promotes survival of mouse pancreatic beta-cell
line in lipotoxic conditions, through the extracellular
signal-related kinase 1/2 pathway. J Diabetes Res.
2016:52940252016. View Article : Google Scholar
|
|
3
|
Natalicchio A, Labarbuta R, Tortosa F,
Biondi G, Marrano N, Peschechera A, Carchia E, Orlando MR,
Leonardini A, Cignarelli A, et al: Exendin-4 protects pancreatic
beta cells from palmitate-induced apoptosis by interfering with
GPR40 and the MKK4/7 stress kinase signalling pathway.
Diabetologia. 56:2456–2466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh YS, Lee YJ, Kang Y, Han J, Lim OK and
Jun HS: Exendin-4 inhibits glucolipotoxic ER stress in pancreatic β
cells via regulation of SREBP1c and C/EBPβ transcription factors. J
Endocrinol. 216:343–352. 2013. View Article : Google Scholar
|
|
5
|
Wu X, Liang W, Guan H, Liu J, Liu L, Li H,
He X, Zheng J, Chen J, Cao X and Li Y: Exendin-4 promotes
pancreatic β-cell proliferation via inhibiting the expression of
Wnt5a. Endocrine. 55:398–409. 2017. View Article : Google Scholar
|
|
6
|
Natalicchio A, Biondi G, Marrano N,
Labarbuta R, Tortosa F, Spagnuolo R, D'Oria R, Carchia E,
Leonardini A, Cignarelli A, et al: Long-term exposure of pancreatic
β-cells to palmitate results in SREBP-1C-dependent decreases in
GLP-1 receptor signaling via CREB and AKT and insulin secretory
response. Endocrinology. 157:2243–2258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Choi PP, Smith WW, Xu W, Ma D,
Cordner ZA, Liang NC and Moran TH: Exendin-4 reduces food intake
via the PI3K/AKT signaling pathway in the hypothalamus. Sci Rep.
7:69362017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwasaki S, Hamada T, Chisaki I, Andou T,
Sano N, Furuta A and Amano N: Mechanism-based
pharmacokinetic/pharmacody-namic modeling of the glucagon-like
peptide-1 receptor agonist exenatide to characterize its
antiobesity effects in diet-induced obese mice. J Pharmacol Exp
Ther. 362:441–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dandona P, Ghanim H, Abuaysheh S, Green K,
Dhindsa S, Makdissi A, Batra M, Kuhadiya ND and Chaudhuri A:
Exenatide increases IL-1RA concentration and induces
Nrf-2Keap-1regulated antioxidant enzymes: Relevance to β-cell
function. J Clin Endocrinol Metab. 103:1180–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varin EM, Wojtusciszyn A, Broca C, Muller
D, Ravier MA, Ceppo F, Renard E, Tanti JF and Dalle S: Inhibition
of the MAP3 kinase Tpl2 protects rodent and human β-cells from
apoptosis and dysfunction induced by cytokines and enhances
anti-inflammatory actions of exendin-4. Cell Death Dis.
7:e20652016. View Article : Google Scholar
|
|
11
|
Chang G, Liu J, Qin S, Jiang Y, Zhang P,
Yu H, Lu K, Zhang N, Cao L, Wang Y, et al: Cardioprotection by
exenatide: A novel mechanism via improving mitochondrial function
involving the GLP-1 receptor/cAMP/PKA pathway. Int J Mol Med.
41:1693–1703. 2018.
|
|
12
|
Kawaguchi T, Itou M, Taniguchi E and Sata
M: Exendin4, a glucagonlike peptide1 receptor agonist, modulates
hepatic fatty acid composition and Δ-5desaturase index in a murine
model of nonalcoholic steatohepatitis. Int J Mol Med. 34:782–787.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SM, Choi SE, Lee JH, Lee JJ, Jung IR,
Lee SJ, Lee KW and Kang Y: Involvement of the TLR4 (Toll-like
receptor4) signaling pathway in palmitate-induced INS-1 beta cell
death. Mol Cell Biochem. 354:207–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi
M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, et al:
Saturated fatty acid and TLR signaling link β cell dysfunction and
islet inflammation. Cell Metab. 15:518–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Ge QM, Bian F, Dong Y and Huang
CM: Inhibition of TLR4 protects rat islets against
lipopolysaccharide-induced dysfunction. Mol Med Rep. 15:805–812.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen X, Yang L, Yan S, Zheng H, Liang L,
Cai X and Liao M: Fetuin A promotes lipotoxicity in β cells through
the TLR4 signaling pathway and the role of pioglitazone in
anti-lipotoxicity. Mol Cell Endocrinol. 412:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma C, Jiang Y, Zhang X, Chen X, Liu Z and
Tian X: Isoquercetin ameliorates myocardial infarction through
anti-inflammation and anti-apoptosis factor and regulating
TLR4-NF-kB signal pathway. Mol Med Rep. 17:6675–6680.
2018.PubMed/NCBI
|
|
18
|
Zhou Y, Ding YL, Zhang JL, Zhang P, Wang
JQ and Li ZH: Alpinetin improved high fat diet-induced
non-alcoholic fatty liver disease (NAFLD) through improving
oxidative stress, inflammatory response and lipid metabolism.
Biomed Pharmacother. 97:1397–1408. 2018. View Article : Google Scholar
|
|
19
|
Xu MX, Wang M and Yang WW: Gold-quercetin
nanoparticles prevent metabolic endotoxemia-induced kidney injury
by regulating TLR4/NF-κB signaling and Nrf2 pathway in high fat
diet fed mice. Int J Nanomedicine. 12:327–345. 2017. View Article : Google Scholar :
|
|
20
|
Xiang JN, Chen DL and Yang LY: Effect of
PANDER in βTC6-cell lipoapoptosis and the protective role of
exendin-4. Biochem Biophys Res Commun. 421:701–706. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo M, Tanabe K, Amo-Shiinoki K,
Hatanaka M, Morii T, Takahashi H, Seino S, Yamada Y and Tanizawa Y:
Activation of GLP-1 receptor signalling alleviates cellular
stresses and improves beta cell function in a mouse model of
Wolfram syndrome. Diabetologia. 61:2189–2201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang G, Lei Y, Inoue A, Piao L, Hu L,
Jiang H, Sasaki T, Wu H, Xu W, Yu C, et al: Exenatide mitigated
diet-induced vascular aging and atherosclerotic plaque growth in
ApoE-deficient mice under chronic stress. Atherosclerosis.
264:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke J, Wei R, Yu F, Zhang J and Hong T:
Liraglutide restores angiogenesis in palmitate-impaired human
endothelial cells through PI3K/Akt-Foxo1-GTPCH1 pathway. Peptides.
86:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen X, Yang L, Yan S, Wei W, Liang L,
Zheng H and Cai X: The effect of FFAR1 on pioglitazone-mediated
attenuation of palmitic acid-induced oxidative stress and apoptosis
in βTC6 cells. Metabolism. 63:335–351. 2014. View Article : Google Scholar
|
|
25
|
Seo JH, Lim JW and Kim H: Differential
role of ERK and p38 on NF-κB activation in helicobacter
pylori-infected gastric epithelial cells. J Cancer Prev.
18:346–350. 2013. View Article : Google Scholar
|
|
26
|
Lu Z, Shen SX, Zhi DJ and Luo FH: The
establishment of 'two-step sequential filtration method' on the
yield rate of purified islets in rats. Zhongguo Dang Dai Er Ke Za
Zhi. 15:572–576. 2013.In Chinese. PubMed/NCBI
|
|
27
|
Kassem M, Niazi ZR, Abbas M, El Habhab A,
Kreutter G, Khemais-Benkhiat S, Auger C, Antal MC, Schini-Kerth VB,
Toti F and Kessler L: Senescence of pancreas in middle-aged rats
with normal vascular function. Ann Transplant. 22:177–186. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen DL, Xiang JN and Yang LY: Role of
ERp46 in β-cell lipoapoptosis through endoplasmic reticulum stress
pathway as well as the protective effect of exendin-4. Biochem
Biophys Res Commun. 426:324–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Lin Y, Wang S, Zhang L and Guo L:
GLP-1 inhibits high-glucose-induced oxidative injury of vascular
endothelial cells. Sci Rep. 7:80082017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schiapaccassa A, Maranhão PA, de Souza
MDGC, Panazzolo DG, Nogueira Neto JF, Bouskela E and Kraemer-Aguiar
LG: 30-days effects of vildagliptin on vascular function, plasma
viscosity, inflammation, oxidative stress, and intestinal peptides
on drug-naive women with diabetes and obesity: A randomized
head-to-head metformin-controlled study. Diabetol Metab Syndr.
11:702019. View Article : Google Scholar
|
|
31
|
Steven S, Jurk K, Kopp M, Kröller-Schön S,
Mikhed Y, Schwierczek K, Roohani S, Kashani F, Oelze M, Klein T, et
al: Glucagon-like peptide-1 receptor signalling reduces
micro-vascular thrombosis, nitro-oxidative stress and platelet
activation in endotoxaemic mice. Br J Pharmacol. 174:1620–1632.
2017. View Article : Google Scholar
|
|
32
|
Shimoda M, Kanda Y, Hamamoto S, Tawaramoto
K, Hashiramoto M, Matsuki M and Kaku K: The human glucagon-like
peptide-1 analogue liraglutide preserves pancreatic beta cells via
regulation of cell kinetics and suppression of oxidative and
endoplasmic reticulum stress in a mouse model of diabetes.
Diabetologia. 54:1098–1108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rebelato E, Mares-Guia TR, Graciano MF,
Labriola L, Britto LR, Garay-Malpartida HM, Curi R, Sogayar MC and
Carpinelli AR: Expression of NADPH oxidase in human pancreatic
islets. Life Sci. 91:244–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu L, Wang K, Wang W, Wen Z, Wang P, Liu L
and Wang DW: Glucagon-like peptide-1 ameliorates cardiac
lipotoxicity in diabetic cardiomyopathy via the PPARα pathway.
Aging Cell. 17:e127632018. View Article : Google Scholar
|
|
35
|
Tesauro M, Schinzari F, Adamo A, Rovella
V, Martini F, Mores N, Barini A, Pitocco D, Ghirlanda G, Lauro D,
et al: Effects of GLP-1 on forearm vasodilator function and glucose
disposal during hyperinsulinemia in the metabolic syndrome.
Diabetes Care. 36:683–689. 2013. View Article : Google Scholar :
|
|
36
|
Ceriello A, Novials A, Canivell S, La Sala
L, Pujadas G, Esposito K, Testa R, Bucciarelli L, Rondinelli M and
Genovese S: Simultaneous GLP-1 and insulin administration acutely
enhances their vasodilatory, antiinflammatory, and antioxidant
action in type 2 diabetes. Diabetes Care. 37:1938–1943. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu XH, Wang YP, Wang LX, Chen Z, Liu XY
and Liu LB: Exendin-4 protects murine MIN6 pancreatic β-cells from
interleukin-1β-induced apoptosis via the NF-κB pathway. J
Endocrinol Invest. 36:803–811. 2013.PubMed/NCBI
|
|
38
|
Chang G, Zhang D, Yu H, Zhang P, Wang Y,
Zheng A and Qin S: Cardioprotective effects of exenatide against
oxidative stress-induced injury. Int J Mol Med. 32:1011–1020. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao H, Zeng Z, Zhang H, Zhou X, Guan L,
Deng W and Xu L: The glucagon-like peptide-1 analogue liraglutide
inhibits oxidative stress and inflammatory response in the liver of
rats with diet-induced non-alcoholic fatty liver disease. Biol
Pharm Bull. 38:694–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka T, Higashijima Y, Wada T and
Nangaku M: The potential for renoprotection with incretin-based
drugs. Kidney Int. 86:701–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim MH, Kim EH, Jung HS, Yang D, Park EY
and Jun HS: EX4 stabilizes and activates Nrf2 via PKCδ,
contributing to the prevention of oxidative stress-induced
pancreatic beta cell damage. Toxicol Appl Pharmacol. 315:60–69.
2017. View Article : Google Scholar
|
|
42
|
Kanoski SE, Hayes MR and Skibicka KP:
GLP-1 and weight loss: Unraveling the diverse neural circuitry. Am
J Physiol Regul Integr Comp Physiol. 310:R885–R895. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wolak M, Staszewska T, Juszczak M,
Gałdyszyńska M and Bojanowska E: Anti-inflammatory and pro-healing
impacts of exendin-4 treatment in Zucker diabetic rats: Effects on
skin wound fibroblasts. Eur J Pharmacol. 842:262–269. 2019.
View Article : Google Scholar
|
|
44
|
Basolo A, Burkholder J, Osgood K, Graham
A, Bundrick S, Frankl J, Piaggi P, Thearle MS and Krakoff J:
Exenatide has a pronounced effect on energy intake but not energy
expenditure in non-diabetic subjects with obesity: A randomized,
double-blind, placebo-controlled trial. Metabolism. 85:116–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jorsal T, Rungby J, Knop FK and Vilsbøll
T: GLP-1 and amylin in the treatment of obesity. Curr Diab Rep.
16:12016. View Article : Google Scholar
|
|
46
|
Camastra S, Astiarraga B, Tura A,
Frascerra S, Ciociaro D, Mari A, Gastaldelli A and Ferrannini E:
Effect of exenatide on postprandial glucose fluxes, lipolysis, and
β-cell function in non-diabetic, morbidly obese patients. Diabetes
Obes Metab. 19:412–420. 2017. View Article : Google Scholar
|
|
47
|
Sun XF, Wang Y, Zhao WJ, Wang L, Bao DQ,
Qu GR, Yao MX, Luan J, Wang YG and Yan SL: Effect of liraglutide on
glucagon secretion in obese type 2 diabetic patients. Zhonghua Nei
Ke Za Zhi. 58:33–38. 2019.In Chinese; Abstract available in Chinese
from the publisher. PubMed/NCBI
|
|
48
|
Opinto G, Natalicchio A and Marchetti P:
Physiology of incretins and loss of incretin effect in type 2
diabetes and obesity. Arch Physiol Biochem. 119:170–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|