Introduction
Long-term hypoxia induced by hemorrhagic shock,
cross-clamping of major vascular structures and extracorporeal
circulation may lead to incomplete ischemic brain injury during the
perioperative period (1-3). Inflammatory cytokines, such as
interleukin-1β (IL-1β) and IL-18, have previously been implicated
in neuronal cell death and functional injuries following incomplete
cerebral ischemia/reperfusion (4). The inactive precursors of IL-1β are
activated into mature inflammatory cytokines by cleaved caspase-1,
which mediates a type of programmed cell death termed pyroptosis
(5). Pyroptosis contributes to
the development of ischemia/reperfusion-related diseases, such as
stoke and acute kidney injury (6,7).
As key strategies for incomplete ischemia/reperfusion therapy,
relieving secondary neuronal pyroptosis and improving surviving
neuronal function are currently in development (8).
Carbon monoxide (CO), which is a type of neurotoxic
molecule, has valuable cytoprotective effects, including positive
and negative alterations of heme-containing enzymatic function and
modulation of numerous cellular targets, such as extracellular
signal-regulated kinase 1/2 (9).
Exogenously administered CO affects cell metabolism and supports
oxidative phosphorylation and mitochondrial respiration, enhancing
ATP production and cellular energy status (10). Carbon monoxide-releasing molecule
(CORM)-3 has emerged as an excellent alternative to CO
administration (11) and has been
demonstrated to provide protection in inflammation and
ischemia/reperfusion injury models by inhibiting the activation of
nucleotide-binding oligomerization domain-like receptors pyrin
domain-3 (NLRP3) inflammasome, which contains NLRP3,
apoptosis-associated speck-like protein containing a CARD domain
(ASC) and pro-caspase-1, in sepsis and metabolic diseases (12,13). IL-1β and IL-18 maturation and
release are controlled by activated NLRP3 inflammasomes (14).
Whether CORM-3 reduces locomotor activity
degeneration in a model of blood loss and re-infusion remains
unclear. The present study aimed to examine whether CO derived from
CORM-3 served a protective role against mitochondrial DNA-induced
neuronal pyroptosis following hemorrhagic shock and resuscitation
(HSR) by inhibiting cellular metabolism and NLRP3 inflammasome
activation.
Materials and methods
Animals
The National Institutes of Health Guidelines for the
Care and Use of Laboratory Animals were followed while performing
all animal experiments in this study. The Animal Review Board of
Cangzhou Central Hospital provided formal approval to conduct the
experiments. Male Sprague-Dawley rats (Charles River Laboratories,
Inc.) aged between 9 and 10 weeks (weight, 350-400 g) were housed
in individual shoebox cages with bedding. The room temperature was
maintained at 25±1°C with a 12-h alternating light/dark cycle. The
health and behavior of rats were monitored every day. The rats were
euthanized by exsanguination from the abdominal aorta under
sevoflurane anesthesia (3-4%) at time points indicated below.
Electrocardiographic monitoring was used to verify death.
HSR protocol
An HSR model was developed as previously described
(15,16). Rats were allowed free access to
water and chow until the start of the experiments. Sevoflurane
(7-8% for induction and 3-4% for maintenance) was used for
anesthetizing tracheally-intubated rats with a volume-controlled
ventilator (tidal volume, 4 ml/100 g; initial respiratory
frequency, 70 bpm; FiO2, 40%; ALC-V; Shanghai Alcott
Biological Technology Inc.). A handheld end-tidal carbon
dioxide/oxygen saturation monitor (PMSH-300; SunLife Science, Inc.)
was used to adjust the respiratory frequency to an end-tidal carbon
dioxide pressure of 35-40 mmHg. The rats underwent cannulation with
a heparinized indwelling needle (22 G) via the left femoral artery
for blood pressure measurements and via the left femoral vein to
induce hemorrhage shock. To maintain a mean arterial pressure of
30±5 mmHg for 60 min, hemorrhagic shock was induced by bleeding
into a heparinized syringe (10 U/ml). Resuscitation was performed
by reperfusion of all shed blood and, if necessary, administration
of sterile saline to reach the baseline arterial pressure as
described in our previous studies (15,16). Next, 4 mg/kg CORM-3 was injected
intravenously (cat. no. S744801; Selleck Chemicals). Catheters to
the left femoral artery and vein were inserted using an indwelling
needle (22 G) as a surgical preparation in the Sham group; the
vehicle control was an equivalent volume of normal saline
injection. Inactive CORM-3 (iCORM-3) was prepared by dissolving
CORM-3 in PBS and incubating it for 24 h under air and light
exposure at 25°C; to remove residual CO, the solution was bubbled
with 100% nitrogen.
Measurement of IL-1β and IL-18
levels
At 3, 6, 12 and 24 h after resuscitation, rats were
euthanized (n=6 per group) and total protein was extracted from the
hippocampal tissues by RIPA Lysis Buffer (cat. no. P0013D; Beyotime
Institute of Biotechnology). ELISA kits (Interleukin-1β Assay kit,
cat. no. H002; Interleukin-18 Assay kit, cat. no. H015; Nanjing
Jiancheng Bioengineering Institute) were used to measure IL-1β and
IL-18 levels according to the manufacturer's protocol.
Immunofluorescence
At 12 h after resuscitation, rats (n=6 per group)
were anesthetized with sevoflurane and perfused with 10%
neutral-buffered formalin to fix the cerebral tissues. The tissues
were embedded in paraffin, cut into 5-µm sections and
underwent immunofluorescence staining to determine the degree of
neuronal pyroptosis. Briefly, the paraffin sections were dewaxed
and hydrated (100% ethanol for 3 min, 95% ethanol for 2 min, 80%
ethanol for 2 min, 75% ethanol for 2 min, H2O for 1 min)
for 10 min at room temperature, followed by incubation with primary
polyclonal rabbit anti-cleaved caspase-1 (1:200; cat. no. ab1872;
Abcam) and polyclonal mouse anti-neuronal nuclei (NeuN; 1:500; cat.
no. ab104224; Abcam) antibodies or NeuN antibody alone overnight at
4°C. The sections were washed three times in PBS and incubated in
the dark for 1 h at 25°C in a blocking solution containing the
secondary antibodies (Cy3-conjugated goat anti-rabbit
IgG; cat. no. A0516; or FITC-conjugated goat anti-mouse IgG; cat.
no. A5608; both from Beyotime Institute of Biotechnology) diluted
1:200 in the blocking solution. Subsequently, 5 µg/ml DAPI
(Beyotime Institute of Biotechnology) was added for 2 min to
identify the cell nuclei. Propidium iodide (PI; Beyotime Institute
of Biotechnology) was added for 2 min to identify dead cells. A
laser scanning confocal microscope (Olympus Corporation) was used
to capture immunofluorescence images; A total of six fields at ×200
magnification were randomly selected from three sections in each
group. The mean densitometry of the fluorescence signal in each
field was analyzed by Image-pro plus 6.0 software (Media
Cybernetics, Inc.). The percentage of cleaved caspase-1 or PI
combined with NeuN- and DAPI-positive cells was calculated.
Measurement of lactate dehydrogenase
(LDH)
At 12 h after resuscitation, homogenates were
prepared using hippocampal tissues (n=6 per group) as described
above; following centrifugation at 1,000 × g and 4°C for 10 min,
the supernatant was used to detect the content of lactic
dehydrogenase (LDH) using an LDH Cytotoxicity Assay kit (cat. no.
C0016, Beyotime Institute of Biotechnology) by spectrophotometry at
492 nm (BioTek Epoch).
MRI study
A clinical 3.0 T MRI scanner (DISCOVERY MR 750; GE
Healthcare) was used to perform MRI with a special coil in rats at
room temperature. The following parameters were used to acquire
coronal fast spin echo T2-weighted images: Repetition time
(TR)/echo time (TE), 3500/85; number of excitations (NEX), 2;
phase, 256; frequency, 320; slices, 21; slice thickness, 1.5 mm;
field of view, 80 mm; and acquisition time, 2 min. At 3, 6, 12, and
24 h after HSR, the rats (n=6 per group) were anesthetized with
sodium pentobarbital (65 mg/kg) and placed prone in an animal
holder, and the coil was positioned and fixed over the head of the
animal. T2W images displaying the CA1 region of the hippocampus
were analyzed. The regions of interest were placed free-hand on the
hippocampus and the signal intensity (SI) was measured in addition
to visual inspection. T2-weighted standardized signal intensity
(SSI) was obtained by calculating the ratios between the average SI
and the temporalis SI to reflect the signal changes on T2WI. As a
measure of the T2W images for each rat, the SSI ratios before/after
indicated stimuli were determined.
1H-magnetic resonance spectroscopy (MRS)
spectra were acquired using multivoxel pattern analysis in the
right hemisphere of the rat brain covering the hippocampus. The
following parameters were used to acquire the 1H-MRS
images: TR/TE, 1500/35; NEX, 1; phase, 18; frequency, 16; and
acquisition time, 7 min 18 sec. The GE Function Tool software (AW
4.5 Workstation; GE Healthcare) was used to perform spectral image
analysis and data processing to analyze the spectrum signal with
MR. For background modeling, the metabolite area under the peak was
quantified using a quantum estimation method with a subtraction
approach. To estimate peak areas, a simulated basis set was used. A
relative quantification method using the Cr peak as the internal
spectral reference was applied to reduce systematic variations
between animals. The N-acetylaspartate (NAA)/creatinine (Cr),
myoinositol (mI)/Cr, and lipid (Lip)/Cr ratios were statistically
evaluated. Each MRS metabolite was identified by the part per
million (ppm) position of the nuclear spectrum, including NAA (2.02
ppm), Cr (3.05 ppm), mI (2.2 ppm) and Lip (0.9 ppm).
Open field test
On day 7 after HSR, open field test was used to
assess locomotor and exploratory activities (n=12 per group). To
track the movement of the rats, the open field apparatus comprised
a square opaque acrylic container (60×60 cm) and a video camera
fixed 1 m above the arena. Prior to testing, the rats were allowed
to acclimatize to the room for 1 h; the open field boxes were
disinfected to remove any smells that could affect behavior. The
tests were performed in the breeding room. Each rat was placed in
the middle of the chamber for each trial. After 1 min of
adaptation, the behavior of the rat was recorded for 5 min prior to
returning to their home cages in the same room during the interval
between trials, and the open field box was cleaned with a damp
cloth. To analyze the images, a computerized tracking system
(XR-XZ301; Shanghai Xinruan Software Co., Ltd.) was used. The
distance, speed, rearing events, time in all corners and grooming
event number were analyzed. After the test, all rats were
euthanized by exsanguination from the abdominal aorta under
sevoflurane anesthesia.
Mitochondria extraction and electron
microscopy
The mitochondria from the hippocampal tissues were
extracted 12 h after HSR (n=6 per group) (17). Briefly, the hippocampal tissue
homogenates were centrifuged twice at 1,000 × g and 4°C for 5 min,
and the supernatant was centrifuged at 15,000 × g and 4°C for 2
min. After carefully eliminating all fat and fluffy layers on the
top of the pellet containing the mitochondria, the supernatant was
removed. The pellets from the two tubes were combined, resuspended
in 1.5 ml ice-cold PBS and centrifuged at 15,000 × g and 4°C for 2
min to eliminate harmful enzymes, nucleases, phospholipases and
proteases. The final pellet was resuspended in ice-cold final
equilibrated buffer (250 mM sucrose, 5 mM
KH2PO4, 10 mM Tris-HCl, 2 mg/ml BSA, pH 7.2)
and fixed in 2.5% glutaraldehyde in 0.1 M sucrose phosphate buffer
(SPB) for 1 h at room temperature. Following washing with 0.1 M
SPB, the mitochondrial extractions were fixed with 1% osmium
tetroxide in 0.1 M SPB for 1 h at room temperature and the pellets
were dehydrated with ethanol at 10, 30, 70 and 99% for 30 min,
infiltrated with LR white resin (Sigma-Aldrich; Merck KGaA),
embedded in capsule beams and polymerized at 65°C for 48 h. Serial
ultrathin slices (50 nm) were cut with an ultramicrotome and
stained with 4% uranyl acetate-lead citrate for 3 min at room
temperature, and transmission electron microscopy (JEM-2000EX;
JEOL, Ltd.) was used to observe the ultrastructure of the
mitochondrial pellets. The dysmorphic mitochondria were examined
and counted at ×3,000 magnification, including swelling and ghost
or normal mitochondria, as described previously (18). Dysmorphic mitochondria were
characterized by the following parameters: i) Degenerative changes,
such as matrix vacuolation, disarrangement of cristae, swelling and
partial cristolysis; and ii) necrosis, characterized by complete
cristolysis or ghost cells as described in our previous study
(15).
Measurement of ATP content
Isolated mitochondria (n=6 animals per group) were
used to measure ATP synthesis with a luciferase/luciferin-based
system as previously described (19). The ATP Assay kit (cat. no. S0027;
Beyotime Institute of Biotechnology) was used to measure ATP
content according to the manufacturer's protocol.
Mitochondrial DNA quantification
At 12 h after resuscitation, the rats (n=6 per
group) were sacrificed as described above. DNA from the hippocampal
tissues was harvested using the Wizard SV Genomic DNA Purification
System (Promega Corporation) according to manufacturer's
instructions. mtDNA levels were measured by normalizing the
mitochondrial cytochrome b gene (MT-CYB; gene ID, Rn03296746_s1) to
the nuclear heat shock protein 70 (Hspa1a; gene ID, Rn00583013_s1)
gene. All samples were replicated three times in a 25-ml reaction
volume containing 50 ng sample DNA and 2X Probe RT-Mastermix
(Qiagen, Inc.) with both probes in each well. The MT-CYB probe was
labeled with hexachloro fluorescein, and the Hspa1a probe was
labeled with carboxyfluorescein. The following amplification
conditions were used: 95°C for 15.05 min, followed by 45 cycles of
95°C for 15 sec and 60°C for 1 min. The 2−∆∆Cq method
was used to quantify the gene expression levels.
Western blotting
At 12 h after HSR, total protein was extracted from
the hippocampal tissues (n=6 per group) as described in the IL-1β
and IL-18 assay. Samples containing 30 µg protein quantified
by bicinchoninic acid (BCA) assay were separated by 10% SDS-PAGE
and transferred onto a PVDF membrane. The membrane was blocked with
5% skimmed milk at 37°C for 2 h, and primary antibodies rabbit
anti-rat polyclonal cleaved caspase-1 (1:1,000; cat. no. ab1872;
Abcam), monoclonal NLRP3 (1:1,000; cat. no. ab210491; Abcam),
polyclonal Gasdermin D (GSDMD; 1:1,000; cat. no. ab219800; Abcam),
polyclonal IL-1b (1:1,000; cat. no. K107559P; Beijing Solarbio
Science & Technology Co., Ltd.) and polyclonal IL-18 (1:1,000;
cat. no. K002143P; Beijing Solarbio Science & Technology Co.,
Ltd.) were applied at 4°C overnight, followed by incubation with a
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (1:2,000; cat. no. sc-2004; Santa Cruz Biotechnology,
Inc.) at 25°C for 1 h. Protein bands in each treatment group were
detected using an enhanced chemiluminescence western blot detection
system ImageJ 1.48u software (National Institutes of Health).
β-actin (1:2,000; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc.) was used as an internal reference (20).
Co-immunoprecipitation (Co-IP)
Total protein was extracted from the hippocampal
tissues (n=6 per group) 12 h after HSR and lysed in tissue lysis
buffer as described above. Following centrifugation at 15,000 × g
and 4°C for 10 min, the supernatant was collected, and the protein
concentration was measured with a BCA protein assay. Rabbit
anti-ASC polyclonal antibodies (3 µg) were added to 500
µg tissue lysate and incubated at 4°C for 60 min with gentle
mixing. After incubation with 20 µl Protein A/G Plus-Agarose
beads (Santa Cruz Biotechnology, Inc.) overnight at 4°C, the
resultant mixture was centrifuged at 4,000 × g and 4°C for 5 min.
The sample buffer was washed with PBS three times and added to the
agarose beads. The samples were analyzed by western blotting as
described above with antibodies against mouse ASC (1:500; cat. no.
ab175449; Abcam), NLRP3 (1:500; cat. no. ab214185; Abcam) and
β-actin (1:2,000; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc.).
Statistical analysis
SPSS 17.0 for Windows (SPSS, Inc.) was used to
conduct all statistical analyses. Data are presented as the mean ±
standard deviation. The Levene test was used to assess the
assumption of homogeneity of variance. When data heteroscedasticity
was identified, it was corrected by logarithmic transformation. A
one-way analysis of variance followed by post hoc Bonferroni test
was used to analyze the differences among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
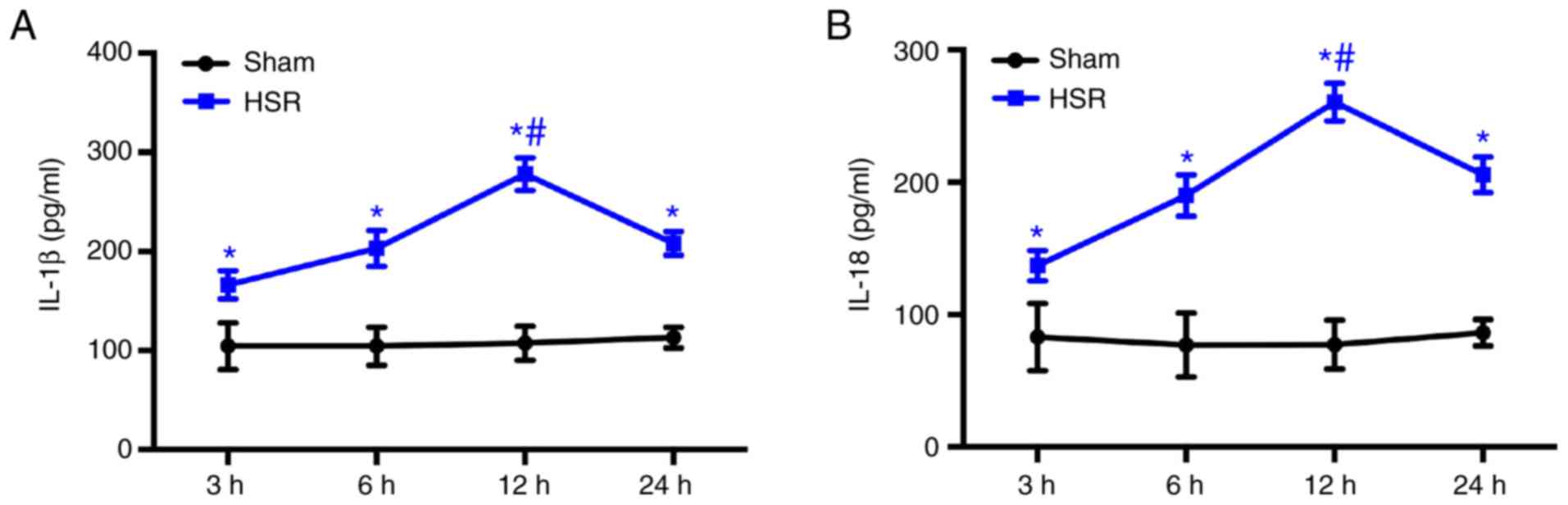
HSR induces increases in IL-1β and IL-18
levels
Rats were treated with hemorrhage shock for 1 h and
resuscitated by blood perfusion to observe the effects of CORM-3 on
HSR exposure. A total of 14 rats died after HSR, and the cause of
death determined by postmortem examination was mainly acute
respiratory distress syndrome or pulmonary embolism. The IL-1β and
IL-18 levels in the hippocampal tissue at different time points
were assessed by ELISA assays. The results demonstrated that HSR
significantly increased the levels of IL-1β and IL-18 at 3, 6, 12
and 24 h post-resuscitation compared with the sham group
(P<0.05; Fig. 1A and B).
Maximum IL-1β and IL-18 levels were observed at 12 h post-HSR
(Fig. 1A and B).
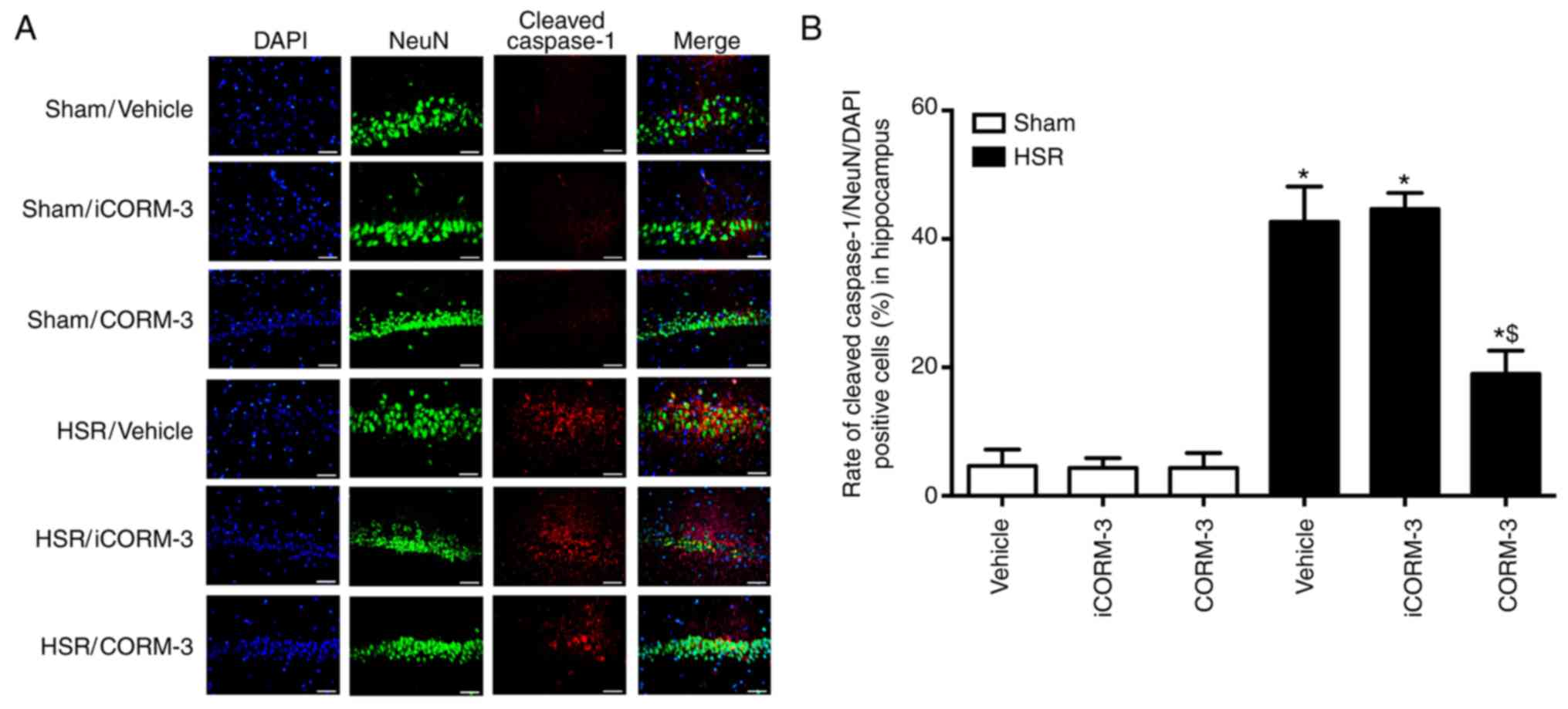
CORM-3 protected against neuronal
pyroptosis in the hippocampus after HSR
Immunofluorescence of cleaved caspase-1 combined
with NeuN and DAPI was used to determine the effects of CORM-3 on
neuronal pyroptosis at 12 h post-HSR (Figs. 2A and 3A). In the sham groups, a small number
of hippocampal neurons exhibited the pyroptotic phenotype in the
CA1 region of the hippocampus (positive for cleaved caspase-1 and
NeuN; Fig. 2A and B). The number
of cells of this type was notably increased in HSR-treated rats
compared with the sham group (P<0.05; Fig. 2A and B). CORM-3-treated rats
exhibited a reduced number of pyroptotic neurons double-stained
with cleaved caspase-1 and NeuN compared with the vehicle and
iCORM-3 groups (P<0.05), whereas the iCORM-3-treated group did
not exhibit a reduction (Fig. 2A and
B). Pyroptosis was also evaluated by determining the expression
levels of cleaved caspase-1 (22 kDa), GSDMD (31 kDa), NLRP3, IL-1β,
and IL-18 using western blotting; the results demonstrated that
rats treated with CORM-3 after HSR exhibited lower expression
levels of hippocampal cleaved caspase-1, GSDMD and NLRP3 compared
with the HSR iCORM-3- and vehicle-treated rats (P<0.05; Fig. 3A-D). As presented in Fig. 3E-F, similar results were observed
for IL-1β and IL-18 expression levels.
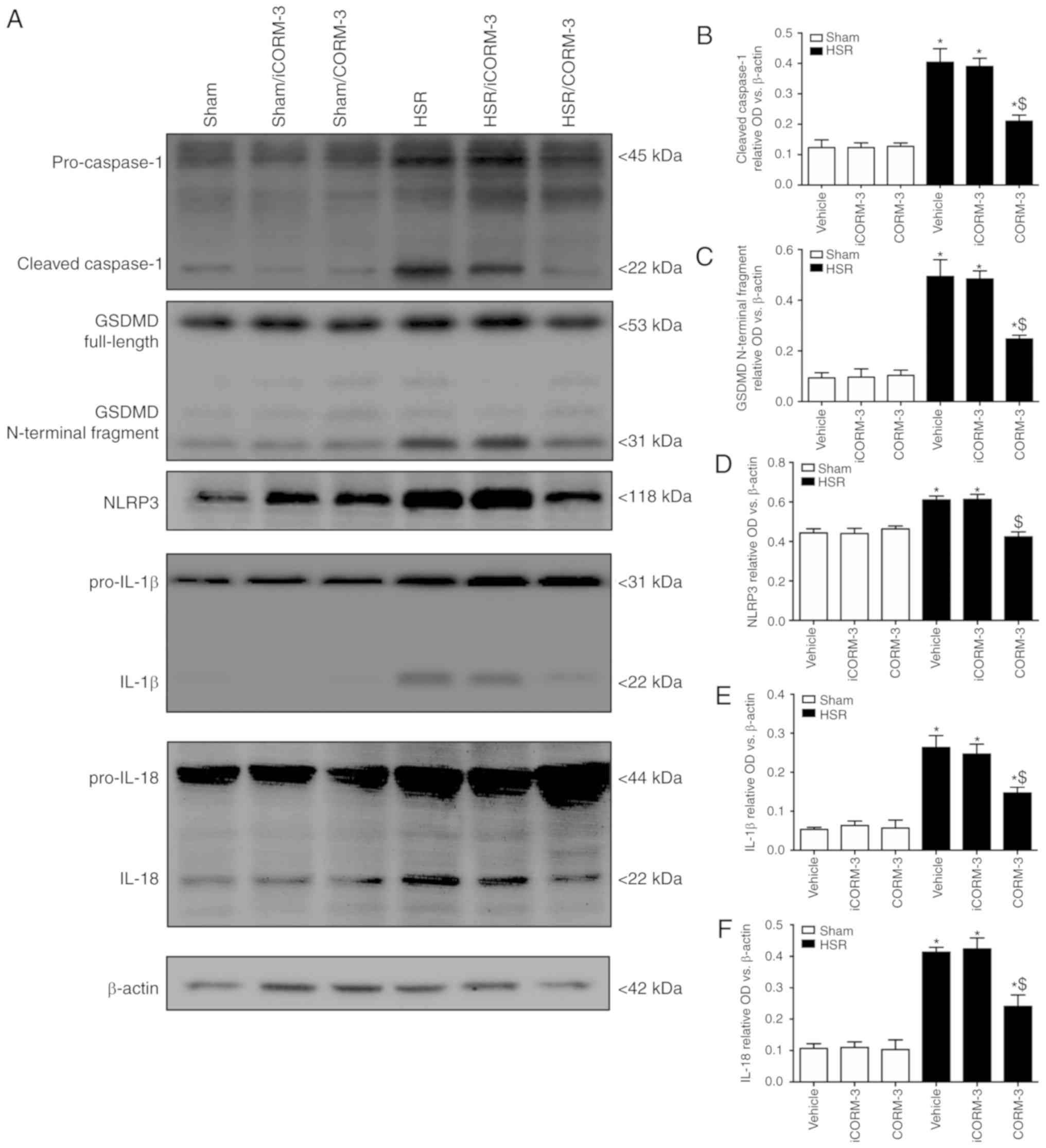 | Figure 3CORM-3 ameliorates upregulation in
pyroptosis-associated protein expression in the hippocampus. (A)
Representative western blots of cleaved caspase-1, GSDMD, NLRP3,
IL-1β and IL-18 in the hippocampal tissue. (B-F) Relative
expression levels of cleaved caspase-1, GSDMD, NLRP3, IL-1β and
IL-18 in the hippocampal tissue evaluated by western blotting. Data
are presented as the mean ± SD (n=6 per group).
*P<0.05 vs. Sham; $P<0.05 vs. HSR
vehicle and HSR iCORM-3. CORM, carbon monoxide-releasing molecule;
HSR, hemorrhage shock and resuscitation; iCORM-3, inactive CORM-3;
NLRP3, nucleotide-binding oligomerization domain-like receptors
pyrin domain-3; IL, interleukin; GSDMD, Gasdermin D. |
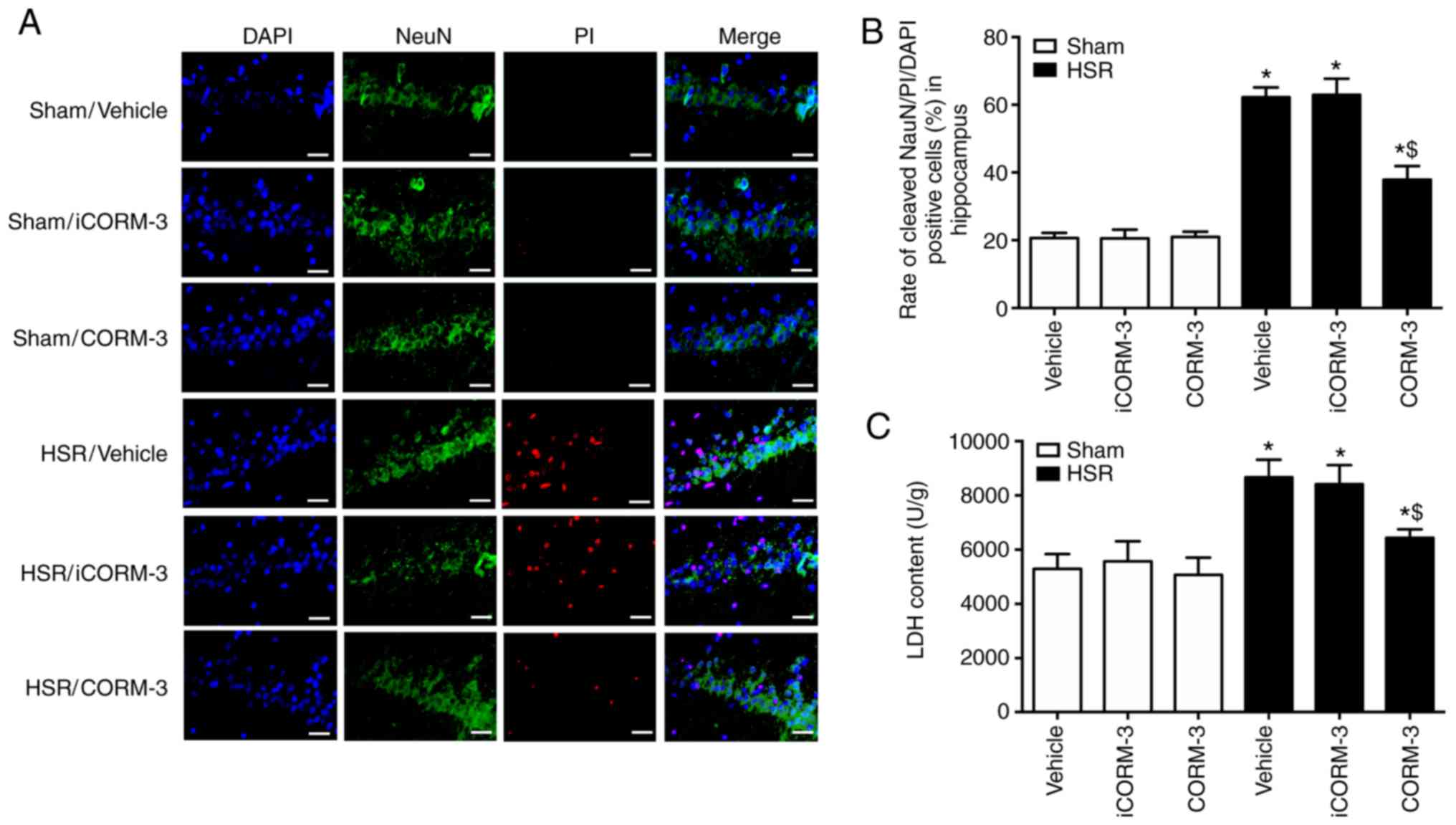
NeuN-FITC/PI/DAPI staining was used for detecting
neuronal death. The results revealed that CORM-3 notably decreased
the number of NeuN-FITC/PI double-positive cells after HSR
(P<0.05; Fig. 4A and B). A
similar effect was observed using the LDH release assay, as LDH
release was lower in HSR-treated rats after CORM-3 treatment
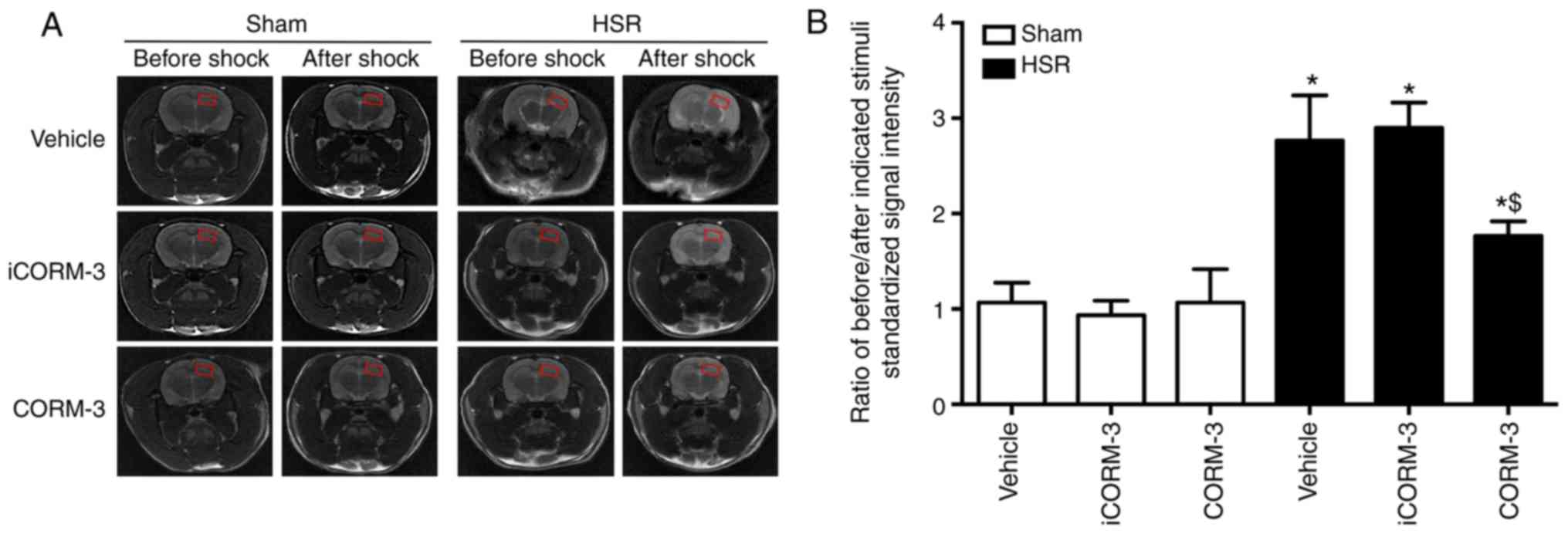
compared with the HSR vehicle and iCORM-3 groups (Fig. 4C). In addition, the results also
revealed that HSR significantly increased the ratio of SSI compared
with the sham group (P<0.05; Fig.
5A and B). A similar effect was observed using the T2-weighted
MRI assay, as demonstrated by the reduced SSI in HSR-treated rats
after CORM-3 treatment compared with the iCORM-3 group (P<0.05;
Fig. 5B). No significant
differences were observed between the HSR vehicle and HSR iCORM-3
groups.
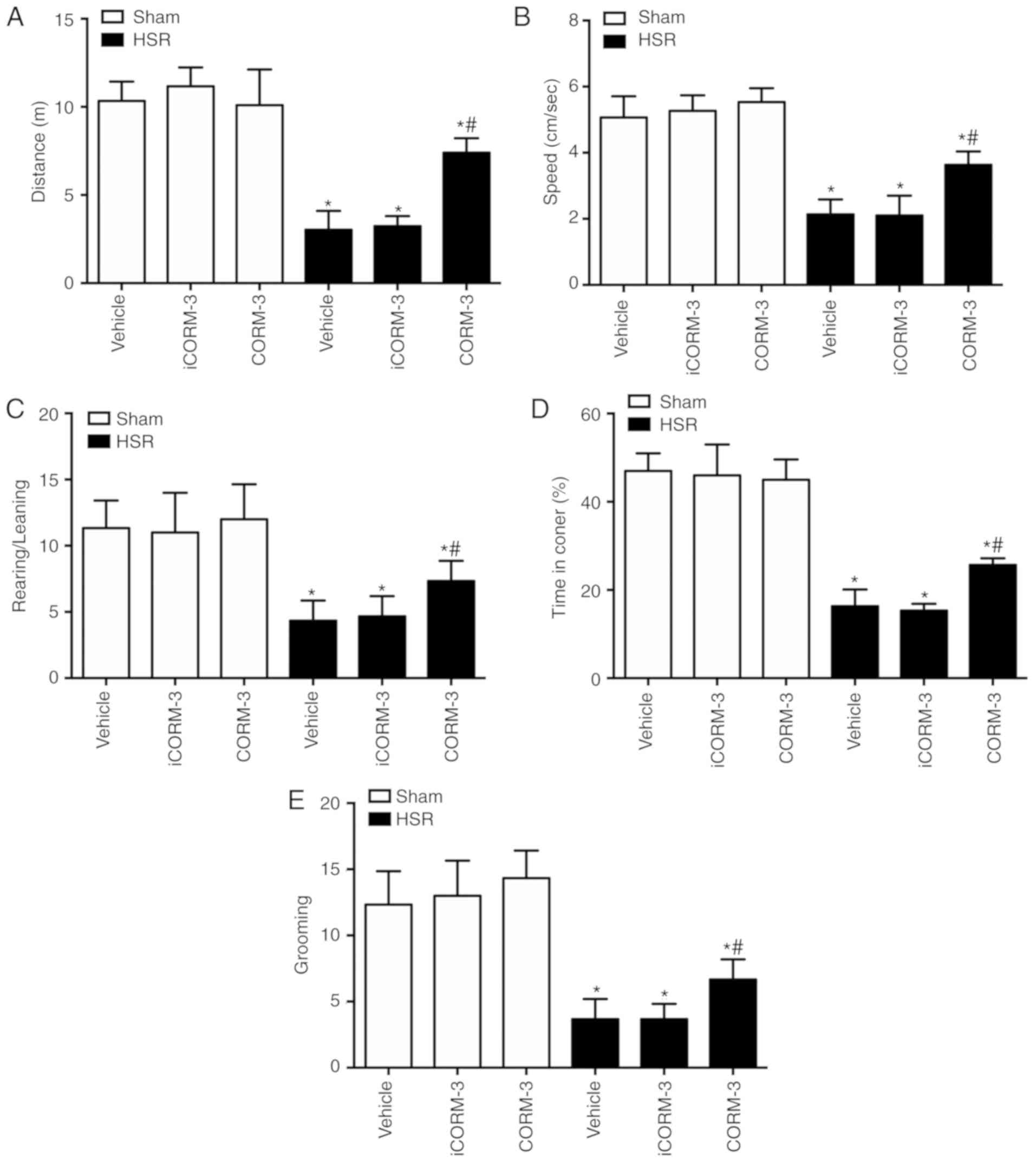
CORM-3 improves behavioral deficits in
HSR-treated rats
To investigate the improvement in behavioral
deficits induced by CORM-3 after HSR, locomotor and exploratory
activities were assessed using an open field test 7 days
post-resuscitation. Locomotion was indicated by total moving
distance and average speed, whereas exploratory activity was
represented by the scores of rearing/leaning and anxiety level
reflected by time spent in the corner and the number of grooming
events. Compared with the sham group, the HSR-treated rats
exhibited a reduced traveling distance, slower moving speed, lower
rearing/leaning scores, spent less time in the corner and grooming
(P<0.05; Fig. 6). Although
iCORM-3 treatment had no effects on these parameters, all values
were improved in the HSR CORM-3 group compared with the HSR iCORM-3
group (P<0.05; Fig. 6). No
significant differences were observed among the sham, sham iCORM-3
and sham CORM-3 groups.
MRS analysis of metabolites in the
hippocampus of rats exposed to HSR
1H MRS was used to detect the metabolite
ratios at 3, 6, 12 and 24 h post-HSR, revealing that significant
changes in the hippocampus were notable at 3 h post-HSR for the
NAA/Cr, mI/Cr and Lip/Cr ratios. The mI/Cr and Lip/Cr ratios
increased, whereas NAA/Cr decreased within 24 h in the HSR iCORM-3
group compared with the Sham group (P<0.05; Fig. 7). HSR treatment induced the
maximum NAA/Cr ratio at 12 h post-HSR (P<0.05; Fig. 7). CORM-3 administration partially
reversed the changes in the NAA/Cr, mI/Cr and Lip/Cr ratios 3-24 h
post-HSR compared with the HSR iCORM-3 group (P<0.05; Fig. 7).
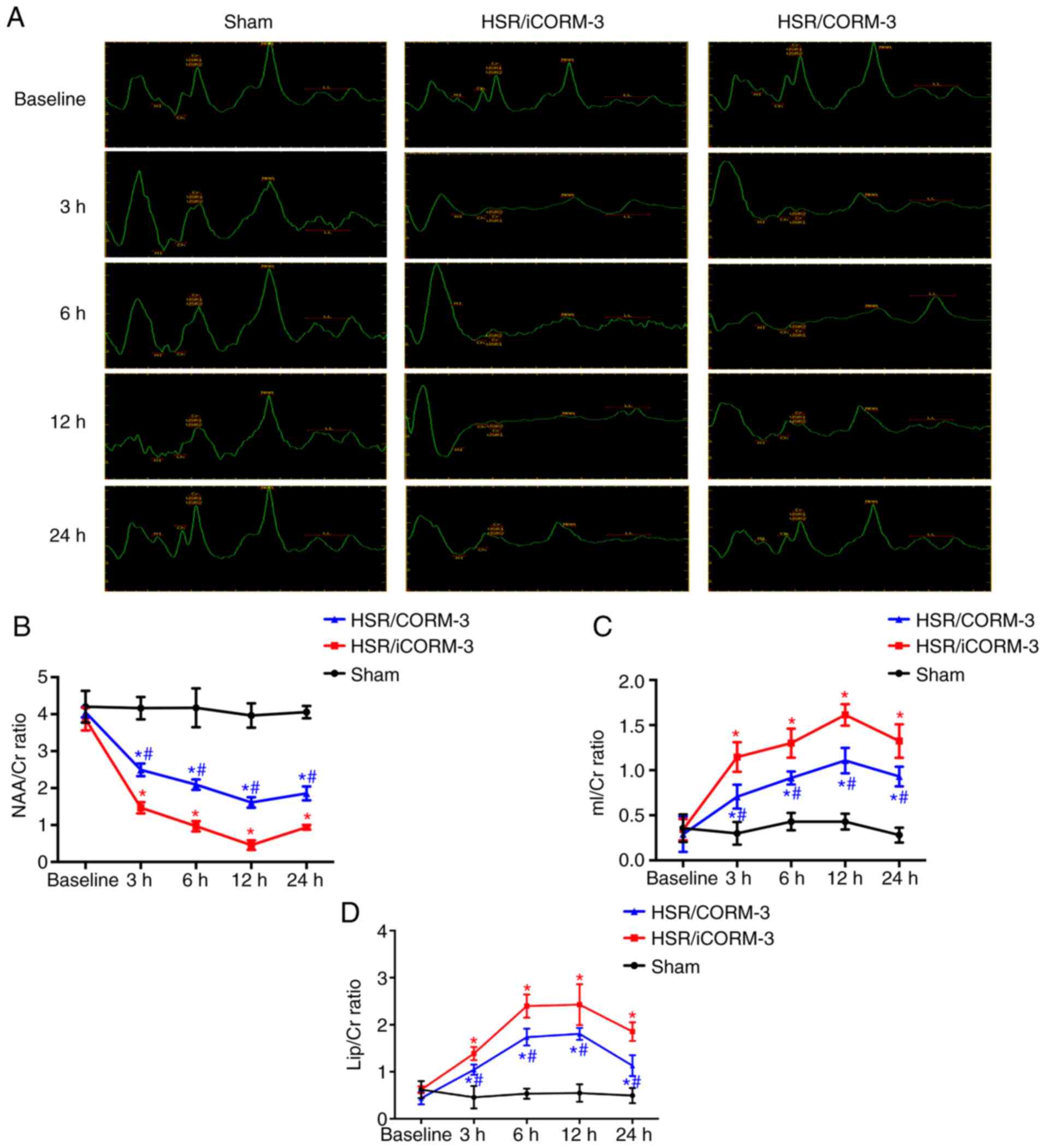 | Figure 7Time course of in vivo
localized 1H MR spectra in the hippocampus induced by
the indicated stimuli. (A) Representative 1H MR spectra
of the hippocampus in the coronal view at 3, 6, 12 and 24 h after
HSR treatment. (B-D) The time course of NAA/Cr, mI/Cr, and Lip/Cr
ratios caused by the indicated stimuli. Data are presented as mean
± SD (n=6 per group). *P<0.05 vs. Sham;
#P<0.05 vs. HSR iCORM-3. CORM, carbon
monoxide-releasing molecule; HSR, hemorrhage shock and
resuscitation; iCORM-3, inactive CORM-3; NAA, N-acetylaspartate;
mI, myoinositol; Lip, lipid; Cr, creatinine. |
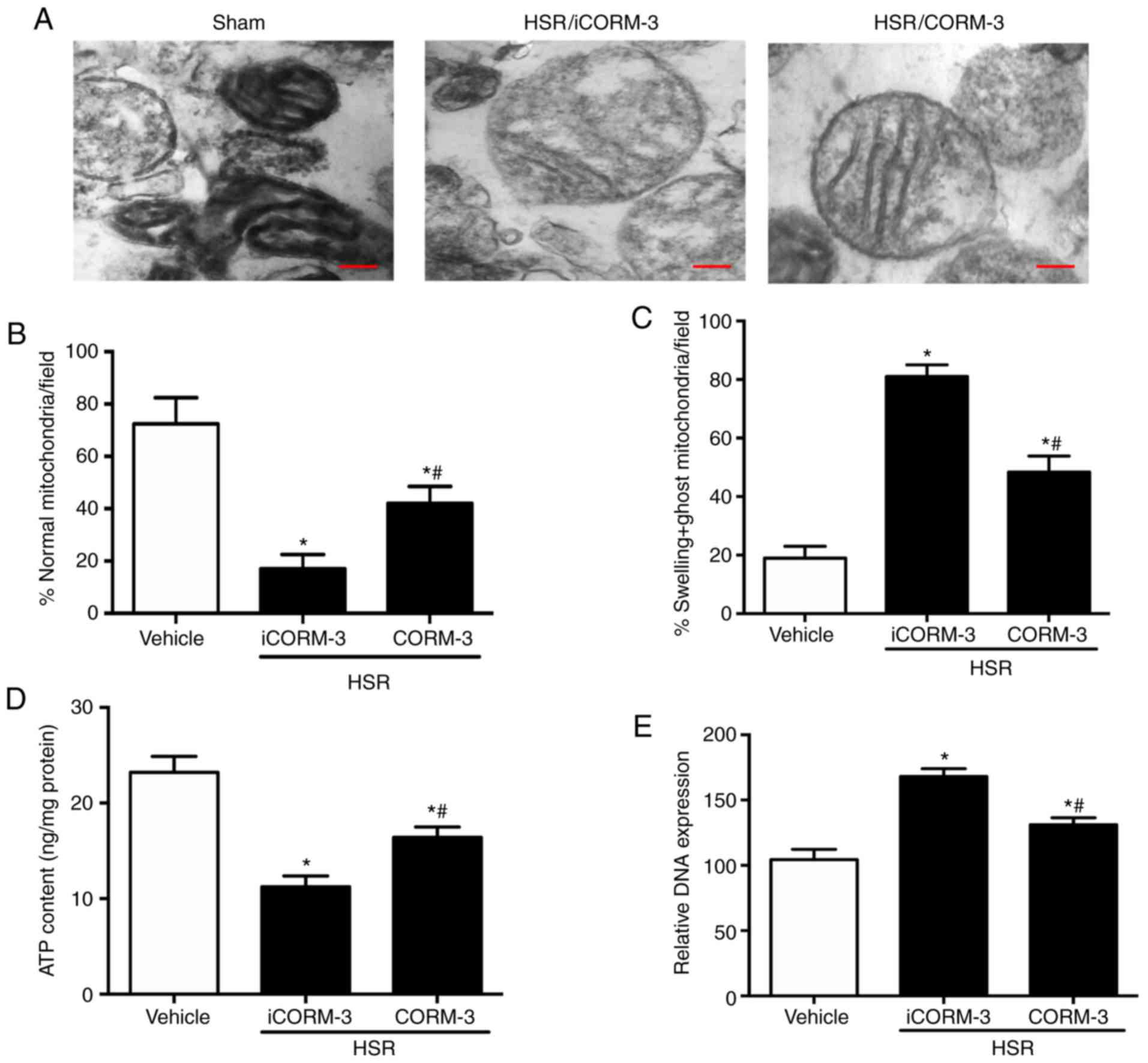
CORM-3 improves mitochondrial dysfunction
in HSR-treated rats
To investigate the improvements in mitochondrial
dysfunction induced by CORM-3 after HSR exposure, the prevalence of
dysmorphic mitochondria (Fig. 8A and
B) and swelling and ghost mitochondria (Fig. 8A and C) were used to determine the
mitochondrial morphology in the hippocampal tissue. A significant
increase in the prevalence of dysmorphic mitochondria and swelling
and ghost mitochondria, as well as a significant decrease in the
level of ATP synthesis, were observed in the HSR iCORM-3 group
compared with the sham group (Fig.
8B-D). CORM-3 treatment after HSR notably improved
mitochondrial morphology and ATP synthesis compared with the HSR
iCORM-3 group. As presented in Fig.
8E, a similar result was also observed for total mtDNA in the
cytosol, which was measured using the mitochondrial cytochrome b
gene. CORM-3 administration after HSR significantly reduced the
level of mtDNA compared with the HSR iCORM-3 group (P<0.05;
Fig. 8E).
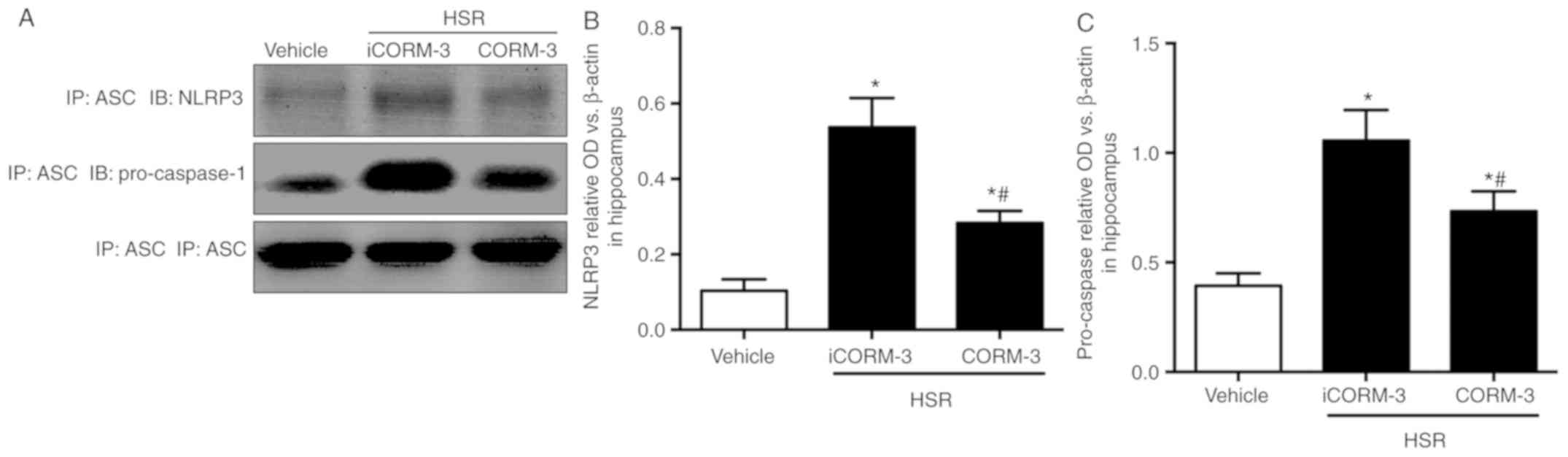
CORM-3 attenuates the interaction of
pro-caspase-1/ NLRP3/ASC after HSR
To determine how CORM-3 inhibits
pro-caspase-1/NLRP3/ASC inflammasome activation, Co-IP was used to
evaluate NLRP3-ASC and pro-caspase-1-ASC interactions. NLRP3 and
ASC interactions were elevated in the HSR iCORM-3 group compared
with the sham group, whereas CORM-3 partially inhibited these
interactions (Fig. 9A). Similar
results were observed for the pro-caspase-1-ASC interactions
(Fig. 9B).
Discussion
The present study determined the effect of CORM-3 on
HSR-induced neuronal dysfunction and provided evidence that
exogenous CO derived from CORM-3 improved rat locomotor and
exploratory activities after HSR. CORM-3 attenuated neuronal
pyroptosis in the hippocampus and prevented subsequent NLRP3
inflammasome activation. In addition, CORM-3 improved mitochondrial
morphology and metabolite ratios and decreased mtDNA levels in the
cytosol.
The cause of death determined by postmortem
examination was mainly acute respiratory distress syndrome or
pulmonary embolism, which was consistent with previous studies
(21,22). Compared with previous study, the
decreased mortality (8%) in this study may be associated with
improved anesthesia and no trauma of laparotomy (22). Incomplete cerebral
ischemia/reperfusion in a rat model of HSR induced severe neuronal
apoptosis and pyroptosis in the hippocampus. Pyroptosis,
characterized as an inflammatory response, occurred in the early
stage after reperfusion (within 24 h post-ischemia), but apoptosis
occurred in the late stage after reperfusion (72 h to 7 days
post-ischemia) in previous studies (15,16). In addition, a high level of
neuronal pyroptosis in the cortical tissue is an important factor
of HSR-induced decrease in learning and memory abilities (15). In the present study, the obtained
results revealed that HSR caused neuronal pyroptosis in the
hippocampus and impaired locomotor and exploratory activities,
revealing that HSR-induced neurological dysfunction may be
associated with neuronal pyroptosis in the hippocampus and may be
characterized by caspase-1 activation and inflammatory factor
release.
CORM-3 alleviated motor functional injury following
spinal cord injury by inhibiting neuronal pyroptosis (23). In addition, CORM-3 attenuated the
degeneration of learning and memory abilities following HSR
mediated by inhibiting neuronal pyroptosis in the cortex (24). NLRP3 inflammasome activation is a
multistep process that includes assembly of ASC and pro-caspase-1
(25). mtDNA from injured
mitochondria has been reported to be fused in response to NLRP3
inflammasome activation (26,27). The results of the present study
revealed that CORM-3 partially reversed the increase in mtDNA
levels and the decreased mitochondrial integrity after HSR compared
with iCORM-3. NLRP3-ASC and pro-caspase-1-ASC interactions were
also inhibited by CORM-3, suggesting that the mechanism of CORM-3
against neuronal pyroptosis may be associated with an improvement
in the dysmorphic mitochondria-induced mtDNA release and an
inhibition of the activation of pro-caspase-1/NLRP3/ASC
inflammasomes.
Ischemia/reperfusion injury induces the release of
neurotoxic amounts of glutamate and causes neuronal calcium
overload, mtDNA leakage and mitochondrial membrane collapse
(28,29). NAA, a surrogate marker of
mitochondrial status, is a general marker of neuronal integrity
viability (30) and a reservoir
that maintains glutamate concentration at a safe level and prevents
it from reaching excitotoxicity (31). Myoinositol (mI), a glial cell
marker, reflects the changes of gliosis (32,33). Regarding the changes of Lip in rat
brains, MRS-detectable Lip/Cr ratio is elevated after ischemic
injury (34). A model of cardiac
arrest has revealed that CO markedly increases ATP and
phosphocreatine levels following reperfusion, and this mechanism
may be associated with the CO-induced improvement of mitochondrial
metabolism (35). In addition,
exogenous CO administration increased the ATP content and improved
oxidative phosphorylation and mitochondrial dysfunction in an
ischemia/reperfusion astrocyte model (36). In the present study, increased
mI/Cr and Lip/Cr ratios, as well as ATP content and a decreased
NAA/Cr ratio were observed in the hippocampus of HSR-treated rats.
CORM-3 significantly partially reversed these changes. Therefore,
the protective effect of CORM-3 against neuronal pyroptosis may be
associated with metabolic reservation after HSR.
There were several limitations to the present study.
First, only one model of incomplete ischemia/reperfusion injury
inducing neuronal pyroptosis in the hippocampus was included. Other
models of incomplete ischemia/reperfusion, such as bilateral common
carotid artery occlusion (37),
should be further studied. Additionally, the present data only
revealed that pro-caspase-1/NLRP3/ASC inflammasomes may be
associated with the neuroprotective effects of CORM-3 against
pyroptosis; the role of NLRP3 activated by mitochondrial
dysfunction should be further studied using NLRP3-knockout mice. In
addition, the present study did not focus on whether pyroptosis
depended on GSDMD and caspase-11 signaling pathways (38). Further studies on other models and
feasible signaling pathways should be performed.
In conclusion, the results of the present study
demonstrated that treatment with CORM-3 ameliorated the impairments
of locomotor and exploratory activities in a rat model of HSR. This
mechanism may be associated with the inhibition of mitochondrial
DNA-induced pyroptosis via improvements in cell metabolism.
Funding
The present study is supported by National Natural
Science Foundation of China (81701296).
Availability of data and materials
All data generated and analyzed during the present
study are included in this published article.
Authors' contributions
LF, LMZ, LQK and FHL designed the study. LQK, LF,
LMZ, YL and YCS edited the manuscript. DXZ and LMZ performed
statistical analysis. LF, DXZ, LMZ, YL, XPW, WCZ, CXG, XDW and XJK
performed the experiments and data collection. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The National Institutes of Health Guidelines for the
Care and Use of Laboratory Animals were followed while performing
all animal experiments in this study. The Animal Review Board of
Cangzhou Central Hospital provided formal approval to conduct the
experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CORM
|
carbon monoxide-releasing molecule
|
|
HSR
|
hemorrhage shock and resuscitation
|
|
NLRP3
|
nucleotide-binding oligomerization
domain-like receptors pyrin domain-3
|
|
MRS
|
magnetic resonance spectroscopy
|
Acknowledgments
Not applicable.
References
|
1
|
Demuro JP, Simmons S, Jax J and Gianelli
SM: Application of the shock index to the prediction of need for
hemostasis intervention. Am J Emerg Med. 31:1260–1263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singhal AK, Abrams JD, Mohara J, Hasz RD,
Nathan HM, Fisher CA, Furukawa S and Goldman BI: Potential
suitability for transplantation of hearts from human
non-heart-beating donors: Data review from the gift of life donor
program. J Heart Lung Transplant. 24:1657–1664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dexter F, Hindman BJ, Cutkomp J and Smith
T: Blood warms as it flows retrograde from a femoral cannulation
site to the carotid artery during cardiopulmonary bypass.
Perfusion. 9:393–397. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshioka M, Itoh Y, Mori K, Ueno K,
Matsumoto M and Togashi H: Effects of an interleukin-1beta analogue
[Lys-D-Pro-Thr], on incomplete cerebral ischemia-induced inhibition
of long-term potentiation in rat hippocampal neurons in vivo.
Neurosci Lett. 261:171–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergsbaken T1, Fink SL, den Hartigh AB,
Loomis WP and Cookson BT: Coordinated host responses during
pyroptosis: caspase-1-dependent lysosome exocytosis and
inflammatory cytokine maturation. J Immunol. 187:2748–2754. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekerdag E, Solaroglu I and Gursoyozdemir
Y: Cell death mechanisms in stroke and novel molecular and cellular
treatment options. Curr Neuropharmacol. 16:1396–1415. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL,
Zhan J, Tong YN, Lin LR and He YN: Ischemia-reperfusion induces
renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J
Physiol Renal Physiol. 306:F75–F84. 2014. View Article : Google Scholar
|
|
8
|
Xia P, Pan Y, Zhang F, Wang N, Wang E, Guo
Q and Ye Z: Pioglitazone confers neuroprotection against
ischemia-induced pyroptosis due to its inhibitory effects on
HMGB-1/RAGE and Rac1/ROS pathway by activating PPAR-γ. Cell Physiol
Biochem. 45:2351–2368. 2018. View Article : Google Scholar
|
|
9
|
Schallner N, Fuchs M, Schwer CI, Loop T,
Buerkle H, Lagrèze WA, van Oterendorp C, Biermann J and Goebel U:
Postconditioning with inhaled carbon monoxide counteracts apoptosis
and neuroinflammation in the ischemic rat retina. PLoS One.
7:e464792012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
R Oliveira S, Queiroga CS and Vieira HL:
Mitochondria and carbon monoxide: Cytoprotection and control of
cell metabolism-a role for Ca(2+) ? J Physiol. 594:4131–4138. 2016.
View Article : Google Scholar
|
|
11
|
Choi EY, Choe SH, Hyeon JY, Choi JI, Choi
IS and Kim SJ: Carbon monoxide-releasing molecule-3 suppresses
Prevotella intermedia lipopolysaccharide-induced production of
nitric oxide and interleukin-1β in murine macrophages. Eur J
Pharmacol. 764:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DW, Shin HY, Jeong JH, Han J, Ryu S,
Nakahira K and Moon JS: Carbon monoxide regulates
glycolysis-dependent NLRP3 inflammasome activation in macrophages.
Biochem Biophys Res Commun. 493:957–963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Tao A, Lan T, Cepinskas G, Kao R,
Martin CM and Rui T: Carbon monoxide releasing molecule-3 improves
myocardial function in mice with sepsis by inhibiting NLRP3
inflammasome activation in cardiac fibroblasts. Basic Res Cardiol.
112:162017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Latz E, Xiao TS and Stutz A: Activation
and regulation of the inflammasomes. Nat Rev Immunol. 13:397–411.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang LM, Zhang DX, Fu L, Li Y, Wang XP,
Qi MM, Li CC, Song PP, Wang XD and Kong XJ: Carbon
monoxide-releasing molecule-3 protects against cortical pyroptosis
induced by hemorrhagic shock and resuscitation via mitochondrial
regulation. Free Radic Biol Med. 141:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang LM and Zhang DX: The dual
neuroprotective-neurotoxic effects of sevoflurane after hemorrhagic
shock injury. J Surg Res. 235:591–599. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ampawong S, Isarangkul D and Aramwit P:
Sericin ameliorated dysmorphic mitochondria in high-cholesterol
diet/streptozotocin rat by antioxidative property. Exp Biol Med
(Maywood). 242:411–421. 2017. View Article : Google Scholar
|
|
18
|
Mariappan N, Soorappan RN, Haque M,
Sriramula S and Francis J: TNF-α-induced mitochondrial oxidative
stress and cardiac dysfunction: Restoration by superoxide dismutase
mimetic Tempol. Am J Physiol Heart Circ Physiol. 293:H2726–H2737.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao P, Liang Z, Piao H, Ji X, Wang Y, Liu
Y, Liu R and Liu J: Conditioned medium of human adipose-derived
mesenchymal stem cells mediates protection in neurons following
glutamate excitotoxicity by regulating energy metabolism and GAP-43
expression. Metab Brain Dis. 29:193–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang DX, Zhang LM, Zhao XC and Sun W:
Neuroprotective effects of erythropoietin against
sevoflurane-induced neuronal apoptosis in primary rat cortical
neurons involving the EPOR-Erk1/2-Nrf2/Bach1 signal pathway. Biomed
Pharmacother. 87:332–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawanishi S, Takahashi T, Morimatsu H,
Shimizu H, Omori E, Sato K, Matsumi M, Maeda S, Nakao A and Morita
K: Inhalation of carbon monoxide following resuscitation
ameliorates hemorrhagic shock-induced lung injury. Mol Med Rep.
7:3–10. 2013. View Article : Google Scholar
|
|
22
|
Chamorro V, Pandolfi R, Moreno L, Barreira
B, Martínez-Ramas A, Morales-Cano D, Ruiz-Cabello J, Lorente JA,
Duarte J, Cogolludo Á, et al: Effects of quercetin in a rat model
of hemorrhagic traumatic shock and reperfusion. Molecules. 21:pii:
E1739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Wang J, Zhang Q, Duan X, Chen Z and
Zhang Y: Postconditioning with sevoflurane ameliorates spatial
learning and memory deficit after hemorrhage shock and
resuscitation in rats. J Surg Res. 206:307–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng G, Zhan Y, Wang H, Luo Z, Zheng F,
Zhou Y, Wu Y, Wang S, Wu Y, Xiang G, et al: Carbon monoxide
releasing molecule-3 alleviates neuron death after spinal cord
injury via inflammasome regulation. EBioMedicine. 40:643–654. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jabaut J, Ather JL, Taracanova A, Poynter
ME and Ckless K: Mitochondria-targeted drugs enhance Nlrp3
inflammasome-dependent IL-1β secretion in association with
alterations in cellular redox and energy status. Free Radic Biol
Med. 60:233–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha
X, Cheng X, Wang J, Qin X, Yu J, et al: Caspase-1 inflammasome
activation mediates homocysteine-induced pyrop-apoptosis in
endothelial cells. Circ Res. 118:1525–1539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahn H, Kim J, Kang SG, Yoon SI, Ko HJ, Kim
PH, Hong EJ, An BS, Lee E and Lee GS: Mercury and arsenic attenuate
canonical and non-canonical NLRP3 inflammasome activation. Sci Rep.
8:136592018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kravcukova P, Danielisova V, Nemethova M,
Burda J and Gottlieb M: Transient forebrain ischemia impact on
lymphocyte DNA damage, glutamic acid level, and SOD activity in
blood. Cell Mol Neurobiol. 29:887–894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lehotský J, Racay P, Pavlíková M,
Tatarková Z, Urban P, Chomová M, Kovalská M and Kaplán P:
Cross-talk of intracellular calcium stores in the response to
neuronal ischemia and ischemic tolerance. Gen Physiol Biophys.
28:F104–F114. 2009.
|
|
30
|
Signoretti S, Marmarou A, Tavazzi B,
Lazzarino G, Beaumont A and Vagnozzi R: N-Acetylaspartate reduction
as a measure of injury severity and mitochondrial dysfunction
following diffuse traumatic brain injury. J Neurotrauma.
18:977–991. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ory-Lavollée L, Blakely RD and Coyle JT:
Neurochemical and immunocytochemical studies on the distribution of
N-acetyl-aspartylglutamate and N-acetyl-aspartate in rat spinal
cord and some peripheral nervous tissues. J Neurochem. 48:895–899.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang C and Jang T: Age-dependent
neurotoxicity of striatal lesions produced by aminooxyacetic acid:
Quantitative in vitro 1H NMR spectroscopic studies. J Neurochem.
65:1192–1198. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mierisová S and Ala-Korpela M: MR
spectroscopy quantitation: A review of frequency domain methods.
NMR Biomed. 14:247–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harada K, Honmou O, Liu H, Bando M, Houkin
K and Kocsis JD: Magnetic resonance lactate and lipid signals in
rat brain after middle cerebral artery occlusion model. Brain Res.
1134:206–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lavitrano M, Smolenski RT, Musumeci A,
Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi
G, Patti M, et al: Carbon monoxide improves cardiac energetics and
safeguards the heart during reperfusion after cardiopulmonary
bypass in pigs. FASEB J. 18:1093–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Almeida AS, Queiroga CS, Sousa MF, Alves
PM and Vieira HL: Carbon monoxide modulates apoptosis by
reinforcing oxidative metabolism in astrocytes: Role of Bcl-2. J
Biol Chem. 287:10761–10770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chai H, Schultz G, Aghaie K and Zhou W: In
vivo assessment of the effects of ginsenoside Rb1 on intimal
hyperplasia in ApoE knockout mice. J Surg Res. 162:26–32. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng S, Fox D and Man SM: Mechanisms of
gasdermin family members in inflammasome signaling and cell death.
J Mol Biol. 430:3068–3080. 2018. View Article : Google Scholar : PubMed/NCBI
|