Introduction
Benign prostatic hyperplasia (BPH), an urologic and
chronic disease, has been regarded as one of the most frequently
occurring diseases among old-aged males, with proportions reaching
≤80% in males aged >70 years old (1,2).
Symptoms of BPH commonly include proliferation of smooth muscle and
epithelial cells inside the prostatic transition area (3). BPH frequently begins as a simple
micronodular hyperplasia that gradually develops into a macroscopic
nodular growth; it can also cause urinary tract symptoms, such as
increased urinary urgency and frequency, nocturia, and weak flow
(2,4). At present, there is an increased
focus on treatments for BPH, such as transurethral resection of the
prostate, pharmacotherapy and phytotherapy, particularly in western
countries (5,6).
Hedgehog refers to a group of proteins that were
first detected in Drosophila, and serves significant roles
in embryonic development (7).
Additionally, the Hedgehog signaling pathway has been highlighted
as crucial not only for maintaining the stem cell compartment, but
also for cell proliferation and differentiation during embryonic
tissue patterning (8). This
signaling pathway, once activated, can also enhance oncogenesis due
to its ability to promote tumor invasion and metastasis in a number
of diseases, such as in prostate and pancreatic cancer, and gastric
carcinoma (9). Gli1, Gli2 and
Gli3 are important transcription factors in the Hedgehog signaling
pathway, and when the Hedgehog signaling pathway is inactivated,
the transcription of Gli1 is inhibited (10). In addition, β transducing repeat
containing 2 mediates the degradation of Gli2, and Gli3 is cleaved
into small fragments in vivo, consequently, Gli proteins
cannot enter the nucleus and exert their function as transcription
factors (11). When Sonic
Hedgehog (Shh) binds to cell membrane receptors and activates the
Hedgehog signaling pathway, Gli proteins translocate into the
nucleus, and mediates the transcription and expression of
downstream target genes (12-14). A previous study revealed that
blocking of Hedgehog signaling could suppress Shh-induced cell
angiogenesis and migration (8).
Cyclopamine is a powerful hedgehog signaling antagonist, and mainly
targets the Smoothened (Smo) proteins (15).
The inhibitory effects of cyclopamine on the
Hedgehog signaling pathway is achieved as follows: Cyclopamine
binds directly to Smo, which affects its function and interferes
with Shh signaling. Cyclopamine also inhibits the Hedgehog
signaling pathway in a Ptch-independent manner and acts to suppress
carcinogenesis. Additionally, it converts Smo into its active form
(16). The mechanism by which
cyclopamine specifically inhibits Shh activation is currently
unclear. It was proposed that cyclopamine interferes with the
reception of Hedgehog signaling in vertebrates; this process
involves receptors with multiple transmembrane domains, namely,
Ptch and Smo (17).
BPH is caused by a non-malignant proliferation of
epithelial and stromal cells in the prostate gland areas (18). It has been reported that
stromal-epithelial associations play central modulator roles in
prostate growth and in homeostasis of the adult prostate (19). Previously, various studies have
demonstrated close associations between cyclopamine and the
Hedgehog signaling pathway, and this compound is commonly employed
in the treatment of prostate cancer (13,14). To further determine its functions
and roles in regulating BPH, the present study investigated the
effects of cyclopamine on the proliferation and apoptosis of
epithelial and stromal cells in rats with BPH by blocking the
Hedgehog signaling pathway.
Materials and methods
Study subjects
A total of 15 normal male SD rats and 50 SD rats
with BPH (6-8 weeks, weighing 400-450 g) were purchased from the
Hunan SLAC Laboratory Animal Co., Ltd. (Changsha, China) and housed
under a 12 h light/dark cycle at 22±2°C with relative humidity at
50±10%. Following 1 week of acclimatization, rats were fasted
overnight with free access to water prior to experiments.
Cyclopamine (0, 10, 20 and 30 mg/kg) was intraperitoneally injected
into rats with BPH (n=5), and BPH tissues was collected for western
blot analysis of Smo protein. The remaining 45 rats were assigned
into the normal group (normal rats, n=15), the BPH group (BPH rats,
n=15) and the cyclopamine group (BPH rats, n=15). Rats in the
cyclopamine group were intraperitoneally injected with 20 mg/kg
cyclopamine. Rats in the normal and BPH groups were fed normally.
After 1 week, rats were sacrificed via CO2 overdose;
prostate tissues were obtained to determine the indexes described
below. Wet weight was measured using an analytical balance,
prostate volume was measured by the volumetric method (20), and prostate index (PI) was
calculated using the formula: PI=wet weight of prostate/total body
weight. All rats were fed in specific-pathogen-free grade chambers,
which was compliant with the Laboratory Animal Requirements of
Environment and Housing Facilities Guidelines (GB 14925-2010).
Hematoxylin and eosin (H&E)
staining
Tissues were sectioned (5-µm thick), laid out
at 45°C, selected and dried at 60°C for 1 h. They were then
deparaffinized with xylene and stained with the H&E solution
(Beijing Solarbio Science & Technology Co., Ltd. Beijing,
China). Then, tissue sections were dehydrated with an ethanol
gradient (50, 70, 80 and 95%), cleared with xylene, mounted using
neutral gum, and observed under a Zeiss fluorescence microscope
(magnification ×400; Axio Observer A1/D1/Z1, Zeiss AG to analyze
histopathological changes in prostate tissues.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) staining
Triton X-100 (0.1%) was added to tissue sections,
which were then placed on ice for 4 min and washed three times with
PBS (2 min each time). TUNEL reaction solution (50 µl,
ZK-8005, Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) was
added for 1 h at 37°C; tissues were then washed three times with
PBS. Diaminobenzidine/H2O2 solution (cat. no.
2014, Invitrogen; Thermo Fisher Scientific, Inc.) was then added
for 5 min at room temperature, after which it was terminated by
washing the sections with PBS. Hematoxylin was applied for
re-staining at 37°C for 10 sec, and sections were dehydrated,
cleared, sealed with anti-fluorescence quenching sealing solution
and observed under a fluorescence microscope (magnification ×400;
BX43; Olympus Optical Co., Ltd.) with five high power fields
randomly selected to count the number of TUNEL positive cells.
Immunohistochemistry
Tissues were fixed using formaldehyde,
paraffin-embedded, and sectioned into 4 µm slices. They were
then dried in a 60°C incubator for 1 h, deparaffinized by xylene
(Mingtou Industry and Trade Co., Ltd., Shanghai, China), dehydrated
an ethanol gradient (50, 70, 80 and 95%), incubated with 3%
H2O2 for 10 min, and finally washed with PBS.
Antigen retrieval was performed under high pressure for 90 sec,
after which samples were cooled down to room temperature.
Non-specific binding was blocked with 5% bovine serum albumin (BSA;
Shanghai Sangon Biological Engineering Technology & Services
Co., Ltd.) blocking buffer at 37°C for 30 min. Tissue sections were
then incubated with the primary antibody (1:200, Ki67 monoclonal
antibody, Biogot Technology, Inc., Nanjing, China) at 4°C
overnight. Goat anti-mouse IgG secondary antibody labeled by biotin
(1:1,000, ab6759, Abcam Inc., Cambridge, MA, USA) was added at 37°C
for 30 min. Subsequently, streptavidin solution (Zhongshan
Goldenbridge Biotechnology, Co. Ltd., Beijing, China) was added at
37°C for 30 min. This was followed by addition of
3,3′-diaminobenzidine (Bioss Biotechnology Co., Ltd., Beijing,
China) for 5 min at room temperature. Tissues were washed with
water for 5 min, and were immersed in the hematoxylin solution
(Yinggong Reagent Co., Ltd., Shanghai, China) for 5 min at 37°C.
Lastly, tissues were washed with running water. PBS was used in
place of the primary antibody in the negative control group. Tissue
sections with positive expression were included in the positive
control group. Tissue sections were observed and imaged under an
optical microscope (XSP-36, Boshida Optical Instrument Co.,
Shenzhen, China). A total of five high power fields (magnification,
×200) were randomly selected, and 100 prostate cells in each field
were analyzed. If the percentage of cells with positive expression
was <10, ≥10 but <50%, or ≥50%, the staining result is
negative, positive, or strongly positive, respectively (15).
Cell treatment
A region of the anterior lobe on the right side and
ventral lobe of the prostate were collected, washed twice with the
D-Hanks solution, and cut into pieces. Any visible macro-vascular
tissue was removed, and the lobes were digested with 2% trypsin at
room temperature for 30 min. Following a 5-min centrifugation at
1,789 × g at 25°C, tissues were digested with 0.06% collagenase
type II at room temperature for 15 min, and were then filtered. The
filtrate was mixed evenly with culture suspension of epithelial
cells, which was then inoculated in a culture flask embedded with
fibronectin (Shanghai Sangon Biological Engineering Technology
& Services Co., Ltd.). Cells were cultured with 10% fetal
bovine serum (FCS500, Excell Bio Company, Shanghai, China) at 5%
CO2 and 37°C. A mixture composed of
insulin-transferrin-selenium (with a volume ratio of 1:100), 0.5
µg/l β-endothelial cell growth factor and 100×103
U/l penicillin-streptomycin double-antibody (B540732; Shanghai
Sangon Biological Engineering Technology & Services Co., Ltd.)
was added.
MTT assay
Epithelial and stromal cells (2.5×105
cells/ml) were seeded into a 96-well plate. Cells were maintained
in an incubator at 5% CO2 and 37°C. The plate was
removed on days 1, 2, 3, 4 and 5 for the MTT assay. Briefly, 10
µl MTT solution (5 g/l) (GD-Y1317, Guduo Biotechnology
Company, Shanghai, China) was added into each well. Cells were then
further incubated at 37°C for 24 h. This was followed by the
addition of 100 µl dimethyl sulfoxide (D2650, Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). The plate was then slightly shaken
and mixed evenly for 10 min. An automatic enzymelabelled reading
meter (BS-1101, Detielab Co., Ltd., Nanjing, China) was used to
measure the optical density value at 490 nm.
Flow cytometry
Cells were collected by 0.25% trypsin at 37°C for 30
min, washed with PBS, and re-centrifuged at 1,145 × g for 5 min at
room temperature. Cell precipitates were fixed using pre-cooled 70%
ethanol at 4°C overnight, and washed twice with PBS. Cell
suspension (100 µl, total number of cells no less than
106 cells/ml) was obtained, to which 1 ml of 50 mg/l
propidium iodide (containing RNAase, Sigma-Aldrich; Merck KGaA) was
added. Staining was conducted for 30 min in the dark. A nylon net
with 100 pores was used for filtration; flow cytometry was
performed with an excitation wavelength of 488 nm for cell cycle
analysis (ModFit LT; version 4.1; Verity Software House, Inc.).
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide double staining was used to examine cell
apoptosis; cells were treated aforementioned for cell cycle
analysis. Cells were then incubated for 48 h at 37°C with 5%
CO2. Subsequently, cells were collected, washed twice
with PBS, centrifuged at 1,145 × g for 5 min at room temperature,
and resuspended in 200 µl binding buff er. As per the
instructions of the Annexin-V-FITC cell apoptosis detection kit
(K201-100, BioVision, Mountain View, CA, USA), Annexin V-FITC, PI
and HEPES were used to make up the Annexin V-FITC/propidium iodide
cocktail in a 1:2:50 ratio. Cells were then incubated in the dark
at room temperature for 20 min. Flow cytometry was used to detect
cell apoptosis at an excitation wavelength of 488 nm. The
experiment was repeated three times.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in prostate cells of all rats were
extracted with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.).
RT-qPCR was carried out according to the instructions provided by
the TaqMan MicroRNA Assays Reverse Transcription kit (cat. no.
4427975, Applied Biosystems Inc.; Thermo Fisher Scientific, Inc.)
using an ABI7500 quantitative PCR instrument (7500, ABI Company,
Oyster Bay, N.Y., USA). The qPCR reaction conditions were as
follows: Pre-denaturation (95°C) for 5 min, denaturation (94°C) for
10 sec, annealing for 30 sec at 30°C, and DNA strand extension for
15 sec at 72°C; 40 cycles were performed in total. GAPDH was used
as the internal reference gene, and relative mRNA expression was
determined by the 2-∆∆Cq method (21), where ΔCt=Cttargeted
gene-CtGAPDH. The experiment was repeated three
times. Primer sequences of RT-qPCR are listed in Table I.
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Premier sequence
(5′-3′) |
|---|
| Shh | Forward:
ACCGAGGGCTGGGACGAAGA |
| Reverse:
ATTTGGCCGCCACCGAGTT |
| Ptch1 | Forward:
TTTGGACTGCTTCTGGGAAGGG |
| Reverse:
TTTTTGTTGGGGGCTGTGGC |
| Gli1 | Forward:
GAAGGTGAAGGTCGGAGT |
| Reverse:
GTCCAGGCTGGCATCCGACA |
| b-FGF | Forward:
GAGGAGTTGTGTCTATCAAAG |
| Reverse:
GTTCGTTTCAGTGCCACATACC |
| TGF-β | Forward:
CAAGAGGCTGTGTTGVTGTGAATC |
| Reverse:
GTTGGTTTGAGAAAATCCATCGG |
| Bax | Forward:
GACCAGGGTGGCTGGGAAGG |
| Reverse:
GATGGTGAGCGAGGCGGTGA |
| Bcl-2 | Forward:
CCGGCATCTGCACACCTGG |
| Reverse:
CACAACTTTGTTTCATGGTCCATCC |
| GAPDH | Forward:
AACGGATTTGGTCGTATTGGG |
| Reverse:
TCGCTCCTGGAAGATGGTGAT |
Western blot analysis
Protein was detected using the bicinchoninic acid
kit (cat. no. 20201ES76; Yeason Biotechnology Co. Ltd.); samples
were diluted in deionized water to achieve a loading quantity of 30
µg protein per lane. Proteins were separated via SDS-PAGE
(12% gel) transferred onto a nitrocellulose membrane, which was
sealed using 5% skim milk powder at 4°C and kept overnight. Diluted
primary anti-rabbit polyclonal antibodies were added to the cells,
and included Shh (1:1,000, ab53281, Abcam), Gli1 (1:1,500,
ab151796, Abcam), Ptch1 (1:1,500, ab53715, Abcam), basic
fibroblastic growth factor (b-FGF; 1:200, ab99979, Abcam),
transforming growth factor β (TGF-β; ab31013, Abcam), B-cell
lymphoma-2 (Bcl-2)-associated X protein (Bax; 1:2,000, ab32503,
Abcam), Bcl-2 (1:500, ab692, Abcam), and GAPDH (1:2,500, ab9485,
Abcam). The rabbit anti-human monoclonal secondary antibody (1:200,
bs-0361R-HRP, BIOSS Company, Beijing, China) was used then added.
Subsequently, protein bands were visualized using electrochemical
luminescence (ECL808-25, Biomiga, Inc., San Diego, CA, USA) and
analyzed by Image J software (version 2.1.4.7; National Institutes
of Health Inc.).
Statistical analysis
All data were analyzed with the SPSS 21.0 software
(IBM Corp., Armonk, NY, USA). Data were presented as the mean ±
standard deviation. Comparisons among multiple groups were
determined by one-way analysis of variance. Pairwise comparison of
mean values was analyzed by the Least Significant Difference
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
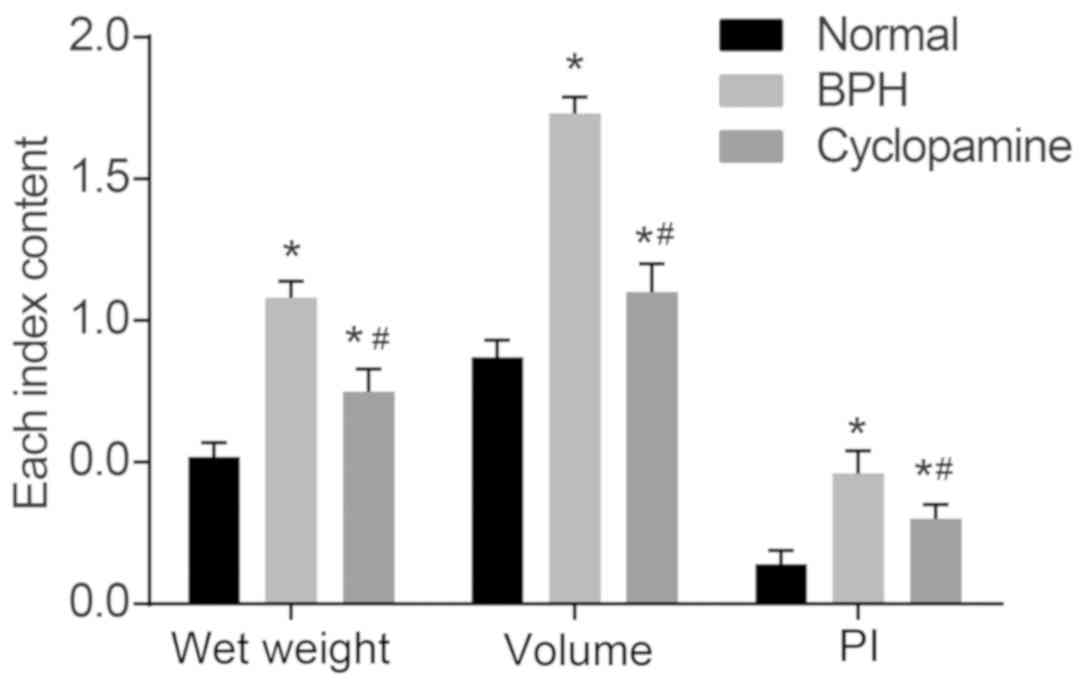
Cyclopamine decreases wet weight, volume,
and PI of prostate in rats with BPH
In our study, we administered 0, 10, 20 and 30 mg/kg
cyclopamine to rats with BPH rats (n=5), and BPH tissues were
collected for determination of wet weight, volume, and PI of
prostate in rats with BPH. The wet weight, volume and PI of
prostate tissues in rats among the three groups were observed
following establishment of the BPH model (Fig. 1). As compared with those in the
normal group, the wet weight, volume and PI of the prostate were
significantly increased in the BPH and cyclopamine groups
(P<0.05). As compared with the BPH group, these parameters in
the cyclopamine group were significantly decreased (P<0.05).
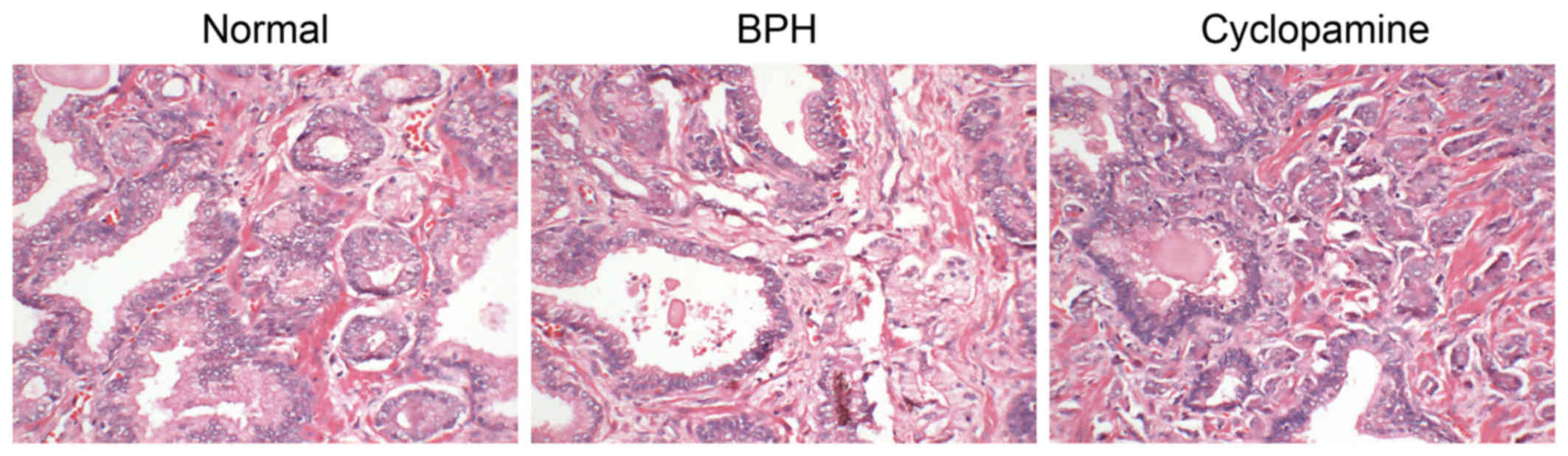
Histopathological alterations in the
prostate tissues after BPH model establishment and in response to
cyclopamine treatment
H&E staining was conducted to observe the
histopathological changes in rat prostate tissues. As presented in
Fig. 2, prostatic epithelial
cells in the normal group showed regular alignment as a columnar
monolayer. Glands were of similar size, and were regularly
arranged; the prostatic cavity was not expanded. In the BPH group,
prostatic epithelia were observed to exhibit papillary hyperplasia
and protrusion into the prostatic cavity in a zigzag fashion. The
mesenchyme and surrounding vessels were expanded, with accompanying
hyperemia and edema. Few glands were expanded, and the prostatic
cavity was also enlarged. Regarding the cyclopamine group,
prostatic epithelial cells were arranged in a columnar monolayer.
The mesenchyme and the surrounding vessels were also expanded,
along with hyperemia and slight edema. Few glands exhibited
expanded cavities with hyperplasia.
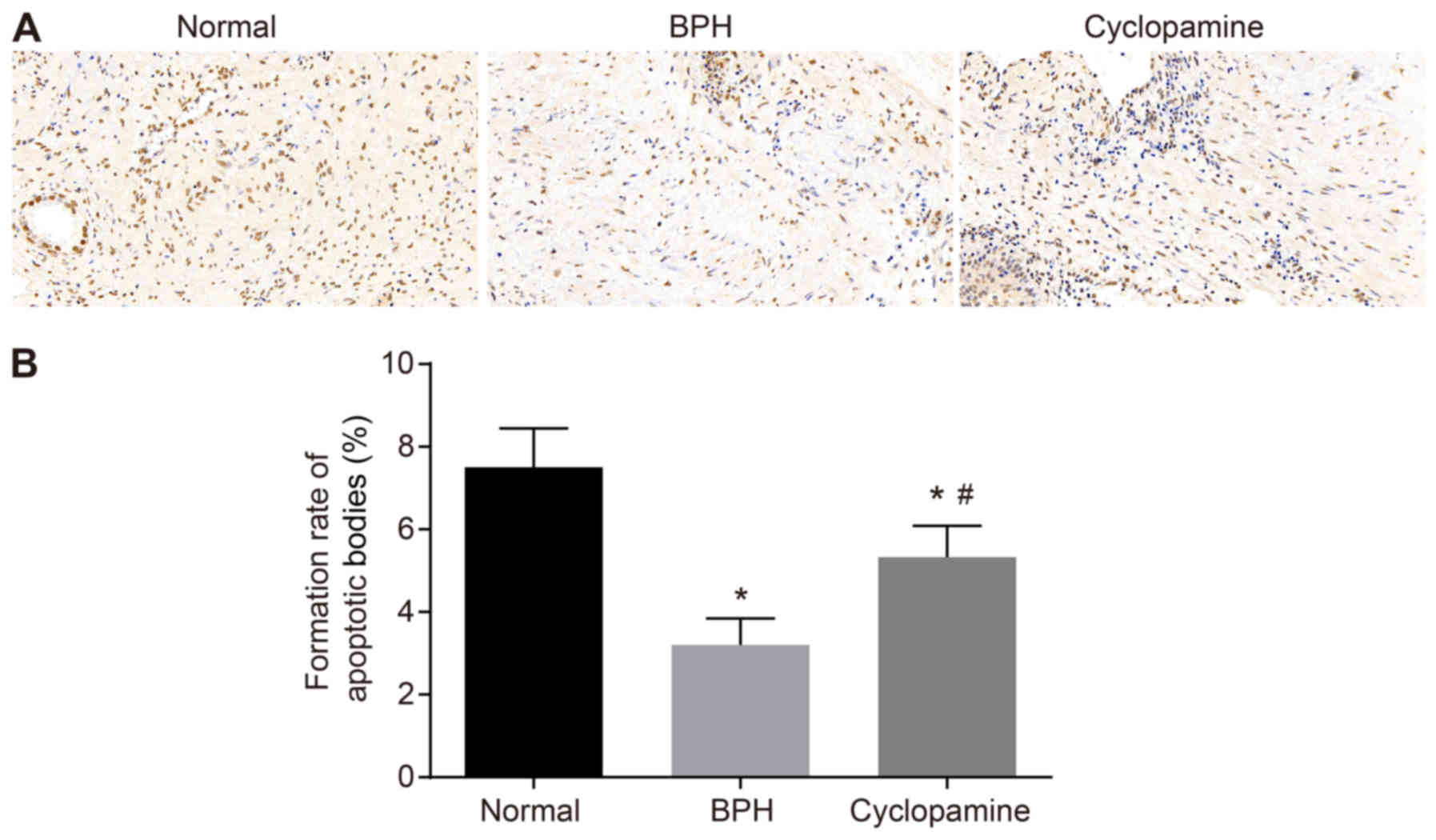
Cyclopamine increases apoptotic body
formation in BPH rats
A TUNEL assay was performed to investigate the
effects of cyclopamine on prostate tissue cell apoptosis in BPH
rats (Fig. 3). Compared with that
of the normal group, apoptotic body formation was significantly
reduced in the BPH and cyclopamine groups (P<0.05).
Additionally, compared with that of the BPH group, apoptotic body
formation was significantly increased in the cyclopamine group
(P<0.05).
As TUNEL staining was enhanced and apoptotic body
formation was increased in the cyclopamine group, these parameters
may serve as indicators in evaluating BPH. Our results suggested
that cyclopamine could upregulate apoptotic body formation in BPH
rats, as presented in Fig.
3B.
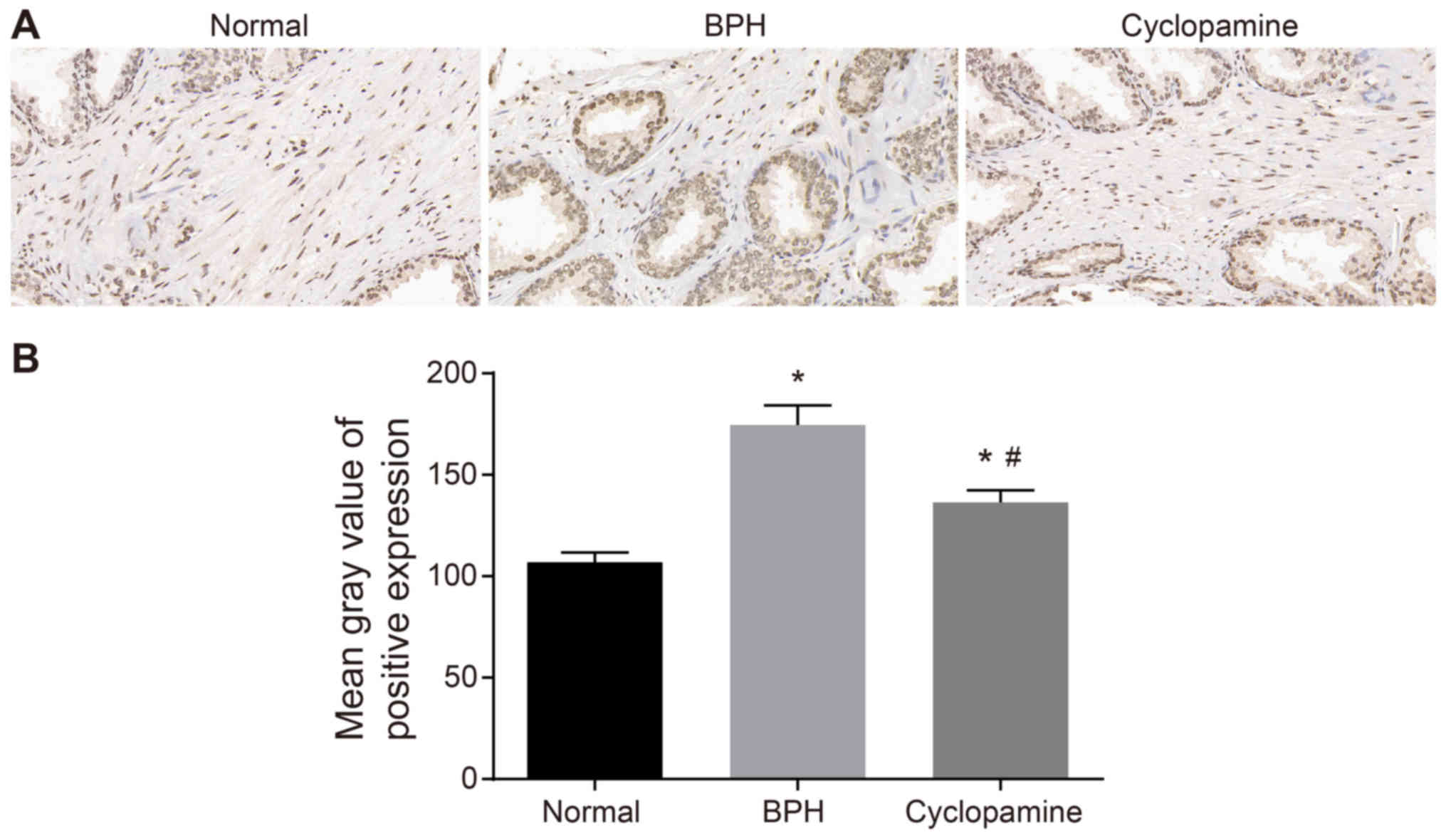
Cyclopamine reduces rate of Ki67 positive
expression in BPH rats
The effects of cyclopamine on Ki67 expression in BPH
rats was assessed by immunohistochemistry (Fig. 4A). As compared with that of the
normal group, the positive Ki67 expression in the BPH and
cyclopamine groups was significantly increased (P<0.05), which
suggested Ki67 as an indicator for evaluating BPH. Compared with
the BPH group, positive Ki67 expression was significantly decreased
in the cyclopamine group (P<0.05). The results further suggested
that cyclopamine could inhibit Ki67 protein expression (Fig. 4B). Collectively, our results
suggested that cyclopamine inhibited BPH in rats by suppressing the
expression of the cell proliferation marker Ki67 in BPH
tissues.
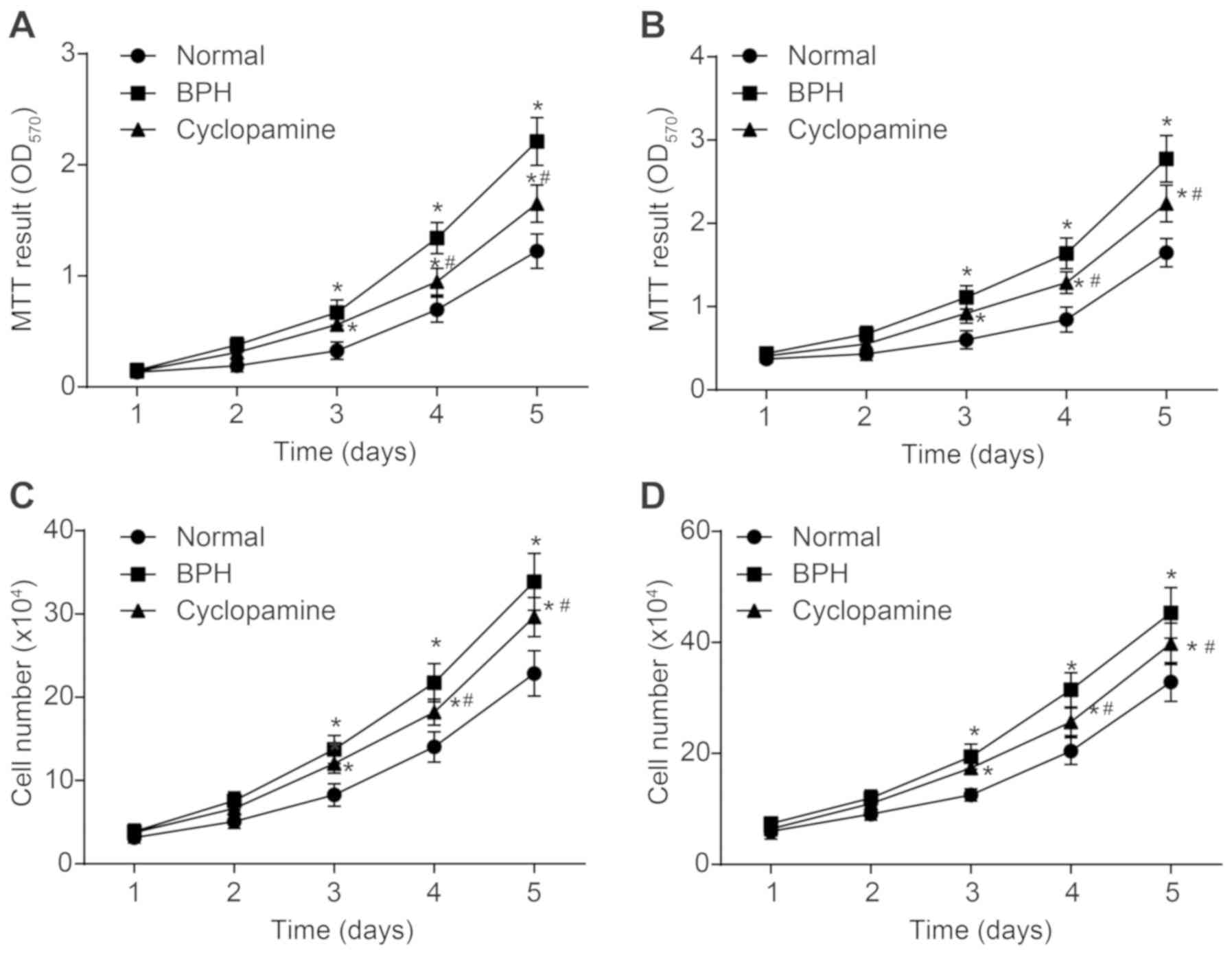
Cyclopamine hinders epithelial and
stromal cell proliferation in BPH rats
An MTT assay was performed to determine the effects
of cyclopamine on the proliferation of stromal (Fig. 5A) and epithelial cells (Fig. 5B) in BPH rats. As compared with
the control group, cell proliferation in the BPH and cyclopamine
groups was significantly increased (P<0.05). Compared with the
BPH group, cell proliferation in the BPH group was significantly
decreased (P<0.05). These findings suggested that cyclopamine
could inhibit proliferation of epithelial and stromal cells in the
prostate. The numbers of epithelial and stromal cells in prostate
tissues were recorded via the cell counting method (Fig. 5C and D). Changes in the numbers of
epithelial and stromal cells were comparable between the MTT assay
and the cell counting method. Hence, cyclopamine contributed to
repressed epithelial and stromal cell proliferation in BPH
rats.
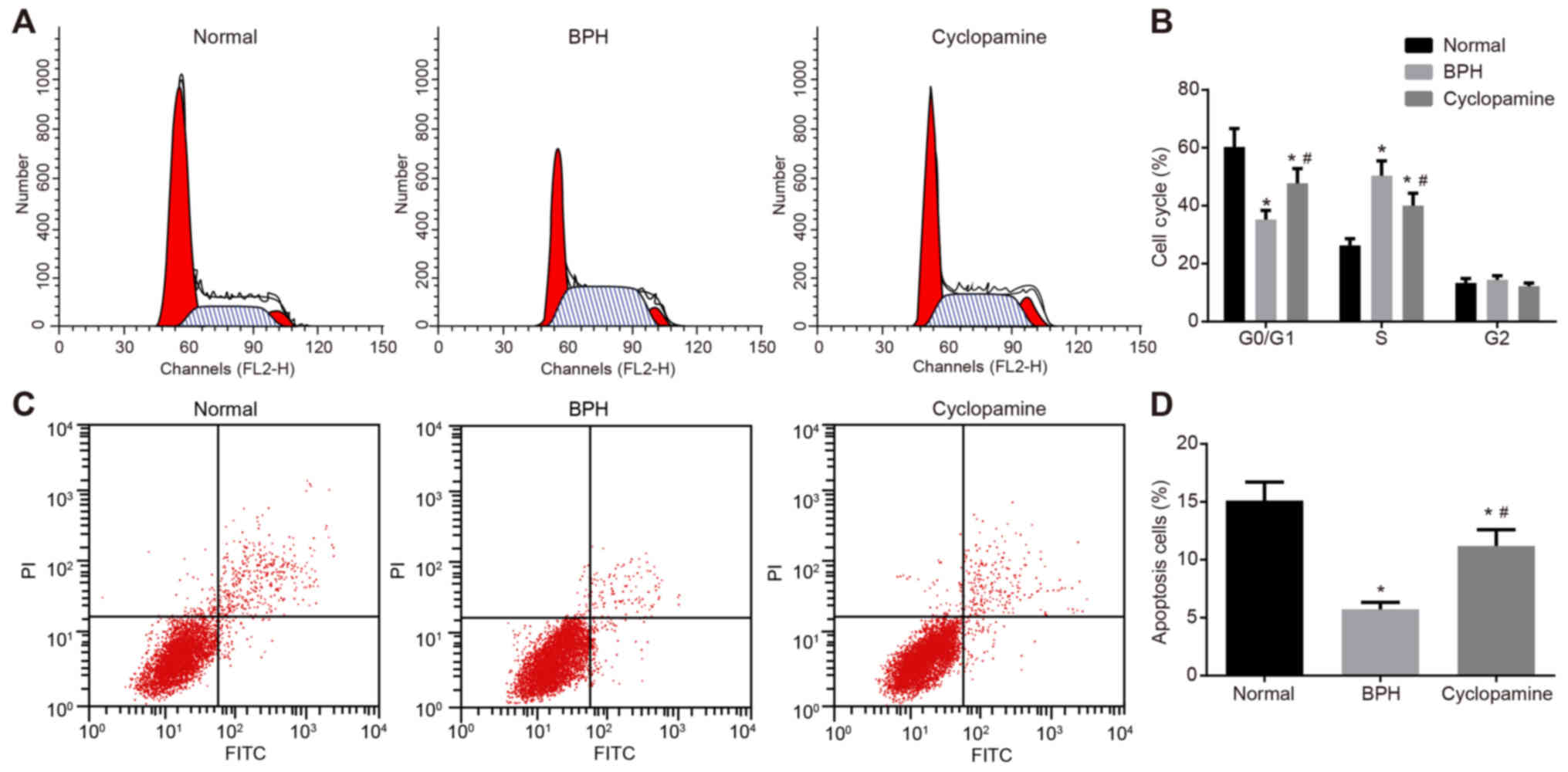
Cyclopamine inhibits epithelial cell
cycle progression and promotes epithelial cell apoptosis in BPH
rats
Propidium iodide staining was adopted to detect
epithelial cell cycle distribution (Fig. 6A-D). As compared with the normal
group, the proportion of cells arrested at the G0/G1 phase in the
BPH and cyclopamine groups was significantly reduced, while the
proportion of cells at arrested the S phase was increased
(P<0.05). Compared with the BPH group, the proportion of cells
arrested at the G0/G1 phase in the cyclopamine group was
significantly increased, while that of cells arrested at the S
phase were reduced (P<0.05). Results indicated that cyclopamine
could suppress cell proliferation and arrest cells at the G0/G1
phase.
Annexin V-FITC/propidium iodide double staining was
adopted to assess epithelial cell apoptosis. The number of
apoptotic cells in the BPH and cyclopamine groups were
significantly reduced compared with the control group (P<0.05).
Conversely, the number of apoptotic cells in the cyclopamine group
was significantly increased (P<0.05) than that of the BPH group.
These results suggested that cyclopamine could induce the apoptosis
of epithelial cells in BPH rats.
Cyclopamine decreases the expression of
Shh, Ptch1, Gli1, b-FGF, TGF-β and Bcl-2, but increases that of Bax
in BPH rats
Finally, RT-qPCR (Fig.
7) and western blot analysis (Fig. 8) were used to measure mRNA and
protein levels of Hedgehog signaling pathway- and
apoptosis-associated genes, as well as b-FGF and TGF-β. Compared
with the normal group, the mRNA and protein levels of Shh, Ptch1,
Gli1, b-FGF, TGF-β, and Bcl-2 were significantly increased in the
BPH and cyclopamine groups; conversely, Bax mRNA and protein levels
were significantly decreased (P<0.05). In addition, as compared
with those of the BPH group, the protein levels of Shh, Ptch1,
Gli1, b-FGF, TGF-β and Bcl-2 were greatly reduced in the
cyclopamine group, while Bax mRNA and protein levels were greatly
increased (P<0.05). These results indicated that cyclopamine
blocks the Hedgehog signaling pathway to inhibit cell proliferation
and enhances apoptosis in BPH rats.
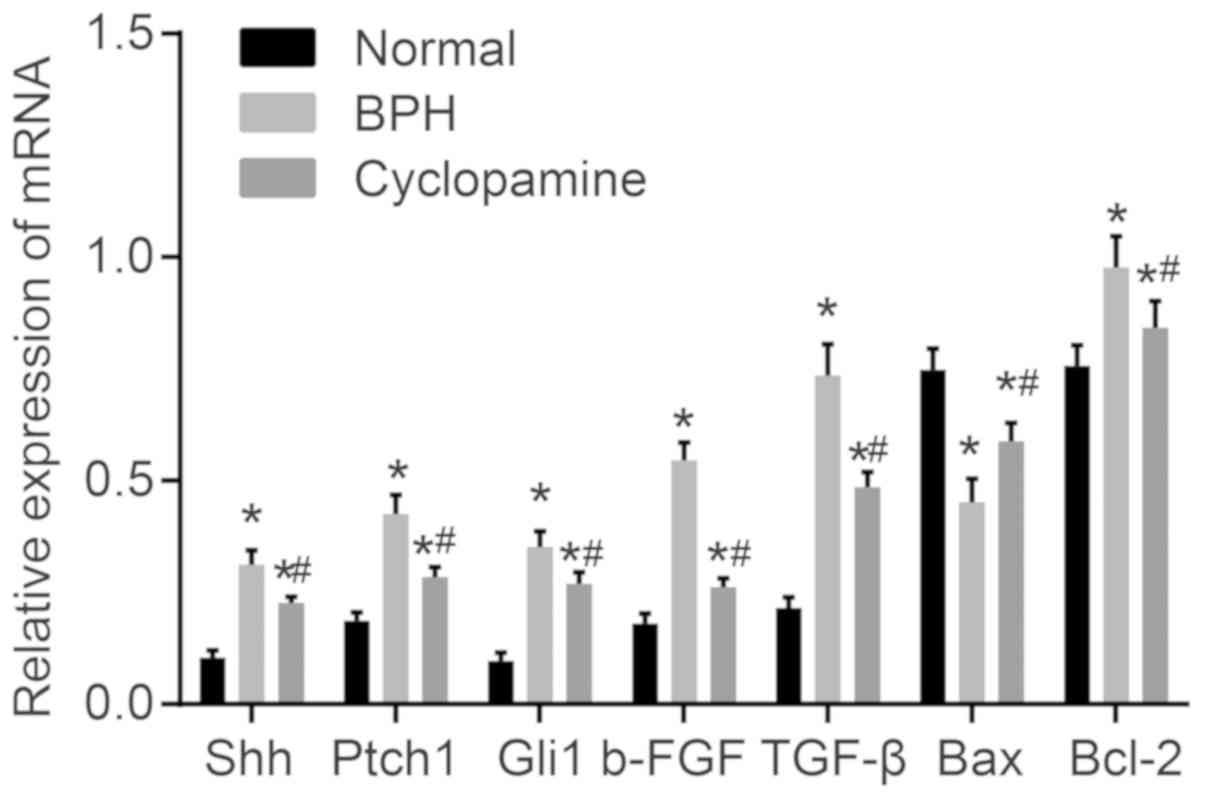 | Figure 7Effect of cyclopamine on mRNA
expression of Shh, Ptch1, Gli1, b-FGF, TGF-β, and Bcl-2 in the
normal, BPH and cyclopamine groups. *P<0.05 vs.
normal group. #P<0.05 vs. BPH group. Shh, sonic
hedgehog; Ptch1, Patched-1; Gli1, glioma-associated oncogene
homolog 1; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein; b-FGF, basic fibroblastic growth factor, TGF-β,
transforming growth factor β; BPH, benign prostatic
hyperplasia. |
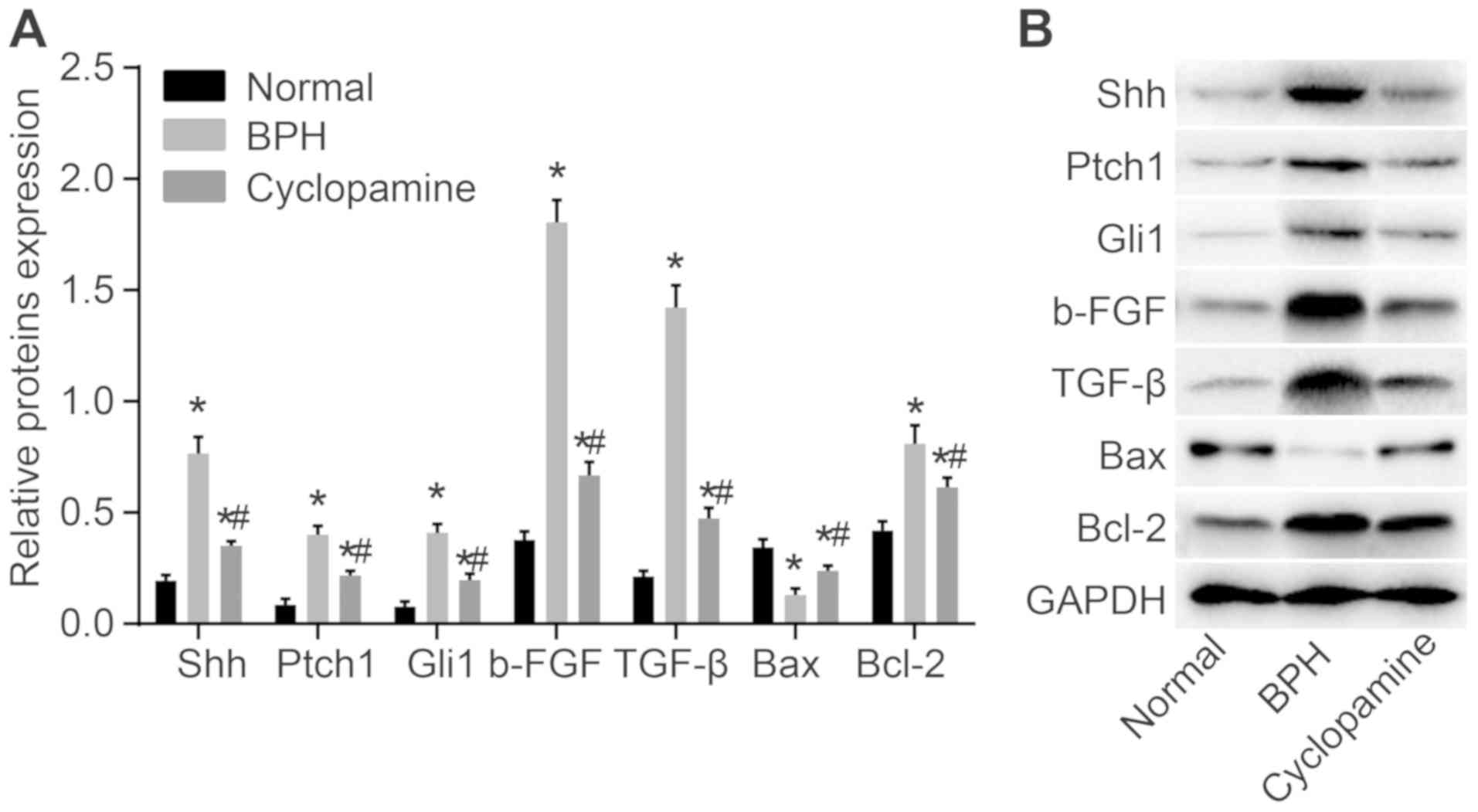 | Figure 8Western blot showing protein levels
of Shh, Ptch1, Gli1, b-FGF, TGF-β and Bcl-2 in BPH rats. (A)
Protein bands of Shh, Ptch1, Gli1, b-FGF, TGF-β, Bax, and Bcl-2.
(B) Quantitative analysis of the relative protein expression levels
of Shh, Ptch1, Gli1, b-FGF, TGF-β, Bax and Bcl-2.
*P<0.05 vs. normal group. #P<0.05 vs.
BPH group. Shh, sonic hedgehog; Ptch1, Patched-1; Gli1,
glioma-associated oncogene homolog 1; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; b-FGF, basic fibroblastic growth
factor, TGF-β, transforming growth factor β; BPH, benign prostatic
hyperplasia. |
Discussion
In recent years, BPH has become a disease that
affects an increasing number of males >50 years old across the
world due to its poorly understood pathogenesis (4). Studies have reported that the
Hedgehog signaling pathway is activated in prostate cancers
(16,22). As an inhibitor of the Hedgehog
signaling pathway, cyclopamine serves a crucial role in suppressing
prostate cancer and improving survival (13); however, the underlying mechanism
of action in BPH remains uncertain. Thus, the present study aimed
to investigate the effects of cyclopamine on the proliferation and
apoptosis of epithelial and stromal cells in BPH rats by blocking
the Hedgehog signaling pathway.
Initially, our results demonstrated that cyclopamine
could decrease the wet weight, the volume and PI of prostate in BPH
rats. In addition, the formation of nodular overgrowth in the
epithelium and fibromuscular tissues inside the transition zone and
the periurethral areas have also been observed (23). Glandular hyperplasia and
interstitial hyperplasia can contribute to enlarged prostates
(24). BPH rats exhibited higher
wet weight and volume of prostate glands in the present study.
Based on the evaluation of these variables, we hypothesized that
cyclopamine may suppress BPH.
Immunohistochemistry analysis showed that
cyclopamine could reduce the rate of positive Ki67 expression in
BPH rats. Ki67 antigen has been reported as an effective tool in
diagnosing and evaluating proliferative activity in normal prostate
tissues, prostatic intraepithelial neoplasia and prostatic tumors
(25,26). Ki67 is associated with the
proliferative phase of cell cycle, and its overexpression can lead
to a robust increase in the number of prostatic cells (27). Therefore, we evaluated Ki67
expression in proliferating prostate cells in all three groups. Our
study revealed that cyclopamine could suppress cell proliferation
and promote cell apoptosis in prostate tissues in BPH rats. Despite
its uncertain pathogenesis, the progression of BPH is highly
associated with decreased cell apoptosis, which leads to an
elevated number of stromal and epithelial cells (28). The Hedgehog signaling pathway has
been reported to be a positive regulator and a proliferative
stimulus during prostate cell growth (29). Inhibition of this signaling
cascade by cyclopamine can lead to increased cell apoptosis
(30,31), which is in consistent with our
findings. BPH is characterized by the hyper-proliferation of
epithelial and stromal prostatic cells, which results in complex
cellular alterations (32). The
development of BPH is concomitant with a decline in stromal and
epithelial cell apoptosis (28).
As cyclopamine can inhibit the Hedgehog signaling pathway, which is
a crucial participator in prostatic cell progression, it may
ultimately inhibit the apoptosis of epithelial cells (15).
Finally, our results indicated that expression of
Shh, Ptch1, Gli1, b-FGF, TGF-β and Bcl-2 increased significantly,
while Bax expression reduced substantially in rat models of BPH.
This was also associated with activated Hedgehog signaling,
increased proliferation and reduced cell apoptosis. In response to
cyclopamine administration, the Hedgehog signaling pathway was
inhibited, cell proliferation was suppressed, and cell apoptosis
was enhanced. Shh/Ptch1/Gli1 are three essential members in the
Hedgehog signaling pathway that can exert notable effects on the
progression of BPH (33). As a
gene encoding angiogenic cytokine, b-FGF was reported to induce
epithelial cell proliferation, which can further promote BPH
(34). A previous study has
confirmed the role of TGF-β in aggravating BPH (23). Bcl-2 and Bax belong to the Bcl-2
family of proteins, with the former serving as an anti-apoptotic
member, while the latter acts as a pro-apoptotic molecule (28,35). When Bcl-2 expression was reduced
and Bax expression was increased, BPH progression was inhibited
(36).
Taken together, the present study is the first to
explore the functional role of cyclopamine in BPH to the best of
our knowledge. Prior to this, clinical research studies have shown
that α1 antagonists, 5α reductase inhibitors, and a combination of
these two molecules can inhibit BPH (37). In addition, silencing of
upregulated gene 11 can also inhibit the proliferation and
epithelial-mesenchymal transition of BPH cells (38). In addition, Wang et al
(39) showed that metformin could
suppress BPH epithelial cell proliferation by inhibiting the
secretion of insulin-like growth factor (IGF)-1 receptor and IGF-1
in stromal cells.
This study demonstrated that cyclopamine reduced the
proliferation of epithelial and stromal cells, and promoted
epithelial cell apoptosis by blocking the Hedgehog signaling
pathway in BPH rats. Future studies are still required to
investigate the mechanism by which inhibition of Hedgehog signaling
by cyclopamine can restrict the progression of BPH. This may also
provide novel insights into potential treatments BPH with
cyclopamine.
Funding
The present study was supported by the Clinical
Medical Technological Innovation Guidance Project of Technological
Innovation Guidance Plan in Hunan Province (grant no. 2017SK50305),
the Scientific Research Project of Traditional Chinese Medicine in
Hunan Province (grant no. 201832), the General Program of National
Natural Science Foundation of China (grant no. 81673984), and the
Construction Project of He Juqiao's National Traditional Chinese
Medicine Expert Inheritance Studio. The authors thank the reviewers
for their helpful comments.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YY, WZ, TL and JQH wrote the paper and conceived and
designed the experiments. XZha collected the data, and QZ and XZho
analyzed the data. YY and JY obtained the results and validated
them. YY, WZ and TL contributed to drafting the manuscript. All
authors have read and approved the final published version of this
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hunan University of Chinese Medicine (Changsha, China)
and conducted in accordance with the Laboratory animal-Requirements
of Environment and Housing Facilities Guidelines (approval no. GB
14925-2010).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Kristal AR, Arnold KB, Schenk JM,
Neuhouser ML, Goodman P, Penson DF and Thompson IM: Dietary
patterns, supplement use, and the risk of symptomatic benign
prostatic hyperplasia: Results from the prostate cancer prevention
trial. Am J Epidemiol. 167:925–934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Nunzio C, Kramer G, Marberger M,
Montironi R, Nelson W, Schröder F, Sciarra A and Tubaro A: The
controversial relationship between benign prostatic hyperplasia and
prostate cancer: The role of inflammation. Eur Urol. 60:106–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McVary KT, Roehrborn CG, Avins AL, Barry
MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan
SA, Penson DF, et al: Update on AUA guideline on the management of
benign prostatic hyperplasia. J Urol. 185:1793–1803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neuhouser ML, Schenk J, Song YJ, Tangen
CM, Goodman PJ, Pollak M, Penson DF, Thompson IM and Kristal AR:
Insulin-like growth factor-I, insulin-like growth factor binding
protein-3 and risk of benign prostate hyperplasia in the prostate
cancer prevention trial. Prostate. 68:1477–1486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaplan SA, Walmsley K and Te AE:
Tolterodine extended release attenuates lower urinary tract
symptoms in men with benign prostatic hyperplasia. J Urol.
174:2273–2275; discussion 2275-2276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keehn A, Taylor J and Lowe FC:
Phytotherapy for benign prostatic hyperplasia. Curr Urol Rep.
17:532016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ok CY, Singh RR and Vega F: Aberrant
activation of the hedgehog signaling pathway in malignant
hematological neoplasms. Am J Pathol. 180:2–11. 2012. View Article : Google Scholar :
|
|
8
|
Yoo YA, Kang MH, Kim JS and Oh SC: Sonic
hedgehog signaling promotes motility and invasiveness of gastric
cancer cells through TGF-beta-mediated activation of the ALK5-Smad
3 pathway. Carcinogenesis. 29:480–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Q, Gao G and Luo S: Hedgehog
signaling pathway and ovarian cancer. Chin J Cancer Res.
25:346–353. 2013.PubMed/NCBI
|
|
10
|
Taylor R, Long J, Yoon JW, Childs R,
Sylvestersen KB, Nielsen ML, Leong KF, Iannaccone S, Walterhouse
DO, Robbins DJ and Iannaccone P: Regulation of GLI1 by cis DNA
elements and epigenetic marks. DNA Repair (Amst). 79:10–21. 2019.
View Article : Google Scholar
|
|
11
|
Skoda AM, Simovic D, Karin V, Kardum V,
Vranic S and Serman L: The role of the Hedgehog signaling pathway
in cancer: A comprehensive review. Bosn J Basic Med Sci. 18:8–20.
2018. View Article : Google Scholar :
|
|
12
|
Bar EE, Chaudhry A, Lin A, Fan X, Schreck
K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A and
Eberhart CG: Cyclopamine-mediated hedgehog pathway inhibition
depletes stem-like cancer cells in glioblastoma. Stem Cells.
25:2524–2533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar SK, Roy I, Anchoori RK, Fazli S,
Maitra A, Beachy PA and Khan SR: Targeted inhibition of hedgehog
signaling by cyclopamine prodrugs for advanced prostate cancer.
Bioorg Med Chem. 16:2764–2768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Atkins D, Reiffen KA, Tegtmeier CL,
Winther H, Bonato MS and Störkel S: Immunohistochemical detection
of EGFR in paraffin-embedded tumor tissues: Variation in staining
intensity due to choice of fixative and storage time of tissue
sections. J Histochem Cytochem. 52:893–901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Harrington N, Moraes RC, Wu MF,
Hilsenbeck SG and Lewis MT: Cyclopamine inhibition of human breast
cancer cell growth independent of Smoothened (Smo). Breast Cancer
Res Treat. 115:505–521. 2009. View Article : Google Scholar
|
|
16
|
Slusarz A, Shenouda NS, Sakla MS,
Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL and
Lubahn DB: Common botanical compounds inhibit the hedgehog
signaling pathway in prostate cancer. Cancer Res. 70:3382–3390.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JK, Taipale J, Cooper MK and Beachy
PA: Inhibition of Hedgehog signaling by direct binding of
cyclopamine to Smoothened. Genes Dev. 16:2743–2748. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palumbo A, Casanova LM, Corrêa MFP, Da
Costa NM, Nasciutti LE and Costa SS: Potential therapeutic effects
of underground parts of Kalanchoe gastonis-bonnieri on benign
prostatic hyperplasia. Evid Based Complement Alternat Med.
2019:63407572019. View Article : Google Scholar :
|
|
19
|
Kogan-Sakin I, Cohen M, Paland N, Madar S,
Solomon H, Molchadsky A, Brosh R, Buganim Y, Goldfinger N, Klocker
H, et al: Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and
IL-8 in response to epithelia-secreted IL-1. Carcinogenesis.
30:698–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XY, Liu X, Xu L, Gui B, Yang QY, Yan
JY and Sun ZY: A mathematical model for predicting putative
association between E2/T ratio and the development of benign
prostate hyperplasia in rats. Biol Reprod. 100:133–138. 2019.
View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Shaw A and Bushman W: Hedgehog signaling
in the prostate. J Urol. 177:832–838. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Yang JR, Yang LY and Liu ZT:
Chronic inflammation in benign prostatic hyperplasia: Implications
for therapy. Med Hypotheses. 70:1021–1023. 2008. View Article : Google Scholar
|
|
24
|
Kobayashi S, Tomiyama Y, Tatemichi S,
Hoyano Y, Kobayashi M and Yamazaki Y: Effects of silodosin and
tamsulosin on the urethra and cardiovascular system in young and
old dogs with benign prostatic hyperplasia. Eur J Pharmacol.
613:135–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adisa JO, Egbujo EC, Ibrahim B, Musa B and
Madukwe J: Expression of some selected cytokeratins and Ki67
protein in prostatic tumor: Can these be used as tumor markers. Pan
Afr Med J. 20:462015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seligson DB, Yu H, Tze S, Said J, Pantuck
AJ, Cohen P and Lee KW: IGFBP-3 nuclear localization predicts human
prostate cancer recurrence. Horm Cancer. 4:12–23. 2013. View Article : Google Scholar :
|
|
27
|
Fonseca-Alves CE, Kobayashi PE, Palmieri C
and Laufer-Amorim R: Investigation of c-KIT and Ki67 expression in
normal, preneoplastic and neoplastic canine prostate. BMC Vet Res.
13:3802017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng H, Xu W, Lin J, Peng J and Hong Z:
Qianliening capsule treats benign prostatic hyperplasia via
induction of prostatic cell apoptosis. Mol Med Rep. 7:848–854.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vezina CM and Bushman AW: Hedgehog
signaling in prostate growth and benign prostate hyperplasia. Curr
Urol Rep. 8:275–280. 2007. View Article : Google Scholar
|
|
30
|
Wang X, Fan G, Wei F, Bu Y and Huang W:
Hyperoside protects rat ovarian granulosa cells against hydrogen
peroxide-induced injury by sonic hedgehog signaling pathway. Chem
Biol Interact. 310:1087592019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma A, De R, Javed S, Srinivasan R, Pal
A and Bhattacharyya S: Sonic hedgehog pathway activation regulates
cervical cancer stem cell characteristics during epithelial to
mesenchymal transition. J Cell Physiol Feb. 4:Epub ahead of
print.
|
|
32
|
Penna G, Fibbi B, Amuchastegui S, Corsiero
E, Laverny G, Silvestrini E, Chavalmane A, Morelli A, Sarchielli E,
Vannelli GB, et al: The vitamin D receptor agonist elocalcitol
inhibits IL-8-dependent benign prostatic hyperplasia stromal cell
proliferation and inflammatory response by targeting the RhoA/Rho
kinase and NF-kappaB pathways. Prostate. 69:480–493. 2009.
View Article : Google Scholar
|
|
33
|
Oue T, Yoneda A, Uehara S, Yamanaka H and
Fukuzawa M: Increased expression of the hedgehog signaling pathway
in pediatric solid malignancies. J Pediatr Surg. 45:387–392. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin J, Zhou J, Xu W, Hong Z and Peng J:
Qianliening capsule inhibits benign prostatic hyperplasia
angiogenesis via the HIF-1α signaling pathway. Exp Ther Med.
8:118–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iacopino F, Angelucci C, Lama G, Zelano G,
La Torre G, D'Addessi A, Giovannini C, Bertaccini A, Macaluso MP,
Martorana G and Sica G: Apoptosis-related gene expression in benign
prostatic hyperplasia and prostate carcinoma. Anticancer Res.
26:1849–1854. 2006.PubMed/NCBI
|
|
36
|
Shariat SF, Ashfaq R, Roehrborn CG, Slawin
KM and Lotan Y: Expression of survivin and apoptotic biomarkers in
benign prostatic hyperplasia. J Urol. 174:2046–2050. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shum CF, Lau W and Teo CPC: Medical
therapy for clinical benign prostatic hyperplasia: α-1 antagonists,
5α reductase inhibitors and their combination. Asian J Urol.
4:185–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang G, Zhu F, Han G, Li Z, Yu Q, Li Z
and Li J: Silencing of URG11 expression inhibits the proliferation
and epithelial-mesenchymal transition in benign prostatic
hyperplasia cells via the RhoA/ROCK1 pathway. Mol Med Rep.
18:391–398. 2018.PubMed/NCBI
|
|
39
|
Wang Z, Xiao X, Ge R, Li J, Johnson CW,
Rassoulian C and Olumi AF: Metformin inhibits the proliferation of
benign prostatic epithelial cells. PLoS One. 12:e01733352017.
View Article : Google Scholar : PubMed/NCBI
|