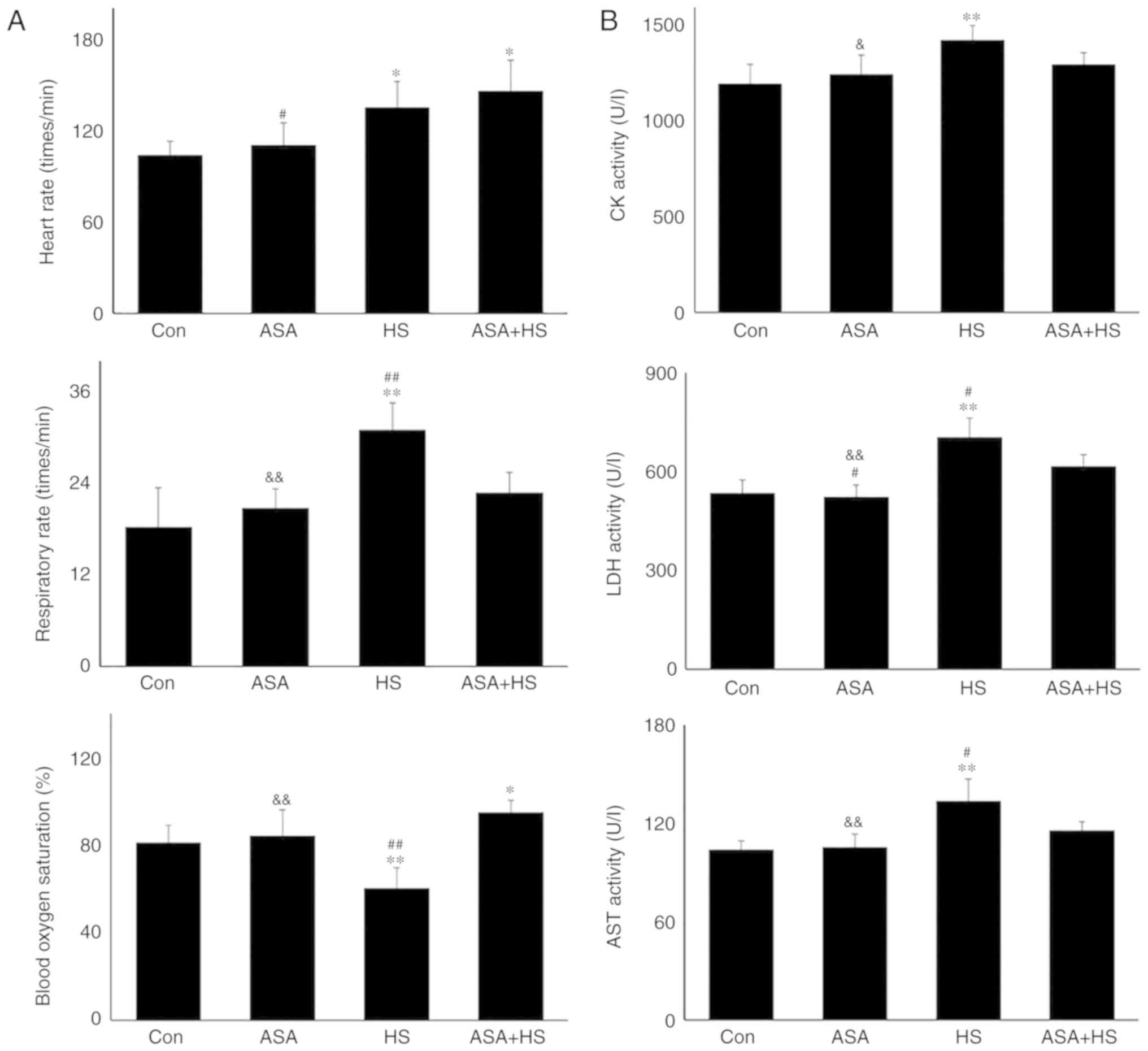
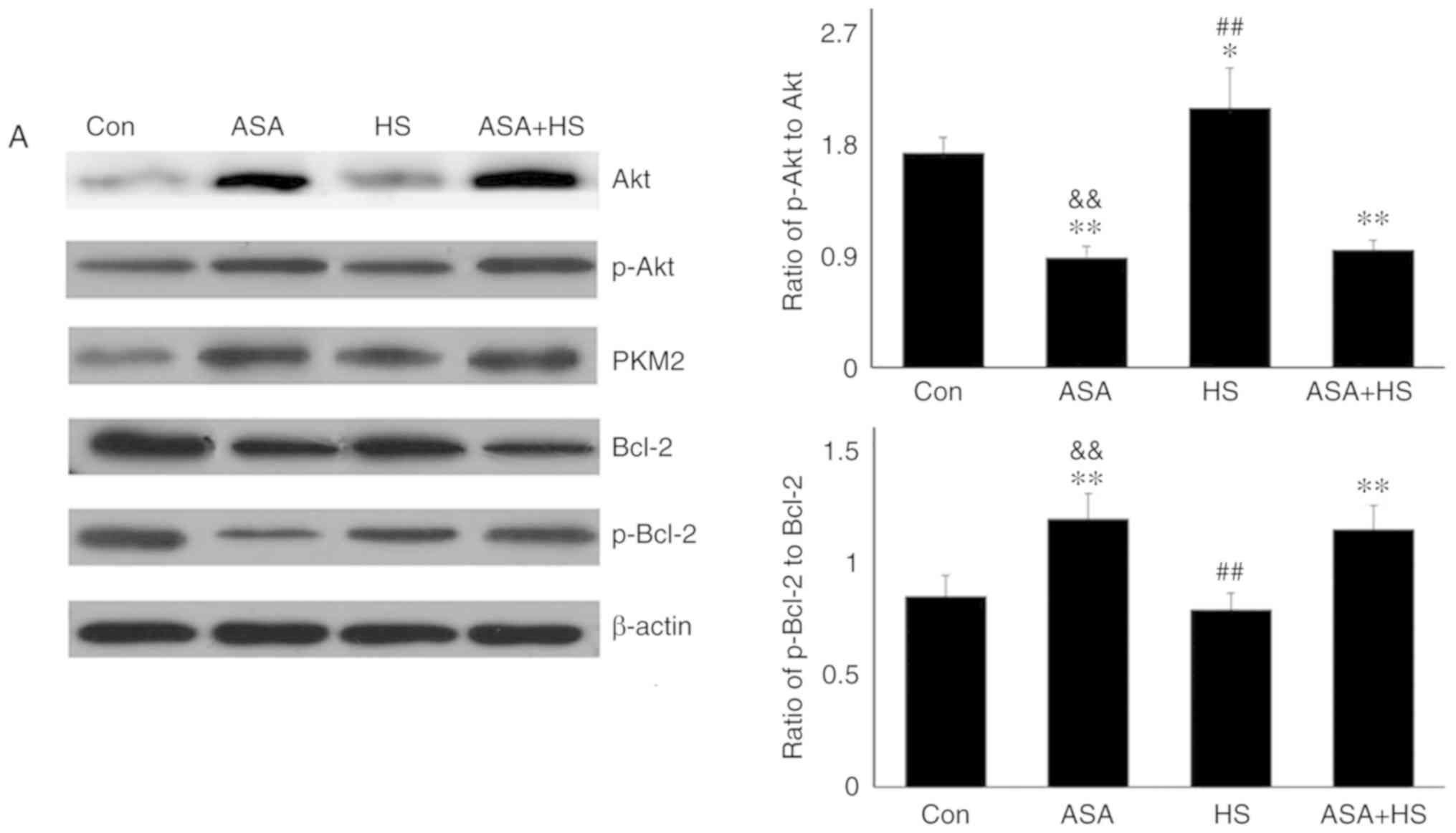
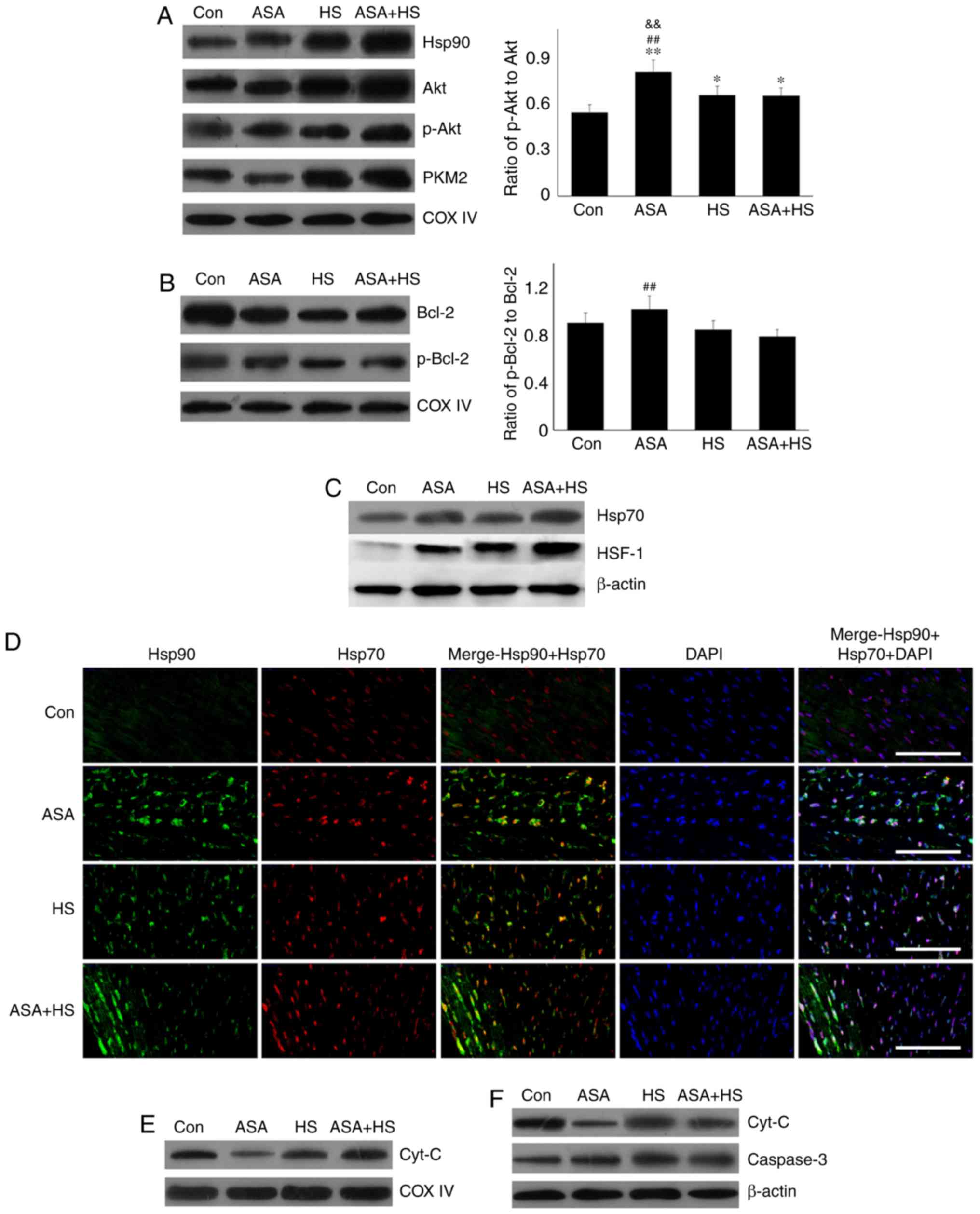
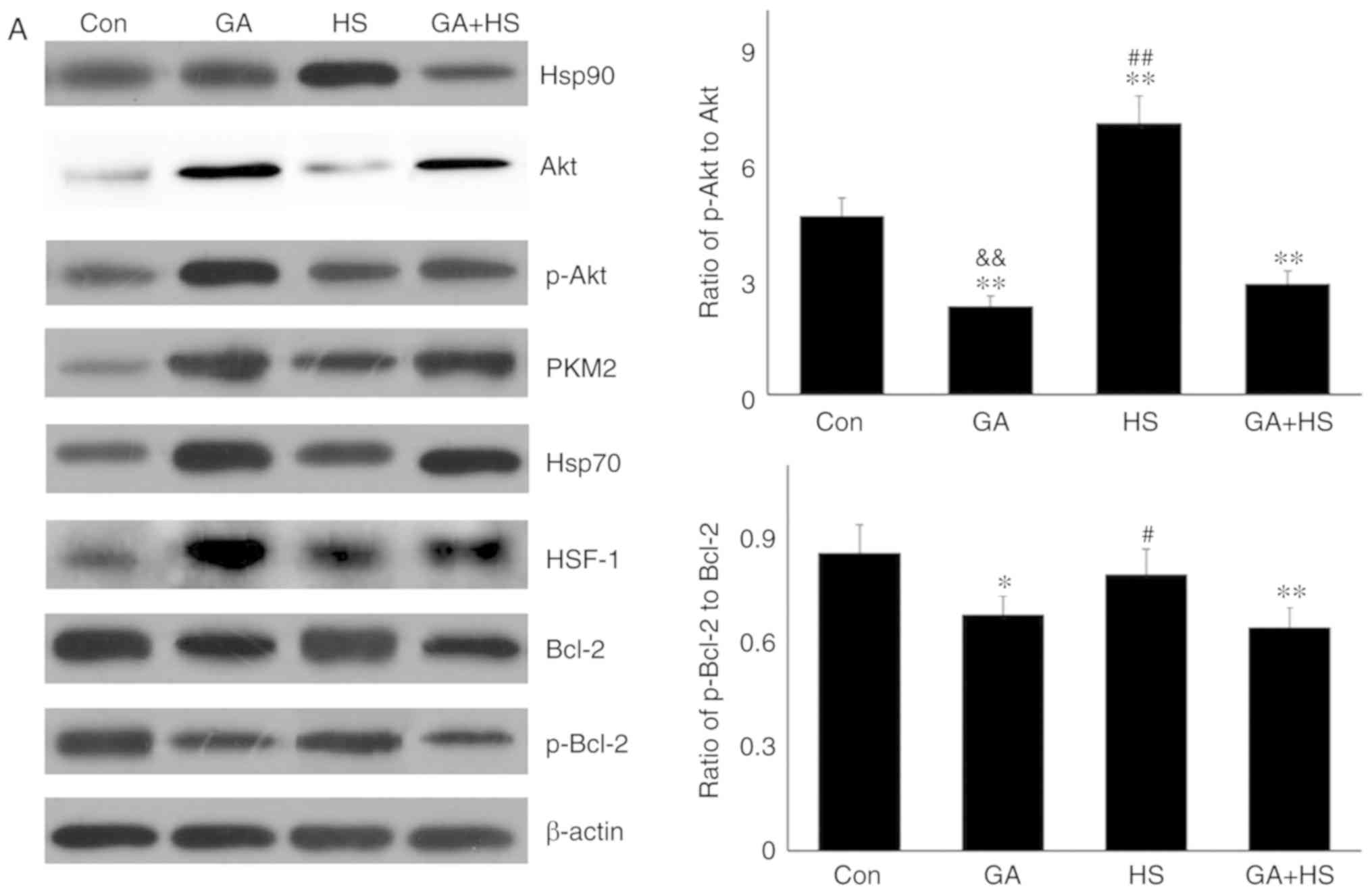
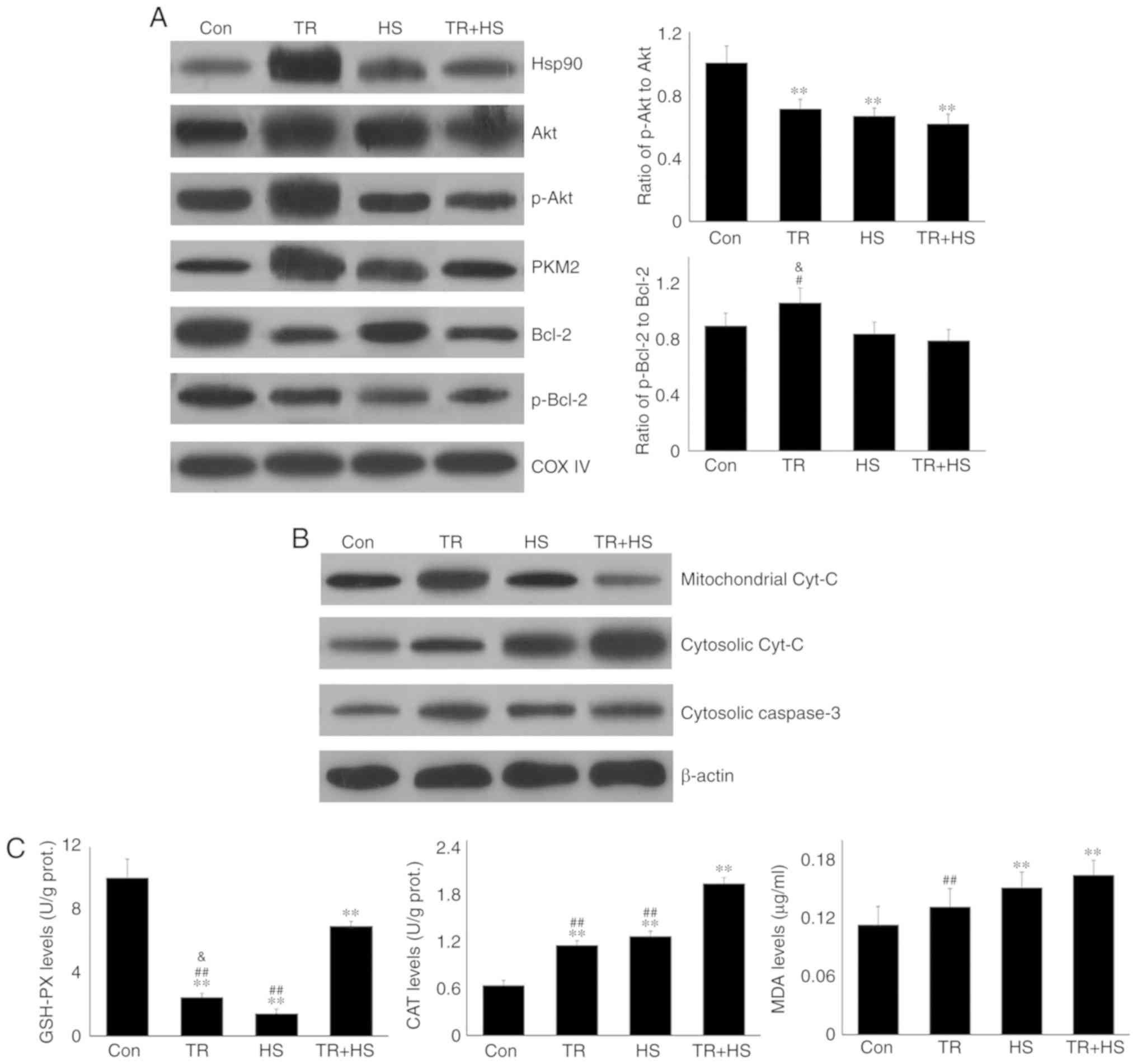
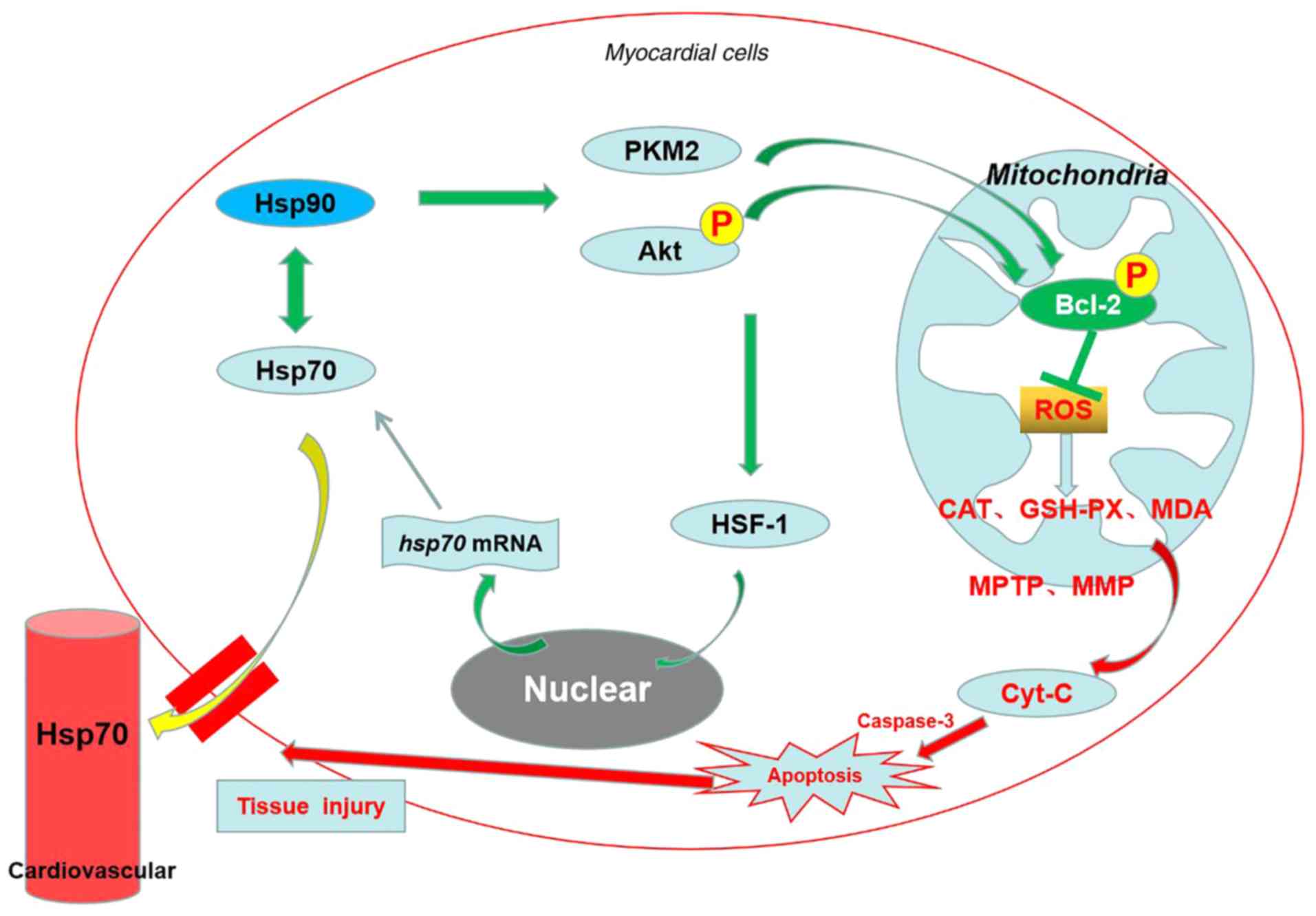
|
1
|
Sandercock DA, Hunter RR, Nute GR,
Mitchell MA and Hocking PM: Acute heat stress-induced alterations
in blood acid-base status and skeletal muscle membrane integrity in
broiler chickens at two ages: Implications for meat quality. Poult
Sci. 80:418–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bettaieb A and Averill-Bates DA:
Thermotolerance induced at a fever temperature of 40 degrees C
protects cells against hyperthermia-induced apoptosis mediated by
death receptor signalling. Biochem Cell Biol. 86:521–538. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst J, Gilbert JD and Byard RW: Urinary
incontinence, hyperthermia, and sudden death. J Forensic Sci.
56:1062–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones TS, Liang AP, Kilbourne EM, Griffin
MR, Patriarca PA, Wassilak SG, Mullan RJ, Herrick RF, Donnell HD
Jr, Choi K and Thacker SB: Morbidity and mortality associated with
the July 1980 heat wave in St Louis and Kansas City, Mo. JAMA.
247:3327–3331. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghaznawi HI and Ibrahim MA: Heat stroke
and heat exhaustion in pilgrims performing the Hajj (annual
pilgrimage) in Saudi Arabia. Ann Saudi Med. 7:323–326. 1987.
View Article : Google Scholar
|
|
6
|
Chang CP, Hsu YC and Lin MT: Magnolol
protects against cerebral ischaemic injury of rat heatstroke. Clin
Exp Pharmacol Physiol. 30:387–392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zak R: Development and proliferative
capacity of cardiac muscle cells. Circ Res. 35(Suppl II): S17–S26.
1974.
|
|
8
|
Islam A, Lv YJ, Abdelnasir A, Rehana B,
Liu ZJ, Zhang M, Tang S, Cheng YF, Chen HB, Hartung J and Bao ED:
The role of Hsp90α in heat-induced apoptosis and cell damage in
primary myocardial cell cultures of neonatal rats. Genet Mol Res.
12:6080–6091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin B, Tang S, Sun J, Zhang X, Xu J, Di L,
Li Z, Hu Y and Bao E: Vitamin C and sodium bicarbonate enhance the
antioxidant ability of H9C2 cells and induce HSPs to relieve heat
stress. Cell Stress Chaperones. 23:735–748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kregel KC: Heat shock proteins: Modifying
factors in physiological stress responses and acquired
thermotolerance. J Appl Physiol (1985). 92:2177–2186. 2002.
View Article : Google Scholar
|
|
11
|
Powers ET, Morimoto RI, Dillin A, Kelly JW
and Balch WE: Biological and chemical approaches to diseases of
proteostasis deficiency. Annu Rev Biochem. 78:959–991. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sonna LA, Wenger CB, Flinn S, Sheldon HK,
Sawka MN and Lilly CM: Exertional heat injury and gene expression
changes: A DNA microarray analysis study. J Appl Physiol (1985).
96:1943–1953. 2004. View Article : Google Scholar
|
|
13
|
Pérez-Salamó I, Papdi C, Rigó G, Zsigmond
L, Vilela B, Lumbreras V, Nagy I, Horváth B, Domoki M, Darula Z, et
al: The heat shock factor A4A confers salt tolerance and is
regulated by oxidative stress and the mitogen-activated protein
kinases MPK3 and MPK6. Plant Physiol. 165:319–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang J, Cao R, Wang X, Zhang Y, Wang P,
Gao H, Li C, Yang F, Zeng R, Wei P, et al: Mitochondrial PKM2
regulates oxidative stress-induced apoptosis by stabilizing Bcl2.
Cell Res. 27:329–351. 2017. View Article : Google Scholar :
|
|
16
|
Evans M, Fored CM, Bellocco R, Fitzmaurice
G, Fryzek JP, McLaughlin JK, Nyrén O and Elinder CG: Acetaminophen,
aspirin and progression of advanced chronic kidney disease. Nephrol
Dial Transplant. 24:1908–1918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wali RK: Aspirin and the prevention of
cardiovascular disease in chronic kidney disease: Time to move
forward? J Am Coll Cardiol. 56:966–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amici C, Rossi A and Santoro MG: Aspirin
enhances thermo-tolerance in human erythroleukemic cells: An effect
associated with the modulation of the heat shock response. Cancer
Res. 55:4452–4457. 1995.PubMed/NCBI
|
|
19
|
Zhang XH, Zhu HS, Qian Z, Tang S, Wu D,
Kemper N, Hartung J and Bao ED: The association of Hsp90 expression
induced by aspirin with anti-stress damage in chicken myocardial
cells. J Vet Sci. 17:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XH, Wu H, Tang S, Li QN, Xu J, Zhang
M, Su YN, Yin B, Zhao QL, Kemper N, et al: Apoptosis in response to
heat stress is positively associated with heat-shock protein 90
expression in chicken myocardial cells in vitro. J Vet Sci.
18:129–140. 2017. View Article : Google Scholar :
|
|
21
|
Yu GW, Chen J, Chen YY, Zheng MZ and Shen
YL: Heat-shock protein 90-dependent translocation of Akt to
mitochondria mediates insulin-like growth factor 1-induced
protection of rat hearts under hypothermic preservation. Chin J
Pathophysiol. 28:1773–1778. 2012.
|
|
22
|
Sato S, Fujita N and Tsuruo T: Modulation
of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA.
97:10832–10837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chrousus GP and Gold PW: The concepts of
stress and stress system disorders. Overview of physical and
behavioral homeostasis. JAMA. 267:1244–1252. 1992. View Article : Google Scholar
|
|
24
|
Laszlo A: The effects of hyperthermia on
mammalian cell structure and function. Cell Prolif. 25:59–87. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yatvin MB and Cramp WA: Role of cellular
membranes in hyperthermia: Some observations and theories reviewed.
Int J Hyperthermia. 9:165–185. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmad N, Wang Y, Haider KH, Wang B, Pasha
Z, Uzun O and Ashraf M: Cardiac protection by mitoKATP channels is
dependent on Akt translocation from cytosol to mitochondria during
late preconditioning. Am J Physiol Heart Circ Physiol.
290:H2402–H2408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chatterjee M, Andrulis M, Stühmer T,
Müller E, Hofmann C, Steinbrunn T, Heimberger T, Schraud H,
Kressmann S, Einsele H and Bargou RC: The PI3K/Akt signaling
pathway regulates the expression of Hsp70, which critically
contributes to Hsp90-chaperone function and tumor cell survival in
multiple myeloma. Haematologica. 98:1132–1141. 2013. View Article : Google Scholar :
|
|
29
|
Shen HY, He JC, Wang Y, Huang QY and Chen
JF: Geldanamycin induces heat shock protein 70 and protects against
MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem.
280:39962–39969. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Chung D, Yang YC, Neely L,
Tsurumoto S, Fan J, Zhang L, Biamonte M, Brekken J, Lundgren K and
Burrows F: Identification of new biomarkers for clinical trials of
Hsp90 inhibitors. Mol Cancer Ther. 5:1256–1264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu D, Xu J, Song E, Tang S, Zhang X,
Kemper N, Hartung J and Bao E: Acetyl salicylic acid protected
against heat stress damage in chicken myocardial cells and may
associate with induced Hsp27 expression. Cell Stress Chaperones.
20:687–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao B, Sun G, Feng G, Duan W, Zhu X, Chen
S, Hou L, Jin Z and Yi D: Carboxy terminus of heat shock protein
(HSP) 70-interacting protein (CHIP) inhibits HSP70 in the heart. J
Physiol Biochem. 68:485–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fontana J, Fulton D, Chen Y, Fairchild TA,
McCabe TJ, Fujita N, Tsuruo T and Sessa WC: Domain mapping studies
reveal that the M domain of hsp90 serves as a molecular scaffold to
regulate Akt-dependent phosphorylation of endothelial nitric oxide
synthase and NO release. Circ Res. 90:866–873. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikeyama S, Kokkonen G, Shack S, Wang XT
and Holbrook NJ: Loss in oxidative stress tolerance with aging
linked to reduced extracellular signal-regulated kinase and Akt
kinase activities. FASEB J. 16:114–116. 2002. View Article : Google Scholar
|
|
35
|
Kaufmann T, Schlipf S, Sanz J, Neubert K,
Stein R and Borner C: Characterization of the signal that directs
Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J
Cell Biol. 160:53–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jackson S: Molecular chaperones. Berlin
Heidelberg, Springer-verlag; pp. 1–272. 2013
|
|
37
|
Pugazhenthi S, Nesterova A, Sable C,
Heidenreich KA, Boxer LM, Heasley LE and Reusch JE: Akt/protein
kinase B up-regulates Bcl-2 expression through cAMP-response
element-binding protein. J Biol Chem. 275:10761–10766. 2000.
View Article : Google Scholar
|
|
38
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ling YH, Liebes L, Zou Y and Perez-Soler
R: Reactive oxygen species generation and mitochondrial dysfunction
in the apoptotic response to Bortezomib, a novel proteasome
inhibitor, in human H460 non-small cell lung cancer cells. J Biol
Chem. 278:33714–33723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Govender J, Loos B, Marais E and
Engelbrecht AM: Mitochondrial catastrophe during
doxorubicin-induced cardio-toxicity: A review of the protective
role of melatonin. J Pineal Res. 57:367–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang S, Wang Y, Zhang Z, Liu Q and Gu J:
Cardioprotective effects of fibroblast growth factor 21 against
doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell
Death Dis. 8:e30182017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Govender J, Loos B and Engelbrecht AM:
Melatonin: A protective role against doxorubicin-induced
cardiotoxicity. Future Oncol. 11:2003–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu D, Ma Z, Di S, Yang Y, Yang J, Xu L,
Reiter RJ, Qiao S and Yuan J: AMPK/PGC1α activation by melatonin
attenuates acute doxorubicin cardiotoxicity via alleviating
mitochondrial oxidative damage and apoptosis. Free Radic Biol Med.
129:59–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Priya LB, Baskaran R, Huang CY and Padma
VV: Neferine ameliorates cardiomyoblast apoptosis induced by
doxorubicin: Possible role in modulating NADPH oxidase/ROS-mediated
NFκB redox signaling cascade. Sci Rep. 7:122832017. View Article : Google Scholar
|
|
45
|
Fan AC, Bhangoo MK and Young JC: Hsp90
functions in the targeting and outer membrane translocation steps
of Tom70-mediated mitochondrial import. J Biol Chem.
281:33313–33324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Daugaard M, Rohde M and Jaattela M: The
heat shock protein 70 family: Highly homologous proteins with
overlapping and distinct functions. FEBS Lett. 581:3702–3710. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chaudhury S, Welch TR and Blagg BSJ: Hsp90
as a target for drug development. ChemMedChem. 1:1331–1340. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dieterle A, Orth R, Daubrawa M, Grotemeier
A, Alers S, Ullrich S, Lammers R, Wesselborg S and Stork B: The Akt
inhibitor triciribine sensitizes prostate carcinoma cells to
TRAIL-induced apoptosis. Int J Cancer. 125:932–941. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang W, Xia Y, Hawke D, Li X, Liang J,
Xing D, Aldape K, Hunter T, Alfred Yung WK and Lu Z: PKM2
phosphorylates histone H3 and promotes gene transcription and
tumorigenesis. Cell. 150:685–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Johnson JD, Campisi J, Sharkey CM, Kennedy
SL, Nickerson M and Fleshner M: Adrenergic receptors mediate
stress-induced elevations in extracellular Hsp72. J Appl Physiol
(1985). 99:1789–1795. 2005. View Article : Google Scholar
|
|
51
|
Kumaraguru U, Pack CD and Rouse BT:
Toll-like receptor ligand links innate and adaptive immune
responses by the production of heat-shock proteins. J Leukoc Biol.
73:574–583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Asea A, Kraeft SK, Kurt-Jones EA,
Stevenson MA, Chen LB, Finberg RW, Koo GC and Calderwood SK: HSP70
stimulates cytokine production through a CD14-dependant pathway,
demonstrating its dual role as a chaperone and cytokine. Nat Med.
6:435–442. 2000. View
Article : Google Scholar : PubMed/NCBI
|