Introduction
GC is the fourth most frequent type of cancer
worldwide, and it is characterized by a high mortality rate
(1). GC is one of the most
serious public health concerns globally, particularly in China
(2). Despite the recent advances
in the treatment of GC, the prognosis of patients with advanced GC
remains poor. Consequently, the investigation and development of
novel therapeutic strategies for GC is of utmost importance.
MicroRNAs (miRNAs or miRs) are a type of small
non-coding RNAs, which function by negatively regulating the
expression of mRNAs by base pairing with their 3′-untranslated
region (UTR) (3,4). miRNAs play a key role in the
proliferation, apoptosis, metastasis and metabolism of cancer cells
(5). The aberrant expression of
miRNAs has been observed in various types of cancer, such as breast
cancer (6), osteosarcoma
(7) and GC (8). In 2018, miR-383-5p was found to act
as a tumor suppressor in hepatocellular carcinoma (9). Since then, several studies on the
function of miR-383-5p have been published in several cancer types.
For example, miR-383-5p has been shown to suppress the
proliferation and metastasis of breast cancer cells (10). LINC01128 has also been shown to
promote the development of cervical cancer by sponging miR-383-5p
(11). RP11-284F21.9 induces the
development of oral squamous cell carcinoma by sponging miR-383-5p
(12). Moreover, the decreased
expression of miR-383-5p has been shown to lead to the
proliferation and migration of GC cells (13), and miR-383-5p has been reported to
suppress the development of GC by targeting HDAC9 (14).
Cancerous inhibitor of PP2A (CIP2A) was first
confirmed to be a tumor-associated auto-antigen in GC and liver
cancer (15), the overexpression
of which was identified in various types of cancer, such as lung
(16), cervical (17) and prostate (18) cancers, as well as GC (19). miR-383-5p has been proven to
regulate the development of lung cancer by targeting CIP2A
(20). However, to date, at least
to the best of our knowledge, there is no study available on the
association between miR-383-5p and CIP2A in GC. Thus, the aim of
the present study was to investigate whether miR-383-5p regulates
the development of GC by targeting CIP2A.
Materials and methods
Cell culture
The 293T cell line, the normal human gastric
epithelial cell line GES-1, and two GC cell lines (AGS and HGC-27)
were purchased from the American Type Culture Collection. The cells
were cultured in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc.), supplemented with 5% fetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc.), in a humidified
incubator with 95% air and 5% CO2.
pcDNA treatment
The full length of CIP2A was amplified from the cDNA
of GES-1 cells and cloned into the pcDNA3.1 plasmid to construct
pcDNA3.1-CIP2A. The cells were then transfected with pcDNA3.1 or
pcDNA3.1-CIP2A (2 µg) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. At 48 h following transfection, the cells were
collected for use in the subsequent experiments.
Transfection with miR mimic
miR-NC mimic and miR-383-5p mimic were synthesized
by Ambion (Thermo Fisher Scientific, Inc.). miR-NC mimic (100 nM)
or miR-383-5p mimic (100 nM) were transfected into cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions, followed by
incubation at 37°C. At 48 h following transfection, the cells were
collected for use in the subsequent experiments.
miR target prediction
TargetScan 7.1 database (http://www.targetscan.org/vert_71/) was used to
identify the potential target mRNAs of miR-383-5p.
Luciferase reporter gene assay
The wild-type (WT) or mutant-type (MT) 3′-UTR of
CIP2A were constructed by RiboBio and inserted into the pmiR-REPORT
vector (Ambion; Thermo Fisher Scientific, Inc.), which was then
transfected into cells using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions, and incubated at 37°C. At 48 h following
transfection, the luciferase activity was determined by the Dual
Luciferase Reporters Assay System (Promega Corporation) on a
LuminoskanTM Ascent Microplate Luminometer (Thermo Fisher
Scientific, Inc.). The luciferase activity was normalized to
Renilla luciferase activity.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Reverse transcription was conducted at
42°C for 15 min followed by incubation at 85°C for 5 sec using the
TaqMan Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
RT-qPCR was conducted using the Applied Biosystems 7300 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with the TaqMan Universal PCR Master Mix (Thermo Fisher Scientific,
Inc.). The primers used were as follows: CIP2A forward, 5′-AATTTA
GTA AAG ACC CTG ATC TG-3′ and reverse, 5′-CAG ATC AGG GTC TTT ACT
AAA TT-3′; GAPDH forward, 5′-GGA GCG AGA TCC CTC CAA AAT-3′ and
reverse, 5′-GGC TGT TGT CAT ACT CTC ATG G-3′; miR-383-5p forward,
5′-TCG GTG TTA GTG GAA GAC TAG AC-3′ and reverse, 5′-GTC TAG TCT
TCC ACT AAC ACC GA -3′; U6 forward, 5′-GTG CTC GCT TCG GCA GCA
CAT-3′ and reverse, 5′-AAT ATG GAA CGC TTC ACG AAT-3′. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 2 min, and 40 cycles of 95°C for 15 sec and 64°C for 30
sec. GAPDH was used as an endogenous control for CIP2A. U6 was used
as an endogenous control for miR-383-5p. The relative expression
levels were calculated using the 2−ΔΔcq method (21).
Western blot analysis
Total protein lysates were prepared by
radioimmunoprecipitation assay (RIPA) lysis buffer (Sigma-Aldrich;
Merck KGaA) at 4°C. The protein concentration was determined using
a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.).
Protein samples (20 µg) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes. The PVDF membranes were then
blocked by 5% BSA (Sigma-Aldrich; Merck KGaA) at room temperature
for 1 h, probed with primary antibodies to CIP2A (dilution 1:1,000;
#14805), p21 (dilution 1:1,000; #2947), CDK4 (dilution 1:1,000;
#12790), Cyclin D1 (dilution 1:1,000; #55506), Bcl-2 (dilution
1:1,000; #4223), BAX (dilution 1:1,000; #5023), β-actin (dilution
1:1,000; #4970) which were purchased form Cell Signaling Technology
at 4°C overnight and horse radish peroxidase-conjugated secondary
antibody (dilution 1:1,000; #7074, Cell Signaling Technology) at
room temperature for 2 h. Finally, the bands were visualized by the
enhanced chemiluminescence detection reagent using the ChemiDoc XRS
system (Bio-Rad Laboratories, Inc.).
Cell proliferation assay
Cell proliferation was assessed by MTT assay
(Sigma-Aldrich; Merck KGaA) as advised in the manufacturer's
manual. Cells (3×103/100 µl) were plated into
each well of the 96-well plates and cultured at 37°C for 3 days.
Subsequently, the dye solution (15 µl) was added to each
well and incubated at 37°C for a further 4 h. Stop solution (100
µl) was then added to each well. The colorimetric absorbance
was recorded with the SpectraMax Plus (Molecular Devices LLC) at
570 nm.
Cell cycle analysis
Cells (1×106) were collected and fixed by
70% ice-cold ethanol at 4°C overnight. The cells were then
re-suspended in PBS (1 ml) supplemented with bovine pancreatic
RNase A (100 µg/ml, Sigma-Aldrich; Merck KGaA) and 40
µg/ml propidium iodide (PI; Sigma-Aldrich; Merck KGaA) for
30 min at 4°C. Subsequently, cell cycle analysis was carried out on
a Becton-Dickinson FACSCalibur cytometer (BD Biosciences) and
analyzed by ModFit software version 3.2.1 (Verity Software
House).
Cell apoptotic analysis
Cell apoptosis was determined using the Annexin
V-fluorescein isothiocyanate (FITC) apop tosis kit (Merck KGaA)
according to the manufacturer's instructions. Cells were incubated
in ice-cold 1X binding buffer (500 µl) containing Annexin
V-FITC (2.3 µl) for 10 min at 4°C, followed by incubation at
room temperature for 10 min in the dark. Subsequently, the cells
were re-suspended in ice-cold 1X binding buffer (500 µl)
supplemented with PI (5 µl), and incubated at room
temperature for 15 min. The signals of Annexin V-FITC and PI were
detected using a flow cytometer (FACSCalibur™; BD Biosciences).
Statistical analysis
All experiments were performed at least 3 times. The
values are expressed as the means ± standard error of the mean.
Differences between 2 groups were analyzed using an unpaired
Student's t-test, while differences among 3 groups were analyzed by
one-way analysis of variance followed by Newman-Keuls test. Values
of P<0.05 were considered to indicate statistically significant
differences.
Results
miR-383-5p expression is decreased in GC
cell lines
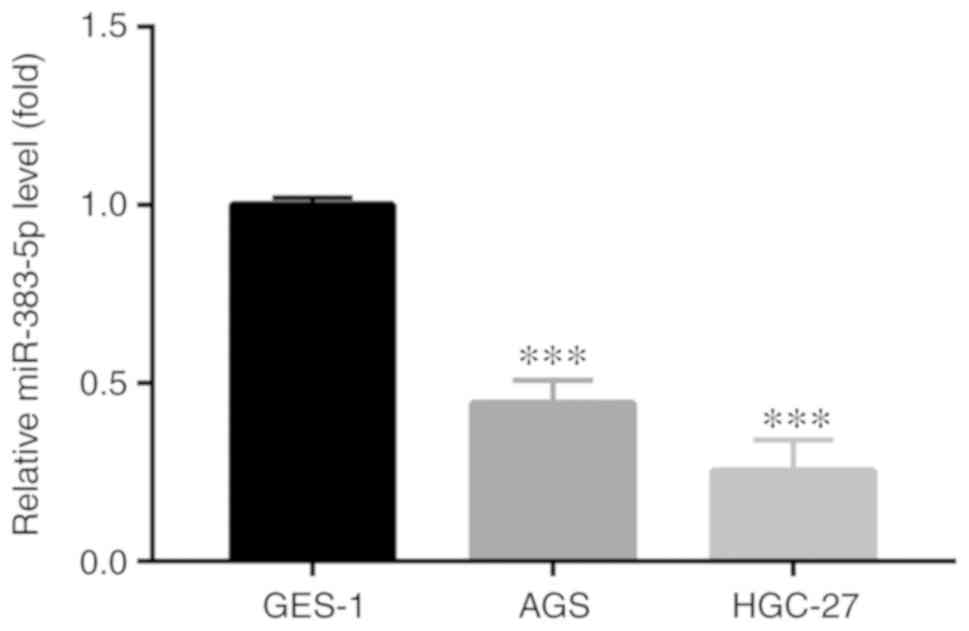
The expression level of miR-383-5p was assessed in
GC cell lines. RT-qPCR analysis revealed that the miR-383-5p
expression level was significantly lower in the GC cell lines (AGS
and HGC-27) compared with the GES-1 normal gastric epithelial cell
line (Fig. 1), which were then
used in the subsequent experiments.
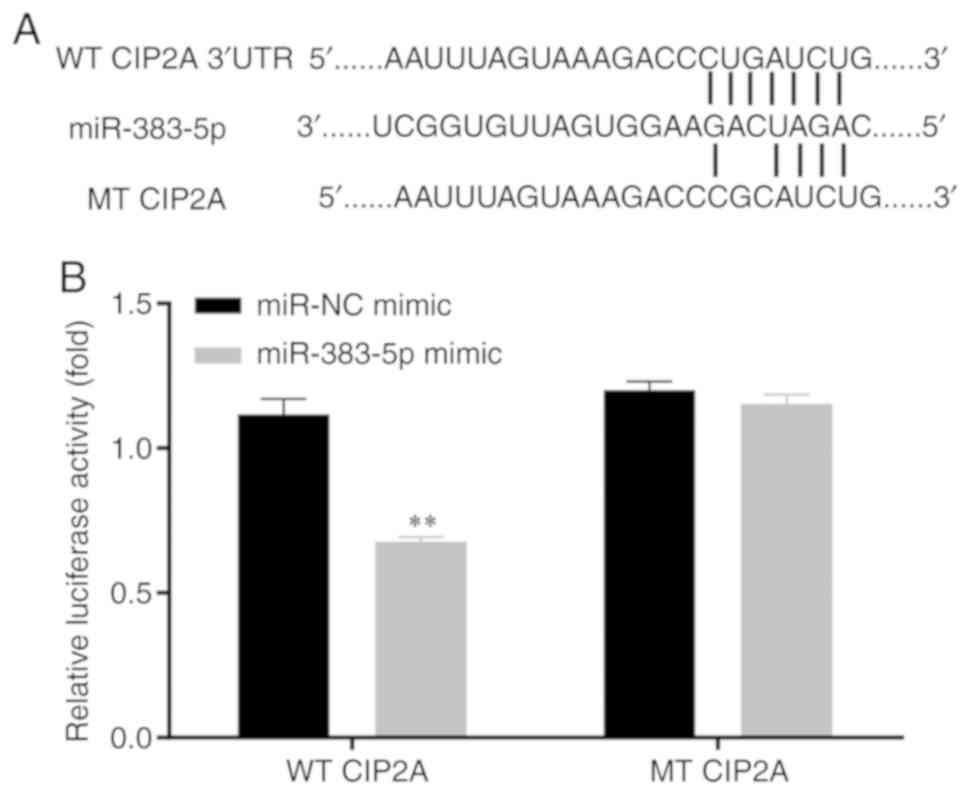
miR-383-5p directly targets CIP2A
Using the online open access database, TargetScan
7.1 (http://www.targetscan.org/vert_71/), CIP2A was
selected as a candidate target mRNA for miR-383-5p. The potential
CIP2A 3′-UTR fragments (WT and MT) are presented in Fig. 2A. In the 293T cell line,
miR-383-5p mimic induced a significantly lower luciferase activity
in the WT CIP2A 3′-UTR luciferase reporter plasmid, whereas no
significant alteration was detected in the luciferase activity of
the MT plasmid (Fig. 2B).
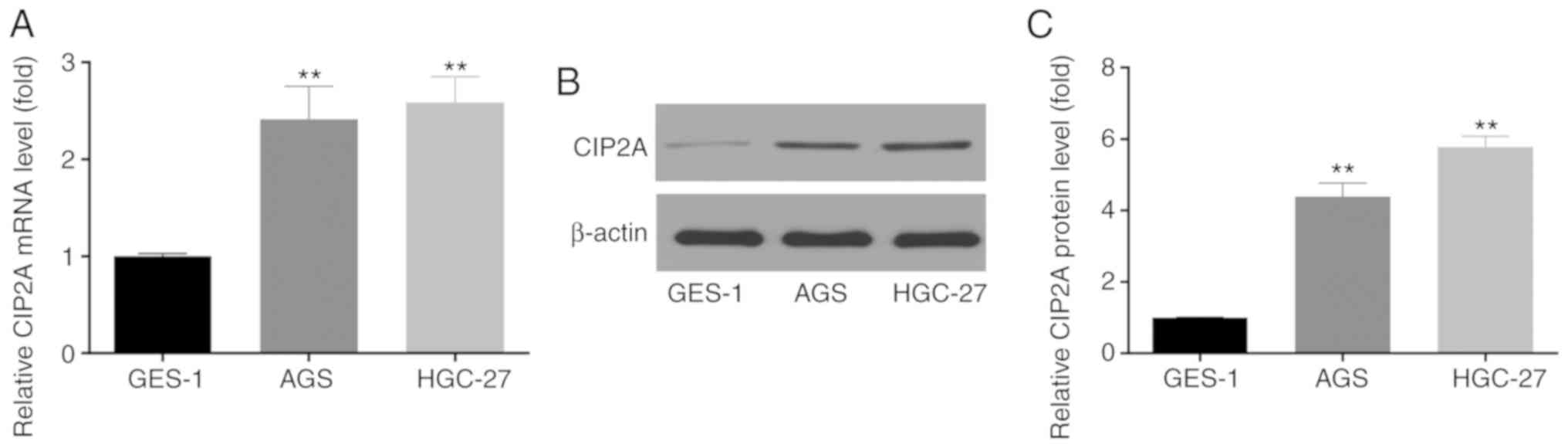
CIP2A expression is increased in GC cell
lines
The expression of CIP2A in the GC cell lines, AGS
and HGC-27, was then investigated by RT-qPCR analysis and western
blot analysis. It was observed that the expression of CIP2A at the
mRNA (Fig. 3A) and protein
(Fig. 3B and C) level was
significantly higher in the AGS and HGC-27 GC cells compared with
that in the normal gastric epithelial cell line, GES-1.
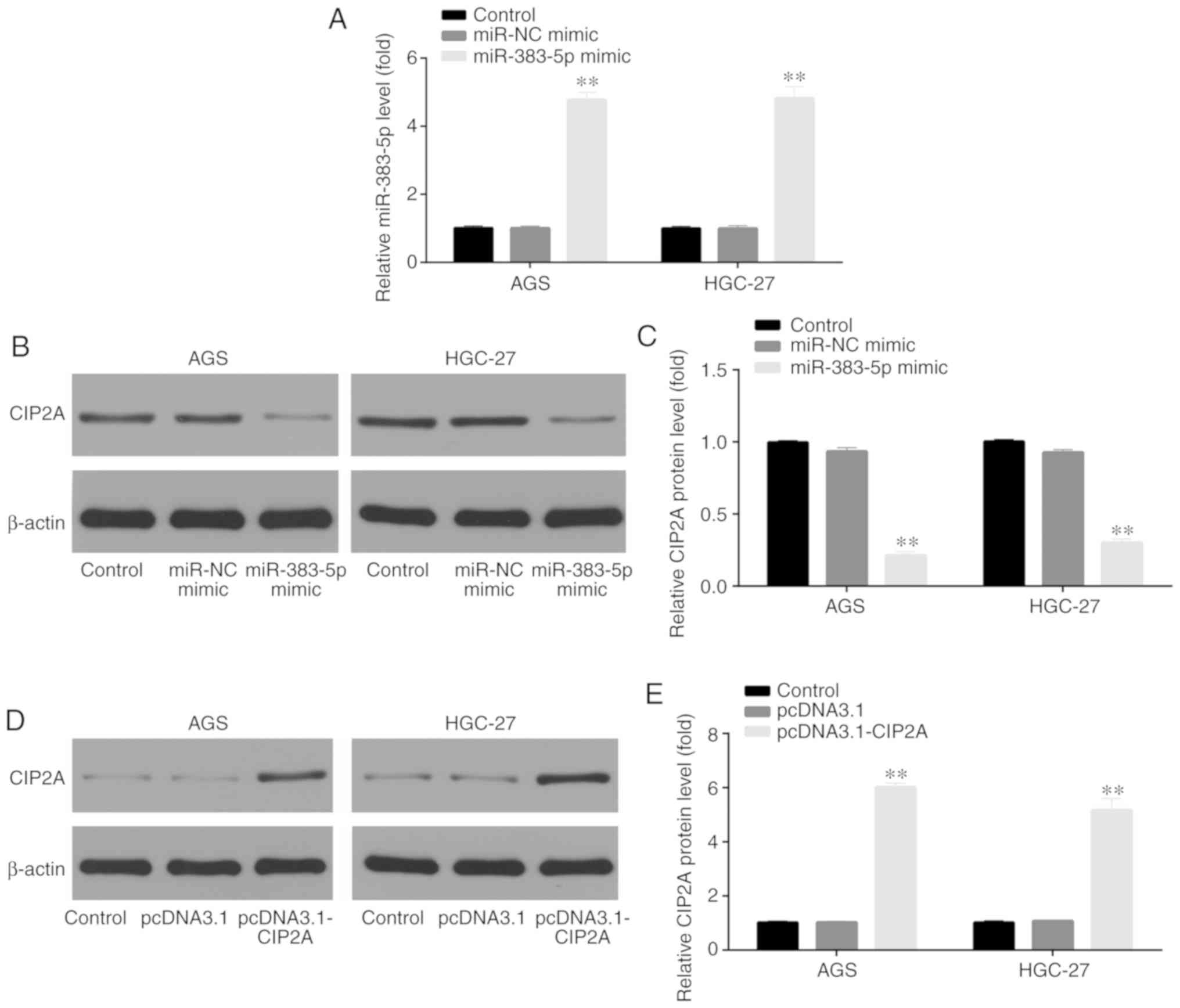
Transfection of miR-383-5p mimic and
pcDNA3.1-CIP2A
RT-qPCR and western blot analysis were used to
verify the transfection of miR-383-5p mimic and pcDNA3.1-CIP2A in
the GC cell lines, AGS and HGC-27. For miR-383-5p, the cells were
divided into 3 groups, including the control, miR-NC mimic and
miR-383-5p mimic groups. The results demonstrated that the
miR-383-5p level was significantly increased by transfection with
miR-383-5p mimic, indicating the successful transfection of
miR-383-5p mimic in the AGS and HGC-27 GC cells (Fig. 4A); furthermore, the CIP2A protein
level was significantly decreased by transfection with miR-383-5p
mimic in the AGS and HGC-27 GC cells (Fig. 4B and C).
For pcDNA3.1-CIP2A, the cells were divided into 3
groups, including the control, pcDNA3.1 and pcDNA3.1-CIP2A groups.
The CIP2A protein level was significantly increased by transfection
with pcDNA3.1-CIP2A in the AGS and HGC-27 GC cells (Fig. 4D and E), indicating the successful
transfection of pcDNA3.1-CIP2A in AGS and HGC-27 GC cells.
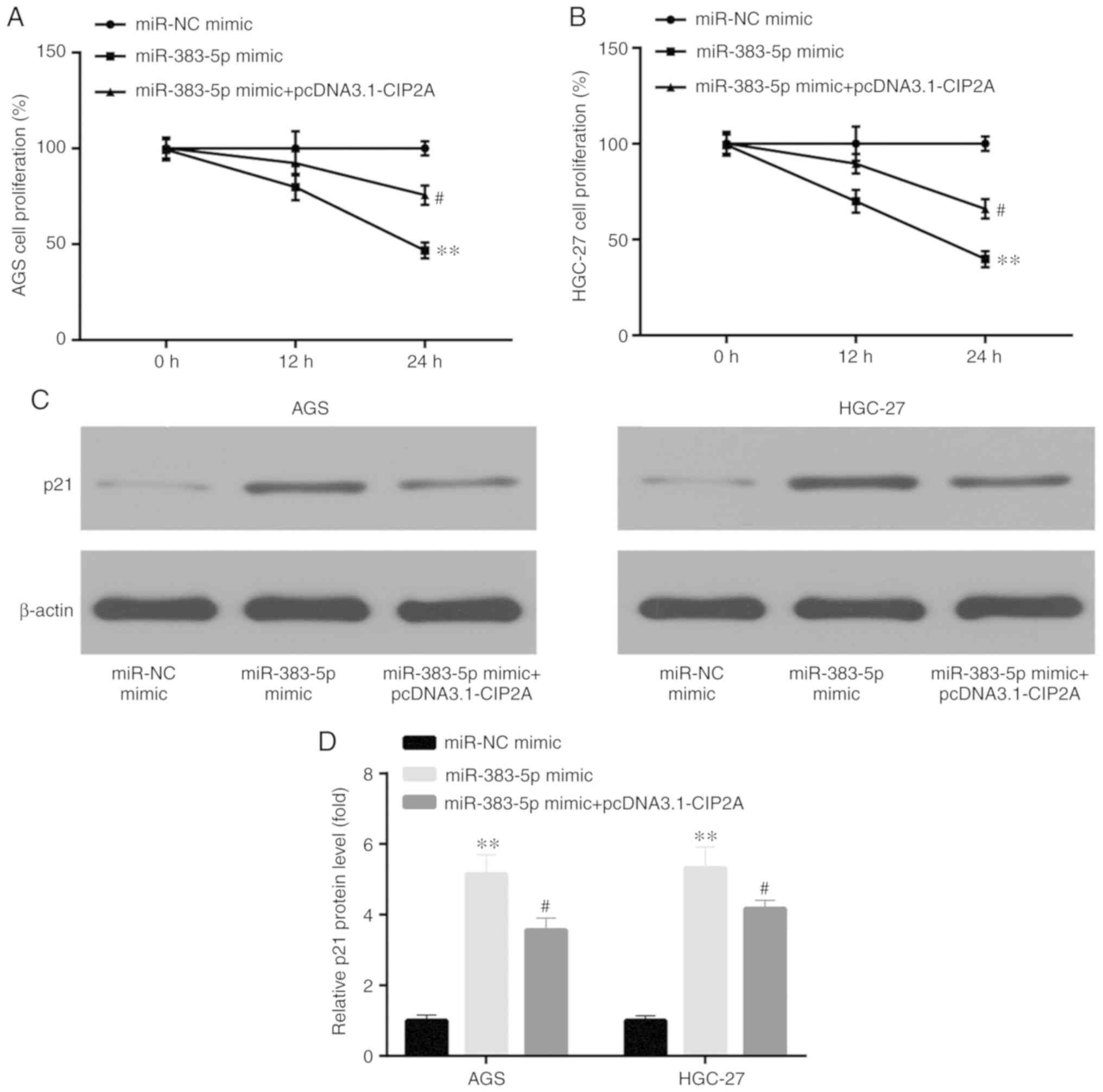
miR-383-5p inhibits the proliferation of
GC cells by targeting CIP2A
When investigating the role of miR-383-5p in human
GC cell proliferation by MTT assay, compared to transfection with
miR-NC mimic, transfection with miR-383-5p mimic was shown to
significantly decrease AGS and HGC-27 cell proliferation, which was
reversed by transfection with pcDNA3.1-CIP2A (Fig. 5A and B). p21 was negatively
correlated with cell proliferation, for example, LincRNAFEZF1-AS1
to induced proliferation of GC cells by repressing p21 (22). Western blot analysis was used to
assess the protein level of p21. Compared to transfection with
miR-NC mimic, miR-383-5p mimic significantly increased the protein
level of p21, which was reversed by pcDNA3.1- CIP2A (Fig. 5C and D).
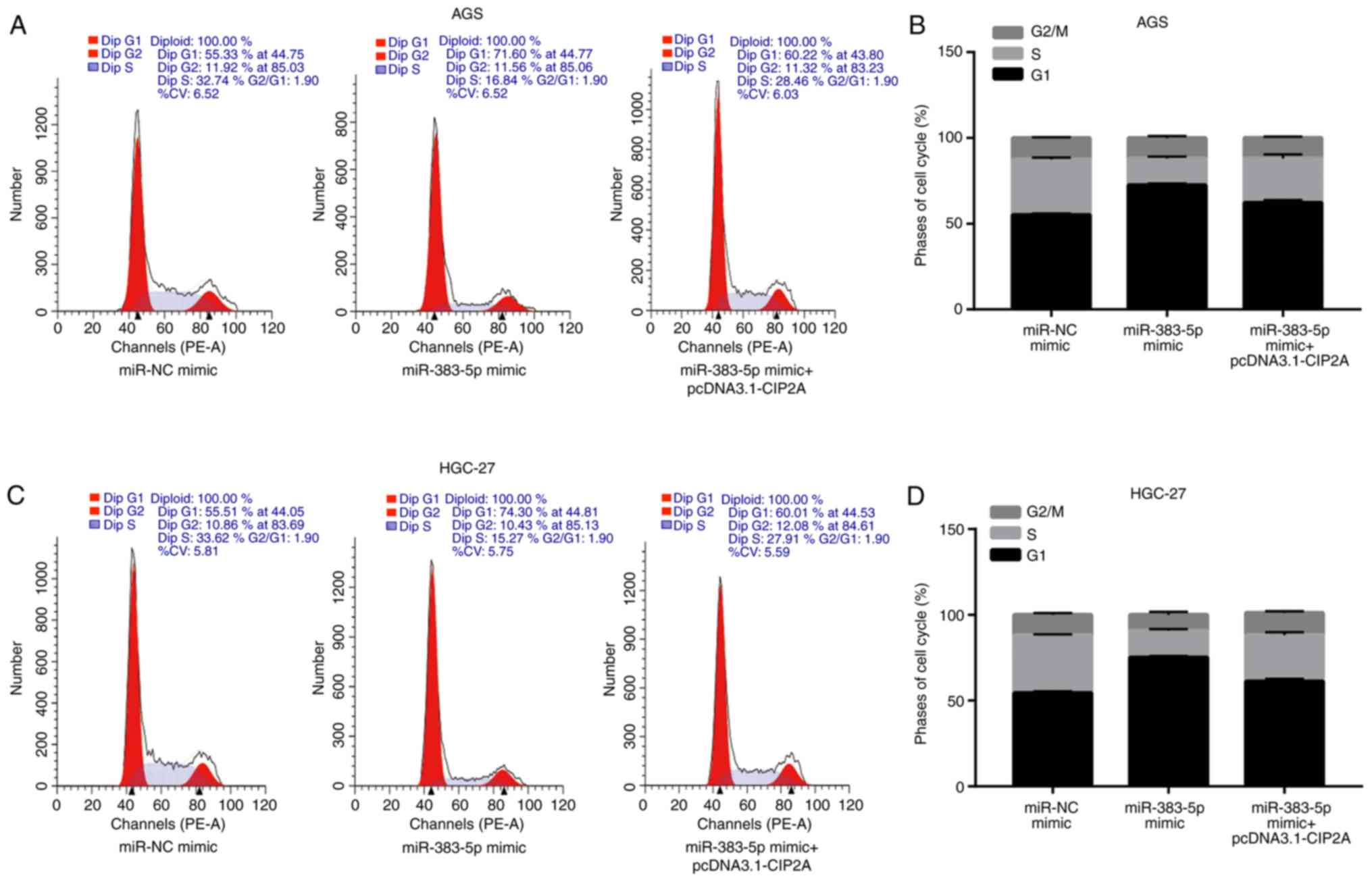
miR-383-5p induces the apoptosis of GC
cells by targeting CIP2A
In order to elucidate the potential
anti-proliferative mechanisms of action of miR-383-5p in the GC
cell lines AGS and HGC-27, cell cycle progression and apoptosis
were analyzed by flow cytometry.
Compared to transfection with miR-NC mimic,
miR-383-5p mimic significantly increased the proportion of cells in
the G1 phase and decreased the proportion of cells in
the S phase, which was reversed by transfection with pcDNA3.1-CIP2A
(Fig. 6A-D). Cyclin D1 and CDK4
are associated with the progression of the cell cycle; for example,
RN181 regulates the activity of cyclin D1-CDK4, thus controlling
the progression from the G1 to the S phase in GC cells
(23). Furthermore, compared to
transfection with miR-NC mimic, transfection with miR-383-5p mimic
significantly increased the protein levels of CDK4 and cyclin D1,
and this effect was reversed by pcDNA3.1-CIP2A (Fig. 6E-G).
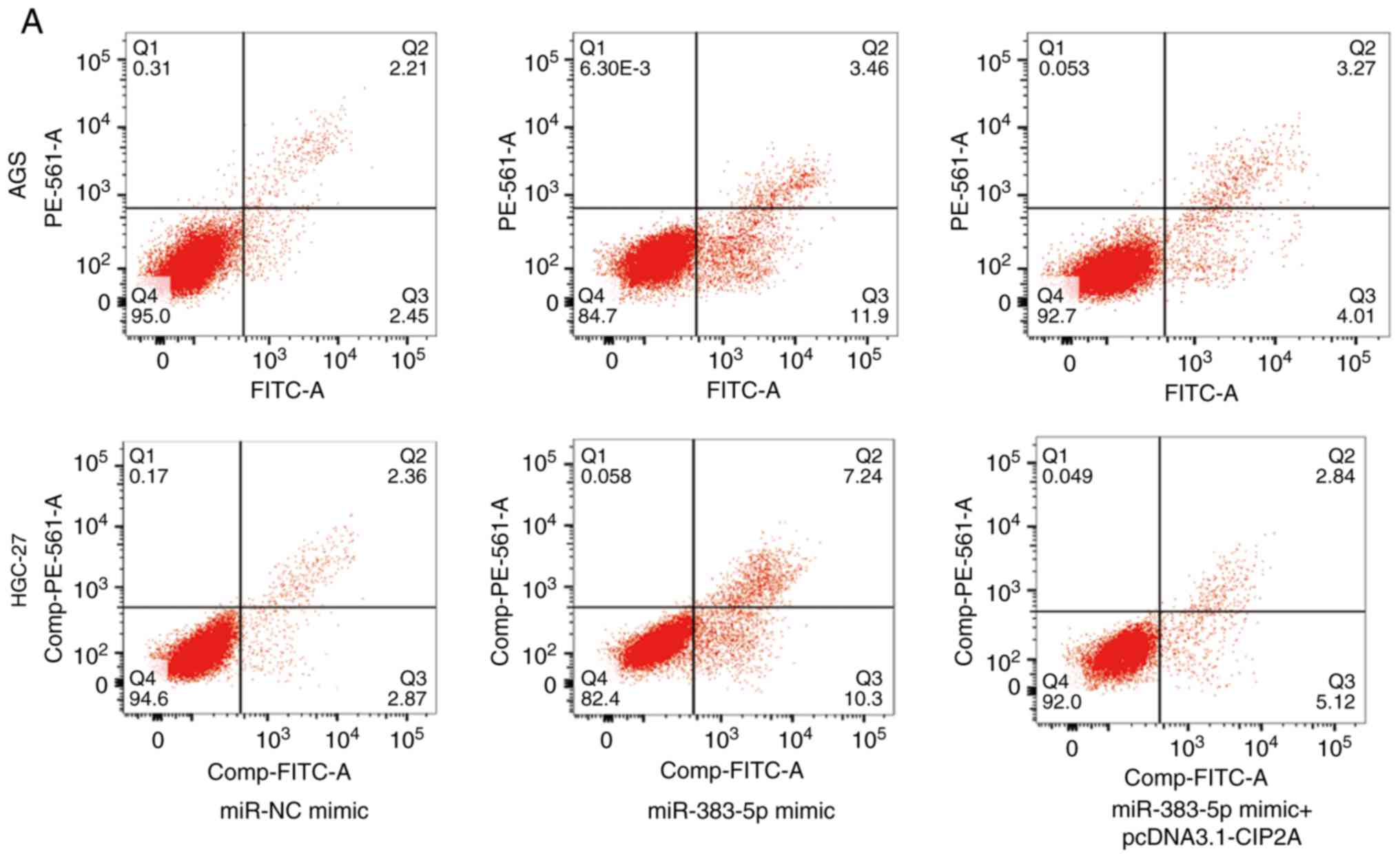
Furthermore, an increased proportion of apoptotic
cells was observed in the GC cell lines, AGS and HGC-27, following
transfection with miR-383-5p mimic compared to miR-NC mimic, which
was reversed by transfection with pcDNA3.1-CIP2A (Fig. 7A and B). The ratio of
pro-apoptotic BAX to anti-apoptotic Bcl-2 was crucial for the
promotion of cell apoptosis (24). In addition, compared to miR-NC
mimic, the BAX/Bcl-2 ratio was increased by miR-383-5p mimic, which
was reversed by pcDNA3.1-CIP2A (Fig.
7C and D).
Discussion
The dysregulation of miR-383 is associated with
various types of cancer, such as hepatocellular carcinoma (25), pancreatic cancer (26) and glioma (27). Consistently, it was observed that
miR-383-5p was also significantly decreased in GC cell lines
compared with the GES-1 normal gastric epithelial cell line.
miRNAs target different mRNAs to control cancer
progression (28,29). For example, the overexpression of
miR-383-5p inhibits ovarian cancer cell proliferation by targeting
and downregulating tripartite motif-containing 27 (30); miR-383-5p reverses hepatocellular
carcinoma cell proliferation by targeting aldoketo reductase family
1 member B10 (9). The present
study demonstrated that miR-383-5p functions as a tumor suppressor
by targeting and downregulating CIP2A, which was also proven by a
previous study on lung cancer (23).
CIP2A overexpression has been detected in GC tumor
samples (19). Consistently, the
present study also demonstrated that CIP2A was significantly
overexpressed in GC cell lines compared with the GES-1 normal
gastric epithelial cell line.
CIP2A and miR-383-5p regulate cell proliferation.
For example, CIP2A was shown to induce cell proliferation and
protect cells from apoptosis in non-small-cell lung cancer
(31,32); furthermore, miR-383-5p reduced
cell proliferation in hepatocellular carcinoma (9) and GC (14). Consistently, it was observed that
miR-383-5p inhibited GC cell proliferation, and induced p21
expression which was negatively related to cell proliferation
(33), by targeting CIP2A. In
addition, miR-383-5p mimic induced G0/G1
arrest and CDK4/cyclin D1 expression which was positively related
to the cell cycle (34), and
increased cell apoptosis and the BAX/Bcl-2 ratio, which was
positively associated with cell apoptosis (35,36), by targeting CIP2A. Therefore, on
the whole, these findings indicate that miR-383-5p inhibited the
proliferation of GC cells by inducing apoptosis and cell cycle
arrest in the G0/G1 phase by targeting CIP2A.
The present study clearly demonstrated that the
restoration of CIP2A expression abrogated the inhibitory effects of
miR-383-5p on GC cell proliferation. Taken together, the findings
of the present study demonstrated that miR-383-5p exerts an
inhibitory effect on GC by inhibiting CIP2A.
Funding
No funding was received.
Availability of data and materials
All the datasets generated and/or analyzed during
the present study are available from the corresponding author on
reasonable request.
Authors' contributions
XL, JY and QC participated in designing and
conducting the experiments; XL, JY and AX analyzed the data; JC
conceived the study, supervised the experiments, performed data
analysis and wrote the manuscript. All the authors have read and
approved the final version of the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY
and Goldie SJ: Development of an empirically calibrated model of
gastric cancer in two high-risk countries. Cancer Epidemiol
Biomarkers Prev. 17:1179–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Guo M, Zhao G, Ma Q, Ma B, Qiu X
and Fan Q: miR-183 inhibits the metastasis of osteosarcoma via
downregulation of the expression of Ezrin in F5M2 cells. Int J Mol
Med. 30:1013–1020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schaefer A, Jung M, Mollenkopf HJ, Wagner
I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G and Jung
K: Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.
|
|
9
|
Wang J, Zhou Y, Fei X, Chen X and Chen Y:
Biostatistics mining associated method identifies AKR1B10 enhancing
hepatocellular carcinoma cell growth and degenerated by miR-383-5p.
Sci Rep. 8:110942018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Kong X, Shi Q and Zhao B:
MicroRNA-383-5p acts as a potential prognostic biomarker and an
inhibitor of tumor cell proliferation, migration, and invasion in
breast cancer. Cancer Biomark. 27:423–432. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y, Ma Y, Liu J, Cai Y, Zhang M and Fang
X: LINC01128 expedites cervical cancer progression by regulating
miR-383-5p/SFN axis. BMC Cancer. 19:11572019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao B, Fu X, Li X, Li Y and Gan N:
RP11-284F21.9 promotes oral squamous cell carcinoma development via
the miR-383-5p/MAL2 axis. J Oral Pathol Med. 49:21–29. 2020.
View Article : Google Scholar
|
|
13
|
Wei C and Gao JJ: Downregulated miR-383-5p
contributes to the proliferation and migration of gastric cancer
cells and is associated with poor prognosis. Peer J. 7:e78822019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu G, Li N, Zhang Y, Zhang J, Xu R and Wu
Y: MicroRNA-383-5p inhibits the progression of gastric carcinoma
via targeting HDAC9 expression. Braz J Med Biol Res. 52:e83412019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soo Hoo L, Zhang JY and Chan EK: Cloning
and characterization of a novel 90 kDa 'companion' auto-antigen of
p62 overexpressed in cancer. Oncogene. 21:5006–5015. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong QZ, Wang Y, Dong XJ, Li ZX, Tang ZP,
Cui QZ and Wang EH: CIP2A is overexpressed in non-small cell lung
cancer and correlates with poor prognosis. Ann Surg Oncol.
18:857–865. 2011. View Article : Google Scholar
|
|
17
|
Huang LP, Adelson ME, Mordechai E and
Trama JP: CIP2A expression is elevated in cervical cancer. Cancer
Biomark. 8:309–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaarala MH, Väisänen MR and Ristimäki A:
CIP2A expression is increased in prostate cancer. J Exp Clin Cancer
Res. 29:1362010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia
J and Xu D: CIP2A is overexpressed in gastric cancer and its
depletion leads to impaired clonogenicity, senescence, or
differentiation of tumor cells. Clin Cancer Res. 14:3722–3728.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao S, Gao X, Zang S, Li Y, Feng X and
Yuan X: MicroRNA-383-5p acts as a prognostic marker and inhibitor
of cell proliferation in lungadenocarcinoma by cancerous inhibitor
of protein phosphatase 2A. Oncol Lett. 14:3573–3579. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Liu YW, Xia R, Lu K, Xie M, Yang F, Sun M,
De W, Wang C and Ji G: LincRNAFEZF1-AS1 represses p21 expression to
promote gastric cancer proliferation through LSD1-mediated H3K4me2
demethylation. Mol Cancer. 16:392017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Wang X, Gao Y, Peng Y, Dong N, Xie
Q, Zhang X, Wu Y, Li M and Li JL: RN181 is a tumour suppressor in
gastric cancer by regulation of the ERK/MAPK-cyclin D1/CDK4
pathway. J Pathol. 248:204–216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: A rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
25
|
Chen L, Guan H, Gu C, Cao Y, Shao J and
Wang F: miR-383 inhibits hepatocellular carcinoma cell
proliferation via targeting APRIL. Tumour Biol. 37:2497–2507. 2016.
View Article : Google Scholar
|
|
26
|
Han S, Cao C, Tang T, Lu C, Xu J, Wang S,
Xue L, Zhang X and Li M: ROBO3 promotes growth and metastasis of
pancreatic carcinoma. Cancer Lett. 366:61–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He Z, Cen D, Luo X, Li D, Li P, Liang L
and Meng Z: Downregulation of miR-383 promotes glioma cell invasion
by targeting insulin-like growth factor 1 receptor. Med Oncol.
30:5572013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Wu J, Jiao K, Wu Q, Ma J, Chen D,
Kang J and Zhao G, Shi Y, Fan D and Zhao G: MicroRNA-495-3p
inhibits multidrug resistance by modulating autophagy through
GRP78/mTOR axis in gastric cancer. Cell Death Dis. 9:10702018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang F, Li K, Pan M, Li W, Wu J, Li M,
Zhao L and Wang H: miR-589 promotes gastric cancer aggressiveness
by a LIFR-PI3K/AKT-c-Jun regulatory feedback loop. J Exp Clin
Cancer Res. 37:1522018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang J, Xie C, Liu Y, Shi Q and Chen Y:
Up-regulation of miR-83-5p suppresses proliferation and enhances
chemosensitivity in ovarian cancer cells by targeting TRIM27.
Biomed Pharmacother. 109:595–601. 2019. View Article : Google Scholar
|
|
31
|
Chao TT, Wang CY, Lai CC, Chen YL, Tsai
YT, Chen PT, Lin HI, Huang YC, Shiau CW, Yu CJ and Chen KF: TD-19,
an erlotinib derivative, induces epidermal growth factor receptor
wild-type nonsmall-cell lung cancer apoptosis through
CIP2A-mediated pathway. J Pharmacol Exp Ther. 351:352–358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lei N, Peng B and Zhang JY: CIP2A
regulates cell proliferation via the AKT signaling pathway in human
lung cancer. Oncol Rep. 32:1689–1694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhai H, Fesler A, Schee K, Fodstad O,
Flatmark K and Ju J: Clinical significance of long intergenic
noncoding RNA-p21 in colorectal cancer. Clin Colorectal Cancer.
12:261–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pozner A, Terooatea TW and Buck-Koehntop
BA: Cell-specific kaiso (ZBTB33) regulation of cell cycle through
cyclin D1 and cyclin E1. J Biol Chem. 291:24538–24550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oda E, Ohki R, Murasawa H, Nemoto J,
Shibue T, Yamashita T, Tokino T, Taniguchi T and Tanaka N: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
LeBlanc H, Lawrence D, Varfolomeev E,
Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D and
Ashkenazi A: Tumor-cell resistance to death receptor-induced
apoptosis through mutational inactivation of the proapoptotic Bcl-2
homolog Bax. Nat Med. 8:274–281. 2002. View Article : Google Scholar : PubMed/NCBI
|