Introduction
Brain senescence is a major risk factor for most
human diseases (1). It commonly
leads to cognitive impairment and several neurological and
neurodegenerative diseases, such as dementia, including Alzheimer's
disease (AD), Parkinson's disease and vascular dementia. It was
previously demonstrated that ~26.9% of the total Chinese population
will be aged >65 years by 2050, placing China among the
countries with the highest proportion of aged individuals worldwide
(2). However, the mechanisms
underlying brain aging remain elusive, and effective prevention
methods are required.
Sodium channel voltage-gated beta 2 (SCN2B), an
aging-associated gene (3), is
expressed in the central nervous system and in cardiac tissue
(4). Previous data demonstrated
that SCN2B is involved in maintaining the normal physiological
functions of the prefrontal cortex and hippocampus, and may be
associated with the decline of aging and memory in the prefrontal
cortex of senescence-accelerated mouse prone 8 (SAMP8) (5). Compared with the control group, the
results from the present study suggested that the expression of
SCN2B in the prefrontal cortex of SAMP8 mice increased with
increases in age, which is the basis of dysfunction. These
increases are accompanied by dysfunction (5). It was also identified that the
downregulation of SCN2B by 60.68% significantly improved
hippocampus-dependent spatial cognitive memory in aged transgenic
mice, and increased the synaptic excitability of the hippocampus by
increasing the number of spinous processes in hippocampal neurons
(6). Furthermore, it was also
observed that sodium current density in hippocampal neurons and
neuronal activity were partially restored following knockdown of
voltage-gated sodium channel β2 (Navβ2), encoded by SCN2B, in
APP/PS1 mice (7). These results
suggested that increased SCN2B levels may be responsible for
aging-associated cognitive decline. However, the regulators of
ectopic SCN2B expression in the aging brain remain unknown.
Previous studies revealed that aging induces various
changes in the expression of multiple genes at the single-neuron
level, which may be attributed to the regulation by microRNAs
(miRNAs/miR). For example, Kadakkuzha et al (8) demonstrated that 1083 Expressed
Sequence Tags (ESTs) were differentially regulated in mature and
older R15 neurons; French et al, found many genes
overexpressed were downregu-lated with age in layer 2/3
glutamatergic neurons (9). miRNAs
are small non-coding RNA molecules measuring ~20-22 nucleotides in
length. By binding to the 3′untranslated region (3′-UTR) of a
target gene, miRNAs induce post-transcriptional regulation of gene
expression and silence the expression of protein-coding genes,
which are involved in cell apoptosis, death and proliferation
(10-14). Recent studies confirmed that
abnormal expression of multiple miRNAs is involved in the
progression of neurodegenerative diseases (15-17).
It was previously demonstrated that the expression
of miR-9 and miR-9* in the hippocampi of SAMP8 mice was decreased
compared with that in the age-matched control
senescence-accelerated mouse-resistant 1 (SAMR1) mice, and SCN2B
was one of the predicted target genes of miR-9 (18). It was also observed that miR-9 and
miR-9* participated in brain aging by targeting the
mitogen-activated protein kinase kinase kinase 3 and cyclin
dependent kinase (CDK) inhibitor 1C genes in SAMP8 mice (18). Based on the aforementioned data,
it was inferred that miRNAs may contribute to the regulation of the
age-associated changes in SCN2B expression. If such a regulatory
mechanism exists, miRNAs may serve a role in the progression of
cognitive decline via targeting the SCN2B gene. An understanding of
the regulatory mechanism underlying the abnormal expression of
SCN2B in the aging process is required to fully elucidate the
potential role and underlying molecular mechanism of SCN2B in the
cognitive decline associated with the aging process.
The aim of the present study was to investigate
whether miRNAs participate in the pathological process of cognitive
decline in the aging brain by regulating the expression of SCN2B.
The results may help identify a miRNA-based therapy that may lead
to an effective and innovative pharmaceutical treatment strategy in
the future.
Materials and methods
Ethics statement
Animal use and care were performed in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health (19) and the Care and Use of Experimental
Animals Guidelines established by the Ministry of Medicine of
Yunnan, China. The ethics committee of Kunming Medical University
approved the study protocol (permit no. km-edw-2013118).
Mouse preparation
Male SAMP8 and SAMR1 mice, aged 4 (SAMP8, n=5;
SAMR1, n=3) and 12 (SAMP8, n=5; SAMR1, n=3) months, respectively,
were purchased from the Tianjin Traditional Chinese Medical College
and maintained at the Kunming Institute of Zoology. The mice were
kept at 21-23°C with a 12:12 h light/dark cycle, and food and
drinking water were available ad libitum. The mice were
sacrificed following anesthesia by 2.5% inhaled isoflurane and
their tissues were harvested rapidly and placed on ice.
SAMP8 is an autogenic senile strain characterized by
early cognitive impairment and age-associated deterioration of
learning and memory; the SAMR1 strain was used as the control.
SAMP8 has become a major biogerontological resource in studies
investigating aging.
Samples of the prefrontal cortex and hippocampus
were collected from 5 4-month-old SAMP8 and 5 12-month-old SAMP8
mice. The age-matched SAMR1 mice were used as control (n=3).
Tissues were then collected and RNA was isolated using
TRIzol® reagent (Gibco; Thermo Fisher Scientific, Inc.).
Total RNA from each sample was quantified by the NanoDrop ND-1000
(NanoDrop Technologies) and RNA integrity was assessed by standard
denaturing agarose gel electrophoresis. Briefly, 10 µg of
RNA sample per lane was prepared in 2 µl 10X MOPS (0.2 mol/l
MOPS pH 7.0; 20 mmol/l sodium acetate; 10 mmol/l EDTA pH 8.0;
Beyotime Institute of Biotechnology), 4 µl 13.3 mol/l
formaldehyde, 10 µl 10 mol/l formamide and 1 µl 200
µg/ml ethidium bromide (EB; Beyotime Institute of
Biotechnology) in a nucleic acid-freed 1.5 ml tube. The RNA sample
was incubated at 55°C for 60 min, cooled at 4°C for 20 min and
centrifuged at 1,000 × g for 5 min. The sample was then mixed with
2 µl BeyoRed DNA loading buffer (D0072; Beyotime Institute
of Biotechnology) with 0.05 U RNase Inhibitor (R0102-2 kU; Beyotime
Institute of Biotechnology). The RNA was analyzed by
electrophoresis using a 1.5% agarose gel stained with EB with 1X
MOPS buffer and visualized using an ultraviolet Bio-Gel imager
(Bio-Rad Laboratories, Inc.). Total intact RNA with 18S and 28S
ribosomes from each sample was used for labeling and array
hybridization.
RNA labeling and array hybridization
The miRNA micro-array experiments were performed
using a Mouse miRNA Expression Array V3.0 chip (Aksomics, Inc.) to
scan the differentially expressed miRNAs in the prefrontal cortex
or hippocampal tissues of SAMP8 and SAMR1 mice aged 4 and 12
months, respectively. Total RNA was extracted by the
TRIzol® reagent (Gibco; Thermo Fisher Scientific, Inc.)
and quantified for purity and concentration using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc.). Following
hybridization, the different fluorescence intensities of miRNA were
obtained by image scanning. Differentially expressed genes were
identified through fold-change filtering. Hierarchical clustering
was performed using the Agilent GeneSpring GX software (Agilent
Technologies, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
miRNAs were quantified using RT-qPCR. RNA from cells
and tissue samples were prepared using TRIzol® reagent
(Gibco; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized from total RNA using
gene-specific primers using the TaqMan MicroRNA Assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Concentrations of RNA samples were
determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies). The 20 µl PCR reaction contained 10 µl
2X PCR Master Mix (Roche Diagnostics), 0.25 µl primer (10
pmol/l), 1 µl cDNA template and 8.5 µl PCR
nuclease-free water. The primer sequences were as follows: U6
forward, 5′-CTC GCT TCG GCA GCA CA-3′ and reverse, 5′-AAC GCT TCA
CGA ATT TGC GT-3′; miR-34a-5p forward, 5′-TCG GAT CTT CCA AGG
CTC-3′ and reverse, 5′-GCT GTC AAC GAT ACG CTA CGT AAC G-3′;
miR-449a forward, 5′-AAT CCG TCT CCC TAA CTC TAG GCT T-3′ and
reverse, 5′-GCT GTC AAC GAT ACG CTA CGT AAC G-3′; miR-9 forward,
5′-CTC TCC GGA TAT CCG TTG CTC CGG TCT C-3′ and reverse, 5′-GCT GTC
AAC GAT ACG CTA CGT AAC G-3′ (Takara Biotechnology Co., Ltd.). The
thermocycling conditions were as follows: 95°C for 3 min, followed
by 40 cycles of 94°C for 35 sec, 56.5°C for 30 sec and 72°C for 30
sec. Samples were normalized to the housekeeping gene U6. Relative
gene expression was determined using the 2−ΔΔCq method
(20) and the experiment was
repeated three times.
Cell culture
The primary mouse hippocampal neuron culture was
performed by using previously described methods (21,22). Briefly, neonatal mice (0 days)
were decapitated following anesthesia with isoflurane, and the
hippocampi were carefully separated. The hippocampi were digested
with 0.05% trypsin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
for 10 min, then centrifuged at 1,000 rpm for 10 min at room
temperature following trypsin digestion. Neurons were resuspended
in complete culture medium containing DMEM-high glucose (HyClone;
GE Healthcare Life Sciences), 15% fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin. Cells at
the required density (5×105 cells/ml) were seeded onto
the PPL-coated culture plate. The cells were cultured in a
humidified incubator at 37°C with 5% CO2. Thereafter,
the culture media from each well were replaced with fresh composite
media every third day.
Bioinformatics predictions
To identify potential miRNAs that may target SCN2B,
multiple computational algorithms, including TargetScan (23-27), miRanda (28) and Diana microT v5.0 (29,30), were used for bioinformatics
predictions as previously described (31). The potential miRNAs were predicted
according to the sequence of the SCN2B 3′-UTR.
Cell transfection
The primary neurons were plated in 6-well culture
plates at a density of 2.0×106 per plate for RIP-Chip
transfections, as described previously (10,32). Briefly, artifi-cial 'Pre-miRNA'
referent tommu-miR-449a was designed and purchased from Ambion;
Thermo Fisher Scientific, Inc. At 24 h after seeding, cells were
transfected with 10 µl of 25 nM each 'Pre-miRNA', or
negative control (NC) miRNA (Ambion; Thermo Fisher Scientific,
Inc.) using RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The sense sequences were
as follows: mmu-miR-449a: GGC AGU GUA UUG UUA GCU G, and NC miRNA:
AGU ACU GCU UAC GAU ACG G. Following transfection, the cells were
cultured for 48 h prior to further experimentation.
Northern blot analysis
To evaluate the effectiveness and specificity of
miRNA transfections, Northern blots were employed according to a
previously described protocol (33). Northern blot analyses were
performed using RNA isolated from cultured neurons 48 h after
transfection with miRNAs.
RNA immunoprecipitation chip
(RIP-Chip)
A total of 3 miRNAs, miR-9, miR-449a and miR-34a-5p,
each of which was predicted by all 3 software programs and were
downregulated in the SAMP8 mouse brain, were selected for further
validation among the numerous miRNAs identified. The anti-AGO
co-immunoprecipitation (co-IP) and downstream microarray analyses
(RIP-Chip) were employed to confirm the results of
computationally-predicted miRNAs targeting SCN2B.
Co-IP of microribonucleoparticles
(miRNPs)
The RIP-Chip co-IP assay protocol employed in the
present study has been previously described in detail (32,34). Briefly, cells were harvested 48 h
after transfection. Cell lysates were subjected to preclearance by
incubation with pre-blocked protein G beads (Invitrogen; Thermo
Fisher Scientific, Inc.) at 4°C for 60 min. Co-IP with either Ago
(cat. no. K004319P; Beijing Solarbio Science and Technology Co.,
Ltd.)-protein G beads or non-immune mouse serum (Pierce; Thermo
Fisher Scientific, Inc.)-protein G beads, was then performed at 4°C
for 90 min using lysate aliquots. Following co-IP, the beads were
washed 3-5 times with lysis buffer at room temperature. Beads and
lysates were then subjected to DNase treatment by gently shaking
and incubating at 37°C for 20 min with 250 µl DNA digestion
solution (Pierce; Thermo Fisher Scientific, Inc.). The
immunoprecipitated RNA and total RNA from lysates were then
extracted using TRIzol LS (Invitrogen; Thermo Fisher Scientific,
Inc.) as described previously (33).
Post-Co-IP microarray analysis
Microarray analysis of RNAs isolated via co-IP was
performed using a Mouse 12×135 K Gene Expression Array manufactured
by Roche NimbleGen, Inc. A total of 8 biological replicates from 3
individual experiments were performed for each transfection
condition. For gene array, slides were scanned at 5 µm/pixel
resolution using an Axon GenePix 4000B scanner (Molecular Devices,
LLC) piloted by GenePix Pro 6.0 software (Axon; Molecular Devices,
LLC). The scanned images were then imported into NimbleScan
software (v.2.5; Roche NimbleGen, Inc.) for grid alignment and
expression data analysis. Expression data were subjected to
quantile normalization and the Robust Multichip Average (RMA)
algorithm included in the NimbleScan software. All gene level files
were imported into Agilent GeneSpring GX software (v.11.5.1;
Agilent Technologies, Inc.) for further analysis. Differentially
expressed genes were identified through fold-change filtering.
Hierarchical clustering was performed using the Agilent GeneSpring
GX 11.0 software (Agilent Technologies, Inc.). The functions of the
differentially expressed miRNAs were analyzed by Gene Ontology (GO)
(35,36) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) (37-39) databases.
RNA preparation and quantification
Following careful rinsing in cooled PBS, cells were
homogenized on ice using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was isolated using TRIzol, according
to the manufacturer's protocol. RNA quality and quantity were
measured using an ND-1000 NanoDrop spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.), and RNA integrity
was determined by gel electrophoresis. An equal amount of RNA (4
µg) was used for each experiment.
RT-qPCR analysis was performed according to the
protocol described previously to detect the expression levels of
the SCN2B gene (32). β-actin was
used as a reference and subtracted for net changes. Gene primers
were synthesized by Takara Bio, Inc. Each data point represents the
results of 3 technical replicates. The primer sequences were as
follows: SCN2B forward, 5′-CTA CAC CGT GAA CCA CAA GCA-3′ and
reverse, 5′-GAC CAC AGC CAG GAA ACC C-3′; β-actin forward, 5′-ATA
TCG CTG CGC TGG TCG TC-3′ and reverse, 5′-AGG ATG GCG TGA GGG AGA
GC-3′.
Dual-luciferase reporter gene assay
The potential target sites for miR-449a on the mouse
SCN2B mRNA 3′-UTR seed regions, including both wild-type (wt) and
mutant (mut) sequences, were cloned. The artificially cloned
sequences were inserted downstream of the luciferase reporter gene,
pGL3 (Guangzhou RiboBio Co., Ltd.), to generate the SCN2B 3′-UTR-wt
and SCN2B 3′-UTR-mut vectors, as described previously (40,41). Briefly, 293T cells (purchased from
Institute of Biochemistry and Cell Biology, Shanghai, China) were
seeded in 96-well plates and co-transfected with 100 ng/ml of each
pGL3-SCN2B 3′-UTR-wt or -mut vector and 35 nM miR-449a mimics or NC
(Guangzhou RiboBio Co., Ltd.). Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used as the
transfection reagent. The effects of miR-449a treatment on
luciferase activity were measured at 48 h post-transfection. The
firefly and Renilla luciferase activities were separately
measured using a Dual Luciferase Reporter Assay System kit (Promega
Corporation) and a Tecan M200 luminescence reader (Tecan Group,
Ltd.) according to the manufacturer's instructions. The relative
transcriptional activity was normal-ized to Renilla
luciferase activity.
Cell treatment
To determine whether miR-449a contributed to
aging-associated pathological events in SAMP8 mice by targeting
SCN2B, miR-449a overexpression and miR-449a inhibition was
established in cultured primary neurons. A lentiviral system was
employed for miR-449a/SCN2B overexpression
(plenti-miR-449a/plenti-SCN2B; Guangzhou RiboBio Co., Ltd.), while
miRNA sponge technology was used to inhibit miR-449a activity
(miR-449a-sponge) as described previously (40,42). The blank plasmid lemiR, referred
to as lemiR, and the non-binding sponge sequence, referred to as
CX-control, were used as controls. The expression of SCN2B was
inhibited by transfection with the pNX-U6 plasmid (Takara
Biotechnology, Co., Ltd.) ligated with small interfering RNA
(siRNA) targeting SCN2B (SCN2B-siRNA; 20 nM; Guangzhou RiboBio Co.,
Ltd.), with the pNX-U6 plasmid alone serving as a blank control
(empty-siRNA) (43).
Additionally, plenti-miR-449a was also co-transfected with
plenti-SCN2B using the aforementioned lentiviral overexpression
system, and was termed 'plenti-miR-449a + plenti-SCN2B'. The
plenti-miR-449a co-transfected with lemiR served as a control,
named 'plenti-miR-1 + SCN2B control'. Similarly, the
miR-449a-sponge was co-transfected with the SCN2B-siRNA plasmid, or
with the empty-siRNA to serve as a control, and referred to as
'miR-449a-sponge + SCN2B-siRNA' and 'miR-449a-sponge +
empty-siRNA', respectively. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used as the
transfection reagent.
The miR-449a expression and SCN2B mRNA and protein
levels were evaluated at 48 h after transfection. Then the neurons
were photographed under a fluorescence LEICA DMI6000B microscope
(LAS AF system; Leica Microsystems GmbH) at ×200 magnification. The
effects of miR-449a overexpression and inhibition on neuronal
growth were additionally evaluated by measuring the axon length,
areas of neurons and cell numbers using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.).
Western blot analysis
Western blot analysis was used to evaluate the SCN2B
protein expression levels in primary neurons transfected with
miR-449a overexpression or sponge vectors, according to a
previously described protocol (6,43).
Briefly, cell samples were lysed on ice for 30 min in CytoBuster
Protein Extraction Buffer (Novagen; Merck KGaA), and 50 µg
protein was used for 10% SDS-PAGE. The proteins were then
transferred to a nitrocellulose membrane and blocked using TBS +
100% Tween-20 (TBST) containing 5% non-fat milk powder for 1 h at
room temperature. The membrane was subsequently incubated with goat
anti-SCN2B (1:800; cat. no. ASC-007; Alomone Labs) and anti-GAPDH
(1:500; cat. no. sc-47724; Santa Cruz Biotechnology, Inc.)
antibodies at 4°C overnight. Following washing in TBST, the
membrane was incubated with horseradish peroxidase-conjugated
anti-mouse secondary antibodies (1:1,000; cat. no. sc-516132; Santa
Cruz Biotechnology, Inc.) for 2 h at 25°C. The bands were
visualized by an electrochemiluminescence technique (Best-Bio Co.,
Ltd.). Images were captured using the JS Gel Imaging System
(Shanghai Peiqing Science & Technology Co., Ltd.), and gray
densities were calculated using SensiAnsys software (version
JS-680D; Shanghai Peiqing Science & Technology Co., Ltd.).
Statistical analyses
Statistical analyses were performed using SPSS
v.16.0 (SPSS, Inc.). Data are presented as the mean ± standard
deviation. The statistical significance among multiple groups was
evaluated by one-way ANOVA followed by Bonferroni's post hoc test,
whereas comparisons between two groups were performed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
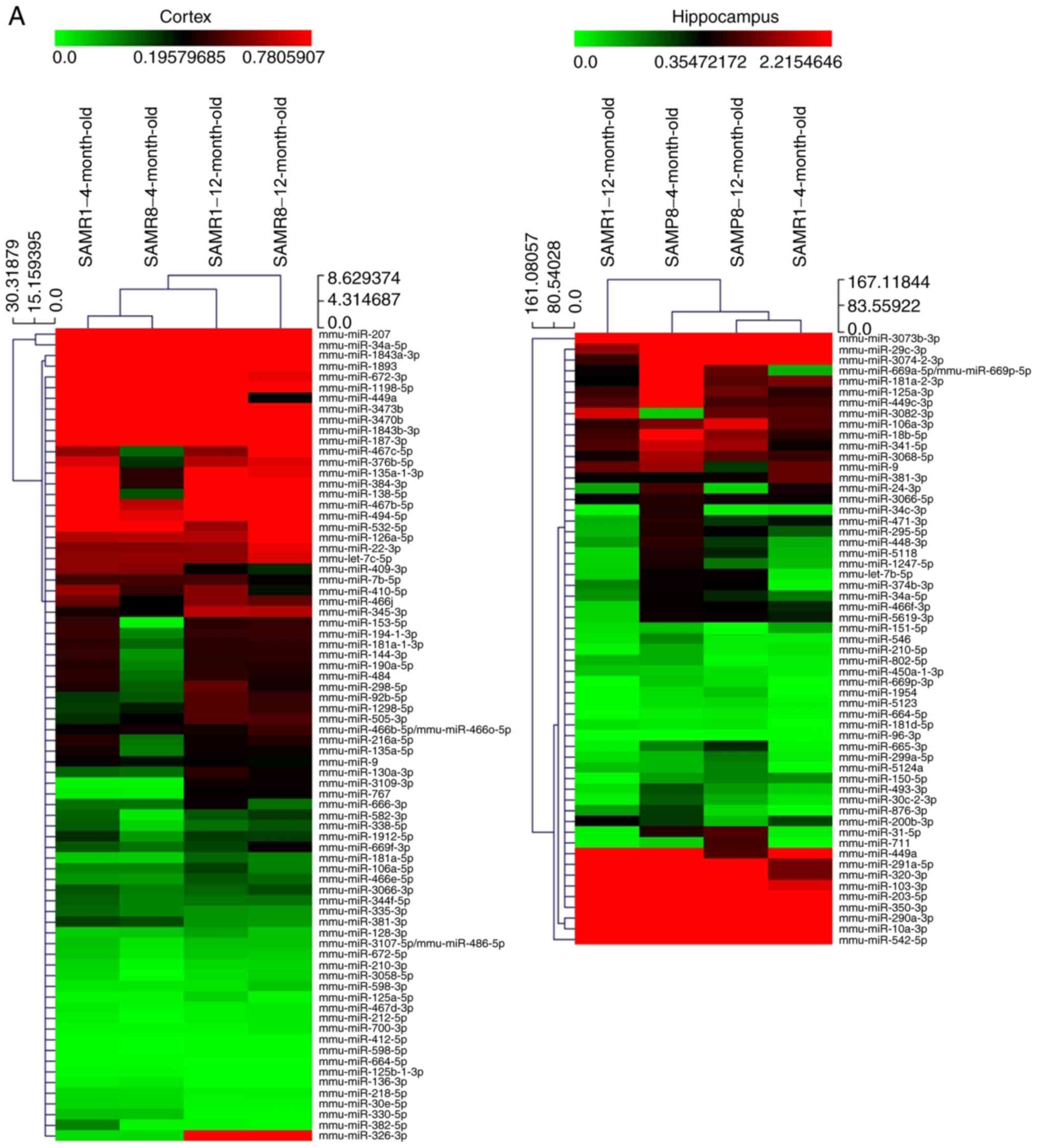
miRNA microarray analysis
In order to elucidate the regulatory mechanism
underlying the increased expression of SCN2B with aging, miRNA
microarray analysis was performed to compare the miRNA expression
profiles in the prefrontal cortex and hippocampus between the SAMP8
and SAMR1 mice aged 4 and 12 months, respectively. Microarray
analysis identified multiple differentially expressed miRNAs in the
prefrontal cortex and hippocampus of SAMP8 mice compared with the
SAMR1 mice (P<0.05; Fig. 1A).
The data suggested that expressional alteration of miRNAs may
contribute to the SCN2B regulation associated with aging in SAMP8
mice. Based on these data, the present study then focused on the
abnormally downregulated miRNAs in SAMP8 mice at 12 months compared
with the age-matched control mice. In order to identify the
potential miRNAs that may target SCN2B, the present study focused
on the downregulated miRNAs, which were also predicted by the
computational algorithms TargetScan (23-27), miRanda (28) and Diana microT v5.0 (29,30) with the presence of 8-mer site in
the 3′-UTR of the SCN2B gene; miR-449a, miR-34a-5p and miR-9a were
identified for subsequent analyses.
RT-qPCR was utilized to validate the expression
levels of miR-449a, miR-34a-5p and miR-9a mRNA in the prefrontal
cortex and hippocampi of the SAMP8 mice. No significant differences
in the expression levels of all 3 miRNAs were observed at the age
of 4 months compared with the SAMR1 mice (Fig. 1B). However, miR-449a was
identified to be significantly decreased in both the prefrontal
cortex and hippocampus, miR-34a-5p was decreased in the prefrontal
cortex, while miR-9a was decreased in the hippocampus of
12-month-old SAMP8 mice compared with the age-matched SAMR1
controls (Fig. 1B).
These results indicated that the downregulation of
miR-449a, miR-34a-5p and miR-9 may be involved in the aging process
of the prefrontal cortex or hippocampus of SAMP8 mice, which may be
mediated by regulating their target genes.
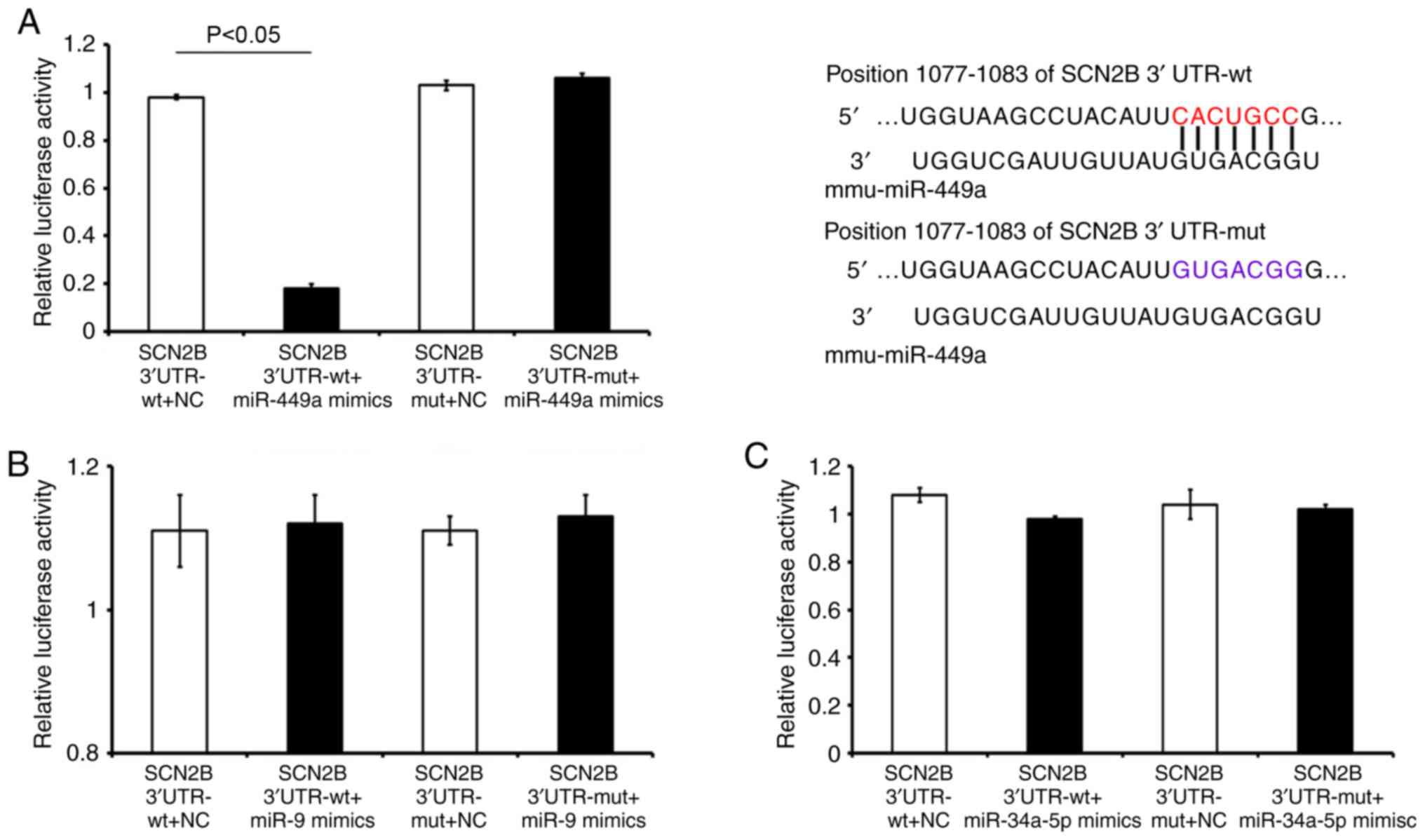
Dual-luciferase reporter assay indicates
SCN2B as a target of mirR-449a
To identify the miRNAs that regulate SCN2B, a
dual-luciferase analysis was performed. SCN2B 3′-UTR-wt and SCN2B
3′-UTR-mut were co-transfected with miR-449a, miR-9, miR-34a-5p
mimics or NC control. The results revealed that the miR-449a mimic
decreased the luciferase activity of the SCN2B 3′-UTR-wt cells, but
exerted no effect on the SCN2B 3′-UTR-mut cells, as compared with
the miR-NC-treated group (Fig.
2A). Furthermore, as demonstrated in Fig. 2B and C, no significant changes in
the luciferase activities of the SCN2B 3′-UTR-wt or SCN2B
3′-UTR-mut cells following transfection with miR-9 or miR-34a-5p,
compared with the matched NC controls. These results indicated that
miR-449a may directly regulate SCN2B 3′-UTR by acting on the seed
region 'CAC UGC CG'.
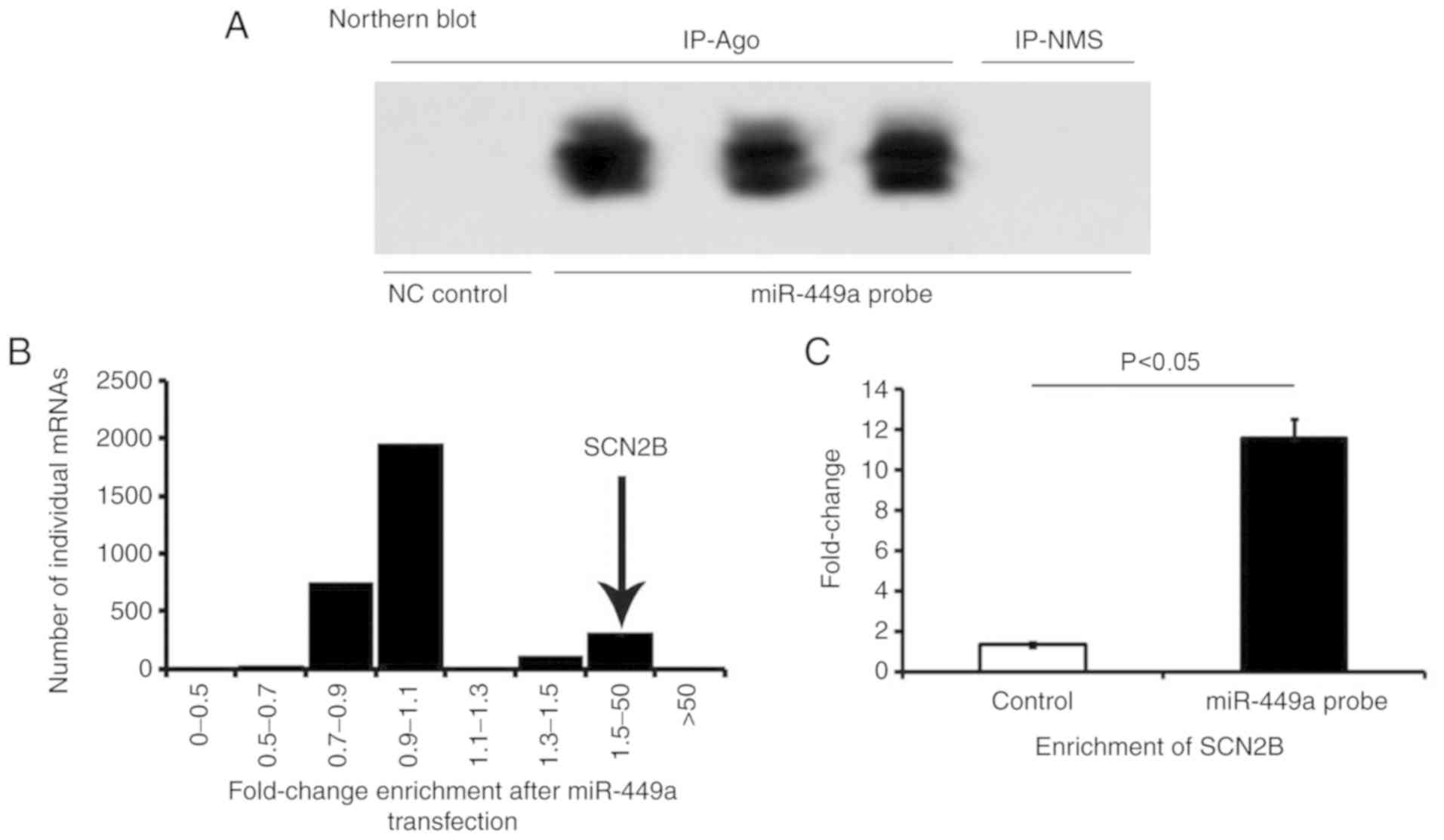
SCN2B is highly enriched in
miR-449a-Ago-miRNPs complex
Northern blots (Fig.
3A) were performed using RNA isolated from neurons 48 h after
transfection with miR-449a, which was performed to examine the
effectiveness and specificity of miRNA transfections. The results
demonstrated that transfected miR-449a was successfully
incorporated into the neurons.
To verify that miR-449a targeted SCN2B, an RIP-Chip
analysis was performed to validate the putative miRNA targets
identified by co-IP miRNPs following the transfection of miR-449a
in primary hippocampal cells of mice. The primary hippocampal cells
incubated with Ago antibody were treated with miR-449a probe, and
target genes bound to the Ago-miRNPs complex were detected. Large
amounts of enriched SCN2B mRNA were detected in the co-precipitated
miR-449a-Ago-miRNPs complex. As demonstrated in Fig. 3B and C, the target mRNA, which was
highly enriched with Ago-miRNPs, was confirmed to be SCN2B. This
result represents important evidence that miR-449a may target the
SCN2B mediated by Ago-miRNPs in cultured hippocampal neurons in
vitro.
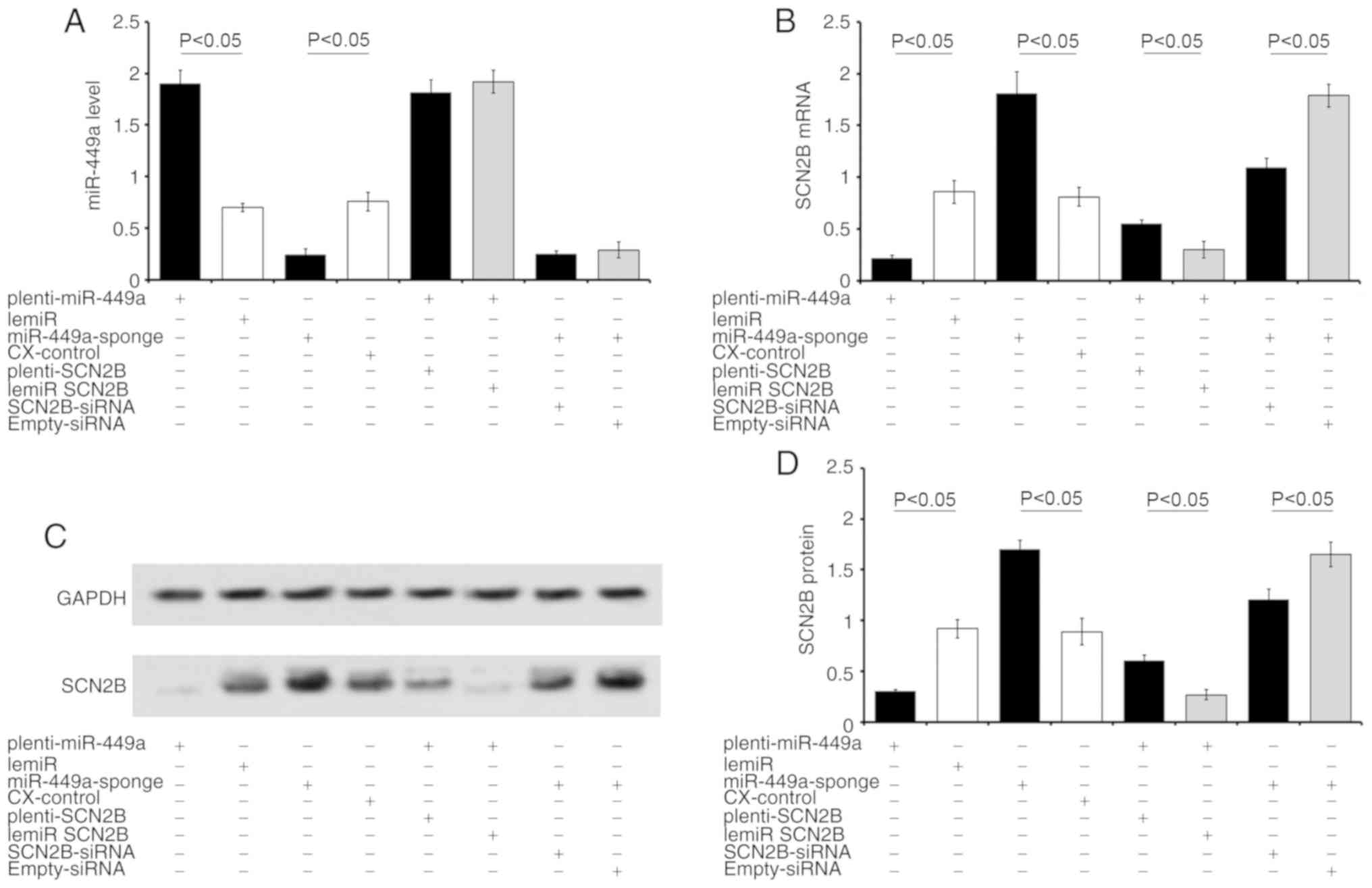
SCN2B is a valid target of miR-449a
In neurons overexpressing miR-449a, treatment with
plenti-miR-449a downregulated the expression of SCN2B mRNA by 75%.
Treatment with plenti-SCN2B partially restored SCN2B expression
compared with the CX-control (Fig.
4A). By contrast, inhibition of miR-449a increased SCN2B mRNA
expression by 2.1-fold in miR-449a-sponge-transfected neurons
compared with the CX-control (Fig.
4B). SCN2B-siRNA treatment partially reversed the miR-449a
inhibition-induced effects. Similar changes in SCN2B protein levels
were also observed in neurons following transfection with various
chemicals (Fig. 4C and D). These
results confirmed SCN2B as a target gene of miR-449a. Considering
the potential roles of SCN2B in the aging-associated cognitive
deficit (5,6), the effects of SCN2B and miR-449a on
the neurite growth of hippocampal neurons in mice was
evaluated.
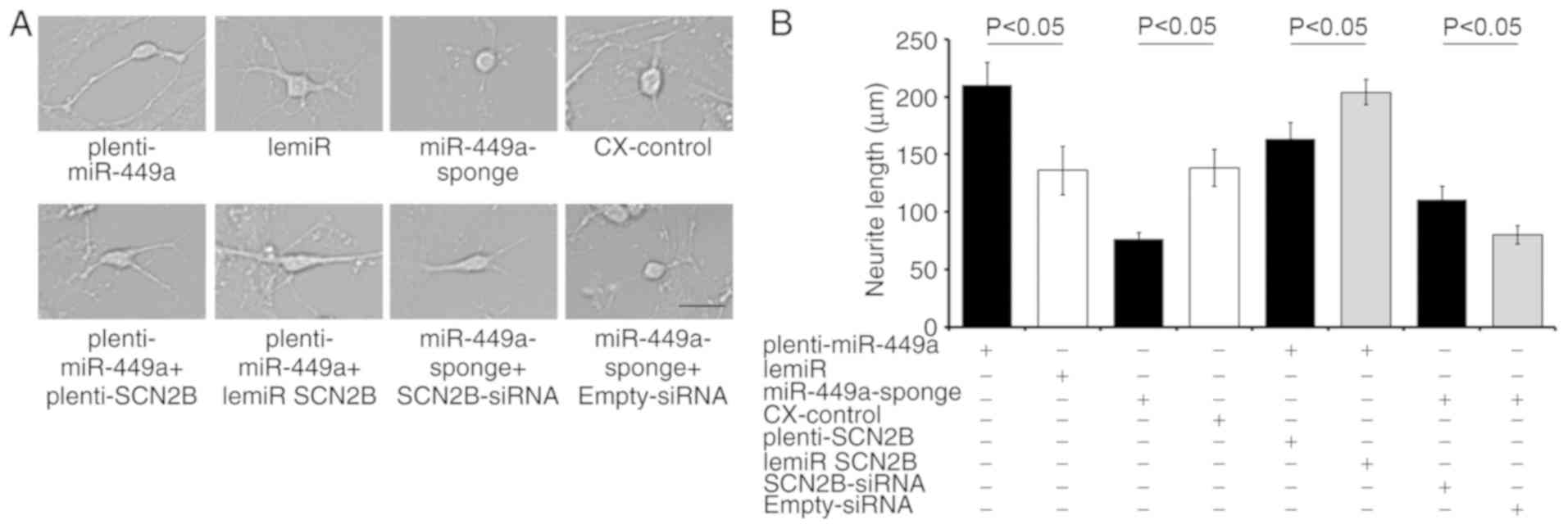
Treatment with plenti-miR-449a increased the mean
neurite length of primary cultured neurons compared with the
matched lemiR-treated control (Fig.
5). By contrast, miR-449a-sponge transfection induced shorter
extension compared with that of the CX-control (Fig. 5). The extension length was
partially decreased in plenti-miR-449a following plenti-SCN2B
transfection when compared with the plenti-miR-449a + lemiR
SCN2B-treated controls (Fig. 5).
SCN2B-siRNA partially restored the decreased neurite extension
induced by the miR-449a-sponge (miR-449a-sponge + SCN2B-siRNA vs.
miR-449a-sponge + empty-siRNA). These results suggested that
miR-449a may serve a key role in neuron growth and the associated
progression of brain aging by targeting SCN2B.
Discussion
In the present study, miRNAs expression profiles in
the prefrontal cortex and hippocampi of 4- and 12-month SAMP8 mice
and age-matched SAMR1 controls were detected by miRNA micro-array.
The SAMP8 strain is an animal model of rapid aging, and is ideal
for studying brain aging and dementia (44). SAMP8 mice not only have
pathological characteristics associated with AD, such as excessive
phosphorylation of tau protein and Aβ deposition, but also exhibit
similar behavioral characteristics to those of elderly humans
(45,46). The present study revealed that
numerous miRNAs detected in the cortex and hippocampus of aged
SAMP8 mice exhibited differences in expression levels from the
aged-matched SAMR1 controls. It was therefore inferred that the
downregulated miRNAs may contribute to the upregulated genes
associated with brain aging. Following consolidation of the results
of miRNA microarray analysis and the predicted lists of target
genes obtained by bioinformatics analysis, 3 miRNAs were selected
for subsequent analysis: miR-449a, miR-34a-5p and miR-9. RT-qPCR
analysis verified the results of the miRNA chip assay, which
demonstrated decreased levels of miR-449a, miR-34a-5p and miR-9 in
the frontal cortex or hippocampus of 12-month old SAMP8 mice. The
subsequent dual-luciferase reporter assay confirmed that miR-449a
targets SCN2B by binding to the 3′-UTR of the SCN2B seed region
'CACUGCCA'. Anti-Ago co-IP combined with Affymetrix microarray
analyses were utilized in the present study as described previously
(32), which verified that the
target mRNA highly enriched in the miR-449a-Ago-miRNPs complex was
SCN2B. These results represent important evidence of the direct
regulation of SCN2B by miR-449a in cultured hippocampal neurons.
The knockout and overexpression experiments demonstrated that SCN2B
was a valid target of miR-449a. It was also revealed that miR-449a
upregulation or SCN2B downregulation promoted the extension of the
processes of cultured neurons. Co-transfection with SCN2B
overexpression or siRNA vectors partially neutralized the effects
induced by miR-449a upregulation or downregulation. These data
suggest that miR-449a may serve an important role in neuron growth
and associated progression of aging by targeting SCN2B.
The crucial role of miR-449a in various diseases,
such as breast and pancreatic cancer, osteosarcoma, gastric
carcinoma, viral hepatitis and hypoxia-induced conditions has been
well-documented (47-51). Previous studies also verified
numerous target genes regulated by miR-449a, including CDKs
(52-54), hepatocyte growth factor/c-Met
receptor system (HGF/MET) (55)
and synaptotagmin 1 (Syt1) (56),
among others. miR-449a was demonstrated to be involved in the
development and progression of AD through a variety of molecular
mechanisms by regulating target genes. CDKs have been confirmed to
be downregulated in AD (57).
HGF/MET was able to induce dendritic arborization and
synaptogenesis when stimulated by N-hexanoic-Tyr-Ile-(6) aminohexanoic amide and it served a
role in facilitating the formation of new functional synaptic
connections and augmenting memory consolidation in animal models of
AD (58). The downregulation of
HGF/MET in the brain functional regions of patients with AD was
associated with a poor prognosis (59). Syt1, as a novel amyloid precursor
protein-interacting protein, promotes Aβ generation and regulates
the Aβ level of the synapse, serving a potentially important role
in the pathogenesis of AD (60,61). The expression of Syt1 was
significantly down-regulated in cerebrospinal fluid and brain
tissue samples of human patients with early-onset AD (62). Therefore, by regulating a series
of target molecules, such as CDKs (57) and/or Syt1 (60-62), miR-449a may be involved in the
synaptogenesis of neurons and the regulation of Aβ levels.
The results of the present study demonstrated that
miR-449a levels decrease with aging in mice and that the
overexpression of miR-449a promotes the extension of neuronal
processes, suggesting that miR-449a may serve an important role in
the occurrence and development of neuro-degenerative diseases.
SCN2B, covalently linked to a subunit, is enriched in the central
nervous system, and has been well characterized as a cell-cell
adhesion protein (63,64). Nav2β serves a key role in
neuropathic pain (65,66), cardiac arrhythmias (67), Brugada syndrome (68), epilepsy (69), multiple sclerosis (70) and perineural invasion in prostate
cancer (71). Studies have
suggested that Navβ2 is a substrate of β-site APP-cleaving enzyme 1
(7,72). Navβ2 knockdown improved cognition
and restored sodium current density in hippocampal neurons and
neuronal activity in APP/PS1 mice (7), whereas presenilin/secretase-mediated
cleavage of the Navβ2-CTF (Navβ2-C-terminal fragment) regulates
cell adhesion and migration (64). SCN2B knockdown significantly
decreases the expression of amyloid precursor protein and the
deposition of Aβ41,42 in the hippocampus of transgenic mice. SCN2B
may be involved in the pathogenesis of AD by changing the stability
and excitability of sodium ion channels. Experiments conducted in
the present study verified that SCN2B is a valid target of
miR-449a, and that miR-449a may regulate the process of brain aging
by targeting SCN2B.
In conclusion, the present study demonstrated that
the age-associated increase in SCN2B is associated with the
regulation of miR-449a, which may serve a crucial role in brain
aging by targeting SCN2B. These data may suggest a novel
pathogenetic mechanism and potential candidate for the treatment of
brain aging.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81960210, 81701212,
81560238 and 31560279), the Yunnan Applied Basic Research
Foundation of Yunnan Province in China (grant no. 2016FB123), the
Foundation of Science and Technology Innovative Team Building of
Kunming Medical University (grant no. CXTD201807) and the Medical
Reserve Talents Cultivation Project of the Health and Family
Planning Commission of Yunnan Province (grant no. H-2017026).
Availability of data and materials
The data sets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YBX, YH, SJ, MNL and YXT designed the study,
analyzed the data and prepared the manuscript. YXT, MNL, SL, BC and
TH conducted mouse experiments, cell culture, cell transfection and
cell treatments. YBX, YH, MNL and SJ performed RNA labeling and
array hybridization, bioinformatics predictions, western blots,
reverse transcription-quantitative PCR, Northern blot experiments
and analysis. BC, LZ, TH, RMa and RMe conducted RIP-Chip, Co-IP,
dual-luciferase, Northern blot experiments and analysis. All
authors were substantially involved in the research, acquisition of
data, analysis and manuscript preparation. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Animal use and care were performed in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health (19) and the Care and Use of Experimental
Animals Guidelines established by the Ministry of Medicine of
Yunnan, China. The Ethics Committee of Kunming Medical University
approved the present study (permit no. km-edw-2013118).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Barzilai N, Cuervo AM and Austad S: Aging
as a biological target for prevention and therapy. JAMA.
320:1321–1322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang EF, Scheibye-Knudsen M, Jahn HJ, Li
J, Ling L, Guo H, Zhu X, Preedy V, Lu H, Bohr VA, et al: A research
agenda for aging in China in the 21st century. Ageing Res Rev.
24:197–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan
J and Yankner BA: Gene regulation and DNA damage in the ageing
human brain. Nature. 429:883–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dhar Malhotra J, Chen C, Rivolta I, Abriel
H, Malhotra R, Mattei LN, Brosius FC, Kass RS and Isom LL:
Characterization of sodium channel alpha- and beta-subunits in rat
and mouse cardiac myocytes. Circulation. 103:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao C, Yanbin X, Jia L, Ping D and Wang
TJ: The expression and changes of SCN2B mrna in frontal lobe and
hippocampus of senescence-accelerated mouse. Chin J Neuroanat.
23:662–665. 2007.
|
|
6
|
XiYang YB, Wang YC, Zhao Y, Ru J, Lu BT,
Zhang YN, Wang NC, Hu WY, Liu J, Yang JW, et al: Sodium channel
voltage-gated beta 2 plays a vital role in brain aging associated
with synaptic plasticity and expression of COX5A and FGF-2. Mol
Neurobiol. 53:955–967. 2016. View Article : Google Scholar
|
|
7
|
Hu T, Xiao Z, Mao R, Chen B, Lu MN, Tong
J, Mei R, Li SS, Xiao ZC, Zhang LF and Xiyang YB: Navβ2 knockdown
improves cognition in APP/PS1 mice by partially inhibiting seizures
and APP amyloid processing. Oncotarget. 8:99284–99295. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kadakkuzha BM, Akhmedov K, Capo TR,
Carvalloza AC, Fallahi M and Puthanveettil SV: Age-associated
bidirectional modulation of gene expression in single identified
R15 neuron of Aplysia. BMC Genomics. 14:8802013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
French L, Ma T, Oh H, Tseng GC and Sibille
E: Age-related gene expression in the frontal cortex suggests
synaptic function changes in specific inhibitory neuron subtypes.
Front Aging Neurosci. 9:1622017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang ZB, Tan YX, Zhao Q, Xiong LL, Liu J,
Xu FF, Xu Y, Bobrovskaya L, Zhou XF and Wang TH: miRNA-7a-2-3p
inhibits neuronal apoptosis in oxygen-glucose deprivation (OGD)
model. Front Neurosci. 13:162019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nohata N, Sone Y, Hanazawa T, Fuse M,
Kikkawa N, Yoshino H, Chiyomaru T, Kawakami K, Enokida H, Nakagawa
M, et al: miR-1 as a tumor suppressive microRNA targeting TAGLN2 in
head and neck squamous cell carcinoma. Oncotarget. 2:29–42. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fabian MR and Sonenberg N: The mechanics
of miRNA-mediated gene silencing: A look under the hood of miRISC.
Nat Struct Mol Biol. 19:586–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Son YH, Ka S, Kim AY and Kim JB:
Regulation of adipocyte differentiation via MicroRNAs. Endocrinol
Metab (Seoul). 29:122–135. 2014. View Article : Google Scholar
|
|
15
|
Saraiva C, Esteves M and Bernardino L:
MicroRNA: Basic concepts and implications for regeneration and
repair of neuro-degenerative diseases. Biochem Pharmacol.
141:118–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin Y, Liang X, Yao Y, Xiao H, Shi Y and
Yang J: Osthole attenuates APP-induced Alzheimer's disease through
up-regulating miRNA-101a-3p. Life Sci. 225:117–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Qin L and Tang B: MicroRNAs in
Alzheimer's disease. Front Genet. 10:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu W, Liu C, Yin B and Peng XZ: Functions
of miR-9 and miR-9* during Aging in SAMP8 mice and their possible
mechanisms. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 37:253–258.
2015.PubMed/NCBI
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Shen Q, Wang Y, Dimos JT, Fasano CA,
Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE and
Temple S: The timing of cortical neurogenesis is encoded within
lineages of individual progenitor cells. Nat Neurosci. 9:743–751.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang F, Qian X, Qin C, Lin Y, Wu H, Chang
L, Luo C and Zhu D: Phosphofructokinase-1 negatively regulates
neurogenesis from neural stem cells. Neurosci Bull. 32:205–216.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal V, Bell GW, Nam J and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
24
|
García DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar :
|
|
26
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Molecular
Cell. 27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C,
Dalamagas T and Hatzigeorgiou AG: DIANA-microT web server v5.0:
Service integration into miRNA functional analysis workflows.
Nucleic Acids Res. 41(Web Server Issue): W169–W173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reczko M, Maragkakis M, Alexiou P, Grosse
I and Hatzigeorgiou AG: Functional microRNA targets in protein
coding sequences. Bioinformatics. 28:771–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang WX, Wilfred BR, Hu Y, Stromberg AJ
and Nelson PT: Anti-Argonaute RIP-Chip shows that miRNA
transfections alter global patterns of mRNA recruitment to
microribonucleoprotein complexes. RNA. 16:394–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang WX, Wilfred BR, Baldwin DA, Isett RB,
Ren N, Stromberg A and Nelson PT: Focus on RNA isolation: Obtaining
RNA for microRNA (miRNA) expression profiling analyses of neural
tissue. Biochim Biophys Acta. 1779:749–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nelson PT, De Planell-Saguer M, Lamprinaki
S, Kiriakidou M, Zhang P, O'Doherty U and Mourelatos Z: A novel
monoclonal antibody against human Argonaute proteins reveals
unexpected characteristics of miRNAs in human blood cells. RNA.
13:1787–1792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
The Gene Ontology Consortium: The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res. 47(D1): D330–D338. 2019. View Article : Google Scholar :
|
|
37
|
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T and
Zhang L: Analyzing the interactions of mRNAs and ncRNAs to predict
competing endogenous RNA networks in osteosarcoma chemo-resistance.
Mol Ther. 27:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai J, Wang H, Pan Z and Su F: A novel
six-microRNA-based model to improve prognosis prediction of breast
cancer. Aging (Albany NY). 11:649–662. 2019. View Article : Google Scholar
|
|
39
|
Lee WJ, Moon J, Jeon D, Shin YW, Yoo JS,
Park DK, Lee ST, Jung KH, Park KI, Jung KY, et al: Possible
epigenetic regulatory effect of dysregulated circular RNAs in
Alzheimer's disease model. Sci Rep. 9:119562019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu T, Chang YF, Xiao Z, Mao R, Tong J,
Chen B, Liu GC, Hong Y, Chen HL, Kong SY, et al: miR-1 inhibits
progression of high-risk papillomavirus-associated human cervical
cancer by targeting G6PD. Oncotarget. 7:86103–86116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ebert MS, Neilson JR and Sharp PA:
MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian
cells. Nat Methods. 4:721–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhalala OG, Pan L, Sahni V, McGuire TL,
Gruner K, Tourtellotte WG and Kessler JA: microRNA-21 regulates
astrocytic response following spinal cord injury. J Neurosci.
32:17935–17947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu T, Lu MN, Chen B, Tong J, Mao R, Li SS,
Dai P, Tan YX and Xiyang YB: Electro-acupuncture-induced
neuroprotection is associated with activation of the IGF-1/PI3K/Akt
pathway following adjacent dorsal root ganglionectomies in rats.
Int J Mol Med. 43:807–820. 2019.
|
|
44
|
Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A,
Shi X, Ji L, Cheng S, Pan B, et al: Trimethylamine-N-oxide promotes
brain aging and cognitive impairment in mice. Aging Cell.
17:e127682018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akiguchi I, Pallàs M, Budka H, Akiyama H,
Ueno M, Han J, Yagi H, Nishikawa T, Chiba Y, Sugiyama H, et al:
SAMP8 mice as a neuropathological model of accelerated brain aging
and dementia: Toshio Takeda's legacy and future directions.
Neuropathology. 37:293–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Manich G, Mercader C, del Valle J,
Duran-Vilaregut J, Camins A, Pallàs M, Vilaplana J and Pelegrí C:
Characterization of amyloid-β granules in the hippocampus of SAMP8
mice. J Alzheimers Dis. 25:535–546. 2011. View Article : Google Scholar
|
|
47
|
Tormo E, Ballester S, Adam-Artigues A,
Burgués O, Alonso E, Bermejo B, Menéndez S, Zazo S, Madoz-Gúrpide
J, Rovira A, et al: The miRNA-449 family mediates doxorubicin
resistance in triple-negative breast cancer by regulating cell
cycle factors. Sci Rep. 9:53162019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li F, Liang J and Bai L: MicroRNA-449a
functions as a tumor suppressor in pancreatic cancer by the
epigenetic regulation of ATDC expression. Biomed Pharmacother.
103:782–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Zhou J, Chen X, Yang B, Wang D,
Yang P, He X and Li H: miRNA-449a is downregulated in osteosarcoma
and promotes cell apoptosis by targeting BCL2. Tumour Biol.
36:8221–8229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sarma NJ, Tiriveedhi V, Crippin JS,
Chapman WC and Mohanakumar T: Hepatitis C virus-induced changes in
microRNA 107 (miRNA-107) and miRNA-449a modulate CCL2 by targeting
the interleukin-6 receptor complex in hepatitis. J Virol.
88:3733–3743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Muth M, Hussein K, Jacobi C, Kreipe H and
Bock O: Hypoxia-induced down-regulation of microRNA-449a/b impairs
control over targeted SERPINE1 (PAI-1) mRNA-a mechanism involved in
SERPINE1 (PAI-1) overexpression. J Transl Med. 9:242011. View Article : Google Scholar
|
|
52
|
Lize M, Pilarski S and Dobbelstein M:
E2F1-inducible microRNA 449a/b suppresses cell proliferation and
promotes apoptosis. Cell Death Differ. 17:452–458. 2010. View Article : Google Scholar
|
|
53
|
Feng M and Yu Q: miR-449 regulates
CDK-Rb-E2F1 through an auto-regulatory feedback circuit. Cell
Cycle. 9:213–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma LP, Li N, He XJ and Zhang Q: miR-449b
and miR-34c on inducing down-regulation of cell cycle-related
proteins and cycle arrests in SKOV3-ipl cell, an ovarian cancer
cell line. Beijing Da Xue Xue Bao Yi Xue Ban. 43:129–133. 2011.In
Chinese. PubMed/NCBI
|
|
55
|
Buurman R, Gürlevik E, Schäffer V, Eilers
M, Sandbothe M, Kreipe H, Wilkens L, Schlegelberger B, Kühnel F and
Skawran B: Histone deacetylases activate hepatocyte growth factor
signaling by repressing microRNA-449 in hepatocellular carcinoma
cells. Gastroenterology. 143:811–820.e15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang B, Dai JX, Pan YB, Ma YB and Chu SH:
Identification of biomarkers and construction of a microRNA-mRNA
regulatory network for ependymoma using integrated bioinformatics
analysis. Oncol Lett. 18:6079–6089. 2019.PubMed/NCBI
|
|
57
|
Lim AC and Qi RZ: Cyclin-dependent kinases
in neural development and degeneration. J Alzheimers Dis.
5:329–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wright JW and Harding JW: The brain
hepatocyte growth Factor/c-Met receptor system: A new target for
the treatment of Alzheimer's disease. J Alzheimers Dis.
45:985–1000. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hamasaki H, Honda H, Suzuki SO, Hokama M,
Kiyohara Y, Nakabeppu Y and Iwaki T: Down-regulation of MET in
hippocampal neurons of Alzheimer's disease brains. Neuropathology.
34:284–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gautam V, D'Avanzo C, Berezovska O, Tanzi
RE and Kovacs DM: Synaptotagmins interact with APP and promote Aβ
generation. Mol Neurodegener. 10:312015. View Article : Google Scholar
|
|
61
|
Kuzuya A, Zoltowska KM, Post KL, Arimon M,
Li X, Svirsky S, Maesako M, Muzikansky A, Gautam V, Kovacs D, et
al: Identification of the novel activity-driven interaction between
synaptotagmin 1 and presenilin 1 links calcium, synapse, and
amyloid beta. BMC Biol. 14:252016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Davidsson P, Jahn R, Bergquist J, Ekman R
and Blennow K: Synaptotagmin, a synaptic vesicle protein, is
present in human cerebrospinal fluid: A new biochemical marker for
synaptic pathology in Alzheimer disease? Mol Chem Neuropathol.
27:195–210. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Isom LL: The role of sodium channels in
cell adhesion. Front Biosci. 7:12–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim DY, Ingano LA, Carey BW, Pettingell WH
and Kovacs DM: Presenilin/gamma-secretase-mediated cleavage of the
voltage-gated sodium channel beta2-subunit regulates cell adhesion
and migration. J Biol Chem. 280:23251–23261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pertin M, Ji RR, Berta T, Powell AJ,
Karchewski L, Tate SN, Isom LL, Woolf CJ, Gilliard N, Spahn DR and
Decosterd I: Upregulation of the voltage-gated sodium channel beta2
subunit in neuropathic pain models: Characterization of expression
in injured and non-injured primary sensory neurons. J Neurosci.
25:10970–10980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lopez-Santiago LF, Pertin M, Morisod X,
Chen C, Hong S, Wiley J, Decosterd I and Isom LL: Sodium channel
beta2 subunits regulate tetrodotoxin-sensitive sodium channels in
small dorsal root ganglion neurons and modulate the response to
pain. J Neurosci. 26:7984–7994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bao Y, Willis BC, Frasier CR,
Lopez-Santiago LF, Lin X, Ramos-Mondragón R, Auerbach DS, Chen C,
Wang Z, Anumonwo J, et al: Scn2b deletion in mice results in
ventricular and atrial arrhythmias. Circ Arrhythm Electrophysiol.
9:pii: e003923. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Riuró H, Beltran-Alvarez P, Tarradas A,
Selga E, Campuzano O, Vergés M, Pagans S, Iglesias A, Brugada J,
Brugada P, et al: A missense mutation in the sodium channel β2
subunit reveals SCN2B as a new candidate gene for Brugada syndrome.
Hum Mutat. 34:961–966. 2013. View Article : Google Scholar
|
|
69
|
Wang JW, Shi XY, Kurahashi H, Hwang SK,
Ishii A, Higurashi N, Kaneko S and Hirose S; Epilepsy Genetic Study
Group Japan: Prevalence of SCN1A mutations in children with
suspected Dravet syndrome and intractable childhood epilepsy.
Epilepsy Res. 102:195–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
O'Malley HA, Shreiner AB, Chen GH,
Huffnagle GB and Isom LL: Loss of Na+ channel beta2
subunits is neuroprotective in a mouse model of multiple sclerosis.
Mol Cell Neurosci. 40:143–155. 2009. View Article : Google Scholar
|
|
71
|
Jansson KH, Castillo DG, Morris JW, Boggs
ME, Czymmek KJ, Adams EL, Schramm LP and Sikes RA: Identification
of beta-2 as a key cell adhesion molecule in PCa cell neurotropic
behavior: A novel ex vivo and biophysical approach. PLoS One.
9:e984082014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huth T, Schmidt-Neuenfeldt K, Rittger A,
Saftig P, Reiss K and Alzheimer C: Non-proteolytic effect of
beta-site APP-cleaving enzyme 1 (BACE1) on sodium channel function.
Neurobiol Dis. 33:282–289. 2009. View Article : Google Scholar
|