Introduction
Esophageal carcinoma is one of the most
life-threatening malignancies worldwide (1,2).
Esophageal squamous cell carcinoma (ESCC) is a major histological
type of esophageal carcinoma, and accounts for ~70-80% of
esophageal cancer cases in China (3). Radiotherapy is one of the preferred
treatment options for patients with advanced ESCC (4). However, the radioresistance of ESCC
hinders the radiotherapeutic effect, which can result in treatment
failure (5). Therefore, the
detailed mechanisms responsible for the radioresistance of ESCC
cells require urgent elucidation.
Long non-coding RNAs (lncRNAs) are a group of highly
conserved transcripts of >200 nucleotides, with no protein
coding ability (6-8). An increasing number of studies have
documented the involvement of lncRNAs in regulating the biological
behaviors of cancer cells (9,10).
lncRNAs can be categorized as oncogenes and/or tumor suppressors
depending on the specific malignancy (11,12), and the aberrant expression of
lncRNAs can be used to evaluate the tumorigenesis, progression and
relapse of malignant tumors. For example, LINC01133 can be used to
predict the progression of ESCC with high sensitivity and
specificity (13), and the
upregulation of lncRNA HOX transcript antisense RNA indicates a
poor prognosis and early-onset metastasis in patients with ESCC
(14). Furthermore, lncRNAs have
been demonstrated to modulate the radiosensitivity of ESCC cells.
Specifically, lncRNA FAM201A induces the radioresistance of ESCC
cells, which results in a reduced overall survival time (15); in addition, LINC00473 may reduce
the radiotherapeutic efficacy in patients with ESCC (16). However, the underlying mechanisms
through which LINC00473 regulates the radiosensitivity of ESCC
cells remain to be elucidated.
MicroRNAs (miRNAs or miRs) are a class of small
non-coding RNAs of 18-25 nucleotides in length that specifically
bind to the 3-untranslated region (3′-UTR) of their target genes,
resulting in translation inhibition or mRNA degradation. In this
manner, miRs are able to regulate the expression of multiple genes
(17,18). The abnormal expression of miRs has
been closely associated with the development and progression of
tumors. miRs have thus been shown to play pivotal roles in the
biological functions of cancer cells, such as differentiation, the
stress response, proliferation, apoptosis and sensitivity to
irradiation (19). Various miRs
of the miR-15/16 family, including miR-15a-3p, miR-16-1-3p and
miR-497, exhibit anticancer behaviors (20-26). For instance, miR-497 has been
shown to suppress the malignant behavior of papillary thyroid
cancer cells by reversely modulating the expression of Yes
associated protein 1 (YAP1) (22); it has also been shown to inhibit
the angiogenesis and metastasis of hepatocellular carcinoma by
impeding the actions of vascular endothelial growth factor A and
astrocyte elevated gene-1 (23).
Notably, miR-497 can influence the DNA damage response (DDR) and
facilitate the radiosensitivity of prostate cancer cells by
inhibiting the expression of Wee1-like protein kinase (24). Nonetheless, the exact role and
mechanisms of action of miR-497-5p in the radio-resistance of ESCC
cells remain elusive.
Cell division cycle 25A (CDC25A), a specific protein
phosphatase, is a key regulator of the cell cycle (27). The human CDC25A protein possesses
524 amino acid residues and has 2 distinctive regions; the
N-terminal domain and the C-terminal catalytic domain (28). CDC25A is also involved in
regulating the radiosensitivity of a variety of malignancies,
including prostate and colorectal cancer (29,30), although the CDC25A-associated
mechanisms of action in ESCC remain unclear.
LINC00473, miR-497-5p and CDC25A conduct crucial
roles in the development of malignancies, and bioinformatics
analyzes suggest that binding sites may exist between them. Based
on these predictions, the present study aimed to elucidate the
effectss of LINC00473 on the radiosensitivity of ESCC cells via
miR-497-3p/CDC25A, the results of which may aid in the optimization
of radiotherapeutic regimens for patients with ESCC.
Materials and methods
Tissue collection
All patients provided written informed consent for
the collection and use of their tissue samples. The present study
was approved by the Ethics Review Committee of the Third People's
Hospital of Linyi (Linyi, China). In total, 46 pairs of ESCC and
adjacent-normal tissues were collected from the Department of
Oncology at the Third People's Hospital of Linyi between April,
2016 and March, 2018. All tumors were pathologically confirmed as
ESCC, and no other malignant tumors were identified. None of the
patients had received neoadjuvant therapy (chemotherapy or
radiotherapy) prior to surgery.
Bioinformatics prediction analysis
The StarBase database (starBase, v2.0, http://starbase.sysu.edu.cn/) was employed to identify
potential miR-lncRNA binding partners. TargetScan (http://www.targetscan.org/vert_72/) was then used
to determine the downstream target genes of these miRs.
Cells and cell culture
Human ESCC cell lines (KYSE-30, KYSE-180, KYSE-150
and TE-5) and a normal esophageal epithelial cell line (Het-1A)
were procured from the Shanghai Institute of Biochemistry and Cell
Biology. All the cell lines were cultured in DMEM (HyClone; GE
Healthcare life Sciences) containing 10% heat-inactivated fetal
bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin
(all Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C (5%
CO2). The media were replaced at 3-4-day intervals, and
the cells were sub-cultured using the trypsinization method (0.25%;
Amerecesco, Inc.).
Transfection
The empty pcDNA vector (NC), pcDNA-LINC00473
(LINC00473), short hairpin (sh)RNA negative control (sh-NC), shRNAs
targeting LINC00473 (sh-LINC00473), miR control (miR-NC),
miR-497-5p mimic and miR-497-5p inhibitor were designed and
constructed by Shanghai GenePharma Co., Ltd. KYSE-30 and TE-5 cells
were seeded into a 24-well plate (3×105 cells/well) and
cultured for 24 h at 37°C (5% CO2), prior to
transfection using Lipofectamine® 3000 (Thermo Fisher
Scientific, Inc.). All transfections were performed using a final
concentration of 60 nM of miR-497-5p mimics and 100 nM of
miR-497-5p inhibitor and sh-LINC00473. Reverse
transcription-quantitative (RT-q)PCR was conducted to confirm
transfection efficiency 24 h post-transfection, and the subsequent
experiments were then performed.
Ionizing irradiation
Transfected ESCC cells were harvested in the
logarithmic phase, and irradiated using a linear accelerator
(Varian Medical Systems) at room temperature. The cells were
exposed to various doses of radiation (0, 2, 4, 6 and 8 Gy) for
24-96 h prior to subsequent analysis. For the time-course
experiment, cells were irradiated with X-rays at a dose of 6 Gy,
and were then collected for immediate RT-qPCR analysis; RT-qPCR was
conducted every 3 h within a 24 h period.
RT-qPCR
Total RNA was isolated from the ESCC tissues and
cultured cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.); 1 μg total RNA was reverse
transcribed into cDNA using the SuperScript™ First Strand cDNA
System (Invitrogen; Thermo Fisher Scientific, Inc.), and qPCR was
subsequently performed. SYBR-Green Premix Ex Taq II (Takara) and
ABI 7500 real-time PCR system (Applied Biosystems) was applied for
qPCR. The qPCR cycling conditions were as follows: 95°C for 2 min,
then 40 cycles at the conditions of 95°C for 15 sec, 60°C for 15
sec, and 68°C for 20 sec. The relative expression levels of
LINC00473 and miR-497-5p were calculated using the
2−ΔΔCq method (31).
The primers sequences were as follows: LINC00473 forward,
5′-GATGGAAAGGAGGGAAGG-3′ and reverse, 5′-CACAGTGGGTCCAGGGTT-3′;
miR-497-5p forward, 5′-CCTTCAGCAGCACACTGTGG-3′ and reverse,
5′-CAGTGCAGGGTCCGAGGTAT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and β-actin forward;
5′-TTCGAGCAAGAGATGGCCA-3′ and reverse, 5′-TACATGGTGGTGCCGCC-3′.
Western blot analysis
The ESCC tissues and cells were lysed with RIPA
buffer (Beyotime Institute of Biotechnology) supplemented with
protease inhibitors. Following high-speed centrifugation (10,000 ×
g, 4°C, 5 min), the supernatants were collected and the proteins
were denatured in a water bath at 100°C for 10 min; the protein
concentrations were determined using the bicinchoninic acid method.
A total of 10 μl total protein extracts were loaded into
each well, and were separated by SDS-PAGE using 12% gels, and then
transferred to polyvinylidene fluoride membranes. The membrane was
washed once in PBS with 0.2% Tween-20 (PBST) and then blocked with
5% non-fat milk for 1 h at room temperature. After washing in TBST
solution, the membranes were incubated overnight with primary
antibodies against CDC25A (cat. no. ab79252; Abcam, 1:1,000) and
β-actin (cat. no. ab20272; Abcam; 1:100) on a shaking platform at
4°C. The membranes were then rinsed with TBS-T solution and
incubated with goat anti-rabbit IgG H&L (cat. no. ab150077;
Abcam; 1:1,000) at room temperature for 1 h. Following a final wash
with TBS-T (3 times), signals were developed with Hypersensitive
ECL Chemiluminescent Substrate (Hubei Biossci Biotechnology Co,
Ltd.) and exposed to X-ray films.
Cell counting kit-8 (CCK-8) assay
The KYSE-30 and TE-5 cells were harvested in the
logarithmic phase and adjusted to a density of 1×104/ml;
100 μl cell suspension was then inoculated into the wells of
a 96-well plate as appropriate, and cultured at 37°C for 24 h.
Subsequently, 10 μl enhanced CCK-8 solution (Hubei Biossci
Biotechnology Co, Ltd.) was added and the plate was returned to the
incubator for a further 1 h. The absorbance (OD value) of each well
was determined at 450 nm using a plate reader (Multiscan FC; Thermo
Fisher Scientific, Inc.) at 24, 48, 72 and 96 h.
Colony formation assay
Transfected ESCC cells were seeded into 6-well
plates at a density of 1×103 cells/well and irradiated
at a specified single dose (0, 2, 4, 6 or 8 Gy). The culture medium
was then discarded and the cells were carefully washed twice with
PBS. Following incubation at 37°C (5% CO2) for 2 weeks,
the cells were fixed with 10% methanol, stained with 0.1% crystal
violet (Sigma-Aldrich; Merck KGaA) for 15 min and dried at room
temperature. Subsequently, the number of colonies was recorded
under a light microscope (Olympus Corporation).
Dual-luciferase reporter gene assay
Wild-type (WT) or mutant-type (MUT) LINC00473 was
subcloned into a pGL3 Basic vector (Promega Corporation). The cells
were then seeded into a 24-well plate at a density of
5×103 cells/well, and the Dual-Luciferase®
Reporter Assay System (Promega Corporation) was used to determine
luciferase activity. miR-497-5p or miR-NC were co-transfected with
WT or MUT reporter vectors into KYSE-30 and TE-5 cells,
respectively, using Lipofectamine® 3000. Luciferase
activity was assessed at 48 h post-transfection. The results were
normalized through comparison with Renilla luciferase
activity.
Statistical analysis
All statistical analyzes were conducted using SPSS
20.0 (IBM Corp.), and all data are presented as the means ±
standard deviation. Whether the data are normally distributed or
not was examined using the Kolmogorov-Smirnov test. For normally
distributed data, an unpaired or paired t-test was used to compare
the data between 2 groups. Comparisons among ≥3 groups were
conducted with one-way ANOVA. If the data exhibited significant
differences, Tukey's post hoc test was then performed to compare
the data between groups. For data that were not normally
distributed, comparisons between 2 groups were performed by a
paired sample Wilcoxon signed-rank test. Pearson's correlation
coefficient was used to evaluate the correlation between the
expression levels of the genes in the ESCC samples. A Chi-squared
test was used to analyze the association between the expression of
LINC00473 and the patient clinicopathological characteristics.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association between the expression levels
of LINC00473, miR-497-5p and CDC25A in ESCC tissues
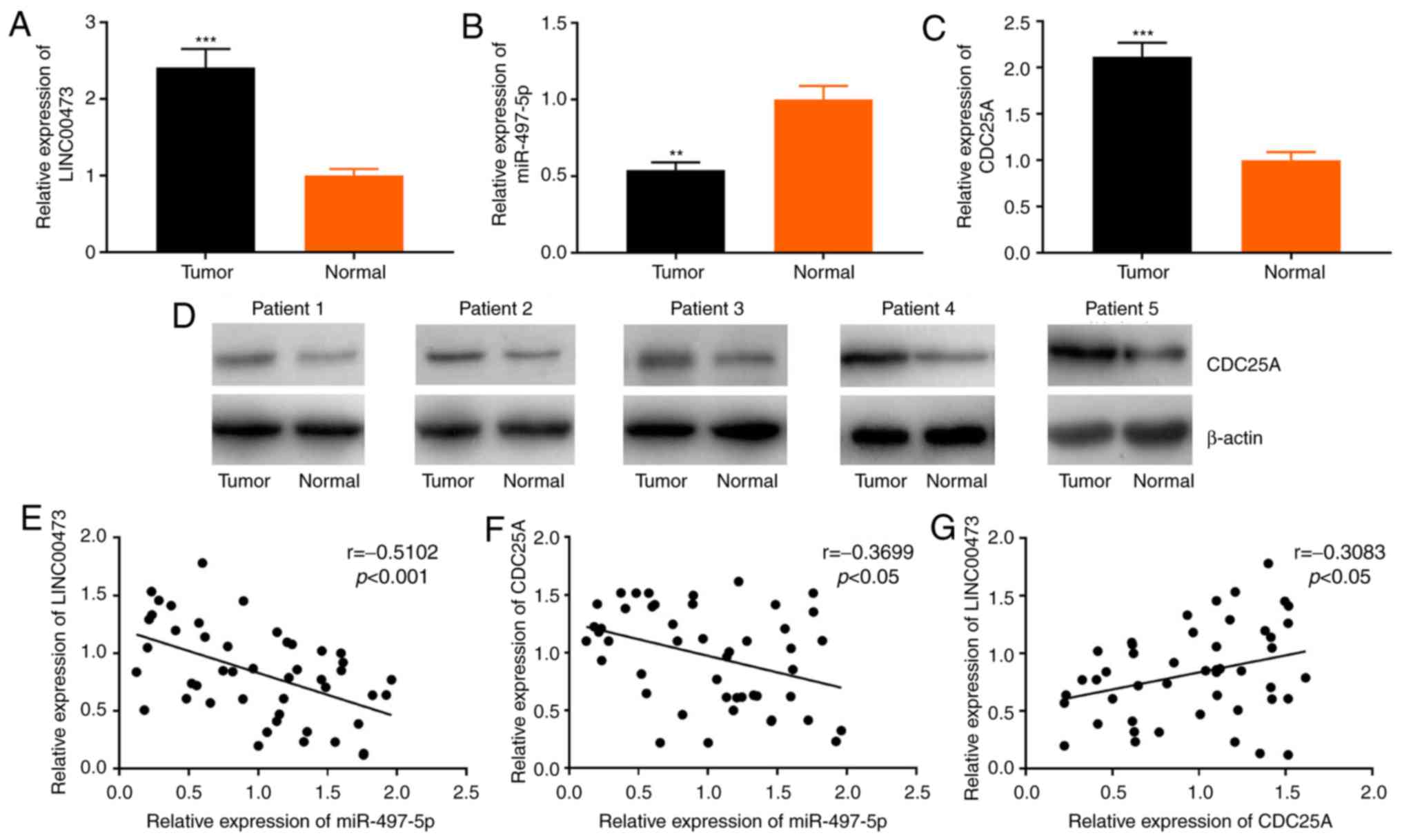
RT-qPCR was conducted to determine the association
between the expression levels of LINC00473, miR-497-5p and CDC25A
mRNA in 46 paired ESCC and adjacent-normal tissue samples. The
expression levels of LINC00473 and CDC25A mRNA were significantly
higher, while miR-497-5p expression was significantly lower in ESCC
tissues, compared with those in the adjacent normal tissues
(Fig. 1A-C). Additionally,
western blot analysis was used to assess CDC25A protein expression
in the ESCC and adjacent tissues of 5 randomly selected patient
samples; the results indicated that CDC25A was also upregulated at
the protein level (Fig. 1D).
LINC00473 expression was also found to inversely correlate with
that of miR-497-5p (Fig. 1E;
r=-0.5102, P<0.001). Moreover, miR-497-5p expression negatively
correlated with CDC25A expression (Fig. 1F; r=−0.3699, P<0.05), while
LINC00473 expression positively correlated with CDC25A expression
(Fig. 1G; r=0.3083, P<0.05).
These data suggest a possible regulatory association among
LINC00473, miR-497-5p and CDC25A.
LINC00473 expression is associated with
multiple patho- logical indicators in patients with ESCC
The association between the expression of LINC00473
and the clinicopathological indexes of patients with ESCC was also
analyzed. High expression levels of LINC00473 in tumor tissues were
found to be significantly associated with local lymph node
metastasis, a low degree of differentiation and a higher T stage in
patients with ESCC; however, they were not associated with sex,
age, tumor size or a history of smoking (Table I). These findings suggest that
high expression of LINC00473 may be involved in the progression of
ESCC.
 | Table IAssociations between LINC00473
expression and the clinicopathological characteristics of patients
with esophageal squamous cell cancer. |
Table I
Associations between LINC00473
expression and the clinicopathological characteristics of patients
with esophageal squamous cell cancer.
| Clinicopathological
indicators | No. of
patients | Relative expression
level of LINC00473
| P-value |
|---|
| High | Low |
|---|
| All cases | 46 | 24 | 22 | |
| Age, years | | | | |
| ≥60 | 35 | 19 | 16 | 0.609 |
| <60 | 11 | 5 | 6 | |
| Sex | | | | |
| Male | 34 | 16 | 18 | 0.242 |
| Female | 12 | 8 | 4 | |
| Smoking | | | | |
| Smoker | 29 | 14 | 15 | 0.489 |
| Never smoked | 17 | 10 | 7 | |
| Tumor size
(d/cm) | | | | |
| >3 cm | 25 | 15 | 10 | 0.246 |
| ≥3 cm | 21 | 9 | 12 | |
| T
classification | | | | |
| T1 + T2 | 20 | 7 | 13 | 0.041 |
| T3 + T4 | 26 | 17 | 9 | |
| Lymph node
metastasis | | | | |
| N0-N1 | 17 | 5 | 12 | 0.018 |
| N2-N3 | 29 | 19 | 10 | |
| Tumor
differentiation | | | | |
| Poor/medium | 24 | 16 | 8 | 0.039 |
| High | 22 | 8 | 14 | |
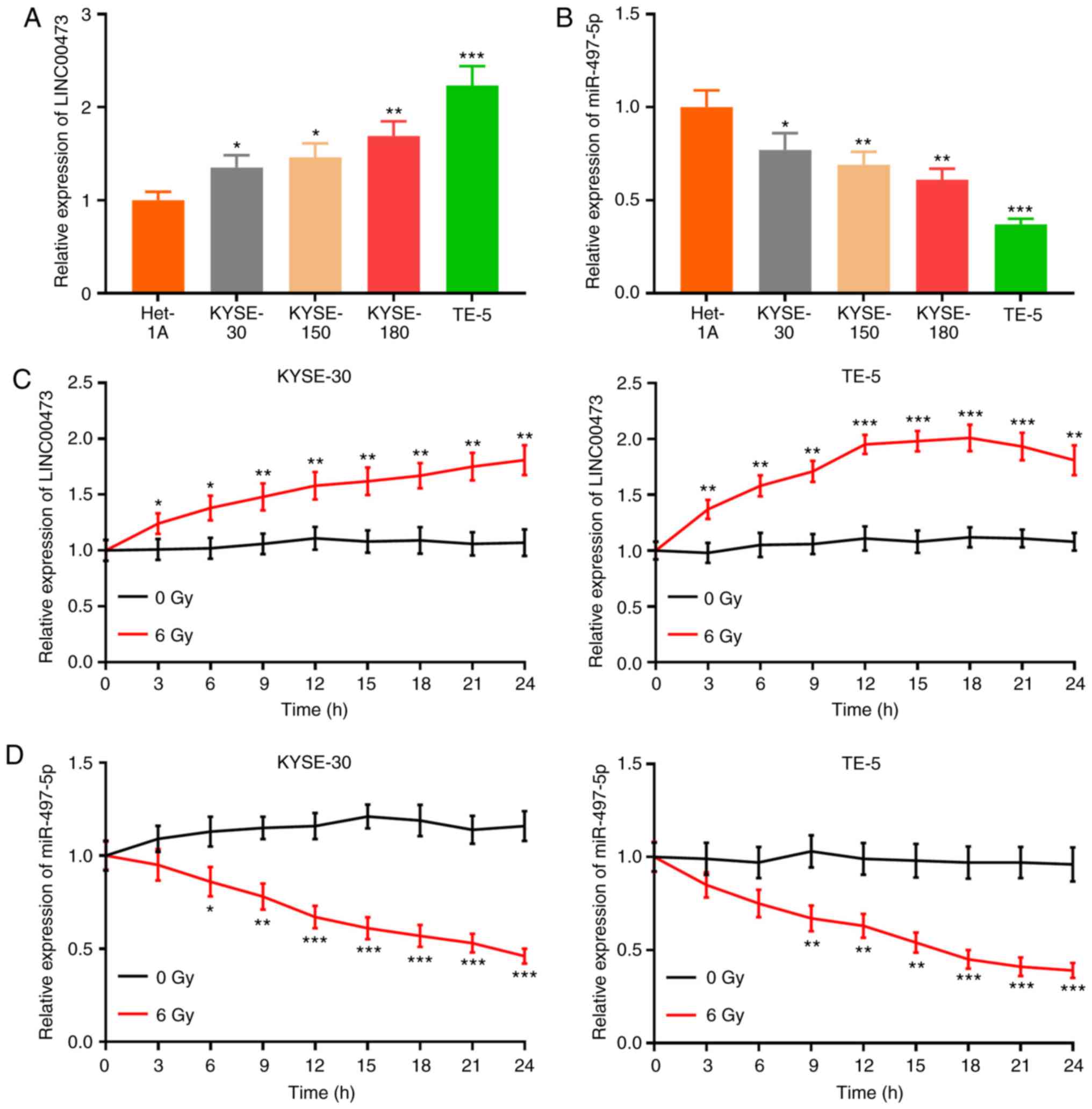
LINC00473 expression is increased and
miR-497-5p expression is decreased in ESCC cells exposed to X-ray
radiation
RT-qPCR was used to quantify the expression levels
of LINC00473 and miR-497-5p in 4 ESCC cell lines (KYSE-30,
KYSE-150, KYSE-180 and TE-5). Compared with Het-1A cells, the
expression of LINC00473 was significantly higher in the ESCC cell
lines, while the expression of miR-497-5p was notably decreased
(Fig. 2A and B). The KYSE-30 and
TE-5 cells were irradiated with 6 Gy X-rays, and the expression of
LINC00473 was detected every 3 h by RT-qPCR. The results revealed
that the expression of LINC00473 in the irradiated KYSE-30 and TE-5
cells was significantly higher than that in the control group (0
Gy) (Fig. 2C and D), and that
following irradiation, the expression of miR-497-5p was markedly
suppressed (Fig. 2E and F). These
results indicated that the expression of LINC00473 and miR-497-5p
was inversely affected by X-ray irradiation.
LINC00473 plays a crucial role in
reducing the radiosensitivity of ESCC cells
The results of the aforementioned experiments
illustrated that the expression of LINC00473 was upregulated in the
irradiated ESCC cells. The expression level of LINC00473 was lowest
in the KYSE-30 cells, and highest in the TE-5 cells; therefore, to
further investigate the role of LINC00473 in the radiosensi-tivity
of ESCC cells, KYSE-30 and TE-5 cells were selected to construct
LINC00473 overexpression and knockdown models, respectively. The
KYSE-30 cells were transfected with a LINC00473 overexpression
plasmid, and the TE-5 cells with LINC00473 shRNA; RT-qPCR indicated
the successful establishment of each model (Fig. 3A). CCK-8 assay was subsequently
performed to assess the proliferative capacity of each cell line;
the results revealed that LINC00473 over-expression significantly
increased KYSE-30 cell proliferation at 48, 72 and 96 h, while
LINC00473 knockdown inhibited the proliferation of TE-5 cells,
demonstrating that LINC00473 enhanced the proliferative ability of
the ESCC cells (Fig. 3B). A
colony formation assay was then performed, and the results
indicated that LINC00473 overexpression and knockdown increased and
decreased the number of colony-forming units, respectively
(Fig. 3C). Collectively, it was
thus concluded that LINC00473 enhances the proliferative ability
and reduces the sensitivity of ESCC cells to irradiation.
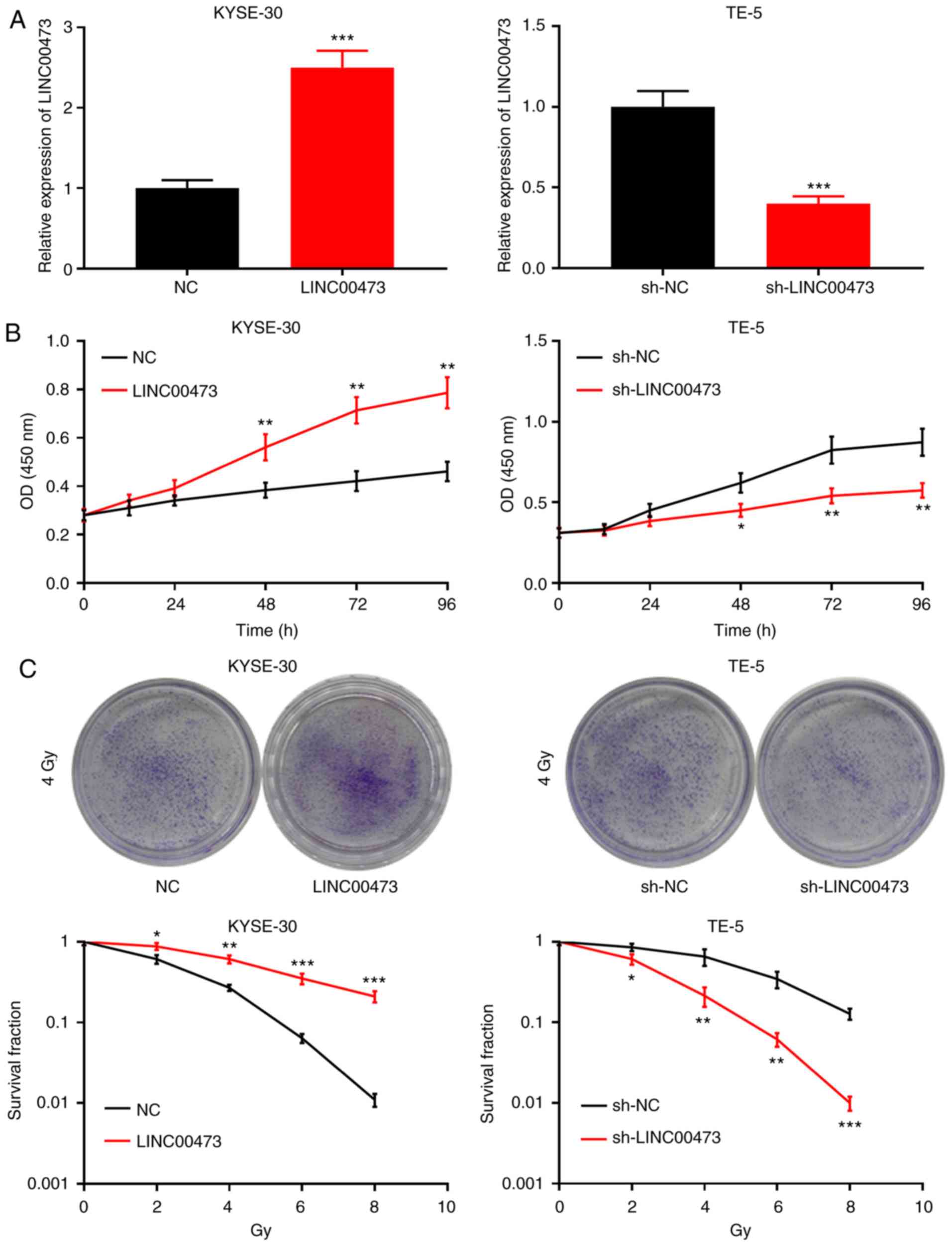 | Figure 3LINC00473 enhances the viability of
esophageal squamous cell cancer cells and promotes resistance to
radiotherapy. (A) KYSE-30 cells were transfected with pcDNA-NC and
pcDNA-LINC00473, and TE-5 cells were transfected with sh-NC and
sh-LINC00473. RT-qPCR was conducted to confirm transfection
efficiency. (B) KYSE-30 and TE-5 cell viability was assessed using
the Cell Counting kit-8 assay at 0, 24, 48 and 96 h. (C) Following
X-ray irradiation (0, 2, 4, 6 and 8 Gy), the colony number was
counted using a colony formation assay. *P<0.05,
**P<0.01 and ***P<0.001 vs. NC. sh,
short hairpin (RNA); NC, negative control. |
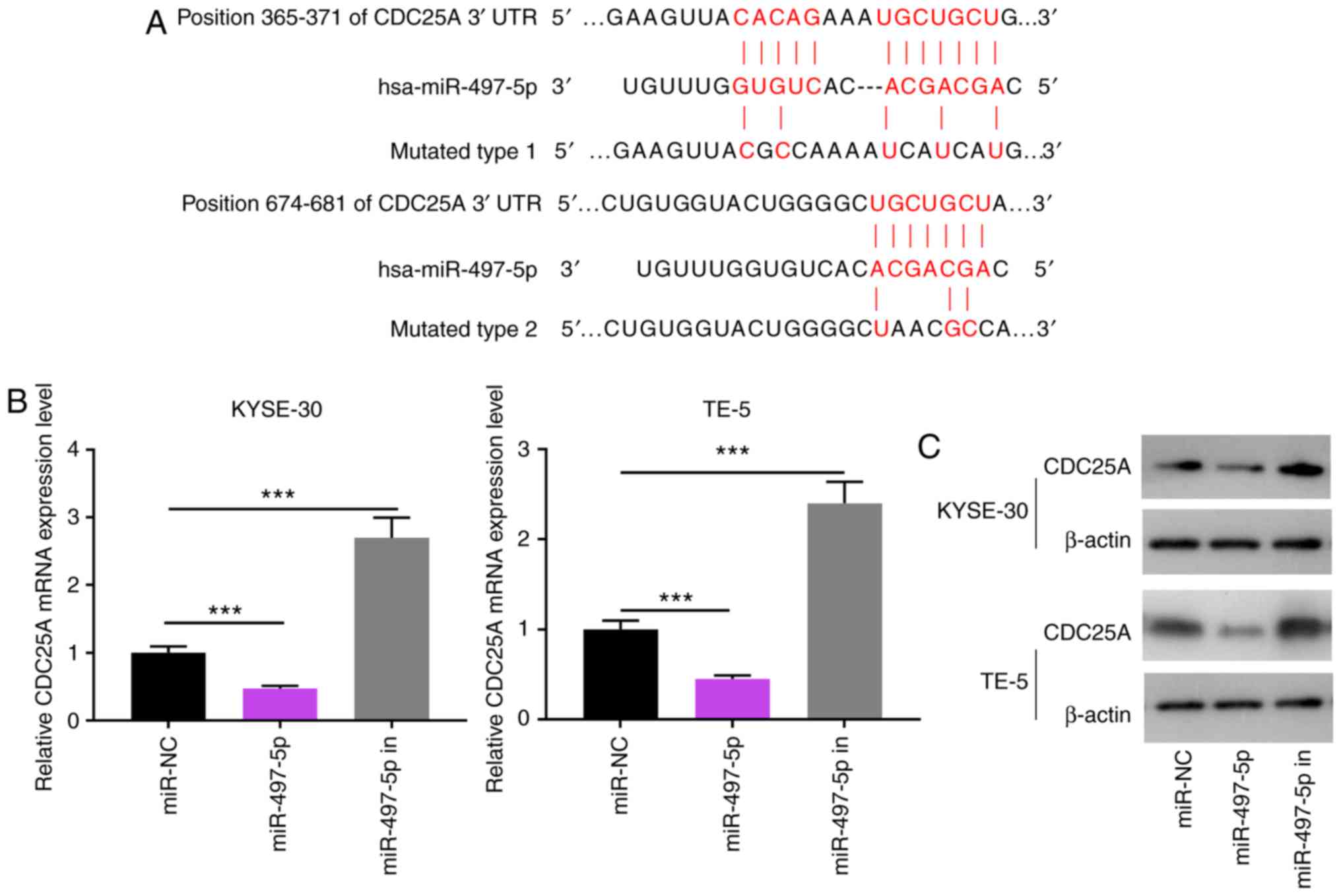
LINC00473 directly interacts with and
negatively regulates the expression of miR-497-5p
Bioinformatics analysis (starBase, v2.0, http://starbase.sysu.edu.cn/) predicted miR-497-5p to
be a candidate target of LINC00473, and the potential binding site
is illustrated in Fig. 4A. Using
a dual-luciferase assay, following the transfection of miR-497
mimics into ESCC cells (Figs. S1
and S2), miR-497-5p
overexpression was shown to markedly decrease the luciferase
activity of pGL3-LINC00473-WT, whereas it did not significantly
affect that of pGL3-LINC00473-MUT (Fig. 4B-D). The results suggested that of
the three predicted binding fragments, the first and third had
similar binding capacities, and the second exhibited the most
statistically significant difference (Fig. 4B-D). In addition, the KYSE-30 and
TE-5 cells were transfected with pcDNA-LINC00473 or sh-LINC00473.
The results suggested that compared with the control group, the
expression of miR-497-5p was notably inhibited by LINC00473
overexpression and increased by LINC00473 knockdown, respectively
(Fig. 4E). Thus, LINC00473 can
directly interact with miR-497-5p and negatively regulate its
expression.
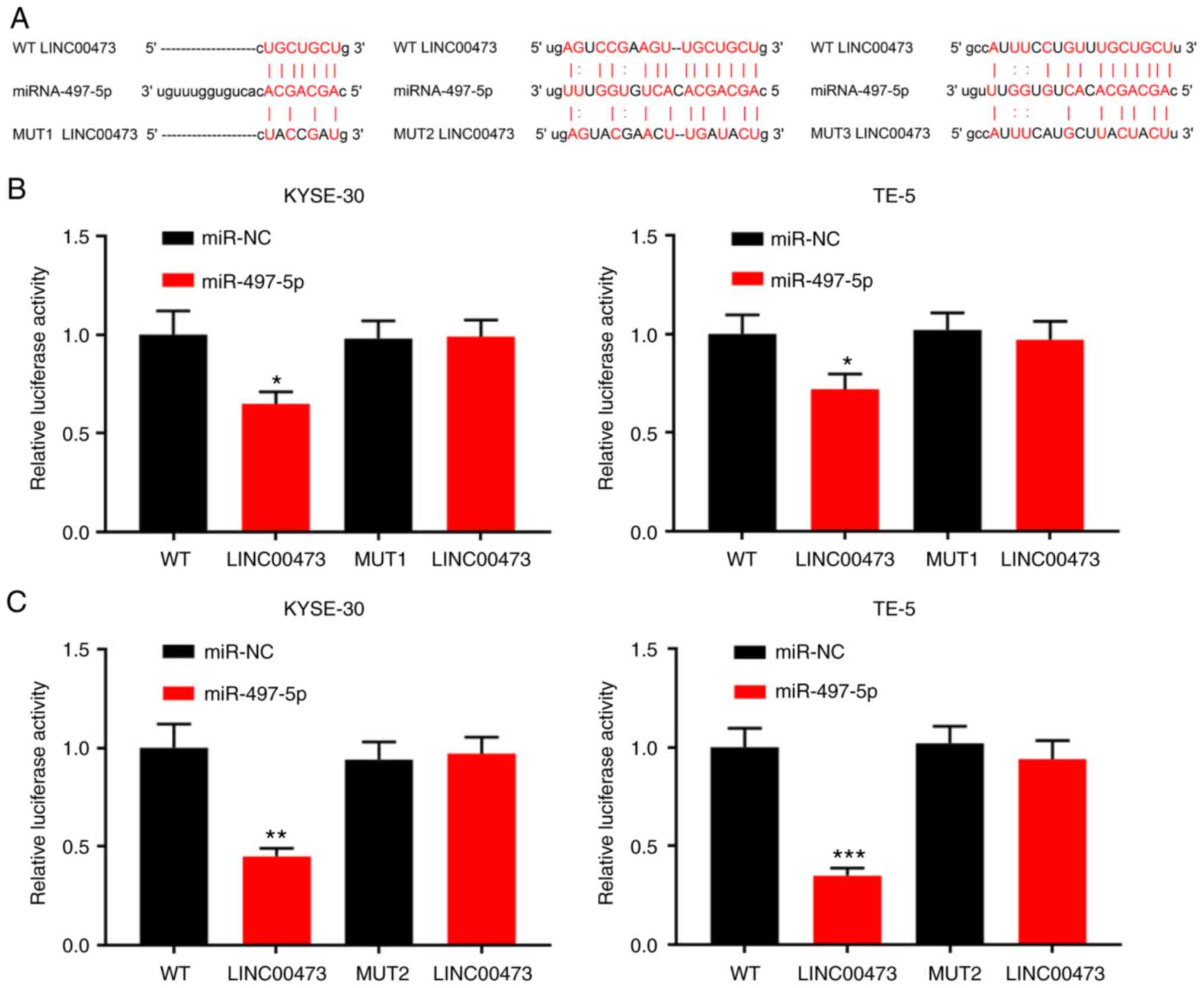 | Figure 4Interaction between LINC00473 and
miR-497-5p. (A) Binding sites between LINC00473 and miR-497-5p were
predicted by bioinformatics analysis. (B-D) KYSE-30 and TE-5 cells
were co-transfected with miR-NC or miR-497-5p and pGL3-LINC00473-WT
or pGL3-LINC00473-MUT, and luciferase activity was detected using a
luciferase reporter gene assay. Of the three predicted binding
fragments, the first and third had similar binding capacities, and
the second exhibited the most statistically significant difference.
(E) KYSE-30 and TE-5 cells were transfected with pcDNA-NC or
pcDNA-LINC00473, and sh-NC or sh-LINC00473, respectively, and the
expression of miR-497-5p was detected by RT-qPCR.
*P<0.05, **P<0.01 and
***P<0.001 vs. NC and sh-NC. miR, microRNA; sh, short
hairpin (RNA); NC, negative control; WT, wildtype; MUT, mutant. |
LINC00473 regulates the radiosensitivity
of ESCC cells via miR-497-5p
To investigate the potential mechanisms responsible
for the radiosensitivity in ESCC cells induced by LINC00473, the
KYSE-30 cells were transfected with pcDNA-NC, pcDNA-LINC00473 or
pcDNA-LINC00473-miR-497-5p mimics; TE-5 cells were concurrently
transfected with sh-NC, sh-LINC00473 or sh-LINC00473-miR-497-5p
inhibitors (Figs. S1 and
S2). The inhibitory effects of
LINC00473 knockdown on ESCC cell viability were weakened by
transfection with miR-497-5p inhibitors, while transfection with
miR-497-5p mimics attenuated the promoting effects of LINC00473 on
cell proliferation (Fig. 5A).
Moreover, transfection with miR-497-5p mimics was shown to
partially counteract the radioresistance of ESCC cells (2, 4, 6 and
8 Gy) induced by LINC00473, while transfection with miR-497-5p
inhibitors exerted the opposite effect (Fig. 5B). Collectively, these data
indicate that LINC00473 reduces the radiosensitivity of ESCC cells
via miR-497-5p.
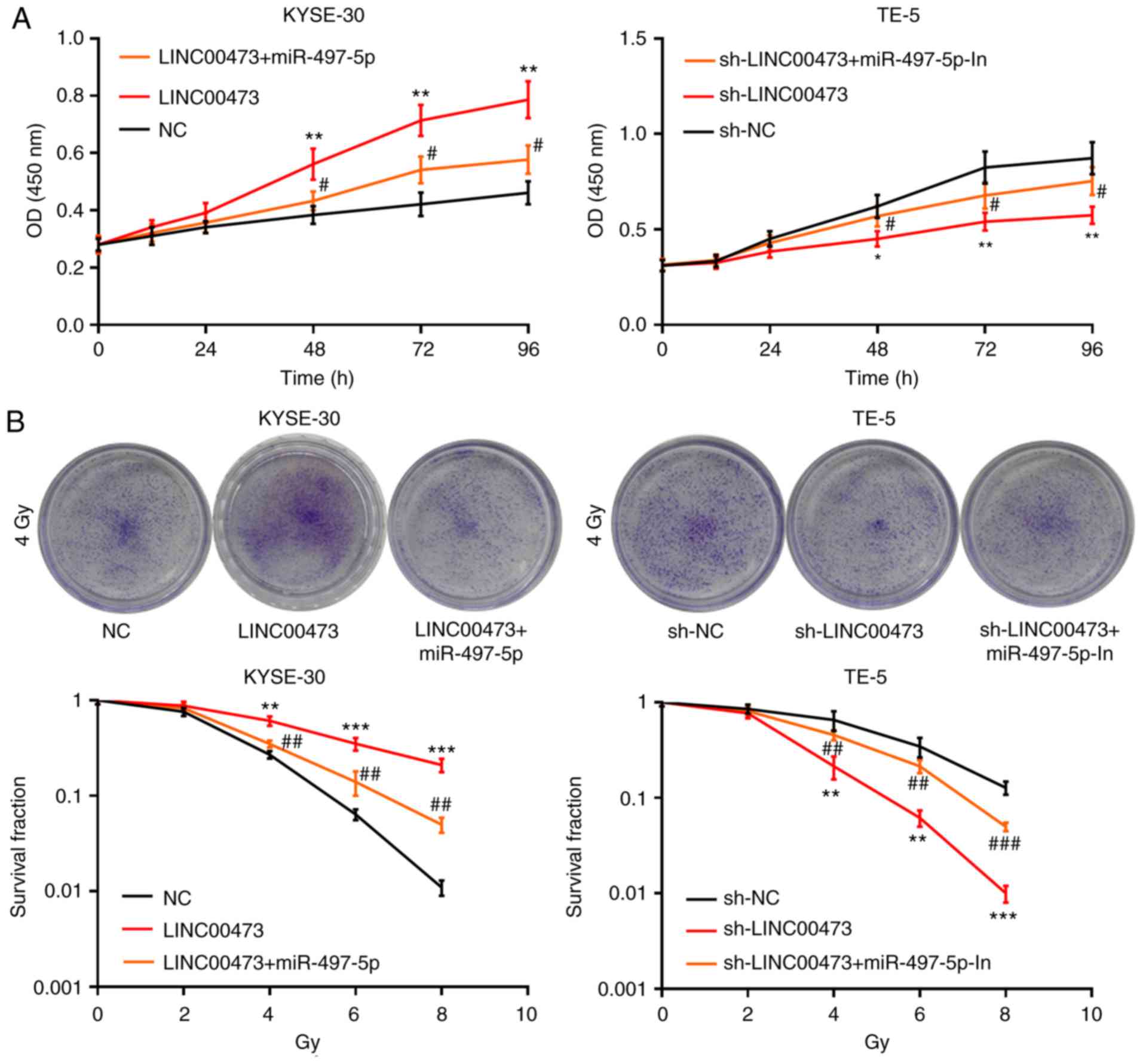 | Figure 5LINC00473 influences the viability
and radiosensitivity of esophageal squamous cell cancer cells by
regulating miR-497-5p. Following pcDNA-NC, pcDNA-LINC00473 or
pcDNA-LINC00473/miR-497-5p mimic transfection into KYSE-30 cells,
and sh-NC, sh-LINC00473 or sh-LINC00473/miR-497-5p inhibitor
transfection into TE-5 cells, cellular viability/proliferation was
detected using the Cell Counting Kit-8 assay (A). Following X-ray
irradiation (0, 2, 4, 6 and 8 Gy) for 2 weeks, the survival ratio
of colony-forming cells was detected using a colony formation assay
(B). *P<0.05, **P<0.01 and
***P<0.001, NC vs. LINC00473 and sh-NC vs.
sh-LINC0473. #P<0.05, ##P<0.01 and
###P<0.001, LINC00473 vs. LINC00473 + miR-497-5p and
sh-LINC00473 vs. sh-LINC00473 + miR-497-5p inhibitors. miR,
microRNA; NC, negative control; sh, short hairpin (RNA). |
miR-497-5p directly targets the 3′-UTR of
CDC25A
TargetScan predicted that CDC25A was one of the
candidate target genes for miR-497-5p, and the speculative binding
sites are displayed in Fig. 6A.
The results of RT-qPCR and western blot analysis revealed that the
mRNA and protein levels of CDC25A were notably decreased in the
KYSE-30 and TE-5 cells following transfection with miR-497-5p
mimics, respectively; on the other hand, miR-497-5p inhibitors
induced the upregulation of CDC25A expression in ESCC cells at both
the mRNA and protein levels (Fig. 6B
and C). The results of the luciferase reporter assay indicated
that miR-497-5p could specifically bind to the 3′UTR of CDC25A
(Fig. 6D). The results of western
blot analysis revealed that the expression of CDC25A was markedly
elevated in the KYSE-30 and TE-5 cells following LINC00473
overexpression, but was reduced after LINC00473 was knocked down
(Fig. 6E). Additionally,
LINC00473 overexpression reversed the inhibitory effect on CDC25A
expression induced by miR-497-5p (Fig. 6F). Furthermore, the expression
level of CDC25A was markedly upregulated in the ESCC cell lines,
compared with the Het-1A cells (Fig.
6G), and its expression in ESCC cells was induced by
irradiation (Fig. 6H).
Collectively, these data confirm that CDC25A is a downstream gene
of miR-497-5p, and that its expression is directly and inversely
regulated by miR-497-5p, though indirectly and positively modulated
by LINC00473.
Discussion
Radiotherapy is extensively used for the treatment
of advanced malignant tumors, including ESCC (32,33). Nevertheless, the radioresistance
of ESCC cells, which is caused by a series of factors, can result
in treatment failure (34).
Consistent with the findings of a previous study, the results of
the present study revealed significantly higher expression levels
of LINC00473 in ESCC tissues and cells, compared with
paired-adjacent tissues and normal esophageal epithelial cells,
respectively (16). In the
present study, a high LINC00473 expression in tumor tissues was
significantly associated with local lymph node metastasis, a low
degree of differentiation and a higher T stage in patients with
ESCC. Through further experimentation, LINC00473 was confirmed to
reduce the radiosensitivity of ESCC cells by modulating the
miR-497-5p/CDC25A axis.
lncRNAs are regarded as key regulators of the
proliferation, apoptosis, metastasis and radioresistance of
multiple types of tumor cells, including ESCC cells (6-8,35).
lncRNAs may act as potential indicators to monitor the progression
of malignant tumors (36); for
example, LINC00657 promotes the proliferation, migration and
invasion, but reduces the radiosensitivity of ESCC cells, and its
high expression level is associated with poor prognosis (35). LINC00473 promotes the progression
of pancreatic cancer by upregulating the expression of programmed
death-ligand 1 (37). LINC00473
knockdown has also been reported to induce the radiosensitization
of ECSS cells (16); this is
consistent with the results of the present study, which also
revealed that X-ray exposure significantly increased the expression
of LINC00473 in ESCC cells, compared with non-irradiated cells. A
CCK-8 assay also revealed that LINC00473 knockdown largely blocked
the viability of ESCC cells, while LINC00473 overexpression
enhanced proliferative capacity. Different doses of X-ray
irradiation also significantly increased the colony-forming
potential of cells overexpressing LINC00473, which was impeded by
LINC00473 knockdown. Taken together, these results indicate that
LINC00473 enhances the proliferative and colony-forming abilities
of ESCC cells, and induces radioresistance.
miRNAs are able to regulate the malignant phenotype
of human tumor cells, including their proliferation, invasiveness
and resistance to X-rays (17-19,38,39). A growing number of studies have
suggested that numerous members of the miR-15/16 family possess an
antitumor role in human malignant tumors (20,40). For example, miR-15b can enhance
the sensitivity of colorectal cancer cells to radiotherapy, which
can be used as a valuable marker for prognosis and treatment
outcome (41). It has been
demonstrated that miRNAs regulate sensitivity to radiotherapy
through the DNA injury mechanism (42). miRNAs are involved in DNA injury
in almost all cell types, including its detection, the transmission
of injury signals, DNA repair, cell cycle activation and inducing
apoptosis (43). This makes
miRNAs a potential target for the early detection of
radioresistance, which may improve the response of cells to
radiotherapy. miR-497 interferes with the cell cycle, DNA synthesis
and function, and activates DDR to induce apoptosis (44). In the present study, miR-497-5p
was expressed at low levels in ESCC tissues and cells; thus,
examining the potential mechanisms of miR-497-5p may provide
possible treatment options for patients with ESCC. Common binding
sites between LINC00473 and miR-497-5p were subsequently predicted
using an online bioinformatics database (StarBase, v2.0, http://starbase.sysu.edu.cn/), and luciferase reporter
gene assays confirmed that LINC00473 could sponge miR-497-5p. In
addition, LINC00473 overexpression was shown to inhibit the
expression of miR-497-5p, while LINC00473 knockdown increased the
expression level of miR-497-5p. The viability of ESCC cells was
also markedly reduced by the transfection of miR-497-5p mimics into
cells overexpressing LINC00473, while an miR-497-5p inhibitor
produced opposing effects. Furthermore, colony formation assays
revealed that miR-497-5p inhibition reverses the enhanced
radiosensitivity caused by LINC00473 knockdown. Thus, it can be
concluded that LINC00473 reduces the radiosensitivity of ESCC cells
by regulating the expression of miR-497-5p.
Previous studies have highlighted the significance
of CDC25A in numerous cellular behaviors, including sensitivity to
radiotherapy (45). For example,
miR-339-5p enhances ESCC radiosensitivity by inhibiting CDC25A
(46). In the present study,
binding sites between miR-497-5p and CDC25A were identified, and
CDC25A expression was markedly downregulated both at the miRNA and
protein levels in ESCC cells transfected with miR-497-5p mimics.
miR-497-5p also decreased the luciferase activity of WT CDC25A, but
not the mutant type. Furthermore, LINC00473 overexpression in ESCC
cells elevated the expression of CDC25A. Hence, CDC25A was
concluded to be directly and reversely regulated by miR-497-5p, as
well as positively and indirectly by LINC00473.
Although previous studies have reported that
LINC000473 in ESCC tissues and cells is significantly upregulated,
and the upregulation of LINC00473 indicated radioresistance and a
poor prognosis of patients with ESCC (16,47), the findings of the present study
highlight a unique lncRNA-regulated mechanism in ESCC, namely that
LINC00473 facilitates the radioresistance of ESCC cells through
miR-497-5p. These results provide a novel theoretical basis for the
diagnosis and treatment of ESCC. There are several limitations in
the present study. Firstly, the conclusion of the present study is
only based on in vitro experiments, and animal models are
required to further validate the results. Secondly, more data are
required to prove that in ESCC, miR-497-5p regulated the
radiosensitivity of cancer cells by suppressing CDC25A.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors' contributions
XW, WHL and YLW designed the present study. WHL,
HYQ, WQW and JX performed the experiments, and analyzed and
interpreted the experimental data. WHL, YLW, XW contributed to the
writing of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent for
the collection and use of their tissue samples. Tissues were
collected during surgery following approval from the Ethics Review
Committee of Third People's Hospital of Linyi (Linyi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang W, Wu D, He X, Hu X, Hu C, Shen Z,
Lin J, Pan Z, He Z, Lin H and Wang M: CCL18-induced HOTAIR
upregulation promotes malignant progression in esophageal squamous
cell carcinoma through the miR-130a-5p-ZEB1 axis. Cancer Lett.
460:18–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao J, Wang Y, Yang J, Zhang W, Meng K,
Sun Y, Li Y and He QY: RNF128 promotes invasion and metastasis via
the EGFR/MAPK/MMP-2 pathway in esophageal squamous cell carcinoma.
Cancers (Basel). 11. pp. E8402019, View Article : Google Scholar
|
|
4
|
Zhao XH, Wang D, Wang F and Zhu SC:
Comparison of the effect of postoperative radiotherapy with surgery
alone for esophagus squamous cell carcinoma patients: A
meta-analysis. Medicine (Baltimore). 97:e131682018. View Article : Google Scholar
|
|
5
|
Luo J, Wang W, Tang Y, Zhou D, Gao Y,
Zhang Q, Zhou X, Zhu H, Xing L and Yu J: mRNA and methylation
profiling of radioresistant esophageal cancer cells: The
involvement of Sall2 in acquired aggressive phenotypes. J Cancer.
8:646–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Sun X, Chi C, Liu Y, Lin C, Xie D,
Shen X and Lin X: Upregulation of long noncoding RNA LINC00152
promotes proliferation and metastasis of esophageal squamous cell
carcinoma. Cancer Manag Res. 11:4643–4654. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Li J, Li Y, Lu Z, Che Y, Mao S,
Lei Y, Zang R, Zheng S, Liu C, et al: Interferon-inducible lncRNA
IRF1-AS represses esophageal squamous cell carcinoma by promoting
interferon response. Cancer Lett. 459:86–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YY, Yan L, Yang S, Xu HN, Chen TT,
Dong ZY, Chen SL, Wang WR, Yang QL and Chen CJ: Long noncoding RNA
AC073284.4 suppresses epithelial-mesenchymal transition by sponging
miR-18b-5p in paclitaxel-resistant breast cancer cells. J Cell
Physiol. 234:23202–23215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu B, Cao W and Ma H: Knockdown of lncRNA
LSINCT5 suppresses growth and metastasis of human glioma cells via
up-regulating miR-451. Artif Cells Nanomed Biotechnol.
47:2507–2515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Ye Y, Chu J, Jia J, Qu Y, Sun T,
Yin H, Ming L, Wan J and He F: Long noncoding RNA FEZF1-AS1
promotes the motility of esophageal squamous cell carcinoma through
Wnt/β-catenin pathway. Cancer Manag Res. 11:4425–4435. 2019.
View Article : Google Scholar :
|
|
12
|
Huang W, Zhou R, Mao L, Deng C and Dang X:
Esophageal cancer related gene-4 inhibits the migration and
proliferation of oral squamous cell carcinoma through BC200
lncRNA/MMP-9 and -13 signaling pathway. Cell Signal. 62:1093272019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang XZ, He QJ, Cheng TT, Chi J, Lei ZY,
Tang Z, Liao QX, Zhang H, Zeng LS and Cui SZ: Predictive value of
LINC01133 for unfavorable prognosis was impacted by alcohol in
esophageal squamous cell carcinoma. Cell Physiol Biochem.
48:251–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song W and Zou SB: Prognostic role of
lncRNA HOTAIR in esophageal squamous cell carcinoma. Clin Chim
Acta. 463:169–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen M, Liu P, Chen Y, Chen Z, Shen M, Liu
X, Li X, Li A, Lin Y, Yang R, et al: Long noncoding RNA FAM201A
mediates the radiosensitivity of esophageal squamous cell cancer by
regulating ATM and mTOR expression via miR-101. Front Genet.
9:6112018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, Zhang Y, Wang H, Pan T, Zhang Y
and Li C: LINC00473/miR-374a-5p regulates esophageal squamous cell
carcinoma via targeting SPIN1 to weaken the effect of radiotherapy.
J Cell Biochem. 120:14562–14572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ruggieri V, Russi S, Zoppoli P, La Rocca
F, Angrisano T, Falco G, Calice G and Laurino S: The role of
MicroRNAs in the regulation of gastric cancer stem cells: A
meta-analysis of the current status. J Clin Med. 8:E6392019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rong D, Lu C, Zhang B, Fu K, Zhao S, Tang
W and Cao H: CircPSMC3 suppresses the proliferation and metastasis
of gastric cancer by acting as a competitive endogenous RNA through
sponging miR-296-5p. Mol Cancer. 18:252019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Wang S, Wang H, Cao J, Huang X,
Chen Z, Xu P, Sun G, Xu J, Lv J and Xu Z: Circular RNA circNRIP1
acts as a microRNA-149-5p sponge to promote gastric cancer
progression via the AKT1/mTOR pathway. Mol Cancer. 18:202019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang T, Hou J, Li Z, Zheng Z, Wei J, Song
D, Hu T, Wu Q, Yang JY and Cai JC: miR-15a-3p and miR-16-1-3p
negatively regulate Twist1 to repress gastric cancer cell invasion
and metastasis. Int J Biol Sci. 13:122–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gajera M, Desai N, Suzuki A, Li A, Zhang
M, Jun G, Jia P, Zhao Z and Iwata J: MicroRNA-655-3p and
microRNA-497-5p inhibit cell proliferation in cultured human lip
cells through the regulation of genes related to human cleft lip.
BMC Med Genomics. 12:702019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng H, Dong H, Feng J, Tian H, Zhang H
and Xu L: miR-497 inhibited proliferation, migration and invasion
of thyroid papillary carcinoma cells by negatively regulating YAP1
expression. Onco Targets Ther. 11:4711–4721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: MiR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatano K, Kumar B, Zhang Y, Coulter JB,
Hedayati M, Mears B, Ni X, Kudrolli TA, Chowdhury WH, Rodriguez R,
et al: A functional screen identifies miRNAs that inhibit DNA
repair and sensitize prostate cancer cells to ionizing radiation.
Nucleic Acids Res. 43:4075–4086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Ye Z, Mei D, Gu H and Zhang J:
Long noncoding RNA DLX6-AS1 promotes tumorigenesis by modulating
miR-497-5p/FZD4/FZD6/Wnt/β-catenin pathway in pancreatic cancer.
Cancer Manag Res. 11:4209–4221. 2019. View Article : Google Scholar :
|
|
26
|
Huang X, Wang L, Liu W and Li F:
MicroRNA-497-5p inhibits proliferation and invasion of non-small
cell lung cancer by regulating FGF2. Oncol Lett. 17:3425–3431.
2019.PubMed/NCBI
|
|
27
|
Shen T and Huang S: The role of Cdc25A in
the regulation of cell proliferation and apoptosis. Anticancer
Agents Med Chem. 12:631–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Jiang M, Cui M, Dong J, Li Y, Xiao H
and Fan S: MiR-365 enhances the radiosensitivity of non-small cell
lung cancer cells through targeting CDC25A. Biochem Biophys Res
Commun. 512:392–398. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao A, Zhao Q, Zhou X, Sun C, Si J, Zhou
R, Gan L and Zhang H: MicroRNA-449a enhances radiosensitivity by
downregulation of c-Myc in prostate cancer cells. Sci Rep.
6:273462016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang MY, Wang JY, Chang HJ, Kuo CW, Tok
TS and Lin SR: CDC25A, VAV1, TP73, BRCA1 and ZAP70 gene
overexpression correlates with radiation response in colorectal
cancer. Oncol Rep. 25:1297–1306. 2011.PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Chen NB, Qiu B, Zhang J, Qiang MY, Zhu YJ,
Wang B, Guo JY, Cai LZ, Huang SM, Liu MZ, et al:
Intensity-modulated radiotherapy versus three-dimensional conformal
radiotherapy in definitive chemoradiotherapy for cervical
esophageal squamous cell carcinoma: Comparison of survival outcomes
and toxicities. Cancer Res Treat. 52:31–40. 2020. View Article : Google Scholar :
|
|
33
|
Zhao Y, Yi J, Tao L, Huang G, Chu X, Song
H and Chen L: Wnt signaling induces radioresistance through
upregulating HMGB1 in esophageal squamous cell carcinoma. Cell
Death Dis. 9:4332018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li C, Wang X, Wang X, Han C, Wang P, Pang
Q, Chen J, Sun X, Wang L, Zhang W, et al: A multicenter phase III
study comparing Simultaneous Integrated Boost (SIB) radiotherapy
concurrent and consolidated with S-1 versus SIB alone in elderly
patients with esophageal and esophagogastric cancer-the 3JECROG
P-01 study protocol. BMC Cancer. 19:3972019. View Article : Google Scholar
|
|
35
|
Sun Y, Wang J, Pan S, Yang T, Sun X, Wang
Y, Shi X, Zhao X, Guo J and Zhang X: LINC00657 played oncogenic
roles in esophageal squamous cell carcinoma by targeting miR-615-3p
and JunB. Biomed Pharmacother. 108:316–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tong YS, Wang XW, Zhou XL, Liu ZH, Yang
TX, Shi WH, Xie HW, Lv J, Wu QQ and Cao XF: Identification of the
long non-coding RNA POU3F3 in plasma as a novel biomarker for
diagnosis of esophageal squamous cell carcinoma. Mol Cancer.
14:32015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou WY, Zhang MM, Liu C, Kang Y, Wang JO
and Yang XH: Long noncoding RNA LINC00473 drives the progression of
pancreatic cancer via upregulating programmed death-ligand 1 by
sponging microRNA-195-5p. J Cell Physiol. 234:23176–23189. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH
and Sun XC: The mechanisms of radioresistance in esophageal
squamous cell carcinoma and current strategies in radiosensitivity.
J Thorac Dis. 9:849–859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong S, Yin H, Dong C, Sun K, Lv P, Meng
W, Ming L and He F: Predictive value of plasma MicroRNA-216a/b in
the diagnosis of esophageal squamous cell carcinoma. Dis Markers.
2016:18570672016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
You C, Liang H, Sun W, Li J, Liu Y, Fan Q,
Zhang H, Yue X, Li J, Chen X and Ba Y: Deregulation of the
miR-16-KRAS axis promotes colorectal cancer. Sci Rep. 6:374592016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhan D, Ji T, Li M, Yao Y, Jia J, Yi H,
Qiao M, Xia J, Zhang Z, Ding H, et al: Enhancement of sensitivity
to chemo/radiation therapy by using miR-15b against DCLK1 in
colorectal cancer. Stem Cell Reports. 11:1506–1522. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu H and Gatti RA: MicroRNAs: New players
in the DNA damage response. J Mol Cell Biol. 3:151–158. 2011.
View Article : Google Scholar :
|
|
43
|
Zhao L, Lu X and Cao Y: MicroRNA and
signal transduction pathways in tumor radiation response. Cell
Signal. 25:1625–1634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Soriano A, Paris-Coderch L, Jubierre L,
Martínez A, Zhou X, Piskareva O, Bray I, Vidal I, Almazán-Moga A,
Molist C, et al: MicroRNA-497 impairs the growth of chemoresistant
neuroblastoma cells by targeting cell cycle, survival and vascular
permeability genes. Oncotarget. 7:9271–9287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nishioka K, Doki Y, Shiozaki H, Yamamoto
H, Tamura S, Yasuda T, Fujiwara Y, Yano M, Miyata H, Kishi K, et
al: Clinical significance of CDC25A and CDC25B expression in
squamous cell carcinomas of the oesophagus. Br J Cancer.
85:412–421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo A, Zhou X, Shi X, Zhao Y, Men Y, Chang
X, Chen H, Ding F, Li Y, Su D, et al: Exosome-derived miR-339-5p
mediates radio-sensitivity by targeting Cdc25A in locally advanced
esophageal squamous cell carcinoma. Oncogene. 38:4990–5006. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He Z: LINC00473/miR-497-5p regulates
esophageal squamous cell carcinoma progression through targeting
PRKAA1. Cancer Biother Radiopharm. 34:650–659. 2019. View Article : Google Scholar : PubMed/NCBI
|