Introduction
Cataracts are one of the most common eye diseases
and are a major cause of blindness worldwide. Ultraviolet radiation
is a risk factor for cataract formation. Human lens epithelial
cells (HLECs) are the most metabolically active cells in the lens,
and they are also an important target tissue of ultraviolet (UV)
radiation-induced damage, which is related to the occurrence and
development of cataracts. The incidence of cataracts markedly
increases at a certain dose of UV radiation to the lens. The UV
radiation-induced apoptosis of HLECs is a common cytological cause
of non-congenital cataract formation (1-3).
A number of studies have attempted to examine the
effect of UV radiation on the human lens to determine the
biochemical mechanisms through which ultraviolet B (UVB)
irradiation induces cataract formation (4-7).
UVB radiation is closely related to cataract formation,
particularly in high elevation locations where individuals are
subjected to increased exposure to UV radiation. Both human and
animal studies have indicated that exposure to UVB causes cortical
and posterior subcapsular cataracts (8-14).
However, the exact association between UVB and HLECs has not yet
been fully elucidated. UVB irradiation may induce cell apoptosis by
activating the mitochondrial initiated programmed cell death
pathway (15-17), which may be regulated by a variety
of molecular processes. The mitochondria can rapidly lose their
transmembrane potential and produce reactive oxygen species (ROS),
both of which may contribute to cells breaking down (18).
Calbindin-D28K (Calb1) is a member of the
Ca2+ binding protein family, whose members have the
EF-hand structure domain (19,20), and its molecular weight is
approximately 28 kDa (21). Calb1
is expressed in a number of organs and tissues, such as in brain
(22), cerebellum (23), pancreatic (24), bone tissue (25,26) and nervous system (19,27). In a previous study by the authors
it was found that Calb1 was also expressed in the lens of SD rats
(28), and that it may play an
important role in reducing and stabilizing the intracellular
Ca2+ levels after the Ca2+ concentrations are
increased in HLECs. It was hypothesized that Calb1 may exert
protective effects on the lens in the presence of excess
Ca2+-mediated damage to HLECs, induced by ionomycin.
Calb1 may maintain calcium homeostasis by buffering excessive
intracellular free Ca2+. The reduced protein and mRNA
expression of Calb1 may lead to increased intracel-lular free
Ca2+ concentrations that are observed in a number of
age-related diseases (29-32).
It has been indicated that Calb1 exerts neuroprotective effects on
ischemic and glutamate toxicity models, which are primarily due to
its ability to chelate Ca2+ (33-36). Calb1 can bind directly to
caspase-3 in osteoblasts and inhibit its activity. Therefore,
calbindin-D28K may prevent apoptosis through various mechanisms
(37).
To verify the hypothesis that Calb1 participates in
HLEC apoptosis and promotes HLEC survival, the present study
examined the changes in Ca2+ levels during HLEC
apoptosis induced by UVB and assessed the protective effects of
Calb1.
Materials and methods
Cell culture and transfection
All experiments were conducted with the approval of
the animal ethics committee of Kunming Medical University. The
human lens epithelial cell line (HLECS-SRA01/04) was obtained from
JCRB (the National Institute for Biomedical Innovation, NIBIO,
Japan). All cultured cells were seeded at a density of
2×104 cells/ml in 6-well and/or 96-well plates that had
been coated with poly-D-lysine and maintained in a 37°C, 5%
CO2 saturated humidity incubator. Cells were maintained
in Dulbecco's modified Eagle medium (DMEM) with 10%
heat-inactivated fetal bovine serum and 1% penicillin-streptomycin
(Life Technologies; Thermo Fisher Scientific). When the SRA01/04
cells reached a density of 80%, they were transported to 12-well
culture plates at a concentration of 2×105 cells per
well in penicillin-streptomycin-free and serum-free medium or
penicillin-streptomycin-free serum-free medium. The SRA01/04 cells
were then transfected with lentiviruses (MOI=30) (GM easy
TMLentiviral Packaging kit; cat. no. Gmeasy-10, Genomeditech)
containing Calb1 cDNA or green fluorescent protein (GFP) cDNA; the
obtained transduced cells are called SRA/Calb1 cells. Treatment
with an siRNA (50 nM) (RiboBio) (Table I) was used to interfere with the
expression of lentiviruses with the fluorescence microscope (BX51,
Olympus Corp.) to further prove the protective effects of Calb1
against apoptosis.
 | Table IThe constructed siRNA sequence. |
Table I
The constructed siRNA sequence.
| Position | SS sequence | AS sequence |
|---|
| 23909 |
GGUUAUAGUUGAUAUGUAACA |
UUACAUAUCAACUAUAACCAG |
Cell groups used in the present
study
The cells were divided into 2 groups as follows: The
SRA group (control SRA01/04 cells) and the SRA-Calb1 group
(SRA/Calb1 cells stably over-expressing Calb1 due to treatment with
lentiviruses). The cells were then further divided into 3 subgroups
as follows: The control subgroup (without UVB irradiation or siRNA
treatment), the UVB subgroup (with UVB irradiation) and the UVB +
siRNA subgroup (with UVB irradiation and siRNA treatment). The
SRA-control represented the control subgroup in the SRA group,
SRA-UVB was the UVB subgroup in the SRA group, SRA-UVB-siRNA was
the UVB + siRNA subgroup in the SRA group, SRA-Calb1 was the
control subgroup in the SRA-Calb1 group, SRA-Calb1-UVB was the UVB
subgroup in the SRA-Calb1 group, and SRA-Calb1-UVB-siRNA was the
UVB + siRNA subgroup in the SRA-Calb1 group.
UVB exposure
The UVB spectrum ranges from 280 nm to 320 nm, and
the peak irradiance is 300 nm. In the present study, UVB lamps
(Sigma-Aldrich; Merck KGaA) were used for UV irradiation. The
intensity of radioactivity was 40 µW/cm2, which
was confirmed by a double-channel UVB illuminance meter (Shanghai
Biaozhi Instrument Co., Ltd.). The exposure duration was 15 min,
and the radiant exposure was 36 mJ/cm2, which was
calculated by the following formula: H=t x Eë [H,
radiant exposure (J/cm2); t, exposure duration (sec);
Eë, measured irradiance (W/cm2)] (38). All groups of irradiated cells were
washed twice with phosphate-buffered saline (PBS, pH 7.4) at 37°C
to remove residual serum and unattached cells prior to UVB
irradiation. Following UVB exposure, the culture medium was added
to each well, and the cells were placed in incubators at 37°C and
5% CO2 for 24 h.
Cell counting kit-8 (CCK-8) cell
viability assay
At 24 h following transfection and UVB irradiation,
the cells were treated with CCK-8 (Dojindo Molecular Technologies,
Inc.) in 96-well plates to evaluate cell viability. CCK-8 solution
(10 µl) was added to each well, and the cells were then
placed in an incubator for 2 h. The absorbance at 450 nm was
determined by ELISA. The survival rate of each transfected cell
group is shown as a percentage of the control group, which is set
at 100%.
Cytosolic Ca2+ measurement and
Ca2+ concentration determination
Intracellular calcium levels were measured using a
Fura-4/AM fluorescence indicator (Beyotime Institute of
Biotechnology). Cells were grown in 6-well plates whose
glass-bottom was coated with poly-D-lysine. They were then
incubated with 5 µM Fura-4/AM dissolved in Hank's balanced
salt solution (HBSS) in which Pluronic F-127 (Beyotime Institute of
Biotechnology) was added to a final concentration of 0.05%. Cells
were incubated for an additional 30 min. Finally, the cells were
imaged at room temperature using a Zeiss LSM 510 META confocal
microscope at a wavelength of 488 nm (Carl Zeiss AG).
The concentration of intracellular calcium ions was
determined by colorimetry (MAK022, Sigma-Aldrich; Merck KGaA). A 5
µM (0.2 µg/ml) CalciuStandard Solution
(Sigma-Aldrich; Merck KGaA) was produced by the addition of 10
µl of 500 µM Calcium Standard Solution to 990
µl water, and it was mixed with pipetting. Dilutions of the
5 µM standard solution (0, 2, 4, 6, 8 and 10 µl) were
added to a 96-well plate, in which an amount of water was added up
to the volume of 50 µl to generate 0 (assay blank), 0.4,
0.8, 1.2, 1.6 and 2.0 µg/well standards, respectively. Cells
in all groups cultured in the 6-well plate were washed with HBSS to
remove residual serum and medium when cells reached 80% confluence.
The cells were then placed on ice, and 200 µl of water were
added to the wells; subsequently, the cells were homogenized by
ultrasonication. The homogenate was centrifuged at 16,097 × g for
15 min at 4°C, and subsequently, 50 µl of the supernatant
was removed to test the Ca2+ concentration. A total of
90 µl of chromogenic reagent (MAK022, Sigma-Aldrich; Merck
KGaA) was added to each well containing standards and was gently
mixed. Subsequently, 60 µl of Calcium Assay Buffer (MAK022,
Sigma-Aldrich) were added to each well and gently mixed. The cells
were then incubated for 5-10 min at room temperature, and the plate
was shielded from light during incubation. The intracellular
Ca2+ concentration was calculated using the following
formula: CCa=Sa/Sv [CCa, concentration of
calcium in the sample; Sa, amount of calcium in unknown sample
(µg) from standard curve; Sv, sample volume (µl)
added into the wells; calcium molecular weight, 40
µg/µmol].
Annexin V/PI staining
Annexin V-FITC/PI staining and flow cytometry were
used to investigate the effects of UVB irradiation on the apoptosis
of treated cells. Flow cytometric analysis was performed using
Annexin+/PI−,
Annexin+/PI+,
Annexin−/PI+, and unlabeled cells
(Annexin−/PI−). Cells were labeled with
Annexin V-FITC and propidium iodide (PI) (Annexin V-FITC apoptosis
assay kit, Dojindo Molecular Technologies, Inc.). A total of
1×105 cells/ml experimental cells were seeded in 6-well
plates for 48 h, washed with 37°C PBS (pH 7.4), exposed to 400
µW/cm2 UVB radiation for 15 min and incubated in
a 5% CO2 incubator for 48 h. Adherent cells were washed
with 37°C sterile PBS 3 times and digested by treatment with 0.5%
pancreatic enzyme for 5 min. Subsequently, a 1×106
cell/ml cell suspension was prepared in Annexin V binding solution
after the pancreatic enzyme was inactivated by the addition of
fetal bovine serum. A 100 µl suspension of cells was added
to 5 µl of Annexin V-FITC and 5 µl of PI, and the
solution was incubated at room temperature for 15 min, while
keeping the solution out of light. A total of 400 µl of
Annexin V Binding solution was then added for flow cytometric
analysis (Accuri C6 flow cytometer, BD Biosciences). The data
collected in each window were designated as the FL-1 channel and
FL-2 channel. The unlabeled cells, the cells labeled with only
Annexin, and the cells labeled with only PI were used as controls
to regulate the compensation between the flow cytometry and the
detector, which then allowed the quadrants to be set. Analysis was
performed on 1×104 cells per sample.
Western blot analysis
Under the same experimental conditions, cells in
each group were used for protein quantification by western blot
analysis. Experimental cells were washed with ice-cold PBS and
lysed for 10 min in a buffer containing 50 mM Tris (pH 7.0), 2 mM
EDTA, 1% Triton X-100, 2 mM PMSF, and 10 µg/ml leupeptin and
aprotinin. The cell lysate was centrifuged at 13,000 × g for 15 min
at 4°C. The protein concentration of the supernatant was measured
using a microvolume UV-Vis spectrophotometer (NanoDrop 2000, Thermo
Fisher Scientific, Inc.). A total of 20 µg of protein from
each sample was separated by 10% SDS-PAGE. The proteins were then
transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad
Laboratories, Inc.). The membranes were incubated overnight at 4°C
with the following primary antibodies: Rabbit anti-polyclonal Calb1
(1:3,000, cat. no. C253L-100ULCN, Merck KGaA), rabbit anti-cleavage
products of caspase-12 (1:1,000, cat. no. ab62484, Abcam), rabbit
anti-Bad (1:1,000, cat. no. ab32445, Abcam), rabbit anti-Bcl-2
(1:1,000, cat. no. ab32124, Abcam) and rabbit anti-monoclonal
β-actin (1:1,000, cat. no. ab115777, Abcam). Subsequently, the
membranes were washed and incubated with the appropriate
HRP-conjugated secondary antibodies (1:2,000, cat. no. ab7090,
Abcam) at room temperature for 60 min, and specific bands in the
membranes were detected using enhanced chemiluminescence (ECL,
CWBio). Background samples from an area near each lane were
subtracted from each band to obtain a mean band density.
Densitometric ratios (ChemiDoc™ XRS+, Bio-Rad Laboratories, Inc.)
between CALB1 and β-actin were calculated to determine the relative
levels of these proteins.
Statistical analysis
All experiments were repeated 3 times, and average
values were obtained. The data were analyzed for significance using
repeated measures, and the data were analyzed using one-way ANOVA
of variance with the Student-Newman-Keuls post hoc test. All values
are represented as the means ± SD, and values of P<0.05 or
P<0.01 were considered to indicate statistically significant and
highly statistically significant differences, respectively.
Results
SRA01/04 cell transfection with
Calb1expression vectors
Both SRA01/04 parental cells and SRA/Calb1 cells
express detectable levels of endogenous Calb1 protein. To confirm
that cells were transfected, SRA01/04 cells were treated with a
lentivirus containing GFP cDNA as an indicator. Subsequently, the
cells in a single field were examined under a fluorescence
microscope. The expression of GFP in the SRA01/04 cells indicated
that the lentivirus carrying Calb1 cDNA was successfully
transfected into the target cells (Fig. 1).
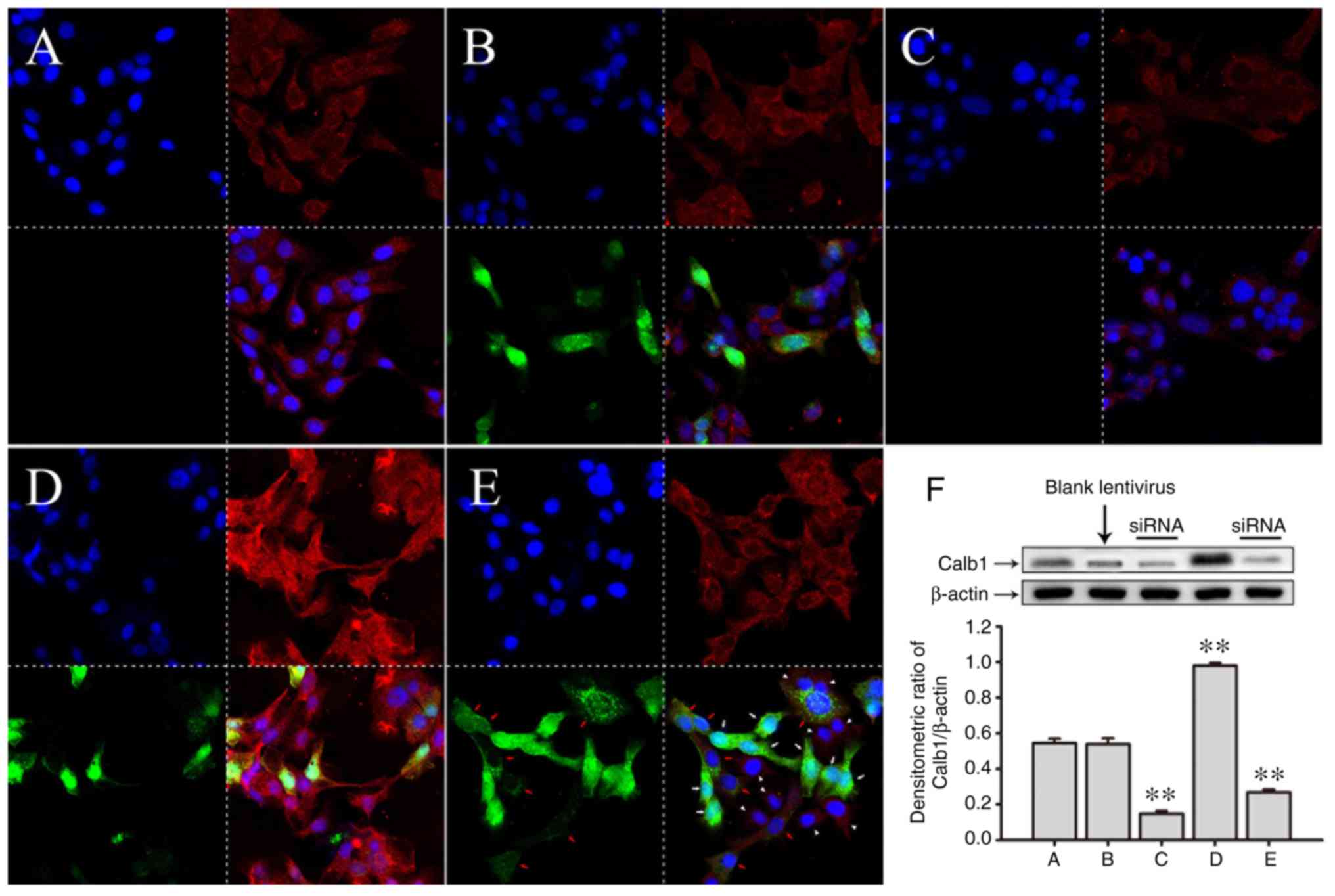 | Figure 1Immunofluorescent staining of
SRA01/04 cells transfected with a lentivirus carrying Calb1 cDNA.
In each image in (A-E) the top left image r the cell nucleus, the
top right shows the Calb1 protein, the bottom left shows the GFP
and the bottom right shows the merged image, respectively. (A)
Control SRA01/04 cells. Calb1 expression was granular (red
fluorescence). (B) SRA01/04 cells transfected with empty lentiviral
vectors carrying green fluorescent protein (GFP). Cells were
successfully transfected. (C) SRA01/04 cells after siRNA-mediated
Calb1 knockdown. The expression and distribution of Calb1 is shown.
SRA01/04 cells exhibited significantly less Calb1 following siRNA
transfection. (D) SRA01/04 cells transfected with lentiviral
vectors carrying Calb1 cDNA and green fluorescent protein (GFP).
These cells overexpressed Calb1 protein (red fluorescence). (E)
Calb1-overexpressing SRA01/04 cells were treated with the siRNA.
The expression of Calb1 was significantly reduced. (F) Quantitative
analysis of Calb1 protein expression in the above-mentioned groups
western blot analysis. Compared with the control group, there was
no significant change in Calb1 expression in the groups transfected
with an empty lentivirus (P>0.05); however, the significant
overexpression of Calb1 was observed in the groups transfected with
lentivirus carrying the Calb1 cDNA (**P<0.01). In
addition, transfection with siRNA significantly decreased the
levels of Calb1 (**P<0.01), n=8. Calb1,
calbindin-D28K. White arrows, completely transfected cells; red
arrows, incompletely transfected cells; white arrowhead,
untransfected cells. |
To further confirm the success of transfection in
these cells, western blot analysis was performed to measure the
expression level of Calb1. The expression of Cabl1 in the SRA01/04
cells transfected with the Calb1-cDNA vector was higher than that
of the SRA01/04 cells transfected with a blank lentivirus vector
(Fig. 1D and F).
In the experiment, lentivirus was added to the
culture plate to transfect the SRA01/04 cells which were marked
with GFP, and there is no marker to indicate the interfered cells
by siRNA. As shown in Fig. 1E, it
can be seen that there are 8 cells which were evidently transfected
with lentivirus (green fluorescence inside the cytoplasm, white
arrows), 7 cells which were transfected incompletely (cytoplasm
with dotted green fluorescence with a red Calb1 expression dot, red
arrows), and there were 9 cells not transfected (no green
fluorescence, and only Calb1 red fluorescence expression in the
cytoplasm, white arrowhead). It was noted that there was almost no
expression of Calb1 (red fluorescence) in the cytoplasm of the
cells that were successfully transfected with lentivirus, and the
expression intensity of Calb1 (red fluorescence) in the cells that
were not thoroughly transfected and those that were not
successfully transfected was superficially the same as that in the
control group. However, the expression intensity of Calb1 in all
cells shown in Fig. 1E was
significantly decreased compared with that of the cells shown in
Fig. 1D. Therefore, in Fig. 1E, the expression of Calb1 in the
lentivirus-transfected cells exhibited an 'all-or-none' phenomenon,
that is, the transfected cells exhibited an expression, whereas the
untransfected cells did not exhibit an expression. Thus, the total
expression of Calb1 protein in all cultured cells was lower than
that in the control group following the quantification of the
western blots.
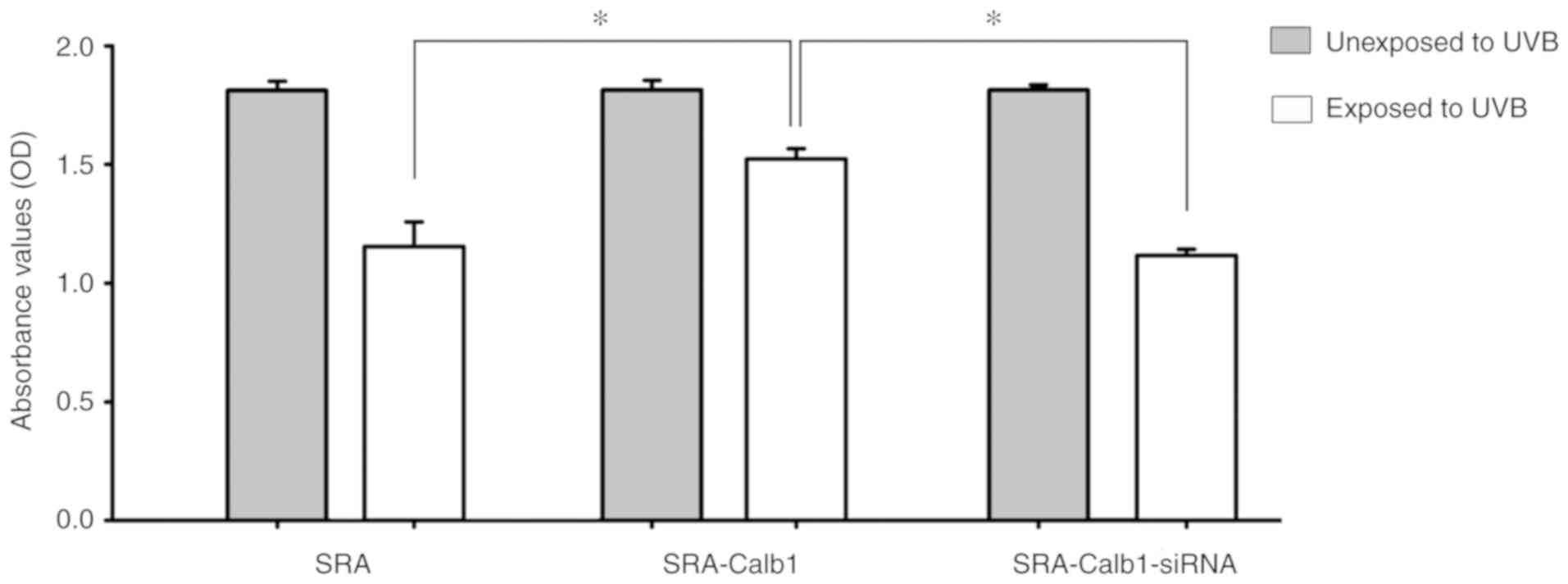
CCK-8 proliferation assay
At 24 h following irradiation with 40
µW/cm2 of UVB, the SRA01/04 cells were
transfected with a lentivirus and a siRNA. Compared with the
control group, no significant change was observed in the
proliferation of the SRA01/04 cells in groups that were not exposed
to UVB, regardless of lentivirus transfection or siRNA knockdown.
Following UVB irradiation, the proliferation of the SRA01/04 cells
overexpressing Calb1 protein was significantly higher than that of
the control group and the siRNA interference group (Fig. 2).
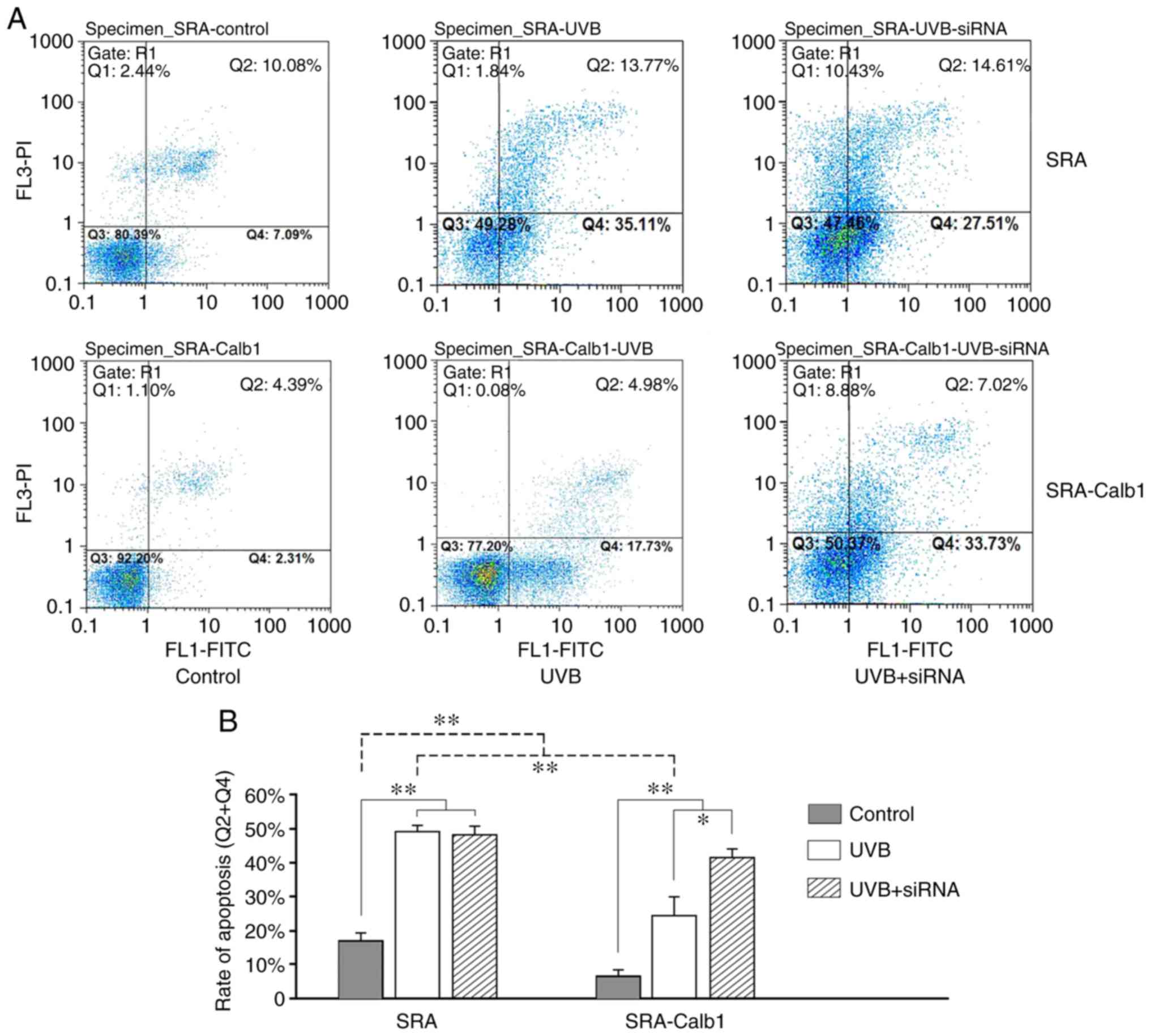
UVB-induced apoptosis detected by flow
cytometry
UVB is known to induce the apoptosis of HLECs
(39,40). Fig.
3A only shows the result of one representative experiment from
each group. Each experiment was repeated 3 times, and 8 samples in
the same subgroup were detected in one experiment. Fig. 3B illustrates the average apoptotic
rate of each group. The average rate of apoptosis in the SRA group
at 24 h was 16.89±2.41% in the control subgroup, 49.42±2.07% in the
UVB subgroup and 48.06±3.17% in the UVB + siRNA subgroup, while the
comparable results in the SRA-Calb1 group were 7.18±1.80% in the
control subgroup, 25.64±4.33% in the UVB subgroup and 42.03±2.57%
in the UVB + siRNA subgroup, respectively. The rate of apoptosis in
the SRA-Calb1 group was low in all subgroups except for the UVB +
siRNA subgroup. The survival of cells treated with 40
µW/cm2 UVB in the SRA group was decreased by
approximately 32-77% at 24 h compared to the control cells
(Fig. 3). The function of Calb1
was investigated by inducing its overexpression and knockdown in
SRA01/04 cells. Transfection with a Calb1 expression vector
resulted in a significant increase in cell survival following
exposure to UVB after 24 h (Fig.
3). The siRNA-mediated inhibition of Calb1 expression
significantly decreased cell survival following exposure to UVB
after 24 h (Fig. 3). These
results indicate that UVB-induced apoptosis is regulated by the
expression of Calb1.
The inhibition of cell apoptosis is not equal to the
increase in cell proliferative activity. As shown in Fig. 2, the cell proliferative activity
of the cells in the control group, which were transfected with
lentivirus overexpressing Calb1, indicated that the transfection
with lentivirus did not affect SRA01/4 cell viability. As shown in
Fig. 3, the decrease in the
apoptotic rate of the cells in the control group, which were
transfected with lentivirus overexpressing Calb1, indicated that
Calb1 could only exert an inhibitory effect on apoptosis, whereas
this does not indicate that the inhibition of apoptosis can
increase cell viability.
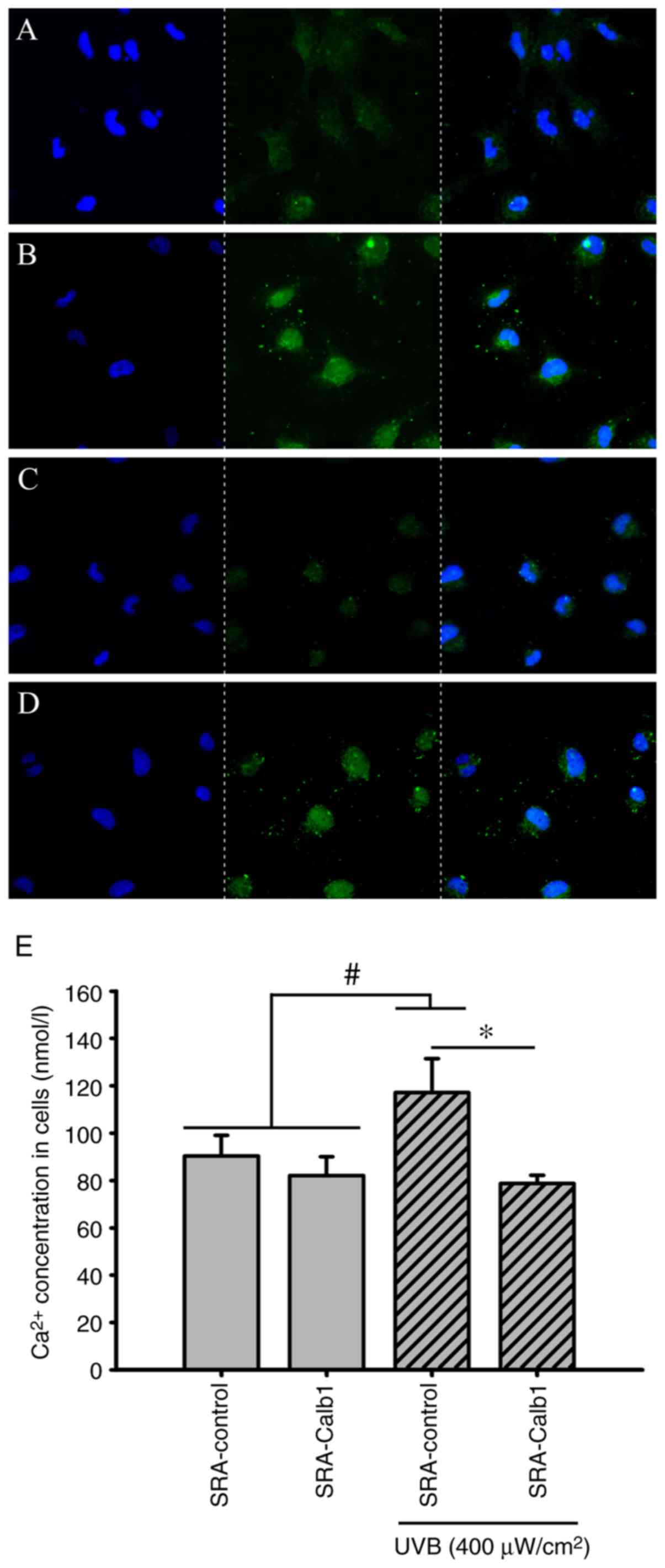
Intracellular Ca2+
distribution and concentrations
The Fluo-4AM Ca2+ fluorescence probe was
used to identify changes in the distribution of Ca2+ in
SRA01/04 cells in vitro. Compared with the control group,
the intracellular concentrations of Ca2+ in the
untransfected groups with UVB irradiation treatment were increased
at 48 h, and in the SRA01/04 cells overexpressing Calb1, a stable
Ca2+ distribution and concentrations at 48 h following
UVB irradiation were found (Fig.
4E). In the present study, the intracellular Ca2+
concentration was approximately 90.39±8.71 nmol/l (Fig. 4E) in the SRA-control group and
approximately 117.12±14.39 nmol/l (Fig. 4E) in the group of untransfected
cells irradiated with UVB. The SRA01/04 cells overexpressing Calb1
were irradiated with UVB for 24 h, and the intracellular
Ca2+ concentration was maintained at 78.68±3.53 nmol/l
(Fig. 4E). The intracellular
Ca2+ concentration in SRA 01/04 cells, which
overexpressed Calb1 and not to be exposed to UVB, was 82.02±7.98
nmol/l (Fig. 4E).
UVB-induced apoptosis is associated with
Bad, Bcl-2 and caspase-12 expression
UVB-induced apoptosis is associated with the
expression of the apoptotic proteins, Bad, Bcl-2 and caspase-12;
thus, in the present study, the expression levels of these proteins
were examined by western blot analysis. In the control group of
untransfected lens epithelial cells, a significant increase in Bad
and caspase-12 protein expression was observed at 24 h following
UVB irradiation for 15 min (Fig. 5A,
B and D). Bcl-2 protein expression was significantly decreased
at the same time point (Fig. 5A and
C). Following exposure to UVB, the group of SRA01/04 cells that
were transfected with the Calb1 overexpression vector exhibited a
significant downregulation in the protein expression of Bad
compared to the untransfected cells, while the expression of Bcl-2
protein was significantly upregulated (Fig. 5A, B and C). Bcl-2 is an inhibitory
apoptotic protein, and it can play an anti-apoptotic role through
the inhibition of the Ca2+ flow crossing the cell
membrane, consequently regulating the intracellular Ca2+
concentration. This is consistent with results of flow cytometric
analysis illustrated in Fig. 3.
However, the expression of caspase-12 did not differ significantly
from that of the control group in which the cells were unexposed to
UVB (grey bars) at the same time point (Fig. 5A and D).
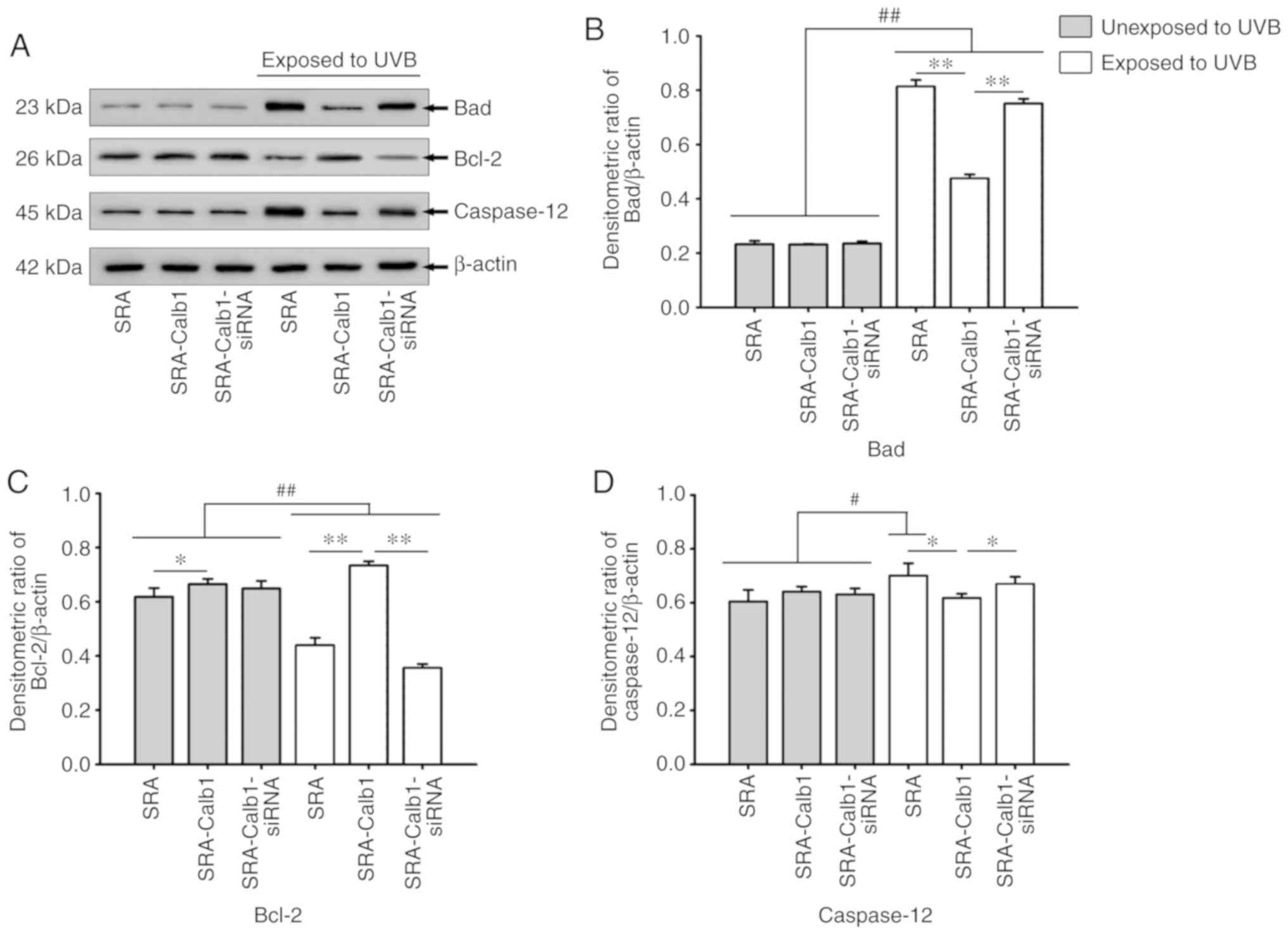 | Figure 5Western blot analysis. The expression
levels of Bad, Bcl-2 and caspase-12 in control SRA01/04 cells and
SRA01/04 cells overexpressing Calb1 after UVB irradiation are
shown. (A) Western blot signals for Bad (23 kDa), Bcl-2 (26 kDa),
caspase-12 (45 kDa) and internal control β-actin (42 kDa). (B)
Changes in Bad protein expression in control SRA01/04 cells and
cells overexpressing Calb1. Compared with cells not exposed to UVB
irradiation, Bad protein expression levels after UVB irradiation
was significantly increased (P<0.01). Bad protein expression in
cells overexpressing Calb1 was significantly lower than that in the
control SRA01/04 cells and in cells transfected with the siRNA
(P<0.01). (C) Change of the Bcl-2 protein expression level.
Compared with cells not exposed to UVB irradiation, Bcl-2 protein
in cells after UVB irradiation was significantly downregulated in
control SRA01/04 cells and cells overexpressing Calb1 that were
also treated with the siRNA (P<0.01), while it was upregulated
in cells overexpressing Calb1 (P<0.05). However, in cells
exposed to UVB irradiation, the Bcl-2 protein expression in the
cells overexpressing Calb1 was significantly higher than it was in
normal SRA01/04 cells and cells overexpressing Calb1 that were also
treated with the siRNA (P<0.01). (D) Changes in caspase-12
protein expression levels. Compared with cells that were not
exposed to UVB irradiation, the expression of caspase-12 protein in
cells that were exposed to UVB irradiation was upregulated in
control SRA01/04 cells (P<0.05), while the expression of
caspase-12 in cells overexpressing Calb1 and the siRNA interference
group was not significantly changed. In the cells exposed to UVB
irradiation, the expression of caspase-12 in the cells
overexpressing Calb1 was lower than it was in the other two groups
(P<0.05). n=8. Calb1, calbindin-D28K. *,#P<0.05;
**,##P<0.01. |
The knockdown of Cabl1 expression increased
UVB-induced apoptosis. Calb1 protein was knocked down by siRNA
transfection to examine the effects of its downregulation on the
apoptosis of SRA01/04 cells. The cells transfected with siRNA
downregulated Calb1 expression in the SRA01/04 cells, and this was
confirmed by western blot analysis (Fig. 1F). Compared with the SRA01/04 cell
control groups, the protein expression of Bad was upregulated
following UVB irradiation and treatment with siRNA to downregulate
Calb1; however, Bcl-2 expression was downregulated under the same
conditions (Fig. 5A, B and C).
The expression of the Caspase-12 protein did not change. These
results suggest that Calb1 may play a protective role in
UVB-mediated HLEC death.
Discussion
HLECs grow in a monolayer under the anterior
subcapsular surface of the lens. As the cornea and aqueous humor do
not filter UV light, the HLECs can be exposed to UVB radiation.
There is some evidence to indicate that UV-induced cataract
formation is caused by damage to the HLECs (14,41,42). The apoptosis of HLECs is a common
cellular basis for non-congenital cataract formation in humans and
animals (3). UVB exhibits strong
radiation energy, and it has the ability to penetrate tissues and
damage cells. Studies have shown that UV-induced cell damage of
lens epithelial cells is strongest at 297 nm, which is in the UVB
range (43). The lens contains
two types of cells: Lens epithelial cells with Ca2+
pumps in the membranes and lens fiber cells without Ca2+
pumps (44). Therefore,
Ca2+ homeostasis in the lens is solely dependent on lens
epithelial cells. Cell membrane calcium ATPase is a high affinity
Ca2+ pump. It uses the energy produced by ATP hydrolysis
to transport Ca2+ from inside to outside cells to
maintain the electrochemical gradient of cells (44). The pump also plays an important
role in cell calcium homeostasis and signal transduction. There are
four subtypes of cell membrane calcium ATPases: 1-4. All four
subtypes are expressed in human lens epithelial cells (45). A previous study found that UVB
irradiation can increase intracellular Ca2+ levels,
inhibit Ca2+-ATPase activity and downregulate PMCA1
expression at the mRNA and protein levels. These findings reveal
that the downregulation of cell membrane calcium ATPase1 levels and
the loss of calcium homeostasis may play important roles in
UVB-induced apoptosis following UVB irradiation (46). UVB irradiation induces cell death
by activating the apoptotic pathway initiated by mitochondria
(15). UVB radiation-induced
apoptosis is regulated by various molecular processes. Mitochondria
rapidly lose transmembrane potential and produce reactive oxygen
species (ROS), and both results may contribute to the breakdown of
cells (18). The concentration of
free intracellular Ca2+ is maintained at a relatively
low level to ensure normal physiological cell function. Studies
have shown that the level of Ca2+ in HLECs is
approximately 78±33 nmol/l or 96±20 nmol/l (47,48). Previous studies have found that
UVB irradiation can apparently increase intracellular
Ca2+ levels (49,50), and the present study also revealed
that an elevation in intracellular Ca2+ levels occurred
following UVB irradiation. Further, we found that SRA01/04 cells
overexpressing Calb1 can effectively prevent the increase of
Ca2+ concentration after UVB irradiation. The results of
the present study demonstrated that UVB irradiation with 40
µW/cm2 for 15 min significantly induced the of
apoptosis of SRA01/04 cells, which was accompanied by a significant
increase in the concentration of Ca2+ in these cells.
The intensity of UVB irradiation with approximately 40
µW/cm2 is known as the appropriate concentration
to affect HLEC apoptosis (16).
In HLECs, intracellular Ca2+ homeostasis is a necessary
condition to maintain the transparency of the lens, and this
homeostasis depends on Ca2+-ATPase pumps. An imbalance
of Ca2+ homeostasis is harmful to cells. Increasing
intracellular Ca2+ concentrations can cause human lens
opacification (51,52). Moreover, UVB irradiation can
interfere with Ca2+ signaling of HLECs in vitro,
resulting in the apoptosis of target cells (53). In the present study, UVB-induced
apoptosis was investigated by flow cytometry, and the expression of
the apoptotic proteins, Bad, Bcl-2 and caspase-12, suggested that
UVB-induced apoptotic signals may be involved in changes in the
Ca2+ concentration.
Ca2+ regulates a number of cellular
functions, including cell proliferation, differentiation and death
(54,55). Ca2+ plays an important
role in apoptosis, and the increased intracellular Ca2+
concentration has been shown to activate apoptotic pathways
(56,57). Increased intracellular
Ca2+ levels trigger the activation of caspase-12,
leading to apoptosis (58,59).
In the present study, to determine the effect of UVB on
Ca2+ homeostasis in SRA01/04 cells, control SRA01/04
cells, cells overexpressing Calb1, and cells subjected to the
siRNA-mediated knockdown of Calb1 were irradiated with UVB;
intracellular Ca2+ levels were then observed to assess
the effect of UVB on apoptosis and its association with the
concentration of intracellular Ca2+. In addition, the
endoplasmic reticulum (ER) plays an important role in maintaining
intracellular Ca2+ homeostasis. UVB-induced apoptosis
may result in ER stress, which is mediated by the mitochondria
(60,61). The increased in intracellular
Ca2+ levels induced by UVB irradiation may be closely
related to the change in Calb1 expression and the increase in ER
stress.
Calb1 is a high-affinity calcium-binding protein
widely expressed in the kidneys, pancreas, brain, bone and lens
(28). It is the main target of
1,25-dihydroxyvitamin D3, which mainly promotes extracellular
Ca2+ transport. Calb1 expression was synchronized with
the expression of calcium channels in epithelial cells (62). It exerts a protective effect on
nerve cells, among other cell types, where it plays a role in the
regulation of apoptosis (63,64). Exogenous Calb1 expression can
reduce oxidative stress and maintain mitochondrial function. Nerve
cells overexpressing Calb1 have been shown to exhibit a low rate of
apoptosis in vitro (65).
Calb1 can also protect kidney cells from PT-induced apoptosis and
cytotoxicity (66). A number of
studies have suggested that the upregulation of intracellular Calb1
expression can reduce the apoptosis induced by different
pro-apoptotic pathways.
The present study confirmed that the expression of
Calb1 inhibited the UVB-induced apoptosis of HLECs. Calb1
overexpression exerted a protective effect on UVB-induced apoptosis
and significantly reduced the expression of Bad and caspase-12. In
addition, using siRNA to knock-down Calb1 expression in SRA01/04
cells significantly decreased the survival rate of SRA01/04 cells
exposed to UVB radiation; additionally, under these conditions, the
protein expression of Bad and caspase-12 was significantly
increased, and the protein expression of Bcl-2 was significantly
downregulated.
In conclusion, the present study demonstrates that
UVB irradiation can increase intracellular Ca2+ levels
directly and/or inhibit Ca2+-ATPase activity and thus
disrupt intracellular Ca2+ homeostasis. The UVB
radiation-induced apoptosis of SRA01/04 cells may be involved in
complex mechanisms, including imbalances in Ca2+
homeostasis. Calb1 protects HLECs from apoptosis by regulating the
concentration of intracellular Ca2+, which mediates the
expression of the pro-apoptotic proteins, Bad, Bcl-2 and
caspase-12.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560160) grant
funded by the Chinese government.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
KL, as the first author, contributed to the
experimental design, the drafting of the manuscript and the
analysis and interpretation of the data for the study. JZ is
responsible for designing the flow cytometry in ensuring that
questions related to the accuracy or integrity had been resolved.
LYa completed the cell culture experiments and transfection, and is
accountable for all aspects of this part of the study. MG designed
and is responsible for the western blot analysis experiments and
the relevant data acquisition. LYu designed and was responsible for
the collection of cellular morphological research data. YG is the
correspondence author and contributed to the experimental
conception and design, the statistical analysis of data, the
critical revision of the manuscript and the final approval of the
manuscript to be published. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jia S, Shi J, Chen X and Tang L:
Ultraviolet radiation-induced apoptosis in human lens epithelial
cells and its effect on Bcl-2 and Bax. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 37:730–736. 2012.In Chinese. PubMed/NCBI
|
|
2
|
McCarty CA and Taylor HR: A review of the
epidemiologic evidence linking ultraviolet radiation and cataracts.
Dev Ophthalmol. 35:21–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li WC, Kuszak JR, Dunn K, Wang RR, Ma W,
Wang GM, Spector A, Leib M, Cotliar AM and Weiss M: Lens epithelial
cell apoptosis appears to be a common cellular basis for
non-congenital cataract development in humans and animals. J Cell
Biol. 130:169–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andley UP, Walsh A, Kochevar IE and Reddan
JR: Effect of ultraviolet-B radiation on protein synthesis in
cultured lens epithelial cells. Curr Eye Res. 9:1099–1106. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andley UP, Malone JP and Townsend RR:
Inhibition of lens photodamage by UV-absorbing contact lenses.
Invest Ophthalmol Vis Sci. 52:8330–8341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee DH, Cho KS, Park SG, Kim EK and Joo
CK: Cellular death mediated by nuclear factor kappa B (NF-kappaB)
translocation in cultured human lens epithelial cells after
ultraviolet-B irradiation. J Cataract Refract Surg. 31:614–619.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao J, Liu Y, Wang X, Shen Y, Yuan S, Wan
Y and Jiang Q: UVB radiation induces human lens epithelial cell
migration via NADPH oxidase-mediated generation of reactive oxygen
species and up-regulation of matrix metalloproteinases. Int J Mol
Med. 24:153–159. 2009.PubMed/NCBI
|
|
8
|
Miyashita H, Hatsusaka N, Shibuya E, Mita
N, Yamazaki M, Shibata T, Ishida H, Ukai Y, Kubo E and Sasaki H:
Association between ultraviolet radiation exposure dose and
cataract in Han people living in China and Taiwan: A
cross-sectional study. PLoS One. 14:e02153382019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lofgren S: Solar ultraviolet radiation
cataract. Exp Eye Res. 156:112–116. 2017. View Article : Google Scholar
|
|
10
|
Galichanin K, Lofgren S and Soderberg P:
Cataract after repeated daily in vivo exposure to ultraviolet
radiation. Health Phys. 107:523–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Yan H, Lofgren S, Tian X and Lou
MF: Ultraviolet radiation-induced cataract in mice: The effect of
age and the potential biochemical mechanism. Invest Ophthalmol Vis
Sci. 53:7276–7285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mody VC Jr, Kakar M, Elfving A, Soderberg
PG and Lofgren S: Ultraviolet radiation-B-induced cataract in
albino rats: Maximum tolerable dose and ascorbate consumption. Acta
Ophthalmol Scand. 84:390–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
West SK, Longstreth JD, Munoz BE, Pitcher
HM and Duncan DD: Model of risk of cortical cataract in the US
population with exposure to increased ultraviolet radiation due to
stratospheric ozone depletion. Am J Epidemiol. 162:1080–1088. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Midelfart A: Ultraviolet radiation and
cataract. Acta Ophthalmol Scand. 83:642–644. 2005. View Article : Google Scholar
|
|
15
|
Jing L, Kumari S, Mendelev N and Li PA:
Coenzyme q10 ameliorates ultraviolet B irradiation induced cell
death through inhibition of mitochondrial intrinsic cell death
pathway. Int J Mol Sci. 12:8302–8315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shui YB, Sasaki H, Pan JH, Hata I, Kojima
M, Yamada Y, Hirai KI, Takahashia N and Sasaki K: Morphological
observation on cell death and phagocytosis induced by ultraviolet
irradiation in a cultured human lens epithelial cell line. Exp Eye
Res. 71:609–618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendelev N, Witherspoon S and Li PA:
Overexpression of human selenoprotein H in neuronal cells
ameliorates ultraviolet irradiation-induced damage by modulating
cell signaling path-ways. Exp Neurol. 220:328–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ricci JE, Gottlieb RA and Green DR:
Caspase-Mediated loss of mitochondrial function and generation of
reactive oxygen species during apoptosis. J Cell Biol. 160:65–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Celio MR: Calbindin D-28k and parvalbumin
in the rat nervous system. Neuroscience. 35:375–475. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Persechini A, Moncrief ND and Kretsinger
RH: The EF-hand family of calcium-modulated proteins. Trends
Neurosci. 12:462–467. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hemmingsen C: Regulation of renal
calbindin-D28k. Pharmacol Toxicol. 87(Suppl 3): S5–S30. 2000.
|
|
22
|
Duncan MJ and Franklin KM: Expression of
5-HT7 receptor mRNA in the hamster brain: Effect of aging and
association with calbindin-D28K expression. Brain Res. 1143:70–77.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iacopino AM, Rhoten WB and Christakos S:
Calcium binding protein (calbindin-D28k) gene expression in the
developing and aging mouse cerebellum. Brain Res Mol Brain Res.
8:283–290. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hall AK and Norman AW: Acute actions of
1,25-dihydroxyvitamin D3 upon chick pancreatic calbindin-D28K.
Biochem Biophys Res Commun. 176:1057–1061. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Margolis DS, Kim D, Szivek JA, Lai LW and
Lien YH: Functionally improved bone in calbindin-D28k knockout
mice. Bone. 39:477–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Faucheux C, Bareille R and Amedee J:
Synthesis of calbi-ndin-D28K during mineralization in human bone
marrow stromal cells. Biochemical J. 333:817–823. 1998. View Article : Google Scholar
|
|
27
|
Baimbridge KG, Celio MR and Rogers JH:
Calcium-Binding proteins in the nervous system. Trends Neurosci.
15:303–308. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geng Y, Lin HT, Chen W, Liu ZC, Xiang W
and Chen WR: Age-Related reduction in calbindin-D28K expression in
the spraguedawley rat lens. Mol Med Rep. 11:422–426. 2015.
View Article : Google Scholar
|
|
29
|
Ahmadian SS, Rezvanian A, Peterson M,
Weintraub S, Bigio EH, Mesulam MM and Geula C: Loss of
calbindin-D28K is associated with the full range of tangle
pathology within basal forebrain cholinergic neurons in Alzheimer's
disease. Neurobiol Aging. 36:3163–3170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang C, Jiang C, Yuan H, Xiao C and Gao D:
Role of calbi-ndin-D28K in estrogen treatment for Parkinson's
disease. Neural Regen Res. 8:702–707. 2013.PubMed/NCBI
|
|
31
|
Ferrante RJ, Kowall NW and Richardson EP
Jr: Proliferative and degenerative changes in striatal spiny
neurons in Huntington's disease: A combined study using the
sectiongolgi method and calbindin D28k immunocytochemistry. J
Neurosci. 11:3877–3887. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kiyama H, Seto-Ohshima A and Emson PC:
Calbindin D28K as a marker for the degeneration of the
striatonigral pathway in Huntington's disease. Brain Res.
525:209–214. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong SM, Chung SY, Park MS, Huh YB, Park
MS and Yeo SG: Immunoreactivity of calcium-binding proteins in the
central auditory nervous system of aged rats. J Korean Neurosurg
Soc. 45:231–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fan Y, Shi L, Gu Y, Zhao Y, Xie J, Qiao J,
Yang GY, Wang Y and Lu CZ: Pretreatment with PTD-calbindin D 28k
alleviates rat brain injury induced by ischemia and reperfusion. J
Cereb Blood Flow Metab. 27:719–728. 2007. View Article : Google Scholar
|
|
35
|
D'Orlando C, Celio MR and Schwaller B:
Calretinin and calbindin D-28k, but not parvalbumin protect against
glutamate-induced delayed excitotoxicity in transfected N18-RE 105
neuroblastomaretina hybrid cells. Brain Res. 945:181–190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meier TJ, Ho DY, Park TS and Sapolsky RM:
Gene transfer of calbindin D28k cDNA via herpes simplex virus
amplicon vector decreases cytoplasmic calcium ion response and
enhances neuronal survival following glutamatergic challenge but
not following cyanide. J Neurochem. 71:1013–1023. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bellido T, Huening M, Raval-Pandya M,
Manolagas SC and Christakos S: Calbindin-D28k is expressed in
osteoblastic cells and suppresses their apoptosis by inhibiting
caspase-3 activity. J Biol Chem. 275:26328–26332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Youn HY, McCanna DJ, Sivak JG and Jones
LW: In vitro ultraviolet-induced damage in human corneal, lens, and
retinal pigment epithelial cells. Mol Vis. 17:237–246.
2011.PubMed/NCBI
|
|
39
|
Okuno T: Ultraviolet action spectrum for
cell killing in a human lens epithelial cell line. Ind Health.
45:137–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Teng H, Huang LY, Tian F, Dong LJ and
Zhang H: Effects of SMP-30 overexpression on apoptosis of human
lens epithelial cells induced by ultraviolet B irradiation.
Zhonghua Yan Ke Za Zhi. 53:835–841. 2017.In Chinese; Abstract
available in Chinese from the publisher. PubMed/NCBI
|
|
41
|
Li WC and Spector A: Lens epithelial cell
apoptosis is an early event in the development of UVB-induced
cataract. Free Radic Biol Med. 20:301–311. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun X, Zou W and Zhao C: Expression of P53
during lens epithe-lial cell apoptosis induced by ultraviolet. J
Tongji Med Univ. 21:263–264. 2001. View Article : Google Scholar
|
|
43
|
Andley UP, Lewis RM, Reddan JR and
Kochevar IE: Action spectrum for cytotoxicity in the UVA- and
UVB-wavelength region in cultured lens epithelial cells. Invest
Ophthalmol Vis Sci. 35:367–373. 1994.PubMed/NCBI
|
|
44
|
Marian MJ, Li H, Borchman D and Paterson
CA: Plasma membrane Ca2+-ATPase expression in the human lens. Exp
Eye Res. 81:57–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Marian MJ, Mukhopadhyay P, Borchman D,
Tang D and Paterson CA: Regulation of sarco/endoplasmic and plasma
membrane calcium ATPase gene expression by calcium in cultured
human lens epithelial cells. Cell Calcium. 41:87–95. 2007.
View Article : Google Scholar
|
|
46
|
Wu Q, Guo D, Bi H, Wang D and Du Y: UVB
irradiation-induced dysregulation of plasma membrane calcium
ATPase1 and intra-cellular calcium homeostasis in human lens
epithelial cells. Mol Cell Biochem. 382:263–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Williams MR, Duncan G, Riach RA and Webb
SF: Acetylcholine receptors are coupled to mobilization of
intracellular calcium cultured human lens cells. Exp Eye Res.
57:381–384. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Riach RA, Duncan G, Williams MR and Webb
SF: Histamine and ATP mobilize calcium by activation of H1 and P2u
receptors in human lens epithelial cells. J Physiol. 486:273–282.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Masaki H, Izutsu Y, Yahagi S and Okano Y:
Reactive oxygen species in HaCaT keratinocytes after UVB
irradiation are triggered by intracellular Ca(2+) levels. J
Investig Dermatol Symp Proc. 14:50–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ding BX and Wang CB: Inhibitory effect of
polypeptides from chlamys farreri on UVB-induced apoptosis and DNA
damage in normal human dermal fibroblasts in vitro. Acta Pharmacol
Sin. 24:1006–1010. 2003.PubMed/NCBI
|
|
51
|
Wackernagel W, Ettinger K, Weitgasser U,
Bakir BG, Schmut O, Goessler W and Faschinger C: Opacification of a
silicone intra-ocular lens caused by calcium deposits on the optic.
J Cataract Refract Surg. 30:517–520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Truscott RJ, Marcantonio JM, Tomlinson J
and Duncan G: Calcium-induced opacification and proteolysis in the
intact rat lens. Invest Ophthalmol Vis Sci. 31:2405–2411.
1990.PubMed/NCBI
|
|
53
|
Hightower KR, Duncan G, Dawson A,
Wormstone IM, Reddan J and Dziedizc D: Ultraviolet irradiation
(UVB) interrupts calcium cell signaling in lens epithelial cells.
Photochem Photobiol. 69:595–598. 1999.PubMed/NCBI
|
|
54
|
Dolmetsch RE, Lewis RS, Goodnow CC and
Healy JI: Differential activation of transcription factors induced
by Ca2+ response amplitude and duration. Nature. 386:855–858. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Richter C and Kass GE: Oxidative stress in
mitochondria: Its relationship to cellular Ca2+ homeostasis, cell
death, proliferation, and differentiation. Chem Biol Interact.
77:1–23. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Martikainen P, Kyprianou N, Tucker RW and
Isaacs JT: Programmed death of nonproliferating
androgen-independent prostatic cancer cells. Cancer Res.
51:4693–4700. 1991.PubMed/NCBI
|
|
57
|
Lynch K, Fernandez G, Pappalardo A and
Peluso JJ: Basic fibroblast growth factor inhibits apoptosis of
spontaneously immortalized granulosa cells by regulating
intracellular free calcium levels through a protein kinase
Cdelta-dependent pathway. Endocrinology. 141:4209–4217. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang Y, Li X, Wang Y, Wang H, Huang C and
Li J: Endoplasmic reticulum stress-induced hepatic stellate cell
apoptosis through calcium-mediated JNK/P38 MAPK and
Calpain/Caspase-12 pathways. Mol Cell Biochem. 394:1–12. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li L, Cao Z, Jia P and Wang Z: Calcium
signals and caspase-12 participated in paraoxon-induced apoptosis
in EL4 cells. Toxicol In Vitro. 24:728–736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jiang L, Liu Y, Ma MM, Tang YB, Zhou JG
and Guan YY: Mitochondria dependent pathway is involved in the
protective effect of bestrophin-3 on hydrogen peroxide-induced
apoptosis in basilar artery smooth muscle cells. Apoptosis.
18:556–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Min SK, Lee SK, Park JS, Lee J, Paeng JY,
Lee SI, Lee HJ, Kim Y, Pae HO, Lee SK and Kim EC: Endoplasmic
reticulum stress is involved in hydrogen peroxide induced apoptosis
in immortalized and malignant human oral keratinocytes. J Oral
Pathol Med. 37:490–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hoenderop JG, Hartog A, Stuiver M, Doucet
A, Willems PH and Bindels RJ: Localization of the epithelial Ca(2+)
channel in rabbit kidney and intestine. J Am Soc Nephrol.
11:1171–1178. 2000.PubMed/NCBI
|
|
63
|
Sun S, Li F, Gao X, Zhu Y, Chen J, Zhu X,
Yuan H and Gao D: Calbindin-D28K inhibits apoptosis in dopaminergic
neurons by activation of the PI3-kinase-Akt signaling pathway.
Neuroscience. 199:359–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Verdes JM, Morana JA, Battes D, Gutiérrez
F, Guerrero F, Goicoa A, Fidalgo LE, Barbeito CG, Zanuzzi CN,
Portiansky EL and Gimeno EJ: Calbindin D28k expression and the
absence of apoptosis in the cerebellum of solanum bonariense
L-intoxicated bovines. Vet Pathol. 47:569–572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Guo Q, Christakos S, Robinson N and
Mattson MP: Calbindin D28k blocks the proapoptotic actions of
mutant presenilin 1: Reduced oxidative stress and preserved
mitochondrial function. Proc Natl Acad Sci USA. 95:3227–3232. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Turner PR, Mefford S, Christakos S and
Nissenson RA: Apoptosis mediated by activation of the G
protein-coupled receptor for parathyroid hormone (PTH)/PTH-related
protein (PTHrP). Mol Endocrinol. 14:241–254. 2000. View Article : Google Scholar : PubMed/NCBI
|