Introduction
Cervical cancer is the most common malignant tumor
of the female reproductive tract, ranking 4th in incidence and
mortality among female malignant tumors and first among
reproductive tract malignancies worldwide (1). In China, the number of patients
diagnosed with cervical cancer increases annually, with the age of
patients decreasing (2). Cervical
cancer seriously endangers the lives, health and safety of Chinese
women. High-risk human papillomavirus (HPV) infection remains a
major risk factor for cervical cancer (2). Clinically, radical surgery,
supplemented radiotherapy or radiotherapy combined with
cisplatin-based chemotherapy is the main treatment for cervical
cancer. However, the most important characteristics of advanced
cervical cancer are high recurrence rate, poor prognosis, local
infiltration and distant metastasis. The development of effective
adjuvant treatments is necessary.
Bone morphogenetic protein (BMP) is a member of the
transforming growth factor-β (TGF-β) superfamily. Over 20 subtypes
of the BMP family have been identified in humans and played
different roles in disease growth and progression (3). Among them, BMP7 is abnormally
expressed in a variety of human tumors and is involved in
regulating the proliferation, invasion and migration of cancer
cells. BMP7 plays different roles in different tumors. In studies
on ovarian cancer, BMP7 was found to be highly expressed in
advanced ovarian cancer (4) and
drug-resistant ovarian cancer cells (5). In digestive tract tumors, BMP7
expression was increased compared with in normal tissues and was
associated with poor prognosis and the promotion of tumor invasion
and metastasis (6,7). In lung cancer, BMP7 could attenuate
the activity and invasiveness of tumor cells, inhibit bone
metastasis, and induce apoptosis and cell cycle arrest (8). In malignant melanoma, BMP7 can
induce mesenchymal-epithelial transformation and inhibit the
metastasis of cancer cells (9).
Studies have found that BMP7 can inhibit epithelial-mesenchymal
transition (EMT)-related genes and cell invasion (10,11), inhibit telomerase, shorten
telomeres, and induce the aging and apoptosis of breast cancer
cells (12). BMP7 has also been
found to increase the cell proliferation and migration potential in
a model of metastatic breast cancer in the bone (13) and prostate cancer (14).
When BMP7 binds to its receptor, it can lead to the
intracellular phosphorylation of mothers against decapentaplegic
homolog (smad)1/5/9 (also known as smad1/5/8) and further exerts
its role in regulating cell proliferation (15). At present, studies on the
association between the BMP7-Smad1/5/9 signaling pathway and
cervical cancer are rare. The purpose of the present study was to
verify the association between the BMP7-Smad1/5/9 signaling pathway
and the occurrence, development and prognosis of cervical cancer,
as well as to provide a new idea for the treatment of cervical
cancer.
Materials and methods
Materials
Cervical cancer patients (n=100) undergoing surgery
in the Department of Gynecology of the Provincial Hospital of
Shandong University between January 2014 and November 2018 were
included in the study, including 95 cases of squamous cell
carcinoma (including 27 cases with corresponding paracancerous
tissues), 4 cases of adenocarcinoma and 1 case of adenosquamous
cell carcinoma (age range of all cases, 29–78 years). In the
control group, 26 cervical tissue specimens were excised due to
benign or precancerous lesions (age range, 30–72 years). All
patients had no other complications. Human cervical cancer HeLa
cells were purchased from the Cell Bank of the Chinese Academy of
Sciences. Dulbecco’s modified Eagle’s medium (DMEM) was from Gibco
(Thermo Fisher Scientific, Inc.), fetal bovine serum (FBS) from
Biological Industries, BMP7 knockdown lentiviral vector (NM_001719)
from GeneChem, Inc., Cell Counting Kit (CCK)-8 reagent from Dojindo
Molecular Technologies, Inc. Transwell chambers and Matrigel were
purchased from Corning Inc. The following antibodies were
purchased: Rabbit anti-human polyclonal antibody to BMP7 (cat. no.
ab56023; Abcam), rabbit anti-human polyclonal antibody to Smad1/5/9
(cat. no. ab66737; Abcam), rabbit anti-human monoclonal antibody to
epithelial (E)-cadherin (cat. no. ab40772; Abcam), rabbit
anti-human monoclonal antibody to neural (N)-cadherin (cat. no.
ab76011; Abcam), rabbit anti-human monoclonal antibody to Vimentin
(cat. no. ab92547; Abcam), rabbit anti-human polyclonal antibody to
Snail + Slug (cat. no. ab85936; Abcam) and rabbit anti-human
monoclonal antibody to GAPDH (cat. no. ab181602; Abcam). Rabbit
anti-human polyclonal antibody to phosphorylated (p)-Smad1/5/9
(cat. no. 12656T; Cell Signaling Technology, Inc.) and horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (cat. no.
ab205718; Abcam) were purchased. The bicinchoninic acid (BCA)
protein concentration assay kit, SDS-PAGE, 3,3′-diaminobenzidine
(DAB) coloring solution and other reagents were obtained from
Beijing Solarbio Science & Technology Co., Ltd.
Immunohistochemistry
Tissue samples were fixed at room temperature in
formaldehyde (~1–7 days) and embedded in paraffin, and then cut
into 4-μm sections, dewaxed with xylene, hydrated with 100–75%
ethanol series, and repaired with sodium citrate (pH 6.0) antigen
in an autoclave (100°C; 2 min). Hydrogen peroxide was used to
remove endogenous peroxidase. The goat serum (cat. no. SL038;
Beijing Solarbio Science & Technology Co., Ltd.) was used for
blocking at 37°C for 30 min and BMP7 primary antibody (1:50) was
then added at 4°C overnight for incubation. The next day, the goat
anti-rabbit secondary antibody (1:200) was added for 30 min at 37°C
and the slice was then stained with DAB at room temperature
(observed until the staining was satisfactory) and hematoxylin for
2 min at room temperature. Images were captured under a cellSens
light microscope (Olympus Corporation). Brown staining was
considered to be BMP7-positive. A total of five fields of view were
randomly selected from each slice, the degree of positive staining
and the proportion of positive cells were recorded and scored
(blind observation by two pathologists), and the positive rate was
evaluated by the product of two items.
Cell culture
Human cervical cancer HeLa cells were inoculated in
DMEM complete culture medium (containing 10% FBS, 1% penicillin and
streptomycin) and cultured at 37°C in a 50-ml/l CO2
saturated humidity incubator, and the liquid was changed every 2
days. When the degree of cell fusion reached 80–90%, cell passage
was carried out using trypsin solution digestion and under strict
aseptic conditions.
Lentivirus infection and grouping
The BMP7 knockdown lentivirus vector (NM_001719) was
achieved by cloning short hairpin RNA (shRNA) using a
self-inactivating lentivirus vector containing a CMV
promoter-driven green fluorescent protein (GFP) reporter gene and a
U6 promoter. The target sequence of BMP7 was
5′-GGATCTACAAGGACTACAT-3′. HeLa cells at the logarithmic growth
stage were inoculated into 96-well plates at 5×104
cells/ml, 100 μl per well. The cells were transfected with
lentivirus vector multiplicity of infection of 100. After 24 h,
normal medium was used to continue culture, passage and screening
with puromycin. The transfection efficiency was monitored by
ImageXpress Micro Confocal High-Content Imaging system and
MetaXpress v6.2.3.733 High-content Image Acquisition and Analysis
software (Molecular Devices, LLC). When the observed GFP expression
reach 95%, the next experiment was started. Cells were divided into
the negative control (sh-NC) and experimental (sh-BMP7) groups.
Western blotting
sh-NC and sh-BMP7 cells at the logarithmic growth
stage were used for experiments. Total cell protein was extracted
by adding lysate [radioimmunoprecipitation assay lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.),
phenylmethanesulfonyl fluoride and phosphatase inhibitor at a ratio
of 100:1:1], and the protein concentration was determined using the
BCA method. The protein (30 μg/lane) was electrophoresed using a 10
or 12% SDS-PAGE gel, transferred using a polyvinylidene fluoride
membrane and blocked with 5% skimmed milk at room temperature for 1
h. The primary antibodies were placed in a shaker at 4°C overnight
for incubation. The primary antibody dilution concentration was as
follows: Rabbit anti-BMP7 (1:2,000), rabbit anti-p-Smad1/5/9
(1:1,000), rabbit anti-E-cadherin (1:1,000), rabbit anti-N-cadherin
(1:1,000), rabbit anti-vimentin (1:2,000), rabbit anti-Snail + Slug
(1:2,000), rabbit anti-cyclinD1 (1:2,000) and rabbit anti-GAPDH
(1:2,000). The membrane was then incubated with horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (1:5,000)
for 1 h at room temperature and exposed to color using ECL reagent
(Immobilon Western Chemiluminescent HRP substrate; EMD Millipore)
and the Amersham Imager 600 (GE Healthcare).
Transwell assay
sh-NC and sh-BMP7 cells at the logarithmic growth
stage were digested and collected, washed 3 times with PBS, and
resuspended in serum-free medium to dilute the cells at
25×104 cells/ml.
In the cell migration assay, 200 μl cell suspension
was added in the upper well and medium containing 10% fetal bovine
serum was added in the lower well. Following culture for 24 h, the
culture medium was discarded and washed 3 times with PBS. Cotton
swabs were used to gently wipe off the cells on the upper well
membrane. The lower well was fixed with 4% paraformaldehyde for 30
min at room temperature. Cells were washed 3 times with PBS again
and stained with hematoxylin for 10 min at room temperature, rinse
with tap water until blue. Following drying, the upper compartment
membrane was cut off and placed on the slide for sealing. Images
were captured under an inverted light microscope and counted using
ImageJ v1.51 software (National Institutes of Health).
In the cell invasion assay, 90 μl pre-diluted
Matrigel (Matrigel: Serum-free medium, 1:7) was added to the upper
well and deposited at 37°C overnight. The uncoagulated Matrigel was
aspirated the next day. The cells were added and cultured for 48 h.
The other steps were the same as the migration experiment.
Cell cycle assay
sh-NC and sh-BMP7 cells were cultured and collected
when the cell density reached ~70% and cells were at the
logarithmic growth stage. The cells were digested into single-cell
suspension with trypsin, washed with PBS once and fixed with 75%
ethanol solution (pre-cooled at −20°C) at 4°C overnight. After
washing the cells, 2 μl RNase (cat. no. R8021; Beijing Solarbio
Science & Technology Co., Ltd.) at a concentration of 1 mg/ml
was added and the cells were bathed in water at 37°C for 40 min.
Next, 100 μl propidium iodide (cat. no. G1021; Wuhan Servicebio
Biotechnology Co., Ltd.) staining solution at a concentration of
100 μg/ml was added and cells were stained in the dark for 20 min
at room temperature. Flow cytometry (CytoFLEX; Beckman Coulter,
Inc.) was used to detect the cell cycle and Modfit Lt version 5.0
(Verity Software House, Inc.) to analyze cell cycle
distribution.
Cell Counting Kit-8 cell proliferation
assay
The assay was performed according to the
manufacturer’s protocol. Briefly, sh-NC and sh-BMP7 cells at the
logarithmic growth stage were inoculated into 96-well plates at
2×104 cells/ml, 100 μl per well, and cultured for 6, 24,
48 and 72 h. The original medium was aspirated and 100 μl medium
containing 10% CCK-8 reagent was added into each well for
incubation in a constant temperature incubator at 37°C for 1 h. The
absorbance of each well was measured at a wavelength of 450 nm and
the absorbance of each hole was recorded to calculate the cell
proliferation activity.
Statistical analysis
GraphPad Prism 7.0 software (GraphPad Software,
Inc.) was used for statistical analysis. Pearson’s χ2
test was used to compare differences in BMP7 expression levels
between cervical cancer and normal cervical or paracancerous
tissues. The t-test was used to compare the differences between the
sh-BMP7 and sh-NC groups. Data are presented as the mean ± standard
deviation of at least three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
BMP7 overexpression in cervical cancer
tissues
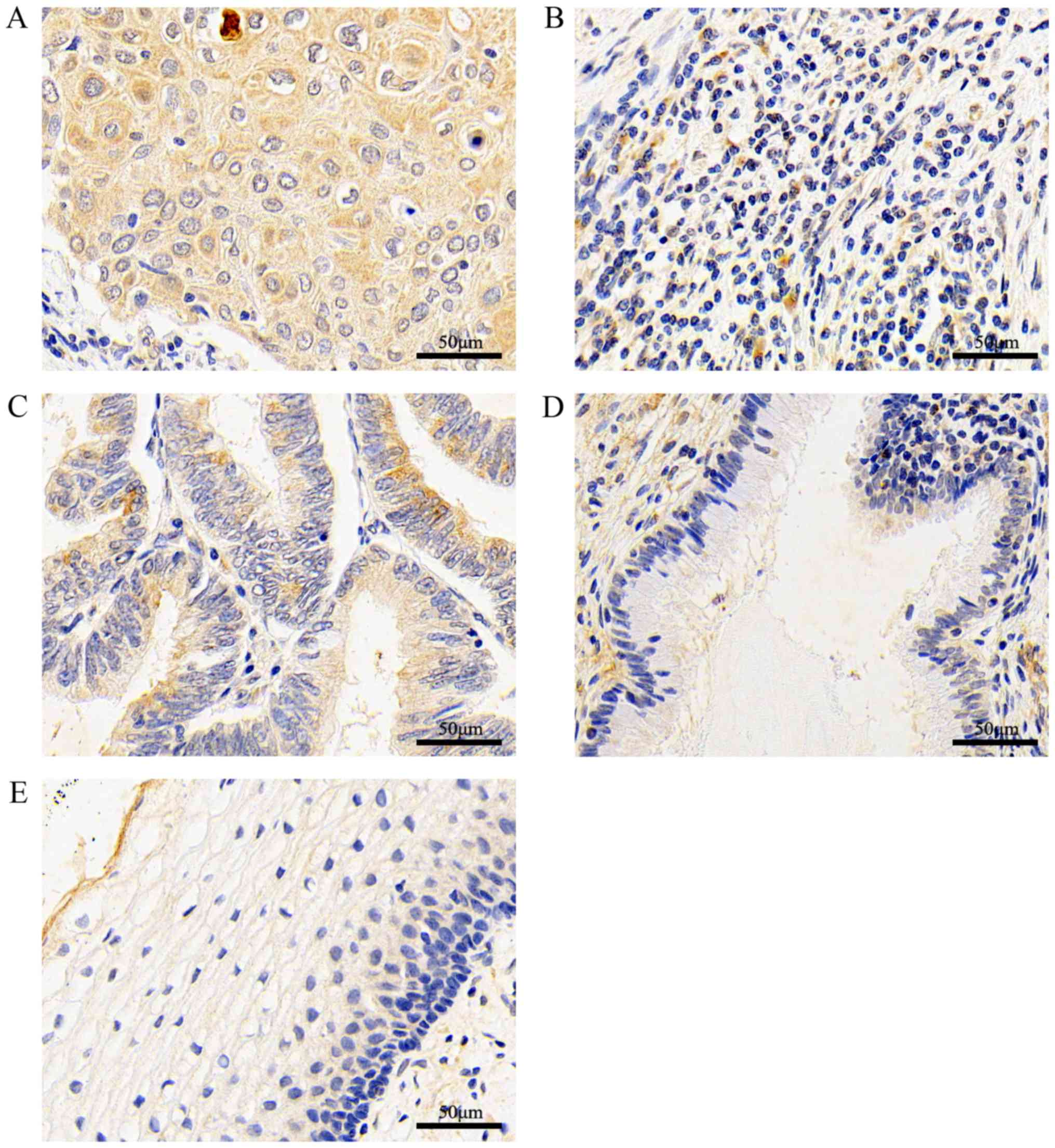
According to the immunohistochemical results, BMP7
staining was mainly located in the cytoplasm and intercellular
substance of the tissue. Cervical squamous cell carcinoma (Fig. 1A) and adenocarcinoma (Fig. 1C) tissues stained darker,
paracancerous tissues (normal cervical tissue within 3 cm from the
cancerous tissue with no cancer cell infiltration) (Fig. 1B) and normal glands (Fig. 1D) stained lighter. There was also
low expression in normal cervical tissues (Fig. 1E). The expression of BMP7 in
cervical cancer tissues (Fig. 1A and
C) was statistically increased compared with in paracancerous
tissues (Fig. 1B; Table I; P<0.05). BMP7 was highly
expressed in cancer tissues (Fig. 1A
and C), as compared with normal cervical epithelial tissues
(Fig. 1D and E; Table II; P<0.05). When comparing the
FIGO stage or pathological grade of cervical cancer tissues with
the expression of BMP7, the difference was not significant.
 | Table IExpression levels of BMP7 in cervical
cancer tissues and paracancerous tissues (control). |
Table I
Expression levels of BMP7 in cervical
cancer tissues and paracancerous tissues (control).
| Tissue | No. | Negative, n | Positive, n | χ2 | P-value |
|---|
| Cancer | 27 | 3 | 24 | 35.9 | <0.0001 |
| Control | 27 | 25 | 2 | | |
 | Table IIExpression levels of BMP7 in cervical
cancer tissues and normal cervical epithelial tissues
(control). |
Table II
Expression levels of BMP7 in cervical
cancer tissues and normal cervical epithelial tissues
(control).
| Tissue | No. | Negative, n | Positive, n | χ2 | P-value |
|---|
| Cancer | 100 | 22 | 78 | 44 | <0.0001 |
| Control | 26 | 24 | 2 | | |
Lentivirus transfection-induced BMP7
knockdown and western blotting verification
The two groups of HeLa cells were transfected by
lentivirus and selected by puromycin for 5 days. The transfection
efficiency was observed under fluorescent microscopy and the GFP
expression was detected (Fig.
2A). The upper panels include light microscopic images and
fluorescence microscopic images of sh-NC, the lower panels include
light microscopic images and fluorescence microscopic images of
sh-BMP7. The transfection efficiency was >95%. The western
blotting results demonstrated that the BMP7 protein expression in
the sh-BMP7 group was significantly decreased compared with in the
sh-NC group. The expression of Smad1/5/9 did not change
significantly in the sh-BMP7 group. In addition, the expression of
p-Smad1/5/9 was significantly decreased in the sh-BMP7 group
(Fig. 2B–D).
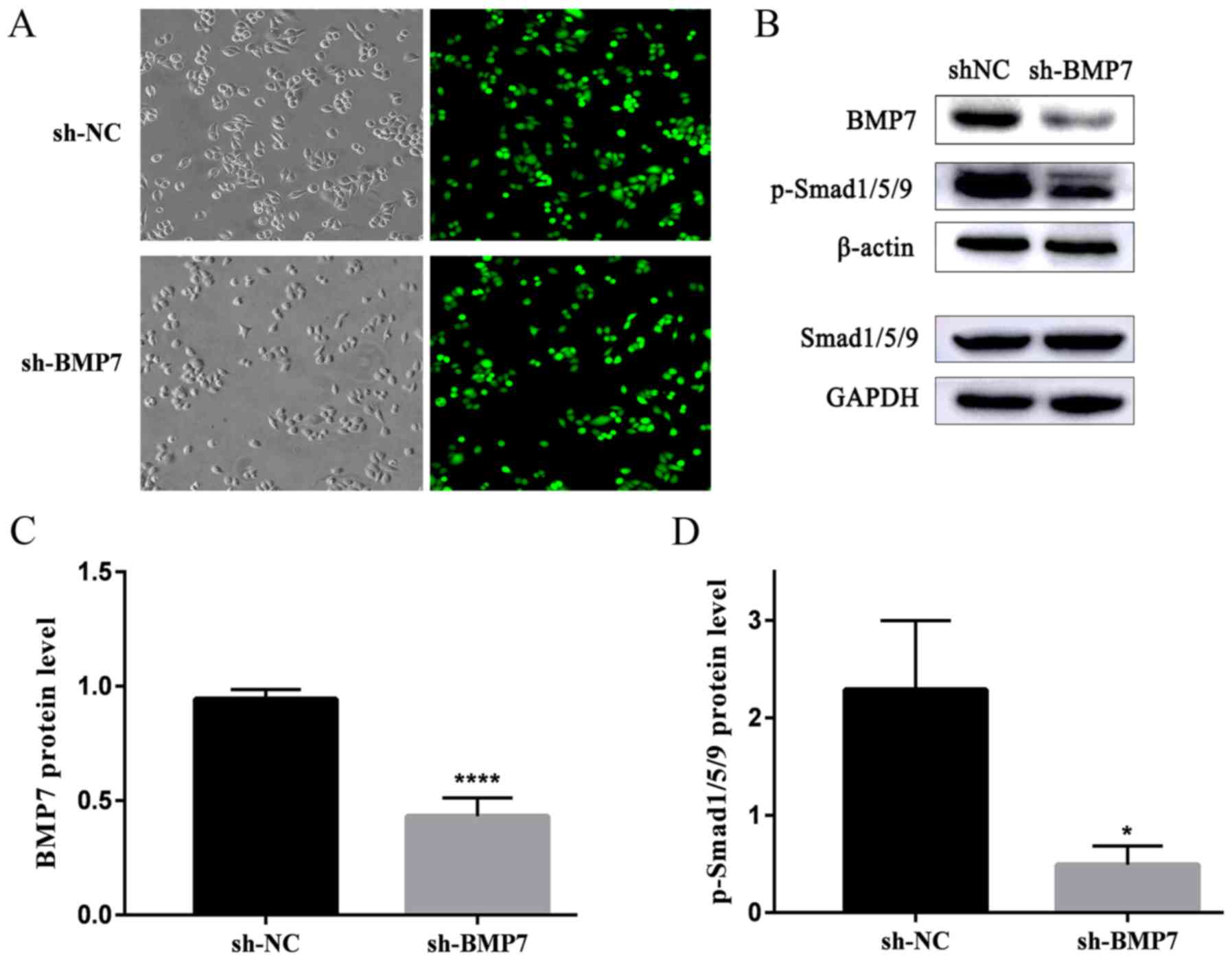 | Figure 2Expression of green fluorescent
protein and BMP7, Smad1/5/9 and p-Smad1/5/9 protein in the sh-BMP7
and sh-NC groups of HeLa cells. (A) Green fluorescent protein GFP
expression in sh-NC and sh-BMP7 groups of HeLa cells detected by
fluorescent microscopy (magnification, ×200). The upper panels are
the light microscopic images and fluorescent microscopic images of
sh-NC, whereas the lower panels include light microscopic images
and fluorescent microscopic images of sh-BMP7. (B) BMP7, Smad1/5/9
and p-Smad1/5/9 protein expression of the two groups, detected by
western blotting. Densitometric analysis of (C) BMP7 and (D)
p-Smad1/5/9 protein expression. *P<0.05 and
****P<0.0001, n≥3.BMP7, bone morphogenetic protein 7;
sh, short hairpin; NC, negative control; smad, mothers against
decapentaplegic homolog; p, phosphorylated. |
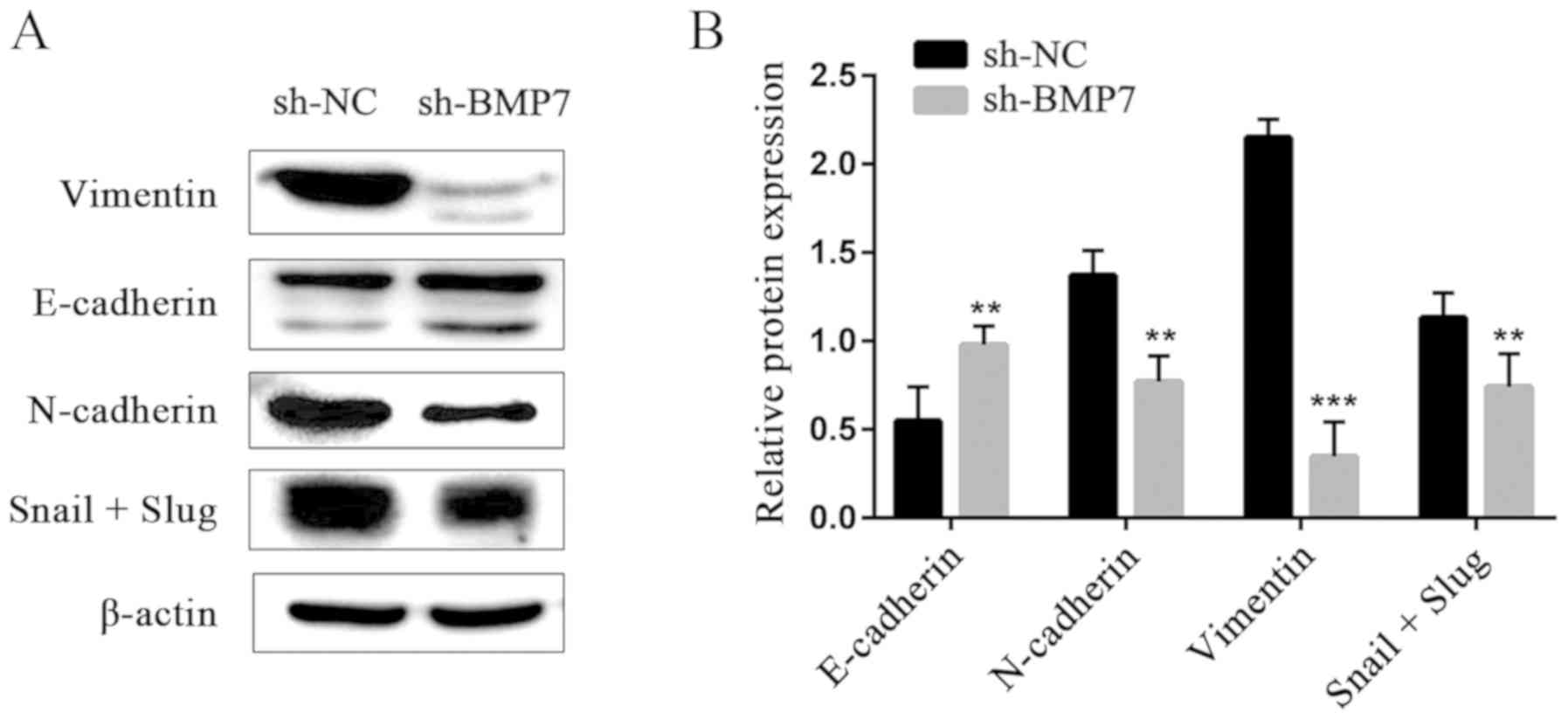
EMT of HeLa cells is inhibited by BMP7
knockdown
Western blotting showed that, compared with the
sh-NC group, the expression of EMT-related E-cadherin protein,
which is an epithelial marker, was significantly increased in the
sh-BMP group, while the expression of the N-cadherin, vimentin,
Snail and Slug proteins, which are mesenchymal markers, was
decreased (Fig. 3), suggesting
that EMT was inhibited in HeLa cells.
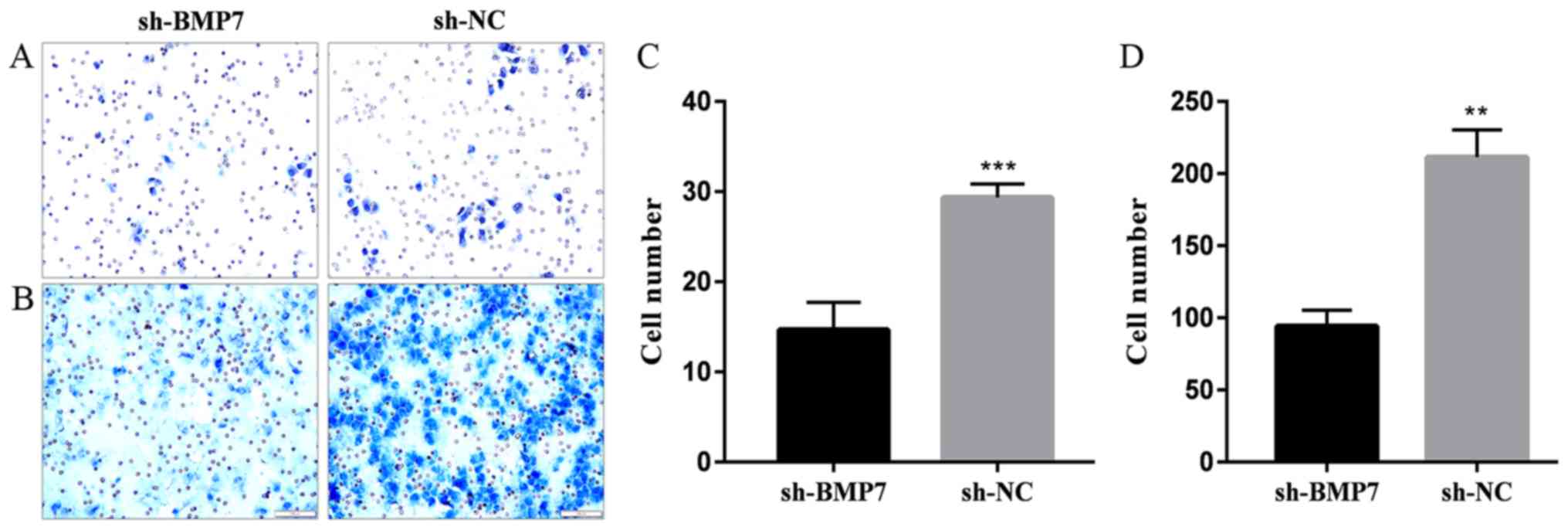
BMP7 knockdown can inhibit the invasion
and migration of HeLa cells
In the Transwell invasion assay, the rate of
Matrigel penetration in the sh-BMP7 group was decreased (Fig. 4A and C). The results of the
Transwell migration assay showed that the cell migration rate was
significantly decreased in the sh-BMP7 compared with in the sh-NC
group (Fig. 4B and D). The
invasion and migration ability of cells was reduced by BMP7
knockdown.
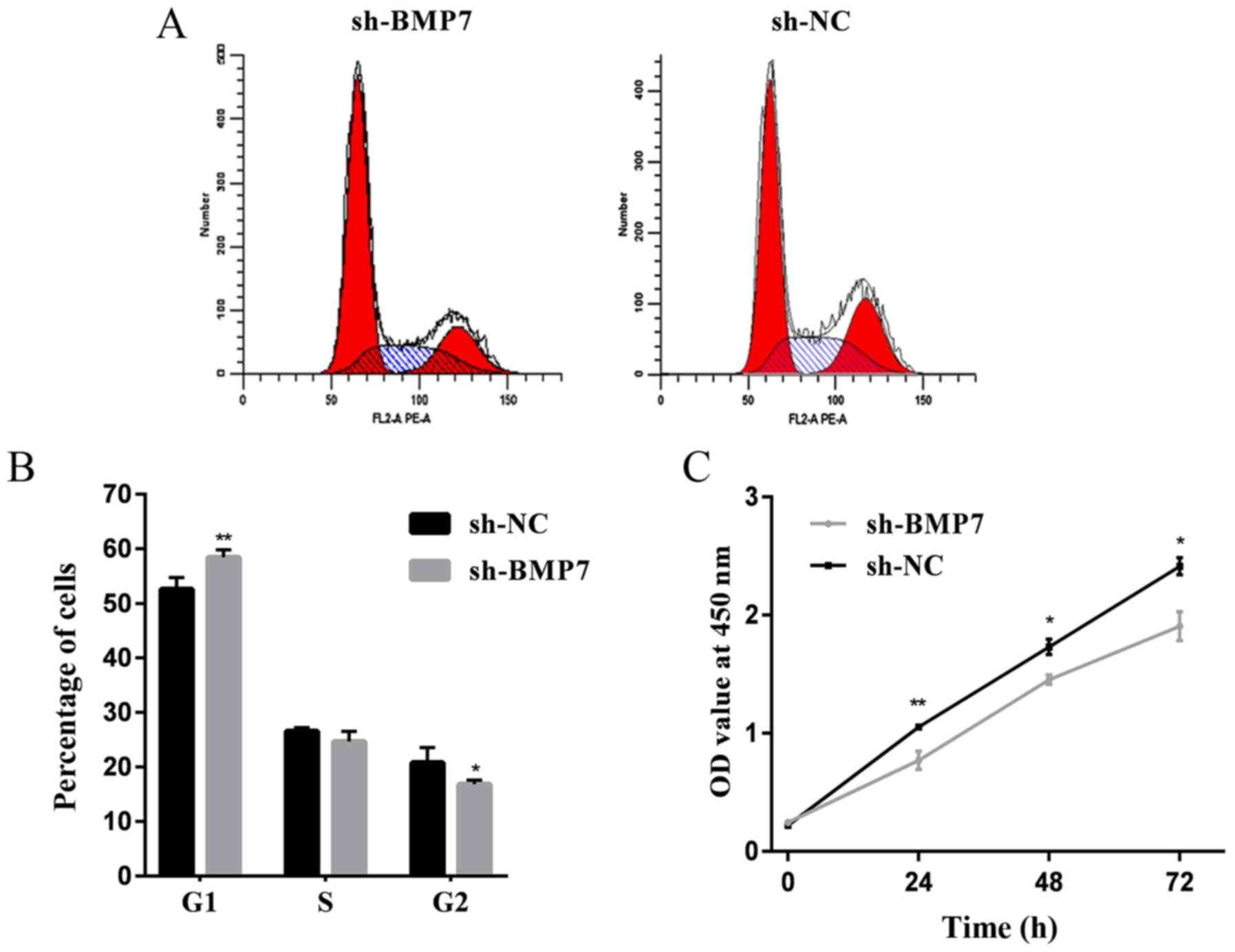
BMP7 knockdown inhibits the proliferation
of HeLa cells
The cell cycle was detected by flow cytometry.
Compared with the sh-NC group, the cell proliferation cycle was
blocked in the sh-BMP7 group. As shown in Fig. 5A and B, the proportion of sh-BMP7
cells at the G1 phase increased and the proportion of cells at the
G2 phase decreased, compared with in the sh-NC group. These results
suggested that the downregulation of BMP7 could cause G1 cell cycle
arrest. Proliferation assay results showed that the cell
proliferation rate of the sh-BMP7 group was decreased compared with
the sh-NC group (Fig. 5C).
Discussion
Cassar et al (16) found that BMP7 could maintain
telomerase activity, negatively regulate telomere maintenance and
induce cervical tumor growth arrest in cervical cancer. However,
the effect of BMP7 on EMT progression in cervical cancer cells has
not been studied. The BMP-Smad signaling pathway plays a role in
the development of a variety of tumors. As a member of the TGF-β
superfamily, BMP binds to type I and II serine/threonine kinase
receptors, resulting in the phosphorylation of Smad, activation of
downstream effectors, and control of cell proliferation,
differentiation, metastasis, and apoptotic gene activation
(17). BMP7 binds to ActR-II/IIB
(type II) and ALK2/3/6 (type I) receptors, resulting in the
phosphorylation of downstream effector Smad1/5/9 (R-Smad).
Subsequently, p-Smad1/5/9 binds to Smad4, and enters and functions
in the nucleus, acting on the promoter of target genes and
initiating the transcription process, thus triggering specific
biological effects (18). The
Smad complex can affect the disease following nuclear entry by
upregulating the inhibitors of differentiation (ID) (19). In previous years, studies have
shown that ID regulate the differentiation of a variety of cells.
In tumor cells, ID can promote angiogenesis and tumor cell
invasion, inhibit apoptosis and promote cell immortality (20). In cervical cancer, elevated ID1 is
associated with HPV infection and poor prognosis (21).
In the present study, based on immunohistochemical
staining of cervical tissue in 125 patients, BMP7 was found to be
highly expressed in cervical cancer tissues, as compared with
normal cervical and paracancerous tissues. The present results are
similar to those of a previous study (22). However, the difference in the
expression intensity of BMP7 by pathological grade or clinical
stage was not significant.
Based on the above conclusions, further cytological
experiments were conducted. First, the expression of BMP7 in HeLa
cells was silenced by lentiviral transfection and the downstream
factors were detected. It was found that the expression of
p-Smad1/5/9 downstream of the BMP7/Smad1/5/9 signaling pathway was
decreased. The low expression of p-Smad1/5/9 may further inhibit
the proliferation and differentiation of cells. EMT research is an
important part of tumor cell invasion and migration research. In
tumor cells, EMT can generate circulating tumor cells and tumor
stem cells, and improve resistance to anticancer drugs (23). Determining how to inhibit EMT in
tumor cells is a major challenge for anti-tumor therapy. EMT is
marked by the loss of the epithelial marker E-cadherin, upregulated
expression of the interstitial cell markers N-cadherin and
vimentin, and zinc finger transcription factors Snail and Slug
(24). EMT verification of HeLa
cells was performed following transfection. EMT in HeLa cells was
inhibited following BMP7 silencing, following which the expression
of E-cadherin was increased and the expression of N-cadherin,
vimentin, Snail and Slug proteins was decreased. In the Transwell
migration and invasion experiments, the cell passage rate of the
sh-BMP7 group was reduced. The invasion and migration ability of
the sh-BMP7 group was weakened, which was the same as the expected
results.
Muthukrishnan et al (25) showed that BMP7 can upregulate the
G1 regulatory gene in renal tissue and Alarmo et al
(26) showed that BMP7 silencing
can lead to G1 growth arrest in breast cancer cells. In the present
study, following BMP7 silencing, HeLa cells showed G1 phase growth
arrest. Cell proliferation experiments showed that the
proliferation rate of the sh-BMP7 group was decreased compared with
the control group, which may be associated with G1 phase
arrest.
In conclusion, BMP7 was highly expressed in cervical
cancer. By knocking down BMP7 in cervical cancer HeLa cells, the
expression of the downstream acting factor p-Smad1/5/9 was reduced.
BMP7 knockdown could also inhibit cervical cancer cell
proliferation, invasion and migration, and reverse the EMT process.
This effect may be caused by the inactivation of the BMP7-Smad1/5/9
signaling pathway. According to the present findings, BMP7 may be a
potential therapeutic target for cervical cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81671434).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
RS wrote the manuscript. RS and HG interpreted the
data and performed experiments. WL collected the data. JL and FW
analyzed the data. CL designed and guided the study. All authors
read and approval the final manuscript.
Ethics approval and consent to
participate
All procedures performed in this study involving
human participants were approved by the Biomedical Research Ethics
Committee of Shandong Provincial Hospital (approval no. 2019-015).
All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu P: Big data evaluation of the clinical
epidemiology of cervical cancer in mainland China. Chin J Pract
Gynecol Obstet. 34:41–45. 2018.
|
|
3
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: A critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar
|
|
4
|
Sunde JS, Donninger H, Wu K, Johnson ME,
Pestell RG, Rose GS, Mok SC, Brady J, Bonome T and Birrer MJ:
Expression profiling identifies altered expression of genes that
contribute to the inhibition of transforming growth factor-beta
signaling in ovarian cancer. Cancer Res. 66:8404–8412. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng L, Lu W, Kulkarni B, Pejovic T, Yan
X, Chiang JH, Hood L, Odunsi K and Lin B: Analysis of chemotherapy
response programs in ovarian cancers by the next-generation
sequencing technologies. Gynecol Oncol. 117:159–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Megumi K, Ishigami S, Uchikado Y, Kita Y,
Okumura H, Matsumoto M, Uenosono Y, Arigami T, Kijima Y, Kitazono
M, et al: Clinicopathological significance of BMP7 expression in
esophageal squamous cell carcinoma. Ann Surg Oncol. 19:2066–2071.
2012. View Article : Google Scholar :
|
|
7
|
Aoki M, Ishigami S, Uenosono Y, Arigami T,
Uchikado Y, Kita Y, Kurahara H, Matsumoto M, Ueno S and Natsugoe S:
Expression of BMP-7 in human gastric cancer and its clinical
significance. Br J Cancer. 104:714–718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen W, Pang H, Xin B, Duan L, Liu L and
Zhang H: Biological effects of BMP7 on small-cell lung cancer cells
and its bone metastasis. Int J Oncol. 53:1354–1362. 2018.PubMed/NCBI
|
|
9
|
Na YR, Seok SH, Kim DJ, Han JH, Kim TH,
Jung H, Lee BH and Park JH: Bone morphogenetic protein 7 induces
mesenchymal to-epithelial transition in melanoma cells, leading to
inhibition of metastasis. Cancer Sci. 100:2218–2225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ying X, Sun Y and He P: Bone Morphogenetic
Protein-7 inhibits EMT-associated genes in breast cancer. Cell
Physiol Biochem. 37:1271–1278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi A, Okuda H, Xing F, Pandey PR,
Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al:
Bone morphogenetic protein 7 in dormancy and metastasis of prostate
cancer stem-like cells in bone. J Exp Med. 208:2641–2655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cassar L, Nicholls C, Pinto AR, Chen R,
Wang L, Li H and Liu JP: TGF-beta receptor mediated telomerase
inhibition, telomere shortening and breast cancer cell senescence.
Protein Cell. 8:39–54. 2017. View Article : Google Scholar :
|
|
13
|
Sakai H, Furihata M, Matsuda C, Takahashi
M, Miyazaki H, Konakahara T, Imamura T and Okada T: Augmented
autocrine bone morphogenic protein (BMP) 7 signaling increases the
metastatic potential of mouse breast cancer cells. Clin Exp
Metastasis. 29:327–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim M, Chuong CM and Roy-Burman P: PI3K,
Erk signaling in BMP7-induced epithelial-mesenchymal transition
(EMT) of PC-3 prostate cancer cells in 2- and 3-dimensional
cultures. Horm Cancer. 2:298–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boon MR, van der Horst G, van der Pluijm
G, Tamsma JT, Smit JW and Rensen PC: Bone morphogenetic protein 7:
A broad-spectrum growth factor with multiple target therapeutic
potency. Cytokine Growth Factor Rev. 22:221–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cassar L, Li H, Pinto AR, Nicholls C,
Bayne S and Liu JP: Bone morphogenetic protein-7 inhibits
telomerase activity, telomere maintenance, and cervical tumor
growth. Cancer Res. 68:9157–9166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyazono K, Kusanagi K and Inoue H:
Divergence and convergence of TGF-beta/BMP signaling. J Cell
Physiol. 187:265–276. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruzinova MB and Benezra R: Id proteins in
development, cell cycle and cancer. Trends Cell Biol. 13:410–418.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roschger C and Cabrele C: The Id-protein
family in developmental and cancer-associated pathways. Cell Commun
Signal. 15:72017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie L, Li J, Zhang Y, Liu B, Peng X, Lin
Y, Xu W and Hu L: Inhibitors of differentiation-1 promotes
nitrosopyrrolidine-induced transformation of HPV 16-immortalized
cervical epithelial cell. Cancer Sci. 105:506–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45(W1):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saitoh M: Involvement of partial EMT in
cancer progression. J Biochem. 164:257–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muthukrishnan SD, Yang X, Friesel R and
Oxburgh L: Concurrent BMP7 and FGF9 signalling governs AP-1
function to promote self-renewal of nephron progenitor cells. Nat
Commun. 6:100272015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alarmo EL, Pärssinen J, Ketolainen JM,
Savinainen K, Karhu R and Kallioniemi A: BMP7 influences
proliferation, migration, and invasion of breast cancer cells.
Cancer Lett. 275:35–43. 2009. View Article : Google Scholar
|