Introduction
The vascular wall consists of endothelial cells,
smooth muscle cells, fibroblasts and extracellular matrix, and is
considered an active and integrated organ (1). The vasculature is sensitive to a
range of stimuli, several of which may contribute to
pathophysiological changes in response to stresses that result in
proliferation of the vasculature (2). The initiating event in vascular
remodeling is endothelial cell damage. Alterations in the presence
of vasoactive substances following endothelial cell dysfunction are
involved in the development and progress of cardiovascular
diseases. A vicious circle is caused by further injury of
endothelial cells following the onset of cardiovascular diseases
(3). This dynamic process
characterizes vascular remodeling, and is defined as any enduring
change in the size and/or composition of an adult blood vessel that
allows blood vessels to adapt and heal (4). Vascular remodeling underlies the
pathogenesis of major cardiovascular disorders, including pulmonary
arterial hypertension (PAH) and atherosclerosis, as well as wound
healing (5). Vascular remodeling
is involved in cells progressing through four cellular processes:
Differentiation, death, migration, and production or degradation of
extracellular matrix (3,6). Endothelial cells are pivotal in the
initiation of vascular remodeling. The increase in mesenchymal or
smooth muscle (SM)-like phenotypes originates from increased
endothelial cell transformation (7). Endothelial cells are the first
barrier of blood vessels and recently they have been regarded as
contributors to vascular remodeling through
endothelial-to-mesenchymal transition (EndMT) (8).
EndMT is the process by which endothelial cells
undergo phenotypic changes and acquire a mesenchymal/SM-like
phenotype (9). Under
pathophysiological conditions, such as transplant atherosclerosis
and restenosis, endothelial-derived mesenchymal cells may
accelerate the development of neointimal lesions (10). Evidence for EndMT has been
demonstrated in the context of cardiac and vascular development,
wound healing and various dysfunctions, including tissue fibrosis
and diabetic nephropathy (11).
Thus, EndMT is regarded as an important mechanism for the
generation of SM-like cells. Endothelial cells facilitate vascular
development and dysfunction, which act through direct and indirect
roles in the vascular remodeling process (12). A previous study has demonstrated
that EndMT is involved in the pathogenesis of vascular remodeling
(13). EndMT contributes to
neointimal formation in interpositional vein grafts (14). Similarly, EndMT contributes to
intimal hyperplasia and early-lesion formation in atherosclerosis
(15). Furthermore, EndMT
contributes to progression of PAH, atherosclerosis and wound
healing (16). Therefore, in the
present study, it was hypothesized that EndMT may accelerate the
pathogenesis of vascular remodeling. However, the role of EndMT and
the mechanisms regulating cell phenotype adaption during vascular
remodeling are poorly understood. Studies have reported that mouse
and human endothelial cells are induced by transforming growth
factor (TGF)-β1 to transform into SM-like cells in which the
expression of α-smooth muscle actin (α-SMA) is upregu-lated
(17,18). Under the synergistic action of
TGF-β1 and the Wnt signaling pathway, EndMT may be induced.
Therefore, in the present study, TGF-β1 was used to develop an
in vitro model of EndMT (19).
Zingiberaceae belongs to the ginger family, which
consists of a large number of aromatic perennial species shown to
exhibit therapeutic potential in a number of cardiac-related
therapies (20). Alpinia
zerumbet (Pers.) Burtt et Smith, termed Yan Shanjiang in China,
is an aromatic plant originating in the East Indies and is widely
used as an herbal medicine in South America, Oceania and Asia
(21,22). It contains several bioactive
constituents and possesses a broad spectrum of pharmacological
properties, such as antihypertensive, anti-inflammatory and
cardiovascular protective effects, as well as antioxidant effects
(23,24), and is widely used as a local Miao
herbal medicine in the Guizhou province. Previous studies have
shown that essential oil from Alpinia zerumbet rhizome (EOFAZ) is
effective against the vasoconstriction induced by release of
norepinephrine and KCl (25). The
mechanisms mediating the beneficial effects of EOFAZ may include
inhibition of oxidative stress and inflammation, and induction of
apoptosis (26). However, the
effects of EOFAZ on EndMT in a range of stress situations has not
been investigated, particularly in relation to TGF-β1 signaling
(22).
In the present study, a cell based in vitro model
treated with TGF-β1 was established to verify the hypothesis that
EOFAZ protects against TGF-β1-induced EndMT, and to determine the
underlying molecular mechanism by which EOFAZ exerts its beneficial
effects. The present study identified a novel molecular mechanism
by which EOFAZ exerts its effects and also provides a theoretical
basis for use of EOFAZ treatment for cardiac disorders.
Materials and methods
Extraction of EOFAZ
The essential oil was extracted from the fruit of
Alpinia zerumbet, which was collected in Zhenfeng County,
Guiyang, China, in October 2018. The fruit was identified by
Professor Qingde Long (Department of Pharmacognosy and
Medico-botany, Guizhou Medical University, Guiyang, China) and a
voucher specimen (no. 20181029) was deposited to the Key Laboratory
of Optimal Utilization of Natural Medicinal Resources, Guizhou
Medical University. The method of extraction/isolation and the
compounds of EOFAZ were identified according to our previous study
(22).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. Cells were cultured in Endothelial
Cell Medium (ScienCell Research Laboratories, Inc.) supplemented
with 5% FBS (ScienCell Research Laboratories, Inc.) and 100 U/ml
penicillin and streptomycin, and maintained in an incubator at 37°C
with 5% CO2. Cells were sub-cultured and seeded into 6-
or 24-well plates for subsequent experiments as required. The cells
were pretreated with EOFAZ and LY2109761 (Selleck Chemicals) for 2
h at 37°C, and TGF-β1 (Peprotech EC Ltd.) was added for 72 h.
HUVECs were trypsinized with 0.25% trypsin and collected for
analysis.
Cell morphological assessment
The HUVECs were plated in 6-well plates at a density
of 3×105 cells/well and were incubated overnight.
Following treatment with 10 ng/ml TGF-β1 for 72 h at 37°C, the
cultured plates were examined and photographs were obtained using
an inverted light microscope (magnification, ×100).
Western blot analysis
Cells were lysed in RIPA lysis buffer containing
protease inhibitors (Beyotime Institute of Biotechnology). The
protein concentration was determined by BCA assay. For western
blotting, a total of 40 μg protein/lane from lysed cells was
separated by 10% SDS-PAGE. The proteins were transferred to a PVDF
membrane and the membrane was blocked with 5% non-fat dry milk at
room temperature (25°C) for 2 h. The membrane was then incubated
with the following primary antibodies overnight at 4°C: Rabbit
anti-Krüppel-like factor 4 (KLF4; 1:1,000; cat. no. 12173S),
anti-vascular endothelial (VE)-cadherin (1:1,000; cat. no. 2500),
anti-α-SMA (1:1,000; cat. no. 19245), anti-snail (1:1,000; cat. no.
5276) and anti-NF-κB phosphorylated (p)-p65 (1:1,000; cat. no.
3033T). All primary antibodies were from Cell Signaling Technology,
Inc. and all were diluted in TBS and 0.2% Tween-20. Subsequently,
the membranes were washed and incubated with a horseradish
peroxidase-conjugated secondary antibody (Cell Signaling
Technology, Inc.; 1:10,000; cat. no. 7076) for 90 min at room
temperature. Signals were visualized using an ECL kit (GE
Healthcare). The expression levels of protein were calculated by
using ImageJ V1.8.0.112 software (National Institutes of Health).
Protein signals were normalized to β-actin (Cell Signaling
Technology, Inc.; 1:1,000; cat. no. 3700).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted using the TransZol Up Plus
RNA kit (Sangon Biotech Co., Ltd.). Total RNA was purified with 75%
ethanol and its concentration was determined using
spectrophotometry. cDNA was synthesized from the purified RNA (200
ng per sample) using a reverse transcription kit (cat. no. DRR037A;
Takara Bio, Inc.), according to the manufacturer's protocol.
Subsequently, qPCR was performed on an ABI 7300 Real-Time PCR
system SYBR Premix ExTaq (Takara Bio, Inc.). The thermocycling
conditions were as follows: Pre-incubation at 94°C for 30 sec
followed by 39 cycles of amplification at 94°C for 5 sec and 56°C
for 30 sec. The sequences of the primers were: Vascular endothelial
(VE)-cadherin forward, 5′-CTTCACCCAGACCAAGTACACA-3′ and reverse,
5′-AGGGCTCATGTATCGGAGGT-3′; α-SMA forward,
5′-ACCATCGGGAATGAACGCTT-3′ and reverse, 5′-CTGTCAGCAATGCCTGGGTA-3′;
snail forward, 5′-GAAGATGCACATCCGAAGCC-3′ and reverse,
5′-TTCACATCCGAGTGGGTCTG-3′; and GAPDH forward,
5′-TGTGAACGGATTTGGCCGTA-3′ and reverse, 5′-GATGGTGATGGGTTTCCCGT-3′.
GAPDH was used as the loading control.
Immunofluorescence staining
Cells at a density of 1×104/ml were
seeded onto slides and fixed with 4% paraformaldehyde (200
μl/well) for 10 min, and washed twice with PBS, 10 min each
time. The cell membrane was treated with 0.1% Triton X-100 in PBS
(200 μl/well) at room temperature for 10 min, and then
washed twice with PBS, 10 min each time. The plates were agitated
gently several times by hand to drain the liquid and cells were
then blocked with 4% goat serum in PBS at room temperature for 30
min. The liquid was gently removed, anti-VE-cadherin and anti-α-SMA
primary antibodies were added, and the cells were incubated at 37°C
for 1 h or at 4°C overnight. The primary antibodies used were:
Anti-VE-cadherin (1:100; Cell Signaling Technology, Inc.; cat. no.
2500) and anti-α-SMA (1:100, Cell Signaling Technology, Inc.; cat.
no. 19245). DAPI (1:1,000; Sigma-Aldrich; Merck KGaA; cat. no.
AC23192) was used to stain the nuclei at room temperature for 10
min. Subsequently, the cells were washed three times with PBS and
then incubated with fluorescein-labeled secondary antibodies (Cell
Signaling Technology, Inc.; 1:100; cat. nos. 4416 and 12877) at
37°C for 60 min. Following washing with 0.01 M PBS three times, 5
min per wash, anti-fade reagent was used to mount the slides at
4°C. Finally, cells were imaged using a fluorescence microscope
(magnification, ×200).
Wound healing assay
HUVECs were seeded in 24-well plates and grown
overnight to confluence. For EndMT induction, cells were treated
with 10 ng/ml TGF-β1, 2 μM LY21097612 and 0.25, 1 or 4
μg/l EOFAZ for 72 h at 37°C. The monolayer of cells was
scratched using a 20-μl pipette tip to create the wound.
Floating cells were removed by washing twice with PBS and
serum-free Endothelial Cell Medium was added. Wound closure rate
was assessed using images captured with a light microscope (Leika
DM3000k magnification, ×100) after 24 h. The motility index=(Mean
of 0 h migration distance-mean of 12 h migration distance)/mean of
0 h migration distance.
Transwell migration assay
Cells were trypsinized, centrifuged at 150 × g for 5
min at room temperature, washed with PBS 1-2 times and resuspended
in serum-free culture medium containing BSA. A cell suspension of
100 μl (2×104 cells) was added to the upper
chamber of the Transwell insert, and 400 μl medium
containing 10% FBS was added to the lower chamber. The cells were
cultured for 12 h, after which the Transwell chamber was removed,
the medium was discarded, and cells were washed twice with PBS and
fixed with paraformaldehyde for 15 min at room temperature.
Subsequently, the cells were stained for 10 min with 0.1% crystal
violet at room temperature. The unmigrated cells in the upper layer
were gently removed with a cotton swab and the chamber was then
washed three times with PBS. Cells were observed under an inverted
light microscope (magnification, ×100) and the number of cells in
five randomly chosen fields were counted. The number of migrated
cells was quantified by manual counting, and the motility index was
determined using the following formula: Motility index=the number
of migrated cells in the experimental group/the number of cells
that migrated in the control group.
Immunoprecipitation
HUVECs were harvested 72 h after treatment with
TGF-β1 or EOFAZ at 37°C for 2 h, the supernatant was removed, the
cells were lysed with NP-40 lysis buffer (Beyotime Institute of
Biotechnology) containing protease inhibitors, and the lysate was
centrifuged on ice at 12,000 × g for 30 min at 4°C. A small
quantity of lysate was used for western blotting, and 1 μg
antibody (anti-KLF4; 1:200; Cell Signaling Technology, Inc.; cat.
no. 12173S) was added to the remaining cell lysate and incubated
overnight at 4°C with gentle agitation. A total of 10 μl
protein A agarose beads were washed with lysis buffer and
centrifuged at 300 × g for 3 min at 4°C. The washed protein A
agarose beads were then added to the cell lysate-antibody solution
and incubated at 4°C with gentle agitation for 2-4 h. Following
immunoprecipitation, agarose beads were pelleted by centrifugation
at 300 × g for 3 min at 4°C. The supernatant was aspirated, and the
agarose beads were washed 3-4 times with 1 ml lysis buffer.
Finally, 15 μl 2x SDS sample buffer was added, and the
samples were boiled for 5 min. Samples were subsequently subjected
to western blotting, as previously described.
Small interference (si)RNA
transfection
HUVECs were plated in 6-well plates at a density of
2×106 cells/well and transfected with siRNA. Briefly, 5
μl KLF4 and control siRNA was mixed with RPMI-1640 media
(Wisent, Inc.). Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was mixed with the RPMI-1640 media and the
mixture was left to stand for 20 min at room temperature. The
Lipofectamine®-siRNA mixture was then added to each well
containing cells and the cells were incubated for 6 h. Medium was
then replaced with antibiotic-free Endothelial Cell Medium
supplemented with 5% FBS for 24 h, after which cells were used for
subsequent experiments. The KLF4 siRNA sequences were: Sense
5′-AGAGTTCCCATCTCAAGGCA-3′ and antisense,
5′-GTCAGTTCATCTGAGCGGG-3′. The sequences of the control
non-specific siRNA were: Sense 5′-CGUUUGUUCGCUUCCUGAGTT-3′ and
antisense, 5′-CUCAGGAAGCGAACAAACGTG-3′.
Statistical analysis
All data are expressed as the mean ± standard
deviation of at least three repeats. SPSS v.21.0 (IBM, Corp.) was
used for statistical analysis. Comparisons among multiple groups
were analyzed using one-way analysis of variance followed by
Bonferroni's post hoc test. Comparisons between two groups were
analyzed using a two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
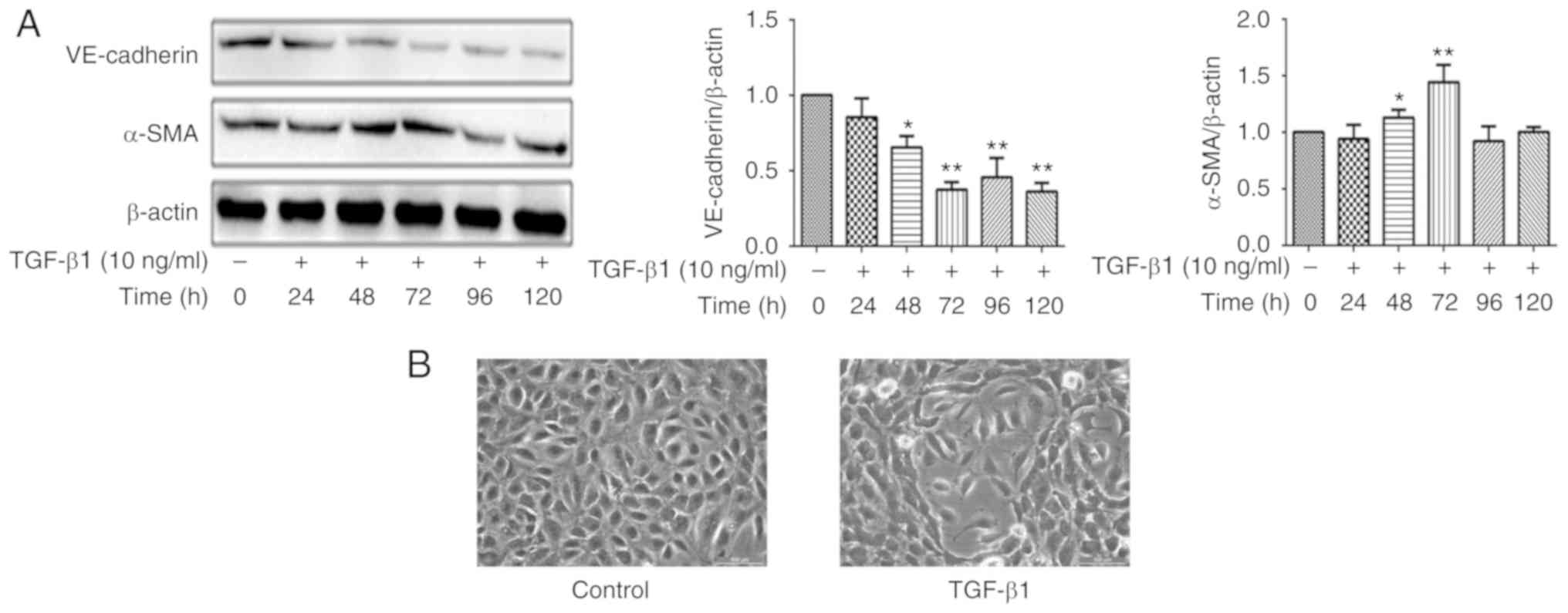
EndMT is induced by TGF-β1 in HUVECs
TGF-β1 is a powerful factor for induction of EndMT,
although the effect depends of the treatment time and dose in
different models (17). Relative
VE-cadherin and α-SMA expression levels were determined by western
blotting. After cells were treated in Endothelial Cell Medium with
or without 10 ng/ml of TGF-β1, the expression levels of VE-cadherin
and α-SMA changed in a time-dependent manner. As presented in
Fig. 1A, the HUVECs were treated
with 10 ng/ml TGF-β1 for various durations, and the VE-cadherin and
α-SMA levels were most significantly changed at 72 h, thus
treatment with 10 ng/ml TGF-β1 for 72 h was selected to establish
the EndMT model in following experiments. In addition, the
morphological EndMT-associated changes in HUVECs by TGF-β1
treatment for 72 h were observed using an inverted microscope
(Fig. 1B). Cells in the control
group demonstrated a cobble-stone like morphology and the junctions
between the cells were dense. The cells induced by TGF-β1 were
primarily fibroblast in morphology, the distance between cells was
increased, and additionally the presence of filamentous pseudopod
protrusions was observed. Taken together, TGF-β1 induced
morphological and phenotypic changes in HUVECs to a more
mesenchymal-like phenotype, suggesting the cells had undergone
EndMT and an EndMT model had been established.
EOFAZ inhibits TGF-β1-induced EndMT in
HUVECs
Subsequently, HUVECs were exposed to 10 ng/ml TGF-β1
for 72 h for EndMT induction. Additionally, the cells were
pretreated with EOFAZ and LY2109761 (a TGF-β1 inhibitor) for 2 h
and then stimulated with TGF-β1. The results of RT-qPCR and western
blotting analysis demonstrated that EOFAZ, like LY2109761,
significantly reversed the TGF-β1-iduced increase in α-SMA and
Snail expression and the TGF-β1-induced decrease in VE-cadherin
expression (Fig. 2A and B).
Similar changes were observed by immunofluorescence (Fig. 2C).
 | Figure 2EOFAZ inhibits TGF-β1-induced EndMT
in HUVECs. (A) Effect of EOFAZ on the mRNA expression levels of
VE-cadherin, α-SMA and Snail, as measured by RT-qPCR and normalized
to β-actin. n=5. (B) Western blots of VE-cadherin, α-SMA and Snail.
β-actin was used as the loading control. (C) Immunofluorescence of
endothelial marker protein VE-cadherin (green fluorescence),
mesenchymal marker protein α-SMA (red fluorescence) and nuclei
(DAPI, blue). *P<0.05, **P<0.01,
***P<0.001. EOFAZ, essential oil from Alpinia
zerumbet rhizome; EndMT, endothelial-to-mesenchymal transition;
TGF-β1, transforming growth factor-β1; HUVEC, human umbilical vein
endothelial cell; α-SMA, α-smooth muscle actin; VE-cadherin,
vascular endothelial-cadherin. |
EOFAZ inhibits the expression of EndMT
markers in cell migration
During the EndMT process initiated in endothelial
cells, the cellular migratory ability is promoted (14). A wound healing assay was used to
observe the effects of EOFAZ on the migration of endothelial cells
treated with TGF-β1. The results demonstrated that the cell
migration rate significantly increased in the TGF-β1-treated group
compared with the control group. In addition, scratch
immunofluorescence staining demonstrated that VE-cadherin was
downregulated, while α-SMA was upregulated in the migrating cells
after TGF-β1 treatment. The number of migrated cells was
significantly reduced in cells pre-treated with LY2109761 and
EOFAZ, and the expression of VE-cadherin was increased and the
expression of α-SMA was decreased compared with the cells only
treated with TGF-β1 (Fig. 3A and
B). These results suggest that the TGF-β1-induced EndMT is
associated with the enhanced migratory capacity of mesenchymal
cells, whereas EOFAZ inhibits this migratory capacity.
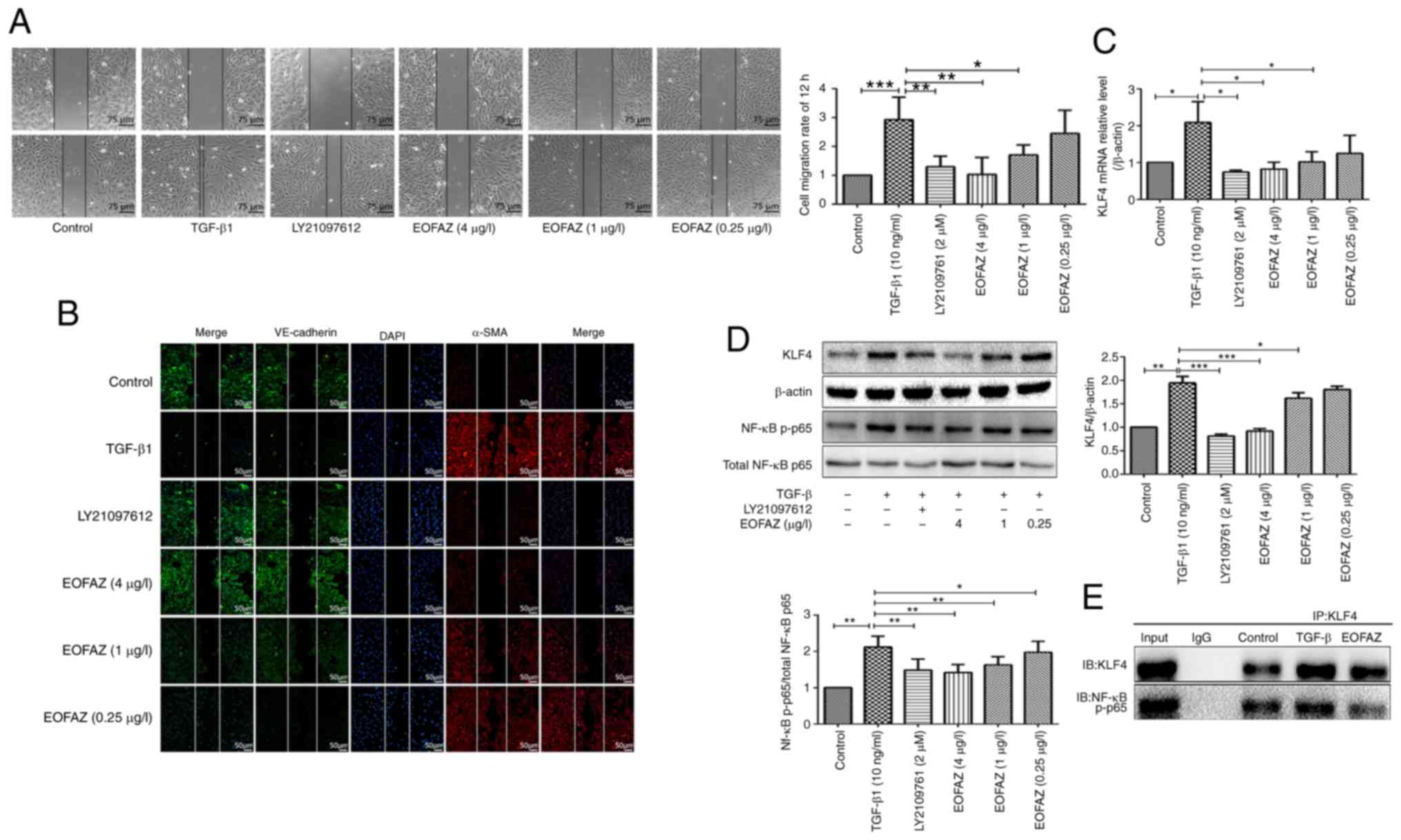 | Figure 3EOFAZ inhibits HUVECs migration and
regulates biomarkers in TGF-β1-induced EndMT. (A) Wound healing
assay of HUVECs treated with TGF-β1 and EOFAZ for the indicated
time intervals. (B) A wound healing assay was used to observe the
effects of EOFAZ on the migration of endothe-lial cells.
Endothelial marker protein VE-cadherin (green fluorescence),
mesenchymal marker protein α-SMA (red fluorescence) and nuclei
(DAPI, blue). (C) Reverse transcription-quantitative PCR analysis
of the mRNA expression of KLF4 in HUVECs treated with TGF-β1 and
EOFAZ. (D) Western blot analysis was performed with treated HUVECs
to analyze the expression of KLF4 and NF-κB p-p65. β-actin was used
as the loading control. (E) Detection of the interaction between
KLF4 and NF-κB by co-immunoprecipitation. *P<0.05,
**P<0.01, ***P<0.001. EOFAZ, essential
oil from Alpinia zerumbet rhizome; EndMT,
endothelial-to-mesenchymal transition; TGF-β1, transforming growth
factor-β1; HUVEC, human umbilical vein endothelial cell; p-,
phosphorylated; KLF4, Krüppel-like factor 4; α-SMA, α-smooth muscle
actin; VE-cadherin, vascular endothelial-cadherin. |
Effect of EOFAZ on the expression of KLF4
and NF-κB p-p65, and on the binding between KLF4 and NF-κB induced
by TGF-β1 in HUVECs
As presented in Fig.
3C and D, KLF4 and NF-κB p-p65 expression levels were
upregulated in the TGF-β1-induced cells compared with the control.
The p-p65/p65 ratio is appropriate to evaluate the activation of
NF-κB (27). The results
demonstrated that EOFAZ and LY2109761 significantly decreased the
TGF-β1-induced increase in p-p65/p65 ratio, which indicated the
inhibitory effects of EOFAZ on NF-κB nuclear translocation and
activation. The TGF-β1-induced increase in KLF4 was also decreased
in the presence of EOFAZ or LY2109761 (Fig. 3C and D). These results suggested
that EOFAZ inhibits TGF-β1-induced KLF4 and NF-κB activation. A
previous study reported that NF-κB is one of key regulators during
EndMT in endothelial cells; activation or inhibition of the NF-κB
signaling pathway resulted in EndMT reversal (28). In addition, it has been reported
that NF-κB is essential for both the induction and maintenance of
EndMT (29).
A previous study suggested that KLF4 inhibits
TNF-α-mediated induction of vascular cell adhesion protein 1
(VCAM1) expression by blocking the binding of p65 to the VCAM1
promoter through the physical association between KLF4 and p65
(30-32). Therefore, the present study
hypothesized that KLF4 similarly regulates the activation of NF-κB
in the TGF-β1-induced EndMT process. To confirm binding between
KLF4 and NF-κB in HUVECs treated with TGF-β1 in the absence or
presence of EOFAZ, co-immunoprecipitation studies were performed.
The results demonstrated that TGF-β1 enhanced the binding between
KLF4 and NF-κB, However, EOFAZ treatment reduced the TGF-β1-induced
interaction between KLF4 and NF-κB, although it was not determined
if this effect was direct or indirect (Fig. 3E).
Effect of KLF4 siRNA transfection on KLF4
expression
Previously, it has been suggested that KLF4 serves
an important role in EndMT, which is involved in the development
and progression of cerebral cavernous malformations. However, to
the best of our knowledge, whether the effect of EOFAZ on EndMT is
mediated by KLF4 in HUVECs has not been assessed. To investigate
the relationship between EOFAZ and KLF4, a KLF4-specific siRNA was
utilized to knockdown the expression of KLF4 (Fig. 4A and B). The results demonstrated
that the green fluorescence-labeled siRNA was successfully
transferred into HUVECs, and western blotting analysis demonstrated
that KLF4 expression was significantly reduced following
transfection with KLF4 siRNA.
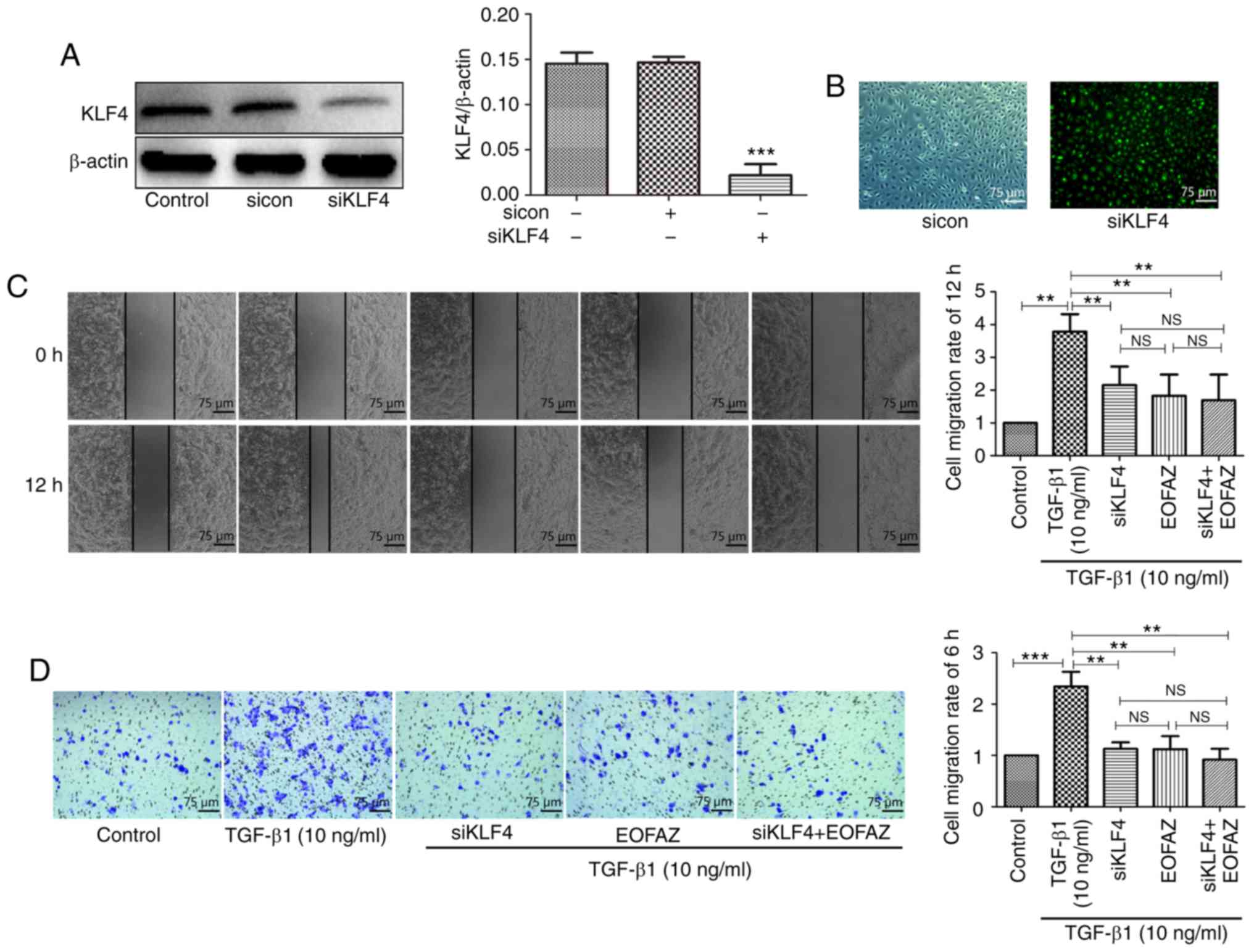 | Figure 4Effect of KLF4-knockdown on the
migration in HUVECs. (A) The efficacy of siKLF4 transfection was
measured by western blotting. (B) Fluorescence microscopy was used
to observe whether green fluorescence-labeled siKLF4 was
effectively transfected into the cells. Magnification, ×100. (C)
Wound healing assay of HUVECs treated with TGF-β1, EOFAZ and
siKLF4. (D) Transwell assay of HUVECs treated with TGF-β1, EOFAZ
and siKLF4. n=5. **P<0.01, ***P<0.001.
NS, not significant; TGF-β1, transforming growth factor-β1; HUVEC,
human umbilical vein endothelial cell; siRNA, small interfering;
KLF4, Krüppel-like factor 4; sicon, control small interfering RNA;
siKLF4, KLF4 small interfering RNA; EOFAZ, essential oil from
Alpinia zerumbet rhizome. |
Effect of EOFAZ on TGF-β1-induced HUVEC
migration following KLF4-knockdown
To evaluate HUVEC migration following transfection
with KLF4 siRNA, wound healing and Transwell assays were performed.
The KLF4 expression was significantly downregulated by siRNA
interference (Fig. 4A and B). As
shown in Fig. 4C, KLF4-knockdown
significantly reduced TGF-β1-induced cell migration. Transwell
assays revealed that the number of migrated cells was significantly
decreased in the KLF4-knockdown cells compared with the TGF-β1
group, and EOFAZ treatment decreased the number of migrated cells
under TGF-β1 stimulation (Fig.
4D). These results suggest that EOFAZ inhibition of
TGF-β1-mediated EndMT induction in HUVECs may occur through
downregulation of KLF4 expression.
EOFAZ inhibits TGF-β1-induced EndMT via
KLF4
To determine the effect of KLF4 siRNA on
EOFAZ-mediated inhibition of TGF-β1-induced EndMT, the expression
of α-SMA and VE-cadherin was determined by immunofluorescence
analysis. As presented in Fig.
5A, VE-cadherin expression was downregulated and α-SMA
expression was upregulated in the TGF-β1 group. However, following
KLF-knockdown and EOFAZ treatment, the effects on VE-cadherin and
α-SMA expression were reversed compared with the TGF-β1-treated
group. Furthermore, the expression levels of α-SMA, Snail,
VE-cadherin and NF-κB p-p65/p65 ratio were determined by western
blotting, and α-SMA, Snail, VE-cadherin were analyzed by RT-qPCR.
Similar results were observed for the changes in VE-cadherin and
α-SMA expression. In addition, the Snail expression and NF-κB
p-p65/p65 ratio levels were significantly repressed when exposed to
siKLF and EOFAZ (Fig. 5B).
Alterations in mRNA expression levels of VE-cadherin, α-SMA and
Snail were consistent with the western blotting (Fig. 5C). Together, these results
demonstrated that KLF4-knockdown inhibited EndMT, and there were no
significant differences between EOFAZ treatment and siKLF4
treatment. These results suggest that EOFAZ regulates EndMT via
KLF4.
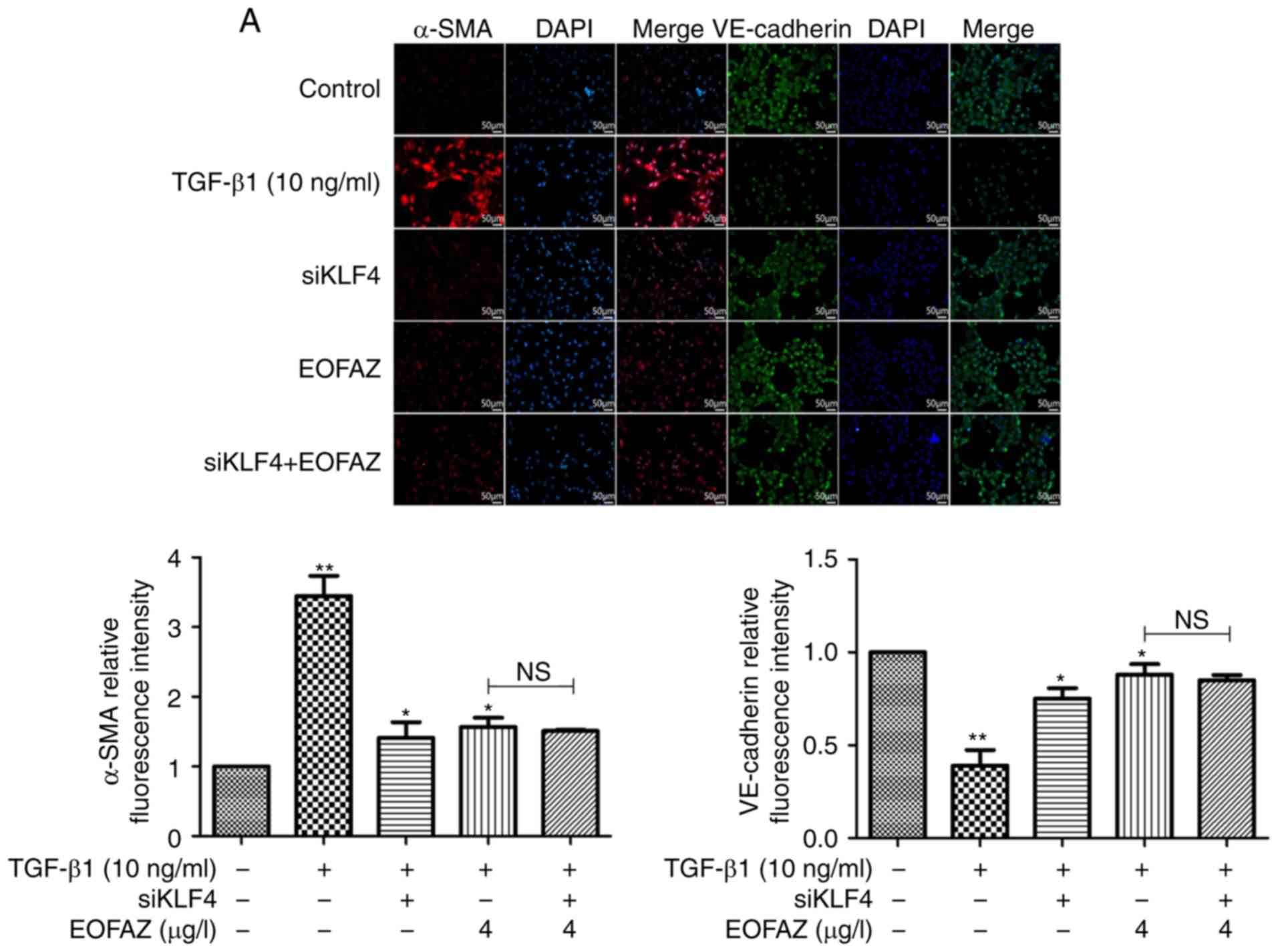 | Figure 5Effect of KLF4 knockdown on
TGF-β1-induced EndMT. (A) Effect of KLF4-knockdown on the
expression levels of VE-cadherin and α-SMA was assessed using
immunofluorescence. Endothelial marker protein VE-cadherin (green
fluorescence), mesenchymal marker protein α-SMA (red fluorescence)
and nuclei (DAPI, blue). Magnification, ×200. (B) Effect of
KLF4-knockdown on the expression of VE-cadherin, α-SMA, NF-κB p-p65
and Snail were evaluated by western blotting. β-actin was used as
the loading control. (C) Effect of KLF4-knockdown on mRNA
expression levels of VE-cadherin and α-SMA, normalized to β-actin.
n=5. *P<0.05, **P<0.01,
***P<0.001. NS, not significant; siKLF4, KLF4 small
interfering RNA; p-, phosphorylated; KLF4, Krüppel-like factor 4;
EOFAZ, essential oil from Alpinia zerumbet rhizome; N.S., not
significant; TGF-β1, transforming growth factor-β1; α-SMA, α-smooth
muscle actin; VE-cadherin, vascular endothelial-cadherin. |
Discussion
Endothelial cells are highly sensitive to stimuli
and physiological cues in the immediate environment (33). Thus, injury of the vascular
endothelium is an initial step in the pathogenesis of vascular
remodeling and contributes to vascular remodeling as a source of
SM-like cells, as endothelial cells can acquire a
fibro-proliferative mesenchymal phenotype through EndMT under
specific pathological conditions, including shear stress, oxidative
stress and atherosclerosis (34).
The present study demonstrated that endothelial cells differentiate
towards a SM-like phenotype via EndMT trans-differentiation when
treated with TGF-β1, and that the KLF4 signaling pathway was
involved in this process. Additionally, it was shown that EOFAZ
could inhibit TGF-β1-induced EndMT via downregulation of KLF4.
EndMT is a newly recognized, fundamental biological
process involved in development and tissue regeneration, as well as
pathological processes, including certain complications of
diabetes, fibrosis, PAH, atherosclerosis (35-38), tumor metastasis and development of
fibrotic lesions in certain vital organs (32). EndMT is tightly controlled by a
series of signaling networks, similar to the
epithelial-to-mesenchymal transition (38). EndMT is involved in intima
formation and pulmonary vascular angiogenesis (16) and may contribute to cardiac
fibrosis (39); similar results
have been observed in diabetes mellitus-induced cardiac fibrosis
(40) and further studies
confirmed that EndMT contributes to fibrogenesis associated with
exposure to TGF-β1 (41).
Consistent with these previous results, the present
study demonstrated that HUVECs underwent phenotypic and biological
behavioral transitions following TGF-β1 exposure consistent with
fibrogenesis. TGF-β1 is involved in a wide range of pathological
conditions, including a number of different types of cancer
(42), myocardial infarction
(43), cerebral cavernous
malformations (44), vascular
remolding and different types of organ fibrosis (40,41). During this process, TGF-β1
activates EndMT via multiple pathways including the canonical
Smad-dependent and non-canonical Smad-independent pathways, and the
bFGF, Wnt and Notch signaling pathways (45). A previous study has demonstrated
the contribution of TGF-β1 to EndMT in the regulation of
cardiovascular diseases (46).
Therefore, any potential target to inhibit or reduce TGF-β1/Snail
signaling may exhibit potential as an anti-proliferative reagent.
The present study demonstrated that TGF-β1 treatment increased the
expression of Snail, α-SMA and NF-κB p-p65/p65 ratio, and reduced
the expression of VE-cadherin, suggesting that TGF-β1 can induce
EndMT. Treatment with EOFAZ inhibited TGF-β1-mediated induction of
EndMT. Additionally, the increases in Snail and α-SMA expression
and NF-κB p-p65/p65 ratio in TGF-β1-treated cells were
significantly decreased following treatment with EOFAZ. These
results suggest that EOFAZ may be a promising agent for
ameliorating EndMT-related diseases.
KLF4 is an evolutionarily conserved zinc
finger-containing transcription factor and is widely expressed in a
range of tissues where it regulates a range of cellular processes,
including cell growth, proliferation and differentiation (47). KLF4 has a critical regulatory
effect on a range of functions, including maintaining intestinal
epithelial homeostasis, proliferation, migration and tube formation
in human retinal microvascular endothelial cells, and inhibiting
NF-κB transcriptional activity and the expression of downstream
prothrombotic, proinflam-matory genes (48-51). KLF4 mediates an increase in the
lesions and the pseudo-normal vasculature of endothelial-specific
CCM1-knockout mice (32). KLF4
also regulates VE-cadherin expression and endothelial barrier
function, angiogenesis via the Notch signaling pathway, vascular
tone and permeability, apoptosis, inflammation and atherothrombosis
(50). KLF4 can contribute to
epithelial-mesenchymal transition during the development and
progression of cancer (51).
Additionally, previous studies have shown that KLF4 is involved in
EndMT in cerebral cavernous malformations (47). In the present study, the results
demonstrated that KLF4 was excessively activated during TGF-β1
exposure, which resulted in the increase in the degree of EndMT in
HUVECs. KLF4-knockdown inhibited EndMT and decreased the protein
and mRNA expression levels of Snail and α-SMA. whilst increased
VE-cadherin expression. Following treatment with EOFAZ, the
processes associated with EndMT were reversed. These results
suggest that EOFAZ may inhibit TGF-β1-induced EndMT through
inhibition of KLF4.
In conclusion, EOFAZ significantly inhibited the
extent of EndMT following TGF-β1 exposure, a protective effect that
was mediated through reduction in KLF4 activity. In sum, a
graphical abstract is proposed and presented in Fig. 6, which summarizes the whole
investigation. The results of the present study contribute to the
understanding of the molecular mechanisms underlying EndMT and
reveal novel targets, which may be the basis of potential
therapeutic strategies.
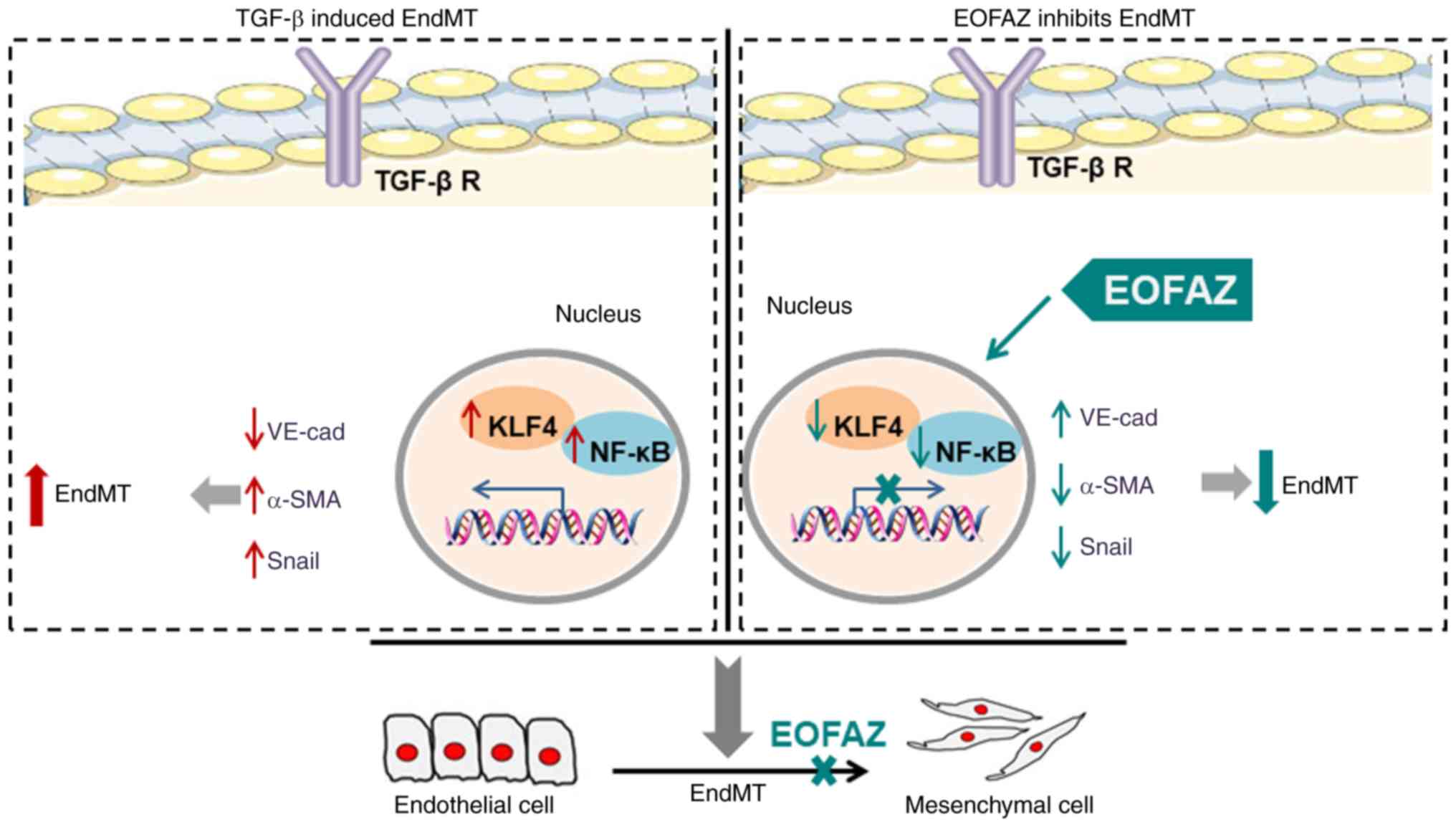 | Figure 6The inhibition effect of EOFAZ on
TGF-β1-induced EndMT. As a crucial factor for induction of EndMT,
TGF-β1 induces downregulation of the endothelial marker VE-cadherin
and upregulation of the mesenchymal marker α-SMA and Snail. In
addition, TGF-β1 enhances the binding between KLF4 and NF-кB. The
present study identified that EOFAZ inhibits the migration of
HUVECs upon TGF-β1-induced EndMT via upregulating α-SMA and Snail,
and downregulating VE-cadherin. Furthermore, the mechanism research
demonstrated that EOFAZ could decrease KLF4 expression and
subsequently prevent the combination between KLF4 and NF-кB, which
could suppress the activation of NF-кB, as well as the stimulation
of TGF-β1. EOFAZ, essential oil from Alpinia zerumbet rhizome;
TGF-β1, transforming growth factor-β1; α-SMA, α-smooth muscle
actin; VE-cadherin, vascular endothelial-cadherin; HUVEC, human
umbilical vein endothelial cell; EndMT, endothelial-to-mesenchymal
transition; TGF-β R, transforming growth factor-β receptor. |
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81560811, 81760725 and
U1812403-4-4), the Guizhou Provincial Key Technology R&D
Program [grant no. (2020)4Y093], the Scientific and Technological
Founding of Guizhou Province [grant no. QKHJ (2016)1128], the
Foundation of Science and Technology Department Cooperation Program
of Guizhou Province (grant no. 2016-7358) and Guizhou Graduate
Research (grant no. 0711007020259).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ performed part of the experiments and wrote the
manuscript. CL performed the experiments. YH designed the study and
analyzed the data. SZ performed the experiments. YX and YC analyzed
the data. FJ and LT extracted the essential oil. XS designed the
experiment and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors wish to thank Dr Di Pan (The State Key
Laboratory of Functions and Applications of Medicinal Plants,
School of Pharmaceutical Sciences, Guizhou Medical University,
Guiyang, China) for their kind linguistic advice and technical
assistance.
References
|
1
|
Chen Q, Zhang H, Liu Y, Adams S, Eilken H,
Stehling M, Corada M, Dejana E, Zhou B and Adams RH: Endothelial
cells are progenitors of cardiac pericytes and vascular smooth
muscle cells. Nat Commun. 7:124222016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gibbons GH and Dzau VJ: The emerging
concept of vascular remodeling. N Engl J Med. 330:1431–1438. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis GE and Senger DR: Endothelial
extracellular matrix: Biosynthesis, remodeling, and functions
during vascular morphogenesis and neovessel stabilization. Circ
Res. 97:1093–1107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karimi Galougahi K, Ashley EA and Ali ZA:
Redox regulation of vascular remodeling. Cell Mol Life Sci.
73:349–363. 2016. View Article : Google Scholar
|
|
5
|
Zhang B, Niu W, Dong HY, Liu ML, Luo Y and
Li ZC: Hypoxia induces endothelial-mesenchymal transition in
pulmonary vascular remodeling. Int J Mol Med. 42:270–278.
2018.PubMed/NCBI
|
|
6
|
Jackson AO, Zhang J, Jiang Z and Yin K:
Endothelial-to-mesenchymal transition: A novel therapeutic target
for cardiovascular diseases. Trends Cardiovasc Med. 27:383–393.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Li YM, Zeng XX, Wang XY, Chen SK,
Gui LX and Lin MJ: Galectin-3-mediated transdifferentiation of
pulmonary artery endothelial cells contributes to hypoxic pulmonary
vascular remodeling. Cell Physiol Biochem. 51:763–777. 2018.
View Article : Google Scholar
|
|
8
|
Wesseling M, Sakkers TR, de Jager SCA,
Pasterkamp G and Goumans MJ: The morphological and molecular
mechanisms of epithelial/endothelial-to-mesenchymal transition and
its involvement in atherosclerosis. Vascul Pharmacol. 106:1–8.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Wu X, Li Y, Zhang H, Li Z, Zhang
Y, Zhang L, Ju J, Liu X, Chen X, et al: Endothelial to mesenchymal
transition contributes to arsenic-trioxide-induced cardiac
fibrosis. Sci Rep. 6:337872016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian D, Zeng X, Wang W, Wang Z, Zhang Y
and Wang Y: Protective effect of rapamycin on
endothelial-to-mesenchymal transition in HUVECs through the Notch
signaling pathway. Vascul Pharmacol. 113:20–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanasaki K, Taduri G and Koya D: Diabetic
nephropathy: The role of inflammation in fibroblast activation and
kidney fibrosis. Front Endocrinol (Lausanne). 4:72013. View Article : Google Scholar
|
|
12
|
Xiong J, Kawagishi H, Yan Y, Liu J, Wells
QS, Edmunds LR, Fergusson MM, Yu ZX, Rovira II, Brittain EL, et al:
A metabolic basis for endothelial-to-mesenchymal transition. Mol
Cell. 69:689–698.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu QC, Song W, Wang D and Zeng YA:
Identification of blood vascular endothelial stem cells by the
expression of protein C receptor. Cell Res. 26:1079–1098. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooley BC, Nevado J, Mellad J, Yang D, St
Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, et al: TGF-β
signaling mediates endothelial-to-mesenchymal transition (EndMT)
during vein graft remodeling. Sci Transl Med. 6:227ra342014.
View Article : Google Scholar
|
|
15
|
Vanchin B, Offringa E, Friedrich J,
Brinker MG, Kiers B, Pereira AC, Harmsen MC, Moonen JA and Krenning
G: MicroRNA-374b induces endothelial-to-mesenchymal transition and
early lesion formation through the inhibition of MAPK7 signaling. J
Pathol. 247:456–470. 2019. View Article : Google Scholar :
|
|
16
|
Suzuki T, Carrier EJ, Talati MH,
Rathinasabapathy A, Chen X, Nishimura R, Tada Y, Tatsumi K and West
J: Isolation and characterization of endothelial-to-mesenchymal
transition cells in pulmonary arterial hypertension. Am J Physiol
Lung Cell Mol Physiol. 314:L118–L126. 2018. View Article : Google Scholar :
|
|
17
|
Li J, Xiong J, Yang B, Zhou Q, Wu Y, Luo
H, Zhou H, Liu N, Li Y, Song Z and Zheng Q: Endothelial cell
apoptosis induces TGF-β signaling-dependent host
endothelial-mesenchymal transition to promote transplant
arteriosclerosis. Am J Transplant. 15:3095–3111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Yan G, Guo H, Zhu Y, Shui X, He Y,
Chen C and Lei W: ITE promotes hypoxia-induced transdifferentiation
of human pulmonary arterial endothelial cells possibly by
activating transforming growth factor-β/Smads and MAPK/ERK
pathways. J Cell Biochem. 120:19567–19577. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zuo XX, Yang Y, Zhang Y, Zhang ZG, Wang XF
and Shi YG: Platelets promote breast cancer cell MCF-7 metastasis
by direct interaction: Surface integrin α2β1-contacting-mediated
activation of Wnt-β-catenin pathway. Cell Commun Signal.
17:1422019. View Article : Google Scholar
|
|
20
|
Shen XC, Tao L, Li WK, Zhang YY, Luo H and
Xia YY: Evidence-based antioxidant activity of the essential oil
from fructus A. Zerumbet on cultured human umbilical vein
endothelial cells' injury induced by ox-LDL. BMC Complement Altern
Med. 12:1742012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tao L, Hu HS and Shen XC:
Endothelium-dependent vasodilatation effects of the essential oil
from Fructus alpiniae zerumbet (EOFAZ) on rat thoracic aortic rings
in vitro. Phytomedicine. 20:387–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Li D, Xu Y, Zhang Y, Tao L, Li S,
Jiang Y and Shen X: Essential oils from fructus A. Zerumbet protect
human aortic endothelial cells from apoptosis induced by Ox-LDL in
vitro. Evid Based Complement Alternat Med. 2014:9568242014.
View Article : Google Scholar
|
|
23
|
Yob NJ, Jofrry SM, Affandi MM, The LK,
Salleh MZ and Zakaria ZA: Zingiber zerumbet (L.) Smith: A review of
its ethnomedicinal, chemical, and pharmacological uses. Evid Based
Complement Alternat Med. 2011:5432162011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chompoo J, Upadhyay A, Fukuta M and Tawata
S: Effect of Alpinia zerumbet components on antioxidant and skin
diseases-related enzymes. BMC Complement Altern Med. 12:1062012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Moura RS, Emiliano AF, de Carvalho LC,
Souza MA, Guedes DC, Tano T and Resende AC: Antihypertensive and
endothelium-dependent vasodilator effects of Alpinia zerumbet, a
medicinal plant. J Cardiovasc Pharmacol. 46:288–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Linghu K, Lin D, Yang H, Xu Y, Zhang Y,
Tao L, Chen Y and Shen X: Ameliorating effects of 1,8-cineole on
LPS-induced human umbilical vein endothelial cell injury by
suppressing NF-κB signaling in vitro. Eur J Pharmacol. 789:195–201.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang H, Fang Z, Qu X, Zhang X and Wang Y:
Procyanidin compound (PC) suppresses lipopolysaccharide-induced
cervical cancer cell proliferation through blocking the TLR4/NF-κB
pathway. Cancer Manag Res. 12:497–509. 2020. View Article : Google Scholar :
|
|
28
|
Shu Y, Liu Y, Li X, Cao L, Yuan X, Li W
and Cao Q: Aspirin-triggered resolvin D1 inhibits TGF-β1-induced
EndMT through increasing the expression of Smad7 and is closely
related to oxidative stress. Biomol Ther (Seoul). 24:132–139. 2016.
View Article : Google Scholar
|
|
29
|
Mahler GJ, Farrar EJ and Butcher JT:
Inflammatory cytokines promote mesenchymal transformation in
embryonic and adult valve endothelial cells. Arterioscler Thromb
Vasc Biol. 33:121–130. 2013. View Article : Google Scholar :
|
|
30
|
Yoshida T, Yamashita M, Iwai M and Hayashi
M: Endothelial Krüppel-like factor 4 mediates the protective effect
of statins against ischemic AKI. J Am Soc Nephrol. 27:1379–1388.
2016. View Article : Google Scholar
|
|
31
|
Cuttano R, Rudini N, Bravi L, Corada M,
Giampietro C, Papa E, Morini MF, Maddaluno L, Baeyens N, Adams RH,
et al: KLF4 is a key determinant in the development and progression
of cerebral cavernous malformations. EMBO Mol Med. 8:6–24. 2016.
View Article : Google Scholar :
|
|
32
|
Zhou Z, Tang AT, Wong WY, Bamezai S,
Goddard LM, Shenkar R, Zhou S, Yang J, Wright AC, Foley M, et al:
Cerebral cavernous malformations arise from endothelial gain of
MEKK3-KLF2/4 signalling. Nature. 532:122–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang N, Xu Y, Zhou H, Lin D, Zhang B,
Zhang Y, Pan D, Tao L, Liu X and Shen X: Essential oil from fructus
alpiniae zerumbet protects human umbilical vein endothelial cells
in vitro from injury induced by high glucose levels by suppressing
nuclear transcription factor-kappa B signaling. Med Sci Monit.
23:4760–4767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu M, Liu L, Xing Y, Yang S, Li H and Cao
Y: Roles and mechanisms of hawthorn and its extracts on
atherosclerosis: A review. Front Pharmacol. 11:1182020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu W, Liu Z, An S, Zhao J, Xiao L, Gou Y,
Lin Y and Wang J: The endothelial-mesenchymal transition (EndMT)
and tissue regeneration. Curr Stem Cell Res Ther. 9:196–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cruz-Solbes AS and Youker K: Epithelial to
mesenchymal transition (EMT) and endothelial to mesenchymal
transition (EndMT): Role and implications in kidney fibrosis.
Results Probl Cell Differ. 60:345–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pardali E, Sanchez-Duffhues G,
Gomez-Puerto MC and Ten Dijke P: TGF-β-induced
endothelial-mesenchymal transition in fibrotic diseases. Int J Mol
Sci. 18:pii: E2157. 2017. View Article : Google Scholar
|
|
38
|
Hao YM, Yuan HQ, Ren Z, Qu SL, Liu LS,
Dang H, Yin K, Fu M and Jiang ZS: Endothelial to mesenchymal
transition in atherosclerotic vascular remodeling. Clin Chim Acta.
490:34–38. 2019. View Article : Google Scholar
|
|
39
|
Geng H and Guan J: MiR-18a-5p inhibits
endothelial-mesen-chymal transition and cardiac fibrosis through
the Notch2 pathway. Biochem Biophys Res Commun. 491:329–336. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen XY, Lv RJ, Zhang W, Yan YG, Li P,
Dong WQ, Liu X, Liang ES, Tian HL, Lu QH and Zhang MX: Inhibition
of myocyte-specific enhancer factor 2A improved diabetic cardiac
fibrosis partially by regulating endothelial-to-mesenchymal
transition. Oncotarget. 7:31053–31066. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guan S and Zhou J: CXCR7 attenuates the
TGF-β-induced endothelial-to-mesenchymal transition and pulmonary
fibrosis. Mol Biosyst. 13:2116–2124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akhurst RJ: Targeting TGF-β signaling for
therapeutic gain. Cold Spring Harb Perspect Biol. 9:pii: a022301.
2017. View Article : Google Scholar
|
|
43
|
Bakhta O, Blanchard S, Guihot AL,
Tamareille S, Mirebeau-Prunier D, Jeannin P and Prunier F:
Cardioprotective role of colchicine against inflammatory injury in
a rat model of acute myocardial infarction. J Cardiovasc Pharmacol
Ther. 23:446–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wetzel-Strong SE, Detter MR and Marchuk
DA: The pathobiology of vascular malformations: Insights from human
and model organism genetics. J Pathol. 241:281–293. 2017.
View Article : Google Scholar
|
|
45
|
Fu Y, Chang A, Chang L, Niessen K, Eapen
S, Setiadi A and Karsan A: Differential regulation of transforming
growth factor beta signaling pathways by Notch in human endothelial
cells. J Biol Chem. 284:19452–19462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cunha SI, Magnusson PU, Dejana E and
Lampugnani MG: Deregulated TGF-β/BMP signaling in vascular
malformations. Circ Res. 121:981–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ghaleb AM and Yang VW: Krüppel-like factor
4 (KLF4): What we currently know. Gene. 611:27–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McConnell BB, Ghaleb AM, Nandan MO and
Yang VW: The diverse functions of Krüppel-like factors 4 and 5 in
epithelial biology and pathobiology. Bioessays. 29:549–557. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Lam O, Nguyen MT, Ng G, Pear WS,
Ai W, Wang IJ, Kao WW and Liu CY: Mastermind-like transcriptional
co-activator-mediated Notch signaling is indispensable for
maintaining conjunctival epithelial identity. Development.
140:594–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Yang C, Gu Q, Sims M, Gu W,
Pfeffer LM and Yue J: KLF4 promotes angiogenesis by activating VEGF
signaling in human retinal microvascular endothelial cells. PLoS
One. 10:e01303412015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ji L, Zhao G, Zhang P, Huo W, Dong P,
Watari H, Jia L, Pfeffer LM, Yue J and Zheng J: Knockout of MTF1
inhibits the epithelial to mesenchymal transition in ovarian cancer
cells. J Cancer. 9:4578–4585. 2018. View Article : Google Scholar : PubMed/NCBI
|