Introduction
It has been well established that malignant glioma
is one of the most common types of brain neoplasms, and is
associated with high mortality and poor 5-year survival rates
worldwide (1). The currently
available treatment for glioma involves surgical removal of the
tumor, chemotherapy and radiotherapy, which can significantly
improve the survival rates of the patients (2). However, for the majority of patients
with glioma, prognosis is poor, with a median survival of ~14
months (3). Temozolomide (TMZ) is
the most effective chemotherapeutic agent for glioma treatment
(3). However, the response rate
to TMZ treatment is accompanied by resistance to chemotherapy and
high rates of toxicity, which may lead to patients experiencing
severe nausea, vomiting and fetotoxic effects (4). Therefore, the development of novel
anticancer agents that may be used to decrease glioma progression
requires further investigation.
Danshen (Salvia miltiorrhiza Bunge) is a
traditional Chinese herb that has been successfully used for the
treatment of cardiovascular disease in Asian countries (5,6).
TS I has been demonstrated to be one of the bioactive components of
Danshen, and has been reported to possess antioxidant,
anti-inflammatory and anticancer properties (7). Recent studies on TS I have focused
on its anticancer activity (8-10).
These results have demonstrated that TS I may induce the apoptosis
of cancer cells in gastric (10),
human breast (11,12) and human colon cancer (13,14). However, to the best of our
knowledge, the exact mechanisms underlying the effects of TS I on
human glioma have not yet been determined.
To determine the mechanisms underlying the
anticancer activity exhibited by TS I in human glioma, the present
study was performed to elucidate the biological mechanisms through
which TS I may induce the inhibition of human glioma U87 MG cell
growth.
Materials and methods
Reagents and antibodies
TS I was purchased from Sigma-Aldrich; Merck KGaA.
The anti-p-AKT (cat. no. 4058), anti-AKT (cat. no. 9272),
anti-cleaved poly(ADP-ribose) polymerase (PARP) (cat. no. 5625),
anti-GADPH (cat. no. 2118), anti-cyclin B1 (cat. no. 4138),
anti-B-cell lymphoma (Bcl)-2 (cat. no. 15071), anti-beclin-1 (cat.
no. 3738), anti-C/EBP homologous protein (CHOP) (cat. no. 2895),
anti-p-eukaryotic initiation factor (eIF)2α (Ser51) (cat. no.
9721), anti-eIF2α (cat. no. 9722), anti-LC3B (cat. no. 2775) and
anti-Bcl-2-associated X protein (Bax) (cat. no. 2774) antibodies
were purchased from Cell Signaling Technology, Inc. The anti-p21
antibody (cat. no. MAB1047) was purchased from R&D Systems,
Inc. LY294002 was purchased from Merck KGaA. The Annexin V-FITC and
propidium iodide (PI) kit was purchased from BD Biosciences;
Becton, Dickinson and Company. N-acetyl-L-cysteine (NAC), a
reactive oxygen species (ROS) scavenger and 3-methyladenine (3-MA;
an inhibitor of autophagy) were purchased from MedChem Express
LLC.
Cell culture
The U87 MG glioma cell line was purchased from
Procell Life Science & Technology Co., Ltd. (cat no. CL-0238).
The cell line was established in the University of Uppsala and was
authenticated using STR profiling. Cells were maintained in DMEM
supplemented with 10% FBS (Procell) and 1X penicillin-streptomycin
solution.
Cell viability assay
U87 MG glioma cell viability was measured using a
Cell Counting Kit-8 (CCK-8) assay. U87 MG cells were then seeded
into a 96-well plate (6×103 cells/well) for 24 h. Cells
were then treated with TS I (0, 0.625, 1.25, 2.5, 5 or 10
µM), and incubated for an additional 24 h. A total of 10
µl CCK-8 solution was subsequently added to each well and
cells were then incubated for 4 h. The absorbance value of each
well was measured using an ELISA reader at a wavelength of 450 nm.
All experiments were performed three times, and results are
expressed as the mean ± standard deviation.
Cell cycle assay
The cell cycle distribution of human glioma U87 MG
cells was analyzed using flow cytometry and PI staining. Cells were
seeded (1×106 cells/well) into 6-well plates and treated
with different TS I concentrations (0, 0.625, 1.25 and 2.5
µM) for 24 h. The treated cell groups were then fixed in
cold 70% ethanol for 2 h. RNase A (60 µg/ml) and PI (50
µg/ml) in PBS were added, and samples were incubated for 30
min in the dark at room temperature. The cell cycle distribution
was analyzed using flow cytometry (FACSCalibur; BD Biosciences;
Becton, Dickinson and Company). A total of 10,000 cells were
collected for each cell group. The percentage of cell populations
at subG0/G1, G2/M and S phases were examined using Modfit LT
version 2.0 software (Verity Software House). Three independent
experiments were performed.
Cell apoptosis assay
Cell apoptosis was assessed using an Annexin
V-FITC/PI kit (BD Pharmingen; BD Biosciences) and the apoptotic
rate was analyzed using a flow cytometer (FACSCalibur; BD
Biosciences; Becton, Dickinson and Company). U87 MG cells were
seeded (1×106 cells/well) into 6-well plates and treated
with different TS I concentrations (0, 0.625, 1.25 and 2.5
µM) for 24 h. Cells were then suspended in a 5-ml culture
tube with 1X binding buffer (provided with the Annexin V-FITC/PI
kit) at a density of 1×106 cells/ml, stained using
Annexin V-FITC and counterstained using PI in binding buffer at
room temperature for 15 min. The number of apoptotic cells was then
determined using a flow cytometer (FACSCalibur; BD Biosciences;
Becton, Dickinson and Company).
Western blot analysis
Cells were seeded onto 35-mm plates
(2×106 cells), with 2 ml complete DMEM for 24 h.
Following TS I treatment, cells were washed with PBS, and lysed
with RIPA lysis buffer (150 mmol/l; NaCl 50 mmol/l Tris-HCl; pH
7.4; 1% Triton X-100; 1% sodium deoxycholate; 0.1% SDS) with 1 mM
sodium orthovanadate, 1 mM MPMSF and 1% cocktail of protease
inhibitors (Sigma-Aldrich; Merck KGaA). Protein concentration was
determined using the BCA method. Equal quantities of protein (40
µg/lane) were separated using 6-12% SDS-PAGE and transferred
onto nitrocellulose membranes (EMD Millipore). The membranes were
blocked with 5% non-fat milk for 2 h at 25°C. The membranes were
incubated with a variety of primary antibodies as follows: AKT
(1:1,000), p-AKT (1:1,000), cyclin B1 (1:1,000), cleaved PARP
(1:1,000), Bcl-2 (1:1,000), Bax (1:1,000) or p21 (1:1,000),
beclin-1 (1:2,000), CHOP (1:1,000), p-eIF2α (Ser51) (1:3,000),
eIF2α (1:4,000) and LC3B (1:2,000), followed by incubation with
anti-rabbit HRP secondary antibody (1:20,000, cat. no. 7074, Cell
Signaling Technology, Inc.) or anti-mouse HRP-conjugated secondary
antibody (1:20,000, cat. no. 7076, Cell Signaling Technology, Inc.)
for 2 h at 25°C. GAPDH (1:3,000) served as a loading control.
Visualization was achieved using SuperSignal West Pico
chemiluminescent Substrate (Pierce; Thermo Fisher Scientific,
Inc.).
Measurement of ROS generation
Intracellular ROS levels were determined using a ROS
assay kit (Keygen Biotech Co., Ltd.). Briefly, the cells were
plated in 6-well plates at a density of 2.0×105
cells/well for 24 h. Following treatment with TS I (0, 0.625, 1.25
and 2.5 µM) for 12 h, cells were then incubated with 10
µM DCFH-DA for 15 min at 37̊C in the dark. Next, the cells
were examined by flow cytometry using a FACScalibur Flow Cytometer
(BD Biosciences) and the data were analyzed by FlowJo 7.6 software
(FlowJo LLC).
Statistical analysis
All data are presented as the mean ± standard
deviation. A Student's t-test and one-way ANOVA followed by
Dunnett's post hoc test were used to determine significance for
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Result
TS I exerts potent cytotoxic effects on
human glioma U87 MG cells
To investigate the potent anticancer effects of TS I
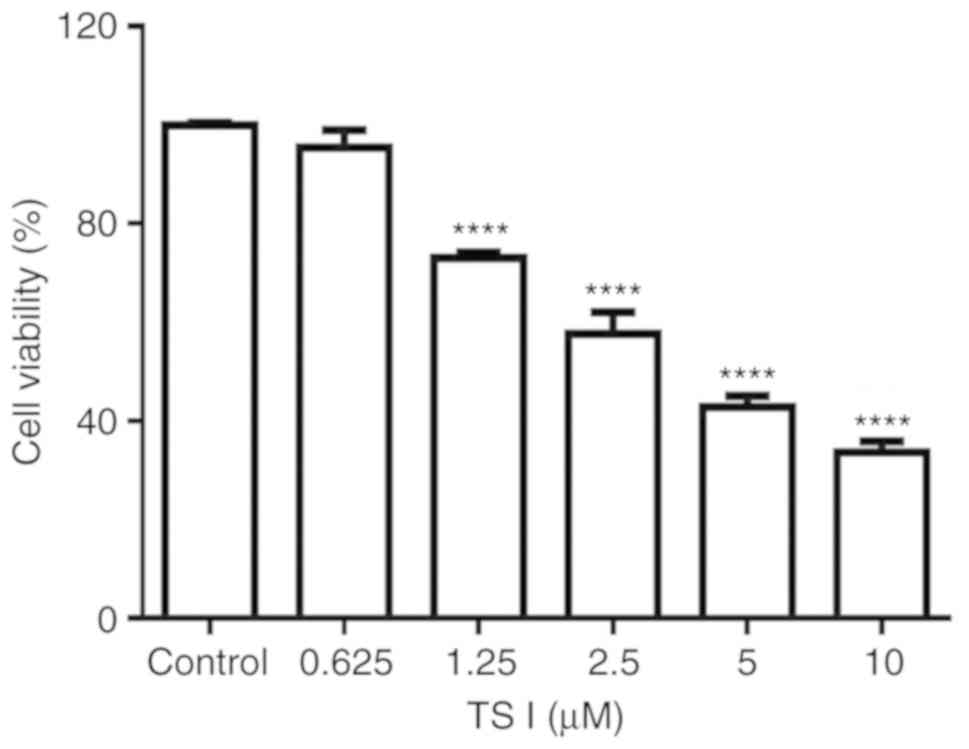
on U87 MG cells, cell viability was analyzed using a CCK-8 assay.
In the present study, U87 MG cells were treated with TS I at
different concentrations (0, 0.625, 1.25, 2.5, 5 and 10 µM)
for 24 h. The results demonstrated that TS I significantly
inhibited U87 MG cell proliferation in a dose-dependent manner.
Cell viability was reduced by 26.92±1.06, 42.37±4.38, 51.17±2.25
and 66.39±2.24% at 1.25, 2.5, 5 and 10 µM TS I,
respectively, after 24 h (Fig.
1). The IC50 value was found to be 3.35±0.44
µM at 24 h. The results demonstrated that TS I exerted
marked cytotoxic effect on U87 MG cells by inhibiting cell
proliferation.
TS I induces cell cycle arrest at the
G2/M phase in human glioma U87 MG cells
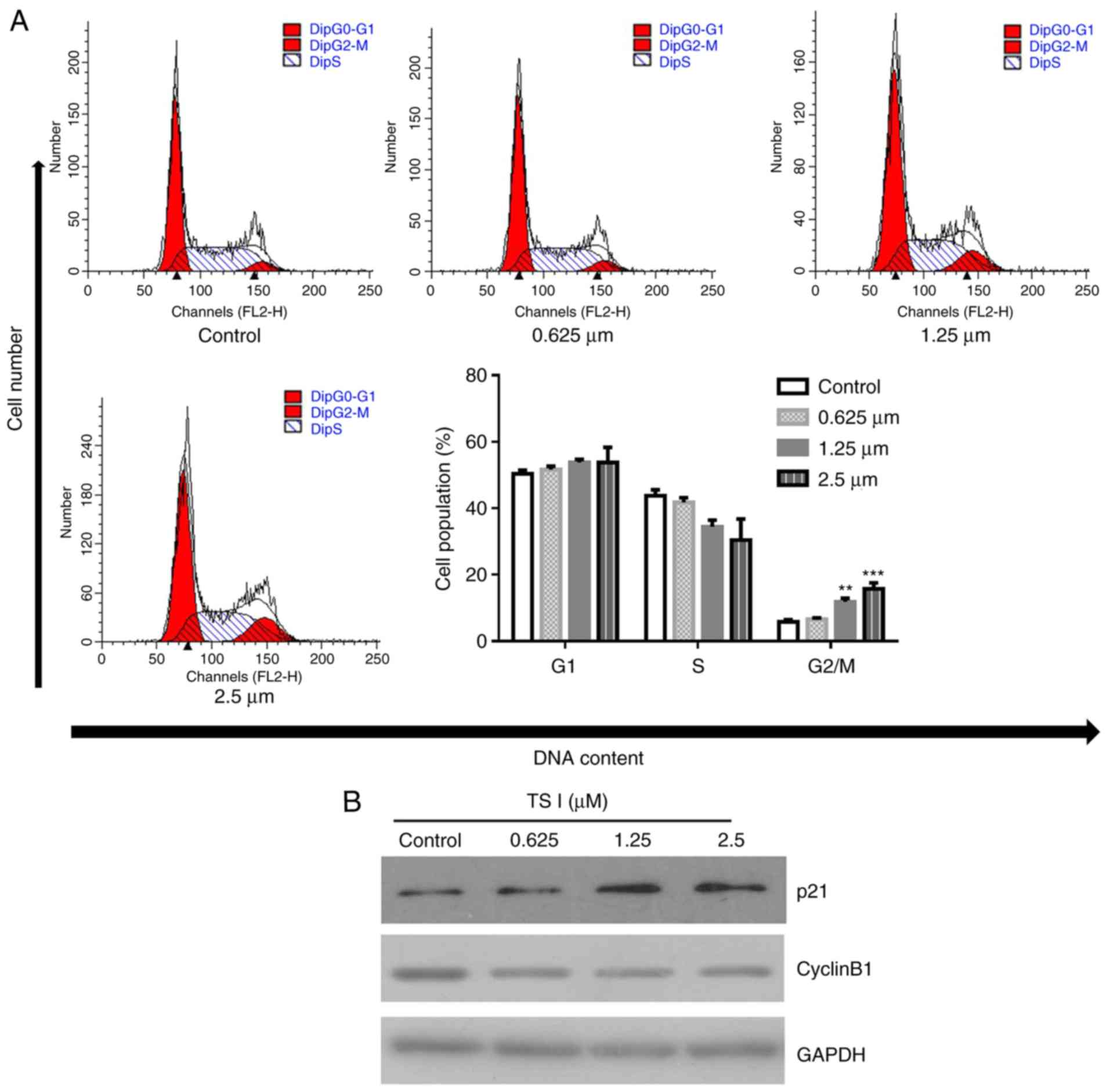
To investigate the mechanisms through which TS I
inhibited U87 MG cell growth, flow cytometry was used to analyze
the distribution of cells within the cell cycle following treatment
with 0, 0.625, 1.25 and 2.5 µM TS I for 24 h. Flow cytometry
indicated that TS I significantly increased the percentage of cells
in the G2/M phase in a dose-dependent manner (6.61±0.40, 11.84±1.08
and 15.79±1.79% in the 0.625, 1.25 and 2.5 µM treatment
groups, respectively) compared with the control group (5.82±0.67%)
(Fig. 2A). Additionally, TS I
treatment significantly decreased the expression of cyclin B1 and
increased the expression of p21 (Fig.
2B). These results indicated that the downregulation of cyclin
B1 expression and the upregulation of p21 expression contributed to
TS I-induced G2/M phase arrest and antiproliferation effect in U87
MG cells.
TS I induces apoptosis in human glioma
U87 MG cells
The present study assessed whether the inhibition of
TS I-induced U87 MG cell growth was associated with apoptosis. Flow
cytometry, which was performed using Annexin V-PI staining,
demonstrated that, following treatment with TS I for 24 h, the
proportion of apoptotic U87 MG cells was markedly increased in a
concentration-dependent manner. The percentage of apoptotic cells
was 6.34±1.54, 10.57±2.28, 21.26±2.48 and 30.33±3.13% in the 0,
0.625, 1.25 and 2.5 µM TS I groups, respectively (Fig. 3A). The levels of
apoptosis-associated proteins were also determined using western
blot analysis. As shown in Fig.
3B, TS I treatment decreased Bcl-2 and increased the cleaved
PARP and Bax expression in a dose-dependent manner. These results
suggested that TS I induces U87 MG cell apoptosis via the
downregulation of Bcl-2 expression and the upregulation of Bax and
cleaved PARP expression.
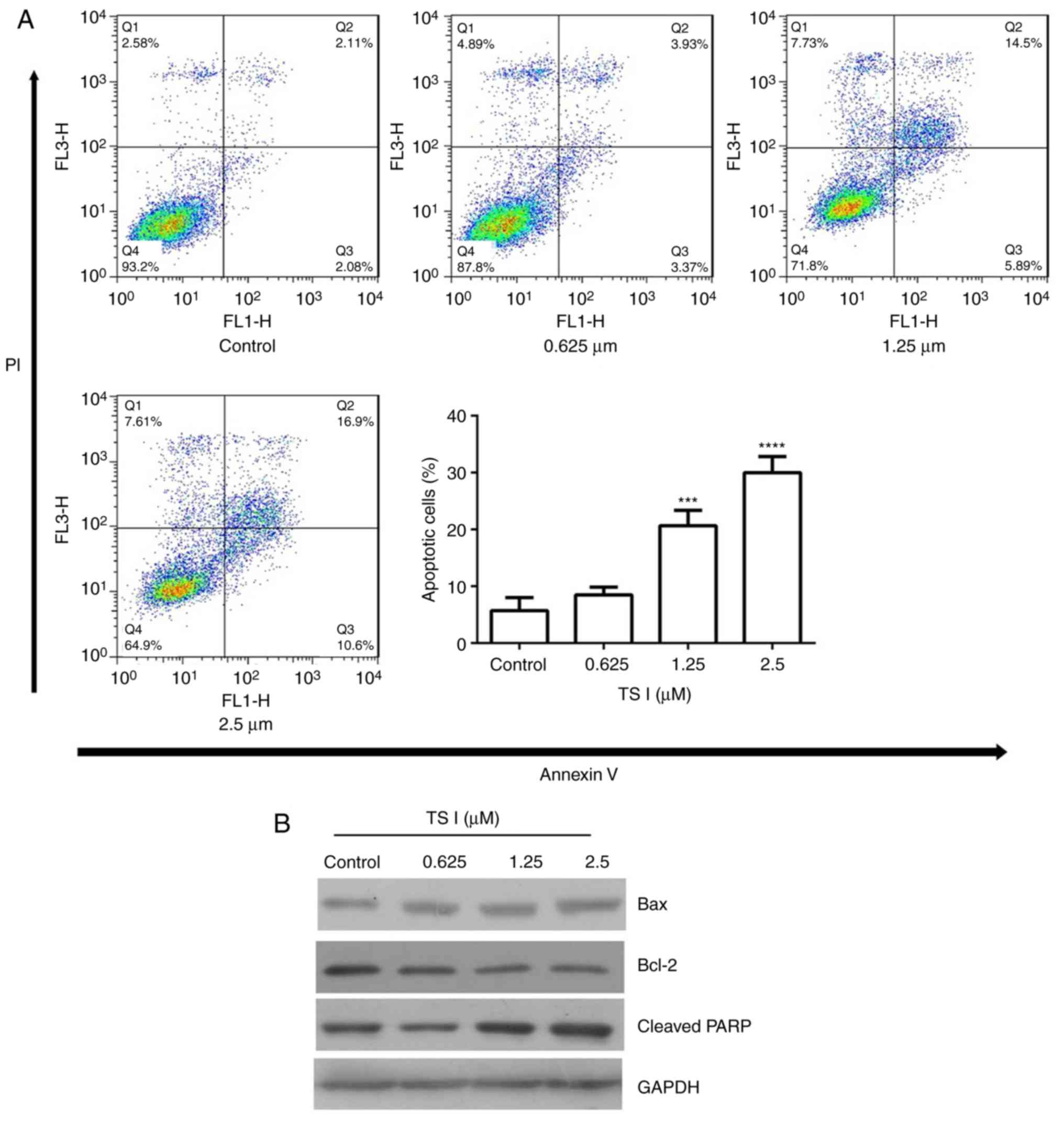 | Figure 3Effects of TS I on U87 MG cell
apoptosis and the expression of cell apoptosis-associated proteins.
(A) Cells were treated with TS I (0, 0.625, 1.25 and 2.5 µm)
for 24 h, and cell apoptosis was analyzed using flow cytometry in
U87 MG cells. (B) Cells were treated with TS I (0, 0.625, 1.25 and
2.5 µm) for 12 h, and the protein levels of Bcl-2, Bax and
cleaved PARP were assessed using western blot analysis in U87 MG
cells. GAPDH was used as a loading control. ***P<0.001;
****P<0.0001. TS-I, tanshinone I; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; PARP, poly(ADP-ribose)
polymerase. |
TS I induces apoptosis via ROS production
in human glioma U87 MG cells
TS I has been previously shown to induce apoptosis
by increasing ROS levels in colon cancer cells (9). Therefore, whether ROS production was
associated with TS I-induced apoptosis of U87 MG cells was assessed
in the present study. Cells were treated with TS I (0, 0.625, 1.25
and 2.5 µM) for 12 h and analyzed using flow cytometry. As
shown in Fig. 4A, ROS generation
was significantly increased in a dose-dependent manner.
Furthermore, cells were pre-treated with 4 mM ROS scavenger NAC for
1 h, and exposed to TS I (1.25 µM) for 24 h. Cell viability
was analyzed using a CCK-8 assay. The results indicated that 4 mM
NAC significantly reduced TS I-induced inhibition of cell growth
(Fig. 4B). Furthermore,
pretreatment with NAC significantly restored the TS I-induced
upregulation of Bax and cleaved PARP expression, and the
downregulation of Bcl-2 expression (Fig. 4C). These data suggest that TS I
promotes ROS generation and this effect is associated with TS
I-induced cell apoptosis.
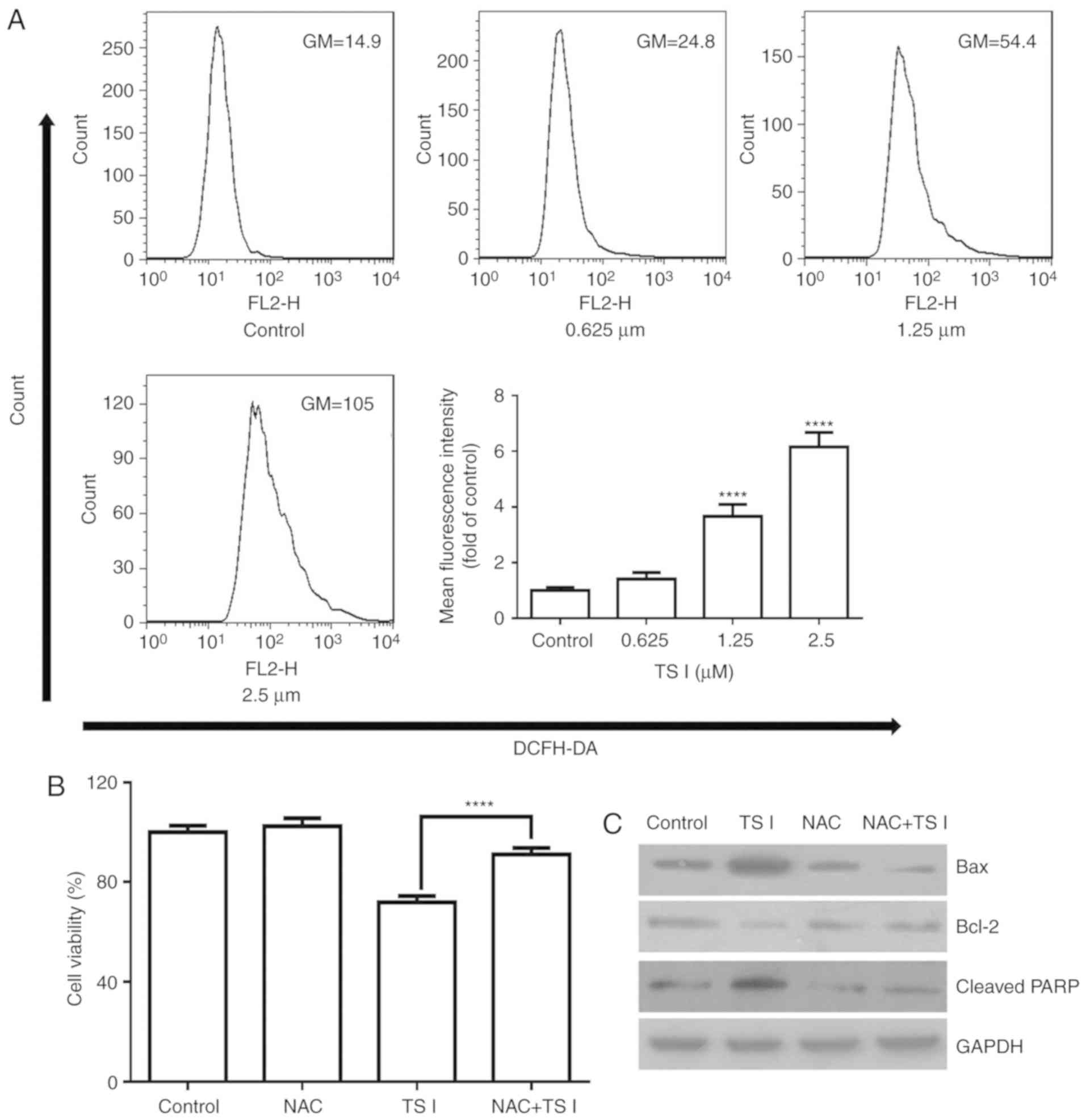 | Figure 4Effects of TS I on intracellular ROS
generation in U87 MG cells. (A) Cells were treated with TS I (0,
0.625, 1.25 and 2.5 µm) for 12 h, and the levels of ROS were
examined using flow cytometry. Bar graphs indicate the
quantification of ROS. (B) U87 MG cells were pretreated with NAC (4
mM) for 1 h, followed by treatment with TS I (1.25 µm) for
24 h. Cell viability was then detected using a Cell Counting Kit-8
assay. (C) U87 MG cells were pretreated with NAC (4 mM) for 1 h,
followed by treatment with TS I (1.25 µm) for 12 h, and the
protein levels of Bcl-2, Bax and cleaved PARP were subsequently
assessed using western blot analysis. GAPDH was used as a loading
control. ****P<0.0001. TS-I, tanshinone I; ROS,
reactive oxygen species; NAC, N-acetyl-L-cysteine; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; PARP, poly(ADP-ribose)
polymerase. |
TS I induces apoptosis by suppressing the
AKT signaling pathway in human glioma U87 MG cells
AKT signaling is a key downstream effector of
phosphoinositide 3-kinase (PI3K) that regulates a variety of
biological processes, including cell cycle progression,
proliferation and apoptosis in cancer cells (15,16). Previous studies have focused on
the role of the AKT signaling pathway in human gliomas (17,18). To determine the molecular
mechanisms underlying the TS I-induced apoptosis of U87 MG cells,
the phosphorylation/activation of AKT in cells treated with TS I
(0, 0.625, 1.25 and 2.5 µM) was assessed for 12 h. The
phosphorylation of AKT was found to decrease following TS I
treatment in a dose-dependent manner. However, TS I exerted no
effect on total AKT expression in U87 MG cells (Fig. 5A). To further elucidate whether TS
I-induced apoptosis was associated with AKT signaling, cells were
treated with 5 µM PI3K inhibitor (LY294002) for 1 h prior to
treatment with TS I (1.25 µM) for 24 h. Cell viability was
measured in U87 MG cells treated with TS I alone or in combination
with LY294002. As shown in Fig.
5B, TS I (1.25 µM) in combination with 5 µM
LY294002 significantly inhibited U87 MG cell proliferation compared
with TS I treatment alone. Additionally, apoptosis-associated
protein expression was also determined using western blot analysis.
As shown in Fig. 5C, LY294002
with TS I significantly decreased Bcl-2 protein expression and
significantly increased the cleaved PARP and Bax protein expression
compared with TS I treatment alone. Furthermore, the ROS-mediated
AKT signaling pathway has been reported to be an important
regulator of apoptosis in cancer cells, and has been shown to
affect cell proliferation (19,20). To elucidate the role of ROS
production in the TS I-mediated inhibition of the AKT signaling
pathway in U87 MG cells, the cells were treated with NAC for 1 h
and subsequently treated with TS I (1.25 µM) for 12 h. As
shown in Fig. 5D, western blot
analysis revealed that TS I-induced reduction of p-AKT was reversed
in the NAC pre-treatment group compared with the TS I alone group.
These data indicated that TS I may induce apoptosis by regulating
the ROS/AKT pathway in human glioma U87 MG cells.
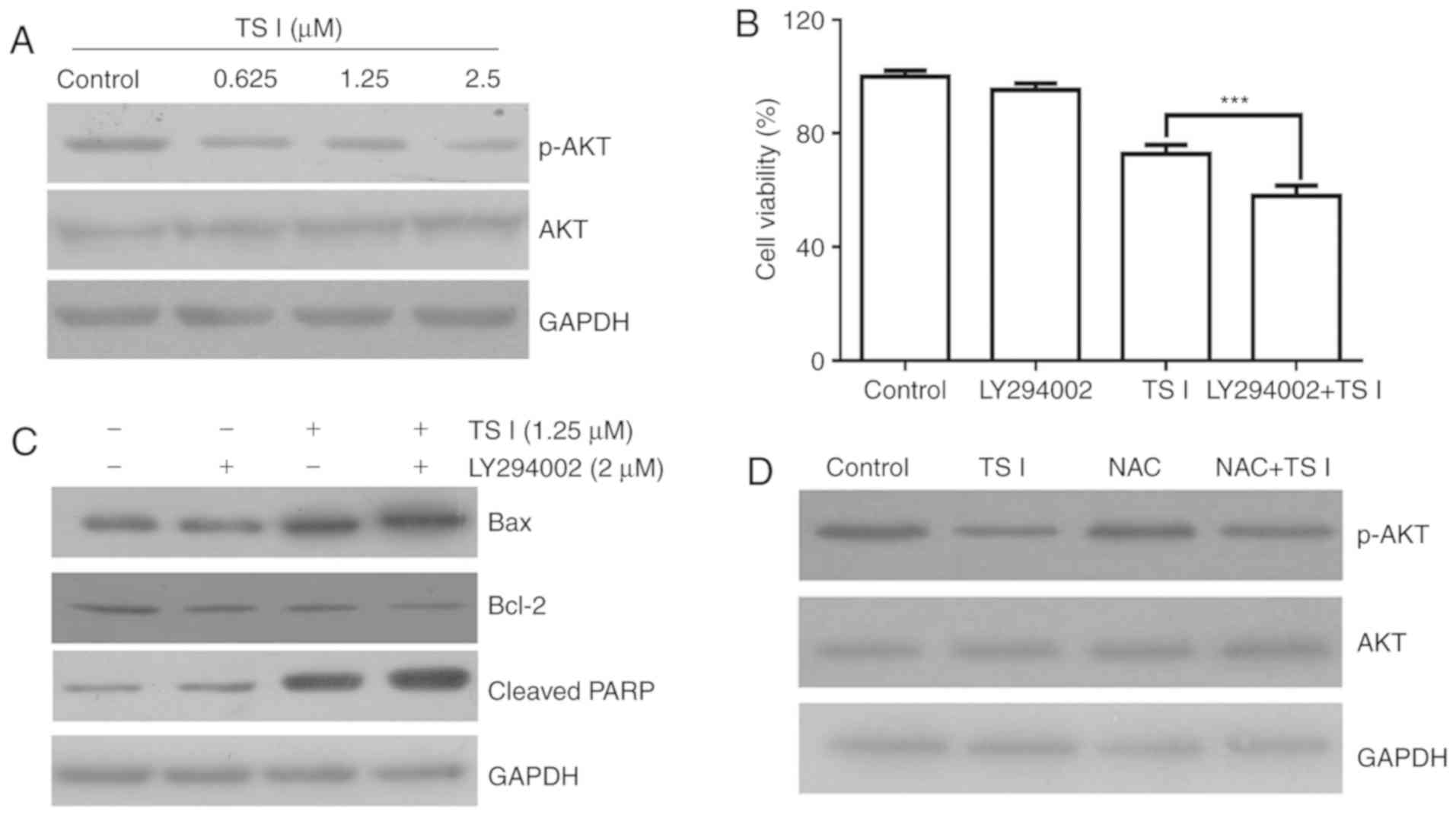 | Figure 5Effects of TS I on the AKT signaling
pathway in U87 MG cells. (A) Cells were treated with TS I (0,
0.625, 1.25 and 2.5 µm) for 24 h, and the expression of
GAPDH, total and phosphorylated AKT were detected using western
blot analysis. (B) U87 MG cells were pre-treated with LY294002 (2
µm) for 1 h, followed by treatment with TS I 1.25 µm
for 24 h, and cell viability was subsequently detected using a cell
counting kit-8 assay. (C) U87 MG cells were pre-treated with
LY294002 (2 µm) for 1 h, followed by treatment with TS I
1.25 µm for 12 h, and protein levels of Bcl-2, Bax and
cleaved PARP were then assessed using western blot analysis. (D)
U87 MG cells were pre-treated with NAC (4 mM) for 1 h, followed by
treatment with TS I 1.25 µm for 12 h, and the protein levels
of GAPDH, total and phosphorylated AKT were then assessed using
western blot analysis. GAPDH was used as a loading control.
***P<0.001; TS-I, tanshinone I; NAC,
N-acetyl-L-cysteine; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; PARP, poly(ADP-ribose) polymerase. |
TS I induces apoptosis via the
ROS-mediated endoplasmic reticulum (ER) stress pathway in human
glioma U87 MG cells
It has been previously reported that increased ROS
generation can increase the expression of unfolded proteins and
activate the ER stress response (19,21). Therefore, in the present study,
the expression of the ER stress-associated proteins p-eIF2α, eIF2α,
activating transcription factor (ATF)4 and CHOP was examined using
western blot analysis. As shown in Fig. 6A, TS I significantly activated ER
stress. To further verify the role of ROS production in the TS
I-mediated activation of ER stress, cells were treated with 4 mM
NAC for 1 h and subsequently treated with TS I (1.25 µM) for
12 h. The results indicated that 4 mM NAC pretreatment
significantly attenuated TS I-mediated activation of ER stress
(Fig. 6B). These results
demonstrated that TS I may induce apoptosis via the ROS-mediated ER
stress pathway in human glioma U87 MG cells.
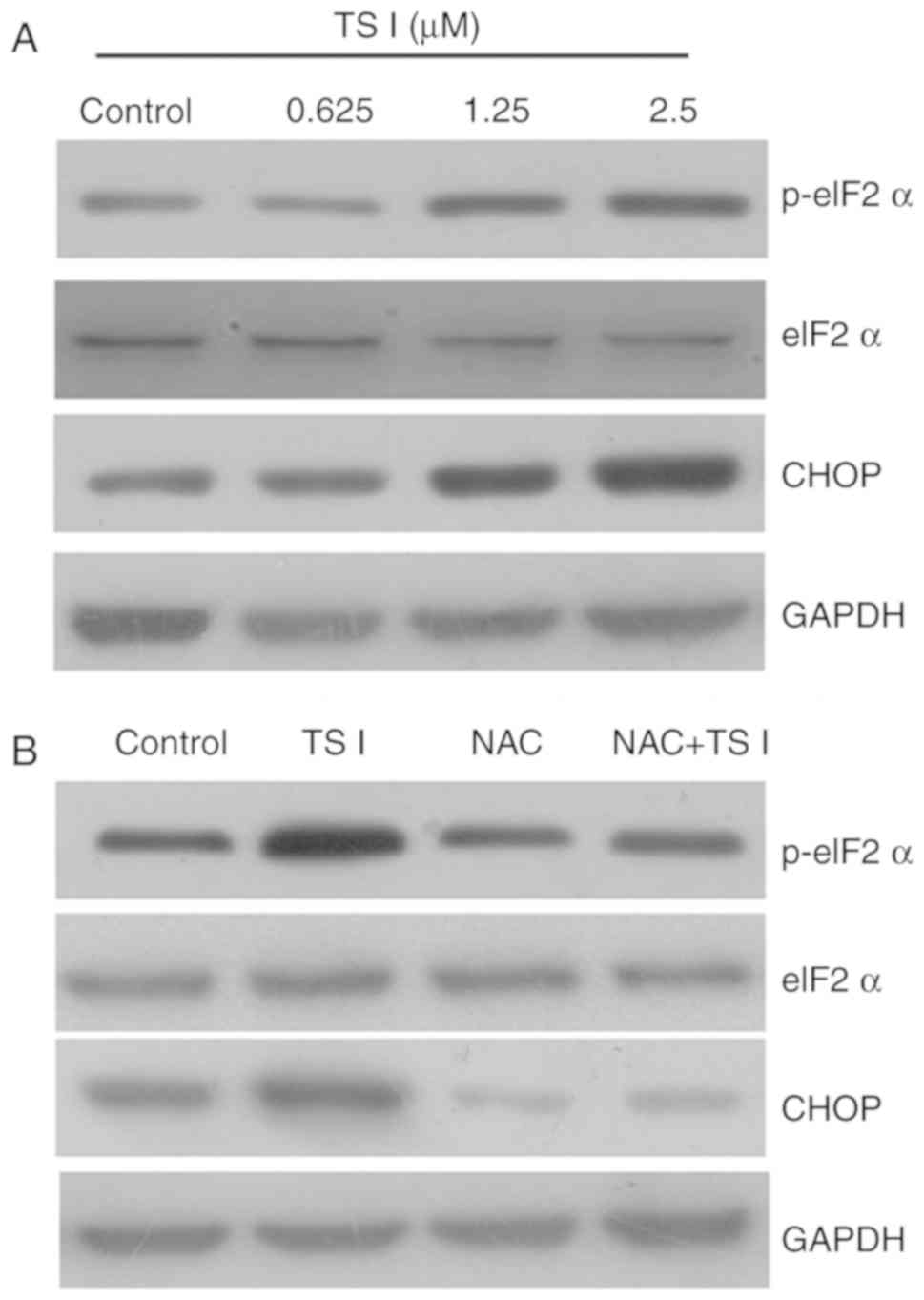 | Figure 6Effects of TS I on the ER stress
signaling pathway in U87 MG cells. (A) Cells were treated with TS I
(0, 0.625, 1.25 and 2.5 µm) for 12 h, and the expressions of
eIF2α, p-eIF2α and CHOP proteins were detected using western blot
analysis. (B) U87 MG cells were pre-treated with NAC (4 mM) for 1
h, followed by treatment with TS I 1.25 µm for 12 h, and the
protein levels of eIF2α, p-eIF2α and CHOP were then assessed using
western blot analysis. GAPDH was used as a loading control. TS-I,
tanshinone I; NAC, N-acetyl-L-cysteine ER, endoplasmic reticulum;
eIF2α, eukaryotic initiation factor; CHOP, C/EBP homologous
protein. |
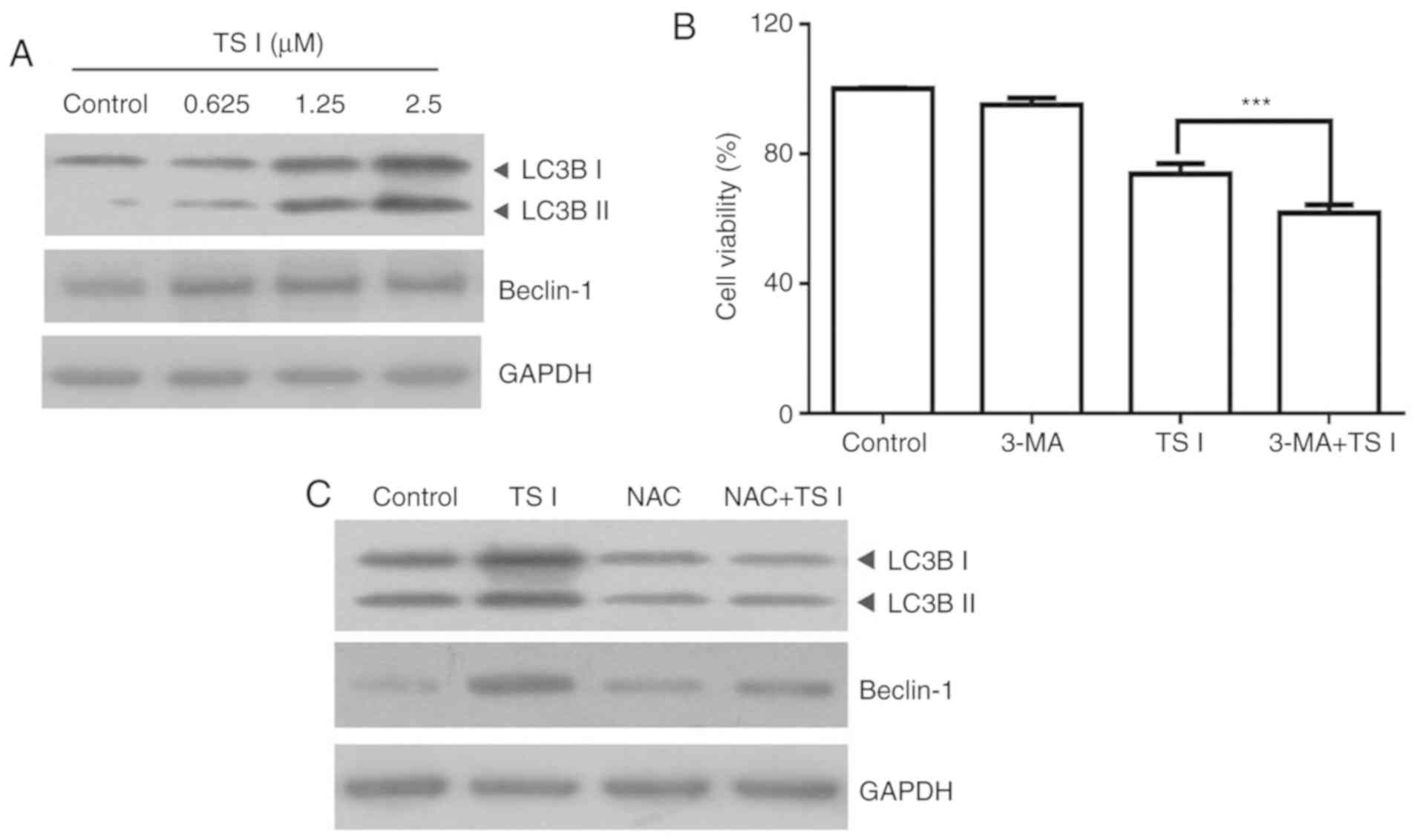
TS I induces autophagy via ROS production
in human glioma U87 MG cells
It has previously been suggested that TS I can
induce autophagy in BGC823 and SGC7901 cells (10). Therefore, the present study
assessed whether TS I induced autophagy in U87 MG cells. The
expression of LC3B and beclin-1 was determined using western blot
analysis. The results demonstrated that the expression of LC3B-II
and beclin-1 were markedly increased in U87 MG cells following
treatment with TS I (Fig. 7A). To
further determine the role of autophagy in TS I-induced cell
apoptosis, cells were treated with 5 mM 3-MA for 1 h and
subsequently treated with TS I (1.25 µM) for 24 h. Cell
viability was analyzed using a CCK-8 assay. As shown in Fig. 6B, co-treatment with 5 mM 3-MA and
TS I significantly enhanced the TS I-induced growth suppression of
U87 MG cells (Fig. 7B).
Additionally, increasing evidence has suggested that autophagy and
ROS accumulation are closely associated events in a variety of
cancer types (22-24). To elucidate the association
between ROS production and TS I-mediated autophagy, cells were
treated with 4 mM NAC for 1 h and subsequently treated with TS I
(1.25 µM) for 12 h. LC3B and beclin-1 expression was
determined using western blot analysis. The results of the present
study indicated that NAC pretreatment significantly attenuated
LC3B-II and beclin-1 expression compared with TS I treatment alone
(Fig. 7C). Therefore, these
findings suggest that TS I may promote protective autophagy via ROS
generation in U87 MG cells.
Discussion
TS I has been demonstrated to exert
antiproliferative effects on gastric cancers cell lines (including
BGC823 and SGC7901) (10), human
breast cancer cell lines (including MCF-7 and MDA-MB-453) (11,12) and human colon cancer cells
(13,14). However, the effect of TS I on
human glioma has not been extensively investigated. The present
study investigated the anticancer effects of TS I on human glioma
U87 MG cells. The data demonstrated that TS I inhibited cell
proliferation, induced G2/M phase arrest and triggered apoptosis in
a dose-dependent manner. The underlying molecular mechanism may
involve increased ROS production, decreased phosphorylation of AKT,
activated autophagy, or an activated ER stress pathway in human
glioma U87 MG cells. TS I was indicated to induce apoptosis via
activating the ER stress pathway and inhibiting AKT signaling, and
was shown to induce protective autophagy via ROS production in U87
MG cells.
It is well established that abnormal cell cycle
progression may cause uncontrolled growth, and that cell cycle
arrest can inhibit the proliferation and induce apoptosis in cancer
cells (25,26). Cell cycle progression is dependent
on the activity of cyclin-dependent kinase (CDK) complexes
(27,28). The downregulation of cyclin B1 may
lead to the inhibition of the cell cycle at the G2/M phase
(29,30). It has also been demonstrated that
p21 is a broad-spectrum CDK inhibitor and may promote cell cycle
arrest by inhibiting the activity of a number of cyclin-CDK
complexes (31,32). Furthermore, a number of studies
have revealed that the upregulated expression of p21 plays a key
role in G2/M arrest (33,34). Additionally, it has been reported
that TS I-induced inhibition of cyclin A and cyclin B decreases
cell cycle progression through the S and G2/M phases (35). Wang et al (11) revealed that TS I inhibited cell
cycle progression by decreasing cyclin B and CDK2 protein levels.
The results of the present study demonstrated that TS I upregulated
the p21 level and decreased the levels of cyclin B1. These data
revealed that TS I caused G2/M arrest by upregulating p21 and
downregulating cyclin B1 expression.
Apoptosis is an important physiological process of
programmed cell death and serves as an important homeostatic
mechanism that balances cell growth and cell death (36,37). The induction of apoptosis in
cancer cells is a strategy that may be used in the screening of new
anticancer agents (38). A
variety of studies have suggested that TS I can induce apoptosis in
a variety of human cancer cells. TS I-induced apoptotic death of
human breast cancer cells was indicated to be mediated by the
activation of caspase 3, the downregulation of Bcl-2 and the
upregulation of Bax expression (12). In human colon cancer COLO-205
cells, TS I was revealed to promote apoptosis by increasing the
expression of Bax and caspase-3 proteins (13). In the present study, treatment
with TS I was demonstrated to significantly induce apoptosis by
upregulating the expression of cleaved PARP and Bax and
downregulating the expression of Bcl-2 in U87 MG cells. These data
indicate that apoptosis plays a key role in TS I-induced U87 MG
cell death.
AKT kinase, which is a serine/threonine kinase,
plays an important role in a number of biological processes,
including cell proliferation, differentiation and apoptosis
(39,40). A number of studies have
demonstrated that natural compounds can induce cancer cell
apoptosis through the suppression of the AKT signaling pathway
(41-43). Additionally, Wang et al
(11) revealed that TS I induced
breast cancer cell apoptosis by regulating the PI3K/AKT/mammalian
target of rapamycin signaling pathway. Furthermore, it has been
demonstrated that the AKT signaling pathway mediates the
mitochondrial apoptotic pathway by increasing the Bax/Bcl-2
expression ratio and activating PARP (44-46). The results of the present study
demonstrated that TS I significantly decreased Bcl-2 and p-AKT
protein expression, and significantly increased cleaved PARP
protein expression. Furthermore, TS I and LY294002 enhanced the
pro-apoptotic effects of TS I. These data suggest that the AKT
pathway was associated with apoptosis in U87 MG cells that were
treated with TS I. A number of studies have demonstrated that ROS
serves as a mediator of apoptosis in a variety of types of
therapeutic drug-induced apoptosis (23,47,48). In human endometrial carcinoma
HEC-1-A cells, TS I has been shown to increase ROS levels (49). In colon cancer cells, TS I has
been demonstrated to induce apoptosis via the ROS-mediated p38
signaling pathway (9). In the
present study, TS I significantly increased the intracellular
levels of ROS in U87 MG cells. Additionally, previous studies have
reported that ROS is a potential upstream regulator of PI3K/AKT
inactivation, which are key molecules in cell apoptosis (19,20,50). In the present study, NAC was
indicated to efficiently reverse the TS I-mediated cell apoptosis
and inhibition of AKT phosphorylation in U87 MG cells. These data
suggested that ROS may act as a mediator in TS I-induced U87 MG
cell apoptosis via the inactivation of the AKT signaling
pathway.
It has been widely reported that ER stress may lead
to ER dysfunction and the activation of the ER stress-related
signaling pathway, which is associated with ROS-mediated cell
apoptosis in a variety of cancer types (51-53). TS IIA inhibited human prostate
cancer cell proliferation via the induction of ER stress in
vitro and in vivo (53). In human lung cancer cells, TS IIA
increased TRAIL-induced cell death via the activation of ER stress
(54). Consistently with these
reports, the results of the present study indicated that TS I
increased the expression of the ER stress-related proteins p-eIF2α,
ATF4 and CHOP. Furthermore, the downregulation of CHOP markedly
attenuated the inhibition of TS I-induced growth in human glioma
U87 MG cells. These data suggested that ER stress may be associated
with ROS-mediated TS I-induced cell apoptosis. To further determine
the role of ROS in the activation of ER stress induced by TS I,
cells were pretreated with NAC. The results revealed that NAC
pretreatment significantly attenuated the TS 1-mediated activation
of ER stress. Overall, the results of the present study revealed
that TS I promotes apoptosis of U87 MG cells via the ROS-mediated
ER stress pathway.
Autophagy can be activated by a variety of different
factors, including ROS accumulation and anticancer drugs, and is
well-known to play an important role in determining cell fate
(23,55,56). In lung cancer 95D cells,
tanshinones induced protective autophagy by increasing ROS
production (57). Jing et
al (10) also reported that
TS I induced protective autophagy in gastric cancer. In the present
study, the results demonstrated that autophagy was activated by TS
I in human glioma U87 MG cells. Furthermore, pretreatment with 3-MA
effectively enhanced the TS I-induced inhibition of U87 MG cells.
Additionally, NAC significantly reduced TS I-induced autophagy.
Therefore, these results indicate that TS I induces protective
autophagy via ROS production in U87 MG cells.
In summary, the results of the present study
demonstrated that TS I inhibited the proliferation of human glioma
U87 MG cells via the induction of apoptosis and G2/M cell cycle
arrest. TS I was shown to mediate G2/M cell cycle arrest in U87 MG
cells by upregulating p21 and downregulating cyclin B1 expression.
TS I induced apoptosis by upregulating the expression of cleaved
PARP and Bax, and downregulating the expression of Bcl-2.
Furthermore, TS I induced apoptosis via the inactivation of the AKT
signaling pathway. Therefore, these findings may be an important
indication that TS I is a potential anticancer drug candidate that
may prove useful in the treatment of human glioma. We aim to
further investigate the specificity of TS I against malignant cells
in a future study.
Funding
The present study was supported in part by a grant
from the National Natural Science Foundation of China (no.
81572485) and the Natural Science Basic Research Plan in Shaanxi
Province of China (program no. 2017JQ8037).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJ, LC and GS conducted the experiments and analyzed
the data. GS made substantial contributions to the design of the
present study and prepared the manuscript. LM, CH, TR, FX and ZB
performed the western blotting and analyzed the data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Jiang Y and Uhrbom L: On the origin of
glioma. Ups J Med Sci. 117:113–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cloughesy TF, Cavenee WK and Mischel PS:
Glioblastoma: From molecular pathology to targeted treatment. Annu
Rev Pathol. 9:1–25. 2014. View Article : Google Scholar
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neyns B, Tosoni A, Hwu WJ and Reardon DA:
Dose-dense temozolomide regimens: Antitumor activity, toxicity, and
immunomodulatory effects. Cancer. 116:2868–2877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fish JM, Welchons DR, Kim YS, Lee SH, Ho
WK and Antzelevitch C: Dimethyl lithospermate B, an extract of
Danshen, suppresses arrhythmogenesis associated with the Brugada
syndrome. Circulation. 113:1393–1400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang PN, Mao JC, Huang SH, Ning L, Wang
ZJ, On T, Duan W and Zhu YZ: Analysis of cardioprotective effects
using purified Salvia miltiorrhiza extract on isolated rat hearts.
J Pharmacol Sci. 101:245–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian XH and Wu JH: Tanshinone derivatives:
A patent review (January 2006-September 2012). Expert Opin Ther
Pat. 23:19–29. 2013. View Article : Google Scholar
|
|
8
|
Wang W, Li J, Ding Z, Li Y, Wang J, Chen S
and Miao J: Tanshinone I inhibits the growth and metastasis of
osteosarcoma via suppressing JAK/STAT3 signalling pathway. J Cell
Mol Med. 23:6454–6465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim DH, Shin EA, Kim B, Shim BS and Kim
SH: Reactive oxygen species-mediated phosphorylation of p38
signaling is critically involved in apoptotic effect of Tanshinone
I in colon cancer cells. Phytother Res. 32:1975–1982. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing X, Xu Y, Cheng W, Guo S, Zou Y and He
L: Tanshinone I induces apoptosis and pro-survival autophagy in
gastric cancers. Cancer Chemother Pharmacol. 77:1171–1181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Wu J, Lu J, Ma R, Sun D and Tang
J: Regulation of the cell cycle and PI3K/Akt/mTOR signaling pathway
by tanshinone I in human breast cancer cell lines. Mol Med Rep.
11:931–939. 2015. View Article : Google Scholar
|
|
12
|
Nizamutdinova IT, Lee GW, Son KH, Jeon SJ,
Kang SS, Kim YS, Lee JH, Seo HG, Chang KC and Kim HJ: Tanshinone I
effectively induces apoptosis in estrogen receptor-positive (MCF-7)
and estrogen receptor-negative (MDA-MB-231) breast cancer cells.
Int J Oncol. 33:485–491. 2008.PubMed/NCBI
|
|
13
|
Su CC, Chen GW and Lin JG: Growth
inhibition and apoptosis induction by tanshinone I in human colon
cancer Colo 205 cells. Int J Mol Med. 22:613–618. 2008.PubMed/NCBI
|
|
14
|
Lu M, Wang C and Wang J: Tanshinone I
induces human colorectal cancer cell apoptosis: The potential roles
of Aurora A-p53 and survivin-mediated signaling pathways. Int J
Oncol. 49:603–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gdowski A, Panchoo M, Treuren TV and Basu
A: Emerging therapeutics for targeting Akt in cancer. Front Biosci
(Landmark Ed). 21:757–768. 2016. View
Article : Google Scholar
|
|
16
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atkins RJ, Dimou J, Paradiso L, Morokoff
AP, Kaye AH, Drummond KJ and Hovens CM: Regulation of glycogen
synthase kinase-3 beta (GSK-3β) by the Akt pathway in gliomas. J
Clin Neurosci. 19:1558–1563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang L, Wang C, Lei F, Zhang L, Zhang X,
Liu A, Wu G, Zhu J and Song L: miR-93 promotes cell proliferation
in gliomas through activation of PI3K/Akt signaling pathway.
Oncotarget. 6:8286–8299. 2015.PubMed/NCBI
|
|
19
|
Ji L, Zhong B, Jiang X, Mao F, Liu G, Song
B, Wang CY, Jiao Y, Wang JP, Xu ZB, Li X and Zhan B: Actein induces
autophagy and apoptosis in human bladder cancer by potentiating
ROS/JNK and inhibiting AKT pathways. Oncotarget. 8:112498–112515.
2017. View Article : Google Scholar
|
|
20
|
Ahn KI, Choi EO, Kwon DH, HwangBo H, Kim
MY, Kim HJ, Ji SY, Hong SH, Jeong JW, Park C, et al: Induction of
apoptosis by ethanol extract of Citrus unshiu Markovich peel in
human bladder cancer T24 cells through ROS-mediated inactivation of
the PI3K/Akt pathway. Biosci Trends. 11:565–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Q, Liu Y, Zhong J, Bi Y, Liu Y, Ren
Z, Li X, Jia J, Yu M and Yu X: Pristimerin induces apoptosis and
autophagy via activation of ROS/ASK1/JNK pathway in human breast
cancer in vitro and in vivo. Cell Death Discov. 5:1252019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Kang X, Niu G, He S, Zhang T, Bai
Y, Li Y, Hao H, Chen C, Shou Z and Li B: Shikonin induces apoptosis
and prosurvival autophagy in human melanoma A375 cells via
ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed
Biotechnol. 47:626–635. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan X, Wang B, Yang L and Zhang Y: The
role of ROS-induced autophagy in hepatocellular carcinoma. Clin Res
Hepatol Gastroenterol. 42:306–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chao JI, Kuo PC and Hsu TS:
Down-regulation of survivin in nitric oxide-induced cell growth
inhibition and apoptosis of the human lung carcinoma cells. J Biol
Chem. 279:20267–20276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Su G, Lin Y, Meng W, Lai JKL,
Qiao L, Li X and Xie X: Targeting cyclin-dependent kinases in
gastrointestinal cancer therapy. Discov Med. 27:27–36.
2019.PubMed/NCBI
|
|
28
|
Bloom J and Cross FR: Multiple levels of
cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol.
8:149–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu CY, Jerry Teng CL, Hung PS, Cheng CC,
Hsu SL, Hwang GY and Tzeng YM: Ovatodiolide isolated from
Anisomeles indica induces cell cycle G2/M arrest and apoptosis via
a ROS-dependent ATM/ATR signaling pathways. Eur J Pharmacol.
819:16–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee MH, Cho Y, Kim DH, Woo HJ, Yang JY,
Kwon HJ, Yeon MJ, Park M, Kim SH, Moon C, et al: Menadione induces
G2/M arrest in gastric cancer cells by down-regulation of CDC25C
and proteasome mediated degradation of CDK1 and cyclin B1. Am J
Transl Res. 8:5246–5255. 2016.
|
|
31
|
Stivala LA, Cazzalini O and Prosperi E:
The cyclin-dependent kinase inhibitor p21CDKN1A as a target of
anti-cancer drugs. Curr Cancer Drug Targets. 12:85–96. 2012.
View Article : Google Scholar
|
|
32
|
Starostina NG and Kipreos ET: Multiple
degradation pathways regulate versatile CIP/KIP CDK inhibitors.
Trends Cell Biol. 22:33–41. 2012. View Article : Google Scholar :
|
|
33
|
Gong FR, Wu MY, Shen M, Zhi Q, Xu ZK, Wang
R, Wang WJ, Zong Y, Li ZL, Wu Y, et al: PP2A inhibitors arrest G2/M
transition through JNK/Sp1- dependent down-regulation of CDK1 and
autophagy-dependent up-regulation of p21. Oncotarget.
6:18469–18483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang LH, Jiang XR, Chen GL, Guo W, Zhang
JY, Cui LJ, Li HH, Li M, Liu X, Yang JY and Wu CF: Anti-tumor
activity of SL4 against breast cancer cells: Induction of
G2/M arrest through modulation of the MAPK-dependent p21
signaling pathway. Sci Rep. 6:364862016. View Article : Google Scholar
|
|
35
|
Tung YT, Chen HL, Lee CY, Chou YC, Lee PY,
Tsai HC, Lin YL and Chen CM: Active component of danshen (Salvia
miltiorrhiza Bunge), Tanshinone I, attenuates lung tumorigenesis
via inhibitions of VEGF, cyclin A, and cyclin B expressions. Evid
Based Complement Alternat Med. 2013:3192472013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu G, Pei F, Yang F, Li L, Amin AD, Liu
S, Buchan JR and Cho WC: Role of autophagy and apoptosis in
non-small-cell lung cancer. Int J Mol Sci. 18:E3672017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Croce CM and Reed JC: Finally, an
apoptosis-targeting therapeutic for cancer. Cancer Res.
76:5914–5920. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng YL, Lee SC, Lin SZ, Chang WL, Chen
YL, Tsai NM, Liu YC, Tzao C, Yu DS and Harn HJ: Anti-proliferative
activity of Bupleurum scrozonerifolium in A549 human lung cancer
cells in vitro and in vivo. Cancer Lett. 222:183–193. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spangle JM, Roberts TM and Zhao JJ: The
emerging role of PI3K/AKT-mediated epigenetic regulation in cancer.
Biochim Biophys Acta Rev Cancer. 1868:123–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kello M, Kulikova L, Vaskova J, Nagyova A
and Mojzis J: Fruit peel polyphenolic extract-induced apoptosis in
human breast cancer cells is associated with ROS production and
modulation of p38MAPK/Erk1/2 and the akt signaling pathway. Nutr
Cancer. 69:920–931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jovaisas E, Koch MA, Schafer A, Stauber M
and Lowenthal D: LAV/HTLV-III in 20-week fetus. Lancet. 2:11291985.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moeinifard M, Hassan ZM, Fallahian F,
Hamzeloo-Moghadam M and Taghikhani M: Britannin induces apoptosis
through AKT-FOXO1 pathway in human pancreatic cancer cells. Biomed
Pharmacother. 94:1101–1110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yaoi X, Lu B, Lu C, Bai Q, Yan D and Xu H:
Taraxerol induces cell apoptosis through A mitochondria-mediated
pathway in HeLa cells. Cell J. 19:512–519. 2017.PubMed/NCBI
|
|
45
|
Li C, Wang Y, Wang C, Yi X, Li M and He X:
Anticancer activities of harmine by inducing a pro-death autophagy
and apoptosis in human gastric cancer cells. Phytomedicine.
28:10–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim MJ, Kwon SB, Kim MS, Jin SW, Ryu HW,
Oh SR and Yoon DY: Trifolin induces apoptosis via extrinsic and
intrinsic pathways in the NCI-H460 human non-small cell lung-cancer
cell line. Phytomedicine. 23:998–1004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Thiagarajan S, Arapoc DJ, Husna Shafie N,
Keong YY, Bahari H, Adam Z and Ei T: Momordica charantia (Indian
and Chinese Bitter Melon) extracts inducing apoptosis in human lung
cancer cell line A549 via ROS-mediated mitochodria injury. Evid
Based Complement Alternat Med. 2019:28215972019. View Article : Google Scholar :
|
|
48
|
N B, Chandrashekar KR, Prabhu A and Rekha
PD: Tetrandrine isolated from Cyclea peltata induces cytotoxicity
and apoptosis through ROS and caspase pathways in breast and
pancreatic cancer cells. In Vitro Cell Dev Biol Anim. 55:331–340.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Q, Zhang J, Liang Y, Mu W, Hou X, Ma X
and Cao Q: Tanshinone l exhibits anticancer effects in human
endometrial carcinoma HEC-1-A cells via mitochondrial mediated
apoptosis, cell cycle arrest and inhibition of JAK/STAT signalling
pathway. J BUON. 23:1092–1096. 2018.PubMed/NCBI
|
|
50
|
Song X, Wang Z, Liang H, Zhang W, Ye Y, Li
H, Hu Y, Zhang Y, Weng H, Lu J, et al: Dioscin induces gallbladder
cancer apoptosis by inhibiting ROS-mediated PI3K/AKT signalling.
Int J Biol Sci. 13:782–793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang H, Xie H, Pan Y, Zheng K, Xia Y and
Chen W: Plumbagin triggers ER stress-mediated apoptosis in prostate
cancer cells via induction of ROS. Cell Physiol Biochem.
45:267–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim SM, Lee HM, Hwang KA and Choi KC:
Benzo(a)pyrene induced cell cycle arrest and apoptosis in human
choriocar-cinoma cancer cells through reactive oxygen
species-induced endoplasmic reticulum-stress pathway. Food Chem
Toxicol. 107:339–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang Y, Zhang Y, Wang L and Lee S:
Levistolide a induces apoptosis via ROS-mediated ER stress pathway
in colon cancer cells. Cell Physiol Biochem. 42:929–938. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao D, Tong L, Zhang L, Li H, Wan Y and
Zhang T: Tanshinone II A stabilizes vulnerable plaques by
suppressing RAGE signaling and NF-κB activation in
apolipoprotein-E-deficient mice. Mol Med Rep. 14:4983–4990. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Geng YD, Zhang L, Wang GY, Feng XJ, Chen
ZL, Jiang L and Shen AZ: Xanthatin mediates G2/M cell
cycle arrest, autophagy and apoptosis via ROS/XIAP signaling in
human colon cancer cells. Nat Prod Res. 27:1–5. Dec 27–2018.Epub
ahead of print. View Article : Google Scholar
|
|
56
|
Wang B, Zhou TY, Nie CH, Wan DL and Zheng
SS: Bigelovin, a sesquiterpene lactone, suppresses tumor growth
through inducing apoptosis and autophagy via the inhibition of mTOR
pathway regulated by ROS generation in liver cancer. Biochem
Biophys Res Commun. 499:156–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gao H, Sun W, Zhao W, Hao W, Leung CH, Lu
J and Chen X: Total tanshinones-induced apoptosis and autophagy via
reactive oxygen species in lung cancer 95D cells. Am J Chin Med.
43:1265–1279. 2015. View Article : Google Scholar : PubMed/NCBI
|