Introduction
Ischaemia-reperfusion (I/R) injury is the most
important and common cause of myocardial damage and subsequent
heart failure worldwide (1,2).
Myocardial I/R injury may induce cell apoptosis and autophagy by
activating oxidative stress and upregulating inflammatory
mediators, ultimately resulting in irreversible fibrotic damage
(3). However, despite numerous
studies on myocardial I/R injury, deeper insight into the
underlying mechanisms of myocardial I/R injury is needed.
MicroRNAs (miRNAs) are small endogenous RNAs that
are associated with tumourigenesis, cell proliferation and
apoptosis (4,5). Generally, the regulation of target
genes by miRNAs occurs through binding with the 3′-untranslated
region (UTR). Recently, miRNAs were also found to be associated
with myocardial I/R injury (6,7).
miR-132 was found to be involved in diseases such as epilepsy
(8), prostate cancer (9) and hepatocellular carcinoma (10). It was previously demonstrated that
targeting of a Na+-Ca2+ exchanger by miR-132
was able to prevent apoptosis of cardiomyocytes under hypoxic
conditions (11). It was also
reported that miR-132 was upregulated in simulated ischaemic injury
in cultured hippocampal neurons (12). However, few studies have focused
on the role of miR-132 in myocardial I/R injury and the underlying
mechanisms.
Sirtuin 1 (SIRT1), a member of the sirtuin family,
regulates the cellular ageing process, participates in metabolic
diseases, such as diabetes, and is related to cancer development
(13). It has been reported that
SIRT1/peroxisome proliferator-activated receptor gamma coactivator
(PGC)-1α/nuclear factor erythroid-2-related factor 2 (Nrf2)
signalling can mediate oxidative stress, which plays an important
role in myocardial I/R injury (14,15). SIRT1 and PGC-1α/Nrf2 signalling
were also reported to be involved in myocardial I/R injury
(16). In addition, miR-132 is
also involved in several cancers, such as lymphocytic leukaemia and
gastric cancer, as well as in Alzheimer’s disease by targeting
SIRT1 (17,18). However, no study has yet focused
on the role of the miR-132/SIRT1 axis in myocardial I/R injury.
Pyroptosis is considered an inherently inflammatory
process of NLRP3/caspase-1-dependent programmed cell death
(19,20). Pyroptosis was found to be
associated with cell apoptosis, oxidative stress and autophagy
(21). An association between
pyroptosis and the PGC-1α/Nrf2 signalling pathway was also
demonstrated a (22). However,
few studies have focused on the role of pyroptosis in myocardial
I/R injury and its possible association with the miR-132/SIRT1
axis.
To the best of our knowledge, the present study was
the first to investigate the role of miR-132 in myocardial I/R
injury and the underlying mechanisms. The aim of the study was to
provide deeper insight into the role of miR-132 in myocardial I/R
and identify new treatment targets for myocardial I/R injury.
Materials and methods
Animals and myocardial I/R model
establishment
A total of 14 C57BL/J6 mice were obtained from the
SJA Laboratory Animal Company (Hunan, China). All mice were aged
>8 weeks and weighed 20-30 g. All animals were housed under
controlled temperature conditions (23-25°C) and had free access to
food and water. Animal handling conformed to the Guidelines for the
Care and Use of Laboratory Animals. The study protocol was approved
by the Institutional Animal Care Committee at Tongji Medical
College of Huazhong University of Science and Technology (Hubei,
China).
After 4 days of feeding, the animals were randomized
into two groups (7 mice per group): The sham and myocardial I/R
groups. To establish the myocardial I/R model, the well-established
left anterior descending (LAD) coronary artery ligation model was
used as described previously (23). Briefly, the mice were
anaesthetized using 4% isoflurane with oxygen for 2 min and then
maintained with 1.5% isoflurane with oxygen throughout the
following surgery, and an incision was made at the fourth
intercostal space. The mice were then subjected to 45 min of
transitory ligation on the LAD coronary artery followed by
reperfusion for 3 h. Occlusion was confirmed by blanching of the
left ventricular myocardium below the suture. The sham operation
was conducted using the same procedure, except for the placement of
the ligature. The animals were then sacrificed by cervical
dislocation, and the myocardial tissues and blood samples were
collected and stored.
Histological analysis
For histological analysis, all myocardial tissues
were fixed with 10% formalin for 24 h at room temperature, embedded
in paraffin, and then cut into 5-µm sections. The sections were
stained with haematoxylin and eosin (H&E) for 15 min at room
temperature and then scanned and photographed using a Leica DFC280
light microscope (Leica Micros Imaging Solutions Ltd.).
Measurement of myocardial enzymes,
inflammatory and oxidative stress factors
The serum levels of the myocardial enzymes creatine
kinase (CK), CK myocardial band isoenzyme (CK-MB), lactate
dehydrogenase (LDH), hydroxybutyrate dehydrogenase (HBDH) and
ischaemia-modified albumin (IMA) were determined using commercial
kits purchased from Maccura Biotechnology. The serum levels of the
inflammatory factors interleukin (IL)-1β, IL-6, and tumour necrosis
factor (TNF)-α were all determined using commercially available
ELISA kits (IL-1β, ab197742; IL-6, ab100713; and TNF-α, ab208348)
according to the manufacturer’s instructions (Abcam). The levels of
malondialdehyde (MDA) and superoxide dismutase (SOD) in myocardial
tissues were measured using MDA and SOD kits (Nanjing Jiancheng
Bio-Technology Co., Ltd.), respectively, as described previously
(24).
Cell culture and transfection
The myocardial cell line H9C2 was purchased from
ATCC. Briefly, cells were cultured in RPMI-1640 (Thermo Fisher
Scientific, Inc.) supplemented with 10% Gibco® foetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C and 5%
CO2. Cells were divided into the following groups: i)
Control group, ii) hypoxia/reoxygenation (H/R) model group, iii)
H/R + inhibitor NC group, iv) H/R + miR-132 inhibitor group, and v)
H/R + miR-132 inhibitor + EX527 group. To establish the in
vitro myocardial I/R model, the H/R method was used. Briefly,
cells were placed in a hypoxia cabin under 2% O2, 93%
N2 and 5% CO2 for 2 h, and then cultured
under normoxic conditions (21% O2, 5% CO2 and
74% N2) for 4 h. For cell transfection, the cells were
transfected with miR-132 mimics or inhibitor (5 nM, GeneChem Corp.)
or their negative control (NC) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer’s instructions. To inhibit the expression of SIRT1,
the SIRT1 inhibitor EX527 (10 µM, Sigma-Aldrich; Merck KGaA) was
used to treat the cells. After 48 h of transfection, the
transfected cells were harvested and subjected to further
experiments. The sequences of miR-132 mimics, mimics NC, miR-132
inhibitor and inhibitor NC were as follows: miR-132 mimics: Sense,
5′-UAACAGUCUACAGCCAUGGUCG-3′ and antisense,
5′-CGACCAUGGCUGUAGACUGUUU-3′; mimics NC: Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. miR-132 inhibitor:
5′-AGUAACAAUCGAAAGCCACGGU-3′; inhibitor NC:
5′-CAGUACUUUUGUGUAGUACAA-3′.
Cell pyroptosis analysis
For cell pyroptosis analysis, the in vitro
FAM-FLICA Caspase-1 Detection kit (ImmunoChemistry Technologies,
LLC) was used according to the manufacturer’s instructions.
Briefly, the cells were harvested and washed with PBS.
Subsequently, the cells were stained with 2 µg/ml PI and 10
µl FAM-FLICA. In each analysis, 20,000 gated events were
recorded. The fluorescence intensity was measured using a
FACSCalibur II flow cytometer and CellQuest software, version 5.1
(BD Biosciences).
Dual luciferase reporter assay
To confirm the SIRT1 3′-UTR as a target of miR-132,
a dual luciferase reporter assay was conducted. Briefly, the
wild-type (WT) or mutant (MUT) 3′-UTR of SIRT1 was sub-cloned into
the pGL4.10 luciferase reporter vector. Cells were then
co-transfected with the vectors, the miR-132 mimics, miR-132
inhibitor, mimics NC or inhibitor NC (5 nM, GeneChem Corp.) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
After transfection for 48 h, a luciferase assay was performed using
the Bright-Glo™ Luciferase Assay System (Promega Corporation). The
luciferase activity was normalized to the Renilla luciferase
activity.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
To determine the expression of miR-132, SIRT1,
IL-1β, IL-6 and TNF-α, RT-qPCR was performed. Total RNA was
extracted from myocardial tissues or H9C2 cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was
synthesized by reverse transcription using the PrimerScript RT
reagent kit (Takara Bio, Inc.). qPCR was performed to quantify
relative mRNA levels using SYBR-Green RT-qPCR SuperMix kit (Thermo
Fisher Scientific, Inc.) with customized primers (Table I) on an AB7300 thermo-cycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR
reaction conditions were as follows: Initial activation step at
95°C for 10 sec, followed by 40 cycles of denaturation at 95°C for
15 sec, annealing at 55°C for 25 sec, and extension at 72°C for 10
sec. GAPDH and U6 small nuclear RNA (U6 snRNA) were used as
internal references for mRNA and miRNA, respectively. Relative
expression levels were calculated using the 2−ΔΔCq
method (25).
 | Table IPrimers used in reverse
transcription-quantitative PCR. |
Table I
Primers used in reverse
transcription-quantitative PCR.
| Genes | Species | Sequences |
|---|
| miR-132 | Mouse | Forward
5′-CGGTGACTCAGCCTAGATGG-3′ |
| Reverse
5′-GGACGGGACAGGGAAGGG-3′ |
| Rat | Forward
5′-GACTGGTCCCGTGGCTTTC-3′, |
| Reverse
[0-9]′-GTGCAGGGTCCGAGGTATTC-[0-9]′ |
| SIRT1 | Mouse | Forward
[0-9]′-GCCTCACATGCAAGCTCTAGTGAC-[0-9]′ |
| Reverse
[0-9]′-TCGAGGATCTGTGCCAATCATAA-[0-9]′ |
| Rat | Forward
[0-9]′-GATCTCCCAGATCCTCAAGCC-[0-9]′ |
| Reverse
[0-9]′-TAGTCCATCAAGGAGCCAC-[0-9]′ |
| IL-1β | Mouse | Forward
[0-9]′-CATGGAATCCGTGTCTTCCT-[0-9]′ |
| Reverse
[0-9]′-GAGCTGTCTGCTCATTCACG-[0-9]′ |
| Rat | Forward
[0-9]′-CCAGGATGAGGACCCAAGCA-[0-9]′ |
| Reverse
[0-9]′-TCCCGACCATTGCTGTTTCC-[0-9]′ |
| IL-6 | Mouse | Forward
[0-9]′-TGACAAAAGAGTTGTGCAATGGC-[0-9]′ |
| Reverse
[0-9]′-GAATGTCCACAAACTGATATGCTT-[0-9]′ |
| Rat | Forward
[0-9]′-GACTTCCAGCCAGTTGCCTTCTTG-[0-9]′ |
| Reverse
[0-9]′-TGGTCTGTTGTGGGTGGTATCCTC-[0-9]′ |
| TNF-α | Mouse | Forward
[0-9]′-TCCCCAAAGGGATGAGAAGTTC-[0-9]′ |
| Reverse
[0-9]′-TCATACCAGGGTTTGAGCTCAG-[0-9]′ |
| Rat | Forward
[0-9]′-AAGCCCGTAGCCCACGTCGTA-[0-9]′ |
| Reverse
[0-9]′-GCC-CGCAATCCAGGCCACTAC-[0-9]′ |
| GAPDH | Mouse | Forward
[0-9]′-CACCCACTCCTCCACCTTTG-[0-9]′ |
| Reverse
[0-9]′-CCACCACCCTGTTGCTGTAG-[0-9]′ |
| Rat | Forward
[0-9]′-GGAGTCCACTGGCGTCTTC-[0-9]′ |
| Reverse
[0-9]′-GGCATTGCTGATGATCTTGAGG-[0-9]′ |
| U6 | Mouse | Forward
[0-9]′-ATGGGTCGAAGTCGTAGCC-[0-9]′ |
| Reverse
[0-9]′-TTCTCGGCGTCTTCTTTCTCG-[0-9]′ |
| Rat | Forward
[0-9]′-CTCGCTTCGGCAGCACA-[0-9]′ |
| Reverse
[0-9]′-AACGCTTCACGAATTTGCGT-[0-9]′ |
Western blot assay
Western blotting was performed to determine the
expression of SIRT1, PGC-1α, Nrf2, endothelial nitric oxide
synthase (eNOS), inducible nitric oxide synthase (iNOS), NLRP3,
caspase-1 and IL-1β. GAPDH was used as a control. Briefly, proteins
were extracted by RIPA buffer (Vazyme Biotech Co., Ltd.) and
quantitated with a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.). Subsequently, 30 µg proteins were loaded
on 10% SDS-PAGE, transferred to PVDF membranes and then blocked
with 5% non-fat milk. Then, membranes were incubated with the
primary antibodies anti-SIRT1 (cat. no. ab110304, 1:2,000),
anti-PGC-1α (cat. no. ab54481, 1:10,000), anti-Nrf2 (cat. no.
ab62352, 1:1,000), anti-eNOS (cat. no. ab76198, 1:500), anti-iNOS
(cat. no. ab15323, 1:500), anti-NLRP3 (cat. no. ab214185, 1:1,000),
anti-caspase-1 (cat. no. ab62698, 1:500), anti-IL-1β (cat. no.
ab200478, 1:1,000) and anti-GAPDH (cat. no. ab8245, 1:2,000) (all
purchased from Abcam) at 4°C overnight, followed by the
corresponding secondary horseradish peroxidase-conjugated
anti-rabbit or anti-mouse IgG antibody (cat. nos. ab205719 and
ab205718, Abcam) for 1 h at room temperature. The films were
scanned using the Pierce ECL Western Blotting Substrate (Pierce;
Thermo Fisher Scientific, Inc.). The proteins were quantified using
Quantity One software, version 4.2.1 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All experiments were repeated at least three times,
and one representative result is presented. All experimental data
are expressed as the mean ± standard deviation. Student’s t-test
(two-tailed) was used for comparison between two groups. One-way
analysis of variance followed by Tukey’s post hoc test was used for
multiple comparisons. SPSS version 13.0 (SPSS, Inc.) was used for
statistical processing. P<0.05 was considered to indicate
statistically significant differences.
Results
Effects of I/R on myocardial injury and
levels of serum myocardial enzymes and inflammatory factors
First, alterations in myocardial tissues and serum
levels of myocardial enzymes and inflammatory factors following I/R
were demonstrated. As shown in Fig.
1A, marked histological myocardial structural abnormalities
were observed in myocardial tissues of the I/R group, such as
tissue necrosis, massive inflammatory infiltration, and perinuclear
vacuolization. In addition, the serum levels of the myocardial
enzymes CK, CK-MB, LDH, HBDH and IMA were all significantly
upregulated after I/R induction compared with the sham group
(Fig. 1B). The serum levels of
IL-1β, IL-6 and TNF-α were also significantly increased in the I/R
group (Fig. 1C). These results
indicated successful establishment of the myocardial I/R injury
model.
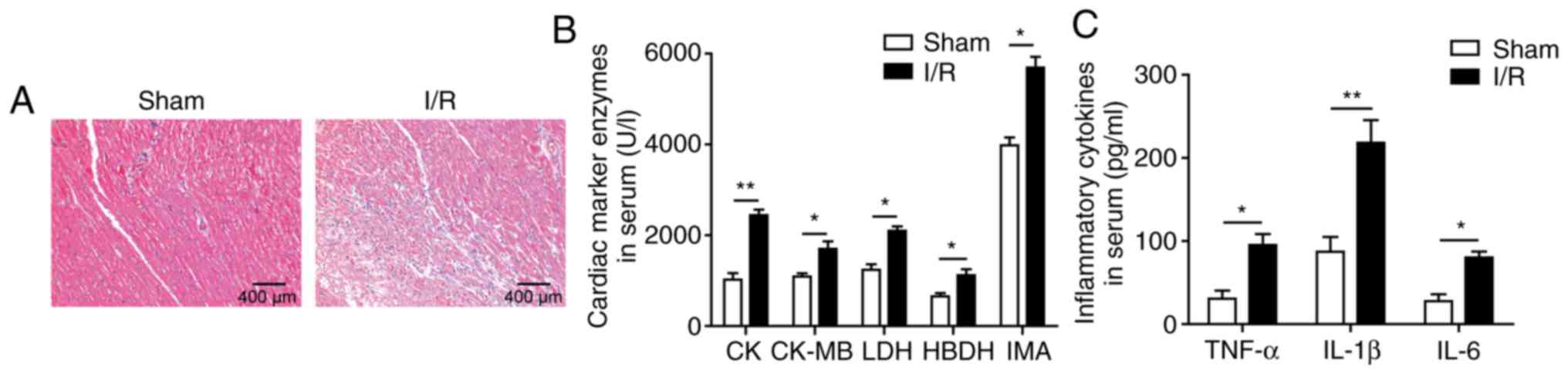 | Figure 1Effects of I/R on myocardial injury
and levels of serum myocardial enzymes and inflammatory factors.
(A) Histological analysis by haematoxylin and eosin staining; scale
bar, 400 µm. (B) Serum levels of the myocardial enzymes CK,
CK-MB, LDH, HBDH and IMA. (C) Levels of the inflammatory factors
IL-1β, IL-6 and TNF-α as determined by ELISA. The results are
representative of three independent experiments. Error bars
represent the mean ± standard deviation. *P<0.05 and
**P<0.01. I/R, ischemia/reperfusion; CK, creatine
kinase; CK-MB, CK myocardial band isoenzyme; LDH, lactate
dehydrogenase; HBDH, hydroxybutyrate dehydrogenase; IMA,
ischaemia-modified albumin; IL, interleukin; TNF, tumour necrosis
factor. |
Effects of I/R on Nrf2-mediated oxidative
stress and pyroptosis
The effects of I/R on oxidative stress and
pyroptosis were next investigated. First, miR-132 was found to be
significantly increased, while the mRNA level of SIRT1 was
significantly decreased in myocardial tissues of I/R mice compared
with the sham group (Fig. 2A and
B). Western blotting demonstrated that the expression of the
PGC-1α/Nrf2 signalling-related proteins PGC-1α, Nrf2 and eNOS was
significantly inhibited by I/R induction, while the expression of
iNOS was significantly upregulated (Fig. 2C and D), suggesting that the
PGC-1α/Nrf2 signalling pathway was inhibited and oxidative stress
was enhanced by I/R. Furthermore, MDA levels were significantly
upregulated and SOD levels were downregulated in myocardial tissues
of I/R mice (Fig. 2F), indicating
the increased oxidative stress in I/R mice. In addition, the
pyroptosis-related proteins NLRP3, caspase-1, and IL-1β were all
significantly upregulated in myocardial tissues of I/R mice,
indicating that pyroptosis was activated (Fig. 2C and E). These results indicate
that I/R induced PGC-1α/Nrf2 signalling pathway-mediated oxidative
stress and downstream signalling-associated pyroptosis in
myocardial tissues.
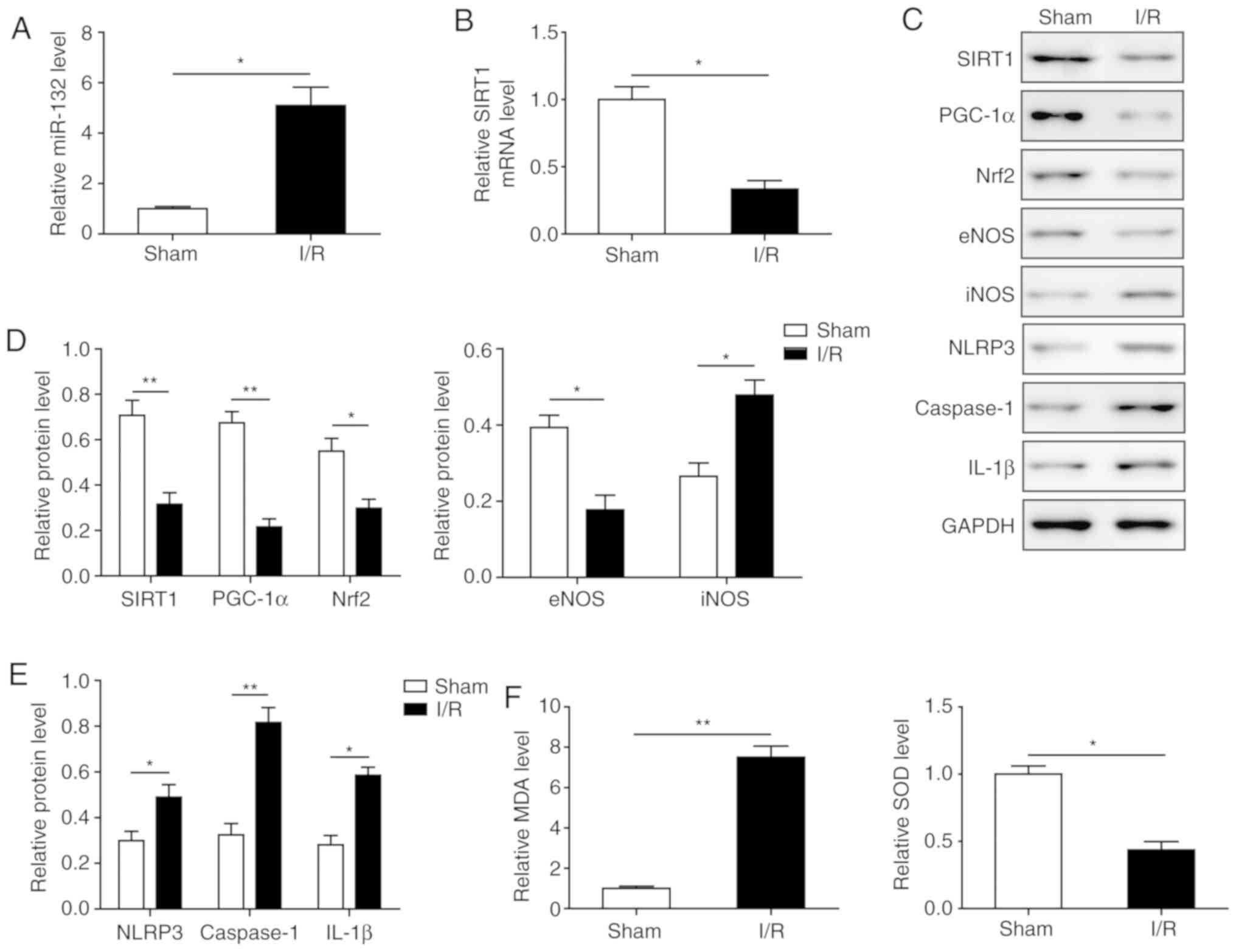 | Figure 2Effects of I/R on Nrf2-mediated
oxidative stress and pyroptosis. (A) Expression of miR-132 in
myocardial tissues in the I/R group and sham group by RT-qPCR. (B)
SIRT1 mRNA level in myocardial tissues in the I/R group and sham
group by RT-qPCR. (C) Protein levels of SIRT1, PGC-1α, Nrf2, eNOS,
iNOS, NLRP3, caspase-1 and IL-1β in myocardial tissues by western
blotting. (D) Quantification of protein expression of SIRT1,
PGC-1α, Nrf2, eNOS and iNOS in C. (E) Quantification of protein
expression of NLRP3, caspase-1, and IL-1β in C. (F) Levels of SOD
and MDA in myocardial tissues. The results are representative of
three independent experiments. Error bars represent the mean ±
standard deviation. *P<0.05 and
**P<0.01. I/R, ischemia/reperfusion; Nrf2, nuclear
factor erythroid-2-related factor 2; RT-qPCR, reverse
transcription-quantitative PCR; SIRT1, sirtuin 1; PGC-1α,
peroxisome proliferator-activated receptor gamma coactivator-1α;
eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric
oxide synthase; IL, interleukin; SOD, superoxide dismutase; MDA,
malondialdehyde. |
miR-132 directly targets SIRT1 and
negatively regulates the expression of SIRT1
To further investigate the roles of miR-132 and
SIRT1 in I/R-induced myocardial injury, a dual luciferase reporter
assay was conducted to confirm the binding between miR-132 and
SIRT1. A binding site for miR-132 was identified in the 3′-UTR of
SIRT1 (Fig. 3A). As shown in
Fig. 3B, the luciferase activity
was significantly decreased when cells were transfected with
miR-132 mimics and significantly elevated when miR-132 was
inhibited in the SIRT1-WT group. However, no significant difference
was observed in the SIRT1-MUT groups after transfection with
miR-132 mimics or inhibitor, suggesting that SIRT1 is a direct
target of miR-132. miR-132 mimics markedly increased the level of
miR-132, and miR-132 inhibitor significantly reduced the level of
miR-132 (Fig. 3C). The mRNA and
protein levels of SIRT1 were found to be significantly upregulated
in H9C2 cells transfected with miR-132 inhibitor and significantly
downregulated when miR-132 was overexpressed (Fig. 3C-E). These results indicated that
SIRT1 is a direct target of miR-132 and is negatively regulated by
miR-132.
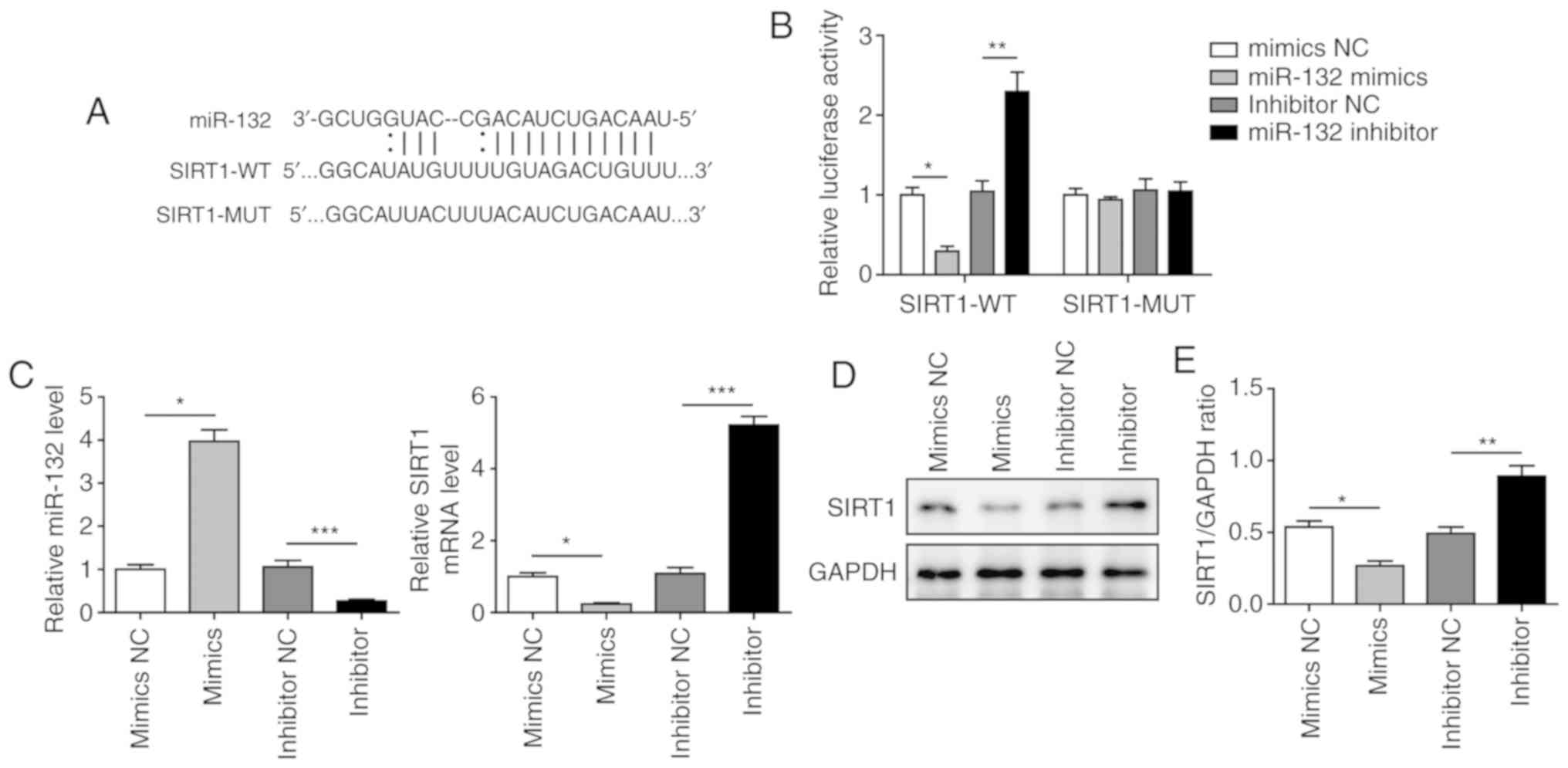 | Figure 3miR-132 directly targeted SIRT1 and
negatively regulated SIRT1 expression. (A) The predicted binding
site of miR-132 and SIRT1. (B) The relative luciferase activity of
SIRT1-WT and SIRT1-MUT groups by dual luciferase reporter assay.
(C) Expression of miR-132 and SIRT1 in cells transfected with
mimics NC, miR-132 mimics, inhibitor NC or miR-132 inhibitor by
RT-qPCR. (D) Protein level of SIRT1 in cells transfected with
mimics NC, miR-132 mimics, inhibitor NC or miR-132 inhibitor by
western blotting. (E) Quantification of SIRT1 protein expression.
The results are representative of three independent experiments.
Error bars represent the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001. SIRT1, sirtuin 1; WT, wild-type; MUT,
mutant; RT-qPCR, reverse transcription-quantitative PCR; NC,
negative control. |
Inhibition of miR-132 inhibits
H/R-induced oxidative stress by activation of PGC-1α/Nrf2
signalling through upregulation of SIRT1
To investigate the effects of miR-132 on H/R-induced
oxidative stress in vitro, a miR-132 inhibitor was applied,
and SIRT1 was inhibited by the SIRT1 inhibitor EX527. The results
demonstrated that the miR-132 inhibitor significantly decreased the
expression of miR-132, which was induced by H/R treatment, while
the expression of SIRT1 was significantly increased compared with
that of the H/R group. Moreover, the aforementioned effects were
markedly reversed by EX527 treatment (Fig. 4A and B), further confirming the
regulatory effect of miR-132 on SIRT1. Analysis of PGC-1α/Nrf2
signalling revealed that PGC-1α, Nrf2 and eNOS were all
significantly upregulated and that iNOS was significantly
downregulated by inhibiting miR-132 compared with the H/R group
(Fig. 4C and D). However, the
inhibition of SIRT1 by EX527 was able to markedly reverse the
effects induced by the miR-132 inhibitor. Similar results were also
observed with MDA and SOD levels. The increased level of MDA and
decreased level of SOD induced by H/R were significantly inhibited
by the miR-132 inhibitor, and these effects were recovered by EX527
treatment (Fig. 4E). These
results suggest that the miR-132 inhibitor suppressed H/R-induced
oxidative stress via activation of PGC-1α/Nrf2 signalling by
upregulating SIRT1.
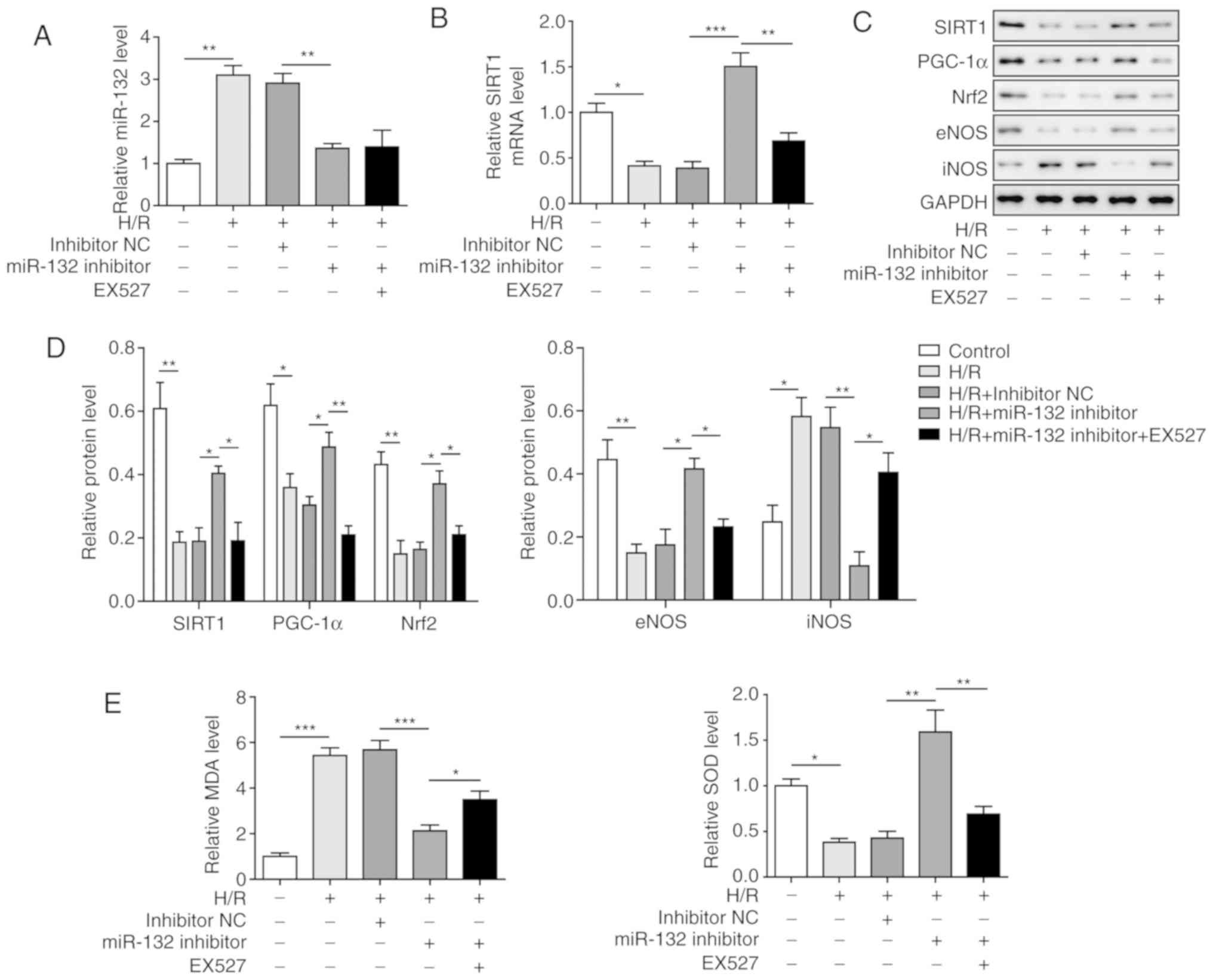 | Figure 4Inhibition of miR-132 suppressed
H/R-induced oxidative stress by activation of PGC-1α/Nrf2
signalling through upregulation of SIRT1. (A) Expression of miR-132
in different groups of cells by RT-qPCR. (B) SIRT1 expression in
different groups of cells by RT-qPCR. (C) Protein levels of SIRT1,
PGC-1α, Nrf2, eNOS and iNOS in different groups of cells by western
blotting. (D) Quantification of SIRT1, PGC-1α, Nrf2, eNOS and iNOS
protein expression. (E) Levels of MDA and SOD in different groups
of cells. The results are representative of three independent
experiments. Error bars represent the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001. H/R, hypoxia/reoxygenation; Nrf2, nuclear
factor erythroid-2-related factor 2; PGC-1α, peroxisome
proliferator-activated receptor gamma coactivator-1α; SIRT1,
sirtuin 1; RT-qPCR, reverse transcription-quantitative PCR; eNOS,
endothelial nitric oxide synthase; iNOS, inducible nitric oxide
synthase; SOD, superoxide dismutase; MDA, malondialdehyde. |
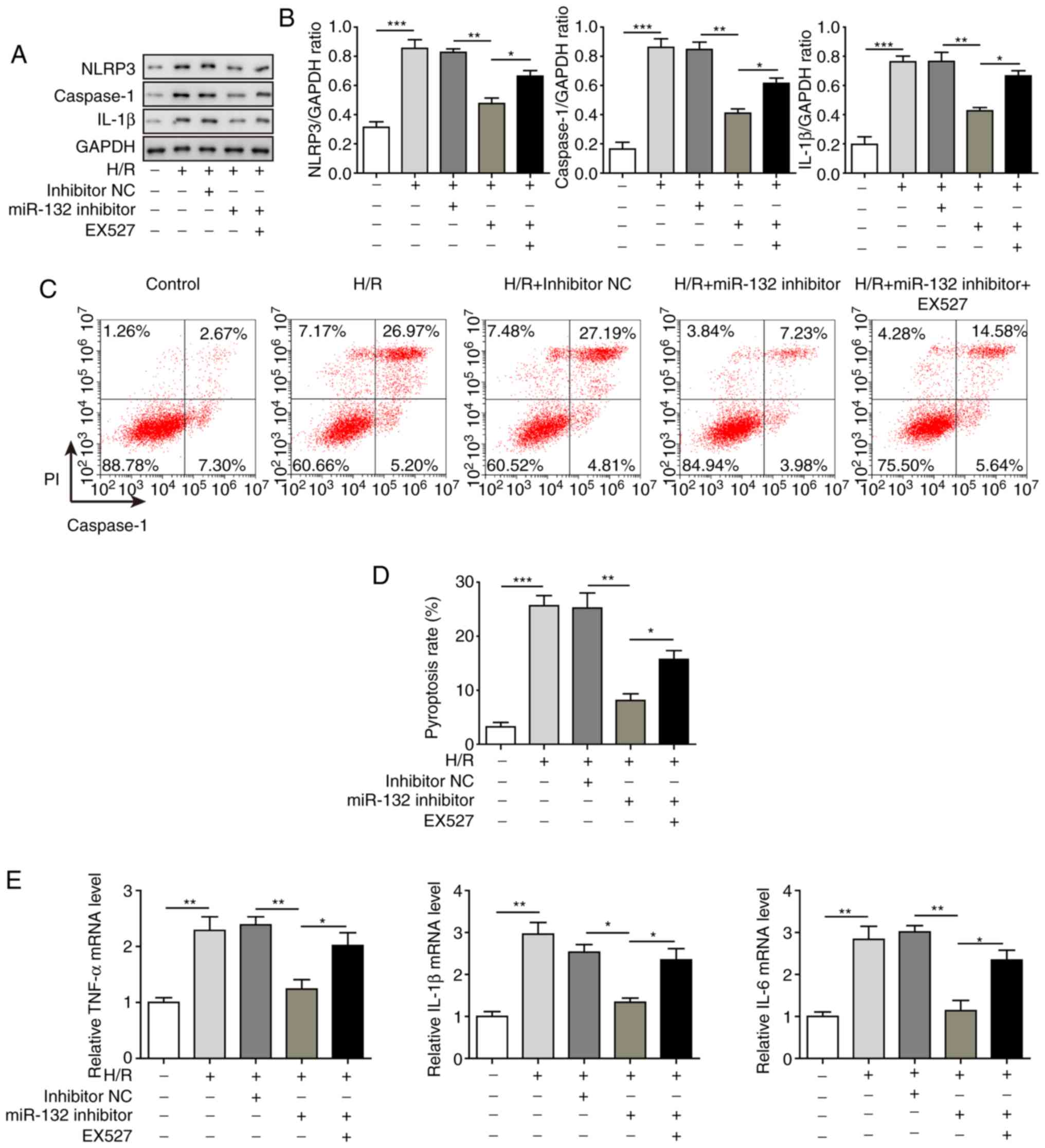
Inhibition of miR-132 suppresses
H/R-induced pyroptosis
Finally, the effects of miR-132 on cell pyroptosis
in the H/R model were determined. As shown in Fig. 5A and B, the expression of the
pyroptosis-related proteins NLRP3, caspase-1 and IL-1β were all
significantly suppressed by miR-132 inhibitor treatment compared
with the H/R model group. However, when SIRT1 was inhibited by
EX527, the inhibitory effects of the miR-132 inhibitor were
significantly recovered, suggesting that the inhibitory effects of
the miR-132 inhibitor on cell pyroptosis were mediated through
regulation of SIRT1. The cell pyroptosis analysis revealed that the
cell pyroptosis induced by H/R was also significantly inhibited by
the miR-132 inhibitor, which was reversed by treatment with EX527
(Fig. 5C and D). Similar results
were observed for the mRNA levels of IL-1β, IL-6 and TNF-α in the
H/R model, which were also significantly decreased by the miR-132
inhibitor and reversed by EX527 treatment (Fig. 5E). These results suggested that
miR-132 inhibition may suppress H/R-induced cell pyroptosis through
regulation of SIRT1.
Discussion
Despite numerous studies on myocardial I/R injury,
the mechanisms underlying the role of miRNAs in myocardial I/R
injury remain unclear. Recently, the role of miRNAs in myocardial
ischaemia has been reported in several studies. He et al
demonstrated that the upregulation of miR-1 and miR-133a decreased
the apoptosis of cardiomyocytes in myocardial ischaemic
post-conditioning (26). Li et
al observed that the miR-939-mediated nitric oxide signalling
pathway was involved in myocardial ischaemia (27). However, no study had focused on
the role of miR-132 and its association with oxidative stress and
pyroptosis in myocardial I/R injury. To the best of our knowledge,
the present study was the first to demonstrate that inhibition of
miR-132 could improve myocardial I/R injury by inhibiting oxidative
stress and pyroptosis through activation of PGC-1α/Nrf2 signalling
by targeting SIRT1.
A role for miR-132 has been reported in several
diseases. Smith et al reported that miR-132 was
downregulated in Alzheimer’s disease, and that deficiency of
miR-132 resulted in increased tau expression, phosphorylation and
aggregation (28). You et
al demonstrated that miR-132 inhibited the migration and
invasion of lung cancer cells by targeting ZEB2 (29). miR-132 is also considered to be
associated with ischaemia-related diseases. Keasey et al
demonstrated that miR-132 was upregulated by ischaemic
preconditioning in cultured hippocampal neurons (12). Huang et al found that the
expression of miR-132 was increased in cardiac fibroblasts
following ischaemia, and inhibition of miR-132 enhanced
angiogenesis and reduced cell apoptosis (30). In the present study, an in
vivo mouse myocardial I/R injury model was successfully
established and miR-132 was found to be upregulated in myocardial
I/R injury, which was consistent with previous studies.
A number of studies have demonstrated the role of
SIRT1 in myocardial I/R injury. Hsu et al demonstrated that
SIRT1 could protect against myocardial I/R injury (31). Fan et al reported that
SIRT1 contributed to the resistance to I/R-induced acute kidney
injury (32). It was also
observed that melatonin may protect against myocardial I/R injury
by activating SIRT1 signalling and inhibiting oxidative stress
(33). In diabetic rats, the
activation of SIRT1 led to activation of eNOS, ultimately resulting
in improvement of myocardial I/R injury (34). The present study demonstrated that
SIRT1 was downregulated in myocardial I/R injury and was a direct
target of miR-132. It was also observed that the overexpression of
miR-132 downregulated SIRT1 levels and that the inhibition of
miR-132 increased SIRT1 expression, indicating that miR-132
negatively regulated SIRT1. Previous studies have also reported the
association between SIRT1 and miR-132. miR-132 was found to affect
aberrant B-cell cytokine regulation by targeting SIRT1 in patients
with relapsing-remitting multiple sclerosis (35). Xiong et al demonstrated
that down-regulation of miR-132 suppressed endoplasmic reticulum
(ER) stress in colitis by activation of SIRT1 (36). The present study was the first to
demonstrate the negative regulatory association between miR-132 and
SIRT1 in myocardial I/R injury.
The association between SIRT1/PGC-1α/Nrf2
signalling-mediated oxidative stress and myocardial I/R injury has
been demonstrated in several studies. Wang et al observed
that SIRT1/Nrf2 signalling was inhibited while ER stress was
activated in an I/R model (16).
Pan et al demonstrated that sulforaphane exerted an
antioxidant effect on myocardial I/R injury through the activation
of the Nrf2/HO-1 antioxidant pathway (37). In the present study, it was also
observed that PGC-1α/Nrf2 signalling and SIRT1 were inhibited in
myocardial I/R injury. The suppression of PGC-1α/Nrf2 signalling
contributed to oxidative stress, which was reflected by the
upregulation of MDA and iNOS and downregulation of SOD and eNOS in
a myocardial injury model. The effects induced by myocardial injury
were reversible by inhibition of miR-132, which caused activation
of PGC-1α/Nrf2 signalling and suppression of oxidative stress.
Pyroptosis is associated with oxidative stress. It
is considered that exacerbated oxidative stress and inflammation
can induce autophagy, apoptosis and pyroptosis (38). Although the role of oxidative
stress in I/R-induced injury has been well established, few studies
have demonstrated the effects of miR-132 on oxidative stress in
myocardial I/R injury and the possible association with pyroptosis.
The present study demonstrated that the levels of NLRP3, caspase-1
and IL-1β and the pyroptosis ratio were markedly increased in
myocardial I/R injury. We first demonstrated that inhibition of
miR-132 may improve myocardial I/R injury by suppressing oxidative
stress-induced pyroptosis by targeting SIRT1. Moreover, inhibition
of SIRT1 by EX527 markedly reversed the effects of miR-132
inhibition, further confirming the role of the miR-132/SIRT1 axis
in myocardial I/R injury. However, further study is required to
gain deeper insight into the association between oxidative stress
and pyroptosis in myocardial I/R injury.
In conclusion, in vivo and in vitro
studies were conducted to investigate the role of miR-132 in
myocardial I/R injury. The results revealed that miR-132 was
upregulated in myocardial I/R injury and that inhibition of miR-132
was able to improve myocardial I/R injury by inhibiting oxidative
stress and pyroptosis through activation of PGC-1α/Nrf2 signalling
by targeting SIRT1. The findings of this study may provide deeper
insight into the role of miR-132 in myocardial I/R injury and help
identify new treatment targets for myocardial I/R injury.
Acknowledgments
Not applicable.
Abbreviations:
|
I/R
|
ischaemia/reperfusion
|
|
miRNA
|
microRNA
|
|
H/R
|
hypoxia/reoxygenation
|
|
LAD
|
left anterior descending
|
|
H&E
|
haematoxylin and eosin
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors’ contributions
ZY and LSL conceived the study. LKS collected the
data. LL analysed the data. ZY and LSL performed the experiments.
ZY and LSL provided the resources and supervised the study. ZY,
LSL, LKS and LL wrote the original draft of the manuscript. ZY and
LSL reviewed and edited the manuscript. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care Committee at Tongji Medical College of Huazhong
University of Science and Technology (Hubei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu P, Zhang J, Yu S, Luo Z, Hua F, Yuan L,
Zhou Z, Liu Q, Du X, Chen S, et al: Protective effect of
sevoflurane post-conditioning against cardiac ischemia/reperfusion
injury via ameliorating mitochondrial impairment, oxidative stress
and rescuing autophagic clearance. PLoS One. 10:e01346662015.
View Article : Google Scholar
|
|
2
|
Pryds K, Nielsen RR, Jorsal A, Hansen MS,
Ringgaard S, Refsgaard J, Kim WY, Petersen AK, Bøtker HE and
Schmidt MR: Effect of long-term remote ischemic conditioning in
patients with chronic ischemic heart failure. Basic Res Cardiol.
112:672017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dang M, Zeng X, Wang H, Li H, Du F and
Chen B: GW28-e0833 Inhibition of myocardial ischemia/reperfusion
apoptosis by soluble receptor for advanced glycation end-product
(sRAGE) via interferon-induced immunoproteasome activity. J Am
College Cardiol. 70:C31–C32. 2017. View Article : Google Scholar
|
|
4
|
Fabbri M, Croce CM and Calin GA:
MicroRNAs. Cancer J. 14:759–774. 2015.
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao J, Zhu X, He B, Zhang Y, Kang B, Wang
Z and Ni X: MiR-204 regulates cardiomyocyte autophagy induced by
ischemia-reperfusion through LC3-II. J Biomedical Sci. 18:352011.
View Article : Google Scholar
|
|
7
|
Pan Z, Sun X, Ren J, Li X, Gao X, Lu C,
Zhang Y, Sun H, Wang Y, Wang H, et al: miR-1 exacerbates cardiac
ischemia-reperfusion injury in mouse models. PLoS One.
7:e505152012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng J, Omran A, Ashhab MU, Kong H, Gan N,
He F and Yin F: Expression patterns of miR-124, miR-134, miR-132,
and miR-21 in an immature rat model and children with mesial
temporal lobe epilepsy. J Mol Neuroscience. 50:291–297. 2013.
View Article : Google Scholar
|
|
9
|
Formosa A, Lena AM, Markert EK, Cortelli
S, Miano R, Mauriello A, Croce N, Vandesompele J, Mestdagh P,
Finazzi-Agrò E, et al: DNA methylation silences miR-132 in prostate
cancer. Oncogene. 32:127–134. 2013. View Article : Google Scholar
|
|
10
|
Wei X, Tan C, Tang C, Ren G, Xiang T, Qiu
Z, Liu R and Wu Z: Epigenetic repression of miR-132 expression by
the hepatitis B virus x protein in hepatitis B virus-related
hepatocellular carcinoma. Cell Signal. 25:1037–1043. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong S, Lee J, Seo HH, Lee CY, Yoo KJ, Kim
SM, Lee S, Hwang KC and Choi E: Na(+)-Ca(2+) exchanger targeting
miR-132 prevents apoptosis of cardiomyocytes under hypoxic
condition by suppressing Ca(2+) overload. Biochem Biophys Res
Commun. 460:931–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keasey MP, Scott HL, Bantounas I, Uney JB
and Kelly S: MiR-132 is upregulated by ischemic preconditioning of
cultured hippocampal neurons and protects them from subsequent OGD
toxicity. J Mol Neurosci. 59:404–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu H, Lin K, Wang H, Wei H, Ji B, Yang Z,
Peng C, Xiao X and Deng H: 1,25(OH)Dimproves cardiac dysfunction,
hypertrophy, and fibrosis through PARP1/SIRT1/mTOR-related
mechanisms in type 1 diabetes. Mol Nutr Food Res. 61:2017.
View Article : Google Scholar
|
|
14
|
Shah SA, Khan M, Jo MH, Min GJ, Amin FU
and Kim MO: Melatonin stimulates the SIRT1/Nrf2 signaling pathway
counteracting lipopolysaccharide (LPS)-induced oxidative stress to
rescue postnatal rat brain. CNS Neurosci Ther. 23:33–44. 2017.
View Article : Google Scholar
|
|
15
|
Huang K, Gao X and Wei W: The crosstalk
between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a
positive feedback loop to inhibit FN and TGF β1 expressions in rat
glomerular mesangial cells. Exp Cell Res. 361:632017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Yuan B, Cheng B, Liu Y, Zhang B,
Wang X, Lin X, Yang B and Gong G: Crocin alleviates myocardial
ischemia/reperfusion-induced endoplasmic reticulum stress via
regulation of MIR-34A/SIRT1/NRF2 Pathway. Shock. 51:123–130. 2019.
View Article : Google Scholar
|
|
17
|
Dal Bo M, D’Agaro T, Gobessi S, Zucchetto
A, Dereani S, Rossi D, Zaja F, Pozzato G, Di Raimondo F, Gaidano G,
et al: The SIRT1/TP53 axis is activated upon B-cell receptor
triggering via miR-132 up-regulation in chronic lymphocytic
leukemia cells. Oncotarget. 6:19102–19117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hadar A, Milanesi E, Walczak M,
Puzianowskakuźnicka M, Kuźnicki J, Squassina A, Niola P, Chillotti
C, Attems J, Gozes I and Gurwitz D: SIRT1, miR-132 and miR-212 link
human longevity to Alzheimer’s Disease. Sci Rep. 8:84652018.
View Article : Google Scholar
|
|
19
|
Bergsbaken T, Fink SL and Cookson BT:
Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol.
7:99–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vande Walle L and Lamkanfi M: Pyroptosis.
Curr Biol. 26:R568–R572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JY, Paton JC, Briles DE, Rhee DK and
Pyo S: Streptococcus pneumoniaeinduces pyroptosis through the
regulation of autophagy in murine microglia. Oncotarget.
6:44161–44178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li R, Zhang LM and Sun WB: Erythropoietin
rescues primary rat cortical neurons from pyroptosis and apoptosis
via Erk1/2-Nrf2/Bach1 signal pathway. Brain Res Bull. 130:236–244.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han Z, Cao J, Song D, Tian L, Chen K, Wang
Y, Gao L, Yin Z, Fan Y and Wang C: Autophagy is involved in the
cardioprotection effect of remote limb ischemic postconditioning on
myocardial ischemia/Reperfusion injury in normal mice, but not
diabetic mice. PLoS One. 9:e868382014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei LF, Zhang HM, Wang SS, Jing JJ, Zheng
ZC, Gao JX, Liu Z and Tian J: Changes of MDA and SOD in brain
tissue after secondary brain injury with seawater immersion in
rats. Turk Neurosurg. 26:384–288. 2016.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
He B, Xiao J, Ren AJ, Zhang YF, Zhang H,
Chen M, Xie B, Gao XG and Wang YW: Role of miR-1 and miR-133a in
myocardial ischemic postconditioning. J Biomed Sci. 18:222011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Liao Y, Gao L, Zhuang T, Huang Z,
Zhu H and Ge J: Coronary serum exosomes derived from patients with
myocardial ischemia regulate angiogenesis through the, 2018
miR-939-mediated Nitric Oxide Signaling Pathway. Theranostics.
8:2079–2093. 2018. View Article : Google Scholar :
|
|
28
|
Smith PY, Hernandez-Rapp J, Jolivette F,
Lecours C, Bisht K, Goupil C, Dorval V, Parsi S, Morin F, Planel E,
et al: miR-132/212 deficiency impairs tau metabolism and promotes
pathological aggregation in vivo. Hum Mol Genet. 24:6721–6735.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You J, Li Y, Fang N, Liu B, Zu L, Chang R,
Li X and Zhou Q: MiR-132 suppresses the migration and invasion of
lung cancer cells via targeting the EMT regulator ZEB2. PLoS One.
9:e918272014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang W, Liang J, Ashraf A, Xu M, Millard
RW, Ashraf M and Wang Y: Abstract 9990: Regulation of miR132 in
cardiac fibroblasts after ischemia enhances angiogenesis and
reduction of apoptosis by targeting sonic hedgehog. Circulation.
124:A99902011.
|
|
31
|
Hsu CP, Zhai P, Yamamoto T, Maejima Y,
Matsushima S, Hariharan N, Shao D, Takagi H, Oka S and Sadoshima J:
Sirt1 protects the heart from ischemia/reperfusion. Circulation.
122:2170–2182. 2011. View Article : Google Scholar
|
|
32
|
Fan H, Yang HC, You L, Wang YY, He WJ and
Hao CM: The histone deacetylase, SIRT1, contributes to the
resistance of young mice to ischemia/reperfusion-induced acute
kidney injury. Kidney Int. 83:404–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai
M, Pei H, Wang X, Zhang H, Meng Q, et al: Melatonin
receptor-mediated protection against myocardial
ischemia/reperfusion injury: Role of SIRT1. J Pineal Res.
57:228–238. 2015. View Article : Google Scholar
|
|
34
|
Ding M, Lei J, Han H, Li W, Qu Y, Fu E, Fu
F and Wang X: SIRT1 protects against myocardial
ischemia-reperfusion injury via activating eNOS in diabetic rats.
Cardiovasc Diabetol. 14:1432015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyazaki Y, Li R, Rezk A, Misirliyan H,
Moore C, Farooqi N, Solis M, Goiry LG, de Faria Junior O, Dang VD,
et al: A novel microRNA-132-surtuin-1 axis underlies aberrant
B-cell cytokine regulation in patients with relapsing-remitting
multiple sclerosis [corrected]. PLoS One. 9:e1054212014. View Article : Google Scholar
|
|
36
|
Xiong Y, Shi L, Wang L, Zhou Z, Wang C,
Lin Y, Luo D, Qiu J and Chen D: Activation of sirtuin 1 by
catalpol-induced downregulation of microRNA-132 attenuates
endoplasmic reticulum stress in colitis. Pharmacol Res. 123:73–82.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan H, He M, Liu R, Brecha NC, Yu AC and
Pu M: Sulforaphane protects rodent retinas against
ischemia-reperfusion injury through the activation of the Nrf2/HO-1
antioxidant pathway. PLoS One. 9:e1141862014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chung SD, Lai TY, Chien CT and Yu HJ:
Activating Nrf-2 signaling depresses unilateral ureteral
obstruction-evoked mitochondrial stress-related autophagy,
apoptosis and pyroptosis in kidney. PLoS One. 7:e472992012.
View Article : Google Scholar : PubMed/NCBI
|