Introduction
The regenerative potential of polyphenols is
attracting increasing attention in the field of oral health, thanks
to their anti-inflammatory and anti-oxidant properties ascribed to
their molecular structure. Flavonoids, a major subclass of
polyphenols, have an anti-inflammatory action and are capable of
modulating the host inflammatory response (1,2).
Clinical evidence has shown that flavonoids have beneficial effects
on periodontitis (1,3), an oral inflammatory disease of
polymicrobial origin that causes the destruction of gingival
connective tissue and the alveolar bone supporting the teeth.
Periodontitis affects 30% of adults (4). Cell biology and in vivo
rodent model studies (5-7) have revealed a multiplicity of
effects exerted by flavonoids on periodontal cells and tissues,
including the regulation of the inflammatory response in
periodontal components, and potential preserving effects on
periodontal ligaments and alveolar bone tissues (8). Potentially beneficial effects of
flavonoids have been reported in various periodontal cells, as well
as alveolar bone-maintaining osteoblasts. Proanthocyanidins have
been shown to exert protective effects against oxidative stress and
periodontitis, both in vitro and in vivo (9,10).
Multiple study findings have shown that poly-phenols can counteract
the shift towards osteoclastogenesis in bone-loss pathologies
(11-14).
In addition to the role played by the molecular
structure of polyphenols in controlling inflammation and related
tissue-protection mechanisms, mounting evidence has suggested their
active involvement in tissue formation. With regards to, for
example, bone tissue, it has been shown that polyphenols exert
effects on osteoblasts by involving different signaling pathways,
such as Wingless-INT/β-catenin (15), insulin-like growth factor
(16), bone morphogenetic
proteins (17), Runt-related
transcription factor 2 (Runx2) (18) and Osterix (19). Furthermore, due to a structural
similarity to mammalian estrogens, some polyphenols, such as
isoflavones, are also called phytoestrogens and are able to bind to
estrogen receptors (ERs) α and β, thus acting as hormone analogs
with different agonistic or antagonistic actions, depending on the
tissue (20). The role of
polyphenols in different mechanisms of bone formation has recently
been reviewed (21,22).
These aforementioned properties make polyphenol
molecules of particular interest to dental materials and devices.
Presently in oral surgery, most dental biomaterials, including bone
fillers, work through a simple scaffolding effect, providing
osteoconduction (i.e., adhesion and growth of osteogenic cells, but
no direct osteoinduction, such as triggering of mechanisms of new
bone formation) (23). In a
clinical setting, dental implants stimulate new bone formation
through a physical effect, namely through a controlled surface
roughness (24). The emerging
field of biomolecular modifications of bone implants and dental
biomaterials aims at enhancing the host tissue response through
biologically active molecules delivered from the device or linked
to the device surface (25-29). The biomolecular modification of
dental devices and materials through polyphenols from different
sources is being investigated (30-38).
A key-issue in the exploitation of polyphenol
properties is their source. Polyphenol-rich pomace extracts
(PRPEs), obtained through straightforward solid-liquid extraction
(39-41), are of particular interest, due to
availability, as well as economical and ethical reasons. The
heterogeneity of the mixture makes these extracts extremely
interesting, since it is possible to take advantage of the
potential of a large number of polyphenolic classes.
The aim of the present study was to investigate the
effects of 2 PRPEs, very different in terms of molecular
composition and content, on hMSC osteogenic commitment. The first
PRPE is obtained from Croatina, a red grape variety very rich in
proanthocyanidins, whereas the second one is obtained from Arneis,
a white grape characterized by the absence of proanthocyanidins and
richness in phenolic acids. Red grapes notoriously contain a
greater amount of polyphenols than white ones (42-44). However, in the making of Arneis
wine (white wine making), once grapes are pressed, skins are
removed in order to obtain a juice fermented without skin contact.
Hence, skin polyphenols are not extracted and remain in the pomace.
Conversely, the making of wine from Croatina (red wine making)
involves the fermentation of pulp together with the grape skins,
which give wine its typical color through the extraction of a
fraction of the polyphenols they contain, rendering the pomace
partially deprived of skin polyphenols.
In particular, it was demonstrated that, through
specific classes of phenolic compounds identified in the 2
grape-pomace extracts, it is possible to induce a specific
biomolecular response in human MSCs (hMSCs). The present study
aimed at setting the basis for conscious design of bone implant
dental devices and materials through biomolecular modification
involving specific PRPEs, obtained from winery byproducts and,
specifically, from the Croatina and Arneis varieties. This aspect
represents the novelty of this study, in fact the use of an
original complex mixture of polyphenols was shown to induce the
same results of osteogenic differentiation in MSCs elicited by
single phenolic standards or a mixture of them (45-50).
Materials and methods
Standards and chemicals
All chemicals were analytical-reagent grade and the
water was distilled prior to use (Milli-Q Adavantage A10; EMD
Millipore). Chemicals, including acetone, acetic acid, hydrochloric
acid, Folin-Ciocalteu (FC) reagent, 2,2-diphenyl-1-picrylhydrazyl
(DPPH), sodium carbonate, sodium bisulphite, gallic acid (GA),
quercetin, rutin, caffeic acid, p-coumaric acid and
malvidin-3-glucoside, were all purchased from Merck KGaA. Grape
pomace was purchased from local winery producers; red grape
(Croatina) from Alemat (Ponzano Monferrato) and white grape
(Arneis) from Tenuta Carretta (Piobesi d'Alba).
PRPE
Grape pomace was collected and stored at −20°C under
vacuum until the beginning of the extraction process. A
multi-residual control was performed on both PRPEs following the
UNI EN 15662:2009 methods, to avoid any collateral effect on cells
due to possible residual pesticides. Prior to extraction, they were
washed with acidified water, dried in a circulating-air oven
(37°C±5°C-UN260; Memmert GmbH + Co.KG) and grinded in a bladed mill
(GM 200; Retsch GmbH). The milled grape pomace (300 g) was
extracted from 2,000 ml of 50:50 acetone:water (v/v) using an
automatic extractor (TIMATIC Micro C, Tecnolab). The extraction
cycle is fully automatic and alternates a dynamic phase, obtained
in programmed pressure, and a static phase, in which a forced
percolation is generated, thus ensuring a continuous solvent flow
to the interior of the plant matrix and avoiding oversaturation,
thanks to the programmable recirculation.
Next, the obtained solution was concentrated in a
rotavap (Laborota 4001) under reduced pressure (and maintained in
the fridge between 2-4°C).
In the present study, two different PRPEs were
prepared: One from white (Arneis) and the other from red (Croatina)
grape pomace. After the total phenolic content measurements were
obtained, PRPEs were diluted in water to reach a common
concentration of 1 mg/ml GA equivalents (GAE).
High-performance liquid chromatography
(HPLC) coupled with diode array detector (DAD)
PRPEs from Arneis and Croatina have been
characterized using HPLC Shimadzu LC 2010 AHT equipped with
Shimadzu SPD-M10AVP DAD. PRPEs were filtered using 0.2 µm
cellulose acetate filters and analyzed using a C8 Luna column
(150×4.6 mm; 5 µm particle size) from Phenomenex operated at
25°C. The mobile phase consisted of 2% (v/v) acetic acid in water
(mobile phase A) and 0.5% acetic acid in water and acetonitrile
(50:50 v/v) (mobile phase B). The gradient program shown in
Table I was used, at a flow rate
of 0.8 ml/min and a total running time of 123 min (51). The injection volume was 10
µl and the DA recorded spectra at 200-600 nm. The
quantification of individual polyphenols was performed using
calibration curves of the corresponding reference compounds. GA
(280 nm), quercetin (370 nm), rutin (355 nm), caffeic acid (320
nm), p-coumaric acid (370 nm) and malvidin-3-glucoside (520 nm)
were dissolved in an ethanol/water solution at concentrations of 1,
5, 10, 50 100, 150 and 200 µg/ml and analyzed using the
aforementioned HPLC method. The quantification was performed by
applying the standard calibration curve.
 | Table IHigh pressure liquid chromatography
gradient method for separation of polyphenols in polyphenol-rich
pomace extracts of Arneis and Croatina. |
Table I
High pressure liquid chromatography
gradient method for separation of polyphenols in polyphenol-rich
pomace extracts of Arneis and Croatina.
| Time (min) | MPA (%) | MPB (%) |
|---|
| 0-35 | 100→95 | 0→5 |
| 35-80 | 95→80 | 5→20 |
| 80-110 | 80→0 | 20→100 |
| 110-113 | 0 | 100 |
| 113-123 | 0→100 | 100→0 |
Phenolic content
The initial phenolic content of Arneis and Croatina
PRPEs was evaluated using the FC method. All extracts were
transferred into a 25-ml volumetric flask and diluted with
distilled water at a ratio of 1:50. Subsequently, 0.5 g FC reagent
was added and mixed for 5 min, and then 1.5 g of 20% anhydrous
sodium carbonate (w/v) solution was added. After 2 h, the
absorbance was measured at 765 nm, using water as the compensation
liquid and a quartz cell (10-mm path length) in a UV-Vis
spectrophotometer (T80+, PG Instruments Limited). The absorbance
value was used to calculate the concentration of polyphenols using
a calibration curve obtained with GA. The results are expressed as
mg/ml of GAE, which means the amount of gallic acid needed to have
the same polyphenolic concentration of the extract.
Calibration curve
A total of 10 mg GA was diluted in 10 ml water to
obtain 1 mg/ml stock solution. Aliquots of stock solution were
transferred into a 25-ml volumetric flask and diluted in water at
the final concentrations of 0.05, 0.025, 0.01 and 0.005 mg/ml. Each
standard solution was prepared according to the procedure described
above for the PRPEs; the absorbance was measured under the same
condition as for PRPEs.
This method was used to calculate the initial
phenolic content and the amount of water needed to dilute the PRPE
at a concentration of 1 mg/ml.
Antioxidant power
The ability of water PRPEs (1 mg/ml of GAE) to
scavenge the DPPH radical was estimated using the method described
by Brand-Williams et al (52). An aliquot of 40 µl PRPEs
was added to 1,600 µl water:ethanol 50:50 (v/v) solution.
Separately, a DPPH solution (0.1 mg/ml..w/v) was prepared in
ethanol and 2 ml of this solution was added to the reaction
mixture. Next, the solution was well-shaken and incubated for 30
min at room temperature in the dark and absorbance was recorded at
525 nm. The blank solution was made up by a solution of
water:ethanol instead of PRPE. The percentage inhibition of the
DPPH radical by the samples was calculated using the following
equation:
Where A0 is the absorbance of control
sample and A1 is the absorbance of the test sample. The
reduction of the absorbance was used as value to indicate the
antioxidant power.
Determination of anthocyanins
The methods of Ribéreau-Gayon and Stonestreet have
been widely used to determine the concentration of
proanthocyanidins in red wine (53). The determination of anthocyanins
of the grape pomace is carried out using two properties stemming
from their molecular features: The modification of their colors
according to the pH and the transformation into colorless
derivatives under the action of certain reagents, such as
bisulphite ions. Thus, the variation of the absorbance read at 520
nm following the addition of excess bisulphite ions is proportional
to the anthocyanin content.
Briefly, solution A was prepared in a test tube of
50 ml, as follows: Mix 1 ml of PRPE with 1 ml of acidified ethanol
solution [0.1% v/v hydrocholoric acid (HCl)] and 20 ml HCl solution
(2% of HCl in distilled water). Subsequently, 5 ml solution A was
mixed in a 25-ml test tube with 2 ml distilled water (solution B);
furthermore, 5 ml solution A was mixed with 2 ml sodium bisulfite
solution in another 25-ml test tube (concentration of 150 g/l;
solution C).
Using an UV-Vis spectrophotometer, absorbance at 520
nm was recorded for both solutions B and C, and the absorbance
variation was used to calculate the amount of anthocyanins
contained in the PRPE. Quantification has been made using a
calibration curve made through the standard anthocyanin
malvidin-3-glucoside dissolved in an ethanol/water solution at
concentrations of 1, 5, 10, 20, 50, 100, 200, 300 and 500
µg/ml. Malvidin-3-glucoside solutions have been analyzed
using the aforementioned method. The linearity of the
absorbance-concentration curve was verified at 0-300 µg/ml
(R2=0.999). Results are expressed as µg/ml of
malvidin-3-glucoside equivalent.
Cell culture
hMSCs, frozen following the first passage in
culture, were purchased from Stemcell Technologies, Inc. (cat. no.
70022; Human Bone Marrow Stromal Cells, Primary human cells, Cryo,
7.5×105 cells). Cells were obtained using Institutional
Review Board (IRB)-approved consent forms and protocols (BIOMED
IRB-Bone marrow collection for therapeutic or non-therapeutic
use-Protocol number 701-01). On receipt, they were cultured at
standard conditions of 37°C and 5% CO2, and maintained
in MesenCult™ Proliferation kit (Human; cat. no. 05411),
supplemented with L-Glutamine (200 mM; cat. no. 07100), both from
Stemcell Technologies, Inc. Experiments were performed with hMSCs
at passage 2 or 3 following receipt.
On day 0 of the preliminary experiments, cells were
seeded in 2 different 12 well-plates (Sarstedt AG & Co. KG) at
a density of ~5×104 cells/ml, placing 2 ml into each
well. After 24 h, the medium was changed and replaced with new
medium. In each plate, the first column (3 wells) of the plate was
used as the control and did not receive any further supplement.
Columns 2 and 3 received 10 µg/ml Arneis and Croatina PRPEs,
respectively. A further control was obtained by culturing hMSC in
osteogenic growth medium (cat. no. 05465, MesenCult™ Osteogenic
Diff kit; Human; Stemcell Technologies, Inc.) in the fourth column
of the plate.
At the first experimental time point (5 days), one
plate was used for RT-qPCR analysis, as described in the next
paragraph. The second plate received 0.5 ml fresh medium and
relevant analysis was performed on day 12.
Based on the obtained results, as described in the
relevant section, a further experiment was planned, using the same
general approach but working at two different PRPE concentrations
(10 and 20 µg/ml). A dedicated 12-well plate for each
concentration and each experimental time point (total 4 12-well
plates) was prepared according to the experimental scheme described
for the preliminary experiment. Measurements were performed after
48 h and 7 days.
RT-qPCR analysis
Total RNA was isolated from hMSCs using the
Maxwell® RSC simply RNA Cells kit (Promega Corporation)
in the Maxwell® RSC instrument (Promega Corporation),
following the manufacturer's protocol. RNA quantification was
performed using the Quantifluor system kit in the Quantus
Fluorometer (both from Promega Corporation) and the obtained total
RNA was reverse-transcribed using a High-Capacity cDNA Reverse
Transcription kit in the Thermal Cycler 2720 (both from Thermo
Fisher Scientific, Inc.) at the following conditions: 10 min at
25°C, 120 min at 37°C, 5 min at 85°C and were maintined at 4°C
until further experimentation. RT-qPCR was performed, following the
Fast running protocol of the TaqMan® FastAdvanced Master
Mix (Thermo Fisher Scientific, Inc.), in the QuantStudio 5 Real
Time PCR System (Thermo Fisher Scientific, Inc.) using designed
TaqMan® assays (Thermo Fisher Scientific, Inc.) to
quantify gene expression of human tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein ζ
(YWHAZ; Hs03044281_g1), a 1 type 1 collagen (Col1a1;
Hs00164004_m1), alkaline phosphatase (ALPL; Hs01029144_m1),
osteocalcin (OCN; Hs00609452_g1), osteonectin (SPARC;
Hs00234160_m1), metalloproteinase (MMP)1 (Hs00899658_m1), tissue
inhibitor of metalloproteinase (TIMP)1 (Hs01092512_g1), Runx2
(Hs01047976_m1), BMP2 (Hs00154192_m1), integrin-binding
sialoprotein (IBSP) (Hs00173720_m1), receptor activator of nuclear
factor κ-B ligand (RANKL; Hs00243519_m1) and osteoprotegerin (OPG;
Hs00900358_m1), and all transcripts were normalized to YWHAZ. Data
have been normalized using the comparative threshold cycle
(ΔΔCq) method (54).
Statistical analysis
Experimental data were analyzed using PAST version
3.18 (55) and results are
presented as the mean ± standard error of the mean from 3
independent experiments (3 wells for each set of data). Statistical
differ-ences between groups were analysed using two-way ANOVA,
followed by Tukey's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Grape pomace extract
characterization
Prior to the in vitro study, a prerequisite
was the identification and quantification of the polyphenolic
pattern by different techniques. Two different types of grape
pomace were used as a source of polyphenols: Arneis and Croatina.
Analysis of phenolic content showed an initial amount of 3.6 mg/ml
of GAE for Croatina PRPE and 5.5 mg/ml of GAE for Arneis PRPE. The
detection of a higher GAE value in white grape pomace is likely the
outcome of the mentioned different technique of white vs. red
wine-making. Starting from that value, the two extracts were
diluted with water to reach the same amount of phenolic content,
equal to 1 mg/ml GAE and then HPLC-DAD analysis and related
antioxidant power were performed.
The analysis of the UV-Vis spectra of the peaks
found in the chromatograms allowed the classification of the
separated peaks (Figs. S1 and
S2) in different classes: Phenolic acid and flavonoids, which
exhibit an absorbance maximum of 277-280 nm, hydroxycinnamic acid
of 313-330 nm with sometimes a shoulder of ~290 nm, flavonols of
350-385 nm and anthocyanidines, which show an absorbance maximum of
280-320 nm with specific absorbance at 525 nm (56).
The different composition of PRPEs (1 mg/ml of GAE)
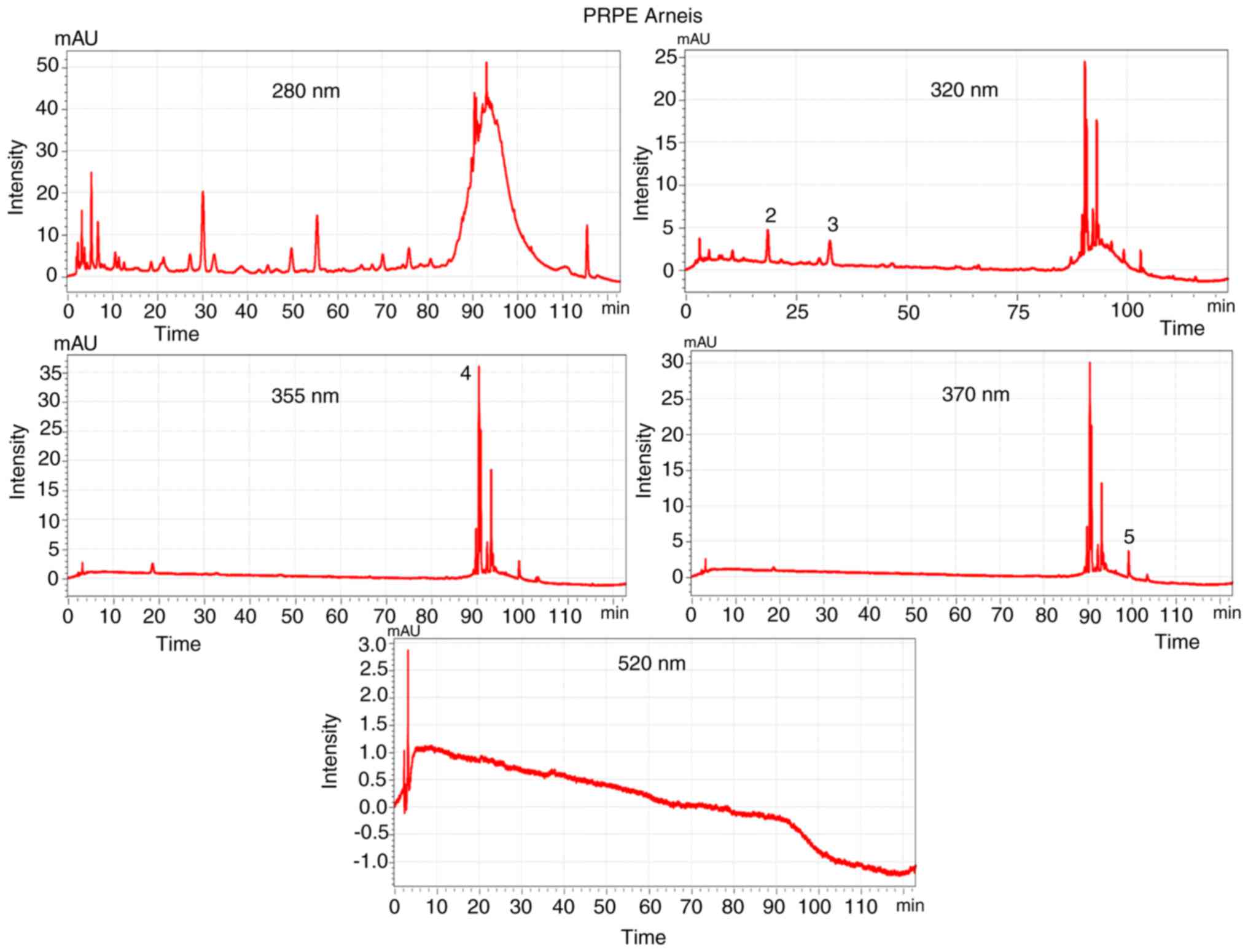
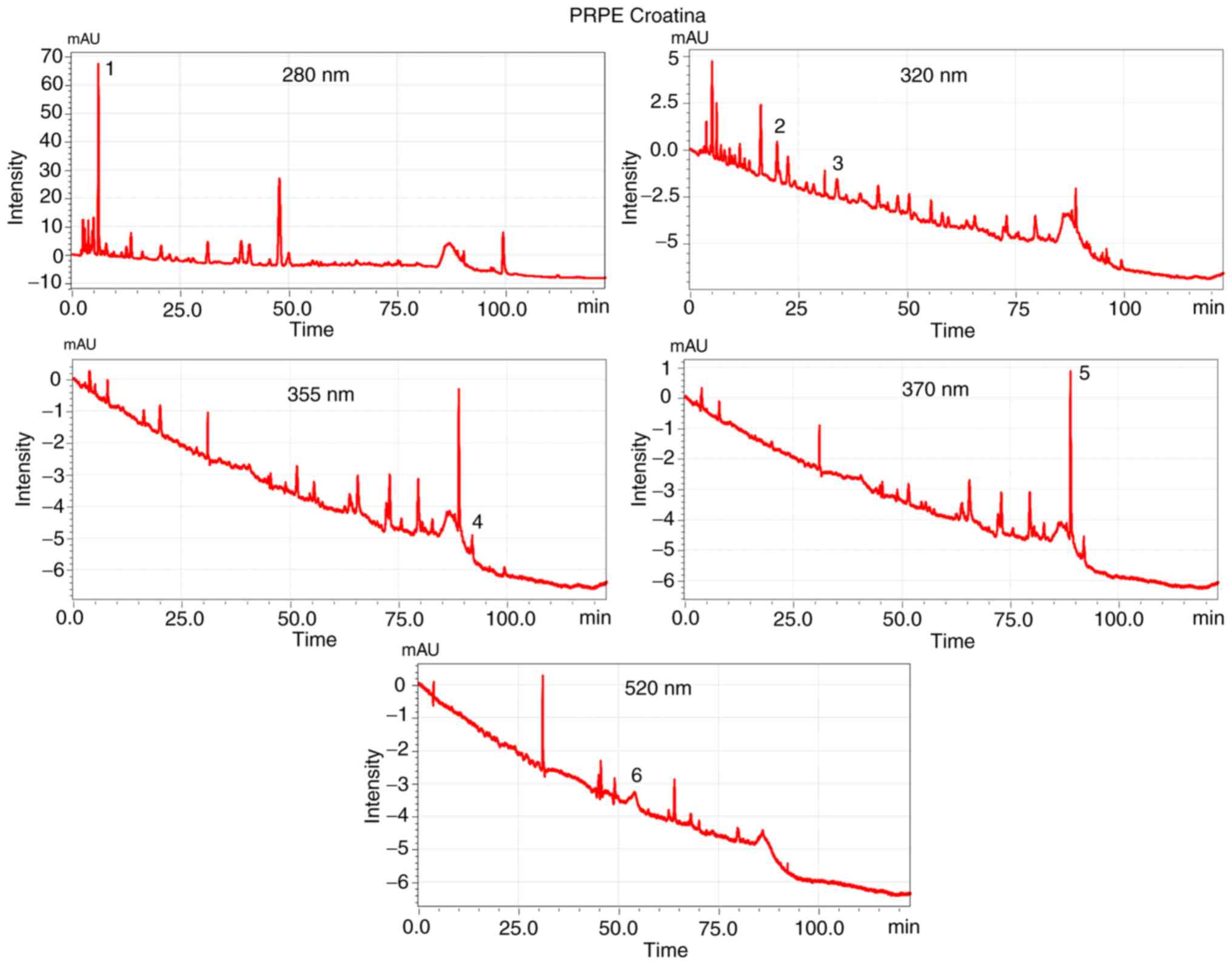
is well represented in Figs. 1
and 2, which contain the
chromatograms of the two extracts, obtained through HPLC-DAD.
The baseline drift is one of the issues of the HPLC
analysis and, aiming at reducing it, the use of a gradient
characterized by the same solvent used both at the beginning and at
the end of the analysis and that has a low absorbance cut-off is
recommended (57,58).
In order to reduce the baseline drift, in the
present analysis acetonitrile was used as solvent, which has a low
cut-off wave-length and is different from the absorbance wavelength
of the compound (>280 nm). This resulted in a very low baseline
drift at the wavelength of 280 nm (Figs. 1 and 2). The chromatograms corresponding to
the Croatina extract (Fig. 2) at
the wavelengths of 320, 355, 370 and 520 nm seem to have a more
evident baseline drift corresponding to the Arneis extract
(Fig. 1) but this is due to the y
axis scale (intensity) which is smaller and, as a consequence,
amplified.
The chromatograms corresponding to the 2 grape PRPEs
showed a particular and distinctive phenolic profile, with a good
separation that created the fingerprint of the extracted residual
phenolics. Using different standard solutions, it was possible to
identify and quantify the specific polyphenols for each extract:
Quercetin, rutin, GA, caffeic acid, p-coumaric acid and
malvidin-3-glucoside (Table
II).
 | Table IIQuantification of different
polyphenol molecules through high pressure liquid
chromatography-diode array detector analysis. |
Table II
Quantification of different
polyphenol molecules through high pressure liquid
chromatography-diode array detector analysis.
| Gallic | Caffeic | Cumaric | Quercetin | Rutin |
Malvidin-3-glucoside |
|---|
| PRPE | acid
(µg/ml) | acid
(µg/ml) | acid
(µg/ml) | (µg/ml) | (µg/ml) | (µg/ml) |
| Croatina | 9.42 | 1.59 | 0.21 | 2.06 | 0.19 | 37.2 |
| Arneis | 2.83 | 1.41 | 0.61 | 1.84 | 18.07 | 0 |
The obtained results showed a significant difference
in the amount of phenolic acid: The amount of GA was 9.42
µg/ml for Croatina and 2.83 µg/ml for Arneis. The
amount of the quercetin flavonol was similar in the 2 extracts,
whereas that of rutin, a flavonoid glycoside, was significantly
increased in the Arneis PRPE, as compared to Croatina PRPE (18.07
and 0.19 µg/ml, respectively).
Both extracts contained a similar amount of caffeic
and coumaric acid (see Table
II). Croatina PRPE is mainly composed of anthocyanidines. As
seen in Table II, the amount of
malvidin-3-glucoside found in the chromatogram of Croatina PRPE is
37.2 µg/ml, as compared with the 0 µg/ml of Arneis
PRPE.
The total amount of anthocyanins was further
determined using the Ribéreau-Gayon and Stonestreet methods
(53), which exploit the
bleaching potential of sodium bisulphite, which reveals that 446.7
µg/ml of anthocyanins are present in Croatina PRPE, whereas
~ 3.7 µg/ml of anthocyanins are present in Arneis PRPE
(Table III).
 | Table IIIAnthocyanins content in PRPEs. |
Table III
Anthocyanins content in PRPEs.
| PRPE | Anthocyanins
(µg/ml) |
|---|
| Croatina | 446.7 |
| Arneis | 3.7 |
The free radical scavenging capacity of both
extracts has also been evaluated by DPPH assay, which is considered
a valid, accurate, easy and economic method to evaluate the radical
scavenging activity of antioxidants, since the radical compound is
stable and needs not to be generated. The antioxidant power for
both PRPEs at 1 mg/ml of GAE was analyzed. Results reported in
Table IV showed that PRPEs with
the same phenolic content can reduce radicals with a different
efficacy. In particular, Arneis PRPE reduces radicals by 41.8%,
whereas Croatina PRPE reduced by 18.5%, suggesting that, with the
same polyphenolic content (1 mg/ml), the molecules contained in
pomace from Arneis exert a higher antioxidant power, as compared
with pomace from Croatina.
 | Table IVReduction (%) of
2,2-diphenyl-1-picrylhydrazyl radical by PRPEs from Arneis and
Croatina. |
Table IV
Reduction (%) of
2,2-diphenyl-1-picrylhydrazyl radical by PRPEs from Arneis and
Croatina.
| PRPE | Reduction % |
|---|
| Croatina | 18.5 |
| Arneis | 41.8 |
Gene expression analysis by RT-qPCR
The qPCR experiment was set up following a series of
preliminary screening tests, in order to determine the best
concentrations of polyphenols extracts at different time points in
terms of cell viability and biological activity (data not shown).
In particular, for the evaluation of cell viability depending on
PRPEs concentrations and time, the MTT assay was used, followed by
RT-qPCR to evaluate gene expression analysis as a response of cells
to the not cytotoxic concentrations of PRPEs.
The expression of genes associated with hMSC
differentiation into osteoblasts was examined by RT-qPCR, however
the absence of experiments investigating both the molecular
mechanisms involved in the observed polyphenols actions and the
protein levels is a limitation of the present study. The first
experiment, at two different time points (5 and 12 days), was
carried out with a low concentration (LC=10 µg/ml) of
extracts, as compared with a control containing hMSCs incubated in
basal growth medium for 5 days. An osteogenic treatment on hMSCs,
grown in osteogenic growth medium (OGM), was also administered to
further investigate the differentiation potential of the two grape
PRPEs. Incubation of hMSCs with both low-concentration (LC) PRPEs
resulted in an increase of the expression levels of genes involved
in the induction of osteogenic differentiation, such as BMP2 and
Runx2 at both time points, as compared with negative control and
osteogenic cells (Fig. 3).
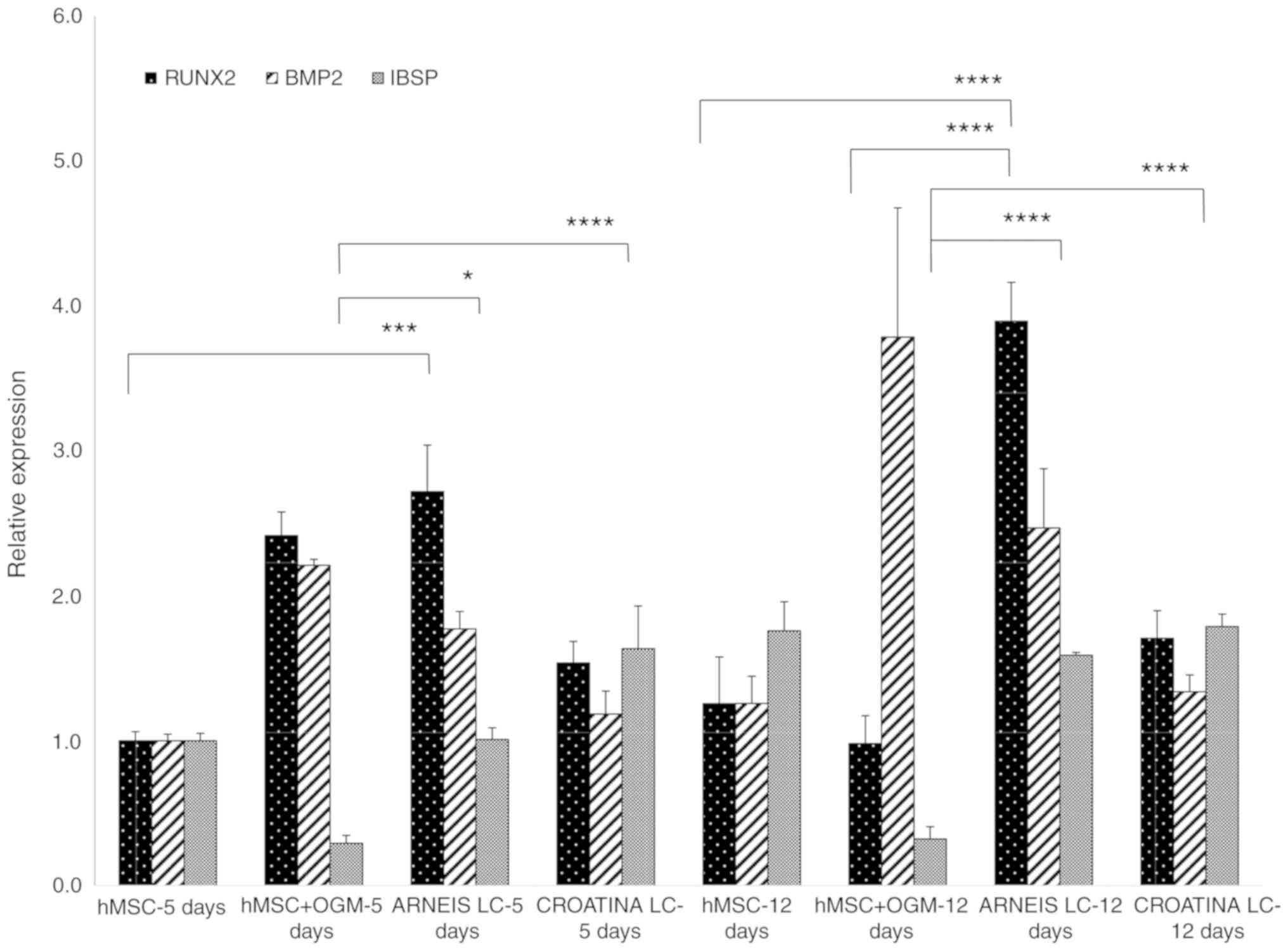 | Figure 3Preliminary reverse
transcription-quantitative PCR experiment. Gene expression analysis
of hMSCs incubated with OGM or LC Arneis and Croatina PRPEs, at 5
and 12 days. Values are expressed as relative expression, compared
with the control group. Data are presented as the mean ± standard
error of the mean. *P≤0.05, ***P≤0.001 and
****P≤0.0001. hMSCs, human mesenchymal cells; OGM,
osteogenic growth medium; LC, low concentration; PRPEs,
polyphenol-rich pomace extracts; iBSP, integrin-binding
sialoprotein; BMP2, bone morphogenetic protein 2; Runx2,
runt-related transcription factor 2. |
Specifically, BMP2 gene expression in hMSCs treated
with LC Arneis PRPE, increased by 1.7 at 5 days and 2.4-fold at 12
days (not significant), whereas the Runx2 expression increased by
2.7-fold (P<0.001) at 5 days and 3.9-fold (P<0.0001) at 12
days, as compared with hMSCs at 5 days. Furthermore, the expression
value of cells with LC Arneis PRPE at 12 days, is also greater than
that of osteogenic cells at 12 days (P<0.0001).
Unexpectedly, for cells incubated with LC Croatina
PRPE, analysis of IBSP showed a significantly increased gene
expression (1.6-fold) at 5 days, as compared with the control
(P<0.05) and osteogenic cells (P<0.0001) at 5 days, and to
osteogenic cells at 12 days (P<0.0001). Furthermore, LC Arneis
PRPE significantly induced IBSP expression at both time points, as
compared with osteogenic cells (P<0.05, at 5 days and
P<0.0001 at 12 days; Fig.
3).
These promising results led the current study to
further investigate additional genes and evaluate whether the
effects seen on hMSCs, following incubation for 48 h and 7 days
with the two tested PRPEs, were dose-dependent; two concentrations,
namely LC and high concentration (HC=20 µg/ml), were
therefore used.
Enhancement of bone matrix protein expression of
genes such as Col1a1 and SPARC can be observed in cells incubated
with LC Arneis PRPE (Col1a1, 1.9-fold at 48 h, P<0.001; 4.7-fold
at 7 days, P<0.0001; SPARC, 1.7-fold at 48 h, P<0.001;
4.7-fold at 7 days, P<0.0001) and in HC Arneis PRPE (Col1a1,
2.1-fold at 48 h, P<0.0001; SPARC, 2.1-fold at 48 h,
P<0.0001), as compared with the control (Fig. 4).
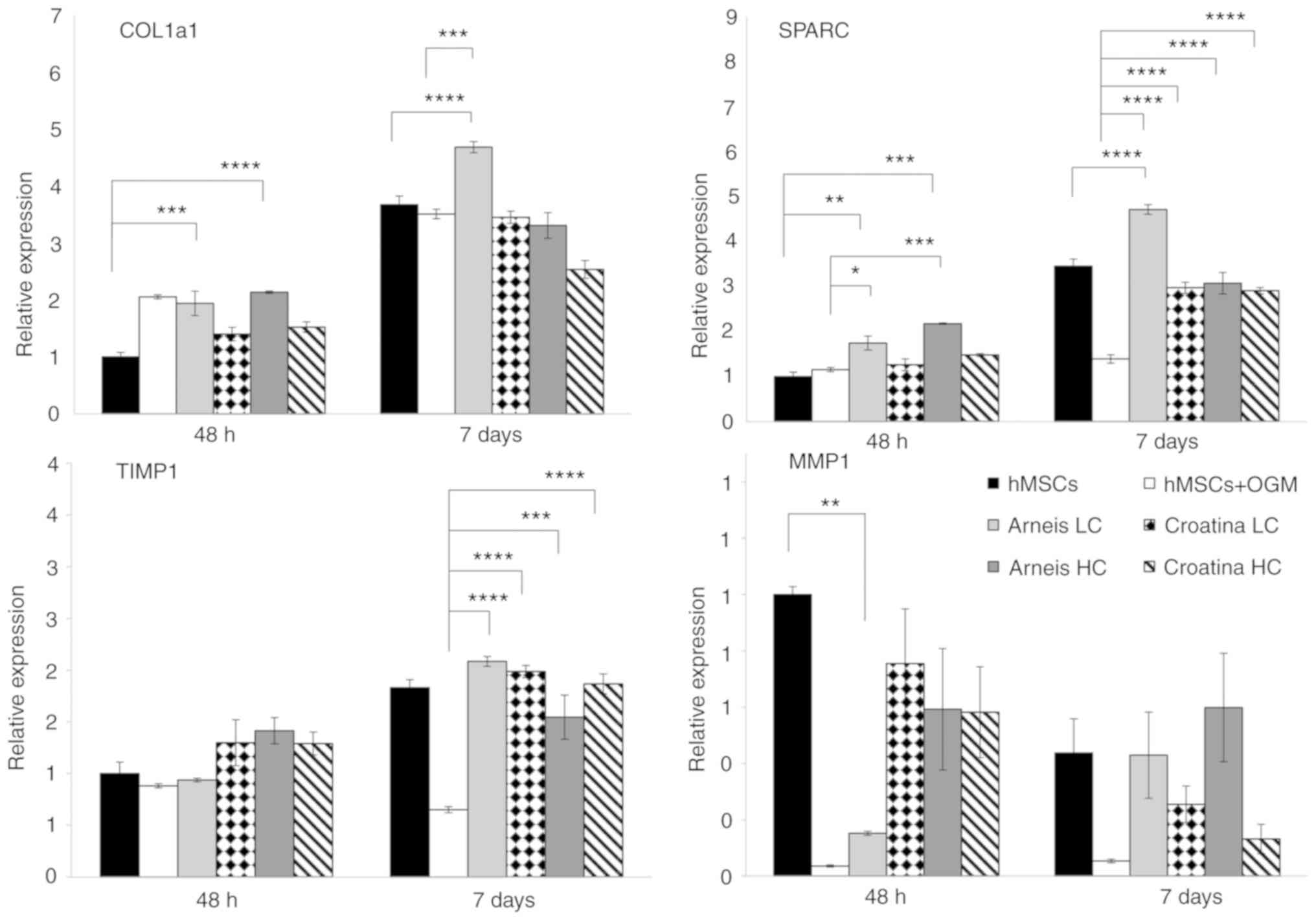 | Figure 4Gene expression analysis of Col1a1,
SPARC, TIMP1 and MMP1 at the mRNA level. Reverse
transcription-quantitative PCR data for Col1a1, SPARC, TIMP1 and
MMP1 genes obtained after 48 h and 7 days for untreated and treated
groups cultured in OGM or Arneis and Croatina PRPEs. Values are
expressed as relative expression, compared with the control group.
Data are presented as the mean ± standard error of the mean.
*P≤0.05, **P≤0.01, ***P≤0.001 and
****P≤0.0001. Col1a1, α 1 type 1 collagen; SPARC,
secreted protein acidic and cysteine rich; TIMP1, tissue inhibitor
of metalloproteinase 1; MMP1, metalloproteinase 1; OGM, osteogenic
growth medium; PRPEs, polyphenol-rich pomace extracts; LC, low
concentration; HC, high concentration. |
Of note, as compared with osteogenic cells, those
incubated with Arneis PRPE had a significantly increased Col1a1
expression (LC at 7 days, P<0.0001) and SPARC levels (LC at 48 h
P<0.05, at 7 days P<0.0001; HC at 48 h P<0.001, at 7 days,
P<0.0001), whereas cells with Croatina PRPE only showed a
significant increase of SPARC (LC and HC at 48 h and 7 days,
P<0.0001; Fig. 4). Analysis of
mineralization-related genes such as ALPL and OCN showed a
significant increase of OCN expression in cells incubated with
Arneis PRPE (HC at 48 h; P<0.001), as compared with the control
(Fig. 5), whereas no differences
were found for ALPL expression (Fig.
S3).
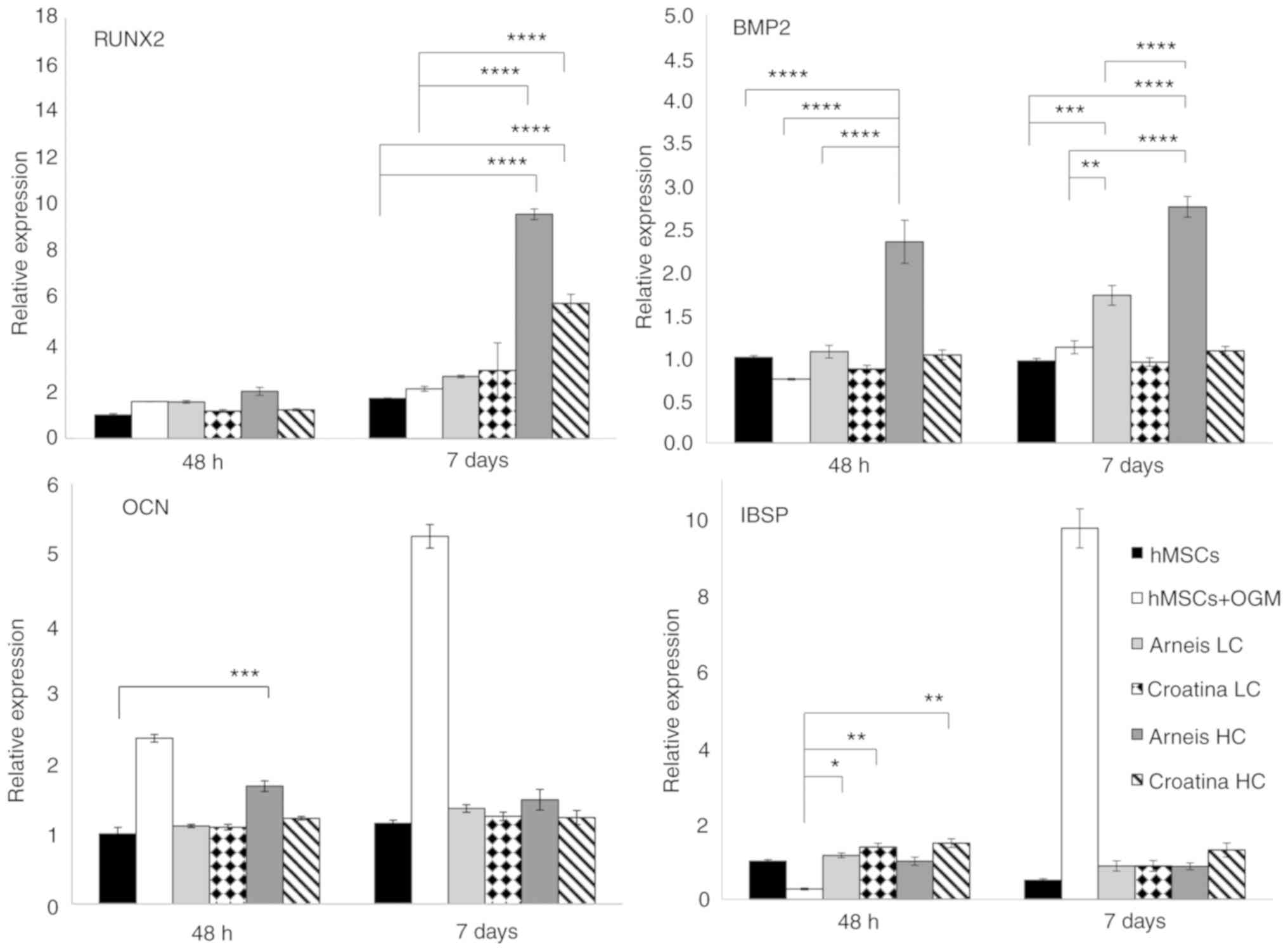 | Figure 5Gene expression analysis of Runx2,
BMP2, OCN and IBSP at the mRNA level. Reverse
transcription-quantitative PCR data for Runx2, BMP2, OCN and IBSP
genes obtained after 48 h and 7 days for untreated and treated
groups cultured in osteogenic differentiation media or Arneis and
Croatina PRPEs. Values are expressed as relative expression,
compared with the control group. Data are presented as the mean ±
standard error of the mean. *P≤0.05,
**P≤0.01, ***P≤0.001 and ****P≤0.0001. Runx2,
runt-related transcription factor 2; BMP2, bone morphogenetic
protein 2; OCN, osteocalcin; IBSP, integrin-binding sialoprotein;
PRPEs, polyphenol-rich pomace extracts; LC, low concentration; HC,
high concentration. |
Given that the osteogenic differentiation process is
also influenced by the perturbation of subtle equilibria, the
opposite actions of MMPs and tissue inhibitor of metalloproteinases
(TIMPs) have been investigated. In particular, as compared with the
control, only LC Arneis PRPE at 48 h showed a reduced MMP1
expression (0.1-fold; P<0.01), whereas the TIMP1 expression was
significantly increased in all PRPEs at 7 days, as compared with
cells grown in OGM (LC Arneis, P<0.0001; LC Croatina,
P<0.0001; HC Arneis, P<0.001; HC Croatina, P<0.0001;
Fig. 4). Particularly interesting
was the analysis of the MMP1/TIMP1 ratio, which the 2 PRPEs
decreased at 48 h (Arneis LC, P<0.0001 and HC, P<0.01;
Croatina LC, P<0.05 and HC, P<0.01; Table V and Fig. 6).
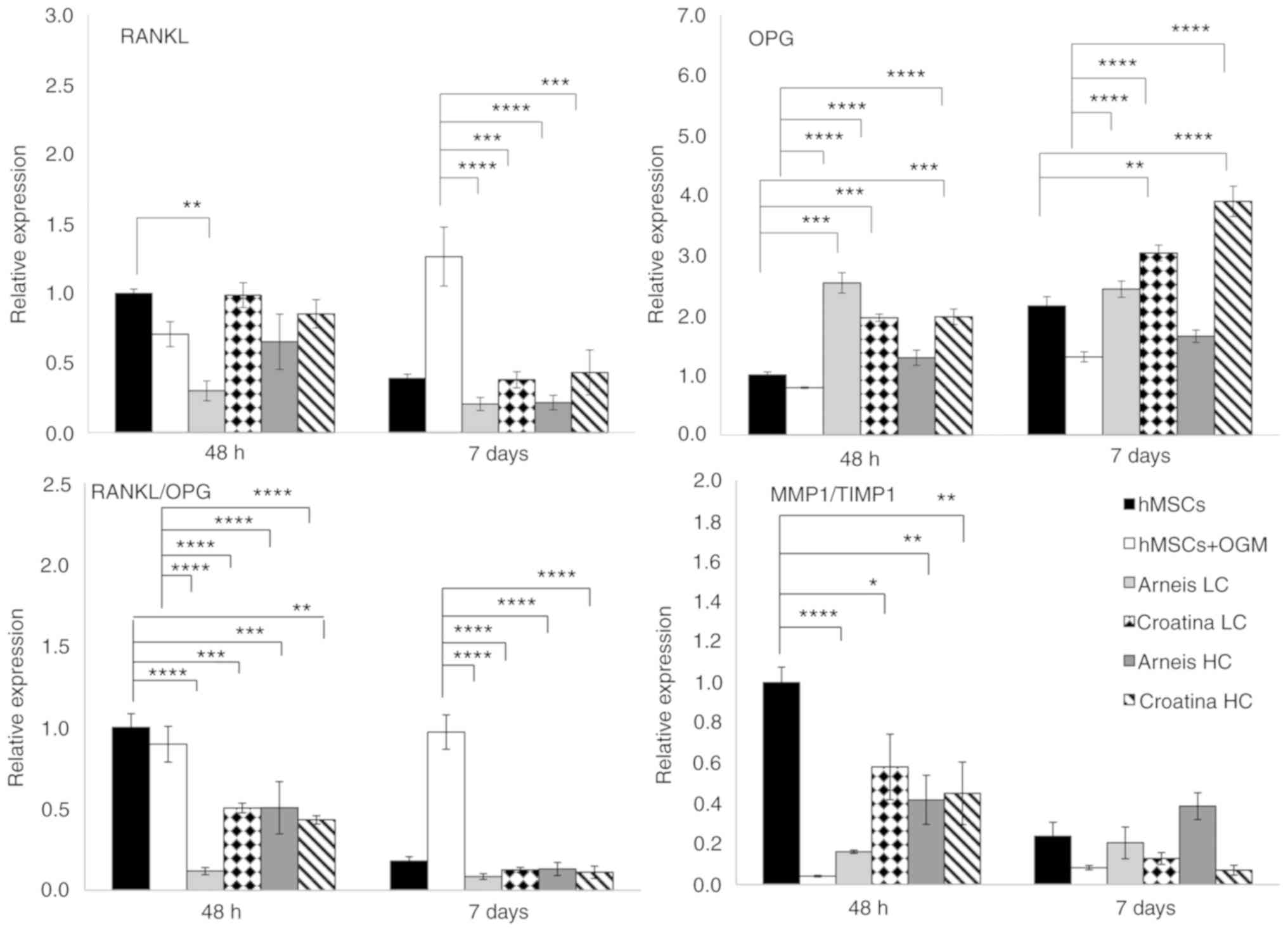 | Figure 6Gene expression analysis of RANKL,
OPG, RANKL/OPG and MMP1/TIMP1 at the mRNA level. Reverse
transcription-quantitative PCR data for RANKL, OPG, RANKL/OPG and
MMP1/TIMP1 genes obtained after 48 h and 7 days for untreated and
treated groups cultured in osteogenic differentiation media or
Arneis and Croatina PRPEs. Values are expressed as relative
expression, compared with the control group. Data are presented as
the mean ± standard error of the mean. *P≤0.05,
**P≤0.01, ***P≤0.001 and
****P≤0.0001. RANKL, receptor activator of nuclear
factor κ-B ligand; OPG, osteoprotegerin; MMP1, metalloproteinase 1;
TIMP1, tissue inhibitor of metalloproteinase 1; PRPEs,
polyphenol-rich pomace extracts. |
 | Table VMMP1/TIMP1 ratio. MMP1 and TIMP1 RQ
ratio. |
Table V
MMP1/TIMP1 ratio. MMP1 and TIMP1 RQ
ratio.
| SAMPLES | RQ values
| MMP1/TIMP1
RATIO |
|---|
| MMP1 | TIMP1 |
|---|
| hMSC-48 h | 1.000 | 1.000 | 1.000 |
| hMSC+OGM-48 h | 0.036 | 0.881 | 0.041 |
| ARNEIS LC-48 h | 0.152 | 0.938 | 0.162 |
| CROATINA LC-48
h | 0.755 | 1.298 | 0.582 |
| ARNEIS HC-48 h | 0.593 | 1.414 | 0.419 |
| CROATINA HC-48
h | 0.582 | 1.290 | 0.451 |
| hMSC-7 days | 0.438 | 1.831 | 0.239 |
| hMSC+OGM-7
days | 0.054 | 0.650 | 0.083 |
| ARNEIS LC-7
days | 0.430 | 2.085 | 0.206 |
| CROATINA LC-7
days | 0.255 | 1.987 | 0.128 |
| ARNEIS HC-7
days | 0.599 | 1.545 | 0.388 |
| CROATINA HC-7
days | 0.131 | 1.866 | 0.070 |
Furthermore, Runx2 analysis showed increased
transcript levels in cells incubated with HC Arneis PRPE (9.5-fold;
P<0.0001) and HC Croatina PRPE (5.7-fold; P<0.0001) at 7
days, as compared with control and osteogenic cells (P<0.0001;
Fig. 5). Another analyzed gene
was BMP2. Only Arneis PRPE induced an increase in the BMP2
expression at LC at 7 days (1.7-fold; P<0.001) and at HC at both
time points (48 h, 2.3-fold; 7 days, 2.7-fold; P<0.0001), as
compared with the control, with a significant difference also when
compared with osteogenic cells, at both time points (48 h,
P<0.01; 7 days, P<0.0001; Fig.
5).
IBSP expression analysis highlighted a tendency of
cells incubated in LC PRPE Arneis and Croatina and in HC PRPE
Croatina (both at 48 h) to maintain a high IBSP expression
(P<0.05 and P<0.01, respectively; Fig. 5), as compared with osteogenic
cells.
Finally, the gene expression of RANKL and its decoy
receptor OPG was evaluated (Fig.
6). In particular, for RANKL levels, only LC Arneis, at 48 h,
induced a statistically significant decrease, as compared with the
control (0.3-fold; P<0.01), whereas all PRPEs, at 7 days and at
both concentrations, reduced RANKL levels, as compared with OGM
cells (LC Arneis, P<0.0001; LC Croatina, P<0.001; HC Arneis,
P<0.0001; HC Croatina, P<0.001). With regard to the OPG
expression, LC Arneis and Croatina at both concentrations at 48 h,
showed increased expression levels (LC Arneis, 2.5-fold; LC
Croatina, 1.9-fold; HC Croatina, 1.9-fold), as compared with the
control (P<0.001 for all), whereas at 7 days, this enhancing
effect was observed only in cells incubated with Croatina at both
concentrations (LC, P<0.01 and HC, P<0.0001; Fig. 6). Of note, as compared with OGM
cells at both time points, these results are of particular
significance (P<0.0001 for all, at both time points) and, by
taking a look at the RANKL/OPG ratio, both concentrations of the 2
PRPEs significantly reduced the RANKL/OPG ratio, as compared with
the control at 48 h (LC Arneis, P<0.0001; LC Croatina,
P<0.001; HC Arneis, P<0.001l HC Croatina, P<0.01; 7 days,
all PRPEs concentrations, P<0.0001) and osteogenic cells at both
time points (P<0.0001; Table
VI and Fig. 6).
 | Table VIRANKL/OPG ratio. RANKL and OPG RQ
ratio. |
Table VI
RANKL/OPG ratio. RANKL and OPG RQ
ratio.
| SAMPLES | RQ values
| RANKL/OPG
RATIO |
|---|
| RANKL | OPG |
|---|
| hMSC-48 h | 1.000 | 1.000 | 1.000 |
| hMSC+OGM-48 h | 0.707 | 0.789 | 0.897 |
| ARNEIS LC-48 h | 0.300 | 2.533 | 0.118 |
| CROATINA LC-48
h | 0.987 | 1.954 | 0.505 |
| ARNEIS HC-48 h | 0.653 | 1.288 | 0.507 |
| CROATINA HC-48
h | 0.852 | 1.970 | 0.433 |
| hMSC-7 days | 0.389 | 2.153 | 0.181 |
| hMSC+OGM-7
days | 1.264 | 1.302 | 0.971 |
| ARNEIS LC-7
days | 0.205 | 2.430 | 0.084 |
| CROATINA LC-7
days | 0.379 | 3.033 | 0.125 |
| ARNEIS HC-7
days | 0.215 | 1.646 | 0.131 |
| CROATINA HC-7
days | 0.432 | 3.894 | 0.111 |
Discussion
Polyphenols have always been recognized as compounds
with health benefits; their well-known anti-oxidant and
anti-inflammatory properties make them attractive therapeutic
agents for different inflammatory conditions (1). Several studies have investigated
their ability to improve and maintain bone health, with different
results showing clear positive effects on osteoblast
differentiation, bone mass regeneration through the enhancement of
osteoblastogenesis and inhibition of osteoclastogenesis and
mitigation of bone loss (59,60).
Direct osteoinductive effects of polyphenols on
osteoblast differentiation, proliferation and protection have been
well documented (21,22) and, as such, several bioactive
polyphenols-coated biomaterials have been engineered (30-38), in order to improve osteogenesis
and bone mineralization. Despite the different content of phenolic
classes in the tested PRPEs, a common trend of hMSC stimulation has
been observed, suggesting that widely available, easy-to-obtain
PRPEs can play a role in the biochemical modification of dental
materials and devices. The PRPEs used in the present study had
different chromatograms, suggesting different molecular
compositions. In particular, extracts from red grape pomace
Croatina are characterized by proanthocyanidins, flavonoids
(flavonols and flavones) and hydroxycinnamic acids. By contrast,
extracts from white grape pomace Arneis are mostly composed by
phenolic and hydroxycinnamic acids and contain a lower number of
anthocyanins, as compared with Croatina PRPE. Proanthocyanidins
were investigated in vivo by Kojima et al (61) and exhibited an increase of total
and cortical bone mass in rat mandibular condyles, in which bone
fragility had been induced.
The importance of polyphenols as bioactive molecules
is represented by their ability to trigger different cell responses
through the activation of diverse biological pathways that
ultimately lead to the modulation of inflammation, oxidative stress
and cell differentiation; thus their application in dental and oral
health could contribute to preventing or treating chronic
pathological conditions, such as periodontal disease. In
particular, polyphenols could enhance periodontal MSC regeneration
properties through both the modulation of the inflammatory
periodontal environment and the stimulation of osteogenic
genes.
In the present study, PRPE actions on hMSCs were
investigated by RT-qPCR, in order to understand the effect of
different groups of polyphenols on osteogenic differentiation; in
fact, in the periodontium, MSCs reside in the periodontal ligament
and alveolar bone and differentiate into bone-forming cells.
Gene expression analysis of hMSCs provided insight
into the effects exerted by PRPEs on the osteogenic differentiation
in hMSCs, with promising results that however need to be further
explored from a mechanistic point of view.
The present results clearly indicated that
polyphenols from Arneis and Croatina extracts display interesting
osteoinductive properties that result in the activation of BMP2 and
Runx2 gene expression, in addition to the stimulation of hMSCs
towards an early osteoblast differentiation stage, as shown by an
increase in the expression of the Col1a1 and SPARC genes [which are
involved in the deposition of extracellular matrix (ECM) that
occurs at the first stages of bone formation] and by the low
transcript levels of the mineralization-related genes alkaline
phosphatase and osteocalcin (OCN; which are expressed at more
mature stages of osteoblast differentiation).
In particular, the BMP2 and Runx2 increase was shown
to be induced by Arneis PRPE, which is particularly rich in the
flavonoid glycoside rutin. Flavonoid glycosides have been shown to
possess a high osteogenic potential related to the stimulation of
BMP2 and Runx2 expression (62-67). More generally, polyphenols are
able to enhance osteoblast activity and differentiation, by
targeting Runx2 through different signaling pathways (16,50,68-70) and by inducing the expression of
bone-matrix related genes (71-74). The different extent of BMP2
expression induced by the 2 PRPEs suggested that their different
phenolic composition might determine the activation of diverse
signaling pathways implicated in the regulation of Runx2
expression. In particular, the higher BMP2 and Runx2 expression in
Arneis PRPE, as compared with Croatina PRPE, could be ascribed to
the higher rutin content in Arneis PRPE, through a possible
mechanism involving the modulation of the ER pathway (75). Indeed, rutin has been shown to
downregulate the RUNX suppressor genes (76) and exert its osteogenic effect
through an ER-mediated mechanism (77), thus providing the basis for
hypothesizing that the increased BMP2 expression is induced by
Arneis PRPE through the activation of the ER (78). Another factor to consider is the
different antioxidant power exhibited by the two extracts. In fact,
given the tendency of flavonoids to interact with each other when
present in a mixture, the global antioxidant power is the result of
synergistic or antagonistic effects between the various possible
combinations of molecules (79).
In light of this, as already shown by Galanakis et al
(80), the lower concentration of
anthocyanidins in Arneis PRPE could account for its higher
antioxidant potential, as compared with Croatina PRPE.
Thanks to their free-radical-scavenging effect,
polyphenols from Arneis and, to a lesser extent, Croatina PRPEs may
exert a pro-osteogenic effect by also regulating mitochondrial
stress; in fact during MSC osteogenic differentiation, mitochondria
require an increased oxygen consumption rate (81).
To investigate the effect of PRPEs on the
inflammation-derived bone resorption, the expression of the key
differentiation factor for osteoclastogenesis, RANKL, was
analyzed.
The present results showed that RANKL is
downregulated by the presence of both PRPEs, with a RANKL/OPG ratio
in favor of OPG. This ratio is decreased, as compared with cells in
both basal medium and OGM. The greater RANKL/OPG ratio observed in
cells with OGM, as compared with cells incubated with PRPEs, can be
explained by both a time-dependent increase of RANKL expression,
induced by different cell maturation stages (82) and by the anti-inflammatory action
of polyphenols (83-86), which determined the RANKL
down-regulation in cells treated with both PRPEs. Notwithstanding
the evidence that RANKL is a member of the tumor necrosis factor
family, which is not only involved in osteoclastogenesis but also
in the regulation of the immune system (87), the absence of data on direct
inflammation is a limitation of this study and needs to be further
investigated.
This is of particular importance because
osteoclastogenesis requires the signaling involving the mediator of
the inflammatory response nuclear factor-κB, activated by RANKL
cytokine, to occur (88).
In addition, both PRPEs exhibited a
concentration-dependent effect on RANKL/OPG ratio, with a different
trend suggesting the presence of multiple and competitive
mechanisms, due to the specific phenolic composition. In
particular, the higher antioxidant power of Arneis PRPE, due to the
different flavonoid: Flavonoid interactions and the presence of
rutin, which has been shown to downregulate the expression of RANKL
cytokine (86), may also account
for the higher RANKL down-regulation, as compared with Croatina
PRPE. These results are in line with those present in the
literature (89,90).
The investigation of MMP expression also elucidated
PRPE anti-inflammatory properties; it was shown that they were
involved in ECM remodeling in both normal (91-93) and pathological (94,95) processes. Along with their
ECM-degrading activity, which gives them a crucial role in
destructive periodontal disease (96), MMPs also display multifaceted
properties involved in the regulation of inflammation; in fact they
have been shown to be implicated in different inflammatory
disorders (97). Furthermore, as
the expression of MMPs and their counteracting inhibitors, TIMPs,
are differentially modulated according to MSC fate commitment
(98), the evaluation of the
MMP/TIMP ratio further clarified PRPE action on hMSCs
differentiation.
In line with previous studies (99-101), polyphenols from both Arneis and
Croatina extracts were able to decrease the MMP/TIMP ratio in the
present study and, in particular, as also shown by Gòmez-Florit
et al (14), the
MMP1/TIMP1 ratio, and to favor hMSCs osteogenic
differentiation.
It is thus not surprising that phenolic compounds
display a potential for their use in the treatment of periodontitis
and, more generally, for restoring and maintaining a healthy oral
bone architecture. In fact, thanks to all these positive
properties, polyphenols are increasingly considered for use in bone
therapeutics, from applications in food and beverages, to
pharmaceuticals, cosmetics and biomedical engineering.
The choice of using a mixture of phenolic compounds
derived from winery wastes aims to give them new life and
valorization, thanks to their content of health-promoting
phytochemicals with proved bioactivity in different physiological
processes (102). In particular,
the grape pomace from a specific grape variety represents a
valuable source of poly-phenols characterized by a distinctive
profile and, possibly, by a specific bioactivity.
It is clear that the different composition of PRPEs
determines different cellular responses in the field of bone
regeneration and specifically, in the treatment of periodontal
disease, with the higher osteogenic potential of Arneis PRPE also
depending on concentration. The use of PRPEs is therefore promising
in the development of biomedical devices which will be
characterized by improved performance, conferred by the presence of
such bioactive phenolic molecules and aimed at enhancing bone
regeneration. Despite the encouraging results, there are some
limitations to this study: First, the preliminary results obtained
in terms of mRNA levels need to be confirmed and made consistent at
in-depth levels through additional investigations, including the
transcriptome and proteome analysis. Second, there is a need to
perform in vivo studies in order to evaluate the
biocompatibility, bioactivity and safety of PRPEs after a detailed
characterization of their biological effects is carried out.
In conclusion, the obtained results showed that
widely different PRPEs, heterogeneous molecular mixtures from
Arneis and Croatina grape varieties, affect hMSC gene expression,
by stimulating differentiation into the osteoblastic lineage.
However, further investigations of the protein levels are needed to
confirm these promising results. In view of the exploitation of
these properties to enhance tissue response to dental implants and
biomaterials, the present results highlighted the main challenges
that will be faced by further studies, be they
surface-immobilization or controlled release.
Supplementary Data
Abbreviations:
|
Col1a1
|
α 1 type 1 collagen
|
|
BMP2
|
bone morphogenetic protein 2
|
|
DPPH
|
2,2-diphenyl-1-picrylhydrazyl
|
|
FC
|
folin-Ciocalteu method
|
|
GAE
|
gallic acid equivalents
|
|
HPLC
|
high-performance liquid
chromatography
|
|
hMSCs
|
human mesenchymal cells
|
|
iBSP
|
integrin-binding sialoprotein
|
|
MMPs
|
metalloproteinases
|
|
OCN
|
osteocalcin
|
|
OPG
|
osteoprotegerin
|
|
PRPEs
|
polyphenol-rich pomace extracts
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
RANKL
|
receptor activator of nuclear factor
κ-B ligand
|
|
Runx2
|
runt-related transcription factor
2
|
|
SPARC
|
secreted protein acidic and cysteine
rich
|
|
TIMPs
|
tissue inhibitor of
metalloproteinases
|
|
YWHAZ
|
tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein ζ
|
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ET, GI, CC, MM and NR conceived of the study; ET,
GI, CC and MM conceived of the methodology; ET and GI performed the
formal analysis; ET, GI, CC and MM performed the investigations; MM
provided the resources; ET and GI performed data curation; ET, GI
and MM wrote and drafted the manuscript; CC wrote, reviewed and
edited the manuscript; MM performed project administration; CC and
MM acquired the funding. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Cells were obtained using Institutional Review Board
(IRB)-approved consent forms and protocols (BIOMED IRB-Bone marrow
collection for therapeutic or non-therapeutic use-Protocol number
701-01).
Patient consent for publication
Not applicable.
Competing interests
MM and CC own shares of the funding company Nobil
Bio Ricerche srl. ET and GI are employees of Nobil Bio Ricerche
srl.
References
|
1
|
Palaska I, Papathanasiou E and Theoharides
TC: Use of polyphenols in periodontal inflammation. Eur J
Pharmacol. 720:77–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sankari SL, Babu NA, Rani V, Priyadharsini
C and Masthan KM: Flavonoids-clinical effects and applications in
dentistry: A review. J Pharm Bioallied Sci. 6(Suppl 1): S26–S29.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bunte K, Hensel A and Beikler T:
Polyphenols in the prevention and treatment of periodontal disease:
A systematic review of in vivo, ex vivo and in vitro studies.
Fitoterapia. 132:30–39. 2019. View Article : Google Scholar
|
|
4
|
Nazir MA: Prevalence of periodontal
disease, its association with systemic diseases and prevention. Int
J Health Sci (Qassim). 11:72–80. 2017.
|
|
5
|
Özden FO, Sakallioğlu EE, Sakallioğlu U,
Ayas B and Erişgin Z: Effects of grape seed extract on periodontal
disease: An experimental study in rats. J Appl Oral Sci.
25:121–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gennaro G, Claudino M, Cestari TM, Ceolin
D, Germino P, Garlet GP and de Assis GF: Green tea modulates
cytokine expression in the periodontium and attenuates alveolar
bone resorption in type 1 diabetic rats. PLoS One. 10:e01347842015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tominari T, Hirata M, Matsumoto C, Inada M
and Miyaura C: Polymethoxy flavonoids, nobiletin and tangeretin,
prevent lipopolysaccharide-induced inflammatory bone loss in an
experimental model for periodontitis. J Pharmacol Sci. 119:390–394.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernández-Rojas B and Gutiérrez-Venegas G:
Flavonoids exert multiple periodontic benefits including
anti-inflammatory, periodontal ligament-supporting, and alveolar
bone-preserving effects. Life Sci. 209:435–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Houde V, Grenier D and Chandad F:
Protective effects of grape seed proanthocyanidins against
oxidative stress induced by lipopolysaccharides of
periodontopathogens. J Periodontol. 77:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Govindaraj J, Emmadi P, Deepalakshmi,
Rajaram V, Prakash G and Puvanakrishnan R: Protective effect of
proanthocyanidins on endotoxin induced experimental periodontitis
in rats. Indian J Exp Biol. 48:133–142. 2010.PubMed/NCBI
|
|
11
|
Shen CL, Wang P, Guerrieri J, Yeh JK and
Wang JS: Protective effect of green tea polyphenols on bone loss in
middle-aged female rats. Osteoporos Int. 19:979–990. 2008.
View Article : Google Scholar
|
|
12
|
Lee JH, Jin H, Shim HE, Kim HN, Ha H and
Lee ZH: Epigallocatechin-3-gallate inhibits osteoclastogenesis by
down-regulating c-Fos expression and suppressing the nuclear
factor-kappaB signal. Mol Pharmacol. 77:17–25. 2010. View Article : Google Scholar
|
|
13
|
Nakamura H, Ukai T, Yoshimura A, Kozuka Y,
Yoshioka H, Yoshinaga Y, Abe Y and Hara Y: Green tea catechin
inhibits lipo-polysaccharide-induced bone resorption in vivo. J
Periodontal Res. 45:23–30. 2010. View Article : Google Scholar
|
|
14
|
Gómez-Florit M, Monjo M and Ramis JM:
Identification of quercitrin as potential therapeutic agent for
periodontal applications. J Periodontol. 85:966–974. 2014.
View Article : Google Scholar
|
|
15
|
Chen JR, Lazarenko OP, Wu X, Kang J,
Blackburn ML, Shankar K, Badger TM and Ronis MJ: Dietary-induced
serum phenolic acids promote bone growth via p38 MAPK/β-catenin
canonical Wnt signaling. J Bone Miner Res. 25:2399–2411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bu SY, Hunt TS and Smith BJ: Dried plum
polyphenols attenuate the detrimental effects of TNF-alpha on
osteoblast function coincident with up-regulation of Runx2, Osterix
and IGF-I. J Nutr Biochem. 20:35–44. 2009. View Article : Google Scholar
|
|
17
|
Trzeciakiewicz A, Habauzit V, Mercier S,
Lebecque P, Davicco MJ, Coxam V, Demigne C and Horcajada MN:
Hesperetin stimulates differentiation of primary rat osteoblasts
involving the BMP signalling pathway. J Nutr Biochem. 21:424–431.
2010. View Article : Google Scholar
|
|
18
|
Byun MR, Sung MK, Kima AR, Lee CH, Jang
EJ, Jeong MG, Noh M, Hwang ES and Hong JH: (-)-Epicatechin gallate
(ECG) stimulates osteoblast differentiation via Runt-related
transcription factor 2 (RUNX2) and transcriptional coactivator with
PDZ-binding motif (TAZ)-mediated transcriptional activation. J Biol
Chem. 289:9926–9935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santiago-Mora R, Casado-Díaz A, De Castro
MD and Quesada-Gómez JM: Oleuropein enhances osteoblastogenesis and
inhibits adipogenesis: The effect on differentiation in stem cells
derived from bone marrow. Osteoporos Int. 22:675–684. 2011.
View Article : Google Scholar
|
|
20
|
Patisaul HB and Jefferson W: The pros and
cons of phytoestrogens. Front Neuroendocrinol. 31:400–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torre E: Molecular signaling mechanisms
behind polyphenol-induced bone anabolism. Phytochem Rev.
16:1183–1226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torre E, Iviglia G, Cassinelli C and Morra
M: Potentials of poly-phenols in bone-implant devices. Polyphenols.
Wong J: IntechOpen; 2018, https://www.intechopen.com/books/polyphe-nols/potentials-of-polyphenols-in-bone-implant-devices.
Accessed April 11, 2018. View Article : Google Scholar
|
|
23
|
Sheikh Z, Sima C and Glogauer M: Bone
replacement materials and techniques used for achieving vertical
alveolar bone augmentation. Materials. 8:2953–2993. 2015.
View Article : Google Scholar
|
|
24
|
Rodriguez Baena RY, Rizzo S, Manzo L and
Lupi SM: Nanofeatured titanium surfaces for dental implantology:
Biological effects, biocompatibility, and safety. J Nanomater.
2017:182017. View Article : Google Scholar
|
|
25
|
Morra M: Biomolecular modification of
implant surfaces. Expert Rev Med Devices. 4:36–372. 2007.
View Article : Google Scholar
|
|
26
|
Brett E, Flacco J, Blackshear C, Longaker
MT and Wan DC: Biomimetics of bone implants: The regenerative road.
Biores Open Access. 6:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morra M, Cassinelli C, Torre E and Iviglia
G: Permanent wettability of a novel, nanoengineered, clinically
available, hyaluronan-coated dental implant. Clin Exp Dent Res.
4:196–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bryers JD, Giachelli CM and Ratner BD:
Engineering biomaterials to integrate and heal: The
biocompatibility paradigm shifts. Biotechnol Bioeng. 109:1898–1911.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Insua A, Monje A, Wang HL and Miron RJ:
Basis of bone metabolism around dental implants during
osseointegration and peri-implant bone loss. J Biomed Mater Res A.
105:2075–2089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Ferraris S, Prenesti E and Verné
E: Surface functionalization of bioactive glasses with natural
molecules of biological significance, part I: Gallic acid as model
molecule. Appl Surf Sci. 2013.
|
|
31
|
Zhang X, Ferraris S, Prenesti E and Verné
E: Surface functionalization of bioactive glasses with natural
molecules of biological significance, part II: Grafting of
polyphenols extracted from grape skin. Appl Surf Sci. 287:341–348.
2013. View Article : Google Scholar
|
|
32
|
Córdoba A, Satué M, Gómez-Florit M,
Hierro-Oliva M, Petzold C, Lyngstadaas SP, González-Martín ML,
Monjo M and Ramis JM: Flavonoid-modified surfaces: Multifunctional
bioactive biomaterials with osteopromotive, anti-inflammatory, and
anti-fibrotic potential. Adv Healthc Mater. 4:540–549. 2015.
View Article : Google Scholar
|
|
33
|
Gomez-Florit M, Pacha-Olivenza MA,
Fernández-Calderón MC, Córdoba A, González-Martín ML, Monjo M and
Ramis JM: Quercitrin-nanocoated titanium surfaces favour gingival
cells against oral bacteria. Sci Rep. 6:224442016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cazzola M, Corazzari I, Prenesti E,
Bertone E, Vernè E and Ferraris S: Bioactive glass coupling with
natural polyphenols: Surface modification, bioactivity and
anti-oxidant ability. Appl Surf Sci. 367:237–248. 2016. View Article : Google Scholar
|
|
35
|
Cazzola M, Vernè E, Cochis A, Sorrentino
R, Azzimonti BC, Prenesti E, Rimondini L and Ferraris S: Bioactive
glasses functionalized with polyphenols: In vitro interactions with
healthy and cancerous osteoblast cells. J Mater Sci. 52:2017.
View Article : Google Scholar
|
|
36
|
Cazzola M, Ferraris S, Boschetto F,
Rondinella A, Marin E, Zhu W, Pezzotti G, Vernè E and Spriano S:
Green tea polyphenols coupled with a bioactive titanium alloy
surface: In vitro characterization of osteoinductive behavior
through a KUSA A1 cell study. Int J Mol Sci. 19:E22552018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuchiya S, Sugimoto K, Kamio H, Okabe K,
Kuroda K, Okido M and Hibi H: Kaempferol-immobilized titanium
dioxide promotes formation of new bone: Effects of loading methods
on bone marrow stromal cell differentiation in vivo and in vitro.
Int J Nanomedicine. 13:1665–1676. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iviglia G, Bollati D, Cassinelli C, Torre
E and Morra M: Dreamer: An Innovative Bone Filler Paste For The
Treatment Of Periodontitis. In: Dreamer: An Innovative Bone Filler
Paste For The Treatment Of Periodontitis. In: Front. Bioeng.
Biotechnol. Conference Abstract: 10th World Biomaterials Congress;
2016
|
|
39
|
Kallithraka S, Garcia-Viguera C, Bridle P
and Bakker J: Survey of solvents for the extraction of grape seed
phenolics. Phytochem Anal. 6:265–267. 1995. View Article : Google Scholar
|
|
40
|
Pekić B, Kovač V, Alonso E and Revilla E:
Study of the extraction of proanthocyanidins from grape seeds. Food
Chem. 61:201–206. 1998. View Article : Google Scholar
|
|
41
|
Shi J, Yu J, Pohorly J, Young JC, Bryan M
and Wu Y: Optimization of the extraction of polyphenols from grape
seed meal by aqueous ethanol solution. J Food Agric Environ.
1:42–47. 2003.
|
|
42
|
Neveu V, Perez-Jiménez J, Vos F, Crespy V,
du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D and
Scalbert A: Phenol-Explorer: An online comprehensive database on
poly-phenol contents in foods. Database (Oxford). 2010. pp.
bap0242010, View Article : Google Scholar
|
|
43
|
Rothwell JA, Urpi-Sarda M, Boto-Ordoñez M,
Knox C, Llorach R, Eisner R, Cruz J, Neveu V, Wishart D, Manach C,
et al: Phenol-Explorer 20: A major update of the Phenol-Explorer
database integrating data on polyphenol metabolism and
pharmacokinetics in humans and experimental animals. Database
(Oxford). 2012. pp. bas0312012, View Article : Google Scholar
|
|
44
|
Rothwell JA, Pérez-Jiménez J, Neveu V,
Medina-Remón A, M'hiri N, García-Lobato P, Manach C, Knox C, Eisner
R, Wishart DS and Scalbert A: Phenol-Explorer 30: A major update of
the Phenol-Explorer database to incorporate data on the effects of
food processing on polyphenol content. Database (Oxford). 2013. pp.
bat0702013, View Article : Google Scholar
|
|
45
|
Arumugam B, Balagangadharan K and
Selvamurugan N: Syringic acid, a phenolic acid, promotes osteoblast
differentiation by stimulation of Runx2 expression and targeting of
Smad7 by miR-21 in mouse mesenchymal stem cells. J Cell Commun
Signal. 12:561–573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gu Q, Cai Y, Huang C, Shi Q and Yang H:
Curcumin increases rat mesenchymal stem cell osteoblast
differentiation but inhibits adipocyte differentiation. Pharmacogn
Mag. 8:202–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin SY, Kang L, Wang CZ, Huang HH, Cheng
TL, Huang HT, Lee MJ, Lin YS, Ho ML, Wang GJ and Chen CH:
(-)-Epigallocatechin-3-gallate (EGCG) enhances osteogenic
differentiation of human bone marrow mesenchymal stem cells.
Molecules. 23:E32212018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang J, Wu K, Xu T, Wu J, Li P, Wang H,
Wu H and Wu G: Epigallocatechin-3-gallate enhances the
osteoblastogenic differentiation of human adipose-derived stem
cells. Drug Des Devel Ther. 13:1311–1321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Song LH, Pan W, Yu YH, Quarles LD, Zhou HH
and Xiao ZS: Resveratrol prevents CsA inhibition of proliferation
and osteoblastic differentiation of mouse bone marrow-derived
mesenchymal stem cells through an ER/NO/cGMP pathway. Toxicol In
Vitro. 20:915–922. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dai Z, Li Y, Quarles LD, Song T, Pan W,
Zhou H and Xiao Z: Resveratrol enhances proliferation and
osteoblastic differentiation in human mesenchymal stem cells via
ER-dependent ERK1/2 activation. Phytomedicine. 14:806–814. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wittenauer J, MäcKle S, Sußmann D,
Schweiggert-Weisz U and Carle R: Inhibitory effects of polyphenols
from grape pomace extract on collagenase and elastase activity.
Fitoterapia. 101:179–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brand-Williams W, Cuvelier ME and Berset
C: Use of a free radical method to evaluate antioxidant activity.
LWT-Food Sci Technol. 28:25–30. 1995. View Article : Google Scholar
|
|
53
|
Ribéreau-Gayon P and Stonestreet E:
Determination of anthocyanins in red wine. Bull Soc Chim Fr.
9:2649–2652. 1965.In French.
|
|
54
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
55
|
Hammer Ø, Harper D and Ryan P: Past:
Paleontological statistics software package for education and data
analysis. Paleontol Electron. 4:92001.
|
|
56
|
Santos J, Oliveira MB, Ibáñez E and
Herrero M: Phenolic profile evolution of different ready-to-eat
baby-leaf vegetables during storage. J Chromatogr A. 1327:118–131.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dolan JW: Gradient Elution, Part V:
Baseline Drift Problems. LCGC North Am. 31:538–543. 2013.
|
|
58
|
Snyder LR, Kirkland JJ and Dolan JW:
Introduction to Modern Liquid Chromatography. 3rd Edition. Wiley;
2010
|
|
59
|
Austermann K, Baecker N, Stehle P and Heer
M: Putative effects of nutritive polyphenols on bone metabolism in
vivo-evidence from human studies. Nutrients. 11:E8712019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Trzeciakiewicz A, Habauzit V and Horcajada
MN: When nutrition interacts with osteoblast function: Molecular
mechanisms of polyphenols. Nutr Res Rev. 22:68–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kojima K, Maki K, Tofani I, Kamitani Y and
Kimura M: Effects of grape seed proanthocyanidins extract on rat
mandibular condyle. J Musculoskelet Neuronal Interact. 4:301–307.
2004.PubMed/NCBI
|
|
62
|
Huang JM, Bao Y, Xiang W, Jing XZ, Guo JC,
Yao XD, Wang R and Guo FJ: Icariin Regulates the Bidirectional
Differentiation of Bone Marrow Mesenchymal Stem Cells through
Canonical Wnt Signaling Pathway. Evid Based Complement Alternat
Med. 2017:80853252017. View Article : Google Scholar
|
|
63
|
Ma HP, Ming LG, Ge BF, Zhai YK, Song P,
Xian CJ and Chen KM: Icariin is more potent than genistein in
promoting osteoblast differentiation and mineralization in vitro. J
Cell Biochem. 112:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hsieh TP, Sheu SY, Sun JS, Chen MH and Liu
MH: Icariin isolated from Epimedium pubescens regulates osteoblasts
anabolism through BMP-2, SMAD4, and Cbfa1 expression.
Phytomedicine. 17:414–423. 2010. View Article : Google Scholar
|
|
65
|
Trzeciakiewicz A, Habauzit V, Mercier S,
Barron D, Urpi-Sarda M, Manach C, Offord E and Horcajada MN:
Molecular mechanism of hesperetin-7-O-glucuronide, the main
circulating metabolite of hesperidin, involved in osteoblast
differentiation. J Agric Food Chem. 58:668–675. 2010. View Article : Google Scholar
|
|
66
|
Sheng H, Zhang G, Wang X, Lee K, Yao X,
Leung K, Li G and Qin L: Phytochemical molecule icariin stimulates
osteogenic but inhibits adipogenic differentiation of mesenchymal
stem cells. Bone. 43(Suppl 1): S42–S43. 2008. View Article : Google Scholar
|
|
67
|
Wei Q, Zhang J, Hong G, Chen Z, Deng W, He
W and Chen MH: Icariin promotes osteogenic differentiation of rat
bone marrow stromal cells by activating the ERα-Wnt/β-catenin
signaling pathway. Biomed Pharmacother. 84:931–939. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh
CF, Kuo ML and Yen ML: Resveratrol promotes osteogenesis of human
mesenchymal stem cells by upregulating RUNX2 gene expression via
the SIRT1/FOXO3A axis. J Bone Miner Res. 26:2552–2563. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dai J, Li Y, Zhou H, Chen J, Chen M and
Xiao Z: Genistein promotion of osteogenic differentiation through
BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci. 9:1089–1098. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yang L, Takai H, Utsunomiya T, Li X, Li Z,
Wang Z, Wang S, Sasaki Y, Yamamoto H and Ogata Y: Kaempferol
stimulates bone sialoprotein gene transcription and new bone
formation. J Cell Biochem. 110:1342–1355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jung WW: Protective effect of apigenin
against oxidative stress-induced damage in osteoblastic cells. Int
J Mol Med. 33:1327–1334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ying X, Sun L, Chen X, Xu H, Guo X, Chen
H, Hong J, Cheng S and Peng L: Silibinin promotes osteoblast
differentiation of human bone marrow stromal cells via bone
morphogenetic protein signaling. Eur J Pharmacol. 721:225–230.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao L, Wang Y, Wang Z, Xu Z, Zhang Q and
Yin M: Effects of dietary resveratrol on excess-iron-induced bone
loss via antioxidative character. J Nutr Biochem. 26:1174–1182.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kim JM, Lee SU, Kim YS, Min YK and Kim SH:
Baicalein stimulates osteoblast differentiation via coordinating
activation of MAP kinases and transcription factors. J Cell
Biochem. 104:1906–1917. 2008.PubMed/NCBI
|
|
75
|
Liu H, Zhong L, Zhang Y, Liu X and Li J:
Rutin attenuates cerebral ischemia/reperfusion injury in
ovariectomized rats via estrogen receptor-mediated BDNF-TrkB and
NGF-TrkA signaling. Biochem Cell Biol. 96:672–681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Abdel-Naim AB, Alghamdi AA, Algandaby MM,
Al-Abbasi FA, Al-Abd AM, Eid BG, Abdallah HM and El-Halawany AM:
Rutin isolated from Chrozophora tinctoria enhances bone cell
proliferation and ossification markers. Oxid Med Cell Longev.
2018:51064692018. View Article : Google Scholar :
|
|
77
|
Rassi CM, Lieberherr M, Chaumaz G,
Pointillart A and Cournot G: Modulation of osteoclastogenesis in
porcine bone marrow cultures by quercetin and rutin. Cell Tissue
Res. 319:383–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhou S, Turgeman G, Harris SE, Leitman DC,
Komm BS, Bodine PV and Gazit D: Estrogens activate bone
morphogenetic protein-2 gene transcription in mouse mesenchymal
stem cells. Mol Endocrinol. 17:56–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hidalgo M, Sánchez-Moreno C and de
Pascual-Teresa S: Flavonoid-flavonoid interaction and its effect on
their antioxidant activity. Food Chem. 121:691–696. 2010.
View Article : Google Scholar
|
|
80
|
Galanakis CM, Kotanidis A, Dianellou M and
Gekas V: Phenolic content and antioxidant capacity of Cypriot
wines. Czech J Food Sci. 33:126–136. 2015. View Article : Google Scholar
|
|
81
|
Gao J, Feng Z, Wang X, Zeng M, Liu J, Han
S, Xu J, Chen L, Cao K, Long J, et al: SIRT3/SOD2 maintains
osteoblast differentiation and bone formation by regulating
mitochondrial stress. Cell Death Differ. 25:229–240. 2018.
View Article : Google Scholar :
|
|
82
|
Giner M, Montoya MJ, Vázquez MA, Rios MJ,
Moruno R, Miranda MJ and Pérez-Cano R: Modifying RANKL/OPG mRNA
expression in differentiating and growing human primary
osteoblasts. Horm Metab Res. 40:869–874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shakibaei M, Shayan P, Busch F, Aldinger
C, Buhrmann C, Lueders C and Mobasheri A: Resveratrol mediated
modulation of Sirt-1/Runx2 promotes osteogenic differentiation of
mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS
One. 7:e357122012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang JF, Li G, Meng CL, Dong Q, Chan CY,
He ML, Leung PC, Zhang YO and Kung HF: Total flavonoids of Herba
Epimedii improves osteogenesis and inhibits osteoclastogenesis of
human mesenchymal stem cells. Phytomedicine. 16:521–529. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Marini H, Minutoli L, Polito F, Bitto A,
Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A,
Frisina N, et al: OPG and sRANKL serum concentrations in
osteopenic, postmenopausal women after 2-year genistein
administration. J Bone Miner Res. 23:715–720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Napimoga MH, Clemente-Napimoga JT, Macedo
CG, Freitas FF, Stipp RN, Pinho-Ribeiro FA, Casagrande R and Verri
WA Jr: Quercetin inhibits inflammatory bone resorption in a mouse
periodontitis model. J Nat Prod. 76:2316–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Papadaki M, Rinotas V, Violitzi F, Thireou
T, Panayotou G, Samiotaki M and Douni E: New insights for RANKL as
a proinflammatory modulator in modeled inflammatory arthritis.
Front Immunol. 10:972019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Boyce BF, Xiu Y, Li J, Xing L and Yao Z:
NF-κB-mediated regulation of osteoclastogenesis. Endocrinol Metab
(Seoul). 30:35–44. 2015. View Article : Google Scholar
|
|
89
|
Bu SY, Lerner M, Stoecker BJ, Boldrin E,
Brackett DJ, Lucas EA and Smith BJ: Dried plum polyphenols inhibit
osteoclastogenesis by downregulating NFATc1 and inflammatory
mediators. Calcif Tissue Int. 82:475–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sheu SY, Tsai CC, Sun JS, Chen MH, Liu MH
and Sun MG: Stimulatory effect of puerarin on bone formation
through co-activation of nitric oxide and bone morphogenetic
protein-2/mitogen-activated protein kinases pathways in mice. Chin
Med J (Engl). 125:3646–3653. 2012.
|
|
91
|
Mauney J and Volloch V: Adult human bone
marrow stromal cells regulate expression of their MMPs and TIMPs in
differentiation type-specific manner. Matrix Biol. 29:3–8. 2010.
View Article : Google Scholar
|
|
92
|
Kobayashi T, Kishimoto J, Ge Y, Jin W,
Hudson DL, Ouahes N, Ehama R, Shinkai H and Burgeson RE: A novel
mechanism of matrix metalloproteinase-9 gene expression implies a
role for keratinization. EMBO Rep. 2:604–608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Philips N, Auler S, Hugo R and Gonzalez S:
Beneficial regulation of matrix metalloproteinases for skin health.
Enzyme Res. 2011:4272852011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Garlet GP, Martins W Jr, Fonseca BA,
Ferreira BR and Silva JS: Matrix metalloproteinases, their
physiological inhibitors and osteoclast factors are differentially
regulated by the cytokine profile in human periodontal disease. J
Clin Periodontol. 31:671–679. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lazăr L, Loghin A, Bud ES, Cerghizan D,
Horváth E and Nagy EE: Cyclooxygenase-2 and matrix
metalloproteinase-9 expressions correlate with tissue inflammation
degree in periodontal disease. Rom J Morphol Embryol. 56:1441–1446.
2015.
|
|
96
|
Sorsa T, Mäntylä P, Tervahartiala T,
Pussinen PJ, Gamonal J and Hernandez M: MMP activation in
diagnostics of periodontitis and systemic inflammation. J Clin
Periodontol. 38:817–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nissinen L and Kähäri VM: Matrix
metalloproteinases in inflammation. Biochim Biophys Acta.
1840:2571–2580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Almalki SG and Agrawal DK: Effects of
matrix metalloproteinases on the fate of mesenchymal stem cells.
Stem Cell Res Ther. 7:1292016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS,
Chai JK, Kim CK and Choi SH: Inhibitory effects of green tea
polyphenol (-)-epigallocatechin gallate on the expression of matrix
metal-loproteinase-9 and on the formation of osteoclasts. J
Periodontal Res. 39:300–307. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang D, Wang Y, Xu S, Wang F, Wang B, Han
K, Sun D and Li L: Epigallocatechin-3-gallate protects against
hydrogen peroxide-induced inhibition of osteogenic differentiation
of human bone marrow-derived mesenchymal stem cells. Stem Cells
Int. 2016:75327982016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gómez-Florit M, Monjo M and Ramis JM:
Quercitrin for periodontal regeneration: Effects on human gingival
fibroblasts and mesenchymal stem cells. Sci Rep. 5:165932015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vauzour D, Rodriguez-Mateos A, Corona G,
Oruna-Concha MJ and Spencer JP: Polyphenols and human health:
Prevention of disease and mechanisms of action. Nutrients.
2:1106–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|