Introduction
Senescence and trauma are major risk factors
contributing to the development of osteoarthritis (OA), a serious
and debilitating chronic disease (1). Arthritis is characterized by the
degeneration of articular cartilage, destruction of the
extracellular matrix (ECM) and even complete loss of function.
Numerous studies have confirmed that increased age is associated
with radiographic changes and high prevalence of OA-related
symptoms in the hands, hip joints, spine and knee joints (2-4).
Articular cartilage undergoes aging-related structural and
biochemical changes, which impair its biomechanical functions and
lead to structural abnormality in the cartilage (5). Senescence-dependent changes in the
cartilage include decreased chondrocyte density and compromised
proliferation/biosynthetic function (6). With increased age, more senescent
cells accumulate in the human body and secrete inflammatory
cytokines, chemokines and proteases, collectively known as
senescence-related secretory phenotypes (7). Senescent cells secrete a
senescence-associated secretory phenotype (SASP) to alter the
tissue microenvironment, attract immune cells and induce malignant
phenotypes in adjacent cells (8).
Constant renewal of cellular components occurs in tissues with high
cellular turnover rates (9).
However, chondrocytes are the only type of cells in the adult
articular cartilage in which regeneration and renewal abilities are
highly limited (10);
additionally, such capability declines with age. The rate of
chondrocyte division after mitosis is hardly detectable (11). OA-related loss of cartilage
homeostasis leads to continued ECM destruction (12). Therefore, studies investigating
the mechanisms of chondrocyte senescence and apoptosis may provide
a new perspective to preserve cartilage integrity and prevent OA
(13).
Autophagy regulates physiological functions, such as
remodeling, differentiation, survival, death and senescence
(14). As a cytoprotective
mechanism, autophagy protects organisms from senescence by
regulating the turnover of proteins and clearance of dysfunctional
organelles. For example, autophagy is activated to sustain highly
energy-dependent processes and low-nutrient energy metabolism,
e.g., growth and differentiation. The regulatory mechanisms of
autophagy are diverse. The regulation of mammalian target of
rapamycin (mTOR), including activation of PI3K and TOR complex 1,
takes a key role in activation of autophagy. The phosphatidyl
inositol 3 kinase (PI3K)-protein kinase B (AKT) pathway transmits a
signal to TORC1, leading to mTOR activation, which then regulates
the activity of autophagy. Therefore, the PI3K-AKT pathway is
considered to be one of the most important upstream pathways of
mTOR (15). It is widely believed
that the PI3K-AKT-mTOR pathway is an essential cellular signaling
pathway that negatively regulates autophagy after its activation.
Autophagy plays a fundamental regulatory role in the pathological
progression of inflammatory diseases (16). It has been observed that the
deficiencies in autophagy and regulation of articular cartilage and
cartilage cells can lead to senescence of the joints and, finally
in turn, OA. Consequently, autophagy is considered to be a key
factor in cartilage degradation and is an important mechanism that
leads to OA (17). Previous
studies have demonstrated that the basal level of autophagy
decreases with age (18,19), suggesting that enhancing autophagy
might be an effective way to delay the degeneration of articular
cartilage. However, the exact mechanism underlying the link between
autophagy and OA has not yet been clarified. Therefore, the purpose
of the present study is to develop possible beneficial treatment
strategies for OA via regulation of the PI3K/AKT pathway.
Panax notoginseng saponins (PNS) is a mixture
that contains ginsenosides such as Rb1, Rc, Rd, Re, Rg1 and Rh1
(20). PNS exerts various
pharmacological functions, including hemostasis maintenance and
anti-leukemia effects, as well as anti-tumor, anti-atherosclerosis,
anti-inflammatory, anti-oxidative and anti-apoptotic effects
(20). The important role of PNS
in preventing cell senescence has been confirmed (21). Numerous components (Re, Rb1 and
Rg1) present in PNS can prevent and treat a variety of diseases,
such as coronary artery disease, neurodegenerative disease,
diabetes and acute pancreatitis, by regulating autophagy (20-24). PNS can inhibit pancreatitis by
inhibiting the PI3K/AKT pathway, promoting mTOR expression and
inhibiting autophagy in pancreatic cells (24). Since the PI3K/AKT/mTOR pathway is
also important in the occurrence and development of OA, reduced
expression of the PI3K/AKT/mTOR pathway components may increase
autophagy in chondrocytes and prevent their entry into senescence,
thus preventing OA. However, the roles of PNS in chondrocyte
senescence and autophagy regulation have not been confirmed. The
aim of the present study was to explore the mechanisms by which PNS
may prevent chondrocyte senescence via increasing autophagy and
reducing apoptosis by regulating the PI3K/AKT/mTOR pathway.
Materials and methods
Experimental animals
All animal experiments were carried out in
accordance with the regulations of the Administration of Laboratory
Animals promulgated by the National Science and Technology
Commission of the People's Republic of China and the Institute of
Medical Ethics of Wuhan University Medical College. Sprague-Dawley
(SD) rats were obtained from the Animal Experimental Center of
Wuhan University (Wuhan, China). The present study was approved by
the Laboratory Animal Welfare & Ethics Committee of the Renmin
Hospital of Wuhan University (Wuhan, China). All efforts were made
to minimize the suffering of the animals used for the present
experiments.
Cell culture and identification
A total of five healthy male SD rats (8 weeks old,
weighing 220-240 g) were used in the present study. The animals
were kept in a stable environment (12-h light/dark cycle, 25±3°C,
35-60% relative humidity with rat feed and tap water available ad
libitum) for 3 weeks prior to sacrifice. The rats were sacrificed
and the articular cartilage was carefully harvested. The specimens
were washed three times with 10 ml PBS and then minced into 1
mm3 sections. The rat cartilage sections were digested
with 3 ml of 0.25% trypsin for 40 min and with 0.2% collagenase II
for further 6 h. Next, the cells were collected by centrifugation
at 1,000 × g for 5 min at room temperature, incubated at 37°C with
5% CO2 for 5 min, mixed with 5 ml DMEM (Gibco; Thermo
Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% mixture of penicillin and streptomycin and
cultured in an incubator at 37°C with 5% CO2. Live
chondrocytes were identified by toluidine blue staining at 37°C for
30 min.
Cell viability at different
concentrations of PNS treatment
Cells were cultured in triplicate in 96-well plates
(8,000 cells/well). To quantify cell viability at a specified time,
10 µl Cell Counting Kit-8 (CCK-8) reagent (Beyotime
Institute of Biotechnology) was added to each well and incubated at
37°C for 2 h according to the manufacturer's protocol. Optical
density (OD) was detected using an automatic microplate reader at
450 nm. The OD450 value is directly proportional to cell viability.
All experiments were performed in triplicate in three independent
experiments.
Experimental grouping of rat
chondrocytes
The rat chondrocytes were divided randomly into 4
groups (n=3): Control, OA, OA + PNS (100 µg/ml) [Kunming
Pharmaceutical Company (Lot no. 12JB09); Patent no. ZL96101652.3]
and OA + PNS (200 µg/ml). The chondrocytes were cultured in
6-well culture plates (1×106 cells/well) for 24 h.
Untreated chondrocytes were used as a control group. The OA model
phenotype was induced by treating normal cells with tumor necrosis
factor (TNF)-α (20 ng/ml) (Peprotech, Inc.). After 36 h of
intervention, different concentrations of PNS (low concentration,
100 µg/ml; high concentration, 200 µg/ml) were added
to the OA chondrocytes, followed by further incubation for 24
h.
SA-β-galactosidase staining
The chondrocytes (1×106 cells/well) were
washed twice and fixed by adding 1 ml of 1X fixative, followed by
incubation for 10 min at 25°C. Subsequently, the fixed cells were
washed three times with PBS, stained using a SA-β-galactosidase
staining kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol and incubated overnight at 37°C. Finally,
the cells were observed under a fluorescence microscope (Olympus
Corporation).
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was extracted from chondrocytes with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) was used to reverse transcribe cDNA from the RNA
at 37°C for 15 min and 85°C for 5 sec. Quantitative PCR was
performed using a StepOnePlus device (Applied Biosystems; Thermo
Fisher Scientific, Inc.) at 95°C for 10 sec, followed by 40 cycles
of 95°C for 5 sec and at 60°C for 20 sec. The following primers
were used: COL2 forward, 5′-AGG GCC AGG ATG TCC GGC A-3′ and
reverse, 5′-GGG TCC CAG GTT CTC CAT CT-3′; MMP-3 forward, 5′-CGG
TGG CTT CAG TAC CTT TC-3′ and reverse, 5′-ACC TCC TCC CAG ACC
TTCA-3′; MMP-13 forward, 5′-GGA GCA TGG CGA CTT CTA C-3′ and
reverse, 5′-GAG TGC TCC AGG GTC CTT-3′; and GADPH forward, 5′-AGG
TCG GTG TGA ACG GAT TTG-3′ and reverse, 5′-GGG GTC GTT GAT GGC AAC
A-3′. GADPH was used as an internal reference. The
2−ΔΔCq method was used to calculate the relative mRNA
expression levels (25).
Flow cytometry to measure mitochondrial
membrane potential (MiMP)
The JC-1 assay kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used to evaluate MiMP. The minced
cartilage pieces were washed with PBS, digested with trypsin and
were incubated with 800 ml the solution for 20 min at 37°C. MiMP
was measured using a FACSCalibur flow cytometer (Becton-Dickinson
and Company) after the cells were washed and suspended in 1 ml 1X
JC-1 staining buffer. FlowJo 10 software was used for analysis
(Becton-Dickinson and Company).
Detection of mitochondrial membrane
permeability conversion (MPTP) openness
A working solution was prepared according to
protocol provided in the MPTP detection kit (Best-Bio Ltd.), and 5
µl fluorescence quencher and 3 µl working solution of
the probe were added to each sample. Staining was carried out in
the dark at 25°C for 20 min. After washing with 1 ml MPTP buffer,
MPTP was measured by flow cytometry as described above. The MPTP
openness was evaluated based on the number of fluorescent
units.
Flow cytometry to assess apoptosis
The rate of apoptosis was assessed by flow
cytometry. Briefly, cells were separated from the culture
supernatant by centrifugation at 2,000 × g for 5 min at room
temperature and then washed twice with pre-chilled PBS. The
chondrocytes were carefully suspended in 500 µl of binding
buffer. Next, 5 µl propidium iodide (PI) and 5 µl
fluorescein isothiocyanate (FITC)-labeled Annexin V
(Becton-Dickinson and Company) were added, followed by staining at
room temperature for 15 min in the dark, and the apoptotic rate was
estimated by flow cytometry (Becton-Dickinson and Company).
Microscopic observation of MiMP
MiMP was evaluated using a Mitochondrial Membrane
Potential Assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's protocol. After washing,
chondrocytes were stained with 800 µl working solution JC-1
at 37°C for 25 min. After removing the staining solution, the cells
were added to 2 ml DMEM with 10% FBS. The red-green fluorescence
ratio was observed under an Olympus fluorescence microscope
(Olympus Corporation).
Immunofluorescence and
immunohistochemistry
After washing with PBS, the specimens were fixed in
4% paraformaldehyde and permeabilized for 10 min at room
temperature. Next, primary antibodies against P62 (1:200; Abcam;
cat. no. ab155686) and COL-II (1:200; Abcam; cat. no. ab34712) were
added and incubated in the dark at 25°C for 1 h. The samples were
then incubated with FITC-conjugated anti-mouse (1:200; Abcam; cat.
no. ab6785) and Cy3-conjugated goat anti-rabbit (1:300; cat. no.
GB21303; Servicebio, Inc.) secondary antibody at 37°C for 1 h and
subsequently incubated with DAPI (Nanjing KeyGen Biotech Co., Ltd.)
at 37°C for 5 min. The cells were observed under an Olympus
fluorescence microscope and positive expression was indicated by
green (p62) and red (COL-II) staining. Image Pro Plus 6.0 analysis
software (Media Cybernetics, Inc.) was used to analyze the
proportions of positive cells in the samples.
The tissue sections were incubated with primary
antibodies against MMP-3 (1:200; Abcam; cat. no. ab52915) or MMP-13
(1:200; Abcam; cat. no. ab39012) overnight at 4°C and subsequently
incubated with a biotinylated Goat anti-rabbit secondary antibody
(1:200; Abcam; cat. no. ab205718). The cells were observed under an
Olympus light microscope and positive expression was indicated by
brown staining. Image Pro Plus 6.0 analysis software (Media
Cybernetics, Inc.) was used to analyze the proportions of positive
cells in the samples.
Animal model and PNS treatment
The rats were randomized into 4 groups (n=5):
control, OA, OA+PI3K-AKT-mTOR pathway agonist IGF-1 (10 mg/kg),
OA+IGF-1+PNS (200 mg/kg). The OA rat model was constructed using
resection of the medial meniscus of the right knee and transection
of the anterior cruciate ligament. The animals were placed in an
electric coil cage for 1 h every 24 h. One month later, IGF-1 (10
mg/kg) and 40 µl PNS (75 µmol/l) were injected into
the joints of rats as per the appropriate treatments of each group
(IGF-1 and PNS were injected into rat joints every three days for a
total of 8 weeks), while OA and control groups received saline
injections. All of the experimental rats were sacrificed 3 months
after surgery.
Ultrastructural chondrocyte
detection
The cartilage sections were sectioned into 1
mm3 pieces, fixed with 2.5% glutaraldehyde for 2 h at
4°C and washed with PBS. A concentration gradient of 1% Ottoman
acid in ethanol (50, 70, 90 and 100%) was used to fix the samples.
The samples were immersed in a mixture of acetone and epoxy resin
twice at 37°C (2:1 for 3 h the first time, 1:2 overnight the second
time). Finally, the tissues were embedded in epoxy resin-filled
capsules and heated at 70°C overnight. Ultrathin sections (60-80
nm) were sliced and observed using a transmission electron
microscope (Hitachi, Ltd.) at ×100,000 magnification.
Western blot analysis
Phosphatase inhibitor: Protease inhibitor: RIPA
lysate was mixed at a ratio of (1:1:50) and added to cells or
cartilage fragments to extract total proteins (Google
Biotechnology). The proteins (40 µg/lane) were separated by
SDS-PAGE on a 12% gel and then transferred to PVDF membranes. The
membranes were blocked with 5% nonfat milk in TBS with 0.05%
Tween-20 (TBST for 1 h at 37°C and incubated overnight at 4°C with
the following primary antibodies: Interleukin (IL)-1β (1:1,000;
cat. no. 12703; Cell Signaling Technology, Inc.), high mobility
group protein B1 (HMGB1; 1:1,000; cat. no. 10829-1-AP; Proteintech,
Inc.), caspase 8 (1:1,000; cat. no. 4790), p16 (1:1,000; cat. no.
80772), PI3K (1:1,000; cat. no. 4249), AKT (1:1,000; cat. no. 4691;
all from Cell Signaling Technology, Inc.), phosphorylated (p-)AKT
(1:500; cat. no. ab38449), p-PI3K (1:500; cat. no. ab182651; both
from Abcam), mTOR (1:1,000; cat. no. 2983; Cell Signaling
Technology, Inc.), p-mTOR (1:500; cat. no. ab84400; Abcam,), Bcl-2
(1:1,000; cat. no. 3498; Cell Signaling Technology, Inc.), Bax
(1:1,000; cat. no. ab32503; Abcam), caspase-3 (1:1,000; cat. no.
9662; Cell Signaling Technology, Inc.), Beclin-1 (1:1,000; cat. no.
3495; Cell Signaling Technology, Inc.), LC3 (1:1,000; cat. no.
14600-1-AP; Proteintech, Inc.) and GAPDH (1:5,000; cat. no.
GB12002; Servicebio, Inc.), Next, the membranes were washed three
times in TBST and incubated with a horseradish
peroxidase-conjugated goat anti-rabbit (1:3,000; cat. no. GB23303;
Servicebio, Inc.) secondary antibody. After washing with TBST, a
chemiluminescence luminol reagent (cat. no. G2014, Servicebio,
Inc.) was used to visualize the protein bands employing the Image
Lab 5.2 quantitative assay system (Bio-Rad Laboratories, Inc.). The
relative protein levels were determined by normalizing to
GAPDH.
Statistical analysis
Data are expressed as the mean ± standard deviation
for each group. Data analysis was conducted using SPSS 21.0 (IBM
Corp.) and GraphPad Prism 6.0 (GraphPad Software, Inc.).
Comparisons among multiple groups were performed using one-way
analysis of variance with Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism software version 5.0 (GraphPad Software, Inc.) was
used to plot the data.
Results
Culture and identification of
chondrocytes and effect of PNS on TNF-α-induced expression of
senescent markers in cultured OA chondrocytes
Chondrocytes are either spindle-shaped, polygonal,
or irregularly shaped with multiple protrusions. Positive toluidine
blue staining can be used to identify the chondrocytes, as they are
stained blue-purple. The cytoplasm of chondrocytes contained
blue-purple heterochromatic granules, indicating that they
expressed proteoglycan (Fig. 1A).
The CCK-8 reagent was used to detect the cell viability of
different concentrations of PNS, thereby determining the PNS
concentration in the experiment was 100 and 200 µg/ml. Cell
viability at different concentrations of PNS. The viability of OA
chondrocytes gradually increased when PNS concentration ranged
between 0 and 200 µg/ml; cell viability was inhibited when
PNS concentration exceeded 200 µg/ml (Fig. 1B). Rat chondrocytes were divided
into four groups: Control, OA, OA+PNS (100 µg/ml) and OA+PNS
(200 µg/ml). Western blotting was used to detect and analyze
the levels of SASP factors (IL-1β, HMGB1, caspase-8 and p16). The
percentages of SASP cells and SA-β-gal-positive cells were highest
in the OA group compared with the other groups. However, PNS
significantly reduced the expression levels of aging-related
markers in OA chondrocytes in a concentration-dependent manner
(Fig. 1C-F).
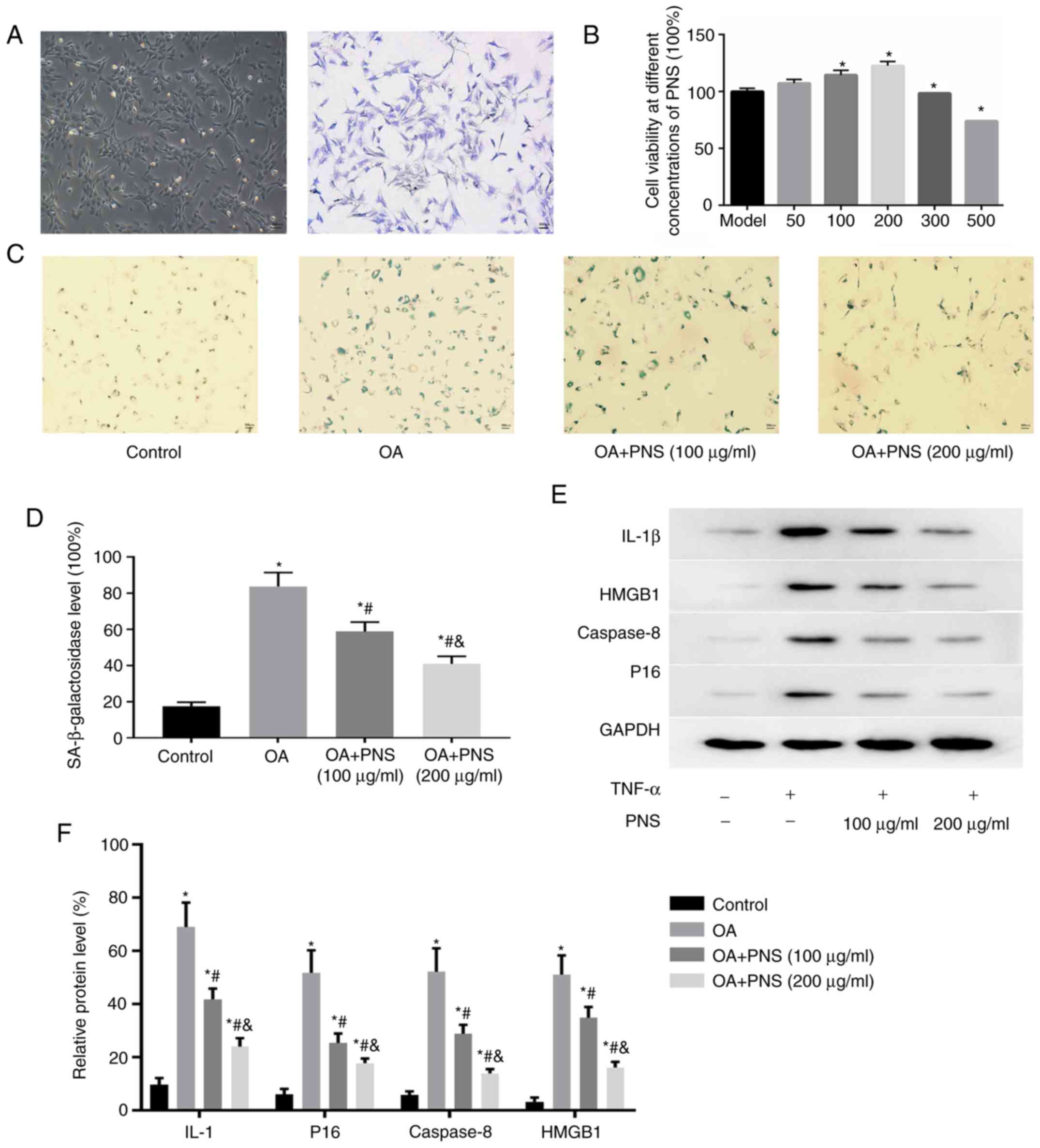 | Figure 1Chondrocytes isolated from rat
cartilage were cultured (magnification, ×40). (A) Identification of
live chondrocytes by toluidine blue staining. Primary chondrocytes
are stained blue-purple. (B) Cell viability at different
concentrations of PNS. The data are expressed as the means ± SD.
*P<0.05 vs. OA model chondrocytes. (C) Positive
staining for vimentin (green), a marker of the senescence marker
SA-β-galactosidase (magnification, ×100) and (D) quantitative
analysis of the numbers of positive cells. (E) Protein levels of
IL-1, p16, caspase-8 and HMGB1 in chondrocytes were assessed by
western blotting. Quantitative analysis of the protein levels of
p16, IL-1, caspase-8 and HMGB1 based on the western blot results
(F). The data are expressed as the means ± SD.
*P<0.05 vs. normal chondrocytes;
#P<0.05 vs. OA and P<0.05 vs. OA+PNS (100
µg/ml) (n=3). OA, osteoarthritis; PNS, panax
notoginseng saponins; SD, standard deviation; IL, interleukin;
HMGB1, High mobility group protein B1. |
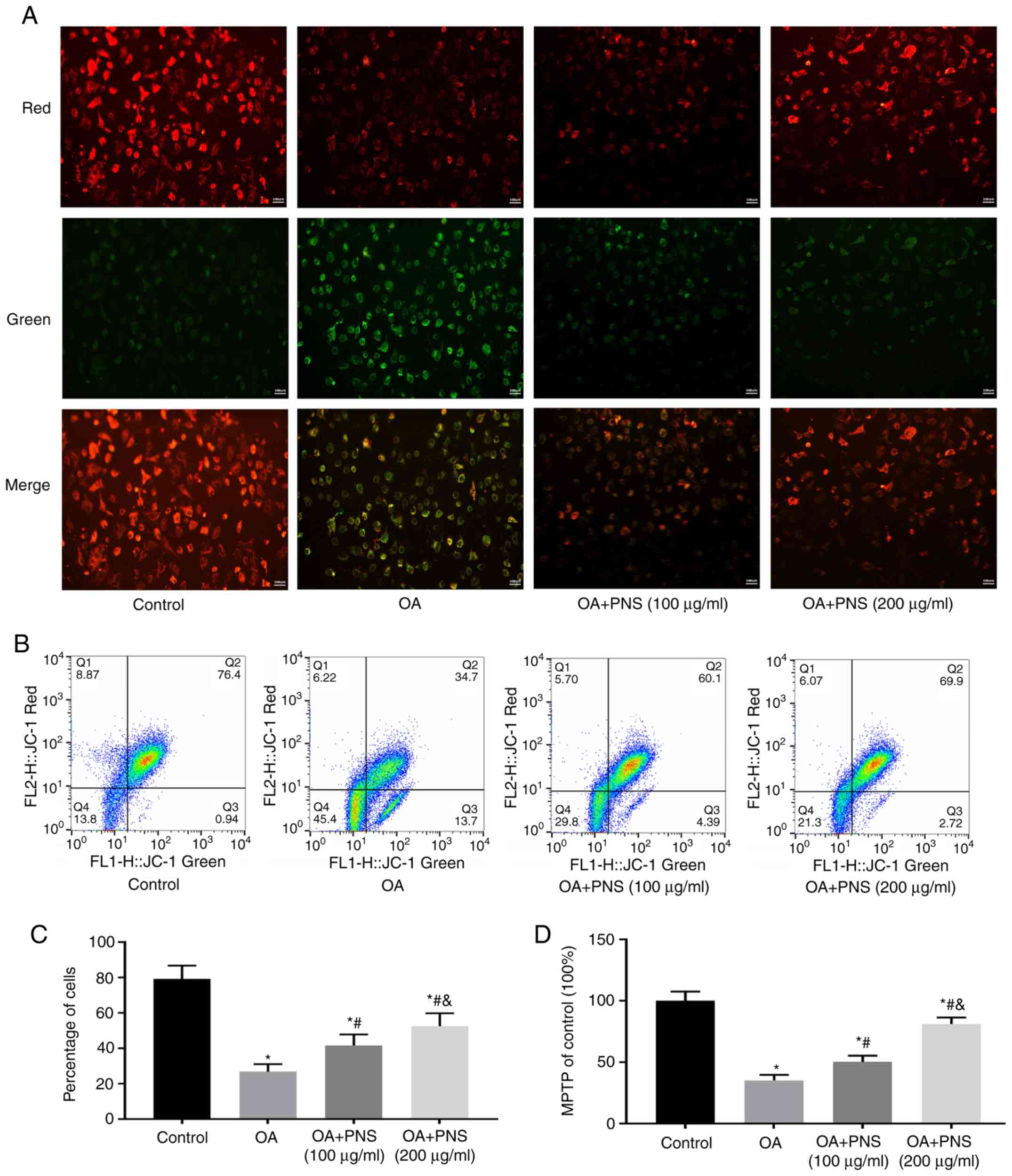
PNS can improve mitochondrial dysfunction
and morphological changes and has an anti-apoptosis effect in OA
chondrocytes
Mitochondria play important roles in energy
production, cell signaling, differentiation, apoptosis and
senescence. Cell death and senescence often cause loss of MiMP and
opening of MPTPs. Therefore, the effects of PNS on MiMP were
examined by fluorescence microscopy (the red/green ratio indicates
the expression of MiMP) (Fig.
2A). Flow cytometry was used to detect changes in MiMP
(Fig. 2B and C), and MPTP
(Fig. 2D). The results of these
experiments showed that the OA group had the lowest MiMP, the
highest MPTP openness and the highest apoptosis rate. PNS increased
MiMP and decreased MPTP opening and apoptosis. These results
indicate that PNS protects mitochondria of the chondrocytes.
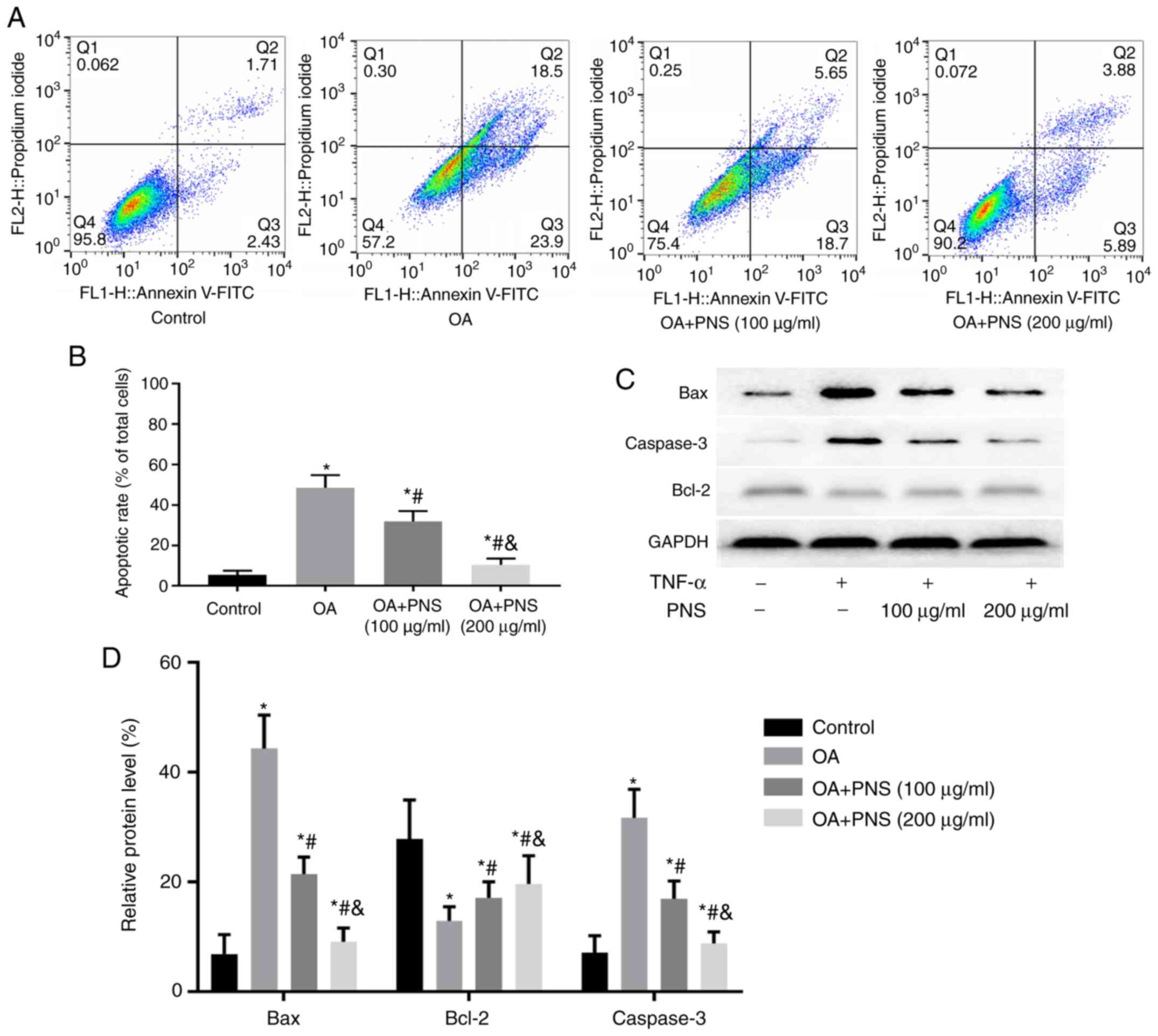
PNS can significantly reduce the level of
apoptosis in OA chondrocytes
Chondrocyte apoptosis is an important process in the
development of OA. In the present research, different methods were
used to detect the degree of chondrocyte apoptosis. Flow cytometry
was used to detect changes in the rate of apoptosis (Fig. 3A and B). Western blotting was used
to detect and analyze the levels of the apoptosis-related factors
caspase-3, Bax and Bcl-2 (Fig. 3C and
D). The results of the flow cytometry experiments were
consistent with those of western blot experiments. The expression
of caspase-3 and Bax was increased in the OA group compared with
those in the control group; however, the level of the
anti-apoptotic protein Bcl-2 was significantly decreased. These
trends were reversed after the addition of PNS. PNS treatment
significantly reduced caspase-3 and Bax levels but enhanced Bcl-2.
These results indicate that PNS counteracts apoptosis.
PNS reduces chondrocyte apoptosis by
regulating the PI3K/AKT/mTOR pathway to increase the expression
levels of autophagy-related proteins
The expression levels of PI3K, p-PI3K, AKT, mTOR,
p-AKT, p-mTOR and the autophagy-related proteins LC3 and Beclin-1
were assessed by western blotting, and the expression of P62 in
chondrocytes was measured by immunofluorescence staining. The
phosphorylation levels of PI3K, AKT and mTOR were significantly
increased in the other groups compared to those in the control
group. In contrast, the expression levels of LC3II/LC3I, Beclin-1
and P62 were significantly decreased. Compared with those in the OA
group, PNS significantly reduced the phosphorylation levels of
p-PI3K, p-AKT and p-mTOR (Fig. 4A and
B) and significantly increased the levels of autophagy-related
proteins (Fig. 4C-G) in a
concentration-dependent manner. Taken together with the previous
experimental results, it can be concluded that PNS can reduce
autophagy in chondrocytes and reduce apoptosis by regulating mTOR
signaling via the PI3K-AKT pathway.
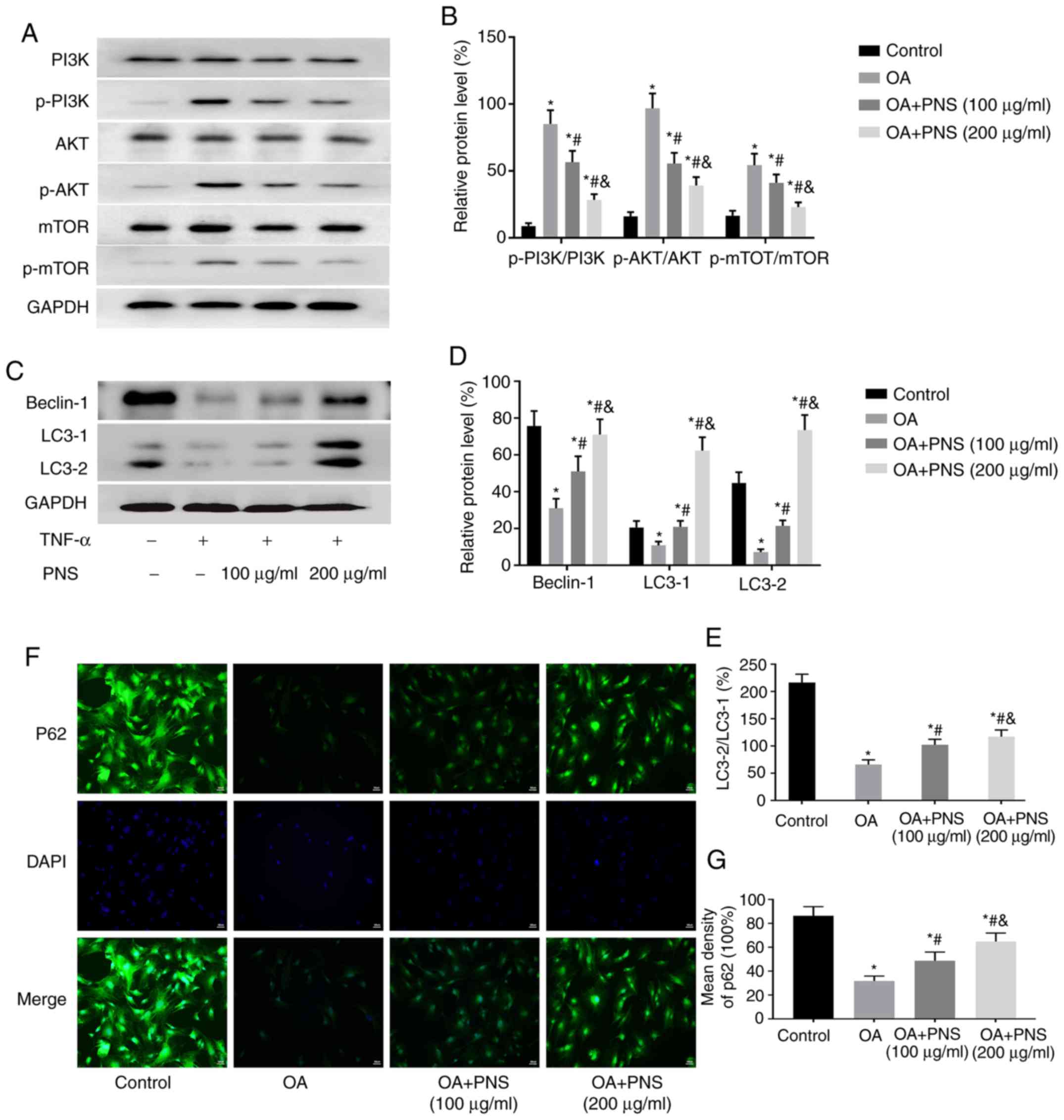 | Figure 4PNS regulates the PI3K/AKT/mTOR
pathway to increase the expression levels of autophagy-related
proteins. (A) Western blotting results showing protein levels of
PI3K, p-PI3K, AKT, p-AKT, mTOR and p-mTOR in chondrocytes and were
(B) quantified. (C) A western blot showing protein levels of LC3
and Beclin-1 in chondrocytes with (D) quantification. (E)
Quantitative analysis of the LC3II/LC3I ratio based on western blot
results. (F) Immunofluorescence staining showing the protein levels
of P62 in chondrocytes along with (G) quantification. The data are
expressed as the means ± standard deviation. *P<0.05
vs. normal chondrocytes; #P<0.05 vs. OA and
&P<0.05 vs. OA+PNS (100 µg/ml) (n=3).
PI3K, phosphatidyl inositol 3 kinase; p, phosphorylated; AKT,
protein kinase B; mTOR, mammalian target of rapamycin; OA,
osteoarthritis; PNS, Panax notoginseng saponins; TNF, tumor
necrosis factor. |
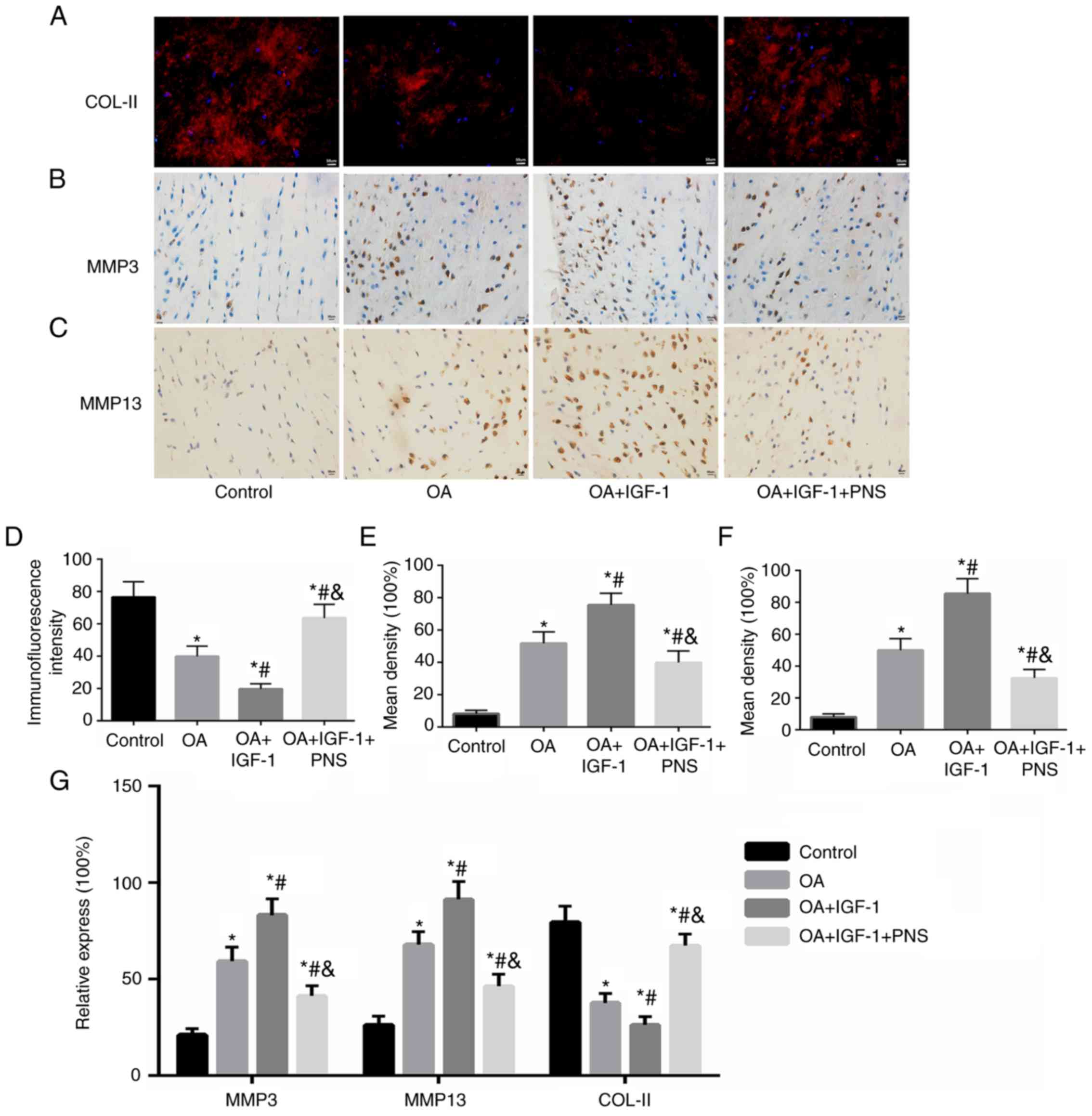
PNS regulates the expression levels of
degeneration-related genes and ultrastructural changes in
cartilage
The effects of PNS on the metabolic activity of the
ECM of cartilage were examined by immunohistochemistry,
immunofluorescence and RT-PCR. As shown in Fig. 5, TNF-α treatment (OA) and
PI3K-AKT-mTOR pathway agonist IGF-1 (OA+IGF-1+PNS) significantly
reduced the mRNA level of the ECM gene, COL-II, and increased the
mRNA level of the ECM degradation genes, MMP-3 and MMP-13 (Fig. 5D). PNS also inhibited the
catabolic activity of ECM. Moreover, PNS antagonized the effect of
the PI3K-Akt-mTOR pathway agonist IGF-1 to significantly inhibit
COL-II degradation (Fig. 5A and
D) and also decreased the expression levels of MMP-3 (Fig. 5B and E) and MMP-13 (Fig. 5C and F); these results were
consistent with the RT-PCR results (Fig. 5G).
PNS regulates the expression levels of
PI3K/AKT/mTOR signaling pathway and ultrastructural changes in
articular cartilage
The phosphorylation levels of PI3K, AKT and mTOR
were significantly increased in the other groups compared to those
in the control group. Compared with those in the OA group, IGF-1
increased the percentages of p-PI3K/PI3K, p-AKT/AKT, p-mTOR/mTOR,
whereas PNS significantly reduced them (Fig. 6A-D). Meanwhile, PNS can reduce the
expression of apoptosis-related proteins and increase the
expression of anti-degradation proteins (Fig. 6E and F). Ultrastructural changes
in the chondrocytes were observed using transmission electron
microscopy. The mitochondria showed a normal shape and the
mitochondrial ridge was clearly visible in the control group;
however, in the OA and OA+IGF-1 groups, the mitochondrial ridge
disappeared and the mitochondria were swollen, indicating that the
mitochondria were damaged and ruptured in these groups. These
effects improved significantly in the PNS-treated experimental
group (Fig. 6G). The present
in vivo experiments showed that compared with those in the
OA group, PNS significantly reduced the autophagy in chondrocytes
and reduce apoptosis by regulating mTOR signaling via the PI3K-AKT
pathway. This is consistent with the results of in vitro
experiments.
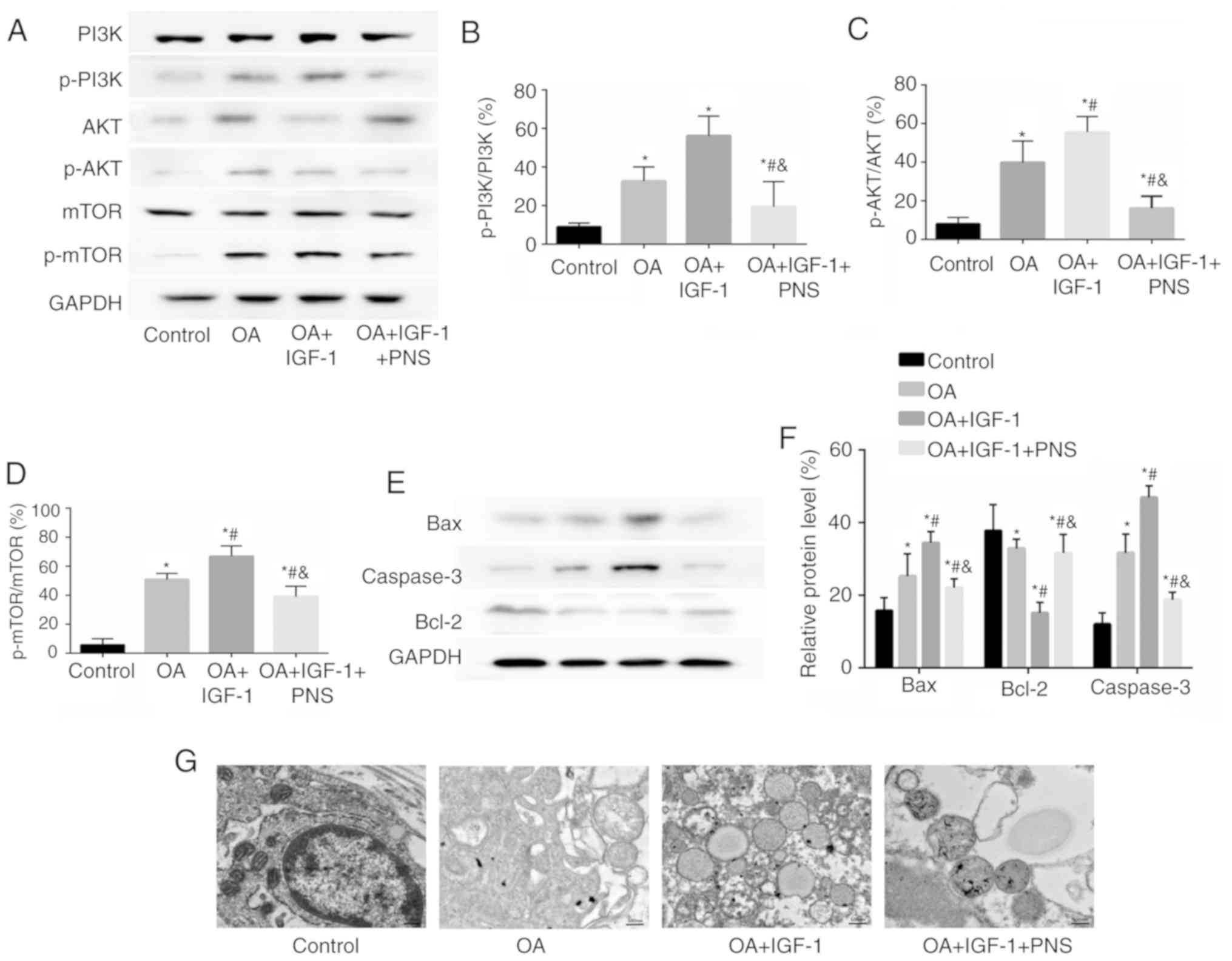 | Figure 6PNS regulates the expression levels
of PI3K/AKT/mTOR signaling pathway and ultrastructural changes in
articular cartilage. (A) Western blotting and quantification of (B)
PI3K, p-PI3K, (C) AKT, p-AKT, (D) mTOR and p-mTOR levels were
estimated using western blotting in rat articular cartilage and
were quantified. (E) The expression of apoptosis-related proteins
caspase-3 and Bax, anti-degradation proteins Bcl-2 and (F)
quantification. (G) Transmission electron microscopy showing
ultrastructural changes in chondrocytes (magnification, ×100,000).
The data are expressed as the means ± standard deviation (n=3).
*P<0.05 vs. control; #P<0.05 vs. OA and
P<0.05 vs. OA+IGF-1. PI3K, phosphatidyl inositol 3 kinase; p,
phosphorylated; Akt, protein kinase B; mTOR, mammalian target of
rapamycin; OA, osteoarthritis; PNS, Panax notoginseng
saponins. |
Discussion
Compounds from some medicinal plants have various
pharmacological effects and studies related to intra-articular
injection of such compounds have become a research hotspot
(26,27). Previous studies have shown that
PNS could inhibit cyclooxidase expression in macrophages and it has
been speculated that PNS has anti-inflammatory and
immunosuppressive effects in vitro (28,29). Intraperitoneal injection of PNS
significantly reduced the levels of TNF-α and IL-6 in the serum of
a liver fibrosis rat model (30).
In another study, it was suggested that PNS had an anti-oxidant
effect due to inhibition of reactive oxygen species formation and
of the Bcl-2/Bax signaling pathway (31). Oxidative stress is an important
factor that leads to cell damage and apoptosis. However, it is not
clear whether the above-mentioned anti-inflammatory effects can
protect articular cartilage from destruction due to excessive
secretion of pro-inflammatory factors during the progression of OA.
In this study, PNS intervention reduced the secretion of cartilage
SASP factors and reduced the extent of OA.
Autophagy and mitochondrial dysfunction are the main
characteristics of cartilage degeneration caused by senescence
(32). Due to cartilage
degeneration and decreased autophagy, adaptability of cartilage
cells to the external environment is significantly decreased,
leading to cell damage, such as abnormal cellular metabolism, and
ultimately to cell death. A study by Zhang et al (33) showed that increased autophagy
signaling in the cartilage had a significant protective effect in
an OA rat model that was constructed using resection of the medial
meniscus of the right knee. This protective effect was related to
degradation of the articular cartilage matrix and a decrease in
apoptosis. In a previous study, the mitochondrial respiratory chain
complex V inhibitor oligomycin was used to stimulate chondrocytes
and the results showed dysfunctions in human chondrocyte
mitochondria that led to reduced autophagy in chondrocytes. These
observations suggest that inhibition of the mitochondrial
respiratory chain inhibits autophagy (34). In the current study, it was
demonstrated that TNF-α intervention promoted senescence in
cartilage cells by inducing mitochondrial dysfunction and reducing
autophagy, and that these effects could be reversed by the addition
of PNS. These findings suggest that PNS may protect cartilage cells
by regulating autophagy and delaying the progress of OA.
mTOR is a receptor of intracellular amino acids, ATP
and hormones in human cells, and it is a major inhibitory regulator
of intracellular autophagy (35,36). In previous years, various studies
have confirmed the important role of mTOR signaling in articular
cartilage homeostasis and OA pathophysiology (37). Caramés et al (38) found that increased autophagy
induced by the inhibition of mTOR signaling was associated with
decreased ADAMTS-5 and IL-1β expression in articular cartilage.
Rapamycin, a specific inhibitor of the mTOR signaling pathway,
increased autophagy in cartilage cells in a mouse OA model and
decreased the severity of OA (39). Rapamycin injection can lead to
numerous adverse reactions, including diarrhea, weight loss,
albuminuria, anemia, allergies, elevated serum cholesterol and
triglycerides. In the current in vitro experiments (40), PNS demonstrated effects similar to
those of rapamycin. The decrease in cartilage autophagy induced by
TNF-α was reversed through the inhibitory effect of PNS on the
PI3K/AKT/mTOR signaling pathway (41). In the present animal model, rats
were treated with the PI3K/AKT/mTOR signaling pathway agonist,
IGF-1; PNS was found have an antagonistic effect towards IGF-1.
Therefore, the current study suggests that PNS can protect
cartilage integrity by inhibiting PI3K/AKT/mTOR signaling
pathway.
Previous studies showed that drugs that suppressed
MiMP or pro-inflammatory factors often did not change the overall
condition of OA (42,43). However, regulating key factors
affecting cell homeostasis, such as autophagy and preventing cell
death and ECM destruction could limit OA progression (44,45). Therefore, the regulation of
autophagy via the mTOR signaling pathway might be a potential
therapeutic target for OA. In this study, it was confirmed that PNS
can reduce mitochondrial damage and senescence in cartilage cells
by increasing autophagy via inhibition of the mTOR signaling
pathway, ultimately delaying the development of OA in an animal
model. Although the clinical implications are not yet clear, this
study will provide a theoretical basis and a new concept for OA
treatment.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81171760) and the Natural
Science Foundation of Hubei Province (grant no.
ZRMS2017000057).
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
YZ and HL designed the study. WC, GH, MC and SZ
performed the experiments and analyzed the data. YZ, JL and GH
contributed the essential reagents or tools and wrote the
manuscript. All authors contributed to revising the manuscript and
approved the final version to be submitted.
Ethics approval and consent to
participate
The animal experiments and procedures were ethically
approved by the Animal Care and Use Committee of Renmin Hospital of
Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Qiong Ding, Dr
Pengchen Yu, Ms. Yingxia Jin and Ms. Lina Zhou (Central laboratory,
Renmin Hospital of Wuhan University) for assistance with the flow
cytometry analysis.
References
|
1
|
Guilak F, Nims RJ, Dicks A, Wu CL and
Meulenbelt I: Osteoarthritis as a disease of the cartilage
pericellular matrix. Matrix Biol. 71-72:40–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahmati M, Mobasheri A and Mozafari M:
Reply to 'Comment on: Inflammatory mediators in osteoarthritis: A
critical review of the state-of-the art, prospects, and future
challenges'. Bone. 105:3112017. View Article : Google Scholar
|
|
3
|
Englund M, Guermazi A, Gale D, Hunter DJ,
Aliabadi P, Clancy M and Felson DT: Incidental meniscal findings on
knee MRI in middle-aged and elderly persons. N Engl J Med.
359:1108–1115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014. View Article : Google Scholar
|
|
5
|
Greene MA and Loeser RF: Aging-related
inflammation in osteoarthritis. Osteoarthritis Cartilage.
23:1966–1971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCulloc K, Litherland GJ and Rai TS:
Cellular senescence in osteoarthritis pathology. Aging Cell.
16:210–218. 2017. View Article : Google Scholar
|
|
7
|
Jeon OH, David N, Campisi J and Elisseeff
JH: Senescent cells and osteoarthritis: A painful connection. J
Clin Invest. 128:1229–1237. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calcinotto A, Kohli J, Zagato E,
Pellegrini L, Demaria M and Alimonti A: Cellular senescence: Aging,
cancer, and injury. Physiol Rev. 99:1047–1078. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wodarz D: Effect of stem cell turnover
rates on protection against cancer and aging. J Theor Biol.
245:449–458. 2007. View Article : Google Scholar
|
|
10
|
Goessler UR, Bieback K, Bugert P, Heller
T, Sadick H, Hörmann K and Riedel F: In vitro analysis of integrin
expression during chondrogenic differentiation of mesenchymal stem
cells and chondrocytes upon dedifferentiation in cell culture. Int
J Mol Med. 17:301–307. 2006.PubMed/NCBI
|
|
11
|
Hou A, Chen P, Tang H, Meng H, Cheng X,
Wang Y, Zhang Y and Peng J: Cellular senescence in osteoarthritis
and anti-aging strategies. Mech Ageing Dev. 175:83–87. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashkavand Z, Malekinejad H and Vishwanath
BS: The patho-physiology of osteoarthritis. J Pharm Res. 7:132–138.
2013.
|
|
13
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: Effective
combinations and clinical considerations. Drug Resist Update.
11:32–50. 2008. View Article : Google Scholar
|
|
16
|
Jones SA, Mills KH and Harris J: Autophagy
and inflammatory diseases. Immunol Cell Biol. 91:250–258. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Jin T and Lu Y: AntimiR-30b
inhibits TNF-α mediated apoptosis and attenuated cartilage
degradation through enhancing autophagy. Cell Physiol Biochem.
40:883–894. 2016. View Article : Google Scholar
|
|
18
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Green DR, Galluzzi L and Kroemer G:
Mitochondria and the autophagy-inflammation-cell death axis in
organismal aging. Science. 333:1109–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duan L, Xiong X, Hu J, Liu Y, Li J and
Wang J: Panax notoginseng saponins for treating coronary artery
disease: A functional and mechanistic overview. Front Pharmacol.
8:7022017. View Article : Google Scholar :
|
|
21
|
Haiping Z, Ziping H, Guangwen L, Sijia Z
and Yumin L: Therapeutic potential and cellular mechanisms of Panax
notoginseng on prevention of aging and cell senescence-associated
diseases. Aging Dis. 8:721–739. 2017. View Article : Google Scholar
|
|
22
|
Xu ZM, Li CB, Liu QL, Li P and Yang H:
Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through
the inhibition of autophagy and endoplasmic reticulum stress in
mice. Int J Mol Sci. 19:E36582018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou P, Weijie X, He S, Sun Y, Meng X, Sun
G and Sun X: Ginsenoside Rb1 as an anti-diabetic agent and its
underlying mechanism analysis. Cells. 8:2042019. View Article : Google Scholar :
|
|
24
|
Liu MW, Wei R, Su MX, Li H, Fang TW and
Zhang W: Effects of Panax notoginseng saponins on severe acute
pancreatitis through the regulation of mTOR/Akt and caspase-3
signaling pathway by upregulating miR-181b expression in rats. BMC
Complement Altern Med. 18:512018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Kikuchi M, Matsuura K, Matsumoto Y,
Inagaki T and Ueda R: Bibliographical investigation of
complementary alternative medicines for osteoarthritis and
rheumatoid arthritis. Geriatr Gerontol Int. 9:29–40. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marcus DM: Therapy: Herbals and
supplements for rheumatic diseases. Nat Rev Rheumatol. 5:299–300.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui
F, Li C, Tang L and Wang Z: Traditional uses, botany,
phytochemistry, pharmacology and toxicology of Panax notoginseng
(Burk) F H Chen: A review. J Ethnopharmacol. 188:234–258. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rhule A, Navarro S, Smith JR and Shepherd
DM: Panax notoginseng attenuates LPS-induced pro-inflammatory
mediators in RAW264.7 cells. J Ethnopharmacol. 106:121–128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng XD, Dai LL, Huang CQ, He CM, Yang B
and Chen LJ: Relationship between anti-fibrotic effect of Panax
notoginseng saponins and serum cytokines in rat hepatic fibrosis.
Biochem Biophys Res Commun. 388:31–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiang H, Zhang C, Shi ZB, Yang HQ and Wang
KZ: Protective effects and mechanism of Panax notoginseng saponins
on oxidative stress-induced damage and apoptosis of rabbit bone
marrow stromal cells. Chin J Integr Med. 16:525–530. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Youle RJ and Narendra DP: Mechanisms of
mitophagy. Nat Rev Mol Cell Biol. 12:9–14. 2011. View Article : Google Scholar
|
|
33
|
Zhang Y, Vasheghani F, Li YH, Blati M,
Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP,
et al: Cartilage-specific deletion of mTOR upregulates autophagy
and protects mice from osteoarthritis. J Ann Rheum Dis.
74:1432–1440. 2015. View Article : Google Scholar
|
|
34
|
López de Figueroa P, Lotz MK, Blanco FJ
and Caramés B: Autophagy activation protects from mitochondrial
dysfunction in human chondrocytes. Arthritis Rheumatol. 67:966–976.
2015. View Article : Google Scholar
|
|
35
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xue JF, Shi ZM, Zou J and Li XL:
Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of
articular chondrocytes and attenuates inflammatory response in rats
with osteoarthritis. Biomed Pharmacother. 89:1252–1261. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pal B, Endisha H, Zhang Y and Kapoor M:
Mtor: A potential therapeutic target in osteoarthritis? Drugs R D.
15:27–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Caramés B, Hasegawa A, Taniguchi N, Miyaki
S, Blanco FJ and Lotz M: Autophagy activation by rapamycin reduces
severity of experimental osteoarthritis. Ann Rheum Dis. 71:575–581.
2012. View Article : Google Scholar :
|
|
39
|
Kapoor M, Martelpelletier J, Lajeunesse D,
Pelletier JP and Fahmi H: Role of proinflammatory cytokines in the
patho-physiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar
|
|
40
|
Gianluigi Z, Paola T, Paolo R, Simona G,
Luigino B and Antonio L: Systemic and nonrenal adverse effects
occurring in renal transplant patients treated with mTOR
inhibitors. Clin Dev Immunol. 2013:4032802013.
|
|
41
|
Fosang AJ and Little CB: Drug insight:
Aggrecanases as therapeutic targets for osteoarthritis. Nat Clin
Pract Rheumatol. 4:420–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Creel PA: Management of mTOR inhibitor
side effects. J Clin J Oncol Nurs. 13(Suppl): S19–S23. 2009.
View Article : Google Scholar
|
|
43
|
Mandl LA: Osteoarthritis year in review
2018: Clinical. Osteoarthr Cartilage. 27:359–364. 2019. View Article : Google Scholar
|
|
44
|
Miyaki S and Lotz MK: Extracellular
vesicles in cartilage homeostasis and osteoarthritis. Curr Opin
Rheumatol. 30:129–135. 2018. View Article : Google Scholar :
|
|
45
|
Rai MF, Cotton R and Sandell LJ:
Chemokines modulate chondrocyte homeostasis: Implications in
osteoarthritis. Osteoarthr Cartilage. 20(Suppl): S2402012.
View Article : Google Scholar
|