Introduction
Intrauterine adhesions (IUAs) were first defined by
Joseph Asherman in 1948, and their presence is also known as
Asherman syndrome (1).
Endometrial fibrosis is caused by various factors, such as trauma
and infection, and it results in impaired endometrial function,
endometrial adhesion, uterine cavity degeneration and progression
to an IUA (2,3). The primary clinical symptoms of IUAs
include decreased menstruation, amenorrhea, repeated spontaneous
abortion and infertility (3),
which have adverse effects on the physical and mental health of the
patients. For infertility patients who wish to remain fertile,
moderate-to-severe IUAs require surgery (3). Hysteroscopic transuterine resection
of adhesion (TCRA) is the most important and commonly used
treatment for moderate-to-severe intrauterine adhesions. TCRA has a
significant role in improving menstruation and increasing pregnancy
rate (4), but the disease
recurrence rate following TCRA is >62% (5). Therefore, the long-term treatment
results remain unsatisfactory. The prevention and decrease
recurrence following TCRA is a major clinical problem.
Transforming growth factor-β1 (TGF-β1) serves as a
multifunctional cellular regulator. Following activation of TGF-β1,
activated TGF-β1 binds to its receptor and activates downstream
Smad signalling, including Smad2 and Smad3, which serves a crucial
role in tissue fibrosis (6,7),
including cardiac (8), liver
(9), pulmonary (10), pancreatic (11) and renal fibrosis (12), and chronic autoimmune disease
Sjögren’s syndrome (SS) (13).
The mechanism of the TGF-β1/Smad signalling pathway involved in the
occurrence of intrauterine adhesions has been described previously
(14,15).
Aspirin is a non-steroidal anti-inflammatory drug
whose primary active component is acetylsalicylic acid (16). Aspirin is clinically used to
decrease fever, inflammation, cardiovascular disease and certain
types of cancer (17–19). Jiang et al (20) described the association between
the use of aspirin and liver fibrosis in 1,856 patients with
chronic liver disease in the USA. The results revealed that the
liver fibrosis index in patients using aspirin was decreased
compared with those who did not use aspirin. A recent study in a
rat liver fibrosis model demonstrated that aspirin may
significantly improve the degree of liver fibrosis in rats
(21). In addition, aspirin has a
positive effect on improving cardiac fibrosis (22). A recent study has demonstrated
that aspirin has a positive effect on the growth and repair of the
endometrium following IUAs (23),
suggesting that this may be associated with the promotion of
endometrial microvascular formation and improvement of local blood
circulation by aspirin, thereby decreasing IUA recurrence,
improving menstruation and increasing the pregnancy rate (23). To the best of our knowledge, there
are no previous studies investigating whether aspirin inhibits
endometrial fibrosis by inhibiting the TGF-β1-Smad2/Smad3 pathway
and decreases postoperative recurrence of IUAs.
Materials and methods
Patient selection
The present study recruited 54 patients with IUAs
who were admitted to the Xiangyang No. 1 People’s Hospital, Hubei
University of Medicine between July 2018 and July 2019. The present
study was reviewed and approved by the Ethics Committee of
Xiangyang No. 1 People’s Hospital, Hubei University of Medicine
(approval no. 2018KYLL). All patients provided written informed
consent prior to the study. The inclusion criteria were: Patients
diagnosed with IUAs by hysteroscopy; patients with a history of
infertility who wished to become pregnant; and patients who were
reviewed again with good compliance. The exclusion criteria were:
Infection; other diseases of the uterus; hormone-dependent or
malignant diseases; and patients who received hormone therapy
within 3 months prior to surgery. All patients underwent
hysteroscopic investigation of the IUAs within 3–7 days following
the end of the menstruation cycle. The IUA scores and grades were
evaluated according to the revised criteria of the American
Fertility Association (AFS) (24).
Postoperative artificial menstrual cycle
therapy and follow-up
Patients with IUAs were randomly divided into two
groups. All patients underwent TCRA surgery and received oral
medication. Patients in group A (observation group; n=26) were
given 4 mg/day oestradiol valerate for 21 days, and 1 mg/day
cyproterone acetate was given for the last 10 of the 21 days for
artificial cycle therapy for a total of 2 cycles. Group B
(combination therapy group; n=28) received 100 mg/day aspirin and 4
mg/day oestradiol valerate for 21 days, and 1 mg/day cyproterone
acetate was given during the last 10 of the 21 days for artificial
cycle therapy for a total of 2 cycles. Patients were treated
continuously for 2 months. All patients from both groups received
TCRA and the placement of an intrauterine-suitable balloon in the
uterus for 1 week. During the postoperative follow-up examinations,
there was no postoperative infection or abdominal pain observed in
any of the patients. The outcomes of the 2 different therapies
after 2 months were assessed using the following indicators:
Uterine length, endometrial thickness, menstrual flow and volume,
postoperative adhesion cases and postoperative adhesion score
according to the AFS standard. Hysteroscopy was performed by the
same senior doctor at the time of admission and 2 months following
surgery.
Histological staining, masson trichrome
staining and immunohistochemistry (IHC)
The endometrial tissues were fixed in 4% formalin
for a minimum of 24 h. The fixed tissues were embedded in paraffin
and cut to 4-μm thick sections for staining. The tissues were
stained using a Masson’s trichrome staining according to the
manufacturer’s protocol (cat. no. G1345; Beijing Solarbio Science
& Technology Co., Ltd.). The sections were immersed in bouin
buffer, incubated at 37°C for 2 h and rinsed three times with PBS.
Samples were then treated with the following agents at room
temperature, with a rinse in warm running water for 3 min after
each application: 0.5% celestine blue staining solution for 3 min;
Mayer (1%) hematoxylin staining solution for 3 min; and 1% acidic
ethanol differentiation solution for 10 sec. The following agents
were then administered, with a triplicate wash with distilled water
between: 1% acid fuchsin solution staining solution for 10 min; 1%
phosphomolybdic acid solution for 10 min; and 2% aniline blue
solution for 5 min. After dehydration with 95% ethanol samples were
treated with absolute ethyl alcohol in triplicate for a duration of
10 sec per treatment. Finally, samples were treated with 50% xylene
solution for 2 min and 100% xylene solution for 2 min. The
percentage of blue staining indicated the extent of endometrial
fibrosis. For IHC (according to the manufacturer’s protocol of the
immunohistochemical staining kit; cat. no. E670016, Sangon Biotech
Co., Ltd.), tissue paraffin sections were deparaffinized in xylene
(100% for 10 min; 50% for 10 min) and rehydrated using an alcohol
gradient (100% for 5 min; 95% for 5 min; 70% for 5 min) at room
temperature. Antigen retrieval was performed by heating in a
high-pressure induction cooker for 100 sec, and endogenous
peroxidase activity was quenched by immersing the samples in 3%
H2O2 for 10 min. Samples were then incubated
with anti-rabbit TGF-β1 (20 μg/ml; cat. no. ab92486; Abcam),
anti-rabbit phosphorylated (p)-Smad2 (1:100; cat. no. ab53100;
Abcam), anti-rabbit p-Smad3 (1:100; cat. no. ab74062; Abcam),
anti-rabbit α-smooth muscle actin (α-SMA; 1:100; cat. no. ab32575;
Abcam), anti-rabbit collagen type I α1 chain (COL1A1; 1
μg/ml; cat. no. LS-C343921; Lifespan BioSciences, Inc.) and
anti-rabbit fibronectin (FN; 1:200; cat. no. ab2413; Abcam) primary
antibodies overnight at 4°C. The slides were washed with PBS and
incubated with normal goat serum (1:50; cat. no. A0208; Beyotime
Institute of Biotechnology) at 37°C for 15 min. The slides were
then stained with 2% 3,3-diaminobenzidine, and cell nuclei were
stained with 1% haematoxylin at room temperature for 3 min. Images
were captured in randomly selected fields of each section at
magnification, ×200, and used for analysis of the positive
staining. The results of the Masson trichrome staining and IHC were
evaluated quantitatively using the ImageJ software [v.1.4.3.67;
National Institutes of Health (NIH)]. Average optical density (AOD)
was used in the present study for the statistical analysis and was
calculated using the following formula: AOD= integrated optical
density (IOD)/area.
Reverse transcriptionquantitative
polymerase chain reaction (RT-qPCR) assay
The total RNA of endometrium tissues was isolated
using a High PureRNA Isolation kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer’s protocol. The
amount of total RNA was estimated by measuring the absorbance at
260 and 280 nm, and the purity of each sample was determined
according to the A260/A280 ratios using a SMA2000 spectrophotometer
(Thermo Fisher Scientific, Inc.). A260/A280 ratio values of ≥1.8
and ≤2 were used an indicator of good quality RNA. For detection of
the α-SMA, COL1A1 and FN mRNA levels, 1 μg total RNA was reverse
transcribed at 37°C for 60 min using the miRcute miRNA First-Strand
cDNA Synthesis kit (Beyotime Institute of Biotechnology) and then
amplified using an Applied Biosystems 7900HT cycler using SuperReal
PreMix Plus (Beyotime Institute of Biotechnology). The qPCR
thermocycling conditions were as follows: Predenaturation at 94°C
for 5 min, 40 cycles of denaturation at 94°C for 20 sec and
annealing at 60°C for 20 sec. GAPDH was used as the normalization
control for qPCR. The relative expression was calculated using the
2−ΔΔCq method (25).
Each reaction was performed in triplicate. The primers used for the
RT-qPCR analysis are presented in Table I.
 | Table ISequences of the reverse
transcription quantitative polymerase chain reaction primers
used. |
Table I
Sequences of the reverse
transcription quantitative polymerase chain reaction primers
used.
| Name | Sequence |
|---|
| α-SMA | Forward:
5′-GCTTCCTCTTCTTCCCTGGAG-3′ |
| Reverse:
5′-AGATGGCTGGAAGAGGGTCTC-3′ |
| COL1A1 | Forward:
5′-GGCATAAAGGGTCATCGTG-3′ |
| Reverse:
5′-GAACCTTCGCTTCCATACTC-3′ |
| FN | Forward:
5′-GAGAGATCTGGAGGTCAT-3′ |
| Reverse:
5′-GGGTGACACCTGAGTTGAA-3′ |
| GAPDH | Forward:
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
| Reverse:
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
Western blot analysis
Endometrial tissues were lysed with a radio immune
precipitation assay buffer containing protease inhibitors. Proteins
were quantified using a BCA protein assay kit. Aliquots of the
lysates (30 μg) were separated via 10% SDS-PAGE, transferred onto
nitrocellulose membranes and blocked with 5% skimmed milk at room
temperature for 2 h. Primary antibodies against TGF-β1 (4 μg/ml;
cat. no. ab92486; Abcam), anti-rabbit p-Smad2 (1:500; cat. no.
ab53100; Abcam), anti-rabbit Smad2 (1:2,000; cat. no. ab40855;
Abcam), anti-rabbit p-Smad3 (1:500; cat. no. ab74062; Abcam),
anti-rabbit Smad3 (1:2,000; cat. no. ab40854; Abcam), anti-rabbit
α-SMA (1:2,000; cat. no. ab32575; Abcam), anti-rabbit COL1A1 (0.1
μg/ml; cat. no. LS-C343921; Lifespan BioSciences, Inc.);
anti-rabbit fibronectin (1:500; cat. no. ab2413; Abcam); and
anti-rabbit GAPDH (1:500; Beyotime Institute of Biotechnology) were
used in the analysis. The membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:1,000; cat. no.
A0208; Beyotime Institute of Biotechnology) for 1 h at 37°C.
Enhanced chemiluminescence (Thermo Fisher Scientific, Inc.) was
used to visualize the bands. ImageJ software (v.1.4.3.67; NIH) was
used to quantify the intensity of the bands, using GAPDH as the
control.
Statistical analysis
Measurement data are expressed as the mean ±
standard deviation. Normally distributed variables (average age,
body mass index and times of labour) were compared using Student’s
t-test between 2 groups, and the Mann-Whitney U test were used for
non-normally distributed variables (times of pregnancy, surgical
abortion, postoperative average AFS score) between 2 groups. All
datasets in which both pre-.vs. postoperative and group A vs. group
B comparisons were statistically compared by two-way
repeated-measures ANOVA, and a Bonferroni’s post hoc test was used
to correct for multiple comparisons. Count data were expressed as
the number of cases as a percentage. Comparisons between groups
were performed using a χ2 test or Fisher’s exact test.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
(v.22.0; IBM Corp.)
Results
Demographic data in the study groups
The baseline demographics and clinical
characteristics, including age, body mass index, times of
pregnancy, times of labour, IUA grade and most probable aetiology
of IUA, of the 54 patients included in the present study were not
significantly different (Table
II).
 | Table IIDemographic data of the study
groups. |
Table II
Demographic data of the study
groups.
| Variable | Group A (n=26) | Group B (n=28) |
|---|
| Average age,
years | 28.15±3.68 | 29.07±4.25 |
| Body mass
index | 22.16±2.18 | 22.01±1.99 |
| Times of
pregnancy | 3.81±1.13 | 3.96±1.07 |
| Times of
labour | 0.88±0.82 | 1.01±0.79 |
| IUA grade (n) |
| Moderate | 15 (57.7) | 16 (57.1) |
| Severe | 11 (42.3) | 12 (42.9) |
| Most probable
etiology of IUA |
| Surgical abortion
(average times) | 2.81±1.33 | 2.86±1.27 |
| Curettage in the
middle and late pregnancy (%) | 4 (15.4) | 3 (10.7) |
| Pelvic
inflammatory disease (%) | 1 (3.8) | 2 (7.1) |
| Other
etiology | No | No |
Patients with IUA receiving a combination
of oestradiol valerate and aspirin therapy exhibit better effects
than those with simple oestradiol valerate therapy
The results revealed that the recurrence rate of
postoperative adhesion and average AFS score in the combination
therapy group was significantly decreased compared with those in
the oestradiol valerate-alone therapy group (Table II). In both treatment groups,
significant differences in uterine length, menstrual flow,
menstrual volume and endometrial thickness between the pre- and
postoperative values within the same group were observed. In
addition, the combination therapy group exhibited greater
improvements, including duration of menstrual period (4.50±0.71
days in group A vs. 5.39±1.20 days in group B; P<0.01),
menstrual volume (55.77±8.45 ml in group A vs. 63.57±12.39 ml in
group B; P<0.01), endometrial thickness (6.88±0.77 mm in group A
vs. 7.54±1.14 mm in group B; P<0.05), no adhesion cases
following operation [34.6% (9/26) in group A vs. 75.0%, 21/28 in
group B; P<0.05] and postoperative average AFS score (5.62±2.42
in group A vs. 3.43±1.73 in group B; P<0.01), with the exception
of uterine length (7.09±0.22 cm in group A vs. 7.13±0.26 cm in
group B (P=0.253) (Table
III).
 | Table IIIOutcome measures in the study
groups. |
Table III
Outcome measures in the study
groups.
| Variable | Group A (n=26) | Group B (n=28) |
|---|
| Uterine length,
cm | | |
| Preoperative | 6.10±0.26 | 6.23±0.37 |
| Postoperative | 7.09±0.22b | 7.13±0.26b |
| Endometrium
thickness, mm | | |
| Preoperative | 5.04±1.04 | 4.96±1.04 |
| Postoperative | 6.88±0.77b | 7.54±1.14a,b |
| Duration of
menstrual period, days | | |
| Preoperative | 2.81±0.75 | 2.71±1.08 |
| Postoperative | 4.50±0.71b | 5.39±1.20a,b |
| Menstrual volume,
ml | | |
| Preoperative | 30.77±9.56 | 31.61±9.72 |
| Postoperative | 55.77±8.45b | 63.57±12.39a,b |
| Postoperative
adhesion cases, n (%) | | |
| No | 9 (34.6) | 21 (75.0)a |
| Mild | 7 (26.9) | 3 (10.7) |
| Moderate | 5 (19.2) | 2 (7.1) |
| Severe | 5 (19.2) | 2 (7.1) |
| Postoperative
average AFS score | 5.62±2.42 | 3.43±1.73a |
Combination therapy with oestradiol
valerate and aspirin inhibits endometrial fibrosis in patients with
IUA
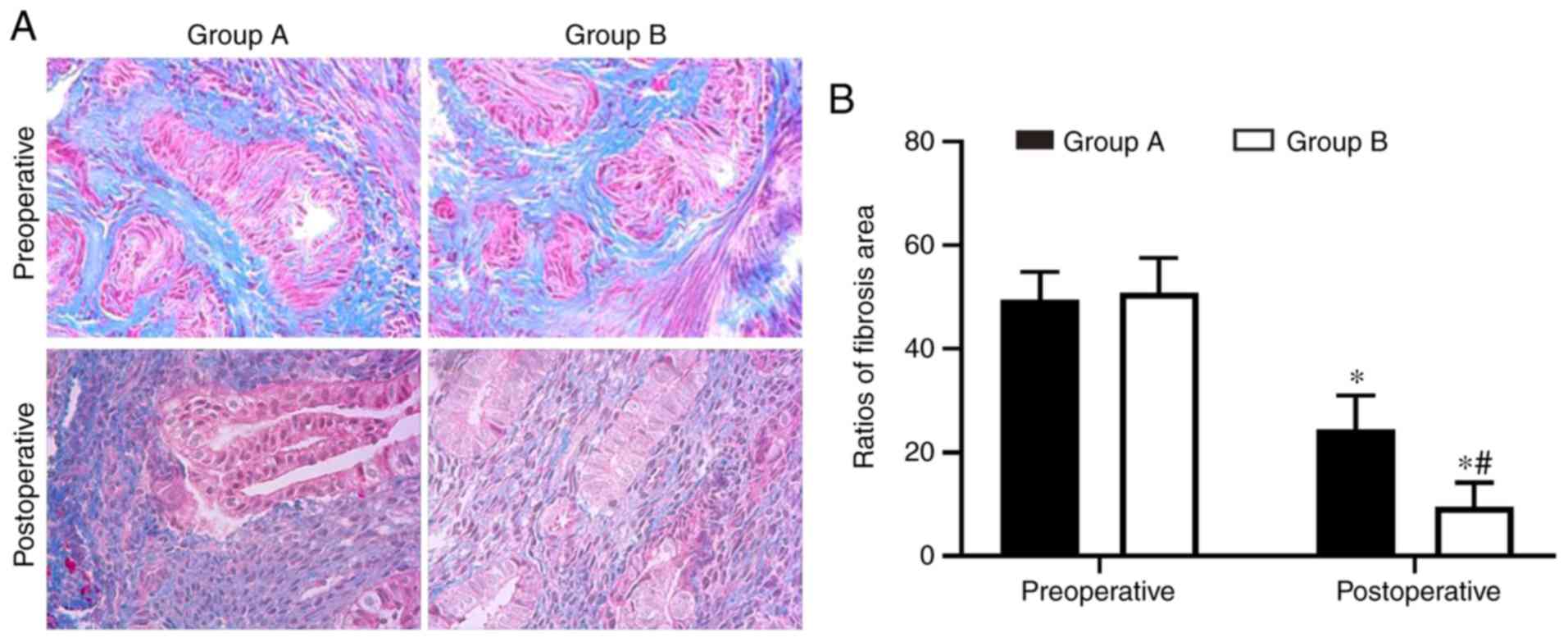
Endometrial fibrosis was measured in the tissues
with Masson’s trichrome staining. The results revealed that the
endometrial tissues from the two postoperative groups exhibited
less fibrosis compared with the tissues from the preoperative
groups. However, the combined group exhibited significantly
decreased levels of endometrial fibrosis compared with the
oestradiol valerate-alone therapy group. The endometrial fibrosis
area was 61% decreased in the combination therapy group compared
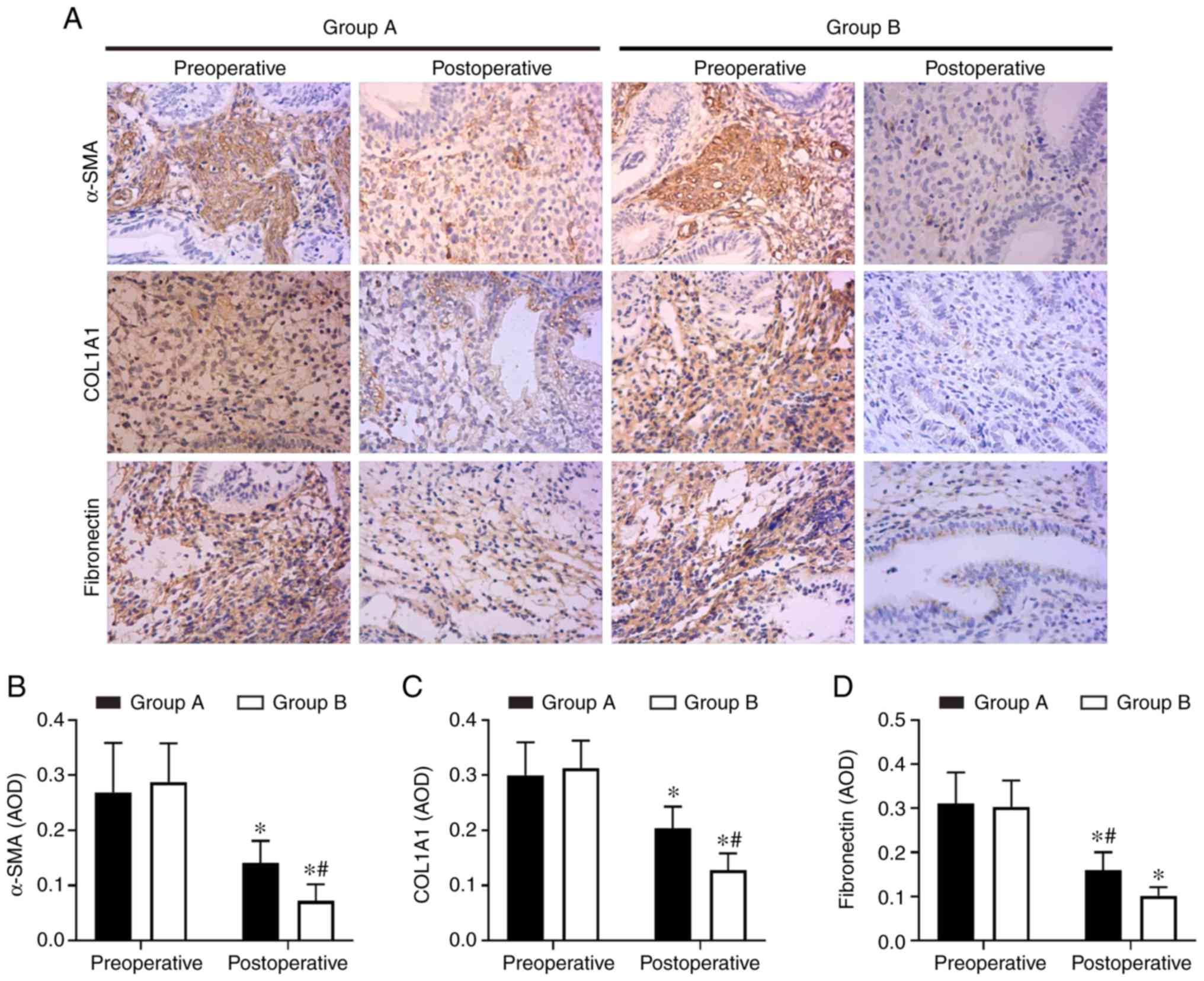
with in the oestradiol valerate-alone therapy group (Fig. 1). In addition, the expression
levels of the fibrosis markers (COL1A1, α-SMA and fibronectin) in
tissues were analysed with IHC staining. COL1A1, α-SMA and
fibronectin exhibited strong staining in the preoperative
endometrial tissues from the two groups. These 3 proteins were
mildly expressed or were undetected in the postoperative tissues.
However, 3 proteins levels were expressed less in the combination
therapy group compared with in the oestradiol valerate-alone
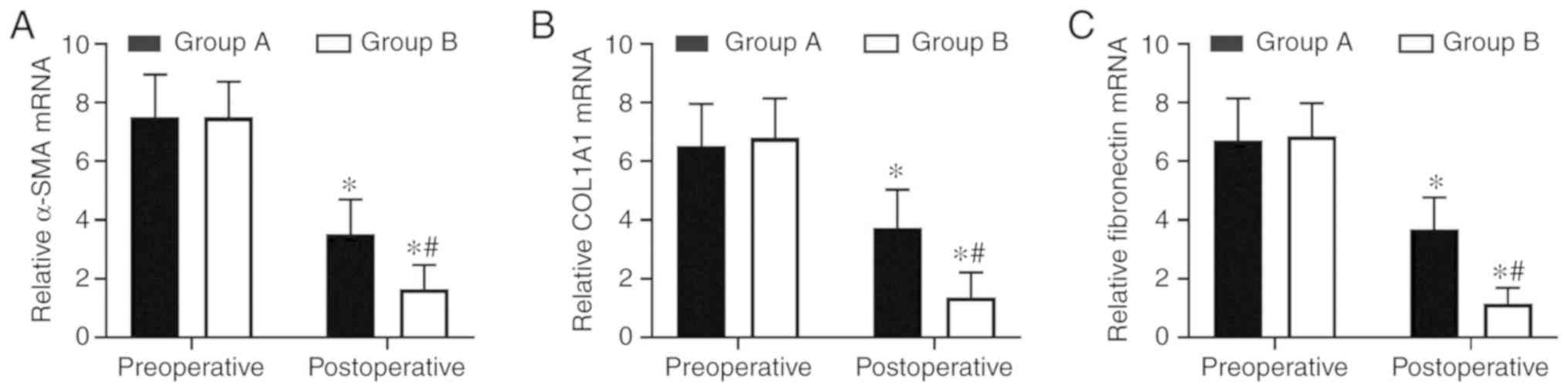
therapy group (Fig. 2). The
levels of 3 fibrotic markers were examined at the mRNA level by
RT-qPCR and at the protein level by western blot analysis in the
pre- and postoperative tissues. There was no significant difference
in preoperative expression levels between the two groups. However,
the expression levels of the two groups were significantly
decreased following therapy compared with the preoperative levels.
In samples from the combination therapy, the mRNA and relative
protein expression levels of α-SMA, COL1A1 and fibronectin were
decreased compared with in the oestradiol valerate-alone therapy
group (Figs. 3 and 4A–D), which were consistent with the IHC
staining results. These data suggest that combination therapy with
oestradiol valerate and aspirin may have a more positive effect on
inhibiting endometrial fibrosis during endometrium rehabilitation
in patients with IUA compared with the oestradiol valerate-alone
therapy group.
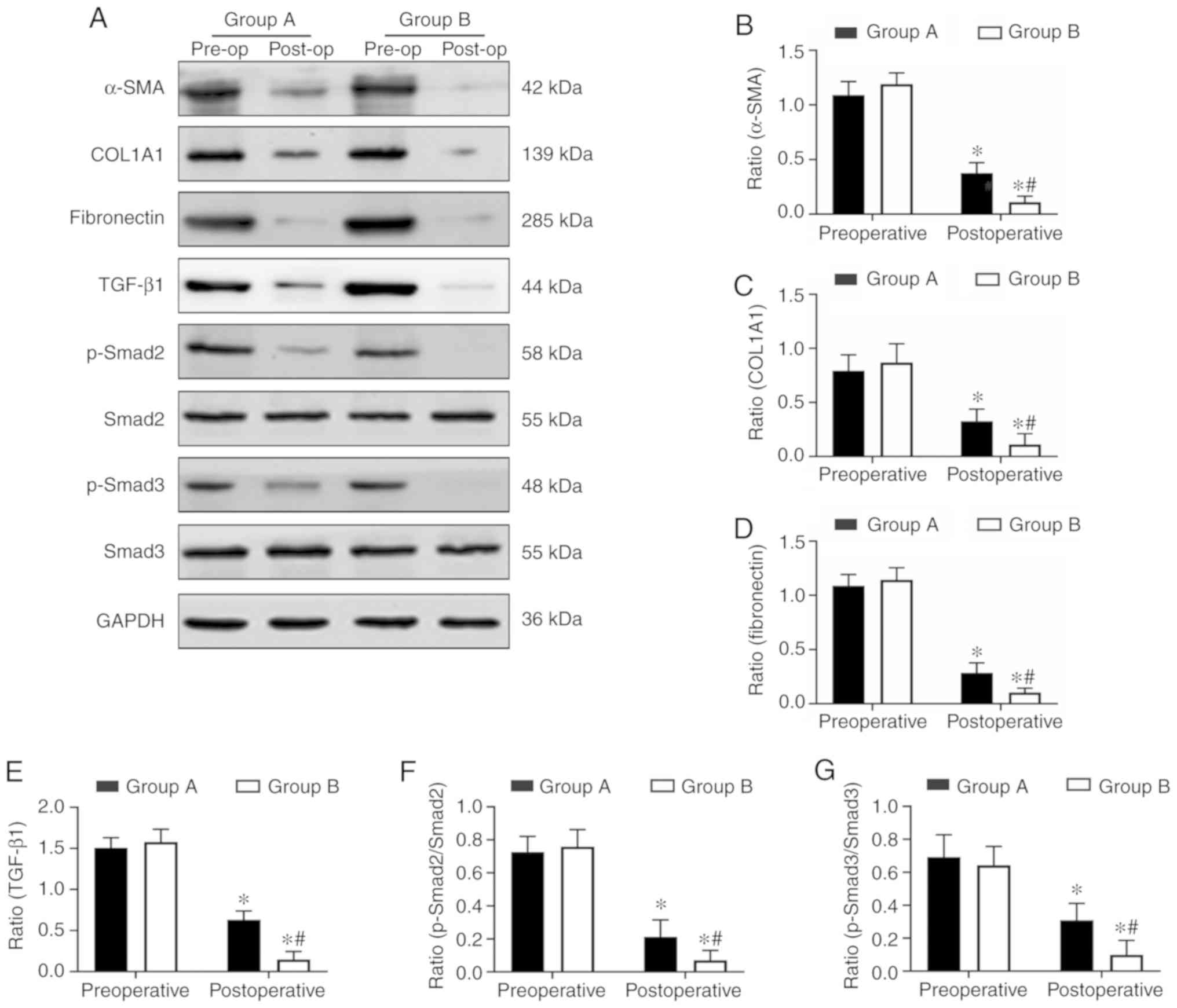 | Figure 4Aspirin inhibits the protein
expression levels of COL1A1, α-SMA, fibronectin and the
TGF-β1-Smad2/Smad3 pathway in endometrial tissues. (A) The
expression levels of COL1A1, α-SMA, fibronectin, TGF-β1, Smad2,
p-Smad2, Smad3 and p-Smad3 in endometrial tissues were determined
at the protein level by western blot analysis. (B–E) The relative
protein quantities (COL1A1, α-SMA, fibronectin, TGF-β1) were
calculated by comparison against the internal control, GAPDH. (F
and G) The relative quantity of p-Smad2 and p-Smad3 were calculated
by comparison against the total Smad2 and Smad3, respectively. The
protein levels of the fibrosis markers (COL1A1, α-SMA,
fibronectin), TGF-β1, p-Smad2/Smad2 and p-Smad3/Smad3, were
significantly inhibited in the endometrium tissues in the aspirin
and oestradiol valerate therapy group (group B; n=8) compared with
the oestradiol valerate therapy group (group A; n=8) following
treatment. *P<0.05 vs. preoperative.
#P<0.05 vs. group A. Pre-op, preoperative; post-op,
postoperative; COL1A1, collagen type I α 1 chain; α-SMA, α-smooth
muscle actin; TGF-β1, transforming growth factor β1; p-,
phosphorylated. |
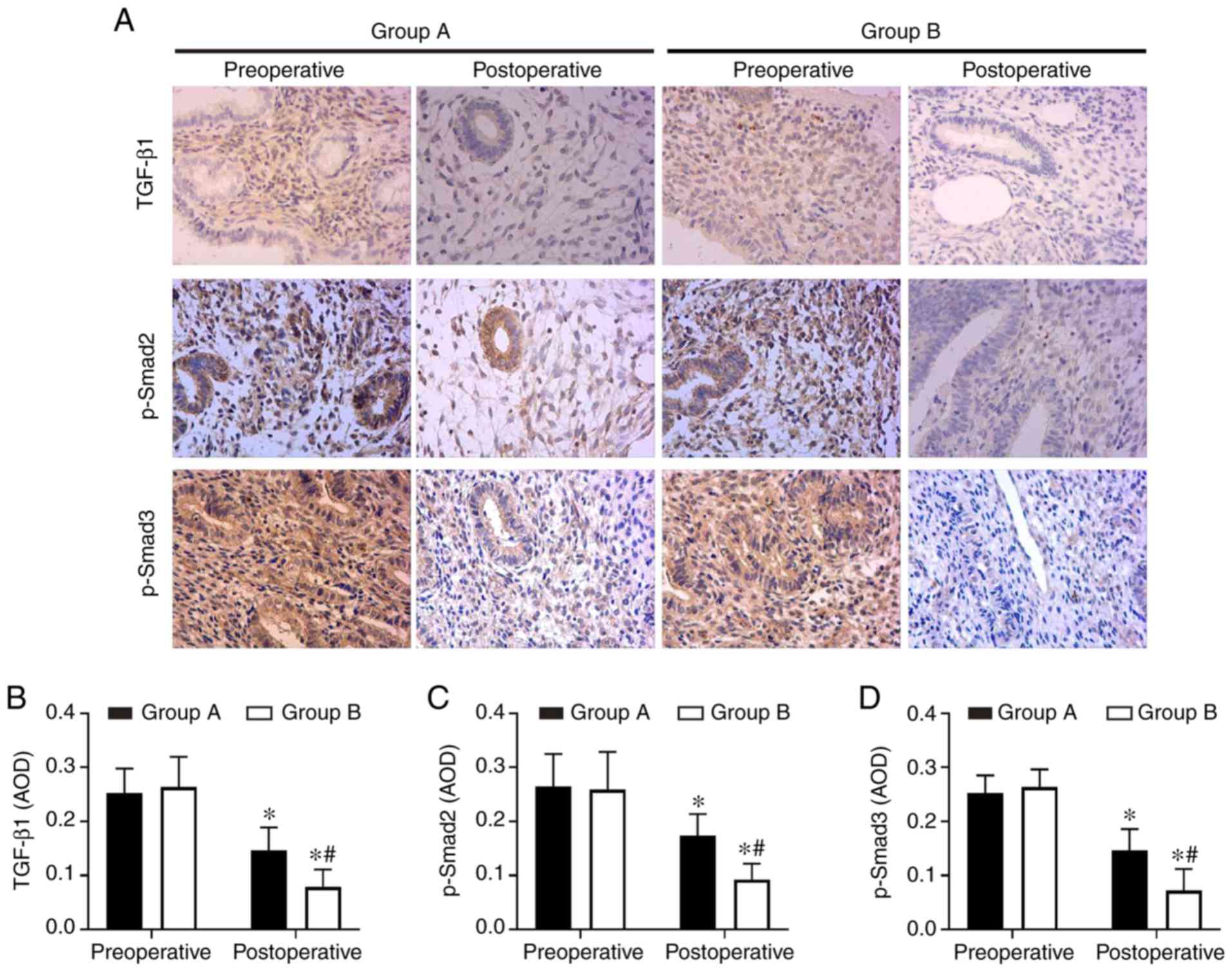
Aspirin inhibits endometrial fibrosis by
suppressing the TGF-β1-Smad2/Smad3 pathway in intrauterine
adhesions
In order to understand the molecular mechanisms of
aspirin inhibition of endometrial fibrosis, the present study
examined changes in TGF-β1, Smad2, p-Smad2, Smad3 and p-Smad3 in
endometrial tissues both prior to and following treatment. The IHC
and western blot analysis results revealed that TGF-β1, p-Smad2 and
p-Smad3 were expressed at high levels in preoperative endometrial
tissues, but that this level decreased following surgery in the two
groups. Combination therapy with aspirin and oestradiol valerate
resulted in a significant inhibition of TGF-β1, p-Smad2 and p-Smad3
expression levels in the uterine tissues compared with oestradiol
valerate alone. However, no significant changes in total Smad2 and
Smad3 levels were observed (Figs. 4A,
E–G and 5). These data
indicated that aspirin may inhibit postoperative endometrial
fibrosis by suppressing the TGF-β1-Smad2/Smad3 pathway in IUAs.
Discussion
IUAs are a major health problem that lead to
abnormal menstrual patterns, repeated spontaneous abortions and
female infertility. In the endometrial tissues of patients with
IUAs, a large amount of fibrous tissue replaces the endometrium,
destroying the basal layer of the endometrium, decreasing the blood
vessels in the tissue and decreasing the perfusion of oestrogen and
progesterone, further aggravating intrauterine adhesions (5). At present, the treatment goals for
IUAs are primarily to restore the normal shape and volume of the
uterine cavity and prevent adhesion recurrence and
treatment-associated symptoms, including infertility and amenorrhea
(26). Although intrauterine
adhesions can be improved to a certain extent through surgery and
treatment, there is currently no effective strategy to prevent
disease recurrence following TCRA surgery.
Extensive studies have been performed regarding this
issue, including oral oestrogen (27), transdermal oestrogen gel (28), placement of an intrauterine
balloon (29) or intrauterine
device (30), intrauterine
injection of sodium hyaluronate (31), amniotic membrane transplantation
(32) and other methods following
TRCA surgery. In order to obtain improved therapeutic effects in
patients with moderate and severe IAUs, it is necessary to use
oestrogen combined with other adjuvant therapy following TCRA
(33). Bosteels et al
(34) statistically analysed the
data on drug therapy to prevent adhesion recurrence and treatment
of infertility following TCRA surgery prior to June 2017, and
suggested that it remains uncertain whether anti-adhesion therapy
improves the critical reproductive outcome or decreases the
severity of IUAs. Of course, due to the blind manner in which the
participants and personnel were involved, as well as the risk of
serious bias associated with indirectness and inaccuracy, the
overall quality of the evidence is low. Conversely, although these
treatments may improve symptoms to a certain extent, the overall
treatment effect remains unsatisfactory.
Aspirin is one of the most widely used drugs in the
world. Aspirin was originally used primarily in patients with
antipyretic and analgesic diseases. At present, there is increasing
evidence that aspirin has a preventative effect in colorectal
cancer and other types of cancer (35). Recent studies have suggested that
aspirin is more effective in preventing adhesion recurrence
following TCRA surgery, as it can increase endometrial angiogenesis
(28). A prospective study
revealed that aspirin combined with an intrauterine balloon and
intrauterine device significantly increased endometrial thickness
following TCRA surgery, and AFS scores and menstrual scores also
improved significantly (23). Chi
et al (28) demonstrated
that compared with transdermal oestrogen alone therapy, transdermal
oestrogen combined with aspirin following TCRA surgery resulted in
an improved effect on increasing the endometrial thickness,
increasing menstrual flow, preventing adhesion recurrence and
improving endometrial receptivity, which may be associated with
increased uterus intimal angiogenesis and decreased resistant index
and pulsatility index in uterine arteries. The endometrial repair
time following TCRA surgery is usually ~2 months (36). Therefore, all patients in the
present study were reviewed 2 months after TCRA to assess for
endometrial recovery. The results of the present study revealed
that patients receiving aspirin combined with oestrogen and
progesterone following TCRA exhibited positive effects regarding
improved menstruation, increased endometrial thickness and improved
AFS scores compared with patients receiving single oestrogen and
progesterone.
Although there are some studies on the application
of aspirin as an anti-fibrosis treatment, the specific
anti-fibrosis mechanism of aspirin remains unclear. Aspirin
decreases cardiac interstitial fibrosis caused by pressure overload
in transverse aortic constriction (TAC) mice, which may be
associated with the inhibition of the p-Erk1/2 and p-Akt/β-catenin
signalling pathways by aspirin, thereby decreasing the expression
levels of α-SMA and collagen I (37). A previous study revealed that
aspirin alleviated cardiac fibrosis in mice by inhibiting autophagy
(22). In a rat liver fibrosis
model, aspirin improved the degree of liver fibrosis in a
dose-dependent manner (21), but
the underlying molecular mechanism remains unclear. Endometrial
fibrosis is considered to be a key pathological event in the
development of IUAs (38).
Previous studies have demonstrated that aspirin may decrease
fibrotic markers, including TGF-β, connective tissue growth factor
(CTGF), collagen I and collagen III, in endometrial tissue
(28), but the specific mechanism
of action remains unclear. The results of the present study
revealed that the expression of COL1A1, α-SMA and FN in the
endometrium of the aspirin group was decreased, thereby indicating
an active role in the anti-fibrosis process.
TGF-β1 is considered to be a key mediator of
fibrosis. It serves a vital role in regulating wound healing and
tissue repair. Activation and overexpression of TGF-β1 are
associated with tissue scarring and fibrosis (39). TGF-β1 activates both classical
(Smad-based) signaling pathways and non-classical (non-Smad-based)
signaling pathways, leading to the activation of myofibroblasts,
overproduction of extracellular matrix (ECM) and inhibition ECM
degradation (40). In Smad-based
signaling pathways, TGF-β1 binds to the TGF-β1 receptor on the cell
membrane to form a ligand-receptor complex, which in turn binds,
phosphorylates and activates Smad2 and Smad3. Smad4 then binds
activated Smad2/3, and the complex enters the nucleus and interacts
with transcription factors to induce transcription of profibrotic
molecules, including α-SMA, collagen I, fibronectin and tissue
inhibitor of matrix metalloproteinases (TIMP) (40). Conversely, Smad3 may induce
transcription of fibrotic fs (miRNAs) and long non-coding RNAs
(lncRNAs), while indirectly inhibiting transcription of
anti-fibrotic miRNAs. In addition, Smad3 can increase the
transcription of fibrotic molecules by affecting epigenetic
modifications of DNA and histones (40). There have been advances in the
progress of various fibrotic diseases treatment options that target
the TGF-β1/Smad signaling pathway, including idiopathic pulmonary
fibrosis and renal fibrosis (41,42). The TGF-β type I receptor kinase
small molecule inhibitor EW-7197 inhibits the TGF-β1/Smad2/3
signalling pathway and has potential for anti-fibrotic therapy
(43). Suberoylanilide hydroxamic
acid, an inhibitor of histone deacetylases that may decrease the
levels of TGF-β1, CTGF, α-SMA, p-Smad2/3, can alleviate rat liver
fibrosis by suppressing TGF-β1 signaling (44). These data indicate that targeting
the TGF-β1-Smad signaling pathway is a potential avenue of
anti-fibrotic therapy.
TGF-β1 has a specific role in the occurrence and
progression of IUAs, and the expression levels of TGF-β1 and Smad3
are positively correlated with the severity of IUAs (15,45). Studies have revealed that
miRNA-29b and miRNA-326 may improve endometrial fibrosis in primary
human endometrial stromal cells, and can significantly decrease
COL1A1, α-SMA and FN expression by inhibiting the TGF-β1/Smad
signalling pathway (14,46). In order to investigate the
potential mechanism underlying the anti-fibrosis effect of aspirin,
the present study examined the expression levels of TGF-β1, Smad2
and Smad3 in the endometrial tissues of two groups. The results of
the present study demonstrated that the expression levels of
COL1A1, α-SMA and FN in the endometrial tissues of the combination
therapy group were decreased compared with those in the oestradiol
valerate group. Aspirin may inhibit the expression of TGF-β1,
p-Smad2 and p-Smad3 by TGF-β1-Smad-based signaling pathways in
endometrial tissues, which may be one of the mechanisms by which
aspirin improves endometrial fibrosis.
It is worth noting that combination therapy with
oestrogen and aspirin may improve the rate of pregnancy in patients
with IUA (28). However, an
additional study indicated that there was no significant
improvement in the pregnancy rate and live birth rate following
additional low dose aspirin (23). Different results may be associated
with the different doses of aspirin, which suggests that aspirin
serves a positive role in improving the pregnancy outcome of
patients with IUAs. The appropriate dose of aspirin required for
endometrial repair following TCRA recovery requires a continuous
follow-up study. In addition, obstetric complications should be
observed, including placentation issues, prematurity and postpartum
hysterectomy following treatment for IUAs (47). For example, intrauterine adhesions
may lead to placenta accreta and the risk of severe postpartum
haemorrhage (48). However, to
the best of our knowledge, whether aspirin may decrease the
obstetric complications with IUAs following surgery has not yet
been described in the literature and requires long-term follow-up
observations.
One potential limitation of the present study is
that there was a relatively small number of cases and a short
follow-up time period. A larger sample size is required in order to
assess the appropriate dose and the duration of aspirin treatment,
and the effect on pregnancy outcomes and obstetric complications,
which will form the basis for future studies. In addition,
endometrial tissue samples from patients with IUAs are rare and
difficult to obtain: A small amount of endometrial tissue can be
valuable for endometrial repair in IUAs, and certain patients with
intrauterine adhesions are reluctant to participate in research for
this reason. Animal models of intrauterine adhesion will be used in
future studies to further investigate the mechanism of
anti-endometrial fibrosis in aspirin.
In conclusion, aspirin combined with oestradiol
valerate exhibited an improved effect on increasing endometrial
thickness and improving menstrual and AFS scores following TCRA
compared with oestradiol valerate alone. This result reveals that
aspirin inhibits endometrial fibrosis by suppressing the
TGF-β1-Smad2/Smad3 pathway in IUAs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Faculty
Development Grants From Xiangyang No. 1 People’s Hospital (grant
no. 28) and the Natural Science Foundation of Hubei Province of
China (grant nos. 2019AHB068 and 2018CFB701).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
ZZ and SL were responsible for protocol/project
development and manuscript writing. JD, SY, ZX, HG and HX performed
data management and analysis. WZ was involved in protocol/project
development and supervision. MS reviewed the final manuscript and
data analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of Xiangyang No. 1 People’s Hospital, Hubei
University of Medicine (approval no. 2018KYLL), and written
informed consent was obtained from all patients prior to their
inclusion in the study.
Patient consent for publication
The patients or their guardians have provided
written informed consent for the publication of any associated data
and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asherman JG: Amenorrhoea traumatica
(atretica). J Obstet Gynaecol Br Emp. 55:23–30. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Healy MW, Schexnayder B, Connell MT, Terry
N, DeCherney AH, Csokmay JM, Yauger BJ and Hill MJ: Intrauterine
adhesion prevention after hysteroscopy: A systematic review and
meta-analysis. Am J Obstet Gynecol. 215:267–275.e7. 2016.
View Article : Google Scholar
|
|
3
|
Deans R and Abbott J: Review of
intrauterine adhesions. J Minim Invasive Gynecol. 17:555–569. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhandari S, Bhave P, Ganguly I, Baxi A and
Agarwal P: Reproductive outcome of patients with Asherman’s
syndrome: A SAIMS experience. J Reprod Infertil. 16:229–235.
2015.
|
|
5
|
Yu D, Wong YM, Cheong Y, Xia E and Li TC:
Asherman syndrome-one century later. Fertil Steril. 89:759–779.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bottinger EP: TGF-beta in renal injury and
disease. Semin Nephrol. 27:309–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu G, Ma C, Yang H and Zhang PY:
Transforming growth factor beta and its role in heart disease. Exp
Ther Med. 13:2123–2128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shevchenko OP, Kurabekova RM and
Tsirulnikova OM: The role of transforming growth factor beta1 under
diseases of liver. Klin Lab Diagn. 62:161–164. 2017.(In Russian).
PubMed/NCBI
|
|
10
|
Zhou LL, Wang M, Liu F, Lu YZ, Song LJ,
Xiong L, Xiang F, He XL, Shuai SY, Xin JB, et al: Cigarette smoking
aggravates bleomycin-induced experimental pulmonary fibrosis.
Toxicol Lett. 303:1–8. 2019. View Article : Google Scholar
|
|
11
|
Liu P, Zhu L, Zou G and Ke H: Matrine
suppresses pancreatic fibrosis by regulating TGF-β/Smad signaling
in rats. Yonsei Med J. 60:79–87. 2019. View Article : Google Scholar
|
|
12
|
Meng XM, Tang PM, Li J and Lan HY:
TGF-β/Smad signaling in renal fibrosis. Front Physiol. 6:822015.
View Article : Google Scholar
|
|
13
|
Sisto M, Lorusso L, Ingravallo G, Tamma R,
Ribatti D and Lisi S: The TGF-β1 signaling pathway as an attractive
target in the fibrosis pathogenesis of sjogren’s syndrome.
Mediators Inflamm. 2018:1965935. 2018. View Article : Google Scholar
|
|
14
|
Ning J, Zhang H and Yang H: MicroRNA 326
inhibits endometrial fibrosis by regulating TGF β1/Smad3 pathway in
intrauterine adhesions. Mol Med Rep. 18:2286–2292. 2018.PubMed/NCBI
|
|
15
|
Salma U, Xue M, Ali Sheikh MS, Guan X, Xu
B, Zhang A, Huang L and Xu D: Role of transforming growth factor-β1
and smads signaling pathway in intrauterine adhesion. Mediators
Inflamm. 2016:4158287. 2016. View Article : Google Scholar
|
|
16
|
Patrono C: Aspirin as an antiplatelet
drug. N Engl J Med. 330:1287–1294. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vonkeman HE and van de Laar MA:
Nonsteroidal anti-inflammatory drugs: Adverse effects and their
prevention. Semin Arthritis Rheum. 39:294–312. 2010. View Article : Google Scholar
|
|
18
|
Albandar HJ, Markert R and Agrawal S: The
relationship between aspirin use and mortality in colorectal
cancer. J Gastrointest Oncol. 9:1133–1137. 2018. View Article : Google Scholar
|
|
19
|
Rosenberg K and Mechcatie E: Low-dose
aspirin use associated with lower ovarian cancer risk. Am J Nurs.
119:502019.
|
|
20
|
Jiang ZG, Feldbrügge L, Tapper EB, Popov
Y, Ghaziani T, Afdhal N, Robson SC and Mukamal KJ: Aspirin use is
associated with lower indices of liver fibrosis among adults in the
United States. Aliment Pharmacol Ther. 43:734–743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li CJ, Yang ZH, Shi XL and Liu DL: Effects
of aspirin and enoxaparin in a rat model of liver fibrosis. World J
Gastroenterol. 23:6412–6419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu PP, Liu HH, Sun SH, Shi XX, Yang WC,
Su GH and Zhao J: Aspirin alleviates cardiac fibrosis in mice by
inhibiting autophagy. Acta Pharmacol Sin. 38:488–497. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Liu L, Luo Y, Chen M, Huan Y and
Fang R: Effects of aspirin and intrauterine balloon on endometrial
repair and reproductive prognosis in patients with severe
intrauterine adhesion: A prospective cohort study. Biomed Res Int.
2017:8526104. 2017.
|
|
24
|
The American Fertility Society
classifications of adnexal adhesions, distal tubal occlusion, tubal
occlusion secondary to tubal ligation, tubal pregnancies, mullerian
anomalies and intrauterine adhesions. Fertil Steril. 49:944–955.
1988. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(−Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Zupi E, Centini G and Lazzeri L: Asherman
syndrome: An unsolved clinical definition and management. Fertil
Steril. 104:1380–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Huang X, Xia E, Zhang X, Li TC and
Liu Y: A cohort study comparing 4 mg and 10 mg daily doses of
postoperative oestradiol therapy to prevent adhesion reformation
after hysteroscopic adhesiolysis. Hum Fertil (Camb). 22:191–197.
2018. View Article : Google Scholar
|
|
28
|
Chi Y, He P, Lei L, Lan Y, Hu J, Meng Y
and Hu L: Transdermal estrogen gel and oral aspirin combination
therapy improves fertility prognosis via the promotion of
endometrial receptivity in moderate to severe intrauterine
adhesion. Mol Med Rep. 17:6337–6344. 2018.PubMed/NCBI
|
|
29
|
Zhu R, Duan H, Gan L and Wang S:
Comparison of intrauterine suitable balloon and foley balloon in
the prevention of adhesion after hysteroscopic adhesiolysis. Biomed
Res Int. 2018:9494101. 2018. View Article : Google Scholar
|
|
30
|
Lin XN, Zhou F, Wei ML, Yang Y, Li Y, Li
TC and Zhang SY: Randomized, controlled trial comparing the
efficacy of intrauterine balloon and intrauterine contraceptive
device in the prevention of adhesion reformation after
hysteroscopic adhesiolysis. Fertil Steril. 104:235–240. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bosteels J, Weyers S, Mol BW and D’Hooghe
T: Anti-adhesion barrier gels following operative hysteroscopy for
treating female infertility: A systematic review and meta-analysis.
Gynecol Surg. 11:113–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amer MI, Abd-El-Maeboud KH, Abdelfatah I,
Salama FA and Abdallah AS: Human amnion as a temporary biologic
barrier after hysteroscopic lysis of severe intrauterine adhesions:
Pilot study. J Minim Invasive Gynecol. 17:605–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johary J, Xue M, Zhu X, Xu D and Velu PP:
Efficacy of estrogen therapy in patients with intrauterine
adhesions: Systematic review. J Minim Invasive Gynecol. 21:44–54.
2014. View Article : Google Scholar
|
|
34
|
Bosteels J, Weyers S, D’Hooghe TM,
Torrance H, Broekmans FJ, Chua SJ and Mol BWJ: Anti-adhesion
therapy following operative hysteroscopy for treatment of female
subfertility. Cochrane Database Syst Rev.
11:CD0111102017.PubMed/NCBI
|
|
35
|
Montinari MR, Minelli S and De Caterina R:
The first 3500 years of aspirin history from its roots-A concise
summary. Vascul Pharmacol. 113:1–8. 2019. View Article : Google Scholar
|
|
36
|
Yang JH, Chen MJ, Chen CD, Chen SU, Ho HN
and Yang YS: Optimal waiting period for subsequent fertility
treatment after various hysteroscopic surgeries. Fertil Steril.
99:2092–2096.e3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Wang G, QiLi M, Liang H, Li T, EX,
Feng Y, Zhang Y, Liu X, Qian M, et al: Aspirin reduces cardiac
interstitial fibrosis by inhibiting Erk1/2-Serpine2 and P-Akt
signalling pathways. Cell Physiol Biochem. 45:1955–1965. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu HY, Ge TX, Pan YB and Zhang SY:
Advanced Role of Hippo Signaling in Endometrial Fibrosis:
Implications for intrauterine adhesion. Chin Med J (Engl).
130:2732–2737. 2017. View Article : Google Scholar
|
|
39
|
Branton MH and Kopp JB: TGF-beta and
fibrosis. Microbes Infect. 1:1349–1365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nature reviews.
Nephrology. 12:325–338. 2016.
|
|
41
|
Ji Y, Dou YN, Zhao QW, Zhang JZ, Yang Y,
Wang T, Xia YF, Dai Y and Wei ZF: Paeoniflorin suppresses TGF-β
mediated epithelial-mesenchymal transition in pulmonary fibrosis
through a Smad-dependent pathway. Acta Pharmacol Sin. 37:794–804.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu J, Yu TT, Zhang K, Li M, Shi HJ, Meng
XJ, Zhu LS and Zhu LK: HGF alleviates renal interstitial fibrosis
via inhibiting the TGF-β1/SMAD pathway. Eur Rev Med Pharmacol Sci.
22:7621–7627. 2018.PubMed/NCBI
|
|
43
|
Park SA, Kim MJ, Park SY, Kim JS, Lee SJ,
Woo HA, Kim DK, Nam JS and Sheen YY: EW-7197 inhibits hepatic,
renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS
signaling. Cell Mol Life Sci. 72:2023–2039. 2015. View Article : Google Scholar
|
|
44
|
Wang Y, Zhao L, Jiao FZ, Zhang WB, Chen Q
and Gong ZJ: Histone deacetylase inhibitor suberoylanilide
hydroxamic acid alleviates liver fibrosis by suppressing the
transforming growth factor-β1 signal pathway. Hepatobiliary
Pancreat Dis Int. 17:423–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li J, Du S, Sheng X, Liu J, Cen B, Huang F
and He Y: MicroRNA-29b inhibits endometrial fibrosis by regulating
the Sp1-TGF-β1/Smad-CTGF axis in a rat model. Reprod Sci.
23:386–394. 2016. View Article : Google Scholar
|
|
46
|
Li J, Cen B, Chen S and He Y: MicroRNA-29b
inhibits TGF-β1-induced fibrosis via regulation of the TGF-β1/Smad
pathway in primary human endometrial stromal cells. Mol Med Rep.
13:4229–4237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deans R, Vancaillie T, Ledger W, Liu J and
Abbott JA: Live birth rate and obstetric complications following
the hysteroscopic management of intrauterine adhesions including
Asherman syndrome. Hum Reprod. 33:1847–1853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Engelbrechtsen L, Langhoff-Roos J, Kjer JJ
and Istre O: Placenta accreta: Adherent placenta due to Asherman
syndrome. Clin Case Rep. 3:175–178. 2015. View Article : Google Scholar : PubMed/NCBI
|