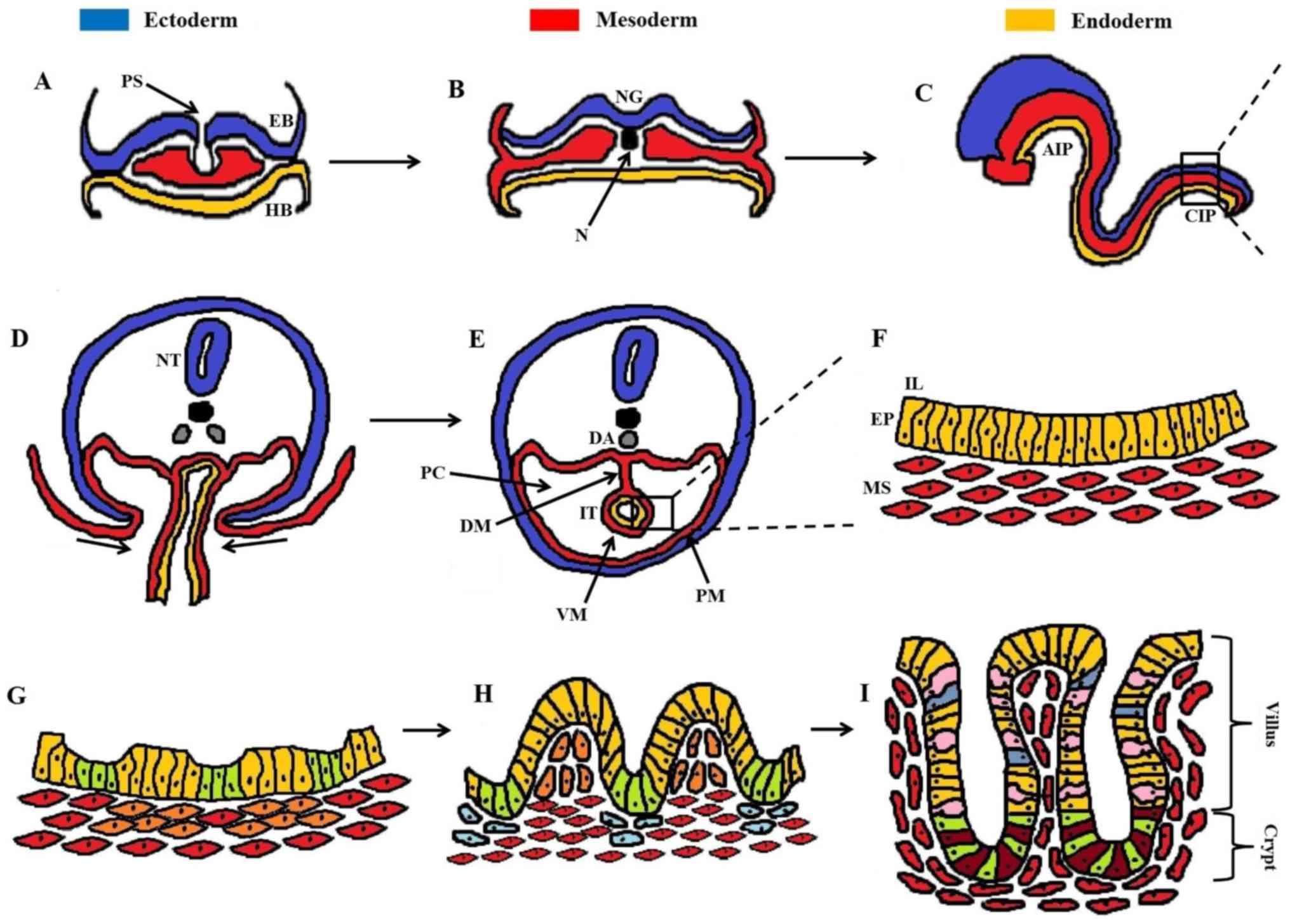
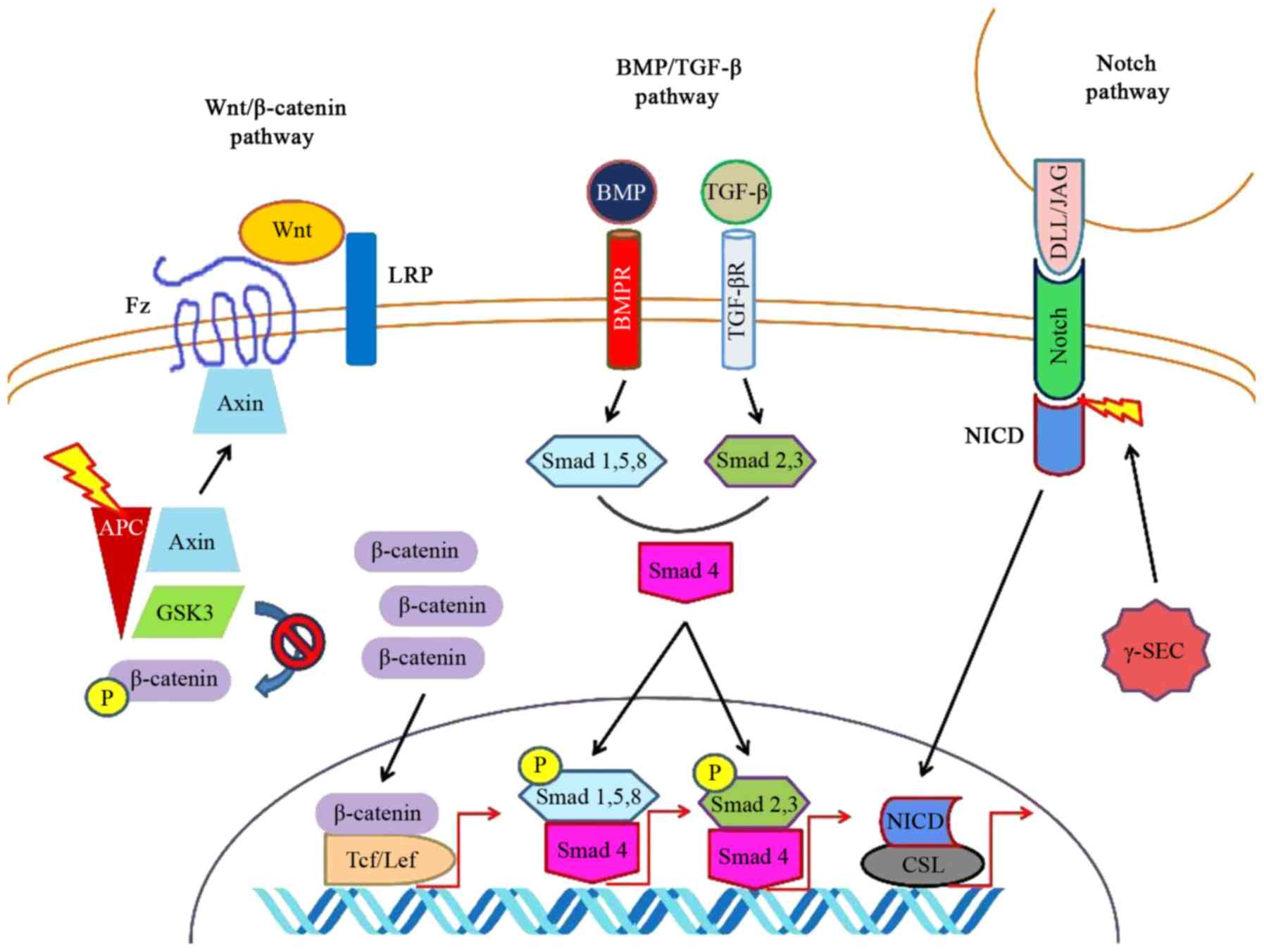
|
1
|
Spence JR, Lauf R and Shroyer NF:
Vertebrate intestinal endoderm development. Dev Dyn. 240:501–520.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts DJ: Molecular mechanisms of
development of the gastrointestinal tract. Dev Dyn. 219:109–120.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Santa Barbara P, Van den Brink GR and
Roberts DJ: Molecular etiology of gut malformations and diseases.
Am J Med Genet. 115:221–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelleher FC, Fennelly D and Rafferty M:
Common critical pathways in embryogenesis and cancer. Acta Oncol.
45:375–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JJ, Wolff BG, Tollefson MK, Walsh EE
and Larson DR: Meckel diverticulum: The mayo clinic experience with
1476 patients (1950-2002). Ann Surg. 241:529–533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kluth D, Jaeschke-Melli S and Fiegel H:
The embryology of gut rotation. Semin Pediatr Surg. 12:275–279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kluth D, Fiegel HC and Metzger R:
Embryology of the hindgut. Semin Pediatr Surg. 20:152–160. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JM and Kim NK: Essential anatomy of
the anorectum for colorectal surgeons focused on the gross anatomy
and histologic findings. Ann Coloproctol. 34:59–71. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uesaka T, Young HM, Pachnis V and Enomoto
H: Development of the intrinsic and extrinsic innervation of the
gut. Dev Biol. 417:158–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bayha E, Jørgensen MC, Serup P and
Grapin-Botton A: Retinoic acid signaling organizes endodermal organ
specification along the entire antero-posterior axis. PLoS One.
4:e58452009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bayliss WM and Starling EH: The movements
and innervation of the small intestine. J Physiol. 24:99–143. 1899.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Timmermans JP, Hens J and Adriaensen D:
Outer submucous plexus An intrinsic nerve network involved in both
secretory and motility processes in the intestine of large mammals
and humans. Anat Rec. 262:71–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen M: Nodal signaling: Developmental
roles and regulation. Development. 134:1023–1034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu P, Wakamiya M, Shea MJ, Albrecht U,
Behringer RR and Bradley A: Requirement for Wnt3 in vertebrate axis
formation. Nat Genet. 22:361–365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haegel H, Larue L, Ohsugi M, Fedorov L,
Herrenknecht K and Kemler R: Lack of beta-catenin affects mouse
development at gastrulation. Development. 121:3529–3537.
1995.PubMed/NCBI
|
|
16
|
Lowe LA, Yamada S and Kuehn MR: Genetic
dissection of nodal function in patterning the mouse embryo.
Development. 128:1831–1843. 2001.PubMed/NCBI
|
|
17
|
Vincent SD, Dunn NR, Hayashi S, Norris DP
and Robertson EJ: Cell fate decisions within the mouse organizer
are governed by graded Nodal signals. Genes Dev. 17:1646–1662.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weng W and Stemple DL: Nodal signaling and
vertebrate germ layer formation. Birth Defects Res C Embryo Today.
69:325–332. 2003. View Article : Google Scholar
|
|
19
|
Tada S, Era T, Furusawa C, Sakurai H and
Nishikawa S, Kinoshita M, Nakao K, Chiba T and Nishikawa S:
Characterization of mesendoderm: A diverging point of the
definitive endoderm and mesoderm in embryonic stem cell
differentiation culture. Development. 132:4363–4374. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horb ME and Thomsen GH: A vegetally
localized T-box transcription factor in Xenopus eggs specifies
mesoderm and endoderm and is essential for embryonic mesoderm
formation. Development. 124:1689–1698. 1997.PubMed/NCBI
|
|
21
|
Hart AH, Hartley L, Sourris K, Stadler ES,
Li R, Stanley EG, Tam PP, Elefanty AG and Robb L: Mixl1 is required
for axial mesendoderm morphogenesis and patterning in the murine
embryo. Development. 129:3597–3608. 2002.PubMed/NCBI
|
|
22
|
Maduro MF, Meneghini MD, Bowerman B,
Broitman-Maduro G and Rothman JH: Restriction of mesendoderm to a
single blastomere by the combined action of SKN-1 and a GSK-3beta
homolog is mediated by MED-1 and -2 in C. elegans. Mol Cell.
7:475–485. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y,
Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP and
Hayashi Y: Depletion of definitive gut endoderm in Sox17-null
mutant mice. Development. 129:2367–2379. 2002.PubMed/NCBI
|
|
24
|
Zhu J, Fukushige T, McGhee JD and Rothman
JH: Reprogramming of early embryonic blastomeres into endodermal
progenitors by a Caenorhabditis elegans GATA factor. Genes Dev.
12:3809–3814. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith JC, Price BM, Van Nimmen K and
Huylebroeck D: Identification of a potent Xenopus mesoderm-inducing
factor as a homologue of activin A. Nature. 345:729–731. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Henry GL, Brivanlou IH, Kessler DS,
Hemmati-Brivanlou A and Melton DA: TGF-beta signals and a pattern
in Xenopus laevis endodermal development. Development.
122:1007–1015. 1996.PubMed/NCBI
|
|
27
|
Lawson A and Schoenwolf GC: Epiblast and
primitive-streak origins of the endoderm in the gastrulating chick
embryo. Development. 130:3491–3501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura W, Yasugi S, Stern CD and Fukuda K:
Fate and plasticity of the endoderm in the early chick embryo. Dev
Biol. 289:283–295. 2006. View Article : Google Scholar
|
|
29
|
Franklin V, Khoo PL, Bildsoe H, Wong N,
Lewis S and Tam PP: Regionalisation of the endoderm progenitors and
morphogenesis of the gut portals of the mouse embryo. Mech Dev.
125:587–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zorn AM and Wells JM: Vertebrate endoderm
development and organ formation. Annu Rev Cell Dev Biol.
25:221–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwon GS, Viotti M and Hadjantonakis AK:
The endoderm of the mouse embryo arises by dynamic widespread
intercalation of embryonic and extraembryonic lineages. Dev Cell.
15:509–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tam PP, Khoo PL, Wong N, Tsang TE and
Behringer RR: Regionalization of cell fates and cell movement in
the endoderm of the mouse gastrula and the impact of loss of
Lhx1(Lim1) function. Dev Biol. 274:171–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lawson KA and Pedersen RA: Cell fate,
morphogenetic movement and population kinetics of embryonic
endoderm at the time of germ layer formation in the mouse.
Development. 101:627–652. 1987.PubMed/NCBI
|
|
34
|
Lewis SL and Tam PP: Definitive endoderm
of the mouse embryo: Formation, cell fates, and morphogenetic
function. Dev Dyn. 235:2315–2329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reed RA, Womble MA, Dush MK, Tull RR,
Bloom SK, Morckel AR, Devlin EW and Nascone-Yoder NM: Morphogenesis
of the primitive gut tube is generated by Rho/ROCK/myosin
II-mediated endoderm rearrangements. Dev Dyn. 238:3111–3125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Molkentin JD, Lin Q, Duncan SA and Olson
EN: Requirement of the transcription factor GATA4 for heart tube
formation and ventral morphogenesis. Genes Dev. 11:1061–1072. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garcia-Garcia MJ, Shibata M and Anderson
KV: Chato, a KRAB zinc-finger protein, regulates convergent
extension in the mouse embryo. Development. 135:3053–3062. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wen J, Chiang YJ, Gao C, Xue H, Xu J, Ning
Y, Hodes RJ, Gao X and Chen YG: Loss of Dact1 disrupts planar cell
polarity signaling by altering dishevelled activity and leads to
posterior malformation in mice. J Biol Chem. 285:11023–11030. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koike T and Yasugi S: In vitro analysis of
mesenchymal influences on the differentiation of stomach epithelial
cells of the chicken embryo. Differentiation. 65:13–25. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sumiya M: Differentiation of the digestive
tract epithelium of the chick embryo cultured in vitro enveloped in
a fragment of the vitelline membrane, in the absence of mesenchyme.
Roux Arch Dev Biol. 179:1–17. 1976. View Article : Google Scholar
|
|
41
|
Aufderheide E and Ekblom P: Tenascin
during gut development: Appearance in the mesenchyme, shift in
molecular forms, and dependence on epithelial-mesenchymal
interactions. J Cell Biol. 107:2341–2349. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kedinger M, Simon-Assmann P, Bouziges F,
Arnold C, Alexandre E and Haffen K: Smooth muscle actin expression
during rat gut development and induction in fetal skin
fibro-blastic cells associated with intestinal embryonic
epithelium. Differentiation. 43:87–97. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yasugi S, Takeda H and Fukuda K: Early
Determination of developmental fate in presumptive intestinal
endoderm of the chicken embryo. Dev Growth Differ. 33:235–241.
1991. View Article : Google Scholar
|
|
44
|
Roberts DJ, Smith DM, Goff DJ and Tabin
CJ: Epithelial-mesenchymal signaling during the regionalization of
the chick gut. Development. 125:2791–2801. 1998.PubMed/NCBI
|
|
45
|
Riddle RD, Johnson RL, Laufer E and Tabin
C: Sonic hedgehog mediates the polarizing activity of the ZPA.
Cell. 75:1401–1416. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan CM and Tessier-Lavigne M: Patterning
of mammalian somites by surface ectoderm and notochord: Evidence
for sclerotome induction by a hedgehog homolog. Cell. 79:1175–1186.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roelink H, Augsburger A, Heemskerk J,
Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T,
Jessell TM, et al: Floor plate and motor neuron induction by vhh-1,
a vertebrate homolog of hedgehog expressed by the notochord. Cell.
76:761–775. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Narita T, Ishii Y, Nohno T, Noji S and
Yasugi S: Sonic hedgehog expression in developing chicken digestive
organs is regulated by epithelial-mesenchymal interactions. Dev
Growth Differ. 40:67–74. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Roberts DJ, Johnson RL, Burke AC, Nelson
CE, Morgan BA and Tabin C: Sonic hedgehog is an endodermal signal
inducing Bmp-4 and Hox genes during induction and regionalization
of the chick hindgut. Development. 121:3163–3174. 1995.PubMed/NCBI
|
|
50
|
Chiang C, Litingtung Y, Lee E, Young KE,
Corden JL, Westphal H and Beachy PA: Cyclopia and defective axial
patterning in mice lacking Sonic hedgehog gene function. Nature.
383:407–413. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pepicelli CV, Lewis PM and McMahon AP:
Sonic hedgehog regulates branching morphogenesis in the mammalian
lung. Curr Biol. 8:1083–1086. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sukegawa A, Narita T, Kameda T, Saitoh K,
Nohno T, Iba H, Yasugi S and Fukuda K: The concentric structure of
the developing gut is regulated by Sonic hedgehog derived from
endodermal epithelium. Development. 127:1971–1980. 2000.PubMed/NCBI
|
|
53
|
Pisano JM, Colón-Hastings F and Birren SJ:
Postmigratory enteric and sympathetic neural precursors share
common, developmentally regulated, responses to BMP2. Dev Biol.
227:1–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chalazonitis A: Neurotrophin-3 in the
development of the enteric nervous system. Prog Brain Res.
146:243–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chawengsaksophak K, de Graaff W, Rossant
J, Deschamps J and Beck F: Cdx2 is essential for axial elongation
in mouse development. Proc Natl Acad Sci USA. 101:7641–7645. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kinkel MD, Eames SC, Alonzo MR and Prince
VE: Cdx4 is required in the endoderm to localize the pancreas and
limit beta-cell number. Development. 135:919–929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sherwood RI, Chen TY and Melton DA:
Transcriptional dynamics of endodermal organ formation. Dev Dyn.
238:29–42. 2009. View Article : Google Scholar
|
|
58
|
Wells JM and Melton DA: Vertebrate
endoderm development. Annu Rev Cell Dev Biol. 15:393–410. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tiso N, Filippi A, Pauls S, Bortolussi M
and Argenton F: BMP signalling regulates anteroposterior endoderm
patterning in zebrafish. Mech Dev. 118:29–37. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Grapin-Botton A: Antero-posterior
patterning of the vertebrate digestive tract: 40 years after Nicole
Le Douarin's PhD thesis. Int J Dev Biol. 49:335–347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Marikawa Y: Wnt/beta-catenin signaling and
body plan formation in mouse embryos. Semin Cell Dev Biol.
17:175–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Johannesson M, Ståhlberg A, Ameri J, Sand
FW, Norrman K and Semb H: FGF4 and retinoic acid direct
differentiation of hESCs into PDX1-expressing foregut endoderm in a
time- and concentration-dependent manner. PLoS One. 4:e47942009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ameri J, Ståhlberg A, Pedersen J,
Johansson JK, Johannesson MM, Artner I and Semb H: FGF2 specifies
hESC- derived definitive endoderm into foregut/midgut cell lineages
in a concentration-dependent manner. Stem Cells. 28:45–56.
2010.
|
|
64
|
Spence JR, Mayhew CN, Rankin SA, Kuhar MF,
Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn
AM, et al: Directed differentiation of human pluripotent stem cells
into intestinal tissue in vitro. Nature. 470:105–109. 2011.
View Article : Google Scholar :
|
|
65
|
Geske MJ, Zhang X, Patel KK, Ornitz DM and
Stappenbeck TS: Fgf9 signaling regulates small intestinal
elongation and mesen-chymal development. Development.
135:2959–2968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang X, Stappenbeck TS, White AC, Lavine
KJ, Gordon JI and Ornitz DM: Reciprocal epithelial-mesenchymal FGF
signaling is required for cecal development. Development.
133:173–180. 2006. View Article : Google Scholar
|
|
67
|
Burns RC, Fairbanks TJ, Sala F, De Langhe
S, Mailleux A, Thiery JP, Dickson C, Itoh N, Warburton D, Anderson
KD and Bellusci S: Requirement for fibroblast growth factor 10 or
fibroblast growth factor receptor 2-IIIb signaling for cecal
development in mouse. Dev Biol. 265:61–74. 2004. View Article : Google Scholar
|
|
68
|
Sala FG, Curtis JL, Veltmaat JM, Del Moral
PM, Le LT, Fairbanks TJ, Warburton D, Ford H, Wang K, Burns RC and
Bellusci S: Fibroblast growth factor 10 is required for survival
and proliferation but not differentiation of intestinal epithelial
progenitor cells during murine colon development. Dev Biol.
299:373–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Grapin-Botton A and Melton DA: Endoderm
development: From patterning to organogenesis. Trends Genet.
16:124–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gao N, White P and Kaestner KH:
Establishment of intestinal identity and epithelial-mesenchymal
signaling by Cdx2. Dev Cell. 16:588–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Grainger S, Savory JG and Lohnes D: Cdx2
regulates patterning of the intestinal epithelium. Dev Biol.
339:155–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Beck F and Stringer EJ: The role of Cdx
genes in the gut and in axial development. Biochem Soc Trans.
38:353–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Allan D, Houle M, Bouchard N, Meyer BI,
Gruss P and Lohnes D: RARgamma and Cdx1 interactions in vertebral
patterning. Dev Biol. 240:46–60. 2001. View Article : Google Scholar
|
|
74
|
Ikeya M and Takada S: Wnt-3a is required
for somite specification along the anteroposterior axis of the
mouse embryo and for regulation of cdx-1 expression. Mech Dev.
103:27–33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Keenan ID, Sharrard RM and Isaacs HV: FGF
signal transduction and the regulation of Cdx gene expression. Dev
Biol. 299:478–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gregorieff A, Grosschedl R and Clevers H:
Hindgut defects and transformation of the gastro-intestinal tract
in Tcf4(-/-)/Tcf1(-/-) embryos. EMBO J. 23:1825–1833. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yokouchi Y, Sakiyama J and Kuroiwa A:
Coordinated expression of Abd-B subfamily genes of the HoxA cluster
in the developing digestive tract of chick embryo. Dev Biol.
169:76–89. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Boulet AM and Capecchi MR: Targeted
disruption of hoxc-4 causes esophageal defects and vertebral
transformations. Dev Biol. 177:232–249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Warot X, Fromental-Ramain C, Fraulob V,
Chambon P and Dollé P: Gene dosage-dependent effects of the Hoxa-13
and Hoxd-13 mutations on morphogenesis of the terminal parts of the
digestive and urogenital tracts. Development. 124:4781–4791.
1997.
|
|
80
|
Zacchetti G, Duboule D and Zakany J: Hox
gene function in vertebrate gut morphogenesis: The case of the
caecum. Development. 134:3967–3973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mortlock DP and Innis JW: Mutation of
HOXA13 in hand-foot-genital syndrome. Nat Genet. 15:179–180. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huelsken J and Birchmeier W: New aspects
of Wnt signaling pathways in higher vertebrates. Curr Opin Genet
Dev. 11:547–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Theodosiou NA and Tabin CJ: Wnt signaling
during development of the gastrointestinal tract. Dev Biol.
259:258–271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
McBride HJ, Fatke B and Fraser SE: Wnt
signaling components in the chicken intestinal tract. Dev Biol.
256:18–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang X, Chen Y, Ye Y, Wang J, Wang H,
Yuan G, Lin Z, Wu Y, Zhang Y and Lin X: Wnt signaling promotes
hindgut fate commitment through regulating multi-lineage genes
during hESC differentiation. Cell Signal. 29:12–22. 2017.
View Article : Google Scholar
|
|
86
|
Behrens J, von Kries JP, Kühl M, Bruhn L,
Wedlich D, Grosschedl R and Birchmeier W: Functional interaction of
beta-catenin with the transcription factor LEF-1. Nature.
382:638–642. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hart M, Concordet JP, Lassot I, Albert I,
del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F,
Benarous R and Polakis P: The F-box protein beta-TrCP associates
with phosphorylated beta-catenin and regulates its activity in the
cell. Curr Biol. 9:207–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hart MJ, de los Santos R, Albert IN,
Rubinfeld B and Polakis P: Downregulation of beta-catenin by human
Axin and its association with the APC tumor suppressor,
beta-catenin and GSK3 beta. Curr Biol. 8:573–581. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Noordermeer J, Klingensmith J, Perrimon N
and Nusse R: Dishevelled and armadillo act in the wingless
signalling pathway in Drosophila. Nature. 367:80–83. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Joo JH, Taxter TJ, Munguba GC, Kim YH,
Dhaduvai K, Dunn NW, Degan WJ, Oh SP and Sugrue SP: Pinin modulates
expression of an intestinal homeobox gene, Cdx2, and plays an
essential role for small intestinal morphogenesis. Dev Biol.
345:191–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
92
|
van Genderen C, Okamura RM, Farinas I, Quo
RG, Parslow TG, Bruhn L and Grosschedl R: Development of several
organs that require inductive epithelial-mesenchymal interactions
is impaired in LEF-1-deficient mice. Genes Dev. 8:2691–2703. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Roose J, Huls G, van Beest M, Moerer P,
van der Horn K, Goldschmeding R, Logtenberg T and Clevers H:
Synergy between tumor suppressor APC and the beta-catenin-Tcf4
target Tcf1. Science. 285:1923–1926. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lickert H, Domon C, Huls G, Wehrle C,
Duluc I, Clevers H, Meyer BI, Freund JN and Kemler R:
Wnt/(beta)-catenin signaling regulates the expression of the
homeobox gene Cdx1 in embryonic intestine. Development.
127:3805–3813. 2000.PubMed/NCBI
|
|
95
|
Takada S, Stark KL, Shea MJ, Vassileva G,
McMahon JA and McMahon AP: Wnt-3a regulates somite and tailbud
formation in the mouse embryo. Genes Dev. 8:174–189. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yamaguchi TP, Bradley A, McMahon AP and
Jones S: A Wnt5a pathway underlies outgrowth of multiple structures
in the vertebrate embryo. Development. 126:1211–1223.
1999.PubMed/NCBI
|
|
97
|
Mlodzik M: Spiny legs and prickled bodies:
New insights and complexities in planar polarity establishment.
Bioessays. 22:311–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li L, Yuan H, Xie W, Mao J, Caruso AM,
McMahon A, Sussman DJ and Wu D: Dishevelled proteins lead to two
signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase
in mammalian cells. J Biol Chem. 274:129–134. 1999. View Article : Google Scholar
|
|
99
|
Winter CG, Wang B, Ballew A, Royou A,
Karess R, Axelrod JD and Luo L: Drosophila Rho-associated kinase
(Drok) links Frizzled-mediated planar cell polarity signaling to
the actin cytoskeleton. Cell. 105:81–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Marlow F, Topczewski J, Sepich D and
Solnica-Krezel L: Zebrafish Rho kinase 2 acts downstream of Wnt11
to mediate cell polarity and effective convergence and extension
movements. Curr Biol. 12:876–884. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Moon RT, Campbell RM, Christian JL, McGrew
LL, Shih J and Fraser S: Xwnt-5A: A maternal Wnt that affects
morphogenetic movements after overexpression in embryos of Xenopus
laevis. Development. 119:97–111. 1993.PubMed/NCBI
|
|
102
|
Kuhl M, Sheldahl LC, Malbon CC and Moon
RT: Ca(2+)/calmodulin-dependent protein kinase II is stimulated by
Wnt and Frizzled homologs and promotes ventral cell fates in
Xenopus. J Biol Chem. 275:12701–12711. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Slusarski DC, Corces VG and Moon RT:
Interaction of Wnt and a Frizzled homologue triggers
G-protein-linked phosphati-dylinositol signalling. Nature.
390:410–413. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xu Q, D'Amore PA and Sokol SY: Functional
and biochemical interactions of Wnts with FrzA, a secreted Wnt
antagonist. Development. 125:4767–4776. 1998.PubMed/NCBI
|
|
105
|
Wang S, Krinks M and Moos M Jr: Frzb-1, an
antagonist of Wnt-1 and Wnt-8, does not block signaling by Wnts
-3A, -5A, or -11. Biochem Biophys Res Commun. 236:502–504. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lee CS, Buttitta LA, May NR, Kispert A and
Fan CM: SHH-N upregulates Sfrp2 to mediate its competitive
interaction with WNT1 and WNT4 in the somitic mesoderm.
Development. 127:109–118. 2000.PubMed/NCBI
|
|
107
|
Cervantes S, Yamaguchi TP and Hebrok M:
Wnt5a is essential for intestinal elongation in mice. Dev Biol.
326:285–294. 2009. View Article : Google Scholar :
|
|
108
|
Matsuyama M, Aizawa S and Shimono A: Sfrp
controls apicobasal polarity and oriented cell division in
developing gut epithelium. PLoS Genet. 5:e10004272009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Capdevila I and Izpisúa Belmonte JC:
Knowing left from right: The molecular basis of laterality defects.
Mol Med Today. 6:112–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Levin M and Mercola M: The compulsion of
chirality: Toward an understanding of left-right asymmetry. Genes
Dev. 12:763–769. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Logan M, Pagan-Westphal SM, Smith DM,
Paganessi L and Tabin CJ: The transcription factor Pitx2 mediates
situs-specific morphogenesis in response to left-right asymmetric
signals. Cell. 94:307–317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Izraeli S, Lowe LA, Bertness VL, Good DJ,
Dorward DW, Kirsch IR and Kuehn MR: The SIL gene is required for
mouse embryonic axial development and left-right specification.
Nature. 399:691–694. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
113
|
Boettger T, Wittler L and Kessel M: FGF8
functions in the specification of the right body side of the chick.
Curr Biol. 9:277–280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Soffers JH, Hikspoors JP, Mekonen HK,
Koehler SE and Lamers WH: The growth pattern of the human intestine
and its mesentery. BMC Dev Biol. 15:312015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hikspoors JPJM, Kruepunga N, Mommen GMC,
Peeters J, Hülsman CJM, Eleonore Köhler S and Lamers WH: The
development of the dorsal mesentery in human embryos and fetuses.
Semin Cell Dev Biol. 92:18–26. 2019. View Article : Google Scholar
|
|
116
|
Mekonen HK, Hikspoors JP, Mommen G, Köhler
SE and Lamers WH: Development of the ventral body wall in the human
embryo. J Anat. 227:673–685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Frazer JE and Robbins RH: On the factors
concerned in causing rotation of the intestine in man. J Anat
Physiol. 50:75–110. 1915.PubMed/NCBI
|
|
118
|
Szpinda M, Paruszewska-Achtel M, Woźniak
A, Badura M, Mila-Kierzenkowska C and Wiśniewski M:
Three-dimensional growth dynamics of the liver in the human fetus.
Surg Radiol Anat. 37:439–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Davis NM, Kurpios NA, Sun X, Gros J,
Martin JF and Tabin CJ: The chirality of gut rotation derives from
left-right asymmetric changes in the architecture of the dorsal
mesentery. Dev Cell. 15:134–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Okada Y, Takeda S, Tanaka Y, Belmonte JI
and Hirokawa N: Mechanism of nodal flow: A conserved symmetry
breaking event in left-right axis determination. Cell. 121:633–644.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ermakov AS: Establishment of visceral
left-right asymmetry in mammals: The role of ciliary action and
leftward fluid flow in the region of Hensen's node. Ontogenez.
44:341–356. 2013.In Russian. PubMed/NCBI
|
|
122
|
Yuan S and Schoenwolf GC: Islet-1 marks
the early heart rudiments and is asymmetrically expressed during
early rotation of the foregut in the chick embryo. Anat Rec.
260:204–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Plageman TF Jr, Zacharias AL, Gage PJ and
Lang RA: Shroom3 and a Pitx2-N-cadherin pathway function
cooperatively to generate asymmetric cell shape changes during gut
morphogenesis. Dev Biol. 357:227–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kurpios NA, Ibanes M, Davis NM, Lui W,
Katz T, Martin JF, Izpisua Belmonte JC and Tabin CJ: The direction
of gut looping is established by changes in the extracellular
matrix and in cell: Cell adhesion. Proc Natl Acad Sci USA.
105:8499–8506. 2008. View Article : Google Scholar
|
|
125
|
Welsh IC, Thomsen M, Gludish DW,
Alfonso-Parra C, Bai Y, Martin JF and Kurpios NA: Integration of
left-right Pitx2 transcription and Wnt signaling drives asymmetric
gut morphogenesis via Daam2. Dev Cell. 26:629–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Gupta R, Soni V, Valse PD, Goyal RB, Gupta
AK and Mathur P: Neonatal intestinal obstruction associated with
situs inversus totalis: Two case reports and a review of the
literature. J Med Case Rep. 11:2642017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Morgan D, Turnpenny L, Goodship J, Dai W,
Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W,
et al: Inversin, a novel gene in the vertebrate left-right axis
pathway, is partially deleted in the inv mouse. Nat Genet.
20:149–156. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
128
|
Mochizuki T, Saijoh Y, Tsuchiya K,
Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H,
Nakatsuji N, et al: Cloning of inv, a gene that controls left/right
asymmetry and kidney development. Nature. 395:177–181. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nonaka S, Tanaka Y, Okada Y, Takeda S,
Harada A, Kanai Y, Kido M and Hirokawa N: Randomization of
left-right asymmetry due to loss of nodal cilia generating leftward
flow of extraembryonic fluid in mice lacking KIF3B motor protein.
Cell. 95:829–837. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hashimoto M, Shinohara K, Wang J, Ikeuchi
S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A
and Hamada H: Planar polarization of node cells determines the
rotational axis of node cilia. Nat Cell Biol. 12:170–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Song H, Hu J, Chen W, Elliott G, Andre P,
Gao B and Yang Y: Planar cell polarity breaks bilateral symmetry by
controlling ciliary positioning. Nature. 466:378–382. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Mahaffey JP, Grego-Bessa J, Liem KF Jr and
Anderson KV: Cofilin and Vangl2 cooperate in the initiation of
planar cell polarity in the mouse embryo. Development.
140:1262–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yoshiba S, Shiratori H, Kuo IY, Kawasumi
A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, et
al: Cilia at the node of mouse embryos sense fluid flow for
left-right determination via Pkd2. Science. 338:226–231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Gottschalk I, Stressig R, Ritgen J,
Herberg U, Breuer J, Vorndamme A, Strizek B, Willruth A, Geipel A,
Gembruch U and Berg C: Extracardiac anomalies in prenatally
diagnosed hetero-taxy syndrome. Ultrasound Obstet Gynecol.
47:443–449. 2016. View Article : Google Scholar
|
|
135
|
Applegate KE, Anderson JM and Klatte EC:
Intestinal malrotation in children: A problem-solving approach to
the upper gastrointestinal series. Radiographics. 26:1485–1500.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Nagel BH, Williams H, Stewart L, Paul J
and Stumper O: Splenic state in surviving patients with visceral
heterotaxy. Cardiol Young. 15:469–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mahalik SK, Khanna S and Menon P:
Malrotation and volvulus associated with heterotaxy syndrome. J
Indian Assoc Pediatr Surg. 17:138–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sedik A, Bar EA and Ismail M: Cecal
volvulus: Case report and review of literature. Saudi Surg J.
3:47–49. 2015. View Article : Google Scholar
|
|
139
|
Tsai EA, Grochowski CM, Falsey AM,
Rajagopalan R, Wendel D, Devoto M, Krantz ID, Loomes KM and Spinner
NB: Heterozygous deletion of FOXA2 segregates with disease in a
family with heterotaxy, panhypopituitarism, and biliary atresia.
Hum Mutat. 36:631–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hagen EM, Sicko RJ, Kay DM, Rigler SL,
Dimopoulos A, Ahmad S, Doleman MH, Fan R, Romitti PA, Browne ML, et
al: Copy-number variant analysis of classic heterotaxy highlights
the importance of body patterning pathways. Hum Genet.
135:1355–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Martin V and Shaw-Smith C: Review of
genetic factors in intestinal malrotation. Pediatr Surg Int.
26:769–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Straus WL Jr: The thoracic and abdominal
viscera of primates with special reference to the orangutan. Proc
Am Philo Soc. 76:1–85. 1936.
|
|
143
|
Bresalier RS, Boland CR and Kim YS:
Regional differences in normal and cancer-associated
glycoconjugates of the human colon. J Natl Cancer Inst. 75:249–260.
1985.PubMed/NCBI
|
|
144
|
Congdon ED, Blumberg R and Henry W:
Fasciae of fusion and elements of the fused enteric mesenteries in
the human adult. Am J Anat. 70:251–279. 1942. View Article : Google Scholar
|
|
145
|
Smith GM: A statistical review of the
variations in the anatomic positions of the caecum and the
processus vermiformis in the infant. Anat Rec. 5:549–566. 1911.
View Article : Google Scholar
|
|
146
|
Uhlenhuth E, Wolfe WM, Smith EM and
Middleton EB: The rectogenital septum. Surg Gynecol Obstet.
76:148–163. 1948.
|
|
147
|
Tobin CE and Benjamin JA: Anatomical and
surgical restudy of Denonvilliers' fascia. Surg Gynec Obst.
80:373–388. 1945.
|
|
148
|
Schumpelick V, Dreuw B, Ophoff K and
Prescher A: Appendix and cecum. Embryology, anatomy, and surgical
applications. Surg Clin North Am. 80:295–318. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Torres AM and Ziegler MM: Malrotation of
the intestine. World J Surg. 17:326–331. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Roux M and Delavierre P: Mesocelial
abscess of probable sigmoid diverticular origin. Hyper-rotation of
the intestinal lopp with the cecum at the left. Concours Med.
87:47–48. 1965.In French. PubMed/NCBI
|
|
151
|
Garude K and Rao S: Mobile cecum: An
incidental finding. Indian J Surg. 75:265–267. 2013. View Article : Google Scholar :
|
|
152
|
Rogers RL and Harford FJ: Mobile cecum
syndrome. Dis Colon Rectum. 27:399–402. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Collins DC: Agenesis of the vermiform
appendix. Am J Surg. 82:689–696. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Karam SM: Lineage commitment and
maturation of epithelial cells in the gut. Front Biosci.
4:D286–D298. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tandler J: Zur entwicklungsgeschichte des
Menschlichen Duodenum in Fruhen Embryonalstadien. Morph Jahrb.
29:187–216. 1900.
|
|
156
|
Cheng W and Tam PK: Murine duodenum does
not go through a 'solid core' stage in its embryological
development. Eur J Pediatr Surg. 8:212–215. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Dalla Vecchia LK, Grosfeld JL, West KW,
Rescorla FJ, Scherer LR and Engum SA: Intestinal atresia and
stenosis: A 25-year experience with 277 cases. Arch Surg.
133:490–497. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Louw JH: Jejunoileal atresia and stenosis.
J Pediatr Surg. 1:8–23. 1966. View Article : Google Scholar
|
|
159
|
Abdullah F, Arnold MA, Nabaweesi R,
Fischer AC, Colombani PM, Anderson KD, Lau H and Chang DC:
Gastroschisis in the United States 1988-2003: Analysis and risk
categorization of 4344 patients. J Perinatol. 27:50–55. 2007.
View Article : Google Scholar
|
|
160
|
Baglaj M, Carachi R and MacCormack B:
Colonic atresia: A clinicopathological insight into its etiology.
Eur J Pediatr Surg. 20:102–105. 2010. View Article : Google Scholar
|
|
161
|
Barnard CN and Louw JH: The genesis of
intestinal atresia. Minn Med. 39:7451956.PubMed/NCBI
|
|
162
|
Louw JH and Barnard CN: Congenital
intestinal atresia; observations on its origin. Lancet.
269:1065–1067. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Black PR, Mueller D, Crow J, Morris RC and
Husain AN: Mesenteric defects as a cause of intestinal volvulus
without malrotation and as the possible primary etiology of
intestinal atresia. J Pediatr Surg. 29:1339–1343. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Stollman TH, Wijnen RM and Draaisma JM:
Investigation for cystic fibrosis in infants with jejunoileal
atresia in the Netherlands: A 35-year experience with 114 cases.
Eur J Pediatr. 166:989–990. 2007. View Article : Google Scholar
|
|
165
|
Erskine JM: Colonic stenosis in the
newborn: The possible thromboembolic etiology of intestinal
stenosis and atresia. J Pediatr Surg. 5:321–333. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Johnson SM and Meyers RL: Inherited
thrombophilia: A possible cause of in utero vascular thrombosis in
children with intestinal atresia. J Pediatr Surg. 36:1146–1149.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Cooley BC, Chen CY and Schmeling G:
Increased venous versus arterial thrombosis in the Factor V Leiden
mouse. Thromb Res. 119:747–751. 2007. View Article : Google Scholar
|
|
168
|
Greer FR: Vitamin K the basics-what's new?
Early Hum Dev. 86(Suppl 1): S43–S47. 2010. View Article : Google Scholar
|
|
169
|
Cortese MG, Morra I, Marchese C,
Costantino S, Forni M and Canavese F: Association between multiple
intestinal atresia and omphalocele: A case report. Pediatr Pathol
Mol Med. 20:203–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Pameijer CR, Hubbard AM, Coleman B and
Flake AW: Combined pure esophageal atresia, duodenal atresia,
biliary atresia, and pancreatic ductal atresia: Prenatal diagnostic
features and review of the literature. J Pediatr Surg. 35:745–747.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Fairbanks TJ, Sala FG, Kanard R, Curtis
JL, Del Moral PM, De Langhe S, Warburton D, Anderson KD, Bellusci S
and Burns RC: The fibroblast growth factor pathway serves a
regulatory role in proliferation and apoptosis in the pathogenesis
of intestinal atresia. J Pediatr Surg. 41:132–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ornitz DM and Itoh N: Fibroblast growth
factors. Genome Biol. 2:REVIEWS30052001. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Botham RA, Franco M, Reeder AL, Lopukhin
A, Shiota K, Yamada S and Nichol PF: Formation of duodenal atresias
in fibroblast growth factor receptor 2IIIb-/- mouse embryos occurs
in the absence of an endodermal plug. J Pediatr Surg. 47:1369–1379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Reeder AL, Botham RA, Franco M, Zaremba KM
and Nichol PF: Formation of intestinal atresias in the Fgfr2IIIb-/-
mice is not associated with defects in notochord development or
alterations in Shh expression. J Surg Res. 177:139–145. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Reeder AL, Zaremba KM, Liebl RM,
Kowalkowski A and Nichol PF: Exogenous Sonic hedgehog protein does
not rescue cultured intestine from atresia formation. J Surg Res.
187:14–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Nichol PF, Tyrrell JD and Saijoh Y:
Retinaldehyde dehydroge-nase 2 is down-regulated during duodenal
atresia formation in Fgfr2IIIb-/- mice. J Surg Res. 175:82–87.
2012. View Article : Google Scholar
|
|
177
|
Reeder AL, Botham RA, Zaremba KM and
Nichol PF: Haploinsufficiency of retinaldehyde dehydrogenase 2
decreases the severity and incidence of duodenal atresia in the
fibroblast growth factor receptor 2IIIb-/- mouse model. Surgery.
152:768–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Ramalho-Santos M, Melton DA and McMahon
AP: Hedgehog signals regulate multiple aspects of gastrointestinal
development. Development. 127:2763–2772. 2000.PubMed/NCBI
|
|
179
|
Mo R, Kim JH, Zhang J, Chiang C, Hui CC
and Kim PC: Anorectal malformations caused by defects in sonic
hedgehog signaling. Am J Pathol. 159:765–774. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Al-Jaroof AH, Al-Zayer F and Meshikhes AW:
A case of sigmoid colon duplication in an adult woman. BMJ Case
Rep. 2014:pii: bcr2014203874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Ramakrishna HK: Intestinal duplication.
Indian J Surg. 70:270–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Favara BE, Franciosi RA and Akers DR:
Enteric duplications. Thirty-seven cases: A vascular theory of
pathogenesis. Am J Dis Child. 122:501–506. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Colony PC, Kois JM and Peiffer LP:
Structural and enzymatic changes during colonic maturation in the
fetal and suckling rat. Gastroenterology. 97:338–347. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Helander HF: Morphological studies on the
development of the rat colonic mucosa. Acta Anat (Basel). 85. pp.
155–176. 1973, View Article : Google Scholar
|
|
185
|
Bell L and Williams L: A scanning and
transmission electron microscopical study of the morphogenesis of
human colonic villi. Anat Embryol (Berl). 165:437–455. 1982.
View Article : Google Scholar
|
|
186
|
Lev R and Orlic D: Histochemical and
radioautographic studies of normal human fetal colon.
Histochemistry. 39:301–311. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Saotome I, Curto M and McClatchey AI:
Ezrin is essential for epithelial organization and villus
morphogenesis in the developing intestine. Dev Cell. 6:855–864.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ratineau C, Duluc I, Pourreyron C,
Kedinger M, Freund JN and Roche C: Endoderm- and
mesenchyme-dependent commitment of the differentiated epithelial
cell types in the developing intestine of rat. Differentiation.
71:163–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Fritsch C, Swietlicki EA, Lefebvre O,
Kedinger M, Iordanov H, Levin MS and Rubin DC: Epimorphin
expression in intestinal myofibroblasts induces epithelial
morphogenesis. J Clin Invest. 110:1629–1641. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Shaker A, Swietlicki EA, Wang L, Jiang S,
Onal B, Bala S, DeSchryver K, Newberry R, Levin MS and Rubin DC:
Epimorphin deletion protects mice from inflammation-induced colon
carcinogenesis and alters stem cell niche myofibroblast secretion.
J Clin Invest. 120:2081–2093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Hirai Y, Takebe K, Takashina M, Kobayashi
S and Takeichi M: Epimorphin: A mesenchymal protein essential for
epithelial morphogenesis. Cell. 69:471–481. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Kolterud A, Grosse AS, Zacharias WJ,
Walton KD, Kretovich KE, Madison BB, Waghray M, Ferris JE, Hu C,
Merchant JL, et al: Paracrine Hedgehog signaling in stomach and
intestine: New roles for hedgehog in gastrointestinal patterning.
Gastroenterology. 137:618–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Madison BB, Braunstein K, Kuizon E,
Portman K, Qiao XT and Gumucio DL: Epithelial hedgehog signals
pattern the intestinal crypt-villus axis. Development. 132:279–289.
2005. View Article : Google Scholar
|
|
194
|
Karlsson L, Lindahl P, Heath JK and
Betsholtz C: Abnormal gastrointestinal development in PDGF-A and
PDGFR-(alpha) deficient mice implicates a novel mesenchymal
structure with putative instructive properties in villus
morphogenesis. Development. 127:3457–3466. 2000.PubMed/NCBI
|
|
195
|
Wang LC, Nassir F, Liu ZY, Ling L, Kuo F,
Crowell T, Olson D, Davidson NO and Burkly LC: Disruption of
hedgehog signaling reveals a novel role in intestinal morphogenesis
and intestinal-specific lipid metabolism in mice. Gastroenterology.
122:469–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Madison BB, McKenna LB, Dolson D, Epstein
DJ and Kaestner KH: FoxF1 and FoxL1 link hedgehog signaling and the
control of epithelial proliferation in the developing stomach and
intestine. J Biol Chem. 284:5936–5944. 2009. View Article : Google Scholar :
|
|
197
|
Kaestner KH, Silberg DG, Traber PG and
Schutz G: The mesenchymal winged helix transcription factor Fkh6 is
required for the control of gastrointestinal proliferation and
differentiation. Genes Dev. 11:1583–1595. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Ormestad M, Astorga J, Landgren H, Wang T,
Johansson BR, Miura N and Carlsson P: Foxf1 and Foxf2 control
murine gut development by limiting mesenchymal Wnt signaling and
promoting extracellular matrix production. Development.
133:833–843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
De Santa Barbara P, Williams J, Goldstein
AM, Doyle AM, Nielsen C, Winfield S, Faure S and Roberts DJ: Bone
morpho-genetic protein signaling pathway plays multiple roles
during gastrointestinal tract development. Dev Dyn. 234:312–322.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
He XC, Zhang J, Tong WG, Tawfik O, Ross J,
Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al: BMP
signaling inhibits intestinal stem cell self-renewal through
suppression of Wnt-beta-catenin signaling. Nat Genet. 36:1117–1121.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
201
|
Howe JR, Bair JL, Sayed MG, Anderson ME,
Mitros FA, Petersen GM, Velculescu VE, Traverso G and Vogelstein B:
Germline mutations of the gene encoding bone morphogenetic protein
receptor 1A in juvenile polyposis. Nat Genet. 28:184–187. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Shroyer NF and Wong MH: BMP signaling in
the intestine: Cross-talk is key. Gastroenterology. 133:1035–1038.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Batts LE, Polk DB, Dubois RN and Kulessa
H: Bmp signaling is required for intestinal growth and
morphogenesis. Dev Dyn. 235:1563–1570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Flentjar N, Chu PY, Ng AY, Johnstone CN,
Heath JK, Ernst M, Hertzog PJ and Pritchard MA: TGF-betaRII rescues
development of small intestinal epithelial cells in Elf3-deficient
mice. Gastroenterology. 132:1410–1419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Jedlicka P and Gutierrez-Hartmann A: Ets
transcription factors in intestinal morphogenesis, homeostasis and
disease. Histol Histopathol. 23:1417–1424. 2008.PubMed/NCBI
|
|
206
|
Kwon MC, Koo BK, Kim YY, Lee SH, Kim NS,
Kim JH and Kong YY: Essential role of CR6-interacting factor 1
(Crif1) in E74-like factor 3 (ELF3)-mediated intestinal
development. J Biol Chem. 284:33634–33641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Pabst O, Zweigerdt R and Arnold HH:
Targeted disruption of the homeobox transcription factor Nkx2-3 in
mice results in postnatal lethality and abnormal development of
small intestine and spleen. Development. 126:2215–2225.
1999.PubMed/NCBI
|
|
208
|
Shikama N, Lutz W, Kretzschmar R, Sauter
N, Roth JF, Marino S, Wittwer J, Scheidweiler A and Eckner R:
Essential function of p300 acetyltransferase activity in heart,
lung and small intestine formation. EMBO J. 22:5175–5185. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Tou L, Liu Q and Shivdasani RA: Regulation
of mammalian epithelial differentiation and intestine development
by class I histone deacetylases. Mol Cell Biol. 24:3132–3139. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Grand RJ, Watkins JB and Torti FM:
Development of the human gastrointestinal tract. A review.
Gastroenterology. 70:790–810. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Kim BM, Mao J, Taketo MM and Shivdasani
RA: Phases of canonical Wnt signaling during the development of
mouse intestinal epithelium. Gastroenterology. 133:529–538. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Pinto D, Gregorieff A, Begthel H and
Clevers H: Canonical Wnt signals are essential for homeostasis of
the intestinal epithelium. Genes Dev. 17:1709–1713. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Kuhnert F, Davis CR, Wang HT, Chu P, Lee
M, Yuan J, Nusse R and Kuo CJ: Essential requirement for Wnt
signaling in proliferation of adult small intestine and colon
revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci
USA. 101:266–271. 2004. View Article : Google Scholar
|
|
214
|
Sinner D, Kordich JJ, Spence JR, Opoka R,
Rankin S, Lin SC, Jonatan D, Zorn AM and Wells JM: Sox17 and Sox4
differentially regulate beta-catenin/T-cell factor activity and
proliferation of colon carcinoma cells. Mol Cell Biol.
27:7802–7815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Wong MH, Huelsken J, Birchmeier W and
Gordon JI: Selection of multipotent stem cells during morphogenesis
of small intestinal crypts of Lieberkuhn is perturbed by
stimulation of Lef-1/beta-catenin signaling. J Biol Chem.
277:15843–15850. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Jensen J, Pedersen EE, Galante P, Hald J,
Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P and Madsen
OD: Control of endodermal endocrine development by Hes-1. Nat
Genet. 24:36–44. 2000. View
Article : Google Scholar
|
|
217
|
Shroyer NF, Helmrath MA, Wang VY, Antalffy
B, Henning SJ and Zoghbi HY: Intestine-specific ablation of mouse
atonal homolog 1 (Math1) reveals a role in cellular homeostasis.
Gastroenterology. 132:2478–2488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
van Es JH, de Geest N, van de Born M,
Clevers H and Hassan BA: Intestinal stem cells lacking the Math1
tumour suppressor are refractory to Notch inhibitors. Nat Commun.
1:182010. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
VanDussen KL and Samuelson LC: Mouse
atonal homolog 1 directs intestinal progenitors to secretory cell
rather than absorptive cell fate. Dev Biol. 346:215–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Koo BK, Lim HS, Chang HJ, Yoon MJ, Choi Y,
Kong MP, Kim CH, Kim JM, Park JG and Kong YY: Notch signaling
promotes the generation of EphrinB1-positive intestinal epithelial
cells. Gastroenterology. 137:145–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Tian H, Biehs B, Chiu C, Siebel CW, Wu Y,
Costa M, de Sauvage FJ and Klein OD: Opposing activities of Notch
and Wnt signaling regulate intestinal stem cells and gut
homeostasis. Cell Rep. 11:33–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Peignon G, Durand A, Cacheux W, Ayrault O,
Terris B, Laurent-Puig P, Shroyer NF, Van Seuningen I, Honjo T,
Perret C and Romagnolo B: Complex interplay between β-catenin
signalling and Notch effectors in intestinal tumorigenesis. Gut.
60:166–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Tsuchiya K, Nakamura T, Okamoto R, Kanai T
and Watanabe M: Reciprocal targeting of Hath1 and beta-catenin by
Wnt glycogen synthase kinase 3beta in human colon cancer.
Gastroenterology. 132:208–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Coant N, Ben Mkaddem S, Pedruzzi E,
Guichard C, Treton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik
Y, Woerther PL, et al: NADPH oxidase 1 modulates WNT and NOTCH1
signaling to control the fate of proliferative progenitor cells in
the colon. Mol Cell Biol. 30:2636–2650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Calvert R, Beaulieu JF and Ménard D:
Epidermal growth factor (EGF) accelerates the maturation of fetal
mouse intestinal mucosa in utero. Experientia. 38:1096–1097. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Griffiths DF, Davies SJ, Williams D,
Williams GT and Williams ED: Demonstration of somatic mutation and
colonic crypt clonality by X-linked enzyme histochemistry. Nature.
333:461–463. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Tian Q, He XC, Hood L and Li L: Bridging
the BMP and Wnt pathways by PI3 kinase/Akt and 143-3zeta. Cell
Cycle. 4:215–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Batlle E, Henderson JT, Beghtel H, van den
Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering
M, Pawson T and Clevers H: Beta-catenin and TCF mediate cell
positioning in the intestinal epithelium by controlling the
expression of EphB/ephrinB. Cell. 111:251–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
van der Flier LG and Clevers H: Stem
cells, self-renewal, and differentiation in the intestinal
epithelium. Annu Rev Physiol. 71:241–260. 2009. View Article : Google Scholar
|
|
230
|
Schemann M: Control of gastrointestinal
motility by the 'gut brain'-the enteric nervous system. J Pediatr
Gastroenterol Nutr. 41(Suppl 1): S4–S6. 2005. View Article : Google Scholar
|
|
231
|
Furness JB: Types of neurons in the
enteric nervous system. J Auton Nerv Syst. 81:87–96. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Goldstein AM, Thapar N, Karunaratne TB and
De Giorgio R: Clinical aspects of neurointestinal disease:
Pathophysiology, diagnosis, and treatment. Dev Biol. 417:217–228.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Kapur RP: Practical pathology and genetics
of Hirschsprung's disease. Semin Pediatr Surg. 18:212–223. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Amiel J and Lyonnet S: Hirschsprung
disease, associated syndromes, and genetics: A review. J Med Genet.
38:729–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Bronner ME and LeDouarin NM: Development
and evolution of the neural crest: An overview. Dev Biol. 366:2–9.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Le Douarin NM and Teillet MA: The
migration of neural crest cells to the wall of the digestive tract
in avian embryo. J Embryol Exp Morphol. 30:31–48. 1973.PubMed/NCBI
|
|
237
|
Yntema CL and Hammond WS: The origin of
intrinsic ganglia of trunk viscera from vagal neural crest in the
chick embryo. J Comp Neurol. 101:515–541. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Kuo BR and Erickson CA: Regional
differences in neural crest morphogenesis. Cell Adh Migr.
4:567–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Burns AJ and Le Douarin NM: Enteric
nervous system development: Analysis of the selective developmental
potentialities of vagal and sacral neural crest cells using
quail-chick chimeras. Anat Rec. 262:16–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Burns AJ, Champeval D and Le Douarin NM:
Sacral neural crest cells colonise aganglionic hindgut in vivo but
fail to compensate for lack of enteric ganglia. Dev Biol.
219:30–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Fu M, Lui VC, Sham MH, Cheung AN and Tam
PK: HOXB5 expression is spatially and temporarily regulated in
human embryonic gut during neural crest cell colonization and
differentiation of enteric neuroblasts. Dev Dyn. 228:1–10. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
De Bellard ME, Rao Y and Bronner-Fraser M:
Dual function of Slit2 in repulsion and enhanced migration of
trunk, but not vagal, neural crest cells. J Cell Biol. 162:269–279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Zuhdi N, Ortega B, Giovannone D, Ra H,
Reyes M, Asención V, McNicoll I, Ma L and de Bellard ME: Slit
molecules prevent entrance of trunk neural crest cells in
developing gut. Int J Dev Neurosci. 41:8–16. 2015. View Article : Google Scholar :
|
|
244
|
Nagy N, Brewer KC, Mwizerwa O and
Goldstein AM: Pelvic plexus contributes ganglion cells to the
hindgut enteric nervous system. Dev Dyn. 236:73–83. 2007.
View Article : Google Scholar
|
|
245
|
Young HM, Bergner AJ, Anderson RB, Enomoto
H, Milbrandt J, Newgreen DF and Whitington PM: Dynamics of neural
crest-derived cell migration in the embryonic mouse gut. Dev Biol.
270:455–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Allan IJ and Newgreen DF: The origin and
differentiation of enteric neurons of the intestine of the fowl
embryo. Am J Anat. 157:137–154. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Young HM and Newgreen D: Enteric neural
crest-derived cells: Origin, identification, migration, and
differentiation. Anat Rec. 262:1–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Wallace AS and Burns AJ: Development of
the enteric nervous system, smooth muscle and interstitial cells of
Cajal in the human gastrointestinal tract. Cell Tissue Res.
319:367–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Burns AJ and Douarin NM: The sacral neural
crest contributes neurons and glia to the post-umbilical gut:
Spatiotemporal analysis of the development of the enteric nervous
system. Development. 125:4335–4347. 1998.PubMed/NCBI
|
|
250
|
Delalande JM, Barlow AJ, Thomas AJ,
Wallace AS, Thapar N, Erickson CA and Burns AJ: The receptor
tyrosine kinase RET regulates hindgut colonization by sacral neural
crest cells. Dev Biol. 313:279–292. 2008. View Article : Google Scholar
|
|
251
|
Burns AJ, Delalande JM and Le Douarin NM:
In ovo transplantation of enteric nervous system precursors from
vagal to sacral neural crest results in extensive hindgut
colonisation. Development. 129:2785–2796. 2002.PubMed/NCBI
|
|
252
|
Uesaka T, Nagashimada M and Enomoto H:
Neuronal differentiation in schwann cell lineage underlies
postnatal neurogenesis in the enteric nervous system. J Neurosci.
35:9879–9888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Nishiyama C, Uesaka T, Manabe T, Yonekura
Y, Nagasawa T, Newgreen DF, Young HM and Enomoto H:
Trans-mesenteric neural crest cells are the principal source of the
colonic enteric nervous system. Nat Neurosci. 15:1211–1218. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Peters-van der Sanden MJ, Kirby ML,
Gittenberger-de Groot A, Tibboel D, Mulder MP and Meijers C:
Ablation of various regions within the avian vagal neural crest has
differential effects on ganglion formation in the fore-, mid- and
hindgut. Dev Dyn. 196:183–194. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Druckenbrod NR and Epstein ML: The pattern
of neural crest advance in the cecum and colon. Dev Biol.
287:125–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Young HM, Bergner AJ, Simpson MJ, McKeown
SJ, Hao MM, Anderson CR and Enomoto H: Colonizing while migrating:
How do individual enteric neural crest cells behave? BMC Biol.
12:232014. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Anderson RB, Turner KN, Nikonenko AG,
Hemperly J, Schachner M and Young HM: The cell adhesion molecule l1
is required for chain migration of neural crest cells in the
developing mouse gut. Gastroenterology. 130:1221–1232. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Druckenbrod NR and Epstein ML:
Age-dependent changes in the gut environment restrict the invasion
of the hindgut by enteric neural progenitors. Development.
136:3195–3203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Simpson MJ, Zhang DC, Mariani M, Landman
KA and Newgreen DF: Cell proliferation drives neural crest cell
invasion of the intestine. Dev Biol. 302:553–568. 2007. View Article : Google Scholar
|
|
260
|
Carmona-Fontaine C, Matthews HK, Kuriyama
S, Moreno M, Dunn GA, Parsons M, Stern CD and Mayor R: Contact
inhibition of locomotion in vivo controls neural crest directional
migration. Nature. 456:957–961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Nagy N and Goldstein AM: Enteric nervous
system development: A crest cell's journey from neural tube to
colon. Semin Cell Dev Biol. 66:94–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Gabriel SB, Salomon R, Pelet A, Angrist M,
Amiel J, Fornage M, Attié-Bitach T, Olson JM, Hofstra R, Buys C, et
al: Segregation at three loci explains familial and population risk
in Hirschsprung disease. Nat Genet. 31:89–93. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Emison ES, McCallion AS, Kashuk CS, Bush
RT, Grice E, Lin S, Portnoy ME, Cutler DJ, Green ED and Chakravarti
A: A common sex-dependent mutation in a RET enhancer underlies
Hirschsprung disease risk. Nature. 434:857–863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Southard-Smith EM, Kos L and Pavan WJ:
Sox10 mutation disrupts neural crest development in Dom
Hirschsprung mouse model. Nat Genet. 18:60–64. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Pattyn A, Morin X, Cremer H, Goridis C and
Brunet JF: The homeobox gene Phox2b is essential for the
development of autonomic neural crest derivatives. Nature.
399:366–370. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
266
|
Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis
V, Takahashi M and Costantini F: Targeted mutation of serine 697 in
the Ret tyrosine kinase causes migration defect of enteric neural
crest cells. Development. 133:4507–4516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Mograbi B, Bocciardi R, Bourget I, Busca
R, Rochet N, Farahi-Far D, Juhel T and Rossi B: Glial cell
line-derived neurotrophic factor-stimulated phosphatidylinositol
3-kinase and Akt activities exert opposing effects on the ERK
pathway: Importance for the rescue of neuroectodermic cells. J Biol
Chem. 276:45307–45319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Natarajan D, Marcos-Gutierrez C, Pachnis V
and de Graaff E: Requirement of signalling by receptor tyrosine
kinase RET for the directed migration of enteric nervous system
progenitor cells during mammalian embryogenesis. Development.
129:5151–5160. 2002.PubMed/NCBI
|
|
270
|
Fu M, Sato Y, Lyons-Warren A, Zhang B,
Kane MA, Napoli JL and Heuckeroth RO: Vitamin A facilitates enteric
nervous system precursor migration by reducing Pten accumulation.
Development. 137:631–640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Simkin JE, Zhang D, Rollo BN and Newgreen
DF: Retinoic acid upregulates ret and induces chain migration and
population expansion in vagal neural crest cells to colonise the
embryonic gut. PLoS One. 8:e640772013. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Taketomi T, Yoshiga D, Taniguchi K,
Kobayashi T, Nonami A, Kato R, Sasaki M, Sasaki A, Ishibashi H,
Moriyama M, et al: Loss of mammalian Sprouty2 leads to enteric
neuronal hyper-plasia and esophageal achalasia. Nat Neurosci.
8:855–857. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
273
|
Zhou R, Niwa S, Homma N, Takei Y and
Hirokawa N: KIF26A is an unconventional kinesin and regulates
GDNF-Ret signaling in enteric neuronal development. Cell.
139:802–813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Barlow A, de Graaff E and Pachnis V:
Enteric nervous system progenitors are coordinately controlled by
the G protein-coupled receptor EDNRB and the receptor tyrosine
kinase RET. Neuron. 40:905–916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Taraviras S, Marcos-Gutierrez CV, Durbec
P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G
and Pachnis V: Signalling by the RET receptor tyrosine kinase and
its role in the development of the mammalian enteric nervous
system. Development. 126:2785–2797. 1999.PubMed/NCBI
|
|
276
|
Schuchardt A, D'Agati V, Larsson-Blomberg
L, Costantini F and Pachnis V: Defects in the kidney and enteric
nervous system of mice lacking the tyrosine kinase receptor Ret.
Nature. 367:380–383. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Uesaka T, Nagashimada M, Yonemura S and
Enomoto H: Diminished Ret expression compromises neuronal survival
in the colon and causes intestinal aganglionosis in mice. J Clin
Invest. 118:1890–1898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Mwizerwa O, Das P, Nagy N, Akbareian SE,
Mably JD and Goldstein AM: Gdnf is mitogenic, neurotrophic, and
chemoattractive to enteric neural crest cells in the embryonic
colon. Dev Dyn. 240:1402–1411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Uesaka T, Nagashimada M and Enomoto H:
GDNF signaling levels control migration and neuronal
differentiation of enteric ganglion precursors. J Neurosci.
33:16372–16382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Flynn B, Bergner AJ, Turner KN, Young HM
and Anderson RB: Effect of Gdnf haploinsufficiency on rate of
migration and number of enteric neural crest-derived cells. Dev
Dyn. 236:134–141. 2007. View Article : Google Scholar
|
|
281
|
Nagy N and Goldstein AM: Endothelin-3
regulates neural crest cell proliferation and differentiation in
the hindgut enteric nervous system. Dev Biol. 293:203–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Baynash AG, Hosoda K, Giaid A, Richardson
JA, Emoto N, Hammer RE and Yanagisawa M: Interaction of
endothelin-3 with endothelin-B receptor is essential for
development of epidermal melanocytes and enteric neurons. Cell.
79:1277–1285. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Carrasquillo MM, McCallion AS,
Puffenberger EG, Kashuk CS, Nouri N and Chakravarti A: Genome-wide
association study and mouse model identify interaction between RET
and EDNRB pathways in Hirschsprung disease. Nat Genet. 32:237–244.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
McCallion AS, Stames E, Conlon RA and
Chakravarti A: Phenotype variation in two-locus mouse models of
Hirschsprung disease: Tissue-specific interaction between Ret and
Ednrb. Proc Natl Acad Sci USA. 100:1826–1831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Hearn CJ, Murphy M and Newgreen D: GDNF
and ET-3 differentially modulate the numbers of avian enteric
neural crest cells and enteric neurons in vitro. Dev Biol.
197:93–105. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Paratore C, Eichenberger C, Suter U and
Sommer L: Sox10 haploinsufficiency affects maintenance of
progenitor cells in a mouse model of Hirschsprung disease. Hum Mol
Genet. 11:3075–3085. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Kapur RP: Early death of neural crest
cells is responsible for total enteric aganglionosis in
Sox10(Dom)/Sox10(Dom) mouse embryos. Pediatr Dev Pathol. 2:559–569.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Lang D, Chen F, Milewski R, Li J, Lu MM
and Epstein JA: Pax3 is required for enteric ganglia formation and
functions with Sox10 to modulate expression of c-ret. J Clin
Invest. 106:963–971. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Zhu L, Lee HO, Jordan CS, Cantrell VA,
Southard-Smith EM and Shin MK: Spatiotemporal regulation of
endothelin receptor-B by SOX10 in neural crest-derived enteric
neuron precursors. Nat Genet. 36:732–737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Anderson RB, Stewart AL and Young HM:
Phenotypes of neural-crest-derived cells in vagal and sacral
pathways. Cell Tissue Res. 323:11–25. 2006. View Article : Google Scholar
|
|
291
|
Sasselli V, Pachnis V and Burns AJ: The
enteric nervous system. Dev Biol. 366:64–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Nagashimada M, Ohta H, Li C, Nakao K,
Uesaka T, Brunet JF, Amiel J, Trochet D, Wakayama T and Enomoto H:
Autonomic neurocristopathy-associated mutations in PHOX2B
dysregulate Sox10 expression. J Clin Invest. 122:3145–3158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
293
|
D'Autréaux F, Morikawa Y, Cserjesi P and
Gershon MD: Hand2 is necessary for terminal differentiation of
enteric neurons from crest-derived precursors but not for their
migration into the gut or for formation of glia. Development.
134:2237–2249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
294
|
D'Autréaux F, Margolis KG, Roberts J,
Stevanovic K, Mawe G, Li Z, Karamooz N, Ahuja A, Morikawa Y,
Cserjesi P, et al: Expression level of Hand2 affects specification
of enteric neurons and gastrointestinal function in mice.
Gastroenterology. 141:576–587. 587.e1–e6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Fu M, Vohra BP, Wind D and Heuckeroth RO:
BMP signaling regulates murine enteric nervous system precursor
migration, neurite fasciculation, and patterning via altered Ncam1
polysialic acid addition. Dev Biol. 299:137–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Chalazonitis A, Pham TD, Li Z, Roman D,
Guha U, Gomes W, Kan L, Kessler JA and Gershon MD: Bone
morphogenetic protein regulation of enteric neuronal phenotypic
diversity: Relationship to timing of cell cycle exit. J Comp
Neurol. 509:474–492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Chalazonitis A, D'Autréaux F, Pham TD,
Kessler JA and Gershon MD: Bone morphogenetic proteins regulate
enteric gliogenesis by modulating ErbB3 signaling. Dev Biol.
350:64–79. 2011. View Article : Google Scholar :
|
|
298
|
Chalazonitis A, Pham TD, Rothman TP,
DiStefano PS, Bothwell M, Blair-Flynn J, Tessarollo L and Gershon
MD: Neurotrophin-3 is required for the survival-differentiation of
subsets of developing enteric neurons. J Neurosci. 21:5620–5636.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
Payette RF, Tennyson VM, Pomeranz HD, Pham
TD, Rothman TP and Gershon MD: Accumulation of components of basal
laminae: Association with the failure of neural crest cells to
colonize the presumptive aganglionic bowel of ls/ls mutant mice.
Dev Biol. 125:341–360. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Parikh DH, Tam PK, Van Velzen D and Edgar
D: Abnormalities in the distribution of laminin and collagen type
IV in Hirschsprung's disease. Gastroenterology. 102:1236–1241.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Akbareian SE, Nagy N, Steiger CE, Mably
JD, Miller SA, Hotta R, Molnar D and Goldstein AM: Enteric neural
crest-derived cells promote their migration by modifying their
microenvironment through tenascin-C production. Dev Biol.
382:446–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Nagy N, Mwizerwa O, Yaniv K, Carmel L,
Pieretti-Vanmarcke R, Weinstein BM and Goldstein AM: Endothelial
cells promote migration and proliferation of enteric neural crest
cells via beta1 integrin signaling. Dev Biol. 330:263–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Breau MA, Dahmani A, Broders-Bondon F,
Thiery JP and Dufour S: Beta1 integrins are required for the
invasion of the caecum and proximal hindgut by enteric neural crest
cells. Development. 136:2791–2801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Soret R, Mennetrey M, Bergeron KF, Dariel
A, Neunlist M, Grunder F, Faure C, Silversides DW and Pilon N: A
collagen VI-dependent pathogenic mechanism for Hirschsprung's
disease. J Clin Invest. 125:4483–4496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Nagy N, Barad C, Graham HK, Hotta R, Cheng
LS, Fejszak N and Goldstein AM: Sonic hedgehog controls enteric
nervous system development by patterning the extracellular matrix.
Development. 143:264–275. 2016. View Article : Google Scholar :
|
|
306
|
Chevalier NR, Gazguez E, Bidault L,
Guilbert T, Vias C, Vian E, Watanabe Y, Muller L, Germain S,
Bondurand N, et al: How tissue mechanical properties affect enteric
neural crest cell migration. Sci Rep. 6:209272016. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Anderson RB: Matrix metalloproteinase-2 is
involved in the migration and network formation of enteric neural
crest-derived cells. Int J Dev Biol. 54:63–69. 2010. View Article : Google Scholar
|
|
308
|
Nagy N, Burns AJ and Goldstein AM:
Immunophenotypic char-acterization of enteric neural crest cells in
the developing avian colorectum. Dev Dyn. 241:842–851. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Jackson SR, Guner YS, Woo R, Randolph LM,
Ford H and Shin CE: L1CAM mutation in association with X-linked
hydrocephalus and Hirschsprung's disease. Pediatr Surg Int.
25:823–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
310
|
Broders-Bondon F, Paul-Gilloteaux P,
Carlier C, Radice GL and Dufour S: N-cadherin and β1-integrins
cooperate during the development of the enteric nervous system. Dev
Biol. 364:178–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Jiang Y, Liu MT and Gershon MD: Netrins
and DCC in the guidance of migrating neural crest-derived cells in
the developing bowel and pancreas. Dev Biol. 258:364–384. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Spouge D and Baird PA: Hirschsprung
disease in a large birth cohort. Teratology. 32:171–177. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Brooks AS, Oostra BA and Hofstra RM:
Studying the genetics of Hirschsprung's disease: Unraveling an
oligogenic disorder. Clin Genet. 67:6–14. 2005. View Article : Google Scholar
|
|
314
|
Griseri P, Lantieri F, Puppo F, Bachetti
T, Di Duca M, Ravazzolo R and Ceccherini I: A common variant
located in the 3'UTR of the RET gene is associated with protection
from Hirschsprung disease. Hum Mutat. 28:168–176. 2007. View Article : Google Scholar
|
|
315
|
Gianino S, Grider JR, Cresswell J, Enomoto
H and Heuckeroth RO: GDNF availability determines enteric neuron
number by controlling precursor proliferation. Development.
130:2187–2198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Bondurand N, Natarajan D, Barlow A, Thapar
N and Pachnis V: Maintenance of mammalian enteric nervous system
progenitors by SOX10 and endothelin 3 signalling. Development.
133:2075–2086. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Hosoda K, Hammer RE, Richardson JA,
Baynash AG, Cheung JC, Giaid A and Yanagisawa M: Targeted and
natural (piebald-lethal) mutations of endothelin-B receptor gene
produce megacolon associated with spotted coat color in mice. Cell.
79:1267–1276. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
318
|
Leibl MA, Ota T, Woodward MN, Kenny SE,
Lloyd DA, Vaillant CR and Edgar DH: Expression of endothelin 3 by
mesenchymal cells of embryonic mouse caecum. Gut. 44:2461999.
View Article : Google Scholar : PubMed/NCBI
|
|
319
|
Stanchina L, Baral V, Robert F, Pingault
V, Lemort N, Pachnis V, Goossens M and Bondurand N: Interactions
between Sox10, Edn3 and Ednrb during enteric nervous system and
melanocyte development. Dev Biol. 295:232–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
320
|
Barlow AJ, Dixon J, Dixon M and Trainor
PA: Tcof1 acts as a modifier of Pax3 during enteric nervous system
development and in the pathogenesis of colonic aganglionosis. Hum
Mol Genet. 22:1206–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
321
|
Yamada K, Yamada Y, Nomura N, Miura K,
Wakako R, Hayakawa C, Matsumoto A, Kumagai T, Yoshimura I, Miyazaki
S, et al: Nonsense and frameshift mutations in ZFHX1B, encoding
Smad-interacting protein 1, cause a complex developmental disorder
with a great variety of clinical features. Am J Hum Genet.
69:1178–1185. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
322
|
Elworthy S, Pinto JP, Pettifer A, Cancela
ML and Kelsh RN: Phox2b function in the enteric nervous system is
conserved in zebrafish and is sox10-dependent. Mech Dev.
122:659–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
323
|
Goldstein AM, Brewer KC, Doyle AM, Nagy N
and Roberts DJ: BMP signaling is necessary for neural crest cell
migration and ganglion formation in the enteric nervous system.
Mech Dev. 122:821–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
324
|
Pingault V, Bondurand N, Kuhlbrodt K,
Goerich DE, Préhu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I,
Legius E, Matthijs G, et al: SOX10 mutations in patients with
Waardenburg-Hirschsprung disease. Nat Genet. 18:171–173. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
325
|
Niederreither K, Vermot J, Le Roux I,
Schuhbaur B, Chambon P and Dollé P: The regional pattern of
retinoic acid synthesis by RALDH2 is essential for the development
of posterior pharyngeal arches and the enteric nervous system.
Development. 130:2525–2534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
326
|
Xiao JB, Leng AM, Zhang YQ, Wen Z, He J
and Ye GN: CUEDC2: Multifunctional roles in carcinogenesis. Front
Biosci (Landmark Ed). 24:935–946. 2019. View Article : Google Scholar
|
|
327
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
328
|
Sokic-Milutinovic A: Appropriate
management of attenuated familial adenomatous polyposis: Report of
a case and review of the literature. Dig Dis. 37:400–405. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
329
|
Schuster-Böckler B and Lehner B: Chromatin
organization is a major influence on regional mutation rates in
human cancer cells. Nature. 488:504–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
330
|
Khavari DA, Sen GL and Rinn JL: DNA
methylation and epigenetic control of cellular differentiation.
Cell Cycle. 9:3880–3883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
331
|
Aranda P, Agirre X, Ballestar E, Andreu
EJ, Román-Gómez J, Prieto I, Martin-Subero JI, Cigudosa JC, Siebert
R, Esteller M and Prosper F: Epigenetic signatures associated with
different levels of differentiation potential in human stem cells.
PLoS One. 4:e78092009. View Article : Google Scholar : PubMed/NCBI
|
|
332
|
Pierce GB: Neoplasms, differentiations and
mutations. Am J Pathol. 77:103–118. 1974.PubMed/NCBI
|
|
333
|
Pierce GB: The cancer cell and its control
by the embryo. Rous-whipple award lecture. Am J Pathol.
113:117–124. 1983.PubMed/NCBI
|
|
334
|
Hu M and Shivdasani RA: Overlapping gene
expression in fetal mouse intestine development and human
colorectal cancer. Cancer Res. 65:8715–8722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
335
|
Kaiser S, Park YK, Franklin JL, Halberg
RB, Yu M, Jessen WJ, Freudenberg J, Chen X, Haigis K, Jegga AG, et
al: Transcriptional recapitulation and subversion of embryonic
colon development by mouse colon tumor models and human colon
cancer. Genome Biol. 8:R1312007. View Article : Google Scholar : PubMed/NCBI
|
|
336
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
337
|
Arney KL and Fisher AG: Epigenetic aspects
of differentiation. J Cell Sci. 117:4355–4363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
338
|
Lemaire P, Darras S, Caillol D and
Kodjabachian L: A role for the vegetally expressed Xenopus gene
Mix.1 in endoderm formation and in the restriction of mesoderm to
the marginal zone. Development. 125:2371–2380. 1998.PubMed/NCBI
|
|
339
|
Costello I, Biondi CA, Taylor JM, Bikoff
EK and Robertson EJ: Smad4-dependent pathways control basement
membrane deposition and endodermal cell migration at early stages
of mouse development. BMC Dev Biol. 9:542009. View Article : Google Scholar : PubMed/NCBI
|
|
340
|
Abdul Khalek FJ, Gallicano GI and Mishra
L: Colon cancer stem cells. Gastrointest Cancer Res. (Suppl 1):
S16–S23. 2010.
|
|
341
|
Leedham SJ, Brittan M, McDonald SA and
Wright NA: Intestinal stem cells. J Cell Mol Med. 9:11–24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
342
|
Gostjeva EV, Zukerberg L, Chung D and
Thilly WG: Bell-shaped nuclei dividing by symmetrical and
asymmetrical nuclear fission have qualities of stem cells in human
colonic embryogenesis and carcinogenesis. Cancer Genet Cytogenet.
164:16–24. 2006. View Article : Google Scholar
|
|
343
|
Manzo G: Similarities between embryo
development and cancer process suggest new strategies for research
and therapy of tumors: A new point of view. Front Cell Dev Biol.
7:202019. View Article : Google Scholar : PubMed/NCBI
|
|
344
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
345
|
Moon BS, Jeong WJ, Park J, Kim TI, Min do
S and Choi KY: Role of oncogenic K-Ras in cancer stem cell
activation by aberrant Wnt/β-catenin signaling. J atl Cancer Inst.
106:djt3732014.
|
|
346
|
Todaro M, Gaggianesi M, Catalano V,
Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S,
Cocorullo G, et al: CD44v6 is a marker of constitutive and
reprogrammed cancer stem cells driving colon cancer metastasis.
Cell Stem Cell. 14:342–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
347
|
Lopes EC, Valls E, Figueroa ME, Mazur A,
Meng FG, Chiosis G, Laird PW, Schreiber-Agus N, Greally JM,
Prokhortchouk E and Melnick A: Kaiso contributes to DNA
methylation-dependent silencing of tumor suppressor genes in colon
cancer cell lines. Cancer Res. 68:7258–7263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
348
|
Ouko L, Ziegler TR, Gu LH, Eisenberg LM
and Yang VW: Wnt11 signaling promotes proliferation,
transformation, and migration of IEC6 intestinal epithelial cells.
J Biol Chem. 279:26707–26715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
349
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
350
|
Takaku K, Oshima M, Miyoshi H, Matsui M,
Seldin MF and Taketo MM: Intestinal tumorigenesis in compound
mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 92:645–656.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
351
|
Lombardo Y, Scopelliti A, Cammareri P,
Todaro M, Iovino F, Ricci-Vitiani L, Gulotta G, Dieli F, de Maria R
and Stassi G: Bone morphogenetic protein 4 induces differentiation
of colorectal cancer stem cells and increases their response to
chemotherapy in mice. Gastroenterology. 140:297–309. 2011.
View Article : Google Scholar
|
|
352
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
353
|
De Craene B, Gilbert B, Stove C, Bruyneel
E, van Roy F and Berx G: The transcription factor snail induces
tumor cell invasion through modulation of the epithelial cell
differentiation program. Cancer Res. 65:6237–6244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
354
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
355
|
Pellegrinet L, Rodilla V, Liu Z, Chen S,
Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J and Radtke F:
Dll1- and dll4-mediated notch signaling are required for
homeostasis of intestinal stem cells. Gastroenterology.
140:1230–1240. e1–e7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
356
|
Hwang WL, Jiang JK, Yang SH, Huang TS, Lan
HY, Teng HW, Yang CY, Tsai YP, Lin CH, Wang HW and Yang MH:
MicroRNA-146a directs the symmetric division of Snail-dominant
colorectal cancer stem cells. Nat Cell Biol. 16:268–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|