Introduction
Tooth enamel is a highly mineralized substance that
acts as a barrier to protect teeth (1). Ameloblasts, which are epithelial
cells, are responsible for amelogenesis (1). Secretory ameloblasts secrete matrix
proteins to organize the entire enamel thickness with approximately
30-35% of the final mineral content (2). During the maturation stage of
amelogenesis, kallikrein-related peptidase 4 (KLK4) is secreted to
degrade enamel proteins. Proteins and water are removed and
specific ions required for the concurrent accretion of mineral are
deposited in the enamel until the mineral content is >95% in
fully formed enamel (3,4).
Ameloblasts are attached to each other at their
lateral membranes by a complex of intercellular junctions during
amelogenesis (5). The most apical
complex of the intercellular junctions is tight junctions (TJs)
(5). TJs generate the
permeability barrier among neighboring cells and control the flux
of ions and non-electrolytes through the paracellular space
(5-8). In addition, TJs can also act as a
'fence' and permit cells to act in a polarized manner, which is
crucial for morphogenesis, protein-membrane trafficking and
transportation (5-8). The TJs of ameloblasts ensure a
suitable microenvironment for enamel deposition and maturation by
determining the para-cellular permeability and selectivity of
solutes (5,9). Claudins (CLDNs) and occludin (OCLN)
are integral membrane proteins of TJ strands (9). CLDN1, CLDN4 and OCLN are expressed
in mature ameloblasts. These proteins may differentially regulate
paracellular permeability against the enamel surface to perform
their functions appropriately (9-13).
Amelogenesis is strictly controlled by genes.
However, if enamel organ cells are exposed to environmental stress
for a long period of time during critical periods of amelogenesis,
enamel defects may occur, such as amelogenesis imperfecta, dental
fluorosis, enamel hypoplasia and molar incisor hypo-mineralization
(MIH) (14-16). Enamel hypomineralization poses an
increased risk for toothaches, caries and concerns about appearance
(17-19). Therefore, it is crucial to prevent
enamel hypomineralization.
The β-lactam antibiotic, amoxicillin, is widely
prescribed as a first-choice antibiotic for common infections in
pediatrics and pedodontics, such as ear, nose and throat
infections, pneumonia and other infections (20). However, certain studies have found
that amoxicillin may affect enamel mineralization (21-27). Epidemiological studies have
demonstrated that amoxicillin administration during pregnancy
and/or early childhood may be associated with MIH (21-23). Furthermore, hypomineralization in
enamel has been found in the offspring of pregnant rats
administered amoxicillin daily (24). In addition, amoxicillin can play a
contributing role in the development of tooth fluorosis (25). It has been reported that
amoxicillin administered to 20- to 24-month-old children increases
the risk of dental fluorosis (26), and an in vitro study
further suggested that amoxicillin and fluoride exert a
potentiation effect on the developing enamel of mouse molars
(27). However, the pathological
mechanisms underlying the effects of amoxicillin on enamel
hypomineralization have not yet been fully elucidated. Furthermore,
to the best of our knowledge, no in vivo studies using
laboratory juvenile animals exposed to amoxicillin, which is
similar to amoxicillin used in early childhood, have been performed
to date. Therefore, the aim of the present study was to assess the
effects in juvenile mice produced by amoxicillin administration on
enamel mineralization, the morphology of ameloblasts and the
expression of KLK4 and TJ proteins, including CLDN1, CLDN4 and
OCLN. The findings of the present study may promote the
understanding of the role of amoxicillin in enamel
hypomineralization and the pathological mechanisms of enamel
hypomineralization.
Materials and methods
Animals
A total of 6 female Kunming mice on gestation day 18
with a body weight of 40±3 g were purchased from the Experimental
Animal Center of Xi'an Jiaotong University. The research was
conducted in accordance with the Declaration of Helsinki and with
the Guide for Care and Use of Laboratory Animals as adopted and
promulgated by the United National Institutes of Health. All
experimental protocols were approved by the Ethics Committee for
the Use of Human or Animal Subjects of Xi'an Jiaotong University.
Mice were kept in cages at room temperature and were provided ad
libitum access to food and water. The temperature of the
environment was maintained at 25±3°C, with the relative humidity
maintained at 40-60%. Artificial lighting was provided for 12 h
each day. The water, food and padding were changed every 24 h.
After birth, the mouse offspring were raised with
their mothers until they were weaned. A total of 6 mice from each
mother, resulting in a total of 36 offspring mice, were randomly
selected and divided into the 0, 50 and 100 mg/kg amoxicillin
treatment groups. Each group included 12 offspring mice, which were
labeled on their back. Since day 3 after birth, the offspring mice
in the control group were intragastrically administered saline at 8
a.m. and 8 p.m, and offspring in the experimental groups were also
intragastrically administered amoxicillin at 50 or 100 mg/kg twice
a day, at 8 a.m. and 8 p.m. Saline and amoxicillin treatment were
administered for 19 days. The mother mice were fed a regular diet.
The offspring mice were weighed daily and were weaned on day 21
after birth. On postnatal day 25, all of the offspring mice were
anesthetized by an intraperitoneal injection of 10% chloral hydrate
at a dose of 400 mg chloral hydrate per kg of animal body weight
(28). No sign of peritonitis was
observed following the intraperitoneal injection. The incisors of
the mice were photographed with a digital camera (Canon; EOS M5)
under natural light. The offspring were then sacrificed by cervical
dislocation for the following experiment. On the same day the
mother mice were administered an intraperitoneal injection of 10%
chloral hydrate at a dose of 400 mg chloral hydrate per kg of
animal body weight, and no signs of peritonitis were observed after
intraperitoneal injection. The mice were then sacrificed by
cervical dislocation and handled according to the Ethics Committee
for the Use of Human or Animal Subjects of Xi'an Jiaotong
University. Direct cardiac palpation was used to confirm the death
of mice.
Tissue preparation
Complete mandibles were harvested and the
surrounding tissues of the bone were gently removed. A total of 12
right hemimandibles in each group were washed with distilled water
and fixed for 24 h in 5% glutaraldehyde at room temperature prior
to performing scanning electron microscopy (SEM) and energy
dispersive X-ray spectroscopy (EDX) analysis. Subsequently, 6 of
the 12 left hemimandibles in each group, which were randomly
selected, were immersed in 1% phosphoric-acid for 60 sec, and were
then washed with distilled water three times. In order to perform
histological and immunohistochemistry analyses, the other 6 left
hemimandi-bles in each group were washed with 0.01%
phosphate-buffered saline (PBS) solution and were then fixed by
immersion in 5% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3)
for 48 h at room temperature. Hemimandibles were then decalcified
in 10% EDTA (pH 7.3) for 4 weeks at 4°C and embedded in paraffin.
Sagittal serial sections at a thickness of 5-10 µm were
prepared and mounted on polyline-coated glass slides.
SEM and EDX analyses
For both SEM and EDX analyses, the incisal/occlusal
half and cervical half of labial/facial surface enamel of the
mandibular incisors and the molars were assessed, respectively. For
the mandibular incisors treated by 1% phosphoric-acid, the incisal
half of distal surface enamel of the mandibular incisors were
assessed. The outer enamel structure of each hemimandible was
examined by SEM (JXA-8100; JEOL, Ltd.) operating in low-vacuum mode
for secondary electron imaging. The JXA-8100 SEM was equipped with
an EDX system (INCA Energy; Oxford Instruments) for qualitative and
quantitative analyses and elemental mapping. Each sample without
phosphoric-acid was randomly observed at three points in each half
of enamel (incisal/occlusal half and cervical half of labial/facial
enamel). Each sample with phosphoric-acid was randomly observed at
three points in each incisal half of labial enamel. A total of 6
micrographs of each sample without phosphoric-acid and 3
micrographs of each sample with phosphoric-acid were obtained at
each magnification. The images were compared among the groups and
representative images were selected. The relative amounts of
calcium (Ca), phosphorus (P) and carbon (C) of samples without
phosphoric-acid were assessed as atom percentage (Atom%) by EDX
analysis, and the mean Atom% of Ca, P, C and Ca/P ratios of each
group was calculated.
Histological analysis
Deparaffinized sections were stained using the
haematoxylin and eosin staining technique. Briefly, the slides were
immersed in haematoxylin for 5 min and then washed. Then the slides
were stained with 1% eosin for 3 min and washed in tap water. The
slides were dehydrated in 95% alcohol and 100% alcohol in turn. The
slides were cleared with xylene twice. Qualitative histology was
performed on the stained sections using a light microscope Leica DM
750 (Leica Microsystems GmbH) with an attached digital camera Leica
ICC50 HD (Leica Microsystems GmbH) at x200 magnification.
Immunohistochemistry
Immunohistochemical staining was performed on
arrayed tissue samples according to a previously described protocol
(29). Briefly, dewaxed sections
were rinsed in PBS and subjected to antigen retrieval in 10 mM
citrate buffer (pH 6.0) for 20 min at 95°C in a microwave oven.
Subsequently, endogenous peroxidase was blocked with 3%
H2O2 for 10 min, and the sections were then
washed with PBS three times. Non-specific staining was blocked for
30 min at 37°C with rabbit serum (cat. no. ab7487; Abcam) in a wet
box, followed by overnight incubation at 4°C with rabbit anti-KLK4
(1:100; cat. no. Ab197657; Abcam), CLDN1 (1:100; cat. no.
13050-1-AP; Proteintech Group, Inc.), CLDN4 (1:200; cat. no.
16195-1-AP; Proteintech Group, Inc.) and OCLN (1:100; cat. no.
13409-1-AP; Proteintech Group, Inc.) antibodies, and non-immune IgG
(1:200; cat. no. ab37415; Abcam) served as a negative control.
Following incubation with primary antibodies, the slides were
washed in PBS three times and antibody binding was visualized using
the Vectastain ABC Elite kit (cat. no. PK-6100; Vector
Laboratories, Inc). The slides were then washed with PBS solution.
This was followed by incubation with biotinylated anti-goat IgG
(1:200; cat. no. PK-6100; Vector Laboratories, Inc.) for 15 min at
37°C. The reaction was amplified with Ultra-Steptavidin conjugated
to horseradish peroxidase (1:50; cat. no. PK-6100; Vector
Laboratories, Inc.) and visualized with diaminobenzidine. Slides
were counterstained with hematoxylin for 3 min at room temperature
prior to microscopic analysis. Immunohistochemical images were
acquired using a Leica DM 750 microscope (Leica Microsystems GmbH)
with an attached digital camera Leica ICC50 HD (Leica Microsystems
GmbH) a ×200 magnification.
Quantification of staining
Following immunohistochemistry staining,
quantitative immunostaining was assessed as previously described
(30). The positive
immunostaining expression of KLK4, CLDN1, CLDN4 and OCLN in mature
ameloblasts of mandibular incisors was subjected to microscopic
analysis. The intensity of the staining signal was measured and
documented using Image-Pro Plus 6.0 image analysis software (Media
Cybernetics, Inc.). The intensity of staining was expressed as the
mean signal density of tissue areas from six randomly selected
visions. The mean signal densities were made relative to the mean
signal density of the 0 mg/kg amoxicillin-treated group.
Statistical analysis
All assays were conducted with three independent
experiments by SPSS 17.0 software (SPSS, Inc.). Data are expressed
as the mean ± standard deviation. One-way analysis of variance
followed by Tukey's method was used to compare differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
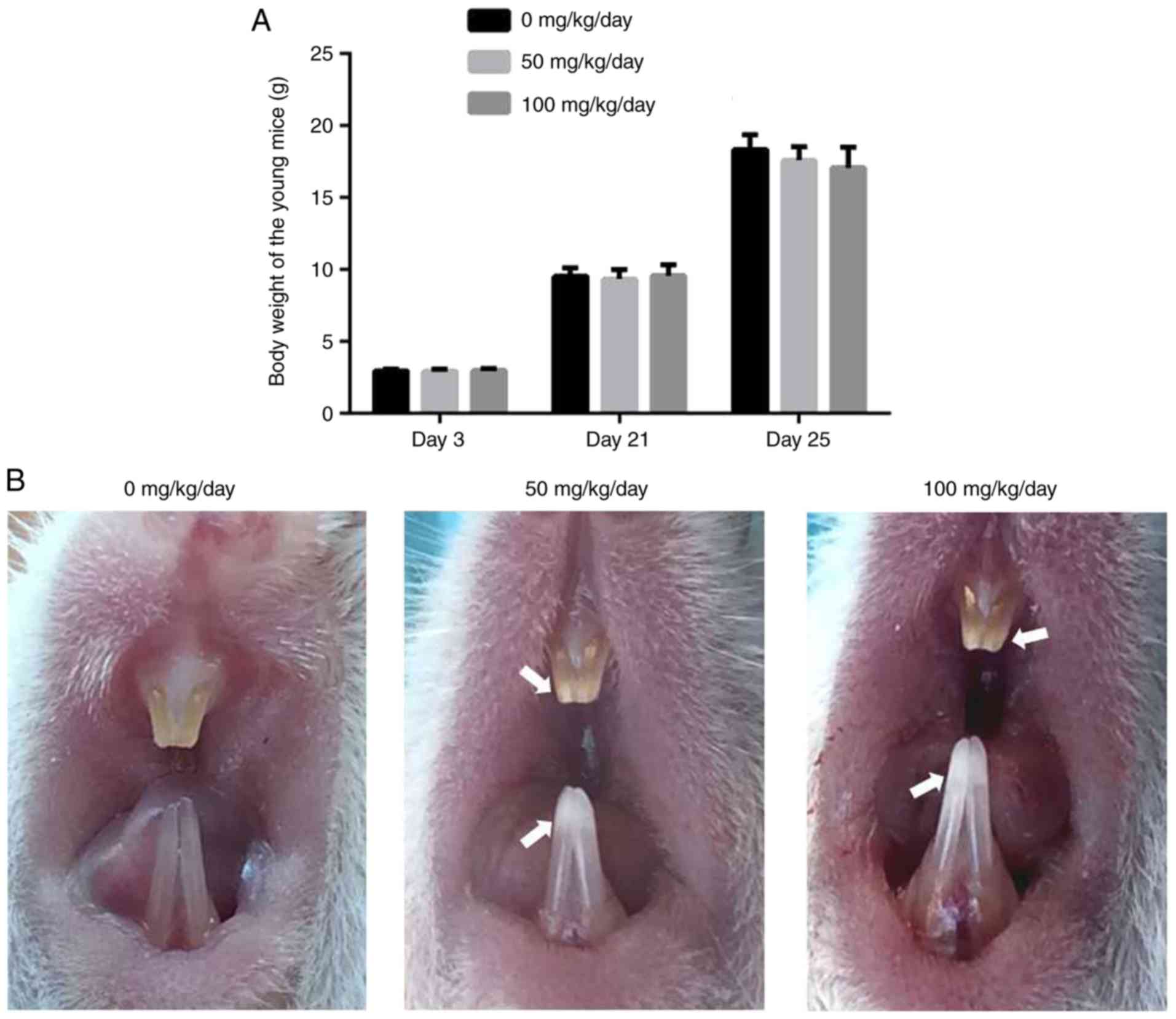
Amoxicillin has no effect on body weight
and induces chalk/white patches on the incisor enamel of juvenile
mice
There was no significant difference in the body
weight of the juvenile mice between the control and
amoxicillin-exposed groups from the 3rd day to the 25th day
(P>0.05; Fig. 1A). However,
the color and translucency of incisor enamel was altered following
amoxicillin exposure (Fig. 1B).
In the control group, the enamel of maxillary and mandibular
incisors was orange in color, and smooth and translucent (Fig. 1B). However, chalk/white patches in
the incisor enamel, particularly the incisal half, were observed in
the amoxicillin-exposed groups (Fig.
1B). Furthermore, 8.3, 58 and 100% of mice exhibited enamel
chalk/white patches in the 0, 50 and 100 mg/kg amoxicillin-exposed
groups, respectively.
Amoxicillin induces enamel
hypomineralization of incisors and molars of juvenile mice
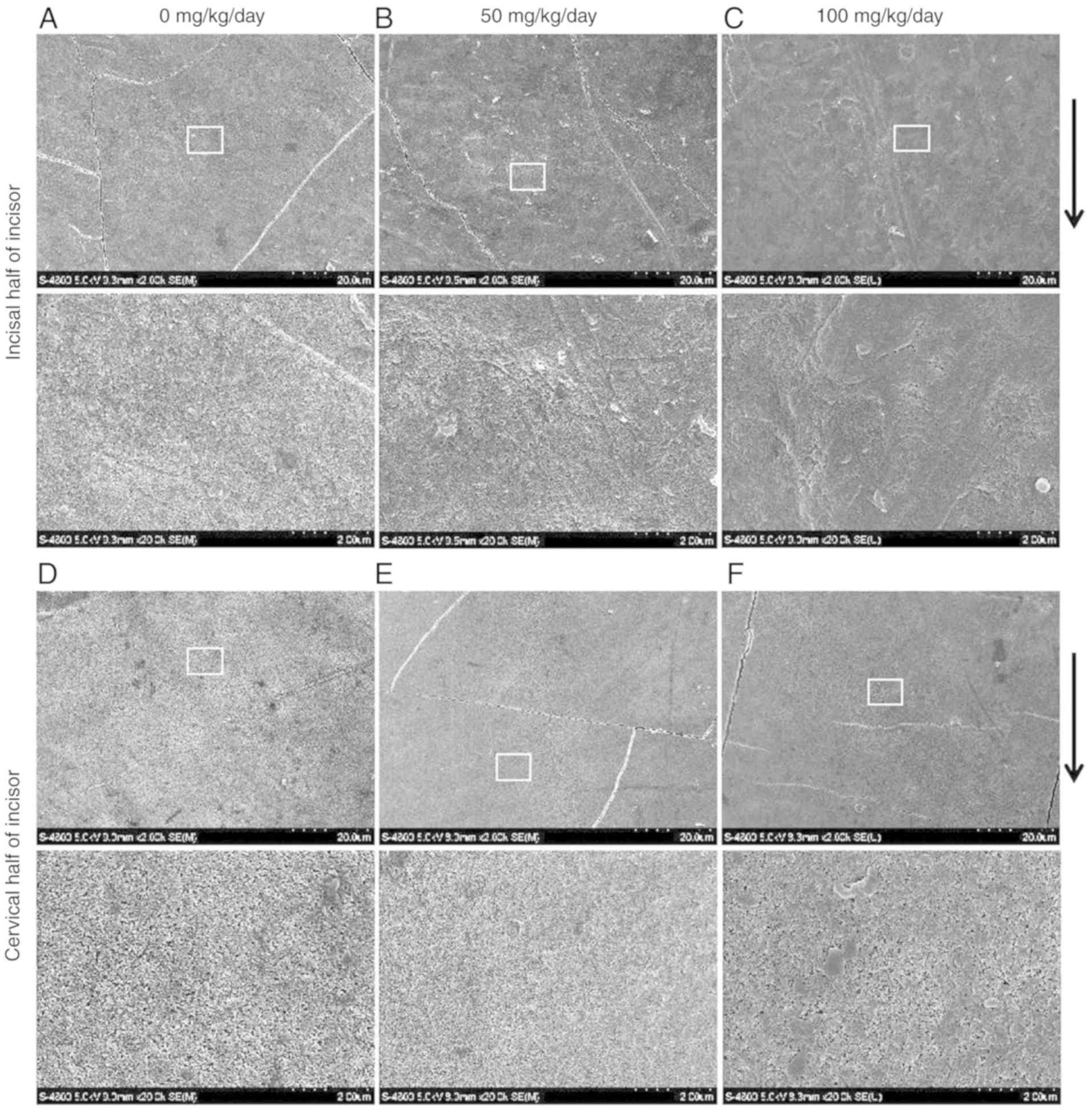
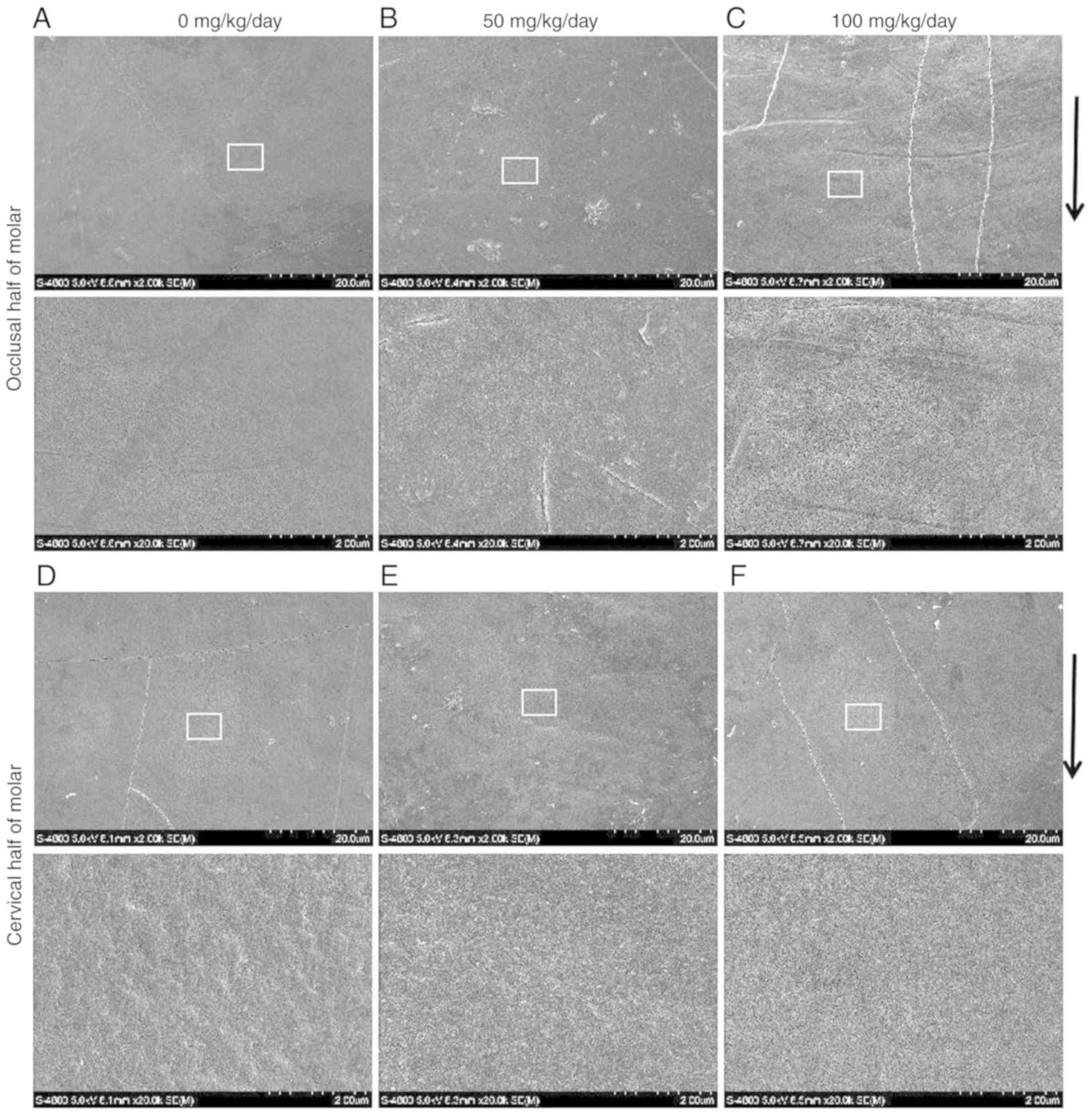
The SEM observations revealed that the enamel
surface of the incisors (Fig. 2A and
D) and molars (Fig. 3A and D)
was smooth and homogeneous with a few visible cracks in the control
group. However, the incisal half of the mandibular incisors
exhibited a rough, irregular and scratched enamel pattern in the 50
mg/kg amoxicillin-exposed group (Fig.
2B). Deeper scratched enamel defects and foam-like enamel with
obvious holes were observed in the 100 mg/kg amoxicillin-exposed
group. The length of enamel defect could be extended to several
microns (Fig. 2C). However, there
were no obvious enamel defects in the cervical half enamel of the
mandibular incisors in the 50 (Fig.
2E) and 100 mg/kg (Fig. 2F)
amoxicillin-exposed groups. Scratched enamel with a different depth
was also often observed in the occlusal half of molars enamel in
the 50 (Fig. 3B) and 100 mg/kg
(Fig. 3C) amoxicillin-exposed
groups; however, the enamel defects were not as severe as those in
the incisal half enamel of the mandibular incisors. Similar to the
mandibular incisors, enamel defects in the cervical half of the
mandibular molars were not obvious in the 50 (Fig. 3E) and 100 mg/kg (Fig. 3F) amoxicillin-exposed groups.
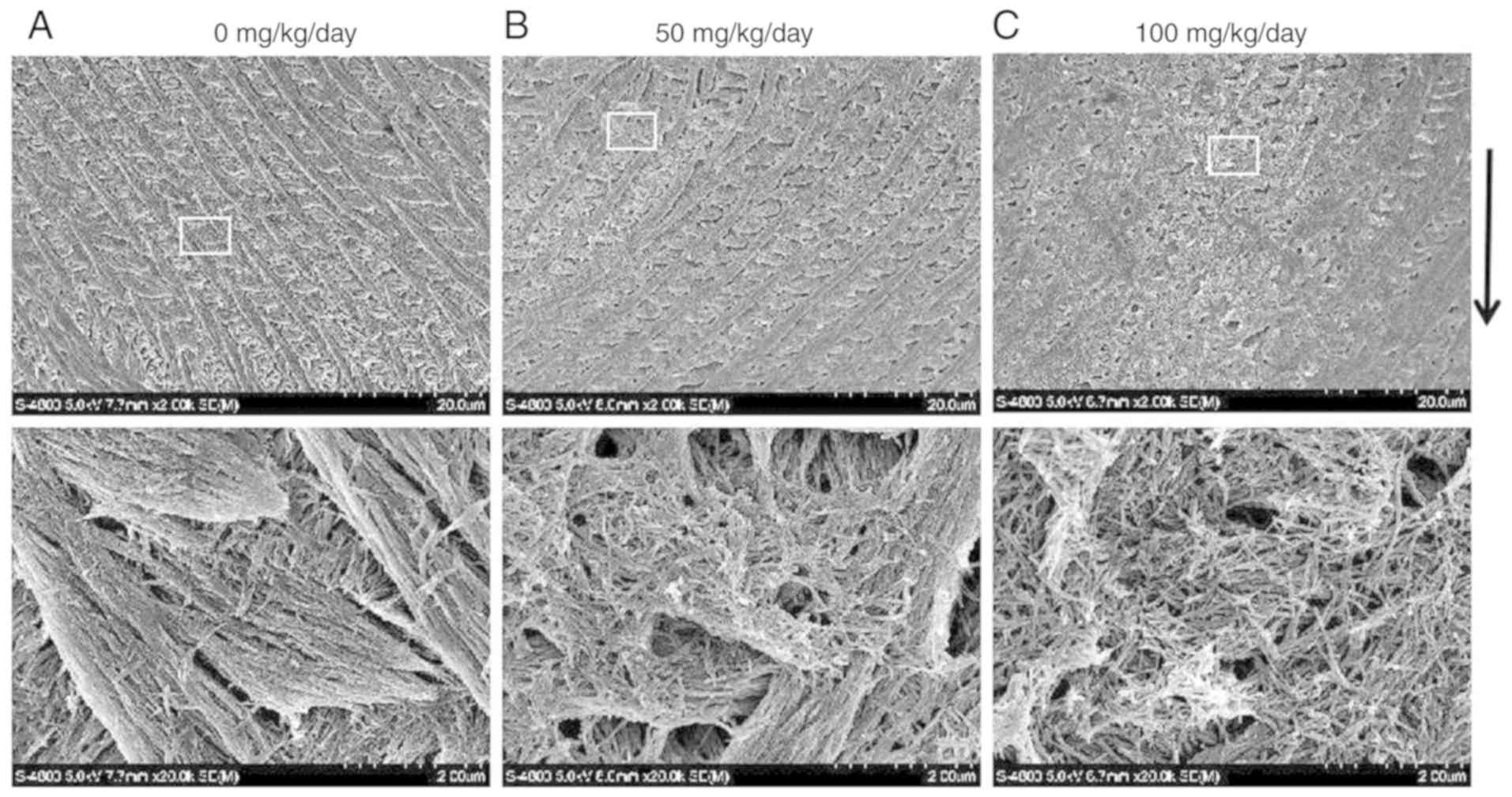
Following phosphoric acid etching, the rods and
interrod enamel were alternately arranged. The enamel rod was
composed of hydroxyapatite crystals whose long axis runs mostly in
the general direction of the longitudinal axis of the rod. The
interrod surrounds each enamel rod, and its crystals are oriented
in a direction different from those making up the rod. The packing
of the crystals was tight and well organized in the control group
(Fig. 4A). The borders between
the enamel rods and inter-rods were indistinct and were hardly
visible in some sites, where the packing of the crystals was less
tight and less well organized in the 50 (Fig. 4B) and 100 (Fig. 4C) mg/kg amoxicillin-exposed
groups.
EDX analysis revealed that, except for the 50 mg/kg
amoxicillin-exposed group, amoxicillin significantly reduced the
mean values of Ca, P and the Ca/P ratio of the incisal half
(Fig. 5A and B) and cervical half
(Fig. 5C and D) of mandibular
incisor enamel (P<0.05), while the mean values of C were not
evidently altered by amoxicillin (P>0.05). No significant
differences were found for the changes in Ca, P and C in the
occlusal half (Fig. 5E and F) and
cervical half (Fig. 5G and H) of
the mandibular first molar enamel between the groups; whereas
amoxicillin significantly decreased the Ca/P ratio in the occlusal
half (P<0.01; Fig. 5F) and in
the cervical half (P<0.05; Fig.
5H) enamel of mandibular first molar compared with the control
group.
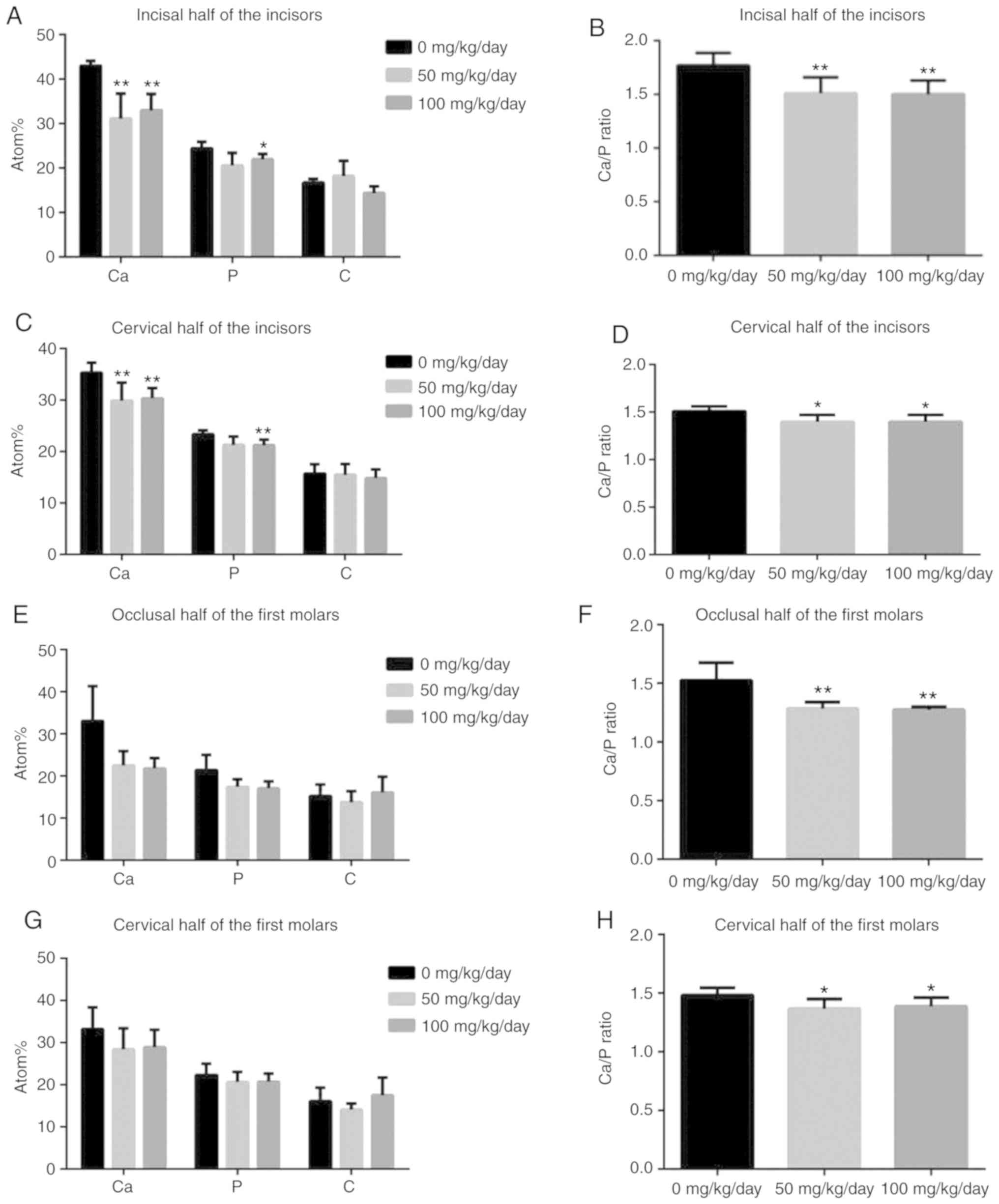 | Figure 5Effects of amoxicillin on enamel Ca,
P and C levels and the Ca/P ratio of the mandibular incisors and
molars, as analyzed by X-ray spectroscopy analysis. Data are
presented as mean ± standard deviation. (A) Ca, P and C atom% of
the enamel surface of the incisors incisal half. (B) The Ca/P ratio
of the enamel surface of the incisors incisal half. (C) Ca, P and C
atom% of the enamel surface of the incisors cervical half. (D) The
Ca/P ratio of the enamel surface of the incisors cervical half. (E)
Ca, P and C atom% of the enamel surface of the first molars
occlusal half. (F) The Ca/P ratio of the enamel surface of the
occlusal half. (G) Ca, P and C atom% of the enamel surface of the
first molars cervical half. (H) The Ca/P ratio of the enamel
surface of the cervical half. *P<0.05, **P<0.01
vs. 0 mg/kg amoxicillin-exposed group. Ca, calcium; P, phosphorous;
C, carbon; Atom%, atom percentage. |
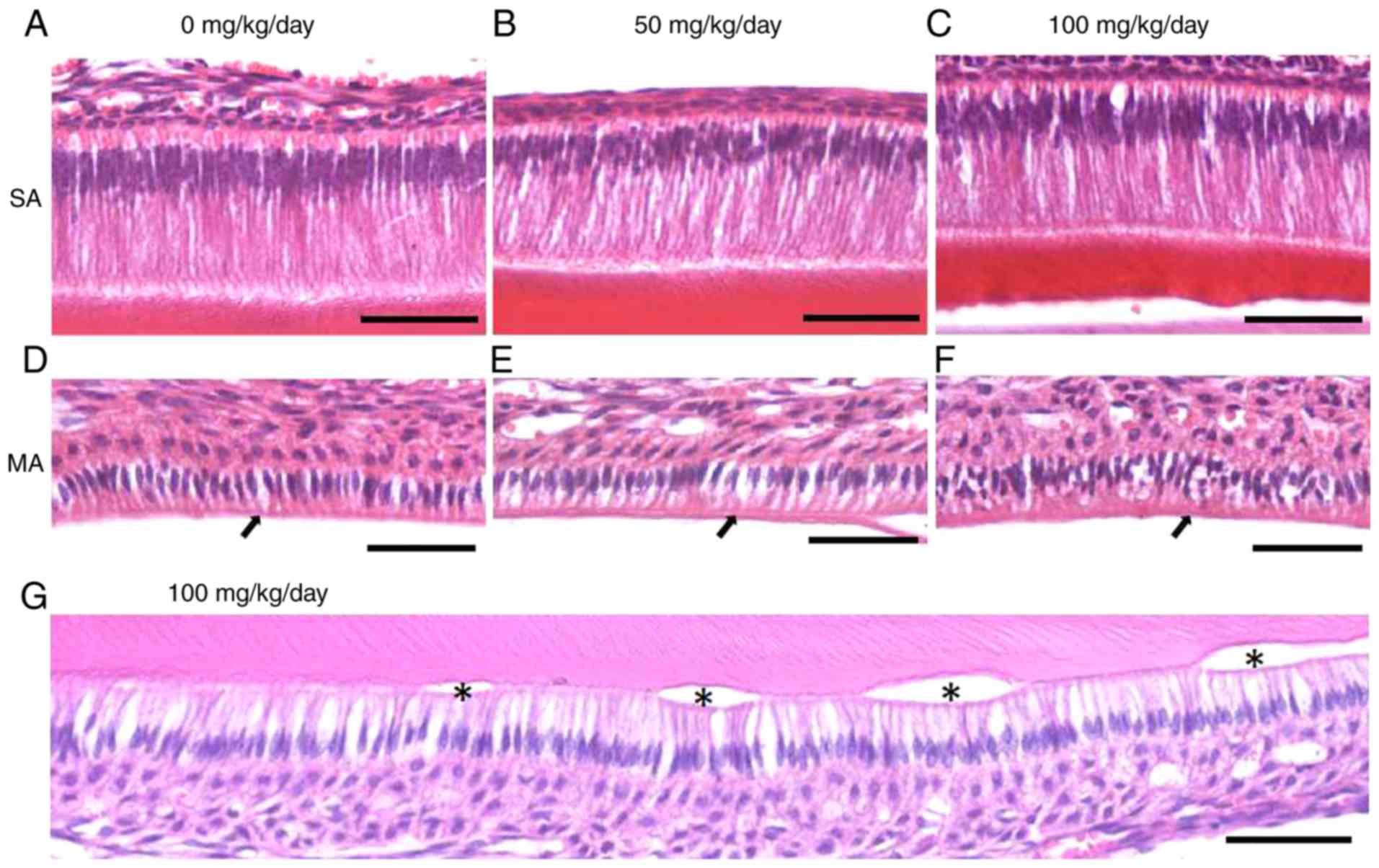
Amoxicillin induces space alterations
among mature ameloblasts and cyst-like lesions in the incisors of
the juvenile mice
Compared with the 0 mg/kg (Fig. 6A) amoxicillin-exposed group,
intercellular spaces among secretory ameloblasts appeared to be
similar in the 50 (Fig. 6B) and
100 mg/kg (Fig. 6C)
amoxicillin-exposed groups. In the control group, the mature
ameloblasts were orderly arranged and obvious gaps among the cells
were occasionally observed (Fig.
6D). However, larger intercellular spaces among mature
ameloblasts could be identified in the 50 (Fig. 6E) and 100 mg/kg (Fig. 6F) amoxicillin-treated groups.
Furthermore, it was demonstrated that some mature ameloblasts
separated from the enamel and formed vesicles in the
amoxicillin-exposed groups (Fig.
6G). In total, 8.3, 50 and 75% of mouse samples exhibited
vesicles in the 0, 50 and 100 mg/kg amoxicillin-exposed groups,
respectively.
Amoxicillin reduces the expression of
KLK4, CLDN1, CLDN4 and OCLN in mature ameloblasts of juvenile
mice
Immunohistochemical staining revealed that brown
granules were found in the cytoplasm of dental epithelial cells of
the maturation stage in the control group, particularly in mature
ameloblasts (Fig. 7A). However,
immunohistochemical staining of KLK4 in the 50 (Fig. 7B) and 100 mg/kg (Fig. 7C) amoxicillin-exposed groups
exhibited a pale yellow color, which was lighter in color than that
in the control group. Weak staining for CLDN1 (Fig. 7D) and OCLN (Fig. 7J) was detected at the distal ends
of mature ameloblasts in the control group. The staining color of
CLDN1 in the 50 (Fig. 7E) and 100
mg/kg (Fig. 7F)
amoxicillin-treated group became a little lighter. The staining
color of OCLN in the 50 (Fig. 7K)
and 100 mg/kg (Fig. 7L)
amoxicillin-treated group appeared to be a little lighter. CLDN4
was detected at both ends of mature ameloblasts and other dental
epithelial cells in the control group (Fig. 7G). The positive staining for CLDN4
in the dental epithelial cells was evidently lesser in the 50
(Fig. 7H) and 100 mg/kg (Fig. 7I) amoxicillin-exposed groups as
compared with the control group (Fig.
7G).
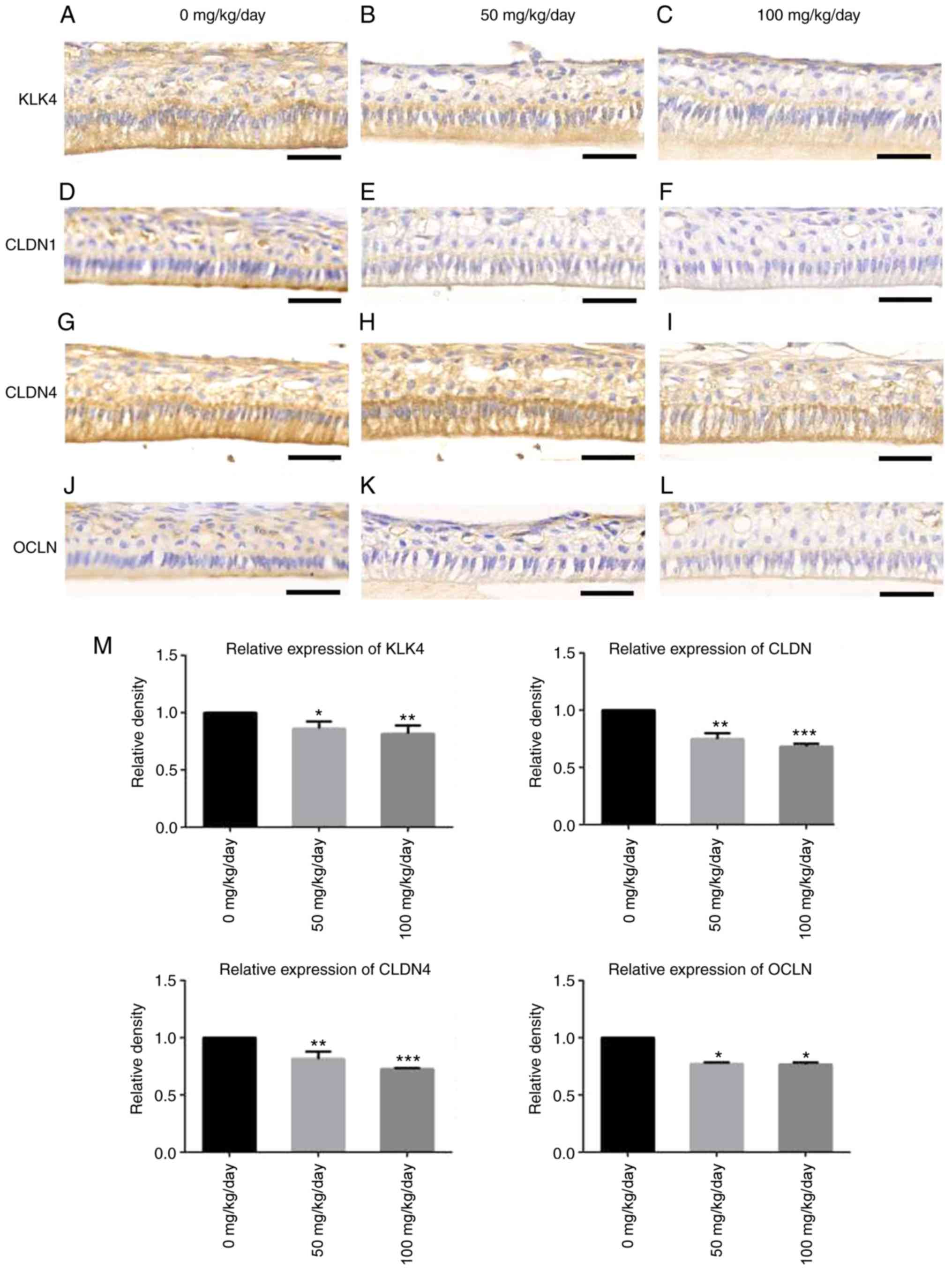 | Figure 7Effects of amoxicillin on the
expression of KLK4, CLDN1, CLDN4 and OCLN in mature ameloblasts of
the incisors, as analyzed by immunohisto-chemical staining. The
expression of KLK4 in (A) 0, (B) 50 and (C) 100 mg/kg/day
amoxicillin-exposed groups. The expression of CLDN1 in (D) 0, (E)
50 and (F) 100 mg/kg/day amoxicillin-exposed groups. The expression
of CLDN4 in (G) 0, (H) 50 and (I) 100 mg/kg/day amoxicillin-exposed
groups. The expression of OLCN in (J) 0, (K) 50 and (L) 100
mg/kg/day amoxicillin-exposed groups. Scale bars, 50 µm. (M)
The quantification of immunohistochemical staining of KLK4, CLDN1,
CLDN4 and OLCN in mature ameloblasts in the 0, 50, 100 mg/kg/day
amoxicillin-exposed groups. *P<0.05,
**P<0.01, ***P<0.001 vs. 0 mg/kg
amoxicillin-exposed group. KLK4, kallikrein-related peptidase 4;
CLDN, claudin; OCLN, occludin. |
The quantification of staining revealed that the
mean densities of KLK4-, CLDN1-, CLDN4- and OCLN-positive staining
in mature ameloblasts in the 50 and 100 mg/kg groups were lower
than that in the 0 mg/kg group, and the differences were
statistically significant (P<0.05; Fig. 7M).
Discussion
Enamel hypomineralization is a qualitative
developmental defect of enamel (14-16) and to the best of our knowledge,
its etiology has not yet been fully determined. Amoxicillin used in
early childhood may be associated with children's MIH, which is one
type of enamel hypomineralization (21-23). Furthermore, amoxicillin use by
children may increase the risk of dental fluorosis (25). In the present study, it was found
that continuous amoxicillin administration (from postnatal days 3
to 21) led to enamel hypomineralization in juvenile mice.
Furthermore, it was found that amoxicillin reduced the expression
of KLK4, CLDN1, CLDN4 and OCLN in mature ameloblasts.
The first mandibular molars of 3-day-old Kunming
mice begin to secrete enamel matrix proteins and they erupt
approximately on postnatal day 20 (31). During postnatal days 3 to 21, the
first mandibular molars of Kunming mice experience both secretory
and maturation periods of amelogenesis. Therefore, the young mice
were exposed to amoxicillin from postnatal days 3 to 21 to
intervene with the whole mineralization period of the first
mandibular enamel.
The dosage of amoxicillin prescribed for children is
≥25 mg/kg (32,33). According to the guideline of drug
conversion from humans to mice provided by the USA Food and Drug
Administration, the dose of 25 mg/kg in humans is ~310 mg/kg in
mice (34). However, some studies
have demonstrated that pregnant rats and adult mice exposed to 50
and 100 mg/kg amoxicillin exhibit enamel defects (24,35), which indicates that the enamel is
quite sensitive to amoxicillin. Furthermore, in the present study,
3-day-old mice were exposed to amoxicillin; thus, the lower doses
of 50 and 100 mg/kg amoxicillin were selected. In addition, 50 and
100 mg/kg amoxicillin did not affect the body weight of young mice,
which suggested that 50 and 100 mg/kg amoxicillin per day may be
relatively safe for the metabolism of the young body.
In the present study, the enamel chalk/white patches
induced by amoxicillin were similar to the clinical manifestation
of enamel hypomineralization (19). It has been reported that enamel
chalk/white patches appear in the enamel of mandibular incisors of
adult mice exposed to amoxicillin for 60 days, while no enamel
patches are found in the maxillary incisors (35). However, in the present study, the
enamel chalk/white patches were also observed in the enamel of
maxillary incisors. Adult mice were used in the previous study,
while the present study used juvenile mice whose drug metabolism
was weaker than that of adult mice. Properties of light reflection
and the transmission of enamel are dependent on its texture, the
orientation of enamel rods and histological characteristics
(36,37). Therefore, chalk/white patches and
a less translucent appearance in the enamel surface suggested that
surface smoothness and/or histological characteristics of the
enamel may be affected by amoxicillin. Furthermore, some enamel
defects in the enamel surface were found by SEM, which confirms the
aforementioned finding. During chewing of mice, the dentin of
maxillary incisor contacts the enamel of mandibular incisors due to
enamel formed only on the labial surface of the mice incisors
(38). Therefore, the enamel of
maxillary incisors bear less friction than that of mandibular
incisors. That may be the reason why the enamel white patches in
maxillary incisors appeared less severe than those in the
mandibular incisors, even if they were similarly affected by
amoxcillin.
In the present study, amoxicillin reduced the ratio
of Ca/P in the mandibular incisors and first molars, which
suggested that amoxicillin indeed caused enamel hypomineralization,
even where there was no obvious enamel chalk/white patches.
However, of note, the SEM observations revealed more enamel defects
in the incisal/occlusal half of the enamel surface in the
amoxicillin-exposed groups, which was a confirmation of the change
in enamel of adult mice in a previous study (35). This may be caused by the bite and
frictional force during chewing. The buccal cusps of mandibular
first molars occluded with opposing central fossa areas of
maxillary first molars; Approximately incisal one third of labial
surface of the mandibular incisors is covered by the maxillary
incisors (39). Therefore, during
chewing, the incisal/occlusal half of the enamel buccal/labial
surface bears a greater friction than the cervical half of enamel
(40). This may cause greater
enamel defects in the incisal/occlusal half of the enamel surface
than in the cervical half of the enamel. Furthermore, when the
enamel is hypomineralized, the mechanical properties of the enamel,
such as pressure and friction resistance, are decreased (41-43). Therefore, enamel defects were more
obvious in the amoxicillin-exposed groups than in the control
group. The enamel defects in the occlusal half of the molars tended
to not be as severe as those in the incisal half of the mandibular
incisor enamel. This may result from the shorter masticating
experience of the mandibular first molar. The first molars of mice
often erupt on postnatal day 20 (31). When samples of the mice were
collected on postnatal day 25, the mandibular first molars
experience mastication for only 5 days, which is shorter than the
mandibular incisors.
The rods and interrod enamel are the fundamental
organizational units of mammalian fully maturation enamel (44-45). In the present study, following
phosphoric acid etching, the featureless and amorphous appearance
of enamel induced by amoxicillin was similar to a SEM study of
human enamel opacities (43). The
different acid response of enamel between the control and
experiment groups suggested that the histological characteristics
and mineralization of enamel may be affected by amoxicillin.
It has been reported that amoxicillin interferes
with the initial stages of amelogenesis by causing structural
changes in ameloblasts (46).
However, in the present study, no obvious changes in secretory
ameloblasts were found among the groups. Some changes in secretory
ameloblasts may have been induced by amoxicillin; however, they
were not detected due to the limitation of the examination method
used in the present study. The widening intercellular spaces
between mature ameloblasts were observed in the amoxicillin-exposed
groups, which suggested that amoxicillin affects the connection
between mature ameloblasts. Several studies have demonstrated that
enamel hypomineralization can be exacerbated by certain
potentiating factors, such as amoxicillin and fluoride, bisphenol A
and fluoride (16,25,27). In the present study, similar to
the changes induced by an overdose of fluoride (29,47), amoxicillin induced more cyst-like
lesions and a decrease in the level of KLK4 in mature ameloblasts,
which indicated that amoxicillin may exacerbate dental fluorosis by
forming cyst-like lesions and decreasing KLK4 expression.
TJs are widely distributed at the top of all
epithelial and endothelial cells (48). Several studies have found that the
TJ proteins, CLDN1, CLDN4 and OCLN, are expressed in mature
ameloblasts. These proteins may regulate paracellular permeability
to create a microenvironment suitable for enamel maturation
(9-13). In the present study, amoxicillin
reduced CLDN1, CLDN4 and OCLN expression in mature ameloblasts,
which indicated that amoxicillin may influence TJs in cells during
enamel maturation, thereby defecting the paracellular permeability
and microenvironment for enamel mineralization. This may also be
associated with the widening intercellular spaces and cyst-like
lesions in mature ameloblasts; however, this warrants further
investigations. In addition, the decreased KLK4 protein expression
induced by amoxicillin indicated that the effect of amoxicillin on
enamel mineralization may be diverse; however, the key mechanism
leading to enamel hypomineralization requires further
investigation.
In conclusion, the present study demonstrated that
amoxicillin led to enamel hypomineralization in young Kunming mice,
and the effect of amoxicillin on hypomineralization may involve
multiple pathways. Due to various factors capable of influencing
the response in vivo, the results of the present study
warrant further investigation in vitro.
Funding
This study was supported by National Natural Science
Foundation of China (grant no. 81602812), Fundamental Research
Funds for Central Universities of China (grant no. xjj2016105), and
the Shaanxi Health Family Planning Research Fund (grant no.
2016D016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG, XLi, LG, HC, BHB, XLiu and HL performed
experiments. JG, XLi and AR assisted with the experiments, and
drafted the article and revised it critically for important
intellectual content. MG and JR designed the study, oversaw the
experiments and provided overall guidance and interpretation of the
results. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee for the Use of Human or Animal Subjects of Xi'an
Jiaotong University (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lacruz RS, Habelitz S, Wright JT and Paine
ML: Dental enamel formation and implications for oral health and
disease. Physiol Rev. 97:939–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frank RM and Nalbandian J: Ultrastructure
of amelogenesis. Structural and Chemical Organization of Teeth.
Miles AEW: 1. Academic Press; New York: pp. 399–466. 1967
|
|
3
|
Robinson C, Kirkham J, Brookes SJ, Bonass
WA and Shore RC: The chemistry of enamel development. Int J Dev
Biol. 39:145–152. 2003.
|
|
4
|
Schmitz JE, Teepe JD, Hu Y, Smith CE,
Fajardo RJ and Chun YH: Estimating mineral changes in enamel
formation by ashing/BSE and microCT. J Dent Res. 93:256–262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bardet C, Ribes S, Wu Y, Diallo MT, Salmon
B, Breiderhoff T, Houillier P, Müller D and Chaussain C: Claudin
loss-of-function disrupts tight junctions and impairs amelogenesis.
Front Physiol. 8:3262017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson JM and Van Itallie CM: Tight
junctions and the molecular basis for regulation of paracellular
permeability. Am J Physiol. 269:G467–G475. 1995.PubMed/NCBI
|
|
7
|
Ma TY, Nighot P and Al-Sadi R: Tight
junctions and the intestinal barrier. Physiology of the
Gastrointestinal Tract. 1-2. 6th edition. Elsevier Inc; pp.
587–639. 2018, View Article : Google Scholar
|
|
8
|
Lumsden AG: Spatial organization of the
epithelium and the role of neural crest cells in the initiation of
the mammalian tooth germ. Development. 103(Suppl): 155–169.
1988.PubMed/NCBI
|
|
9
|
Hata M, Kawamoto T, Kawai M and Yamamoto
T: Differential expression patterns of the tight
junction-associated proteins occludin and claudins in secretory and
mature ameloblasts in mouse incisor. Med Mol Morphol. 43:102–106.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki H, Tani K, Tamura A, Tsukita S and
Fujiyoshi Y: Model for the architecture of claudin-based
paracellular ion channels through tight junctions. J Mol Biol.
427:291–297. 2015. View Article : Google Scholar
|
|
11
|
Kirschner N, Rosenthal R, Furuse M, Moll
I, Fromm M and Brandner JM: Contribution of tight junction proteins
to ion, macromolecule, and water barrier in keratinocytes. J Invest
Dermatol. 133:1161–1169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elmadih A, Wan MW, Downey D, Elliott R,
Swain JE and Abel KM: Natural variation in maternal sensitivity is
reflected in maternal brain responses to infant stimuli. Behav
Neurosci. 130:500–510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inai T, Sengoku A, Hirose E, Iida H and
Shibata Y: Differential expression of the tight junction proteins,
claudin-1, claudin-4, occludin, ZO-1, and PAR3, in the ameloblasts
of rat upper incisors. Anat Rec (Hoboken). 291:577–585. 2008.
View Article : Google Scholar
|
|
14
|
Jeremias F, Koruyucu M, Küchler EC, Bayram
M, Tuna EB, Deeley K, Pierri RA, Souza JF, Fragelli CM, Paschoal
MA, et al: Genes expressed in dental enamel development are
associated with molar-incisor hypomineralization. Arch Oral Biol.
58:1434–1442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeremias F, Pierri RA, Souza JF, Fragelli
CMB, Restrepo M, Finoti LS, Bussaneli DG, Cordeiro RC, Secolin R,
Maurer-Morelli CV, et al: Family-based genetic association for
molar-incisor hypomineralization. Caries Res. 50:310–318. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jedeon K, Houari S, Loiodice S, Thuy TT,
Le Normand M, Berdal A and Babajko S: Chronic exposure to bisphenol
A exacerbates dental fluorosis in growing rats. J Bone Miner Res.
31:1955–1966. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hubbard MJ, Mangum JE, Perez VA, Nervo GJ
and Hall RK: Molar hypomineralisation: A call to arms for enamel
researchers. Front Physiol. 8:5462017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeremias F, de Souza JF, Silva CM,
Cordeiro Rde C, Zuanon AC and Santos-Pinto L: Dental caries
experience and molar-incisor hypomineralization. Acta Odontol
Scand. 71:870–876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mast P, Rodrigueztapia MT, Daeniker L and
Krejci I: Understanding MIH: Definition, epidemiology, differential
diagnosis and new treatment guidelines. Eur J Paediatr Dent.
14:204–208. 2013.PubMed/NCBI
|
|
20
|
Mill C, Primeau MN, Medoff E, Lejtenyi C,
O'Keefe A, Netchiporouk E, Dery A and Ben-Shoshan M: Assessing the
diagnostic properties of a graded oral provocation challenge for
the diagnosis of immediate and nonimmediate reactions to
amoxicillin in children. JAMA Pediatr. 170:e1600332016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weerheijm KL: Molar incisor
hypomineralization (MIH): Clinical presentation, aetiology and
management. Dent Update. 31:9–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laisi S, Ess A, Sahlberg C, Arvio P,
Lukinmaa PL and Alaluusua S: Amoxicillin may cause molar incisor
hypomineralization. J Dent Res. 88:132–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wuollet E, Laisi S, Salmela E, Ess A and
Alaluusua S: Molar–incisor hypomineralization and the association
with childhood illnesses and antibiotics in a group of Finnish
children. Acta Odontol Scand. 74:416–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gottberg B, Berné J, Quiñónez B and
Solórzano E: Prenatal effects by exposing to amoxicillin on dental
enamel in Wistar rats. Med Oral Patol Oral Cir Bucal. 19:e38–e43.
2014. View Article : Google Scholar :
|
|
25
|
Hong L, Levy SM, Warren JJ, Bergus GR,
Dawson DV, Wefel JS and Broffitt B: Primary tooth fluorosis and
amoxicillin use during infancy. J Public Health Dent. 64:38–44.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong L, Levy SM, Warren JJ and Broffitt B:
Amoxicillin use during early childhood and fluorosis of later
developing tooth zones. J Public Health Dent. 71:229–235.
2011.PubMed/NCBI
|
|
27
|
Sahlberg C, Pavlic A, Ess A, Lukinmaa PL,
Salmela E and Alaluusua S: Combined effect of amoxicillin and
sodium fluoride on the structure of developing mouse enamel in
vitro. Arch Oral Biol. 58:1155–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Xu G, Qiao T, Yuan S and Zhuang X:
Effects of CpG oligo-deoxynucleotide 1826 on acute
radiation-induced lung injury in mice. Biol Res. 49:82016.
View Article : Google Scholar
|
|
29
|
Gao J, Ruan J and Gao L: Excessive
fluoride reduces Foxo1 expression in dental epithelial cells of the
rat incisor. Eur J Oral Sci. 122:317–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu J, Liang Y, Qiao L, Li X, Li X, Lu Y
and Zheng Q: Expression analysis of URI/RMP gene in endometrioid
adenocarcinoma by tissue microarray immunohistochemistry. Int J
Clin Exp Pathol. 6:2396–2403. 2013.PubMed/NCBI
|
|
31
|
Gao Y, Wang W, Sun Y, Zhang J, Li D, Wei Y
and Han T: Distribution of amelotin in mouse tooth development.
Anat Rec (Hoboken). 293:135–140. 2010. View Article : Google Scholar
|
|
32
|
Jeske AH: Mosby's Dental Drug Reference.
12th edition. Elsevier Inc; St. Louis, Mo: 2018
|
|
33
|
Lexicomp Online, Pediatric and Neonatal
Lexi-Drugs Online. Wolters Kluver Clinical Drug Information.
https://www.woltersklu-wercdi.com/drug-data/drug-screening/.
|
|
34
|
Dept. of Health and Human Services, Food
and Drug Administration, Center for Drug Evaluation and Research:
Guidance for industry estimating the maximum safe starting dose in
initial clinical trials for therapeutics in adult healthy.
Rockville, MD: 2005
|
|
35
|
Mihalaş E, Matricala L, Chelmuş A, Gheţu
N, Petcu A and Paşca S: The role of chronic exposure to
amoxicillin/clavulanic acid on the developmental enamel defects in
mice. Toxicol Pathol. 44:61–70. 2016. View Article : Google Scholar
|
|
36
|
Villarroel M, Fahl N, De Sousa AM and De
Oliveira OB Jr: Direct esthetic restorations based on translucency
and opacity of composite resins. J Esthet Restor Dent. 23:73–87.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Winter R: Visualizing the natural
dentition. J Esthet Dent. 5:103–118. 1993. View Article : Google Scholar
|
|
38
|
Møinichen CB, Lyngstadaas SP and Risnes S:
Morphological characteristics of mouse incisor enamel. J Anat.
189(Pt 2): 325–333. 1996.PubMed/NCBI
|
|
39
|
Davies S and Gray RM: What is occlusion?
Br Dent J. 191:235–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
d'Incau E, Couture C and Maureille B:
Human tooth wear in the past and the present: Tribological
mechanisms, scoring systems, dental and skeletal compensations.
Arch Oral Biol. 57:214–229. 2012. View Article : Google Scholar
|
|
41
|
Mahoney E, Ismail FS, Kilpatrick N and
Swain M: Mechanical properties across hypomineralized/hypoplastic
enamel of first permanent molar teeth. Eur J Oral Sci. 112:497–502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mahoney EK, Rohanizadeh R, Ismail FS,
Kilpatrick NM and Swain MV: Mechanical properties and
microstructure of hypomineralised enamel of permanent teeth.
Biomaterials. 25:5091–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fagrell TG, Dietz W, Jälevik B and Norén
JG: Chemical, mechanical and morphological properties of
hypomineralized enamel of permanent first molars. Acta Odontol
Scand. 68:215–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nanci A and Smith CE: Development and
calcification of enamel. Calcification in biological systems.
313–343. 1992.
|
|
45
|
Weiner S: Organization of extracellularly
mineralized tissues: A comparative study of biological crystal
growt. CRC Crit Rev Biochem. 20:365–408. 1986. View Article : Google Scholar
|
|
46
|
de Souza JF, Gramasco M, Jeremias F,
Santos-Pinto L, Giovanini AF, Cerri PS and Cordeiro Rde C:
Amoxicillin diminishes the thickness of the enamel matrix that is
deposited during the secretory stage in rats. Int J Paediatr Dent.
26:199–210. 2016. View Article : Google Scholar
|
|
47
|
Bronckers AL, Lyaruu DM and DenBesten PK:
The impact of fluoride on ameloblasts and the mechanisms of enamel
fluorosis. J Dent Res. 88:877–893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zihni C, Mills C, Matter K and Balda MS:
Tight junctions: From simple barriers to multifunctional molecular
gates. Nat Rev Mol Cell Biol. 17:564–580. 2016. View Article : Google Scholar : PubMed/NCBI
|